ABSTRACT
Ferroptosis is a novel form of programmed cell death. We found that the ferroptosis sensitivity in renal and ovarian cancers are regulated by cell density through TAZ-EMP1-NOX4 and TAZ-ANGPTL4-NOX2 pathway, respectively. These findings reveal TAZ as a novel genetic determinant of ferroptosis. Triggering ferroptosis may have therapeutic potential for TAZ-activated tumors.
KEYWORDS: Ferroptosis, Hippo pathway, transcriptional coactivator with PDZ-binding motif (TAZ), Yes-associated protein 1 (YAP1), NADPH Oxidase 4 (NOX4), Renal Cell Carcinoma, Ovarian Cancer, Erastin, Epithelial Membrane Protein 1 (EMP1), Angiopoietin-Like 4 (ANGPTL4), NADPH Oxidase 2 (NOX2)
Ferroptosis is a recently defined form of programmed cell death1 characterized by the accumulation of lipid peroxidation. The relevance of ferroptosis for various human diseases is now beginning to be appreciated.2 The lipid peroxidation results from oxidative stresses generated by NADPH oxidase(s) (NOXs) that is often repaired by glutathione peroxidase 4 (GPX4) using the glutathione as a co-factor. Therefore, ferroptosis can be induced by the removal of cystine (limiting component for glutathione synthesis), inhibition of GPX4, or activation of NOXs. The canonical ferroptosis inducer, erastin, is an inhibitor of cystine-glutamate transporter (xCT) that reduces cystine import and depletes glutathione.1 While ferroptosis may have therapeutic potential toward cancer,3 much remains unknown about the genetic determinants and underlying mechanisms of ferroptosis to select tumors which are most likely to respond to these ferroptosis-inducing agents and predict potential resistant mechanisms against such approach.
Many studies have identified various genetic determinants of ferroptosis involved in the GSH/lipid metabolisms,4 oncogenic somatic mutations, regulation of iron levels5 and process of epithelial–mesenchymal transitions.6 In tumor microenvironment, there are also many non-genetic factors that may impact tumor progression, metastasis, and treatment response. These factors include physical, chemical and mechanic stresses, such as tissue hypoxia, lactic acidosis, nutrient deprivation, osmotic pressure, tissue tension, and stiffness. Unlike cell-intrinsic genetic factors, it is not clear whether these non-genetic factors also affect ferroptosis. Recently, we and other independent research groups have observed that vulnerability to ferroptosis is highly influenced by a non-genetic factor, cell density.7–9 While the renal cell carcinoma and ovarian tumor cells are highly sensitive to ferroptosis when grown at low density; they become highly resistant to ferroptosis when grown in confluent conditions. Since the cell density-dependent phenotypes can be sensed and regulated by the evolutionarily conserved Hippo pathway effectors, YAP (Yes-associated protein 1)/TAZ (transcriptional coactivator with PDZ-binding motif), we wondered if Hippo pathway effectors are involved in such density-dependent ferroptosis response. The activities and functions of YAP/TAZ are regulated by the state of phosphorylation and intracellular localization. That is, in high cell density, YAP/TAZ are phosphorylated, restricted in the cytosol, and subjected to proteasome-mediated degradation. On the other hand, YAP/TAZ are dephosphorylated and localized in the nucleus to associate with transcriptional factors, such as TEAD (transcriptional-enhanced associate domain) family proteins, to drive expressions of proliferation and metastasis genes.
Our data show that TAZ, instead of YAP, is abundantly expressed in both renal and ovarian cancer cells and undergoes density-dependent nuclear/cytosolic translocation.7,9 TAZ removal confers ferroptosis resistance, while overexpression of constitutively active form of TAZ, TAZS89A, sensitizes cells to ferroptosis. We have found that ovarian cancers including both clear cell and serous subtypes are sensitive to ferroptosis induced by cystine deprivation. In addition, we found that lower TAZ level in the recurrent ovarian cancer is responsible for reduced ferroptosis susceptibility. We further investigated the mechanisms and found that TAZ regulates ferroptosis through an epithelial membrane protein 1 (EMP1)- NADPH oxidase 4 (NOX4) axis in renal cancers7 and Angiopoietin-like 4 (ANGPTL4)- NADPH oxidase 2 (NOX2) axis in ovarian cancers.9 An independent study also identified that YAP as a novel regulator of ferroptosis sensitivity in mesothelioma.8 Collectively, these three studies have shown the relevance of Hippo pathway effectors for ferroptosis and suggest that ferroptosis-inducing agents may be used to target the YAP/TAZ-activated tumors.
Our studies7,9 have provided novel insights into ferroptosis, a novel form of cell death, firstly reporting on the link between the Hippo pathway effector TAZ and ferroptosis sensitivities in renal and ovarian cancers. These studies have several implications for the fields of ferroptosis and cancer biology. First, our results indicate a model (Figure 1) in which outside environmental cues in the tumor microenvironments are sensed by the Hippo pathway and its effectors, resulting in the translocation of YAP or TAZ to associate with TEAD transcriptional factor and regulate the transcriptional outputs. The YAP/TAZ target genes encode proteins involved in the signaling relay and amplification to affect lipid/iron metabolism (ACSL4: acyl-CoA synthetase long-chain family member 4/TFRC: transferrin receptor) or reactive oxygen species (ROS) productions (NOX2/NOX4) as part of the adaptive responses to changes in tumor microenvironments. These Hippo-driven changes affects the life vs. death decisions when the cancer cells are placed under ferroptosis-inducing conditions, such as erastin or cystine deprivation. Second, while Hippo pathway effectors, YAP/TAZ, regulate ferroptosis in multiple biological contexts, the specific effectors, transcriptional targets and ferroptosis executors may be different in each cell types. For example, NOXs are involved in the TAZ-regulated ferroptosis in both renal cell carcinoma and ovarian cancers. However, different NOXs are involved in renal vs. ovarian cancer cells. NOX4 is the predominant NOX family protein in the renal cells and NOX2 is the highest-expressed transcripts in ovarian cancer patients. Third, since Hippo pathway effectors are involved in the proliferation and metastasis of many human tumors, our results suggest that the YAP/TAZ activation status may predict the sensitivity to ferroptosis-inducing agents. Furthermore, the Hippo pathway integrates a wide variety of non-genetic factors, such as mechanical properties and tissue stiffness.10 Therefore, our findings may suggest many non-genetic factors may also regulate ferroptosis sensitivities in the setting of “stiff” tumor environment known to activate the YAP/TAZ and promote ferroptosis. Finally, since the higher activity of YAP/TAZ promotes invasion and metastases and often associate with poor prognosis, our findings suggest that triggering ferroptosis may be valuable in a combinational therapy for these tumors which tend to become resistant to standard treatments. Taken together, including ferroptosis in the current cancer therapeutics may improve the response rate and clinical outcomes of patients, especially with YAP/TAZ-activated tumors.
Figure 1.
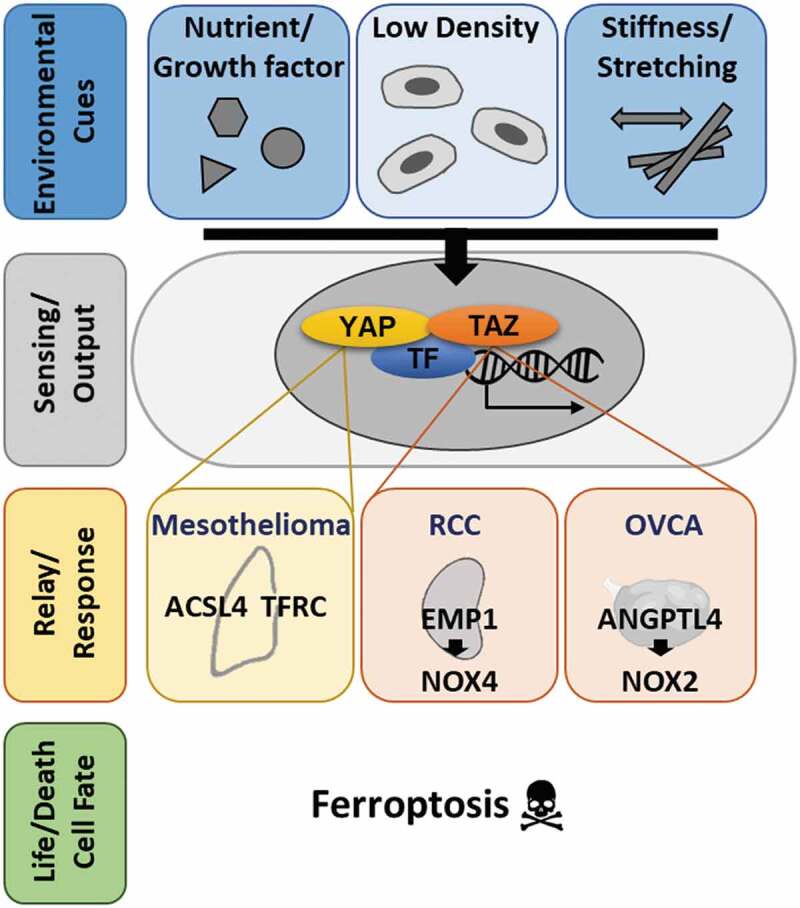
Hippo pathway effectors, YAP/TAZ, integrates various environmental cues and regulates ferroptosis through affecting the expression of different tissue-specific target genes. In response to various non-genetic environmental cues, Hippo pathway effectors, YAP/TAZ, regulates ferroptosis by affecting the expression of different sets of tissue-specific targets genes encoding lipid/iron metabolism and ROS production by NOXs (YAP1: Yes-associated protein 1; TAZ: transcriptional coactivator with PDZ-binding motif; TF: transcriptional factor(s); RCC: renal cell carcinoma; OVCA: ovarian cancer; ROS: reactive oxygen species; NOX: NADPH Oxidase; ACSL4: encoding acyl-CoA synthetase long-chain family member 4; TFRC: encoding transferrin receptor; EMP1: encoding epithelial membrane protein 1; ANGPTL4: encoding angiopoietin-Like 4).
Funding Statement
This work was supported by the Foundation for the National Institutes of Health [1R01GM124062]; Foundation for the National Institutes of Health [1R01NS111588]; U.S. Department of Defense [W81XWH-19-1-0842]; U.S. Department of Defense [W81XWH-15-1-0486]; U.S. Department of Defense [W81XWH-17-1-0143].
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
References
- 1.Dixon SJ, Lemberg KM, Lamprecht MR, Skouta R, Zaitsev EM, Gleason CE, Patel DN, Bauer AJ, Cantley AM, Yang WS, et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 2012;149(5):1–3. doi: 10.1016/j.cell.2012.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stockwell BR, Friedmann Angeli JP, Bayir H, Bush AI, Conrad M, Dixon SJ, Fulda S, Gascon S, Hatzios SK, Kagan VE, et al. Ferroptosis: a regulated cell death nexus linking metabolism, redox biology, and disease. Cell. 2017;171(2):273–285. doi: 10.1016/j.cell.2017.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cramer SL, Saha A, Liu J, Tadi S, Tiziani S, Yan W, Triplett K, Lamb C, Alters SE, Rowlinson S, et al. Systemic depletion of L-cyst(e)ine with cyst(e)inase increases reactive oxygen species and suppresses tumor growth. Nat Med. 2017;23(1):120–127. doi: 10.1038/nm.4232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xie Y, Hou W, Song X, Yu Y, Huang J, Sun X, Kang R, Tang D.. Ferroptosis: process and function. Cell Death Differ. 2016;23(3):369–379. doi: 10.1038/cdd.2015.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen PH, Wu J, Ding CC, Lin CC, Pan S, Bossa N, Xu Y, Yang WH, Mathey-Prevot B, Chi JT.. Kinome screen of ferroptosis reveals a novel role of ATM in regulating iron metabolism. Cell Death Differ. 2019. doi: 10.1038/s41418-019-0393-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Viswanathan VS, Ryan MJ, Dhruv HD, Gill S, Eichhoff OM, Seashore-Ludlow B, Kaffenberger SD, Eaton JK, Shimada K, Aguirre AJ, et al. Dependency of a therapy-resistant state of cancer cells on a lipid peroxidase pathway. Nature. 2017;547(7664):453–457. doi: 10.1038/nature23007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang WH, Ding CC, Sun T, Rupprecht G, Lin CC, Hsu D, Chi JT. The Hippo pathway effector TAZ regulates ferroptosis in renal cell carcinoma. Cell Rep. 2019;28(10):2501–2508.e4. doi: 10.1016/j.celrep.2019.07.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu J, Minikes AM, Gao M, Bian H, Li Y, Stockwell BR, Chen ZN, Jiang X. Intercellular interaction dictates cancer cell ferroptosis via NF2-YAP signalling. Nature. 2019. doi: 10.1038/s41586-019-1426-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang WH, Huang Z, Wu J, Ding CC, Murphy SK, Chi JT. A TAZ–ANGPTL4–NOX2 Axis regulates ferroptotic cell death and chemoresistance in epithelial ovarian cancer. Mol Cancer Res. 2019. doi: 10.1158/1541-7786.MCR-19-0691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zanconato F, Cordenonsi M, Piccolo S. YAP/TAZ at the roots of cancer. Cancer Cell. 2016;29(6):783–803. doi: 10.1016/j.ccell.2016.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]


