To the Editor:
Obstructive sleep apnea (OSA) is associated with systemic hypertension and cardiovascular comorbidities (1), but the underlying pathophysiology is not well understood. In our SOX (Supplemental Oxygen in OSA) randomized controlled trial, recently published in this journal, supplemental oxygen virtually abolished the morning rise in blood pressure normally seen during continuous positive airway pressure (CPAP) withdrawal (2). This suggests that intermittent hypoxia (IH) is the dominant cause of morning blood pressure elevations during CPAP withdrawal, rather than recurrent arousals. However, after CPAP withdrawal, we did not observe differences in secondary outcome measures of systemic inflammation (C-reactive protein) and sympathetic activation (urinary normetadrenaline). The role of IH-mediated oxidative stress and inflammation in the development of OSA-mediated cardiovascular disease is controversial (3, 4).
In the present study, we used a hypothesis-free approach to identify potential unique transcriptomic profiles from whole-blood leukocytes that were activated by IH, such as that occurring when CPAP is withdrawn, and to explore the effects of supplemental oxygen (which largely attenuated this IH) on these profiles.
We used samples from the SOX trial. Briefly, patients with known moderate to severe OSA, previously established on CPAP, were withdrawn from CPAP onto supplemental oxygen or supplemental air (sham) for 14 nights in a randomized crossover design. Supplemental oxygen markedly attenuated IH; the median (first and third quartiles) oxygen desaturation index was 32.5/h (25.6 and 47.0) during sham and 6.4/h (4.0 and 14.7) during supplemental oxygen.
Morning blood samples were collected before and after 14 nights in both groups. Whole-blood leukocytes were extracted and stored for subsequent RNA extraction, using LeukoLOCK kits. After leukocyte RNA extraction, 3′ RNA sequencing was performed (Lexogen) on the Hiseq 4000 platform (Illumina) with an average of 5 million reads per sample. Reads were aligned using Htseq v0.6.1 with DESEQ2 v1.10.1 for normalization of data and differential expression (http://www.bioconductor.org/packages/release/bioc/html/DESeq2.html). Gene set enrichment analysis was performed using the Hallmark Molecular Signatures Database (MsigDB) within the JavaGSEA version 2 platform (http://software.broadinstitute.org/gsea/index.jsp). These are 50 of the most well-defined biological processes or states.
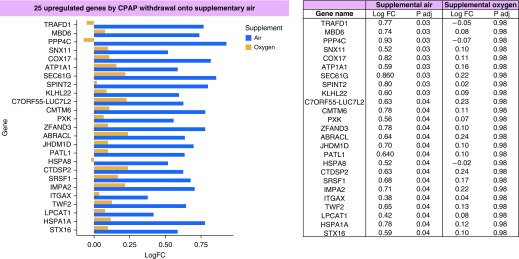
After false discovery rate correction (Benjamin-Hochberg), 25 genes were significantly upregulated after CPAP withdrawal onto sham, compared with baseline (Figure 1). These included several genes (TRAFD1, PPP4C, ZFAND3, HPSA8, ITGAX, and HSPA1A) involved in NFκB (nuclear factor kappa B) signaling, which is an IH-driven inflammatory pathway (5, 6). In contrast, there were no significantly differentially expressed genes after CPAP withdrawal onto supplemental oxygen.
Figure 1.
Bar chart showing the 25 genes that were significantly upregulated after continuous positive airway pressure (CPAP) withdrawal onto supplemental air (sham) compared with baseline. Blue bars represent the log fold change for CPAP withdrawal onto air; yellow bars represent the log fold change for the same genes with CPAP withdrawal onto supplemental oxygen. The log fold changes and adjusted P values are shown on the right of this figure. LogFC = log fold change to the base 2; P adj = P value after adjustment for false discoveries.
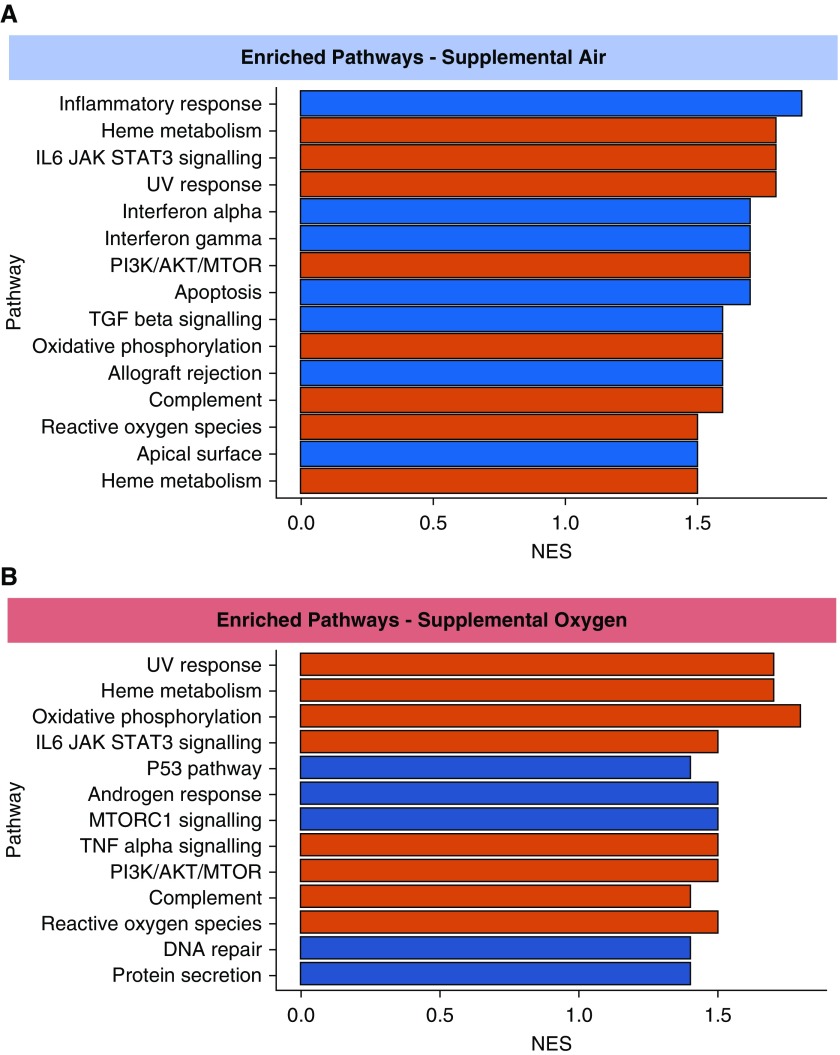
Next, we looked at whether any Hallmark MsigDB gene pathways were enriched in our data sets. As shown in Figure 2, 15 pathways were upregulated after CPAP withdrawal onto sham. Of these, seven pathways were only upregulated with CPAP withdrawal onto sham and not supplemental oxygen. These included the inflammatory response, IFNα response, IFNγ response, and TGFβ signaling. IFNα and IFNγ pathways are upstream modulators of NFκB. Eight pathways were significantly upregulated after CPAP withdrawal in both groups, including pathways relating to oxidative stress (reactive oxygen species and oxidative phosphorylation) and inflammatory pathways (TNFA signaling, IL6/JAK/STAT3 signaling). There were five pathways that were only upregulated with CPAP withdrawal onto supplemental oxygen and not sham. These included MTORC1 signaling and DNA repair pathways.
Figure 2.
Bar charts showing (A) significantly enriched (upregulated) pathways of genes with continuous positive airway pressure withdrawal onto supplemental air and (B) significantly enriched (upregulated) pathways of genes with continuous positive airway pressure withdrawal onto supplemental oxygen. Red bars signify significantly enriched pathways common to both groups. Because of the small number of samples, a stringent false discovery rate corrected q value <0.05 was considered significant. NES = normalized enrichment score; UV = ultraviolet.
We report for the first time that after CPAP withdrawal the return of OSA results in upregulation of several genes in circulating leukocytes, and importantly, that no single gene was upregulated after CPAP withdrawal in the presence of supplemental oxygen (that attenuates IH), suggesting that the IH is the driving factor. Further pathway analysis demonstrated enrichment of several proinflammatory pathways, such as inflammatory response, IFNα, and IFNγ with sham, that was not seen in the supplemental oxygen group. The upregulation of these genes and gene pathways with sham, but not with supplemental oxygen, suggests that IH may lead to cardiovascular disease through the activation of inflammatory processes, possibly through NFκB signaling, as previously suggested (3). There were also inflammatory NFκB-mediated pathways that were enriched in both groups (TNFA signaling and IL6/JAK/STAT3 signaling), and although it was not a direct comparison, the normalized enrichment scores were lower in the supplemental oxygen group, again suggesting IH-driven, NFκB-mediated inflammation in OSA.
In contrast, pathways relating to oxidative stress were similarly enriched with CPAP withdrawal in both groups. There are several possible explanations for this result. First, it could be that oxidative stress is caused by non–hypoxia-mediated mechanisms, such as sleep deprivation (7) or sleep fragmentation, which occurred to an equal extent in both groups. Second, although supplemental oxygen attenuated the IH, it did not fully abolish it. Therefore, it could be that smaller oscillations in oxygen saturations still lead to cyclical deoxygenation and reoxygenation sufficient to trigger oxidative stress (8). Third, there could be different mechanisms inducing oxidative stress, with intermittent hyperoxia in the supplemental oxygen group and intermittent hypoxia in the sham group both causing oxidative stress.
Some pathways were only significantly upregulated after CPAP withdrawal onto supplemental oxygen, including the DNA repair pathway. This pathway has been implicated in hyperoxic damage (9), and supplemental oxygen may have potentially deleterious effects, as has been shown in the context of acute myocardial infarction (10).
In summary, our results have identified that there are discernible alterations in the transcriptome of circulating leukocytes after CPAP withdrawal. Some caveats must be applied to our conclusions; principally, transcriptomic changes were identified in leukocytes, and it would be unwise to extrapolate these findings to other tissues. Nevertheless, the changes observed in gene expression support a proinflammatory role of IH in OSA and suggest that supplemental oxygen may attenuate inflammation in OSA. The role of the transcriptional changes we observed in the large blood pressure effect seen in the main SOX study is uncertain (2). In any case, the IH-mediated inflammation demonstrated in this study could contribute to the development of atherosclerosis and related cardiovascular morbidities known to be associated with uncontrolled OSA. Further confirmatory work is needed to fully understand the physiological effects, long-term efficacy, safety, and tolerability of supplemental oxygen to establish whether it is a viable treatment option when CPAP is not tolerated.
Supplementary Material
Footnotes
Supported by the Oxford Radcliffe Hospital Charitable Funds and ResMed UK and by the National Institute for Health Research (NIHR) Oxford Biomedical Research Centre. The views expressed are those of the authors and not necessarily of the NHS, the NIHR, or the Department of Health. N.P. was supported by a NIHR Academic Clinical Lectureship.
Author Contributions: C.D.T., J.S., and N.P. were responsible for study design; C.D.T., D.S., and N.P. performed data collection; C.D.T., L.Y.W.L., T.S. and N.P. performed data analysis; C.D.T. performed literature searches; and all authors contributed to data interpretation and manuscript writing.
Originally Published in Press as DOI: 10.1164/rccm.201904-0832LE on September 13, 2019
Author disclosures are available with the text of this letter at www.atsjournals.org.
References
- 1.Marin JM, Carrizo SJ, Vicente E, Agusti AG. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365:1046–1053. doi: 10.1016/S0140-6736(05)71141-7. [DOI] [PubMed] [Google Scholar]
- 2.Turnbull CD, Sen D, Kohler M, Petousi N, Stradling JR. Effect of supplemental oxygen on blood pressure in obstructive sleep apnea (SOX): a randomized continuous positive airway pressure withdrawal trial. Am J Respir Crit Care Med. 2019;199:211–219. doi: 10.1164/rccm.201802-0240OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ryan S, McNicholas WT. Intermittent hypoxia and activation of inflammatory molecular pathways in OSAS. Arch Physiol Biochem. 2008;114:261–266. doi: 10.1080/13813450802307337. [DOI] [PubMed] [Google Scholar]
- 4.Kohler M, Stradling JR. Mechanisms of vascular damage in obstructive sleep apnea. Nat Rev Cardiol. 2010;7:677–685. doi: 10.1038/nrcardio.2010.145. [DOI] [PubMed] [Google Scholar]
- 5.Liang KC, Suzuki Y, Kumagai Y, Nakai K. Analysis of changes in transcription start site distribution by a classification approach. Gene. 2014;537:29–40. doi: 10.1016/j.gene.2013.12.038. [DOI] [PubMed] [Google Scholar]
- 6.Yang Y, Wu J, Wang J. A database and functional annotation of NF-κB target genes. Int J Clin Exp Med. 2016;9:7986–7995. [Google Scholar]
- 7.Villafuerte G, Miguel-Puga A, Rodríguez EM, Machado S, Manjarrez E, Arias-Carrión O. Sleep deprivation and oxidative stress in animal models: a systematic review. Oxid Med Cell Longev. 2015;2015:234952. doi: 10.1155/2015/234952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Neri M, Riezzo I, Pascale N, Pomara C, Turillazzi E. Ischemia/reperfusion injury following acute myocardial infarction: a critical issue for clinicians and forensic pathologists. Mediators Inflamm. 2017;2017:7018393. doi: 10.1155/2017/7018393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lingappan K, Maity S, Jiang W, Wang L, Couroucli X, Veith A, et al. Role of cytochrome P450 (CYP)1A in hyperoxic lung injury: analysis of the transcriptome and proteome. Sci Rep. 2017;7:642. doi: 10.1038/s41598-017-00516-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stub D, Smith K, Bernard S, Nehme Z, Stephenson M, Bray JE, et al. AVOID Investigators. Air versus oxygen in ST-segment-elevation myocardial infarction. Circulation. 2015;131:2143–2150. doi: 10.1161/CIRCULATIONAHA.114.014494. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.