The long-distance signals 12-OPDA and KODA activate induced systemic resistance (ISR) triggered by the root-colonizing beneficial fungal endophyte Trichoderma virens.
Abstract
Multiple long-distance signals have been identified for pathogen-induced systemic acquired resistance, but mobile signals for symbiont-induced systemic resistance (ISR) are less well understood. We used ISR-positive and -negative mutants of maize (Zea mays) and the beneficial fungus Trichoderma virens and identified 12-oxo-phytodienoic acid (12-OPDA) and α-ketol of octadecadienoic acid (KODA) as important ISR signals. We show that a maize 13-lipoxygenase mutant, lox10, colonized by the wild-type T. virens (TvWT) lacked ISR response against Colletotrichum graminicola but instead displayed induced systemic susceptibility. Oxylipin profiling of xylem sap from T. virens–treated plants revealed that 12-OPDA and KODA levels correlated with ISR. Transfusing sap supplemented with 12-OPDA or KODA increased receiver plant resistance in a dose-dependent manner, with 12-OPDA restoring ISR of lox10 plants treated with TvWT or T. virens Δsm1, a mutant unable to induce ISR. Unexpectedly, jasmonic acid (JA) was not involved, as the JA-deficient opr7 opr8 mutant plants retained the capacity for T. virens–induced ISR. Transcriptome analysis of TvWT-treated maize B73 revealed upregulation of 12-OPDA biosynthesis and OPDA-responsive genes but downregulation of JA biosynthesis and JA response genes. We propose a model that differential regulation of 12-OPDA and JA in response to T. virens colonization results in ISR induction.
INTRODUCTION
Within the rhizosphere, plants must discern beneficial microbes from pathogens and respond accordingly by dampening host defenses when colonized by beneficial microbes or heightening defenses against pathogens (Heil and Bostock, 2002; Katagiri and Tsuda, 2010). One such response to beneficial microorganisms is heightened resistance to a wide range of pathogens, a phenomenon termed induced systemic resistance (ISR). ISR is triggered following root colonization by beneficial microbes, and it results in priming of rapid and robust defense responses against future pathogen infections (Pieterse et al., 2014b). While the primed plants display strong systemic resistance upon infection, they display little to no discernable changes in their defense status in the absence of pathogens (Wang et al., 2005; Conrath et al., 2006; Pieterse et al., 2014a). The mechanisms by which these beneficial microbes enhance both plant growth and defenses remain a mystery (Havko et al., 2016). The specific signaling mechanisms of ISR are much less understood compared with the pathogen-triggered systemic acquired resistance. While systemic acquired resistance (SAR) relies mostly on salicylic acid (SA) signaling (Pieterse et al., 2014b), the mobile signals responsible include azelaic acid, pipecolic acid, methyl salicylate, glycerol-3-P, and dehydroabietinal (Klessig et al., 2018). ISR induction is postulated to require jasmonic acid (JA) and ethylene signaling in an SA-independent manner (Pieterse et al., 2014a); however, SA has also been implicated in ISR signaling for certain species (Knoester et al., 1999; Korolev et al., 2008; Pozo et al., 2008; Salas-Marina et al., 2011).
Trichoderma spp are soil-borne filamentous fungi found ubiquitously, including agriculturally relevant Trichoderma virens and Trichoderma harzianum. They have been studied extensively for their beneficial effects on plants, such as enhancing growth of shoots and roots, interacting directly via antimicrobial activity against soil-borne pathogens through antibiosis and mycoparasitism, and triggering ISR (Yedidia et al., 1999; Howell et al., 2000; Lorito et al., 2010; Druzhinina et al., 2011; Hermosa et al., 2012). T. virens–induced ISR against diverse pathogens was demonstrated in several plant species including Arabidopsis (Arabidopsis thaliana), cotton (Gossypium hirsutum), tomato (Solanum lycopersicum), and maize (Zea mays; Djonović et al., 2006, 2007; Contreras-Cornejo et al., 2011).
The signal communication between Trichoderma and host plants that results in ISR is poorly understood; however, a plethora of fungal molecules have been identified that trigger or suppress ISR. Sm1, a small secreted protein from T. virens, plays an integral role in ISR signaling. The Δsm1 mutant can no longer trigger ISR in either maize or cotton, while the strains overexpressing SM1 enhance plant resistance against pathogens to levels greater than the wild-type T. virens (TvWT; Djonović et al., 2006, 2007). Similarly, Epl1, a homolog of Sm1 in Trichoderma atroviride, induces ISR in tomato and improves resistance against bacterial and fungal pathogens (Salas-Marina et al., 2015). T. virens also secretes several other peptides that function as negative regulators of ISR. For example, deletion of Suppressor of Induced Resistance (SIR1; protein ID 77560) greatly enhanced ISR of maize against a necrotrophic pathogen, Cochliobolus heterostrophus, the causal agent of Southern corn leaf blight (Lamdan et al., 2015). The specific mechanisms by which these secreted peptides regulate ISR remain unknown. We previously showed that one function of Sm1 is to regulate synthesis of maize oxylipins with signaling properties (Constantino et al., 2013).
Oxylipins are a large group of oxidized lipid signals that regulate many physiological processes, such as growth and development, defense responses to pathogens and herbivores, and abiotic stresses (Borrego and Kolomiets, 2016). They are mainly produced through enzymatic oxygenation of polyunsaturated fatty acids, linoleic acid (C18:2), and linolenic acid (C18:3) at the carbon position 9 or 13 by 9- or 13-lipoxygenase (LOX), respectively (Feussner and Wasternack, 2002; Andreou et al., 2009). The primary products of LOX reactions are fed into several enzyme branches, resulting in the biosynthesis of more than 650 structurally and functionally diverse oxylipins (Borrego and Kolomiets, 2016). Undoubtedly, the best-characterized oxylipin in terms of relevance to ISR is JA. JA biosynthesis begins in plastids, where 13-LOX, allene oxide synthase (AOS), and allene oxide cyclase (AOC) produce JA precursor 12-oxo-phytodienoic acid (12-OPDA). 12-OPDA is transported to the peroxisome and converted to JA. JA is further conjugated to Ile by JAR1 to produce JA-Ile, the biologically active jasmonate that binds as a ligand to the CORONATINE INSENSITIVE1 (COI1)-JASMONATE ZIM DOMAIN (JAZ) receptor complex to activate downstream JA responses.
While the functions of JA are well documented, the physiological roles of the vast majority of other oxylipins, especially the 9-LOX–derived metabolites, collectively called 9-oxylipins, are largely unknown (Borrego and Kolomiets, 2016). The negative role of 9-LOX activity in regulating ISR has been demonstrated by examination of the maize lox3-4 mutant. This strong loss-of-function mutant demonstrated increased resistance against various seed, stalk, root, and foliar pathogens (Gao et al., 2007; Isakeit et al., 2007). Roots of lox3-4 mutant over-accumulated defense hormones JA, ethylene, and SA and overexpressed many defense genes (Gao et al., 2008). Expression of LOX3 has been observed exclusively in maize roots and has been shown to be suppressed by T. virens in a Sm1-dependent manner (Constantino et al., 2013). Furthermore, stem transfusion with xylem sap derived from lox3-4 roots to the wild-type maize confers systemic resistance against Colletotrichum graminicola, proving that a systemic resistance signal(s) originates from roots and travels systemically through the xylem (Constantino et al., 2013). Because LOX3-deficient mutants overexpress several JA biosynthesis genes including LOX10 (Gao et al., 2008), we hypothesized that constitutively active ISR in the lox3-4 mutant is due to overexpression of LOX10 and overproduction of JA or other oxylipins. Maize lox10 mutants are green leaf volatile deficient and accumulate significantly lower wound-induced levels of 12-OPDA and JA in leaves (Christensen et al., 2013).
In support of this hypothesis, here we showed that LOX10 function is indeed required to establish proper ISR, as instead of increased resistance to leaf pathogens, colonization of lox10 mutant roots by T. virens resulted in increased susceptibility. Oxylipin and hormone profiling of xylem sap from the wild-type maize, lox3, and lox10 mutants treated with either the wild type or ISR-perturbed T. virens mutants, along with pharmacological treatments indicated that 12-OPDA, produced by LOX10, and the α-ketol 9-hydroxy-10-oxo-12(Z),15(Z)-octadecadienoic acid (KODA), produced by an unknown 9-LOX, are major ISR long-distance signals. Unexpectedly, the JA-deficient opr7 opr8 double mutant displayed normal ISR response induced by T. virens, suggesting that JA is dispensable for ISR signaling in maize. Taken together, our results present evidence that the JA precursor, 12-OPDA, and KODA, but not JA, are xylem-resident signals required for ISR induction in maize.
RESULTS
T. virens Induces LOX10 Expression at the Early Stages of Interaction
To assess the involvement of LOX10 in maize–T. virens interactions, B73 seedlings were grown in hydroponic conditions and subsequently treated with TvWT, Δsm1, or Δsir1. These three strains have different abilities to induce ISR in maize. Compared with TvWT, Δsm1 is unable to induce ISR (Djonovic et al., 2007), whereas Δsir1 induces exceptionally strong ISR against C. heterostrophus, a necrotrophic pathogen and causal agent of Southern corn leaf blight (Lamdan et al., 2015). Treatment with the TvWT strain resulted in the upregulation of LOX10 expression as early as 3 and 6 h, ranging between three- and fourfold induction compared to untreated plants (Figure 1A). This effect was diminished by 9 h, suggesting that LOX10 induction is transient. Interestingly, Δsir1 induced LOX10 earlier than TvWT at 2 h after treatment. By contrast, the Δsm1 strain was unable to induce transcript accumulation at any time point, suggesting that LOX10 expression is dependent on functional Sm1 protein.
Figure 1.
LOX10 Expression Is Induced by T. virens in a Sm1-Dependent Manner.
(A) Expression of LOX10 by qPCR was determined in seedling roots of B73 after treatment with T. virens strains, TvWT, ISR-deficient strain Δsm1, or ISR-enhancing strain Δsir1, at 0, 1, 2, 3, 6, and 9 h after treatment and compared with untreated plants. Expression was calculated from cycle threshold values using the 2−ΔΔCt method and normalized to transcript levels of α-Tubulin, and relative expression is the fold change relative to untreated plants at time 0 h. Points represent means ± se (n = 3 biological replicates of plants grown in hydroponic jars, each consisting of five maize seedlings). Statistical significance was determined with Tukey’s HSD test compared with untreated plants (*P < 0.05, **P < 0.01).
(B) Confocal microscopic detection of accumulation of LOX10-YFP fusion protein, driven by the endogenous ZmLOX10 promoter, in the newly formed lateral roots of transgenic maize after treatment with TvWT compared with untreated roots. Bars = 100 μm.
(C) Expression of SM1 and SIR1 by qPCR was determined in T. virens hyphal tissue grown in MS medium. Expression was calculated from cycle threshold values using the 2−ΔΔCt method and normalized to transcript levels of Actin. Bars represent means ± se (n = 3 biological replicates of T. virens mycelia grown in hydroponic jars) relative to TvWT. Statistical significance was determined with Tukey’s HSD test (*P < 0.05). These experiments were repeated at least two times with similar results.
We further tested accumulation of LOX10 protein by taking advantage of a publicly available transgenic maize line expressing stable LOX10-yellow fluorescent protein (YFP) driven by the native LOX10 promoter (Mohanty et al., 2009). When the transgenic seedlings were treated with TvWT, they accumulated substantially greater levels of the fusion protein in the developing lateral roots compared to untreated plants (Figure 1B). Together, these results suggested that LOX10 expression was induced at both the transcript and protein levels and that induction is positively regulated by Sm1 but negatively regulated by Sir1.
To determine whether the more robust induction of LOX10 expression by Δsir1 was due to altered expression of SM1, transcript accumulation of SM1 and SIR1 was measured by qPCR in TvWT and both mutants at 6 h after transfer to hydroponic conditions. Expression of SM1 was threefold greater in Δsir1 mutant compared to TvWT, whereas SIR1 expression in Δsm1 was not altered (Figure 1C). Expression of SM1 and SIR1 at 30 h mirrored that seen at 6 h (data not shown). These results indicate that a likely reason for Δsir1 being more effective in induction of LOX10 is due to increased expression of SM1 in this mutant and confirm that LOX10 is indeed one host target positively regulated by Sm1 to promote ISR.
LOX10 Is Required for T. virens–Mediated ISR
To further define the role of LOX10 in regulating maize–T. virens interactions, the B73 inbred line and near-isogenic lox10 mutant alleles, lox10-2 and lox10-3, were treated with TvWT and assessed for ISR response against pathogen infection. As expected, TvWT-treated B73 displayed characteristic ISR response, as evidenced by significant reduction of lesion areas and chlorosis caused by C. graminicola (Figures 2A and 2B). As previously reported, both untreated lox10-3 and lox10-2 mutant alleles were significantly more resistant to C. graminicola (Christensen, 2009). Surprisingly, instead of the ISR observed with B73, T. virens treatment of both lox10 mutants resulted in significantly increased susceptibility, which we termed induced systemic susceptibility (ISS; Figures 2C and 2D). To test whether the ISS phenotype can be observed in a different genetic background of the maize inbred line, W438 inbred and near-isogenic line lox10-3 mutant in the W438 background were treated with TvWT and challenged with C. graminicola. The results reflected those observed in B73; specifically, untreated lox10-3 was more resistant than untreated W438, but TvWT-treated lox10-3 became significantly more susceptible (Figures 2E and 2F).
Figure 2.
LOX10 Acts as a Positive Regulator of T. virens-Triggered ISR against the Hemibiotrophic Pathogen C. graminicola.
(A) and (B) Visual representation of disease development (A) and lesion area (B) caused by C. graminicola on the leaves of untreated or TvWT-treated B73 or lox10-3 mutant plants.
(C) and (D) Visual representation of disease development (C) and lesion area (D) caused by C. graminicola on the leaves of untreated or TvWT-treated B73 or lox10-2 mutant plants.
(E) and (F) Visual representation of disease development (E) and lesion area (F) caused by C. graminicola on the leaves of untreated or TvWT-treated W438 or lox10-3 mutant plants.
Infected leaves were scanned and measured using ImageJ software to determine mean lesion area. Bars represent means ± se of the mean (n = 5 maize plants as biological replicates), with letters indicating significant differences among all treatments (Tukey’s HSD test, P < 0.05). These experiments were repeated at least three times with similar results.
ISR is effective against a broad range of pathogens. Because C. graminicola is a hemibiotroph, we tested whether LOX10 is required for ISR against a necrotroph. For this, B73 and both lox10 mutant alleles were treated with TvWT and infected with C. heterostrophus. Similar to C. graminicola, disease severity caused by C. heterostrophus was reduced in T. virens–colonized B73 and untreated lox10-3 and lox10-2 (Figures 3A to 3D). Furthermore, TvWT-treated lox10-3 and lox10-2 mutants displayed ISS, as evidenced by significantly larger lesions compared to untreated plants. ISS was also observed in TvWT-treated lox10-3 mutant in the W438 genetic background (Figures 3E and 3F). These results suggest that LOX10 plays an essential positive regulatory role in T. virens–triggered ISR against both hemibiotrophic and necrotrophic pathogens.
Figure 3.
LOX10 Acts as a Positive Regulator of T. virens–Triggered ISR against the Necrotrophic Pathogen C. heterostrophus.
(A) and (B) Visual representation of disease development (A) and lesion area (B) caused by C. heterostrophus on the leaves of untreated or TvWT-treated B73 or lox10-3 mutant plants.
(C) and (D) Visual representation of disease development (C) and lesion area (D) caused by C. heterostrophus on the leaves of untreated or TvWT-treated B73 or lox10-2 mutant plants.
(E) and (F) Visual representation of disease development (E) and lesion area (F) caused by C. heterostrophus on the leaves of untreated or TvWT-treated W438 or lox10-3 mutant plants.
Infected leaves were scanned and measured using ImageJ software to determine mean lesion area. Bars represent means ± se of the mean (n = 5 maize plants as biological replicates), with letters indicating significant differences among all treatments (Tukey’s HSD test, P < 0.05). These experiments were repeated at least three times with similar results.
To determine whether the lox10 ISS phenotype may be due to insufficient root colonization by T. virens, we quantified maize root colonization by T. virens by plating TvWT-treated B73 and lox10-3 roots on selective media and enumerating colony-forming units per total root biomass. Surprisingly, lox10-3 roots were colonized at a greater level compared to B73 roots (Supplemental Figure 1A). Next, we tested whether increased colonization of lox10 mutant roots had detrimental effects on growth promotion by T. virens. In spite of the over-colonization, lox10-3 plants treated with TvWT, Δsm1, or Δsir1 were not negatively impacted in growth or development by any of these strains. We concluded that T. virens plant growth promotion was not affected by the LOX10 mutation (Supplemental Figures 1B and 1C).
T. virens–Triggered ISS in lox10 Mutant Is Mediated by Both Host LOX3 and Fungal Sir1 Genes
Because the lox3-4 mutant overexpressed LOX10 in root tissue (Gao et al., 2007) and displayed enhanced resistance against C. graminicola in either the presence or absence of the TvWT treatment (Constantino et al., 2013), we hypothesized that overexpression of LOX10 was responsible for constitutive ISR in lox3 mutant. To test this hypothesis, we created the lox3 lox10 double mutant (lox3-4 lox10-3) in B73 background at the backcross seven (BC7) genetic stage. The lox3 lox10 double mutant was morphologically indistinguishable from the lox3-4 mutant (data not shown). While the untreated double mutant displayed similar to lox3-4 resistance level, TvWT-treated lox3 lox10 did not display the ISS phenotype observed in TvWT-treated lox10-3, suggesting that ISS in lox10 mutants was dependent on functional LOX3 (Figure 4).
Figure 4.
ISR against C. graminicola in B73, lox3-4, lox10-3, and lox3 lox10 Double Mutant in Response to TvWT, Δsm1, and Δsir1 T. virens Strain Treatments.
Lesion area caused by C. graminicola on the leaves of untreated or TvWT-, Δsm1-, or Δsir1-treated B73, lox3-4, lox10-3, or lox3 lox10 (lox3-4 lox10-3 double mutant). Infected leaves were scanned and measured using ImageJ software to determine mean lesion area. Bars represent means ± se of the mean (n = 5 maize plants as biological replicates), with letters indicating significant differences among all treatments (Tukey’s HSD test, P < 0.05). This experiment was repeated at least three times with similar results.
Additionally, we tested the role of the fungal peptide regulators of ISR, Sm1 and Sir1, in conferring the ISS phenotype of lox10-3 mutants. As expected, there was no observed ISR in B73 colonized by Δsm1 (Figure 4), while Δsir1 treatment conferred enhanced ISR against C. graminicola to greater levels than TvWT. Surprisingly, treatment with either Δsm1 or Δsir1 increased susceptibility of lox3-4, suggesting that Sm1 may be involved in more than suppression of LOX3 expression (Constantino et al., 2013). While both TvWT and Δsm1 treatments resulted in increased susceptibility in lox10-3, Δsir1 had no such effect. The loss of ISS triggered by Δsir1 may suggest that functional Sir1 in T. virens is the primary elicitor of ISS in lox10-3 mutant. The phenotypes of Δsm1- and Δsir1-treated lox3 lox10 resembled that of B73, suggesting that an imbalance of either LOX3 or LOX10 may be causing ISS.
Metabolite Profiling of Xylem Sap Identifies 12-OPDA and KODA as Potential ISR Signals
A previous study revealed that injecting xylem sap from lox3-4 conferred systemic resistance to B73 receiver plants, thus confirming that xylem sap of the donor lox3-4 plants contains ISR-relevant signals (Constantino et al., 2013). To determine whether saps from T. virens–treated plants were altered in the ability to enhance resistance, we have taken advantage of the sap transfusion methodology developed by Constantino et al. (2013) to circumvent the inability to graft monocots. B73, lox3-4, and lox10-3 plants were left untreated or treated with TvWT, Δsm1, or Δsir1, and xylem saps were collected at 14 d after treatment. Aliquots of 10 μL of 2× diluted sap were transfused into untreated B73 receiver plants prior to infection with C. graminicola. Corroborating the efficacy of the transfusion method, the xylem sap–treated receiver plants mostly phenocopied the donor plants ISR (Figure 5A). Specifically, the receiver plants treated with sap collected from TvWT-treated B73 or Δsir1-treated B73 showed enhanced systemic resistance, as lesions were significantly smaller compared to those that developed on plants treated with sap from untreated B73 or Δsm1-treated B73 (Figure 5A). Transfusion with xylem sap from untreated or TvWT-treated lox3-4 plants also resulted in significantly enhanced resistance, consistent with the findings of Constantino et al. (2013), while sap from Δsm1-treated lox3-4 did not. Transfusion with sap from untreated or Δsir1-treated lox10-3 enhanced resistance, thus mimicking the increased resistance of untreated lox10 mutant. However, transfusion with the sap from TvWT-treated or Δsm1-treated lox10-3 resulted in increased susceptibility. Interestingly, the sap transfusion results were not restricted to B73, as the sap receiver plants of another inbred line, Tx714, also displayed ISR phenotypes (Figure 5B). These results confirm that the ISR signal(s) are root derived, transported through xylem to above-ground organs to confer systemic resistance, and require functional LOX10. Furthermore, xylem sap from TvWT-treated lox10-3 conferred ISS to lox10-3 receiver plants, suggesting that sap from TvWT-treated lox10-3 contains an as-yet-unknown signal(s) responsible for ISS (Figure 5C). These results suggested that xylem sap from ISR-positive B73 (TvWT- and Δsir1-treated plants) and lox3-4 (untreated and TvWT-treated plants) were enriched with ISR positive signal(s) and therefore were ideal for metabolite profiling in our search for the ISR long-distance signal. These analyses also identified saps lacking ISR activity, which included B73 (untreated and Δsm1-treated plants) and lox3-4 (Δsm1- and Δsir1-treated plants).
Figure 5.
Treatment with Xylem-Derived Sap from the Wild Type and Mutants Treated with T. virens Phenocopies the ISR of the Respective Mutants.
(A) Lesion areas caused by C. graminicola on B73 receiver plants transfused with xylem sap from untreated or TvWT-, Δsm1-, or Δsir1-treated B73, lox3-4, or lox10-3 plants.
(B) Lesion areas caused by C. graminicola on Tx714 receiver plants after transfusion with xylem sap from untreated or TvWT-treated B73 or lox10-3 plants.
(C) Lesion areas caused by C. graminicola on lox10-3 (B73 background) receiver plants after transfusion with xylem sap from untreated or TvWT-treated B73, lox3-4, or lox10-3 plants.
Infected leaves were scanned and measured using ImageJ software to determine mean lesion area. Bars represent means ± se of the mean (n = 5 maize plants as biological replicates), with letters indicating significant differences among all treatments (Tukey’s HSD test, P < 0.05). These experiments were repeated at least two times with similar results.
The collected xylem saps were analyzed by liquid chromatography–tandem mass spectrometry (LC-MS/MS) to quantify ∼60 diverse oxylipins and several phytohormones, as presented in Supplemental Table 1 and Supplemental Data Set 1. To reduce the number of ISR-relevant candidate metabolites, the following criteria were used for selection of an ISR signal candidate. This molecule(s) had to accumulate at (1) increased levels in the saps from TvWT- and Δsir1-treated B73 and untreated and TvWT-treated lox3-4; (2) reduced levels in the untreated B73, Δsm1-treated B73, and Δsm1- and Δsir1-treated lox3-4; and (3) statistically lower levels in lox10-3 plants regardless of treatment compared with TvWT-treated B73. Of the metabolites and phytohormones detected, only 12-OPDA and KODA, a 9-LOX– and 9-AOS–derived α-ketol (Vick and Zimmerman, 1984; Yokoyama et al., 2000), met all three criteria (Figures 6A to 6C). Specifically, the results showed that these two oxylipins were enriched in the saps with potent ISR activity, which include TvWT- and Δsir1-treated B73 and untreated and TvWT-treated lox3-4 (Figures 6B and 6C). Xylem sap from the plants not displaying ISR, which consisted of untreated B73, Δsm1-treated B73, and Δsm1- and Δsir1-treated lox3-4, accumulated 12-OPDA and KODA at much lower levels. Surprisingly, neither JA nor JA-Ile met the three criteria, as their levels did not correlate with the sap ISR activity. Specifically, JA levels were significantly elevated in B73 colonized by Δsm1 and Δsir1 (Figure 6D). Interestingly, JA-Ile levels were not elevated in the saps from TvWT-treated B73 and untreated lox3-4 (Figure 7E), both of which induced strong ISR (Figure 4). Not surprisingly, low levels of 12-OPDA, JA, and JA-Ile were detected in all lox10-3 plants regardless of treatment (Figure 6), consistent with previous report that lox10 mutants were unable to accumulate the three jasmonates in wounded leaves (Christensen et al., 2013).
Figure 6.
Metabolites and Phytohormones Detected in Xylem Sap from Diverse T. virens–Treated Maize.
(A) Heatmap of accumulation of metabolite and phytohormone levels in xylem sap collected from untreated or TvWT-, Δsm1-, or Δsir1-treated B73, lox3-4, or lox10-3 14 d after the treatments, represented by Z-score transformed concentrations (number of standard deviations above/below the mean of the column). Combinations of maize and T. virens that lack ISR are represented in blue font, while combinations that are positive for ISR are represented in red font. Both 12-OPDA and KODA are boxed as the candidate ISR signals due to higher accumulation in ISR-positive saps and lower accumulation in ISR-negative saps. Full names of measured metabolites and phytohormones are listed in Supplemental Table 1.
(B) to (G) Concentrations of (B) 12-OPDA, (C) KODA, (D) JA, (E) JA-Ile, (F) traumatic acid, and (G) SA in xylem sap collected from untreated, TvWT-, Δsm1-, or Δsir1-treated B73, lox3-4, or lox10-3 plants. Bars represent means ± se of the mean (n = 5 maize plants as biological replicates), with letters indicating significant differences among all treatments (Tukey’s HSD test, P < 0.05). This experiment was repeated two times with similar results.
Figure 7.
Transfusion with the Sap from Untreated B73 Supplemented with 12-OPDA or KODA Increased Resistance in Receiver Plants in a Dose-Dependent Manner, While Sap Supplemented with JA-Ile Increased Susceptibility.
(A) to (E) Lesion area caused by C. graminicola on the leaves of B73 plants transfused with saps supplemented with different concentrations of (A) 12-OPDA (1, 10, 25, 100, or 1000 nM), (B) KODA (1, 10, 100, or 1000 nM), (C) PGA1 (1, 10, 100, or 1000 nM), (D) JA-Ile (1, 10, 100, or 1000 nM), and (E) 12-OPDA and KODA combined (10 nM). Positive control (+) treatment was transfusion with ISR-positive sap from TvWT-treated B73, while negative control (−) treatment was transfusion with ISR-negative sap from untreated B73. Infected leaves were scanned and measured using ImageJ software to determine mean lesion area. Bars represent means ± se of the mean (n = 5 maize plants as biological replicates), with letters indicating significant differences among all treatments (Tukey’s HSD test, P < 0.05). These experiments were repeated at least two times with similar results.
Because traumatic acid synthesis requires functional LOX10 in leaves (Christensen et al., 2013), this molecule was considered a potential ISR signal. However, contents of this molecule were reduced in sap from B73 treated with all T. virens strains (Figure 6F). Lastly, SA accumulation levels did not correlate with the ISR phenotypes conferred by the diverse sap samples and were not altered greatly between the different treatments (Figure 6G). These data suggest that of all the known LOX10 products, only 12-OPDA appears to be relevant for ISR. Levels of KODA, a 9-LOX product, appears to be influenced, but not directly produced, by LOX10, which is predominantly a 13-LOX (Nemchenko et al., 2006). Experiments are currently underway to identify which of the six maize 9-LOXs is responsible for the biosynthesis of KODA.
12-OPDA and KODA, but Not JA, Increase Plant Resistance in a Dose-Dependent Manner
To directly test the effect of 12-OPDA and KODA, B73 plants were transfused with different, but biologically relevant, concentrations of 12-OPDA or KODA to identify any effect on maize systemic resistance. To mimic the sap transfusion methods used previously, 12-OPDA or KODA was added to sap from untreated B73 plants and transfused to B73 receiver plants grown without T. virens. Positive control treatment for ISR activity was transfusion with the sap from TvWT-treated B73 plants, while negative control treatment was transfusion with the sap from untreated B73. After transfusion, the leaves of treated B73 plants were challenged with C. graminicola. Transfusion with the sap supplemented with 12-OPDA enhanced resistance in a dose-dependent manner, with significant reduction of lesion area conferred by 25 nM, 100 nM, and higher 12-OPDA (Figure 7A). Similarly, transfusion with KODA in a similar concentration range also resulted in a dose-dependent effect on enhancing resistance, with the strongest effect occurring between 100 and 1000 nM (Figure 7B).
As 12-OPDA is a reactive electrophilic species (RES), which contain a α,β-unsaturated carbonyl structure and have been shown to activate expression of many defense genes in Arabidopsis and maize (Alméras et al., 2003; Christensen et al., 2015), one possibility is that enhanced maize resistance associated with 12-OPDA transfusion was due to RES activity. In order to differentiate 12-OPDA–specific activity from more general RES activity, B73 were transfused with saps supplemented with equivalent to 12-OPDA range of concentrations of the mammalian hormone prostaglandin A1 (PGA1), a RES not produced in plants (Mueller et al., 2008). None of the concentrations of PGA1 used resulted in enhanced resistance, suggesting that 12-OPDA effect on maize resistance to C. graminicola was not due to RES activity (Figure 7C).
While JA-Ile was not an ISR signal candidate from our metabolite profiling of xylem saps, JA-Ile has been implicated in ISR regulation in other species. Therefore, we tested JA-Ile as a supplement in the sap transfusion experiments with the same range of concentrations. JA-Ile concentrations from 1 to 100 nM had no impact on lesion area (Figure 7D). Unexpectedly, JA-Ile at 1000 nM led to enhanced susceptibility. Furthermore, transfusion with the JA-Ile precursor, JA, at 1000 nM concentration also increased susceptibility (data not shown). Lastly, while transfusion with 10 nM 12-OPDA or KODA alone had no significant effect on B73 resistance, simultaneous application of both signals at 10 nM concentration resulted in stronger resistance in receiver plants, suggesting an additive or synergistic effect (Figure 7E). Taken together, these results indicate that 12-OPDA and KODA, not JA or JA-Ile, are the oxylipin signals that positively regulate ISR at physiologically relevant nanomolar concentrations.
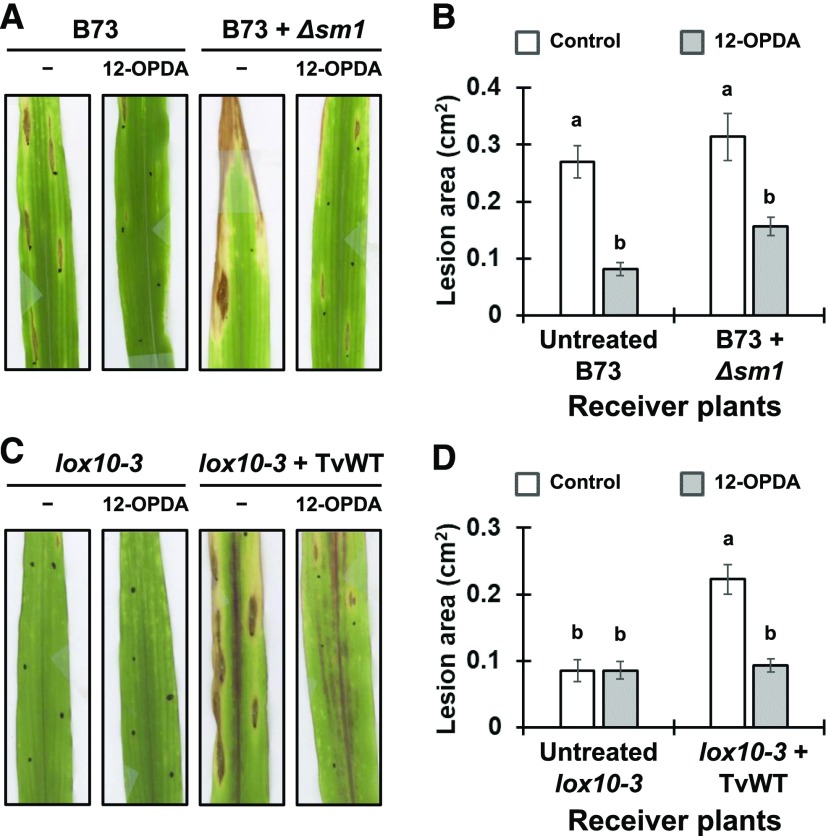
To test whether ISR deficiency of Δsm1-treated B73 can be rescued by exogenous application of 12-OPDA, we supplemented the saps from untreated B73 (no ISR signal) and Δsm1-treated B73 (no ISR signal) with 1 µM 12-OPDA. This treatment to both the untreated and Δsm1-treated B73 significantly increased resistance to C. graminicola (Figures 8A and 8B). These results suggest that 12-OPDA can complement the lack of ISR activity in both ISR-negative saps. To test whether 12-OPDA can reverse the ISS phenotype of TvWT-treated lox10-3, sap samples from untreated or TvWT-treated lox10-3 were supplemented with 12-OPDA and transfused into untreated lox10-3 or TvWT-treated lox10-3. The results showed that 12-OPDA restored TvWT-treated lox10-3 resistance to levels found in lox10-3 not colonized by TvWT, as evidenced by significantly decreased lesion area (Figures 8C and 8D). This suggested that TvWT-treated lox10-3 ISS is due to reduced 12-OPDA.
Figure 8.
Transfusion with the Sap Supplemented with Exogenous 12-OPDA Enhanced Resistance in B73 and Δsm1-Treated B73 and Restored Resistance of TvWT-Treated lox10-3.
(A) and (B) Visual representation of disease development (A) and lesion area (B) caused by C. graminicola on the leaves of untreated B73 or Δsm1-treated B73 receiver plants transfused with the sap from untreated B73 or Δsm1-treated B73 donor plants supplemented with 0 (control) or 1000 nM 12-OPDA.
(C) and (D) Visual representation of disease development (C) and lesion area (D) caused by C. graminicola on the leaves of untreated lox10-3 or TvWT-treated lox10-3 receiver plants transfused with the sap from untreated lox10-3 or TvWT-treated lox10-3 donor plants supplemented with 0 nM (control) or 1000 nM 12-OPDA.
Infected leaves were scanned and measured using ImageJ software to determine mean lesion area. Bars represent means ± se of the mean (n = 5 maize plants as biological replicates), with letters indicating significant differences among all treatments (Tukey’s HSD test, P < 0.05). This experiment was repeated two times with similar results.
JA Is Not Required for T. virens–Mediated ISR in Maize
The unexpected results from JA-Ile–enriched sap transfusion posed the question of whether JA-Ile was relevant to T. virens–triggered ISR in maize. To directly address this question, we utilized the JA-deficient opr7 opr8 double mutant, which is devoid of JA biosynthesis in every organ tested (Yan et al., 2012). This mutant was treated with TvWT and infected with C. graminicola. The untreated opr7 opr8 mutants were significantly more resistant to anthracnose leaf blight than untreated B73 plants (Figures 9A and 9B). Surprisingly, in response to TvWT colonization, lesion area was further significantly decreased on opr7 opr8 leaves, indicating that this mutant was still capable of ISR (Figures 9A and 9B). This was in stark contrast to lox3-4 plants, whose resistance did not benefit further with TvWT treatment (Constantino et al., 2013). These results provide strong genetic evidence that JA is not required for T. virens–triggered ISR in maize.
Figure 9.
JA Is Dispensable for ISR Induction by T. virens Treatment in the JA-Deficient opr7 opr8 Double Mutant.
(A) and (B) Visual representation of disease development (A) and lesion area (B) caused by C. graminicola on the leaves of untreated or TvWT-treated B73 or opr7 opr8 mutant plants.
Infected leaves were scanned and measured using ImageJ software to determine mean lesion area. Bars represent means ± se of the mean (n = 5 maize plants as biological replicates), with letters indicating significant differences among all treatments (Tukey’s HSD test, P < 0.05). This experiment was repeated at least three times with similar results.
Transcriptomic Analysis Reveals Induction of the Genes Involved in 12-OPDA Biosynthesis or Signaling but Suppression of Downstream JA Biosynthesis and Response Genes
Our findings prompted the hypothesis that JA biosynthesis and 12-OPDA biosynthesis genes may be differentially regulated by T. virens colonization. We tested this hypothesis by performing RNA sequencing (RNA-seq) analysis on the roots of B73 seedlings grown in hydroponic conditions and treated with TvWT at 0, 6, and 54 h. The time points represented fungal recognition at 6 h and advanced colonization at 54 h (Malinich et al., 2019). Importantly, the 54-h time point reflects 48 h after the initial 6-h harvest to avoid any transcriptome changes associated with the circadian clock.
In maize, 12-OPDA biosynthesis begins in the plastid with 13-LOXs (LOX7, LOX8, LOX9, LOX10, LOX11, and LOX13) activity, which converts C18:3 to 13(S)-hydroperoxylinolenic acid (Figure 10; Borrego and Kolomiets, 2016). As measured by fragments per kilobase of transcript per million mapped reads (FPKM), only LOX10 expression was induced in response to TvWT recognition, with approximately sixfold increase in transcript abundance compared with untreated plants, consistent with qPCR results (Figure 1A). LOX7 and LOX13 transcripts were not detected in any samples. LOX8 and LOX9 were expressed only in untreated plants, but they were suppressed by TvWT at both time points. While expression of LOX11 was unchanged between untreated and TvWT-treated B73 at 6 h, its expression was downregulated at 54 h. These data point to LOX10 as the sole 13-LOX that was rapidly induced by TvWT even before root colonization took place and support the previous data that LOX10 is induced by T. virens in a Sm1-dependent manner (Figure 1A).
Figure 10.
Maize-T. virens Interactions Upregulates Genes for 12-OPDA Biosynthesis and Response, but Not Genes Downstream of 12-OPDA for JA or JA-Ile Biosynthesis and Signaling.
(A) to (D) Heatmaps of transcript accumulation measured by RNA-seq of (A) the AOS pathway branch genes, (B) 12-OPDA–responsive genes, (C) JA downstream signaling genes, and (D) SA biosynthesis and response genes, represented by Z-score–transformed FPKM (number of standard deviations above/below the mean of the row). Values represent average FPKM, with asterisks (*) denoting significant differences compared with untreated plants (Tukey’s HSD test, P < 0.05). Treatments comprised of untreated B73 plants (n = 4), and TvWT-treated plants (n = 10) at 6 and 54 h. Gene IDs and functions of each gene are listed in Supplemental Table 2. n is the number of biological replicates consisting of plants grown in hydroponic jars, each consisting of five maize seedlings.
Expression of AOS1c and AOC1, which encode enzymes involved in conversion of 13(S)-hydroperoxylinolenic acid to 12-OPDA, was upregulated at 54 h. Multiple studies have identified genes that are specifically regulated by 12-OPDA, but not by other jasmonates (Taki et al., 2005). Several of these 12-OPDA marker genes were differentially expressed in response to TvWT treatment (Figure 10B). More specifically, a glycosyl hydrolase (GH9B8), known to be downregulated in response to 12-OPDA, was repressed after TvWT treatment. On the other hand, expression of several 12-OPDA–upregulated genes, such as Calcium binding EF-hand family protein (CML41), zinc finger transcription factor (ZBF4), and glutathione transferase (GST5), were all significantly increased at 54 h after treatment.
In agreement with the notion that JA is not a major player in ISR induction, expression of both OPR7 and OPR8, the only OPRs required for JA biosynthesis (Yan et al., 2012), were downregulated following colonization by TvWT (Figure 10). Furthermore, expression of several genes (ACX, MFP, and KAT) involved in β-oxidation were also downregulated at 54 h. Lastly, among the five JAR1 maize homologs responsible for the conjugation of Ile to JA (Borrego and Kolomiets, 2016), only JAR1b was expressed in the untreated roots, and its transcripts were not detected at 54 h. Taken together, these results suggested that some of the genes responsible for 12-OPDA biosynthesis were upregulated in response to TvWT colonization, but the genes responsible for converting 12-OPDA to JA-Ile were downregulated. qPCR validation of AOS1c, AOC1, and OPR7 showed similar trends of expression (Supplemental Figure 2). Our conclusions of induction of 12-OPDA biosynthesis and lack of induction of JA were further corroborated with metabolite profiling of TvWT-treated B73 seedlings roots, which showed significantly increased accumulation of 12-OPDA and KODA at 54 h, but not of JA or JA-Ile (Figures 11A to 11D).
Figure 11.
Metabolite Profiling of TvWT-Treated Maize Seedling Roots Reveal Increased Accumulation of 12-OPDA and KODA.
(A) to (E) Accumulation of (A) 12-OPDA, (B) KODA, (C) JA, (D) JA-Ile, and (E) SA in the root tissues of untreated or TvWT-treated B73 seedlings grown under hydroponic conditions at 6, 30, and 54 h after TvWT treatment. Bars represent means ± se of the mean (n = 5 biological replicates of plants grown in hydroponic jars, each consisting of five maize seedlings). Statistical significance was determined with Tukey’s HSD test (*P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001).
JA-Ile interaction with the COI1-JAZ coreceptor complex results in the degradation of JAZ proteins, negative regulators of JA responses, to allow transcriptional activation of MYC and WRKY transcription factors and induction of JA downstream signaling (Song et al., 2013). Of the six maize COI1 genes, five genes were expressed in untreated roots, two were rapidly induced at 6 h, and all three were substantially downregulated at 54 h (Figure 10C). Expression of transcription factors MYC7 and WRKY46, implicated in positive regulation of JA-responsive genes (Borrego and Kolomiets, 2016), were repressed at 54 h. Of the JAZ genes expressed in roots, JAZ5, JAZ6, and JAZ8 transcripts significantly increased at 54 h. Additionally, CYP94B3B1, which is involved in JA-Ile catabolism and also known as tasselseed5 (Lunde et al., 2019), was significantly upregulated by 54 h after TvWT treatment. In agreement with the repression of transcription factors and induction of JA negative regulators, expression of most JA-dependent genes, which include 9-LOX genes (LOX1 and LOX5), OPR genes (OPR2, OPR3, and OPR5), and a phospholipase, PLC (Yan et al., 2012), were downregulated at 54 h. Overall, upregulation of several JAZ genes, which encode JA-response repressors, and downregulation of JA-dependent genes suggest that T. virens colonization results in suppression of JA responses.
In agreement with observed suppression of JA signaling, several SA biosynthesis and signaling genes are significantly upregulated at the time of colonization, likely due to the well-documented SA-JA antagonism (Pieterse et al., 2012). Specifically, expression of all phenylalanine ammonia lyase (PAL) genes were strongly upregulated at 54 h, with the exception of PAL4 (Figure 10D). Similarly, expression of SA-responsive genes, PR1, PR5, and PR10, was significantly upregulated at 54 h as well. qPCR validation of PR1 and PR5 expression was in agreement with the RNA-seq results, showing that both transcripts were increased incrementally from 6 to 54 h (Supplemental Figure 2). Surprisingly, expression of NPR1, a major component of SAR signaling, was significantly downregulated at 54 h. While the transcriptome data suggest upregulation of SA biosynthesis and downstream signaling, metabolite profiling of TvWT-treated B73 seedlings roots actually showed no significant change in SA levels over the time course (Figure 11E).
DISCUSSION
12-OPDA Is a Mobile Signal for ISR Induction
Multiple long-distance signaling molecules that regulate SAR have been identified (Klessig et al., 2018). By contrast, long-distance signals responsible for establishing ISR remain less characterized, with most studies pointing to JA and ethylene as the two signaling pathways involved (Pieterse et al., 2014b). In this study, we utilized genetic, biochemical, and pharmacological approaches with ISR-positive and -negative mutants of both T. virens (Δsm1 and Δsir1) and maize (lox3 and lox10) to identify the JA precursor 12-OPDA as a major signal responsible for regulating T. virens–triggered ISR in maize. The lox10 mutants, reduced in 12-OPDA content (Christensen et al., 2013), not only lost the capacity for T. virens–induced ISR against both hemibiotrophic and necrotrophic pathogens but also displayed ISS in response to TvWT treatment (Figures 2 and 3). Importantly, 12-OPDA transfusion restored ISR in TvWT-treated lox10-3 to the levels observed with TvWT-treated B73 plants (Figure 8), indicating that 12-OPDA deficiency contributed to the lack of ISR in lox10 mutants. Furthermore, 12-OPDA levels in xylem sap were higher in plants that displayed ISR and lower in those that did not (Figures 6A and 6B). Transfusion with xylem sap supplemented with low doses of 12-OPDA conferred robust ISR phenotype, with increasing concentrations of 12-OPDA resulting in stronger systemic resistance against C. graminicola (Figure 7A). The dose-dependent effect of 12-OPDA in triggering maize systemic resistance is a characteristic property of a hormone. This enhanced resistance was not due to RES activity and was 12-OPDA specific, as transfusion with PGA1 had no effect on resistance against infection (Figure 7C). This was further supported by the lack of induction of RES-response marker genes, such as GST25 (Zm00001d048558), TolB (Zm00001d022277), and CYP81D11 (Zm00001d030856), in response to TvWT treatment (Supplemental Figure 3). One interesting observation is that despite the low levels of 12-OPDA, the untreated lox10 mutants were significantly more resistant compared with untreated B73 (Figures 2 and 3).
These results raise the question of how previous research did not identify 12-OPDA as an ISR signal. While 12-OPDA is a precursor of JA, there is growing evidence that the signaling functions of 12-OPDA are vastly different from those of JA (Dave and Graham, 2012; Christensen et al., 2015; Maynard et al., 2018). Microarray analysis of Arabidopsis treated with 12-OPDA, JA, or methyl-JA revealed a set of genes that responded only to 12-OPDA in a COI1-independent manner (Taki et al., 2005; Mueller et al., 2008). Recent studies revealed that there exists OPDA-Ile conjugate, lending credence to 12-OPDA being an independent signal in the same manner as JA (Floková et al., 2016). A recent maize study revealed that resistance to aphids (Rhopalosiphum maidis) is mediated by 12-OPDA, while JA-Ile was not required for defense against these sucking insects (Varsani et al., 2019). Additionally, Arabidopsis resistance to root-knot nematodes (Meloidogyne hapla) was reliant on the accumulation of 12-OPDA, not JA, as mutants deficient in the production of 12-OPDA and JA were hyper-susceptible, but mutants deficient in JA only were as resistant as the wild-type plants (Gleason et al., 2016). OPR3-silenced tomato mutants showed dramatically lower levels of 12-OPDA and downstream JA products; however, treatment with 12-OPDA, not JA, had a significant effect in restoring plant basal resistance against Botrytis cinerea (Scalschi et al., 2015). In AOC overexpressor lines of rice (Oryza sativa), higher accumulation of 12-OPDA led to increased resistance against brown planthoppers (Nilaparvata lugens), a piercing-sucking insect (Guo et al., 2014). Additionally, treatment with 12-OPDA, but not JA or JA-Ile, led to enhanced resistance against the planthoppers in rice. Supporting our findings, tomato JA-deficient mutant def1 lost the capacity for ISR against Fusarium infection when treated with T. virens (Jogaiah et al., 2018). While the authors assigned the lack of ISR to loss of JA in def1, they did not examine whether 12-OPDA may have been responsible for the loss of ISR, as the mutant was determined to be disrupted in both 12-OPDA and JA biosynthesis (Howe et al., 1996). Increase in 12-OPDA, but not JA, content also correlated with tendril coiling in Bryonia dioica (Stelmach et al., 1998; Blechert et al., 1999).
Interestingly, the liverwort Marchantia polymorpha accumulated 12-OPDA in response to wounding, but it was unable to produce JA (Yamamoto et al., 2015). Similarly, the moss Physcomitrella patens lacks the enzymes that convert 12-OPDA to JA, but it still maintained the components for JA perception and signaling (Stumpe et al., 2010; Ponce De León et al., 2012). Furthermore, a recent study found that M. polymorpha COI1 was functionally conserved with AtCOI1 but recognized dinor-OPDA as a ligand rather than JA-Ile (Monte et al., 2018). A single amino acid substitution between MpCOI1 and AtCOI1 was responsible for the switch in ligand specificity, suggesting that 12-OPDA is the more ancient jasmonate signal and that JA-Ile arose later. These revelations on COI1 also raise the possibility that, unlike the single COI1 in Arabidopsis, some of the six distinct COI1 proteins of maize, especially COI2, which is unable to complement a coi1 mutant of Arabidopsis (An et al., 2018), may perceive 12-OPDA rather than JA-Ile.
A possible explanation for why 12-OPDA is overlooked is that most research has focused specifically on JA. Since JA downstream signaling also results in a positive feedback loop for JA biosynthesis (Wasternack, 2007), JA-insensitive mutants would lack that positive feedback and be unable to trigger upregulation of both JA and 12-OPDA biosynthesis. Tomato spr2, the mutant in a fatty acid desaturase, is impaired in wound-induced JA biosynthesis due to more than 90% decreased C18:3 content in leaves (Li et al., 2003), and it is unable to establish mycorrhiza-induced resistance (Song et al., 2015). It was not reported whether this mutant also lacked 12-OPDA in roots, although 12-OPDA levels were significantly reduced in seeds (Goetz et al., 2012).
Furthermore, treating cucumber (Cucumis sativus) roots with the 12-OPDA and JA biosynthesis inhibitor diethyldithiocarbamic acid (Farmer et al., 1994), blocked Trichoderma asperellum–triggered ISR (Shoresh et al., 2005). In both cases, the focus of the studies was on the end product, JA, and not the intermediaries such as 12-OPDA.
9-Oxylipin KODA Is a Novel Candidate Long-Distance ISR Signal
Another key finding of this study is the identification of KODA as another potential ISR signal. While accumulation of KODA, a 9-LOX and 9-AOS pathway product (Yokoyama et al., 2000; Howe and Schilmiller, 2002; Sakamoto et al., 2010), in xylem sap may be affected by LOX10 function, LOX10 is a 13-LOX (Nemchenko et al., 2006) and therefore is not likely to be responsible for KODA production. The function of KODA has been studied only rudimentarily, with several reports indicating that KODA has flowering-promotion effects in duckweed (Lemna paucicostata), apple (Malus domestica) trees, and Japanese pear (Pyrus pyrifolia; Yokoyama et al., 2000; Kittikorn et al., 2010; Sakamoto et al., 2010). KODA was also reported to accumulate at high levels in duckweed after exposure to abiotic stresses such as drought, heat, or osmotic stresses (Yokoyama et al., 2000). Wheat treated with KODA displayed enhanced abiotic stress tolerance by promoting root elongation, increased germination rate and seedling growth, and improved drought tolerance (Haque et al., 2016). One study demonstrated that exogenous application of KODA enhanced grape resistance against Glomerella cingulata and induced high levels of LOX and AOS expression (Wang et al., 2016), which may suggest that KODA induced 12-OPDA biosynthesis. Interestingly, application of KODA had no impact on pathogen growth on agar media, suggesting that KODA had no antimicrobial properties (Wang et al., 2016). This is in stark contrast to 12-OPDA, which has been reported to inhibit growth of several fungal pathogens (Prost et al., 2005). This brings up the question of whether sap-transfused 12-OPDA in this study may have direct antifungal activity against C. graminicola in leaves. However, the concentration used in the Prost et al. (2005) study was 100 μM, a concentration 100 times higher than the highest concentration of 1 μM used in this study. Additionally, while we did observe higher levels of 12-OPDA and KODA in B73 roots in response to TvWT treatment, levels of both metabolites in shoot tissue were not significantly altered (Supplemental Figure 4). It is equally unlikely that sap-transfused 12-OPDA had direct antifungal activity, since untreated lox10 contained significantly lower levels of 12-OPDA in the leaves (Christensen et al., 2013), yet was significantly more resistant to infection (Figures 2 and 3). While JA and 12-OPDA have been reported to inhibit root growth, it is possible that increased KODA production in response to T. virens may be behind simultaneous T. virens–promoted growth promotion and ISR induction. Efforts are currently being taken to screen the available 9-LOX maize mutants to identify which is responsible for KODA production.
Is JA a Long-Distance Signal for ISR?
JA signaling is well established as essential for ISR in the Pseudomonas fluorescens WCS417r-triggered ISR in Arabidopsis Columbia 0 (Col-0), as the jar1 mutant lacked the ISR response against Pseudomonas syringae pv tomato (Pst; Pieterse et al., 1998, 2014b). Surprisingly, our study showed that JA and JA-Ile levels in xylem sap did not correlate with the ISR expression in diverse maize–T. virens genotype combinations (Figure 6). Rather, transfusion with sap supplemented with JA-Ile resulted in enhanced susceptibility (Figure 7D). Importantly, the analysis of ISR competency of the JA-deficient mutant opr7 opr8 (Yan et al., 2012) showed that they were capable of mounting ISR (Figure 9A). Moreover, transcriptome analysis of TvWT-treated B73 plants showed upregulation of plastid-localized 12-OPDA biosynthesis genes (LOX10, AOS1c, and AOC1) and several 12-OPDA–specific marker genes but downregulation of JA biosynthesis genes downstream of 12-OPDA (OPR7, OPR8, β-oxidation genes, and JAR1) during colonization (Figure 10). Several JA-Ile repressor or catabolism genes, which include JAZ genes and CYP94B1 (Lunde et al., 2019), respectively, were significantly upregulated, indicating suppression of JA signaling upon TvWT colonization. In support of the transcriptional changes, metabolite profiling of TvWT-treated B73 roots showed increased 12-OPDA and KODA and either low or undetected levels of JA and JA-Ile (Figure 11). Taken together, these results implicate 12-OPDA and KODA, not JA, as the mobile ISR signals in maize. In support of JA being dispensable for ISR, the MiSSP7 effector-mediated suppression of JA was shown to be essential for forming symbiosis between the ectomycorrhizal fungus Laccaria bicolor and poplar trees (Populus sp; Plett et al., 2011). This effector was shown to stabilize poplar JAZ6 protein, thus repressing JA signaling and allowing for better root colonization (Plett et al., 2014). Similarly, interactions between gray poplar (Populus canescens) and the ectomycorrhizal fungus Paxillus involutus were reported to increase SA and decrease JA in the roots (Luo et al., 2009).
It is possible that JA was not necessary for T. virens–triggered ISR in maize but may be required for interactions between other plant hosts and beneficial microbes. While P. fluorescens strain WCS417r-triggered ISR in Arabidopsis Col-0 against Pst infection (Pieterse et al., 1998), P. fluorescens strain CHA0r could not (Iavicoli et al., 2003). Instead, CHA0r-triggered ISR protected Col-0 against Peronospora parasitica infection, but it could not protect the Wassilewskija (Ws)-0 ecotype against the same pathogen. Similarly, T. harzianum–triggered ISR in Col-0, but not in the other ecotypes such as Landsberg erecta, Ws-4, or Nossen (Korolev et al., 2008). Furthermore, while the bacterium Paenibacillus alvei K165 conferred systemic resistance to Arabidopsis against Verticillium dahliae infection, it did not rely on JA signaling, as the jar1 mutant retained ISR (Tjamos et al., 2005). Rather, eds5/sid1 and sid2 mutants lost ISR when treated with K165, suggesting that SA played a prominent role in this system. Another example of the complexity of ISR signaling is the study showing the beneficial fungus Penicillium viridicatum was able to trigger ISR in both jar1-1 and npr1-1 mutants against Pst infection (Hossain et al., 2017). While these studies do not address the role of 12-OPDA or KODA, they strongly suggest that there is no consensus ISR signaling pathway and that ISR arises based on specific plant host, beneficial microbe, and pathogen combinations.
SA Roles in Maize–T. virens Interactions
Our data showed that while SA biosynthesis and responsive genes were strongly upregulated in response to TvWT colonization (Figure 10), SA levels in TvWT-treated B73 roots remained unchanged over time (Figure 11E). This may indicate that there exists a fine-tuned balance in the regulation of this defense hormone to accommodate symbiotic interactions. This balance may be regulated not only by the host but also by the beneficial microbes. For example, a study has shown that induction of SA-mediated defenses is necessary to limit Trichoderma colonization of roots to the apoplast and exclude colonization of the vascular tissue (Alonso-Ramírez et al., 2014). When colonized by T. harzianum, the Arabidopsis SA-induction-deficient mutant sid2 displayed stunted growth and root rot, and T. harzianum was able to spread systemically through the vascular tissue and into the leaves. Additionally, several Trichoderma species were shown to degrade SA (Navarro-Meléndez and Heil, 2014; Martínez-Medina et al., 2017; Jogaiah et al., 2018). Our data do not clarify whether SA is required for T. virens–triggered ISR in maize, as we did not observe SA increase in xylem sap samples from TvWT-treated plants (Figure 6G) or in the roots (Figure 11E). Interestingly, while SA levels in roots were not altered by TvWT colonization, SA levels in shoot did increase at 54 h (Supplemental Figure 4E). Recently, several salicylate monooxygenase/hydroxylase genes, which degrade SA, were identified in T. virens as differentially expressed genes in the presence of maize (Malinich et al., 2019). Therefore, it is possible that T. virens may use these enzymes to prevent elevation of host SA levels during colonization of roots. SA-deficient plants will be required to establish the function of SA in ISR.
T. virens Does Not Produce 12-OPDA, KODA, or JA Itself, but Uses Peptide Signals to Differentially Regulate Host Oxylipin Synthesis for ISR
As reported in this study, LOX10 transcriptional activation as early as 3 to 6 h during interaction between maize roots and T. virens required functional fungal peptide signal Sm1 (Figure 1A). Such early activation is consistent with a previous report showing Sm1 is constitutively expressed and secreted continuously by fungal hyphae even in the absence of a host (Djonović et al., 2006). Here, we provided additional evidence that Sm1 is a major peptide signal for ISR by demonstrating that the enhanced ISR activation by Δsir1 mutant is likely due to a threefold greater expression of SM1 (Figure 1C). The antagonistic interactions between Sm1 and Sir1 indicate that these peptides may be responsible for different aspects of regulating maize–fungal symbiosis, although the exact mode of action on plant cells by Sm1 or Sir1 remains to be explored.
We had also considered the possibility that T. virens may itself be producing the 12-OPDA or KODA that we detected in xylem sap. There is a precedent for plant-associated microbes producing oxylipins such as 12-OPDA and JA. The plant pathogenic fungi Lasiodiplodia theobromae (Tsukada et al., 2010) and Fusarium oxysporum f. sp tulipae (Oliw and Hamberg, 2017) were shown to produce 12-OPDA and other jasmonates. Additionally, several endophytic bacteria isolated from sunflowers (Helianthus annuus) produce 12-OPDA and JA in growth media and under drought conditions (Forchetti et al., 2007). With regard to T. virens, however, we were unable to detect any discernable chromatograph signals for 12-OPDA, KODA, or JA within the fungus or in the surrounding media.
Does the Balance between TvWT-Induced Growth Promotion and ISR Involve Differential Regulation of 12-OPDA and JA?
In the field of symbiont–host interactions, one of the mysteries that remains to be explored is the mechanism by which beneficial microbes induce ISR defense and promote plant growth simultaneously. Plant growth and defense trade-offs can occur depending on environmental cues, prompting the plant to devote more resources toward defense responses or toward growth in the absence of stresses (Huot et al., 2014). There is overwhelming evidence that increased JA can result in impaired plant growth (Wasternack and Hause, 2013; Huang et al., 2017; Dubois et al., 2018). Exogenous JA treatment results in reduced root growth, leaf expansion, and hypocotyl growth in Arabidopsis (Wasternack and Hause, 2013). Most recently, overexpression of a plastid lipase gene was shown to result in significantly higher accumulation of JA, reducing vegetative growth even when Arabidopsis was grown in nutrient-rich media (Wang et al., 2018).
In maize, improper balancing between plant growth and defense could be best observed with lox3 mutants, which overproduce defense hormones SA, JA, and ethylene in the roots and display constitutive ISR, but have reduced germination rate, reduced height by ∼25 to 30%, and prematurely senesce (Gao et al., 2008). If JA is a major signal for ISR induction by beneficial microorganisms, but inhibits growth, then it remains unclear how the symbionts could also promote plant growth. The identification of 12-OPDA and KODA, rather than JA, as ISR signals provides an intriguing venue to explore how this widely reported growth-defense balance by T. virens is achieved. Currently, studies on the effect of 12-OPDA on plant growth suggest an inhibitory role similar to that of JA, although the concentrations used in those studies may not have been biologically relevant (25 and 50 μM; Yamamoto et al., 2015). While 12-OPDA was shown to promote Arabidopsis (Col-0 and Ws backgrounds) seed dormancy, Yamamoto et al. (2015) and Dave et al. (2011) used much higher concentrations (10 and 50 μM) in the growth media. Our results showed that despite triggering ISS and over-colonizing lox10 roots (Supplemental Figure 1A), T. virens had no detrimental effect on lox10 plant biomass (Supplemental Figures 1B and 1C). The different levels of 12-OPDA between B73 and lox10-3 mutant plants did not correlate with plant growth, suggesting that growth promotion and ISR are distinct pathways. Supporting this notion is the previous report showing that T. harzianum was able to enhance growth of Arabidopsis mutants that were unable to mount ISR (Korolev et al., 2008).
Working Model of Oxylipin Regulation of ISR Response
A summary of the maize ISR process induced by T. virens is presented in the working model presented in Figure 12. In short, T. virens root colonization results in the suppression of the negative regulator of ISR, LOX3, in a Sm1-dependent manner (Constantino et al., 2013), which allows the upregulation of LOX10 (Gao et al., 2008; Constantino et al., 2013). Upregulation of LOX10 and the 12-OPDA biosynthesis genes (AOS1c and AOC1) results in increased accumulation of 12-OPDA. Simultaneously, JA biosynthesis and signaling are downregulated, which may help accommodate root colonization by T. virens. It is possible that increased 12-OPDA leads to increased biosynthesis of yet additional mobile signal, KODA, produced in the 9-LOX/9-AOS branch of the LOX pathway. Both 12-OPDA and KODA are transported through xylem to confer systemic resistance throughout the rest of the plant.
Figure 12.
Model of T. virens–Triggered ISR in Maize.
The below-ground interactions between maize roots and T. virens result in the transcriptional and metabolic reprogramming of the roots that lead to synthesis of a long-distance ISR signal(s) that travels systemically within the xylem to confer resistance throughout the plant against a broad range of pathogen infection. T. virens produces the small peptides Sm1, which promotes ISR, and Sir1, which suppresses expression of SM1, resulting in suppression of ISR. The fungus suppresses LOX3 and induces LOX10 expression, either sequentially or concurrently, in a Sm1-dependent manner. The ISR signals, 12-OPDA and KODA, are able to significantly improve systemic resistance and rescue susceptibility.
METHODS
Plant and Fungal Material
The maize (Zea mays) inbred lines B73, W438, and Tx714 and mutator transposon knockout mutants lox3-4 (Gao et al., 2007), lox10-2, lox10-3 (Christensen, 2009), and double mutant opr7 opr8 (opr7-5 opr8-2; Yan et al., 2012) were used in this study. The mutant maize lines are all near-isogenic at the BC7 genetic stage in the B73 or W438 genetic backgrounds. The double mutant lox3 lox10 was generated by crossing single mutants lox3-4 and lox10-3 at the BC7 stage and identified from the segregating populations of BC7F2 by using PCR primers as described previously by Gao et al. (2007) and Christensen et al. (2013).
Strains Gv29-8 (TvWT; Baek and Kenerley, 1998), Δsm1 (Djonović et al., 2006), and Δsir1 (formerly Δ77560; Lamdan et al., 2015) of Trichoderma virens were grown on Potato Dextrose Agar (catalog no. 213200, BD Difco Laboratories) at 27°C from glycerol stock cultures maintained at −80°C. Chlamydospores of T. virens were obtained from cultures grown in molasses-yeast extract medium (Mukherjee and Kenerley, 2010). After 2 weeks, the incubated cultures were vacuum filtered and air dried, and the mycelial mat containing chlamydospores was ground to fine powder. Colletotrichum graminicola (1.001 strain) on Potato Dextrose Agar and Cochliobolus heterostrophus on complete medium with Xyl (substituted for Glc to improve conidiation) were grown at room temperature (21 to 23°C) under fluorescent lights (Tzeng et al., 1992).
Soil Growth Conditions
Maize seeds were surface sterilized with 70% (v/v) ethanol for 5 min, followed with a 0.6% (v/v) sodium hypochlorite wash for 5 min, and then rinsed three to five times with sterile water. The seeds were planted in sterilized (steam sterilized for 1 h, cooled overnight, and sterilized again for 1 h) soil (MetroMix 360 Rsi, Sun Gro Horticulture) in long cone-tainers (catalog no. SC10R, Stuewe and Sons). The plants were grown on light shelves at room temperature (21 to 23°C) under three 6500K blue and three 3000K red fluorescent lights (catalog nos. 901618 and 901619, respectively; Spectralux) with a 14-h light period.
ISR Assay
Infection with C. graminicola and C. heterostrophus was performed in this study as described by Gao et al. (2007). Seven days after maize seeds were planted, similarly developed seedlings were either left untreated or treated with 0.1 g of T. virens chlamydospores (added to the soil at a depth of 4 to 5 cm). When the plants reached vegetative stage 4, the third true leaves were infected with C. graminicola or C. heterostrophus. Briefly, the plants were placed in trays (78.5 × 63 × 7 cm) lined with paper towels with the third leaves taped down flat. To harvest spores, 5 mL of sterile water was added to C. graminicola or C. heterostrophus plates that were then scraped with an inoculating loop to free the conidia. The suspension was filtered through sterile cheesecloth into a 50-mL Falcon tube to remove mycelia. The conidial suspension was centrifuged twice at 3000 rpm for 3 min, with the water being replaced each time with fresh sterile water. The initial conidia concentration was determined using a hemocytometer. The suspension was finally diluted to a concentration of 106 spores/mL. Ten microliters of the spore suspension (10,000 spores) was drop inoculated to six individual areas of the middle part of the leaf on both sides of the midvein. Paper towels were moistened with sterile water and were then covered with plastic wrap (GLAD Press’n’Seal) to create a humid chamber. The humidity chambers were placed in darkness for 24 h at 25°C. The plants were then positioned upright and returned to light shelves. Leaves were scanned 4 d after infection, and lesion area was determined using the ImageJ software (https://imagej.nih.gov/ij/).
Root Colonization Assay
Surface-sterilized maize seeds were germinated on Luria-Bertani (LB; catalog no. 244510, BD Difco Laboratories) agar plates for 3 d and then transplanted 2.5 cm deep into sterile, capped test tubes with 30 g of sterile soil (2:3 ratio of sand to loam) mixed with sterile water (ratio, 1 mL/10 g of soil) and T. virens chlamydospores (0.03 g chlamydospore/1 g of soil) with a hand mixer for 1 min. After 7 d, the plants were gently removed from the test tubes, and soil was gently rinsed off the roots. The roots were washed by submerging in 1% (w/v) sodium metaphosphate solution and shaking at 50 rpm for 30 min. The roots were subsequently washed with sterile water at 50 rpm for 30 min and then surface sterilized by submerging in 1% (v/v) bleach and shaking at 50 rpm for 1 min. The roots were washed with sterile water until the bleach smell was removed. The sterilized roots were measured for total biomass, cut into small pieces, and laid flat on Trichoderma-selective GVSM media (Park et al., 1992). After 48-h incubation at 27°C, colony-forming units emerging from the roots were counted. Root colonization data are expressed as colonies per gram of root weight.
Xylem-Enriched Sap Collection
Xylem-enriched sap was collected from B73, lox3-4, and lox10-3 plants treated with the wild type, Δsm1, or Δsir1 strains of T. virens or left untreated as described above. Seven days after maize seeds were planted, similarly developed seedlings were either left untreated or treated with 0.1 g of T. virens chlamydospores and placed on the growth shelves as described above. After 14 d when the plants reached vegetative stage 4, the plants were kept well saturated with water (Constantino et al., 2013). The plants were then decapitated by a diagonal cut with a scalpel above the first leaf. The first droplet of sap was discarded to avoid collecting wounding-related signals. The droplets after that were collected for 3 h and stored on ice. The plants were also periodically recut when sap flow declined, with the first new droplet being discarded. The saps were spun down to remove debris, and the final sap collections were stored in −80°C until needed for the experiments.
Xylem Sap Transfusion Assay
Collected xylem-enriched saps were transfused into a small cut along the pseudo-stem of receiver plants as described by Constantino et al. (2013). In short, to perform sap transfusion, receiver plants were placed on their sides in trays lined with paper towels with the third true leaves taped down flat as previously described by Constantino et al. (2013). One incision was made with a scalpel between the first and second leaves, and 10 μL of water or xylem sap diluted 1:1 with water was added into each incision. For transfusion assays with saps supplemented with 12-OPDA (catalog no. 88520, Cayman Chemical), KODA (catalog no. 13-1828, Larodan AB), PGA1 (catalog no. 10010, Cayman Chemical), or JA-Ile (catalog no. 10740, Cayman Chemical), the protocol for xylem sap transfusion assay was slightly modified. Xylem sap from untreated B73 was diluted 1:1 with water for control or different concentrations of the metabolites. Three hours after sap transfusion, plant leaves were drop inoculated with C. graminicola as described above.
Quantification of Xylem Sap Metabolites
Xylem-enriched sap and fresh maize tissue metabolites were quantified using LC-MS/MS. For analysis of metabolites in xylem-enriched sap samples, 90 μL of a 1:1 diluted sap solution was mixed with 10 μL of 5 μM isotopically labeled internal standards [d-ABA ([2 H6](+)-cis,trans-abscisic acid, OlChemIm), d-IAA ([2 H5]indole-3-acetic acid, OlChemIm), d-JA (2,4,4-d3; acetyl-2,2-d2 JA, CDN Isotopes), and d-SA (d6-SA, Sigma-Aldrich)]. For analysis of metabolites in fresh tissue, 100 mg of root tissue was finely ground under liquid nitrogen and mixed with 500 μL of phytohormone extraction buffer (1-propanol/water/HCl [2:1:0.002 v/v/v]) containing 5 μM of the isotopically labeled internal standards listed above. The samples were agitated for 30 min at 4°C, and 500 μL of dichloromethane was added to each sample. The samples were agitated again for 30 min at 4°C and then centrifuged at 14,000g for 5 min. The lower layer of each sample was transferred to a glass vial for evaporation under a nitrogen gas stream. The samples were resuspended in 150 μL of methanol, transferred to a 1.5-mL microcentrifuge tube, and centrifuged at 14,000g for 2 min to pellet any debris. Supernatant (90 µL) of each sample was transferred into autosampler vials with glass inserts. For both sample-types, a 15-μL aliquot was injected into an API 3200 LC-MS/MS system (Sciex) using electrospray ionization in negative mode with multiple reaction mentoring. After integration with Analyst v1.6.3 (Sciex), the concentrations of endogenous metabolites were determined by comparing peak areas against isotopically labeled internal standards using appropriate response factors. The chromatography was performed with an Ascentis Express C-18 column (3 cm × 2.1 mm, 2.7 µm; Sigma-Aldrich). The mobile phase was set at 400 mL/min consisting of solution A (0.02% [v/v] acetic acid in water) and solution B (0.02% acetic acid in acetonitrile) with a gradient consisting of the following (time in minutes – % [v/v] solution B): 0.5 – 10%, 1.0 – 20%, 21.0 – 70%, 24.6 – 100%, 24.8 – 10%, 29 – stop. All hormones and oxylipins were quantified by comparing levels of endogenous metabolites to isotopically labeled standards from Sigma-Aldrich, Cayman Chemical, and Larodan AB, with appropriate response factors (Müller and Munné-Bosch, 2011; Christensen et al., 2014). The metabolite and phytohormone m/z values and retention times are listed in Supplemental Table 1.
Hydroponic Growth Conditions for RNA-Seq
To study the effects of colonization by T. virens on maize root transcriptome, a hydroponic system as described by Djonovic et al. (2007) was used to prevent contamination and minimize mechanical damage. Maize seeds were surface sterilized with a 70% (v/v) ethanol wash for 5 min, rinsed with sterile water, washed with 10% (v/v) hydrogen peroxide for 2 h, and finally rinsed with sterile water. The seeds were plated on LB agar plates and incubated at 28°C in humidity chambers. The seeds were checked every day for signs of bacterial or fungal contamination, and clean seeds were carefully transferred to fresh LB plates. After 7 d, clean and uniformly germinated seeds were selected and placed in hydroponic jars consisting of a Ball wide mouth canning jar (16 oz) with a 125-mL shaker clamp (catalog no. EW-51706-78, Thermo Fisher Scientific) supporting a plastic mesh stage, and filled with 200 mL of half-strength Murashige and Skoog (MS) media with Gamborg vitamins (catalog no. M0404, Sigma-Aldrich) at pH 5.6. Each jar contained five seedlings positioned so that the tap roots threaded through the mesh and were in contact with the MS media. The jars were capped with the bottom of sterile plastic Petri plates (100 × 15 mm) and placed on shakers (50 rpm) at 25 to 27°C with a 16-h-light/8-h-dark photoperiod for 4 d and light intensity of 150 μmol m−2 s−1. Twenty-four hours before treatment, TvWT was cultured in 1 L of Potato Dextrose Broth in a Fernbach flask at a concentration of 105 spores/mL. The mycelia were collected by filtration with sterile nylon and washed with sterile water. One gram of fresh weight of the mycelial inoculum was added to the jars, after which a second mason jar (24-oz wide mouth canning jar [Ball]) was placed atop the first jar and held in place with parafilm to allow unimpeded shoot growth. Root tissue was harvested at 6 and 54 h after adding T. virens inoculum to the hydroponic jars with maize seedlings. The shoot tissue included all parts above the maize mesocotyl, while the root tissue included the radicle, lateral, and seminal roots. The roots were not rinsed with water when collected, especially at 54 h when the T. virens tissue was inextricable from maize root tissue. All the tissue samples collected were immediately flash frozen in liquid nitrogen and preserved at −80°C until RNA extraction.
RNA Extraction and RNA-Seq Analysis
RNA was extracted from root using a modified method for the RNeasy Plant Mini kit (catalog no. 74903, QIAGEN). The tissue samples were first ground in liquid nitrogen, and 1 μg of the tissue was aliquoted for RNA extraction. While the samples were still chilled in liquid nitrogen, 1 mL of TRI reagent (catalog no. TR118, Molecular Research Center) was added to each sample and mixed well. The samples were left at room temperature for 5 min before 200 μL of chloroform was added and mixed well. The samples were stored at room temperature for 10 min before being centrifuged at 13,000g at 4°C for 15 min. The supernatant was transferred to a new tube containing 500 μL of isopropanol. The samples were gently mixed and stored at room temperature for 10 min prior to transfer into the RNeasy spin columns (Qiagen). The samples were then processed following the manufacturer’s instructions. For each sample, 3 μg of RNA was treated with DNase I (catalog no. EN0521, Thermo Fisher Scientific) following the manufacturer’s instructions. RNA-seq analysis was performed at the Texas A&M AgriLife Genomics and Bioinformatics Service. The cDNA libraries were created with the NEXTflex Rapid Illumina Directional RNA-Seq Library Prep kit (catalog no. NOVA-5138-07, Bioo Scientific). Sequencing parameters were 50-bp paired end reads, to a depth of 250 million reads, on a NovaSeq 6000 system (catalog no. 20012850, Illumina).
RNA-Seq Data Analysis
Base-calling, quality checking, and removal of adaptor sequences was performed by the Texas A&M AgriLife Genomic and Bioinformatics Service as per their standard operating procedure. Raw, paired end, 50-bp reads were then aligned back to the B73 reference genome sequence (AGPv4 release 38) via the TopHat2 v2.1.0 pipeline (Kim et al., 2013). Uniquely aligned reads were counted with the HT-Seq 0.6.1 pipeline (Anders et al., 2015) using the Ensembl GCA_000005005.6 Zm-B73-REFERENCE-GRAMENE-4.0 for annotation. The FPKM values were determined using the Ballgown (v2.10.0) pipeline (https://ccb.jhu.edu/software.shtml). The raw and processed RNA-seq data from this study have been deposited at Gene Expression Omnibus (https://www.ncbi.nlm.nih.gov/geo/).
qPCR Analysis
RNA-seq results were validated with qPCR. The qPCR reactions were set up using the Verso 1-step RT-qPCR kit, SYBR Green, ROX (catalog no. AB4106A, Thermo Fisher Scientific) following the manufacturer’s procedure with 10-μL volume reactions. The samples were run in the StepOne Real-Time PCR system (catalog no. 4376357, Applied Biosystems) using the following conditions: 48°C for 30 min, 95°C for 10 min, followed by 40 cycles of 95°C for 15 s, 54°C for 30 s, 72°C for 30 s. Melt curve conditions were 95°C for 15 s, 60°C for 1 min, 95°C for 15 s. After normalizing to maize α-TUBULIN, relative expression was determined as fold change between untreated B73 and TvWT-treated B73 using the 2−ΔΔCT method (Czechowski et al., 2005; Xia et al., 2014). Expression of LOX10 in B73 roots in response to treatment with TvWT, Δsm1, or Δsir1 was performed in a similar manner. Expression of T. virens SM1 and SIR1 was normalized to Actin. Primers used are listed in Supplemental Table 3.
Confocal Microscopy for LOX10-YFP
Transgenic maize producing LOX10-YFP fusion proteins (Mohanty et al., 2009) were grown under hydroponic conditions as stated previously. After treatment with TvWT, roots were harvested every 12 h for 72 h, and images were collected using the Nikon FN1 C1si confocal microscope.
Statistical Analysis
Statistically significant differences were determined by one-way ANOVA followed by post hoc tests. Student’s t test was used for comparisons between two sample groups, while Tukey honestly significant difference (HSD) test was used for other comparisons. A value of P < 0.05 was considered to be statistically significant. The number of biological replicates and significance thresholds for each experiment are indicated in the figure legends. Details of statistical results are in Supplemental Data Set 2.
Accession Numbers
RNA-seq data from this study can be found at Gene Expression Omnibus under accession number GSE137826. Maize gene accession numbers are listed in Supplemental Table 2.
Supplemental Data
Supplemental Figure 1. Increased root colonization of lox10-3 by T. virens has no detrimental effects on plant growth and development (supports Figure 2 and 3).
Supplemental Figure 2. qPCR validation of RNA-seq transcriptomic analysis (supports Figure 10).
Supplemental Figure 3. Maize-T. virens interactions do not upregulate RES response genes (supports Figures 7 and 10).
Supplemental Figure 4. Metabolite profiling of seedling shoot tissue from TvWT-treated maize reveals significantly increased SA, but not 12-OPDA, KODA, JA, or JA-Ile (supports Figures 10 and 11).
Supplemental Table 1. Common and formal names, m/z, and retention times of oxylipins and phytohormones in this study.
Supplemental Table 2. IDs, names, and functions of genes in this study for the RNA-seq results.
Supplemental Table 3. List of the genes and the primer sequences for RT-qPCR used in this study.
Supplemental Data Set 1. Detected metabolites and phytohormones in collected xylem sap samples. LC-MS/MS.
Supplemental Data Set 2. Statistical Report of ANOVA Results for the data presented in each figure.
DIVE Curated Terms
The following phenotypic, genotypic, and functional terms are of significance to the work described in this paper:
Acknowledgments
We thank Pei-Cheng Huang, Zack Gorman, Nasie Constantino, Ramadhika Damarwinasis, John Bennet, James Taylor, and Robert Dorosky (Texas A&M University) for help in preparing hydroponic growth of maize and tissue harvesting. Brianna Hankinson, Joseph Vasselli, and Andrew Horgan (Texas A&M University) are acknowledged for contributions in ISR assays, xylem sap collection, xylem sap transfusions, and RNA extractions. We thank Kacey Wilson (Texas A&M University) for contributions in root colonization assays. Jordan Tolley (Texas A&M University) is acknowledged for LOX10-YFP fusion protein imaging using confocal microscopy. We thank Elizabeth Malinich for guidance on RNA-seq data analysis. We acknowledge the Texas A&M AgriLife Genomics and Bioinformatics Service for their sequencing services and the Texas A&M High Computing Research Center for providing supercomputing capability for RNA-seq analysis. This work was supported by the USDA-National Institute of Food and Agriculture (NIFA grant 2016-67013-24730 to C.M.K. and M.V.K and 2017-67013-26524 to M.V.K.).
AUTHOR CONTRIBUTIONS
K.-D.W., E.J.B., C.M.K., and M.V.K. designed the research and analyzed data; K.-D.W. performed most of the experiments; E.J.B. quantified metabolites via LC-MS/MS; and K.-D.W., C.M.K., and M.V.K. wrote the article with input from and revisions by E.J.B., C.M.K., and M.V.K.
References
- Alméras E., Stolz S., Vollenweider S., Reymond P., Mène-Saffrané L., Farmer E.E. (2003). Reactive electrophile species activate defense gene expression in Arabidopsis. Plant J. 34: 205–216. [DOI] [PubMed] [Google Scholar]
- Alonso-Ramírez A., Poveda J., Martín I., Hermosa R., Monte E., Nicolás C. (2014). Salicylic acid prevents Trichoderma harzianum from entering the vascular system of roots. Mol. Plant Pathol. 15: 823–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An L., Ahmad R.M., Ren H., Qin J., Yan Y. (2018). Jasmonate signal receptor gene family ZmCOIs restore male fertility and defense response of Arabidopsis mutant coi1–1. J. Plant Growth Regul. 38: 479–493. [Google Scholar]
- Anders S., Pyl P., Huber W. (2015). HTSeq—a Python framework to work with high-throughput sequencing data. Bioinformatics 31: 166–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreou A., Brodhun F., Feussner I. (2009). Biosynthesis of oxylipins in non-mammals. Prog. Lipid Res. 48: 148–170. [DOI] [PubMed] [Google Scholar]
- Baek J.M., Kenerley C.M. (1998). The arg2 gene of Trichoderma virens: Cloning and development of a homologous transformation system. Fungal Genet. Biol. 23: 34–44. [DOI] [PubMed] [Google Scholar]
- Blechert S., Bockelmann C., Fusslein M., Von Schrader T., Stelmach B., Niesel U., Weiler E.W. (1999). Structure-activity analyses reveal the existence of two separate groups of active octadecanoids in elicitation of the tendril-coiling response of Bryonia dioica Jacq. Planta 207: 470–479. [Google Scholar]
- Borrego E.J., Kolomiets M.V. (2016). Synthesis and functions of jasmonates in maize. Plants (Basel) 5: 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen, S.A. (2009). The Function of the Lipoxygenase ZmLOX10 in Maize Interactions with Insects and Pathogens. PhD dissertation (College Station: Texas A&M University).
- Christensen S.A., et al. (2015). Maize death acids, 9-lipoxygenase-derived cyclopente(a)nones, display activity as cytotoxic phytoalexins and transcriptional mediators. Proc. Natl. Acad. Sci. USA 112: 11407–11412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen S.A., et al. (2013). The maize lipoxygenase, ZmLOX10, mediates green leaf volatile, jasmonate and herbivore-induced plant volatile production for defense against insect attack. Plant J. 74: 59–73. [DOI] [PubMed] [Google Scholar]
- Christensen S.A., Nemchenko A., Park Y.S., Borrego E., Huang P.C., Schmelz E.A., Kunze S., Feussner I., Yalpani N., Meeley R., Kolomiets M.V. (2014). The novel monocot-specific 9-lipoxygenase ZmLOX12 is required to mount an effective jasmonate-mediated defense against Fusarium verticillioides in maize. Mol. Plant Microbe Interact. 27: 1263–1276. [DOI] [PubMed] [Google Scholar]
- Conrath U., et al. ; Prime-A-Plant Group (2006). Priming: Getting ready for battle. Mol. Plant Microbe Interact. 19: 1062–1071. [DOI] [PubMed] [Google Scholar]
- Constantino N.N., Mastouri F., Damarwinasis R., Borrego E.J., Moran-Diez M.E., Kenerley C.M., Gao X., Kolomiets M.V. (2013). Root-expressed maize lipoxygenase 3 negatively regulates induced systemic resistance to Colletotrichum graminicola in shoots. Front. Plant Sci. 4: 510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contreras-Cornejo H.A., Macías-Rodríguez L., Beltrán-Peña E., Herrera-Estrella A., López-Bucio J. (2011). Trichoderma-induced plant immunity likely involves both hormonal- and camalexin-dependent mechanisms in Arabidopsis thaliana and confers resistance against necrotrophic fungi Botrytis cinerea. Plant Signal. Behav. 6: 1554–1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czechowski T., Stitt M., Altmann T., Udvardi M.K., Scheible W.-R. (2005). Genome-wide identification and testing of superior reference genes for transcript normalization in Arabidopsis. Plant Physiol. 139: 5–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dave A., Graham I.A. (2012). Oxylipin signaling: A distinct role for the jasmonic acid precursor cis-(+)-12-oxo-phytodienoic acid (cis-OPDA). Front. Plant Sci. 3: 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dave A., Hernández M.L., He Z., Andriotis V.M., Vaistij F.E., Larson T.R., Graham I.A. (2011). 12-Oxo-phytodienoic acid accumulation during seed development represses seed germination in Arabidopsis. Plant Cell 23: 583–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djonović S., Pozo M.J., Dangott L.J., Howell C.R., Kenerley C.M. (2006). Sm1, a proteinaceous elicitor secreted by the biocontrol fungus Trichoderma virens induces plant defense responses and systemic resistance. Mol. Plant Microbe Interact. 19: 838–853. [DOI] [PubMed] [Google Scholar]
- Djonovic S., Vargas W.A., Kolomiets M.V., Horndeski M., Wiest A., Kenerley C.M. (2007). A proteinaceous elicitor Sm1 from the beneficial fungus Trichoderma virens is required for induced systemic resistance in maize. Plant Physiol. 145: 875–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Druzhinina I.S., Seidl-Seiboth V., Herrera-Estrella A., Horwitz B.A., Kenerley C.M., Monte E., Mukherjee P.K., Zeilinger S., Grigoriev I.V., Kubicek C.P. (2011). Trichoderma: The genomics of opportunistic success. Nat. Rev. Microbiol. 9: 749–759. [DOI] [PubMed] [Google Scholar]
- Dubois M., Van den Broeck L., Inzé D. (2018). The pivotal role of ethylene in plant growth. Trends Plant Sci. 23: 311–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer E.E., Caldelari D., Pearce G., Walker-Simmons M., Ryan C.A. (1994). Diethyldithiocarbamic acid inhibits the octadecanoid signaling pathway for the wound induction of proteinase inhibitors in tomato leaves. Plant Physiol. 106: 337–342. [Google Scholar]
- Feussner I., Wasternack C. (2002). The lipoxygenase pathway. Annu. Rev. Plant Biol. 53: 275–297. [DOI] [PubMed] [Google Scholar]
- Floková K., Feussner K., Herrfurth C., Miersch O., Mik V., Tarkowská D., Strnad M., Feussner I., Wasternack C., Novák O. (2016). A previously undescribed jasmonate compound in flowering Arabidopsis thaliana - The identification of cis-(+)-OPDA-Ile. Phytochemistry 122: 230–237. [DOI] [PubMed] [Google Scholar]
- Forchetti G., Masciarelli O., Alemano S., Alvarez D., Abdala G. (2007). Endophytic bacteria in sunflower (Helianthus annuus L.): Isolation, characterization, and production of jasmonates and abscisic acid in culture medium. Appl. Microbiol. Biotechnol. 76: 1145–1152. [DOI] [PubMed] [Google Scholar]
- Gao X., Shim W.B., Göbel C., Kunze S., Feussner I., Meeley R., Balint-Kurti P., Kolomiets M. (2007). Disruption of a maize 9-lipoxygenase results in increased resistance to fungal pathogens and reduced levels of contamination with mycotoxin fumonisin. Mol. Plant Microbe Interact. 20: 922–933. [DOI] [PubMed] [Google Scholar]
- Gao X., Starr J., Göbel C., Engelberth J., Feussner I., Tumlinson J., Kolomiets M. (2008). Maize 9-lipoxygenase ZmLOX3 controls development, root-specific expression of defense genes, and resistance to root-knot nematodes. Mol. Plant Microbe Interact. 21: 98–109. [DOI] [PubMed] [Google Scholar]
- Gleason C., Leelarasamee N., Meldau D., Feussner I. (2016). OPDA has key role in regulating plant susceptibility to the root-knot nematode Meloidogyne hapla in Arabidopsis. Front. Plant Sci. 7: 1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goetz S., Hellwege A., Stenzel I., Kutter C., Hauptmann V., Forner S., McCaig B., Hause G., Miersch O., Wasternack C., Hause B. (2012). Role of cis-12-oxo-phytodienoic acid in tomato embryo development. Plant Physiol. 158: 1715–1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo H.M., Li H.C., Zhou S.R., Xue H.W., Miao X.X. (2014). Cis-12-oxo-phytodienoic acid stimulates rice defense response to a piercing-sucking insect. Mol. Plant 7: 1683–1692. [DOI] [PubMed] [Google Scholar]
- Haque E., Osmani A.A., Ahmadi S.H., Ogawa S., Takagi K., Yokoyama M., Ban T. (2016). KODA, an α-ketol derivative of linolenic acid provides wide recovery ability of wheat against various abiotic stresses. Biocatal. Agric. Biotechnol. 7: 67–75. [Google Scholar]
- Havko N.E., Major I.T., Jewell J.B., Attaran E., Browse J., Howe G.A. (2016). Control of carbon assimilation and partitioning by jasmonate: An accounting of growth–defense tradeoffs. Plants (Basel) 5: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heil M., Bostock R.M. (2002). Induced systemic resistance (ISR) against pathogens in the context of induced plant defences. Ann. Bot. 89: 503–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermosa R., Viterbo A., Chet I., Monte E. (2012). Plant-beneficial effects of Trichoderma and of its genes. Microbiology 158: 17–25. [DOI] [PubMed] [Google Scholar]
- Hossain M.M., Sultana F., Hyakumachi M. (2017). Role of ethylene signalling in growth and systemic resistance induction by the plant growth‐promoting fungus Penicillium viridicatum in Arabidopsis. J. Phytopathol. 165: 432–441. [Google Scholar]
- Howe G.A., Lightner J., Browse J., Ryan C.A. (1996). An octadecanoid pathway mutant (JL5) of tomato is compromised in signaling for defense against insect attack. Plant Cell 8: 2067–2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe G.A., Schilmiller A.L. (2002). Oxylipin metabolism in response to stress. Curr. Opin. Plant Biol. 5: 230–236. [DOI] [PubMed] [Google Scholar]
- Howell C.R., Hanson L.E., Stipanovic R.D., Puckhaber L.S. (2000). Induction of terpenoid synthesis in cotton roots and control of rhizoctonia solani by seed treatment with Trichoderma virens. Phytopathology 90: 248–252. [DOI] [PubMed] [Google Scholar]
- Huang H., Liu B., Liu L., Song S. (2017). Jasmonate action in plant growth and development. J. Exp. Bot. 68: 1349–1359. [DOI] [PubMed] [Google Scholar]
- Huot B., Yao J., Montgomery B.L., He S.Y. (2014). Growth-defense tradeoffs in plants: A balancing act to optimize fitness. Mol. Plant 7: 1267–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iavicoli A., Boutet E., Buchala A., Métraux J.-P. (2003). Induced systemic resistance in Arabidopsis thaliana in response to root inoculation with Pseudomonas fluorescens CHA0. Mol. Plant Microbe Interact. 16: 851–858. [DOI] [PubMed] [Google Scholar]
- Isakeit T., Gao X., Kolomiets M. (2007). Increased resistance of a maize mutant lacking the 9‐lipoxygenase gene, ZmLOX3, to root rot caused by Exserohilum pedicellatum. J. Phytopathol. 155: 758–760. [Google Scholar]
- Jogaiah S., Abdelrahman M., Tran L.P., Ito S.I. (2018). Different mechanisms of Trichoderma virens-mediated resistance in tomato against Fusarium wilt involve the jasmonic and salicylic acid pathways. Mol. Plant Pathol. 19: 870–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katagiri F., Tsuda K. (2010). Understanding the plant immune system. Mol. Plant Microbe Interact. 23: 1531–1536. [DOI] [PubMed] [Google Scholar]
- Kim D., Pertea G., Trapnell C., Pimentel H., Kelley R., Salzberg S. (2013). TopHat2: Accurate alignment of transcriptomes in the presence of insertions, deletions and gene fusions. Genome biology 14: R36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kittikorn M., Shiraishi N., Okawa K., Ohara H., Yokoyama M., Ifuku O., Yoshida S., Kondo S. (2010). Effect of fruit load on 9,10-ketol-octadecadienoic acid (KODA), GA and jasmonic acid concentrations in apple buds. Sci. Hortic. 124: 225–230. [Google Scholar]
- Klessig D.F., Choi H.W., Dempsey D.A. (2018). Systemic acquired resistance and salicylic acid: Past, present, and future. Mol. Plant Microbe Interact. 31: 871–888. [DOI] [PubMed] [Google Scholar]
- Knoester M., Pieterse C.M.J., Bol J.F., Van Loon L.C. (1999). Systemic resistance in Arabidopsis induced by rhizobacteria requires ethylene-dependent signaling at the site of application. Mol. Plant Microbe Interact. 12: 720–727. [DOI] [PubMed] [Google Scholar]
- Korolev N., David D.R., Elad Y. (2008). The role of phytohormones in basal resistance and Trichoderma-induced systemic resistance to Botrytis cinerea in Arabidopsis thaliana. BioControl 53: 667–683. [Google Scholar]
- Lamdan N.L., Shalaby S., Ziv T., Kenerley C.M., Horwitz B.A. (2015). Secretome of Trichoderma interacting with maize roots: Role in induced systemic resistance. Mol. Cell. Proteomics 14: 1054–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C., Liu G., Xu C., Lee G.I., Bauer P., Ling H.Q., Ganal M.W., Howe G.A. (2003). The tomato suppressor of prosystemin-mediated responses2 gene encodes a fatty acid desaturase required for the biosynthesis of jasmonic acid and the production of a systemic wound signal for defense gene expression. Plant Cell 15: 1646–1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorito M., Woo S.L., Harman G.E., Monte E. (2010). Translational research on Trichoderma: From ’omics to the field. Annu. Rev. Phytopathol. 48: 395–417. [DOI] [PubMed] [Google Scholar]
- Lunde C., Kimberlin A., Leiboff S., Koo A.J., Hake S. (2019). Tasselseed5 overexpresses a wound-inducible enzyme, ZmCYP94B1, that affects jasmonate catabolism, sex determination, and plant architecture in maize. Commun. Biol 2: 114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Z.-B., Janz D., Jiang X., Göbel C., Wildhagen H., Tan Y., Rennenberg H., Feussner I., Polle A. (2009). Upgrading root physiology for stress tolerance by ectomycorrhizas: Insights from metabolite and transcriptional profiling into reprogramming for stress anticipation. Plant Physiol. 151: 1902–1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malinich E.A., Wang K., Mukherjee P.K., Kolomiets M., Kenerley C.M. (2019). Differential expression analysis of Trichoderma virens RNA reveals a dynamic transcriptome during colonization of Zea mays roots. BMC Genomics 20: 280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-Medina A., Appels F.V.W., van Wees S.C.M. (2017). Impact of salicylic acid- and jasmonic acid-regulated defences on root colonization by Trichoderma harzianum T-78. Plant Signal. Behav. 12: e1345404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maynard D., Gröger H., Dierks T., Dietz K.J. (2018). The function of the oxylipin 12-oxophytodienoic acid in cell signaling, stress acclimation, and development. J. Exp. Bot. 69: 5341–5354. [DOI] [PubMed] [Google Scholar]
- Mohanty A., Luo A., DeBlasio S., Ling X., Yang Y., Tuthill D.E., Williams K.E., Hill D., Zadrozny T., Chan A., Sylvester A.W., Jackson D. (2009). Advancing cell biology and functional genomics in maize using fluorescent protein-tagged lines. Plant Physiol. 149: 601–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monte I., et al. (2018). Ligand-receptor co-evolution shaped the jasmonate pathway in land plants. Nat. Chem. Biol. 14: 480–488. [DOI] [PubMed] [Google Scholar]
- Mueller S., Hilbert B., Dueckershoff K., Roitsch T., Krischke M., Mueller M.J., Berger S. (2008). General detoxification and stress responses are mediated by oxidized lipids through TGA transcription factors in Arabidopsis. Plant Cell 20: 768–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee P.K., Kenerley C.M. (2010). Regulation of morphogenesis and biocontrol properties in Trichoderma virens by a VELVET protein, Vel1. Appl. Environ. Microbiol. 76: 2345–2352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller M., Munné-Bosch S. (2011). Rapid and sensitive hormonal profiling of complex plant samples by liquid chromatography coupled to electrospray ionization tandem mass spectrometry. Plant Methods 7: 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro-Meléndez A.L., Heil M. (2014). Symptomless endophytic fungi suppress endogenous levels of salicylic acid and interact with the jasmonate-dependent indirect defense traits of their host, lima bean (Phaseolus lunatus). J. Chem. Ecol. 40: 816–825. [DOI] [PubMed] [Google Scholar]
- Nemchenko A., Kunze S., Feussner I., Kolomiets M. (2006). Duplicate maize 13-lipoxygenase genes are differentially regulated by circadian rhythm, cold stress, wounding, pathogen infection, and hormonal treatments. J. Exp. Bot. 57: 3767–3779. [DOI] [PubMed] [Google Scholar]
- Oliw E.H., Hamberg M. (2017). An allene oxide and 12-oxophytodienoic acid are key intermediates in jasmonic acid biosynthesis by Fusarium oxysporum. J. Lipid Res. 58: 1670–1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park Y.-H., Stack J., Kenerley C. (1992). Selective isolation and enumeration of Gliocladium virens and G. roseum from soil. Plant Dis. 76: 230–235. [Google Scholar]
- Pieterse C.M., Van der Does D., Zamioudis C., Leon-Reyes A., Van Wees S.C. (2012). Hormonal modulation of plant immunity. Annu. Rev. Cell Dev. Biol. 28: 489–521. [DOI] [PubMed] [Google Scholar]
- Pieterse C.M., van Wees S.C., van Pelt J.A., Knoester M., Laan R., Gerrits H., Weisbeek P.J., van Loon L.C. (1998). A novel signaling pathway controlling induced systemic resistance in Arabidopsis. Plant Cell 10: 1571–1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pieterse C.M., Zamioudis C., Berendsen R.L., Weller D.M., Van Wees S.C., Bakker P.A. (2014b). Induced systemic resistance by beneficial microbes. Annu. Rev. Phytopathol. 52: 347–375. [DOI] [PubMed] [Google Scholar]
- Pieterse C.M., Zamioudis C., Does D., Van Wees S.C. (2014a). Signalling networks involved in induced resistance In . In Induced Resistance for Plant Defence: A Sustainable Approach to Crop Protection, Walters D., Newton A.C., and Lyon G., eds, 2nd ed, (New York: John Wiley and Sons; ), pp. 58–80. [Google Scholar]
- Plett J.M., Daguerre Y., Wittulsky S., Vayssières A., Deveau A., Melton S.J., Kohler A., Morrell-Falvey J.L., Brun A., Veneault-Fourrey C., Martin F. (2014). Effector MiSSP7 of the mutualistic fungus Laccaria bicolor stabilizes the Populus JAZ6 protein and represses jasmonic acid (JA) responsive genes. Proc. Natl. Acad. Sci. USA 111: 8299–8304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plett J.M., Kemppainen M., Kale S.D., Kohler A., Legué V., Brun A., Tyler B.M., Pardo A.G., Martin F. (2011). A secreted effector protein of Laccaria bicolor is required for symbiosis development. Curr. Biol. 21: 1197–1203. [DOI] [PubMed] [Google Scholar]
- Ponce De León I., Schmelz E.A., Gaggero C., Castro A., Álvarez A., Montesano M. (2012). Physcomitrella patens activates reinforcement of the cell wall, programmed cell death and accumulation of evolutionary conserved defence signals, such as salicylic acid and 12-oxo-phytodienoic acid, but not jasmonic acid, upon Botrytis cinerea infection. Mol. Plant Pathol. 13: 960–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pozo M.J., Van Der Ent S., Van Loon L.C., Pieterse C.M. (2008). Transcription factor MYC2 is involved in priming for enhanced defense during rhizobacteria-induced systemic resistance in Arabidopsis thaliana. New Phytol. 180: 511–523. [DOI] [PubMed] [Google Scholar]
- Prost I., et al. (2005). Evaluation of the antimicrobial activities of plant oxylipins supports their involvement in defense against pathogens. Plant Physiol. 139: 1902–1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto D., Nakamura Y., Sugiura H., Sugiura T., Asakura T., Yokoyama M., Moriguchi T. (2010). Effect of 9-hydroxy-10-oxo-12(Z), 15(Z)-octadecadienoic acid (KODA) on endodormancy breaking in flower buds of japanese pear. HortScience 45: 1470–1474. [Google Scholar]
- Salas-Marina M.A., Isordia-Jasso M.I., Islas-Osuna M.A., Delgado-Sánchez P., Jiménez-Bremont J.F., Rodríguez-Kessler M., Rosales-Saavedra M.T., Herrera-Estrella A., Casas-Flores S. (2015). The Epl1 and Sm1 proteins from Trichoderma atroviride and Trichoderma virens differentially modulate systemic disease resistance against different life style pathogens in Solanum lycopersicum. Front. Plant Sci. 6: 77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salas-Marina M.A., Silva-Flores M.A., Uresti-Rivera E.E., Castro-Longoria E., Herrera-Estrella A., Casas-Flores S. (2011). Colonization of Arabidopsis roots by Trichoderma atroviride promotes growth and enhances systemic disease resistance through jasmonic acid/ethylene and salicylic acid pathways. Eur. J. Plant Pathol. 131: 15–26. [Google Scholar]
- Scalschi L., Sanmartín M., Camañes G., Troncho P., Sánchez-Serrano J.J., García-Agustín P., Vicedo B. (2015). Silencing of OPR3 in tomato reveals the role of OPDA in callose deposition during the activation of defense responses against Botrytis cinerea. Plant J. 81: 304–315. [DOI] [PubMed] [Google Scholar]
- Shoresh M., Yedidia I., Chet I. (2005). Involvement of jasmonic acid/ethylene signaling pathway in the systemic resistance induced in cucumber by Trichoderma asperellum T203. Phytopathology 95: 76–84. [DOI] [PubMed] [Google Scholar]
- Song S., Qi T., Fan M., Zhang X., Gao H., Huang H., Wu D., Guo H., Xie D. (2013). The bHLH subgroup IIId factors negatively regulate jasmonate-mediated plant defense and development. PLoS Genet. 9: e1003653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Y., Chen D., Lu K., Sun Z., Zeng R. (2015). Enhanced tomato disease resistance primed by arbuscular mycorrhizal fungus. Front. Plant Sci. 6: 786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stelmach B.A., Müller A., Hennig P., Laudert D., Andert L., Weiler E.W. (1998). Quantitation of the octadecanoid 12-oxo-phytodienoic acid, a signalling compound in plant mechanotransduction. Phytochemistry 47: 539–546. [DOI] [PubMed] [Google Scholar]
- Stumpe M., Göbel C., Faltin B., Beike A.K., Hause B., Himmelsbach K., Bode J., Kramell R., Wasternack C., Frank W., Reski R., Feussner I. (2010). The moss Physcomitrella patens contains cyclopentenones but no jasmonates: Mutations in allene oxide cyclase lead to reduced fertility and altered sporophyte morphology. New Phytol. 188: 740–749. [DOI] [PubMed] [Google Scholar]
- Taki N., et al. (2005). 12-Oxo-phytodienoic acid triggers expression of a distinct set of genes and plays a role in wound-induced gene expression in Arabidopsis. Plant Physiol. 139: 1268–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tjamos S.E., Flemetakis E., Paplomatas E.J., Katinakis P. (2005). Induction of resistance to Verticillium dahliae in Arabidopsis thaliana by the biocontrol agent K-165 and pathogenesis-related proteins gene expression. Mol. Plant Microbe Interact. 18: 555–561. [DOI] [PubMed] [Google Scholar]
- Tsukada K., Takahashi K., Nabeta K. (2010). Biosynthesis of jasmonic acid in a plant pathogenic fungus, Lasiodiplodia theobromae. Phytochemistry 71: 2019–2023. [DOI] [PubMed] [Google Scholar]
- Tzeng T.H., Lyngholm L.K., Ford C.F., Bronson C.R. (1992). A restriction fragment length polymorphism map and electrophoretic karyotype of the fungal maize pathogen Cochliobolus heterostrophus. Genetics 130: 81–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varsani S., Grover S., Zhou S., Koch K.G., Huang P.-C., Kolomiets M.V., Williams W.P., Heng-Moss T., Sarath G., Luthe D.S., Jander G., Louis J. (2019). 12-Oxo-phytodienoic acid acts as a regulator of maize defense against corn leaf aphid. Plant Physiol. 179: 1402–1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vick B.A., Zimmerman D.C. (1984). Biosynthesis of jasmonic acid by several plant species. Plant Physiol. 75: 458–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K., Guo Q., Froehlich J.E., Hersh H.L., Zienkiewicz A., Howe G.A., Benning C. (2018). Two abscisic acid-responsive plastid lipase genes involved in jasmonic acid biosynthesis in Arabidopsis thaliana. Plant Cell 30: 1006–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S., Saito T., Ohkawa K., Ohara H., Shishido M., Ikeura H., Takagi K., Ogawa S., Yokoyama M., Kondo S. (2016). α-Ketol linolenic acid (KODA) application affects endogenous abscisic acid, jasmonic acid and aromatic volatiles in grapes infected by a pathogen (Glomerella cingulata). J. Plant Physiol. 192: 90–97. [DOI] [PubMed] [Google Scholar]
- Wang Y., Ohara Y., Nakayashiki H., Tosa Y., Mayama S. (2005). Microarray analysis of the gene expression profile induced by the endophytic plant growth-promoting rhizobacteria, Pseudomonas fluorescens FPT9601-T5 in Arabidopsis. Mol. Plant Microbe Interact. 18: 385–396. [DOI] [PubMed] [Google Scholar]
- Wasternack C. (2007). Jasmonates: An update on biosynthesis, signal transduction and action in plant stress response, growth and development. Ann. Bot. 100: 681–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasternack C., Hause B. (2013). Jasmonates: Biosynthesis, perception, signal transduction and action in plant stress response, growth and development. An update to the 2007 review in Annals of Botany. Ann. Bot. 111: 1021–1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia A., Feng-ying D., Song G., Fan-jun C., Li-Xing Y., Ri-Liang G. (2014). Transcriptional regulation of expression of the maize aldehyde dehydrogenase 7 gene (ZmALDH7B6) in response to abiotic stresses. J. Integr. Agric. 13: 1900–1908. [Google Scholar]
- Yamamoto Y., Ohshika J., Takahashi T., Ishizaki K., Kohchi T., Matusuura H., Takahashi K. (2015). Functional analysis of allene oxide cyclase, MpAOC, in the liverwort Marchantia polymorpha. Phytochemistry 116: 48–56. [DOI] [PubMed] [Google Scholar]
- Yan Y., Christensen S., Isakeit T., Engelberth J., Meeley R., Hayward A., Emery R.J., Kolomiets M.V. (2012). Disruption of OPR7 and OPR8 reveals the versatile functions of jasmonic acid in maize development and defense. Plant Cell 24: 1420–1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yedidia I., Benhamou N., Chet I. (1999). Induction of defense responses in cucumber plants (Cucumis sativus L.) by the biocontrol agent trichoderma harzianum. Appl. Environ. Microbiol. 65: 1061–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoyama M., Yamaguchi S., Inomata S., Komatsu K., Yoshida S., Iida T., Yokokawa Y., Yamaguchi M., Kaihara S., Takimoto A. (2000). Stress-induced factor involved in flower formation of Lemna is an α-ketol derivative of linolenic acid. Plant Cell Physiol. 41: 110–113. [DOI] [PubMed] [Google Scholar]