Abstract
Context
Foam rolling (FR) is considered an effective postexercise modality for reducing delayed-onset muscle soreness and enhancing recovery of muscle function. However, the effects of FR on muscle and joint proprioception have not been investigated.
Objective
To examine the effects of FR on muscle and joint proprioception after an intense exercise protocol.
Design
Controlled laboratory study.
Setting
University-based laboratory.
Patients or Other Participants
A total of 80 healthy, physically active male students were randomly assigned to either the FR (n = 40; age = 22.8 ± 3.3 years, height = 176.4 ± 5.3 cm, mass = 74.2 ± 6.4 kg) or passive-recovery (PR; n = 40; age = 23.0 ± 3.2 years, height = 178.1 ± 5.5 cm, mass = 74.6 ± 6.2 kg) group.
Intervention(s)
Participants in both groups performed 4 sets of 25 repetitions of voluntary maximal eccentric contractions at 60°/s from 20° to 100° of knee flexion to induce exercise-induced muscle damage. The exercise was followed by either PR or 2 minutes of FR immediately (1 hour) and 24, 48, and 72 hours postexercise.
Main Outcome Measure(s)
Muscle soreness, pressure-pain threshold, quadriceps-muscle strength, joint position sense, isometric force sense, and threshold to detect passive movement at baseline and immediately, 24, 48, and 72 hours postexercise after FR.
Results
Foam rolling resulted in decreased muscle pain, increased pressure-pain threshold, improved joint position sense, attenuated force loss, and reduced threshold to detect passive movement compared with PR at 24 and 48 hours postexercise.
Conclusions
Foam rolling postexercise diminished delayed-onset muscle soreness and improved recovery of muscle strength and joint proprioception. These results suggested that FR enhanced recovery from exercise-induced damage.
Keywords: athletic performance, manual medicine, pain therapy, sports medicine
Key Points
Proprioception was reduced immediately after muscle-damaging exercise and returned to pre-exercise levels after 72 hours of passive recovery.
Muscle strength was decreased at 24 and 48 hours after muscle-damaging exercise and had not returned to pre-exercise levels after 72 hours of passive recovery.
Foam rolling attenuated the perceived muscle soreness at 24 and 48 hours after exercise-induced muscle damage.
Foam rolling facilitated the recovery of muscle and joint proprioception and muscle strength at 24 and 48 hours after exercise-induced muscle damage.
Unaccustomed, intensive high-volume exercise typically results in myofibrillar damage and disturbances in the extracellular matrix, sarcomeres, and excitation-contraction coupling.1,2 Exercise-induced muscle damage (EIMD) can be triggered by a sudden acute stretch of the muscle-tendon unit during unaccustomed or excessive exercise.3 Athletes often experience EIMD after starting a new workout or increasing their workout intensity, which not only results in discomfort and disability but also temporarily impedes performance and prevents training.4
Delayed-onset muscle soreness (DOMS) usually begins a few hours after strength exercise, reaches its peak 24 to 48 hours later, and may last for several days.2,3 It is associated with loss of muscle strength and endurance,5,6 decreased range of joint motion,7–9 impaired proprioception,5,10,11 increased muscle stiffness,10 and unsteady limbs and clumsiness during precision movements.12
Errors during position- and force-matching tasks increase postexercise and increase more after eccentric than concentric exercise,13 probably due to more EIMD after eccentric exercise. The reduced force-generating capacity after eccentric exercise persists for at least 24 to 48 hours,6 and substantial matching errors are still reported after 24 hours.14 Perhaps a greater contributor to error in force-matching tasks and increased clumsiness after eccentric exercise is impaired proprioception. Limb position and movement are registered by muscle spindles and joint and skin-stretch receptors,15,16 and proprioception is indeed substantially reduced during EIMD. This reduction may, in part, be due to the increased stiffness of the muscle17 or to loss of muscle-receptor input after damage to the intrafusal fibers of the muscle spindles.5,10 Dynamic tasks require accurate proprioceptive information and sufficient muscular strength18 to control posture and movement; a lack of proprioception (body awareness) can increase the chance of sport injury.18 Maintaining proprioceptive function, therefore, may be an effective strategy to prevent sport injuries.
Different strategies have been proposed to speed recovery from EIMD, such as foam rolling (FR), neuromuscular electrical stimulation, cryotherapy, and nutritional supplements,19 but only Shin and Sung10 have investigated the benefits of massage for proprioception. Foam rolling reduced the self-perceived intensity of muscle soreness20–22 and speeded recovery of joint range of motion,7–9,21 neuromuscular control,23 running speed, and jump height after EIMD.21
Some of these improvements may occur via acute decreases in passive tissue stiffness9 that reduce neural inhibition11 or other neurologic effects, such as reduced pain, even when the rolling was performed on the contralateral limb.20,21 To our knowledge, no researchers have investigated the effects of FR on muscle and joint proprioception after EIMD. Therefore, the purpose of our study was to examine the effects of FR on muscle and joint proprioception after an intense exercise protocol. We hypothesized that FR would accelerate the recovery of muscle and joint proprioception after EIMD and, thereby, improve neuromuscular function.24
METHODS
Participants
Eighty male sport-science students participated in our study (Table). Volunteers were included if they participated in mild- to moderate-intensity physical activities (ie, walking, jogging, or endurance activities) 2 to 3 times per week (9.9 ± 3.5 h/wk) for ≥6 months before recruitment; answered no to all questions on the Physical-Activity Readiness Questionnaire (PAR-Q)25; did not take any medications in the 48 hours before the study; did not have a lower extremity injury; did not have a musculoskeletal disorder; did not have psychiatric, neurologic, pulmonary, cardiovascular, renal, or inflammatory disease; and did not have a history of FR in the 30 days before the study. Exclusion criteria were participation in another study in which muscle damage was induced or the use of supplements, caffeine, nicotine, or alcohol in the 24 hours before the study or during the study period.
Table.
Participant Characteristics
| Characteristic |
Group, Mean ± SD |
t Value |
P Value |
|
| Foam Rolling (n = 40) |
Passive Recovery (n = 40) |
|||
| Age, y | 22.8 ± 3.3 | 23.0 ± 3.2 | 0.33 | .68 |
| Height, cm | 176.4 ± 5.3 | 178.1 ± 5.5 | 1.43 | .18 |
| Mass, kg | 74.2 ± 6.4 | 74.6 ± 6.2 | 0.72 | .86 |
| Body mass index, kg/m2 | 23.9 ± 2.1 | 23.3 ± 2.3 | 0.54 | .34 |
| No. of exercise sessions/wk | 3.8 ± 0.8 | 3.7 ± 0.7 | 0.25 | .76 |
| Exercise time/wk, min | 120 ± 20 | 115 ± 17 | 0.63 | .47 |
| Target side, No. left/right | 5/35 | 7/33 | NA | NA |
Abbreviation: NA, not applicable.
Sample-size calculations (G*Power, version 3.1 software,26 Heinrich-Heine-Universität Düsseldorf, Germany) were based on the effect sizes of massage on ankle-joint proprioception reported by Shin and Sung.10 With an effect size of 0.62, a 2-tailed α level of .05, and a desired power (1–β) of 0.80, a sample size of 33 in each group was needed. Given that we anticipated a drop-out rate of 20%, we enrolled 40 participants in each group. All participants provided written informed consent, and the study was approved by the Institutional Review Board of Shahrood University of Technology.
Procedures
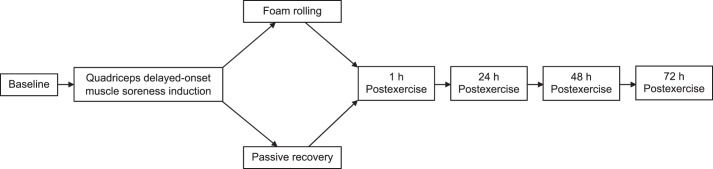
The experimental design is illustrated in Figure 1. After inducing DOMS with an eccentric quadriceps exercise protocol, we assessed the effects of FR on the time course of recovery from muscle soreness, pressure-pain threshold, joint position sense (JPS), isometric force sense, and threshold to detect passive movement. The participants were randomly allocated to the passive-recovery (PR) or FR group using a computer-generated random-allocation sequence (version 2.0; Random Allocation Software, Isfahan University of Medical Sciences, Isfahan, Iran).
Figure 1.
Experimental design of the study.
The participants visited the laboratory 5 times. During the first session, we obtained informed consent, collected anthropometric information, familiarized the participants with the experimental protocol, conducted the testing procedures in the order presented in the Methods section, and induced DOMS. During the second session, participants in the FR group performed 4 sets of 120-second rolling cycles of the right quadriceps muscle, whereas the PR group participated in a time-matched PR. This was repeated at 24, 48, and 72 hours postexercise. At baseline and immediately (1 hour), 24, 48, and 72 hours postexercise, a series of neuromuscular evaluations were performed on the right lower extremity.
Eccentric Exercise Protocol
Before muscle damage was induced, the participants performed a 5-minute warm-up, including brisk walking on a treadmill for 2 minutes, followed by a series of aerobic movements, including leg swings, high knee jog, and muscle stretches of the lower limbs (quadriceps stretch, hamstrings stretch, calf stretch, and twisting reverse lunge). The participants then sat on an adjustable isokinetic dynamometer (version 3 Pro; Biodex Medical Systems Inc, Shirley, NY) with their hips flexed to 120° and knees flexed to 90°. The axis of rotation (tibiofemoral joint) of the knee was aligned with the axis of rotation of the dynamometer. The participants were secured with straps across the chest and hips to minimize body movement during contractions. To further limit movement, the tested limb was secured with a hook-and-loop strap to the lever arm of the dynamometer, and participants were instructed to hold on to the lateral support of the chair. The resistance pad was placed as distally as possible on the tibia while still allowing full dorsiflexion of the ankle. The eccentric exercise protocol for inducing muscle damage consisted of 4 sets of 25 maximal voluntary eccentric contractions of the knee extensors at 60°/s from 20° to 100° of knee flexion, with a 2-minute rest between sets. We provided visual feedback on the generated force and oral encouragement to maintain the force during each repetition.
Intervention
Foam rolling was conducted using polypropylene groove standard foam rollers (BlackRoll AG, Bottighofen, Switzerland). The rollers had a material stiffness of 23.46 N/mm, a length of 29.5 cm, and a circumference of 14.8 cm. This small foam roll was chosen because it is easy to transport and widely used by professional athletes. A sport-science specialist supervised the FR. Starting in a prone position with the roller approximately 8 cm inferior to the anterior-superior iliac spine, participants crossed 1 limb over the other (Figure 2). They rolled down to a position superior to the patellar tendon and back using their elbows to guide movement. We instructed participants to exert as much pressure as tolerable on the foam roller at all times because pressure that causes pain reduced the benefits of FR.21,22 The application frequency was 30 rolling cycles per minute for 2 minutes.7 To help participants roll at this frequency, we instructed them to change the rolling direction with every tap of a metronome.
Figure 2.

Foam rolling.
Foam rolling was performed immediately and at 24, 48, and 72 hours after the EIMD protocol. We chose FR immediately after the DOMS-inducing exercise because DOMS intensity increases during the first 24 hours and peaks around 48 hours postexercise.2,21,22
Measures
Immediately after completing each FR session, measurements were performed in the following order.
Passive Recovery
During PR, the participants sat on a bench for 2 minutes. We instructed them not to engage in any other form of recovery procedure (eg, massage, cold-water immersion) during the study period.
Muscle Soreness
Muscle soreness was rated with a visual analogue scale that consisted of a 100-mm line, with 0 indicating no pain and 100 representing extreme pain. Participants marked their muscle soreness on the 100-mm line while 5 kg of pressure was applied by an algometer probe (model Algometer Commander; J Tech Medical Industries Inc, Midvale, UT) to a 1.0-cm2 area on the midline of the quadriceps muscle midway between the iliac crest and the superior border of the patella.
Pressure-Pain Threshold
The pressure-pain threshold, or the minimal amount of pressure that causes pain, was used to assess muscle tenderness.20 A higher pressure-pain threshold indicates less muscle tenderness. To assess the quadriceps pressure-pain threshold, an algometer device with a 1-cm-diameter circular and flat probe (Algometer Commander) was placed on the midline of the quadriceps muscle midway between the iliac crest and the superior border of the patella while the participant was in a relaxed standing position. The pressure was gradually increased at a constant rate of 50 to 60 kPa/s until the participants indicated pain. Four trials with 30-second intervals between measurements were completed, and the data were recorded in kilopascals. The average of the 4 trials was used for analysis.
Joint Position Sense
Joint position sense was evaluated with the participant seated in the dynamometer chair as described in the Eccentric Exercise Protocol subsection with his eyes closed. He held a “hold” button to stop movement of the lever arm when he thought it had reached the target angle. For each test, the leg was passively moved at a speed of 10°/s and held still for approximately 10 seconds at 30°, 45°, and 60° to help the participant memorize these target positions. Next, we instructed him to actively position the leg to these angles, starting from 100° of knee flexion. The order of selected target angles was random. For each target angle, 4 trials were completed to determine the repositioning error.
Threshold to Detect Passive Movement
The threshold to detect passive movement of the knee joint was evaluated with the participant seated in the dynamometer chair as described in the “Eccentric Exercise Protocol” subsection with eyes closed and wearing ear plugs to eliminate audiovisual cues. As for the JPS evaluation, the participant held a “hold” button that he was instructed to push as soon as he felt a change in the knee angle. To maximize the stimulation of joint receptors and minimize stimulation of cutaneous and muscle receptors, the test was performed at a low knee-extension angular velocity (0.25°/s). Four trials were performed from the starting positions of 30° and 70° of knee flexion, and the threshold angle of perception of passive movement was the average angle in these consecutive tests. If the wrong rotational direction was detected, the trial was repeated.
Isometric Force Sense
To assess isometric force sense, the participant was seated in the dynamometer chair as described in the “Eccentric Exercise Protocol” subsection and were instructed to maintain torques of 10%, 30%, and 50% of maximal voluntary isometric contraction (MVIC) during a 7-second isometric contraction. We tested isometric force sense to minimize the influence of movement and assess the ability to control force.12 Low loads were chosen to minimize fatigue. We randomized the order of the target forces and instructed the participant to complete each percentage of the selected MVIC torque 4 times. Visual feedback of force was provided via a monitor. Next, he was instructed to reproduce the same target force while blindfolded. For each torque percentage, 4 trials were completed with 60-second rest intervals. The difference between target torque and produced torque was the absolute value. The average of 4 trials was used for statistical analysis.
Isokinetic Muscle Strength
A dynamometer (Biodex System 3 Pro) was used to assess quadriceps muscle strength. Before each measurement, we performed a gravity correction. To perform the measurements, the participant sat on the chair as described in the “Eccentric Exercise Protocol” subsection. Before testing, he performed 3 submaximal contractions to become familiar with the isokinetic device. The maximal voluntary force was measured during a set of 5 isokinetic concentric contractions at both 60°/s and 120/s.27 The participant received visual feedback and oral encouragement to reach the maximum torque. The testing started at the lower speed, and a 2-minute rest was given between each set of contractions.
Statistical Analysis
The Shapiro-Wilk test showed that all data were normally distributed. A 2 (group: FR, PR) × 5 (time: baseline and immediately, 24, 48, and 72 hours postexercise) mixed-model analysis of variance was used to evaluate the main and interaction effects of variables. Follow-up Bonferroni-corrected t tests for multiple comparisons were conducted where appropriate. To assess the effect size of the range of training gains between baseline and postexercise times, we calculated Cohen d and interpreted the sizes as trivial (≤0.19), small (0.2–0.49), moderate (0.50–0.79), or large (≥0.80).28 The α level was set at .05 for all statistical analyses. We used SPSS (version 18; SPSS Inc, Chicago, IL) for statistical analyses.
RESULTS
Age, height, mass, and body mass index were not different between groups (Table).
For muscle soreness, we observed main effects of time (F1,78 = 1448.7, P = .001, ηp2 = 0.94) and group (F1,78 = 38.5, P = .001, ηp2 = 0.33) and a time × group interaction (F1,78 = 43.8, P = .001, ηp2 = 0.36). Post hoc tests showed that muscle soreness was lower in the FR group than in the PR group at 24 hours (34.3% versus 45.7%; d = 1.2, P = .001) and 48 hours (45.8% versus 62.3%; d = 1.3, P = .001) after the eccentric exercise (Figure 3A).
Figure 3.
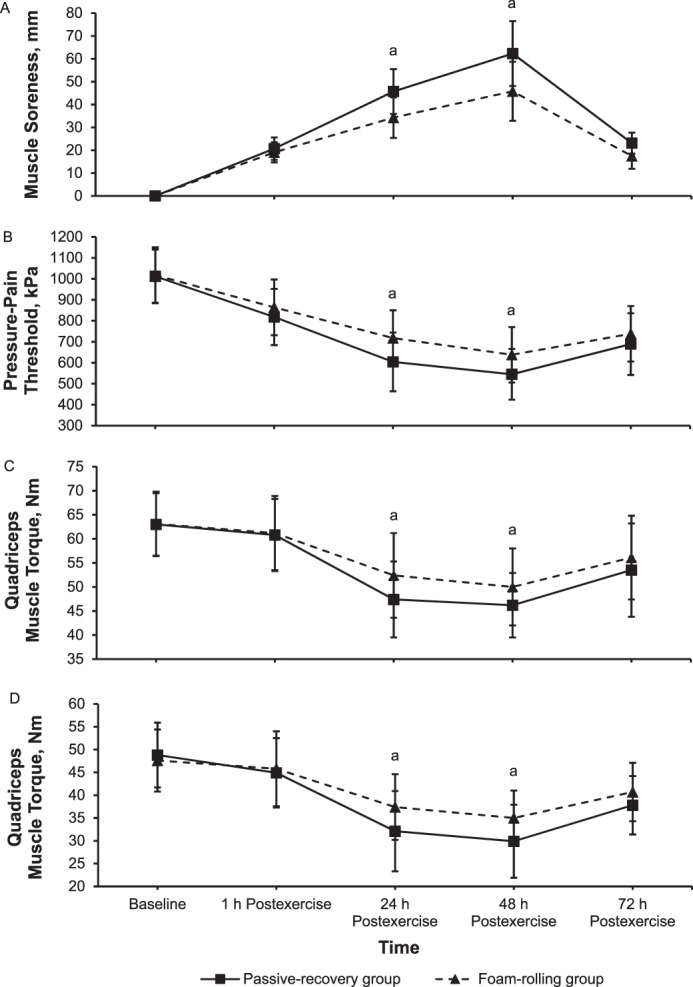
Mean ± SD, A, muscle soreness rated with a visual analogue scale (range = 0–100; B, pressure-pain threshold; C, quadriceps muscle torque at 60°/s; and D, quadriceps muscle torque at 120°/s at baseline and immediately (1 h) to 72 h after eccentric exercise for the foam-rolling and passive-recovery groups. a Difference between groups (P < .05).
For the pressure-pain threshold, we found main effects of time (F1,78 = 1251.6, P = .001, ηp2 = 0.94) and group (F1,78 = 5.4, P = .02, ηp2 = 0.07) and a time × group interaction (F1,78 = 7.6, P = .01, ηp2 = 0.09). Post hoc tests showed that the pressure-pain threshold was higher in the FR group than in the PR group at 24 hours (70.5% versus 60%; d = 0.82, P = .001) and 48 hours (63% versus 54%; d = 0.72, P = .01) after the eccentric exercise, indicating less tenderness in the FR group (Figure 3B).
For muscle strength, we noted main effects of time (F1,78 = 152.3, P = .001, ηp2 = 0.66 and F1,78 = 107.3, P = .001, ηp2 = 0.58) and time × group interactions (F1,78 = 6.5, P = .01, ηp2 = 0.08 and F1,78 = 7.5, P = .01, ηp2 = 0.9) at 60°/s and 120°/s, respectively. Follow-up comparisons showed that muscle strength was higher in the FR than in the PR group at 24 hours (83% versus 75%; d = 0.59, P = .01 and 79% versus 66%; d = 0.66, P = .01) and 48 hours (79% versus 66%; d = 0.49, P = .03 and 73% versus 61%; d = 0.69, P = .01) after eccentric exercise at 60°/s and 120°/s, respectively (Figures 3C and 3D, respectively).
For JPS, only a main effect of time was present (F1,78 = 106.8, P = .001, ηp2 = 0.58; Figure 4A) at 30°. We demonstrated main effects of time (F1,78 = 70.3, P = .001, ηp2 = 0.47 and F1,78 = 93.6, P = .001, np2 = 0.55) and group (F1,78 = 12.3, P = .001, ηp2 = 0.14 and F1,78 = 10.6, P = .002, ηp2 = 0.12) and a time × group interaction (F1,78 = 3.1, P = .02, ηp2 = 0.04 and F1,78 = 3.7, P = .01, ηp2 = 0.05) for JPS at 45° and 60°, respectively. Follow-up comparisons showed that the absolute repositioning error was smaller in the FR than in the PR group at 24 hours (282% versus 366%; d = 0.93, P = .001 and 203% versus 303%; d = 0.90, P = .001) and 48 hours (212% versus 283%; d = 0.81, P = .001 and 148% versus 203%; d = 0.55, P = .01) after eccentric exercise at 45° and 60°, respectively (Figures 4B and 4C, respectively).
Figure 4.
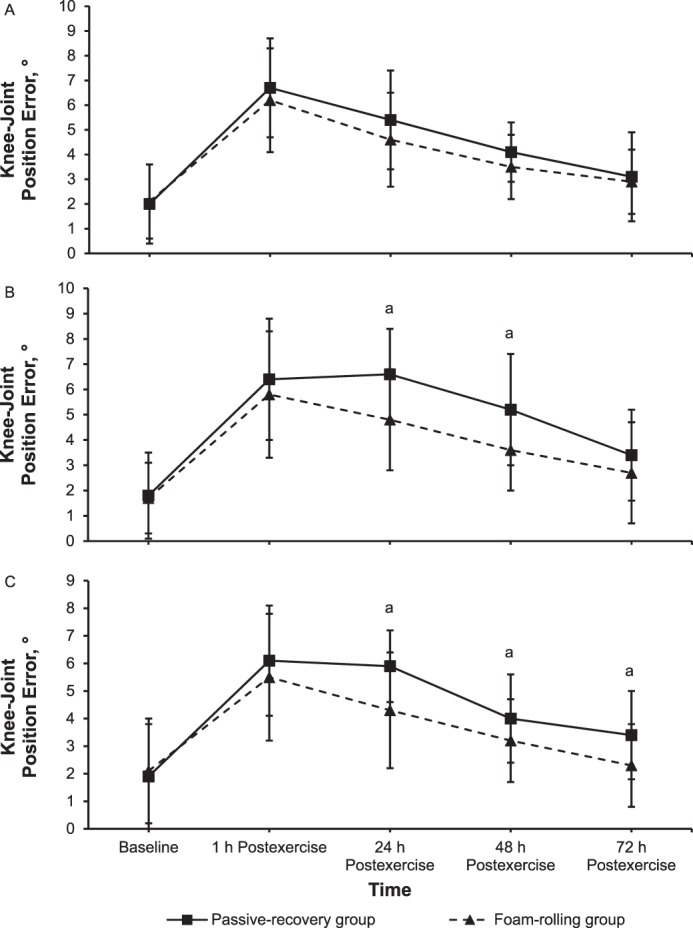
Position error of the knee joint (mean ± SD) at A, 30°; B, 45°; and C, 60° in absolute values during knee flexion at baseline and immediately (1 h) to 72 h after eccentric exercise for the foam-rolling and passive-recovery groups. a Difference between groups (P < .05).
We found main effects of time (F1,78 = 29.3, P = .001, ηp2 = 0.28 and F1,78 = 39.7, P = .001, ηp2 = 0.34) and group (F1,78 = 10.5, P = .002, ηp2 = 0.12 and F1,78 = 5.7, P = .02, ηp2 = 0.07) and a time × group interaction (F1,78 = 3.1, P = .02, ηp2 = 0.04 and F1,78 = 3.7, P = .01, ηp2 = 0.05) for the threshold to detect passive movement at 30° and 70° of knee flexion, respectively. Follow-up comparisons showed that the threshold to detect passive movement was smaller for the FR than for the PR group at 24 hours (210% versus 305%; d = 0.50, P = .03; Figure 5A) and 48 hours (228% versus 341%; d = 0.63, P = .01 and 233% versus 297%; d = 0.43, P = .03, respectively; Figures 5A and 5B, respectively) after eccentric exercise.
Figure 5.
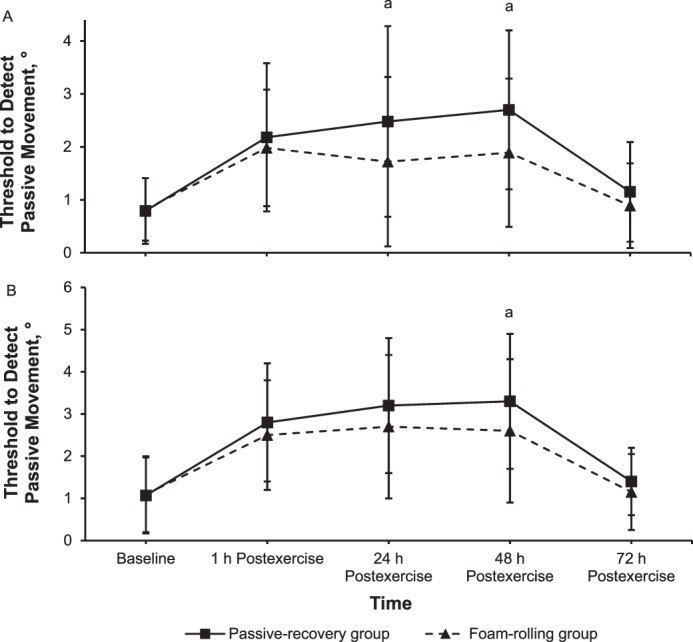
Threshold to detect passive movement (mean ± SD) at A, 30° and B, 70° in degrees at baseline of knee flexion and immediately (1 h) to 72 h after eccentric exercise for the foam-rolling and passive-recovery groups. a Difference between groups (P < .05).
For force sense during knee flexion, main effects of time (F1,78 = 111.9, P = .001, ηp2 = 0.59; F1,78 = 65.2, P = .001, ηp2 = 0.46; and F1,78 = 137.9, P = .001, ηp2 = 0.64) and group (F1,78 = 29.9, P = .001, ηp2 = 0.28; F1,78 = 12.1, P = .001, ηp2 = 0.13; and F1,78 = 26.7, P = .001, ηp2 = 0.27) and time × group interactions (F1,78 = 3.4, P = .01, ηp2 = 0.04; F1,78 = 2.9, P = .03, ηp2 = 0.03; and F1,78 = 4.9, P = .004, ηp2 = 0.06) were found at 10%, 30%, and 50% MVIC, respectively. Follow-up comparisons showed that the absolute error of the force match was smaller in the FR than in the PR group at 24 hours (159% versus 213%; d = 0.94, P = .001; 176% versus 213%; d = 0.59, P = .02; and 221% versus 279%; d = 0.58, P = .02) and 48 hours (205% versus 254%; d = 0.72, P = .01; 178% versus 220%; d = 0.72, P = .01; and 232% versus 336%; d = 1.02, P = .001) after eccentric exercise for 10%, 30%, and 50% of MVIC, respectively (Figures 6A through 6C, respectively).
Figure 6.
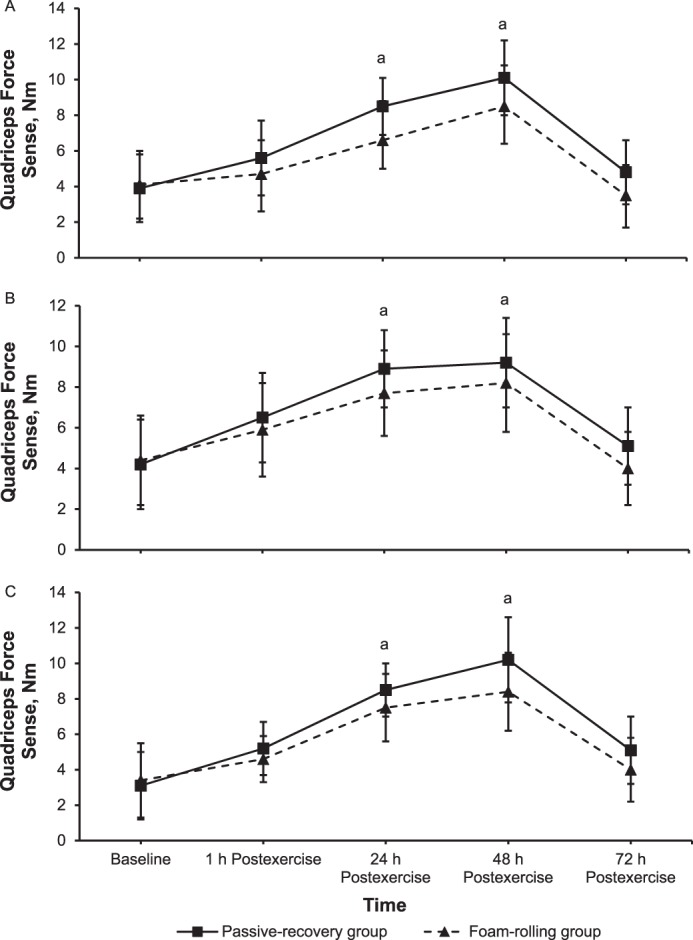
Quadriceps force sense (mean ± SD) at A, 10%; B, 30%; and C, 50% of the maximal voluntary isometric contraction in absolute values of torque during knee flexion at baseline and immediately (1 h) to 72 h after eccentric exercise for the foam-rolling and passive-recovery groups. a Difference between groups (P < .05).
DISCUSSION
The main observation of our study was that FR attenuated muscle soreness and facilitated the recovery from loss of proprioception and muscle strength induced by muscle-damaging exercise.
Muscle Soreness
Consistent with previous researchers,21,22 we found that FR reduced DOMS at 24 and 48 hours postexercise, even in the absence of an immediate effect. Therefore, FR can help in the recovery from or attenuate the development of DOMS. The faster recovery from DOMS with FR may be related to a central modulation of pain perception.20,21 A further contribution of FR could be a suppression of the Hoffmann reflex23 that may underlie the increased pressure-pain threshold. Other physiological (eg, increased circulation and skin temperature and decreased interstitial inflammatory mediators),8,29 mechanical (eg, decreased stiffness of the fascia),7,8 or metabolic (eg, decreased edema, enhanced tissue healing, and enhanced blood lactate removal)8 factors may also contribute.
Joint and Muscle Proprioception
During voluntary movements, limb position and movement are mainly sensed by muscle spindles, with some contributions from joint and skin-stretch receptors.15,16 We observed that proprioception was reduced immediately after eccentric exercise and had returned to pre-exercise levels only after 72 hours. Part of the apparent reduction in proprioception may be related to pain that diverts attention from proprioception. However, pain cannot be the sole explanation for reduced sensorimotor control after eccentric exercise, as muscle soreness and tenderness peaked at 48 hours and errors in perceived joint angles peaked at 24 hours postexercise. In addition, pain control is not sufficient to restore proprioception30 or postural control31 unless the pain site has a critical proprioceptive role in the joint involved in the task.30 Although muscle-receptor dysfunction may play a role, this is unlikely, as researchers32 showed that JPS was also disturbed after concentric exercise, when no disruption of muscle spindles is expected. After eccentric exercise, the increased muscle stiffness may alter the responses of proprioceptive receptors such as muscle spindles and, thus, position sense at the limb.17
We found that FR effectively restored muscle and joint position sensing after eccentric exercise. A possible mechanism is that FR promotes a faster return to more normal muscle-fiber alignment and, hence, facilitates recovery of muscle function.1 In addition, the fascia that play a functional part in stability and locomotion24 are populated with 3 groups of mechanoreceptors: type Ib Golgi tendon organs, type II Pacini corpuscles and Ruffini endings, and type III and IV interstitial myofascial tissue receptors.24 The most abundant of all intrafascial mechanoreceptors, type III and IV sensory nerves, are responsive to the mechanical pressure delivered by FR and may change neural excitability, thereby reducing muscle tension and pain.24
Muscle Strength
A decrease in muscle strength at 24 and 48 hours after unaccustomed eccentric exercise, which we observed, is considered the most reliable indicator of muscle damage.2 Reductions in muscle strength after unaccustomed eccentric exercise may be due to a combination of factors, including (1) damage to sarcomeres, such as Z-line tearing and myofibrillar damage, especially of type II muscle fibers3,6; (2) acute fatigue6; (3) decreased joint range of motion due to increased muscle stiffness; (4) inflammation6; and (5) fear caused by pain during movement.33 Whereas damage is intuitively an important factor, the decrease in force was most pronounced at 24 and 48 hours rather than immediately after eccentric exercise. The loss and recovery of force after the eccentric exercise session followed a similar time course as muscle soreness. Allen6 suggested that pain-induced impaired muscle activation, rather than muscle damage, caused the loss of force postexercise.
As discussed, FR likely acts by reducing neural inhibition11 due to accelerated recovery of the connective tissue, which results from decreased inflammation and reduced pain8,29 and decreased nociceptor activation,8 thus permitting better communication with afferent receptors in the connective tissue.11 Better communication with afferent receptors may allow natural muscle sequencing, agonist-antagonist coordination, or activation-ratio and motor-unit synchronization and recruitment patterns11 to be preserved so that muscle strength is maintained. Foam rolling may also reduce muscle stiffness9 and thereby improve the capacity of the musculotendinous unit to store elastic energy that does not improve maximal muscle force10 but can improve performance.
LIMITATIONS
Any improvement in proprioception may be due to a learning effect. However, this bias was limited in our study, as the measures were repeated with the PR group. No participants had an injury or comorbidity, and it remains to be seen whether FR would have similar benefits in patients with such conditions.
CONCLUSIONS
Foam rolling resulted in a quicker recovery from muscle soreness induced by eccentric exercise, loss of muscle strength, and loss of proprioception than PR. Foam rolling is a simple and inexpensive intervention that can be easily applied in the field and clinical settings to address DOMS.
ACKNOWLEDGMENTS
We thank the participants for their time and effort.
REFERENCES
- 1.Stupka N, Tarnopolsky MA, Yardley N, Phillips SM. Cellular adaptation to repeated eccentric exercise-induced muscle damage. J Appl Physiol (1985) 2001;91(4):1669–1678. doi: 10.1152/jappl.2001.91.4.1669. [DOI] [PubMed] [Google Scholar]
- 2.Clarkson PM, Hubal MJ. Exercise-induced muscle damage in humans. Am J Phys Med Rehabil. 2002;81(suppl 11):S52–S69. doi: 10.1097/00002060-200211001-00007. [DOI] [PubMed] [Google Scholar]
- 3.Cheung K, Hume PA, Maxwell L. Delayed onset muscle soreness: treatment strategies and performance factors. Sports Med. 2003;33(2):145–164. doi: 10.2165/00007256-200333020-00005. [DOI] [PubMed] [Google Scholar]
- 4.Burnett D, Smith K, Smeltzer C, Young K, Burns S. Perceived muscle soreness in recreational female runners. Int J Exerc Sci. 2010;3(3):108–116. doi: 10.70252/CDGZ8195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vila-Chã C, Riis S, Lund D, Moller A, Farina D, Falla D. Effect of unaccustomed eccentric exercise on proprioception of the knee in weight and non-weight bearing tasks. J Electromyogr Kinesiol. 2011;21(1):141–147. doi: 10.1016/j.jelekin.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 6.Allen DG. Eccentric muscle damage: mechanisms of early reduction of force. Acta Physiol Scand. 2001;171(3):311–319. doi: 10.1046/j.1365-201x.2001.00833.x. [DOI] [PubMed] [Google Scholar]
- 7.Kelly S, Beardsley C. Specific and cross-over effects of foam rolling on ankle dorsiflexion range of motion. Int J Sports Phys Ther. 2016;11(4):544–551. [PMC free article] [PubMed] [Google Scholar]
- 8.Macgregor LJ, Fairweather MM, Bennett RM, Hunter AM. The effect of foam rolling for three consecutive days on muscular efficiency and range of motion. Sports Med. 2018;4(1):26. doi: 10.1186/s40798-018-0141-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wilke J, Niemeyer P, Niederer D, Schleip R, Banzer W. Influence of foam rolling velocity on knee range of motion and tissue stiffness: a randomized, controlled crossover trial. 2019] doi: 10.1123/jsr.2018-0041. [published online ahead of print January 30. J Sport Rehabil. doi: [DOI] [PubMed]
- 10.Shin MS, Sung YH. Effects of massage on muscular strength and proprioception after exercise-induced muscle damage. J Strength Cond Res. 2015;29(8):2255–2260. doi: 10.1519/JSC.0000000000000688. [DOI] [PubMed] [Google Scholar]
- 11.Saxton JM, Clarkson PM, James R, et al. Neuromuscular dysfunction following eccentric exercise. Med Sci Sports Exerc. 1995;27(8):1185–1193. [PubMed] [Google Scholar]
- 12.Docherty CL, Arnold BL. Force sense deficits in functionally unstable ankles. J Orthop Res. 2008;26(11):1489–1493. doi: 10.1002/jor.20682. [DOI] [PubMed] [Google Scholar]
- 13.Brockett C, Warren N, Gregory J, Morgan DL, Proske U. A comparison of the effects of concentric versus eccentric exercise on force and position sense at the human elbow joint. Brain Res. 1997;771(2):251–258. doi: 10.1016/s0006-8993(97)00808-1. [DOI] [PubMed] [Google Scholar]
- 14.Sanchís-Gomar F, Pareja-Galeano H, Pérez-Quilis C, et al. Effects of allopurinol on exercise-induced muscle damage: new therapeutic approaches? Cell Stress Chaperones. 2015;20(1):3–13. doi: 10.1007/s12192-014-0543-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Proske U, Gandevia SC. The proprioceptive senses: their roles in signaling body shape, body position and movement, and muscle force. Physiol Rev. 2012;92(4):1651–1697. doi: 10.1152/physrev.00048.2011. [DOI] [PubMed] [Google Scholar]
- 16.Allen TJ, Proske U. Effect of muscle fatigue on the sense of limb position and movement. Exp Brain Res. 2006;170(1):30–38. doi: 10.1007/s00221-005-0174-z. [DOI] [PubMed] [Google Scholar]
- 17.Gregory JE, Morgan DL, Proske U. Aftereffects in the responses of cat muscle spindles and errors of limb position sense in man. J Neurophysiol. 1988;59(4):1220–1230. doi: 10.1152/jn.1988.59.4.1220. [DOI] [PubMed] [Google Scholar]
- 18.Riemann BL, Lephart SM. The sensorimotor system, part II: the role of proprioception in motor control and functional joint stability. J Athl Train. 2002;37(1):80–84. [PMC free article] [PubMed] [Google Scholar]
- 19.Rey E, Padrón-Cabo A, Barcala-Furelos R, Casamichana D, Romo-Perez V. Practical active and passive recovery strategies for soccer players. Strength Cond J. 2018;40(3):45–57. [Google Scholar]
- 20.Aboodarda SJ, Spence AJ, Button DC. Pain pressure threshold of a muscle tender spot increases following local and non-local rolling massage. BMC Musculoskelet Disord. 2015;16(1):265. doi: 10.1186/s12891-015-0729-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.MacDonald GZ, Button DC, Drinkwater EJ, Behm DG. Foam rolling as a recovery tool after an intense bout of physical activity. Med Sci Sports Exerc. 2014;46(1):131–142. doi: 10.1249/MSS.0b013e3182a123db. [DOI] [PubMed] [Google Scholar]
- 22.Pearcey GE, Bradbury-Squires DJ, Kawamoto JE, Drinkwater EJ, Behm DG, Button DC. Foam rolling for delayed-onset muscle soreness and recovery of dynamic performance measures. J Athl Train. 2015;50(1):5–13. doi: 10.4085/1062-6050-50.1.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Young JD, Spence AJ, Behm DG. Roller massage decreases spinal excitability to the soleus. J Appl Physiol (1985) 2018;124(4):950–959. doi: 10.1152/japplphysiol.00732.2017. [DOI] [PubMed] [Google Scholar]
- 24.Schleip R. Fascial plasticity–a new neurobiological explanation: part 1. J Bodyw Mov Ther. 2003;7(1):11–19. [Google Scholar]
- 25.Thomas S, Reading J, Shephard RJ. Revision of the Physical Activity Readiness Questionnaire (PAR-Q) Can J Sport Sci. 1992;17(4):338–345. [PubMed] [Google Scholar]
- 26.Faul F, Erdfelder E, Buchner A, Lang AG. Statistical power analyses using G*Power 3.1: tests for correlation and regression analyses. Behav Res Methods. 2009;41(4):1149–1160. doi: 10.3758/BRM.41.4.1149. [DOI] [PubMed] [Google Scholar]
- 27.Luna NMS, Alonso AC, Brech GC, Mochizuki L, Nakano EY, Greve JM. Isokinetic analysis of ankle and ground reaction forces in runners and triathletes. Clinics (Sao Paulo) 2012;67(9):1023–1028. doi: 10.6061/clinics/2012(09)07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cohen J. Statistical Power Analysis for the Behavioral Sciences 2nd ed. Hillsdale, NJ: Lawrence Erlbaum Associates; 1988. [Google Scholar]
- 29.Mense S. Neurobiological concepts of fibromyalgia—the possible role of descending spinal tracts. Scand J Rheumatol Suppl. 2000;113:24–29. doi: 10.1080/030097400446599. [DOI] [PubMed] [Google Scholar]
- 30.Weerakkody NS, Blouin JS, Taylor JV, Gandevia SC. Local subcutaneous and muscle pain impairs detection of passive movements at the human thumb. J Physiol. 2008;586(13):3183–3193. doi: 10.1113/jphysiol.2008.152942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hirata RP, Arendt-Nielsen L, Graven-Nielsen T. Experimental calf muscle pain attenuates the postural stability during quiet stance and perturbation. Clin Biomech (Bristol, Avon) 2010;25(9):931–937. doi: 10.1016/j.clinbiomech.2010.06.001. [DOI] [PubMed] [Google Scholar]
- 32.Allen TJ, Leung M, Proske U. The effect of fatigue from exercise on human limb position sense. J Physiol. 2010;588(8):1369–1377. doi: 10.1113/jphysiol.2010.187732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.George SZ, Dover GC, Fillingim RB. Fear of pain influences outcomes after exercise-induced delayed onset muscle soreness at the shoulder. Clin J Pain. 2007;23(1):76–84. doi: 10.1097/01.ajp.0000210949.19429.34. [DOI] [PubMed] [Google Scholar]