Abstract
Following the success of the inaugural games, the Microbial Olympics return with a new series of events and microbial competitors. The games may have moved to a new hosting venue, but the dedication to training, fitness, competition (and yes, education and humour) lives on.
Four years have passed since the London games1, where phage burst through to take sprint glory and Rhodobacter dominated in the pool. Where Pseudomonas’s disgrace made MRSA mighty and the common cold took relay gold. Winners have become legend, while valiant losers fade from memory. The next generation comes along rapidly in the world of microbial sport though, and a fresh cohort of competitors now rises. Training hard for selection, our new crop of microbial athletes have been honing their fitness and acquiring the skills needed to compete. With operons switched on and secretion systems sharpened, our heroes are ready to do battle once more. The drums are beating, the torch is lit, let the carnival begin.
As the sun rises over the newly constructed Nature Microbiology stadium, we welcome you to the Microbial Olympics 2016.
Marathon
To win the marathon, athletes need a combination of endurance and speed. Unlike the human marathon of 26.2 miles, the microbial marathon extends across continents and ocean basins. All the participants in this event move by passive means; a flagellum provides no advantage on these spatial scales. A diverse set of contestants toes the starting line, getting ready to conquer this rigorous and exhausting race.
BANG! The starting gun goes off and Zika virus, a late entrant to the event, sprints out to an early lead. This high-profile virus has a home field advantage in Brazil and is a superlight athlete, expressing only one polyprotein. Zika utilizes its mosquito vector to rapidly spread through the human population. Wow, they really can fly! However, the Zika virus fails to outrun public awareness as real-time genomic data (ZEST data portal: https://zika.labkey.com/project/OConnor/begin.view ) slows this public-health emergency. Dispersal of the Zika virus is hampered by strong prevention efforts, and its chances in this marathon fortunately begin to falter.
As Zika begins to stall, the marine thermophilic endospores take the lead at the halfway mark. These Firmicutes thrive in ocean hydrothermal vents, but travel vast distances in cold ocean currents. Once surfing a cold current at speeds up to 9 kph, they form dormant spores. The high endurance capacity of this bacterium proves advantageous in this race, and they maintain a steady lead over the other competitors. But the greatest advantage of these heat-loving bacteria is also their Achilles’ heel. After travelling thousands of kilometres, most are deposited in cold sediments where they can lie dormant for over 4,000 years. While this makes them a useful tracer for bacterial dispersal, without new reproduction they hit the wall2. In the end, the thermophilic endospores lack the finishing kick to break the tape.
The final contestant breaks away towards the finish line by drafting on the trade winds, often topping speeds of 40 kph. Like Kenyan and Ethiopian athletes, this bacterium trains at high altitude. Members of the genus Polaromonas dominate in glacial ice and sediments at high elevations where their diverse metabolic capabilities allow them to capitalize on the available resources. These extreme environments are linked through the upper atmosphere by the movement of air masses. As a result, many Polaromonas phylotypes are cosmopolitan, and the genus displays as much genetic diversity within an environment as across the globe3. Polaromonas captures the top spot on the podium with the thermophilic endospores taking home the silver and Zika, the bronze.
Canoe slalom
We can now go live to our correspondent at the white-water canoe slalom complex, for a report on the day’s events:
There you have it, ladies and gentle-microbes, the 2016 canoe slalom medallists. We’ve seen bacteria from all corners of our watery planet compete in this exceptionally tumultuous Olympic course. And my golly, amidst all the rumours of new strategies to be unveiled at these games, the field did not disappoint. Indeed, these novel adaptations led to surprising upsets. The long-time motility hero, Escherichia coli, twiddled away its chances at the podium, losing this contest of speed and manoeuvrability to a wild-card bug that can’t even swim. Here’s our Microbe Spy News team with this year’s Olympic Champion, Vibrio coralliilyticus, to analyse tonight’s events!
Microbe Spy: Congrats, V. coralliilyticus, on your gold medal. Hey, may we call you V. cor for short?
V. cor: Why, you can even call me Hard Core — a nickname from my fans.
Microbe Spy: Ha ha, would you pose with your medal for us? Flex your flagella, perhaps?
V. cor: Well, unlike the eukaryotic models, bacterial flagella are rigid. I can flex my hook for you, though (wink).
Microbe Spy: Ah yes, the flexible hook! That’s the secret behind ‘the flick’ technique you use to turn4.
V. cor: Exactly, I reverse really hard, forcing my hook to buckle5, in turn causing the flagellar deformation that reorients my banana-shaped canoe body.
Microbe Spy: The flick was initially developed by your Motile Marine teammate, Vibrio alginolyticus, right?
V. cor: [SNP-ily] Sure, V. al was the first to get international recognition with the flick4. But that doesn’t mean others haven’t been using it. In fact, the majority of the Motile Marine team are run-and-reverse flickers5.
Microbe Spy: And most of you competed today with only one paddle, too!
V. cor: Yep, about 90% of the Motile Marine team are devoted to a belief in mono-flagellation6.
Microbe Spy: So, this use of a bacterial flagellum as a propeller and rudder4 exists all over the ocean!
V. cor: Everything is everywhere after all.
Microbe Spy: Alright, let’s zoom in on your specific strategy. Your teammate and silver medallist, Pseudoalteromonas haloplanktis, paddled an average speed of 40 body lengths per second, with burst speeds of up to 5 times that7! Your own split times, V cor, don’t compare… how did you come out on top?
V. cor: Speed is nothing without control. For this reason, marine bacteria train daily in turbulent waters.
Microbe Spy: I understand that P. halo runs long practice sets, tracking algae7 and marine particles8 with great endurance and accuracy. What focuses you so strongly on the finish line?
V. cor: Determination, and mucus. As a coral pathogen, I push everyday against the turbulent flows corals create with their ciliated surfaces9. That attractive smell of the coral mucus layer10 really gets me going.
Microbe Spy: No wonder the local favourite, Azospirillum brasilense, tumbled off the podium. The rooty underground doesn’t seem nearly as vicious as a whirling wall of cilia.
V. cor: Who knows? Fourth place is still an impressive feat.
Microbe Spy: Hey, what did you think of today’s bronze medallist, Xylella fastidiosa. Only with careful visual observation could one see the retractable pili on what otherwise looks like a canoe without a paddle.
V. cor: Yeah, I was surprised to see a non-swimming plant pathogen in the finals. Like, what could olive trees have in common with white-water? But the way that X. fast grabbed the bottom surface with pili and then hauled herself upstream11, against eddies and fast streamlines. that’s some Xceptionally fast twitching!
Microbe Spy: But still not as fast as your final gate. Here’s the video we caught on the Spy Cam (Fig. 1). Gosh, that’s an elegant flick! It’s so much easier to appreciate in slo-mo when in real-time the turn is over in 10 milliseconds5.
Figure 1 |.

Whitewater winner. Gold medallist, Vibrio coralliilyticus, flicks into first, overcoming a strategically diverse field of white-water slalom contenders.
V. cor: Aw, shucks. You know, for all this talk about training. it helps when you end up at just the right place at just the right time.
Microbe Spy: Congratulations again, V. cor, on a stellar series of runs today. We certainly are excited to see the next generation of tricks emerge over these next four years!
V. cor: Thanks, guys! The sport is constantly evolving — keep an eye out for it!
(Biogeochemical) cycling
And it’s over to the velodrome now, where the action is about to get underway.
In the biogeochemical cycling event, teams of two cycle between the two oxidation states of a single element. Of course, speed alone will not bring home the gold, since competitors must power themselves over steep thermodynamic hills throughout the course. This year’s favourites are iron, nitrogen, and sulfur. Carbon was disqualified in the initial heats because too many competitors were on the course. They started out well, with methane being oxidized to CO2 by Methanomirabilis oxyfera12, using nitrate as its electron acceptor, but hundreds of phototrophs, lithoautotrophs, and fermentative organisms with different carbon substrate preferences crowded the exchange zone in advance of the reduction phase. Each was unwilling to yield to Methanosarcina barkeri, which could have reduced CO2 to methane on its own, had its teammates not crowded it out.
Team sulfur, the underdogs, begin slowly as Desulfosarcina variabilis makes the most of the small amount of energy available from the reduction of sulfate to sulfide. However, the team’s secret weapon is the behemoth Beggiatoa sp., whose bulging vacuoles and strong filaments (gained after its winter training regime), rocket the team back into medal contention.
However, team sulfur is easily overtaken by team iron, who pass the baton back and forth easily between Shewanella oneidensis performing iron reduction, and Mariprofundus ferrooxydans13 performing iron oxidation. They go so quickly, it’s almost as if they have a direct electrical connection14! However, neither sulfur nor iron gain as much power from their redox cycling as team nitrogen, for whom Paracoccus denitrificans reduces nitrate and then Nitrosopumilus maritimus15 oxidizes the ammonium back to nitrate.
Wait! In a shocking turn of events, a spectator’s camera reveals that Paracoccus denitrificans is only reducing nitrate to nitrogen, while using a cyanobacterial helper to ‘fix’ the nitrogen (and the race). Team nitrogen is immediately disqualified. Team iron, the new gold medal winners, express their shock at nitrogen’s breach of the public’s trust. However, this team show the true meaning of irony when, a few months later, sales receipts surface for excess sulfide, proving that team iron had doped their race with the abiotic reduction of iron.
Therefore, sulfur stands as the sole medal winning team of this year’s biogeochemical cycling event. However, neither team member is available for a quote, since each is buried under layers of marine sediments.
In response to the cheating scandals, iron and nitrogen have announced that they will forfeit their amateur status to join the professional limnological and oceanographic leagues, where this sort of behaviour is not only condoned, but is essential to the healthy functioning of Earth.
Synchronized swarming
Off to the swarming rink now for an event that, while often maligned as a microbial sport, is both athletically demanding and aesthetically delightful. We leave you in the hands of our enthusiastic team of reviewers.
Reviewer 1: Welcome everyone to the 2016 synchronized swarming! We are very excited for this event to get started, despite the humidity.
Reviewer 2: Today’s contestants will be judged based on how quickly they move atop a semi-solid agar surface. The participating microorganisms are Vibrio parahaemolyticus, Proteus mirabilis and Bacillus subtilis. Each contestant will start in the middle of the arena and will move outwards towards the finish line.
Reviewer 3: Are these organisms even found in the same environment? And where do you find perfectly poured soft agar in nature? This competition has no scientific merit.
Reviewer 2: Let’s overrule reviewer 3 and get back to today’s event. The contestants are gathering in the middle of the arena forming a highly dense centre. Are you ready? Set! Swarm!!!!!
Reviewer 1: Bacillus is off to an early start, doubling its number of flagella and decreasing in cell size16. Bacillus is sweating a surface-wetting agent from all those changes.
Reviewer 2: Bacillus is beginning to swarm! Taking a closer look, Bacillus appears to be forming highly-motile, aligned rafts of cells that push the colony outward. Meanwhile, Proteus is elongating to many times its normal cell length and making too many flagella to count17. They’ve lined up in rafts as well and are now moving quickly across the surface.
Reviewer 1: Is Vibrio giving up?! It’s single polar flagellum is failing under these conditions! Wait, no it seems not. The Vibrio cells are elongating as well and expressing a second flagellar gene system to make lots of lateral flagella18. This competition is really starting to heat up.
Reviewer 2: It certainly is, so much so that the arena is starting to dry out. Look, Bacillus is beginning to struggle on the harder surface; it’s slowing to a crawl.
Proteus cruises past Bacillus! Hold on, what’s this? Proteus just stopped moving19 and appears to be enjoying the scenery? Reviewer 1: Vibrio parahaemolyticus, quickly catches up and takes the lead for the gold!
Reviewer 2: Proteus mirabilis has a bulls-eye on Bacillus, suddenly restarts swarming and takes silver.
Reviewer 1: Bacillus subtilis fights to the finish line for the bronze.
Reviewer 2: Amazing, what a competition. The grace with which these competitors moved across that plate was really something to behold, this year’s competition will become a citation classic.
Fencing
Meanwhile, over in the mixed microbial arts centre, today’s three contestants, Pseudomonas aeruginosa, Vibrio cholerae and Acinetobacter baylyi, are getting ready for the historic final match in bacterial fencing by growing happily in rich media, where they gain strength for the upcoming bouts. All three contestants possess the type six secretion systems (T6SS), which can quickly propel their needle-like blades into competitors20. Making the competition even more interesting is the fact that these blades are also covered with various toxins, not fully disclosed to us. Additionally, each contestant has a set of shields, which may block some of the opponent’s toxins21,22. Since the whole cell is a valid target for these poisoned swords, the match will be an épée discipline. To win a round, the opponent has to éithér die or stop fighting.
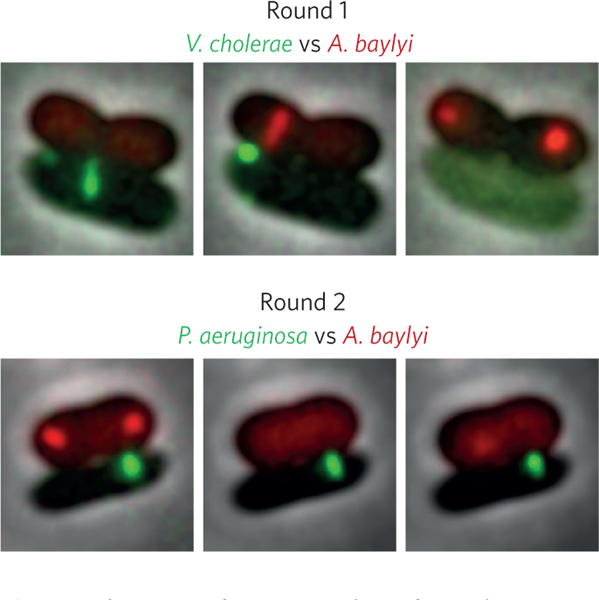
The first round is held between V. cholerae in green and A. baylyi in red (Fig. 2, top). Both combatants are very agile and use up to 1-μm-long weapons. A few minutes into the fight it is clear that their tactics are very similar. These fencers behave as if they were blindfolded and have no way of knowing where their target really is. They poke their swords in all directions all the time23. Clearly they will have to rely on their defence mechanisms to win. The duel has already taken about 30 minutes with both contestants managing to launch about one attack per minute. But now, V cholerae’s defence seems to be weakening. It manages to keep fighting for a while but as A. baylyi continues its attacks with unfading speed and brutality, V. cholerae’s finally succumbs. A. baylyi claims the victory and moves on to the next round.
Figure 2 |.
Fencing foes. Snapshots from the fencing. Top, in round 1 A. baylyi (red) outmuscles V. cholerae (green). Bottom, the swift and accurate counter attacks from P. aeruginosa (green) are too much for A. baylyi (red) in round 2.
For the duel with P aeruginosa in green (Fig. 2, bottom), A. baylyi (again in red) chooses to use the same tactics; surprise its opponent, attack quickly and try to inflict as many wounds as possible. P aeruginosa’s tactics seems to be completely different. At first, it seemed as if P. aeruginosa was passive and did not want to engage in the fight. But after getting hit by A. baylyi’s blade a couple of times, P aeruginosa fiercely responds with an extremely well-coordinated counterattack after just a few tens of seconds. P aeruginosa hits A. baylyi with a fast sequence of precisely aimed strokes, inflicting profound damage24. Numerous similar exchanges follow, A. baylyi constantly attacking without attempting to aim its attacks and P. aeruginosa waiting for the hits and responding with decisive counterattacks. After about half an hour, the fight seems to be drawing to a close. A. baylyi’s defence is fading and eventually it stops fighting completely.
The winner of the tournament is P. aeruginosa! This fencer showed an incredible talent to combine strong defence and calm fighting tactics while waiting for the opponent’s moves but also a devastating ability to strike back quickly and precisely. For this performance, P. aeruginosa deserves the gold medal. A. baylyi takes silver for beating V. cholerae, which leaves with the bronze.
Equestrian
At today’s individual dressage equestrian finals, the gold went to the competitor who showed peerless control over its host’s behaviour and physiology. The crowd was electric after the jumping event where Brazilian native Ophiocordyceps unilateralis hopped between ants, earning top marks25. However, Ophiocordyceps faced steep competition from Wolbachia and Toxoplasma gondii, who both effortlessly performed evolutionarily challenging host jumps.
The intestinal microbiota impressed judges by lowering anxiety, increasing sociability, and reducing depressive symptoms in its murine host26–29. However, the judges followed their gut feelings and disqualified the microbiome, agreeing that the community should have competed in the team event instead of the individual round.
Batrachochytrium dendrobatidis made its Japanese tree frog produce extended and premature mating calls, causing early reproduction30. Amphibious spectators protested with “Save the Frogs!” banners, as Batrachochytrium afflicted lethal effects on their relatives31. Mortality running counter to the Olympic spirit; the controversy and the frog’s early calls resulted in artistic penalties.
Rio’s hometown hero Ophiocordyceps had exquisite form, infecting the ant through spores, guiding the zombie-ant to climb a tree, causing a strong bite on a leaf vein, and sprouting a stroma32. This bold routine caught the judges’ attentions, especially the precise mandibular control, but the ant was rendered immobile. Since liveliness is central to dressage, Ophiocordyceps went home with the bronze.
Toxoplasma gondii got off to a fantastic start, infiltrating the brain of its murine steed by lysing infected vascular endothelial cells33. During judging, Toxoplasma drove the mouse to lose aversion to a feline fan34. The cat devoured the mouse, and Toxoplasma reproduced in the cat’s intestine35. Despite this unbelievable cat- and-mouse game, the technically difficult routine earned a high score for crossing the blood-brain barrier, and Toxoplasma walked away with a silver medal.
Looking back at Wolbachia’s routine, this bacterium infected a crustacean and, incredibly, inhibited male endocrine gland development36. In a dramatic change, the crustacean transitioned into a functional female! Recall the equestrian event is the only Olympic event where both genders compete equally; the crustacean also produced twice as many offspring as uninfected females. Considering the high technical difficulty and artistic appeal to the judges, Wolbachia is the gold medallist today. Away from the show ring, Wolbachia’s pandemic influence cannot be denied. The worthy sportsman, er, sportswoman, infects more than 106 insect species, and is a true humanitarian, reducing dengue virus susceptibility in mosquitos while promoting females in sports and science37,38.
Triathlon
A series of physical and biochemical challenges await our competitors in the triathlon, where the gold medal goes to the bacterium which swims, cycles, and runs to the finish line first. Candidatus Ovobacter propellens is a favourite due to its world record swimming speed conferred by an effortlessly cool pompadour of several hundred flagella39. Escherichia coli and Bacillus subtilis will be wondering if too many generations in cosy lab conditions have weakened their competitive instincts.
Nearly anoxic conditions await the competitors at the starting line for the swim, so aerotaxis — swimming towards higher oxygen concentrations — will be important in this stage. Ovobacter starts quickly, swimming several hundred body lengths in just a few seconds, but struggles with its transition into the oxygen saturated cycling event. E. coli and B. subtilis simultaneously reach the swim exit, leaving the oxygen shy Ovobacter in their wake.
In the Krebs cycling stage, carbon must be pushed through the gears of metabolism. Glucose flows to each competitor and just a few turns of the cycle lead to a build-up of reducing equivalents. Both cells realize that biomass synthesis is the ideal electron sink; with new progeny cycling more carbon this is now a race of populations.
Just as a new generation of cells emerge the growth medium is changed, forcing the cycling populations to shift gears from glucose to acetate metabolism! This should favour E. coli, which can make biomass via the glyoxylate shunt while B. subtilis cannot. However, only a fraction of E. coli cells actually shift gears to new population growth on acetate, those which are growing slightly slower on glucose40. Phenotypic heterogeneity in metabolic flux — variation that exists between genetically identical cells under identical environmental conditions — lets the population hedge its bet, with subsets of cells both growing and persisting during this environmental change. The final burst of population growth for E. coli on acetate puts it into the lead as the species transition to the final running stage.
Both E. coli and B. subtilis populations use a swarming strategy on the solid agar run41, but as seen in the pool for the synchronized swarming event, they have trouble as the agar becomes dehydrated. Slowly a few B. subtilis cells appear to slide, and this time phenotypic variation within a clonal population is benefiting B. subtilis. Some cells secrete surfactant, others produce matrix, and the two cell types divide labour to build ‘van Gogh’ bundles, tightly aligned chains of cells that migrate42. A combination of growth and matrix production push B. subtilis across the surfactant-slicked surface to a gold medal finish! E. coli gets the silver for making it further than Ovobacter, whose fastidiousness leaves it in bronze medal position. From these results, it is clear the collaboration of phenotypically distinct individuals in a clonal population can help bacteria achieve more than was possible alone!
Long jump
It’s a windy day here at the Olympic long jump event, and unfortunately we’re still waiting for some action. So far, it’s a near tie between all eight contestants, with a maximum jumping distance of 1 (± 1) micrometre. We’ve seen some good efforts so far, but the judges aren’t impressed.
Escherichia coli is at the starting line for its third and final attempt. It looks determined — it’s not just here for a random walk. Final attempt… pressure’s on… E. coli is running toward the pit, and oh no, it seems to have rotated its peritrichous flagella clockwise, tumbling off the track entirely. That’s got to be disappointing.
Next up is Myxococcus xanthus. This is the last chance for Myxo, after disqualifications for both adventurous and social gliding motility. Judges have agreed that since Myxo fails to lose contact with the ground while gliding, this can’t be considered a jump. Those are the only techniques we’ve seen it use in practice, so what will it try next?
It seems to be forming some kind of fruiting body! That’s an unusual technique, but I don’t see any objection from the judges — maybe they’re using the wrong objective lens. Oh goodness, what a tragedy; the spores are flying in the wrong direction! And they’re really going far, they’ve been picked up by a trade wind — now this is remarkable, our experts extrapolate that within a few days the spores may well have travelled thousands of kilometres. Well, looks like Myxo will set a new record for long-jumping in the negative direction. Extraordinary, but not what we’re looking for here.
And now for the final contestant. In its two previous attempts, Shigella flexneri tried hiding in chicken and raw vegetables hoping to be carried across the jumping pit, but that strategy hasn’t worked too well for it in this competition. Here’s an exciting turn of events, Shigella has used its type IV conjugative pilus to transfer a virulence plasmid to E. coli, just moments before E. coli ran and tumbled into the judge’s carelessly untended coffee mug. The judge doesn’t look so good after E. coli (or is it Shigella?) activates its new Shiga toxins. This contest isn’t over yet, as our competitor is ejected a full metre ahead of the foul line, easily securing the gold!
Uh oh, is yet another twist underway? The judges have ruled that Shigella (or is it E. coli?) has used performance enhancing genes! Now the judges will have to test all of the contestants for horizontal gene transfer (HGT), which is clearly prohibited by the Olympic rules set forth by the eukaryotes. And this is unprecedented… all the contestants have tested positive and are disqualified as a result, except for the intracellular parasite Buchnera aphidicola, which wins the gold, silver and bronze medals. It’s a short-lived victory though, as Buchnera succumbs to the antimicrobial properties of the silver medal, and its inability to adapt via HGT.
Closing ceremony
As the sporting events reach their conclusion, the torch is extinguished bringing the games to a close. To the victors go our congratulations, while our respect and admiration for the commitment and dedication to constant self-improvement is shared amongst all of our valiant competitors. The Microbial Olympics are not just about sporting entertainment however, but also about education and outreach. Using a sporting backdrop is just one way that it is possible to convey often complex details about the microbiological world in language accessible to the layperson. Too often scientists fail to find understandable and creative ways to capture the imagination of the general public and inspire the next generation of scientists and science enthusiasts. If this year’s games are to have a legacy, let it be hoped that anyone who reads the above record considers a little more how science is communicated with the wider public, and how they might encourage greater engagement, despite the often complex nature of the subject matter.
Contributor Information
Michaeline B. Nelson, Department of Ecology and Evolutionary Biology, University of California, Irvine, California, USA.
Alexander B. Chase, Department of Ecology and Evolutionary Biology, University of California, Irvine, California, USA.
Jennifer B. H. Martiny, Department of Ecology and Evolutionary Biology, University of California, Irvine, California, USA.
Roman Stocker, Institute of Environmental Engineering, Department of Civil, Environmental and Geomatic Engineering, ETH Zurich, 8093 Zurich, Switzerland..
Jen Nguyen, Institute of Environmental Engineering, Department of Civil, Environmental and Geomatic Engineering, ETH Zurich, 8093 Zurich, Switzerland..
Karen Lloyd, Department of Microbiology, M409 Walters Life Sciences, Knoxville,Tennessee 37996, USA..
Reid T. Oshiro, Department of Biology, Indiana University, Bloomington, Indiana 47405, USA.
Daniel B. Kearns, Department of Biology, Indiana University, Bloomington, Indiana 47405, USA
Johannes P. Schneider, Focal Area Infection Biology, Biozentrum, University of Basel, Basel, Switzerland.
Peter D. Ringel, Focal Area Infection Biology, Biozentrum, University of Basel, Basel, Switzerland
Marek Basler, Focal Area Infection Biology, Biozentrum, University of Basel, Basel, Switzerland..
Christine A. Olson, Department of Integrative Biology & Physiology, University of California Los Angeles, Los Angeles, California 90095, USA.
Helen E. Vuong, Department of Integrative Biology & Physiology, University of California Los Angeles, Los Angeles, California 90095, USA.
Elaine Y. Hsiao, Department of Integrative Biology & Physiology, University of California Los Angeles, Los Angeles, California 90095, USA.
Benjamin R. K. Roller, ETH Zurich, Department of Environmental Systems Sciences, Zurich, Switzerland and Eawag, Department of Environmental Microbiology, Dubendorf, Switzerland. ETH Zurich, Center for Adaptation to a Changing Environment, Zurich, Switzerland.
Martin Ackermann, ETH Zurich, Department of Environmental Systems Sciences, Zurich, Switzerland and Eawag, Department of Environmental Microbiology, Dubendorf, Switzerland..
Chris Smillie, Center for Microbiome Informatics and Therapeutics, Department of Biological Engineering, Massachusetts Institute of Technology, Cambridge, Massachusetts, USA..
Diana Chien, Center for Microbiome Informatics and Therapeutics, Department of Biological Engineering, Massachusetts Institute of Technology, Cambridge, Massachusetts, USA..
Eric Alm, Center for Microbiome Informatics and Therapeutics, Department of Biological Engineering, Massachusetts Institute of Technology, Cambridge, Massachusetts, USA..
Andrew J. Jermy, Nature Microbiology, 4 Crinan Street, London N1 9XW, UK.
References
- 1.Youle M. et al. Nature Rev. Microbiol 10, 583–588 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Müller AL et al. ISME J 8, 1153–1165 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Darcy JL, Lynch RC, King AJ, Robeson MS & Schmidt SK PLoS ONE 6, e23742 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xie L, Altindal T, Chattopadhyay S. & Wu X. Proc. Natl Acad. Sci. USA 108, 2246–2251 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Son K, Guasto JS & Stocker R. Nature Physics 9, 494–498 (2013). [Google Scholar]
- 6.Leifson E, Cosenza BJ, Murchelano R & Cleverdon RC J. Bacteriol 87, 652–666 (1964). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barbara GM & Mitchell JG FEMS Microbiol. Ecol 44, 79–87 (2003). [DOI] [PubMed] [Google Scholar]
- 8.Stocker R, Seymour JR, Samadani A, Hunt DE & Polz MF Proc. Natl Acad. Sci. USA 105, 4209–4214 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shapiro OH et al. Proc. Natl Acad. Sci. USA 111, 13391–13396 (2014). [Google Scholar]
- 10.Garren M. et al. ISME J 8, 999–1007 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meng YZ et al. J. Bacteriol 187, 5560–5567 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ettwig KF et al. Nature 464, 543–548 (2010). [DOI] [PubMed] [Google Scholar]
- 13.Emerson D. et al. PLoS ONE 8, e667 (2007). [Google Scholar]
- 14.Pirbadian S. et al. Proc. Natl Acad. Sci. USA 111, 12883–12888 (2014). [Google Scholar]
- 15.Könneke M. et al. Nature 437, 543–546 (2005). [DOI] [PubMed] [Google Scholar]
- 16.Mukherjee S. et al. Proc. Natl Acad. Sci. USA 112, 250–255 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoeniger JF M. J. Gen. Microbiol 40, 29–42 (1965). [Google Scholar]
- 18.McCarter L, Hilmen M. & Silverman M. Cell 54, 345–351 (1988). [DOI] [PubMed] [Google Scholar]
- 19.Rauprich O. et al. J. Bacteriol 178, 6525–6538 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ho BT, Dong TG & Mekalanos JJ Cell Host Microbe 15, 9–21 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Durand E, Cambillau C, Cascales E. & Journet L. VgrG,Tae, Tle, and beyond: the versatile arsenal of Type VI secretion effectors. Trends Microbiol 22, 498–507 (2014). [DOI] [PubMed] [Google Scholar]
- 22.Russell AB, Peterson SB & Mougous JD Nature Rev. Microbiol 12, 137–148 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Basler M, Pilhofer M, Henderson GP, Jensen GJ& Mekalanos JJ. Nature 483, 182–186 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Basler M, Ho BT & Mekalanos JJ Cell 152, 884–894 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Evans HC, Elliot SL & Hughes DP PLoS ONE 6, e17024 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Luna RA & Foster JA Curr. Opin. Biotechnol 32, 35–41 (2015). [DOI] [PubMed] [Google Scholar]
- 27.Parashar A. & Udayabanu M. Eur. Neuropsychopharm 26, 78–91 (2016). [DOI] [PubMed] [Google Scholar]
- 28.Desbonnet L, Clarke G, Shanahan F, Dinan TG & Cryan JF Mol. Psychiatry 19, 146–148 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bravo JA et al. Proc. Natl Acad. Sci. USA 108, 16050–16055 (2011). [Google Scholar]
- 30.An D. & Waldman B. Biol. Lett 12, 20160018 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Crawford AJ, Lips KR & Bermingham E. Proc. Natl Acad. Sci. USA 107, 13777–13782 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.de Bekker C, Merrow M. & Hughes DP Integr. Comp. Biol 54, 166–176 (2014). [DOI] [PubMed] [Google Scholar]
- 33.Konradt C. et al. Nature Microbiol 1, 16001 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vyas A, Kim SK, Giacomini N, Boothroyd JC& Sapolsky RM. Proc. Natl Acad. Sci. USA 104, 6442–6447 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dubey JP, Lindsay DS & Speer CA Clin. Microbiol. Rev 11, 267–299 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Merçot H. & Poinsot DC R. Biol 332, 284–297 (2009). [DOI] [PubMed] [Google Scholar]
- 37.Ye YH et al. PLoS Neglect. Trop. Dis 9, e0003894 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Werren JH, Baldo L. & Clark ME Nature Rev. Microbiol 6, 741–751 (2008). [DOI] [PubMed] [Google Scholar]
- 39.Fenchel T. & Thar R. FEMS Microbiol. Ecol 48, 231–238 (2004). [DOI] [PubMed] [Google Scholar]
- 40.Kotte O, Volkmer B, Radzikowski JL & Heinemann M. Mol. Syst. Biol 10, 736–736 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kearns DB Nature Rev. Microbiol 8, 634–644 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.van Gestel J, Vlamakis H. & Kolter R. PLoS Biol 13, e1002141 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]