Abstract
Background:
Several bone graft materials are popularized in the treatment of intrabony defects. Demineralized freeze-dried bone allograft (DFDBA) is widely used in the treatment of intrabony defects. Platelet-rich fibrin (PRF) is autologous blood preparation which helps in wound healing and regeneration. Hence, this study focuses on evaluation of PRF, DFDBA, and their combination in the regeneration of intrabony defects.
Materials and Methods:
A total of 39 sites with intrabony defects were randomly assigned into three groups: (Group I - Open flap debridement, Group II - DFDBA alone, and Group III- DFDBA + PRF). Parameters such as probing pocket depth (PPD), relative attachment level (RAL), and radiographic bone fill were measured at baseline, 3 months, and 6 months. Intragroup comparison at various study intervals was made using one-way ANOVA test. Intergroup comparison was made using Tukey's multiple post hoc test.
Results:
Reduction in the PPD and greater difference in RAL was observed over the study period in all the three groups with greater reduction in DFDBA + PRF group. Reduction in the radiographic defect depths was observed over the study period in all the three groups with the greatest reduction of 38.99% in the DFDBA + PRF group. However, no statistically significant difference was reported by DFDBA versus DFDBA + PRF group.
Conclusion:
Combination of DFDBA and PRF improved the clinical and radiographic parameters compared to PRF and DFDBA alone. PRF was combined with DFDBA to produce a synergistic effect for treating intrabony defects in chronic periodontitis patients.
Key words: Demineralized freeze-dried bone allograft, intrabony defects, periodontal surgery, platelet-rich fibrin
INTRODUCTION
Chronic periodontitis is an infectious disease, resulting in inflammation within the supporting tissues of the teeth which lead to periodontal pocket deepening, loss of attachment and bone. The osseous defects occurring as a result of periodontitis can be treated employing various regeneration methods. Periodontal reconstruction surgery uses a combination of a variety of biologic agents and methods,[1] which enhance the success of regeneration by helping in cell migration, adherence, growth, and differentiation.[2] Autogenous bone, bone substitutes play vital role in regeneration.[3] The autogenous bone grafting, though considered “gold standard” in bone grafting procedures has certain limitations such as, limited availability and complications pertaining to donor site. Such limitations with regard to the autogenous bone grafts encouraged the use of bone graft substitutes in periodontal regeneration.[1] Demineralized freeze-dried bone allograft (DFDBA) is an allograft that exhibits osteoinductive property because of bone morphogenetic proteins (BMPs-2, 4, 7).[4] Platelet-rich fibrin (PRF) is an autologous platelet concentrate possessing higher amounts of growth factors (GFs) that aid in regeneration.[1] It consists of different GFs, leukocytic cells, and their cytokines (interleukins-1,64 and tumor necrosis factor-α). Studies reported that PRF as membrane alone or combination with bone graft materials enhances regeneration.[5] Hence, the present study was designed to compare the efficacy of PRF alone or as an adjunct to DFDBA grafting in the treatment of intrabony defects in chronic periodontitis patients.
MATERIALS AND METHODS
A randomized, controlled clinical trial was designed to evaluate and compare the treatment outcome of intrabony defects with PRF as an adjunct to DFDBA. The participants for the study were selected from the outpatient's section after obtaining the institutional ethical committee approval. Informed consent of the patient was obtained after explaining the study design in the patient's vernacular language.
Patients with chronic periodontitis within 25–55 years of age with at least three teeth with probing pocket depth (PPD) >6 mm and evidence of angular defects as determined by intraoral periapical (IOPA) were included in the study.
Patients with aggressive periodontitis, systemic diseases, known drug history in the past 6 months who have had no active periodontal therapy in the past, smokers, pregnant, and lactating women were excluded from the study.
A total of 39 sites with intrabony defects in chronic periodontitis patients were randomly assigned into three groups, based on the treatment modality rendered to them. They were Group I - Open flap debridement (OFD), Group II - DFDBA alone, and Group III - DFDBA + PRF. A simple randomization technique was used where the three treatment groups were assigned as A, B, C, and numbers were written from 1 to 9 where the number picked will decide the treatment procedure 1–3-treatment A, 4–6 – treatment B, and 7–9 – treatment C. Scaling and root planning was performed in all patients. The clinical parameters recorded were relative attachment level (RAL), PPD [Figure 1a], and radiographic changes like reduction in defect depth were recorded at baseline, 3 months, and 6 months.
Figure 1.
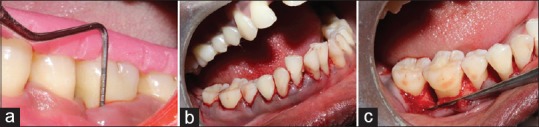
(a) Measurement of clinical parameters by using stent; (b) sulcular incision; (c) Intrabony defect after debridement
Surgical procedure
After anesthetizing the area with 2% lignocaine with adrenaline (1: 80,000) solution, a sulcular incision was given and a full-thickness mucoperiosteal flap was elevated (Kirkland Flap) [Figure 1b]. Then, thorough debridement was performed using area-specific curettes (Hu-Friedy, USA) curettes (Hu-Friedy, USA) [Figure 1c], and the anatomy of the intrabony defect was clinically confirmed and if the defect was two and three wall defect, it was filled either with DFDBA or DFDBA with PRF or the defect was left alone after debridement, according to the group assigned.
Platelet-rich fibrin preparation
A volume of 10 ml of whole venous blood was drawn by venipuncturing the antecubital vein. Blood was collected in a sterile glass tube without any anti-coagulant. The test tubes were then placed in a centrifugal machine [Figure 2] at 3000 revolutions per minute for 10 min. The middle fraction containing the fibrin clot along with a small red blood cell layer at the end of PRF clot was removed [Figure 3a and b]. It was used along with the bone graft (DFDBA) according to the procedure rendered [Figure 3c]. The mixture of PRF and bone graft was placed in the defect in Group-III. The buccal and lingual flaps were approximated using a 3–0 (nonresorbable) suture [Figure 4a and b].
Figure 2.

Centrifuge
Figure 3.
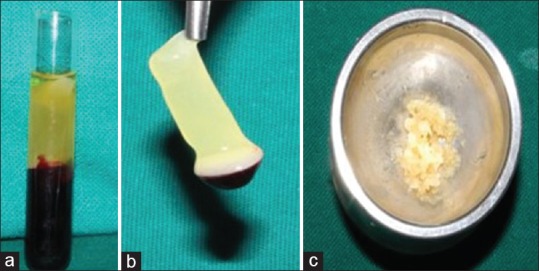
(a) Platelet-rich fibrin in test tube; (b) platelet-rich fibrin; (c) platelet-rich fibrin mixed with demineralized freeze-dried bone allograft
Figure 4.
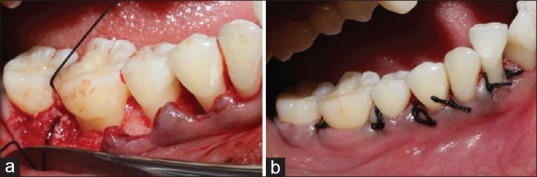
(a) Graft placement into the bone defect; (b) sutures placed
All patients received systemic antibiotic therapy (Amoxicillin 500 mg thrice daily) for 7 days and analgesic therapy (ketorol DT twice daily) for 3 days to alleviate postoperative pain and edema. Sutures and pack were checked and removed 10 days after the surgery. Local plaque control was maintained by 0.2% chlorhexidine rinse thrice daily. No attempt to probe was made before the 3 months follow-up examination.
Radiographic evaluation
Radiographic evaluation was done at baseline, 3rd month, and 6th month. Radiographically, the measurements were recorded from a fixed reference point (adjacent tooth cusp tip) to base of the defect. The radiographs were standardized by using radiovisiography with the long cone paralleling technique and using film holders (RINN XCP™, DENTSPLY). Measurements were done using special software (University of Texas Health Science Center at San Antonio [UTHSCSA] Image Tool)™ to evaluate the bone fill. Standardization of the radiographs was done using a metallic ball of known diameter incorporated into the IOPA. This was done using a metal ball whose diameter was measured using digital Vernier calipers and the metal ball was incorporated into the sleeve of the PSP plate, and IOPA was taken. The UTHSCSA software measures the difference in actual diameter of the metallic ball and the diameter as measured in the radiograph and calibrates accordingly. Subsequent measurements were adjusted using these calibrations automatically by making up for the foreshortening or magnification if any as noticed in the IOPA [Figure 5a and b].
Figure 5.
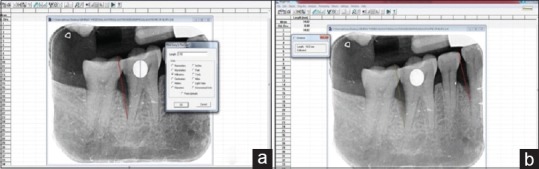
(a) Calibration of metal ball; (b) measurement of defect depth
Statistical analysis
The intragroup analysis was performed using one-way ANOVA test, whereas the intergroup analysis was performed using Tukey's multiple post hoc test. Differences were considered statistically significant at P < 0.05*. The data collected were analyzed using the SPSS software 19.00 program (SPSS Inc., Chicago, IL, USA).
RESULTS
All the participants completed 6 months study period with the recall visits. No postoperative complications were seen with any of the participants during the study. On intragroup comparison gradual reduction in the radiographic defect depths were observed over the study period in all the three groups which were found to be significant from baseline to 3rd month and baseline to 6th month in all the three groups with greatest reduction of 38.99% in the DFDBA + PRF group and intergroup comparisons were statistically significant at 6th month study interval between OFD versus DFDBA + PRF group (P = 0.0438) and from baseline to 3rd month and baseline to 6th month among OFD versus DFDBA and OFD versus DFDBA + PRF. However, no statistically significant difference was reported by DFDBA versus DFDBA + PRF group [Table 1] [Graphs 1–3]. On intragroup comparison, reduction in the PPD and greater difference in RAL was observed over the study period in all the three groups with greater reduction in DFDBA + PRF group which was found to be significant and intergroup comparisons of PPD showed statistically significant results at baseline and 3rd month interval between OFD versus DFDBA group (P < 0.05) and from baseline to 6th month interval in OFD versus DFDBA + PRF group in comparison to other groups [Table 2 and Graphs 4 and 5]. Intergroup comparisons of RAL showed statistically significant results at 6th month study interval of OFD versus DFDBA + PRF group (P < 0.05) [Table 3 and Graph 6].
Table 1.
Comparison of three groups with radiographic defect depth by one-way ANOVA
| Baseline | 3rd month | 6th month | Changes from baseline to | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 3rd month | 6th month | |||||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| OFD | 12.74 | 1.94 | 10.92 | 1.78 | 10.16 | 1.88 | 1.82 | 1.68 | 2.58 | 1.47 |
| DFDBA | 14.54 | 3.24 | 10.40 | 2.47 | 9.14 | 2.12 | 4.13 | 2.04 | 5.39 | 2.07 |
| DFDBA + PRF | 13.71 | 1.55 | 9.65 | 1.53 | 8.37 | 1.40 | 4.06 | 1.92 | 5.35 | 2.05 |
| Percentage of change in OFD | 14.26%# | P=0.0021* | 20.24%# | P=0.00001* | ||||||
| Percentage of change in DFDBA | 28.45%# | P=0.00001* | 37.11%# | P=0.00001* | ||||||
| Percentage of change in DDBA + PRF | 29.64%# | P=0.00001* | 38.99%# | P=0.00001* | ||||||
| F | 1.8951 | 1.3736 | 3.8134 | 6.3631 | 9.5123 | |||||
| P | 0.1650 | 0.2661 | 0.0500* | 0.0043* | 0.0005* | |||||
| Pair wise comparisons by Tukey’s multiple post hoc procedures | ||||||||||
| OFD versus DFDBA (P) | 0.1411 | 0.7799 | 0.3409 | 0.0094* | 0.0016* | |||||
| OFD versus DFDBA + PRF (P) | 0.5478 | 0.2390 | 0.0438* | 0.0120* | 0.0019* | |||||
| DFDBA versus DFDBA + PRF (P) | 0.6504 | 0.5978 | 0.5311 | 0.9952 | 0.9979 | |||||
*P<0.05, #Applied paired t-test. OFD – Open flap debridement; DFDBA – Demineralized freeze-dried bone allograft; PRF – Platelet-rich fibrin; SD – Standard deviation
Graph 1.
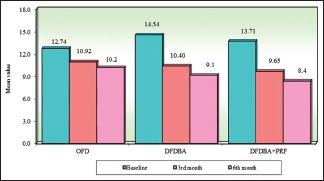
Intragroup comparison of three groups with respect to radiographic defect depth at all the study intervals. OFD – Open flap debridement; DFDBA – Demineralized freeze-dried bone allograft; PRF – Platelet-rich fibrin
Graph 3.
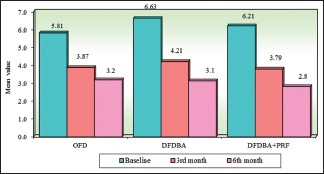
Intragroup comparison of three groups with respect to relative attachment level at all the study intervals. OFD – Open flap debridement; DFDBA – Demineralized freeze-dried bone allograft; PRF – Platelet-rich fibrin
Table 2.
Comparison of three groups with probing pocket depth by one-way ANOVA
| Groups | Baseline | 3rd month | 6th month | Changes from baseline to | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 3rd month | 6th month | |||||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| OFD | 5.21 | 1.13 | 3.37 | 0.66 | 2.85 | 0.40 | 1.85 | 1.13 | 2.37 | 1.12 |
| DFDBA | 6.63 | 1.82 | 4.13 | 0.99 | 3.08 | 0.64 | 2.50 | 1.12 | 3.56 | 1.56 |
| DFDBA + PRF | 6.25 | 1.26 | 3.54 | 0.48 | 2.65 | 0.40 | 2.71 | 1.28 | 3.60 | 1.42 |
| Percentage of change in OFD | 35.42%# | P=0.0001* | 45.39%# | P=0.00001* | ||||||
| Percentage of change in DFDBA | 37.68%# | P=0.00001* | 53.62%# | P=0.00001* | ||||||
| Percentage of change in DDBA + PRF | 43.38%# | P=0.00001* | 57.54%# | P=0.00001* | ||||||
| F | 3.4246 | 3.8815 | 2.3843 | 1.9127 | 3.3434 | |||||
| P | 0.0435* | 0.0298* | 0.1066 | 0.1624 | 0.0466* | |||||
| Pair wise comparisons by Tukey’s multiple post hoc procedures | ||||||||||
| OFD versus DFDBA (P) | 0.0413* | 0.0309* | 0.4671 | 0.3429 | 0.0843 | |||||
| OFD versus DFDBA + PRF (P) | 0.1695 | 0.8224 | 0.5872 | 0.1604 | 0.0500* | |||||
| DFDBA versus DFDBA + PRF (P) | 0.7745 | 0.1132 | 0.0883 | 0.8910 | 0.9973 | |||||
*P<0.05, #Applied paired t-test. OFD – Open flap debridement; DFDBA – Demineralized freeze-dried bone allograft; PRF – Platelet-rich fibrin; SD – Standard deviation
Graph 4.
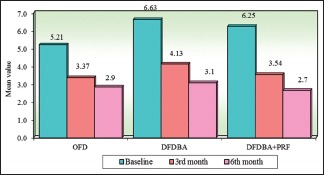
Intragroup comparison of three groups with respect to probing pocket depth at all the study interval. OFD – Open flap debridement; DFDBA – Demineralized freeze-dried bone allograft; PRF – Platelet-rich fibrin
Graph 5.
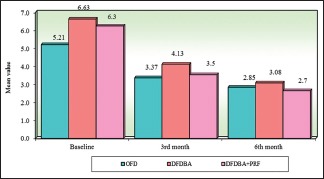
Intergroup comparison of three groups with respect to probing pocket depth at all the study intervals. OFD – Open flap debridement; DFDBA – Demineralized freeze-dried bone allograft; PRF – Platelet-rich fibrin
Table 3.
Comparison of three groups with relative attachment level by one-way ANOVA
| Groups | Baseline | 3rd month | 6th month | Changes from baseline to | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 3rd month | 6th month | |||||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| OFD | 5.81 | 1.10 | 3.87 | 0.67 | 3.19 | 0.46 | 1.94 | 1.09 | 2.62 | 1.16 |
| DFDBA | 6.63 | 1.65 | 4.21 | 0.96 | 3.13 | 0.42 | 2.42 | 0.87 | 3.50 | 1.48 |
| DFDBA + PRF | 6.21 | 1.53 | 3.79 | 0.50 | 2.81 | 0.33 | 2.42 | 1.48 | 3.40 | 1.65 |
| Percentage of change in OFD | 33.44%# | P=0.00001* | 45.03%# | P=0.00001* | ||||||
| Percentage of change in DFDBA | 36.52%# | P=0.00001* | 52.75%# | P=0.00001* | ||||||
| Percentage of change in DDBA + PRF | 39.01%# | P=0.0001* | 54.80%# | P=0.00001* | ||||||
| F | 1.0658 | 1.2165 | 3.4328 | 0.7249 | 1.4672 | |||||
| P | 0.3551 | 0.3081 | 0.0432* | 0.4913 | 0.2440 | |||||
| P | 0.3551 | 0.3081 | 0.0432* | 0.4913 | 0.2440 | |||||
| OFD versus DFDBA (P) | 0.3220 | 0.4622 | 0.9297 | 0.5553 | 0.2755 | |||||
| OFD versus DFDBA + PRF (P) | 0.7575 | 0.9618 | 0.0500* | 0.5553 | 0.3561 | |||||
| DFDBA versus DFDBA + PRF (P) | 0.7375 | 0.3200 | 0.1117 | 0.9999 | 0.9843 | |||||
*P<0.05, #Applied paired t-test. DFDBA – Demineralized freeze-dried bone allograft; PRF – Platelet-rich fibrin; OFD – Open flap debridement; SD – Standard deviation
Graph 6.
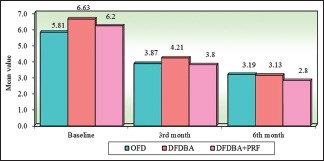
Inter group comparison of three groups with respect to relative attachment level at all the study intervals. OFD – Open flap debridement; DFDBA – Demineralized freeze-dried bone allograft; PRF – Platelet-rich fibrin
Graph 2.
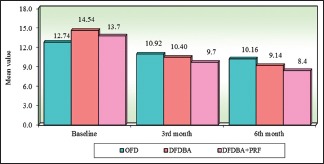
Intergroup comparison of three groups with respect to radiographic defect depth at all the study intervals. OFD – Open flap debridement; DFDBA – Demineralized freeze-dried bone allograft; PRF – Platelet-rich fibrin
DISCUSSION
One of the significant characteristic symptoms of destructive periodontal disease is alveolar bone loss, whose visualization not only is necessary its successful management but also for understanding its etiology. The biggest therapeutic challenge in periodontal therapy still remains to be intrabony defects. Various techniques are employed in treating intrabony defects, such as osseous surgery, OFD, guided tissue regeneration (GTR) and using bone graft materials. OFD alone provides only access to the defect. Bone replacement grafts permit the attachment and proliferation of osteoblasts that are anchorage-dependent by acting as structural scaffolds and hence are widely used for bone formation and periodontal regeneration. Autogenous bone though considered “gold standard” has limitations, that are by-passed by employing bone allograft as an alternative.[6] DFDBA, a bone allograft intensifies the endochondral bone formation due to its osteoinductive ability rendered by BMPs.[4] Various in vitro studies have shown a beneficial effect of PRF on proliferation and differentiation of osteoblasts.[7,8] When mixed with the graft, PRF fragments form a biological connector between bone particles. There are various clinical implications of PRF and due to the extended duration release of the various GFs, it would be expected that treating intrabony defects with PRF may boost the wound healing and periodontal regeneration. Platelet and leukocyte derived GFs are released in a sustained manner as the dense fibrin matrix of PRF takes greater time to resorb.[9] The present study compares the clinical and radiographic parameters in the treatment of intrabony defects using PRF as an adjunct to bone grafting. The radiographic techniques used in this study include paralleling technique using film holders in combination with digital processing of the images to measure bony changes. Standardization of the radiographs was done using a metallic ball of known diameter incorporated into the IOPA. The UTHSCSA software measures the difference in actual diameter of the metallic ball and the diameter as measured and in the radiograph and calibrates accordingly. OFD has been conventionally included as the control procedure in clinical trials evaluating regenerative techniques, such as GTR [10,11] and use of biologic factors, including enamel matrix derivative.[12] In the present study, OFD was used as a control and compared with DFDBA and DFDBA + PRF. In a study, Pietruska et al. concluded that there was no clinical and radiographic improvement when nanocrystalline hydroxyapatite bone substitute material was used additionally.[13] Several authors have advocated the use of OFD, both with and without intramarrow penetration, resulted in a significant reduction in Probing depth and gain in clinical attachment levels. Radiographically, both treatments resulted in a significant decrease in radiographic defect depth. These results are consistent with the reported outcomes of studies on OFD efficacy as surgical therapy for intrabony defects.[14] Intergroup comparisons of PPD at all study intervals in the present study showed statistical significance at baseline and 3rd month study interval between OFD versus DFDBA groups. These results are correlated with studies where DFDBAs have consistently demonstrated improvements in soft- and hard-tissue parameters for the treatment of intraosseous periodontal defects.[15] Several studies have been published, in which DFDBA has been used in combination with other materials such as Bone Derived Xenograft,[16] Enamel Matrix Derivatives,[17] Freeze Dried Bone Allograft,[18] PRP,[19] and all the studies showed improvement in the clinical and radiographic parameters when DFDBA is used in combination with other materials. In contrast to the above studies when DFDBA is used in combination with PRP,[15] GTR,[20,21] bioactive glass,[22] EMD,[23] no statistically significant difference in radiographic defect fill was observed in both the groups. Mellonig reported the greater bone repair in defects treated with DFDBA as compared to ungrafted controls.[24] A study by Masters et al. compared DFDBA + tetracycline with DFDBA alone and OFD and found improvement in the clinical parameters, but the radiographic defect fill was greater with DFDBA alone when compared to other groups,[25] these observations are correlating with the observations of the present study where defect fill with DFDBA was better than OFD alone. These results compare well with other studies treating human intrabony defects with DFDBA alone.[20,26,27,28]
The novelty of the present study is that DFDBA alone was compared with DFDBA + PRF and (OFD). The results of the present study showed improvement in clinical and radiographic parameters with significantly greater improvement in the DFDBA + PRF group. There could be a synergistic interaction when DFDBA is used in combination with PRF in the treatment of periodontal intrabony defects.[15]
Various studies were conducted using PRF as an individual grafting material [29,30] or combining PRF with other materials and showing better results.[31] In the present study, better results were found when PRF was adjunctively used with DFDBA. Comparison of autologous PRF with a conventional OFD alone showed the greatest reduction in PD, more RAL gain and greater intrabony defect fill at sites treated with PRF than the OFD alone. Greater intrabony defect fill attributed to the GFs that enhance periodontal healing.[32,33] Pradeep et al. studied the efficacy of PRF and PRP in chronic periodontitis concluded that there was a similar reduction of probing depth, gain in clinical attachment level, and bone fill in the sites treated with PRF or PRP with conventional open flap debridement. PRF can be preferred to PRP as the preparation time is reduced considerably and technique of preparation is less sensitive.[34] Reduction in PPD, gain in clinical attachment, and significant radiographic bone fill was observed when PRF was used along with alloplastic bone mineral in the treatment of intrabony defects.[35,36,37] These observations were similar to the results of the present study where PRF showed better results but in combination with DFDBA.
The present study showed a gradient reduction in the radiographic defect depths over the study period in all the groups with greater reduction in the DFDBA + PRF group. The postsurgical healing of the patients in the present study can be correlated to the previous studies,[32,33] thus supporting the periodontal wound healing property of PRF.[30] The best possible outcome of periodontal regenerative techniques has been achieved with bone grafts. GFs usage in adjunct to bone grafts holds an important position for the purpose of complete regeneration of periodontium. The results of the present study showed improvement in all the three groups at all the study intervals with greater improvement in the DFDBA + PRF group.
To the best of our knowledge, this is the first study compared OFD, DFDBA, and PRF + DFDBA in the treatment of intrabony defects.
CONCLUSION
The adjunctive use of PRF to DFDBA could improve the clinical and radiographic parameters. PRF was combined with DFDBA to produce a synergistic effect for treating intrabony defects in chronic periodontitis patients. Long-term, randomized, controlled clinical trials will be required to quantify these effects over other treatment modalities with better standardized radiographic techniques like digital subtraction radiography to evaluate the bone fill.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Parimala M, Mehta DS. Comparative evaluation of bovine porous bone mineral. J Indian Soc Periodontol. 2010;14:126–31. doi: 10.4103/0972-124X.70834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Camargo PM, Lekovic V, Weinlaender M, Divnic-Resnik T, Pavlovic M, Kenney EB. A surgical reentry study on the influence of platelet-rich plasma in enhancing the regenerative effects of bovine porous bone mineral and guided tissue regeneration in the treatment of intrabony defects in humans. J Periodontol. 2009;80:915–23. doi: 10.1902/jop.2009.080600. [DOI] [PubMed] [Google Scholar]
- 3.Trombelli L, Farina R. Clinical outcomes with bioactive agents alone or in combination with grafting or guided tissue regeneration. J Clin Periodontol. 2008;35:117–35. doi: 10.1111/j.1600-051X.2008.01265.x. [DOI] [PubMed] [Google Scholar]
- 4.Committee on Research, Science and Therapy of the American Academy of Periodontology. Tissue banking of bone allografts used in periodontal regeneration. J Periodontol. 2001;72:834–8. doi: 10.1902/jop.2001.72.6.834. [DOI] [PubMed] [Google Scholar]
- 5.Gurbuzer B, Pikdoken L, Tunali M, Urhan M, Kucukodaci Z, Ercan F. Scintigraphic evaluation of osteoblastic activity in extraction sockets treated with platelet-rich fibrin. J Oral Maxillofac Surg. 2010;68:980–9. doi: 10.1016/j.joms.2009.09.092. [DOI] [PubMed] [Google Scholar]
- 6.Reynolds MA, Aichelmann-Reidy ME, Branch-Mays GL. Regeneration of periodontal tissue: bone replacement grafts. Dent Clin North Am. 2010;54:55–71. doi: 10.1016/j.cden.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 7.He L, Lin Y, Hu X, Zhang Y, Wu H. A comparative study of Platelet-Rich Fibrin (PRF) and Platelet-Rich Plasma (PRP) on the effect of proliferation and differentiation of rat osteoblasts in vitro. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;108:707–13. doi: 10.1016/j.tripleo.2009.06.044. [DOI] [PubMed] [Google Scholar]
- 8.Dohan Ehrenfest DM, Diss A, Odin G, Doglioli P, Hippolyte MP, Charrier JB. In vitro effects of Choukrounf PRF (Platelet-Rich Fibrin) on human gingival fibroblasts, dermal prekeratinocytes, preadipocytes, and maxillofacial osteoblasts in primary cultures. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;108:341–52. doi: 10.1016/j.tripleo.2009.04.020. [DOI] [PubMed] [Google Scholar]
- 9.Dohan DM, Choukroun J, Diss A, Dohan SL, Dohan AJ, Mouhyi J, et al. Platelet-Rich Fibrin (PRF): A second-generation platelet concentrate. Part II: platelet-related biologic features. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101:45–50. doi: 10.1016/j.tripleo.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 10.Cortellini P, Pini Prato GP, Tonetti MS. Periodontal regeneration of human intrabony defects with bioresorbable membranes. A controlled clinical trial. J Periodontol. 1996;67:217–23. doi: 10.1902/jop.1996.67.3.217. [DOI] [PubMed] [Google Scholar]
- 11.Needleman I, Tucker R, Giedrys-Leeper E, Worthington H. Guided tissue regeneration for periodontal intrabony defects defcochrane systematic review. Periodontol 2000. 2005;37:106–23. doi: 10.1111/j.1600-0757.2004.37101.x. [DOI] [PubMed] [Google Scholar]
- 12.Heijl L, Heden G, Svardstrom G, Ostgren A. Enamel matrix derivative (EMDOGAIN) in the treatment of intrabony periodontal defects. J Clin Periodontol. 1997;24:705–14. doi: 10.1111/j.1600-051x.1997.tb00253.x. [DOI] [PubMed] [Google Scholar]
- 13.Pietruska M, Skurska A, Pietruski J, DoliERLI E, Arweiler N, Milewski R, et al. Clinical and radiographic evaluation of intrabony periodontal defect treatment by open flap debridement alone or in combination with nanocrystalline hydroxyapatite bone substitute. Ann Anat. 2012;194:533–7. doi: 10.1016/j.aanat.2012.03.009. [DOI] [PubMed] [Google Scholar]
- 14.Graziani F, Gennai S, Cei S, Cairo F, Baggiani A, Miccoli M, et al. Clinical performance of access flap surgery in the treatment of the intrabony defect. A systematic review and meta-analysis of randomized clinical trials. J Clin Periodontol. 2012;39:145–56. doi: 10.1111/j.1600-051X.2011.01815.x. [DOI] [PubMed] [Google Scholar]
- 15.Piemontese M, Aspriello SD, Rubini C, Ferrante L, Procaccini M. Treatment of periodontal intrabony defects with demineralized freeze-dried bone allograft in combination with platelet-rich plasma: A comparative clinical Trial. J Periodontol. 2008;79:802–10. doi: 10.1902/jop.2008.070436. [DOI] [PubMed] [Google Scholar]
- 16.Richardson CR, Mellonig JT, Brunsvold MA, McDonnell HT, Cochran DL. Clinical evaluation of Bio-Oss: A bovine-derived xenograft for the treatment of periodontal osseous defects in humans. J Clin Periodontol. 1999;26:421–8. doi: 10.1034/j.1600-051x.1999.260702.x. [DOI] [PubMed] [Google Scholar]
- 17.Aspriello SD, Ferrante L, Rubini C, Piemontese M. Comparative study of DFDBA in combination with enamel matrix derivative versus DFDBA alone for treatment of periodontal intrabony defects at 12 months post-surgery. Clin Oral Investig. 2011;15:225–32. doi: 10.1007/s00784-009-0369-y. [DOI] [PubMed] [Google Scholar]
- 18.Rummelhart JM, Mellonig JT, Gray JL, Towle HJ. A comparison of freeze-dried bone allograft and demineralized freeze-dried bone allograft in human periodontal osseous defects. J Periodontol. 1989;60:655–63. doi: 10.1902/jop.1989.60.12.655. [DOI] [PubMed] [Google Scholar]
- 19.de Obarrio JJ, Arauz-Dutari JI, Chamberlain TM, Croston A. The use of autologous growth factors in periodontal surgical therapy: Platelet gel biotechnology d=11203586” 586”. Int J Periodont Restor Dent. 2000;20:486–97. [PubMed] [Google Scholar]
- 20.Parashis A, Andronikaki-Faldami A, Tsiklakis K. Comparison of 2 regenerative procedures bmed?ded Tissue regeneration and demineralized-freeze-dried bone allograft 851” stry.” us+DFDBA+aintrabony defects: A clinical and radiographic study. J Periodontol. 1998;69:751–8. doi: 10.1902/jop.1998.69.7.751. [DOI] [PubMed] [Google Scholar]
- 21.Kothiwale S, Bhimani R, Kaderi M, Ajbani J. Comparative study of DFDBA and FDBA block grafts in combination with chorion membrane for the treatment of periodontal intra-bony defects at 12 months post surgery. Cell Tissue Bank. 2019. Available from: https://doi.org/10.1007/s10561-018-09744-5 . [DOI] [PubMed]
- 22.Lovelace TB, Mellonig JT, Meffert RM, Jones AA, Nummikoski PV, Cochran DL. Clinical evaluation of bioactive glass in the treatment of periodontal osseous defects in humans. J Periodontol. 1998;69:1027–35. doi: 10.1902/jop.1998.69.9.1027. [DOI] [PubMed] [Google Scholar]
- 23.Hoidal MJ, Grimard BA, Mills MP, Schoolfield JD, Mellonig JT, Mealey BL. Clinical evaluation of demineralized freeze-dried bone allograft with and without enamel matrix derivative for the treatment of periodontal osseous defects in humans. J Periodontol. 2008;79:2273–80. doi: 10.1902/jop.2008.080259. [DOI] [PubMed] [Google Scholar]
- 24.Meadows CL, Gher ME, Quintero G, Lafferty TA. A comparison of polylactic acid granules and decalcified freeze-dried bone allograft in human periodontal osseous defects. J Periodontol. 1993;64:103–9. doi: 10.1902/jop.1993.64.2.103. [DOI] [PubMed] [Google Scholar]
- 25.Masters LB, Mellonig JT, Brunsvold MA, Nummikoski PV. A clinical evaluation of demineralized freeze-dried bone allograft in combination With tetracycline in the treatment of periodontal osseous defects. J Periodontol. 1996;67:770–81. doi: 10.1902/jop.1996.67.8.770. [DOI] [PubMed] [Google Scholar]
- 26.Persson GR, Falk H, Laurell L. A retrospective radiographic outcome assessment study of intra-bony defects treated by osseous surgery or by bone graft procedures. J Clin Periodontol. 2000;27:104–8. doi: 10.1034/j.1600-051x.2000.027002104.x. [DOI] [PubMed] [Google Scholar]
- 27.Mellonig JT. Decalcified freeze-dried bone allograft as an implant material in human periodontal defects. Int J Periodont Restor Dent. 1984;4:40–55. [PubMed] [Google Scholar]
- 28.Quintero G, Mellonig JT, Gambill VM, Pelleu GB., Jr A six-month clinical evaluation of decalcified freeze-dried bone allografts in periodontal osseous defects. J Periodontol. 1982;53:726–30. doi: 10.1902/jop.1982.53.12.726. [DOI] [PubMed] [Google Scholar]
- 29.Chang YC, Wu KC, Zhao JH. Clinical application of platelet-rich fibrin as the sole grafting material in periodontal intrabony defects. J Dent Sci. 2011;6:181–8. [Google Scholar]
- 30.Patel GK, Gaekwad SS, Gujjari SK, Veerendra Kumar SC. Platelet-rich fibrin in regeneration of intra-bony defects: A randomized controlled trial. J Periodontol. 2017;88:1192–9. doi: 10.1902/jop.2017.130710. [DOI] [PubMed] [Google Scholar]
- 31.Chandradas ND, Ravindra S, Rangaraju VM, Jain S, Dasappa S. Efficacy of platelet rich fibrin in the treatment of human intrabony defects with or without bone graft: A randomized controlled trial. J Int Soc Prevent Community Dent. 2016;6:S153–9. doi: 10.4103/2231-0762.189753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sharma A, Pradeep AR. Treatment of 3-wall intrabony defects in patients with chronic periodontitis with autologous platelet-rich fibrin: A randomized controlled clinical trial. J Periodontol. 2011;82:1705–12. doi: 10.1902/jop.2011.110075. [DOI] [PubMed] [Google Scholar]
- 33.Thorat MK, Pradeep AR, Pallavi B. Clinical effect of autologous platelet-rich fibrin in the treatment of intra-bony defects: A controlled clinical trial. J Clin Periodontol. 2011;38:925–32. doi: 10.1111/j.1600-051X.2011.01760.x. [DOI] [PubMed] [Google Scholar]
- 34.Pradeep AR, Rao NS, Agarwal E, Bajaj P, Kumari M, Naik SB. Comparative evaluation of autologous platelet-rich fibrin and platelet-rich plasma in the treatment of 3-wall intrabony defects in chronic periodontitis: A randomized controlled clinical trial. J Periodontol. 2012;83:1499–507. doi: 10.1902/jop.2012.110705. [DOI] [PubMed] [Google Scholar]
- 35.Lekovic V, Milinkovic I, Aleksic Z, Jankovic S, Stankovic P, Kenney EB, Camargo PM. Platelet-rich fibrin and bovine porous bone mineral vs. platelet-rich fibrin in the treatment of intrabony periodontal defects. J Periodont Res. 2012;47:409–17. doi: 10.1111/j.1600-0765.2011.01446.x. [DOI] [PubMed] [Google Scholar]
- 36.Panda S, Ramamoorthi S, Jayakumar ND, Sankari M, Varghese SS. Platelet rich fibrin and alloplast in the treatment of intrabony defect. J Pharm Bioallied Sci. 2014;6:127–31. doi: 10.4103/0975-7406.129178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bodhare GH, Kolte AP, Kolte RA, Shirke PY. Clinical and radiographic evaluation and comparison of bioactive bone alloplast morsels when used alone and in combination with platelet-rich fibrin in the treatment of periodontal intrabony defects deA randomized controlled trial. J Periodontol. 2019;90:584–94. doi: 10.1002/JPER.18-0416. [DOI] [PubMed] [Google Scholar]


