To the Editor:
Interstitial lung disease (ILD) encompasses a large number of diffuse parenchymal lung diseases that commonly result in pulmonary fibrosis (PF). The most common forms of ILD include idiopathic PF (IPF), connective tissue disease–associated ILD (CTD-ILD), chronic hypersensitivity pneumonitis (CHP), and unclassifiable ILD (U-ILD). Various circulating plasma biomarkers, including CXCL13 (1), CA-125 (2), MMP7 (3), SP-D (2), YKL-40 (4), and VCAM-1 (3), have been linked to differential survival in patients with IPF, but their utility in other ILD subtypes is unclear. In this investigation, we hypothesized that increased concentrations of the aforementioned plasma biomarkers would predict reduced survival in patients with CTD-ILD, CHP, or U-ILD.
Methods
Consecutive patients with longitudinal clinic follow-up and a multidisciplinary diagnosis of CTD-ILD, CHP, or U-ILD who consented to a research blood draw at the University of California, Davis (UC-Davis) (institutional review board #875917) and University of Chicago (institutional review board #14163) were included. Blood was collected from May 2016 to December 2018 at UC-Davis and from March 2010 to August 2016 at the University of Chicago. CTD-ILD subtypes included rheumatoid arthritis, systemic sclerosis, myositis, Sjogren’s syndrome, systemic lupus erythematosus, and mixed connective tissue disease. Vital status was assessed by medical record review and telephone communication.
Stored frozen plasma in ethylenediaminetetraacetic acid aliquots were thawed and processed at UC-Davis in institutional batches. CA-125, CXCL13, MMP7, SP-D, YKL-40, and VCAM-1 concentrations were determined using a Luminex magnetic bead-based custom multiplex assay (R&D Systems) according to the manufacturer’s protocol. Biomarker values above and below threshold detection limits were imputed using the highest and lowest detectable levels, respectively, with <1% of data imputed. Biomarkers were measured in duplicate in a subset of patients, and a high correlation was observed (R2 ≥ 0.98).
The primary endpoint was 2-year progression-free survival, defined as death, lung transplant, or ≥10% relative decline in FVC. A preliminary survival analysis was conducted using a web-based tool (http://molpath.charite.de/cutoff/index.jsp) that identifies optimal biomarker thresholds using iterative univariable Cox proportional hazards regression. Dichotomized biomarkers associated with survival at P < 0.008, which adjusted for multiple testing, were advanced for final analysis using a multivariable Cox model adjusted for center, sex/age/physiology-ILD index (5), race, smoking history, and immunosuppressive exposure time. Statistical analyses were performed using Stata (Release 15; StataCorp. 2015) with statistical significance otherwise set at P < 0.05.
Results
Among the ILD subtypes, 148 patients had CTD-ILD, 98 had CHP, and 159 had U-ILD. Cohort characteristics, outcomes, and preliminary biomarker analysis are shown in Table 1. Age, sex, race, and immunosuppression exposure varied substantially by ILD subtype, whereas lung function was similar among the cohorts. Among the CTD subtypes, rheumatoid arthritis (32%) and myositis (28%) predominated.
Table 1.
Baseline Characteristics, Outcomes, and Preliminary Biomarker Analysis by Interstitial Lung Disease Subtype
| Baseline Characteristics | CTD-ILD* (n = 148) | CHP† (n = 98) | U-ILD‡ (n = 159) | |||
|---|---|---|---|---|---|---|
| Center, n (%) | |
|
|
|||
| University of California, Davis | 83 (56.1) |
60 (61.2) |
93 (58.5) |
|||
| University of Chicago | 65 (43.9) |
38 (38.8) |
66 (41.5) |
|||
| Age, mean ± SD | 61 ± 13.8 |
67.9 ± 9.7 |
68.5 ± 11.1 |
|||
| Male sex, n (%) | 48 (32.4) |
49 (50) |
81 (50.9) |
|||
| Race, n (%) | |
|
|
|||
| White | 80 (54) |
71 (72.4) |
120 (75.4) |
|||
| African American | 28 (18.9) |
4 (4.1) |
14 (8.8) |
|||
| Hispanic | 26 (17.6) |
11 (11.2) |
6 (3.8) |
|||
| Asian | 8 (5.4) |
3 (3.1) |
6 (3.8) |
|||
| Other/unknown | 6 (4.1) |
9 (9.2) |
13 (8.2) |
|||
| Ever smoker, n (%) | 69 (46.6) |
52 (53.1) |
90 (57) |
|||
| Lung function | |
|
|
|||
| FVC% predicted, mean ± SD | 67.2 ± 19 |
66.5 ± 20 |
69.1 ± 20 |
|||
| DlCO% predicted, mean ± SD | 50.4 ± 18.9 |
55.1 ± 20.3 |
52.3 ± 21.1 |
|||
| Immunosuppressive therapy, n (%)§ | 90 (60.8) |
42 (42.9) |
53 (33.3) |
|||
| Immunosuppressive exposure months, median (IQR) | 24 (11–24) |
11.5 (5.5–24) |
12 (6.7–24) |
|||
| GAP-ILD score, mean ± SD | 1.4 ± 1.3 |
1.8 ± 1.6 |
3.7 ± 1.6 |
|||
| |
|
|
||||
| Outcomes | |
|
|
|||
| Death, n (%) | 17 (11.5) |
21 (21.4) |
37 (23.3) |
|||
| Lung transplant, n (%) | 2 (1.4) |
2 (2.1) |
3 (1.9) |
|||
| ≥10% FVC decline, n (%) | 21 (14.2) |
32 (32.7) |
29 (18.2) |
|||
| Follow-up months, median (IQR) | 12.7 (6.1–23.8) | 11 (6.8–19) | 11.2 (4.6–20.8) | |||
| CTD-ILD* (n = 148) |
CHP† (n = 98) |
U-ILD‡ (n = 159) |
||||
|---|---|---|---|---|---|---|
| Preliminary biomarker analysis | Threshold | HR (95% CI) | Threshold | HR (95% CI) | Threshold | HR (95% CI) |
| CXCL13 | 525 pg/ml | 4.78 (2.23–10.22)‖ | 140 pg/ml | 2.2 (1.22–3.96)‖ | 107 pg/ml | 3.17 (1.63–6.15)‖ |
| CA-125 | 90 pg/ml | 3.31 (1.71–6.42)‖ | 46 pg/ml | 1.83 (1.01–3.29) | 117 pg/ml | 3.42 (1.66–7.07)‖ |
| MMP7 | 7.9 ng/ml | 2.27 (1.0–5.17) | 6.5 ng/ml | 2.33 (1.27–4.28)‖ | 4.7 ng/ml | 2.26 (1.32–3.85)‖ |
| YKL-40 | 80 ng/ml | 2.18 (1.12–4.24) | 58 ng/ml | 2.05 (1.14–3.71) | 30 ng/ml | 1.97 (0.96–4.03) |
| SP-D | 34 ng/ml | 0.41 (0.15–1.16) | 25 ng/ml | 1.7 (0.89–3.25) | 21 ng/ml | 2.42 (1.27–4.59)‖ |
| VCAM-1 | 2,116 ng/ml | 3.66 (1.6–8.4)‖ | 1,277 ng/ml | 4.12 (2.14–7.95)‖ | 1,887 ng/ml | 2.34 (1.0–5.49) |
Definition of abbreviations: CHP = chronic hypersensitivity pneumonitis; CI = confidence interval; CTD-ILD = connective tissue disease–associated interstitial lung disease; GAP-ILD = gender, age, physiology ILD score; HR = hazard ratio; IQR = interquartile range; U-ILD = unclassifiable interstitial lung disease.
n when values were missing (FVC = 140; DlCO = 135; GAP-ILD n = 140).
n when values were missing (DlCO = 90).
n when values were missing (ever smoker = 158; FVC = 156; DlCO = 144; GAP-ILD = 156).
Mycophenolate mofetil, azathioprine, cyclophosphamide, and/or rituximab.
Advanced for final analysis based on Cox model Wald P < 0.008, which adjusted for multiple testing.
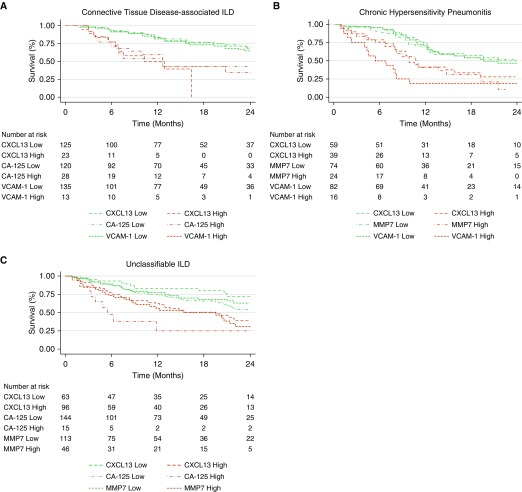
Within the CTD-ILD cohort, increased concentrations of all biomarkers except SP-D were associated with reduced survival, and CXCL13, CA-125, and VCAM-1 were advanced for final analysis after adjustment for multiple testing. In multivariable modeling, CXCL13 (hazard ratio [HR], 4.44; 95% confidence interval [CI], 2.0–9.84; P < 0.001), CA-125 (HR, 2.69; 95% CI, 1.30–5.57; P = 0.008), and VCAM-1 (HR, 3.07; 95% CI, 1.21–7.77; P = 0.018) maintained a survival association (Figure 1A) with a consistent effect size across centers.
Figure 1.
Relationship between biomarker levels and progression-free survival in patients with (A) connective tissue disease–associated interstitial lung disease (ILD), (B) chronic hypersensitivity pneumonitis, or (C) unclassifiable ILD.
Within the CHP cohort, increased concentrations of all biomarkers were associated with reduced survival, and CXCL13, MMP7, and VCAM-1 were advanced for final analysis after adjustment for multiple testing. In multivariable modeling, CXCL13 (HR, 2.08; 95% CI, 1.11–3.92; P = 0.023), MMP7 (HR, 2.84; 95% CI, 1.30–6.20; P = 0.009), and VCAM-1 (HR, 6.08; 95% CI, 2.73–13.51; P < 0.001) maintained a survival association (Figure 1B) with a consistent effect size across centers.
Within the U-ILD cohort, increased concentrations of all biomarkers were associated with reduced survival, and CXCL13, CA-125, MMP7, and SP-D were advanced for final analysis after adjustment for multiple testing. In multivariable modeling, CXCL13 (HR, 2.69; 95% CI, 1.36–5.30; P = 0.004), CA-125 (HR, 3.74; 95% CI, 1.71–8.16; P = 0.001), MMP7 (HR, 2.91; 95% CI, 1.54–5.51; P = 0.001), and SP-D (HR, 2.07; 95% CI, 1.11–3.86; P = 0.023) maintained a survival association (Figure 1C), with each demonstrating a consistent effect size across centers.
Discussion
In this investigation, we found circulating plasma biomarkers of IPF survival to be relevant predictors of outcome in other ILD subtypes. Increased CXCL13 predicted reduced survival in three ILD subtypes, and MMP7, CA-125, and VCAM-1 predicted reduced survival in two subtypes each. A consistent effect size was observed across two geographically diverse ILD centers and after adjustment for relevant confounders. To our knowledge, this study is among the first to validate circulating plasma biomarkers of IPF survival in other ILD subtypes, and highlights shared molecular pathways that underpin progressive PF.
Our CXCL13 findings support the work of others in IPF (1). CXCL13 is a chemokine that promotes B lymphocyte migration, and increased levels have been associated with ectopic germinal centers in autoimmune disease (6) and may be involved in T-cell–mediated alveolitis and granuloma formation in patients with HP (7). The CXCL13 threshold variability may reflect differences in the underlying biology, with inflammatory phenotypes having higher baseline levels. Whether CXCL13 could identify an immunosuppression-responsive population remains unclear, but anti-CXCL13 monoclonal antibodies (8) may be worthy of investigation in inflammatory ILDs.
Our data also extend previous findings with regard to the role of CA-125 and SP-D (2) and MMP7 and VCAM-1 (3) in IPF. In addition, although it fell below our multiple testing threshold, YKL-40 predicted survival across cohorts, which is consistent with studies of IPF (4), CHP (9), and myositis-associated ILD (10). Interestingly, CA-125 predicted survival when measured in cross-section in subjects with CTD-ILD and U-ILD. This supports the findings of Maher and colleagues, who showed that an increase in CA-125 over time was a strong predictor of progressive IPF (2).
This study had several limitations. First, despite our multicenter approach, the sample sizes were relatively small, especially for CTD subtypes, which undoubtedly left some biomarker analyses underpowered. Next, batch effects and protein degradation may have influenced our results, as plasma storage time varied substantially between centers. Finally, biomarker threshold precision could not be ascertained given the multiplex platform used for this analysis. Prospective studies using validated ELISA platforms are needed.
Conclusions
Circulating plasma biomarkers are among the most readily measurable biomarkers in the human body. A wealth of biomarker data have emerged in IPF, and our findings suggest that molecular markers of progressive IPF may in fact be markers of progressive PF, irrespective of ILD subtype. Additional research is needed to validate these findings and determine how such biomarkers can inform clinical decision-making across diverse forms of ILD.
Supplementary Material
Footnotes
Supported by the NHLBI (K23HL138190), the American College of Chest Physicians, and the Keith and Geraldine Hamilton Endowment.
Author Contributions: Clinical data acquisition: S.A., A.A., C.C., I.N., M.E.S., and J.M.O. Biologic sample processing: A.L., C.H., S.-F.M., A.S., and J.M.O. Study design: A.A., A.L., I.N., M.E.S., and J.M.O. Data analysis: J.M.O. and A.A. Interpretation of results: S.A., A.A., A.L., C.H., C.C., S.-F.M., I.N., M.E.S., and J.M.O. Manuscript preparation: S.A., A.A., A.L., I.N., M.E.S., and J.M.O. All authors reviewed, revised, and approved the manuscript for submission.
Originally Published in Press as DOI: 10.1164/rccm.201907-1343LE on September 16, 2019
Author disclosures are available with the text of this letter at www.atsjournals.org.
References
- 1.Vuga LJ, Tedrow JR, Pandit KV, Tan J, Kass DJ, Xue J, et al. C-X-C motif chemokine 13 (CXCL13) is a prognostic biomarker of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2014;189:966–974. doi: 10.1164/rccm.201309-1592OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maher TM, Oballa E, Simpson JK, Porte J, Habgood A, Fahy WA, et al. An epithelial biomarker signature for idiopathic pulmonary fibrosis: an analysis from the multicentre PROFILE cohort study. Lancet Respir Med. 2017;5:946–955. doi: 10.1016/S2213-2600(17)30430-7. [DOI] [PubMed] [Google Scholar]
- 3.Richards TJ, Kaminski N, Baribaud F, Flavin S, Brodmerkel C, Horowitz D, et al. Peripheral blood proteins predict mortality in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2012;185:67–76. doi: 10.1164/rccm.201101-0058OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Korthagen NM, van Moorsel CH, Barlo NP, Ruven HJ, Kruit A, Heron M, et al. Serum and BALF YKL-40 levels are predictors of survival in idiopathic pulmonary fibrosis. Respir Med. 2011;105:106–113. doi: 10.1016/j.rmed.2010.09.012. [DOI] [PubMed] [Google Scholar]
- 5.Ryerson CJ, Vittinghoff E, Ley B, Lee JS, Mooney JJ, Jones KD, et al. Predicting survival across chronic interstitial lung disease: the ILD-GAP model. Chest. 2014;145:723–728. doi: 10.1378/chest.13-1474. [DOI] [PubMed] [Google Scholar]
- 6.Ansel KM, Ngo VN, Hyman PL, Luther SA, Förster R, Sedgwick JD, et al. A chemokine-driven positive feedback loop organizes lymphoid follicles. Nature. 2000;406:309–314. doi: 10.1038/35018581. [DOI] [PubMed] [Google Scholar]
- 7.Agostini C, Calabrese F, Poletti V, Marcer G, Facco M, Miorin M, et al. CXCR3/CXCL10 interactions in the development of hypersensitivity pneumonitis. Respir Res. 2005;6:20. doi: 10.1186/1465-9921-6-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Klimatcheva E, Pandina T, Reilly C, Torno S, Bussler H, Scrivens M, et al. CXCL13 antibody for the treatment of autoimmune disorders. BMC Immunol. 2015;16:6. doi: 10.1186/s12865-015-0068-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Long X, He X, Ohshimo S, Griese M, Sarria R, Guzman J, et al. Serum YKL-40 as predictor of outcome in hypersensitivity pneumonitis. Eur Respir J. 2017;49:1501924. doi: 10.1183/13993003.01924-2015. [DOI] [PubMed] [Google Scholar]
- 10.Hozumi H, Fujisawa T, Enomoto N, Nakashima R, Enomoto Y, Suzuki Y, et al. Clinical utility of YKL-40 in polymyositis/dermatomyositis-associated interstitial lung disease. J Rheumatol. 2017;44:1394–1401. doi: 10.3899/jrheum.170373. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.