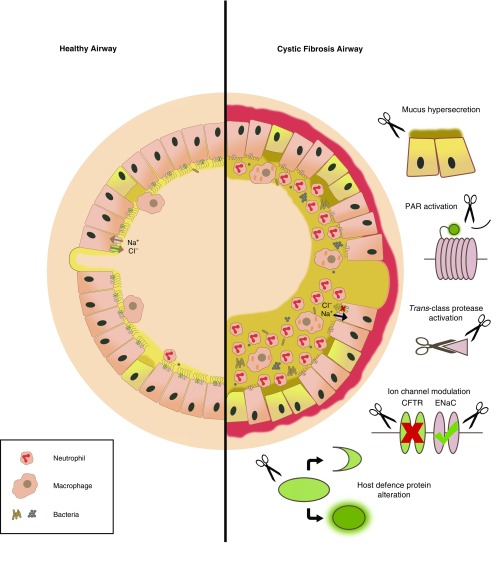
Cystic fibrosis (CF) is an autosomal recessive genetic condition, predominantly of white populations, that impacts multiple organ systems. However, it is the chronic progressive lung disease of CF that causes the greatest morbidity and mortality. The disease is caused by mutations of the CFTR (cystic fibrosis transmembrane conductance regulator) gene. Consequently, the function of epithelial CFTR anion (chloride and bicarbonate) channels is compromised, leading to impaired anion and fluid secretion and airway surface dehydration, which in turn result in highly viscous airway mucus and impaired mucociliary clearance, setting the stage for mucus plugging, chronic inflammation, and polymicrobial infection (1). Such a state causes progressive and irreversible damage of the airways and lung parenchyma because recruited immune cells (predominantly neutrophils) release proteases, DNA, and reactive oxygen species and promote further immune cell recruitment by cytokine signaling.
The introduction of CFTR modulators (potentiators, correctors, and amplifiers) in recent years has transformed the treatment of CF. Phase 2 trials of triple-combination therapy suggest that a CFTR modulator therapy approach may be effective in up to 90% of patients with CF (2, 3). Although the emerging therapies show immense promise, there are still patients with CF whose specific genotypes may not be amenable to these therapies. Furthermore, CFTR modulation alone may be insufficient to allow complete and lasting clearance of chronic airway infection and resolution of pulmonary inflammation, especially in the context of chronic CF lung disease with established structural lung damage (4). Importantly, it is unknown whether, or to what extent, these CFTR-directed therapies decrease protease activity. Until such a decrease has been demonstrated, novel antiprotease strategies are still highly relevant to limit tissue damage in CF lung disease.
The protease–antiprotease hypothesis is a simple paradigm that attempts to explain certain disease states as a product of an imbalance of proteases and cognate antiproteases, resulting in elevated protease activity and damaging consequences for lung homeostasis (5). It is now well established that proteases play a significant role in the pathobiology of the CF lung (6), regardless of whether they are derived from immune cells or indeed the cells of the lung itself. The perception of these enzymes’ roles has moved far beyond the terminal degradation of proteins; it is now recognized that proteases are key signaling molecules and that specific substrate cleavage can have myriad effects (7), beneficial or detrimental, in the CF lung (Figure 1). The use of protease inhibitor therapy may offer an alternative option for mitigating the disease state to regain homeostasis, which may go hand in hand with pharmacological rescue of mutant CFTR by emerging modulator therapies.
Figure 1.
A model of the cystic fibrosis (CF) airway and associated protease-mediated pathologies. A healthy airway maintains a thin layer of well-hydrated mucus covering the airway surface. Invading pathogens and particulates are trapped and subsequently removed from the airway by mucociliary clearance. In CF airways, malfunction of CFTR anion channels and increased activity of epithelial sodium channels (ENaCs) result in airway surface dehydration, altered viscoelastic properties of airway mucus, and impaired mucociliary clearance, which makes the airway susceptible to chronic infection and inflammation. Neutrophils recruited to the airway, together with macrophages and epithelial cells, secrete proteases that aggravate key aspects of the pathophysiology of CF. Active proteases compromise the structural integrity of the airway through the degradation of elastin and collagen, leading to bronchiectasis. In addition, other protease roles in CF include (top to bottom): the enhancement of mucin/mucus production and secretion, the activation of PARs (protease-activated receptors) leading to proinflammatory signaling, the trans-activation of other proteases by cleaving prodomains and degrading cognate antiproteases, the aggravation of basic CF ion transport defects by the proteolytic degradation of CFTR and activation of ENaC, and the cleavage of various host protein substrates precipitating either activation (in the case of some proinflammatory cytokines) or inactivation (in the case of some antimicrobial peptides and surfactant proteins). CFTR = cystic fibrosis transmembrane conductance regulator.
So far, the serine protease class has drawn the most attention in CF, in particular neutrophil elastase (NE), with its free extracellular form previously believed to be the major player in CF lung disease pathogenesis. Indeed, free NE in sputum has long been known to correlate with FEV1 in children with CF (8), and elevated NE activity in BAL fluid at 3 months of age was found to be associated with persistent bronchiectasis by the Australian Respiratory Early Surveillance Team for Cystic Fibrosis (9). However, several novel concepts unfolding in pulmonary protease biology have led investigators to broaden their view beyond NE. These concepts include the redundancy of function between proteases (Table 1), the trans-class activation of proteases, the discovery of highly active membrane-bound proteases, and the emergence of new key players from the cysteine and MMP (matrix metalloprotease) classes. This review summarizes the current knowledge of host protease function in CF lung disease and how this may inform therapeutic intervention. The role of bacterial proteases in CF lies beyond the scope of this review; we direct readers to two recent publications for information on that topic (10, 11).
Table 1.
Protease Functions and Redundancy in Cystic Fibrosis Lung Disease
| Function | Protease Class Involved | References |
|---|---|---|
| Matrix degradation | Serine, cysteine, MMP | 52, 54, 55, 70 |
| Cytokine processing | Serine, cysteine, MMP | 14, 18, 46, 56 |
| Cytokine upregulation | Serine, cysteine, MMP | 15, 48, 54, 55 |
| PAR activation | Serine, cysteine, MMP | 48, 71 |
| Trans-class protease activation | Serine, cysteine | 9, 12, 26, 27 |
| Host defense protein degradation (including antiproteases) | Serine, cysteine | 21–23, 29, 42–45 |
| ENaC activation | Serine, cysteine | 38, 49, 50 |
| CFTR degradation | Serine | 37 |
| Mucus modulation | Serine | 33–36, 48 |
| Iron liberation | Serine | 24, 25 |
Definition of abbreviations: CFTR = cystic fibrosis transmembrane conductance regulator; ENaC = epithelial sodium channel; MMP = matrix metalloprotease; PAR = protease-activated receptor.
The Actions of Serine Proteases in CF
The neutrophilic nature of CF airway inflammation gives intuitive significance to the group of proteases known collectively as the NSPs (neutrophil serine proteases). Its members are NE; proteinase 3; cathepsin G; and the more recently discovered, less studied NSP-4. The NSPs may be activated by the CTSC (cysteine protease cathepsin C) (12) and are involved in the intracellular degradation of neutrophil-phagocytosed microbes, a particularly important process in the chronically infected CF lung. The NSPs are harbored in primary neutrophil granules, and their exocytosis is increased in CF, even during early CF disease before the onset of bronchiectasis (13). Extracellular NSPs have been shown to actively mediate the recruitment of immune cells to the site of inflammation by processing an array of cytokines including members of the IL-1 family (14), upregulating neutrophil chemoattractants such as IL-8 (15), and triggering the release of damage-associated molecular patterns such as HMGB1 (high mobility group box 1 protein), which can act as a biomarker for CF lung disease severity (16, 17). This modulation of neutrophil chemotaxis leads to enhanced inflammatory cell infiltration, continuing the vicious cycle of CF inflammation. Furthermore, highly chemotactic, truncated forms of various chemokines including IL-8 can be produced by NSP-mediated cleavage (18).
Neutrophil extracellular traps, the complex matrix of secreted DNA, proteases, and other cellular contents released by neutrophils in CF airways (19), are important reservoirs of NSPs in CF. It has been demonstrated that this DNA-based matrix effectively maintains protease activity by preventing interactions with cognate endogenous or administered antiproteases (20). Although intended as a protective mechanism, NSP activity can adversely affect the body’s innate response mechanisms to infection, including antimicrobial peptides (AMPs) and surfactant proteins. A number of proteases, including NE, can cleave AMPs such as lactoferrin and midkine (21, 22) and degrade surfactant proteins (23), thereby compromising the host response and/or susceptibility to infection. NE also cleaves extracellular heme-containing proteins such as ferritin, liberating sequestered iron into the airway. Not only does this increase oxidative stress in the airway epithelium, but it also promotes bacterial proliferation and biofilm formation because iron is made accessible for microbial nutrition (24, 25).
NSPs may also play an important role as regulators of other proteases, particularly the MMPs. This role is especially relevant when considering the tissue-destructive nature of the proteases: NSP-activated MMP-9 and MMP-12, as well as the NSPs themselves, contribute directly to extracellular matrix (ECM) remodeling and bronchiectasis that are characteristic of CF (9, 26, 27). The protease-mediated loss of elastin limits elastic recoil, whereas the loss of collagen creates a structural deficit, leading to the emphysematous phenotype that can occur in adolescent and adult patients with CF (28). The body’s endogenous protection against aberrant NSP activity is also compromised because NE inactivates tissue-protective antiproteases (some of which also possess antimicrobial properties) such as secretory leukocyte protease inhibitor (29).
The inability of such endogenous antiproteases, even when intact, to perform their inhibitory function has also been highlighted in recent years. This may be due, in part, to membrane association of NSPs such as NE and cathepsin G (30). More recently, novel Förster resonance energy transfer–based probes were used to analyze membrane-bound activity of proteases such as NE on the surface of neutrophils (31). In this surface-bound form, proteases are less accessible for their prospective inhibitors, which are unable to access the enzyme’s active site. Indeed, surface-bound NE has been found to correlate with severity of lung disease and various inflammatory markers in CF (32).
In addition to influencing both inflammatory cell recruitment and tissue destruction, NE contributes to increased mucus production in the CF lung by upregulating mucin expression and inducing goblet cell metaplasia, a process believed to be mediated through tumor necrosis factor-α–converting enzyme (33, 34). In addition, NE induces secretion of mucins from airway epithelial cells, augmenting mucus plugging in the CF lung (35). NE has also been shown to decrease the ciliary beat frequency and damage the airway epithelium (36), which may contribute to impaired mucociliary clearance and hence mucus plugging. Furthermore, NE may directly impact airway ion transport by degrading CFTR (37) and activating epithelial sodium channel (ENaC) (38), thereby aggravating the basic ion transport defect and airway surface dehydration in CF airways. Although CF is caused by mutations in CFTR, CF airways are characterized by increased ENaC-mediated sodium absorption in addition to deficient CFTR-mediated chloride secretion. Mimicking the hyperactivity of ENaC by airway-specific overexpression in mice can produce a phenotype that is strikingly similar to that found in patients with CF, demonstrating that airway surface dehydration is a key disease mechanism in CF lung disease (1, 39) and that increased ENaC activity contributes critically to this abnormality. In this context, proteolytic activation of ENaC by NE and other proteases may be a key mechanism leading to increased ENaC activity that aggravates airway surface dehydration in CF airways.
Collectively, these studies show that NE is a key mediator in each of the major pathologies contributing to CF lung disease. However, the roles of the other NSPs have been less well studied, and more research into these and their relative importance in CF is warranted.
The Actions of Cysteine Proteases in CF
The predominant group of cysteine proteases in CF is the cysteine cathepsins. These papain-like proteases are lysosomally derived and hence display optimal activity in a reducing and acidic environment; only CTSS (cathepsin S) is believed to maintain its activity in the neutral–alkaline pH range (40). In the intracellular context, cathepsins are involved in the degradation of host and pathogen proteins as well as the processing and presentation of antigens. These functions are crucial in homeostatic protein turnover, fighting infection, and the development of adaptive immune responses to infections. However, like the NSPs, certain members can be found in the extracellular milieu of the CF lung. Cathepsins are secreted by macrophages but may also be sourced from neutrophils, other antigen-presenting cells, and lung epithelial and endothelial cells; this secretion may be associated with acidification of the pericellular space (41).
Although only recently recognized as major players in CF, the cysteine proteases mirror many of the actions of the NSPs. Like all classes of proteases, the cysteine cathepsins are capable of degrading various ECM components, contributing to the tissue-destructive web of proteases involved in CF. A series of studies demonstrated the potential of cathepsins B, L, and S to compromise mucosal immunity in the CF lung via mechanisms similar to those mentioned already for the NSPs. They were shown to cleave AMPs including lactoferrin, LL-37, surfactant protein A, and the human β-defensins (42–45). Thus, by the loss of active airway AMPs, the ability to maintain a pathogen-free airway may be undermined in CF. Several cathepsins have also demonstrated the ability to process chemokine (C-X-C motif) ligands in vitro, though it has yet to be determined whether these modifications occur or are highly relevant in vivo (46). The role of CTSC in the activation of NSPs makes it an interesting candidate for therapeutic intervention in the context of neutrophilic CF lung disease, and although inhibitors are in early-phase clinical trials, their efficacy and potential for CF remains to be determined (12).
CTSS is emerging as an important player in early CF lung disease with extracellular CTSS concentrations correlating significantly with lung function decline and neutrophil recruitment into the airways (47). A recent study using the β-ENaC–overexpressing mouse with CF-like lung disease elucidated roles for CTSS in the in vivo pathogenesis of several key CF pathologies (48). In this study, genetic and pharmacological knockdown of CTSS was associated with a reduction in neutrophil recruitment and amelioration of airway mucus obstruction and lung tissue destruction. It also highlighted that CTSS may mediate inflammatory cell recruitment and mucin expression via protease-activated receptor 2. In relation to airway ion transport, both CTSS and cathepsin B have been reported to activate ENaC (49, 50). As such, in concert with NE-mediated CFTR degradation, the cysteine cathepsins may accentuate the mucus dehydration intrinsic to CF airway pathology.
The Actions of Matrix Metalloproteases in CF
The members of the MMP class are not abundant in the healthy lung; however, they are produced by lung and inflammatory cells in response to inflammatory chemokines, noxious stimuli, and free oxygen radicals (51). These zinc- and calcium-dependent endopeptidases are loosely numbered in order of discovery up to MMP-28 and, as their name suggests, are potent ECM-degrading enzymes (52). Although some MMPs are mainly tissue derived, MMP-8 and MMP-9 are predominantly derived from neutrophils, making them proteases of particular interest in CF (53). The degradation of interstitial collagen is key to the development of bronchiectasis and other aberrant structural formations of the CF lung. In addition, this cleavage process generates matrix fragments, which can produce secondary downstream effects. During airway inflammation, the proline-glycine-proline (PGP; a potent neutrophil chemoattractant) fragments produced by collagenase activity are not matched by a concomitant rise in PGP degradation by leukotriene A4 hydrolase, causing PGP accumulation that contributes to CF neutrophilia (54, 55). In addition, MMP-9 is capable of truncating IL-8 into a highly chemoattractive form (56).
Although it is clear that the dominant immune cell population in the CF lung is the neutrophil, macrophage-derived proteases are gaining a reputation in CF, particularly as regards their membrane-associated activity. A noteworthy example of this is macrophage elastase (also known as MMP-12). Recent studies in β-ENaC–overexpressing mice with CF-like lung disease and pediatric patients with CF suggest that mucostatic conditions in the CF airways may trigger elevated membrane-associated MMP-12 activity via macrophage activation (57). Interestingly, a functional polymorphism in the MMP12 promoter (rs2276109) that decreases MMP12 expression was positively associated with FEV1 percent predicted in patients with CF (57). This work opens interesting lines of inquiry: What are the specific signals that precipitate protease release from specific cell types? Are they to be found in CF mucus? Can they be targeted therapeutically? Can protease gene expression be targeted?
It is worth noting that there is evidence of positive roles for macrophage MMPs in the inflamed lung. MMP-10, for example, is highly expressed in macrophages of patients with CF and appears to have a protective role in acute bacterial infection by moderating macrophage inflammatory responses (58). MMPs continue to draw most attention for their contribution to lung tissue damage, though there is an emerging sense that this may not be the limit of their influence in CF.
Therapeutic Strategies Targeting the Protease–Antiprotease Imbalance
The combined contribution of proteases to the pathology of CF makes them promising targets for novel therapeutics. Endogenous protease inhibitors are overpowered as a consequence of quantitatively elevated concentrations of secreted “free” protease and have limited efficacy in the inhibition of surface-bound activity in CF lung disease. Importantly, current CF therapy relies heavily on mucolytic agents such as dornase alfa that are known to markedly increase NE activity in CF sputum (59). Therefore, antiproteases may constitute an important adjunct therapy to help limit further lung injury. Indeed, it has been shown that certain antiproteases are most effective when used together with mucolytics (59). Increased protease secretion and membrane-associated activity are likely already initiated during infancy and early childhood, even in the absence of detectable bacterial infection (5, 9), strengthening the case for early antiprotease treatment.
To directly redress the protease–antiprotease imbalance, two principal strategies may be employed: antiprotease replacement/augmentation and pharmacological protease inhibition. There is an attraction to using antiprotease-based therapies such as alpha-1 antitrypsin augmentation, especially considering the success of this strategy in alpha-1 antitrypsin deficiency. In 2015, a long-term randomized controlled trial was reported with weekly alpha-1 antitrypsin administration for up to 48 months (60). RAPID (Randomized, Placebo-controlled Trial of Augmentation Therapy in Alpha-1 Proteinase Inhibitor Deficiency) demonstrated the slowing of lung parenchymal damage after redressing the protease–antiprotease imbalance and, significantly, that this effect was most evident over the course of months and years rather than in short-term improvements over weeks (60). This was not a surprising finding, considering that antiprotease therapy is predicted to slow the progression of irreversible lung damage and bronchiectasis rather than to produce short-term improvements in lung function; short trials are therefore unlikely to capture these therapeutic benefits. Alpha-1 antitrypsin augmentation has also been tested in CF, though this has been limited to short trials, predominantly powered to establish safety (61). Endogenous antiprotease augmentation is not without its pitfalls, given the propensity of antiproteases to be degraded by proteases (host or pathogen). Recombinant variants of the endogenous antiproteases, such as secretory leukocyte protease inhibitor, with decreased susceptibility to protease cleavage have shown efficacy in reducing inflammation (62). However, the size and complexity of these proteins, their generally broad antiprotease activity, and the quantities required to address the substantial protease burden in CF are all factors to be overcome. These molecules have also yet to demonstrate efficacy against surface-bound proteases.
With these considerations in mind, perhaps it is the synthetic, low-molecular-weight, specific chemical inhibitors that hold the answer? As with all drugs, walking the tightrope between specificity and bioreactivity has proved to be a challenge. For various reasons (including safety, a propensity for hapten formation, and the struggle for target selectivity), it is no longer the consensus that rapid, irreversible inhibition is necessarily the gold standard for these compounds (63). A new generation of highly specific, reversible inhibitors of NE or the emerging proteases (CTSC, CTSS, and MMP-12) might help to shape the future of antiprotease therapy in CF. Many synthetic inhibitors have demonstrated potency in vitro and in the preclinical in vivo setting. However, NE inhibition, which has been the focus of clinical antiprotease work, has so far not proved overly effective in reducing key measures of disease in CF or other inflammatory lung diseases (64). Therefore, it may be that the inhibition of other proteases or a spectrum of proteases in combination with conventional therapies produces more promising results. Comprehensive studies will be required to ensure that patient susceptibility to infection is not increased by protease inhibition, though there is little preclinical evidence that this will be the case. Interestingly, the genetic ablation of NE in β-ENaC–overexpressing mice did not increase susceptibility to spontaneous airway infection in this model with CF-like lung disease (33). NSP-deficient mice have exhibited weakened host defense against certain respiratory pathogens (65, 66), though it should be noted that inhibitor-treated and full-knockout mice are not direct corollaries, and, as such, further research is required to assess the effects of therapeutically relevant protease knockdown on host immunity.
An interesting alternative to the use of canonical antiproteases is inhaled heparin, which has been shown to improve pulmonary function in chronic obstructive pulmonary disease (67). 2-O, 3-O-desulfated heparin, a modified polysulfated molecule, possesses both anti-NSP and antiinflammatory properties with minimal anticoagulant activity (16, 68). In some instances, repurposed drugs might offer a simpler and faster route to protease inhibition than the development of novel inhibitors, especially regarding their safety profile. One drug that has emerged is the tetracycline antibiotic doxycycline. A 2017 study in patients with CF highlighted FEV1 improvements after doxycycline treatment during acute pulmonary exacerbations, seemingly independent of doxycycline’s antibiotic properties, via MMP-9 neutralization and TIMP-1 enhancement (69). Currently, there are no other licensed drugs that are known to fall into this category.
Summary and Outlook
What is evident from the research to date is that proteases play a role in many of the most damaging facets of CF lung disease and as such could be targeted in combination with current antibiotic, mucolytic, bronchodilator, and CFTR modulator therapies. A return to protease–antiprotease parity may indeed facilitate the breaking of the inflammatory cycle and slow the rate of structural and functional decline in CF. For proteases such as NE and CTSS, which are elevated from an early stage in the pathogenesis of CF lung disease, the age of patients with CF at the start of protease inhibitor therapy and the frequency and duration of treatments may well be crucial factors to consider for the design of clinical trials. A significant challenge remains in developing protease inhibitors that retain specificity, stability, and efficacy in the complex milieu of the CF lung and that are well tolerated over longer courses of treatment.
Knowledge of the role and functions of proteases continues to evolve through the development and use of new experimental tools, reagents, and pathobiological models. Because of their differential expression and activity profiles in CF lung disease, proteases (and their endogenous inhibitors) may serve as useful biomarkers for diagnostic and monitoring purposes to enable, for example, detection of lung disease severity and prediction of progression or response to treatment. Nonetheless, further work is needed to extensively characterize the lung degradome in addition to the status of endogenous antiproteases, activators, substrates, and cleavage products in the CF lung.
Supplementary Material
Footnotes
Supported by the Northern Ireland Department for the Economy (studentship [M.C.M.]), the Cystic Fibrosis Foundation (WELDON18G0), the Medical Research Council (MR/P022847/1 [C.C.T. and S.W.]), the Rosetrees Trust (grant A2450 [C.C.T. and S.W.]), the German Federal Ministry of Education and Research (82DZL004A1 [M.A.M.]), the German Research Foundation (SFB-TR84TP B08 [M.A.M.]), and the Einstein Foundation Berlin (EP-2017-393 [M.A.M.]).
Author Contributions: Conception, design, and drafting of the article or critical revision for important intellectual content: M.C.M., S.W., D.F.M., M.A.M., and C.C.T.
Originally Published in Press as DOI: 10.1164/rccm.201906-1190PP on October 18, 2019
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Mall MA, Hartl D. CFTR: cystic fibrosis and beyond. Eur Respir J. 2014;44:1042–1054. doi: 10.1183/09031936.00228013. [DOI] [PubMed] [Google Scholar]
- 2.Davies JC, Moskowitz SM, Brown C, Horsley A, Mall MA, McKone EF, et al. VX16-659-101 Study Group. VX-659-tezacaftor-ivacaftor in patients with cystic fibrosis and one or two Phe508del alleles. N Engl J Med. 2018;379:1599–1611. doi: 10.1056/NEJMoa1807119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Keating D, Marigowda G, Burr L, Daines C, Mall MA, McKone EF, et al. VX16-445-001 Study Group. VX-445-tezacaftor-ivacaftor in patients with cystic fibrosis and one or two Phe508del alleles. N Engl J Med. 2018;379:1612–1620. doi: 10.1056/NEJMoa1807120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hisert KB, Heltshe SL, Pope C, Jorth P, Wu X, Edwards RM, et al. Restoring cystic fibrosis transmembrane conductance regulator function reduces airway bacteria and inflammation in people with cystic fibrosis and chronic lung infections. Am J Respir Crit Care Med. 2017;195:1617–1628. doi: 10.1164/rccm.201609-1954OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Birrer P, McElvaney NG, Rüdeberg A, Sommer CW, Liechti-Gallati S, Kraemer R, et al. Protease-antiprotease imbalance in the lungs of children with cystic fibrosis. Am J Respir Crit Care Med. 1994;150:207–213. doi: 10.1164/ajrccm.150.1.7912987. [DOI] [PubMed] [Google Scholar]
- 6.Voynow JA, Fischer BM, Zheng S. Proteases and cystic fibrosis. Int J Biochem Cell Biol. 2008;40:1238–1245. doi: 10.1016/j.biocel.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Turk B, Turk D, Turk V. Protease signalling: the cutting edge. EMBO J. 2012;31:1630–1643. doi: 10.1038/emboj.2012.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sagel SD, Sontag MK, Wagener JS, Kapsner RK, Osberg I, Accurso FJ. Induced sputum inflammatory measures correlate with lung function in children with cystic fibrosis. J Pediatr. 2002;141:811–817. doi: 10.1067/mpd.2002.129847. [DOI] [PubMed] [Google Scholar]
- 9.Sly PD, Gangell CL, Chen L, Ware RS, Ranganathan S, Mott LS, et al. AREST CF Investigators. Risk factors for bronchiectasis in children with cystic fibrosis. N Engl J Med. 2013;368:1963–1970. doi: 10.1056/NEJMoa1301725. [DOI] [PubMed] [Google Scholar]
- 10.Reihill JA, Moreland M, Jarvis GE, McDowell A, Einarsson GG, Elborn JS, et al. Bacterial proteases and haemostasis dysregulation in the CF lung. J Cyst Fibros. 2017;16:49–57. doi: 10.1016/j.jcf.2016.10.006. [DOI] [PubMed] [Google Scholar]
- 11.Molloy K, Smith SG, Cagney G, Dillon ET, Greene CM, McElvaney NG. Characterisation of the major extracellular proteases of Stenotrophomonas maltophilia and their effects on pulmonary antiproteases. Pathogens. 2019;8:92. doi: 10.3390/pathogens8030092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Korkmaz B, Caughey GH, Chapple I, Gauthier F, Hirschfeld J, Jenne DE, et al. Therapeutic targeting of cathepsin C: from pathophysiology to treatment. Pharmacol Ther. 2018;190:202–236. doi: 10.1016/j.pharmthera.2018.05.011. [DOI] [PubMed] [Google Scholar]
- 13.Margaroli C, Garratt LW, Horati H, Dittrich AS, Rosenow T, Montgomery ST, et al. Elastase exocytosis by airway neutrophils is associated with early lung damage in children with cystic fibrosis. Am J Respir Crit Care Med. 2019;199:873–881. doi: 10.1164/rccm.201803-0442OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clancy DM, Sullivan GP, Moran HBT, Henry CM, Reeves EP, McElvaney NG, et al. Extracellular neutrophil proteases are efficient regulators of IL-1, IL-33, and IL-36 cytokine activity but poor effectors of microbial killing. Cell Reports. 2018;22:2937–2950. doi: 10.1016/j.celrep.2018.02.062. [DOI] [PubMed] [Google Scholar]
- 15.Devaney JM, Greene CM, Taggart CC, Carroll TP, O’Neill SJ, McElvaney NG. Neutrophil elastase up-regulates interleukin-8 via toll-like receptor 4. FEBS Lett. 2003;544:129–132. doi: 10.1016/s0014-5793(03)00482-4. [DOI] [PubMed] [Google Scholar]
- 16.Griffin KL, Fischer BM, Kummarapurugu AB, Zheng S, Kennedy TP, Rao NV, et al. 2-O, 3-O-desulfated heparin inhibits neutrophil elastase–induced HMGB-1 secretion and airway inflammation. Am J Respir Cell Mol Biol. 2014;50:684–689. doi: 10.1165/rcmb.2013-0338RC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liou TG, Adler FR, Keogh RH, Li Y, Jensen JL, Walsh W, et al. Sputum biomarkers and the prediction of clinical outcomes in patients with cystic fibrosis. PLoS One. 2012;7:e42748. doi: 10.1371/journal.pone.0042748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Padrines M, Wolf M, Walz A, Baggiolini M. Interleukin-8 processing by neutrophil elastase, cathepsin G and proteinase-3. FEBS Lett. 1994;352:231–235. doi: 10.1016/0014-5793(94)00952-x. [DOI] [PubMed] [Google Scholar]
- 19.Gray RD, Hardisty G, Regan KH, Smith M, Robb CT, Duffin R, et al. Delayed neutrophil apoptosis enhances NET formation in cystic fibrosis. Thorax. 2018;73:134–144. doi: 10.1136/thoraxjnl-2017-210134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dubois AV, Gauthier A, Bréa D, Varaigne F, Diot P, Gauthier F, et al. Influence of DNA on the activities and inhibition of neutrophil serine proteases in cystic fibrosis sputum. Am J Respir Cell Mol Biol. 2012;47:80–86. doi: 10.1165/rcmb.2011-0380OC. [DOI] [PubMed] [Google Scholar]
- 21.Britigan BE, Hayek MB, Doebbeling BN, Fick RB., Jr Transferrin and lactoferrin undergo proteolytic cleavage in the Pseudomonas aeruginosa-infected lungs of patients with cystic fibrosis. Infect Immun. 1993;61:5049–5055. doi: 10.1128/iai.61.12.5049-5055.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nordin SL, Jovic S, Kurut A, Andersson C, Gela A, Bjartell A, et al. High expression of midkine in the airways of patients with cystic fibrosis. Am J Respir Cell Mol Biol. 2013;49:935–942. doi: 10.1165/rcmb.2013-0106OC. [DOI] [PubMed] [Google Scholar]
- 23.Rubio F, Cooley J, Accurso FJ, Remold-O’Donnell E. Linkage of neutrophil serine proteases and decreased surfactant protein-A (SP-A) levels in inflammatory lung disease. Thorax. 2004;59:318–323. doi: 10.1136/thx.2003.014902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fischer BM, Domowicz DAL, Zheng S, Carter JL, McElvaney NG, Taggart C, et al. Neutrophil elastase increases airway epithelial nonheme iron levels. Clin Transl Sci. 2009;2:333–339. doi: 10.1111/j.1752-8062.2009.00151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ghio AJ, Roggli VL, Soukup JM, Richards JH, Randell SH, Muhlebach MS. Iron accumulates in the lavage and explanted lungs of cystic fibrosis patients. J Cyst Fibros. 2013;12:390–398. doi: 10.1016/j.jcf.2012.10.010. [DOI] [PubMed] [Google Scholar]
- 26.Garratt LW, Sutanto EN, Ling K-M, Looi K, Iosifidis T, Martinovich KM, et al. Australian Respiratory Early Surveillance Team for Cystic Fibrosis (AREST CF) Matrix metalloproteinase activation by free neutrophil elastase contributes to bronchiectasis progression in early cystic fibrosis. Eur Respir J. 2015;46:384–394. doi: 10.1183/09031936.00212114. [DOI] [PubMed] [Google Scholar]
- 27.Guyot N, Wartelle J, Malleret L, Todorov AA, Devouassoux G, Pacheco Y, et al. Unopposed cathepsin G, neutrophil elastase, and proteinase 3 cause severe lung damage and emphysema. Am J Pathol. 2014;184:2197–2210. doi: 10.1016/j.ajpath.2014.04.015. [DOI] [PubMed] [Google Scholar]
- 28.Wielpütz MO, Eichinger M, Biederer J, Wege S, Stahl M, Sommerburg O, et al. Imaging of cystic fibrosis lung disease and clinical interpretation. Rofo. 2016;188:834–845. doi: 10.1055/s-0042-104936. [DOI] [PubMed] [Google Scholar]
- 29.Weldon S, McNally P, McElvaney NG, Elborn JS, McAuley DF, Wartelle J, et al. Decreased levels of secretory leucoprotease inhibitor in the Pseudomonas-infected cystic fibrosis lung are due to neutrophil elastase degradation. J Immunol. 2009;183:8148–8156. doi: 10.4049/jimmunol.0901716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Owen CA, Campbell MA, Sannes PL, Boukedes SS, Campbell EJ. Cell surface-bound elastase and cathepsin G on human neutrophils: a novel, non-oxidative mechanism by which neutrophils focus and preserve catalytic activity of serine proteinases. J Cell Biol. 1995;131:775–789. doi: 10.1083/jcb.131.3.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gehrig S, Mall MA, Schultz C. Spatially resolved monitoring of neutrophil elastase activity with ratiometric fluorescent reporters. Angew Chem Int Ed Engl. 2012;51:6258–6261. doi: 10.1002/anie.201109226. [DOI] [PubMed] [Google Scholar]
- 32.Dittrich AS, Kühbandner I, Gehrig S, Rickert-Zacharias V, Twigg M, Wege S, et al. Elastase activity on sputum neutrophils correlates with severity of lung disease in cystic fibrosis. Eur Respir J. 2018;51:1701910. doi: 10.1183/13993003.01910-2017. [DOI] [PubMed] [Google Scholar]
- 33.Gehrig S, Duerr J, Weitnauer M, Wagner CJ, Graeber SY, Schatterny J, et al. Lack of neutrophil elastase reduces inflammation, mucus hypersecretion, and emphysema, but not mucus obstruction, in mice with cystic fibrosis-like lung disease. Am J Respir Crit Care Med. 2014;189:1082–1092. doi: 10.1164/rccm.201311-1932OC. [DOI] [PubMed] [Google Scholar]
- 34.Park JA, Sharif AS, Shiomi T, Kobzik L, Kasahara DI, Tschumperlin DJ, et al. Human neutrophil elastase-mediated goblet cell metaplasia is attenuated in TACE-deficient mice. Am J Physiol Lung Cell Mol Physiol. 2013;304:L701–L707. doi: 10.1152/ajplung.00259.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shao MX, Nadel JA. Neutrophil elastase induces MUC5AC mucin production in human airway epithelial cells via a cascade involving protein kinase C, reactive oxygen species, and TNF-α-converting enzyme. J Immunol. 2005;175:4009–4016. doi: 10.4049/jimmunol.175.6.4009. [DOI] [PubMed] [Google Scholar]
- 36.Bingle L, Richards RJ, Fox B, Masek L, Guz A, Tetley TD. Susceptibility of lung epithelium to neutrophil elastase: protection by native inhibitors. Mediators Inflamm. 1997;6:345–354. doi: 10.1080/09629359791488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Le Gars M, Descamps D, Roussel D, Saussereau E, Guillot L, Ruffin M, et al. Neutrophil elastase degrades cystic fibrosis transmembrane conductance regulator via calpains and disables channel function in vitro and in vivo. Am J Respir Crit Care Med. 2013;187:170–179. doi: 10.1164/rccm.201205-0875OC. [DOI] [PubMed] [Google Scholar]
- 38.Caldwell RA, Boucher RC, Stutts MJ. Neutrophil elastase activates near-silent epithelial Na+ channels and increases airway epithelial Na+ transport. Am J Physiol Lung Cell Mol Physiol. 2005;288:L813–L819. doi: 10.1152/ajplung.00435.2004. [DOI] [PubMed] [Google Scholar]
- 39.Mall M, Grubb BR, Harkema JR, O’Neal WK, Boucher RC. Increased airway epithelial Na+ absorption produces cystic fibrosis-like lung disease in mice. Nat Med. 2004;10:487–493. doi: 10.1038/nm1028. [DOI] [PubMed] [Google Scholar]
- 40.Kirschke H, Wiederanders B, Brömme D, Rinne A. Cathepsin S from bovine spleen: purification, distribution, intracellular localization and action on proteins. Biochem J. 1989;264:467–473. doi: 10.1042/bj2640467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Reddy VY, Zhang QY, Weiss SJ. Pericellular mobilization of the tissue-destructive cysteine proteinases, cathepsins B, L, and S, by human monocyte-derived macrophages. Proc Natl Acad Sci USA. 1995;92:3849–3853. doi: 10.1073/pnas.92.9.3849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rogan MP, Taggart CC, Greene CM, Murphy PG, O’Neill SJ, McElvaney NG. Loss of microbicidal activity and increased formation of biofilm due to decreased lactoferrin activity in patients with cystic fibrosis. J Infect Dis. 2004;190:1245–1253. doi: 10.1086/423821. [DOI] [PubMed] [Google Scholar]
- 43.Andrault PM, Samsonov SA, Weber G, Coquet L, Nazmi K, Bolscher JGM, et al. Antimicrobial peptide LL-37 is both a substrate of cathepsins S and K and a selective inhibitor of cathepsin L. Biochemistry. 2015;54:2785–2798. doi: 10.1021/acs.biochem.5b00231. [DOI] [PubMed] [Google Scholar]
- 44.Lecaille F, Naudin C, Sage J, Joulin-Giet A, Courty A, Andrault PM, et al. Specific cleavage of the lung surfactant protein A by human cathepsin S may impair its antibacterial properties. Int J Biochem Cell Biol. 2013;45:1701–1709. doi: 10.1016/j.biocel.2013.05.018. [DOI] [PubMed] [Google Scholar]
- 45.Taggart CC, Greene CM, Smith SG, Levine RL, McCray PB, Jr, O’Neill S, et al. Inactivation of human β-defensins 2 and 3 by elastolytic cathepsins. J Immunol. 2003;171:931–937. doi: 10.4049/jimmunol.171.2.931. [DOI] [PubMed] [Google Scholar]
- 46.Repnik U, Starr AE, Overall CM, Turk B. Cysteine cathepsins activate ELR chemokines and inactivate non-ELR chemokines. J Biol Chem. 2015;290:13800–13811. doi: 10.1074/jbc.M115.638395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Weldon S, McNally P, McAuley DF, Oglesby IK, Wohlford-Lenane CL, Bartlett JA, et al. miR-31 dysregulation in cystic fibrosis airways contributes to increased pulmonary cathepsin S production. Am J Respir Crit Care Med. 2014;190:165–174. doi: 10.1164/rccm.201311-1986OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Small DM, Brown RR, Doherty DF, Abladey A, Zhou-Suckow Z, Delaney RJ, et al. Targeting of cathepsin S reduces cystic fibrosis-like lung disease. Eur Respir J. 2019;53:1801523. doi: 10.1183/13993003.01523-2018. [DOI] [PubMed] [Google Scholar]
- 49.Tan CD, Hobbs C, Sameni M, Sloane BF, Stutts MJ, Tarran R. Cathepsin B contributes to Na+ hyperabsorption in cystic fibrosis airway epithelial cultures. J Physiol. 2014;592:5251–5268. doi: 10.1113/jphysiol.2013.267286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Haerteis S, Krappitz M, Bertog M, Krappitz A, Baraznenok V, Henderson I, et al. Proteolytic activation of the epithelial sodium channel (ENaC) by the cysteine protease cathepsin-S. Pflugers Arch. 2012;464:353–365. doi: 10.1007/s00424-012-1138-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Greenlee KJ, Werb Z, Kheradmand F. Matrix metalloproteinases in lung: multiple, multifarious, and multifaceted. Physiol Rev. 2007;87:69–98. doi: 10.1152/physrev.00022.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bonnans C, Chou J, Werb Z. Remodelling the extracellular matrix in development and disease. Nat Rev Mol Cell Biol. 2014;15:786–801. doi: 10.1038/nrm3904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gaggar A, Hector A, Bratcher PE, Mall MA, Griese M, Hartl D. The role of matrix metalloproteinases in cystic fibrosis lung disease. Eur Respir J. 2011;38:721–727. doi: 10.1183/09031936.00173210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Akthar S, Patel DF, Beale RC, Peiró T, Xu X, Gaggar A, et al. Matrikines are key regulators in modulating the amplitude of lung inflammation in acute pulmonary infection. Nat Commun. 2015;6:8423. doi: 10.1038/ncomms9423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Turnbull AR, Pyle CJ, Patel DF, Jackson PL, Hilliard TN, Regamey N, et al. Abnormal pro-gly-pro pathway and airway neutrophilia in pediatric cystic fibrosis. J Cyst Fibros. doi: 10.1016/j.jcf.2019.05.017. [online ahead of print] 5 June 2019; DOI: 10.1016/J.JCF.2019.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Van den Steen PE, Proost P, Wuyts A, Van Damme J, Opdenakker G. Neutrophil gelatinase B potentiates interleukin-8 tenfold by aminoterminal processing, whereas it degrades CTAP-III, PF-4, and GRO-α and leaves RANTES and MCP-2 intact. Blood. 2000;96:2673–2681. [PubMed] [Google Scholar]
- 57.Trojanek JB, Cobos-Correa A, Diemer S, Kormann M, Schubert SC, Zhou-Suckow Z, et al. Airway mucus obstruction triggers macrophage activation and matrix metalloproteinase 12-dependent emphysema. Am J Respir Cell Mol Biol. 2014;51:709–720. doi: 10.1165/rcmb.2013-0407OC. [DOI] [PubMed] [Google Scholar]
- 58.McMahan RS, Birkland TP, Smigiel KS, Vandivort TC, Rohani MG, Manicone AM, et al. Stromelysin-2 (MMP10) moderates inflammation by controlling macrophage activation. J Immunol. 2016;197:899–909. doi: 10.4049/jimmunol.1600502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kummarapurugu AB, Afosah DK, Sankaranarayanan NV, Navaz Gangji R, Zheng S, Kennedy T, et al. Molecular principles for heparin oligosaccharide-based inhibition of neutrophil elastase in cystic fibrosis. J Biol Chem. 2018;293:12480–12490. doi: 10.1074/jbc.RA118.002644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chapman KR, Burdon JGW, Piitulainen E, Sandhaus RA, Seersholm N, Stocks JM, et al. RAPID Trial Study Group. Intravenous augmentation treatment and lung density in severe α1 antitrypsin deficiency (RAPID): a randomised, double-blind, placebo-controlled trial. Lancet. 2015;386:360–368. doi: 10.1016/S0140-6736(15)60860-1. [DOI] [PubMed] [Google Scholar]
- 61.Brennan S. Revisiting α1-antitrypsin therapy in cystic fibrosis: can it still offer promise? Eur Respir J. 2007;29:229–230. doi: 10.1183/09031936.00159606. [DOI] [PubMed] [Google Scholar]
- 62.Camper N, Glasgow AMA, Osbourn M, Quinn DJ, Small DM, McLean DT, et al. A secretory leukocyte protease inhibitor variant with improved activity against lung infection. Mucosal Immunol. 2016;9:669–676. doi: 10.1038/mi.2015.90. [DOI] [PubMed] [Google Scholar]
- 63.Scott CJ, Taggart CC. Biologic protease inhibitors as novel therapeutic agents. Biochimie. 2010;92:1681–1688. doi: 10.1016/j.biochi.2010.03.010. [DOI] [PubMed] [Google Scholar]
- 64.Polverino E, Rosales-Mayor E, Dale GE, Dembowsky K, Torres A. The role of neutrophil elastase inhibitors in lung diseases. Chest. 2017;152:249–262. doi: 10.1016/j.chest.2017.03.056. [DOI] [PubMed] [Google Scholar]
- 65.Hirche TO, Benabid R, Deslee G, Gangloff S, Achilefu S, Guenounou M, et al. Neutrophil elastase mediates innate host protection against Pseudomonas aeruginosa. J Immunol. 2008;181:4945–4954. doi: 10.4049/jimmunol.181.7.4945. [DOI] [PubMed] [Google Scholar]
- 66.Hahn I, Klaus A, Janze AK, Steinwede K, Ding N, Bohling J, et al. Cathepsin G and neutrophil elastase play critical and nonredundant roles in lung-protective immunity against Streptococcus pneumoniae in mice. Infect Immun. 2011;79:4893–4901. doi: 10.1128/IAI.05593-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shute JK, Calzetta L, Cardaci V, di Toro S, Page CP, Cazzola M. Inhaled nebulised unfractionated heparin improves lung function in moderate to very severe COPD: a pilot study. Pulm Pharmacol Ther. 2018;48:88–96. doi: 10.1016/j.pupt.2017.10.001. [DOI] [PubMed] [Google Scholar]
- 68.Rao NV, Argyle B, Xu X, Reynolds PR, Walenga JM, Prechel M, et al. Low anticoagulant heparin targets multiple sites of inflammation, suppresses heparin-induced thrombocytopenia, and inhibits interaction of RAGE with its ligands. Am J Physiol Cell Physiol. 2010;299:C97–C110. doi: 10.1152/ajpcell.00009.2010. [DOI] [PubMed] [Google Scholar]
- 69.Xu X, Abdalla T, Bratcher PE, Jackson PL, Sabbatini G, Wells JM, et al. Doxycycline improves clinical outcomes during cystic fibrosis exacerbations. Eur Respir J. 2017;49:1601102. doi: 10.1183/13993003.01102-2016. [DOI] [PubMed] [Google Scholar]
- 70.Fonović M, Turk B. Cysteine cathepsins and extracellular matrix degradation. Biochim Biophys Acta. 2014;1840:2560–2570. doi: 10.1016/j.bbagen.2014.03.017. [DOI] [PubMed] [Google Scholar]
- 71.Heuberger DM, Schuepbach RA. Protease-activated receptors (PARs): mechanisms of action and potential therapeutic modulators in PAR-driven inflammatory diseases. Thromb J. 2019;17:4. doi: 10.1186/s12959-019-0194-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.