Abstract
Increased gamma-hydroxybutyric acid in urine and blood are metabolic hallmarks of succinic semialdehyde dehydrogenase deficiency, a defect of 4-aminobutyric acid metabolism. Here, we examined the hypothesis that succinic semialdehyde dehydrogenase deficiency could be identified via measurement of gamma-hydroxybutyric acid in newborn and post-newborn dried bloodspots. Quantitation of gamma-hydroxybutyric acid using liquid chromatography-tandem mass spectrometry in twelve archival newborn patient dried bloodspots was 360 ± 57 μM (mean, standard error; range 111–767), all values exceeding the previously established cutoff for newborn detection of 78 μM established from 2,831 dried bloodspots derived from newborns, neonates and children. Gamma-hydroxybutyric acid in post-newborn dried bloodspots (n=19; ages 0.8–38 years) was 191 ± 65 μM (mean, standard error; range 20–1218), exceeding the aforementioned GHB cutoff for patients approximately 10 years of age or younger. Further, gamma-hydroxybutyric acid in post-newborn dried bloodspots displayed a significant (p<0.0001) inverse correlation with age. This preliminary study suggests that succinic semialdehyde dehydrogenase deficiency may be identified in newborn and post-newborn dried bloodspots via quantitation of gamma-hydroxybutyric acid, while forming the platform for more extensive studies extended in affected and unaffected dried bloodspots.
Keywords: Gamma-hydroxybutyric acid, dried bloodspots, newborn screening, GABA metabolism
1. Introduction
Succinic semialdehyde dehydrogenase (SSADH) deficiency (SSADHD) is caused by mutations in the gene encoding aldehyde dehydrogenase 5A1 (ALDH5A1; OMIM 271980) [1] (Fig. 1) and manifests a phenotype of static encephalopathy featuring global delays, hypotonia, absence of speech and variable epilepsy [1]. The biochemical hallmark includes elevation of gamma-hydroxybutyric acid (GHB) in physiological fluids and tissues. Interventional approaches primarily target neuropsychiatric morbidity and seizures [2, 3]. Following the development of an animal model [4], elucidation of pathomechanisms and the development of new preclinical therapeutics have been the primary focus of research efforts paving the way to a recently completed trial (outcomes pending) with the GABAb receptor antagonist SGS-742 (www.clinicaltrials.gov; NCT02019667) and an NIH-supported natural history study [R01 HD 91142].
Fig. 1. GABA metabolism.
Abbreviations: GABA, gamma-aminobutyric acid; SSA, succinic semialdehyde; SSADH, succinic semialdehyde dehydrogenase (deficiency of which leads to gamma-hydroxybutyric aciduria; shaded grey box); GHB, gamma-hydroxybutyric acid.
Currently, SSADHD has not yet been nominated for newborn screening (NBS) in the US (www.hrsa.gov/advisorycommittees/mchbadvisory/heritabledisorders/index.html). Yet, NBS for SSADHD is clinically relevant considering the potential benefits of early intervention with targeted therapeutics. Currently, the median age at disease onset is 1 year whereas the median age at diagnosis is 3 years, a loss of 2 treatment-years at a critical developmental age (data from a 2018 survey of fifty five SSADHD families worldwide; personal communication from the SSADH Association), and a significant stress on parents in anticipation of a diagnosis. In addition, the nonspecific neurological symptoms of SSADHD when combined with a mild clinical phenotype has resulted in an increasing number of patients identified in adulthood [5]. These observations and reports strongly underscore the need for newborn screening of the disease, a pre-requisite for early therapeutic interventions, early identification of milder cases, and a full understanding of the natural history of this ultra-rare condition. In the current study, we have collected both newborn and post-newborn dried bloodspots (DBS) from confirmed patients with SSADHD to determine if measurement of GHB could eventually be applicable for newborn detection [6].
2. Material and Methods
Archival newborn DBS were obtained with parental consent from nine patients (12 unique DBS) obtained from California, Texas, Maryland, and Ireland. Six of twelve samples included both 1st and routine 2nd screens from three patients (approximate age at collection, 48 and 340 hrs). California stores archival DBS at 4 °C, while other US States store archival DBS at room temperature.
DBSs from post-newborn SSADHD patients were collected with informed consent (WSU IRB 15901). Nineteen teen samples included: 11M/8F, ages 0.8–38 years (median, 8.7), and 4 sibships (total, 8 patients). Confirmation of SSADHD for patients contributing post-newborn DBS was previously confirmed through a combination of GHB measurement, ALDH5A1 molecular analyses, and assay of SSADH in white cells for older patients (data not shown) [7–9]. DBS were obtained using standard finger lance and blood collected onto 903TM five spot blood cards (Eastern Business Cards, Greenville, SC, USA).
An earlier study developing methodology for measurement of GHB using UPLC-tandem mass spectrometry assessed 2831 archival DBS derived from newborns, neonates and children to develop both a control range and a cutoff for potential use in newborn screening [6]. In that study, the mean and standard error was 8 + 0.1 μM (n=2831; standard deviation, 5), and the preliminary cutoff at the 99.99%-ile for detection of SSADHD was determined as a GHB concentration of 78 μM. Because of the large number of controls evaluated in the pilot study [6], we assessed only limited control samples in the current study to verify overlap with the previous control range. In the current study, we quantified GHB in DBS derived from four unaffected control individuals (3M, 1F; ages 4, 15, 56 and 63 years) and a previously reported patient with SSADHD [10].
3. Results
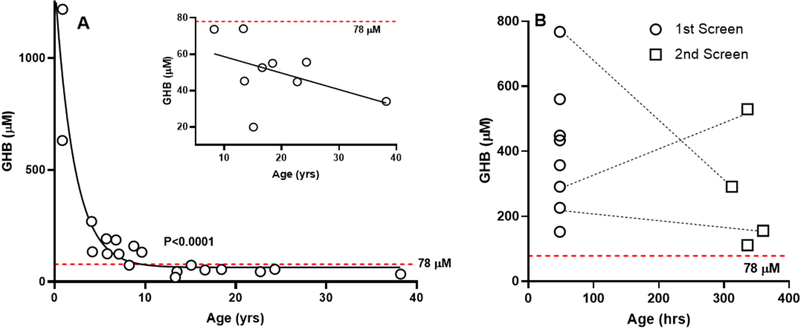
GHB content in post-newborn and newborn DBS derived from patients with SSADHD, as well as control DBS simultaneously characterized in addition to a previously evaluated SSADHD DBS [6], are summarized in Table 1. GHB content for nineteen post-newborn DBSs (Fig. 2A) demonstrated a significant age-dependent negative correlation (p<0.0001, Spearman exact test). Nine of these patients displayed GHB concentrations below the threshold of 78 μM previously developed for newborn detection [6]. The GHB content of these nine DBS was replotted against age (inset, Fig. 2A), revealing a trend toward a negative correlation that failed to reach significance. For these patients (n=9), the mean ± standard deviation of GHB content was 51 + 17 μM (range 20–74, median 55 μM), still significantly above the previously reported value of 8 ± 5 μM (p<0.0001, two-tailed t test).
Table 1:
Concentration of Gamma-Hydroxybutyric Acid in Dried Bloodspots of Newborn and Post-Newborn Dried Bloodspots derived from Patients with Succinic Semialdehyde Dehydrogenase Deficiencya
| Post-Newborn Bloodspots | Newborn Bloodspots | Control Post-Newborn Bloodspots | |||
|---|---|---|---|---|---|
| Age (yrs) | GHB (μM) | Age (hrs) | GHB (μM) | Age (yrs) | GHB (μM) |
| 0.8 | 631 | 48 | 226 | 4 | 5 |
| 0.9 | 1218 | 48 | 767 | 15 | 18 |
| 4.1 | 269 | 48 | 388 | 56 | 8 |
| 4.2 | 134 | 48 | 152 | 63 | LODc |
| 5.7 | 192 | 48 | 560 | 3d | 545d |
| 5.8 | 125 | 48 | 434 | ||
| 6.8 | 187 | 48 | 357 | ||
| 7.1 | 124 | 48 | 448 | ||
| 8.2 | 74 | 312 | 291b | ||
| 8.7 | 160 | 336 | 111b | ||
| 9.6 | 132 | 336 | 529b | ||
| 13.3 | 20 | 360 | 116b | ||
| 13.5 | 45 | ||||
| 15.1 | 74 | ||||
| 16.7 | 53 | ||||
| 18.4 | 55 | ||||
| 22.7 | 45 | ||||
| 24.3 | 56 | ||||
| 38.2 | 34 | ||||
The mean ± standard deviation for GHB in 2,831 control dried bloodspots (newborn, neonates, children) previously reported was 8 ± 5 μM, range 0–78 μM [6]
second screen
limit of detection
previously reported patient with combined succinic semialdehyde dehydrogenase deficiency and Rett syndrome, for whom the originally measured GHB content in dried bloodspot was 673 μM [10].
Fig. 2. Content of gamma-hydroxybutyric acid in post-newborn and newborn dried bloodspots from patients with succinic semialdehyde dehydrogenase deficiency.
(A) GHB in post-newborn dried bloodspots derived from patients with SSADHD as a function of age. The figure shows the DBS GHB content for all nineteen patients characterized. The dotted red line represents the cutoff of 78 μM established earlier [6] for newborn detection of SSADHD. Statistical analysis, Spearman exact test. The insert shows the relationship between DBS GHB content and age for those patients whose GHB content fell below the 78 μM threshold. Statistical analysis, Pearson correlation (p=ns). (B) GHB in newborn DBS obtained from nine patients with confirmed SSADHD. These samples included five 1st screens, one 2nd screen (1st screen sample unavailable), and three patients for whom 1st and 2nd screens were obtained (dashed lines). The newborn detection cutoff value of 78 μM is again represented by the horizontal red dashed line.
Because of the large number of control samples previously evaluated for GHB content in DBS (n=2831), we opted to focus effort in the current report on SSADHD DBS and simultaneously only analyzed five control DBS (Table 1). One of these samples represented reassessment of an SSADHD DBS previously shown to have a content of 673 μM [10], and in the current study a level of 545 μM, providing additional methodological validation.
GHB determinations in DBS of nine SSADHD patients (eight 1st screens, four 2nd screens) revealed a range of 111–767 μM (median, 388), all above the cutoff threshold of 78 μM (Table 1; Fig 2B) [6]. We observed a trend toward lower GHB values in 2nd screens, but the mean values were not significantly different (417 μM 1st screens; 262 μM 2nd screens; p=ns, t test). For one sibship that contributed multiple DBSs (female, 2nd newborn screen (GHB=111 μM) and again at 8.7 years (GHB=160 μM); male, 1st screen (GHB=226 μM), 2nd screen (GHB=116 μM), and again at 6.8 years (GHB=187 μM)), there was no clear trend with age.
4. Discussion and conclusions
The preliminary data presented suggest that SSADHD can be detected in newborn DBS via measurement of GHB content. Further, the results underscore a powerful negative correlation of GHB with age, consistent with earlier data from plasma [11]. An omission in our earlier study [6] was the absence of patient age and gender for DBS derived from patients with SSADHD. Further, a concern with the current study is the inability to control for storage conditions of archival DBS. However, for seven DBS retrieved from room temperature storage, the mean ± SD for GHB was 347 ± 238 (range 111–767 μM) while that for three DBS retrieved from 4 °C storage was 382 ± 209 (range 152–560; p=ns, t test), suggesting GHB long-term stability regardless of storage conditions. Details of the conditions of storage for the two newborn DBS obtained from Ireland was not available.
Our assay for GHB in DBS represents a standalone method that is not multiplexed with other newborn screening markers, and is currently of limited throughput as it requires a liquid chromatography step to separate GHB from other isomers. On the other hand, the method may lend itself to integration into pilot newborn screening studies in different States in which liquid chromatography-tandem mass spectrometry may be undertaken in the near future, especially States with smaller newborn populations. Although it is currently in data analysis, evidence for efficacy of SGS-742 in SSADHD would strengthen the argument for adding SSADHD into a pilot newborn screening program utilizing liquid chromatography in combination with mass spectrometry.
In terms of our current GHB assay in DBS, future work will focus on redefining the range of normative data using the UPLC-MS/MS methodology with only newborn samples [6]. Although there was no evidence for an age-dependent decrease in DBS GHB in our earlier study [6], there remains a remote chance that we might observe an age-dependent decrease in control DBS GHB employing an age-matched control cohort for our post-newborn patient samples. Nonetheless, we still predict that patient DBS below the 78 μM cutoff would still be elevated with respect to GHB using an age-matched control cohort, but this needs to be experimentally evaluated. In sum, the data presented in the current study suggests that quantitation of GHB in DBS has utility as a second-tier screening method. This will augment our ongoing efforts to develop a robust first-tier screening method for SSADHD employing metabolites currently measured in multiplexed fashion in State newborn screening programs, including amino acids, acylcarnitines, and guanidino (creatine, creatinine, guanidinoacetate) species.
Acknowledgments
This work was supported by the National Institutes of Health HD 91142. The authors thank Drs. Yilmiz Yildaz, Gabriella Horvath, Hilary Vallance, Johannes Haberle, Francois Feillet, Saadet Andrews, and Ellen Crushell, as well as multiple parents, for supplying dried bloodspots of patients with SSADH deficiency. The assistance of the Departments of Health, Newborn Screening Sections, of Texas, California and Maryland, and the newborn screening program affiliated with Cork, Ireland, in providing newborn dried bloodspots is gratefully acknowledged.
Abbreviations employed
- GHB
gamma-hydroxybutyric acid
- DBS
dried bloodspot
- NBS
newborn screening
- SSADH
succinic semialdehyde dehydrogenase
- SSADHD
SSADH deficiency
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Attri SV, Singhi P, Wiwattanadittakul N, Goswami JN, Sankhyan N, Salomons GS, Roullet J-B, Hodgeman R, Parviz M, Gibson KM, Pearl PL (2017) Incidence and Geographic Distribution of Succinic Semialdehyde Dehydrogenase (SSADH) Deficiency. JIMD Rep 34:111–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Malaspina P, Roullet J-B, Pearl PL, Ainslie GR, Vogel KR, Gibson KM (2016) Succinic semialdehyde dehydrogenase deficiency (SSADHD): Pathophysiological complexity and multifactorial trait associations in a rare monogenic disorder of GABA metabolism. Neurochem. Int. 99: 72–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Parviz M, Vogel K, Gibson KM, Pearl PL (2014) Disorders of GABA metabolism: SSADH and GABA-transaminase deficiencies. J. Pediatr Epilepsy 3: 217–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Vogel KR, Ainslie GR, Walters DC, McConnell A, Dhamne SC, Rotenberg A, Roullet JB, Gibson KM (2018) Succinic semialdehyde dehydrogenase deficiency, a disorder of GABA metabolism: an update on pharmacological and enzyme-replacement therapeutic strategies. J Inherit Metab Dis 41: 699–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Lapalme-Remis S, Lewis EC, De Meulemeester C, Chakraborty P, Gibson KM, Torres C, Guberman A, Salomons GS, Jakobs C, Ali-Ridha A, Parviz M, Pearl PL (2015) Natural history of succinic semialdehyde dehydrogenase deficiency through adulthood. Neurology 85: 861–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Forni S, Pearl PL, Gibson KM, Yu Y, Sweetman L (2013) Quantitation of gamma-hydroxybutyric acid in dried blood spots: feasibility assessment for newborn screening of succinic semialdehyde dehydrogenase (SSADH) deficiency. Mol. Genet. Metab 109: 255–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Akaboshi S, Hogema BM, Novelletto A, Malaspina P, Salomons GS, Maropoulos GD, Jakobs C, Grompe M, Gibson KM (2003) Mutational spectrum of the succinate semialdehyde dehydrogenase (ALDH5A1) gene and functional analysis of 27 novel disease-causing mutations in patients with SSADH deficiency. Hum Mutat 22: 442–50. [DOI] [PubMed] [Google Scholar]
- [8].Gibson KM, Christensen E, Jakobs C, Fowler B, Clarke MA, Hammersen G, Raab K, Kobori J, Moosa A, Vollmer B, Rossier E, Iafolla AK, Matern D, Brouwer OF, Finkelstein J, Aksu F, Weber HP, Bakkeren JA, Gabreels FJ, Bluestone D, Barron TF, Beauvais P, Rabier D, Santos C, Lehnert W (1997) The clinical phenotype of succinic semialdehyde dehydrogenase deficiency (4-hydroxybutyric aciduria): case reports of 23 new patients. Pediatrics 99: 567–74. [DOI] [PubMed] [Google Scholar]
- [9].Gibson KM, Lee CF, Chambliss KL, Kamali V, Francois B, Jaeken J, Jakobs C (1991) 4-Hydroxybutyric aciduria: application of a fluorometric assay to the determination of succinic semialdehyde dehydrogenase activity in extracts of cultured human lymphoblasts. Clin Chim Acta 196: 219–21. [DOI] [PubMed] [Google Scholar]
- [10].Brown M, Ashcraft P, Arning E, Bottiglieri T, McClintock W, Giancola F, Lieberman D, Hauser NS, Miller R, Roullet JB, Pearl P, Gibson KM (2019) Rett syndrome (MECP2) and succinic semialdehyde dehydrogenase (ALDH5A1) deficiency in a developmentally delayed female. Mol Genet Genomic Med Mar 4:e629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Jansen EE, Vogel KR, Salomons GS, Pearl PL, Roullet JB, Gibson KM (2016) Correlation of blood biomarkers with age informs pathomechanisms in succinic semialdehyde dehydrogenase deficiency (SSADHD), a disorder of GABA metabolism. J Inherit Metab Dis 39: 795–800. [DOI] [PMC free article] [PubMed] [Google Scholar]