Abstract
IL-6 is a pro-inflammatory cytokine upregulated in some autoimmune diseases. The role of IL-6 in the development of type 1 diabetes (T1D) is unclear. Clinical studies are investigating whether tocilizumab (anti-IL-6 receptor) can help preserve beta cell function in patients recently diagnosed with T1D. However, in some rodent models and isolated human islets, IL-6 has been found to have a protective role for beta cells by reducing oxidative stress. Hence, we systematically investigated local tissue expression of IL-6 in human pancreata from nondiabetic, auto-antibody positive donors and donors with T1D and T2D. IL-6 was constitutively expressed by beta and alpha cells regardless of the disease state. However, expression of IL-6 was highly reduced in insulin-deficient islets of donors with T1D, and the expression was then mostly restricted to alpha cells. Our findings suggest that the implication of IL-6 in T1D pathogenesis might be more complex than previously assumed.
Introduction
The cytokine interleukin-6 (IL-6) is mostly known as a pro-inflammatory molecule associated with both immunity and autoimmunity [1]. IL-6 primarily signals through two modes based on whether it binds to soluble or membrane bound IL-6 receptor (IL-6R). Antiinflammatory effects of IL-6 are attributed to classical signaling (through membrane bound IL-6R and gp130), whereas pro-inflammatory effects are induced by trans-signaling (through soluble IL-6R and gp130) [2]. The pathological effects of IL-6 in autoimmunity are often associated with phosphorylation of STAT3 [3]. Signaling via this pathway is essential for T helper 17 (Th17) cell differentiation and inhibition of regulatory T (Treg) cell development [4].
Mice deficient in IL-6 are protected from autoimmune diseases such as experimental autoimmune encephalomyelitis [5]. Elevated IL-6 serum/tissue concentrations are a feature of autoimmune diseases such as rheumatoid arthritis, systemic lupus erythematosus and multiple sclerosis, with the concentration of IL-6 often correlating with disease severity [6–8]. It is therefore thought that IL-6 may drive autoimmunity and could be a uniquely attractive therapeutic target. In humans, rheumatoid arthritis, juvenile idiopathic arthritis and Castleman’s disease have all been successfully treated with tocilizumab, an antibody that targets the IL-6R, demonstrating the value of targeting the IL-6/IL-6R pathway in humans [9].
The role for IL-6 in the development of type 1 diabetes (T1D) is unclear. Evidence for an association between IL-6 and T1D came originally from the non-obese diabetic/Wehi mouse model which showed a significantly reduced incidence of diabetes following IL-6 inhibition [10] and the RIP-LCMV model which showed induction of diabetes following beta-cell specific production of IL-6 [11]. Overexpression of IL-6 in mouse beta cells (RIP-IL-6) has led to an increased infiltration of B cells and other immune cells in islets, however this infiltration was not sufficient to precipitate T1D [12]. More recent studies in humans with diabetes appear to have confirmed this link: increased IL-6 signaling pathway and IL-6R expression was found in monocytes from subjects with T1D [13, 14], increased numbers of Th17 cells were present in subjects with new-onset T1D [15], and there is an association between T1D and a genetic variant in the IL-6R gene [16]. In contrast, IL-6 has been shown to have various functions in metabolic regulation such as induction of GLP-1 secretion and expansion in alpha cells [17, 18], regulation of glucose homeostasis [19] and exerciseinduced loss of visceral fat [20]. In addition, IL-6 has been shown to be essential in exercisemediated protection of beta cells from cytokine induced death [21]. There are contradicting evidences about the role of IL-6 in insulin sensitivity: acute treatment with recombinant IL-6 has been shown to improve insulin-mediated glucose disposal in humans [22], while blocking the IL-6 pathway using Tocilizumab has improved insulin sensitivity in patients with rheumatoid arthritis [23]. More recently, it was shown that IL-6 exerts a protective role in beta cells by linking autophagy to anti-oxidant responses [24]. The EXTEND trial (Preserving Beta-Cell Function with Tocilizumab in New Onset Type 1 Diabetes) is currently investigating whether tocilizumab (anti-IL-6R) can slow disease progression and help maintain natural insulin production in adults with new-onset T1D [25].
One of the limitations of previous studies investigating the link between human T1D and IL-6 was that peripheral blood or isolated islets were studied, whereas the impact of the disease is at the islets and pancreatic lymph nodes. We therefore aimed to systematically investigate local tissue expression of IL-6 within human pancreata to assess whether IL-6 may play a role in the pathogenesis of T1D.
Research Design and Methods
Patients
Pancreatic sections from a total of 37 cadaveric donors were obtained through the nPOD (network of pancreatic organ donor) consortium. Age- and BMI-matched cases from donors without diabetes (n=3), auto-antibody positive donors (Aab+, n=3), donors with T1D (n=3) and donors with type 2 diabetes (T2D, n=3) were stained (Figure 1B). In another experiment, age- and BMI- matched pancreatic sections from donors without diabetes (n=8), Aab+ donors (n=11), donors with T1D (n=11) and T2D (n=5) were stained (Suppl. Fig 2). Both experiments had 10 cases in common. Pancreatic sections from live recent onset T1D (n=6) donors were obtained from the DiViD (Diabetes Virus Detection) study. Detailed demographic and clinical information of all the donors are presented in Table 1 and Table 2. Research conducted for this study was performed in accordance with approvals from the Institutional Review Board at the La Jolla Institute protocol #DI3-054-0315.
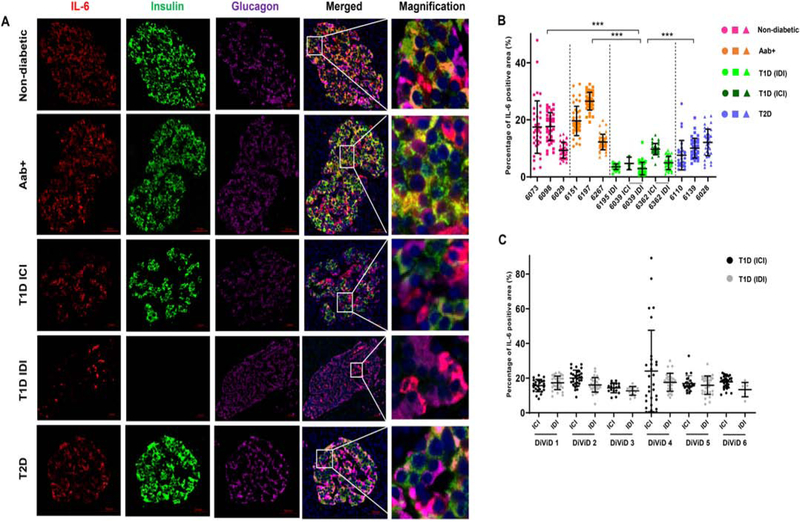
Figure 1. IL-6 is expressed on pancreatic beta cells and alpha cells and expression is reduced in insulin-deficient islets of donors with T1D.
(A) FFPE sections of human pancreata were stained with anti-IL-6 (red), anti-insulin (green), anti-glucagon (magenta) and Hoechst (blue). Representative images of islets from a non-diabetic donor (nPOD 6098), GAD auto-antibody positive (Aab+) donor (nPOD 6151), insulin-containing islet (ICI) of a T1D donor (nPOD 6362), insulin-deficient islet (IDI) of a T1D donor (nPOD 6195) and a donor with T2D (nPOD 6110) are shown. Images were acquired using Axioscan with a 20x objective. Scale bar – 50 μm. Systematic quantification of IL-6 staining was performed using Image Pro Premier software. (B) Percentages of the total islet area that stained positive for IL-6 in 3 non-diabetic (pink) donors, 3 auto-antibody positive (Aab+, orange) donors, insulin-containing islets (ICI, dark green) and insulin-deficient islets (IDI, light green) of 3 donors with T1D and 3 donors with T2D (blue). (C) Percentages of the total islet area positive for IL-6 staining in ICI (black) and IDI (gray) of recent onset T1D cases from the DiViD study. Graphs show mean ± SD of more than 30 islets per case. Each data point represents an islet. ***P<0.0001.
Table 1.
Demographic and Clinical Information of Pancreatic Organ Donors.
| nPOD Case Number | Diagnosis | Autoantibody Status | Age | Sex | Race | BMI | Diabetes Duration (years) | C-peptide | Figure used |
|---|---|---|---|---|---|---|---|---|---|
| 6029 | Non-diabetic | not tested | 24 | F | Hispanic/Latino | 22.6 | N/A | not known | 1B, S2 |
| 6073 | Non-diabetic | negative | 19.2 | M | Caucasian | 36 | N/A | 0.69 | 1B, S2 |
| 6098 | Non-diabetic | negative | 17.8 | M | Caucasian | 22.8 | N/A | 1.41 | 1B, S2 |
| 6165 | Non-diabetic | negative | 45.8 | F | Caucasian | 25 | NA | 4.45 | S2 |
| 6251 | Non-diabetic | negative | 33 | F | Caucasian | 29.5 | NA | 1.92 | S2 |
| 6290 | Non-diabetic | negative | 58 | M | Caucasian | 22.5 | NA | 7.46 | S2 |
| 6295 | Non-diabetic | negative | 47 | F | African American | 30.4 | NA | 10.91 | S2 |
| 6101 | Aab+ | GADA | 64.8 | M | Caucasian | 34.3 | NA | 26.18 | S2 |
| 6123 | Aab+ | GADA | 23.2 | F | Caucasian | 17.6 | NA | 2.01 | S2 |
| 6147 | Aab+ | GADA | 23.8 | F | Caucasian | 32.9 | NA | 3.19 | S2 |
| 6151 | Aab+ | GADA | 30 | M | Caucasian | 24.2 | NA | 5.49 | 1B, S2 |
| 6171 | Aab+ | GADA | 4.4 | F | Caucasian | 14.8 | NA | 8.95 | S2 |
| 6184 | Aab+ | GADA | 47.6 | F | Hispanic/Latino | 27 | NA | 3.42 | S2 |
| 6301 | Aab+ | GADA | 26 | M | African American | 32.1 | NA | 3.92 | S2 |
| 6080 | Aab+ | GADA, mIAA | 69.2 | F | Caucasian | 21.3 | NA | 1.84 | S2 |
| 6158 | Aab+ | GADA, mIAA | 40.3 | M | Caucasian | 29.7 | NA | 0.51 | S2 |
| 6167 | Aab+ | IA2A, ZnT8A | 37 | M | Caucasian | 26.3 | NA | 5.43 | S2 |
| 6197 | Aab+ | GADA, IA2A | 22 | M | African American | 28.2 | NA | 17.48 | 1B |
| 6267 | Aab+ | GADA, IA2A | 23 | F | Caucasian | 23.5 | NA | 16.59 | 1B, S2 |
| 6038 | T1D | negative | 37.2 | F | Caucasian | 30.9 | 20 | 0.2 | S2 |
| 6039 | T1D | GADA, IA2A, mIAA, ZnT8A | 28.7 | F | Caucasian | 23.4 | 12 | <0.05 | 1B, S2 |
| 6040 | T1D | mIAA | 50 | F | Caucasian | 31.6 | 20 | <0.05 | S2 |
| 6069 | T1D | not tested | 22.9 | M | African American | 28.8 | 7 | not known | S2 |
| 6076 | T1D | GADA, mIAA | 25.8 | M | Caucasian | 18.8 | 15 | <0.05 | S2 |
| 6081 | T1D | negative | 31.4 | M | Hispanic/Latino | 28 | 15 | 0.24 | S2 |
| 6084 | T1D | mIAA | 14.2 | M | Caucasian | 26.3 | 4 | <0.05 | S2 |
| 6173 | T1D | negative | 44.1 | M | Caucasian | 23.9 | 15 | <0.05 | S2 |
| 6195 | T1D | GADA, IA2A, mIAA, ZnT8A | 19.3 | M | Caucasian | 23.7 | 5 | <0.05 | 1B, S2 |
| 6198 | T1D | GADA, IA2A, mIAA, ZnT8A | 22 | F | Hispanic/Latino | 23.1 | 3 | <0.05 | S2 |
| 6212 | T1D | mIAA | 20 | M | Caucasian | 29.1 | 5 | <0.05 | S2 |
| 6247 | T1D | mIAA | 24 | M | Caucasian | 24.3 | 0.6 | 0.47 | S2 |
| 6362 | T1D | GADA | 25 | M | Caucasian | 28.5 | <1 | 0.38 | 1B |
| 6028 | T2D | negative | 33.2 | M | African Am | 30.2 | 17 | 22.4 | 1B, S2 |
| 6109 | T2D | mIAA | 48.8 | F | Hispanic/Latino | 32.5 | unknown | <0.05 | S2 |
| 6110 | T2D | negative | 20.7 | F | African American | 49 | unknown | 0.58 | 1B, S2 |
| 6139 | T2D | negative | 37.2 | F | Hispanic/Latino | 45.4 | 1.5 | 0.6 | 1B, S2 |
| 6149 | T2D | GADA | 39.3 | F | African American | 29.1 | 16 | 11.55 | S2 |
Aab, autoantibody; F, female; GADA, GAD autoantibody; IA-2A, Insulinoma-2-associated autoantibody; mIAA, microinsulin autoantibody; M, male; ZnT8A, Zinc transporter-8 autoantibodies; NA, not applicable; T1D, type 1 diabetes; T2D, type 2 diabetes.
Table 2.
Demographic and clinical information of recent onset T1D donors from the DiViD study.
| Case number | Diagnosis | Autoantibody status | Age | Sex | Race | BMI | Diabetes duration (weeks) | C-Peptide |
|---|---|---|---|---|---|---|---|---|
| Divid 1 | T1D | GADA, IA2A, mIAA, ZnT8A | 25 | F | Caucasian | 21 | 4 | 0.46 |
| Divid 2 | T1D | GADA, IA2A, ZnT8A | 24 | M | Caucasian | 20.9 | 3 | 0.35 |
| Divid 3 | T1D | GADA, IA2A, ZnT8A | 34 | F | Caucasian | 23.7 | 9 | 0.74 |
| Divid 4 | T1D | GADA, IA2A, mIAA | 31 | M | Caucasian | 25.6 | 5 | unknown |
| Divid 5 | T1D | GADA, IA2A, mIAA | 24 | F | Caucasian | 28.6 | 5 | unknown |
| Divid 6 | T1D | GADA, mIAA | 35 | M | Caucasian | 26.7 | 5 | 0.24 |
F, female; GADA, GAD autoantibody; IA2A, Insulinoma-2-associated autoantibody; mIAA, microinsulin autoantibody; M, male; ZnT8A, Zinc transporter-8 autoantibodies; T1D, type 1 diabetes
Tissue Samples
Formalin fixed paraffin embedded (FFPE) tissue sections of 6 μm thickness from the tail region of the pancreas were obtained from nPOD or DiViD. In each experiment, an optimization slide from an nPOD donor without diabetes was stained for IL-6 (positive control) or with only secondary antibody (negative control). FFPE sections of human tonsils were used for optimizing staining conditions.
Validation of the anti-IL-6 antibody
Human umbilical vein endothelial cells (HUVEC) were obtained as a gift from the Sharma laboratory at the La Jolla Institute for Immunology. Cells were cultured in EBM-2 media (Lonza) supplemented with EGM-2 Single quots (Lonza). Cells were treated with either LPS derived from E. Coli (100 ng/ml) or vehicle control (DMSO) for 24 hours. Brefeldin A (5 μg/ml) was added during the last 4 hours of culture. Cell-free supernatants were analyzed by human IL-6 ELISA kit (R&D systems). Cells were cytospun on poly-lysine slides and fixed with ice cold acetone for 10 minutes. Slides were then stained with rabbit polyclonal anti-IL-6 (Proteintech or Abcam) followed by goat anti-rabbit AF555 (Invitrogen).
Immunofluorescence staining
Pancreatic sections (Figure 1B) were stained for insulin, glucagon and IL-6 (Proteintech). Alternatively, pancreatic sections (Figure 1C and Suppl. Fig. 2) were stained for insulin and IL-6 (Abcam). After deparaffinization and rehydration, slides were unmasked by boiling them in citrate buffer of pH 6 at 95°C for 20 min. After blocking with Avidin-Biotin block (Vector Laboratories), slides were incubated with rabbit polyclonal anti-human IL-6 (Proteintech or Abcam) overnight at 4°C. Detection was performed using goat anti-rabbit Biotin (Vector Laboratories) and Streptavidin-AF647 (Molecular Probes). Thereafter, slides were incubated with mouse anti-insulin-AF488 (eBioscience) and mouse anti-Glucagon (Abcam) conjugated in-house with AF555 (Invitrogen) for 1 hour. After counterstaining with Hoechst (Molecular Probes), slides were mounted using Prolong Gold antifade mountant (Life Technologies). A list of antibodies and dilutions used can be found in Suppl. Table 1.
Image acquisition and quantitative analysis
A whole tissue scan was performed using Axio Scan Z.1 (Zeiss) and images were acquired using Zen 2 (Zeiss). Islets were randomly cropped across the whole section using Qupath (GitHub Inc) [26]. Quantitative analysis was performed using Imagepro Premier 9.1 (Media Cybernetics Inc). For each islet, negative areas were defined by the mean intensity of IL-6 staining in nuclear regions (MIN). Positive threshold for IL-6 was set above MIN+3SD. The percentage of positive area (PPA) of IL-6 was calculated as a measure of IL-6 positive area divided by the islet area. For each islet, the proportions of the IL-6 positive area that overlapped with insulin or glucagon staining were calculated. Likewise, the proportions of beta cell area and alpha cell area that stained positive for IL-6 were calculated. Statistical analyses were performed using Prism 7 software (Graphpad). Kruskal-Wallis or Mann-Whitney test were used to calculate P values and Dunn’s multiple comparisons test was used as a posthoc test, when appropriate. P < 0.05 was considered significant.
Culture of human islets
Human islets from a non-diabetic cadaveric donor were obtained through IIDP (Integrated Islet Distribution Program). Islets were handpicked and cultured in human islet maintenance medium (Insphero, CS-07-005-01) for atleast 24 hours in a 96-well U bottom plate at 37°C before assays
Glucose stimulated insulin secretion
Low and high glucose solutions were prepared in Krebs-Ringer buffer (Insphero, CS-07-051-01) with 0.5% BSA. Briefly, islets were rested in low (2.8 mM) glucose solution for 1 hr. Islets were then incubated with low (2.8 mM) glucose solution for 2 hours and then with high (16.6 mM) glucose solution for 2 hours. Supernatants were collected in both steps and analysed for insulin secretion using Human Insulin ELISA kit (80-INSHU-E01.1, ALPCO).
Induction of stress in islets
20 islets were seeded in each well of a 96-well plate. To induce metabolic stress, islets were cultured in media containing 25 mM glucose and 0.5 mM Palmitic acid. To induce proinflammatory immune stress, islets were cultured in media containing TNF (10 ng/ml), IL-1β (2 ng/ml) and IFN-γ (10 ng/ml). After 2.5 days, supernatants were collected and tested for IL-6 secretion using human IL-6 Duoset ELISA (DY206, R&D systems).
Staining of islet cultures
Islets were fixed with 4% Paraformaldehyde (PFA) in PBS for 2 h at 4°C, and then permeabilized with 0.5% Triton X in goat serum dilution buffer (containing 30% goat serum) for 2 h at room temperature with shaking. Islets were blocked with human FcR block (BD biosciences) at 1:15 dilution for 10 min at room temperature. Islets were them incubated with primary antibodies (rabbit anti-human IL-6 and mouse anti-Glucagon overnight at 4°C), followed by incubation with goat anti-rabbit-AF647 and goat mouse-AF555 for 2 h at room temperature, followed by staining with directly conjugated anti-Insulin-AF488 and Hoechst. After staining, islets were picked and mounted on coverslips along with Prolong Gold antifade mounting media. Images were acquired at high resolution using confocal LSM-780.
Results
IL-6 is expressed on beta cells and alpha cells of human pancreata
We first validated our anti-IL-6 antibodies (Proteintech or Abcam) using HUVEC cells (Suppl. Fig. 1). We then systematically investigated the localization of IL-6 in human pancreata. Expression of IL-6 was mostly localized in the endocrine compartment, overlapping with both insulin and glucagon staining (Fig. 1A). We performed a systematic analysis of IL-6 expression in a total of 498 islets from 12 cases (at least 30 islets from each case) to account for tissue heterogeneity [27]. We found that the expression of IL-6 was reduced in islets of donors with T1D. In particular, insulin-deficient islets (IDI) had further reduced expression of IL-6 (3.7 ± 2.1%), compared with non-diabetic (14.8 ± 7.3%) and Aab+ (19.5 ± 7.0%) donors (Fig.1B). This reduction in IL-6 expression is possibly a consequence of beta loss in T1D cases. Interestingly, the proportion of IL-6 expressing cells was also significantly reduced in insulin-containing islets (ICI, 9.3 ± 2.4%) of donors with T1D and in donors with T2D (10.1 ± 4.7%). In another set of experiment, performed using a different antibody for IL-6 (Abcam), we analyzed a total of 1150 islets from 35 cases. We also observed a similar reduction in IL-6 expression in ICI (9.9 ± 4.3%) and IDI (8.17 ± 4.5%) of donors with T1D, compared to non-diabetic (13.14 ± 4.6%) and Aab+ (14.4 ± 5.6%) donors (Suppl. Fig. 2). However, in this experiment we did not observe a reduction of IL-6 in donors with T2D compared with non-diabetic and Aab+ donors. In recent onset T1D cases from DiViD, stained with this IL-6 (Abcam) antibody, we did not observe a significant difference in IL-6 expression between ICI (18.5 ± 11.0%) and IDI (16.1 ± 4.7%) islets (Fig. 1C). In the recent onset T1D cases from the DiViD study, 36% of all islets had insulin and 74% of these islets had completely normal levels of insulin and only 11% of all islets had insulitis [28]. Hence, many islets in DiViD cases may be having normal beta cell mass and in very early stages of immune destruction, explaining the lack of IL-6 reduction.
Beta cells are the major source of IL-6 in insulin-containing islets
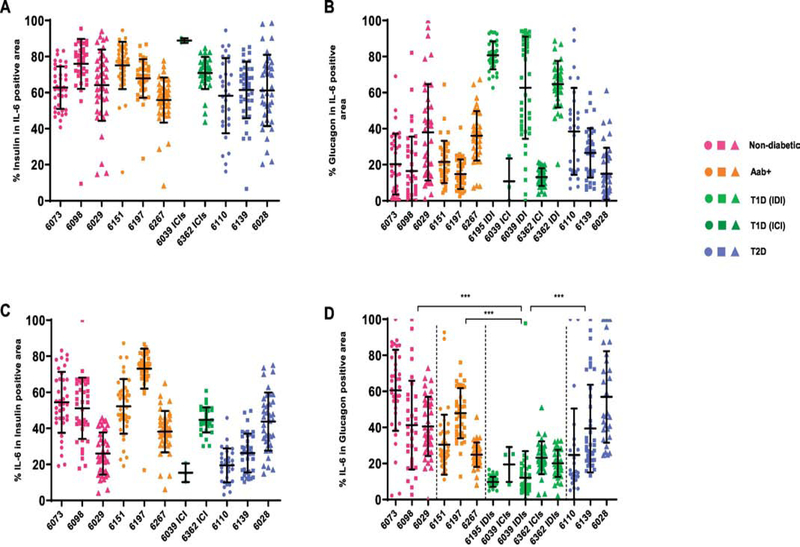
Next, we determined the cellular source of IL-6 in the islets by measuring the IL-6 positive area that overlapped with insulin or glucagon staining. We found that beta cells are the source of majority of IL-6 (65.7 ± 16.4%) in all insulin-containing islets, irrespective of the disease status (Fig. 2A). However, in IDI, the alpha cells contain the majority of IL-6 (69.2 ± 20.6%) expressed in the islets (Fig. 2B). This finding is not surprising, because alpha cells occupy the majority of islet area in IDI. The percentage of IL-6 expression in beta cells varied widely within each group, with values in the range of 43.3 ± 20.1% (Fig. 2C). There were also notable variations between cases in the proportion of alpha cells that express IL-6. However, both ICI and IDI from subjects with T1D had significantly reduced expression of IL-6 in alpha cells (16.0 ± 11.1%), compared with non-diabetic (47.7 ± 23.0%), Aab+ (34.3 ± 16.3%) and donors with T2D (40.7 ± 27.7%), Fig. 2D.
Figure 2. Beta cells are the major source of IL-6 in insulin-containing islets, irrespective of diabetes status.
Quantification of insulin, glucagon and IL-6 positive area were performed using Image Pro Premier software. (A) Percentages of IL-6 positive area that overlap with insulin positive area. (B) Percentages of IL-6 positive area that overlap with glucagon positive area. (C) Percentages of insulin positive area that stained positive for IL-6 (D) Percentages of glucagon positive area that stained positive for IL-6. Shown are values of mean ± SD of more than 30 islets per case. Each data point represents an islet. ***P<0.0001.
Next, we assessed if islet cells express IL-6R and pSTAT3 (Ser 727), a signature of IL-6R activation. We found strong expression of IL-6R on both beta and alpha cells (Suppl. Fig. 3) in a non-diabetic nPOD retired case (6007). We also found nuclear staining of pSTAT3 (Ser 727) in a few beta and alpha cells of a non-diabetic nPOD retired case (6373) (Suppl. Fig. 4), suggesting the possibility of IL-6 having an autocrine or paracrine role.
IL-6 is secreted by human islet cultures
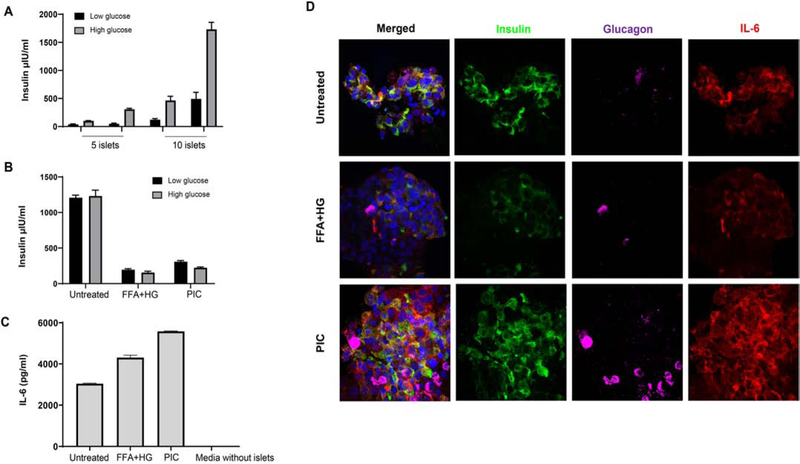
To confirm the secretion of IL-6 in live islets, we obtained human islets from a non-diabetic cadaveric donor through IIDP. First, we tested the functionality of islets by GSIS (Glucose stimulated insulin secretion) assay. Islets were functional as evidenced by higher secretion of insulin upon stimulation with high glucose (Fig 3A). We then induced metabolic stress (high glucose and fatty acid) or immune stress (pro-inflammatory cytokine cocktail containing TNF, IL-1β and IFN-γ) to simulate the kind of stress in type 2 diabetes and type 1 diabetes, respectively. Insulin secretion was highly reduced after metabolic and immune stress (Fig 3B). Human islets secreted IL-6 in the range of 3043.4 ± 22.9 pg/ml, which was further enhanced to 4309.2 ± 117.2 pg/ml upon metabolic stress and 5581.9 ± 18.4 pg/ml upon immune stress (Fig 3C). We then stained these islets for Insulin, Glucagon and IL-6 using immunofluorescence imaging to confirm the source of IL-6. We observed a clear cytoplasmic and membrane staining of IL-6 on both beta and alpha cell in human islets with and without induction of metabolic and immune stress (Fig 3D).
Figure 3. IL-6 is secreted by human islet cultures and increased upon metabolic and immune stress.
Human islets were cultured in 96-well plates. Functional GSIS assay showing insulin secretion upon low and high glucose (A), Insulin secretion upon induction of metabolic (free fatty acid and high glucose – FFA+HG) and immune stress (Proinflammatory cytokine cocktail – PIC) (B). Secretion of IL-6 by human islets with and without metabolic and immune stress (C). Multiplex immunofluorescence imaging of islets with different stressors, showing Insulin (green), Glucagon (magenta), IL-6 (red) and Hoechst (blue). Images were acquired using Zeiss LSM 780 confocal microscope.
Discussion
This study is the first detailed and systematic histopathological evaluation of IL-6 in the human pancreata obtained from donors with T1D. Expression of IL-6 was previously reported in major endocrine glands, including the pancreatic islets [29, 30]. Initial studies examining the role of IL-6 in diabetes used supernatants from islet cell cultures, in which IL-6 expression was enhanced upon exposure to TNF-α and IFN-γ [31]. IL-6 by itself did not alter islet cell viability [32], however, in combination with other inflammatory cytokines, IL-6 enhanced cytotoxicity of insulin secreting cell lines, suggesting a pathogenic role of IL-6 for beta cells [33]. Contrarily, IL-6 at physiologically relevant doses exerted a positive effect on beta cell health by enhancing insulin secretion and maintaining redox status in mouse islets and insulin secreting cell lines [32]. More recently, Marasco et al. have shown that IL-6 protects human islets from inflammatory stress-induced apoptosis, by reduction of reactive oxygen species [24, 34]. Our finding that IL-6 is endogenously expressed in islets of human pancreata points towards a physiological role, contrary to the previously assumed pathogenic role of IL-6 within the islets. We measured protein expression of IL-6 by immunofluorescence. mRNA levels measured by in-situ hybridization (ISH) did not correlate with protein expression in our previous study [27]. We found that the expression of IL-6 was reduced in islets of T1D donors, particularly in insulin-deficient islets. This finding suggests that the loss of IL-6 may be a consequence of beta cell loss, because IL-6 expression was retained in auto-antibody positive cases, where beta cell mass is conserved [35]. Additionally, we observed a reduction in the percentage of alpha cells expressing IL-6 in donors with T1D (Fig. 3D). This observation is particularly intriguing and suggests the possibility of a dysregulation in alpha cells in T1D. In this study, we used a percentage of positive area and refrained from using mean intensities. Comparing mean intensities between different cases and experiments has many potential caveats, since even minor variations in organ retrieval, fixation, staining and imaging conditions can lead to huge variations in mean intensities.
Next, to confirm our histological findings of IL-6 expression in live islets, we cultured human islets for 60 hours. IL-6 could be measured in the supernatants of islet cultures and levels are increased following metabolic and immune stress (Fig 3C), which is in line with previous reports where IL-6 secretion in human islets was increased following treatment with free fatty acid and high glucose or upon infection with coxsackie virus B [36–38]. Our observation confirms that human islets endogenously produce IL-6 and their levels can be altered during immune or metabolic stress. This suggests the possibility of beta cells secreting more IL-6 to combat the stress and maintain the redox status.
Anti-cytokine treatments, especially anti-TNFα, have been very successful in treating autoimmune diseases such as rheumatoid arthritis. Tocilizumab has been shown to be effective in adult rheumatoid arthritis by modulating T cell responses towards regulatory T cells and by blocking polarization to Th17 cells [39]. In consideration that cytokines may not always only be pathogenic and can have physiological role in islet homeostasis [40], the right balance in fine-tuning immune responses and islet cell homeostasis is essential. Further mechanistic in-vitro functional assays will help in better understanding of the role of IL-6 in islet cell biology and in T1D.
Supplementary Material
Highlights.
IL-6 is expressed on human pancreatic beta and alpha cells
Beta cells are the source of majority of IL-6 in pancreatic islets
IL-6 expression is reduced in islets of donors with type 1 diabetes, particularly in insulin-deficient islets
IL-6 is secreted by live human islet cultures and increased upon metabolic and immune stress
Acknowledgements
This research was performed with the support of the Network for Pancreatic Organ donors with Diabetes (nPOD; RRID:SCR_014641), a collaborative type 1 diabetes research project sponsored by JDRF (nPOD: 5-SRA-2018-557-Q-R) and The Leona M. & Harry B. Helmsley Charitable Trust (Grant #2018PG-T1D053). Organ Procurement Organizations (OPO) partnering with nPOD to provide research resources are listed at http://www.jdrfnpod.org//for-partners/npod-partners/. The authors thank Zbigniew Mikulski and Bill Kiosses of the imaging core facility at La Jolla Institute for Immunology for helping with image acquisition and analysis. We acknowledge Dr. Amelia Linnemann for constructive suggestions. The authors thank Ellie Ling for editorial assistance. This research was supported by National Institute of Health grant R01AI134971-02.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Duality of interest
M.G.v.H. and F.A. are employees of Novo Nordisk. No other potential conflicts of interest relevant to this article were reported.
Prior Presentation
Parts of this study were presented at the JDRF nPOD 8th, 10th and 11th Annual Scientific Meetings, Fort Lauderdale, FL, 2016, 2018 and 2019, Cytokines Meeting, San Francisco, CA, 2016 and Immunology of Diabetes Society Meeting, San Francisco, CA, 2016.
Reference List
- 1.Kishimoto T, The biology of interleukin-6. Blood, 1989. 74(1): p. 1–10. [PubMed] [Google Scholar]
- 2.Scheller J, et al. , The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochim Biophys Acta, 2011. 1813(5): p. 878–88. [DOI] [PubMed] [Google Scholar]
- 3.Chen Q, et al. , Inducible microRNA-223 down-regulation promotes TLR-triggered IL-6 and IL-1beta production in macrophages by targeting STAT3. PLoS One, 2012. 7(8): p. e42971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bettelli E, et al. , Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature, 2006. 441(7090): p. 235–8. [DOI] [PubMed] [Google Scholar]
- 5.Samoilova EB, et al. , IL-6-deficient mice are resistant to experimental autoimmune encephalomyelitis: roles of IL-6 in the activation and differentiation of autoreactive T cells. J Immunol, 1998. 161(12): p. 6480–6. [PubMed] [Google Scholar]
- 6.Hirano T, et al. , Excessive production of interleukin 6/B cell stimulatory factor-2 in rheumatoid arthritis. Eur J Immunol, 1988. 18(11): p. 1797–801. [DOI] [PubMed] [Google Scholar]
- 7.Linker-Israeli M, et al. , Elevated levels of endogenous IL-6 in systemic lupus erythematosus. A putative role in pathogenesis. J Immunol, 1991. 147(1): p. 117–23. [PubMed] [Google Scholar]
- 8.Maimone D, Guazzi GC, and Annunziata P, IL-6 detection in multiple sclerosis brain. J Neurol Sci, 1997. 146(1): p. 59–65. [DOI] [PubMed] [Google Scholar]
- 9.Tanaka T, Narazaki M, and Kishimoto T, Therapeutic targeting of the interleukin-6 receptor. Annu Rev Pharmacol Toxicol, 2012. 52: p. 199–219. [DOI] [PubMed] [Google Scholar]
- 10.Campbell IL, et al. , Essential role for interferon-gamma and interleukin-6 in autoimmune insulin-dependent diabetes in NOD/Wehi mice. J Clin Invest, 1991. 87(2): p. 739–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Van Belle TL, et al. , Beta-cell specific production of IL6 in conjunction with a mainly intracellular but not mainly surface viral protein causes diabetes. J Autoimmun, 2014. 55: p. 24–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Campbell IL, et al. , Islet inflammation and hyperplasia induced by the pancreatic islet-specific overexpression of interleukin-6 in transgenic mice. The American journal of pathology, 1994. 145(1): p. 157–166. [PMC free article] [PubMed] [Google Scholar]
- 13.Bradshaw EM, et al. , Monocytes from patients with type 1 diabetes spontaneously secrete proinflammatory cytokines inducing Th17 cells. J Immunol, 2009. 183(7): p. 4432–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hundhausen C, et al. , Enhanced T cell responses to IL-6 in type 1 diabetes are associated with early clinical disease and increased IL-6 receptor expression. Sci Transl Med, 2016. 8(356): p. 356ra119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marwaha AK, et al. , Cutting edge: Increased IL-17-secreting T cells in children with new-onset type 1 diabetes. J Immunol, 2010. 185(7): p. 3814–8. [DOI] [PubMed] [Google Scholar]
- 16.Ferreira RC, et al. , Functional IL6R 358Ala Allele Impairs Classical IL-6 Receptor Signaling and Influences Risk of Diverse Inflammatory Diseases. PLOS Genetics, 2013. 9(4): p. e1003444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ellingsgaard H, et al. , Interleukin-6 enhances insulin secretion by increasing glucagon-like peptide-1 secretion from L cells and alpha cells. Nat Med, 2011. 17(11): p. 1481–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ellingsgaard H, et al. , Interleukin-6 regulates pancreatic alpha-cell mass expansion. Proc Natl Acad Sci U S A, 2008. 105(35): p. 13163–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Timper K, et al. , IL-6 Improves Energy and Glucose Homeostasis in Obesity via Enhanced Central IL-6 trans-Signaling. Cell Rep, 2017. 19(2): p. 267–280. [DOI] [PubMed] [Google Scholar]
- 20.Wedell-Neergaard A-S, et al. , Exercise-Induced Changes in Visceral Adipose Tissue Mass Are Regulated by IL-6 Signaling: A Randomized Controlled Trial. Cell Metabolism, 2019. 29(4): p. 844–855.e3. [DOI] [PubMed] [Google Scholar]
- 21.Paula FM, et al. , Exercise increases pancreatic beta-cell viability in a model of type 1 diabetes through IL-6 signaling. FASEB J, 2015. 29(5): p. 1805–16. [DOI] [PubMed] [Google Scholar]
- 22.Carey AL, et al. , Interleukin-6 increases insulin-stimulated glucose disposal in humans and glucose uptake and fatty acid oxidation in vitro via AMP-activated protein kinase. Diabetes, 2006. 55(10): p. 2688–97. [DOI] [PubMed] [Google Scholar]
- 23.Schultz O, et al. , Effects of Inhibition of Interleukin-6 Signalling on Insulin Sensitivity and Lipoprotein (A) Levels in Human Subjects with Rheumatoid Diseases. PLOS ONE, 2010. 5(12): p. e14328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marasco MR, et al. , Interleukin-6 Reduces beta-Cell Oxidative Stress by Linking Autophagy With the Antioxidant Response. Diabetes, 2018. 67(8): p. 1576–1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.ITN opens extend type 1 diabetes, 2019, Online source.
- 26.Bankhead P, et al. , QuPath: Open source software for digital pathology image analysis. Scientific reports, 2017. 7(1): p. 16878–16878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Anquetil F, et al. , Alpha cells, the main source of IL-1β in human pancreas. Journal of autoimmunity, 2017. 81: p. 68–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Krogvold L, et al. , Insulitis and characterisation of infiltrating T cells in surgical pancreatic tail resections from patients at onset of type 1 diabetes. Diabetologia, 2016. 59(3): p. 492–501. [DOI] [PubMed] [Google Scholar]
- 29.Jablonowska M, et al. , Immunohistochemical localization of interleukin-6 in human pancreatitis. Appl Immunohistochem Mol Morphol, 2008. 16(1): p. 40–3. [DOI] [PubMed] [Google Scholar]
- 30.Kontogeorgos G, et al. , Immunohistochemical localization of interleukin-6 in peripheral human endocrine glands. Endocrine, 2002. 17(2): p. 135–40. [DOI] [PubMed] [Google Scholar]
- 31.Campbell I, et al. , Evidence for IL-6 production by and effects on the pancreatic β-cell. Vol. 143 1989. 1188–91. [PubMed] [Google Scholar]
- 32.da Silva Krause M, et al. , Physiological concentrations of interleukin-6 directly promote insulin secretion, signal transduction, nitric oxide release, and redox status in a clonal pancreatic beta-cell line and mouse islets. J Endocrinol, 2012. 214(3): p. 301–11. [DOI] [PubMed] [Google Scholar]
- 33.Russell MA, et al. , Differential effects of interleukin-13 and interleukin-6 on Jak/STAT signaling and cell viability in pancreatic β-cells. Islets, 2013. 5(2): p. 95–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Linnemann AK, et al. , Interleukin 6 protects pancreatic beta cells from apoptosis by stimulation of autophagy. FASEB J, 2017. 31(9): p. 4140–4152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rodriguez-Calvo T, et al. , Increase in Pancreatic Proinsulin and Preservation of beta-Cell Mass in Autoantibody-Positive Donors Prior to Type 1 Diabetes Onset. Diabetes, 2017. 66(5): p. 1334–1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schulte BM, et al. , Cytokine and chemokine production by human pancreatic islets upon enterovirus infection. Diabetes, 2012. 61(8): p. 2030–2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ehses JA, et al. , Increased Number of Islet-Associated Macrophages in Type 2 Diabetes. Diabetes, 2007. 56(9): p. 2356. [DOI] [PubMed] [Google Scholar]
- 38.Boni-Schnetzler M, et al. , Free fatty acids induce a proinflammatory response in islets via the abundantly expressed interleukin-1 receptor I. Endocrinology, 2009. 150(12): p. 5218–29. [DOI] [PubMed] [Google Scholar]
- 39.Shetty A, et al. , Tocilizumab in the treatment of rheumatoid arthritis and beyond. Drug Des Devel Ther, 2014. 8: p. 349–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kristiansen OP and Mandrup-Poulsen T, Interleukin-6 and diabetes: the good, the bad, or the indifferent? Diabetes, 2005. 54 Suppl 2: p. S114–24. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.