Abstract
Microbiology has been largely developed thanks to the discovery and optimization of culture media. The first liquid artificial culture medium was created by Louis Pasteur in 1860. Previously, bacterial growth on daily materials such as some foods had been observed. These observations highlighted the importance of the bacteria's natural environment and their nutritional needs in the development of culture media for their isolation. A culture medium is essentially composed of basic elements (water, nutrients), to which must be added different growth factors that will be specific to each bacterium and necessary for their growth.
The evolution of bacterial culture through the media used for their culture began with the development of the first solid culture medium by Koch, allowing not only the production of bacterial colonies, but also the possibility of purifying a bacterial clone. The main gelling agent used in solid culture media is agar. However, some limits have been observed in the use of agar because of some extremely oxygen-sensitive bacteria that do not grow on agar media, and other alternatives were proposed and tested. Then, the discovery of antimicrobial agents and their specific targets prompted the emergence of selective media. These inhibiting agents make it possible to eliminate undesirable bacteria from the microbiota and select the bacteria desired. Thanks to a better knowledge of the bacterial environment, it will be possible to develop new culture media and new culture conditions, better adapted to certain fastidious bacteria that are difficult to isolate.
Keywords: Culture media, Enriched media, Gelling agents, Liquid and solid media, Selective media
Introduction
The discovery of culture media allowed the development of microbiology in the nineteenth century [1]. Bacterial culture was the first method developed to study the human microbiota [2], using an artificial medium that allows growth and isolation of bacteria. The first to have cultured a bacterium in a reproducible way was Louis Pasteur in 1860 thanks to the development of the first so-called artificial culture medium [3]. Recently, after the emergence of molecular techniques in the 1970s, such as PCR, sequencing and more particularly metagenomics, microbiologists have favoured these innovative techniques to the detriment of culture. Nevertheless, metagenomics presents certain disadvantages and in particular a depth bias, due to the lack of sensitivity of the primers used, because it does not detect bacteria present at concentrations <105 bacteria per gram of stool [2]. Moreover, these techniques only detect DNA: it is impossible with these techniques to differentiate DNA belonging to living bacteria from that of the transient bacteria of the microbiota studied, or from that of dead bacteria.
A few years ago, culturomics, a new culture technique that uses a very large number of culture media and culture conditions to extend the repertoire of bacteria, was developed in our laboratory [2]. This technique demonstrates the complementarity between metagenomics and culturomics. Therefore, the metagenomic identification of bacterial species existing in a given microbiota can be exploited by culturomics through the optimization of new specific culture media for the isolation of these species. This complementarity allows culturomics to become a targeted technique.
New culture media today mimic the natural environment of bacteria by adding different elements in culture medium to cultivate bacteria that were previously uncultivated.
We propose here a bibliographical review of culture media and the evolution of techniques through the development of microbiology over time.
Empirical approach of microbiology
Observational microbiology
Microbiology is defined not only by the organisms it studies, but also by the tools used to study them. The first observation of a bacterium was made around 1673 by the Dutch microscopist Anton van Leeuwenhoek thanks to the microscopes he had developed. Those enlarged from 50 to 300 times what he observed [1,3].
In the course of his research, he highlighted small structures that he called ‘animalcules’ [4], because he thought he was observing small animals [5]. Throughout his observations, he described and drew yeast cells, filiform fungi, microscopic algae and protozoa [4]. Leeuwenhoek was also the first to observe a parasite, Giardia lamblia [3]. However, microscopy alone cannot address all the questions about the microorganisms studied. For about 200 years microbiology was stagnating until the development of microbial isolation techniques in pure cultures. This is another important milestone in the history of microbiology.
Cultural microbiology
The birth of culture broth
In the thirteenth century, 400 years before Leeuwenhoek, a blood-like substance appeared on the communion bread. In line with Christian beliefs, this red substance was assumed to be the blood of Christ. Bartholomeo Bizio, an Italian pharmacist, solved this mystery in 1817 thanks to advances in microbiology and showed that it was not blood, but a microorganism that he named Serratia marcescens. This bacterium appeared as red colonies on bread when stored in a warm and humid atmosphere [6]. It is one of the first natural cultures of a bacterium. The origins of culture media date back to the nineteenth century. Many bacteriologists have tried, with varying degrees of success, to grow bacteria on the food or material on which the microorganism was first developed.
Evolution of microbiology was made possible by challenging the theory of spontaneous generation, according to which living organisms could develop from dead or decaying matter and so spontaneously appear in cultures. Hence, it was inconceivable to obtain pure cultures. In 1861, Louis Pasteur, a French microbiologist [3], solved this problem through an experiment. He placed nutrient solutions in flasks, heated their necks with flame and stretched them in various ways, keeping the end open to the air. Pasteur boiled the solutions for a few minutes and cooled them. No growth appeared even if the contents of the flasks had been exposed to air. Pasteur noticed that there was no growth because dust and germs had been trapped on the walls of the curved tubes. If the tubes were broken, growth began immediately. In this way, Pasteur not only demonstrated that spontaneous generation was nonsense, but also found ways to keep sterile solutions [3].
The first to have cultured bacteria reproducibly in a liquid culture medium was Louis Pasteur. In 1860, he developed a culture medium containing ‘yeast soup’, ashes, sugar and ammonium salts [7,8]. His objective was to create a fermentation medium to demonstrate that each fermentation (alcoholic, acetic, lactic …) was associated with the development of a particular microorganism [9]. The presence of these different elements in the medium allowed him to observe that some of these components could promote or inhibit the growth of certain bacteria and that they could also allow the emergence of certain bacteria compared with others [8].
Indeed, in the course of the study of fermentation of beer, he was confronted with a problem: when the beer was healthy, the microscope only showed brewer's yeast, but when the beer was acid, Pasteur noticed that the tanks contained ‘tiny rod-shaped objects’ producing lactic acid. This fermentation medium therefore made it possible to highlight the multiplication of this bacterium [1,3].
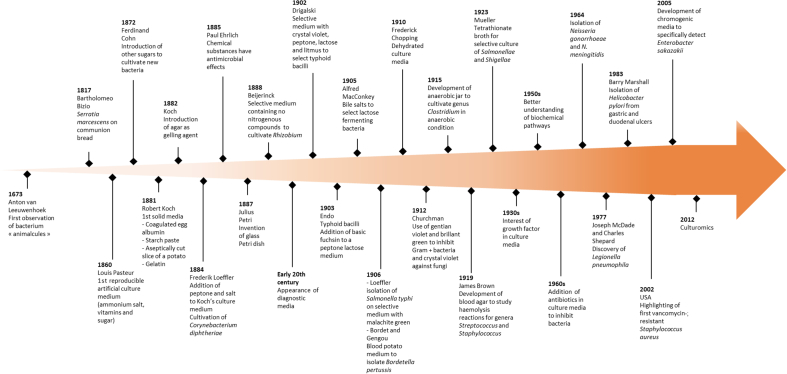
In 1881, Robert Koch demonstrated optimal growth of bacteria when they were incubated in a broth composed of fresh beef serum or meat extract. However, the use of a liquid culture medium did not produce pure bacterial cultures. Koch therefore sought a way to solidify the medium [8] (Fig. 1).
Fig. 1.
Evolution of culture media: from the first bacterial culture (1673) to culturomics.
Emergence of pure cultures in solid media
First, he tested coagulated egg albumin, starch paste or an aseptically cut slice of potato. However, not all of these techniques allowed him to isolate colonies. Koch then added gelatin to his broth and poured it all over a flat glass plate. However, gelatin had disadvantages as it liquefied at temperatures above 25°C and could be consumed by gelatinase, an enzyme produced by certain bacteria. Thanks to the wife of one of his assistants, Fannie Hesse, who used agar to solidify her jams, he replaced gelatin with agar, which allowed him to obtain firm agars and isolate bacteria. In 1887, Julius Richard Petri replaced the glass plate with a circular culture box, the Petri box was created, and is still used today [8]. This allowed him to obtain and observe isolated colonies and to limit contamination.
How to design an enriched culture medium?
An enriched culture medium is a medium to which have been added elements essential for the growth of bacteria.
The nutrients
In order to grow, bacteria need a minimum of nutrients: water, a carbon source, a nitrogen source and some mineral salts [10].
Water
Water plays a fundamental role in solubilizing nutrients, transporting them and ensuring hydrolysis reactions. Some bacteria need free water for their growth. If evaporation occurs during the incubation of the agar, there may be a loss of this water, resulting in a decrease in colony size and inhibition of bacterial growth [10,11].
Carbon sources
Carbon is the most abundant constituent element in bacteria. It is essential for bacteria to produce carbon molecules, such as fats, carbohydrates, proteins and nucleic acids. Bacteria can use inorganic carbon sources, such as carbon dioxide, or organic sources such as sugars and alcohols [10,12].
Nitrogen sources
As for nitrogen sources, they are numerous and can be found in a large number of compounds used in the composition of a culture medium. It is found in the organic form, corresponding to protein hydrolysates, particularly in case of hydrolysate, proteose-peptone or tryptone [12], but also in an inorganic form, nitrates [13]. Nitrogen allows bacteria to synthesize their proteins.
Finally, among the common mineral salts, phosphate, sulphate, magnesium or calcium [11] are regularly found.
Energy sources
There are two types of bacteria, phototrophic bacteria, such as Thiocapsa roseopersicina [14], which uses light as an energy source by transforming it into an electrochemical gradient of protons [14], and chemotrophic bacteria, which use the energy of oxidation of mineral or organic compounds as energy sources [9,15]. Among these bacteria, we can find Listeria monocytogenes [16].
Growth factors
The use of a minimal medium does not allow the growth of certain bacteria that need specific elements to grow. It is sometimes necessary to add growth factors to culture media to boost the multiplication of bacteria. Growth factors are elements that bacteria are unable to synthesize from the nutrients available in the environment [10,17]. Growth factors are required in small quantities in the culture medium and their need is justified by the absence or blocking of a metabolic pathway in the bacterium.
Purine and pyrimidine bases
There are different categories of growth factors among which we find purine and pyrimidine bases. They are necessary for synthesis of nucleic acids. Indeed, some lactic acid bacteria need adenine, guanine, thymine or uracil for growth [17]. This is the case in particular for the bacterium Leuconostoc mesenteroides, for which guanine is essential for its growth [18].
Amino acids
Amino acids are also growth factors and are used for protein synthesis. Dunn et al. [19] showed in 1946 that only two amino acids were essential for Leuconostoc mesenteroides, glutamic acid and valine, whereas for Lactobacillus brevis, no less than 15 amino acids were necessary for its growth. This high requirement for amino acids can be explained by the fact that the base medium used at the time was not rich in common nutrients. Indeed, nowadays, Lactobacillus brevis grows on a COS agar (Columbia Blood Agar) (Biomérieux, Marcy l’Étoile, France), not supplemented with amino acids [20], but composed of casein hydrolysate and peptone proteose, themselves sources of amino acids [11].
Vitamins
Vitamins are also part of growth factors. They are coenzymes or precursors of coenzymes. A vitamin is an organic substance, necessary in small quantities for the metabolism of a living organism, which cannot be synthesized in sufficient quantity by that organism. Faecalibacterium prausnitzii, which is a fastidious bacterium, requires a large number of vitamins to grow, such as biotin, folic acid, riboflavin or vitamin B12 [21]. This bacterium also requires the presence of other growth factors such as volatile fatty acids (acetic acid, propionic acid or valeric acid) [21].
Blood use and its derivatives
Blood and its derivatives will also boost the growth of certain bacteria. In culture media, it is common to use sheep's blood or horse's blood. The role of blood is to act as a protective agent against toxic oxygen radicals [22], but also as a nutritional supplement. For instance, cooked sheep's blood provides the factor X (haem) necessary for the growth of many pathogenic species, including Haemophilus influenzae [23]. Most often, 5% blood is added to the culture media [22]. Serum, such as fetal calf or lamb serum, can also be used as a growth factor by providing a large number of elements such as lipids, vitamins, triglycerides, minerals and others [24]. This blood has been cleared of cells, platelets and clotting factors. Fetal calf serum, which is most commonly used in microbiology [25,26], must be inactivated by heat before use (56°C for 30 min) to inactivate all components of the complement system present in this serum [27,28]. It must be stored at –20°C and heated to room temperature rather than in a water bath to avoid deterioration of the proteins it contains.
Rumen fluid
Finally, it is also possible to use the rumen fluid [2,29] to promote the growth of certain bacterial species by mimicking their natural environment. The rumen fluid used corresponds to the rumen of the sheep. To prepare it, it is first necessary to recover the juice from the fermented plants in the stomach by filtering it through a fine cloth. This juice is then centrifuged (10 000 rpm for 90 min) and the supernatant is collected. Then, it undergoes three successive filtrations at 0.8 μm, 0.45 μm and 0.22 μm [2,30]. 5% rumen juice is usually added to the culture media. The rumen fluid can promote the growth of certain species of Treponema, such as Treponema hyodysenteriae and Treponema innocens [31].
Antioxidants
Some anaerobic bacteria are fastidious to grow and new culture strategies have been developed to isolate them. Indeed, anaerobic bacteria are most abundant in the human intestinal microbiota, accounting for up to 99.9% of total bacteria [32] and are extremely sensitive to oxygen, which is toxic to them. Antioxidants have therefore been added to culture media to allow the culture of strict anaerobic bacteria under aerobic conditions [33,34]. A number of antioxidants have been tested and have shown satisfactory results. This is the case for ascorbic acid and glutathione [34] or uric acid [35]. The addition of these antioxidants to the culture medium and its incubation under aerobic conditions allowed the growth of 135 strict anaerobic bacteria, 12 microaerophilic bacteria and 22 strict aerobic bacteria [35].
How can we have a selective culture medium?
A selective culture medium is used to isolate a particular bacterial species or genus. After the addition of a number of inhibitors to the culture medium, the objective of this type of medium is to eliminate unwanted microbial flora. The selective medium is composed of a basic medium to which antibiotics, chemicals, dyes, antiseptics, sodium salts or phages can be added [36].
Antibiotics
Antibiotics are the most commonly used selective agents. Their spectrum of action being well known, it is easier to anticipate their action on bacteria. There are a large number of antibiotics that can be used in culture media, some of which are called antibacterial because they target bacteria and others antifungal because they eliminate fungi and yeasts [37]. Some molecules target Gram-positive bacteria, such as penicillin G, bacitracin or vancomycin, whereas others target Gram-negative bacteria, such as colistin or polymixin B. Amphotericin B, cycloheximide or nystatin have an action against fungi and yeasts (Table 1) [10,39]. Several antibiotics can be combined to obtain a more selective medium [37].
Table 1.
Antibiotics agents used in bacterial culture
| Inhibitors | Microorganisms |
Example of culture media | References | |||
|---|---|---|---|---|---|---|
| Gram-positive | Gram-negative | Fungi, yeast | ||||
| Penicillins | Penicillin G | ✓ | χ | χ | *A7 Agar modified | ECN Pilly 2018 [38] Power et al., 2009 [11] |
| Cephalosporins | Cefalotin (first-generation) | ✓ | ✓Entrobacteria | χ | *Cefalotin-buffered dextrin broth | Sachan and Agarwal, 2000 [40] Corry et al., 2012 [41] |
| Cefamandole (second-generation) | ✓ | ✓Enterobacteria | χ | *BCYE supplemented with Cefamandole | Bartram et al., 2007 [42] | |
| Cefixime (third-generation) | ✓ Cocci | ✓ Bacillus | χ | *MacConkey Sorbitol agar (CT-SMAC) | Delarras, 2014 [39] | |
| Ceftazidime (third-generation) | ✓ | χ | *Palcam medium | Delarras, 2014 [39] | ||
| Carboxypenicillins | Ticarcillin | ✓ | ✓ Aerobic bacillus | χ | *PC agar | Power et al., 2009 [11] |
| Diaminopyrimidines | Trimethoprim | ✓ | ✓ | χ | *Bolton broth *Preston Agar and Broth |
Delarras, 2014 [39] |
| Nitrofurane | Nitrofurantoin |
✓ Especially those of the urinary tract |
✓ Especially those of the zurinary tract |
χ | *Differentiation test | Delarras, 2014 [39] |
| Phenicoles | Chloramphenicol | ✓ | ✓ | χ | *Yeast Extract Glucose Chloramphenicol Agar *Chloramphenicol Sabouraud Agar |
Delarras, 2014 [39] |
| Rifamycines | Rifampicin | ✓ | ✓ Other than Enterobacteria | χ | *Preston Agar and Broth | Delarras, 2014 [39] |
| Tetracyclines | Oxytetracycline | ✓ | ✓ | χ | *OGYE Agar Base | Power et al., 2009 [11] |
| Macrolides | Erythromycin | ✓ | ✓ | χ | Gallery API Campy Biomérieux | Delarras, 2014 [39] |
| Polypeptides | Polymyxin B | χ | ✓ Aerobic | χ | *PALCAM Medium Base *SPS Agar *TSN Agar |
Power et al., 2009 [11] |
| Colistin | χ | ✓ Aerobic | χ | * Oxford Agar *Campylosel Agar *Thayer–Martin Selective Agar |
Power et al., 2009 [11] | |
| Bacitracin | ✓ | χ | *Chocolate II Agar with Bacitracin *Haemophilus Isolation Agar with Bacitracin |
Power et al., 2009 [11] | ||
| Quinolones | Nalidixic acid | χ | ✓Enterobacteria | χ | *Todd–Hewitt Broth with Gentamicin and Nalidixic Acid *UVM Modified Listeria Enrichment Broth *Columbia CNA Agar |
Power et al., 2009 [11] |
| Aminosides | Neomycin | ✓ Aerobic | ✓ Aerobic | χ | *Lecithin Lactose Agar *Neomycin Blood Agar *TSN Agar |
Power et al., 2009 [11] |
| Gentamycin | ✓ Aerobic | ✓ Aerobic | χ | *Todd–Hewitt Broth with Gentamicin and Nalidixic Acid *Trypticase™ Soy Agar with 5% Sheep Blood (TSA II) with Gentamicin |
Power et al., 2009 [11] | |
| Glycopeptides | Vancomycin | ✓ | χ | χ | *Vancomycin Screen Agar *Anaerobe Laked Sheep Blood Agar with Kanamycin and Vancomycin |
Power et al., 2009 [11] |
| Phosphomycin (natural antibiotic) | ✓ Aerobic | ✓Enterobacteria | χ | *Oxford Agar | Power et al., 2009 [11] | |
| Novobiocin | ✓ | ✓ Cocci | χ | *CIN Agar *Yersinia Antimicrobic Supplement CN *EC Medium, Modified |
Power et al., 2009 [11] | |
| Antibiotics/antifungal | Amphotericin B | χ | χ | ✓ | *Campylobacter Antimicrobic Supplement Blaser *Butzler Agar *Campylosel Agar |
Power et al., 2009 [11] Delarras, 2014 [39] |
| Cycloheximide | χ | χ | ✓ | *Brain–Heart CC Agar *Dermatophyte Test Medium Base *m E Agar |
Power et al., 2009 [11] | |
| Nystatin | χ | χ | ✓ | *Thayer–Martin Selective Agar | Power et al., 2009 [11] | |
✓ Action against bacteria or fungi; χ No action against bacteria or fungi.
Antiseptics
Antiseptics are more rarely used in culture media. However, cetrimide [43,44] or acriflavin [10] can be found in some culture media. Chlorhexidine can be used to select Mycobacterium tuberculosis [45,46] (Table 2). Ethanol can also select bacterial species, including sporulated bacteria [47,48] such as Clostridioides difficile [47].
Table 2.
Antiseptics used in bacterial culture
| Inhibitors | Microorganisms |
Example of culture media | References | ||
|---|---|---|---|---|---|
| Gram-positive | Gram-negative | Fungi, yeast | |||
| Chlorhexidine | ✓ | ✓ | χ | *Selection of Mycobacterium tuberculosis | Asmar et al., 2015 [44] Asmar et al., 2016 [45] |
| Cetrimide | ✓ | ✓ | χ | *Cetrimide Agar Base *CN Agar |
Power et al., 2009 [11] Delarras, 2014 [39] |
| Acriflavin | ✓ | χ | χ | *Fraser Broth Base *Listeria Enrichment Broth *PALCAM Medium Base |
Power et al., 2009 [11] |
✓ Action against bacteria or fungi; χ No action against bacteria or fungi.
Cetrimide Agar Base is a culture medium used to selectively isolate and identify Pseudomonas aeruginosa. Cetrimide is a quaternary ammonium that inhibits a large number of bacteria, including those of the genus Pseudomonas, other than Pseudomonas aeruginosa [10].
Sodium salts
Sodium salts are known for their inhibitory properties. The best known is sodium chloride, used to select halophilic bacteria that resist very high amounts of salts [49]. In addition, sodium deoxycholate has a strong solvent action on bacteria [50] (Table 3).
Table 3.
Sodium salts
| Inhibitors | Microorganisms |
Example of culture media | References | ||
|---|---|---|---|---|---|
| Gram-positive | Gram-negative | Fungi, yeast | |||
| Sodium azide | χ | ✓ | χ | *Azide Blood Agar Base *Azide Dextrose Broth *m E Agar *EVA Broth |
Power et al., 2009 [11] |
| Sodium chloride |
✓ Except halophilic and halotolerant bacteria |
✓ Except halophilic and halotolerant bacteria |
χ | *Fraser Broth Base *Mannitol Salt Agar *Marine Agar 2216 |
Power et al., 2009 [11] |
| Sodium deoxycholate | ✓ | ✓ | χ | *m TEC Agar *TT Broth Base, Hajna *XLD Agar |
Power et al., 2009 [11] |
| Sodium citrate | ✓ | χ | χ | *APT Agar *DCLS Agar *Desoxycholate Citrate Agar |
Power et al., 2009 [11] |
| Sodium selenite | ✓ | ✓ Other than Salmonella | χ | *Selenite Cystine Broth *Selenite Broth |
Power et al., 2009 [11] |
| Sodium tetrathionate | χ | ✓ Other than Salmonella and Proteus | χ | *MKTTn Broth | Delarras, 2014 [39] |
✓ Action against bacteria or fungi; χ No action against bacteria or fungi.
The Marine Agar 2216E culture medium is used to enumerate marine heterotrophic bacteria. It is composed of a high concentration of salt, which eliminates a large number of bacteria and preserves marine bacteria of interest [51].
Chemical substances
Chemical substances can be added to culture media to inhibit certain bacteria. These inhibiting substances include potassium tellurite and bile salts, which inhibit Gram-positive bacteria [10,39,52] or lithium chloride [10,39], which eliminates Gram-negative bacteria (Table 4).
Table 4.
Chemical substances
| Inhibitors | Microorganisms |
Example of culture media | References | ||
|---|---|---|---|---|---|
| Gram-positive | Gram-negative | Fungi, yeast | |||
| Bile salts | ✓ | χ | χ | *Bile Esculin Agar *EC Medium *m FC Agar and Broth |
Power et al., 2009 [11] |
| Ox gall | ✓ | χ | χ | *Bile Esculin Agar *Brilliant Green Bile Agar |
Power et al., 2009 [11] |
| Lithium chloride | χ | ✓ | χ | *VJ Agar *Baird–Parker Agar Base *Giolitti–Cantoni Broth Base |
Power et al., 2009 [11] |
| d-cycloserine | ✓ | ✓ | χ | *Cycloserine-cetoxitin-fructose agar *Tryptose Sulphite Cycloserine Agar |
Power et al., 2009 [11] |
| Irgasan | ✓ | ✓ Other than Pseudomonas | χ | *CIN Agar *Pseudomonas Isolation Agar |
Power et al., 2009 [11] |
| Tergitol 7 | ✓ | ✓ | χ | *TTC and Tergitol 7 Lactose Agar | Delarras, 2014 [39] |
| Potassium tellurite | ✓ | χ | χ | *Serum Tellurite Agar *Tellurite Glycine Agar |
Power et al., 2009 [11] |
| Lauryl sulphates | ✓ | χ | χ | *m Endo Agar LES *Lauryl Tryptose Broth *Lauryl Sulphate Broth |
Power et al., 2009 [11] |
✓ Action against bacteria or fungi; χ No action against bacteria or fungi.
Brayton et al. [53] have created a selective culture medium for Vibrio vulnificus, which is a pathogenic halophilic bacterium. This medium, VV agar, consists, among other things, of potassium tellurite as selective agent for inhibiting Enterobacteriaceae.
Dyes
Dyes can be used as a colour indicator in a culture medium or as a selective agent against certain bacteria. Crystal violet is one of the most commonly used dyes to inhibit bacteria [37,54]. Malachite green and methylene blue are also used to inhibit Gram-positive and Gram-negative bacteria and Gram-positive bacteria, respectively [10,55] (Table 5).
Table 5.
Dyes
| Inhibitors | Microorganisms |
Example of culture media | References | ||
|---|---|---|---|---|---|
| Gram-positive | Gram-negative | Fungi, yeast | |||
| Methylene blue | ✓ | χ | χ | *Eosin Methylene Blue Agar *Levine EMB Agar |
Power et al., 2009 [11] Delarras., 2014 [39] |
| Eosin | ✓ | χ | χ | *Eosin Methylene Blue Agar *Levine EMB Agar |
Power et al., 2009 [11] Delarras, 2014 [39] |
| Crystal violet | ✓ Cocci | χ | χ | *MacConkey Agar *Mitis Salivarius Agar *Drigalski medium |
Power et al., 2009 [11] Delarras, 2014 [39] |
| Ethyl violet | ✓ | χ | χ | *EVA Broth *Litsky Broth |
Power et al., 2009 [11] Delarras, 2014 [39] |
| Brilliant green | ✓ | ✓ | χ | *Brilliant Green Bile Broth *SS Agar |
Power et al., 2009 [11] Delarras, 2014 [39] |
| Malachite green | ✓ | ✓ | χ | *Mycobacteria 7H11 Agar *Wallenstein Medium |
Power et al., 2009 [11] Delarras, 2014 [39] |
✓ Action against bacteria or fungi; χ No action against bacteria or fungi.
A selective medium of Streptococcus pneumoniae has been developed, containing crystal violet. This dye is used to select streptococci and inhibit staphylococci as well as other Gram-positive bacteria [54].
Phages
Bacteriophages are specific viruses of bacteria that can infect and even destroy bacteria, in the case of lytic phages. In order to isolate Mycobacterium tuberculosis, the use of phage lysin decontaminates the sputum of other bacteria present in the pulmonary microbiota [56].
Sillankorva et al. [57] worked on permanent urinary tract infections due to Escherichia coli. In order to treat these infections, they tested different phages (T1, T4 and φX174-like phages) against E. coli. After 2 hours of treatment, phage T1 reduced the E. coli population by 45%, demonstrating the efficacy of this selective agent.
Disadvantages of gelling agents use
Liquid culture media versus solid culture media
There are two main types of culture media, liquid and solid.
In liquid culture media, also called culture broths, nutrients are dissolved in water. The growth of bacteria in this type of medium can be demonstrated by the appearance of a turbidity in the medium, although this is not always the case. It is difficult to isolate a bacterium specifically in this type of medium. Indeed, the bacteria obtained from a sample inoculated into the culture broth are all mixed with each other. In addition, this type of culture medium does not allow the morphological characteristics of bacterial species to be identified [9].
However, liquid culture media facilitate access to nutrients for bacteria. These nutrients are all the more accessible as the culture media are incubated under agitation, allowing a renewal of nutrients for bacteria.
Solid culture media are obtained by adding a gelling agent, such as agar, to the culture broth. They make it possible to obtain isolated colonies of different bacterial species, which can be identified. The different morphological characteristics of the bacterium can be described from these cultures [9,58]. However, in solid culture media, access to nutrients for bacteria may be limited. Media with high gel content, such as agar, will form smaller colonies than low gel content media because nutrient flow and toxin removal are reduced [7].
In addition, it has been shown that agar, in excessive quantities, can inhibit the growth of certain bacteria, highlighting the need to find other gelling agents [9].
Different gelling agents
One of the first gelling agents used in culture media was gelatin. The problem with this gelling agent is that it melts at 37°C, which is the incubation temperature of most bacteria. Moreover, the presence of an enzyme in certain bacteria, gelatinase, causes the digestion of gelatin and therefore its degradation. Agar was then used in culture media. However, over-consumption of agar has led to a reduction in its source, red algae [59], which has increased costs. In addition, agarase, present in some bacteria, destroys agar, preventing the isolation of these bacteria [60]. In addition, agar can inhibit the growth of some anaerobic bacteria because inhibitory growth compounds can be produced from autoclaving phosphate with agar [61]. Finally, agar can cause inhibition of PCR of fungal DNA when extracted directly from the solid culture medium [62]. For all these reasons, new gelling agents have been sought. These gelling agents include κ-carrageenan, ι-carrageenan, sodium alginate, high-methoxyl and low-methoxyl pectins and gellan gum [60,63]. All these gelling agents have different properties and particular needs to gel (Table 6).
Table 6.
| Origin | Type | Gel texture | Necessary ions | Gelling temperature | Melting temperature |
|---|---|---|---|---|---|
| Red seaweed extracts | Agar | Firm, brittle, transparent Acid-resistant (up to pH 3.5) |
<35°C | >80°C | |
| Red seaweed extracts | Carrageenans (kappa, iota) | Kappa Elastic or firm (depending on concentration), transparent, glossy. Very fast gel setting |
K+ | <40°C | >65°C |
| Iota Elastic, transparent, reformable |
Na+ or K+ | ||||
| Brown seaweed extracts | Sodium alginate | Flexible gel | Ca2+ | Whatever the temperature | Thermo-irreversible |
| Extracts of vegetable by-products | HM Pectin | Gels in an acidic environment (pH < 3) and in the presence of sugar Slow gel setting ‘Spreadable’ gel |
<65°C | Thermo-irreversible | |
| Extracts of vegetable by-products | LM Pectine | Brittle, transparent | Ca2+ | Thermo-reversible | |
| Biosynthetics | Gellan gum | Transparent, shiny, firm Stable up to pH 3 |
<90°C | >90°C | |
| Animal | Gelatin | Elastic gel, transparent | <20°C | >40°C | |
| Xanthomonas campestris bacteria | Xanthan gum (+carob bean gum) | Stable over a wide temperature and pH range Soft elastic gel in combination with carob bean gum and in the presence of salts |
270°C thermo-reversible | ||
| Plant exudates | Arabic gum | Soft gel (>10% of final volume) Stable in acidic medium Heating must be limited in time as there is a risk of loss of the gum's gelification capacity |
|||
| Animal | Egg | Thermo-irreversible |
Carrageenan gums
κ-carrageenan and ι-carrageenan are part of the carrageenan gums.
The first will form a firm gel with a rapid mass build-up when combined with potassium ions. It allows the growth of some bacteria. This gelling agent resists very alkaline pH values above 12.5, so can isolate very highly alkaliphilic bacteria [64]. It can be used to replace agar because many bacteria grow on κ-carrageenan-based media [65].
ι-carrageenan is rarely used because it gives elastic gels that make bacterial culture difficult [65].
Sodium alginate
This gelling agent is produced from brown seaweed extract and forms a flexible gel in the presence of calcium ions. However, this gelling agent does not provide a gel firm enough to grow bacteria [63].
High methoxyl and low methoxyl pectins
High-methoxyl pectins require sugar and high acidity to gel. Gel setting is slow and results in the formation of a ‘spreadable’ gel.
Low-methoxyl pectins form brittle gels in the presence of Ca2+ [62].
Gellan gum
Gellan gum is a polysaccharide produced by a bacterial genus, Sphingomonas spp. According to Tamaki et al. [66], 108 bacteria tested on media with gellan gum as gelling agent showed growth.
The use of these different gelling agents could allow the culture of new bacteria, which do not grow on the agar, because of the presence of an agarase for example or because the agar forms a network too dense to allow motility and optimal growth of certain bacteria [9].
Colony size
The amount of nutrients available in a culture medium will determine the size of bacterial colonies [58]. An overly firm culture medium, due to a high concentration of gelling agent, causes a decrease in the flow of nutrients and so a decrease in the access to these nutrients by bacteria [7,9]. On the other hand, in some culture media, the amount of nutrients available is too high and can be toxic for certain bacteria that require a poor culture medium to grow [68]. Microcolonies are colonies that are barely visible to the naked eye (between 100 and 300 μm in diameter). To obtain larger colonies, it is sometimes necessary to mimic the bacterium's natural environment by providing it with specific elements. This is the case, for example, for Phascolarctobacterium faecium and Phascolarctobacterium succinatutens, which form microcolonies. However, when the medium is supplemented with succinate, the colonies have a diameter ranging from 0.8 to 1.2 mm [69,70].
Conclusion
After stagnation in the development of new culture techniques, due to the rapid evolution of new microbiological methods such as metagenomics, bacterial culture is experiencing a new boom. In recent years, culturomics, with the use of new culture media and new culture conditions, has enabled the enrichment of the bacterial repertoire through the isolation of new bacterial species. This shows that, despite the abandonment of culture by a large number of microbiologists, culture media remain a fundamental tool for bacteriologists for the isolation of commensal but also pathogenic bacteria.
Studying the natural environment of bacteria that have remained uncultivated to date would be interesting because it would provide the essential elements for the bacteria to grow. Indeed, although there are many enriched culture media, each bacterium is unique and has specific requirements. The use of new gelling agents could also allow the isolation of new species for which agar was not suitable for their growth. Although many gelling agents have been tested, few are still used in commercial culture media and therefore in laboratories. Many developments in bacterial culture are therefore still to come, making it possible to enrich the bacterial repertoire and gain a better understanding of certain diseases.
In addition, intracellular bacteria such as Coxiella burnetii or Tropheryma whipplei require a host cell to survive and multiply. Some of these bacteria cause severe diseases and pose a diagnostic problem because of their fastidious growth or lack of growth on conventional media [71]. It would be interesting to develop culture media that allow faster and easier detection of these bacteria. In addition, as the microbiota plays an increasingly important role in human health [72,73], the development of probiotics is on the rise [74]. The use of targeted culture media to select certain bacteria with an important medical role therefore remains a priority.
Funding sources
This research is funded by the Agence Nationale de la Recherche as part of the Méditerranée Infection 10-IAHU-03 project.
Conflict of interest
No conflict of interest has been declared.
References
- 1.Wainwright M., Lederberg J. History of microbiology. Encyclop Microbiol. 1992;2:419–437. [Google Scholar]
- 2.Lagier J.C., Hugon P., Khelaifia S., Fournier P.E., La Scola B., Raoult D. The rebirth of culture in microbiology through the example of culturomics to study human gut microbiota. Clin Microbiol Rev. 2015;28:237–264. doi: 10.1128/CMR.00014-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Prescott L.M., Willey J.M., Sherwood L.M., Woolverton C.J. 5ème edition. De Boeck Supérieur; Louvain-la-Neuve: 2018. Microbiologie. [Google Scholar]
- 4.Pommerville J.C. 10th ed. Jones & Bartlett; Burlington: 2013. Fundamentals of microbiology. [Google Scholar]
- 5.Weeks B. 3rd ed. Jones & Bartlett; Sudbury: 2012. Alcamo's microbes and society. [Google Scholar]
- 6.Yu V.L. Serratia marcescens: historical perspective and clinical review. N Engl J Med. 1979;300:887–893. doi: 10.1056/NEJM197904193001604. [DOI] [PubMed] [Google Scholar]
- 7.Murray P.R., Rosenthal K.S., Pfaller M.A. 8th ed. Elsevier Health Sciences; Philadelphia: 2015. Medical microbiology. [Google Scholar]
- 8.Sandle T. History and development of microbiological culture media. J Inst Sci Technol. 2011:10–14. [Google Scholar]
- 9.Vallery-Radot P. Masson et compagnie; Paris: 1922. Les œuvres de Pasteur; p. 2. [Google Scholar]
- 10.Kumar S. JP Medical Ltd; New Delhi: 2012. Textbook of microbiology. [Google Scholar]
- 11.Power D.A., Johnson J.A. 2nd ed. Becton, Dickinson and Company; Sparks: 2009. Difco™ & BBL™ manual. [Google Scholar]
- 12.Atlas R.M. 3rd ed. CRC Press; Boca Raton, FL: 2010. Handbook of microbiological media. [Google Scholar]
- 13.Latge J.P. Croissance et sporulation de 6 espèces d'Entomophthorales II. Influence de diverses sources d'azote. Mycopathologia. 1975;57:53–57. doi: 10.1007/BF00431180. [DOI] [PubMed] [Google Scholar]
- 14.Yurkov V.V., Beatty J.T. Aerobic anoxygenic phototrophic bacteria. Microbiol Mol Biol Rev. 1998;62:695–724. doi: 10.1128/mmbr.62.3.695-724.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thauer R.K., Jungermann K., Decker K. Energy conservation in chemotrophic anaerobic bacteria. Bacteriol Rev. 1977;41:100. doi: 10.1128/br.41.1.100-180.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Van der Horst M.A., Key J., Hellingwerf K.J. Photosensing in chemotrophic, non-phototrophic bacteria: let there be light sensing too. Trends Microbiol. 2007;15:554–562. doi: 10.1016/j.tim.2007.09.009. [DOI] [PubMed] [Google Scholar]
- 17.Werkman C.H., Wilson P.W. Academic Press Inc.; New-York: 1951. Bacterial physiology. [Google Scholar]
- 18.Snell E.E., Mitchell H.K. Purine and pyrimidine as growth substances for lactic acid bacteria. Proc Natl Acad Sci USA. 1941;27:1. doi: 10.1073/pnas.27.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dunn M.S., Shankman S., Camien M.N., Block H. The amino acid requirements of twenty-three lactic acid bacteria. J Biol Chem. 1947;168:1–22. [PubMed] [Google Scholar]
- 20.Magdoub M.N., Hassan Z.M., Effat B.A., Sadek Z.I., Tawfik N.F., Mabrouk A.M. Probiotic properties of some lactic acid bacteria isolated from Egyptian dairy products. Int J Curr Microbiol Appl Sci. 2015;4:758–766. [Google Scholar]
- 21.Duncan S.H., Hold G.L., Harmsen H.J., Stewart C.S., Flint H.J. Growth requirements and fermentation products of Fusobacterium prausnitzii, and a proposal to reclassify it as Faecalibacterium prausnitzii gen. nov., comb. nov. Int J Syst Evol Microbiol. 2002;52:2141–2146. doi: 10.1099/00207713-52-6-2141. [DOI] [PubMed] [Google Scholar]
- 22.Collins C.H., Lyne P.M. 3rd ed. Arnold; London: 1970. Microbiological methods. [Google Scholar]
- 23.Vastine D.W., Dawson C.R., Hoshiwara I., Yoneda C., Daghfous T., Messadi M. Comparison of media for the isolation of Haemophilus species from cases of seasonal conjunctivitis associated with severe endemic trachoma. Appl Environ Microbiol. 1974;28:688–690. doi: 10.1128/am.28.4.688-690.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cézard F. 2nd édition. Dunod; Paris: 2013. Biotechnologies en 27 fiches. [Google Scholar]
- 25.Albertson N., Wenngren I., Sjöström J.E. Growth and survival of Helicobacter pylori in defined medium and susceptibility to Brij 78. J Clin Microbiol. 1998;36:1232–1235. doi: 10.1128/jcm.36.5.1232-1235.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Northoff H., Flegel W.A. Fetal calf serum. In: Northoff H., Flegel W.A., editors. Encyclopedia of immunology. Elsevier Health Sciences; Philadelphia: 1998. pp. 896–897. [Google Scholar]
- 27.Meenakshi A. Cell culture media: a review. Mater Methods. 2013;3:175. [Google Scholar]
- 28.Rahman H., Qasim M., Schultze F.C., Oellerich M., Asif A.R. Fetal calf serum heat inactivation and lipopolysaccharide contamination influence the human T lymphoblast proteome and phosphoproteome. Proteome Sci. 2011;9:71. doi: 10.1186/1477-5956-9-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lagier J.C., Armougom F., Million M., Hugon P., Pagnier I., Robert C. Microbial culturomics: paradigm shift in the human gut microbiome study. Clin Microbiol Infect. 2012;18:1185–1193. doi: 10.1111/1469-0691.12023. [DOI] [PubMed] [Google Scholar]
- 30.Goodman A.L., Kallstrom G., Faith J.J., Reyes A., Moore A., Dantas G. Extensive personal human gut microbiota culture collections characterized and manipulated in gnotobiotic mice. Proc Natl Acad Sci USA. 2011;108:6252–6257. doi: 10.1073/pnas.1102938108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Szynkiewicz Z.M., Binek M. Evaluation of selective media for primary isolation of Treponema hyodysenteriae and Treponema innocens. Comp Immunol Microbiol Infect Dis. 1986;9:71–77. doi: 10.1016/0147-9571(86)90077-9. [DOI] [PubMed] [Google Scholar]
- 32.Bennett J.E., Dolin R., Blaser M.J. 8th ed. vol. 1. Elsevier Health Sciences; Philadelphia: 2014. (Mandell, Douglas, and Bennett's principles and practice of infectious diseases). [Google Scholar]
- 33.Lagier J.C., Edouard S., Pagnier I., Mediannikov O., Drancourt M., Raoult D. Current and past strategies for bacterial culture in clinical microbiology. Clin Microbiol Infect. 2015;28:208–236. doi: 10.1128/CMR.00110-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.La Scola B., Khelaifia S., Lagier J.C., Raoult D. Aerobic culture of anaerobic bacteria using antioxidants: a preliminary report. Eur J Clin Microbiol Infect Dis. 2014;33:1781–1783. doi: 10.1007/s10096-014-2137-4. [DOI] [PubMed] [Google Scholar]
- 35.Dione N., Khelaifia S., La Scola B., Lagier J.C., Raoult D. A quasi-universal medium to break the aerobic/anaerobic bacterial culture dichotomy in clinical microbiology. Clin Microbiol Infect. 2016;22:53–58. doi: 10.1016/j.cmi.2015.10.032. [DOI] [PubMed] [Google Scholar]
- 36.Harrigan W.F. 3rd ed. Academic Press; California: 1998. Laboratory methods in food microbiology. [Google Scholar]
- 37.Entis P. The Food Processor Institute; Washington D.C: 2002. Food microbiology: the laboratory. [Google Scholar]
- 38.Pilly E.C.N. 5th ed. Alinéa; Paris: 2018. Maladies infectieuses et tropicales; p. 324. [Google Scholar]
- 39.Delarras C. Lavoisier; Paris: 2014. Pratique en microbiologie de laboratoire? Recherche de bactéries et de levures-moisissures. [Google Scholar]
- 40.Sachan N., Agarwal R.K. Selective enrichment broth for the isolation of Aeromonas sp. from chicken meat. Int J Food Microbiol. 2000;60:65–74. doi: 10.1016/s0168-1605(00)00322-6. [DOI] [PubMed] [Google Scholar]
- 41.Corry J.E., Curtis G.D., Baird R.M. 3rd ed. Royal Society of Chemistry; Cambridge: 2011. Handbook of culture media for food and water microbiology. [Google Scholar]
- 42.Bartram J., Chartier Y., Lee J.V., Pond K., Surman-Lee S. World Health Organization; Geneva: 2007. Legionella and the prevention of legionellosis. [Google Scholar]
- 43.Brown V.I., Lowbury E.J.L. Use of an improved cetrimide agar medium and other culture methods for Pseudomonas aeruginosa. J Clin Pathol. 1965;18:752–756. doi: 10.1136/jcp.18.6.752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Oa O., Eabomo O. Antimicrobial activities of chlorhexidine gluconate and cetrimide against pathogenic microorganisms isolated from slaughter houses in Rivers State, Nigeria. Int J Pharm Pharm Sci. 2015;7:322–328. [Google Scholar]
- 45.Asmar S., Drancourt M. Chlorhexidine decontamination of sputum for culturing Mycobacterium tuberculosis. BMC Microbiol. 2015;15:155. doi: 10.1186/s12866-015-0479-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Asmar S., Chatellier S., Mirande C., van Belkum A., Canard I., Raoult D. A chlorhexidine-agar plate culture medium protocol to complement standard broth culture of Mycobacterium tuberculosis. Front Microbiol. 2016;7:30. doi: 10.3389/fmicb.2016.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Browne H.P., Forster S.C., Anonye B.O., Kumar N., Neville B.A., Stares M.D. Culturing of ‘unculturable’ human microbiota reveals novel taxa and extensive sporulation. Nature. 2016;533(7604):543. doi: 10.1038/nature17645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Koransky J.R., Allen S.D., Dowell V.R. Use of ethanol for selective isolation of sporeforming microorganisms. Appl Environ Microbiol. 1978;35:762–765. doi: 10.1128/aem.35.4.762-765.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Seck E.H., Dufour J.C., Raoult D., Lagier J.C. Halophilic & halotolerant prokaryotes in humans. Future Microbiol. 2018;13:799–812. doi: 10.2217/fmb-2017-0237. [DOI] [PubMed] [Google Scholar]
- 50.Leifson E. New culture media based on sodium desoxycholate for the isolation of intestinal pathogens and for the enumeration of colon bacilli in milk and water. J Pathol Bacteriol. 1935;40:581–599. [Google Scholar]
- 51.Patrick F.M. The use of membrane filtration and marine agar 2216E to enumerate marine heterotrophic bacteria. Aquaculture. 1978;13:369–372. [Google Scholar]
- 52.Hoyle L. A tellurite Blood-Agar Medium for the rapid diagnosis of diphtheria. Lancet. 1941:175–176. [Google Scholar]
- 53.Brayton P.R., West P.A., Russek E., Colwell R.R. New selective plating medium for isolation of Vibrio vulnificus biogroup 1. J Clin Microbiol. 1983;17:1039–1044. doi: 10.1128/jcm.17.6.1039-1044.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nichols T., Freeman R. A new selective medium for Streptococcus pneumoniae. J Clin Pathol. 1980;33:770–773. doi: 10.1136/jcp.33.8.770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Arroyo G., Arroyo J.A. Selective action of inhibitors used in different culture media on the competitive microflora of Salmonella. J Appl Microbiol. 1995;78:281–289. doi: 10.1111/j.1365-2672.1995.tb05027.x. [DOI] [PubMed] [Google Scholar]
- 56.Subramanyam B., Sivaramakrishnan G.N., Dusthackeer A., Nagamiah S., Kumar V. Phage lysin as a substitute for antibiotics to detect Mycobacterium tuberculosis from sputum samples with the BACTEC MGIT 960 system. Clin Microbiol Infect. 2012;18:497–501. doi: 10.1111/j.1469-0691.2011.03601.x. [DOI] [PubMed] [Google Scholar]
- 57.Sillankorva S., Oliveira D., Moura A., Henriques M., Faustino A., Nicolau A. Efficacy of a broad host range lytic bacteriophage against E. coli adhered to urothelium. Curr Microbiol. 2011;62:1128–1132. doi: 10.1007/s00284-010-9834-8. [DOI] [PubMed] [Google Scholar]
- 58.Denyer S.P., Hodges N.A., Gorman S.P. 7th ed. Blackwell Science; Massachusetts: 2008. Hugo and Russell's pharmaceutical microbiology. [Google Scholar]
- 59.McLachlan J. Biosalinity in action: bioproduction with saline water. Springer; Dordrecht: 1985. Macroalgae (seaweeds): industrial resources and their utilization; pp. 137–157. [Google Scholar]
- 60.Das N., Triparthi N., Basu S., Bose C., Maitra S., Khurana S. Progress in the development of gelling agents for improved culturability of microorganisms. Front Microbiol. 2015;6:698. doi: 10.3389/fmicb.2015.00698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tanaka T., Kawasaki K., Daimon S., Kitagawa W., Yamamoto K., Tamaki H. A hidden pitfall in the preparation of agar media undermines microorganism cultivability. Appl Environ Microbiol. 2014;80:7659–7666. doi: 10.1128/AEM.02741-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schmidt D., Rath P.M. Faster genetic identification of medically important aspergilli by using gellan gum as gelling agent in mycological media. J Med Microbiol. 2003;52:653–655. doi: 10.1099/jmm.0.05135-0. [DOI] [PubMed] [Google Scholar]
- 63.Doublier J.L. 2001. Les systèmes alimentaires gélifiés: structure et propriétés fonctionnelles. 9ème Colloque de l'Alliance 7/CEDUS : le sucre et la conservation des produits à base de fruits. [Google Scholar]
- 64.Datta S., Mody K., Gopalsamy G., Jha B. Novel application of κ-carrageenan: as a gelling agent in microbiological media to study biodiversity of extreme alkaliphiles. Carbohydr Polym. 2011;85:465–468. [Google Scholar]
- 65.Abbott I.A., Chapman F.A. Evaluation of kappa carrageenan as a substitute for agar in microbiological media. Arch Microbiol. 1981;128:355–359. doi: 10.1007/BF00405912. [DOI] [PubMed] [Google Scholar]
- 66.Tamaki H., Hanada S., Sekiguchi Y., Tanaka Y., Kamagata Y. Effect of gelling agent on colony formation in solid cultivation of microbial community in lake sediment. Environ Microbiol. 2009;11:1827–1834. doi: 10.1111/j.1462-2920.2009.01907.x. [DOI] [PubMed] [Google Scholar]
- 67.Tako M. The principle of polysaccharide gels. Adv Biosci Biotechnol. 2015;6:22. [Google Scholar]
- 68.Ferrari B.C., Binnerup S.J., Gillings M. Microcolony cultivation on a soil substrate membrane system selects for previously uncultured soil bacteria. Appl Environ Microbiol. 2005;71:8714–8720. doi: 10.1128/AEM.71.12.8714-8720.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Del Dot T., Osawa R., Stackebrandt E. Phascolarctobacterium faecium gen. nov, spec. nov., a novel taxon of the Sporomusa group of bacteria. Syst Appl Microbiol. 1993;16:380–384. [Google Scholar]
- 70.Watanabe Y., Nagai F., Morotomi M. Characterization of Phascolarctobacterium succinatutens sp. nov., an asaccharolytic, succinate-utilizing bacterium isolated from human feces. Appl Environ Microbiol. 2012;78:511–518. doi: 10.1128/AEM.06035-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lamoth F., Schrenzel J., Greub G. Approches diagnostiques des bactéries intracellulaires et des germes fastidieux. Rev Med Suisse. 2014;10:2130–2136. [PubMed] [Google Scholar]
- 72.Sekirov I., Russell S.L., Antunes L.C.M., Finlay B.B. Gut microbiota in health and disease. Physiol Rev. 2010;90:859–904. doi: 10.1152/physrev.00045.2009. [DOI] [PubMed] [Google Scholar]
- 73.Guinane C.M., Cotter P.D. Role of the gut microbiota in health and chronic gastrointestinal disease: understanding a hidden metabolic organ. Therap Adv Gastroenterol. 2013;6:295–308. doi: 10.1177/1756283X13482996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kerry R.G., Patra J.K., Gouda S., Park Y., Shin H.S., Das G. Benefaction of probiotics for human health: a review. J Food Drug Anal. 2018;26:927–939. doi: 10.1016/j.jfda.2018.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]