A comprehensive review of all plant genes currently known to be required for symbiotic nitrogen fixation in two model legume species, Medicago truncatula and Lotus japonicus, and two crop species, soybean and common bean.
Abstract
Since 1999, various forward- and reverse-genetic approaches have uncovered nearly 200 genes required for symbiotic nitrogen fixation (SNF) in legumes. These discoveries advanced our understanding of the evolution of SNF in plants and its relationship to other beneficial endosymbioses, signaling between plants and microbes, the control of microbial infection of plant cells, the control of plant cell division leading to nodule development, autoregulation of nodulation, intracellular accommodation of bacteria, nodule oxygen homeostasis, the control of bacteroid differentiation, metabolism and transport supporting symbiosis, and the control of nodule senescence. This review catalogs and contextualizes all of the plant genes currently known to be required for SNF in two model legume species, Medicago truncatula and Lotus japonicus, and two crop species, Glycine max (soybean) and Phaseolus vulgaris (common bean). We also briefly consider the future of SNF genetics in the era of pan-genomics and genome editing.
INTRODUCTION
Fabaceae (legumes) is the third largest family of flowering plants, with members spread around the globe (Doyle and Luckow, 2003). Part of their success is due to N-fixing symbiosis with soil bacteria called rhizobia, which enables legumes to grow on atmospheric nitrogen (N2) in soils lacking mineral and organic N. Their natural ability to inject fixed N into soils make them keystone species for natural and agricultural ecosystems. Although once the primary source of N for agricultural systems, symbiotic nitrogen fixation (SNF) in legumes (approximately 50 million tons per annum) now contributes less than half that provided by chemical fertilizer N (Canfield et al., 2010). Unfortunately, manufactured fertilizer N is costly both economically and environmentally (Sutton et al., 2011). By contrast, SNF in legumes is seen as a sustainable source of N for agriculture. Research on the genetics of SNF, especially over the last 20 years, has revealed the great complexity of this process in plants as well as aspects of its evolution in different plant lineages. This knowledge, reviewed here, will underpin future efforts to improve SNF in legumes via conventional plant breeding and is already guiding more audacious efforts to engineer SNF in nonlegumes (Charpentier and Oldroyd, 2010).
During the 1980s, it was thought that a few dozen genes might be required for SNF in legumes based on the number of nodule-specific genes, or nodulin genes, known at the time. With the genomic revolution over the past 20 years, it has become clear that thousands of genes are expressed at relatively high levels in legume nodules (Benedito et al., 2008; Mun et al., 2016). Over the same period, genetic approaches have revealed many genes with large effects on SNF, with mutations in these genes causing defects in nodule development and/or plant growth in the absence of soil N (Figure 1). Reverse genetics, which begins with a gene of interest and ends with a phenotype, in contrast to classical/forward genetics, has uncovered both large- and moderate-effect genes for SNF. Together, forward and reverse genetics have revolutionized our understanding of the molecular and cellular processes underpinning SNF, through the discovery of at least 196 genes required for effective symbiosis (Supplemental Data Set; https://nobleapps.noble.org/legumegenetics).
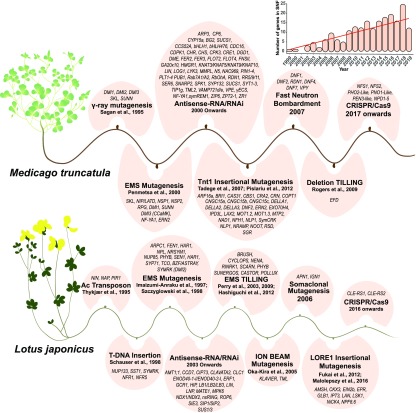
Figure 1.
Historical Timeline of Gene Discovery in M. truncatula and L. japonicus.
Mutant resources developed using a variety of mutagens have been utilized since 1995 in both species. Mutagens are indicated in boldface. The inset shows the number of SNF genes discovered by mutational analyses each year over the past 20 years. Refer to the text and the Supplemental Data Set for more information on individual genes.
Genetic Resources Developed for Legume Research
Mutations in genes are the “bread and butter” of genetics, and natural mutations in genes of Pisum sativum (garden pea) were instrumental in the development of genetics as a scientific discipline (Mendel, 1866). However, natural rates of mutation are low and generally mitigated by cellular DNA repair mechanisms. To accelerate genetic discovery, model species have been subjected to chemical and physical mutagens, including EMS and/or fast neutron bombardment, in Lotus japonicus, Medicago (Medicago truncatula), soybean (Glycine max), and common bean (Phaseolus vulgaris; Szczyglowski et al., 1998; Penmetsa and Cook, 2000; Perry et al., 2003; Bolon et al., 2011; Espina et al., 2018). The resulting mutant populations have been instrumental in the discovery of genes required for SNF (Figures 1 and 2; Supplemental Data Set). Over time, mutant populations that were easier to work with were developed using transposons as mutagens. These include an L. japonicus mutant population resulting from T-DNA insertions (Schauser et al., 1998), an M. truncatula mutant population containing nearly a million genomic insertions of the tobacco (Nicotiana tabacum) retrotransposon Tnt1 (Tadege et al., 2008; Pislariu et al., 2012), and an L. japonicus mutant population resulting from mobilization of the endogenous retrotransposon 1 (LORE1; Fukai et al., 2013; Małolepszy et al., 2016). The genomic DNA sequences adjacent to sites of transposon insertion, called flanking sequence tags, can be used to identify the causative mutation for both forward and reverse genetics. These strategies are particularly potent in diploid in-breeding species such as M. truncatula (2n = 16), L. japonicus (2n = 12), and P. vulgaris (2n = 22) but less so in paleopolyploid species such as soybean (2n = 40).
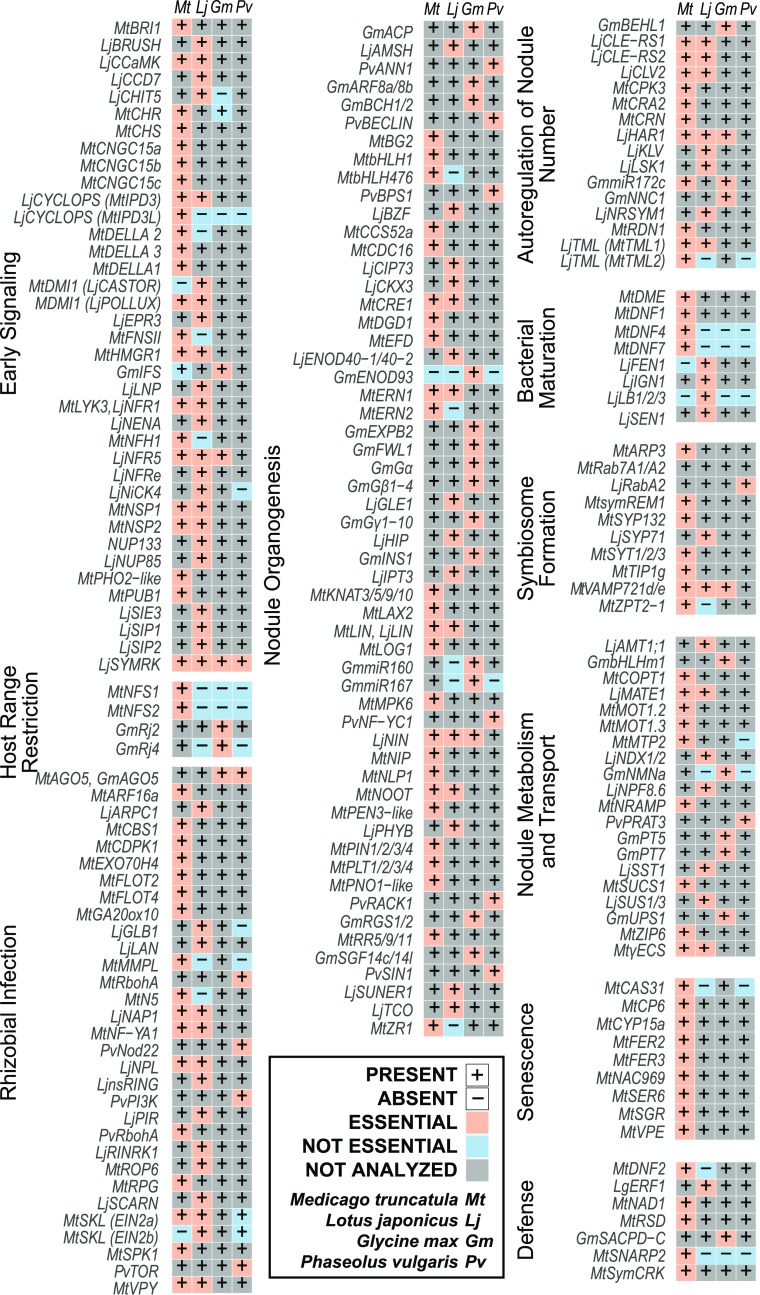
Figure 2.
Comparison of SNF Genes in Different Species.
Presence or absence of orthologous genes is indicated by + or −. Requirement for SNF is specified by cell color: coral (essential), blue (not essential), gray (not analyzed). Data are based on published literature, if available, and sequence information in public data repositories. Also see the Supplemental Data Set.
Overview of SNF
SNF is the culmination of a complex series of chemical and physical interactions between legumes and compatible rhizobia, including signaling processes that trigger changes in gene expression in both partners, shape partner selection, and suppress plant defenses. These signals also provide bacterial access to plant epidermal and cortical cells, induce root cell division and nodule meristem formation, and eventually produce thousands of specialized cellular organelles called “symbiosomes,” each containing one or more nitrogen-fixing bacteroids. Along the way, differentiation of plant tissues in nodules provides an environment rich in nutrients for the bacteria, with low levels of free oxygen compatible with nitrogen reduction by the oxygen-labile nitrogenase enzyme complex yet sufficient for respiration to energize the process. In essence, SNF is a metabolic symbiosis built upon the trading of reduced carbon from the plant for reduced nitrogen from the bacterial symbionts. The following sections describe and contextualize the genetic discoveries (Figure 2) that have transformed our understanding of how legume-rhizobia symbioses develop.
EARLY SIGNALING AND PARTNER SELECTION
Legume-rhizobia interactions typically begin in N-limited soils with legumes secreting a class of metabolites called flavonoids (Peters et al., 1986; Redmond et al., 1986). Rhizobia recognize these as signals, which trigger the synthesis and release of lipochitooligosaccharides or nodulation (Nod) factors via rhizobial nod genes/proteins (Dénarié and Cullimore, 1993). Nod factors serve as a “calling card” to inform the plant of a potentially beneficial symbiont. Subsequent perception of Nod factors by plant receptors leads to recognition of the symbiont, triggering a series of plant responses that roll out a welcome mat to the bacteria (D’Haeze and Holsters, 2002).
Flavonoids as Signals to Rhizobia
Flavonoids in legume root exudates act as chemotactic signals to rhizobia under low-N conditions (reviewed in Liu and Murray, 2016). Together with isoflavonoids, they confer host specificity. For example, the isoflavones genistein and diadzein found in root exudates of G. max and P. vulgaris induce nod genes in their compatible rhizobial symbionts, Bradyrhizobium japonicum and Rhizobium leguminosarum bv phaseoli, respectively (Bolaños-Vásquez and Werner, 1997). Host-specific flavonoids are thought to interact with rhizobial NodD protein, a transcription factor (TF) that induces the expression of “common” nod(ulation) genes, leading to the production of lipochitooligosaccharide Nod factors with a conserved core structure (Peters et al., 1986; Lerouge et al., 1990). A variety of side groups produced by enzymes encoded by “host-specific” nod genes further contribute to partner selection (Long, 1996).
Suppression of plant flavonoid production by RNA interference (RNAi) of MtCHS (CHALCONE SYNTHASE) substantially decreases nodulation in M. truncatula (Wasson et al., 2006). Knockdown of other flavonoid biosynthetic genes, including MtCHR (CHALCONE REDUCTASE) and MtFNS (FLAVONE SYNTHASE) but not MtIFS (ISOFLAVONE SYNTHASE), inhibits nodulation to varying degrees (Zhang et al., 2009). However, GmIFS is required for nodulation in soybean, highlighting the differing requirements of isoflavonoids between legumes (Subramanian et al., 2006).
Nod Factor Signaling and the Common Symbiotic Pathway
Rhizobial Nod factor is necessary and sufficient to induce nodule development on legume roots, even in the absence of rhizobia (Truchet et al., 1991). Plant symbiotic signaling genes, including Nod factor receptor genes, were among the first SNF genes to be isolated, in part because of their dramatic phenotypes: the absence of nodulation (Nod−) and nitrogen fixation (Fix−), poor growth on low-N soils, and defects in Nod factor responses. Analysis of plant Nod− mutants that are unable to perceive Nod factors enabled the discovery of Nod factor receptor genes, including the orthologous pairs LjNFR1 (NOD FACTOR RECEPTOR)/MtLYK3 (LysM RECEPTOR KINASE) and LjNFR5/MtNFP (NOD FACTOR PERCEPTION; Limpens et al., 2003; Madsen et al., 2003; Radutoiu et al., 2003; Arrighi et al., 2006; Smit et al., 2007). These genes encode receptor kinases with three extracellular lysin motif (LysM) domains that form homomeric and heteromeric complexes at the plasma membrane and the infection thread (IT) membrane ( Figure 3), which bind Nod factors (Haney et al., 2011; Broghammer et al., 2012; Moling et al., 2014). M. truncatula transformed with LjNFR1 and LjNFR5 can interact with Mesorhizobium loti, a natural symbiont of L. japonicus, and activate early nodulin gene expression as well as nodule organogenesis, in addition to its natural partner Sinorhizobium meliloti, indicating that these receptors are crucial for partner selection/host range determination (Radutoiu et al., 2007). LjNFR5 also forms heteromers with a plasma membrane leucine-rich repeat receptor-like kinase (LRR-RLK), the symbiotic receptor-like kinase LjSYMRK (Antolín-Llovera et al., 2014). LjSYMRK/MtDMI2 were the first “common symbiotic” genes isolated and found to be required for both rhizobial and mycorrhizal symbioses (Endre et al., 2002; Stracke et al., 2002). Ljsymrk mutants form no ITs or nodules but have excessive root hair branching responses to Nod factor. Interestingly, Ljsymrk-14, a mutant with defects in an essential GPDC tetra-amino acid motif in the extracellular domain of the receptor, supports nodule organogenesis but not infection (Kosuta et al., 2011). Recently, epidermal Nod Factor Receptor (LjNFR), another LysM-RLK, was found to phosphorylate LjNFR5 and support calcium spiking, nodule initiation, and activation of the TF NIN (Schauser et al., 1999; Murakami et al., 2018).
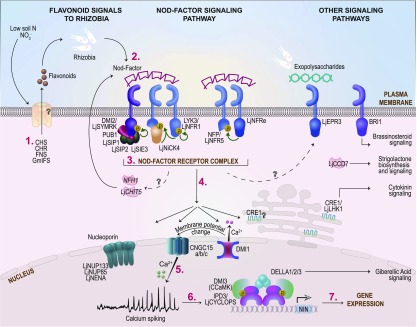
Figure 3.
Genes and Processes Involved in Early Signaling during Nodulation.
(Iso)flavonoids produced under low soil N (1) trigger the production of bacterial Nod factors (2) that, together with other signals, are perceived by receptors at the plasma membrane of epidermal cells (3). This triggers biochemical and physiological responses (4–6) that lead to changes in nuclear gene expression (7). See main text for key to gene/protein names and further explanation of early signaling pathways and components. M. truncatula protein names are provided unless otherwise specified.
Downstream of these receptors is a signaling pathway that shares several components with the arbuscular mycorrhizal (AM) signaling pathway. This “common symbiosis” signaling pathway has been reviewed extensively (Oldroyd et al., 2011; Oldroyd, 2013; Geurts et al., 2016). Common symbiotic signaling components include a Nod factor-induced E3 ubiquitin ligase called MtPUB1 (PLANT U-BOX PROTEIN1) that is phosphorylated by the LjSYMRK ortholog MtDMI2 as well as the entry receptor MtLYK3 (Mbengue et al., 2010; Vernié et al., 2016). Mtpub1-1 mutants have increased numbers of nodules and infection events, indicating that MtPUB1 is a negative regulator of nodulation (Vernié et al., 2016). Silencing of MtPUB1 allowed efficient nodulation by an S. meliloti nodFnodL mutant strain that produced altered Nod factors and poorly nodulated wild-type plants, suggesting that MtPUB1 plays a role in Nod factor discrimination (Mbengue et al., 2010). Mutants affecting three other SYMRK INTERACTING PROTEINS (SIPs) that presumably act in early signaling, LjSIP1, a mitogen-activated protein kinase kinase, LjSIP2, an ARID domain-containing protein, and SYMRK INTERACTING E3 UBIQUITIN LIGASE (LjSIE3), are defective in nodule organogenesis (Zhu et al., 2008; Chen et al., 2012; Yuan et al., 2012; Wang et al., 2013).
The perception of Nod factors leads to depolarization of cell membranes and changes in ion fluxes. These changes include oscillations in calcium concentrations called “calcium spiking” in the nuclei of epidermal root hair cells, which appear to drive changes in gene expression associated with nodule development and infection (Figure 3; Charpentier and Oldroyd, 2013). This is followed by deformation of root hairs, early nodulin gene expression, and, subsequently, the formation of nodule primordia. Nod− mutants defective in calcium spiking were used to identify various nuclear envelope proteins that help generate this signal, including the calcium channels LjCASTOR and LjPOLLUX/MtDMI1 (Ané, 2004; Imaizumi-Anraku, 2005; Charpentier et al., 2008; Kim et al., 2019); nucleoporin subunits LjNUCLEOPORIN85 (LjNUP85) and LjNUP133 (Kanamori et al., 2006; Saito et al., 2007); and calcium channels MtCNGC (CYCLIC NUCLEOTIDE GATED CHANNELS) a/b/c (Figure 3; Charpentier et al., 2016). Interestingly, MtDMI1 seems to serve the purposes of both LjCASTOR and LjPOLLUX, showing how evolution has come up with different solutions to the same problem in different species (Supplemental Data Set; Ané et al., 2004; Charpentier et al., 2008). Mutations in the nucleoporin-localized protein LjNENA lead to defects in infection by both rhizobial and mycorrhizal symbionts. Ljnena mutants do not exhibit calcium spiking in response to Nod factor and show clear defects in rhizobial infections without any changes in nodule organogenesis (Groth et al., 2010). However, nodules that form on Ljnena are empty, with all infections blocked at the microcolony stage. An unknown calcium pump and the known calcium channels (CNGCa/b/c) are believed to work in concert to generate nuclear calcium spiking (Charpentier et al., 2016). A gain-of-function mutation in LjBRUSH, another CNGC gene, impairs rhizobial infection but only at high temperatures (Maekawa-Yoshikawa et al., 2009; Chiasson et al., 2017). Analysis of other Nod− mutants revealed that the nuclear calcium-calmodulin kinase MtDMI3/CCaMK acts as an intermediary between Nod factor perception and nodule development (Gleason et al., 2006; Tirichine et al., 2006), presumably as a sensor of the signal encoded in nuclear calcium spiking. The TF LjCYCLOPS/INTERACTING PROTEIN OF DMI3 (MtIPD3) regulates gene expression, leading to nodulation (Yano et al., 2008; Singh et al., 2014). Importantly, this TF can be phosphorylated by CCaMK, apparently tying calcium spiking to gene transcription (Figure 3; Singh et al., 2014). Two other TFs, MtNSP1 and MtNSP2 (NODULATION SIGNALING PATHWAY), of the GIBBERELLIC ACID INSENSITIVE (GAI), REPRESSOR of GAI, and SCARECROW (GRAS) family were also discovered from forward genetics analysis of Nod− mutants (Kaló et al., 2005; Smit et al., 2005). NSP1 and NSP2 interact to form heterodimers that are required for binding to and activating Nod factor-responsive genes (Hirsch et al., 2009).
Downstream of the Nod factor signaling pathway, paralogous Nod factor hydrolases MtNFH1 (NOD FACTOR HYDROLASE1) and LjCHIT5 (CHITINASE5) cleave the lipochitooligosaccharidic backbone of Nod factors produced by their respective symbionts (Figure 3). This degradation of Nod factors appears to be crucial for rhizobial infection, colonization, and nodule formation (Cai et al., 2018; Malolepszy et al., 2018). Interestingly, delays in nodule formation by aberrant Nod factor-producing strains were abolished in Ljchit5 mutants, implicating Nod factor hydrolysis in partner recognition (Malolepszy et al., 2018).
Other Signaling Pathways
In addition to Nod factor, bacterial surface exopolysaccharides (EPS) are recognized by plant plasma membrane receptors (Figure 3). The exopolysaccharide receptor LjEPR3 (EXOPOLYSACCHARIDE RECEPTOR) is a LysM domain-containing entry receptor that is dispensable for early responses to Nod factor but is required for infection progression beyond the microcolony stage (Kawaharada et al., 2015). LjEPR3 is differentially regulated in the epidermis and the nodule primordia (Kawaharada et al., 2017b). An Ljepr3 LORE1-insertion mutant identified in a suppressor screen with the EPS-defective M. loti exoU mutant overcame the strain incompatibility and formed infrequent but mature pink nodules in addition to some small, uncolonized nodule primordia like those that form on wild-type plants (Kawaharada et al., 2015). Further studies are required to uncover components downstream of this receptor and understand how EPS signaling complements Nod factor signaling in rhizobial partner selection.
Several hormone signaling pathways crosstalk with Nod factor signaling to control nodulation and SNF at various stages (López-Ráez et al., 2017; Liu et al., 2018). LjCCD7 (CAROTENOID CLEAVAGE DIOXYGENASE7), encoding an enzyme required for strigolactone biosynthesis, and MtBRI1 (BRASSINOSTEROID INSENSITIVE1), encoding an LRR-RLK that perceives the brassinosteroid hormone brassinolide, both act early during nodulation (Liu et al., 2013; Cheng et al., 2017). Silencing of either gene has a mild negative effect on the number of nodules formed. Analysis of Tnt1 insertion mutants in MtDELLA1, MtDELLA2, and MtDELLA3, encoding negative regulators of gibberellic acid signaling, revealed that these DELLA proteins control Nod factor-induced gene expression and rhizobial infection in the epidermis as well as nodule number and density (Fonouni-Farde et al., 2016; Liu and Murray, 2016). DELLAs appear to do this by interacting directly with the TFs MtIPD3 and MtNSP2 (Fonouni-Farde et al., 2016; Jin et al., 2016). Cytokinin orchestrates nodule initiation and development together with auxin, which also appears to play roles in the progression of rhizobial infection (Figure 4; see Organogenesis). Lastly, the knockdown of MtHMGR1 (3-HYDROXY-3-METHYLGLUTARYL COA REDUCTASE1), encoding an enzyme involved in the production of isoprenoid compounds in the mevalonate pathway that likely interacts with MtDMI2, suppressed nodule formation (Kevei et al., 2007). It remains unclear how this fits into early signaling pathways leading to nodulation.
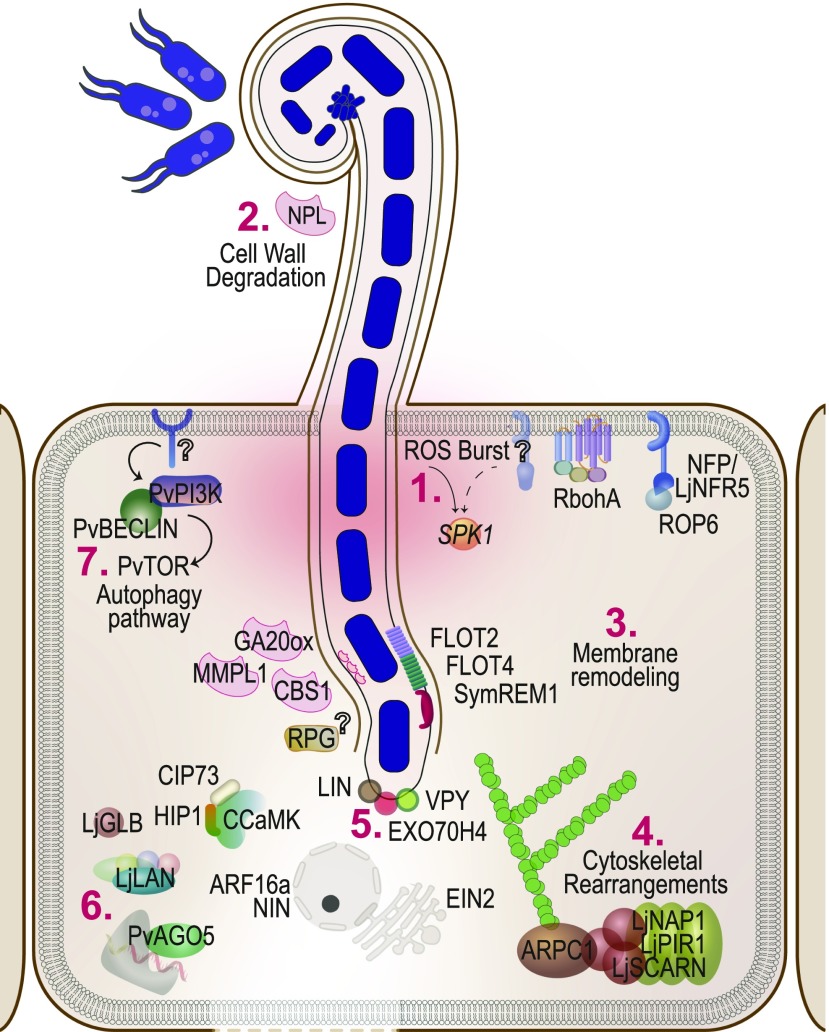
Figure 4.
Rhizobial Infection.
The entry of rhizobia into the plant cell via tubular ITs triggers a transient ROS burst (1). This is accompanied by cell wall degradation (2), membrane remodeling (3), and cytoskeletal rearrangements (4). The IT is led by a complex at its tip called the infectosome (5). In parallel, several other transcriptional and posttranslational changes (6) and the autophagy pathway (7) ensure accommodation of the symbiont within the plant host.
PLANT IMMUNITY AND HOST RANGE RESTRICTION
In parallel with Nod factor-dependent partner selection, the plant immune system helps exclude other soil microorganisms from legume roots (Zipfel and Oldroyd, 2017). Partner selection is a continuous process. The host legume employs multiple checkpoints throughout this process to discriminate between symbionts and pathogens. Defensive receptor kinase complexes including LRR-RLKs and LysM-RLKs recognize microbial molecules at the surfaces of plant cells, while microbial “effectors” injected into cells to disarm plant defenses are recognized and neutralized by NBS-LRR (nucleotide binding site leucine-rich repeat) R (Resistance) proteins (Cao et al., 2017). Consequently, the immune system limits the number of rhizobial strains that a legume can associate with. Genes for “host-range restriction” include the NBS-LRR-encoding Rj2/Rfg1 (Rhizobium japonicum2/Rhizobium fast-growing1) and Rj4, encoding a member of the pathogenesis-related protein family 5 (Yang et al., 2010; Tang et al., 2016). Rj2-carrying cultivars of G. max form active N-fixing symbioses with many strains of Bradyrhizobium but not with B. japonicum USDA 122. Rfg1 restricts symbiosis with certain Sinorhizobium fredii strains (Fan et al., 2017). Rj2 and Rfg1 are alleles of a TIR-NBS-LRR gene, with variant nucleotides affecting seven amino acids (Yang et al., 2010). Rj2 recognizes the effector protein NopP secreted by the incompatible strain B. japonicum USDA 122 type III secretion system, which triggers the plant immune system and blocks rhizobial infection (Sugawara et al., 2018). Rj4 is a dominant allele encoding a thaumatin-like protein, which restricts nodulation with ineffective strains of B. japonicum and Bradyrhizobium elkanii (Tang et al., 2016). Soybean cultivars with the alternate allele, rj4, are nodulated by many ineffective strains of B. elkanii (Sadowsky and Cregan, 1992). The restriction of host range helps ensure effective SNF. Editing of Rj4 using CRISPR/Cas9 led to interactions with the incompatible rhizobial strain, B. japonicum Is-34 (Hayashi et al., 2014; Tang et al., 2016). Both Rj2 and Rj4 plants have fewer infections with incompatible rhizobial strains compared with rj2 and rj4 plants. The nodules that occasionally form are developmentally arrested, with only a few cortical divisions and signs of defense responses (Yang et al., 2010; Hayashi et al., 2014). It remains unknown which bacterial molecule is recognized by Rj4.
Nodule cysteine-rich (NCR) peptides also affect partner selection. Editing of two NCR genes in M. truncatula ecotype Jemalong A17 allowed colonization by Sinorhizoboim meliloti strain Rm41, normally a poor nodulator of A17. NCR peptide-encoding loci MtNFS1 (NITROGEN FIXATION SPECIFICITY) and MtNFS2 were identified in a recombinant inbred line population derived from ecotypes A17 and DZA315 (Wang et al., 2017; Yang et al., 2017). These functionally dominant peptides ensure that A17 plants are colonized preferentially by efficient N-fixing symbionts.
Interestingly, OsCERK1 (CHITIN ELICITOR RECEPTOR KINASE1), a rice (Oryza sativa) LysM-RLK that recognizes chitin oligosaccharides of fungal pathogens, also perceives signals from beneficial AM fungi (Miyata et al., 2014; Zhang et al., 2015), suggesting that some symbiotic receptors might participate in receptor complexes that recognize pathogens. This is an exciting, emerging area of research.
RHIZOBIAL INFECTION
Attachment of rhizobia to root hair cells leads to root hair curling, which “traps” rhizobia in so-called infection pockets that eventually contain microcolonies of dividing rhizobia. The most common route of rhizobial entry into root cells in the four legumes under discussion is via tubular ITs in epidermal root hair cells, although rhizobia are also known to enter via cracks on the root surface (Sprent and James, 2007). Both modes of entry are Nod factor dependent. However, rhizobia can also enter legume roots via intercellular spaces between cells in a Nod factor-independent manner (reviewed by Oldroyd et al., 2011). ITs originate at infection pockets as the plant cell wall is deconstructed, and the adjacent plasma membrane invaginates to allow the entry of multiplying rhizobia. Simultaneous plant cell divisions in the root cortex and pericycle trigger nodule organogenesis in cells undergoing successful infections (Xiao et al., 2014).
Hormonal Regulation
One of the first plant mutants identified with defective rhizobial infection was Mtskl (sickle). This mutant is impaired in EIN2 (ETHYLENE INSENSITIVE2), an endoplasmic reticulum-tethered protein crucial for ethylene signaling (Penmetsa and Cook, 1997; Penmetsa et al., 2003). The hormone ethylene coordinates the biosynthesis and signaling of several other phytohormones at multiple stages of SNF while also modulating defense pathways (Larrainzar et al., 2015). This central role for ethylene is exemplified by the pleiotropic phenotypes caused by mutated EIN2 in Mtskl plants. Mtskl exhibits massive proliferation of ITs and nodule primordia, although these rarely develop into mature nodules. This role of EIN2 is conserved in L. japonicus but requires two copies of EIN2 such that the double mutant ein2a ein2b does not fix nitrogen (Miyata et al., 2013; Reid et al., 2018). Not only is Mtskl hypersensitive to beneficial bacteria but it is also hypersusceptible to infection by fungal pathogens, suggesting that ethylene plays a crucial role in fine-tuning interactions with microbes (Penmetsa et al., 2008).
Auxin positively regulates rhizobial infection while gibberellic acid appears to be a negative regulator of this process. A mutation in MtARF16a (AUXIN RESPONSE FACTOR), which is required for IT elongation but not organogenesis, implicates auxin in the infection process (Breakspear et al., 2014). Mutations in MtARF16a reduced the number of infection events, although a few productive ITs resulted in nodule colonization (Breakspear et al., 2014). The biosynthesis and degradation of gibberellic acid play distinct roles in SNF in addition to signaling mediated by DELLA proteins (see Early Signaling and Partner Selection). New research indicates that the catabolism of gibberellic acid by MtGA2ox10 (GIBBERELLIC ACID OXIDASE10) plays a positive role in infection (Kim et al., 2019), which is in line with the negative correlation between both gibberellic acid and DELLA-mediated signaling and rhizobial infection (Fonouni-Farde et al., 2016; McAdam et al., 2018). Targeted editing that disrupted MtGA2ox10 reduced the number of infections and the number and size of nodules formed on hairy roots. Interestingly, MtGA2ox10 is not only induced in the maturation zone of the root in response to infection by rhizobia but also in the meristem, infection, and fixation zones of mature nodules, suggesting that it plays a role in later stages of nodule functioning (Kim et al., 2019).
Role of Plant Cytoskeleton
Many components of the cytoskeleton are required for reorientation of root hair tip growth and IT development (Figure 4; Timmers, 2008). Mutations in LjNap1 (Nck-associated protein1) and LjPir1 (121F-specific p53 inducible RNA), encoding components of the SCAR/WAVE complex, affect rhizobial infection but not nodule organogenesis (Yokota et al., 2009). SCAR/WAVE proteins drive actin assembly and consequently control cell growth by initiating the formation of actin complexes from which filaments can extend. Ljnap1 and Ljpir1 mutants initiate fewer infections, which are also delayed compared with the wild type (Yokota et al., 2009). Like the Ljnap1 and Ljpir1 mutants, most ITs that form on Ljscarn (scar-nodulation) mutants abort prematurely and release bacteria within the root hair (Qiu et al., 2015). Epidermally expressed LjSCARN is required for actin nucleation without which small, white nodules generally form (Qiu et al., 2015). SCAR proteins bind to and activate the Actin-Related Protein2/3 (ARP2/3) complex, a subunit of which, LjARPC1 (ACTIN-RELATED PROTEIN COMPONENT1), is also required for infection. Ljarpc1 mutants produce few microcolonies and ITs, which abort/disintegrate prematurely (Hossain et al., 2012; Qiu et al., 2015), resulting in mostly uncolonized nodules. Interestingly, in addition to abnormalities in root hair growth due to actin rearrangements, a knockdown of MtCDPK1 (CALCIUM DEPENDENT PROTEIN KINASE1) resulted in constitutive root hair bending and branching, even in the absence of rhizobia. Only 50% of all incipient infections on CDPK RNAi lines were successful, and nodules rarely formed (Ivashuta et al., 2005).
Cell Wall and Cell Membrane Components
IT formation and extension require the plant cell wall to degrade to allow entry of rhizobia (van Spronsen et al., 1994). Consistent with this notion, the mutation of the pectate lyase-encoding gene LjNPL (NODULE PECTATE LYASE) resulted in a drastic reduction in the number of ITs in L. japonicus roots (Xie et al., 2012). Purified LjNPL protein degrades both polygalacturonic acids and pectin. Like other mutants defective in rhizobial infection, Ljnpl mostly produced small, white, uninfected nodules (Xie et al., 2012; Liu et al., 2019b).
Membrane trafficking proteins contribute to the development of the IT as the plant plasma membrane invaginates and extends inward (Figure 4). Studies on other integral membrane proteins, including MtFLOTILLIN2 (MtFLOT2), MtFLOT4, and MtSYMREM1 (SYMBIOTIC REMORIN1), demonstrated the importance of membrane processes during infection progression (Haney and Long, 2010; Lefebvre et al., 2010). FLOTILLINs are lipid raft markers present in membrane microdomains (Glebov et al., 2006). MtFLOT4 is a nodulin that accumulates at the tips of root hairs upon inoculation with rhizobia (Winzer et al., 1999). Mtflot4 RNAi resulted in fewer initiating and elongating ITs, which were more likely to abort in root hairs compared with the wild type (Haney and Long, 2010). Like Mtflot4, knockdown of Mtflot2 by RNAi resulted in fewer ITs and nodules, while Mtflot2 mutations are lethal in the homozygous state, indicating that Mtflot2 plays a broader, essential role in the plant (Liang et al., 2018). Together, FLOTILLINs are thought to play a role in invagination of ITs into root hairs, with MtFLOT4 playing a specialized role in IT elongation (Haney and Long, 2010). MtFLOT4 colocalizes to membrane nanodomains within the root hair cells with the symbiotic remorin protein, MtSYMREM1. Upon rhizobial inoculation, MtSYMREM1 and MtFLOT4 interact with the plant-encoded bacterial entry receptor MtLYK3 to mediate endocytosis of the receptor protein and allow infection progression (Lefebvre et al., 2010; Haney et al., 2011). The density of MtSYMREM1 on root epidermal cells is reduced sixfold in Mtflot4 mutants, suggesting that MtFLOT4 is required for the recruitment of MtSYMREM1. Like Mtflot4, Mtsymrem1 mutants have increased numbers of infection foci but very few elongating ITs (Haney and Long, 2010; Lefebvre et al., 2010; Liang et al., 2018). Reduced expression levels of MtFLOT4 in MtN5 (NODULIN5) RNAi lines could explain the infection defects observed by silencing this nonsulfated lipid transfer protein gene (Pii et al., 2012).
The Infectosome
Recent research points to the existence of an exocyst complex at the tip of the IT, termed the “infectosome,” which ensures polar growth of the IT toward the nodule cortex (Figure 4; Liu et al., 2019a). The infectosome comprises the common symbiotic protein MtVPY (MtVAPYRIN) and its two interacting partners, MtLIN (LUMPY INFECTION) and the exocyst complex subunit MtEXO70 H4 (EXOCYST subunit H4; (Pumplin et al., 2010; Murray, 2011; Liu et al., 2019a). Mutating any of the three components causes defects in IT elongation (Liu et al., 2019c). Both Mtvpy and Mtlin mutants produce few nodules, which are small, white, and uninfected, while Mtexo70 h4 mutants form normal, functional nodules, possibly reflecting genetic redundancy (Murray, 2011; Liu et al., 2019a).
The Autophagy Pathway
Components of the autophagy pathway play positive roles in IT formation, such as a common symbiosis kinase of the phosphatidylinositol 3-kinase (PI3K) family identified using RNAi in P. vulgaris. PvPI3K is expressed in root hairs, and its expression is further enhanced by infection with Rhizobium tropici (Estrada-Navarrete et al., 2016). Downregulating PvPI3K decreased root hair length and the frequency with which root hairs responded to rhizobia. Most infections were arrested at the bases of root hairs. The resulting nodules were devoid of intracellular infections and unable to fix nitrogen. Knockdown of PvPI3K suppressed multiple autophagy genes, including PvBECLIN. Independent knockdown of PvBECLIN and another autophagy-related kinase gene, PvTOR (TARGET OF RAPAMYCIN), phenocopied the PvPI3K-silenced lines and led to a drastic reduction in nodule number (Estrada-Navarrete et al., 2016; Nanjareddy et al., 2016).
Reactive Oxygen Species
Nod factor-triggered reactive oxygen species (ROS) production is correlated with the induction of the nodulin gene RIP1 (RHIZOBIUM INDUCED PEROXIDASE1) in M. truncatula and is dependent on DMI1 (Ramu et al., 2002). RNAi uncovered a positive role for the transient ROS burst during rhizobial infection. Silencing of the protein kinase gene MtSPK1 (SYMBIOTIC PROTEIN KINASE1), LjROP6 (Rho family of small GTPases in plants), and two nonorthologous NADPH oxidase genes, PvRBOHa and MtRBOHa (for RESPIRATORY BURST OXIDASE HOMOLOGS), affected SNF to varying degrees. MtSPK1 expression is induced by both Nod factor and H2O2, primarily in infected root hair cells and the infection zones of nodules (Andrio et al., 2013). Artificial microRNA Mtspk1 lines had fewer nodules than the wild type. L. japonicus ROP6 RNAi lines had fewer ITs in the root epidermis, cortex, and nodule primordia and fewer nodules than wild-type plants (Ke et al., 2012). Interfering with NADPH oxidase-catalyzed ROS production by silencing either MtRBOHa or PvRBOHa strongly suppressed SNF, underlining a positive role for ROS in SNF (Marino et al., 2011; Arthikala et al., 2017).
The Roles of Transcription and Posttranslational Machinery in Rhizobial Infection
Components of the general transcriptional machinery have been implicated in the infection process, including LjLAN (LACK OF SYMBIONT ACCOMMODATION), encoding a putative component of a mediator complex required as a cofactor for the recruitment of RNA polymerase II (Suzaki et al., 2019). The EMS-induced Ljlan mutant was Nod− 2 weeks after inoculation, although intracellular crack entry of rhizobia led to nodulation during later stages. Analysis of nodule sections confirmed the presence of bacterial clusters in the absence of any cortical ITs. Curiously, LORE1-insertion mutants and CRISPR/Cas9 mutations in the C-terminal domain of LjLAN did not reproduce this phenotype, indicating that this domain is dispensable for its function (Suzaki et al., 2019). Silencing PvAGO5, encoding a scaffolding Argonaute protein of the RNA-silencing machinery, reduced root hair bending in response to rhizobia and resulted in fewer, smaller nodules (Reyero-Saavedra et al., 2017).
Ancillary Genes
Several genes that support rhizobial infection have been identified via genetics but cannot be placed into any particular process/pathway. Defects in MtRPG (RHIZOBIUM DIRECTED POLAR GROWTH) result in abnormally thick cell IT walls and impaired infection, although early Nod factor responses are normal (Arrighi et al., 2008). By contrast, a Tnt1 insertion in MtCBS1 (Cystathionine-β-Synthase-like Domain-Containing) resulted in more microcolonies and fewer ITs but normal IT wall thickness (Sinharoy et al., 2016).
Infection and organogenesis are interdependent but genetically separable processes; this often complicates the identification of mutants affecting either process alone. For example, the expression of LjnsRING (nodule-specific REALLY INTERESTING NEW GENE), a component of the ubiquitination pathway, correlates with bacterial infection into nodule cells but ceases as nodule development progresses (Shimomura et al., 2006). LjnsRING transcript levels are significantly reduced in nonfunctional nodules induced by a Fix− ΔnifH M. loti mutant. Yet, not only do a limited number of ITs form on LjnsRING RNAi lines, but nodule development is also severely affected, with some transgenic roots infrequently forming small bumps (Shimomura et al., 2006). Genetics has uncovered many other genes required for infection; see Figure 2 and the Supplemental Data Set for more information.
ORGANOGENESIS
Nodule organogenesis is driven by three distinctive processes that occur simultaneously: bacterial colonization, nodule inception, and autoregulation of nodule number. Legumes such as M. truncatula have persistent nodule meristems, which generate elongated, sometimes multi-lobed “indeterminate” nodules, whereas “determinate,” mostly spherical nodules are formed by transiently active meristems in the nodules of species such as L. japonicus, P. vulgaris, and G. max.
Cell Division and Nodule Inception
As root hairs curl, entrap compatible rhizobia, and develop ITs for bacterial transport into root tissues, root pericycle and cortical cells reenter the cell cycle and start dividing, which initiates nodule primordia formation and vascularization (Oldroyd et al., 2011; Guan et al., 2013). Although organogenesis of both indeterminate and determinate types of nodules requires the initiation of cell division in the pericycle, indeterminate nodules emerge from the inner cortex, while determinate nodules emerge from the middle and outer cortex (Hadri et al., 1998). Nodule tissues consist of outer cortex, cortical endodermis, vascular bundles, and inner cortex, which encase the central, mostly infected region. In mature indeterminate nodules, this region is organized into developmental zones: the meristem (zone I), infection zone (zone II), nitrogen fixation zone (zone III), and senescence zone (zone IV). In mature determinate nodules, the central region consists only of nitrogen fixation and, eventually, senescence zones (Xiao et al., 2014). Development of the various tissues and zones of nodules results from successive anticlinal and periclinal divisions in the pericycle, endodermis, and C3 to C5 cortical layers (Xiao et al., 2014). For functional nodules, it is important that some meristematic cells differentiate into large, polyploid cells, which will accommodate the symbionts. Endoreduplication involves DNA replication without cell division, a process facilitated by ubiquitin-dependent proteolysis of mitotic cyclins. MtCCS52A, encoding an anaphase-promoting complex activator, controls endoreduplication during infected cell differentiation in indeterminate nodules. Mtccs52a mutant nodule cells remain small with low ploidy, leading to symbiont and host cell death (Cebolla et al., 1999; Vinardell et al., 2003). The fate of nodule meristem cells was explored by downregulating MtPLETHORA (MtPLT) genes using an RNAi approach. Distinct domains were identified in which MtPLT1 to MtPLT4 genes control differentiation into either cells of the central region (MtPLT3 and MtPLT4) or cells of the nodule periphery (MtPLT1 and MtPLT2; Franssen et al., 2015). Nodule morphogenesis is promoted by class 2 KNOX homeobox KNAT3/4/5-like TFs. RNAi silencing of MtKNAT3/4/5-like genes resulted in fused nodules, although a number of nodules and infection events were not affected (Di Giacomo et al., 2017). Although infection and nodule organogenesis are distinct, genetically separate programs, proper nodule development is dependent on rhizobial infection (Voroshilova et al., 2009). Analysis of lin-4, a weak mutant allele of the MtLIN gene, revealed that normal nodule development requires the initiation of ITs. Moreover, lin-4 nodules that develop 2 to 3 months postinoculation have centrally located vascular bundles. This is reminiscent of nodule formation on N-fixing actonirrhizal plants, which nodulate in association with actinobacteria called Frankia (Kuppusamy et al., 2004; Kiss et al., 2009; Guan et al., 2013). Maintenance of root nodule identity is controlled by the M. truncatula NODULE ROOT (MtNOOT) gene, an ortholog of the Arabidopsis (Arabidopsis thaliana) BLADE-ON-PETIOLE transcriptional activator gene (Couzigou et al., 2012). In noot mutants, roots emerge from the nodule meristem, suggesting that these genes were recruited to repress root identity in nodules.
Transcriptional Control of Nodule Organogenesis
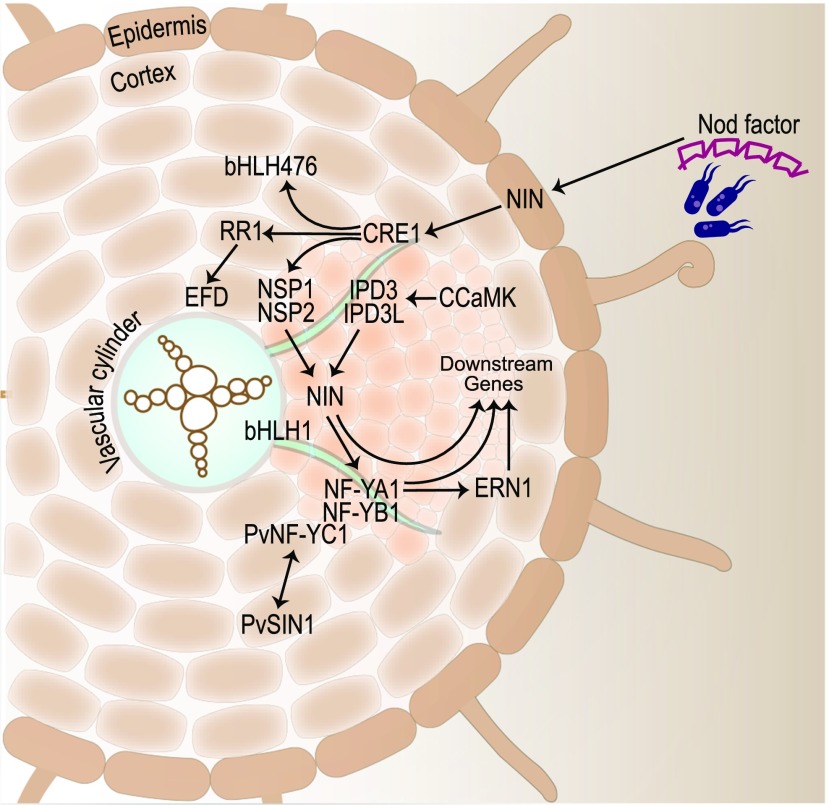
The very first symbiotic gene to be discovered, named Nodule Inception (NIN), was predicted to be a TF gene in L. japonicus (Schauser et al., 1999). A transposon-tagged nin mutant was defective in bacterial recognition, displaying excessive root hair curling without infection or nodulation (Schauser et al., 1999). NIN, which encodes an RWP-RK-containing TF, is a master regulator of multiple genes/processes that have positive or negative effects on nodule organogenesis, depending on the developmental time point, including IT initiation, nodule organogenesis, and the control of nodule number (Figure 4; Yano, 2008; Soyano et al., 2013, 2014; Liu, 2019b).
NIN triggers IT initiation by inducing the LjNPL (see Cell Wall and Cell Membrane Components) and nuclear transcription factor Y subunit A-1 (MtNF-YA1) genes (Figure 5; Xie et al., 2012; Laporte et al., 2014). In response to Ca2+ oscillations triggered by Nod factor signaling, the calcium/calmodulin-dependent protein kinase LjCCamK/MtDMI3 phosphorylates LjCYCLOPS/MtIPD3, which activates NIN expression, leading to IT formation and nodule inception (Yano et al., 2008; Figures 1 and 5). A functionally redundant IPD3-like (MtIPD3L) gene was recently found to act downstream of MtDMI3 to control nodule organogenesis (Jin et al., 2018). NIN expression is also induced by the NSP1 GRAS-type TF, as part of a heterodimer with NSP2 (Hirsch et al., 2009).
Figure 5.
TFs Involved in Nodule Organogenesis.
Cell divisions opposite protoxylem poles initiate nodules. Cytokinin signaling downstream of the CRE1 receptor activates the GRAS domain TFs NSP1 and NSP2, leading to the activation of NIN. LjCYCLOPS/IPD3 bind to the promoter of NIN, leading to the activation of downstream TFs NF-YA1, ERN1, and ERN2. Medicago gene names are displayed unless otherwise indicated.
IT formation and nodule organogenesis in Medicago are dependent on Ethylene Response Factor Required for Nodulation1 (ERN1), which encodes an APETALA2/Ethylene Responsive Factor (AP2/ERF) TF. Mtern1 mutants form infection pockets with limited cortical cell divisions that produce empty nodule “bumps” (Middleton et al., 2007). Similar to NIN, ERN1 is induced by the CCaMK/CYCLOPS complex (Cerri et al., 2017; Kawaharada et al., 2017a) and controls the expression of cell wall-associated ENOD11 and ENOD12, which are critical for IT development (Andriankaja et al., 2007). When MtERN2, a close homolog of MtERN1, is disrupted, functional nodules develop, but they senesce early (Cerri et al., 2016). Since an ern1-1 ern2-1 double mutant was found to be devoid of both infection and nodule organogenesis, it was postulated that, although ERN1 and ERN2 work together in the root epidermis, only ERN1 is required for nodule organogenesis. The induction of LjERN1 by Nod factor is dependent on the Nod factor receptor LjNFR1 and the TFs LjNSP2 and LjCYCLOPS, but not LjNIN or LjNF-YA1, in the epidermis (Kawaharada et al., 2017b). Interestingly, MtNIN controls the extent of infection by limiting the expression of MtENOD11 (EARLY NODULIN11) in the root epidermis, thus acting as an ERN1 antagonist (Vernié et al., 2015). Analysis of a weak nin allele termed daphne-like, with an insertion 7 kb upstream of the NIN start site, further suggested that LjNIN plays a negative role in rhizobial infection but not organogenesis (Yoro et al., 2014).
Like NIN, the Nuclear Factor Y complex (a CCAAT-box binding heterotrimeric TF complex consisting of NF-YA, NF-YB, and NF-YC) controls multiple steps of nodule organogenesis. MtNF-YA1 is critical for nodule meristem initiation and persistence (Combier et al., 2006, 2008). Furthermore, LjNF-YA1 and LjNF-YB1 promote cortical cell division in an LjNIN-dependent manner (Soyano et al., 2013). In P. vulgaris, the NF-Y heterotrimer of PvNF-YAa, PvNF-YB7, and PvNF-YC1 is required not only for infection and organogenesis, but it also plays a role in symbiotic partner selection for improved nitrogen fixation efficiency (Zanetti et al., 2010; Rípodas et al., 2019). The GRAS domain TF PvSIN1 (Scarecrow-like13 Involved in Nodulation) interacts with PvNF-YC1 to control nodule number, size, and IT formation (Figure 5; Battaglia et al., 2014).
Hormonal Regulation
Hormones have long been known to control nodule organogenesis (Figure 6; Grunewald et al., 2009). The concentrations and ratios of auxins and cytokinins determine where and when cells divide. Cytokinins regulate cell division leading to nodule primordium formation, and their signals are mainly perceived by the cytokinin receptor, MtCRE1/LjLHK1 (Gonzalez-Rizzo et al., 2006; Murray et al., 2007; Tirichine et al., 2007). The cytokinin receptors LjLHK1A and LjLHK3 share partially redundant functions with LjLHK1 during nodule development (Held et al., 2014). Direct targets of cytokinin signaling include MtNSP2 and the basic helix-loop-helix TF gene MtbHLH476, both encoding positive regulators of nodule organogenesis (Ariel et al., 2012). Genes for cytokinin biosynthesis and homeostasis are required for normal nodule development, including LjIPT3, LjCKX3, and MtLOG1. Cytokinin biosynthesis is mediated by isopentenyl transferase (encoded by LjIPT3), and cytokinin homeostasis during nodule development is maintained by cytokinin oxidase/dehydrogenase3 (encoded by LjCKX3) in L. japonicus and the cytokinin-activating enzyme riboside 5′-monophosphate phosphoribohydrolase (encoded by the LONELY GUY gene MtLOG1) in M. truncatula (Chen et al., 2014; Mortier et al., 2014; Reid et al., 2016). The involvement of the key TF NIN in cytokinin signaling during nodule organogenesis was recently demonstrated by Liu et al., 2019c. A careful analysis of the NIN promoter revealed that a 2.2-kb segment can reverse the excessive root hair curling and restore infection pocket formation, a 5-kb segment can restore IT formation, but only the presence of 10 cytokinin-responsive promoter elements 18 kb upstream of the NIN translation site can restore nodule organogenesis.
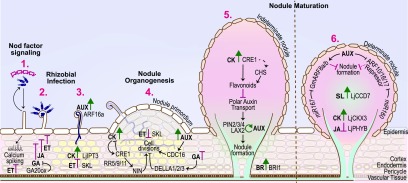
Figure 6.
Hormonal Control of Symbiotic Nitrogen Fixation.
Ethylene (ET) represses calcium spiking induced upon recognition of a compatible Nod factor (1). Rhizobial infection is countered by the hormones ET, jasmonic acid (JA), and gibberellic acid (GA). By contrast, the hormone cytokinin (CK; whose biosynthesis is mediated by IPT3) and auxin (AUX) signaling (via ARF16a) positively regulate IT development (2 and 3). Multiple hormones control cell divisions at the site of infection that lead to the formation of the nodule primordium (4). As the primordium develops into a mature nodule, hormone requirements differ between indeterminate and determinate nodules (5 and 6). Positive and negative roles are shown in green and magenta, respectively. Medicago gene names are shown unless otherwise indicated.
Although auxin response genes are induced at the site of initiation of both determinate and indeterminate nodules, the inhibition of polar auxin transport is crucial for indeterminate but not determinate nodule development (Figure 6; Ng and Mathesius, 2018). Medicago PINFORMED (PIN) auxin exporters MtPIN2, MtPIN3, and MtPIN4 and the auxin influx carrier MtLAX2 (LIKE AUXIN2) promote nodule organogenesis (Huo et al., 2006; Roy et al., 2017). Auxin-mediated organogenesis of determinate nodules also involves the microRNA miR167 and the auxin response factors GmARF8a and GmARF8b in soybean. miR167 acts upstream of NIN, NSP1, NF-YA1, NF-YA2, and ENOD40 and positively regulates nodule formation by inhibiting GmARF8a and GmARF8b expression (Wang et al., 2015). Another microRNA, miR160, plays a crucial role in maintaining the auxin/cytokinin balance during soybean nodule inception and maturation (Nizampatnam et al., 2015). Auxin-induced transcriptional regulation of nodule development is also regulated by the basic helix-loop-helix TF MtbHLH1 (Godiard et al., 2011). Finally, M. truncatula CELL DIVISION CYCLE16 (MtCDC16), a presumed component of the anaphase-promoting complex, participates in auxin-dependent nodule organogenesis. The cdc16 mutant has fewer lateral roots and slightly more nodules than the wild type but shows an auxin-resistant root elongation phenotype (Kuppusamy et al., 2009).
Carotenoid cleavage products, including two plant hormones, strigolactone and abscisic acid, also play roles in nodule organogenesis. The β-carotene hydroxylases GmBCH1 and GmBCH2 catalyze the conversion of β-carotene to β-zeaxanthin in soybean. RNAi silencing of GmBCH1/2 impaired nodule development and SNF (Kim et al., 2013). Silencing of P. vulgaris BYPASS1 (PvBPS1) resulted in defective nodule development, which was partially rescued by the carotenoid biosynthesis inhibitor fluridone (Arthikala et al., 2018).
Additional Genes Required for Nodule Organogenesis
Defects in MtNPF1.7 of the Nitrate and Peptide Transporter Family account for the mutant phenotypes of Mtnip-1, Mtnip-3, and Mtlatd (numerous infections and polyphenolics/lateral root-organ defective; Yendrek et al., 2010). These mutants have nodules and lateral roots that lack a persistent meristem, with the more severe Mtnip-1 and Mtlatd mutants showing rhizobia trapped within ITs (Veereshlingam et al., 2004; Teillet et al., 2008). Although MtNPF1.7 transported nitrate in Xenopus laevis oocytes at high affinity (Bagchi et al., 2012), the in vivo substrate and precise role of MtNPF1.7 remain unclear, given that some NPFs also transport hormones (Zhang et al., 2014).
Nodule organogenesis requires other genes involved in cellular events and processes in addition to those described above. Refer to Figure 2 and the Supplemental Data Set for more information on these genes.
AUTOREGULATION OF NODULATION
Autoregulation of nodulation (AON) is a systemic, long-distance signaling pathway involving signals from developing nodules that are transported to the shoot, where they trigger retrograde signaling from the shoot to control (suppress) further nodulation (Caetano-Anollés and Gresshoff, 1991; Krusell et al., 2002). Nodule numbers are also controlled by local feedback confined to roots, as described below (Figure 7).
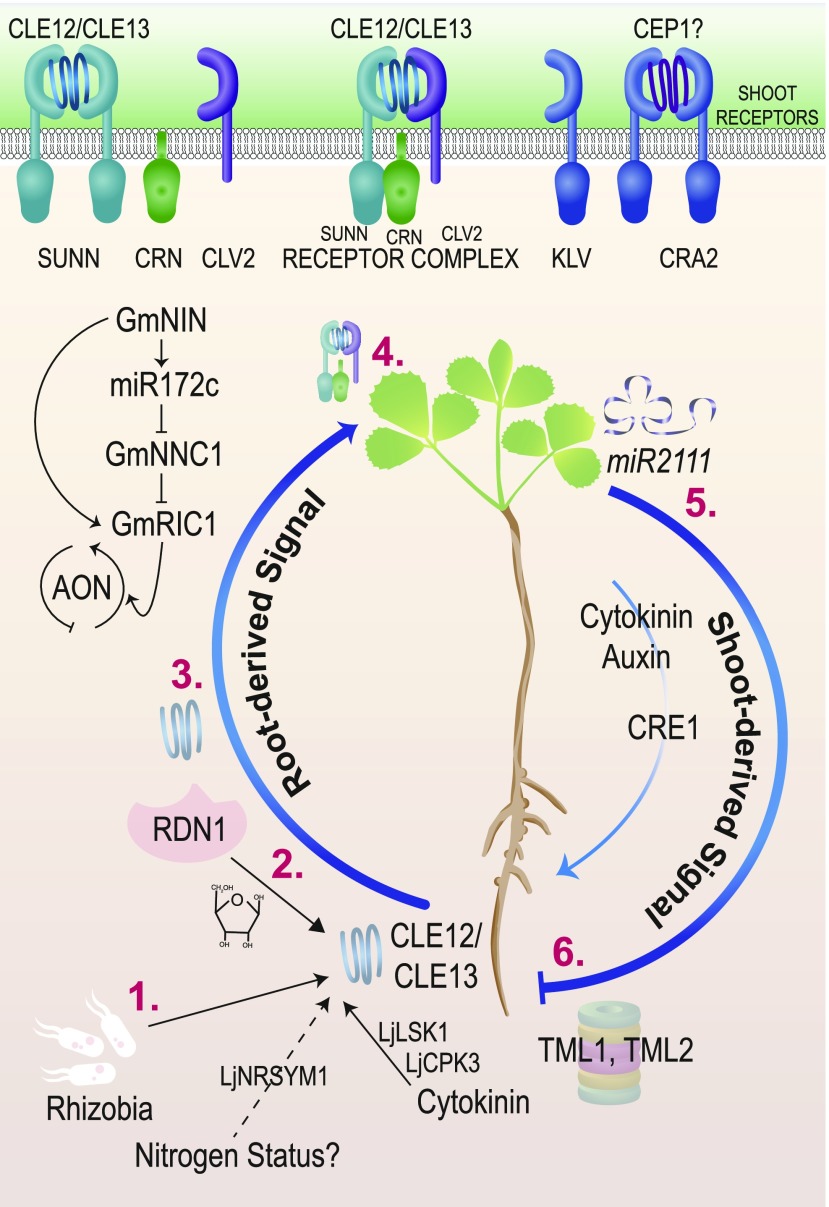
Figure 7.
Autoregulation of Nodulation.
Upon perception of rhizobia, the activation of cytokinin signaling results in increased expression of the genes encoding CLE12 and CLE13 peptides (1), which are then modified with arabinose residues by the RDN1 enzyme (2) and transported through the xylem to the shoot (3). Perception of the peptides in the shoot requires the receptor kinase SUNN, CLV2 receptor-like protein, and CRN pseudo-kinase (4). A second pathway involving the CRA2 receptor also controls nodule number by responding to a CEP peptide. Receptors in the shoot increase auxin and cytokinin transport to the root and the transport of miR2111(5), which affects TML expression (6).
Shoot-root grafting of hypernodulating mutant and wild-type soybean showed that the root supernodulation of the nts382 (nitrate tolerant symbiosis382) soybean mutant is determined by the shoot genotype (Delves et al., 1986), implicating a long-distance signaling pathway. The causative mutation in nts382 is present in the GmNARK (NODULE AUTOREGULATION RECEPTOR KINASE) gene, which is orthologous to the LjHAR1 (HYPERNODULATION AND ABERRANT ROOT) and MtSUNN genes (Krusell et al., 2002; Searle et al., 2003; Schnabel et al., 2005). LjHAR1/GmNARK/MtSUNN, which are homologous to Arabidopsis CLAVATA1 (CLV1), a gene involved in meristem maintenance, encode class XI LRR-RLKs containing a short N-terminal plasma membrane-targeting signal, LRRs, a transmembrane domain, and a cytoplasmic Ser/Thr kinase domain (Figure 7; Krusell et al., 2002; Nishimura et al., 2002; Schnabel et al., 2005).
Constitutive expression of the nodulation-induced peptide-coding gene MtCLE12/LjCLE-RS1 or MtCLE13/LjCLE-RS2 eliminates nodulation (Okamoto et al., 2009; Mortier et al., 2010). However, overexpression of MtCLE12 or MtCLE13 in the sunn mutant background reduces but does not eliminate nodulation, indicating that SUNN is required for responses to these two peptides (Mortier et al., 2012). LjCLE-RS2 binds to LjHAR1, and the application of the peptide through vascular “feeding” showed that LjCLE-RS2 is sufficient to halt nodulation in wild-type L. japonicus but not in a har1 mutant, indicating that the LjHAR1 receptor kinase is required for the control of the AON pathway by LjCLE (Okamoto et al., 2013). These data point to CLE peptides as the root-to-shoot signal in the AON pathway. Counterparts of these CLEs include the rhizobia-induced GmRIC1 and GmRIC2 in G. max (Lim et al., 2011; Reid et al., 2011; Ferguson et al., 2014). Some CLE peptides in Arabidopsis must be arabinosylated at Hyp residues in order to function (Ogawa-Ohnishi et al., 2013; Imin et al., 2018). The Mtrdn1/Ljplenty mutants, carrying a disrupted Hyp arabinosyl transferase enzyme, also hypernodulate, but unlike MtSUNN, MtRDN1 exerts its effect in roots (Yoshida et al., 2010; Schnabel et al., 2011; Yoro et al., 2019). Wild-type plants exhibit reduced nodule number in the presence of abundant available nitrogen (e.g., 10 mM KNO3), but most hypernodulation mutants have nitrate-resistant nodulation, implying that nitrogen status functions as an input to AON. While LjCLE-RS1 and LjCLE-RS2 genes were first identified as induced by rhizobia, LjCLE-RS2 is also induced by nitrate (Okamoto et al., 2009), connecting nodulation, CLEs, and nitrate sensing. The nitrate-induced expression of LjCLE-RS1 and LjCLE-RS2 is dependent on NITRATE UNRESPONSIVE SYMBIOSIS1 (LjNRSYM1), a NIN-like protein (Nishida et al., 2018). IT formation and nodulation are insensitive to the application of high nitrate in Ljnrsym1 mutants. However, analysis of the Ljhar1-7 Ljnrsym1-1 double mutant indicated that while LjHAR1 may be involved in regulating nodule number under N-deficient conditions, the control of IT number, nodule cell size, and N fixation is independent of LjHAR1 (Nishida et al., 2018). Thus, the control of SNF by LjNRSYM1 under high-N conditions likely occurs in parallel to AON.
Given the findings described above, legume CLV2 homologs were also pursued for a role in AON. RNAi and EMS mutagenesis of LjCLV2 produced a hypernodulation phenotype (Krusell et al., 2011). MtCLV2 interacts with the MtSUNN receptor kinase and MtCORYNE (CRN), a pseudokinase that causes hypernodulation when mutated (Crook et al., 2016). The Mtcrn mutant behaves like a Mtsunn mutant in grafting experiments: the effect is shoot derived (Crook et al., 2016), and Mtcrn mutants do not respond to overexpression of MtCLE12 or MtCLE13 (Nowak et al., 2019). Together, mutational and physical evidence suggests crosstalk between AON receptors in the shoot (Figure 7, top).
Mutants in other receptors affecting nodule number yielded information about more potential interactions for AON receptors. The Ljklavier (Ljklv) mutant is a shoot-controlled hypernodulation mutant (Oka-Kira et al., 2005). LjKLV encodes a receptor-like kinase placed genetically in the AON pathway alongside LjHAR1, based upon mutant responses to CLE-RS1 and CLE-RS2 overexpression and physical interaction between the KLV and HAR1 proteins (Miyazawa et al., 2010). Interestingly, M. truncatula has two MtKLV sequence homologs (Supplemental Data Set), which remain to be tested for roles in AON. Another receptor kinase identified by mutation, MtCRA2, regulates nodule number systemically from the shoot, independently of MtSUNN, to limit nodule development in the presence of nitrate (Huault et al., 2014; Mohd-Radzman et al., 2016). The C-terminally encoded peptide (CEP) family act as nitrogen “hunger” signals in plants and are transported systemically from root to shoot (Okamoto et al., 2016). Overexpression of MtCEP1 enhances nodulation (Imin et al., 2013), and CRA2 is required for CEP activity (Laffont et al., 2019), but analysis of a cra2 sunn double mutant suggested that these nodule regulatory pathways are independent (Laffont et al., 2019; Nowak et al., 2019).
Exciting progress has also been made on genes/proteins that act in roots in response to the shoot-derived inhibitor and on this mobile inhibitor itself. TOO MUCH LOVE (TML), a gene identified via analysis of a hypernodulating mutant of L. japonicus, regulates nodule number from the root and is active after the signal is sent from the shoot (Magori and Kawaguchi, 2009). LjTML encodes a Kelch-repeat F-box protein possibly involved in proteosomal degradation of target proteins as a late step in AON (Takahara et al., 2013). Medicago has two TML genes, MtTML1 and MtTML2. Knockdown of these genes by RNAi increased relative nodule number, implicating them in AON (Gautrat et al., 2019). Interestingly, homologous sequences in Arabidopsis and G. max are targeted for degradation by miR2111, a conserved microRNA that may be transported systemically (Ferguson et al., 2019). In fact, the expression of miR2111 regulates TML in L. japonicus in a shoot-controlled manner dependent on har1 (Tsikou et al., 2018), which would be expected of the AON shoot-derived inhibitor.
NODULE NUMBER CONTROL1 (GmNNC1) gene transcripts, encoding an AP2 TF, are cleaved by miR172c, which enables nodule development in soybean (Figure 7). Expression of miR172c is increased in Gmnark nodule primordia, making miR172c another potential player in AON (Wang et al., 2014). GmNNC1 physically interacts with the TF BES1/BZR1 HOMOLOG LIKE1 (GmBEHL1), a soybean homolog of Arabidopsis BES1/BZR1 HOMOLOG1 (BEH1) involved in brassinosteroid signaling (Yan et al., 2018). Knockdown of GmBEHL1 expression doubled the number of nodules formed, concomitant with the induction of GmmiR172c expression.
Cytokinin and AON-Independent Control of Nodulation
Ljlhk1 cytokinin receptor mutants exhibit suppressed AON, leading to increased nodulation in L. japonicus (Murray et al., 2007). By contrast, mutations in MtCRE1, the Medicago ortholog of LjLHK1, reduce nodule number, which in an Mtsunn mutant background brings nodule number closer to wild-type levels, indicating that two separate pathways control nodule number (Gonzalez-Rizzo et al., 2006). CRE1 is one of several genes that interact with AON signals to affect nodule number locally. In M. truncatula, the induction of MtCLE12 and MtCLE13 in the nodule meristem requires an active MtCRE1 cytokinin receptor (Mortier et al., 2012). Similarly, LjCLE-RS1 and LjCLE-RS3 accumulation during infection is reduced in lhk1-1 mutant roots (Tsikou et al., 2018), suggesting that early cytokinin signaling is required to send the AON signal. Evidence from tissue-specific expression of LjLHK1 supports the notion that cortical cell cytokinin signaling functions as a local link to the systemic AON pathway (Miri et al., 2019). Other genes affecting nodule number are also tied to cytokinin signaling, but less directly to AON. Please see Figure 2 and the Supplemental Data Set for more information.
BACTERIAL RELEASE AND SYMBIOSOME FORMATION
When the IT reaches the incipient nodule cortex, it branches and releases bacteria into plant cells via unwalled infection “droplets,” resulting in a unique “organelle” called the symbiosome (Figure 8). Bacteria within symbiosomes grow and divide, along with the plant-produced symbiosome membrane (SM), until the plant cell is more or less packed with thousands of symbiosomes, each containing one or a few bacteria (Roth and Stacey, 1989).
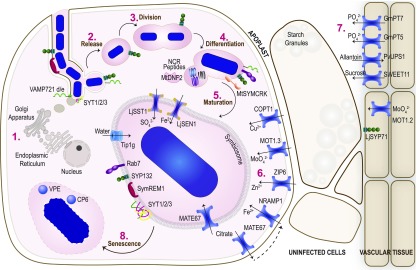
Figure 8.
Transport and Metabolism during Symbiotic Nitrogen Fixation.
Bacteria are released into the host cell in infection droplets (1) derived from Golgi deposits at the site of bacterial release (6), where they divide (2) and differentiate (3). Mature symbiosomes have multiple transporters on the symbiosome membrane and other membrane proteins that are essential for symbiosome membrane biogenesis (4). Other transporters on the cell membrane and from the vasculature (7) also import elements required for bacterial metabolism and optimal N fixation before undergoing senescence (8).
Role of the Exocytotic Pathway
Although early studies proposed that symbiosome formation occurs through an endocytic process (Mellor, 1989; Verma, 1992), recent genetic evidence implicates the secretory/exocytotic pathway, which delivers membrane vesicles to the plasma membrane, in this process (Limpens et al., 2009). Membrane fusion during exocytosis is mediated by SNARE (soluble N-ethylmaleimide-sensitive factor attachment protein receptor) complexes, which are of two types: vesicle membrane-localized SNAREs (v-SNAREs) and target membrane-localized SNAREs (t-SNAREs). Downregulation of two v-SNARE genes in M. truncatula, MtVAMP721d and MtVAMP721e (VESICLE-ASSOCIATED MEMBRANE PROTEIN), blocked bacterial release from ITs and symbiosome formation without affecting nodule development (Ivanov et al., 2012). Similar results were found in G. max and L. japonicus (Gavrin et al., 2016; Sogawa et al., 2019). MtVAMP721d and MtVAMP721e are localized to the site of bacterial release and on the SM (Ivanov et al., 2012; Gavrin et al., 2016). Interestingly, reducing MtVAMP721d and MtVAMP721e expression also blocked arbuscule formation but not fungal colonization during AM symbiosis, indicating that these genes have conserved functions in intracellular symbioses in plants (Ivanov et al., 2012). As described above, LjNPL is required for the degradation of plant cell walls during rhizobium infection (Xie et al., 2012). The encapsulation of its ortholog, GmNPL1, in GmVAMP721d-positive vesicles indicates that exocytosis also plays a role in loosening cell walls prior to bacterial release into host cells (Gavrin et al., 2016). Another member of the M. truncatula VAMP72 family, MtVAMP721a, which may be involved in plant defense, is repressed by the nodule-enhanced C2H2-type TF, MtRSD (REGULATOR OF SYMBIOSOME DEVELOPMENT), and Mtrsd mutants are defective in symbiosome development and nitrogen fixation (Sinharoy et al., 2013). Whether VAMP721a interferes or competes with VAMP721d/e-mediated vesicle trafficking and/or directs different types of vesicles (e.g., those containing defense molecules) to sites of bacterial entry in Mtrsd mutants remains to be determined.
The exocytotic pathway involves the t-SNARE protein MtSYP132 (SYNTAXIN OF PLANTS132), which is localized to the IT membrane, infection droplets, and SM in M. truncatula (Catalano et al., 2007; Limpens et al., 2009). RNAi of MtSYP132A impairs symbiosome differentiation and SNF (Pan et al., 2016). MtSYP132A is also required for arbuscule development during AM symbiosis, indicating that it plays conserved roles in plant-microbe interactions (Pan et al., 2016). LjSYP71 has also been implicated in symbiosome differentiation, with Ljsyp71 mutants exhibiting enlarged symbiosomes and a Fix− phenotype (Hakoyama et al., 2012b).
Synaptotagmins bind to t-SNAREs and v-SNAREs and facilitate the fusion of vesicles to target membranes. MtSYT1, MtSYT2, and MtSYT3 are nodule synaptotagmins that are expressed from the meristematic to fixation zones. RNAi of the corresponding genes delayed rhizobium release, blocked symbiosome differentiation, and resulted in Fix− nodules (Gavrin et al., 2017).
Role of the Endocytic Pathway
Although exocytosis plays a pivotal role in rhizobium release and symbiosome formation, two endocytic pathway proteins, MtFLOT2/4 and MtSYMREM1, are found in the SM (Haney and Long, 2010; Lefebvre et al., 2010). RNAi of MtFLOT2/4 caused the formation of small, white nodules with reduced SNF capacity, whereas an Mtsymrem1 mutant was defective in bacterial release. Thus, MtFLOT2/4, MtSYMREM1, and endocytosis may participate in signal transduction and/or membrane trafficking associated with bacterial release and/or symbiosome development. Symbiosome maturation is also impaired upon knockdown of the late endosomal marker GTPase MtRab7 (Ras-related protein in brain), resulting in early nodule senescence (Limpens et al., 2009).
Additional Components Involved in Symbiosome Differentiation
The cytoskeleton, including microtubules and actin filaments, plays a crucial role in intracellular transport (Petrásek and Schwarzerová, 2009). Actin undergoes dynamic rearrangement during bacterial release and symbiosome differentiation in nodules (Zhang et al., 2019). RNAi of MtARP3 (ACTIN RELATED PROTEIN), encoding an actin nucleation complex component required for actin filament branching, impaired symbiosome development, resulting in small, nonfunctional symbiosomes (Gavrin et al., 2015). TFs also affect symbiosome differentiation. Silencing a Kruppel-like zinc finger protein gene (MtZPT2-1) impaired early bacterial differentiation and SNF (Frugier et al., 2000). Additionally, the aquaporin MtTIP1g, which is required for water transport, is redirected from the vacuolar membrane to the SM in the nodule fixation zone. Repression of MtTIP1g arrested symbiosome elongation prior to maturation (Gavrin et al., 2014).
HOST CONTROL OF BACTERIAL MATURATION
Rhizobia within symbiosomes differentiate into N-fixing bacteroids that release ammonia into the host cell in exchange for reduced carbon and other nutrients from the plant, with exchange mediated by transporters in the SM (Udvardi and Poole, 2013). In certain legumes, primarily galegoid (temperate) legumes including M. truncatula, bacterial differentiation involves changes in volume and shape (e.g., elongation), DNA content (endoreduplication), and loss of viability as a free-living organism (Mergaert et al., 2006). Bacteroid differentiation is accompanied (and in part controlled) by a decrease in free oxygen concentration in infected cells, which prevents the inactivation of oxygen-labile nitrogenase. Microaerobic conditions within nodules are achieved by restricted gaseous diffusion into nodules and high rates of respiration facilitated by oxygen-binding and oxygen-transporting leg(ume)hemoglobin in the cytoplasm of plant cells (Ott et al. 2005).
Activation of Bacterial Nitrogenase
The induction of nitrogenase genes in rhizobia is controlled by oxygen. Free-oxygen concentrations in nodule cells are as low as 10−8 M in the fixation zone, permitting nitrogenase to function (Tjepkema and Yocum, 1974). However, plant mitochondria and bacteroids require high oxygen flux to sustain respiration for ATP supply to nitrogenase. Leghemoglobins are essential for buffering oxygen in nodules at low levels while transporting it rapidly to rhizobia. RNAi and CRISPR/Cas9 of LjLB1, LjLB2, and LjLB3 resulted in nodules with elevated oxygen concentrations but reduced ATP:ADP ratios and defective SNF (Ott et al., 2005; Wang et al., 2019). Importantly, nif genes (nifH) were not induced in these nodules (Ott et al., 2009).
Interestingly, bacterial nitrogenase activity is also dependent on plant supply of the cofactor homocitrate, at least in rhizobia that lack nifE and cannot make homocitrate. Mutations in LjFEN1, which encodes homocitrate synthase, impair SNF, but this can partially be overcome by adding homocitrate (Hakoyama et al., 2009).
Terminal Differentiation
Indeterminate nodule-forming legumes belonging to the inverted repeat-lacking clade, including M. truncatula, require their symbionts to undergo terminal differentiation, including loss of motility, elongation, and endoreduplication, before they fix nitrogen. This process is controlled by the Cys-rich NCR peptides, which are cleaved into their biologically active forms from prepropeptides by peptidases (Figure 8). Mutations in two enzymes controlling NCR peptide maturation cause severe defects in nitrogen fixation. DNF1 (DEFECTIVE IN NITROGEN FIXATION1) encodes a signal peptidase enzyme required for cleaving the N-terminal signal peptide directing NCR peptides to the symbiosome. Unprocessed prepropeptide NCRs in dnf1 mutants do not induce terminal differentiation of bacteria, blocking SNF (Van de Velde et al., 2010; Wang et al., 2010). Nonetheless, infection and nodule organogenesis of dnf1 are normal. A DNA methylase required for maturation of at least 82 NCR peptides, encoded by DEMETER (MtDME; Satgé et al., 2016), is also required for bacteroid endoreduplication, elongation, and SNF.
Mature 35- to 70-amino acid NCR peptides are targeted to the symbiosome, where they induce bacteroid differentiation. The NCR peptide family has at least 700 members in M. truncatula (de Bang et al., 2017), making it difficult to study them using conventional genetic tools. NCR coding sequences are short, making it hard to retrieve insertion mutants by reverse genetics. Furthermore, functional redundancy is expected to stymie genetic studies. Surprisingly, therefore, single mutations in MtNCR211 (dnf4) or MtNCR169 (dnf7) impair SNF (Horváth et al., 2015; Kim et al., 2015). Thus, their roles appear to be unique. Irregularly shaped undifferentiated bacteroids in dnf7 mutant lines appear to be incompletely surrounded by the SM, while bacteroid-containing cells in dnf4 mutants appear degenerated (Horváth et al., 2015; Kim et al., 2015). These observations demonstrate the importance of the NCR family in establishing effective symbiosis in M. truncatula.
Although determinate nodule-forming legumes do not possess NCR peptides, the Fix− phenotype of an Ljapn1 (aspartic peptidase nodule induced1) mutant hints at the idea that other peptides control bacterial persistence within nodules (Yamaya-Ito et al., 2018). Further studies of the roles of plant peptides during SNF are clearly warranted.
SYMBIOTIC METABOLISM AND TRANSPORT
SNF is a metabolic symbiosis based upon the exchange of reduced C derived from plant photosynthesis for reduced N from rhizobial nitrogen fixation. Genetic studies have shown that effective SNF requires the coordination of multiple metabolic and transport pathways in both the plant and the microbe (Figure 8).
Carbon Metabolism and Transport
Sucrose derived from photosynthesis is transported through the vascular system to nodules, where it is hydrolyzed by Suc synthases or invertases that are typically nodule-enhanced (Morell and Copeland, 1984; Hohnjec et al., 2003; Flemetakis et al., 2006). The reduced Suc synthase activity in P. sativum Pssus1 and L. japonicus Ljsus1 Ljsus3 double mutants impairs SNF, as does RNAi silencing of MtSucS (Gordon et al., 1999; Baier et al., 2007; Horst et al., 2007). Built-in metabolic redundancy seems to be a theme in nodule carbon metabolism and transport. Both a Tnt1-insertion mutant of the nodule-enhanced Suc transporter gene MtSWEET11 and a null mutation of LjSUS3, encoding the major nodule-induced isoform of Suc synthase, are uncompromised in SNF (Horst et al., 2007; Kryvoruchko et al., 2016).
Redox Metabolism
High rates of respiration result in the production of ROS, which are harmful to nodule metabolism (Chang et al., 2009). Antioxidants, including ascorbate, reduced GSH, and homoglutathione, can scavenge ROS and protect cells from oxidative damage. In M. truncatula, γ-glutamylcysteine synthetase (MtγECS) is required for GSH biosynthesis; silencing MtγECS impairs nodule growth and SNF (El Msehli et al., 2011).
Nitrogen Metabolism and Transport
Plant ammonia transport and assimilation and organic N export are central aspects of nodule function. The Gln synthetase-glutamate synthase (GOGAT) cycle catalyzes the assimilation of ammonium into amino acids. RNAi of MsNADH-GOGAT reduced nodule amino acid content (primarily Glu, Gln, and Ala) and impaired SNF in alfalfa (Medicago sativa; Cordoba et al. 2003). Amino acids, especially Gln and Asn, are exported from nodules of temperate legumes, including M. truncatula and L. japonicus, whereas ureides, especially allantoin and allantoic acid, are synthesized and transported from nodules to shoots in tropical legumes, including G. max and P. vulgaris (Smith and Atkins, 2002). In plants, ureides are synthesized by purine oxidation. Glutamine phosphoribosyl pyrophosphate amidotransferase (PRAT) catalyzes the first step of de novo purine synthesis. Silencing of nodule-enhanced PvPRAT3 severely reduced ureide production and slightly reduced SNF (Coleto et al., 2016).
Several transporters of nitrogen-containing compounds are required for effective SNF. These include LjAMT1;1, GmUPS1-1 and GmUPS1-2, LjNPF8.6, and MtNPF1.7. RNAi of two nodule cortical and vascular plasma membrane-localized ureide permease genes, GmUPS1-1 and GmUPS1-2, resulted in increased nodule ureide levels but reduced plant growth, reflecting decreased translocation of N from nodules to shoots (Collier and Tegeder, 2012). LjAMT1;1 encodes a plasma membrane-localized high-affinity ammonium transporter. RNAi of LjAMT1;1 resulted in less effective SNF, suggesting that this ammonium transporter might function in recovering ammonium that diffuses out of nodule cells before it is incorporated into amino acids for export to the shoot (Rogato et al., 2008). LjNPF8.6, a nodule-induced member of the NPF, showed dual-affinity nitrate transport activity in X. laevis oocytes, and SNF was reduced in an LjNPF8.6 mutant (Valkov et al., 2017).
Phosphate and Sulfate Transport
The macronutrient phosphate is required by developing and functional nodules to sustain growth and metabolism. Low soil phosphate is an important limiting factor of nodule initiation and growth (Le Roux et al., 2009). PHOSPHATE STARVATION RESPONSE25 (GmPHR25)-regulated phosphate transporters GmPT5 (PHOSPHATE TRANSPORTER5) and GmPT7, which likely control phosphorus uptake into nodules, control nodule number and size (Qin et al., 2012; Xue et al., 2017; Chen et al., 2019). Sulfate is also important for SNF. Mutants of L. japonicus Symbiotic Sulfate Transporter1 (LjSST1) are defective in SNF (Krusell et al., 2005). A proteomics study placed LjSST1 on the SM (Wienkoop and Saalbach, 2003), indicating that it plays a role in sulfate transport from infected cells to rhizobia (Krusell et al., 2005).
Metal Ion Transport
Metal ions play crucial roles in SNF as cofactors of metalloenzymes, including (Fe)-hemoglobins, (Fe-Mo)- and (Fe-S)-nitrogenases, and (Cu-Fe)-cytochromes, as well as TFs, including zinc finger TFs (Figure 8; Rubio et al., 2007; González-Guerrero et al., 2014).
Iron is delivered by the vascular system to nodules, where it is released to the apoplasm before being taken up by plant cells and delivered to bacteroids (Rodríguez-Haas et al., 2013). The M. truncatula plasma membrane iron transporter, Natural Resistance-Associated Macrophage Protein1 (MtNRAMP1), transports iron from the apoplast into infected nodule cells where it is essential for SNF, as shown using Mtnramp1 Tnt1-insertion mutants (Tejada-Jiménez et al., 2015). Iron is often chelated to citrate to ensure its solubility and movement throughout the plant. The plasma membrane-localized, iron-activated citrate efflux transporter MtMATE67 is required for iron uptake from the apoplasm of nodule cells and for effective SNF (Kryvoruchko et al., 2018). Similarly, RNAi of Ljmate1 altered iron distribution in nodules and impaired SNF in L. japonicus (Takanashi et al., 2013).
LjSEN1 is required for rhizobial differentiation into N-fixing bacteroids in L. japonicus nodules (Suganuma et al., 2003). LjSEN1 is homologous to Arabidopsis VACUOLAR IRON TRANSPORTER1 and is expressed specifically in nodule-infected cells. LjSEN1 is thought to transport iron, but this remains to be demonstrated (Hakoyama et al., 2012a).
Molybdenum is crucial for iron-molybdenum nitrogenase activity. Two molybdenum transporters, MtMOT1.2 and MtMOT1.3, are essential for SNF in M. truncatula. MtMOT1.2 is located on the plasma membrane of endodermal cells surrounding nodule vascular vessels and mediates molybdate uptake and distribution into nodule cells (Gil-Díez et al., 2019). MtMOT1.3, which is located on the plasma membrane of infected cells, mediates molybdate uptake into these cells (Tejada-Jiménez et al., 2017). The mutation of either MtMOT1.2 or MtMOT1.3 impairs SNF (Tejada-Jiménez et al., 2017; Gil-Díez et al., 2019). How molybdate is transported across the SM remains to be determined.
Zinc is a cofactor of superoxide dismutases that detoxify ROS and is present in zinc-finger motif TFs (Rubio et al., 2007; González-Guerrero et al., 2014). Two zinc transporters, MtZIP6 (Zinc-Iron Permease6) and MtMTP2, are essential for SNF. RNAi of MtZIP6, encoding an enzyme in the plasma membrane of infected cells responsible for zinc uptake from the apoplast, impaired SNF (Abreu et al., 2017). M. truncatula Metal Tolerance Protein2 (MtMTP2), which is localized to the endomembrane system, is a zinc efflux protein (León-Mediavilla et al., 2018). Mutation of Mtmtp2 resulted in aberrant bacteroid differentiation and early senescence, suggesting a defect in intracellular zinc allocation (León-Mediavilla et al., 2018).
Copper is a cofactor of several enzymes, including cytochrome oxidases. M. truncatula Copper Transporter1 (MtCOPT1), which is localized to the plasma membrane, imports copper from the apoplast into nodule cells. Mtcopt1 mutants displayed lower SNF and lower copper-dependent cytochrome oxidase activity than wild-type plants (Senovilla et al., 2018).
SENESCENCE AND DEFENSE
Nodule senescence is a controlled process that enables nutrient recycling and can be triggered by developmental and environmental cues such as drought and high nitrate levels. During age-related senescence, bacteroids lyse, forming numerous vesicles within the cytosol of infected cells, followed by senescence of infected cells (Van de Velde et al., 2006). Typically, senescence is mediated by TFs and involves proteases and lipases that degrade proteins and lipids, respectively, as well as transporters that export breakdown products (Schippers et al., 2015). The TF gene MtNAC969 (NAM/ATAF/CUC-encoding969) is induced in the nitrogen fixation zone upon nitrate treatment (de Zélicourt et al., 2012). RNAi of MtNAC969 did not alter nodule formation but it activated senescence genes, including two senescence-induced cysteine proteinase (CP) genes, and caused premature nodule aging (de Zélicourt et al., 2012). Therefore, MtNAC969 appears to act as a suppressor of nodule senescence that is executed, at least in part, by CPs. RNAi of two CP genes expressed in the nodule senescence zone, MtCP6, encoding a papain-type proteinase, and MtVPE (VACUOLAR PROCESSING ENZYME), encoding a protein of the legumain proteinase subclass, delayed nodule senescence and prolonged nodule growth and nitrogen fixation (Pérez Guerra et al., 2010; Pierre et al., 2014). MtCP6 and MtVPE are localized to the SM, suggesting that they are involved in symbiosome degradation. Conversely, knockdown of the CP inhibitor gene MtSERPIN6 accelerated nodule aging (Dhanushkodi et al., 2018). Interestingly, mutations in the STAY-GREEN gene MtSGR led to aberrant shoot senescence and delayed nodule senescence (Zhou et al., 2011).
Iron liberated from leghemoglobin, especially during senescence, is a potential source of ROS, which can interfere with nodule metabolism. Iron storage proteins that scavenge free iron include ferritin multimeric complexes (Theil, 2003). Silencing of two ferritin genes, MtFer2 and MtFer3, increased free iron levels in the nodule and induced early nodule senescence (Dhanushkodi et al., 2018). Therefore, preventing leghemoglobin degradation might delay nodule senescence. Accordingly, nodules of the Mtcas31 (cold acclimation specific31) mutant, which is defective in a dehydrin protein that interacts with the leghemoglobin MtLB120-1, had elevated transcript levels of senescence genes and reduced nitrogenase activity under drought stress (Li et al., 2018). Although nodule senescence enables plants to recover part of their investment in nodules via the remobilization of nutrients, premature nodule senescence compromises SNF and N supply to the plant. Therefore, it would be interesting to identify key signaling components and regulators of senescence as a step toward improving SNF by delaying nodule senescence.
Defense phenotypes often overlap with those exhibited by senescent nodules. For example, knockdown of MtSNARP2 (SMALL NODULIN ACIDIC RNA BINDING PROTEIN2) resulted in defense responses such as the development of a thick nodule outer cortex and a closed endodermal layer (Laporte et al., 2010). On the other hand, Mtsnarp2 mutants senesce prematurely without accumulating brown pigments often associated with defense. In nodule with activated defenses1(Mtnad1) mutant nodules, cells of both symbiotic partners are disrupted and the defense marker gene PR10 is upregulated (Wang et al., 2016; Domonkos et al., 2017). Bacteria in Mtnad1 mutants have an unorganized bacteroid cell wall showing degradation. Consequently, SNF is arrested and does not progress to the N-fixation stage.
Interfering with SNF progression at any stage also elicits defense-like responses. Fix− mutants of the DOES NOT FIX2 (MtDNF2) gene, encoding a phosphatidylinositol phospholipase C-like protein, and the coregulated SYMBIOTIC CYSTEINE RICH RECEPTOR-LIKE KINASE (MtSymCRK), which do not belong to known defense pathways, exhibit defense-like phenotypes such as brown-pigmented, necrotic nodules, defense marker gene induction, a block in bacterial terminal differentiation, and no N-fixing activity (Bourcy et al., 2013). Interestingly, these defense-like reactions in the dnf2 mutant manifest only when grown on agar containing impurities, further supporting the idea that defense responses triggered by an external elicitor can impair SNF progression (Berrabah et al., 2014). Additional research is warranted to understand whether such molecular players are direct repressors of defense pathways or if the associated mutant phenotypes are secondary responses to interrupted SNF.
CONCLUSIONS AND FUTURE DIRECTIONS
The last 20 years have seen amazing progress in the discovery of genes and mechanisms underlying SNF. While most SNF genes have homologs in nonlegumes, many of these genes have evolved roles in SNF via gene family expansion and/or neofunctionalization, often resulting in nodule-specific expression. Mycorrhizal root symbiosis evolved in land plants hundreds of millions of years before legume-rhizobia SNF, and work over the last 20 years has uncovered several common symbiotic genes that are required for SNF that first evolved for mycorrhizal symbiosis. Genetic studies in legumes have focused on one or two genotypes per species, but genome sequencing of multiple genotypes per species has revealed that the pan-genome, which encompasses sequences of all individuals of a species, is much larger than any single genome. This raises questions about the functions of additional (or missing) sequences in specific genotypes and the extent to which they might contribute to SNF. New genetic tools, especially genome editing (Curtin et al., 2017) that enables targeted genetic changes in novel genotypes, should help to answer such questions. Genome editing will also be useful in addressing the functions of multigene families, whose roles in SNF have thus far been shrouded by genetic redundancy, as well as the roles of small genes that are less frequently mutated by conventional approaches. While genomics is beginning to illuminate the sequence diversity present within individual plant species, plant breeders have been using such diversity through phenotypic selection for improved plant traits for many years. However, breeding programs have rarely if ever selected directly for SNF effectiveness in crop or forage legumes. Doing so now with the benefit of the genome sequences of hundreds or thousands of diverse genotypes for a given species opens up the possibility of identifying genes and alleles of those genes that contribute to SNF effectiveness. This, in turn, may facilitate breeding to enhance SNF in legumes via marker-assisted or genomic-selection approaches, for instance.
Despite the remarkable progress of the last 20 years, much remains to be learned about the genetics and molecular biology of SNF in legumes and how the various pieces fit together. The next 20 years will likely to be as exciting as the past, especially as we endeavor to translate genetic knowledge into plant breeding programs that optimize SNF in legumes and possibly extend it into nonlegumes of agricultural importance.
Supplemental Data
Supplemental Data Set. List of functionally validated SNF genes in legumes.
DIVE Curated Terms
The following phenotypic, genotypic, and functional terms are of significance to the work described in this paper:
Acknowledgments
We thank Marcus Griffiths and Josh Meo for help with figure preparation and Yinbing Ge and Jody Beard for developing the accompanying webpage (https://nobleapps.noble.org/legumegenetics), Nick Krom for sequence information, and the three anonymous reviewers for thorough and constructive feedback. We thank the creators of the Reactome icon library, which we used extensively (Sidiropoulos et al., 2017). This work was funded by the National Science Foundation Plant Genome Research Program (grants DBI-1127155 and DBI-1733470) and by the Noble Research Institute.
AUTHOR CONTRIBUTIONS
S.R. and W.L. curated gene information; R.S.N. performed database searches; S.R. prepared all figures with input from W.L.; S.R, W.L, R.S.N, A.C., C.I.P., K.S.M., J.F., and R.D. contributed to the writing of the article; M.K.U wrote, edited and supervised all aspects of the article.
Footnotes
Articles can be viewed without a subscription.
References
- Abreu I., Saéz Á., Castro-Rodríguez R., Escudero V., Rodríguez-Haas B., Senovilla M., Larue C., Grolimund D., Tejada-Jiménez M., Imperial J., González-Guerrero M. (2017). Medicago truncatula Zinc-Iron Permease6 provides zinc to rhizobia-infected nodule cells. Plant Cell Environ. 40: 2706–2719. [DOI] [PubMed] [Google Scholar]
- Andriankaja A., Boisson-Dernier A., Frances L., Sauviac L., Jauneau A., Barker D.G., de Carvalho-Niebel F. (2007). AP2-ERF transcription factors mediate Nod factor dependent Mt ENOD11 activation in root hairs via a novel cis-regulatory motif. Plant Cell 19: 2866–2885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrio E., Marino D., Marmeys A., de Segonzac M.D., Damiani I., Genre A., Huguet S., Frendo P., Puppo A., Pauly N. (2013). Hydrogen peroxide-regulated genes in the Medicago truncatula-Sinorhizobium meliloti symbiosis. New Phytol. 198: 179–189. [DOI] [PubMed] [Google Scholar]
- Ané J.-M., et al. (2004). Medicago truncatula DMI1 required for bacterial and fungal symbioses in legumes. Science 303: 1364–1367. [DOI] [PubMed] [Google Scholar]
- Antolín-Llovera M., Ried M.K., Parniske M. (2014). Cleavage of the SYMBIOSIS RECEPTOR-LIKE KINASE ectodomain promotes complex formation with Nod factor receptor 5. Curr. Biol. 24: 422–427. [DOI] [PubMed] [Google Scholar]
- Ariel F., et al. (2012). Two direct targets of cytokinin signaling regulate symbiotic nodulation in Medicago truncatula. Plant Cell 24: 3838–3852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrighi J.-F., et al. (2006). The Medicago truncatula lysin [corrected] motif-receptor-like kinase gene family includes NFP and new nodule-expressed genes. Plant Physiol. 142: 265–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrighi J.-F., Godfroy O., de Billy F., Saurat O., Jauneau A., Gough C. (2008). The RPG gene of Medicago truncatula controls Rhizobium-directed polar growth during infection. Proc. Natl. Acad. Sci. USA 105: 9817–9822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arthikala M.-K., Montiel J., Sánchez-López R., Nava N., Cárdenas L., Quinto C. (2017). Respiratory Burst Oxidase Homolog Gene A is crucial for rhizobium infection and nodule maturation and function in common bean. Front. Plant Sci. 8: 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arthikala M.-K., Nanjareddy K., Lara M. (2018). In BPS1 downregulated roots, the BYPASS1 signal disrupts the induction of cortical cell divisions in bean-Rhizobium symbiosis. Genes (Basel) 9: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagchi R., Salehin M., Adeyemo O.S., Salazar C., Shulaev V., Sherrier D.J., Dickstein R. (2012). Functional assessment of the Medicago truncatula NIP/LATD protein demonstrates that it is a high-affinity nitrate transporter. Plant Physiol. 160: 906–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baier M.C., Barsch A., Küster H., Hohnjec N. (2007). Antisense repression of the Medicago truncatula nodule-enhanced sucrose synthase leads to a handicapped nitrogen fixation mirrored by specific alterations in the symbiotic transcriptome and metabolome. Plant Physiol. 145: 1600–1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battaglia M., Rípodas C., Clúa J., Baudin M., Aguilar O.M., Niebel A., Zanetti M.E., Blanco F.A. (2014). A nuclear factor Y interacting protein of the GRAS family is required for nodule organogenesis, infection thread progression, and lateral root growth. Plant Physiol. 164: 1430–1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedito V.A., et al. (2008). A gene expression atlas of the model legume Medicago truncatula. Plant J. 55: 504–513. [DOI] [PubMed] [Google Scholar]
- Berrabah F., Bourcy M., Eschstruth A., Cayrel A., Guefrachi I., Mergaert P., Wen J., Jean V., Mysore K.S., Gourion B., Ratet P. (2014). A nonRD receptor-like kinase prevents nodule early senescence and defense-like reactions during symbiosis. New Phytol. 203: 1305–1314. [DOI] [PubMed] [Google Scholar]
- Bolaños-Vásquez M.C., Werner D. (1997). Effects of Rhizobium tropici, R. etli, and R. leguminosarum bv. phaseoli on nod gene-inducing flavonoids in root exudates of Phaseolus vulgaris. Mol. Plant Microbe Interact. 10: 339–346. [Google Scholar]
- Bolon Y.-T., et al. (2011). Phenotypic and genomic analyses of a fast neutron mutant population resource in soybean. Plant Physiol. 156: 240–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourcy M., Brocard L., Pislariu C.I., Cosson V., Mergaert P., Tadege M., Mysore K.S., Udvardi M.K., Gourion B., Ratet P. (2013). Medicago truncatula DNF2 is a PI-PLC-XD-containing protein required for bacteroid persistence and prevention of nodule early senescence and defense-like reactions. New Phytol. 197: 1250–1261. [DOI] [PubMed] [Google Scholar]
- Breakspear A., Liu C., Roy S., Stacey N., Rogers C., Trick M., Morieri G., Mysore K.S., Wen J., Oldroyd G.E., Downie J.A., Murray J.D. (2014). The root hair “infectome” of Medicago truncatula uncovers changes in cell cycle genes and reveals a requirement for auxin signaling in rhizobial infection. Plant Cell 26: 4680–4701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broghammer A., et al. (2012). Legume receptors perceive the rhizobial lipochitin oligosaccharide signal molecules by direct binding. Proc. Natl. Acad. Sci. USA 109: 13859–13864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caetano-Anollés G., Gresshoff P.M. (1991). Plant genetic control of nodulation. Annu. Rev. Microbiol. 45: 345–382. [DOI] [PubMed] [Google Scholar]
- Cai J., et al. (2018). Role of the Nod factor hydrolase MtNFH1 in regulating Nod factor levels during rhizobial infection and in mature nodules of Medicago truncatula. Plant Cell 30: 397–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canfield D.E., Glazer A.N., Falkowski P.G. (2010). The evolution and future of Earth’s nitrogen cycle. Science 330: 192–196. [DOI] [PubMed] [Google Scholar]
- Cao Y., Halane M.K., Gassmann W., Stacey G. (2017). The role of plant innate immunity in the legume-rhizobium symbiosis. Annu. Rev. Plant Biol. 68: 535–561. [DOI] [PubMed] [Google Scholar]
- Catalano C.M., Czymmek K.J., Gann J.G., Sherrier D.J. (2007). Medicago truncatula syntaxin SYP132 defines the symbiosome membrane and infection droplet membrane in root nodules. Planta 225: 541–550. [DOI] [PubMed] [Google Scholar]
- Cebolla A., Vinardell J.M., Kiss E., Oláh B., Roudier F., Kondorosi A., Kondorosi E. (1999). The mitotic inhibitor ccs52 is required for endoreduplication and ploidy-dependent cell enlargement in plants. EMBO J. 18: 4476–4484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerri M.R., Frances L., Kelner A., Fournier J., Middleton P.H., Auriac M.-C., Mysore K.S., Wen J., Erard M., Barker D.G., Oldroyd G.E., de Carvalho-Niebel F. (2016). The symbiosis-related ERN transcription factors act in concert to coordinate rhizobial host root infection. Plant Physiol. 171: 1037–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerri M.R., et al. (2017). The ERN1 transcription factor gene is a target of the CCaMK/CYCLOPS complex and controls rhizobial infection in Lotus japonicus. New Phytol. 215: 323–337. [DOI] [PubMed] [Google Scholar]
- Chang C., Damiani I., Puppo A., Frendo P. (2009). Redox changes during the legume-rhizobium symbiosis. Mol. Plant 2: 370–377. [DOI] [PubMed] [Google Scholar]
- Charpentier M., Bredemeier R., Wanner G., Takeda N., Schleiff E., Parniske M. (2008). Lotus japonicus CASTOR and POLLUX are ion channels essential for perinuclear calcium spiking in legume root endosymbiosis. Plant Cell 20: 3467–3479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charpentier M., Oldroyd G. (2010). How close are we to nitrogen-fixing cereals? Curr. Opin. Plant Biol. 13: 556–564. [DOI] [PubMed] [Google Scholar]
- Charpentier M., Oldroyd G.E. (2013). Nuclear calcium signaling in plants. Plant Physiol. 163: 496–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charpentier M., Sun J., Vaz Martins T., Radhakrishnan G.V., Findlay K., Soumpourou E., Thouin J., Véry A.-A., Sanders D., Morris R.J., Oldroyd G.E. (2016). Nuclear-localized cyclic nucleotide-gated channels mediate symbiotic calcium oscillations. Science 352: 1102–1105. [DOI] [PubMed] [Google Scholar]
- Chen L., et al. (2019). A nodule-localized phosphate transporter GmPT7 plays an important role in enhancing symbiotic N2 fixation and yield in soybean. New Phytol. 221: 2013–2025. [DOI] [PubMed] [Google Scholar]
- Chen T., Zhu H., Ke D., Cai K., Wang C., Gou H., Hong Z., Zhang Z. (2012). A MAP kinase kinase interacts with SymRK and regulates nodule organogenesis in Lotus japonicus. Plant Cell 24: 823–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Chen W., Li X., Jiang H., Wu P., Xia K., Yang Y., Wu G. (2014). Knockdown of LjIPT3 influences nodule development in Lotus japonicus. Plant Cell Physiol. 55: 183–193. [DOI] [PubMed] [Google Scholar]
- Cheng X., Gou X., Yin H., Mysore K.S., Li J., Wen J. (2017). Functional characterisation of brassinosteroid receptor MtBRI1 in Medicago truncatula. Sci. Rep. 7: 9327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiasson D.M., Haage K., Sollweck K., Brachmann A., Dietrich P., Parniske M. (2017). A quantitative hypermorphic CNGC allele confers ectopic calcium flux and impairs cellular development. eLife 6: e25012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleto I., Trenas A.T., Erban A., Kopka J., Pineda M., Alamillo J.M. (2016). Functional specialization of one copy of glutamine phosphoribosyl pyrophosphate amidotransferase in ureide production from symbiotically fixed nitrogen in Phaseolus vulgaris. Plant Cell Environ. 39: 1767–1779. [DOI] [PubMed] [Google Scholar]
- Collier R., Tegeder M. (2012). Soybean ureide transporters play a critical role in nodule development, function and nitrogen export. Plant J. 72: 355–367. [DOI] [PubMed] [Google Scholar]
- Combier J.P., de Billy F., Gamas P., Niebel A., Rivas S. (2008). Trans-regulation of the expression of the transcription factor MtHAP2-1 by a uORF controls root nodule development. Genes Dev. 22: 1549–1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Combier J.-P., Frugier F., de Billy F., Boualem A., El-Yahyaoui F., Moreau S., Vernié T., Ott T., Gamas P., Crespi M., Niebel A. (2006). MtHAP2-1 is a key transcriptional regulator of symbiotic nodule development regulated by microRNA169 in Medicago truncatula. Genes Dev. 20: 3084–3088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordoba E., Shishkova S., Vance C.P., Hernández G. (2003). Antisense inhibition of NADH glutamate synthase impairs carbon/nitrogen assimilation in nodules of alfalfa (Medicago sativa L.). Plant J. 33: 1037–1049. [DOI] [PubMed] [Google Scholar]
- Couzigou J.-M., et al. (2012). NODULE ROOT and COCHLEATA maintain nodule development and are legume orthologs of Arabidopsis BLADE-ON-PETIOLE genes. Plant Cell 24: 4498–4510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crook A.D., Schnabel E.L., Frugoli J.A. (2016). The systemic nodule number regulation kinase SUNN in Medicago truncatula interacts with MtCLV2 and MtCRN. Plant J. 88: 108–119. [DOI] [PubMed] [Google Scholar]
- Curtin S.J., Tiffin P., Guhlin J., Trujillo D.I., Burghart L.T., Atkins P., Baltes N.J., Denny R., Voytas D.F., Stupar R.M., Young N.D. (2017). Validating genome-wide association candidates controlling quantitative variation in nodulation. Plant Physiol. 173: 921–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bang T.C., et al. (2017). Genome-wide identification of Medicago peptides involved in macronutrient responses and nodulation. Plant Physiol. 175: 1669–1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delves A.C., Mathews A., Day D.A., Carter A.S., Carroll B.J., Gresshoff P.M. (1986). Regulation of the soybean-Rhizobium nodule symbiosis by shoot and root factors. Plant Physiol. 82: 588–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dénarié J., Cullimore J. (1993). Lipo-oligosaccharide nodulation factors: A new class of signaling molecules mediating recognition and morphogenesis. Cell 74: 951–954. [DOI] [PubMed] [Google Scholar]
- de Zélicourt A., Diet A., Marion J., Laffont C., Ariel F., Moison M., Zahaf O., Crespi M., Gruber V., Frugier F. (2012). Dual involvement of a Medicago truncatula NAC transcription factor in root abiotic stress response and symbiotic nodule senescence. Plant J. 70: 220–230. [DOI] [PubMed] [Google Scholar]
- D’Haeze W., Holsters M. (2002). Nod factor structures, responses, and perception during initiation of nodule development. Glycobiology 12: 79R–105R. [DOI] [PubMed] [Google Scholar]
- Dhanushkodi R., Matthew C., McManus M.T., Dijkwel P.P. (2018). Drought-induced senescence of Medicago truncatula nodules involves serpin and ferritin to control proteolytic activity and iron levels. New Phytol. 220: 196–208. [DOI] [PubMed] [Google Scholar]
- Di Giacomo E., Laffont C., Sciarra F., Iannelli M.A., Frugier F., Frugis G. (2017). KNAT3/4/5-like class 2 KNOX transcription factors are involved in Medicago truncatula symbiotic nodule organ development. New Phytol. 213: 822–837. [DOI] [PubMed] [Google Scholar]
- Domonkos Á., et al. (2017). NAD1 controls defense-like responses in Medicago truncatula symbiotic nitrogen fixing nodules following rhizobial colonization in a BacA-independent manner. Genes (Basel) 8: 387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle J.J., Luckow M.A. (2003). The rest of the iceberg: Legume diversity and evolution in a phylogenetic context. Plant Physiol. 131: 900–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Msehli S., Lambert A., Baldacci-Cresp F., Hopkins J., Boncompagni E., Smiti S.A., Hérouart D., Frendo P. (2011). Crucial role of (homo)glutathione in nitrogen fixation in Medicago truncatula nodules. New Phytol. 192: 496–506. [DOI] [PubMed] [Google Scholar]
- Endre G., Kereszt A., Kevei Z., Mihacea S., Kaló P., Kiss G.B. (2002). A receptor kinase gene regulating symbiotic nodule development. Nature 417: 962–966. [DOI] [PubMed] [Google Scholar]
- Espina M.J., Ahmed C.M.S., Bernardini A., Adeleke E., Yadegari Z., Arelli P., Pantalone V., Taheri A. (2018). Development and phenotypic screening of an ethyl methane sulfonate mutant population in soybean. Front. Plant Sci. 9: 394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estrada-Navarrete G., Cruz-Mireles N., Lascano R., Alvarado-Affantranger X., Hernández-Barrera A., Barraza A., Olivares J.E., Arthikala M.-K., Cárdenas L., Quinto C., Sanchez F. (2016). An autophagy-related kinase is essential for the symbiotic relationship between Phaseolus vulgaris and both rhizobia and arbuscular mycorrhizal fungi. Plant Cell 28: 2326–2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Y., Liu J., Lyu S., Wang Q., Yang S., Zhu H. (2017). The soybean Rfg1 gene restricts nodulation by Sinorhizobium fredii USDA193. Front. Plant Sci. 8: 1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson B.J., Li D., Hastwell A.H., Reid D.E., Li Y., Jackson S.A., Gresshoff P.M. (2014). The soybean (Glycine max) nodulation-suppressive CLE peptide, GmRIC1, functions interspecifically in common white bean (Phaseolus vulgaris), but not in a supernodulating line mutated in the receptor PvNARK. Plant Biotechnol. J. 12: 1085–1097. [DOI] [PubMed] [Google Scholar]
- Ferguson B.J., Mens C., Hastwell A.H., Zhang M., Su H., Jones C.H., Chu X., Gresshoff P.M. (2019). Legume nodulation: The host controls the party. Plant Cell Environ. 42: 41–51. [DOI] [PubMed] [Google Scholar]
- Flemetakis E., Efrose R.C., Ott T., Stedel C., Aivalakis G., Udvardi M.K., Katinakis P. (2006). Spatial and temporal organization of sucrose metabolism in Lotus japonicus nitrogen-fixing nodules suggests a role for the elusive alkaline/neutral invertase. Plant Mol. Biol. 62: 53–69. [DOI] [PubMed] [Google Scholar]
- Fonouni-Farde C., Tan S., Baudin M., Brault M., Wen J., Mysore K.S., Niebel A., Frugier F., Diet A. (2016). DELLA-mediated gibberellin signalling regulates Nod factor signalling and rhizobial infection. Nat. Commun. 7: 12636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franssen H.J., Xiao T.T., Kulikova O., Wan X., Bisseling T., Scheres B., Heidstra R. (2015). Root developmental programs shape the Medicago truncatula nodule meristem. Development 142: 2941–2950. [DOI] [PubMed] [Google Scholar]
- Frugier F., Poirier S., Satiat-Jeunemaître B., Kondorosi A., Crespi M. (2000). A Krüppel-like zinc finger protein is involved in nitrogen-fixing root nodule organogenesis. Genes Dev. 14: 475–482. [PMC free article] [PubMed] [Google Scholar]
- Fukai E., Stougaard J., Hayashi M. (2013). Activation of an endogenous retrotransposon associated with epigenetic changes in Lotus japonicus: A tool for functional genomics in legumes. Plant Genome 6. [Google Scholar]
- Gautrat P., Mortier V., Laffont C., De Keyser A., Fromentin J., Frugier F., Goormachtig S. (2019). Unraveling new molecular players involved in the autoregulation of nodulation in Medicago truncatula. J. Exp. Bot. 70: 1407–1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavrin A., Chiasson D., Ovchinnikova E., Kaiser B.N., Bisseling T., Fedorova E.E. (2016). VAMP721a and VAMP721d are important for pectin dynamics and release of bacteria in soybean nodules. New Phytol. 210: 1011–1021. [DOI] [PubMed] [Google Scholar]
- Gavrin A., Jansen V., Ivanov S., Bisseling T., Fedorova E. (2015). ARP2/3-mediated actin nucleation associated with symbiosome membrane is essential for the development of symbiosomes in infected cells of Medicago truncatula root nodules. Mol. Plant Microbe Interact. 28: 605–614. [DOI] [PubMed] [Google Scholar]
- Gavrin A., Kaiser B.N., Geiger D., Tyerman S.D., Wen Z., Bisseling T., Fedorova E.E. (2014). Adjustment of host cells for accommodation of symbiotic bacteria: Vacuole defunctionalization, HOPS suppression, and TIP1g retargeting in Medicago. Plant Cell 26: 3809–3822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavrin A., Kulikova O., Bisseling T., Fedorova E.E. (2017). Interface symbiotic membrane formation in root nodules of Medicago truncatula: The role of synaptotagmins MtSyt1, MtSyt2 and MtSyt3. Front. Plant Sci. 8: 201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geurts R., Xiao T.T., Reinhold-Hurek B. (2016). What does it take to evolve a nitrogen-fixing endosymbiosis? Trends Plant Sci. 21: 199–208. [DOI] [PubMed] [Google Scholar]
- Gil-Díez P., Tejada-Jiménez M., León-Mediavilla J., Wen J., Mysore K.S., Imperial J., González-Guerrero M. (2019). MtMOT1.2 is responsible for molybdate supply to Medicago truncatula nodules. Plant Cell Environ. 42: 310–320. [DOI] [PubMed] [Google Scholar]
- Gleason C., Chaudhuri S., Yang T., Muñoz A., Poovaiah B.W., Oldroyd G.E. (2006). Nodulation independent of rhizobia induced by a calcium-activated kinase lacking autoinhibition. Nature 441: 1149–1152. [DOI] [PubMed] [Google Scholar]
- Glebov O.O., Bright N.A., Nichols B.J. (2006). Flotillin-1 defines a clathrin-independent endocytic pathway in mammalian cells. Nat. Cell Biol. 8: 46–54. [DOI] [PubMed] [Google Scholar]
- Godiard L., Lepage A., Moreau S., Laporte D., Verdenaud M., Timmers T., Gamas P. (2011). MtbHLH1, a bHLH transcription factor involved in Medicago truncatula nodule vascular patterning and nodule to plant metabolic exchanges. New Phytol. 191: 391–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Guerrero M., Matthiadis A., Sáez Á., Long T.A. (2014). Fixating on metals: New insights into the role of metals in nodulation and symbiotic nitrogen fixation. Front. Plant Sci. 5: 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Rizzo S., Crespi M., Frugier F. (2006). The Medicago truncatula CRE1 cytokinin receptor regulates lateral root development and early symbiotic interaction with Sinorhizobium meliloti. Plant Cell 18: 2680–2693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon A.J., Minchin F.R., James C.L., Komina O. (1999). Sucrose synthase in legume nodules is essential for nitrogen fixation. Plant Physiol. 120: 867–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groth M., Takeda N., Perry J., Uchida H., Dräxl S., Brachmann A., Sato S., Tabata S., Kawaguchi M., Wang T.L., Parniske M. (2010). NENA, a Lotus japonicus homolog of Sec13, is required for rhizodermal infection by arbuscular mycorrhiza fungi and rhizobia but dispensable for cortical endosymbiotic development. Plant Cell 22: 2509–2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunewald W., van Noorden G., Van Isterdael G., Beeckman T., Gheysen G., Mathesius U. (2009). Manipulation of auxin transport in plant roots during Rhizobium symbiosis and nematode parasitism. Plant Cell 21: 2553–2562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan D., et al. (2013). Rhizobial infection is associated with the development of peripheral vasculature in nodules of Medicago truncatula. Plant Physiol. 162: 107–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadri A.-E., Spaink H.P., Bisseling T., Brewin N.J. (1998). Diversity of root nodulation and rhizobial infection processes In The Rhizobiaceae, Spaink H.P., Kondorosi A., and Hooykaas P.J.J., eds (Dordrecht, The Netherlands: Springer; ), pp. 347–360. [Google Scholar]
- Hakoyama T., et al. (2009). Host plant genome overcomes the lack of a bacterial gene for symbiotic nitrogen fixation. Nature 462: 514–517. [DOI] [PubMed] [Google Scholar]
- Hakoyama T., et al. (2012a). The integral membrane protein SEN1 is required for symbiotic nitrogen fixation in Lotus japonicus nodules. Plant Cell Physiol. 53: 225–236. [DOI] [PubMed] [Google Scholar]
- Hakoyama T., et al. (2012b). The SNARE protein SYP71 expressed in vascular tissues is involved in symbiotic nitrogen fixation in Lotus japonicus nodules. Plant Physiol. 160: 897–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haney C.H., Long S.R. (2010). Plant flotillins are required for infection by nitrogen-fixing bacteria. Proc. Natl. Acad. Sci. USA 107: 478–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haney C.H., Riely B.K., Tricoli D.M., Cook D.R., Ehrhardt D.W., Long S.R. (2011). Symbiotic rhizobia bacteria trigger a change in localization and dynamics of the Medicago truncatula receptor kinase LYK3. Plant Cell 23: 2774–2787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi M., et al. (2014). A thaumatin-like protein, Rj4, controls nodule symbiotic specificity in soybean. Plant Cell Physiol. 55: 1679–1689. [DOI] [PubMed] [Google Scholar]
- Held M., Hou H., Miri M., Huynh C., Ross L., Hossain M.S., Sato S., Tabata S., Perry J., Wang T.L., Szczyglowski K. (2014). Lotus japonicus cytokinin receptors work partially redundantly to mediate nodule formation. Plant Cell 26: 678–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch S., Kim J., Muñoz A., Heckmann A.B., Downie J.A., Oldroyd G.E. (2009). GRAS proteins form a DNA binding complex to induce gene expression during nodulation signaling in Medicago truncatula. Plant Cell 21: 545–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohnjec N., Perlick A.M., Pühler A., Küster H. (2003). The Medicago truncatula sucrose synthase gene MtSucS1 is activated both in the infected region of root nodules and in the cortex of roots colonized by arbuscular mycorrhizal fungi. Mol. Plant Microbe Interact. 16: 903–915. [DOI] [PubMed] [Google Scholar]
- Horst I., Welham T., Kelly S., Kaneko T., Sato S., Tabata S., Parniske M., Wang T.L. (2007). TILLING mutants of Lotus japonicus reveal that nitrogen assimilation and fixation can occur in the absence of nodule-enhanced sucrose synthase. Plant Physiol. 144: 806–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horváth B., et al. (2015). Loss of the nodule-specific cysteine rich peptide, NCR169, abolishes symbiotic nitrogen fixation in the Medicago truncatula dnf7 mutant. Proc. Natl. Acad. Sci. USA 112: 15232–15237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hossain M.S., Liao J., James E.K., Sato S., Tabata S., Jurkiewicz A., Madsen L.H., Stougaard J., Ross L., Szczyglowski K. (2012). Lotus japonicus ARPC1 is required for rhizobial infection. Plant Physiol. 160: 917–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huault E., Laffont C., Wen J., Mysore K.S., Ratet P., Duc G., Frugier F. (2014). Local and systemic regulation of plant root system architecture and symbiotic nodulation by a receptor-like kinase. PLoS Genet. 10: e1004891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huo X., Schnabel E., Hughes K., Frugoli J. (2006). RNAi phenotypes and the localization of a protein:GUS fusion imply a role for Medicago truncatula PIN genes in nodulation. J. Plant Growth Regul. 25: 156–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imaizumi-Anraku H., et al. (2005). Plastid proteins crucial for symbiotic fungal and bacterial entry into plant roots. Nature 433: 527–531. [DOI] [PubMed] [Google Scholar]
- Imin N., Mohd-Radzman N.A., Ogilvie H.A., Djordjevic M.A. (2013). The peptide-encoding CEP1 gene modulates lateral root and nodule numbers in Medicago truncatula. J. Exp. Bot. 64: 5395–5409. [DOI] [PubMed] [Google Scholar]
- Imin N., Patel N., Corcilius L., Payne R.J., Djordjevic M.A. (2018). CLE peptide tri-arabinosylation and peptide domain sequence composition are essential for SUNN-dependent autoregulation of nodulation in Medicago truncatula. New Phytol. 218: 73–80. [DOI] [PubMed] [Google Scholar]
- Ivanov S., Fedorova E.E., Limpens E., De Mita S., Genre A., Bonfante P., Bisseling T. (2012). Rhizobium-legume symbiosis shares an exocytotic pathway required for arbuscule formation. Proc. Natl. Acad. Sci. USA 109: 8316–8321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivashuta S., Liu J., Liu J., Lohar D.P., Haridas S., Bucciarelli B., VandenBosch K.A., Vance C.P., Harrison M.J., Gantt J.S. (2005). RNA interference identifies a calcium-dependent protein kinase involved in Medicago truncatula root development. Plant Cell 17: 2911–2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Y., Chen Z., Yang J., Mysore K.S., Wen J., Huang J., Yu N., Wang E. (2018). IPD3 and IPD3L function redundantly in rhizobial and mycorrhizal symbioses. Front. Plant Sci. 9: 267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Y., Liu H., Luo D., Yu N., Dong W., Wang C., Zhang X., Dai H., Yang J., Wang E. (2016). DELLA proteins are common components of symbiotic rhizobial and mycorrhizal signalling pathways. Nat. Commun. 7: 12433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaló P., et al. (2005). Nodulation signaling in legumes requires NSP2, a member of the GRAS family of transcriptional regulators. Science 308: 1786–1789. [DOI] [PubMed] [Google Scholar]
- Kanamori N., et al. (2006). A nucleoporin is required for induction of Ca2+ spiking in legume nodule development and essential for rhizobial and fungal symbiosis. Proc. Natl. Acad. Sci. USA 103: 359–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaharada Y., James E.K., Kelly S., Sandal N., Stougaard J. (2017a). The ethylene responsive factor required for nodulation 1 (ERN1) transcription factor is required for infection-thread formation in Lotus japonicus. Mol. Plant Microbe Interact. 30: 194–204. [DOI] [PubMed] [Google Scholar]
- Kawaharada Y., et al. (2015). Receptor-mediated exopolysaccharide perception controls bacterial infection. Nature 523: 308–312. [DOI] [PubMed] [Google Scholar]
- Kawaharada Y., Nielsen M.W., Kelly S., James E.K., Andersen K.R., Rasmussen S.R., Füchtbauer W., Madsen L.H., Heckmann A.B., Radutoiu S., Stougaard J. (2017b). Differential regulation of the Epr3 receptor coordinates membrane-restricted rhizobial colonization of root nodule primordia. Nat. Commun. 8: 14534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ke D., Fang Q., Chen C., Zhu H., Chen T., Chang X., Yuan S., Kang H., Ma L., Hong Z., Zhang Z. (2012). The small GTPase ROP6 interacts with NFR5 and is involved in nodule formation in Lotus japonicus. Plant Physiol. 159: 131–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kevei Z., et al. (2007). 3-Hydroxy-3-methylglutaryl coenzyme A reductase 1 interacts with NORK and is crucial for nodulation in Medicago truncatula. Plant Cell 19: 3974–3989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim G.-B., Son S.-U., Yu H.-J., Mun J.-H. (2019). MtGA2ox10 encoding C20-GA2-oxidase regulates rhizobial infection and nodule development in Medicago truncatula. Sci. Rep. 9: 5952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M., Chen Y., Xi J., Waters C., Chen R., Wang D. (2015). An antimicrobial peptide essential for bacterial survival in the nitrogen-fixing symbiosis. Proc. Natl. Acad. Sci. USA 112: 15238–15243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y.-K., et al. (2013). Functional implication of β-carotene hydroxylases in soybean nodulation. Plant Physiol. 162: 1420–1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiss E., Oláh B., Kaló P., Morales M., Heckmann A.B., Borbola A., Lózsa A., Kontár K., Middleton P., Downie J.A., Oldroyd G.E., Endre G. (2009). LIN, a novel type of U-box/WD40 protein, controls early infection by rhizobia in legumes. Plant Physiol. 151: 1239–1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosuta S., Held M., Hossain M.S., Morieri G., Macgillivary A., Johansen C., Antolín-Llovera M., Parniske M., Oldroyd G.E., Downie A.J., Karas B., Szczyglowski K. (2011). Lotus japonicus symRK-14 uncouples the cortical and epidermal symbiotic program. Plant J. 67: 929–940. [DOI] [PubMed] [Google Scholar]
- Krusell L., et al. (2005). The sulfate transporter SST1 is crucial for symbiotic nitrogen fixation in Lotus japonicus root nodules. Plant Cell 17: 1625–1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krusell L., et al. (2002). Shoot control of root development and nodulation is mediated by a receptor-like kinase. Nature 420: 422–426. [DOI] [PubMed] [Google Scholar]
- Krusell L., et al. (2011). The Clavata2 genes of pea and Lotus japonicus affect autoregulation of nodulation. Plant J. 65: 861–871. [DOI] [PubMed] [Google Scholar]
- Kryvoruchko I.S., Routray P., Sinharoy S., Torres-Jerez I., Tejada-Jiménez M., Finney L.A., Nakashima J., Pislariu C.I., Benedito V.A., González-Guerrero M., Roberts D.M., Udvardi M.K. (2018). An iron-activated citrate transporter, MtMATE67, is required for symbiotic nitrogen fixation. Plant Physiol. 176: 2315–2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kryvoruchko I.S., Sinharoy S., Torres-Jerez I., Sosso D., Pislariu C.I., Guan D., Murray J., Benedito V.A., Frommer W.B., Udvardi M.K. (2016). MtSWEET11, a nodule-specific sucrose transporter of Medicago truncatula. Plant Physiol. 171: 554–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuppusamy K.T., Endre G., Prabhu R., Penmetsa R.V., Veereshlingam H., Cook D.R., Dickstein R., Vandenbosch K.A. (2004). LIN, a Medicago truncatula gene required for nodule differentiation and persistence of rhizobial infections. Plant Physiol. 136: 3682–3691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuppusamy K.T., Ivashuta S., Bucciarelli B., Vance C.P., Gantt J.S., Vandenbosch K.A. (2009). Knockdown of CELL DIVISION CYCLE16 reveals an inverse relationship between lateral root and nodule numbers and a link to auxin in Medicago truncatula. Plant Physiol. 151: 1155–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laffont C., Huault E., Gautrat P., Endre G., Kalo P., Bourion V., Duc G., Frugier F. (2019). Independent regulation of symbiotic nodulation by the SUNN negative and CRA2 positive systemic pathways In Plant Physiol., Volume 180, pp. 559–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laporte P., Lepage A., Fournier J., Catrice O., Moreau S., Jardinaud M.-F., Mun J.-H., Larrainzar E., Cook D.R., Gamas P., Niebel A. (2014). The CCAAT box-binding transcription factor NF-YA1 controls rhizobial infection. J. Exp. Bot. 65: 481–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laporte P., Satiat-Jeunemaître B., Velasco I., Csorba T., Van de Velde W., Campalans A., Burgyan J., Arevalo-Rodriguez M., Crespi M. (2010). A novel RNA-binding peptide regulates the establishment of the Medicago truncatula-Sinorhizobium meliloti nitrogen-fixing symbiosis. Plant J. 62: 24–38. [DOI] [PubMed] [Google Scholar]
- Larrainzar E., et al. (2015). Deep sequencing of the Medicago truncatula root transcriptome reveals a massive and early interaction between nodulation factor and ethylene signals. Plant Physiol. 169: 233–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefebvre B., et al. (2010). A remorin protein interacts with symbiotic receptors and regulates bacterial infection. Proc. Natl. Acad. Sci. USA 107: 2343–2348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- León-Mediavilla J., Senovilla M., Montiel J., Gil-Díez P., Saez Á., Kryvoruchko I.S., Reguera M., Udvardi M.K., Imperial J., González-Guerrero M. (2018). MtMTP2-facilitated zinc transport into intracellular compartments is essential for nodule development in Medicago truncatula. Front. Plant Sci. 9: 990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerouge P., Roche P., Faucher C., Maillet F., Truchet G., Promé J.C., Dénarié J. (1990). Symbiotic host-specificity of Rhizobium meliloti is determined by a sulphated and acylated glucosamine oligosaccharide signal. Nature 344: 781–784. [DOI] [PubMed] [Google Scholar]
- Le Roux M., Khan S., Valentine A. (2009). Nitrogen and carbon costs of soybean and lupin root systems during phosphate starvation. Symbiosis 48: 102–109. [Google Scholar]
- Li X., Feng H., Wen J., Dong J., Wang T. (2018). MtCAS31 aids symbiotic nitrogen fixation by protecting the leghemoglobin MtLb120-1 under drought stress in Medicago truncatula. Front. Plant Sci. 9: 633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang P., Stratil T.F., Popp C., Marín M., Folgmann J., Mysore K.S., Wen J., Ott T. (2018). Symbiotic root infections in Medicago truncatula require remorin-mediated receptor stabilization in membrane nanodomains. Proc. Natl. Acad. Sci. USA 115: 5289–5294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim C.W., Lee Y.W., Hwang C.H. (2011). Soybean nodule-enhanced CLE peptides in roots act as signals in GmNARK-mediated nodulation suppression. Plant Cell Physiol. 52: 1613–1627. [DOI] [PubMed] [Google Scholar]
- Limpens E., Franken C., Smit P., Willemse J., Bisseling T., Geurts R. (2003). LysM domain receptor kinases regulating rhizobial Nod factor-induced infection. Science 302: 630–633. [DOI] [PubMed] [Google Scholar]
- Limpens E., Ivanov S., van Esse W., Voets G., Fedorova E., Bisseling T. (2009). Medicago N2-fixing symbiosomes acquire the endocytic identity marker Rab7 but delay the acquisition of vacuolar identity. Plant Cell 21: 2811–2828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C.-W., et al. (2019a). A protein complex required for polar growth of rhizobial infection threads. Nat Commun 10: 2848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C.-W., et al. (2019b). NIN acts as a network hub controlling a growth module required for rhizobial infection. Plant Physiol. 179: 1704–1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C.-W., Murray J.D. (2016). The role of flavonoids in nodulation host-range specificity: An update. Plants (Basel) 5: 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H., Zhang C., Yang J., Yu N., Wang E. (2018). Hormone modulation of legume-rhizobial symbiosis. J. Integr. Plant Biol. 60: 632–648. [DOI] [PubMed] [Google Scholar]
- Liu J., Novero M., Charnikhova T., Ferrandino A., Schubert A., Ruyter-Spira C., Bonfante P., Lovisolo C., Bouwmeester H.J., Cardinale F. (2013). Carotenoid cleavage dioxygenase 7 modulates plant growth, reproduction, senescence, and determinate nodulation in the model legume Lotus japonicus. J. Exp. Bot. 64: 1967–1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Rutten L., Limpens E., van der Molen T., van Velzen R., Chen R., Chen Y., Geurts R., Kohlen W., Kulikova O., Bisseling T. (2019c). A remote cis-regulatory region is required for NIN expression in the pericycle to initiate nodule primordium formation in Medicago truncatula. Plant Cell 31: 68–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long S.R. (1996). Rhizobium symbiosis: Nod factors in perspective. Plant Cell 8: 1885–1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Ráez J.A., Shirasu K., Foo E. (2017). Strigolactones in plant interactions with beneficial and detrimental organisms: The yin and yang. Trends Plant Sci. 22: 527–537. [DOI] [PubMed] [Google Scholar]
- Madsen E.B., Madsen L.H., Radutoiu S., Olbryt M., Rakwalska M., Szczyglowski K., Sato S., Kaneko T., Tabata S., Sandal N., Stougaard J. (2003). A receptor kinase gene of the LysM type is involved in legume perception of rhizobial signals. Nature 425: 637–640. [DOI] [PubMed] [Google Scholar]
- Maekawa-Yoshikawa M., Müller J., Takeda N., Maekawa T., Sato S., Tabata S., Perry J., Wang T.L., Groth M., Brachmann A., Parniske M. (2009). The temperature-sensitive brush mutant of the legume Lotus japonicus reveals a link between root development and nodule infection by rhizobia. Plant Physiol. 149: 1785–1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magori S., Kawaguchi M. (2009). Long-distance control of nodulation: Molecules and models. Mol. Cells 27: 129–134. [DOI] [PubMed] [Google Scholar]
- Malolepszy A., et al. (2018). A plant chitinase controls cortical infection thread progression and nitrogen-fixing symbiosis. eLife 7: e38874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Małolepszy A., et al. (2016). The LORE1 insertion mutant resource. Plant J. 88: 306–317. [DOI] [PubMed] [Google Scholar]
- Marino D., Andrio E., Danchin E.G.J., Oger E., Gucciardo S., Lambert A., Puppo A., Pauly N. (2011). A Medicago truncatula NADPH oxidase is involved in symbiotic nodule functioning. New Phytol. 189: 580–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mbengue M., Camut S., de Carvalho-Niebel F., Deslandes L., Froidure S., Klaus-Heisen D., Moreau S., Rivas S., Timmers T., Hervé C., Cullimore J., Lefebvre B. (2010). The Medicago truncatula E3 ubiquitin ligase PUB1 interacts with the LYK3 symbiotic receptor and negatively regulates infection and nodulation. Plant Cell 22: 3474–3488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAdam E.L., Reid J.B., Foo E. (2018). Gibberellins promote nodule organogenesis but inhibit the infection stages of nodulation. J. Exp. Bot. 69: 2117–2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellor R.B. (1989). Bacteroids in the Rhizobium-legume symbiosis inhabit a plant internal lytic compartment: implications for other microbial endosymbioses. J. Exp. Bot. 40: 831–839. [Google Scholar]
- Mendel G. (1866). Experiments in plant hybridization In Proceedings of the Natural History Society of Brno, Volume IV (Biodiversity Heritage Library; ), pp. 3–47. [Google Scholar]
- Mergaert P., Uchiumi T., Alunni B., Evanno G., Cheron A., Catrice O., Mausset A.-E., Barloy-Hubler F., Galibert F., Kondorosi A., Kondorosi E. (2006). Eukaryotic control on bacterial cell cycle and differentiation in the Rhizobium-legume symbiosis. Proc. Natl. Acad. Sci. USA 103: 5230–5235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middleton P.H., et al. (2007). An ERF transcription factor in Medicago truncatula that is essential for Nod factor signal transduction. Plant Cell 19: 1221–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miri M., Janakirama P., Huebert T., Ross L., McDowell T., Orosz K., Markmann K., Szczyglowski K. (2019). Inside out: Root cortex-localized LHK1 cytokinin receptor limits epidermal infection of Lotus japonicus roots by Mesorhizobium loti. New Phytol. 222: 1523–1537. [DOI] [PubMed] [Google Scholar]
- Miyata K., Kawaguchi M., Nakagawa T. (2013). Two distinct EIN2 genes cooperatively regulate ethylene signaling in Lotus japonicus. Plant Cell Physiol. 54: 1469–1477. [DOI] [PubMed] [Google Scholar]
- Miyata K., et al. (2014). The bifunctional plant receptor, OsCERK1, regulates both chitin-triggered immunity and arbuscular mycorrhizal symbiosis in rice. Plant Cell Physiol. 55: 1864–1872. [DOI] [PubMed] [Google Scholar]
- Miyazawa H., et al. (2010). The receptor-like kinase KLAVIER mediates systemic regulation of nodulation and non-symbiotic shoot development in Lotus japonicus. Development 137: 4317–4325. [DOI] [PubMed] [Google Scholar]
- Mohd-Radzman N.A., Laffont C., Ivanovici A., Patel N., Reid D., Stougaard J., Frugier F., Imin N., Djordjevic M.A. (2016). Different pathways act downstream of the CEP peptide receptor CRA2 to regulate lateral root and nodule development. Plant Physiol. 171: 2536–2548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moling S., Pietraszewska-Bogiel A., Postma M., Fedorova E., Hink M.A., Limpens E., Gadella T.W., Bisseling T. (2014). Nod factor receptors form heteromeric complexes and are essential for intracellular infection in Medicago nodules. Plant Cell 26: 4188–4199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morell M., Copeland L. (1984). Enzymes of sucrose breakdown in soybean nodules: Alkaline invertase. Plant Physiol. 74: 1030–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortier V., Den Herder G., Whitford R., Van de Velde W., Rombauts S., D’Haeseleer K., Holsters M., Goormachtig S. (2010). CLE peptides control Medicago truncatula nodulation locally and systemically. Plant Physiol. 153: 222–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortier V., De Wever E., Vuylsteke M., Holsters M., Goormachtig S. (2012). Nodule numbers are governed by interaction between CLE peptides and cytokinin signaling. Plant J. 70: 367–376. [DOI] [PubMed] [Google Scholar]
- Mortier V., Wasson A., Jaworek P., De Keyser A., Decroos M., Holsters M., Tarkowski P., Mathesius U., Goormachtig S. (2014). Role of LONELY GUY genes in indeterminate nodulation on Medicago truncatula. New Phytol. 202: 582–593. [DOI] [PubMed] [Google Scholar]
- Mun T., Bachmann A., Gupta V., Stougaard J., Andersen S.U. (2016). Lotus Base: An integrated information portal for the model legume Lotus japonicus. Sci. Rep. 6: 39447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami E., et al. (2018). Epidermal LysM receptor ensures robust symbiotic signalling in Lotus japonicus. eLife 7: e33506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray J.D., Karas B.J., Sato S., Tabata S., Amyot L., Szczyglowski K. (2007). A cytokinin perception mutant colonized by Rhizobium in the absence of nodule organogenesis. Science 315: 101–104. [DOI] [PubMed] [Google Scholar]
- Murray J.D., et al. (2011). Vapyrin, a gene essential for intracellular progression of arbuscular mycorrhizal symbiosis, is also essential for infection by rhizobia in the nodule symbiosis of Medicago truncatula. Plant J. 65: 244–252. [DOI] [PubMed] [Google Scholar]
- Nanjareddy K., Blanco L., Arthikala M.-K., Alvarado-Affantranger X., Quinto C., Sánchez F., Lara M. (2016). A legume TOR protein kinase regulates rhizobium symbiosis and is essential for infection and nodule development. Plant Physiol. 172: 2002–2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng J.L.P., Mathesius U. (2018). Acropetal auxin transport inhibition is involved in indeterminate but not determinate nodule formation. Front. Plant Sci. 9: 169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishida H., Tanaka S., Handa Y., Ito M., Sakamoto Y., Matsunaga S., Betsuyaku S., Miura K., Soyano T., Kawaguchi M., Suzaki T. (2018). A NIN-LIKE PROTEIN mediates nitrate-induced control of root nodule symbiosis in Lotus japonicus. Nat. Commun. 9: 499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura R., Hayashi M., Wu G.-J., Kouchi H., Imaizumi-Anraku H., Murakami Y., Kawasaki S., Akao S., Ohmori M., Nagasawa M., Harada K., Kawaguchi M. (2002). HAR1 mediates systemic regulation of symbiotic organ development. Nature 420: 426–429. [DOI] [PubMed] [Google Scholar]
- Nizampatnam N.R., Schreier S.J., Damodaran S., Adhikari S., Subramanian S. (2015). microRNA160 dictates stage-specific auxin and cytokinin sensitivities and directs soybean nodule development. Plant J. 84: 140–153. [DOI] [PubMed] [Google Scholar]
- Nowak S., Schnabel E., Frugoli J. (2019). The Medicago truncatula CLAVATA3-LIKE CLE12/13 signaling peptides regulate nodule number depending on the CORYNE but not the COMPACT ROOT ARCHITECTURE2 receptor. Plant Signal. Behav. 14: 1598730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa-Ohnishi M., Matsushita W., Matsubayashi Y. (2013). Identification of three hydroxyproline O-arabinosyltransferases in Arabidopsis thaliana. Nat. Chem. Biol. 9: 726–730. [DOI] [PubMed] [Google Scholar]
- Oka-Kira E., et al. (2005). klavier (klv), a novel hypernodulation mutant of Lotus japonicus affected in vascular tissue organization and floral induction. Plant J. 44: 505–515. [DOI] [PubMed] [Google Scholar]
- Okamoto S., Ohnishi E., Sato S., Takahashi H., Nakazono M., Tabata S., Kawaguchi M. (2009). Nod factor/nitrate-induced CLE genes that drive HAR1-mediated systemic regulation of nodulation. Plant Cell Physiol. 50: 67–77. [DOI] [PubMed] [Google Scholar]
- Okamoto S., Shinohara H., Mori T., Matsubayashi Y., Kawaguchi M. (2013). Root-derived CLE glycopeptides control nodulation by direct binding to HAR1 receptor kinase. Nat. Commun. 4: 2191. [DOI] [PubMed] [Google Scholar]
- Okamoto S., Tabata R., Matsubayashi Y. (2016). Long-distance peptide signaling essential for nutrient homeostasis in plants. Curr. Opin. Plant Biol. 34: 35–40. [DOI] [PubMed] [Google Scholar]
- Oldroyd G.E. (2013). Speak, friend, and enter: Signalling systems that promote beneficial symbiotic associations in plants. Nat. Rev. Microbiol. 11: 252–263. [DOI] [PubMed] [Google Scholar]
- Oldroyd G.E., Murray J.D., Poole P.S., Downie J.A. (2011). The rules of engagement in the legume-rhizobial symbiosis. Annu. Rev. Genet. 45: 119–144. [DOI] [PubMed] [Google Scholar]
- Ott T., Sullivan J., James E.K., Flemetakis E., Günther C., Gibon Y., Ronson C., Udvardi M. (2009). Absence of symbiotic leghemoglobins alters bacteroid and plant cell differentiation during development of Lotus japonicus root nodules. Mol. Plant Microbe Interact. 22: 800–808. [DOI] [PubMed] [Google Scholar]
- Ott T., van Dongen J.T., Günther C., Krusell L., Desbrosses G., Vigeolas H., Bock V., Czechowski T., Geigenberger P., Udvardi M.K. (2005). Symbiotic leghemoglobins are crucial for nitrogen fixation in legume root nodules but not for general plant growth and development. Curr. Biol. 15: 531–535. [DOI] [PubMed] [Google Scholar]
- Pan H., Oztas O., Zhang X., Wu X., Stonoha C., Wang E., Wang B., Wang D. (2016). A symbiotic SNARE protein generated by alternative termination of transcription. Nat. Plants 2: 15197. [DOI] [PubMed] [Google Scholar]
- Penmetsa R.V., Cook D.R. (1997). A legume ethylene-insensitive mutant hyperinfected by its rhizobial symbiont. Science 275: 527–530. [DOI] [PubMed] [Google Scholar]
- Penmetsa R.V., Cook D.R. (2000). Production and characterization of diverse developmental mutants of Medicago truncatula. Plant Physiol. 123: 1387–1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penmetsa R.V., Frugoli J.A., Smith L.S., Long S.R., Cook D.R. (2003). Dual genetic pathways controlling nodule number in Medicago truncatula. Plant Physiol. 131: 998–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penmetsa R.V., et al. (2008). The Medicago truncatula ortholog of Arabidopsis EIN2, sickle, is a negative regulator of symbiotic and pathogenic microbial associations. Plant J. 55: 580–595. [DOI] [PubMed] [Google Scholar]
- Pérez Guerra J.C., Coussens G., De Keyser A., De Rycke R., De Bodt S., Van De Velde W., Goormachtig S., Holsters M. (2010). Comparison of developmental and stress-induced nodule senescence in Medicago truncatula. Plant Physiol. 152: 1574–1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry J.A., Wang T.L., Welham T.J., Gardner S., Pike J.M., Yoshida S., Parniske M. (2003). A TILLING reverse genetics tool and a web-accessible collection of mutants of the legume Lotus japonicus. Plant Physiol. 131: 866–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters N.K., Frost J.W., Long S.R. (1986). A plant flavone, luteolin, induces expression of Rhizobium meliloti nodulation genes. Science 233: 977–980. [DOI] [PubMed] [Google Scholar]
- Petrásek J., Schwarzerová K. (2009). Actin and microtubule cytoskeleton interactions. Curr. Opin. Plant Biol. 12: 728–734. [DOI] [PubMed] [Google Scholar]
- Pierre O., Hopkins J., Combier M., Baldacci F., Engler G., Brouquisse R., Hérouart D., Boncompagni E. (2014). Involvement of papain and legumain proteinase in the senescence process of Medicago truncatula nodules. New Phytol. 202: 849–863. [DOI] [PubMed] [Google Scholar]
- Pii Y., Molesini B., Masiero S., Pandolfini T. (2012). The non-specific lipid transfer protein N5 of Medicago truncatula is implicated in epidermal stages of rhizobium-host interaction. BMC Plant Biol. 12: 233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pislariu C.I., et al. (2012). A Medicago truncatula tobacco retrotransposon insertion mutant collection with defects in nodule development and symbiotic nitrogen fixation. Plant Physiol. 159: 1686–1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pumplin N., Mondo S.J., Topp S., Starker C.G., Gantt J.S., Harrison M.J. (2010). Medicago truncatula Vapyrin is a novel protein required for arbuscular mycorrhizal symbiosis. Plant J. 61: 482–494. [DOI] [PubMed] [Google Scholar]
- Qin L., Zhao J., Tian J., Chen L., Sun Z., Guo Y., Lu X., Gu M., Xu G., Liao H. (2012). The high-affinity phosphate transporter GmPT5 regulates phosphate transport to nodules and nodulation in soybean. Plant Physiol. 159: 1634–1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu L., Lin J.S., Xu J., Sato S., Parniske M., Wang T.L., Downie J.A., Xie F. (2015). SCARN a novel class of SCAR protein that is required for root-hair infection during legume nodulation. PLoS Genet. 11: e1005623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radutoiu S., Madsen L.H., Madsen E.B., Felle H.H., Umehara Y., Grønlund M., Sato S., Nakamura Y., Tabata S., Sandal N., Stougaard J. (2003). Plant recognition of symbiotic bacteria requires two LysM receptor-like kinases. Nature 425: 585–592. [DOI] [PubMed] [Google Scholar]
- Radutoiu S., Madsen L.H., Madsen E.B., Jurkiewicz A., Fukai E., Quistgaard E.M., Albrektsen A.S., James E.K., Thirup S., Stougaard J. (2007). LysM domains mediate lipochitin-oligosaccharide recognition and Nfr genes extend the symbiotic host range. EMBO J. 26: 3923–3935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramu S.K., Peng H.-M., Cook D.R. (2002). Nod factor induction of reactive oxygen species production is correlated with expression of the early nodulin gene rip1 in Medicago truncatula. Mol. Plant Microbe Interact. 15: 522–528. [DOI] [PubMed] [Google Scholar]
- Redmond J.W., Batley M., Djordjevic M.A., Innes R.W., Kuempel P.L., Rolfe B.G. (1986). Flavones induce expression of nodulation genes in Rhizobium. Nature 323: 632–635. [Google Scholar]
- Reid D., Liu H., Kelly S., Kawaharada Y., Mun T., Andersen S.U., Desbrosses G., Stougaard J. (2018). Dynamics of ethylene production in response to compatible Nod factor. Plant Physiol. 176: 1764–1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid D.E., Ferguson B.J., Hayashi S., Lin Y.-H., Gresshoff P.M. (2011). Molecular mechanisms controlling legume autoregulation of nodulation. Ann. Bot. 108: 789–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid D.E., Heckmann A.B., Novák O., Kelly S., Stougaard J. (2016). CYTOKININ OXIDASE/DEHYDROGENASE3 maintains cytokinin homeostasis during root and nodule development in Lotus japonicus. Plant Physiol. 170: 1060–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyero-Saavedra M.D.R., Qiao Z., Sánchez-Correa M.D.S., Díaz-Pineda M.E., Reyes J.L., Covarrubias A.A., Libault M., Valdés-López O. (2017). Gene silencing of argonaute5 negatively affects the establishment of the legume-rhizobia symbiosis. Genes (Basel) 8: 352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rípodas C., Castaingts M., Clúa J., Villafañe J., Blanco F.A., Zanetti M.E. (2019). The PvNF-YA1 and PvNF-YB7 subunits of the heterotrimeric NF-Y transcription factor influence strain preference in the Phaseolus vulgaris-Rhizobium etli symbiosis. Front. Plant Sci. 10: 221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez-Haas B., Finney L., Vogt S., González-Melendi P., Imperial J., González-Guerrero M. (2013). Iron distribution through the developmental stages of Medicago truncatula nodules. Metallomics 5: 1247–1253. [DOI] [PubMed] [Google Scholar]
- Rogato A., D’Apuzzo E., Barbulova A., Omrane S., Stedel C., Simon-Rosin U., Katinakis P., Flemetakis M., Udvardi M., Chiurazzi M. (2008). Tissue-specific down-regulation of LjAMT1;1 compromises nodule function and enhances nodulation in Lotus japonicus. Plant Mol. Biol. 68: 585–595. [DOI] [PubMed] [Google Scholar]
- Roth L.E., Stacey G. (1989). Bacterium release into host cells of nitrogen-fixing soybean nodules: The symbiosome membrane comes from three sources. Eur. J. Cell Biol. 49: 13–23. [PubMed] [Google Scholar]
- Roy S., et al. (2017). MtLAX2, a functional homologue of the Arabidopsis auxin influx transporter AUX1, is required for nodule organogenesis. Plant Physiol. 174: 326–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubio M.C., Becana M., Sato S., James E.K., Tabata S., Spaink H.P. (2007). Characterization of genomic clones and expression analysis of the three types of superoxide dismutases during nodule development in Lotus japonicus. Mol. Plant Microbe Interact. 20: 262–275. [DOI] [PubMed] [Google Scholar]
- Sadowsky M.J., Cregan P.B. (1992). The soybean Rj4 allele restricts nodulation by Bradyrhizobium japonicum serogroup 123 strains. Appl. Environ. Microbiol. 58: 720–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito K., et al. (2007). NUCLEOPORIN85 is required for calcium spiking, fungal and bacterial symbioses, and seed production in Lotus japonicus. Plant Cell 19: 610–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satgé C., Moreau S., Sallet E., Lefort G., Auriac M.-C., Remblière C., Cottret L., Gallardo K., Noirot C., Jardinaud M.-F., Gamas P. (2016). Reprogramming of DNA methylation is critical for nodule development in Medicago truncatula. Nat. Plants 2: 16166. [DOI] [PubMed] [Google Scholar]
- Schauser L., Handberg K., Sandal N., Stiller J., Thykjaer T., Pajuelo E., Nielsen A., Stougaard J. (1998). Symbiotic mutants deficient in nodule establishment identified after T-DNA transformation of Lotus japonicus. Mol. Gen. Genet. 259: 414–423. [DOI] [PubMed] [Google Scholar]
- Schauser L., Roussis A., Stiller J., Stougaard J. (1999). A plant regulator controlling development of symbiotic root nodules. Nature 402: 191–195. [DOI] [PubMed] [Google Scholar]
- Schippers J.H., Schmidt R., Wagstaff C., Jing H.-C. (2015). Living to die and dying to live: The survival strategy behind leaf senescence. Plant Physiol. 169: 914–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnabel E., Journet E.-P., de Carvalho-Niebel F., Duc G., Frugoli J. (2005). The Medicago truncatula SUNN gene encodes a CLV1-like leucine-rich repeat receptor kinase that regulates nodule number and root length. Plant Mol. Biol. 58: 809–822. [DOI] [PubMed] [Google Scholar]
- Schnabel E.L., Kassaw T.K., Smith L.S., Marsh J.F., Oldroyd G.E., Long S.R., Frugoli J.A. (2011). The ROOT DETERMINED NODULATION1 gene regulates nodule number in roots of Medicago truncatula and defines a highly conserved, uncharacterized plant gene family. Plant Physiol. 157: 328–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Searle I.R., Men A.E., Laniya T.S., Buzas D.M., Iturbe-Ormaetxe I., Carroll B.J., Gresshoff P.M. (2003). Long-distance signaling in nodulation directed by a CLAVATA1-like receptor kinase. Science 299: 109–112. [DOI] [PubMed] [Google Scholar]
- Senovilla M., Castro-Rodríguez R., Abreu I., Escudero V., Kryvoruchko I., Udvardi M.K., Imperial J., González-Guerrero M. (2018). Medicago truncatula copper transporter 1 (MtCOPT1) delivers copper for symbiotic nitrogen fixation. New Phytol. 218: 696–709. [DOI] [PubMed] [Google Scholar]
- Shimomura K., Nomura M., Tajima S., Kouchi H. (2006). LjnsRING, a novel RING finger protein, is required for symbiotic interactions between Mesorhizobium loti and Lotus japonicus. Plant Cell Physiol. 47: 1572–1581. [DOI] [PubMed] [Google Scholar]
- Sidiropoulos K., et al. (2017). Reactome enhanced pathway visualization. Bioinformatics 33: 3461–3467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh S., Katzer K., Lambert J., Cerri M., Parniske M. (2014). CYCLOPS, a DNA-binding transcriptional activator, orchestrates symbiotic root nodule development. Cell Host Microbe 15: 139–152. [DOI] [PubMed] [Google Scholar]
- Sinharoy S., Liu C., Breakspear A., Guan D., Shailes S., Nakashima J., Zhang S., Wen J., Torres-Jerez I., Oldroyd G., Murray J.D., Udvardi M.K. (2016). A Medicago truncatula cystathionine-β-synthase-like domain-containing protein is required for rhizobial infection and symbiotic nitrogen fixation. Plant Physiol. 170: 2204–2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinharoy S., Torres-Jerez I., Bandyopadhyay K., Kereszt A., Pislariu C.I., Nakashima J., Benedito V.A., Kondorosi E., Udvardi M.K. (2013). The C2H2 transcription factor regulator of symbiosome differentiation represses transcription of the secretory pathway gene VAMP721a and promotes symbiosome development in Medicago truncatula. Plant Cell 25: 3584–3601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smit P., Limpens E., Geurts R., Fedorova E., Dolgikh E., Gough C., Bisseling T. (2007). Medicago LYK3, an entry receptor in rhizobial nodulation factor signaling. Plant Physiol. 145: 183–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smit P., Raedts J., Portyanko V., Debellé F., Gough C., Bisseling T., Geurts R. (2005). NSP1 of the GRAS protein family is essential for rhizobial Nod factor-induced transcription. Science 308: 1789–1791. [DOI] [PubMed] [Google Scholar]
- Smith P.M., Atkins C.A. (2002). Purine biosynthesis: Big in cell division, even bigger in nitrogen assimilation. Plant Physiol. 128: 793–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sogawa A., Yamazaki A., Yamasaki H., Komi M., Manabe T., Tajima S., Hayashi M., Nomura M. (2019). SNARE proteins LjVAMP72a and LjVAMP72b are required for root symbiosis and root hair formation in Lotus japonicus. Front. Plant Sci. 9: 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soyano T., Hirakawa H., Sato S., Hayashi M., Kawaguchi M. (2014). Nodule Inception creates a long-distance negative feedback loop involved in homeostatic regulation of nodule organ production. Proc. Natl. Acad. Sci. USA 111: 14607–14612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soyano T., Kouchi H., Hirota A., Hayashi M. (2013). NODULE INCEPTION directly targets NF-Y subunit genes to regulate essential processes of root nodule development in Lotus japonicus. PLoS Genet. 9: e1003352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprent J.I., James E.K. (2007). Legume evolution: Where do nodules and mycorrhizas fit in? Plant Physiol. 144: 575–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stracke S., Kistner C., Yoshida S., Mulder L., Sato S., Kaneko T., Tabata S., Sandal N., Stougaard J., Szczyglowski K., Parniske M. (2002). A plant receptor-like kinase required for both bacterial and fungal symbiosis. Nature 417: 959–962. [DOI] [PubMed] [Google Scholar]
- Subramanian S., Stacey G., Yu O. (2006). Endogenous isoflavones are essential for the establishment of symbiosis between soybean and Bradyrhizobium japonicum. Plant J. 48: 261–273. [DOI] [PubMed] [Google Scholar]
- Suganuma N., Nakamura Y., Yamamoto M., Ohta T., Koiwa H., Akao S., Kawaguchi M. (2003). The Lotus japonicus Sen1 gene controls rhizobial differentiation into nitrogen-fixing bacteroids in nodules. Mol. Genet. Genomics 269: 312–320. [DOI] [PubMed] [Google Scholar]
- Sugawara M., Takahashi S., Umehara Y., Iwano H., Tsurumaru H., Odake H., Suzuki Y., Kondo H., Konno Y., Yamakawa T. (2018). Variation in bradyrhizobial NopP effector determines symbiotic incompatibility with Rj2-soybeans via effector-triggered immunity. Nat. Commun. 9: 3139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton M.A., Oenema O., Erisman J.W., Leip A., van Grinsven H., Winiwarter W. (2011). Too much of a good thing. Nature 472: 159–161. [DOI] [PubMed] [Google Scholar]
- Suzaki T., Takeda N., Nishida H., Hoshino M., Ito M., Misawa F., Handa Y., Miura K., Kawaguchi M. (2019). LACK OF SYMBIONT ACCOMMODATION controls intracellular symbiont accommodation in root nodule and arbuscular mycorrhizal symbiosis in Lotus japonicus. PLoS Genet. 15: e1007865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szczyglowski K., Shaw R.S., Wopereis J., Copeland S., Hamburger D., Kasiborski B., Dazzo F.B., de Bruijn F.J. (1998). Nodule organogenesis and symbiotic mutants of the model legume Lotus japonicus. Mol. Plant Microbe Interact. 11: 684–697. [Google Scholar]
- Tadege M., Wen J., He J., Tu H., Kwak Y., Eschstruth A., Cayrel A., Endre G., Zhao P.X., Chabaud M., Ratet P., Mysore K.S. (2008). Large-scale insertional mutagenesis using the Tnt1 retrotransposon in the model legume Medicago truncatula. Plant J. 54: 335–347. [DOI] [PubMed] [Google Scholar]
- Takahara M., et al. (2013). TOO MUCH LOVE, a novel Kelch repeat-containing F-box protein, functions in the long-distance regulation of the legume-Rhizobium symbiosis. Plant Cell Physiol. 54: 433–447. [DOI] [PubMed] [Google Scholar]
- Takanashi K., Yokosho K., Saeki K., Sugiyama A., Sato S., Tabata S., Ma J.F., Yazaki K. (2013). LjMATE1: A citrate transporter responsible for iron supply to the nodule infection zone of Lotus japonicus. Plant Cell Physiol. 54: 585–594. [DOI] [PubMed] [Google Scholar]
- Tang F., Yang S., Liu J., Zhu H. (2016). Rj4, a gene controlling nodulation specificity in soybeans, encodes a thaumatin-like protein but not the one previously reported. Plant Physiol. 170: 26–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teillet A., Garcia J., de Billy F., Gherardi M., Huguet T., Barker D.G., de Carvalho-Niebel F., Journet E.-P. (2008). api, a novel Medicago truncatula symbiotic mutant impaired in nodule primordium invasion. Mol. Plant Microbe Interact. 21: 535–546. [DOI] [PubMed] [Google Scholar]
- Tejada-Jiménez M., Castro-Rodríguez R., Kryvoruchko I., Lucas M.M., Udvardi M., Imperial J., González-Guerrero M. (2015). Medicago truncatula Natural Resistance-Associated Macrophage Protein1 is required for iron uptake by rhizobia-infected nodule cells. Plant Physiol. 168: 258–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tejada-Jiménez M., Gil-Díez P., León-Mediavilla J., Wen J., Mysore K.S., Imperial J., González-Guerrero M. (2017). Medicago truncatula Molybdate Transporter type 1 (MtMOT1.3) is a plasma membrane molybdenum transporter required for nitrogenase activity in root nodules under molybdenum deficiency. New Phytol. 216: 1223–1235. [DOI] [PubMed] [Google Scholar]
- Theil E.C. (2003). Ferritin: At the crossroads of iron and oxygen metabolism. J. Nutr. 133 (suppl. 1): 1549S–1553S. [DOI] [PubMed] [Google Scholar]
- Timmers A.C. (2008). The role of the plant cytoskeleton in the interaction between legumes and rhizobia. J. Microsc. 231: 247–256. [DOI] [PubMed] [Google Scholar]
- Tirichine L., et al. (2006). Deregulation of a Ca2+/calmodulin-dependent kinase leads to spontaneous nodule development. Nature 441: 1153–1156. [DOI] [PubMed] [Google Scholar]
- Tirichine L., Sandal N., Madsen L.H., Radutoiu S., Albrektsen A.S., Sato S., Asamizu E., Tabata S., Stougaard J. (2007). A gain-of-function mutation in a cytokinin receptor triggers spontaneous root nodule organogenesis. Science 315: 104–107. [DOI] [PubMed] [Google Scholar]
- Tjepkema J.D., Yocum C.S. (1974). Measurement of oxygen partial pressure within soybean nodules by oxygen microelectrodes. Planta 119: 351–360. [DOI] [PubMed] [Google Scholar]
- Truchet G., Roche P., Lerouge P., Vasse J., Camut S., de Billy F., Promé J.-C., Dénarié J. (1991). Sulphated lipo-oligosaccharide signals of Rhizobium meliloti elicit root nodule organogenesis in alfalfa. Nature 351: 670–673. [Google Scholar]
- Tsikou D., Yan Z., Holt D.B., Abel N.B., Reid D.E., Madsen L.H., Bhasin H., Sexauer M., Stougaard J., Markmann K. (2018). Systemic control of legume susceptibility to rhizobial infection by a mobile microRNA. Science 362: 233–236. [DOI] [PubMed] [Google Scholar]
- Udvardi M., Poole P.S. (2013). Transport and metabolism in legume-rhizobia symbioses. Annu. Rev. Plant Biol. 64: 781–805. [DOI] [PubMed] [Google Scholar]
- Valkov V.T., Rogato A., Alves L.M., Sol S., Noguero M., Léran S., Lacombe B., Chiurazzi M. (2017). The nitrate transporter family protein LjNPF8.6 controls the N-fixing nodule activity. Plant Physiol. 175: 1269–1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van de Velde W., Guerra J.C., De Keyser A., De Rycke R., Rombauts S., Maunoury N., Mergaert P., Kondorosi E., Holsters M., Goormachtig S. (2006). Aging in legume symbiosis: A molecular view on nodule senescence in Medicago truncatula. Plant Physiol. 141: 711–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van de Velde W., et al. (2010). Plant peptides govern terminal differentiation of bacteria in symbiosis. Science 327: 1122–1126. [DOI] [PubMed] [Google Scholar]
- van Spronsen P.C., Bakhuizen R., van Brussel A.A., Kijne J.W. (1994). Cell wall degradation during infection thread formation by the root nodule bacterium Rhizobium leguminosarum is a two-step process. Eur. J. Cell Biol. 64: 88–94. [PubMed] [Google Scholar]
- Veereshlingam H., Haynes J.G., Penmetsa R.V., Cook D.R., Sherrier D.J., Dickstein R. (2004). nip, a symbiotic Medicago truncatula mutant that forms root nodules with aberrant infection threads and plant defense-like response. Plant Physiol. 136: 3692–3702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma D. (1992). Signals in root nodule organogenesis and endocytosis of Rhizobium. Plant Cell 4: 373–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernié T., et al. (2016). PUB1 interacts with the receptor kinase DMI2 and negatively regulates rhizobial and arbuscular mycorrhizal symbioses through its ubiquitination activity in Medicago truncatula. Plant Physiol. 170: 2312–2324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernié T., Kim J., Frances L., Ding Y., Sun J., Guan D., Niebel A., Gifford M.L., de Carvalho-Niebel F., Oldroyd G.E. (2015). The NIN transcription factor coordinates diverse nodulation programs in different tissues of the Medicago truncatula root. Plant Cell 27: 3410–3424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinardell J.M., Fedorova E., Cebolla A., Kevei Z., Horvath G., Kelemen Z., Tarayre S., Roudier F., Mergaert P., Kondorosi A., Kondorosi E. (2003). Endoreduplication mediated by the anaphase-promoting complex activator CCS52A is required for symbiotic cell differentiation in Medicago truncatula nodules. Plant Cell 15: 2093–2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voroshilova V.A., Demchenko K.N., Brewin N.J., Borisov A.Y., Tikhonovich I.A. (2009). Initiation of a legume nodule with an indeterminate meristem involves proliferating host cells that harbour infection threads. New Phytol. 181: 913–923. [DOI] [PubMed] [Google Scholar]
- Wang C., et al. (2016). NODULES WITH ACTIVATED DEFENSE 1 is required for maintenance of rhizobial endosymbiosis in Medicago truncatula. New Phytol. 212: 176–191. [DOI] [PubMed] [Google Scholar]
- Wang C., Zhu H., Jin L., Chen T., Wang L., Kang H., Hong Z., Zhang Z. (2013). Splice variants of the SIP1 transcripts play a role in nodule organogenesis in Lotus japonicus. Plant Mol. Biol. 82: 97–111. [DOI] [PubMed] [Google Scholar]
- Wang D., Griffitts J., Starker C., Fedorova E., Limpens E., Ivanov S., Bisseling T., Long S. (2010). A nodule-specific protein secretory pathway required for nitrogen-fixing symbiosis. Science 327: 1126–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Rubio M.C., Xin X., Zhang B., Fan Q., Wang Q., Ning G., Becana M., Duanmu D. (2019). CRISPR/Cas9 knockout of leghemoglobin genes in Lotus japonicus uncovers their synergistic roles in symbiotic nitrogen fixation. New Phytol. 224: 818–832. [DOI] [PubMed] [Google Scholar]
- Wang Q., et al. (2017). Host-secreted antimicrobial peptide enforces symbiotic selectivity in Medicago truncatula. Proc. Natl. Acad. Sci. USA 114: 6854–6859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Li K., Chen L., Zou Y., Liu H., Tian Y., Li D., Wang R., Zhao F., Ferguson B.J., Gresshoff P.M., Li X. (2015). MicroRNA167-directed regulation of the auxin response factors GmARF8a and GmARF8b is required for soybean nodulation and lateral root development. Plant Physiol. 168: 984–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Wang L., Zou Y., Chen L., Cai Z., Zhang S., Zhao F., Tian Y., Jiang Q., Ferguson B.J., Gresshoff P.M., Li X. (2014). Soybean miR172c targets the repressive AP2 transcription factor NNC1 to activate ENOD40 expression and regulate nodule initiation. Plant Cell 26: 4782–4801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasson A.P., Pellerone F.I., Mathesius U. (2006). Silencing the flavonoid pathway in Medicago truncatula inhibits root nodule formation and prevents auxin transport regulation by rhizobia. Plant Cell 18: 1617–1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wienkoop S., Saalbach G. (2003). Proteome analysis: Novel proteins identified at the peribacteroid membrane from Lotus japonicus root nodules. Plant Physiol. 131: 1080–1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winzer T., Bairl A., Linder M., Linder D., Werner D., Müller P. (1999). A novel 53-kDa nodulin of the symbiosome membrane of soybean nodules, controlled by Bradyrhizobium japonicum. Mol. Plant Microbe Interact. 12: 218–226. [DOI] [PubMed] [Google Scholar]
- Xiao T.T., Schilderink S., Moling S., Deinum E.E., Kondorosi E., Franssen H., Kulikova O., Niebel A., Bisseling T. (2014). Fate map of Medicago truncatula root nodules. Development 141: 3517–3528. [DOI] [PubMed] [Google Scholar]
- Xie F., Murray J.D., Kim J., Heckmann A.B., Edwards A., Oldroyd G.E., Downie J.A. (2012). Legume pectate lyase required for root infection by rhizobia. Proc. Natl. Acad. Sci. USA 109: 633–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue Y.-B., Xiao B.-X., Zhu S.-N., Mo X.-H., Liang C.-Y., Tian J., Liao H., Miriam G. (2017). GmPHR25, a GmPHR member up-regulated by phosphate starvation, controls phosphate homeostasis in soybean. J. Exp. Bot. 68: 4951–4967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaya-Ito H., Shimoda Y., Hakoyama T., Sato S., Kaneko T., Hossain M.S., Shibata S., Kawaguchi M., Hayashi M., Kouchi H., Umehara Y. (2018). Loss-of-function of ASPARTIC PEPTIDASE NODULE-INDUCED 1 (APN1) in Lotus japonicus restricts efficient nitrogen-fixing symbiosis with specific Mesorhizobium loti strains. Plant J. 93: 5–16. [DOI] [PubMed] [Google Scholar]
- Yan Q., Wang L., Li X. (2018). GmBEHL1, a BES1/BZR1 family protein, negatively regulates soybean nodulation. Sci. Rep. 8: 7614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S., Tang F., Gao M., Krishnan H.B., Zhu H. (2010). R gene-controlled host specificity in the legume-rhizobia symbiosis. Proc. Natl. Acad. Sci. USA 107: 18735–18740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S., Wang Q., Fedorova E., Liu J., Qin Q., Zheng Q., Price P.A., Pan H., Wang D., Griffitts J.S., Bisseling T., Zhu H. (2017). Microsymbiont discrimination mediated by a host-secreted peptide in Medicago truncatula. Proc. Natl. Acad. Sci. USA 114: 6848–6853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yano K., et al. (2008). CYCLOPS, a mediator of symbiotic intracellular accommodation. Proc. Natl. Acad. Sci. USA 105: 20540–20545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yendrek C.R., et al. (2010). A putative transporter is essential for integrating nutrient and hormone signaling with lateral root growth and nodule development in Medicago truncatula. Plant J. 62: 100–112. [DOI] [PubMed] [Google Scholar]
- Yokota K., et al. (2009). Rearrangement of actin cytoskeleton mediates invasion of Lotus japonicus roots by Mesorhizobium loti. Plant Cell 21: 267–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoro E., Nishida H., Ogawa-Ohnishi M., Yoshida C., Suzaki T., Matsubayashi Y., Kawaguchi M. (2019). PLENTY, a hydroxyproline O-arabinosyltransferase, negatively regulates root nodule symbiosis in Lotus japonicus. J. Exp. Bot. 70: 507–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoro E., Suzaki T., Toyokura K., Miyazawa H., Fukaki H., Kawaguchi M. (2014). A positive regulator of nodule organogenesis, NODULE INCEPTION, acts as a negative regulator of rhizobial infection in Lotus japonicus. Plant Physiol. 165: 747–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida C., Funayama-Noguchi S., Kawaguchi M. (2010). plenty, a novel hypernodulation mutant in Lotus japonicus. Plant Cell Physiol. 51: 1425–1435. [DOI] [PubMed] [Google Scholar]
- Yuan S., Zhu H., Gou H., Fu W., Liu L., Chen T., Ke D., Kang H., Xie Q., Hong Z., Zhang Z. (2012). A ubiquitin ligase of symbiosis receptor kinase involved in nodule organogenesis. Plant Physiol. 160: 106–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanetti M.E., Blanco F.A., Beker M.P., Battaglia M., Aguilar O.M. (2010). A C subunit of the plant nuclear factor NF-Y required for rhizobial infection and nodule development affects partner selection in the common bean-Rhizobium etli symbiosis. Plant Cell 22: 4142–4157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C., Bousquet A., Harris J.M. (2014). Abscisic acid and LATERAL ROOT ORGAN DEFECTIVE/NUMEROUS INFECTIONS AND POLYPHENOLICS modulate root elongation via reactive oxygen species in Medicago truncatula. Plant Physiol. 166: 644–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Subramanian S., Stacey G., Yu O. (2009). Flavones and flavonols play distinct critical roles during nodulation of Medicago truncatula by Sinorhizobium meliloti. Plant J. 57: 171–183. [DOI] [PubMed] [Google Scholar]
- Zhang X., Dong W., Sun J., Feng F., Deng Y., He Z., Oldroyd G.E., Wang E. (2015). The receptor kinase CERK1 has dual functions in symbiosis and immunity signalling. Plant J. 81: 258–267. [DOI] [PubMed] [Google Scholar]
- Zhang X., Han L., Wang Q., Zhang C., Yu Y., Tian J., Kong Z. (2019). The host actin cytoskeleton channels rhizobia release and facilitates symbiosome accommodation during nodulation in Medicago truncatula. New Phytol. 221: 1049–1059. [DOI] [PubMed] [Google Scholar]
- Zhou C., et al. (2011). From model to crop: Functional analysis of a STAY-GREEN gene in the model legume Medicago truncatula and effective use of the gene for alfalfa improvement. Plant Physiol. 157: 1483–1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu H., Chen T., Zhu M., Fang Q., Kang H., Hong Z., Zhang Z. (2008). A novel ARID DNA-binding protein interacts with SymRK and is expressed during early nodule development in Lotus japonicus. Plant Physiol. 148: 337–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zipfel C., Oldroyd G.E. (2017). Plant signalling in symbiosis and immunity. Nature 543: 328–336. [DOI] [PubMed] [Google Scholar]