Abstract
Rationale: Alveolar epithelial cell (AEC) injury and dysregulated repair are implicated in the pathogenesis of pulmonary fibrosis. Endoplasmic reticulum (ER) stress in AEC has been observed in idiopathic pulmonary fibrosis (IPF), a disease of aging.
Objectives: To investigate a causal role for ER stress in the pathogenesis of pulmonary fibrosis (PF) and therapeutic potential of ER stress inhibition in PF.
Methods: The role of ER stress in AEC dysfunction and fibrosis was studied in mice with tamoxifen (Tmx)-inducible deletion of ER chaperone Grp78, a key regulator of ER homeostasis, in alveolar type II (AT2) cells, progenitors of distal lung epithelium, and in IPF lung slice cultures.
Measurements and Main Results: Grp78 deletion caused weight loss, mortality, lung inflammation, and spatially heterogeneous fibrosis characterized by fibroblastic foci, hyperplastic AT2 cells, and increased susceptibility of old and male mice, all features of IPF. Fibrosis was more persistent in more severely injured Grp78 knockout (KO) mice. Grp78 KO AT2 cells showed evidence of ER stress, apoptosis, senescence, impaired progenitor capacity, and activation of TGF-β (transforming growth factor-β)/SMAD signaling. Glucose-regulated protein 78 is reduced in AT2 cells from old mice and patients with IPF, and ER stress inhibitor tauroursodeoxycholic acid ameliorates ER stress and fibrosis in Grp78 KO mouse and IPF lung slice cultures.
Conclusions: These results support a causal role for ER stress and resulting epithelial dysfunction in PF and suggest ER stress as a potential mechanism linking aging to IPF. Modulation of ER stress and chaperone function may offer a promising therapeutic approach for pulmonary fibrosis.
Keywords: ER stress, alveolar epithelial cell dysfunction, pulmonary fibrosis
At a Glance Commentary
Scientific Knowledge on the Subject
A major challenge to drug development in idiopathic pulmonary fibrosis is that mechanisms underlying fibrogenesis remain unclear. Endoplasmic reticulum (ER) stress caused by SFTPC mutations has been linked to alveolar epithelial cell (AEC) injury and pulmonary fibrosis; however, a more generalized role for ER stress in AEC dysfunction and subsequent fibrosis remains to be established.
What This Study Adds to the Field
We generated a novel mouse model with inducible knockout of the ER chaperone Grp78 specifically in alveolar epithelial type II cells. GRP78 loss led to an ER stress/unfolded protein response, apoptosis, senescence and impaired progenitor capacity of alveolar epithelial type II cells, and age-linked lung fibrosis. These findings strongly support a more generalized role for epithelial ER stress in the pathogenesis of lung fibrosis. Inducible Grp78 knockout mice provide a unique model to study mechanism(s) whereby ER stress in AEC drives a profibrotic response through aberrant cross-talk with other cell types (e.g., macrophages and fibroblasts), as well as for discovery of drugs that target ER stress signaling pathways to advance therapy for this devastating disease.
The endoplasmic reticulum (ER) plays a critical role in protein synthesis, folding, and quality control (1). Compromised ER function causes misfolded proteins to accumulate, triggering activation of an unfolded protein response (UPR). The UPR initially protects cells; however, it can also trigger apoptosis when ER stress is excessive and/or prolonged. ER stress/UPR activation is associated with many human diseases including fibrosis (2).
Idiopathic pulmonary fibrosis (IPF) is an age-linked, progressive, usually lethal disorder of unknown etiology characterized by alveolar epithelial cell (AEC) injury, accumulation of fibroblasts/myofibroblasts, and extracellular matrix deposition (3, 4). Chronic/recurrent epithelial injury and dysregulated repair are implicated in disease pathogenesis (5–7); however, underlying mechanism(s) have not been fully elucidated (3, 8). Mutations of surfactant proteins and viral infection leading to ER stress in AEC have been associated with IPF pathogenesis (9, 10). In mice, conditional expression of SFTPC (surfactant protein C) mutant L188Q induced ER stress in alveolar epithelial type II (AT2) cells, and mice developed exaggerated fibrosis after bleomycin (11). Mice with inducible expression of SFTPC mutant I73T demonstrated dysregulated autophagy in AT2 cells and spontaneous fibrosis without ER stress, whereas inducible expression of SFTPC mutant C121G in AT2 cells caused ER stress and spontaneous fibrosis (12, 13). Because SFTPC mutations are rare (<5%) in IPF (14), a more generalized role for ER stress in AEC dysfunction and subsequent fibrosis remains to be established.
The chaperone protein GRP78 (glucose-regulated protein 78) (also known as BiP/HSPA5) is a “master regulator” of ER homeostasis (15). It represses the UPR by interacting with three transmembrane ER stress sensors (i.e., PERK [protein kinase R-like endoplasmic reticulum kinase], IRE1α [inositol-requiring kinase 1α], and ATF6 [activating transcription factor 6]). GRP78 expression is downregulated in some tissues of old rodents, suggesting possible involvement of GRP78 in aging (16, 17). GRP78 reduction leads to ER stress/UPR activation (15, 18), and liver-specific Grp78 deletion exacerbates fibrosis after injury (19). GRP78 is required for AT2 cell survival during lung development (20), raising the possibility that ER stress may also be an important determinant of AT2 cell dysfunction and survival in the adult lung.
We generated mice with tamoxifen (Tmx)-inducible Grp78 knockout (KO) specifically in AT2 cells, distal lung epithelial progenitors. Grp78 loss resulted in ER stress/UPR response, apoptosis, senescence, decreased stem/progenitor cell capacity, and activation of TGF-β (transforming growth factor-β)/SMAD signaling, inflammation, and age-linked pulmonary fibrosis. These results support a causal role for ER stress in epithelial cell dysfunction and subsequent fibrotic responses. Some of the results have been previously reported in abstract form (21–23).
Methods
Generation of Inducible AT2 Cell-Specific Grp78 Knockout Mice
Grp78SCE mice with Tmx-inducible knockout of Grp78 (Grp78 KO) in AT2 cells (genotype Sftpc+/creERT2;Grp78f/f) were developed by breeding SCE (genotype Sftpc+/creERT2) mice (from H. A. Chapman, University of California, San Francisco) (24) with Grp78f/f mice (18). Grp78hSCE mice with inducible AT2 cell-specific Grp78 KO (genotype SftpccreERT2/creERT2;Grp78f/f) were also generated, in which Cre is homozygous. To generate Grp78 KO reporter mice, ROSATm/Tm reporter mice (Tomato [Tm] reporter gene knocked into the ubiquitously expressed ROSA [reverse orientation with splice acceptor] 26 locus, originally identified by gene trapping using the ROSA vector) (25) were used in crosses. Lungs from Grp78 KO or control mice were harvested for hematoxylin and eosin (H&E), trichrome and Sirius red staining, and immunofluorescence. Lungs or AT2 cells from Grp78 KO mice with >10% but ≤30% weight loss were harvested for further analysis by Sircol assay, Western analysis, BAL, terminal deoxynucleotide transferase–mediated deoxyuridine triphosphate nick end label (TUNEL) assay, and β-galactosidase staining. All animal protocols were approved by the Institutional Animal Care and Use Committee at the University of Southern California. Detailed methods are provided in the online supplement.
Statistical Analysis
Values are shown as mean ± SD. Significance (P < 0.05) was determined by: 1) two-tailed t tests for comparison of two group means, using either parametric analysis for n > 3 or nonparametric analysis (Wilcoxon or Mann-Whitney) for n = 3; 2) Dunnett’s test for multiple comparisons to control for time courses of Tmx dose effects; 3) the Kaplan-Meier method and log-rank tests for survival analyses; and 4) one-way and two-way ANOVA with Holm-Sidak’s correction for three or more group means with one and two factors.
Results
Generation of Tmx-Inducible AT2 Cell-Specific Grp78 KO Mice
Tmx-inducible AT2 cell-specific Grp78 KO mice, referred to as Grp78SCE (genotype Sftpc+/creERT2;Grp78f/f; Cre is heterozygous) or Grp78hSCE (genotype SftpccreERT2/creERT2;Grp78f/f; Cre is homozygous), respectively, and corresponding reporter mice, Grp78SCE;ROSAtm/tm and Grp78hSCE;ROSAtm/tm (25), were generated (Figure E1 in the online supplement). Grp78hSCE mice allowed use of lower Tmx doses, thereby minimizing potential side effects (26). Tmx (50, 80, 100, 140, and 200 mg/kg on two consecutive days) was administered to young (2–4 mo) Grp78SCE mice by intraperitoneal injection. At 80 mg/kg and above, Tmx induced dose-dependent weight loss and mortality (Figures 1A and E2 and Table E1). Grp78SCE mice demonstrated an average maximal weight loss of 5 ± 2% at 80 mg/kg, 14 ± 2% at 100 mg/kg, 16 ± 2% at 140 mg/kg, and 17 ± 3% at 200 mg/kg of Tmx and increased mortality (12% at 80 mg/kg, 18% at 100 mg/kg, 43% at 140 mg/kg, and 80% at 200 mg/kg of Tmx), compared with control Grp78SCE mice without Tmx (Figure 1A) within 14 days. Mortality was greater in male than female Grp78SCE mice (Figure E2). Based on these initial studies, we selected 100 mg/kg Tmx for male and 140 mg/kg for female Grp78SCE mice (Table E2). Perhaps due to background differences, the same doses of Tmx for three consecutive days were required for induction of Grp78 KO and Tomato (Tm) expression in Grp78SCE;ROSAtm/tm mice (Table E2). Similarly, we optimized the dose of Tmx in Grp78hSCE mice by administering 20, 25, 30, 40, and 50 mg/kg on two consecutive days and found that 20 mg/kg induced an average maximal weight loss of 18 ± 1% and 46% mortality within 14 days (Figure E3A). We therefore chose 20 mg/kg Tmx on two consecutive days for Grp78 deletion (Table E2). Twenty milligrams per kilogram for three consecutive days was required for induction of Grp78 KO and Tm expression in Grp78hSCE;ROSAtm/tm mice (Table E2).
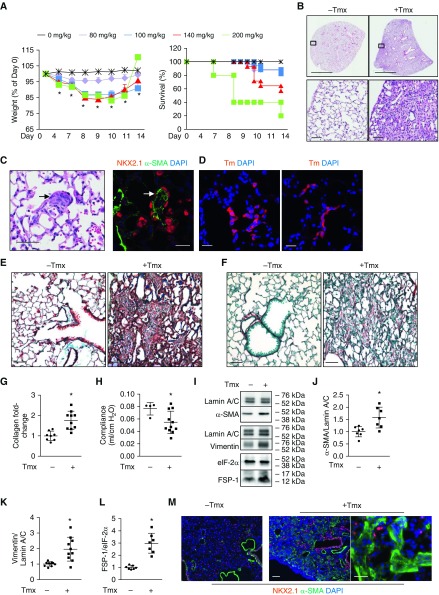
Figure 1.
Grp78 (glucose-regulated protein 78) knockout (KO) induces fibrosis in Grp78SCE mice. (A) Tamoxifen (Tmx) injection (80, 100, 140, and 200 mg/kg for two consecutive days i.p.) into Grp78SCE mice (∼2 mo old) induces weight loss and mortality. Controls are mice without Tmx. n = 24, 17, 14, 5, and 29 mice for 80, 100, 140, and 200 mg/kg of Tmx and no Tmx, respectively. Dunnett’s test: *P < 0.05 for 100 and 140 mg/kg of Tmx at all time points and for 200 mg/kg at Days 4 and 7 compared with control. Kaplan-Meier and log-rank test: P < 0.05 for 200 mg/kg compared with control for mortality. (B) Hematoxylin and eosin staining shows heterogeneous parenchymal remodeling with mesenchymal expansion and increased alveolar wall thickness 14 days after Tmx injection. n = 3 for control (−Tmx) and n = 23 (+Tmx). Scale bars: upper panels, 2 mm; lower panels, 50 μm. (C) Hematoxylin and eosin (left panel) and NKX2.1 (red)/α-SMA (α-smooth muscle actin) (green) double staining (right panel) show areas resembling fibroblastic foci (arrows) in Grp78 KO mice 14 days after Tmx injection. n = 3. Scale bars, 50 μm. (D) Both left and right panels show that Tomato-positive (Tm+) cells resembling hyperplastic alveolar epithelial type II cells line alveoli in Grp78 KO reporter mice 14 days after Tmx injection into Grp78SCE;ROSAtm/tm (Tm reporter gene knocked into the ubiquitously expressed ROSA [reverse orientation with splice acceptor] 26 locus, originally identified by gene trapping using the ROSA vector) mice. n = 4. Scale bars, 25 μm. (E and F) Trichrome (collagen [green] and counterstain [brown]) (E) and Sirius red staining (collagen [red] and counterstain [green]) (F) show increased collagen deposition in Grp78SCE mice 14 days after Tmx. For trichrome staining, n = 22 for KO and n = 6 for control mice. For Sirius red staining, n = 12 for KO and n = 3 for control mice. Scale bars, 50 μm. (G) Sircol assay shows increased collagen content in Grp78 KO lungs 14 days after Grp78SCE mice were injected with Tmx compared with control mice without Tmx. n = 10 for KO and n = 8 for control mice. Data are shown as normalized to control mice. Unpaired two-tailed t test: *P < 0.05. (H) Grp78 KO reduced lung compliance in Grp78SCE mice 14 days after Tmx. n = 11 for KO, and n = 4 for control. Unpaired two-tailed t test: *P < 0.05. (I–L) Representative Western blot (I) and quantitative analysis (J–L) using whole-lung lysates show increased expression of α-SMA, vimentin, and FSP-1 (fibroblast-specific protein-1) in Grp78SCE mice 14 days after Tmx injection. eIF-2α and lamin A/C are loading controls. n = 7. Data are shown as normalized to control mice (no Tmx). Unpaired two-tailed t test: *P < 0.05. (M) Double staining shows increased α-SMA–positive (green) and decreased NKX2.1-positive (red) cells in fibrotic regions of Grp78SCE lungs after Tmx. Scale bars: left and middle panels, 100 μm; right panel, 50 μm.
Grp78SCE and Grp78hSCE Mice Develop Fibrosis
H&E-stained sections from both Grp78SCE (Figure 1B) and Grp78hSCE mice (Figure E3B) demonstrated spatially heterogeneous areas of parenchymal remodeling with subpleural predominance, mesenchymal expansion, increased alveolar wall thickness, and features of fibroblastic foci with hyperplastic epithelial cells overlying aggregates of α-SMA+ myofibroblasts (Figures 1C and E3C) 2 weeks after Tmx. Clusters of Tomato+ (Tm+) Grp78 KO AT2 cells resembling hyperplastic AT2 cells (Figure 1D) were observed in Grp78 KO reporter mice, that were prosurfactant protein B+ and CC10−, with some cells also expressing aquaporin-5 (AQP5)+ (Figures E4A–E4C). No p63+ cells were detected in distal lung (Figure E4D). Tmx had no effect on SCE (genotype Sftpc+/creER), hSCE (genotype SftpccreERT2/creERT2), and Grp78f/f control mice (Figures E3B and E5). Trichrome and Sirius red staining demonstrated increased collagen deposition (Figures 1E and 1F and E3D), Sircol assay showed approximately twofold increases in soluble collagen in both Grp78SCE and Grp78hSCE mice after Tmx (Figures 1G and E3E), and lung compliance was reduced by 22 ± 0.86% (Figure 1H) in Grp78SCE mice after Tmx. Mesenchymal markers α-SMA (α-smooth muscle actin), vimentin, and FSP-1 (fibroblast-specific protein-1) increased in Grp78SCE and Grp78hSCE mice after Tmx (Figures 1I–1L and E6A–E6D). Double immunostaining showed increased α-SMA and decreased NKX2.1- and AQP5-expressing cells in fibrotic areas (Figures 1M and E6E). These data demonstrate that Grp78 KO induces fibrosis in both Grp78SCE and Grp78hSCE mice. Although insertion of the creER2T, rtTA, and Neo cassette into the stop codon of the endogenous Sftpc gene (24) results in decreased Sftpc expression (Figure E7), 15-month-old hSCE mice are normal histologically (Figure E8), indicating that fibrosis is likely not simply the result of reduction in Sftpc expression. However, that does not completely exclude the possibility that low Sftpc expression alters AT2 cell sensitivity to injury.
Knockout of Grp78 Induces ER Stress, Apoptosis, and Senescence in AT2 Cells and Lung Inflammation
We next examined whether reduction of GRP78 activates UPR signaling as previously reported (15, 27). GRP78 protein was decreased 33.1 ± 0.97% accompanied by increases in CHOP (238 ± 53.60%) and GRP94 (341 ± 15.26%) in AT2 cells isolated from Grp78SCE mice 1 week after Tmx (Figures 2A–2D). Because CRE efficiency is ∼60% (Figure E9), we also evaluated GRP78 expression in sorted Tm+ AT2 cells from Grp78SCE;ROSAtm/tm and SCE;ROSAtm/tm mice 5 days after Tmx. In KO AT2 cells, GRP78 was undetectable, and GRP94 and CHOP levels were increased (Figure 2E). Similarly, GRP78 was decreased (58.1 ± 5.9%), and GRP94 (304 ± 20%) and CHOP (799 ± 54%) were increased in AT2 cells from Grp78hSCE mice 1 week after Tmx (Figures E10A–E10E). Additionally, qRT-PCR showed an ∼8.4-fold reduction of the transcript encoding GRP78 (Hspa5), whereas transcripts encoding ER stress markers GRP94 (Hsp90b1) (∼8.0-fold), CHOP (Ddit3) (∼16.3-fold), ATF6 (Atf6) (∼4.1-fold), calreticulin (Calr) (∼5.2-fold), ATF4 (Atf4) (∼3.4-fold), and PDI (P4hb) (∼3.1-fold) were increased in Grp78hSCE lungs after Tmx (Figures E10F–E10G). RNA sequencing (Figures E11A and E11B and Table E3) demonstrated that the UPR and ER stress pathways are among the top significantly altered pathways in Grp78 KO AT2 cells (Figures E11C and E11D and Table E4). Western analysis showed increases in the apoptotic mediator-cleaved caspase 3 and senescence markers p53, γ-H2A.X, and p21 in Grp78 KO AT2 cells (Figures 2F and 2G and E12A–E12C). qRT-PCR showed increased Cip1 (encoding p21), but not Cdkn2a (encoding p16) in Grp78 KO AT2 cells (Figure E13A). Increased apoptosis was confirmed by TUNEL assay (Figures 2H and E12D), although the majority of TUNEL+ cells are Tm− (or NKX2.1−), perhaps due to loss of Tomato and AT2 cell markers in cells undergoing apoptosis. Additionally, senescence-associated β-galactosidase staining was present (Figure E13B), and genes involved in the senescence-associated secretory phenotype (e.g., Mmp and Cxcl family members, Serpine1 and Fgf2) were increased in Grp78 KO AT2 cells (Figure 2I). Grp78 KO increased BAL cell number, protein concentration, and both macrophages and neutrophils (Figures 3A–3D and E14A–E14C). CD68, Ly6G (Figure 3E), and H&E staining (Figure E14D) further confirm increased inflammatory cells in Grp78 KO lungs. A trend toward increased proinflammatory cytokine IL 6 and monocyte chemoattractant protein 1 (MCP-1) in Grp78 KO BAL (Figure 3F) and increases of Il6 (∼3.1-fold) and Ccl2 (encoding MCP-1) (∼20.7-fold) mRNA in Grp78 KO AT2 cells (Figure E14E) were detected. Although TGF-β was not increased in BAL, p-SMAD2 and p-SMAD3 protein were increased in Grp78 KO AT2 cells (Figure 3G–3I). Ingenuity Pathway Analysis identified TGF-β signaling as one of the top upstream regulators responsible for alterations of the gene expression profile in Grp78 KO AT2 cells (Table E5).
Figure 2.
Loss of GRP78 (glucose-regulated protein 78) induces epithelial endoplasmic reticulum stress, senescence, and apoptosis in Grp78SCE mice. (A–D) Representative Western blot (A) and quantitative analysis (B–D) show decreased GRP78 and increased GRP94 and CHOP in alveolar epithelial type II (AT2) cells (that include a mixture of Grp78 knockout [KO] and wild-type cells) isolated from Grp78SCE mice 7–14 days after injection with tamoxifen (Tmx) (100–140 mg/kg) compared with no Tmx. eIF-2α is a loading control. Data are shown as normalized to control (no Tmx). For GRP78 and GRP94, n = 3 for no Tmx, and n = 4 for with Tmx. For CHOP, n = 6. Unpaired two-tailed t test: *P < 0.05. (E) Western blot shows decreased GRP78 and increased GRP94 and CHOP in purified Tomato-positive (Tm+) Grp78 KO AT2 cells sorted from Grp78SCE;ROSAtm/tm (Tm reporter gene knocked into the ubiquitously expressed ROSA [reverse orientation with splice acceptor] 26 locus, originally identified by gene trapping using the ROSA vector) mice (lanes 3 and 4) compared with Tm+ AT2 cells sorted from control SCE;ROSAtm/tm mice (lanes 1 and 2) 5 days after injection with Tmx (100–140 mg/kg). β-Actin is loading control. n = 2. (F and G) Representative Western blot (F) and quantitative analysis (G) show increased apoptotic and senescence markers in AT2 cells isolated from Grp78SCE mice 10–14 days after injection with Tmx (100–140 mg/kg) compared with no Tmx. n = 7 for caspase 3, and n = 4 for p53, γ-H2A.X, and p21. Unpaired two-tailed t test: *P < 0.05. (H) Terminal deoxynucleotide transferase–mediated deoxyuridine triphosphate nick end label staining shows increased numbers of apoptotic cells (green) (upper panels) in lungs of Grp78 KO reporter mice (Grp78SCE;ROSAtm/tm) compared with control reporter mice (SCE;ROSAtm/tm) at 14 days after Tmx injection. Higher magnification with individual (lower panels, i–iii) and merged channels (lower panel, iv) shows Tm+ apoptotic cells (arrows) in Grp78 KO reporter mice. n = 3. Scale bars, 25 μm. DAPI (blue) is the nuclear counterstain. (I) RNA sequencing shows altered expression of genes involved in the senescence-associated secretory phenotype in AT2 cells isolated from Grp78SCE mice 10 days after injection with Tmx (100 mg/kg) compared with no Tmx. n = 3. *False discovery rate < 0.05. CHOP = CCAAT-enhancer–binding protein homologous protein.
Figure 3.
Grp78 (glucose-regulated protein 78) knockout (KO) induces inflammation in Grp78SCE mice. (A and B) Increased cell number (A) and protein concentration (B) in BAL from Grp78SCE mice 14 days after tamoxifen (Tmx) (100–140 mg/kg i.p. on two consecutive days). n = 8 for KO, and n = 5 for control (no Tmx) mice. Unpaired two-tailed t test: *P < 0.05. (C and D) Differential staining (C) and quantitative analysis (D) show increased macrophages and neutrophils in BAL from Grp78SCE mice 14 days after Tmx injection. n = 8 for KO, and n = 5 for control (no Tmx) mice. Unpaired two-tailed t test: *P < 0.05. Scale bars, 100 μm. (E) CD68 and Ly6G staining (green) show increased numbers of macrophages (left panels) and neutrophils (right panels) in Grp78SCE lungs 14 days after Tmx injection. DAPI and propidium iodide are nuclear counterstains, respectively. n = 3. Scale bars, 25 μm. (F) ELISA shows a trend toward increased IL-6 and MCP-1 (monocyte chemoattractant protein 1) in BAL 14 days after Tmx (100–140 mg/kg i.p. on two consecutive days). n = 3. (G and H) Representative Western blot (G) and quantitative analysis (H) show increased p-SMAD2 and p-SMAD3 in alveolar type II cells isolated from Grp78SCE mice 1 week after injection with Tmx compared with no Tmx. n = 7. Unpaired two-tailed t test: *P < 0.05, significantly different from mice without Tmx injection.
Fibrosis Persists in More Severely Injured Grp78 KO Mice
Fibrosis was assessed in lungs harvested 3 months after Tmx from six young (∼3 mo) Grp78SCE mice. Two mice with the greatest weight loss (maximum 23 and 20%) showed persistent morphologic abnormalities and fibrosis (although less extensive than at earlier time points) (Figure 4A and Table E6), whereas lungs from the remaining four mice (maximal weight loss between 12 and 18%) appeared normal (not shown). Similarly, lungs of three mice with the highest weight loss (maximum ∼30%) harvested 3 months after Tmx from four young (∼3 mo) Grp78hSCE mice showed persistent morphologic abnormalities and fibrosis (also less extensive than at earlier time points), whereas the fourth mouse (Grp78hSCE1; maximum weight loss ∼12%) appeared normal (Figure E15). These data suggest that fibrosis persists in mice with the greatest initial ER stress. Mortality with higher doses of Tmx limited our ability to fully evaluate dose dependence of Grp78 KO on persistence of fibrosis.
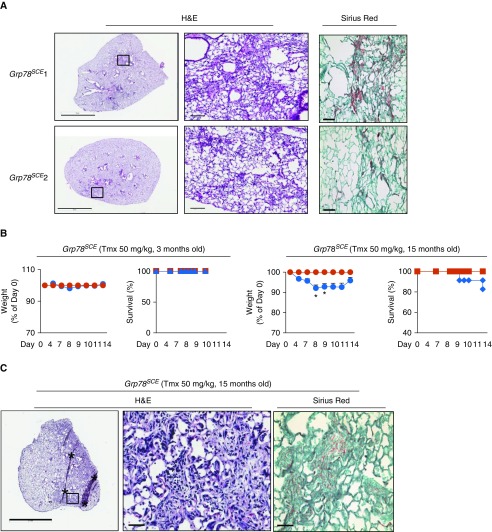
Figure 4.
Fibrosis persists in some young Grp78SCE mice, and old mice are more sensitive to endoplasmic reticulum stress–mediated fibrosis. (A) Lungs were harvested 3 months after tamoxifen (Tmx) from six young (∼3 mo) Grp78SCE mice (with weight loss >10% within 2 weeks after Tmx injection). Two mice (Grp78SCE 1 and 2) with the highest weight loss (maximum 23 and 20%) still show abnormal lung morphology and patchy fibrosis, whereas others appear normal (not shown). For Sirius red staining, collagen is red, and counterstain is green. n = 6. Scale bars: hematoxylin and eosin (H&E) staining, 3 mm for the left panels and 100 μm for the right panels, which show higher-magnification images for the boxed areas in the left panels; Sirius red, 50 μm. (B) Fifty milligrams per kilogram Tmx (three consecutive days) results in weight loss and decreased survival in old (15 mo) but not young (3 mo) Grp78SCE mice at 14 days after Tmx (blue line). Mice without Tmx are control (red line). n = 23 for old knockout, n = 5 for old control, n = 22 for young knockout, and n = 4 for young control mice. Unpaired two-tailed t test: *P < 0.05 at Days 8 and 9. (C) Representative H&E and Sirius red staining show fibrosis 2 weeks after Tmx (50 mg/kg) in old Grp78SCE mice. n = 6 out of 22, with weight loss greater than 10%. The middle panel shows higher-magnification images for the boxed area in the left panel. Scale bars: left panel, 2 mm; middle and right panels, 50 μm. Asterisks are artifacts produced during histological processing. Grp78 = glucose-regulated protein 78.
Old Mice Are More Sensitive to ER Stress–mediated Fibrosis
Older Grp78SCE mice (∼15 mo) were injected with Tmx (50 mg/kg, three consecutive days) (Table E2), a dose that did not induce weight loss or mortality in young mice (∼2–4 mo). We observed 8 ± 2% average maximal weight loss and 17% mortality) in old Grp78SCE mice, with six mice showing greater than 10% weight loss among 23 old mice tested, but with very minor effects in younger mice (∼3 mo) (2 ± 0% average maximal weight loss and no mortality) (Figure 4B and Table E7), with only three mice showing greater than 10% weight loss among 22 young mice tested. All six old Grp78 KO mice (Figure 4C) and two of three young mice (data not shown) with maximal weight loss greater than 10% demonstrated fibrosis.
Decreased GRP78 Expression and Increased ER Stress in AT2 Cells from Old Mice and IPF Lungs
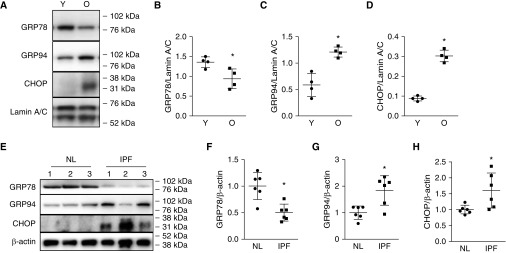
Age-related reductions of GRP78 could potentially be a crucial factor underlying age-dependent increases of ER stress and resulting fibrosis. To test this, we evaluated GRP78 expression in isolated AT2 cells from old (17–19 mo) and young (2 mo) C57BL/6 mice. AT2 cells from old mice showed decreased expression of GRP78, whereas ER stress markers GRP94 and CHOP were increased compared with younger AT2 cells (Figures 5A–5D). Importantly, AT2 cells isolated from IPF lungs (Table E8) showed increased GRP94 and CHOP accompanied by decreased GRP78 expression (Figures 5E–5H) compared with normal donors.
Figure 5.
Decreased expression of GRP78 (glucose-regulated protein 78) and increased GRP94 and CHOP in old mice. (A–D) Representative Western blot (A) and quantitative analysis (B–D) show that alveolar epithelial type II (AT2) cells from old C57BL/6 mice (O, 17–19 mo) express less GRP78 than AT2 cells from younger mice (Y, 2 mo), whereas CHOP and GRP94, markers of endoplasmic reticulum stress, are increased. n = 4. Unpaired two-tailed t test: *P < 0.05. (E–H) Representative Western blot (E) and quantitative analysis (F–H) show decreased expression of GRP78 and increased expression of CHOP and GRP94 in idiopathic pulmonary fibrosis (IPF) compared with normal (NL) AT2 cells. Data are normalized to normal human AT2 cells. n = 6. Unpaired two-tailed t test: *P < 0.05. CHOP = CCAAT-enhancer–binding protein homologous protein.
Grp78 KO Impairs AT2 Progenitor Cell Proliferation and Colony-Forming Efficiency
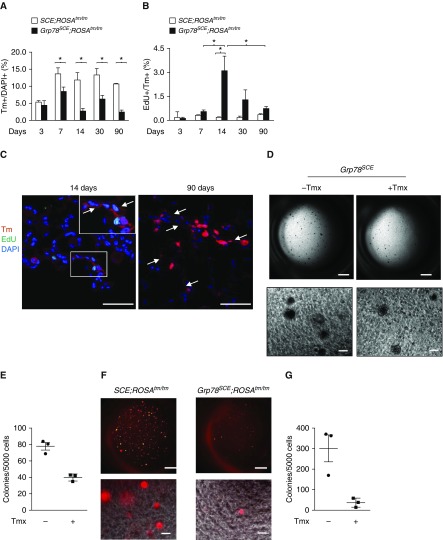
We evaluated proliferation and differentiation of AT2 cells from control (SCE;ROSAtm/tm) and Grp78 KO reporter mice (Grp78SCE;ROSAtm/tm) over time (3, 7, 14, 30, and 90 d) after Tmx (Table E2). At Day 3, the number of Tm+ AT2 cells as a percentage of all cells is similar (∼5%) in both genotypes, whereas it is significantly lower at 7 days and all subsequent time points in Grp78 KO compared with control lungs (7 d [control 13.6 ± 0.3% vs. KO 8.8 ± 0.6%]; 14 d [control 11.8 ± 0.2% vs. KO 2.8 ± 3.1%]; 30 d [control 13.9 ± 0.2% vs. KO 6.3 ± 0.2%]; and 90 d [control 10.7 ± 0.4% vs. KO 2.5 ± 0.7%]) (Figures 6A and E16A and E16B). Similar results were observed in Grp78hSCE;ROSAtm/tm mice (Figures E17A and E17B). The number of Tm+EdU+ cells was increased in KO mice, with EdU labeling peaking at 2 weeks and a decrease to basal levels at 30–90 days after Tmx (Figures 6B, E16C, E17C, and E17D). In addition, Tm+ AT1 cells expressing AQP5, but not prosurfactant protein B (and without Edu labeling), appear in KO lungs 2 weeks after Tmx (Figures 6C and E17E–E18), and Tm+ AT1 cell foci are enlarged at 90 days compared with 14 days after Tmx (Figure 6C), suggesting that Grp78 KO induces transient AT2 cell proliferation and accelerated transdifferentiation of Tm+ AT2 to AT1 cells. However, overall Tm+ AT2 cell numbers remained reduced (Figures 6A and E17B), and Tm+ AT1 cells covered only small portions of the alveolar surface at 3–6 months after Tmx (data not shown), indicating that increased Tm+ cell proliferation and differentiation are insufficient to compensate for Tm+ cell loss due to Grp78 KO. To investigate effects on progenitor function, AT2 cells (EPCAMhiCD24−Sca1−CD45−CD34−CD31−) were sorted from Grp78 KO and control mice and cultured in Matrigel (28). Colony-forming efficiency of Grp78 KO AT2 cells shows a trend toward reduction (Figures 6D–6E) compared with control cells. This trend, specifically in AT2 cells lacking GRP78, was further confirmed in sorted Tm+ AT2 cells (Figures 6F and 6G).
Figure 6.
Grp78 (glucose-regulated protein 78) knockout (KO) decreases alveolar epithelial type II (AT2) cell proliferation and colony-forming efficiency. (A and B) Tm+ AT2 cells decrease (A) and Tm+ Edu+ cells increase (B) at 1 week, peak at 2 weeks, and decrease over time after tamoxifen (Tmx) (100–140 mg/kg on three consecutive days) injection into Grp78SCE;ROSATm/Tm (Tm reporter gene knocked into the ubiquitously expressed ROSA [reverse orientation with splice acceptor] 26 locus, originally identified by gene trapping using the ROSA vector) mice (2–4 mo old) compared with SCE;ROSATm/Tm mice after Tmx. n = 3 for each group. Two-way ANOVA: *P < 0.05. (C) Tm+ AT1 cells (arrows) appear in Grp78 KO lungs at 14 days, and a cluster of Tm+ AT1 cells is enlarged at 90 days after Tmx. n = 3. Scale bars, 50 μm. Inset shows higher-magnification views of rectangular area. (D and E) Representative image (D) and quantitative analysis (E) show a trend toward decreased number of colonies of AT2 cells isolated 1 week after Tmx injection (100–140 mg/kg) into Grp78SCE mice compared with AT2 cells from mice without Tmx injection. n = 3. Scale bars: upper panels, 1 mm; lower panels, 100 μm. (F and G) Representative image (F) and quantitative analysis (G) show that Tm+ Grp78 KO AT2 cells sorted 5 days after Tmx injection into Grp78SCE;ROSATm/Tm mice form very few colonies compared with cells from SCE;ROSATm/Tm mice. n = 3. Scale bars: upper panels, 1 mm; lower panels, 100 μm. EdU = 5-ehtynyl-2′-deoxyuridine; Tm = Tomato.
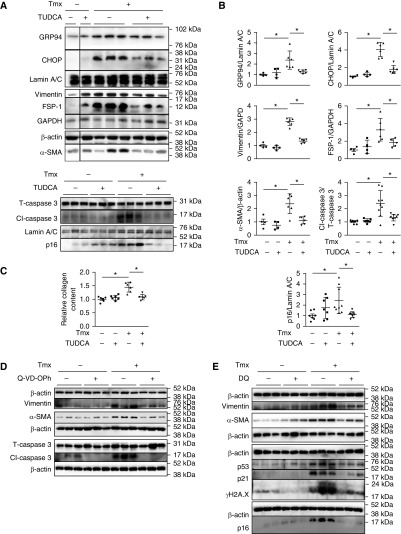
Tauroursodeoxycholic Acid, Q-VD-OPh, and Dasatinib and Quercetin Inhibit Mesenchymal Maker Expression in Grp78 KO Lungs in Ex Vivo Culture
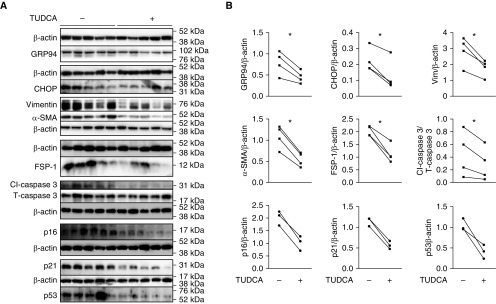
Lungs were harvested from Grp78SCE mice with >10% weight loss at 2 weeks after Tmx and cultured ex vivo (29) for 5 days. Tauroursodeoxycholic acid (TUDCA) (30) decreased expression of ER stress markers GRP94 and CHOP, mesenchymal markers vimentin, α-SMA, and FSP-1, apoptotic marker Cl-caspase 3, senescence marker p16, and collagen deposition (Figures 7A–7C). Additionally, pan-caspase inhibitor Q-VD-OPh (Figures 7D and E19A) and senolytic drugs dasatinib (D) and quercetin (Q) (DQ) (29, 31) (Figures 7E and E19B) reduced expression of mesenchymal markers, whereas DQ reduced expression of senescence markers p21, p53, γ-H2A.X, and p16. Importantly, similar inhibitory effects of TUDCA were also observed in IPF slices cultured ex vivo (Figure 8).
Figure 7.
Inhibitors of endoplasmic reticulum (ER) stress, apoptosis, and senescence reduce fibrotic responses in Grp78 knockout (KO) lungs cultured ex vivo. (A and B) Representative Western blot (A) and quantitative analysis (B) show reduced expression of ER stress, mesenchymal, apoptotic, and senescence markers in Grp78 KO lung slices (isolated from Grp78SCE mice 14 d after tamoxifen [Tmx; 100–140 mg/kg i.p. on two consecutive days]) after 5 days of treatment with tauroursodeoxycholic acid (TUDCA) (500 μM) in ex vivo culture. TUDCA had no effect on control lungs (Grp78SCE mice without Tmx). Each lane represents one slice from the same lung. n = 6 for Grp78 KO, and n = 4 for control lungs for ER stress and mesenchymal markers; n = 9 for Grp78 KO, and n = 7 for control lungs for apoptotic and senescence markers. Two-way ANOVA: *P < 0.05. (C) TUDCA (500 μM, 5 d treatment) reduces Grp78 KO-induced collagen deposition in lung slices in ex vivo culture. n = 6 lungs. Two-way ANOVA: *P < 0.05. (D) Representative Western blot shows pan-caspase inhibitor Q-VD-OPh treatment (36.4 μg/ml, 5 d) reduced expression of mesenchymal and apoptotic markers in Grp78 KO lung slices cultured ex vivo. Each lane represents one slice from the same lung. n = 6 for Grp78 KO lungs, and n = 6 for control lungs. (E) Western blot shows dasatinib and quercetin (DQ) treatment (25 μM for D and 250 nM for Q, 5 d) reduced expression of mesenchymal and senescence markers in Grp78 KO slices cultured ex vivo. Each lane represents one slice from the same lung. n = 3 for Grp78 KO lungs, and n = 3 for control lungs. CHOP = CCAAT-enhancer–binding protein homologous protein; Grp78 = glucose-regulated protein 78.
Figure 8.
Tauroursodeoxycholic acid (TUDCA) reduces fibrotic responses in idiopathic pulmonary fibrosis lungs cultured ex vivo. (A and B) Representative Western blot (A) and quantitative analysis (B) show that TUDCA (500 μM) reduces expression of endoplasmic reticulum stress and mesenchymal and apoptotic markers with a trend toward reduction of senescence markers in idiopathic pulmonary fibrosis lung slices after 5 days of treatment in ex vivo culture. Each lane represents one slice from the same lung. n = 4 lungs for endoplasmic reticulum stress and mesenchymal and apoptotic markers; n = 3 for senescence markers with and without TUDCA. Paired t test: *P < 0.05.
Discussion
ER stress is induced by both exogenous factors (e.g., viral infections) and intracellular processes (e.g., aging and genetic mutations) (32, 33). We generated mice with inducible AT2 cell-specific KO of the ER chaperone GRP78. Grp78 KO caused activation of ER stress/UPR signaling. Grp78 KO AT2 cells displayed apoptosis, senescence, and impaired stem cell capacity. Grp78 KO mice developed lung fibrosis with several features of IPF, including increased sensitivity of old mice. Fibrosis of both Grp78 KO and IPF lungs was ameliorated in ex vivo culture with ER stress inhibitor TUDCA. Furthermore, GRP78 was reduced in AT2 cells from old mice and IPF patients, suggesting GRP78 reduction as a potential mechanism linking aging to IPF. Together these results strongly support a causal role for ER stress in the pathogenesis of lung fibrosis.
There is growing evidence supporting a link between AT2 cell dysfunction and lung fibrosis. Telomere dysfunction in AT2 cells leads to fibrotic lung remodeling (34). Expression of the SR39TK mutant of Herpes simplex viral thymidine kinase in AT2 cells led to collagen deposition and AT2 cell depletion (35), whereas mice expressing the human diphtheria toxin receptor on AT2 cells develop pulmonary fibrosis after administration of diphtheria toxin (36). Mice with inducible expression of I73T mutant SFTPC in AT2 cells develop spontaneous lung fibrosis (12), whereas inducible expression of C121G mutant SFTPC caused ER stress in AT2 cells, and mice developed spontaneous fibrosis (13). In this study, we report spontaneous lung fibrosis after loss of GRP78 in AT2 cells, further establishing a general role for ER stress–induced AT2 cell dysfunction in driving pulmonary fibrosis.
Apoptosis, senescence, and ER stress–induced UPR signaling may be associated with production of proinflammatory/profibrotic molecules (37, 38); however, cellular mechanisms whereby dysfunctional AEC contribute to fibrogenesis have not been fully elucidated. Expression of mesenchymal markers was reduced by ER stress inhibitor TUDCA, pan-caspase inhibitor QV-D-OPh, and senolytic drugs DQ, suggesting ER stress–mediated apoptosis and senescence are involved in the development of fibrosis in Grp78 KO mice. Both p21 and p16 increased, whereas only Cip1, but not Cdkn2a, increased, suggesting differential regulation of their responses to Grp78 KO. It will be interesting to further investigate differential regulation of these responses to ER stress in Grp78 KO mice. Increased p-SMAD2/p-SMAD3 and prediction of TGF-β signaling as one of the top upstream regulators responsible for altered gene expression profile in Grp78 KO AT2 cells suggest local activation of TGF-β1/SMAD signaling (39), implicating TGF-β/SMAD signaling is an important regulator of AEC dysfunction and lung fibrosis, consistent with findings in TGF-β receptor type II KO mice (40). Increased MCP-1 and macrophages in Grp78 KO BAL also suggest activation of lung macrophages via MCP-1/CCR2 signaling (41), leading to fibrotic remodeling. Mechanisms linking AT2 cell dysfunction and fibrosis could therefore include either direct epithelial–mesenchymal interactions and/or involvement of macrophages.
Grp78 KO increased both proliferation and differentiation of AT2 cells, consistent with a recent report after LPS injury (42). Overall, numbers of Tm+ Grp78 KO AT2 cells decreased, indicating that the response of Grp78 KO AT2 cells is insufficient to compensate for AT2 cell loss. Grp78 KO AT2 cells showed reduced colony-forming efficiency, suggesting that ER stress–induced AT2 stem cell dysfunction may also contribute to the fibrotic response in Grp78 KO mice (43). Old mice were more sensitive to ER stress–induced fibrosis, and fibrosis was more persistent in more severely injured Grp78 KO mice, suggesting that the balance between the degree of AEC stress and regenerative capacity determines development of fibrosis. Because the UPR has been recognized in cell fate decisions (44), resolution of fibrosis likely results from regeneration of remaining wild-type AT2 cells or other stem/progenitor cells (e.g., bronchioalveolar stem cells, club cells, or lineage-negative epithelial progenitors [45]).
Several aging-related changes have been linked to IPF (46–48). In Grp78 KO mice, ER stress in AT2 cells led to senescence and apoptosis and decreased regenerative capacity, all of which are features of aging, suggesting ER stress as a potential mechanism linking aging to the pathogenesis of IPF. ER chaperones including GRP78 are reduced in tissues of older rodents (49). Here we show that GRP78 expression is decreased, whereas ER stress is increased in old AT2 cells, suggesting that impaired ER stress/UPR responses may compromise the function of old AT2 cells. Whereas our data support activation of ER stress in IPF AT2 cells, GRP78 protein was decreased in IPF AT2 cells in contrast to previous results (10). This difference may perhaps be due to the heterogeneity of epithelial responses induced by various environmental factors and the patchy nature of fibrosis, as well as distinct AT2 populations in IPF lungs leading to differences between areas sampled. It will be interesting to further investigate mechanisms underlying GRP78 reduction in IPF AT2 cells and its contribution to the aging phenotype and aging-associated diseases such as IPF.
In summary, our studies strongly support a central role for ER stress in AEC dysfunction and subsequent fibrosis. Grp78 KO mice demonstrate several features of IPF, and Grp78 KO AT2 cells exhibit some pivotal features of the “aging phenotype,” suggesting utility of these mice for developing novel therapies for IPF. Treatment with ER stress inhibitors such as TUDCA may offer a promising therapeutic approach for IPF.
Supplementary Material
Acknowledgments
Acknowledgment
The authors thank Lynn Fukushima, R.N., for collating information describing the human cohorts and facilitating collection of human lung explants, and Juan R. Alvarez for isolation of AT2 cells.
Footnotes
This work was supported by the Hastings Foundation; research grants HL114959 (B.Z.), R35HL135747, HL112638, and HL126877 (Z.B.), CA027607 (A.S.L.), and HL119346 (M.F.B.) from the NIH; and VA Merit Review I01BX001176 (M.F.B.). Z.B. is Edgington Chair in Medicine and Hastings Professor. M.F.B. is an Albert Rose Established Investigator of the Pulmonary Fibrosis Foundation. Histology and microscopy services were provided by the Cell and Tissue Imaging Core of the University of Southern California (USC) Research Center for Liver Diseases (P30 DK048522 and S10 RR022508), Norris Comprehensive Cancer Center Core (P30 CA014089), and the Core Facility of the Broad CIRM Center (USC). Flow cytometry was performed in Core Facilities at USC (P30CA014089 and the USC Office of the Provost, Dean’s Development Funds, Keck School of Medicine of USC) and Children’s Hospital Los Angeles.
Author Contributions: Z.B. conceived the study, directed experiments, interpreted experimental data, and edited the manuscript. M.H. performed RNA sequencing and statistical analyses. P.F. conceived experiments, interpreted data, and edited the manuscript. H.W. maintained mice and performed histology, immunostaining, and Western blotting. Y.L. performed ex vivo and three-dimensional cultures, Western blotting, and staining. S.G. facilitated procurement of idiopathic pulmonary fibrosis lungs. A.S.L. provided the floxed Grp78 mouse strain and edited the manuscript. A.L.F. facilitated procurement of idiopathic pulmonary fibrosis lungs and edited the manuscript. P.M., C.L., and M.F.B. interpreted experimental data and edited the manuscript. B.Z. conceived the study, directed experiments, analyzed and interpreted experimental data, and wrote the manuscript.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1164/rccm.201902-0451OC on November 18, 2019
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Luo B, Lee AS. The critical roles of endoplasmic reticulum chaperones and unfolded protein response in tumorigenesis and anticancer therapies. Oncogene. 2013;32:805–818. doi: 10.1038/onc.2012.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhu G, Lee AS. Role of the unfolded protein response, GRP78 and GRP94 in organ homeostasis. J Cell Physiol. 2015;230:1413–1420. doi: 10.1002/jcp.24923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Selman M, Pardo A. Role of epithelial cells in idiopathic pulmonary fibrosis: from innocent targets to serial killers. Proc Am Thorac Soc. 2006;3:364–372. doi: 10.1513/pats.200601-003TK. [DOI] [PubMed] [Google Scholar]
- 4.Albert RK, Smith B, Perlman CE, Schwartz DA. Is progression of pulmonary fibrosis due to ventilation-induced lung injury? Am J Respir Crit Care Med. 2019;200:140–151. doi: 10.1164/rccm.201903-0497PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Selman M, Pardo A. Revealing the pathogenic and aging-related mechanisms of the enigmatic idiopathic pulmonary fibrosis: an integral model. Am J Respir Crit Care Med. 2014;189:1161–1172. doi: 10.1164/rccm.201312-2221PP. [DOI] [PubMed] [Google Scholar]
- 6.Ahluwalia N, Shea BS, Tager AM. New therapeutic targets in idiopathic pulmonary fibrosis: aiming to rein in runaway wound-healing responses. Am J Respir Crit Care Med. 2014;190:867–878. doi: 10.1164/rccm.201403-0509PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hewlett JC, Kropski JA, Blackwell TS. Idiopathic pulmonary fibrosis: epithelial-mesenchymal interactions and emerging therapeutic targets. Matrix Biol. 2018;71-72:112–127. doi: 10.1016/j.matbio.2018.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Borok Z. Alveolar epithelium: beyond the barrier. Am J Respir Cell Mol Biol. 2014;50:853–856. doi: 10.1165/rcmb.2014-0089PS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lawson WE, Crossno PF, Polosukhin VV, Roldan J, Cheng DS, Lane KB, et al. Endoplasmic reticulum stress in alveolar epithelial cells is prominent in IPF: association with altered surfactant protein processing and herpesvirus infection. Am J Physiol Lung Cell Mol Physiol. 2008;294:L1119–L1126. doi: 10.1152/ajplung.00382.2007. [DOI] [PubMed] [Google Scholar]
- 10.Korfei M, Ruppert C, Mahavadi P, Henneke I, Markart P, Koch M, et al. Epithelial endoplasmic reticulum stress and apoptosis in sporadic idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2008;178:838–846. doi: 10.1164/rccm.200802-313OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lawson WE, Cheng DS, Degryse AL, Tanjore H, Polosukhin VV, Xu XC, et al. Endoplasmic reticulum stress enhances fibrotic remodeling in the lungs. Proc Natl Acad Sci USA. 2011;108:10562–10567. doi: 10.1073/pnas.1107559108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nureki SI, Tomer Y, Venosa A, Katzen J, Russo SJ, Jamil S, et al. Expression of mutant Sftpc in murine alveolar epithelia drives spontaneous lung fibrosis. J Clin Invest. 2018;128:4008–4024. doi: 10.1172/JCI99287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Katzen J, Wagner BD, Venosa A, Kopp M, Tomer Y, Russo SJ, et al. An SFTPC BRICHOS mutant links epithelial ER stress and spontaneous lung fibrosis. JCI Insight. 2019;4:126125. doi: 10.1172/jci.insight.126125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wolters PJ, Collard HR, Jones KD. Pathogenesis of idiopathic pulmonary fibrosis. Annu Rev Pathol. 2014;9:157–179. doi: 10.1146/annurev-pathol-012513-104706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee AS. Glucose-regulated proteins in cancer: molecular mechanisms and therapeutic potential. Nat Rev Cancer. 2014;14:263–276. doi: 10.1038/nrc3701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Paton AW, Beddoe T, Thorpe CM, Whisstock JC, Wilce MC, Rossjohn J, et al. AB5 subtilase cytotoxin inactivates the endoplasmic reticulum chaperone BiP. Nature. 2006;443:548–552. doi: 10.1038/nature05124. [DOI] [PubMed] [Google Scholar]
- 17.Wolfson JJ, May KL, Thorpe CM, Jandhyala DM, Paton JC, Paton AW. Subtilase cytotoxin activates PERK, IRE1 and ATF6 endoplasmic reticulum stress-signalling pathways. Cell Microbiol. 2008;10:1775–1786. doi: 10.1111/j.1462-5822.2008.01164.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Luo S, Mao C, Lee B, Lee AS. GRP78/BiP is required for cell proliferation and protecting the inner cell mass from apoptosis during early mouse embryonic development. Mol Cell Biol. 2006;26:5688–5697. doi: 10.1128/MCB.00779-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ji C, Kaplowitz N, Lau MY, Kao E, Petrovic LM, Lee AS. Liver-specific loss of glucose-regulated protein 78 perturbs the unfolded protein response and exacerbates a spectrum of liver diseases in mice. Hepatology. 2011;54:229–239. doi: 10.1002/hep.24368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Flodby P, Li C, Liu Y, Wang H, Marconett CN, Laird-Offringa IA, et al. The 78-kD glucose-regulated protein regulates endoplasmic reticulum homeostasis and distal epithelial cell survival during lung development. Am J Respir Cell Mol Biol. 2016;55:135–149. doi: 10.1165/rcmb.2015-0327OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhou B, Wang H, Liu Y, Flodby P, Crandall ED, Lee A, et al. Inducible alveolar epithelial type II (AT2) cell-specific Grp78 knockout mice develop endoplasmic reticulum (ER) stress and reversible pulmonary fibrosis [abstract] Am J Respir Crit Care Med. 2018;197:A6206. [Google Scholar]
- 22.Zhou B, Liu Y, Wang H, Flodby P, Lee A, Borok Z. ER stress-induced stem cell failure and lung fibrosis are reversed by TUDCA [abstract] Am J Respir Crit Care Med. 2019;199:A5880. [Google Scholar]
- 23.Zhou B, Horie M, Flodby P, Wang H, Liu Y, Lee A, et al. Transcriptomic analysis identifies pathways mediating alveolar epithelial type II (AT2) cell dysfunction and fibrosis in response to ER stress following Grp78 knockout (KO) [abstract] Am J Respir Crit Care Med. 2019;199:A7233. [Google Scholar]
- 24.Chapman HA, Li X, Alexander JP, Brumwell A, Lorizio W, Tan K, et al. Integrin α6β4 identifies an adult distal lung epithelial population with regenerative potential in mice. J Clin Invest. 2011;121:2855–2862. doi: 10.1172/JCI57673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arenkiel BR, Hasegawa H, Yi JJ, Larsen RS, Wallace ML, Philpot BD, et al. Activity-induced remodeling of olfactory bulb microcircuits revealed by monosynaptic tracing. PLoS One. 2011;6:e29423. doi: 10.1371/journal.pone.0029423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koitabashi N, Bedja D, Zaiman AL, Pinto YM, Zhang M, Gabrielson KL, et al. Avoidance of transient cardiomyopathy in cardiomyocyte-targeted tamoxifen-induced MerCreMer gene deletion models. Circ Res. 2009;105:12–15. doi: 10.1161/CIRCRESAHA.109.198416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heijmans J, van Lidth de Jeude JF, Koo BK, Rosekrans SL, Wielenga MC, van de Wetering M, et al. ER stress causes rapid loss of intestinal epithelial stemness through activation of the unfolded protein response. Cell Reports. 2013;3:1128–1139. doi: 10.1016/j.celrep.2013.02.031. [DOI] [PubMed] [Google Scholar]
- 28.Chen H, Matsumoto K, Brockway BL, Rackley CR, Liang J, Lee JH, et al. Airway epithelial progenitors are region specific and show differential responses to bleomycin-induced lung injury. Stem Cells. 2012;30:1948–1960. doi: 10.1002/stem.1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lehmann M, Korfei M, Mutze K, Klee S, Skronska-Wasek W, Alsafadi HN, et al. Senolytic drugs target alveolar epithelial cell function and attenuate experimental lung fibrosis ex vivo. Eur Respir J. 2017;50:1602367. doi: 10.1183/13993003.02367-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nakada EM, Bhakta NR, Korwin-Mihavics BR, Kumar A, Chamberlain N, Bruno SR, et al. Conjugated bile acids attenuate allergen-induced airway inflammation and hyperresponsiveness by inhibiting UPR transducers. JCI Insight. 2019;4:98101. doi: 10.1172/jci.insight.98101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schafer MJ, White TA, Iijima K, Haak AJ, Ligresti G, Atkinson EJ, et al. Cellular senescence mediates fibrotic pulmonary disease. Nat Commun. 2017;8:14532. doi: 10.1038/ncomms14532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Burman A, Kropski JA, Calvi CL, Serezani AP, Pascoalino BD, Han W, et al. Localized hypoxia links ER stress to lung fibrosis through induction of C/EBP homologous protein. JCI Insight. 2018;3:99543. doi: 10.1172/jci.insight.99543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mora AL, Bueno M, Rojas M. Mitochondria in the spotlight of aging and idiopathic pulmonary fibrosis. J Clin Invest. 2017;127:405–414. doi: 10.1172/JCI87440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Naikawadi RP, Disayabutr S, Mallavia B, Donne ML, Green G, La JL, et al. Telomere dysfunction in alveolar epithelial cells causes lung remodeling and fibrosis. JCI Insight. 2016;1:e86704. doi: 10.1172/jci.insight.86704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Garcia O, Hiatt MJ, Lundin A, Lee J, Reddy R, Navarro S, et al. Targeted type 2 alveolar cell depletion: a dynamic functional model for lung injury repair. Am J Respir Cell Mol Biol. 2016;54:319–330. doi: 10.1165/rcmb.2014-0246OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Osterholzer JJ, Olszewski MA, Murdock BJ, Chen GH, Erb-Downward JR, Subbotina N, et al. Implicating exudate macrophages and Ly-6C(high) monocytes in CCR2-dependent lung fibrosis following gene-targeted alveolar injury. J Immunol. 2013;190:3447–3457. doi: 10.4049/jimmunol.1200604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Justice JN, Nambiar AM, Tchkonia T, LeBrasseur NK, Pascual R, Hashmi SK, et al. Senolytics in idiopathic pulmonary fibrosis: results from a first-in-human, open-label, pilot study. EBioMedicine. 2019;40:554–563. doi: 10.1016/j.ebiom.2018.12.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hotamisligil GS. Endoplasmic reticulum stress and the inflammatory basis of metabolic disease. Cell. 2010;140:900–917. doi: 10.1016/j.cell.2010.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Neurohr C, Nishimura SL, Sheppard D. Activation of transforming growth factor-beta by the integrin alphavbeta8 delays epithelial wound closure. Am J Respir Cell Mol Biol. 2006;35:252–259. doi: 10.1165/rcmb.2006-0013OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li M, Krishnaveni MS, Li C, Zhou B, Xing Y, Banfalvi A, et al. Epithelium-specific deletion of TGF-β receptor type II protects mice from bleomycin-induced pulmonary fibrosis. J Clin Invest. 2011;121:277–287. doi: 10.1172/JCI42090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Young LR, Gulleman PM, Short CW, Tanjore H, Sherrill T, Qi A, et al. Epithelial-macrophage interactions determine pulmonary fibrosis susceptibility in Hermansky-Pudlak syndrome. JCI Insight. 2016;1:e88947. doi: 10.1172/jci.insight.88947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Riemondy KA, Jansing NL, Jiang P, Redente EF, Gillen AE, Fu R, et al. Single cell RNA sequencing identifies TGFβ as a key regenerative cue following LPS-induced lung injury. JCI Insight. 2019;5:123637. doi: 10.1172/jci.insight.123637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Alder JK, Barkauskas CE, Limjunyawong N, Stanley SE, Kembou F, Tuder RM, et al. Telomere dysfunction causes alveolar stem cell failure. Proc Natl Acad Sci USA. 2015;112:5099–5104. doi: 10.1073/pnas.1504780112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.van Lidth de Jeude JF, Meijer BJ, Wielenga MCB, Spaan CN, Baan B, Rosekrans SL, et al. Induction of endoplasmic reticulum stress by deletion of Grp78 depletes Apc mutant intestinal epithelial stem cells. Oncogene. 2017;36:3397–3405. doi: 10.1038/onc.2016.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tata PR, Rajagopal J. Plasticity in the lung: making and breaking cell identity. Development. 2017;144:755–766. doi: 10.1242/dev.143784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Selman M, Rojas M, Mora AL, Pardo A. Aging and interstitial lung diseases: unraveling an old forgotten player in the pathogenesis of lung fibrosis. Semin Respir Crit Care Med. 2010;31:607–617. doi: 10.1055/s-0030-1265901. [DOI] [PubMed] [Google Scholar]
- 47.Thannickal VJ. Mechanistic links between aging and lung fibrosis. Biogerontology. 2013;14:609–615. doi: 10.1007/s10522-013-9451-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Torres-González E, Bueno M, Tanaka A, Krug LT, Cheng DS, Polosukhin VV, et al. Role of endoplasmic reticulum stress in age-related susceptibility to lung fibrosis. Am J Respir Cell Mol Biol. 2012;46:748–756. doi: 10.1165/rcmb.2011-0224OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pfaffenbach KT, Lee AS. The critical role of GRP78 in physiologic and pathologic stress. Curr Opin Cell Biol. 2011;23:150–156. doi: 10.1016/j.ceb.2010.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.