Abstract
Aging is a progressive loss of physiological integrity and functionality process which increases susceptibility and mortality to diseases. Vascular aging is a specific type of organic aging. The structure and function changes of endothelial cells (ECs) and vascular smooth muscle cells (VSMCs) are the main cause of vascular aging, which could influence the threshold, process, and severity of vascular related diseases. Accumulating evidences demonstrate that exosomes serve as novel intercellular information communicator between cell to cell by delivering variety biologically active cargos, especially exosomal non-coding RNAs (ncRNAs), which are associated with most of aging-related biological and functional disorders. In this review, we will summerize the emerging roles and mechanisms of exosomal ncRNAs in vascular aging and vascular aging related diseases, focusing on the role of exosomal miRNAs and lncRNAs in regulating the functions of ECs and VSMCs. Moreover, the relationship between the ECs and VSMCs linked by exosomes, the potential diagnostic and therapeutic application of exosomes in vascular aging and the clinical evaluation and treatment of vascular aging and vascular aging related diseases will also be discussed.
Keywords: exosomes, endothelial cells, vascular smooth muscle cells, vascular aging, vascular diseases
Review
1.Introduction
Vascular aging is a specific type of organic aging, leading to major adverse cardiovascular events including restenosis, atherosclerosis, vascular calcification (VC) and pulmonary hypertension. It could influence the threshold, process, and severity of various cardiovascular diseases. Vascular aging occurs mainly in the inner and medial layers of vessel wall [1]. Therefore, the dysfunctions of endothelial cells (ECs) and vascular smooth muscle cells (VSMCs) including cellular apoptosis, senescence, proliferation, inflammation, and the phenotypic change of VSMCs play an important role in vascular aging [2]. In terms of the cells,ECs and VSMCs may undergo both replicative and induced senescence in vivo. At the level of vascular tissue, progenitor cells may be involved in damage and replacement of senescent cells. If progenitor cells are depleted, and ECs and VSMCs are less self-renewing, it may lead to vascular aging. In terms of function, it is featured by increased stiffness, reduced sensitivity to vasodilators, increased sensitivity to vasoconstrictors and decreased angiogenic capacity.
Exosomes are endosomal origin from multivesicular bodies with the smallest diameter of 30-100nm. Exosomes derived from different cells transport distinct proteins, lipids, nucleic acids such as mRNA, microRNAs (miRNAs) and long noncoding RNAs (lncRNAs) [3, 4]. Because of the variety and abundance of specific cargos, exosomes keep a great potential application for diagnostic and prognostic biomarkers and therapeutic tools. Exosomes, exploited as gene regulators and information communicators, contributed to many essential physiological and pathophysiological processes including proliferation, differentiation, apoptosis, homeostasis, and migration [5, 6]. Accordingly, increasing evidence has demonstrated that aging-related biological and functional disorders are associated with alterations in exosomes, suggesting that exosomes are potential regulators for vascular aging [7-10].
Non-coding RNAs (ncRNAs) are mainly including miRNAs, lncRNAs as well as circular RNAs(cirRNAs) [11]. Recently, several ncRNAs have emerged as important molecules regulating the complexity of vascular aging. A large number of studies dealing with circulating exosomes and their cargos prove that exosomal miRNAs and lncRNAs were involved in regulating ECs and VSMCs dysfunction, which significantly affecting the process of vascular aging [12, 13]. However, there are fewer researches to explore the role of cirRNAs incorporated into exosomes in regulating vascular aging. Therefore, in this review, we summarize the current knowledge about the exosomal ncRNAs focusing on the role of miRNAs and lncRNAs in vascular aging, especially targeting at the effects on ECs and VSMCs and the information communication between these cells.
2.The role of exosomes in vascular aging
Vascular aging is tightly associated to alterations in the physiological functions and structural properties of the vascular wall, mainly in ECs and VSMCs. Emerging evidence suggest that exosomes acted as natural vehicles for the delivery of nucleic acids and signaling molecules [14, 15]. The important role of exosomes in modulating vascular aging is exploited with growing interests in exosomal cargo studies, especially the exosomal miRNAs and lncRNAs. Hence, better understanding of vascular biological and functional changes with aging is vital to against vascular aging related diseases.
2.1 Exosomal ncRNAs and ECs functions
ECs, the monolayer lining blood vessels, regulate the vascular integrity and homeostasis [16, 17]. An extensive surge of abnormalities of ECs, collectively termed “endothelial dysfunction”, are fundamental contributors to the pathogenesis of vascular diseases. When the endothelium was exposed to micro-environmental stimuli, such as oxidative stress, hypoxia, inflammatory mediators, hypertension, hyperglycemia, or shear stress, the ECs would undergo alterations including proliferation, apoptosis, migration, senescence, angiogenesis, inflammation, and functional networks which eventually affect vascular aging [18]. In this part, we focused on the relationship between specific contents of exosomal ncRNAs and ECs functions (Table 1).
Table 1.
Exosomal non-coding RNAs implicated in ECs functions.
| Exosomal non-coding RNAs | Cargos | Targets | ECs functions | Reference |
|---|---|---|---|---|
| Exosomal miRNAs | miR-122-5p | HGF | pro-proliferation, pro-migration | [23] |
| miR-210-3p | HGF | pro-proliferation, pro-migration | [23] | |
| miR-296-5p | HGF | pro-proliferation, pro-migration | [23] | |
| miR-376c-3p | HGF | pro-proliferation, pro-migration | [23] | |
| miR-214 | ATM | pro-proliferation, anti-senescence, pro-angiogenesis | [24] | |
| miR-17 | ANGPT1 STAT3 |
pro-proliferation, pro-migration, anti-inflammation |
[25] [35] | |
| miR-19 | ANGPT1 | pro-proliferation, pro-migration | [25] | |
| miR-20a | ANGPT1 | pro-proliferation, pro-migration | [25] | |
| miR-30c | ANGPT1 | pro-proliferation, pro-migration | [25] | |
| miR-126 | ANGPT1 | pro-proliferation, pro-migration | [25] | |
| miR-92a | SOCS5 | anti- proliferation, pro-inflammation | [26] [30] | |
| miR-21 | RhoB | anti- proliferation, pro-inflammation | [27] [31] | |
| miR-24 | Sp1 | anti- proliferation | [28] | |
| miR-15a | VEGF, NF-kB | pro-inflammation | [32, 33] | |
| miR-27a | VEGF, EGFR | pro-inflammation | [32, 33] | |
| miR-34a | BCL2, SIRT1 | pro-inflammation | [32, 33] | |
| miR-223 | ICAM-1 | anti-inflammation | [34] | |
| miR-125a | DLL4 | pro-angiogenesis | [36] | |
| miR-181b-5p | TRPM7 | pro-angiogenesis | [37] | |
| miR-106b-5p | ANG2 | anti- angiogenesis | [38] | |
| miR-320 | IGF-1, Hsp20, Ets2 | anti- angiogenesis | [39] | |
| Exosomal lncRNAs | HOTTIP | cyclin D1, PCNA | pro-proliferation, pro-migration | [48] |
| POU3F3 | bFGF, VEGF | pro-proliferation, pro-migration, pro-angiogenesis |
[49] | |
| MALAT1 | IL-6, TNF-α, SAA3 | anti- proliferation, pro-migration, pro-inflammation |
[50] [59, 60] |
|
| HOTAIR | VEGF | pro-angiogenesis | [53] | |
| H19 | Unknown | pro-angiogenesis | [54] | |
| CCAT2 | TGFβ, Bcl-2 | pro-angiogenesis, anti-apoptosis | [55] | |
| Meg3 | Unknown | anti- angiogenesis | [57] | |
| GAS5 | P53, Caspase 3, Caspase 7 | pro-apoptosis | [58] |
HGF: hepatocyte growth factor; ATM: ataxia telangiectasia mutated; ANGPT1: angiopoietin-1; STAT3: signal transducer and activator of transcription 3; SOCS5: suppressor of cytokine signaling 5; RhoB: ras homologue family member B; Sp1: specificity protein 1; VEGF: vascular endothelial growth factor; NF-kB: nuclear factor-kappa B; EGFR epidermal growth factor receptor; BCL2: B-cell CLL/lymphoma 2; SIRT1: silent information regulator 1; ICAM-1: intercellular adhesion molecule-1; DLL4: delta-like 4; TRPM7: transient receptor potential melastatin 7; ANG2: Angiopoietin 2; IGF-1: insulin-like growth factor-1; Hsp20: small heat-shock protein 20; Ets2: E26 transformation-specific 2; PCNA: proliferating cell nuclear antigen; bFGF: basic fibroblast growth factor; IL-6: interleukin 6; TNF-α: tumor necrosis factor alpha; SAA3: serum amyloid antigen 3;TGFβ:transforming growth factor β.
2.1.1 Exosomal miRNAs and ECs functions
One of striking aspects of the content of exosomes is that they are highly enriched in miRNAs that could target the 3’untranslated region (3’UTR) of specific mRNAs to inhibit, in most cases, their translation. Numerous lines of evidence supported that exosomal miRNAs were involved in regulating ECs proliferation,apoptosis, senescence, angiogenesis, and inflammation [19-21].
ECs proliferation is vital to promote endothelial healing. Accumulating evidence had indicated that exosomal miRNAs acted as critical regulators to participate in EC proliferation [22, 23]. It had been reported that exosomes carried miR-122-5p, miR-210-3p, miR-296-5p, and miR-376c-3p were significantly increased in human umbilical endothelia cell (HUVEC) and may account for the different regulation of ECs proliferation and migration [23]. Besides, miR-214 was incorporated into exosomes, leading to ECs proliferation and angiogenesis both in vitro and in vivo [24]. Garcia et al. proved that exosomes containing an enrichment of proangiogenic miR-17, miR-19, miR-20a, miR-30c and miR-126 from cardiomyocytes could promote the proliferation and migration of ECs [25]. On the contrary, a large number of researches reported that miRNAs had an effect of inhibiting the proliferation of ECs, including miR-92a [26], miR-21 [27] and miR-24 [28]. It had been shown that miR-92a could modulate KLF4 and MKK4 expression in ECs and inhibition of miR-92a might increase ECs proliferation and migration [26].
ECs inflammation induced by stimuli such as shear stress, inflammatory cytokines, or hypoxia, is more susceptible to cardiovascular diseases. Recent reports had shown that several exosomal miRNAs were involved in the regulatory mechanisms of cellular inflammation by controlling leukocyte activation and infiltration through the vascular wall [29]. As the proinflammatory regulator in ECs, miR-92a could activate inflammatory cytokines and chemokines and promote monocyte adhesion [30] while miR-21 could induce the expression of C-C motif chemokine 2 (CCL2) and vascular cell adhesion protein 1 (VCAM-1) by enhancing the activity of the transcription factor AP-1 [31]. It had been demonstrated that some miRNAs from exosomes (miR-15a, miR-27a and miR-34a) were increased in patients with sepsis, which could modulate the inflammatory response [32, 33]. Oppositively, Li et al. discovered that exosomes containing miR-223 inhibited intercellular adhesion molecule-1 (ICAM-1) expression during inflammation through regulating the NF-κB and MAPK pathways [34]. It had been confirmed that exosomes derived from mesenchymal stem cells contained miR-17 superfamily that played an anti-inflammatory role in pulmonary ECs through the suppression of signal transducer and activator of transcription 3 (STAT3) [35].
ECs angiogenesis is responsible for the progression of vascular aging, and emerging evidence suggest that exosomal miRNAs are signaling molecules that modulate the microenvironment and promote angiogenesis of ECs [36]. Several miRNAs are responsible for angiogenesis while others are the so-called antiangiogenic miRNAs. Liang et al. indicated that exosomes could transfer miR-125a to ECs and promote angiogenesis by repressing angiogenic inhibitor delta-like 4 (DLL4) [36]. Yang et al. showed that exosomes could promote the ECs angiogenesis induced by oxygen-glucose deprivation via miR-181b-5p/TRPM7 axis [37]. On the contrary, it had been proven that up-regulation of exosomal miR-106b-5p suppressed angiogenesis in ECs by overexpression of angiopoietin 2 [38]. Besides, it had been indicated that exosomes exerted an anti-angiogenic function through transfer of miR-320 into ECs [39].
ECs apoptosis and senescence results in a disruption of the endothelium barrier and creating leaks that destroy the vascular wall integrity, which contribute to the development of atherosclerosis [40]. Exosomes containing miR-214 repressed the expression of ataxia telangiectasia mutated in recipient cells, thereby preventing senescence and inducing angiogenesis and migration [24].
2.1.2 Exosomal lncRNAs and ECs function
LncRNAs contains a class of transcripts longer than 200 nucleotides [41]. They regulate gene expression at transcription, epigenetic, and translation levels, coordinating and integrating multiple signaling pathways involved in cellular proliferation, differentiation, and homeostasis [42, 43]. Several lines of evidence had demonstrated that lncRNAs were involved in different aspects during the process of vascular aging including cellular differentiation, proliferation, apoptosis, and inflammation [44-47]..
Recent report showed that exosomal lncRNA HOTTIP promoted ECs proliferation and migration via activation of the Wnt/β-catenin pathway [48]. Another study demonstrated that exosomal lncRNA POU3F3 could increase ECs proliferation and migration [49]. Besides, it had been reported that silencing of exosomal lncRNA MALAT1 by GapmeRs or small interfering RNAs induced a switch of the ECs phenotype to a promigratory but antiproliferative state that resulted in impaired ECs proliferation and reduced vessel growth [50].
A large number of studies had reported that several lncRNAs played an important role in regulating angiogenesis of ECs [51, 52]. For instance, the lncRNA HOTAIR was packed into exosomes secreted by glioma cells and conveyed to ECs. Then, HOTAIR stimulated angiogenesis by increasing the expression of VEGFA [53]. In addition, it had been reported that CD90+ cells modulated ECs angiogenesis through the release of exosomes containing lncRNA H19 by upregulating VEGF production [54]. Besides, exosomal lncRNA POU3F3 could promote ECs angiogenesis in vivo [49] and glioma cells could also enhance ECs angiogenesis by activating VEGFA and TGFβ through the release of exosomes containing lncRNA CCAT2 [55]. Moreover, Kaneko et al demonstrated that Meg3 epigenetically regulated gene expression via interacting with Ezh2 and Jarid2 [56], and Boon et al. further reported that Meg3-mediated changes in epigenetic regulation of gene expression contributed to endothelial dysfunction in angiogenesis [57].
Except from the role in regulating angiogenesis of ECs, CCAT2 could also inhibit ECs apoptosis by decreasing Bax and caspase-3 expression [55]. Conversely, recent report had shown that high expression of lncRNA GAS5 could increase the apoptosis of vascular ECs [58]. Meanwhile, MALAT1, involved in modulating ECs proliferation as mentioned before [50], had been also identified as a regulator of inflammatory cytokine production and affected inflammation of ECs through SAA3 [59, 60].
2.2 Exosomal ncRNAs and VSMCs functions
VSMCs, the main cells of the media vessel wall, can control blood flow and keep vascular tension maintenance. VSMCs phenotypic conversion from a contractile to synthetic state with the progress of aging and in response to various pathological stimuli, contributes to vascular pathologies including atherosclerosis, restenosis, and VC. Investigators indicated that exosomes acted as a specific signaling mechanism during VSMC phenotypic modulation was taken part in regulating cellular proliferation, migration, apoptosis, calcification, and differentiation [61]. In this part, we summarized the role of exosomal ncRNAs in the regulation of VSMCs functions (Table 2).
Table 2.
Exosomal non-coding RNAs implicated in VSMCs functions.
| Exosomal non-coding RNAs | Cargos | Targets | ECs functions | Reference |
|---|---|---|---|---|
| Exosomal miRNAs | miR-31 | MAPK/ERK, LATS2 | promote phenotype transition | [63] |
| miR-133 | Sp1 | promote phenotype transition | [64] | |
| miR-223 | MEF2C, RhoB | promote phenotype transition | [65] | |
| miR-26a | Smad1 | inhibit phenotype transition | [66] | |
| miR-21 | BCL2 | pro-proliferation | [67] | |
| miR-130a | BMP2, TGFβ1 | pro-proliferation | [68] | |
| miR-221/222 | p27, p57 | pro-proliferation | [69, 70] | |
| miR-152 | DNMT1 | anti-proliferation | [73] | |
| miR-155 | Ets1 | anti-proliferation | [74] | |
| miR-223 | PDGFRβ | anti-proliferation | [75] | |
| miR-339 | PDGFRβ | anti-proliferation | [75] | |
| miR-21 | PDGFRβ | anti-proliferation | [75] | |
| miR-29b | DNMT3b | pro-migration | [78] | |
| miR-143-3p | TGFβ | pro-migration, pro-angiogenesis | [79] | |
| miR-712 | NCKX4 | pro-calcification | [80] | |
| miR-714 | PMCA1 | pro-calcification | [80] | |
| miR-762 | NCX1 | pro-calcification | [80] | |
| miR-29a/b | ADAMTS-7 | anti-calcification | [83] | |
| miR-125b | Ets1, Osterix | anti-transdifferentiation, anti-calcification |
[84, 85] | |
| miR-126-3p | VEGF, ANG1, ANG2, MMP9, TSP1 | pro-angiogenesis | [86] | |
| miR-92a | MKK4 | anti-apoptosis, anti-senescence | [88] | |
| miR-34a | SIRT1 | pro-senescence | [89] | |
| Exosomal lncRNAs | MALAT1 | Unknown | pro-proliferation, pro-migration | [92] |
| MEG3 | p53 | anti-proliferation, anti-migration | [93] | |
| lncRNA-p21 | p53 | anti-proliferation, pro-apoptosis | [94] | |
| HOTAIR | ALPL, BMP2 | anti-calcification | [95] |
MAPK/ERK: mitogen-activated protein kinase/extracellular regulated kinase; LATS2: large tumor suppressor homolog 2; Sp1: specificity protein 1; MEF2C: myocyte enhancer factor-2c; RhoB: ras homologue family member B; Smad1: SMAD family member 1; BCL2: B-cell CLL/lymphoma 2; BMP2: bone morphogenetic protein 2; TGFβ1: transforming growth factor beta 1; DNMT1: DNA methyltransferase 1; Ets1: E26 transformation-specific 1; PDGFRβ: platelet-derived growth factor receptor-beta; DNMT3b: DNA methyltransferase 3b; TGFβ:transforming growth factor β; NCKX4: sodium/calcium exchanger 4; PMCA1: plasma membrane calcium ATPase 1; NCX1: sodium/calcium exchanger 1; ADAMTS-7: a disintegrin and metalloproteinase with thrombospondin motifs-7; VEGF: vascular endothelial growth factor; ANG1: angiopoietin 1; ANG2: angiopoietin 2; MMP9: matrix metallopeptidase 9; TSP1: thrombospondin 1; MKK4: mitogen-activated protein kinase 4; SIRT1: silent information regulator 1; ALPL: alkaline phosphatase; BMP2: bone morphogenetic Protein 2.
2.2.1 Exosomal miRNAs and VSMCs functions
Previous studies had indicated that several exosomal miRNAs were involved in the regulation of VSMCs phenotypic modulation. The contractile phenotype of VSMCs with non-proliferative and non-migratory switched to a proliferative, migratory, apoptotic, and differentiation phenotype [61, 62].
Liu et al. had proved that exosomal miR-31 could promote the VSMCs contractile phenotype by repressing cellular repressor of E1A-stimulated genes (CREG) expression [63]. Besides, exosomes containing miR-133 had been shown to regulate the differentiation of VSMCs by inhibiting the transcription factor, specificity protein-1 (Sp-1) [64]. In addition, it had been reported that miR-223 regulated VSMCs phenotype transition from contractile to synthesis [65]. On the contrary, miR-26a inhibited the differentiation of VSMCs and governed phenotypic shifting by directly targeting SMAD1 and SMAD4 [66].
An increasing number of studies had demonstrated that miRNAs played a pivotal role in the progress of VSMCs proliferation through post-transcriptional mechanisms [67, 68]. For example, some exosomal miRNAs had been found to promote the proliferation of VSMCs such as miR-21, miR-130a, miR-221/222, et al [67-69]. MiR-21 is one of the most abundant miRNAs in the vascular wall which can enhance the VSMCs proliferation [67]. miR-221 and miR-222 had been shown to regulate the proliferation of VSMCs by targeting p27 and p57 [69, 70]. While, others had been proven to inhibit the proliferation of VSMCs [71, 72]. MiR-152 inhibited cell proliferation in VSMCs by decreasing DNA methyltransferase 1 (DNMT1) expression and downregulating the methylation level of ERα gene promoter region [73]. MiR-155 induced by oxidized low-density lipoprotein (ox-LDL) was considered to inhibit the proliferation of VSMCs by suppression of certain members of the matrix metalloproteinase (MMP) family [74]. Besides, Tan et al. indicated that activated platelet-derived exosomes containing miR-223, miR-339 and miR-21 could be transferred into VSMCs and inhibit VSMCs proliferation [75].
VSMCs can migrate from the medial wall to the intimal lining in response to vascular injury. Some miRNAs involved in the regulatory mechanisms of cellular migration are promoters while others are inhibitors [76, 77]. For instance, Chen et al. indicated that miR-29b promoted VSMCs migration by regulating the expression of MMP-2/MMP-9 genes and targeting DNA methyltransferase 3b (DNMT3b) [78]. In addition, miR-143-3p could modulate exosome-mediated responses in pulmonary VSMCs and Deng at al. proved that miR-143-3p enriched exosomes had the VSMCs promigratory and proangiogenic effect [79].
VC is a tightly regulated process actually driven by VSMCs osteogenic conversion and related to the activation of sphingomyelin phosphodiesterase 3 (SMPD3) and cytoskeletal remodeling. As the process of VSMCs calcification is tightly regulated by the genetic reprogramming, it is not surprising that there are accumulating evidence to support an integral role of miRNAs involved in this process [80-82]. One study reported that several exosomal miRNAs could disrupt calcium transporters and promote calcium deposition of VSMCs including miR-712, miR-714 and miR-762 [80]. On the contrary, Du et al. identified that miR-29a/b inhibited VSMCs calcification by suppressing the expression of a disintegrin and metalloproteinase with thrombospondin motifs-7 (ADAMTS-7) [83]. Moreover, the expression of miR-125b suppressed VSMCs transdifferentiation and calcification [84, 85].
Angiogenesis of VSMCs is responsible for the progression of vascular aging. MiR-126-3p enhanced VSMCs angiogenesis by suppressing the expression of its known target, SPRED1, simultaneously modulating the expression of genes involved in angiogenic pathways including vascular endothelial growth factor (VEGF), angiopoietin 1 (ANG1), angiopoietin 2 (ANG2), matrix metallopeptidase 9 (MMP9), thrombospondin 1 (TSP1) and so on [86]. Apoptosis and senescence of VSMCs had been proven as important processes in vascular diseases [87]. It had been shown that miR-92a overexpression inhibited VSMCs apoptosis and senescence by suppressing mitogen-activated protein kinase 4 (MKK4) and JNK1 pathways [88]. In contrast, miR-34a could promote VSMCs senescence through SIRT1 downregulation [89].
2.2.2 Exosomal lncRNAs and VSMCs functions
LncRNAs are capable of modulating target DNA, RNA, and proteins at the pre- and posttranscriptional level. An increasing number of studies had demonstrated that lncRNAs played a pivotal role in the regulation of VSMC phenotype, functions, perhaps also in the development of VSMCs-related diseases [90, 91]. This chapter described the emerging roles of exosomal lncRNAs in VSMCs proliferation, migration, apoptosis, and calcification.
As mentioned above, MALAT1 could regulate the proliferative and promigratory functions of ECs [50], Brock et al. [92] proved that MALAT1 could also significantly enhance proliferation and migration of pulmonary VSMCs, probably through hypoxia-inducible factor 1α. In addition, Sun et al. [93] found that inhibition of MEG3 regulated the cell cycle progression and promoted more VSMCs from the G0/G1 phase to the G2/M+S phase. Therefore, lncRNA MEG3 downregulation triggers pulmonary VSMCs proliferation and migration. Exosomal lncRNA-p21 was a p53-induced lncRNA [45], which recently was proven to control VSMCs proliferation [94]. Importantly, subsequent studies showed that inhibition of lncRNA-p21 could also reduce apoptosis of VSMCs by interfering with p53 [94]. Besides, Carrion et al. showed that silencing of lncRNA HOTAIR elevated expressions of calcification-related genes, such as BMP2 and ALP, which eventually played a key role in calcification of VSMCs [95].
3. The role of exosomes in the network of information communication between ECs and VSMCs
Exosomes, a newly identified natural nanocarrier and intercellular messenger, played a pivotal role in regulating cell-to-cell communication. Active interactions between ECs and VSMCs are critical to modulate the process of vascular aging. Accumulating evidence showed that the close communication between ECs and VSMCs were achieved via the release of exosomes [96, 97]. However, little is known about the mechanisms that regulate ECs and VSMCs crosstalk. In this part, we aimed to discuss the mechanisms underlying the regulation of the ECs and VSMCs through exosomes especially exosomal ncRNAs (Figure 1).
Figure 1.
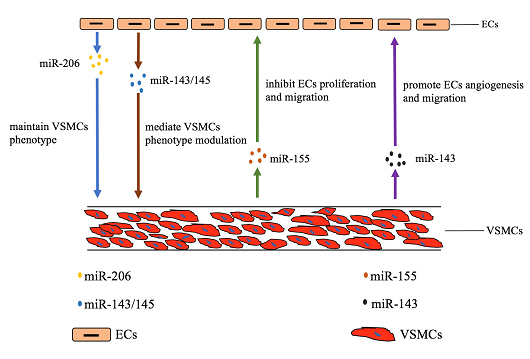
The communication between ECs and VSMCs via exosomal ncRNAs. The blue and brown arrows indicate that exosomal miR206 and miR-143/145 secreted by ECs regulate the functions of VSMCs. The green and purple arrows indicate that exosomal miR-155 and miR-143 secreted by VSMCs regulate the functions of ECs.
Several reports showed that ECs could regulate the functions of VSMCs through the release of exosomes containing miRNAs. For example, the expression of the miR-143/145 cluster was regulated by shear-responsive transcription factor KLF2 in ECs [97] and then miR-143/145 could mediate VSMCs phenotype modulation by transporting to VSMCs via exosomes [98, 99]. In addition, miR-206 derived from HUVEC maintains the contractile phenotype of VSMCs by suppressing exosomes secretion [100].
A large line of reports found that VSMCs could also modulate ECs functions through exosomes. For instance, miR-155, significantly expressed in Krüppel-like factor 5 (KLF5)-overexpressing VSMCs, was a potent regulator of endothelium barrier function. VSMCs-derived exosomes mediated the transfer of miR-155 from VSMCs to ECs, which, in turn, inhibited EC proliferation and migration, thus eventually destroying the tight junctions and the integrity of endothelial barriers [101]. Deng et al. had discovered that a crucial exosomal communication between pulmonary VSMCs and ECs in pulmonary artery hypertension (PAH) [79]. It had been proven that exosomes secreted by pulmonary VSMCs were enriched with miR-143, which could be transported to ECs, inducing ECs migration and angiogenesis [79].
4.The role of exosomes in vascular aging related diseases
Vascular aging occurs earlier than clinical diseases. It is a high-risk factor of vascular aging-related diseases. Vascular aging and vascular diseases interact with each other. The aging blood vessels provide an environment for the occurrence and development of vascular diseases, whereas vascular diseases accelerate the process of vascular aging. Exosomal non-coding RNAs are recently regarded as a critical factor in vascular diseases. It has been demonstrated that a number of vascular aging related diseases like heart diseases, hypertension, cerebro-vascular diseases, kidney diseases, and peripheral artery diseases (PAD) are highly influenced by exosomes (Table 3).
Table 3.
Roles of exosomal miRNAs in vascular aging related diseases.
| Cargo | Disease | Functions | ||
|---|---|---|---|---|
| Heart Diseases | miR-221/222 | CAD | Promote the progression of CAD | [103] |
| miR-208a | CAD | Promote the progression of CAD | [104] | |
| miR-126 | CAD | Inhibit the progression of CAD | [105] | |
| miR-17-92 | CAD | Inhibit the progression of CAD | [106] | |
| miR-22 | AMI | Protect against CMCs apoptosis | [108] | |
| miR-133 | AMI | Promote the progression of AMI | [109] | |
| miR-21 | AMI, HF | Promote CMCs loss | [110, 113] | |
| miR-24 | HF | Promote the progression of HF | [114] | |
| miR-214 | HF | Promote the progression of HF | [115] | |
| Hypertension | miR-211 | EH | Activate the axis of RAAS | [119] |
| miR-615 | EH | Activate the axis of RAAS | [119] | |
| miR-155 | EH | Regulate the progression of VC | [99] | |
| Cerebrovascular Diseases | miR-126 | AIS | Inhibit microglial activation and inflammatory response | [122] |
| miR-30d-5p | AIS | Inhibit autophagy-mediated microglial polarization to M1 | [123] | |
| miR-181b-5p | AIS | promoted BMEC angiogenesis | [37] | |
| miR-146a | VCID | Inhibit inflammatory effects on damaged astrocytes | [124] | |
| Kidney Diseases | miR-200b | CKD | Regulate the progression of renal fibrosis | [126] |
| miR-16 | CKD | Regulate the progression of CKD | [127] | |
| PAD | miR-92a | Hind Limb Ischemia | Inhibit functional recovery | [129] |
CAD: coronary artery disease; AMI: acute myocardial infarction; CMCs: cardiomyocytes; HF: heart failure; VC: vascular calcification; EH: essential hypertension; RAAS: rennin-angiotensin-aldosterone system; AIS: acute ischemic stroke; BMEC: brain microvascular endothelial cell; VCID: vascular cognitive impairment and dementia; CKD: chronic kidney disease; PAD: peripheral artery disease.
4.1 The role of exosomes in vascular aging related heart diseases
Intercellular communication mediated by exosomes is vital for preserving vascular integrity and in the development of cardiovascular diseases. A recent surge of reports suggested that major heart diseases like coronary artery disease (CAD), acute myocardial infarction (AMI) and heart failure (HF) were highly influenced by the cargoes in exosomes.
The present study demonstrated that vessel wall and inflammatory cell-derived miRNAs could be detected in the blood and were regulated in patients with CAD. The communication among the involved cells, leading to promote the formation of plaque, were regulated by exosomes in CAD [102]. Bazan et al. found that exosomes derived from VSMCs exhibited more pro-atherosclerotic miR-221/222 in patients with CAD [103]. MiR-208a,which was enriched in serum-derived exosomes,tended to be elevated with stable CAD [104]. Vascular miRNAs, including miR-126 [105] and miR-17-92 cluster [106], were downregulated in patients with CAD.
AMI, a detrimental consequence of acute coronary occlusion, is characterized by cardiomyocyte loss and myocardial necrosis [107]. It had been revealed that exosomes contained the increased amount of miR-22 under ischemic condition, and could be centralized to cardiomyocytes to protect against apoptosis by targeting methyl-CpG-binding protein 2 [108]. On the contrary, it had been proved that exosomal miR-133 [109] and miR-21 [110] could promote the progression of AMI.
HF is a general term for the heart’s inability to pump sufficiently and maintain blood flow to meet the body’s needs. In general, significant cardiac ultrastructural, biochemical and molecular abnormalities, contribute to the pathophysiology of all forms of HF [111]. A number of exosomal miRNAs were recurrent [112] and confirmed to be altered in various forms of HF. It had been reported that miR-21 [113], miR-24 [114] and miR-214 [115] were consistently affected in patients with varying origins and degrees of HF.
4.2 The role of exosomes in vascular aging related primary hypertension
Primary hypertension is characterized by decreased compliance and increased rigidity of vessels. The interaction between vascular aging and hypertension contributes to arterial stiffness, while arterial stiffness and hypertension are mutually causal. On the one hand, hypertension causes vessel wall injury, leading to arterial stiffness. On the other hand, arterial stiffness is the main cause of high systolic blood pressure, especially in the elderly [116]. Activation of rennin-angiotensin-aldosterone system (RAAS) is the bedrock in hypertension [117]. Recently, it had been demonstrated that exosomal miRNAs were altered by RAAS activation and thus correlated with hypertension [118]. The miRNome analysis of exosomes from patients with hypertension found that miR-211 and miR-615 were fluctuated with blood pressure [119]. Besides, VC, the hydroxyapatite deposition in the intimal and medial layers of the vascular wall, can lead to hypertension. Chen et al. reported that miR-155 could regulate VC procession [99].
4.3 The role of exosomes in vascular aging related cerebrovascular diseases
Vascular aging leads to the development of hemodynamic aging syndrome [120]. It may cause cerebrovascular diseases and cognitive impairment because of the high pulsation blood flow.
Acute ischemic stroke and lacunar infarction are associated with endothelial dysfunction. Vascular aging may play an important role in the pathogenesis of stroke and serve as a potential marker of the risk of stroke [121]. Geng et al. found that miR-126 was significantly reduced in patients with ischemic stroke, miR-126+ exosomes could inhibit microglial activation and inflammatory response induced by ischemic stroke and promote neurogenesis and vasculogenesis after ischemic stroke [122]. Jiang et al. revealed that exosomes enriched with miR-30d-5p had a protective effect on AIS by inhibiting autophagy-mediated microglial polarization to M1 [123]. Yang et al. suggested that the expression of miR-181b-5p were remarkably enhanced in exosomes, which indicated that miR-181b-5p may involve in the progress of AIS [37].
Vascular cognitive impairment and dementia is a type of cognitive disorder induced by vascular abnormalities. It had been reported that exosomal miR-146a exerted anti-inflammatory effects on damaged astrocytes and prevented diabetes-induced cognitive impairment [124].
4.4 The role of exosomes in vascular aging related kidney diseases
The interaction of vascular aging and arteriosclerosis can ultimately contribute to chronic kidney disease (CKD) [125]. Arteriosclerosis is common in end-stage renal disease (ESRD). The incidence of plaque calcification and arterial stiffness is increased in patients with ESRD. VC is a strong predictor and common complication in patients with ESRD. Yu et al. found that the renal tubule-derived urinary exosomal miR-200b could regulate the progression of renal fibrosis, eventually leading to CKD [126]. Lange et al. concluded that miR-16 derived from urinary exosomes was the most stable endogenous reference gene in patients with CKD [127].
4.5 The role of exosomes in vascular aging related PAD
PAD is a vascular abnormity of diffuse atherosclerosis and is a major cause of cardiovascular mortality [128]. To date, less attention were paid to the association of miRNAs with vascular integrity in PAD. Bonauer et al. revealed that the expression of exosomal miR-92a was significantly increased in ischemic injury. Besides, inhibition of miR-92a promoted functional recovery in mice with ischemic damage to limb muscle [129].
5.Diagnosis and clinical evaluation of vascular aging
The specific cargoes of exosomes make them to be promising novel biomarkers and diagnostic tool for vascular aging. It especially concerns exosomes-associated miRNAs because of the significantly different levels between physiological and pathological conditions. In patients affected with AMI, the level of p53 responsive miRs (miR-192, miR-194, miR-34a) inside in exosomes were associated with development of HF, which suggesting a diagnostic value of these miRNAs [130]. The miRNome analysis of exosomes from hypertensive patients proved that several miRNAs including miR-615, miR-211 were sensitive to blood pressure, which exhibited a promising prognostic biomarker on hypertension [119]. Nevertheless, evaluation of exosomes as a source of diagnostic and prognostic biomarkers of vascular diseases is still in its infancy. Further studies should be done to validate diagnostic and prognostic value of exosome-associated biomarkers.
Clinical evaluation of vascular aging is generally divided into invasive and non-invasive methods. Invasive evaluations are rarely used in clinical practice because of their complex requirements, high cost and damage. Currently, non-invasive methods are commonly used in clinical assessment of vascular aging mainly include: (1) Framingham formula; (2) non-invasive detection of vascular function and structure; (3) biomarkers of vascular senescence cells [131].
-
(1)
Framingham formula: Framingham heart study establishes a vascular age formula based on risk factors for cardiovascular events, such as men, hyperlipidemia, hypertension, diabetes, and smoking. It proposes the concept of vascular age, which is still widely used in clinical vascular age assessment and guidance of cardiovascular risk prevention [132].
-
(2)
Non-invasive detection of vascular function and structure: (i) Pulse wave velocity (PWV) is the conduction velocity of the arterial pulse wave along the arterial wall from the proximal to the distal end when the heart pump hemorrhage occurs. The ratio of the distance between the two recorded parts and the conduction time of the pulse wave is measured. PWV is based on the mechanism that the speed of conduction of pulse waves in the blood output from the heart increases when the arteriosclerosis is hardened. The more severe arterial stiffness becomes, the greater the PWV is. The elastic components of the aorta decrease, the vessel wall thickens, the arteries expand progressively, the arterial stiffness increases, and the PWV increases with increasing aging. Moreover, the PWV of central elastic artery increased more significantly than that of peripheral artery [133]. PWV is positively correlated with Framingham and has predictive value for the diagnosis of coronary heart disease [134]. (ii) Intima-media thickness (IMT) is the distance between the arterial intima to media that measured by high-frequency B-mode ultrasound probe. IMT thickening is the landmark of vascular aging. The carotid IMT is an independent predictor of cardiovascular and cerebrovascular risk. For each 0.1 mm increase in carotid IMT, the risk of myocardial infarction increased by 11% [135]. IMT in healthy people is increasing gradually with the increase of age. Besides, vascular aging is accelerated as well as IMT is growing faster in the populations with atherosclerotic cardiovascular risk factors. (iii) The principle of vasodilation function is to measure the temporal change of brachial artery diameter under shear stress by ultrasound. The impaired diastolic function of brachial artery was associated with aging [136].
-
(3)
Biomarkers of vascular senescence cells: Currently, endothelial microparticles (EMPs) and endothelial progenitor cells (EPCs) are widely recognized and detected as cell markers. EMPs are microparticles carrying certain antigenic properties of ECs when they are activated, damaged or apoptotic. The increase of EMPs represents vascular endothelial injury, indicating vascular aging [137]. When vascular endothelial injury occurs, EPCs, derived from bone marrow, could be differentiated into mature ECs to repair damage blood vessels. Besides, EPCs can activate the proliferation and migration of ECs around the injured region through paracrine secretion and it is an important mechanism of endogenous vascular endothelial repair [138]. The decreasing of EPCs is closely related to the prognosis of atherosclerosis and cardiovascular diseases, which suggesting vascular aging [139].
6.Treatment and clinic intervention of vascular aging
In addition to act as potential biomarkers, exosomes could also be served as a therapeutic tool for treatment of vascular diseases. The main promise of using exosomes as a therapeutic tool is their property to stably maintain and carry a variety of biomolecules. Specific gene expression can be modulated by genetic approaches including silencing or overexpression of the prospective miRNAs or lncRNAs. The promising miRNA triad (miR-126, miR-145 and miR-155) could be primarily suggested for anti-atherogenic therapy. Moreover, since exosomes possess some properties such as biological barrier permeability, biocompatibility, low immunogenicity and low toxicity, which are suitable for therapeutic delivery [140, 141]. Thus, many researches investigate the function of exosomes as vehicles for exogenous miRNAs, lncRNAs, and even drugs delivery. Notably, although targeting miRNAs or lncRNAs shows promising therapeutic strategies, careful monitoring and studying of these interactions is essential and important in order to guarantee a safe application in humans.
Vascular aging is not only a natural physiological process, but also a pathological process involving a variety of risk factors, among which genetics, environment and lifestyle are involved in. It is an effective means of anti-vascular aging in clinic that regulating the reversible mechanism of vascular aging and strengthening the prevention and control of risk factors. The patients with vascular aging had better improve the lifestyle through healthy eating, weight control, smoking cessation and reasonable exercise. Drugs target for risk factors such as angiotensin-converting enzyme inhibitors, calcium antagonists or endothelial function like beraprost sodium have been found to improve vascular function, ameliorate arterial stiffness, and regulate the process of vascular aging. Although aging is irreversible, early detection and intervention of premature vascular failure is a new direction for prevention and treatment of cardiovascular, cerebrovascular and other vascular diseases.
7.Perspectives and Conclusions
The accumulation of the changes in the structure and function of aging vessels constitute the basis of vascular aging, while vascular aging is the basis of a variety of vascular diseases. Therefore, better understanding of the mechanism, evaluation and management of vascular aging will provide new research targets for vascular diseases. The cargos of exosomes especially ncRNAs play a crucial role in regulating aging processes and vascular aging related diseases. It also offers promising opportunities for treating and assessing vascular diseases. Despite interesting promises in implication, exosomal ncRNAs-based therapies still require more preclinical studies and many obstacles still need to be overcome in the future.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 81770833 and 81501212); the Hunan Province Special Health Research Projects (A2015-04).
Footnotes
Conflict of interest
Not applicable. The authors declare that they have no competing interests.
References
- [1].Wu M, Rementer C, Giachelli CM (2013). Vascular calcification: an update on mechanisms and challenges in treatment. Calcif Tissue Int, 93: 365-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Lin X, Zhan JK, Wang YJ, Tan P, Chen YY, Deng HQ, et al. (2016). Function, Role, and Clinical Application of MicroRNAs in Vascular Aging. Biomed Res Int, 2016: 6021394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Jenjaroenpun P, Kremenska Y, Nair VM, Kremenskoy M, Joseph B, Kurochkin IV (2013). Characterization of RNA in exosomes secreted by human breast cancer cell lines using next-generation sequencing. PeerJ, 1: e201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Lai RC, Chen TS, Lim SK (2011). Mesenchymal stem cell exosome: a novel stem cell-based therapy for cardiovascular disease. Regen Med, 6: 481-92. [DOI] [PubMed] [Google Scholar]
- [5].Liao XB, Zhang ZY, Yuan K, Liu Y, Feng X, Cui RR, et al. (2013). MiR-133a modulates osteogenic differentiation of vascular smooth muscle cells. Endocrinology, 154: 3344-52. [DOI] [PubMed] [Google Scholar]
- [6].Cui RR, Li SJ, Liu LJ, Yi L, Liang QH, Zhu X, et al. (2012). MicroRNA-204 regulates vascular smooth muscle cell calcification in vitro and in vivo. Cardiovasc Res, 96: 320-9. [DOI] [PubMed] [Google Scholar]
- [7].Liu FJ, Wen T, Liu L (2012). MicroRNAs as a novel cellular senescence regulator. Ageing Res Rev, 11: 41-50. [DOI] [PubMed] [Google Scholar]
- [8].Dimmeler S, Nicotera P (2013). MicroRNAs in age-related diseases. EMBO Mol Med, 5: 180-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Kapustin AN, Shanahan CM (2016). Emerging roles for vascular smooth muscle cell exosomes in calcification and coagulation. J Physiol, 594: 2905-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Zhang C, Zhang K, Huang F, Feng W, Chen J, Zhang H, et al. (2018). Exosomes, the message transporters in vascular calcification. J Cell Mol Med, 22: 4024-4033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Yan B, Wang Z (2012). Long noncoding RNA: its physiological and pathological roles. DNA Cell Biol, 31 Suppl 1: S34-41. [DOI] [PubMed] [Google Scholar]
- [12].Gupta SK, Piccoli MT, Thum T (2014). Non-coding RNAs in cardiovascular ageing. Ageing Res Rev, 17: 79-85. [DOI] [PubMed] [Google Scholar]
- [13].Maiese K (2017). Harnessing the Power of SIRT1 and Non-coding RNAs in Vascular Disease. Curr Neurovasc Res, 14: 82-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].He C, Zheng S, Luo Y, Wang B (2018). Exosome Theranostics: Biology and Translational Medicine. Theranostics, 8: 237-255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].O'Loughlin AJ, Woffindale CA, Wood MJ (2012). Exosomes and the emerging field of exosome-based gene therapy. Curr Gene Ther, 12: 262-74. [DOI] [PubMed] [Google Scholar]
- [16].Tabas I, Garcia-Cardena G, Owens GK (2015). Recent insights into the cellular biology of atherosclerosis. J Cell Biol, 209: 13-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Gimbrone MA Jr, Garcia-Cardena G (2016). Endothelial Cell Dysfunction and the Pathobiology of Atherosclerosis. Circ Res, 118: 620-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Staszel T, Zapala B, Polus A, Sadakierska-Chudy A, Kiec-Wilk B, Stepien E, et al. (2011). Role of microRNAs in endothelial cell pathophysiology. Pol Arch Med Wewn, 121: 361-6. [PubMed] [Google Scholar]
- [19].Das S, Halushka MK (2015). Extracellular vesicle microRNA transfer in cardiovascular disease. Cardiovasc Pathol, 24: 199-206. [DOI] [PubMed] [Google Scholar]
- [20].Kishore R, Garikipati VN, Gumpert A (2016). Tiny Shuttles for Information Transfer: Exosomes in Cardiac Health and Disease. J Cardiovasc Transl Res, 9: 169-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Santulli G (2016). MicroRNAs and Endothelial (Dys) Function. J Cell Physiol, 231: 1638-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Araldi E, Suarez Y (2016). MicroRNAs as regulators of endothelial cell functions in cardiometabolic diseases. Biochim Biophys Acta, 1861: 2094-2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Jia L, Zhou X, Huang X, Xu X, Jia Y, Wu Y, et al. (2018). Maternal and umbilical cord serum-derived exosomes enhance endothelial cell proliferation and migration. Faseb j, 32: 4534-4543. [DOI] [PubMed] [Google Scholar]
- [24].van Balkom BW, de Jong OG, Smits M, Brummelman J, den Ouden K, de Bree PM, et al. (2013). Endothelial cells require miR-214 to secrete exosomes that suppress senescence and induce angiogenesis in human and mouse endothelial cells. Blood, 121: 3997-4006, s1-15. [DOI] [PubMed] [Google Scholar]
- [25].Garcia NA, Ontoria-Oviedo I, Gonzalez-King H, Diez-Juan A, Sepulveda P (2015). Glucose Starvation in Cardiomyocytes Enhances Exosome Secretion and Promotes Angiogenesis in Endothelial Cells. PLoS One, 10: e0138849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Iaconetti C, Polimeni A, Sorrentino S, Sabatino J, Pironti G, Esposito G, et al. (2012). Inhibition of miR-92a increases endothelial proliferation and migration in vitro as well as reduces neointimal proliferation in vivo after vascular injury. Basic Res Cardiol, 107: 296. [DOI] [PubMed] [Google Scholar]
- [27].Jin C, Zhao Y, Yu L, Xu S, Fu G (2013). MicroRNA-21 mediates the rapamycin-induced suppression of endothelial proliferation and migration. FEBS Lett, 587: 378-85. [DOI] [PubMed] [Google Scholar]
- [28].Svensson D, Gidlof O, Turczynska KM, Erlinge D, Albinsson S, Nilsson BO (2014). Inhibition of microRNA-125a promotes human endothelial cell proliferation and viability through an antiapoptotic mechanism. J Vasc Res, 51: 239-45. [DOI] [PubMed] [Google Scholar]
- [29].Qin G, Dong Z, Zeng P, Liu M, Liao X (2013). Association of vitamin D receptor BsmI gene polymorphism with risk of osteoporosis: a meta-analysis of 41 studies. Mol Biol Rep, 40: 497-506. [DOI] [PubMed] [Google Scholar]
- [30].Loyer X, Potteaux S, Vion AC, Guerin CL, Boulkroun S, Rautou PE, et al. (2014). Inhibition of microRNA-92a prevents endothelial dysfunction and atherosclerosis in mice. Circ Res, 114: 434-43. [DOI] [PubMed] [Google Scholar]
- [31].Zhou J, Wang KC, Wu W, Subramaniam S, Shyy JY, Chiu JJ, et al. (2011). MicroRNA-21 targets peroxisome proliferators-activated receptor-alpha in an autoregulatory loop to modulate flow-induced endothelial inflammation. Proc Natl Acad Sci U S A, 108: 10355-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Goodwin AJ, Guo C, Cook JA, Wolf B, Halushka PV, Fan H (2015). Plasma levels of microRNA are altered with the development of shock in human sepsis: an observational study. Crit Care, 19: 440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Real JM, Ferreira LRP, Esteves GH, Koyama FC, Dias MVS, Bezerra-Neto JE, et al. (2018). Exosomes from patients with septic shock convey miRNAs related to inflammation and cell cycle regulation: new signaling pathways in sepsis? Crit Care, 22: 68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Li J, Tan M, Xiang Q, Zhou Z, Yan H (2017). Thrombin-activated platelet-derived exosomes regulate endothelial cell expression of ICAM-1 via microRNA-223 during the thrombosis-inflammation response. Thromb Res, 154: 96-105. [DOI] [PubMed] [Google Scholar]
- [35].Lee C, Mitsialis SA, Aslam M, Vitali SH, Vergadi E, Konstantinou G, et al. (2012). Exosomes mediate the cytoprotective action of mesenchymal stromal cells on hypoxia-induced pulmonary hypertension. Circulation, 126: 2601-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Liang X, Zhang L, Wang S, Han Q, Zhao RC (2016). Exosomes secreted by mesenchymal stem cells promote endothelial cell angiogenesis by transferring miR-125a. J Cell Sci, 129: 2182-9. [DOI] [PubMed] [Google Scholar]
- [37].Yang Y, Cai Y, Zhang Y, Liu J, Xu Z (2018). Exosomes Secreted by Adipose-Derived Stem Cells Contribute to Angiogenesis of Brain Microvascular Endothelial Cells Following Oxygen-Glucose Deprivation In Vitro Through MicroRNA-181b/TRPM7 Axis. J Mol Neurosci, 65: 74-83. [DOI] [PubMed] [Google Scholar]
- [38].Li Y, Liang J, Hu J, Ren X, Sheng Y (2018). Down-regulation of exosomal miR-106b-5p derived from cholesteatoma perimatrix fibroblasts promotes angiogenesis in endothelial cells by overexpression of Angiopoietin 2. Cell Biol Int, 42: 1300-1310. [DOI] [PubMed] [Google Scholar]
- [39].Wang X, Huang W, Liu G, Cai W, Millard RW, Wang Y, et al. (2014). Cardiomyocytes mediate anti-angiogenesis in type 2 diabetic rats through the exosomal transfer of miR-320 into endothelial cells. J Mol Cell Cardiol, 74: 139-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Chen Y, Li D, Xu Y, Zhang Y, Tao L, Li S, et al. (2014). Essential Oils from Fructus A. zerumbet Protect Human Aortic Endothelial Cells from Apoptosis Induced by Ox-LDL In Vitro. Evid Based Complement Alternat Med, 2014: 956824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Hung T, Chang HY (2010). Long noncoding RNA in genome regulation: prospects and mechanisms. RNA Biol, 7: 582-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Rinn JL, Chang HY (2012). Genome regulation by long noncoding RNAs. Annu Rev Biochem, 81: 145-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Wang KC, Chang HY (2011). Molecular mechanisms of long noncoding RNAs. Mol Cell, 43: 904-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Khaitan D, Dinger ME, Mazar J, Crawford J, Smith MA, Mattick JS, et al. (2011). The melanoma-upregulated long noncoding RNA SPRY4-IT1 modulates apoptosis and invasion. Cancer Res, 71: 3852-62. [DOI] [PubMed] [Google Scholar]
- [45].Huarte M, Guttman M, Feldser D, Garber M, Koziol MJ, Kenzelmann-Broz D, et al. (2010). A large intergenic noncoding RNA induced by p53 mediates global gene repression in the p53 response. Cell, 142: 409-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Tian D, Sun S, Lee JT (2010). The long noncoding RNA, Jpx, is a molecular switch for X chromosome inactivation. Cell, 143: 390-403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Yin DD, Zhang EB, You LH, Wang N, Wang LT, Jin FY, et al. (2015). Downregulation of lncRNA TUG1 affects apoptosis and insulin secretion in mouse pancreatic beta cells. Cell Physiol Biochem, 35: 1892-904. [DOI] [PubMed] [Google Scholar]
- [48].Liao B, Chen R, Lin F, Mai A, Chen J, Li H, et al. (2018). Long noncoding RNA HOTTIP promotes endothelial cell proliferation and migration via activation of the Wnt/beta-catenin pathway. J Cell Biochem, 119: 2797-2805. [DOI] [PubMed] [Google Scholar]
- [49].Lang HL, Hu GW, Chen Y, Liu Y, Tu W, Lu YM, et al. (2017). Glioma cells promote angiogenesis through the release of exosomes containing long non-coding RNA POU3F3. Eur Rev Med Pharmacol Sci, 21: 959-972. [PubMed] [Google Scholar]
- [50].Michalik KM, You X, Manavski Y, Doddaballapur A, Zornig M, Braun T, et al. (2014). Long noncoding RNA MALAT1 regulates endothelial cell function and vessel growth. Circ Res, 114: 1389-97. [DOI] [PubMed] [Google Scholar]
- [51].He C, Yang W, Yang J, Ding J, Li S, Wu H, et al. (2017). Long Noncoding RNA MEG3 Negatively Regulates Proliferation and Angiogenesis in Vascular Endothelial Cells. DNA Cell Biol, 36: 475-481. [DOI] [PubMed] [Google Scholar]
- [52].Ruan W, Zhao F, Zhao S, Zhang L, Shi L, Pang T (2018). Knockdown of long noncoding RNA MEG3 impairs VEGF-stimulated endothelial sprouting angiogenesis via modulating VEGFR2 expression in human umbilical vein endothelial cells. Gene, 649: 32-39. [DOI] [PubMed] [Google Scholar]
- [53].Nakamura K, Taguchi E, Miura T, Yamamoto A, Takahashi K, Bichat F, et al. (2006). KRN951, a highly potent inhibitor of vascular endothelial growth factor receptor tyrosine kinases, has antitumor activities and affects functional vascular properties. Cancer Res, 66: 9134-42. [DOI] [PubMed] [Google Scholar]
- [54].Conigliaro A, Costa V, Lo Dico A, Saieva L, Buccheri S, Dieli F, et al. (2015). CD90+ liver cancer cells modulate endothelial cell phenotype through the release of exosomes containing H19 lncRNA. Mol Cancer, 14: 155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Lang HL, Hu GW, Zhang B, Kuang W, Chen Y, Wu L, et al. (2017). Glioma cells enhance angiogenesis and inhibit endothelial cell apoptosis through the release of exosomes that contain long non-coding RNA CCAT2. Oncol Rep, 38: 785-798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Kaneko S, Bonasio R, Saldana-Meyer R, Yoshida T, Son J, Nishino K, et al. (2014). Interactions between JARID2 and noncoding RNAs regulate PRC2 recruitment to chromatin. Mol Cell, 53: 290-300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Boon RA, Hofmann P, Michalik KM, Lozano-Vidal N, Berghauser D, Fischer A, et al. (2016). Long Noncoding RNA Meg3 Controls Endothelial Cell Aging and Function: Implications for Regenerative Angiogenesis. J Am Coll Cardiol, 68: 2589-2591. [DOI] [PubMed] [Google Scholar]
- [58].Chen L, Yang W, Guo Y, Chen W, Zheng P, Zeng J, et al. (2017). Exosomal lncRNA GAS5 regulates the apoptosis of macrophages and vascular endothelial cells in atherosclerosis. PLoS One, 12: e0185406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Gutschner T, Hammerle M, Diederichs S (2013). MALAT1 -- a paradigm for long noncoding RNA function in cancer. J Mol Med (Berl), 91: 791-801. [DOI] [PubMed] [Google Scholar]
- [60].Puthanveetil P, Chen S, Feng B, Gautam A, Chakrabarti S (2015). Long non-coding RNA MALAT1 regulates hyperglycaemia induced inflammatory process in the endothelial cells. J Cell Mol Med, 19: 1418-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Maegdefessel L, Rayner KJ, Leeper NJ (2015). MicroRNA regulation of vascular smooth muscle function and phenotype: early career committee contribution. Arterioscler Thromb Vasc Biol, 35: 2-6. [DOI] [PubMed] [Google Scholar]
- [62].Robinson HC, Baker AH (2012). How do microRNAs affect vascular smooth muscle cell biology? Curr Opin Lipidol, 23: 405-11. [DOI] [PubMed] [Google Scholar]
- [63].Liu X, Cheng Y, Chen X, Yang J, Xu L, Zhang C (2011). MicroRNA-31 regulated by the extracellular regulated kinase is involved in vascular smooth muscle cell growth via large tumor suppressor homolog 2. J Biol Chem, 286: 42371-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Torella D, Iaconetti C, Catalucci D, Ellison GM, Leone A, Waring CD, et al. (2011). MicroRNA-133 controls vascular smooth muscle cell phenotypic switch in vitro and vascular remodeling in vivo. Circ Res, 109: 880-93. [DOI] [PubMed] [Google Scholar]
- [65].Rangrez AY, M'Baya-Moutoula E, Metzinger-Le Meuth V, Henaut L, Djelouat MS, Benchitrit J, et al. (2012). Inorganic phosphate accelerates the migration of vascular smooth muscle cells: evidence for the involvement of miR-223. PLoS One, 7: e47807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Yang X, Dong M, Wen H, Liu X, Zhang M, Ma L, et al. (2017). MiR-26a contributes to the PDGF-BB-induced phenotypic switch of vascular smooth muscle cells by suppressing Smad1. Oncotarget, 8: 75844-75853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Ji R, Cheng Y, Yue J, Yang J, Liu X, Chen H, et al. (2007). MicroRNA expression signature and antisense-mediated depletion reveal an essential role of MicroRNA in vascular neointimal lesion formation. Circ Res, 100: 1579-88. [DOI] [PubMed] [Google Scholar]
- [68].Calvier L, Chouvarine P, Legchenko E, Hoffmann N, Geldner J, Borchert P, et al. (2017). PPARgamma Links BMP2 and TGFbeta1 Pathways in Vascular Smooth Muscle Cells, Regulating Cell Proliferation and Glucose Metabolism. Cell Metab, 25: 1118-1134.e7. [DOI] [PubMed] [Google Scholar]
- [69].Liu X, Cheng Y, Zhang S, Lin Y, Yang J, Zhang C (2009). A necessary role of miR-221 and miR-222 in vascular smooth muscle cell proliferation and neointimal hyperplasia. Circ Res, 104: 476-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Davis BN, Hilyard AC, Nguyen PH, Lagna G, Hata A (2009). Induction of microRNA-221 by platelet-derived growth factor signaling is critical for modulation of vascular smooth muscle phenotype. J Biol Chem, 284: 3728-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Albinsson S, Sessa WC (2011). Can microRNAs control vascular smooth muscle phenotypic modulation and the response to injury? Physiol Genomics, 43: 529-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Yu X, Li Z (2014). MicroRNAs regulate vascular smooth muscle cell functions in atherosclerosis (review). Int J Mol Med, 34: 923-33. [DOI] [PubMed] [Google Scholar]
- [73].Wang YS, Chou WW, Chen KC, Cheng HY, Lin RT, Juo SH (2012). MicroRNA-152 mediates DNMT1-regulated DNA methylation in the estrogen receptor alpha gene. PLoS One, 7: e30635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Ma X, Ma C, Zheng X (2013). MicroRNA-155 in the pathogenesis of atherosclerosis: a conflicting role? Heart Lung Circ, 22: 811-8. [DOI] [PubMed] [Google Scholar]
- [75].Tan M, Yan HB, Li JN, Li WK, Fu YY, Chen W, et al. (2016). Thrombin Stimulated Platelet-Derived Exosomes Inhibit Platelet-Derived Growth Factor Receptor-Beta Expression in Vascular Smooth Muscle Cells. Cell Physiol Biochem, 38: 2348-65. [DOI] [PubMed] [Google Scholar]
- [76].Salomon C, Yee S, Scholz-Romero K, Kobayashi M, Vaswani K, Kvaskoff D, et al. (2014). Extravillous trophoblast cells-derived exosomes promote vascular smooth muscle cell migration. Front Pharmacol, 5: 175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Cho JR, Lee CY, Lee J, Seo HH, Choi E, Chung N, et al. (2015). MicroRNA-761 inhibits Angiotensin II-induced vascular smooth muscle cell proliferation and migration by targeting mammalian target of rapamycin. Clin Hemorheol Microcirc, 63: 45-56. [DOI] [PubMed] [Google Scholar]
- [78].Chen KC, Wang YS, Hu CY, Chang WC, Liao YC, Dai CY, et al. (2011). OxLDL up-regulates microRNA-29b, leading to epigenetic modifications of MMP-2/MMP-9 genes: a novel mechanism for cardiovascular diseases. Faseb j, 25: 1718-28. [DOI] [PubMed] [Google Scholar]
- [79].Deng L, Blanco FJ, Stevens H, Lu R, Caudrillier A, McBride M, et al. (2015). MicroRNA-143 Activation Regulates Smooth Muscle and Endothelial Cell Crosstalk in Pulmonary Arterial Hypertension. Circ Res, 117: 870-883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Gui T, Zhou G, Sun Y, Shimokado A, Itoh S, Oikawa K, et al. (2012). MicroRNAs that target Ca(2+) transporters are involved in vascular smooth muscle cell calcification. Lab Invest, 92: 1250-9. [DOI] [PubMed] [Google Scholar]
- [81].Kapustin AN, Chatrou ML, Drozdov I, Zheng Y, Davidson SM, Soong D, et al. (2015). Vascular smooth muscle cell calcification is mediated by regulated exosome secretion. Circ Res, 116: 1312-23. [DOI] [PubMed] [Google Scholar]
- [82].Kapustin AN, Schoppet M, Schurgers LJ, Reynolds JL, McNair R, Heiss A, et al. (2017). Prothrombin Loading of Vascular Smooth Muscle Cell-Derived Exosomes Regulates Coagulation and Calcification. Arterioscler Thromb Vasc Biol, 37: e22-e32. [DOI] [PubMed] [Google Scholar]
- [83].Du Y, Gao C, Liu Z, Wang L, Liu B, He F, et al. (2012). Upregulation of a disintegrin and metalloproteinase with thrombospondin motifs-7 by miR-29 repression mediates vascular smooth muscle calcification. Arterioscler Thromb Vasc Biol, 32: 2580-8. [DOI] [PubMed] [Google Scholar]
- [84].Wen P, Cao H, Fang L, Ye H, Zhou Y, Jiang L, et al. (2014). miR-125b/Ets1 axis regulates transdifferentiation and calcification of vascular smooth muscle cells in a high-phosphate environment. Exp Cell Res, 322: 302-12. [DOI] [PubMed] [Google Scholar]
- [85].Goettsch C, Rauner M, Pacyna N, Hempel U, Bornstein SR, Hofbauer LC (2011). miR-125b regulates calcification of vascular smooth muscle cells. Am J Pathol, 179: 1594-600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Mathiyalagan P, Liang Y, Kim D, Misener S, Thorne T, Kamide CE, et al. (2017). Angiogenic Mechanisms of Human CD34(+) Stem Cell Exosomes in the Repair of Ischemic Hindlimb. Circ Res, 120: 1466-1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Tan P, Wang YJ, Li S, Wang Y, He JY, Chen YY, et al. (2016). The PI3K/Akt/mTOR pathway regulates the replicative senescence of human VSMCs. Mol Cell Biochem, 422: 1-10. [DOI] [PubMed] [Google Scholar]
- [88].Zhang L, Zhou M, Wang Y, Huang W, Qin G, Weintraub NL, et al. (2014). miR-92a inhibits vascular smooth muscle cell apoptosis: role of the MKK4-JNK pathway. Apoptosis, 19: 975-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Badi I, Burba I, Ruggeri C, Zeni F, Bertolotti M, Scopece A, et al. (2015). MicroRNA-34a Induces Vascular Smooth Muscle Cells Senescence by SIRT1 Downregulation and Promotes the Expression of Age-Associated Pro-inflammatory Secretory Factors. J Gerontol A Biol Sci Med Sci, 70: 1304-11. [DOI] [PubMed] [Google Scholar]
- [90].He JY, Tu C, Liu YS (2018). Role of lncRNA in aging and age-related diseases. Aging Medicine, 1: 158-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Wang Y, Song X, Li Z, Liu B (2018). Long non-coding RNAs in coronary atherosclerosis. Life Sci, 211: 189-197. [DOI] [PubMed] [Google Scholar]
- [92].Brock M, Schuoler C, Leuenberger C, Buhlmann C, Haider TJ, Vogel J, et al. (2017). Analysis of hypoxia-induced noncoding RNAs reveals metastasis-associated lung adenocarcinoma transcript 1 as an important regulator of vascular smooth muscle cell proliferation. Exp Biol Med (Maywood), 242: 487-496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Sun Z, Nie X, Sun S, Dong S, Yuan C, Li Y, et al. (2017). Long Non-Coding RNA MEG3 Downregulation Triggers Human Pulmonary Artery Smooth Muscle Cell Proliferation and Migration via the p53 Signaling Pathway. Cell Physiol Biochem, 42: 2569-2581. [DOI] [PubMed] [Google Scholar]
- [94].Wu G, Cai J, Han Y, Chen J, Huang ZP, Chen C, et al. (2014). LincRNA-p21 regulates neointima formation, vascular smooth muscle cell proliferation, apoptosis, and atherosclerosis by enhancing p53 activity. Circulation, 130: 1452-1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Carrion K, Dyo J, Patel V, Sasik R, Mohamed SA, Hardiman G, et al. (2014). The long non-coding HOTAIR is modulated by cyclic stretch and WNT/beta-CATENIN in human aortic valve cells and is a novel repressor of calcification genes. PLoS One, 9: e96577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Zhao L, Luo H, Li X, Li T, He J, Qi Q, et al. (2017). Exosomes Derived from Human Pulmonary Artery Endothelial Cells Shift the Balance between Proliferation and Apoptosis of Smooth Muscle Cells. Cardiology, 137: 43-53. [DOI] [PubMed] [Google Scholar]
- [97].Hergenreider E, Heydt S, Treguer K, Boettger T, Horrevoets AJ, Zeiher AM, et al. (2012). Atheroprotective communication between endothelial cells and smooth muscle cells through miRNAs. Nat Cell Biol, 14: 249-56. [DOI] [PubMed] [Google Scholar]
- [98].Cheng Y, Liu X, Yang J, Lin Y, Xu DZ, Lu Q, et al. (2009). MicroRNA-145, a novel smooth muscle cell phenotypic marker and modulator, controls vascular neointimal lesion formation. Circ Res, 105: 158-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Chen NX, Kiattisunthorn K, O'Neill KD, Chen X, Moorthi RN, et al. (2013). Decreased microRNA is involved in the vascular remodeling abnormalities in chronic kidney disease (CKD). PLoS One, 8: e64558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Lin X, He Y, Hou X, Zhang Z, Wang R, Wu Q (2016). Endothelial Cells Can Regulate Smooth Muscle Cells in Contractile Phenotype through the miR-206/ARF6&NCX1/Exosome Axis. PLoS One, 11: e0152959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Zheng B, Yin WN, Suzuki T, Zhang XH, Zhang Y, Song LL, et al. (2017). Exosome-Mediated miR-155 Transfer from Smooth Muscle Cells to Endothelial Cells Induces Endothelial Injury and Promotes Atherosclerosis. Mol Ther, 25: 1279-1294. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [102].Pfeifer P, Werner N, Jansen F (2015). Role and Function of MicroRNAs in Extracellular Vesicles in Cardiovascular Biology. Biomed Res Int, 2015: 161393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Bazan HA, Hatfield SA, O'Malley CB, Brooks AJ, Lightell D Jr, Woods TC (2015). Acute Loss of miR-221 and miR-222 in the Atherosclerotic Plaque Shoulder Accompanies Plaque Rupture. Stroke, 46: 3285-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Fichtlscherer S, De Rosa S, Fox H, Schwietz T, Fischer A, Liebetrau C, et al. (2010). Circulating microRNAs in patients with coronary artery disease. Circ Res, 107: 677-84. [DOI] [PubMed] [Google Scholar]
- [105].Mocharla P, Briand S, Giannotti G, Dorries C, Jakob P, Paneni F, et al. .(2013). AngiomiR-126 expression and secretion from circulating CD34(+) and CD14(+) PBMCs: role for proangiogenic effects and alterations in type 2 diabetics. Blood, 121: 226-36. [DOI] [PubMed] [Google Scholar]
- [106].Jaiswal R, Luk F, Gong J, Mathys JM, Grau GE, Bebawy M (2012). Microparticle conferred microRNA profiles--implications in the transfer and dominance of cancer traits. Mol Cancer, 11: 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Feld S, Kjellgren O, Smalling RW (1995). Aggressive interventional treatment of acute myocardial infarction. Lessons from the animal laboratory applied to the catheterization suite. Cardiology, 86: 365-73. [DOI] [PubMed] [Google Scholar]
- [108].Feng Y, Huang W, Wani M, Yu X, Ashraf M (2014). Ischemic preconditioning potentiates the protective effect of stem cells through secretion of exosomes by targeting Mecp2 via miR-22. PLoS One, 9: e88685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].De Rosa S, Fichtlscherer S, Lehmann R, Assmus B, Dimmeler S, Zeiher AM (2011). Transcoronary concentration gradients of circulating microRNAs. Circulation, 124: 1936-44. [DOI] [PubMed] [Google Scholar]
- [110].Zile MR, Mehurg SM, Arroyo JE, Stroud RE, DeSantis SM, Spinale FG (2011). Relationship between the temporal profile of plasma microRNA and left ventricular remodeling in patients after myocardial infarction. Circ Cardiovasc Genet, 4: 614-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Liew CC, Dzau VJ (2004). Molecular genetics and genomics of heart failure. Nat Rev Genet, 5: 811-25. [DOI] [PubMed] [Google Scholar]
- [112].Melman YF, Shah R, Das S(2014). MicroRNAs in heart failure: is the picture becoming less miRky? Circ Heart Fail, 7: 203-14. [DOI] [PubMed] [Google Scholar]
- [113].Ikeda S, Pu WT (2010). Expression and function of microRNAs in heart disease. Curr Drug Targets, 11: 913-25. [DOI] [PubMed] [Google Scholar]
- [114].Matkovich SJ, Van Booven DJ, Youker KA, Torre-Amione G, Diwan A, Eschenbacher WH, et al. (2009). Reciprocal regulation of myocardial microRNAs and messenger RNA in human cardiomyopathy and reversal of the microRNA signature by biomechanical support. Circulation, 119: 1263-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Naga Prasad SV, Duan ZH, Gupta MK, Surampudi VS, Volinia S, Calin GA, et al. (2009). Unique microRNA profile in end-stage heart failure indicates alterations in specific cardiovascular signaling networks. J Biol Chem, 284: 27487-99. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [116].Wang M, Kim SH, Monticone RE, Lakatta EG (2015). Matrix metalloproteinases promote arterial remodeling in aging, hypertension, and atherosclerosis. Hypertension, 65: 698-703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Su SA, Xie Y, Fu Z, Wang Y, Wang JA, Xiang M (2017). Emerging role of exosome-mediated intercellular communication in vascular remodeling. Oncotarget, 8: 25700-25712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Qi Y, Wang X, Rose KL, MacDonald WH, Zhang B, Schey KL, et al. (2016). Activation of the Endogenous Renin-Angiotensin-Aldosterone System or Aldosterone Administration Increases Urinary Exosomal Sodium Channel Excretion. J Am Soc Nephrol, 27: 646-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Gildea JJ, Carlson JM, Schoeffel CD, Carey RM, Felder RA (2013). Urinary exosome miRNome analysis and its applications to salt sensitivity of blood pressure. Clin Biochem, 46: 1131-1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Cunha PG, Boutouyrie P, Nilsson PM, Laurent S (2017). Early Vascular Ageing (EVA): Definitions and Clinical Applicability. Curr Hypertens Rev, 13: 8-15. [DOI] [PubMed] [Google Scholar]
- [121].Blum A, Vaispapir V, Keinan-Boker L, Soboh S, Yehuda H, Tamir S (2012). Endothelial dysfunction and procoagulant activity in acute ischemic stroke. J Vasc Interv Neurol, 5: 33-9. [PMC free article] [PubMed] [Google Scholar]
- [122].Geng W, Tang H, Luo S, Lv Y, Liang D, Kang X, et al. (2019). Exosomes from miRNA-126-modified ADSCs promotes functional recovery after stroke in rats by improving neurogenesis and suppressing microglia activation. Am J Transl Res, 11: 780-792. [PMC free article] [PubMed] [Google Scholar]
- [123].Jiang M, Wang H, Jin M, Yang X, Ji H, Jiang Y, et al. (2018). Exosomes from MiR-30d-5p-ADSCs Reverse Acute Ischemic Stroke-Induced, Autophagy-Mediated Brain Injury by Promoting M2 Microglial/Macrophage Polarization. Cell Physiol Biochem, 47: 864-878. [DOI] [PubMed] [Google Scholar]
- [124].Kubota K, Nakano M, Kobayashi E, Mizue Y, Chikenji T, Otani M, et al. (2018). An enriched environment prevents diabetes-induced cognitive impairment in rats by enhancing exosomal miR-146a secretion from endogenous bone marrow-derived mesenchymal stem cells. PLoS One, 13: e0204252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Safar ME, London GM, Plante GE (2004). Arterial stiffness and kidney function. Hypertension, 43: 163-8. [DOI] [PubMed] [Google Scholar]
- [126].Yu Y, Bai F, Qin N, Liu W, Sun Q, Zhou Y, et al. (2018). Non-Proximal Renal Tubule-Derived Urinary Exosomal miR-200b as a Biomarker of Renal Fibrosis. Nephron, 139: 269-282. [DOI] [PubMed] [Google Scholar]
- [127].Lange T, Stracke S, Rettig R, Lendeckel U, Kuhn J, Schluter R, et al. (2017). Identification of miR-16 as an endogenous reference gene for the normalization of urinary exosomal miRNA expression data from CKD patients. PLoS One, 12: e0183435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Vogiatzi G, Oikonomou E, Deftereos S, Siasos G, Tousoulis D (2018). Peripheral artery disease: a micro-RNA-related condition? Curr Opin Pharmacol, 39: 105-112. [DOI] [PubMed] [Google Scholar]
- [129].Bonauer A, Carmona G, Iwasaki M, Mione M, Koyanagi M, Fischer A, et al. (2009). MicroRNA-92a controls angiogenesis and functional recovery of ischemic tissues in mice. Science, 324: 1710-3. [DOI] [PubMed] [Google Scholar]
- [130].Matsumoto S, Sakata Y, Suna S, Nakatani D, Usami M, Hara M, et al. (2013). Circulating p53-responsive microRNAs are predictive indicators of heart failure after acute myocardial infarction. Circ Res, 113: 322-6. [DOI] [PubMed] [Google Scholar]
- [131].Geriatric Cardiology Group of Chinese Geriatric Society(2018). Expert consensus on clinical assessment and intervention of vascular aging in China. Chin J Geriatr, 37: 1177-1184. [Google Scholar]
- [132].D'Agostino RB Sr, Vasan RS, Pencina MJ, Wolf PA, Cobain M, Massaro JM, et al. (2008). General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation, 117: 743-53. [DOI] [PubMed] [Google Scholar]
- [133].Khoshdel AR, Thakkinstian A, Carney SL, Attia J (2006). Estimation of an age-specific reference interval for pulse wave velocity: a meta-analysis. J Hypertens, 24: 1231-7. [DOI] [PubMed] [Google Scholar]
- [134].Li SS, Zhang CT, Zhou HL, Huang K, Fsn QP (2012). Correlation of brachial ankle pulse wave velocity with cardiovascular risks and Framingham score. Chin J Mult Organ Dis Elderly, 11: 912-916. [Google Scholar]
- [135].Xu Y, Arora RC, Hiebert BM, Lerner B, Szwajcer A, McDonald K, et al. (2014). Non-invasive endothelial function testing and the risk of adverse outcomes: a systematic review and meta-analysis. Eur Heart J Cardiovasc Imaging, 15: 736-46. [DOI] [PubMed] [Google Scholar]
- [136].Celermajer DS, Sorensen KE, Spiegelhalter DJ, Georgakopoulos D, Robinson J, Deanfield JE (1994). Aging is associated with endothelial dysfunction in healthy men years before the age-related decline in women. J Am Coll Cardiol, 24: 471-6. [DOI] [PubMed] [Google Scholar]
- [137].Wang Y, Tao J, Yang Z, Tu C, Xu MG, Wang JM, et al. (2005). Tumor necrosis factor-α induces release of endothelial microparticle from endothelial cells. Chin J Cardiol, 32: 1137-1140. [PubMed] [Google Scholar]
- [138].Xia WH, Li J, Su C, Yang Z, Chen L, Wu F, et al. (2012). Physical exercise attenuates age-associated reduction in endothelium-reparative capacity of endothelial progenitor cells by increasing CXCR4/JAK-2 signaling in healthy men. Aging Cell, 11: 111-9. [DOI] [PubMed] [Google Scholar]
- [139].Tao J, Wang Y, Yang Z, Tu C, Xu MG, Wang JM (2006). Circulating endothelial progenitor cell deficiency contributes to impaired arterial elasticity in persons of advancing age. J Hum Hypertens, 20: 490-5. [DOI] [PubMed] [Google Scholar]
- [140].Xitong D, Xiaorong Z (2016). Targeted therapeutic delivery using engineered exosomes and its applications in cardiovascular diseases. Gene, 575: 377-384. [DOI] [PubMed] [Google Scholar]
- [141].Boukouris S, Mathivanan S (2015). Exosomes in bodily fluids are a highly stable resource of disease biomarkers. Proteomics Clin Appl, 9: 358-67. [DOI] [PMC free article] [PubMed] [Google Scholar]


