Abstract
Glioblastoma multiforme (GBM) and other central nervous system (CNS) cancer have poor long-term prognosis and there is significant need for improved treatments. GBM initiation and progression are mediated, in part, by microRNA (miRNA), which are endogenous posttranscriptional gene regulators. Misregulation of miRNAs is a potential target for therapeutic intervention in GBM. In this work, a micelle-like nanoparticle delivery system based upon the block copolymer poly(ethylene glycol-b-lactide-b-arginine) was designed with and without a reducible linkage between the lactide and RNA-binding peptide, R15, to assess the ability of the micelle-like particles to disassemble. Using confocal live cell imaging, intracellular dissociation was pronounced for the reducible micelleplexes. This dissociation was also supported by higher efficiency in a dual luciferase assay specific for the miRNA of interest, miR-21. Notably, micelleplexes were found to have significantly better stability and high anti-miRNA activity in cerebrospinal fluid (CSF) than in human plasma, suggesting the advantage of applying micelleplexes for CNS disease and in vivo CNS therapeutics. The reducible delivery system was determined a promising delivery platform for the treatment of CNS disease with miRNA therapy.
Keywords: micelles, micelleplexes, miRNAs, miRNA-21, glioblastoma, anti-miRNAs, cell penetrating peptide
Graphical Abstract
INTRODUCTION
Glioblastoma multiforme (GBM) is a fatal brain tumor with an annual incidence of approximately 5 in 100,000 people with a median survival of 15 months despite aggressive intervention, specifically neurosurgery, radiation, and chemotherapy.1 Unfortunately, few effective therapeutic advances have reached the clinic over the last several decades to improve the survival of patients.2 New treatment options, including delivery system and therapeutic molecules, are need to treat this disease. The blood-brain barrier limits drug transport into the brain3, 4 and tumors diffusely invade within the brain5, 6 challenging the development of novel therapeutics. These characteristics limit systemic therapy but also allow for the development of intracranial administered therapeutics.7, 8
Short noncoding RNAs, miRNAs, are gene regulators of multiple target genes through seed pairing with 3’ untranslated region (UTR) of mRNA.9–12 MiRNAs play critical roles in many steps of the tumorigenic process, including cellular proliferation, invasion, apoptosis, angiogenesis, and stem-like properties of various types of cancer including: GBM.13, 14 In GBM and other cancers, miRNA misregulation can result in the up regulation or down regulation of miRNAs in the diseased cells. Due to targets in apoptotic, proliferation, and angiogenic networks, miR-21 has been suggested as a potential miRNA target for anti-miRNA therapy.15–20 Tumor suppressor genes, including programmed cell death 4 (PDCD4) and serpin peptidase inhibitor clade B (ovalbumin) member 5 (SERPINB5), are transcriptionally regulated by miR-21.17, 20–22 When miR-21 is present, the mRNA of PDCD4 and SERPINB5 are degraded and the proteins downregulated, leading to cell growth and proliferation. Our previous research has demonstrated that PDCD4 and SERPINB5 mRNAs level increased after anti-miR-21 delivered to glioblastoma cells via cell penetrating peptide resulting in decreased migration.23
The negatively charged nature and instability of nucleic acids require the development of effective delivery vehicles to achieve cellular uptake and protect nucleic acids against enzymatic degradation in vivo.12 Micelleplexes have attracted significant scientific attention for nucleic acid delivery. Micelles can be formed before loading nucleic acids via electrostatic interactions. Thermodynamically speaking, micelleplexes are more stable than polyplexes since hydrophobic and electrostatic interactions are entropically driven processes, which cooperatively contribute to micelleplexes formation and stability.24
However, micelle-based drug delivery has been plagued by stability issues following systemic administration.25 Upon injection, charged nanocarriers are confronted with the complex biologic components of blood, particularly proteins, polysaccharides, and lipids, which causes premature release of nucleic acids and system disassembly.26 Cerebrospinal fluid (CSF) is a clear, colorless body fluid present in the brain and spine.27, 28 The protein concentration in human CSF is 0.2 mg/mL, much lower than the protein concentration of 6.0 mg/mL in human serum.29 In this study, we hypothesized that micelleplexes have better stability in CSF than human plasma and that the micelleplexes would actively deliver miRNA to the cells in the CNS.
A micelleplex system was designed with the hydrophilic arginine-rich cell penetrating peptide (R15) conjugated to the carboxy-terminal of methoxy-poly(ethylene glycol-b-lactide) (PEG-b-PLA) block copolymer, similar to a micellar system in clinical trials.30–33 The anti-miRNAs were complexed with the micelles, forming micelleplexes, with the expectation that the anti-miRNA would complex with R15 in the hydrophilic corona through electrostatic interactions.34, 35 To facilitate the dissociation of the micelleplexes after cellular entry, we sought to exploit the reducing potential of the cell used to defend against oxidative stress.36, 37 In particular, intracellular glutathione (GSH) concentration (~10 mM) is significantly higher than the level in extracellular environment (less than 2 μM),38 leading to significant glutathione-based reduction intracellularly serving as a trigger for drug delivery. A reducible disulfide bond was incorporated between PEG-b-PLA and R15 to enhance anti-miRNAs release by promoting micelle disassembly in GSH rich cytosol (Figure 1).
Figure 1. Schematic illustration of the chemical synthesis and intracellular dissociation of (A) reducible and (B) nonreducible micelleplexes.
MATERIALS AND METHODS
Materials.
The block copolymer, α-methoxy, ω-carboxy-poly(ethylene glycol-b-lactide) (PEG-PLA-COOH; MWPEG~2,000 g/mol; MWPLA ~ 3,000 g/mol), was purchased from Advanced Polymer Materials (Montreal, Canada). Peptides, Ac-CR15-NH2 and CR15K(FITC)NH2, were synthesized by VCPBIO (Shenzhen, China). N-(3-dimethylaminopropyl)-N′-ethylcarbodiimide hydrochloride (EDC), N-hydroxysuccinimide (NHS), 1,1′-Dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate (DiI), 3,3′-dioctadecyloxacarbocyanine perchlorate (DiO) and N-(2-aminoethyl) maleimide trifluoroacetate salt (AEM) were purchased from Sigma Aldrich. Crosslinker, 3-(2-pyridyldithio) proprionyl hydrazide (PDPH), was obtained from Fisher Scientific. Single stranded anti-miRNA, Cy-5 labeled, was obtained from Invitrogen. MirVana™miRNA-21 inhibitor (miRBase accession # MIMAT0000076, mature miRNA sequence UAGCUUAUCA-GACUGAUGUUGA) and miRNA inhibitor negative control #1 (anti-miRc) was purchased from Ambion (Austin, TX). The miR-21 luciferase reporter vector was obtained from Signosis, Inc. (Santa Clara, CA). Dr. Yu Hou of the University of Illinois Cancer Center kindly provided the Renilla vector.
The human glioblastoma U251 cell line was obtained from Dr. Lena Al-Harthi (Rush University). Cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM; Gibco) supplemented with 10% fetal bovine serum (FBS), 1 mM sodiumpyruvate, 1% nonessential amino acids, 100 U/mL streptomycin, and 100 U/mL penicillin. Cells were incubated in humidified air and 5% CO2 at 37°C.
Cerebrospinal Fluid (CSF, Cat No# 991–19-S-5) was obtained from Lee Biosolutions, Inc (St. Louis, MO). Anti-coagulated (citrate) human plasma was purchased from Innovative Research (Novi, MI). The University of Illinois at Chicago Institutional Review Board (IRB) reviewed the protocol and approved the study as exempt human research.
Preparation of reducible and nonreducible block copolymers.
Reducible block copolymer, PEG-PLA-SS-R15, was synthesized using disulfide exchange reaction. Carboxy-terminal PEG-PLA, EDC and NHS (100, 40 and 24 mg, respectively) were dissolved in DMSO and stirred for 30 min, after which 14 mg PDPH crosslinker was added, thus modifying PEG-PLA with a pyridyldisulfide functional group at the previous carboxyl termini (Figure 1A). Unreacted reagents were removed by dialysis with a membrane with molecular weight cut off of 1,000 g/mol, against deionized water for 24 h. The PEG-PLA-PDPH was recovered from the dialysis tube and lyophilized to a white powder. Successful PDPH modification was confirmed with 1H NMR (Figure S1) in CDCl3 using a 400 MHz Bruker DPX-400 spectrometer (Bruker BioSpin Corp., Billerica, MA). To couple PEG-PLA-PDPH with CR15, 40 mg of PEG-PLA-PDPH and 10 mg CR15 were dissolved in argon degased DMSO and reacted for 6 hours in an argon environment before purification with a Sep-Pak C18 column. Successful coupling, producing PEG-PLA-SS-R15, was confirmed by 1H NMR in DMSO-d6 (Figure 2).39 The guanidine group (-NHCH=NHNH2-, δ 7.43) in CR15 and the proton (-CO-CH(CH3)-O-, δ 5.19) in the PLA segment were present in the NMR spectrum of the final product. The R15 substitution was calculated based on the intensity ratio between the proton peak of the guanidine group and that of the PLA segment, which was approximately 30%.
Figure 2. 1H NMR spectra of (A) CR15 (B) PEG-PLA-COOH, (C) PEG-PLA-R15, and (D) PEG-PLA-SS-R15.

The purified products have characteristic peaks from both guanidine group (δ 7.43, 4H) in R15 and PLA segment (δ 5.19, 1H).
Nonreducing control copolymer, PEG-PLA-R15, was synthesized using thiol-maleimide coupling. Carboxyl terminal PEG-PLA, EDC and NHS (100, 40 and 24 mg, respectively) were dissolved in DMSO and stirred for 30 min, after which 16 mg AEM crosslinker was added, thus modifying PEG-PLA with a maleimide functional group at the previous carboxyl termini (Figure S2). The purification, coupling and confirmation steps were the same as the reducing copolymer. Successful preparation of the PEG-PLA-AEM (Figure S2) and PEG-PLA-R15 copolymers (Figure 2) was confirmed with 1H NMR spectra.
Micelleplex and micelleplex preparation.
Micelles and micelleplexes were prepared as previously reported by our group.34 Briefly, block copolymer, 10 mg, was dissolved in 0.5 mL acetonitrile. Solvent was removed by rotovap to form a polymer film. RNAse free water (0.5 mL) was added to rehydrate the polymer film followed by sonication for 5 min to form empty micelles. To prepare micelleplexes, micelles were diluted by dropwise addition of RNA in RNAse free water (10 μM), sonication for 5 minutes and incubation for 15 min at room temperature.
Micelle and micelleplex characterization.
Diameter and ζ-potential of micelles and micelleplexes were measured in 10 mM HEPES buffer (pH 7.4) with a Nicomp 380 Zeta Potential/Particle Sizer (Particle Sizing Systems, Santa Barbara, CA), the buffer system was chosen due to similarity to cell-culture conditions used in the experiments. Additionally, the morphology of micelles and micelleplexes were characterized by transmission electron microscopy (TEM, JEM-1220, JEOL Ltd., Japan). A drop of micelles (1 mg/mL) was placed on a carbon-coated 300 mesh copper grid, followed by staining with 2% (w/v) uranyl acetate solution prior to drying at room temperature.
Micelle-anti miRNA association.
The interaction of anti-miRNAs with micelles was confirmed by a gel shift assay.34 Micelleplexes were prepared at predetermined positive to negative (+/−) charge ratios, where the charge ratio is calculated by dividing the number of positively charged arginine guanidine groups by the number of negatively charged phosphate groups of the anti-miRNA. The resulting micelleplexes were analyzed by electrophoresis using a 20% non-denaturing polyacrylamide gel for 1 h at 80 V in TBE buffer (89 mM Tris-borate, 2 mM EDTA). Following SYBR gold staining, the gel was visualized using a gel documentation system (GelDoc 2000, Bio-Rad, Hercules, CA).
Stability of micelles and micelleplexes in cerebrospinal fluid.
The stability of micelleplexes was examined utilizing a Förster resonance energy transfer (FRET) based method.40 The FRET dye pair, DiO and DiI, were encapsulated in the hydrophobic core of the micelles by dissolving 1% w/w of DiO and DiI in acetonitrile with block copolymers. Solvent was removed by rotovap to form a polymer matrix, and deionized water was added to rehydrate the polymer film followed by sonication for 5 min to form DiO/DiI-loaded micelles. The un-encapsulated dye precipitate was removed by filtering the micelle solution with 0.45 μm syringe filter. Based on the balance between cellular association efficiency and vehicle cytotoxicity, micelleplexes were then prepared as described above at charge ratio 30. The DiO/DiI-loaded micelleplexes (100 μg/mL, final concentration) were incubated with PBS, CSF and human plasma at 37°C. The volume ratio between micelleplexes solution and medium was 1:9 and the total volume was 1 mL. Time-resolved spectra were collected on a spectrofluorophotometer (RF 1501, Shimadzu, Japan) at excitation wavelength of 484 nm and emission wavelength from 480 to 600 nm. To monitor the relative peak shift between the emission of DiO at a wavelength of 499 nm, I499, and the emission of DiI at a wavelength of 561 nm, I561, the FRET ratio, λ, was calculated (Equation 1).
| Equation 1 |
To predict the extracellular dissociation of anti-miRNA, PEG-PLA-SS-R15/Cy3-anti-miRNA micelleplexes were prepared at charge ratio of 30 in RNAse free water. Micelleplexes containing 50 pmoles of anti-miRNA were added to PBS, human CSF or human plasma at 37 °C, respectively, in a final total volume of 100 μL. MiRNA or micelles, a molar equivalent of what was present in the micelleplexes, were added to PBS, CSF or human plasma and used as positive and negative controls, respectively. The fluorescence was normalized against fluorescence intensity of Cy3-anti-miRNA in the same medium. Corning Black 96 well microplates were sealed and kept in a 37°C incubator between measurements on BioTek Synergy2 Multi-Mode Microplate Reader (Winooski, VT) at an excitation wavelength of 543 nm and emission wavelength of 570 nm.
Cellular association and intracellular anti-miRNA dissociation from micelleplexes.
Cellular association was measured as previously reported.23 Cells (2×105) were seeded on 12-well plates prior to overnight culture in a total media volume 1 mL. Cells were incubated for 4 h in the presence of micelleplexes before washing with cold PBS twice followed by trypsinization and collection by centrifugation at 1500 rpm for 5 min. Cell pellets were washed twice with cold PBS before suspension in 200 μL of 1% formaldehyde and analysis on a Becton Dickinson Fortessa flow cytometer (San Jose, CA).
To monitor the anti-miRNA dissociation from micelleplexes in cytoplasm, U251 cells were seeded in 4-chamber 35-mm glass bottom dish (In Vitro Scientific, Sunnyvale, CA) at 1.5×105 cells per chamber in 500 μL growth media 24 hours before an experiment. The media was exchanged with OPTI MEM and micelleplexes formed from CR15K(FITC)NH2 micelles and Cy3-anti-miRNA were applied to the chamber. Four hours later, cells were rinsed twice with PBS and the medium was replaced with OPTI MEM. After another twenty hours, the nuclei were stained with Hoechst 33258 for 5 min before confocal laser scanning microscopy (CLSM) using Zeiss LSM 510 META (Carl Zeiss, Germany) equipped with a water immersion 63× objective (C-Apochromat, Carl Zeiss). Excitation wavelengths were 405 nm (Diode 405), 488 nm (argon laser) and 543 nm (HeNe laser) for Hoechst 33258, FITC and Cy3, respectively. In order to obtain the 3-dimensional dissociation efficiency, z-stacks comprised of 20 slices wee made through 30 cells and the overlapping signal of the dyes (FITC for the peptide and Cy3 for anti-miRNA) quantified as previously reported.41, 42 The colocalization of Cy3-labeled anti-miRNA and FITC-labeled micelles was using the Mender’s coefficient, □, which describes the of red pixel (Cy3-labeled anti-miRNA) colocalizing with green pixels (FITC-labeled micelles) with an automatic threshold43 in ImageJ. The anti-miRNA dissociation efficiency, εd, was then calculated, Equation 2.
| Equation 2 |
Micelleplex activity.
The efficiency of anti-miRNA delivery was assessed using a miR-21 luciferase reporter assay.44 A miR-21 complementary sequence was engineered in the 3΄-untranslated region of the firefly luciferase gene. In the presence of miR-21, firefly luciferase mRNA is degraded, thereby producing low levels of firefly luciferase. Depending on the delivery efficiency of anti-miR-21, firefly luciferase protein activity increases due to miR-21 inhibition. As a transfection control, a constitutively translated Renilla luciferase expression vector was cotransfected.45, 46 Firefly luciferase and Renilla luciferase selectively convert their substrates, luciferin and coelenterazine respectively, which have non-overlapping luminescent wavelength.
Using this assay system, U251 cells were seeded in white 96 well plates at 10,000 per well. Cells were cultured for 24 to 48 hour to achieve at least 80% confluence at the time of transfection. Prior to transfection, the medium was removed and replaced with CSF containing the micelleplexes. Culturing U251 cells with CSF up to 4 hours did not affect cell viability (data not shown). Four hours after the micelleplex transfection, miR-21 firefly luciferase vector and Renilla control vector was cotransfected with Dharmacon DharmaFECT™ Duo Transfection reagents according to the protocol. Luminescence was measured with Dual-Luciferase® Reporter Assay System (Promega) by following the reagent protocol in a BioTek Synergy2 Multi-Mode Microplate Reader (Winooski, VT).
Statistical analysis.
For all experiments, the data represent the mean plus or minus (±) the standard deviation (SD) from at least three independent experiments, unless otherwise noted For statistical comparison, 1-way ANOVA was applied with Tukey’s post-hoc test to determine groups with statistically significant differences. When appropriate and as noted, 2-way ANOVA was applied to determine which variables were significant.
RESULTS AND DISCUSSION
Characterization of micelleplexes.
We previously showed that the triblock copolymer, PEG-PLA-SS-R15, will form micelles with a core-shell conformation in aqueous environment where the hydrophobic block PLA collapse and the PEG and R15 block intertwine to constitute a hydrophilic corona.34 The oligonucleotides were loaded onto the micelles through electrostatic interaction with the oligoarginine block on the surface. It was expected that both the reducing and nonreducing copolymers form similar micelles with the miRNA loaded in the surface R15/PEG layer.
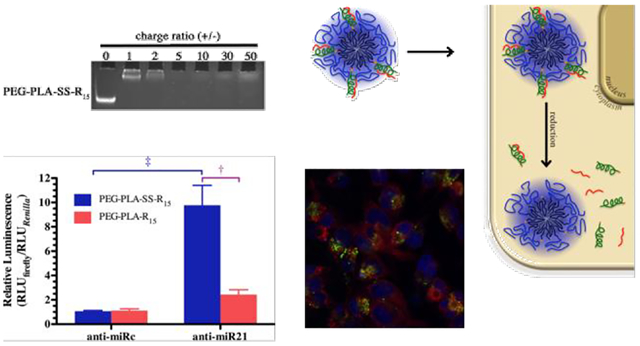
As expected, reducible and nonreducible micelles demonstrated similar anti-miRNA condensing ability due to their similar oligoarginine conjugation rate. Micelleplexes were formed at and above a charge ratio of 5 for both micelles (Figure 3A). Although micelleplexes were formed at charge ratio of 5 for both micelleplex types, we chose to work with a charge ratio of 30 to remain consistent with our previous work.34 We hope to examine a full range of charge ratios in future work.
Figure 3. Micelleplex characterization.
(A) PEG-PLA-SS-R15 and PEG-PLA-R15 micelles complex with anti-miRNA at charge ratios of 1, 2, 5, 10, 30 and 50. (B) Transmission electron micrographs of PEG-PLA-SS-R15 and PEG-PLA-R15 micelles and micelleplexes showing the size and morphology, the scale bar is 100 nm.
The diameter of micelleplexes formed were similar (Table 1) and similar to the size of micelles without anti-miRNA.34 These values are used primarily to compare the systems as the ionic strength of the media is less than would be present in biologic media, i.e. cell culture media, blood, and cerebral spinal fluid. It is well known that the size and ζ-potential are related to the media in which they are measured, and these estimates would change in other conditions. Although the ζ-potential for the nonreducible micelles was significantly lower than the reducible micelles (p = 0.006), both micelleplexes were moderately positively charged. It is not clear why this is the case, although we speculate that both the linker chemistry and small differences in conjugation rate are the cause. Despite this difference, the morphology of reducing and nonreducing micelles before and after loading anti-miRNA retained a spherical shape and sizes that match the size obtained by dynamic light scattering (Figure 3B).
Table 1.
Particle size and zeta potential of PEG-PLA-SS-R15 and PEG-PLA-R15 micelles.
| Diameter (nm) | ζ-Potential (mV) | ||
|---|---|---|---|
| micelle | PEG-PLA-SS-R15 | 20.4±1.5 | 30.5±1.9 |
| PEG-PLA-R15 | 18.2±1.6 | 23.9±1.0 | |
| micelleplex | PEG-PLA-SS-R15 | 19.9±3.0 | 4.95±0.43 |
| PEG-PLA-R15 | 23.3* | 3.11* |
N=1
Stability of micelleplexes in Cerebrospinal Fluid.
Clinical translation of micelles for drug delivery is largely hindered by the stability in biologic fluids.25 Small molecules encapsulated within micelles tend to be rapidly released upon injection in the body due to dilution below critical micelle concentration and interaction with serum components.47 In the case of micelleplexes, the nucleic acids often suffer from early release from the complex due to competition from components in the blood serum.24, 25 Since we have developed the micelleplexes for cerebral administration, we sought to examine micelleplex stability in human CSF as opposed to plasma. To accomplish this, a FRET-based method was used to measure the micelleplex stability. The FRET phenomenon will dissipate upon the release of DiO and/or DiI or during micelleplex disassembly due to separation of the two dyes; FRET is position dependent with optimal transfer at less than 10 nm.48 The FRET ratio, λ, was used to monitor the relative shift in intensity to conventional fluorescence as the DiI and DiO separate, evidenced by a drop in FRET ratio.40 For micelles and micelleplexes incubated in PBS, there was little decrease in FRET ratio, suggesting that the system is stable (Figure 4). In plasma, micelle and micelleplex disassembly occurred immediately after the micelleplexes were mixed with a drop to almost half the original FRET ratio. However, micelles and micelleplexes demonstrated significantly better (p < 0.0001) stability in CSF than in human plasma, with only around 10% lower FRET ratio than in PBS. It should be noted that the 4-hour time point the difference is significant, but the timeframe for augmented stability is not yet known. The stability of reducible micelles, i.e. without anti-miRNA loading, was similar to the reducible micelleplex stability, suggesting anti-miRNA loading did not influence micelle/micelleplex stability (Figure 4B).
Figure 4. Stability of reducible micelleplexes and micelles in different media.

(A) Reducible micelle stability (λ) in PBS, CSF and human plasma, (B) reducible micelleplex stability (λ) in PBS, CSF and human plasma, and (C) stability of the association between Cy3-anti-miRNA from micelleplexes in PBS, CSF and human plasma expressed in relative fluorescence relative to the anti-miRNA in the appropriate biologic fluid. Data points represent the mean plus or minus (±) the standard deviation. Three independent replicates (n=3) of each experiment were performed.
Due to the fact that the DiO/DiI pair was loaded in the micelle core, the FRET-based assay only indicates stability of micelle assembly, but not the electrostatic association between anti-miRNA and micelles. One of the critical challenges facing micelleplexes is the loss of nucleic acids due to competition from complex components in plasma before reaching the disease site.23, 49 To determine the release of anti-miRs, Cy3-anti-miRNA was loaded on the micelleplexes, which resulted in quenching. There was limited background fluorescence when no Cy3-anti-miRNA was present: 8.0±1.0, 6.0±2.6, and 74±7.5 for PBS, CSF, and plasma, respectively. When anti-miRNA released from the micelleplexes, the fluorescence intensity of Cy3-anti-miRNA increased when the nucleic acids dissociated and quenching was diminished due to separation of the Cy3 molecules. The fluorescence intensity of Cy3-anti-miRNA/micelleplexes in PBS was 68.4% that of free Cy3-anti-miRNA in PBS. Fluorescence quenching remained relatively constant, suggesting the anti-miRNAs were tightly condensed by micelleplexes in PBS and was not disrupted in this simple buffer (Figure 4C). The fluorescence intensity of Cy3-anti-miRNA/micelleplexes recovered immediately after mixing with the human plasma, suggesting Cy3-anti-miRNA release from the micelleplexes due to competition of the complex components in plasma. Surprisingly, the fluorescence intensity of Cy3-anti-miRNA/micelleplexes in CSF was observed to be similar to the intensity in PBS at 2 h. Due to the significantly lower protein concentration in CSF, competition was not significant enough to cause anti-miRNA release or micelle disassembly. We believe this is a significant finding as not only will the micelleplex be more stable at the same concentration in CSF, but total CSF volume (approximately 150 mL) in the human body is much lower than blood in the human body (approximately 5 L). This will result in less dilution following injection and even greater relative stability. We plan to further explore the phenomena in the future, but it appears that micelleplexes have a significant level of stability in CSF.
Anti-miRNA dissociation from micelleplexes.
Since the formation and disassembly of micelleplexes require diametrically opposed properties, mechanisms for selective intracellular disassembly of micelleplexes are thought to be necessary to allow stable micelles before cell entry.50, 51 To enhance anti-miRNA release from the micelleplexes after cellular uptake, we designed the micelleplexes with and without a disulfide bond between the PEG-PLA block of the micelles and polyarginine block necessary for anti-miRNA complexation. We hypothesized that the polyarginine block would leave the micelle in the reducing environment of the cytoplasm and facilitate anti-miRNA dissociation from the delivery system.
To test the hypothesis, we fluorescently labeled the micelles on the polyarginine block with FITC and prepared micelleplexes with Cy3-labeled anti-miRNA. The dissociation of Cy3-anti-miRNA from the micelleplexes was monitored with confocal laser live cell microscopy at 4 and 24 hours post transfection. Based upon the images, a similar proportion of the cells contained or interacted with micelleplexes and anti-miRNA (Figure 5). This was further supported by flow cytometry where at a charge ratio of 30:1 reducible and nonreducible micelleplexes associated with 90.0% and 89.7% of cells, respectively. This trend was similar at a charge ratio of 15:1, with 62.2% and 65.7% of cells associating with reducible and nonreducible micelleplexes, respectively. This is compared to 0.3% of cells expressing background florescence and naked Cy3-anti-miRNA associating with 0.2% of cells. However, cell-associated anti-miRNA may not enter the cell or be released from the micelleplex. Therefore, confocal microscopy was used to examine cellular entry and dissociation.
Figure 5. Anti-miRNA dissociates from micelleplexes.
(A) Representative pseudocolored CLSM micrographs of anti-miRNA (Red; Cy3 label) loaded PEG-PLA-R15 and PEG-PLA-SS-R15 micelles (Green; FITC-labeled R15) within cells, where the nucleus (blue) is stained, 4 (top two rows) and 24 h (bottom two rows) after transfection. Micelleplexes were formed at 100 nM anti-miRNA in association with micelles at a charge ratio of 30. The scale bar is 20 μm. (B) Quantitative analysis of micelleplex, anti-miRNA dissociation, εd, for Cy3-anti-miRNA/PEG-PLA-SS-R15 micelleplexes (blue bars) and Cy3-anti-miRNA/PEG-PLA-R15 micelleplexes (red bars) at 4 and 24 h after transfection. Dissociation was calculated for 30 individual cells. Data points represent the average plus or minus (±) the standard deviation were performed where † indicates statistical difference, p < 0.001, between the indicated fluids.
Based upon previous research with reducible dithiol bonds, we expected the bonds to be cleaved on the order of minutes,52–54 although we did not directly measure the reduction of the dithiol bond. The reducing and nonreducing micelleplexes had similar (p > 0.05) RNA dissociation at 4 hours but reducible micelleplexes continued to dissociate (approaching 90% dissociated) over the next 24 hours while the nonreducible micelleplexes remained approximately 50% associated (Figure 5). Whether this dissociation (polyarginine from anti-miRNA) is due to solely to reduction or other mechanisms was not be determined at this time.
Anti-miR-21/micelleplexes efficiency in cerebrospinal fluid.
As an initial assessment of the suitability of the micelleplex system for glioblastoma treatment, we examined anti-miR-21/micelleplexes efficiency using the dual luciferase assay in the U251 glioblastoma cell line. Glioblastoma cells were incubated with micelleplexes or appropriate controls in the presence of CSF for 4 hours before cells were returned to complete media. The treatment regime with micelleplexes exhibited no toxicity (Figure 6). Using CSF as culturing media, reducible micelleplexes expressed greater anti-miR-21 activity than the nonreducible micelleplexes (Figure 7). Taken together, these results clearly demonstrate the influence of the reducing environment on the separation and activity of anti-miRNA from the micelleplex.
Figure 6. Cytotoxicity following reducible and nonreducible micelle treatment of U251 malignant glioma cells.
Varying amounts of free peptide (R15; ●), or equivalent molar peptide amounts of reducible micelles, PEG-PLA-SS-R15 (■), and nonreducible, PEG-PLA-R15 (▲), micelles were incubated with U251 malignant glioma cells and cultured in CSF for 4 hours followed by further incubation in full growth medium for 48 hours, as described for the dual luciferase assay. Data points represent the average plus or minus (±) the standard deviation. Three independent replicates (n=3) of each experiment were performed.
Figure 7. Anti-miR-21/micelleplex efficiency measured with the dual luciferase assay.
The efficiency of reducible micelleplexes (blue bars; PEG-PLA-SS-R15) and nonreducing micelleplexes (red bars; PEG-PLA-R15) were compared following complexation with anti-miR-21 or inactive control anti-miRNA, anti-miRc. U251 glioblastoma cells were then transfected with the micelleplexes in cerebrospinal fluid. The miR-21 sensitive firefly luciferase activity was normalized to the Renilla luciferase activity. Data represent the mean plus or minus (±) the standard deviation of three independent replicates (n=3) for each experiment were performed where † and ‡ indicate statistical difference, p < 0.01, between the indicated micelleplexes or between the control and active miRNA for the same micelleplexes, respectively.
Release from delivery carrier is a rate-limiting step for oligonucleotide delivery, and our results demonstrate that reducible micelleplexes exhibit selective intracellular disassembly. Three-dimension quantification with confocal microscopy supports our hypothesis that dithiol reduction leads to increased anti-miRNAs dissociation from micelleplexes within the cells. However, the enhanced anti-miRNAs efficiency of reducible micelleplexes observed in the dual luciferase assay may not be exclusively due to increased anti-miRNAs release. When the reducible micelleplexes responded to the reducing milieu in cells, it is possible that the anti-miRNA localize to specific sub-cellular regions that allow greater activity. The fact that the control anti-miRNA does not elicit activity clearly shows that release oligoarginine does not have activity.
While it is well established that reducible delivery systems, including micelles, can be used for intracellular dissociation,36, 37 we demonstrated, for the first time to our knowledge, stability and transfection efficiency of micelleplexes in CSF. Upon contact with biologic fluids, nanoparticles are confronted with complex proteins, which form a coating on nanoparticle surface known as the protein corona.55 It is now well accepted that a bare nanoparticle does not exist in vivo.56, 57 The corona forms rapidly after mixing with human plasma. The corona formed influences hemolysis, thrombocyte activation, nanoparticle phagocytosis, and endothelial cell survival immediately after exposure.58 This corona also disrupts surface-bound materials, including DNA and RNA. To better understand the potential clinical impact, we initially examined this system in blood (plasma and serum) and observed rapid dissociation of the micelles and micelleplexes, confirming observations by many others. However, the more relevant environment, CSF, has not been examined and the influence on this unique biologic fluid on stability and transfection warrants examination. Since GBM treatment can be intracerebral, we believe that the results obtained suggest that this route of administration may yield stabile, active particles following administration. Future investigations will focus on the stability of micellar, and other nanoparticulate systems, in CSF and in live brains in vivo.
4. CONCLUSIONS
A reducible micelle-like delivery system for miRNA therapy was designed and characterized. The reducible micelleplexes were found to contribute significantly to efficiency due to enhanced cargo release and separation from the cationic peptides in the cells. In addition to augmented miRNA silencing efficiency, the micelleplexes were nontoxic in the conditions tested and are stable and effective when administered in CSF, suggesting potential for in vivo delivery. For the first time, we demonstrated that micelleplexes have better stability in CSF than in human plasma, suggesting potential advantage of applying such delivery system for brain disease and circumventing the stability challenge that have plagued systemically administered micelle-based drug delivery.
Supplementary Material
Acknowledgements
This investigation was conducted in a facility constructed with support from Research Facilities Improvement Program Grant (C06 RR015482) from the National Centre for Research Resources of the National Institutes of Health (NIH). This research has been funded, in part, by the University of Illinois at Chicago Center for Clinical and Translational Science (CCTS) award supported by the NCRR (UL1 TR000050, RAG & JSB), the Chicago Biomedical Consortium with support from the Searle funds at the Chicago Community Trust (JER), and the University of Illinois at Chicago Graduate Fellowships (JER, JSB, YZ). Additionally, the authors thank Dr. Hayat Onyuksel for use of equipment.
Footnotes
Supporting Information. The Supporting Information is available free of charge on the ACS Publications website. 1H NMR spectra of mPEG-PLA-PDP and mPEG-PLA-AEM (PDF) and flow cytometry analysis of Cy3-labelled anti-miRNA association with cells.
The authors declare no competing financial interest.
References
- 1.Omuro A; DeAngelis LM Glioblastoma and other malignant gliomas: a clinical review. JAMA-J. Am. Med. Assoc 2013, 310, (17), 1842–50. [DOI] [PubMed] [Google Scholar]
- 2.Mrugala MM; Adair J; Kiem HP Temozolomide: Expanding its role in brain cancer. Drugs Today. 2010, 46, (11), 833–46. [DOI] [PubMed] [Google Scholar]
- 3.Blanchette M; Fortin D Blood-brain barrier disruption in the treatment of brain tumors. Methods Mol Biol. 2011, 686, 447–63. [DOI] [PubMed] [Google Scholar]
- 4.Fine HA New strategies in glioblastoma: exploiting the new biology. Clin Cancer Res. 2015, 21, (9), 1984–8. [DOI] [PubMed] [Google Scholar]
- 5.Gill BJ; Pisapia DJ; Malone HR; Goldstein H; Lei L; Sonabend A; Yun J; Samanamud J; Sims JS; Banu M; Dovas A; Teich AF; Sheth SA; McKhann GM; Sisti MB; Bruce JN; Sims PA; Canoll P MRI-localized biopsies reveal subtype-specific differences in molecular and cellular composition at the margins of glioblastoma. Proc Natl Acad Sci U S A. 2014, 111, (34), 12550–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen L; Chaichana KL; Kleinberg L; Ye X; Quinones-Hinojosa A; Redmond K Glioblastoma recurrence patterns near neural stem cell regions. Radiother Oncol. 2015, 116, (2), 294–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morshed RA; Cheng Y; Auffinger B; Wegscheid ML; Lesniak MS The potential of polymeric micelles in the context of glioblastoma therapy. Front Pharmacol. 2013, 4, 157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lesniak MS; Brem H Targeted therapy for brain tumours. Nat. Rev. Drug Discov 2004, 3, (6), 499–508. [DOI] [PubMed] [Google Scholar]
- 9.Fire A; Xu S; Montgomery MK; Kostas SA; Driver SE; Mello CC Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature. 1998, 391, (6669), 806–11. [DOI] [PubMed] [Google Scholar]
- 10.Pushparaj PN; Aarthi JJ; Manikandan J; Kumar SD siRNA, miRNA, and shRNA: in vivo Applications. J Dent Res. 2008, 87, (11), 992–1003. [DOI] [PubMed] [Google Scholar]
- 11.Tong AW; Nemunaitis J Modulation of miRNA activity in human cancer: a new paradigm for cancer gene therapy? Cancer Gene Ther. 2008, 15, (6), 341–355. [DOI] [PubMed] [Google Scholar]
- 12.Zhang Y; Wang ZJ; Gemeinhart RA Progress in microRNA Delivery. J. Control. Release 2013, 172, (3), 962–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garzon R; Marcucci G; Croce CM Targeting microRNAs in cancer: rationale, strategies and challenges. Nat Rev Drug Discov. 2010, 9, (10), 775–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bader AG; Brown D; Winkler M The promise of microRNA replacement therapy. Cancer Res. 2010, 70, (18), 7027–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chan JA; Krichevsky AM; Kosik KS MicroRNA-21 is an antiapoptotic factor in human glioblastoma cells. Cancer Res. 2005, 65, (14), 6029–33. [DOI] [PubMed] [Google Scholar]
- 16.Gabriely G; Wurdinger T; Kesari S; Esau CC; Burchard J; Linsley PS; Krichevsky AM MicroRNA 21 promotes glioma invasion by targeting matrix metalloproteinase regulators. Mol Cell Biol. 2008, 28, (17), 5369–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gaur AB; Holbeck SL; Colburn NH; Israel MA Downregulation of Pdcd4 by mir-21 facilitates glioblastoma proliferation in vivo. Neuro Oncol. 2011, 13, (6), 580–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hermansen SK; Dahlrot RH; Nielsen BS; Hansen S; Kristensen BW MiR-21 expression in the tumor cell compartment holds unfavorable prognostic value in gliomas. J Neurooncol. 2013, 111, (1), 71–81. [DOI] [PubMed] [Google Scholar]
- 19.Moore LM; Zhang W Targeting miR-21 in glioma: a small RNA with big potential. Expert Opin Ther Targets. 2010, 14, (11), 1247–57. [DOI] [PubMed] [Google Scholar]
- 20.Papagiannakopoulos T; Shapiro A; Kosik KS MicroRNA-21 targets a network of key tumor-suppressive pathways in glioblastoma cells. Cancer Res. 2008, 68, (19), 8164–72. [DOI] [PubMed] [Google Scholar]
- 21.Asangani IA; Rasheed SA; Nikolova DA; Leupold JH; Colburn NH; Post S; Allgayer H MicroRNA-21 (miR-21) post-transcriptionally downregulates tumor suppressor Pdcd4 and stimulates invasion, intravasation and metastasis in colorectal cancer. Oncogene. 2008, 27, (15), 2128–36. [DOI] [PubMed] [Google Scholar]
- 22.Frankel LB; Christoffersen NR; Jacobsen A; Lindow M; Krogh A; Lund AH Programmed cell death 4 (PDCD4) is an important functional target of the microRNA miR-21 in breast cancer cells. J Biol Chem. 2008, 283, (2), 1026–33. [DOI] [PubMed] [Google Scholar]
- 23.Zhang Y; Köllmer M; Buhrman JS; Tang MY; Gemeinhart RA Arginine-rich, Cell Penetrating Peptide-anti-microRNA Complexes Decrease Glioblastoma Migration Potential. Peptides. 2014, 58, (1), 83–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Navarro G; Pan J; Torchilin VP Micelle-like nanoparticles as carriers for DNA and siRNA. Mol Pharmaceutics. 2015, 12, (2), 301–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim S; Shi Y; Kim JY; Park K; Cheng JX Overcoming the barriers in micellar drug delivery: loading efficiency, in vivo stability, and micelle-cell interaction. Expert Opin Drug Deliv. 2010, 7, (1), 49–62. [DOI] [PubMed] [Google Scholar]
- 26.Pearson RM; Hsu H.-j.; Bugno J; Hong S Understanding nano-bio interactions to improve nanocarriers for drug delivery. MRS Bull. 2014, 39, (03), 227–237. [Google Scholar]
- 27.Brunzel NA, Fundamentals of Urine and Body Fluid Analysis. 3rd ed.; Saunders: St. Louis, MI, USA, 2013. [Google Scholar]
- 28.Manchester IR; Andersson KS; Andersson N; Shiriaev AS; Eklund A A nonlinear observer for on-line estimation of the cerebrospinal fluid outflow resistance. Automatica. 2008, 44, (5), 1426–1430. [Google Scholar]
- 29.Kabat EA; Moore DH; Landow H An Electrophoretic Study of the Protein Components in Cerebrospinal Fluid and Their Relationship to the Serum Proteins. J Clin Invest. 1942, 21, (5), 571–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim DW; Kim SY; Kim HK; Kim SW; Shin SW; Kim JS; Park K; Lee MY; Heo DS Multicenter phase II trial of Genexol-PM, a novel Cremophor-free, polymeric micelle formulation of paclitaxel, with cisplatin in patients with advanced non-small-cell lung cancer. Ann Oncol. 2007, 18, (12), 2009–14. [DOI] [PubMed] [Google Scholar]
- 31.Kim SC; Kim DW; Shim YH; Bang JS; Oh HS; Kim SW; Seo MH In vivo evaluation of polymeric micellar paclitaxel formulation: toxicity and efficacy. J Control Release. 2001, 72, (1–3), 191–202. [DOI] [PubMed] [Google Scholar]
- 32.Lee JL; Ahn JH; Park SH; Lim HY; Kwon JH; Ahn S; Song C; Hong JH; Kim CS; Ahn H Phase II study of a cremophor-free, polymeric micelle formulation of paclitaxel for patients with advanced urothelial cancer previously treated with gemcitabine and platinum. Invest New Drugs. 2012, 30, (5), 1984–90. [DOI] [PubMed] [Google Scholar]
- 33.Lee KS; Chung HC; Im SA; Park YH; Kim CS; Kim SB; Rha SY; Lee MY; Ro J Multicenter phase II trial of Genexol-PM, a Cremophor-free, polymeric micelle formulation of paclitaxel, in patients with metastatic breast cancer. Breast Cancer Res Treat. 2008, 108, (2), 241–50. [DOI] [PubMed] [Google Scholar]
- 34.Zhang Y; Liu Y; Sen S; Král P; Gemeinhart RA Charged Group Surface Accessibility Determines Micelleplexes Formation and Cellular Interaction. Nanoscale. 2015, 7, (17), 7559–7564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bazile D; Prud’homme C; Bassoullet MT; Marlard M; Spenlehauer G; Veillard M Stealth Me. PEG-PLA nanoparticles avoid uptake by the mononuclear phagocytes system. J Pharm Sci. 1995, 84, (4), 493–8. [DOI] [PubMed] [Google Scholar]
- 36.Banerjee R Redox outside the box: linking extracellular redox remodeling with intracellular redox metabolism. J Biol Chem. 2012, 287, (7), 4397–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moriarty-Craige SE; Jones DP Extracellular thiols and thiol/disulfide redox in metabolism. Annu Rev Nutr. 2004, 24, 481–509. [DOI] [PubMed] [Google Scholar]
- 38.Cheng R; Feng F; Meng F; Deng C; Feijen J; Zhong Z Glutathione-responsive nano-vehicles as a promising platform for targeted intracellular drug and gene delivery. Journal of controlled release: official journal of the Controlled Release Society. 2011, 152, (1), 2–12. [DOI] [PubMed] [Google Scholar]
- 39.Zhao ZX; Gao SY; Wang JC; Chen CJ; Zhao EY; Hou WJ; Feng Q; Gao LY; Liu XY; Zhang LR; Zhang Q Self-assembly nanomicelles based on cationic mPEG-PLA-b-Polyarginine(R15) triblock copolymer for siRNA delivery. Biomaterials. 2012, 33, (28), 6793–807. [DOI] [PubMed] [Google Scholar]
- 40.Lu J; Owen SC; Shoichet MS Stability of Self-Assembled Polymeric Micelles in Serum. Macromolecules. 2011, 44, (15), 6002–6008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Akita H; Ito R; Khalil IA; Futaki S; Harashima H Quantitative three-dimensional analysis of the intracellular trafficking of plasmid DNA transfected by a nonviral gene delivery system using confocal laser scanning microscopy. Mol Ther. 2004, 9, (3), 443–51. [DOI] [PubMed] [Google Scholar]
- 42.El-Sayed A; Khalil IA; Kogure K; Futaki S; Harashima H Octaarginine- and octalysine-modified nanoparticles have different modes of endosomal escape. J Biol Chem. 2008, 283, (34), 23450–61. [DOI] [PubMed] [Google Scholar]
- 43.Bolte S; Cordelieres FP A guided tour into subcellular colocalization analysis in light microscopy. J Microsc. 2006, 224, (Pt 3), 213–32. [DOI] [PubMed] [Google Scholar]
- 44.Robertson B; Dalby AB; Karpilow J; Khvorova A; Leake D; Vermeulen A Specificity and functionality of microRNA inhibitors. Silence. 2010, 1, (1), 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zeng Y; Cullen BR Sequence requirements for micro RNA processing and function in human cells. RNA. 2003, 9, (1), 112–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Grentzmann G; Ingram JA; Kelly PJ; Gesteland RF; Atkins JF A dual-luciferase reporter system for studying recoding signals. RNA. 1998, 4, (4), 479–86. [PMC free article] [PubMed] [Google Scholar]
- 47.Owen SC; Chan DPY; Shoichet MS Polymeric micelle stability. Nano Today. 2012, 7, (1), 53–65. [Google Scholar]
- 48.Valeur B; Berberan-Santos MN, Molecular fluorescence: principles and applications. Second edition. ed.; Wiley-VCH: Weinheim, Germany, 2012; p xxi, 569 pages. [Google Scholar]
- 49.Zheng M; Pavan GM; Neeb M; Schaper AK; Danani A; Klebe G; Merkel OM; Kissel T Targeting the blind spot of polycationic nanocarrier-based siRNA delivery. ACS Nano. 2012, 6, (11), 9447–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hwang HS; Kang HC; Bae YH Bioreducible polymers as a determining factor for polyplex decomplexation rate and transfection. Biomacromolecules. 2013, 14, (2), 548–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen C-K Biodegradable Polymeric Materials for Gene and Drug Delivery. Ph.D., State University of New York; at Buffalo, Ann Arbor, 2013. [Google Scholar]
- 52.Sun H; Guo B; Cheng R; Meng F; Liu H; Zhong Z Biodegradable micelles with sheddable poly(ethylene glycol) shells for triggered intracellular release of doxorubicin. 2009, 30, (31), 6358–66. [DOI] [PubMed] [Google Scholar]
- 53.Meng F; Hennink WE; Zhong Z Reduction-sensitive polymers and bioconjugates for biomedical applications. 2009, 30, (12), 2180–98. [DOI] [PubMed] [Google Scholar]
- 54.Phillips DJ; Patterson JP; O’Reilly RK; Gibson MI Glutathione-triggered disassembly of isothermally responsive polymer nanoparticles obtained by nanoprecipitation of hydrophilic polymers. 2014, 5, (1), 126–131. [Google Scholar]
- 55.Docter D; Westmeier D; Markiewicz M; Stolte S; Knauer SK; Stauber RH The nanoparticle biomolecule corona: lessons learned - challenge accepted? Chemical Society reviews. 2015, 44, (17), 6094–121. [DOI] [PubMed] [Google Scholar]
- 56.Masserini M Nanoparticles for brain drug delivery. ISRN Biochem. 2013, 2013, 238428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pearson RM; Juettner VV; Hong S Biomolecular corona on nanoparticles: a survey of recent literature and its implications in targeted drug delivery. Front. Chem 2014, 2, Article 108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tenzer S; Docter D; Kuharev J; Musyanovych A; Fetz V; Hecht R; Schlenk F; Fischer D; Kiouptsi K; Reinhardt C; Landfester K; Schild H; Maskos M; Knauer SK; Stauber RH Rapid formation of plasma protein corona critically affects nanoparticle pathophysiology. Nat Nanotechnol. 2013, 8, (10), 772–81. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.







