Abstract
In aquatic toxicology, perhaps no pharmaceutical has been investigated more intensely than 17alpha-ethinylestradiol (EE2), the active ingredient of the birth control pill. At the turn of the century, the fields of comparative endocrinology and endocrine disruption research witnessed the emergence of omics technologies, which were rapidly adapted to characterize potential hazards associated with exposures to environmental estrogens, such as EE2. Since then, significant advances have been made by the scientific community, and as a result, much has been learned about estrogen receptor signaling in fish from environmental xenoestrogens. Vitellogenin, the egg yolk precursor protein, was identified as a major estrogen-responsive gene, establishing itself as the premier biomarker for estrogenic exposures. Omics studies have identified a plethora of estrogen responsive genes, contributing to a wealth of knowledge on estrogen-mediated regulatory networks in teleosts. There have been ~40 studies that report on transcriptome responses to EE2 in a variety of fish species (e.g., zebrafish, fathead minnows, rainbow trout, pipefish, mummichog, stickleback, cod, and others). Data on the liver and testis transcriptome dominate in the literature and have been the subject of many EE2 studies, yet there remain knowledge gaps for other tissues, such as the spleen, kidney, and pituitary. Inter-laboratory genomics studies have revealed transcriptional networks altered by EE2 treatment in the liver; networks related to amino acid activation and protein folding are increased by EE2 while those related to xenobiotic metabolism, immune system, circulation, and triglyceride storage are suppressed. EE2-responsive networks in other tissues are not as comprehensively defined which is a knowledge gap as regulated networks are expected to be tissue-specific. On the horizon, omics studies for estrogen-mediated effects in fish include: (1) Establishing conceptual frameworks for incorporating estrogen-responsive networks into environmental monitoring programs; (2) Leveraging in vitro and computational toxicology approaches to identify chemicals associated with estrogen receptor-mediated effects in fish (e.g., male vitellogenin production); (3) Discovering new tissue-specific estrogen receptor signaling pathways in fish; and (4) Developing quantitative adverse outcome pathway predictive models for estrogen signaling. As we look ahead, research into EE2 over the past several decades can serve as a template for the array of hormones and endocrine active substances yet to be fully characterized or discovered.
Keywords: Endocrine disruption, pharmaceutical, teleost, computational toxicology, hormone action
1. Introduction
The past twenty years of endocrine disruption research has revealed that several veterinary and human pharmaceuticals are present in aquatic environments at concentrations sufficient enough to elicit adverse effects in a range of species, from the smallest microorganisms up to the largest of aquatic mammals. These waterborne pharmaceuticals can be detrimental to relationships within aquatic food webs [15] and can impact populations for generations [4, 113]. Arguably, no pharmaceutical has received as much scientific nor public attention as 17alpha-ethinylestradiol (EE2), which is used in the pharmaceutical industry as a surrogate for 17beta-estradiol (E2) in birth control pills. In the early 1990s, researchers became increasingly aware of how ubiquitous this pharmaceutical was in water systems, and over the past twenty years, significant efforts have been made in characterizing aquatic EE2 exposure, as well as improving its removal from wastewater treatment facilities in order to protect aquatic wildlife in receiving waters.
There is compelling evidence that EE2 exposure can lead to tissue damage [121], reproductive dysfunction [55], disrupted tissue steroidogenesis [69, 104], altered spawning [23], behavioral changes [94], and population level consequences, as noted by Kidd et al. (2007) in their seminal work in the Experimental Lakes Area [63]. Many of these aforementioned effects were noted at environmentally relevant concentrations of EE2 (less than 5 ng/L). While apical responses in fish to EE2 are well documented, omics studies continue to reveal new molecular and cellular insights into underlying mechanisms. Both research avenues highlight the legacy of EE2 and its negative effects in aquatic organisms, prompting a movement to address other pharmaceuticals present in the aquatic environment. Acknowledging this is important, we continue to produce new pharmaceuticals that not only impact reproduction, but also those that control blood pressure, impede cancer cell growth (i.e. antineoplastics), regulate lipids, and manage depression, to name but a few. In this mini review, we highlight research conducted on the omics of EE2-mediated toxicity in fish to identify knowledge gaps and future directions, and to define directions that research from which other pharmaceuticals can benefit.
2. A problem discovered: A brief history of EE2 in ecotoxicology
In the late 1980s, researchers recognized that estrogenic chemicals were present in various watersheds. Studies associated adverse effects of waterborne estrogenic chemicals with changes in gonad size and reproductive development in fish near pulp mills in Sweden [97] and Canada [82]. Initial studies investigating effluents demonstrated impacts at several points along the pituitary-gonad axis in fish such as the white sucker (Catostomus commersoni) [112], and estrogen receptor activation assays in cell lines revealed estrogenic responses to pulp and paper mill effluent [122]. Additional studies determined that some of these biological responses could be replicated with exposure to β-sitosterol, a plant sterol with estrogen-like activity [70], although the responses were not always identical to those from estrogens as sterols with estrogen-like activities also interfere with other hormonal systems in fishes, depending on the concentration and reproductive maturation of the individual [83]. Studies first started to look at linkages to vitellogenin (described below) and pulp and paper mill effluent in Finland in the late 1990s [103].
Parallel to the studies in Canada on pulp mills, studies in the United Kingdom were finding intersex in fish associated with sewage effluent discharges. A low incidence of intersex had been noticed back to the mid-1980s [111]. While early studies suspected estrogens (reviewed in [90]), initial attention focused largely on the role of detergents and alkylphenols [59], although it quickly expanded to examine other chemicals. By 1996–98, attention was shifting to estrogens in the effluent [110] and studies found widespread evidence of intersex in fish near sewage outfalls [40, 58]. Concerns are now global, agreed upon by many that xenoestrogens, specifically the pharmaceutical EE2, can exert widespread reproductive effects in aquatic wildlife [77].
The question morphed from incidence to biomarker development for estrogenic exposure and potential endocrine activity. Importantly, teleost fish produce vitellogenin in the liver, which travels through the blood to the ovary. Vitellogenin is largely under the control of estrogens and is eventually incorporated into growing oocytes via receptor-mediated actions [31, 100]. Vitellogenin and other lipids in the yolk sac provide a rich source of nutrients for embryos, and the early development of fish depends upon an adequate source of lipids for energy. Sumpter and Jobling [105] pointed out that this endogenous reproductive process in female fish could be used as a biomarker for estrogenic exposures in environments. This was a breakthrough in ecotoxicology - males also carry the genes to naturally produce vitellogenin, albeit at relatively low levels compared to females. However, when males are exposed to weak estrogens or estrogens at low concentrations, vitellogenin can be rapidly and actively transcribed at elevated levels. Thus, vitellogenin induction, both at the messenger and protein level, became a reliable and robust biomarker for estrogenic exposures; even today it remains the gold standard as a molecular indicator of exposure in aquatic organisms [3, 10, 25, 34, 109, 123]. Building upon this, researchers realized that additional estrogen-responsive genes needed to be identified to understand the molecular basis of estrogenic actions [54]. Over the past decade, a repertoire of estrogen-responsive genes has been revealed in teleost fish, and these responses have advanced our understanding of endogenous processes regulated by estrogens, as well as the potential health impacts of exogenous endocrine active substances.
3. Transcriptomics and adverse outcome pathways
Toxicology research over the years has shifted from measuring lethality using high doses of contaminants to investigating sub-lethal doses that potentially impact development, reproduction, health, and susceptibility to disease. This focus on mechanistic models of toxicology expanded at the same time that “omics” technologies were being developed in human medicine to provide a more comprehensive analysis of molecular impairments that lead to disease. The holistic approach that these methods provide spurred the idea of being able to link a molecular initiating event to downstream changes at the cellular, tissue, organismal, and population level for aquatic organisms. This new linkage paradigm, referred to as “Adverse Outcome Pathways (AOP)” [5], has reshaped how ecotoxicologists think about pharmaceuticals in the environment. In fact, it can be thought of as a new way of approaching effects-based toxicology.
Omics technologies today include broad methods that evaluate whole genomes (DNA), transcriptomes (mRNAs and non-coding RNAs), proteomes (proteins) and metabolomes (metabolites) that are altered upon interaction with a chemical substance. Omics technologies deliver specific and relevant information at the molecular level about how a compound interacts with its target, and many of the earlier case studies in environmental science involved endocrine active substances that were known to interact with nuclear receptors and induce gene transcription. The estrogen receptor itself is induced in the liver, along with a large number of other genes that are regulated by estrogens or estrogen mimics. The initial binding of an estrogen to the estrogen receptor sets a particular pathway in motion. Thus, it has been a broadly applied tool to identify estrogen responsive genes in multiple species and tissues.
Transcriptomics methods rely on genome-wide measurements of changes in the levels of mRNAs in tissues of exposed animals. For example, microarrays are made commercially, with the probes printed directly to the glass slides with knowledge of the coordinates for each. RNA that is extracted from controls and chemical treated organisms is obtained, copied into cDNA and labeled with a fluorescent probe and then hybridized to the arrays. The amount of fluorescent probe per spot on the array is used to quantify the amount of the message present in the tissue of origin. The ability to print thousands of cDNA or oligonucleotides onto a glass slide paved the way to generate a wealth of molecular data, increasing understanding of E2-responsive genes and networks in a variety of fish tissues. Only recently and within the past few years, researchers have moved toward the use of RNA sequencing (RNAseq) to study endocrine-mediated responses. RNAseq is a non-targeted method for determining changes in the transcriptome. In this method, total RNA is prepared, and mRNAs with poly A tails are sequestered from the total RNA by binding to poly dT oligonucleotides attached to magnetic beads. The mRNAs are then converted to cDNAs, fragmented and prepared for sequencing by Next Generation Sequencers. For this process, the Illumina sequencer is most often selected today due to sample cost and experimental throughput, although other platforms (e.g. PacBio) are becoming more popular. The analysis of the data includes matching the reads to a reference genome and then counting how many copies of each transcript is present in the output. Put together, both microarray and RNAseq technologies have advanced our understanding of EE2 action in fish, and we outline some of these efforts below.
4. Omics, 17alpha-ethinylestradiol, and fish
At the turn of the century, researchers began to apply gene array technology to study xenoestrogens. These earlier efforts involved the printing of cDNA molecules onto nylon membranes; radiolabeled pieces of cDNA would find their complementary targets on the nylon membrane, yielding a signal that was proportional to the expression levels of the cDNAs present in the sample. Typically, these membranes, or macroarrays, contained less than 50 genes and included transcripts expected to be responsive to xenoestrogens. These early macroarrays were applied to screen estrogens and EE2 in sheepshead minnow (Cyprinodon variegatus variegatus) [68] and plaice (Pleuronectes platessa) [16] and opened the door for more elaborate printing technologies onto glass slides (i.e. microarrays). In teleost fish, microarrays were also quickly leveraged to study the effects of EE2 in the mid-2000s. Microarrays containing 15,000 to 64,000 probes were used to assess environmental estrogens, quantifying molecular responses in context of phenotypic anchors. Taking a step back, we conducted a search using Pubmed in June of 2019 using the terms “fish + ethinyl estradiol + transcriptomics or microarray” and identified 39 published transcriptomic studies in fish; some of these first studies were published in 2006 and as of 2019, there were new reports published on the actions of EE2 at the transcriptome level (Table 1). These new studies leverage RNA-seq approaches, a more robust technology with higher sensitivity and accuracy compared to microarrays. Due to increased depth, RNAseq is expected to reveal new pathways and E2-responsive interactomes involving EE2 and other xenoestrogens.
Table 1:
Studies investigating 17alpha-ethinylestradiol in fish tissues using transcriptomics approaches (i.e. specifically microarray and next generation sequencing).
| Species | Scientific Name | Year | Tissue | Sex | Marine or Freshwater | Life stage | Type of study | Method | Publication |
|---|---|---|---|---|---|---|---|---|---|
| Chub mackerel | Scomber japonicus | 2019 | liver | male | saltwater | prob adult based on size | lab | RNA-seq | [91] |
| Japanese medaka | Oryzias latipes | 2019 | whole body | male | freshwater | embryos-larvae | lab | RNA-seq | [1] |
| Pacific sardine | Sardinops sagax | 2019 | liver | male | saltwater | prob adult based on size | lab | RNA-seq | [91] |
| Rainbow trout | Oncorhynchus mykiss | 2019 | posterior kidney | unknown | freshwater | adults (160 days at least) | lab | RNA-seq | [9] |
| Atlantic cod | Gadus morhua | 2018 | liver slice exposure | male, female | saltwater | juveniles | lab | RNA-seq | [120] |
| Zebrafish | Danio rerio | 2018 | liver | male | freshwater | adult | lab | microarray and RNA-seq | [52] |
| Zebrafish | Danio rerio | 2018 | testis | male | freshwater | adult | lab | RNA-seq | [87] |
| Fathead minnow | Pimephales promelas | 2017 | liver | male | freshwater | adult | lab | microarray | [38] |
| Guppy | Poecilia reticulata | 2017 | whole brain | male, female | freshwater | adult | lab | RNA-seq | [96] |
| Rare minnow | Gobiocypris rarus | 2017 | gonads | male, female | freshwater | adult | lab | RNA-seq | [41] |
| Three-spined stickleback | Gasterosteus aculeatus | 2017 | liver | male | freshwater | likely mixed | wild-caught, lab exp. | microarray | [119] |
| Zebrafish | Danio rerio | 2017 | whole brain | male, female | freshwater | adult | lab | RNA-seq | [88] |
| Fathead minnow | Pimephales promelas | 2016 | testis | male | freshwater | adult | lab | microarray | [37] |
| Three-spined stickleback | Gasterosteus aculeatus | 2016 | testis | male | freshwater | adult | lab | microarray | [89] |
| Gulf pipefish | Syngnathus scovelli | 2015 | liver | male, female | saltwater | adult | wild-caught, lab exp. | RNA-seq | [92] |
| Korean rose bitterling | Rhodeus uyekii | 2015 | liver, skin | male | freshwater | adult? | wild-caught, lab exp. | RNA-seq | [65] |
| Rainbow trout | Oncorhynchus mykiss | 2015 | hepatocytes | unknown | freshwater | juvenile | lab | microarray | [53] |
| Rainbow trout | Oncorhynchus mykiss | 2015 | testis | male | freshwater | juvenile | lab | microarray | [27] |
| Rainbow trout | Oncorhynchus mykiss | 2015 | testis | male | freshwater | juvenile | lab | microarray | [26] |
| Largemouth bass | Micropterus salmoides | 2014 | liver, ovary | female | freshwater | adult | lab | microarray | [20] |
| Yellow catfish | Pelteobagrus fulvidraco | 2014 | gonads | male, female, super male | 1 year old | lab | RNA-seq | [57] | |
| Coho salmon | Oncorhynchus kisutch | 2013 | pituitary | female | saltwater | sub-adult | lab | RNA-seq | [46] |
| Mummichog | Fundulus heteroclitus | 2013 | liver | female | estuarine | adult | lab | microarray | [32] |
| Zebrafish | Danio rerio | 2013 | embryo | unknown | freshwater | embryos | lab | microarray | [99] |
| Japanese Medaka | Oryzias latipes | 2012 | testis | male | freshwater | adult | lab | microarray | [79] |
| Japanese Medaka | Oryzias latipes | 2012 | testis | male | freshwater | adult | lab | microarray | [48] |
| Stickleback | Gasterosteus aculeatus | 2010 | liver | male | freshwater | adult | wild-caught, lab exp. | microarray | [60] |
| Zebrafish | Danio rerio | 2010 | liver | male, female | freshwater | adults | lab | microarray | [24] |
| Fathead minnow | Pimephales promelas | 2009 | testis | male | freshwater | adult | lab | microarray | [42] |
| Rainbow trout | Oncorhynchus mykiss | 2008 | liver | unknown | freshwater | immature | lab | microarray | [49] |
| Zebrafish (lab wild-type strain) | Danio rerio | 2008 | brain, gonads | male, female | freshwater | mature | lab | microarray | [118] |
| Zebrafish | Danio rerio | 2008 | brain, gonads | male, female | freshwater | adult | lab | microarray | [117] |
| Common carp | Cyprinus carpio | 2007 | liver | unknown | freshwater | immature | lab | microarray | [81] |
| Rainbow trout | Oncorhynchus mykiss | 2007 | hepatocytes | male | freshwater | adult? | lab | microarray | [39] |
| Rainbow trout | Oncorhynchus mykiss | 2007 | liver | male, female | freshwater | juvenile | lab | microarray | [45] |
| Zebrafish | Danio rerio | 2007 | liver, telencephalon | male | freshwater | adult | lab | microarray | [74] |
| Zebrafish | Danio rerio | 2007 | gonads | male, female | freshwater | adult | lab | microarray | [98] |
| Common carp | Cyprinus carpio | 2006 | liver | unknown | freshwater | juvenile | lab | microarray | [80] |
| Goldfish | Carassius auratus | 2006 | brain | male | freshwater | adult? | lab | microarray | [75] |
| Rainbow trout | Oncorhynchus mykiss | 2006 | hepatic | male | freshwater | adult, juvenile | lab | microarray | [101] |
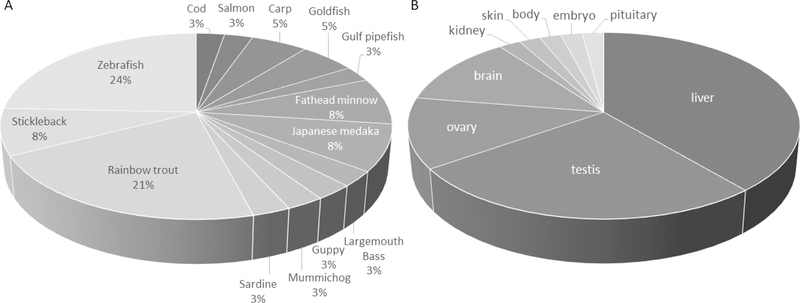
The species that have been studied for their response to EE2 exposure include both freshwater and marine, for example goldfish (Carassius auratus), zebrafish (Danio rerio), flounder (Platichthys flesus), Atlantic cod (Gadus morhua), stickleback (Gasterosteus aculeatus), mummichog (Fundulus heteroclitus), rainbow trout (Oncorhynchus mykiss), and largemouth bass (Micropterus salmoides), among others (Figure 1A; Table 1). However, more than 85% of the transcriptomics studies with EE2 have been conducted in freshwater species (e.g., zebrafish (Danio rerio), guppy (Poecilia reticulata), rare minnow (Gobiocypris rarus), and largemouth bass (Micropterus salmoides) compared to saltwater species such as Pacific sardine (Sardinops sagax), Atlantic cod (Gadus morhua), gulf pipefish (Syngnathus scovelli), sheepshead minnow (Cyprinodon variegatus), and mummichog (Fundulus heteroclitus). Moving forward, additional data to address how saltwater species are affected by pharmaceutical estrogens like EE2 are required to fill gaps in our understanding of estrogen-mediated gene networks. Physiological differences in ion regulation and hormone signaling are reported between freshwater and saltwater fish and this may translate into sensitivity differences in gene expression to pharmaceutical estrogens [2, 50]. What is impressive is that an array of species has been investigated at the transcriptomics level, and this presents a rich comparative perspective of EE2-induced gene expression. Moreover, there have been a range of tissues investigated at the molecular level following exposure to EE2, the most predominant tissues being the liver and the testes (Figure 1B). Moving forward, additional data on spleen, kidney [9], pituitary [46] and early embryogenesis would be needed to strengthen understanding of the molecular pathways altered by EE2 in addition to revealing novel mechanisms for estrogens. A last point to make is that, while the majority of studies are in male fish, there are some data available on female fish, which offer a unique perspective on sex-specific responses. EE2 has been studied in female zebrafish, Atlantic cod, largemouth bass and mummichog (listed in Table 1), which provides useful information on sex differentiation, as well as sex-specific biomarkers for estrogenic exposures.
Figure 1:
Percent of studies reporting on transcriptomics responses to 17alpha-ethinyestradiol (EE2) in (A) fish and (B) tissues. Rainbow trout and zebrafish have been the dominant species studied, while the liver and the testis are often the most studied tissues when investigating molecular responses to EE2.
5. Transcriptomics reveals mechanisms of 17alpha-ethinylestradiol action in fish
EE2 induces both intersex and sex change in fish [28, 121], but the mechanisms by which EE2 induces these conditions, both in the laboratory and in the field, have been a significant question by scientists globally [8]. In one study, Feswick et al. [37] used a short-term (96 hour) exposure of male fathead minnows to environmentally-relevant levels (15 ng/L) of EE2, to identify early transcriptional changes potentially related to the initiation of intersex. Exposed males did not exhibit any significant change in testes morphology nor gonadosomatic index during the short exposure, but they did show a reduction in both testosterone and 11keto-testosterone. Transcriptomic profiling in the testes revealed that gene networks associated with male reproduction (e.g. sperm motility, insemination, male sex determination) were rapidly suppressed with EE2 exposure while gene networks related to female reproduction (e.g. ovary function, ovary follicle development, and granulosa cell development) were rapidly increased following exposure to EE2. Moreover, networks involved in steroid biosynthesis and steroid metabolism were suppressed. Gene networks centered around key transcription factors such as foxl2, which signals ovarian follicle development and granulosa cell development, as well as dmrt1 and sox9, two key proteins in male sex determination, were rapidly regulated by EE2. Studies such as these are important because they begin to identify molecular initiating events prior to the appearance of intersex.
Feswick and colleagues [36, 38] also conducted a broad scale study using EE2 to compare interlaboratory reliability and reproducibility for gene expression data. Laboratories were tasked to identify estrogen-responsive genes and networks in fathead minnow liver following exposure to EE2. The objective was to identify E2-responsive genes in the liver that could yield new insights into the regulation of hepatic physiology and to identify reliable biomarkers for EE2 exposure (i.e., those identified in all laboratories). Microarrays revealed EE2 exposure increased processes such as protein folding and amino acid activation in the liver, whereas gene networks associated with blood clotting and coagulation, as well as the alternative and classical complement activation pathways, triglycerides storage, and xenobiotic clearance and metabolism were decreased by EE2. Laboratories were consistent in identifying a number of estrogen-responsive genes in the liver, including apolipoprotein E, apolipoprotein A1, insulin growth factor 1, x-box binding protein 1, and estrogen receptor alpha. Notably, there was high variability, both biological and technical, for vitellogenin, which can impede interpretation of the data [38]. High variability in vitellogenin has been observed by others [11]. Jastrow and colleagues [56] recently presented some guidelines for addressing variability in the vitellogenin biomarker with estrogenic exposures.
From an environmental perspective, the realization that specific genes show consistent and reproducible responses to EE2 leads to the question of whether E2-responsive gene networks are more appropriate for biomonitoring programs, compared to individual biomarkers such as vitellogenin. Based on these data from the interlaboratory study, and those collected from the Comparative Toxicogenomics Database, an estrogen-responsive network was developed (including estrogen receptor alpha, transferrin, myeloid cell leukemia 1, insulin like growth factor 1, and methionine adenosyltransferase 2A, among other genes) [38]. Thus, considering multiple lines of evidence, we continue to hone our knowledge of estrogen-responsive gene networks, and these networks have been proposed in environmental monitoring programs to improve decision-making and to increase our ability to detect estrogens in the environment [73]. However, in order to better characterize risk from endocrine active substances, including xenoestrogens, an increasing reliance on integrative and computational approaches may be needed within the context of the adverse outcome pathway framework.
Knowledge as to the role of xenoestrogens in the process of both intersex and sex reversal is not limited to the gonads, and researchers now appreciate the role that estrogens play in shaping the central nervous system of fishes. In the guppy, RNASeq was used to document the effect of 8 ng/L and 38 ng/L EE2 on the brain transcriptome of both males and females [95]. Not surprisingly, the male brains exposed to EE2 exhibited gene expression changes which were more similar to female brains, suggesting a feminizing effect. While the researchers did not conduct pathway analysis nor gene set enrichment, the study nevertheless revealed that a significant number of genes affected in the brain were related to glutamate and nuclear receptor signaling, chromatin organization, and LINE transposable elements, which showed treatment and sex specific responses.
Exposure of males to EE2 during development has revealed that even transient, low-level (environmentally relevant) exposure during critical developmental periods can have irreversible reproductive consequences into adulthood. For example, using RNAseq, a host of genes associated with spermatogenesis, steroid synthesis, and testis development and function were differentially expressed in zebrafish exposed to 1.2 and 1.5 ng/L EE2 from fertilization to 80 days of age followed by depuration for 82 days [87]. Other biological processes affected by EE2 in the study included lipid and carbohydrate metabolic process, protein and nucleic acid metabolic processes, gene regulation, response to hormone, response to stress, and circadian rhythm. These processes are therefore hypothesized to be related to lower fertility in male adult zebrafish that persist over time, in the absence of EE2. Many of the these biological processes are consistent with those reported in other studies investigating effects of EE2 [37]. The study by Porseryd et al. [87] suggests that non-coding sequence perturbations by EE2 is a potential mechanism of disruption in fish, and this would not have been revealed without transcriptome data. The role of non-long coding RNA in estrogen-mediated responses is anticipated to be a new avenue of research in fish endocrinology.
6. Non-genomic signaling by estrogens
There is a body of evidence showing that vertebrates, including fish, have membrane receptors for estradiol and other sex steroids, in addition to soluble nuclear receptors [106]. These receptors bind their ligands with very high affinity (Kd’s in the 1–5 nM range) and have very specific functions in reproduction. Data on the characterization of specific membrane receptors for estradiol, progestins, and testosterone have been presented previously [19, 61, 62, 86].
Initial studies by the Thomas laboratory characterized the progestin membrane receptor alpha (mPRa) and showed that it regulated oocyte maturation and in fact was the maturation inducing steroid (MIS) 20β–S receptor [124]. In addition to effects in the ovary, MIS also is responsible for inducing sperm hypermotility in males, and thus the mPRa receptor has also been found in the membranes of sperm. Specific membrane receptors for testosterone were also identified [13].
Membrane estradiol receptors have also been characterized in fish. The receptor identified by the Thomas group was GPER, also known as GPR30 [85]. This receptor is found on the surface of fish oocytes and when it is bound by estradiol shows inhibitory action on oocyte maturation. It is possible that a truncated version of ERa may also be expressed tethered to membranes.
A general characteristic of the membrane receptors is that they are composed of 7 transmembrane segments and are part of a G-protein superfamily. They signal through activation of intracellular second messenger pathways and the signaling occurs very quickly. Through in situ hybridization, membrane steroid receptors have been found in several tissues, including the brain [71].
In a recent experiment, fathead minnow were exposed to 5 ng/L EE2 or 100 ng/L levonorgestrel (the progestin portion of the birth control pill) for 30 min to evaluate signaling cascades using a phosphoproteomics approach [102]. Changes in phosphorylation patterns of brain proteins were distinct for the two chemicals, with some overlap. Both estradiol and the progestin altered phosphorylation patterns in proteins generally involved in neurogenesis and synaptic activity, but the specific group of proteins affected by each was different. In some cases, the directionality of phosphorylation on the same protein was in opposite directions by the two chemicals. Each chemical also showed some unique phosphorylation pattern cascades. EE2 was involved mostly in neuronal processes and neuroprotection, while levonorgestrel altered phosphorylation patterns for proteins involved in axon cargo transport and calcium signaling.
It is intriguing to understand how soluble nuclear receptors and their membrane counterparts work together to signal tissues in fish brains and reproductive tissues. Clearly, more research is required to get the complete picture of how they support each other to maintain homeostasis and control reproduction.
7. Computational endocrinology: New view of estrogens
Various data sources are available to examine estrogen-mediated effects, and it is important to leverage data incorporating reliable endpoints with direct mechanistic and/or (sub)population relevance. In fish, these endpoints are primarily derived from reproductive parameters in chronic studies. For example, there are parameters with high mechanistic specificity for estrogen activity, such as male vitellogenin production. On the contrary, there are parameters affected by several modes of action. For example, fecundity is an apical endpoint regulated by estrogen, but systemic toxicity can also affect reproductive output (ECHA/EFSA, 2018). Thus, it is important to consider the mechanistic specificity of these parameters, as they have different levels of utility to identify potential estrogens.
Fortunately, many guideline studies include informative parameters, and there are various resources to access these data, as well as non-guideline studies. For example, the fish short term reproduction assay (OECD TG 229), 21-day fish assay (OECD TG 230), fish sexual development test (OECD TG 234), and medaka extended one-generation reproduction test (OECD TG 240) are harmonized guideline studies with parameters indicative of estrogen activity. In addition, the fish life cycle toxicity test (OPPTS 850.1500) is a common assay that can be modified to include mechanistic parameters, such as vitellogenin. There are several major data sources to access this information, including the eChemPortal database from REACH, the ECOTOX database from USEPA, the METI database from the Japanese Ministry of the Environment, the Pesticide Ecotoxicity Database from USEPA, and peer-reviewed literature, to name a few. A recent effort has consolidated these data sources, as well as others, into a curated database called EnviroTox (https://envirotoxdatabase.org/) that contains >91,000 aquatic toxicity records for >1,500 species and >4,000 CAS numbers [21]. Ultimately, these resources are valuable to identify ecotoxicology studies for risk assessment and other applications. Not only are these data useful to identify toxicity thresholds for individual substances, they may also be used to compare effects between species and chemicals. For example, probabilistic approaches such as species sensitivity distributions have been used to identify sensitive taxa and predicted-no-effect concentrations (i.e., HC5 values) for estrogens [18], and chemical toxicity distributions have been used to compare the sensitivities of common in vitro and in vivo estrogen agonist assays [30]. Thus, data mining offers a useful approach to address certain information needs related to estrogenic effects in fish.
The molecular targets of estrogens are similar between fish and other vertebrates, and the growth of new assessment methodologies (NAMs), including omics, has offered several data-driven approaches to better understand how molecular diversity affects responses to estrogens. Contrary to most vertebrates, which have 2 nuclear estrogen receptors, there are 3 nuclear estrogen receptors in teleost fish: esr1, esr2a, and esr2b, the latter of which arose from esr2 in a whole genome duplication event that occurred approximately 350 million years ago [44, 47]. Indeed, these nuclear receptors have unique effects, primarily via genomic mechanisms, and these activities complement those by membrane estrogen receptors, which act via intracellular signaling cascades [84]. While these targets have been susceptible to molecular evolution, there remains a high level of molecular and functional conservation for estrogen receptors among vertebrates, including fish. One way to examine this conservation, and by extension taxonomic susceptibility to estrogens, is to leverage omics data to compare molecular target sequence similarity.
This concept has been materialized in the SeqAPASS (Sequence Alignment to Predict Across Species Susceptibility) tool by USEPA, which quantitatively compares protein sequence/structural similarity across species to identify taxonomic sensitivity for a given target. The tool uses 3 levels of analysis to determine susceptibility: 1) primary amino acid sequence similarity, 2) functional domain sequence similarity, 3) amino acid residue similarity. Put together, this information is evaluated to set a susceptibility “cut-off” that is compared across taxa [67]. In the case of estrogens, this approach has been used to identify susceptible taxa based on the human estrogen receptor. Indeed, it was found that fish (class Actinopterygii) met the “cut-off”, and this susceptibility was confirmed with reproductive toxicity data associated with estrogen receptor activity [66]. While this current approach examines susceptibility at a broad taxonomic level, in the long term, omics data will become further useful to refine the relative sensitivity of species to estrogens through more advanced comparative bioinformatics approaches.
For ecotoxicologists, evidence of estrogen receptor conservation supports the use of read across approaches that complement or expand our understanding of estrogen-mediated effects in fish. For example, many high-throughput screening assays in the ToxCast/Tox21 programs [29, 107] have been used to screen chemicals for endocrine activity, including estrogen receptor agonism/antagonism [51, 93]. While these in vitro assays are based on mammalian models, they have screened thousands of compounds and offer useful data to characterize estrogen activity. Furthermore, this information has been used to develop computational models that integrate multiple assay responses to predict in vivo estrogen-mediated responses, such as those from the mammalian uterotrophic assay [17]. Likewise, since these assays screen large chemical libraries with high structural diversity, this information is also useful to construct robust quantitative structure-activity relationship (QSAR) models that predict estrogen receptor binding and activity [72]. In the near future, these assays are expected to include transcriptomics [78], which will offer high-dimensional response profiling for many chemicals, including potential estrogens. These datasets will be useful to identify chemicals affecting estrogen signaling pathways and genes, among others.
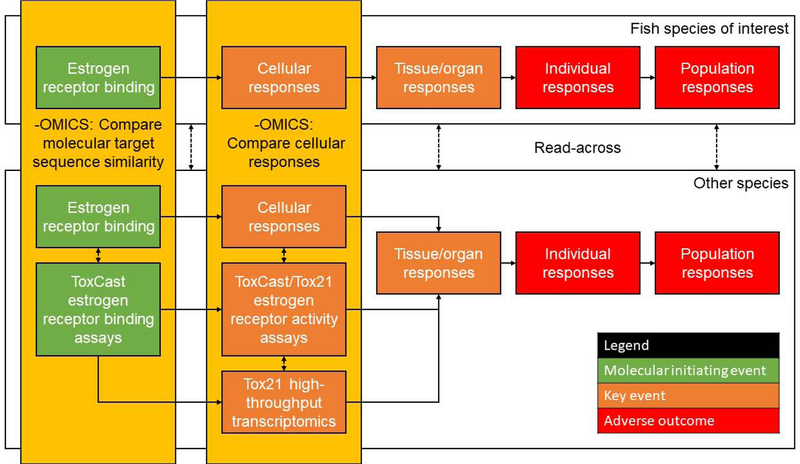
Certainly, these NAMs offer valuable information, and it remains important to validate these approaches to assess estrogen-mediated effects in fish. It is evident that there is strong structural conservation of ERα among species, which allows similar compounds to bind to fish and human ERα [7], although at different affinities [76]. Still, mammalian in vitro models have been useful to predict non-mammalian responses. For example, there is a significant relationship between the relative potency of compounds in ToxCast/Tox21 estrogen agonist assays, the ToxCast ER bioactivity model, and in vivo vitellogenin induction in male fish [33]. Likewise, in Tier 1 Screening of the Endocrine Disruptor Screening Program, there are similar outcomes between in vivo mammalian and fish assays [6]. Thus, there is growing evidence that comparisons across approaches, especially those utilizing NAMs (e.g., omics/HTS), will become increasing useful to predict estrogenic responses in non-target species, such as fish (Figure 2).
Figure 2:
A framework for assessing environmental estrogens in the context of adverse outcome pathways. Omics can be leveraged to support read across from other taxa, including mammalian data (e.g., high-throughput in vitro assays).
Considering the growing complexity of ecotoxicological data – especially omics data – adverse outcome pathways (AOPs) offer a useful framework to organize and integrate numerous lines of evidence. An AOP consists of a series of key events (KE) linking a molecular initiating event (MIE) to an adverse outcome (AO) through a series of causal key event relationships (KERs) [114]. In this framework, omics data primarily describe cellular KEs, but they are also useful to inform on MIEs. For example, proteomics data are useful to identify molecular target sequences that are conserved across species (e.g., SeqAPASS), and this information can define the taxonomic domain of applicability for an AOP [14]. Accordingly, QSAR models may be useful to identify structural features, and thus related chemicals, that trigger a MIE in a particular AOP [35, 108]. Downstream, omics data offer a useful perspective to identify cellular departures from homeostasis. Given sufficient magnitude, these responses may trigger subsequent KEs and potentially lead to an AO. In addition, gene expression profiles may also be associated with a particular mode of action. For example, gene set classifiers have been developed in zebrafish for endocrine disrupting chemicals, including estrogen agonists (e.g., EE2) [116]. Likewise, estrogen-responsive interactomes have been developed to complement vitellogenin as a diagnostic biomarker for estrogenicity in fish [38]. Thus, omics data are useful for several applications in the AOP framework, and this information will be important to improve AOPs for estrogen-mediated effects in fish.
A major goal of the AOP framework is to identify causal pathways between mechanistic and apical responses. In many cases, these pathways are not linear and may involve larger networks that include several MIEs and/or AOs [64]. Thus, it will be important to define critical paths in these networks to identify those associated with estrogen versus alternative or confounding paths [115]. In addition, a major goal is to better define our quantitative understanding of these biological relationships. To this end, quantitative AOPs (qAOPs) have been developed for reproductive outcomes in fish [22], and additional efforts will be required to consider compensatory and recovery processes. While recovery processes have been a research topic for estrogens and fish [12, 43], it remains a challenge to integrate this information into AOPs. Regardless, the AOP framework has been useful to organize effects data, and these efforts will ultimately lead to a better understanding of estrogen-mediated effects in fish to support ecological risk assessment.
8. Conclusions
The past several years has yielded a rich source of comparative data for EE2 in various teleost fishes. Studies investigating the pharmaceutical EE2 have yielded important clues into estrogen action. Major steps needed moving forward include: (1) Establishing conceptual frameworks for incorporating estrogenic-responsive networks into environmental monitoring programs; (2) Data mining (ECOTOX, EnviroTox) to identify effect thresholds for estrogens in fish; (3) Characterizing novel estrogen receptor signaling pathways in fish, including both nuclear and membrane receptor activity; (4) Leveraging comparative bioinformatics to identify susceptible taxa; (5) Integrating comparative lines of evidence (e.g., mammalian data, in vitro assays) to identify chemicals likely to affect estrogen-mediated endpoints in fish; (6) Incorporating various lines of evidence (described above) to construct qAOPs for estrogen-related effects in fish that include compensatory/recovery processes. As we look ahead, research into EE2 and other environmental estrogens can serve as a template for other potential endocrine active substances.
Highlights.
17alpha-ethinylestradiol (EE2) is one of the most widely studied pharmaceuticals in fish.
Transcriptome studies have revealed mechanisms of action in numerous fish species and tissues.
While data are prevalent for liver, brain, and gonad, less is known about EE2 action in kidney and pituitary.
Transcriptomics will contribute to quantitative adverse outcome pathways for estrogen signaling.
Acknowledgements
The authors would like to thank the many undergraduate and graduate students that researched questions about the effects of estrogenic chemicals in fish over the past several years. Without this effort, Funding for these studies were provided by Natural Sciences and Engineering Research Council (NSERC 386275-2010, C.J.M), Canada Research Chair Program (C.J.M and K.R.M), National Institutes of Health Pathway to Independence Award (K99 ES016767-01A1, C.J.M), Superfund Basic Research Program from the National Institute of Environmental Health Sciences (R01 ES015449, N.D.), and NSF Graduate Fellowship (D.A.D.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Abdelmoneim A, Abdu A, Chen S, Sepúlveda MS, Molecular signaling pathways elicited by 17α-ethinylestradiol in Japanese medaka male larvae undergoing gonadal differentiation. Aquatic toxicology. 208 (2019) 187–195. [DOI] [PubMed] [Google Scholar]
- [2].Al-Jandal NJ, Whittamore JM, Santos EM, Wilson RW, The influence of 17β-estradiol on intestinal calcium carbonate precipitation and osmoregulation in seawater-acclimated rainbow trout (Oncorhynchus mykiss). Journal of Experimental Biology. 214 (2011) 2791–2798. [DOI] [PubMed] [Google Scholar]
- [3].Amano H, Uno S, Koyama J, Hiramatsu N, Todo T, Hara A, Development of specific enzyme-linked immunosorbent assays for multiple vitellogenins in marbled sole, Pleuronectes yokohamae. Gen Comp Endocrinol. 281 (2019) 67–72. [DOI] [PubMed] [Google Scholar]
- [4].Andreozzi R, Raffaele M, Nicklas P, Pharmaceuticals in STP effluents and their solar photodegradation in aquatic environment. Chemosphere. 50 (2003) 1319–1330. [DOI] [PubMed] [Google Scholar]
- [5].Ankley GT, Bennett RS, Erickson RJ, Hoff DJ, Hornung MW, Johnson RD, et al. , Adverse outcome pathways: a conceptual framework to support ecotoxicology research and risk assessment. Environ Toxicol Chem 29 (2010) 730–741. [DOI] [PubMed] [Google Scholar]
- [6].Ankley GT, Gray LE, Cross-species conservation of endocrine pathways: a critical analysis of tier 1 fish and rat screening assays with 12 model chemicals. Environ Toxicol Chem 32 (2013) 1084–1087. [DOI] [PubMed] [Google Scholar]
- [7].Ankley GT, LaLone CA, Gray LE, Villeneuve DL, Hornung MW, Evaluation of the scientific underpinnings for identifying estrogenic chemicals in nonmammalian taxa using mammalian test systems. Environ Toxicol Chem 35 (2016) 2806–2816. [DOI] [PubMed] [Google Scholar]
- [8].Bahamonde PA, Munkittrick KR, Martyniuk CJ, Intersex in teleost fish: are we distinguishing endocrine disruption from natural phenomena? Gen Comp Endocrinol 192 (2013) 25–35. [DOI] [PubMed] [Google Scholar]
- [9].Bailey C, von Siebenthal EW, Rehberger K, Segner H, Transcriptomic analysis of the impacts of ethinylestradiol (EE2) and its consequences for proliferative kidney disease outcome in rainbow trout (Oncorhynchus mykiss). Comparative Biochemistry and Physiology Part C: Toxicology & Pharmacology. 222 (2019) 31–48. [DOI] [PubMed] [Google Scholar]
- [10].Barber LB, Rapp JL, Kandel C, Keefe SH, Rice J, Westerhoff P, et al. , Integrated Assessment of Wastewater Reuse, Exposure Risk, and Fish Endocrine Disruption in the Shenandoah River Watershed. Environ Sci Technol 53 (2019) 3429–3440. [DOI] [PubMed] [Google Scholar]
- [11].Biales AD, Bencic DC, Flick RW, Lazorchak J, Lattier DL, Quantification and associated variability of induced vitellogenin gene transcripts in fathead minnow (Pimephales promelas) by quantitative real-time polymerase chain reaction assay. Environmental toxicology and chemistry. 26 (2007) 287–296. [DOI] [PubMed] [Google Scholar]
- [12].Blanchfield PJ, Kidd KA, Docker MF, Palace VP, Park BJ, Postma LD, Recovery of a wild fish population from whole-lake additions of a synthetic estrogen. Environ Sci Technol 49 (2015) 3136–3144. [DOI] [PubMed] [Google Scholar]
- [13].Braun AM, Thomas P, Biochemical characterization of a membrane androgen receptor in the ovary of the Atlantic croaker (Micropogonias undulatus). Biology of reproduction. 71 (2004) 146–155. [DOI] [PubMed] [Google Scholar]
- [14].Brockmeier EK, Hodges G, Hutchinson TH, Butler E, Hecker M, Tollefsen KE, et al. , The Role of Omics in the Application of Adverse Outcome Pathways for Chemical Risk Assessment. Toxicol Sci 158 (2017) 252–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Brodin T, Piovano S, Fick J, Klaminder J, Heynen M, Jonsson M, Ecological effects of pharmaceuticals in aquatic systems—impacts through behavioural alterations. Philosophical Transactions of the Royal Society B: Biological Sciences. 369 (2014) 20130580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Brown M, Davies IM, Moffat CF, Robinson C, Redshaw J, Craft JA, Identification of transcriptional effects of ethynyl oestradiol in male plaice (Pleuronectes platessa) by suppression subtractive hybridisation and a nylon macroarray. Marine environmental research. 58 (2004) 559–563. [DOI] [PubMed] [Google Scholar]
- [17].Browne P, Judson RS, Casey WM, Kleinstreuer NC, Thomas RS, Screening Chemicals for Estrogen Receptor Bioactivity Using a Computational Model. Environ Sci Technol 49 (2015) 8804–8814. [DOI] [PubMed] [Google Scholar]
- [18].Caldwell DJ, Mastrocco F, Anderson PD, Lange R, Sumpter JP, Predicted-no-effect concentrations for the steroid estrogens estrone, 17beta-estradiol, estriol, and 17alpha-ethinylestradiol. Environ Toxicol Chem 31 (2012) 1396–1406. [DOI] [PubMed] [Google Scholar]
- [19].Charles NJ, Thomas P, Lange CA, Expression of membrane progesterone receptors (mPR/PAQR) in ovarian cancer cells: implications for progesterone-induced signaling events. Horm Cancer. 1 (2010) 167–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Colli-Dula RC, Martyniuk CJ, Kroll KJ, Prucha MS, Kozuch M, Barber DS, et al. , Dietary exposure of 17-alpha ethinylestradiol modulates physiological endpoints and gene signaling pathways in female largemouth bass (Micropterus salmoides). Aquat Toxicol 156 (2014) 148–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Connors KA, Beasley A, Barron MG, Belanger SE, Bonnell M, Brill JL, et al. , Creation of a Curated Aquatic Toxicology Database: EnviroTox. Environ Toxicol Chem 38 (2019) 1062–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Conolly RB, Ankley GT, Cheng W, Mayo ML, Miller DH, Perkins EJ, et al. , Quantitative Adverse Outcome Pathways and Their Application to Predictive Toxicology. Environ Sci Technol 51 (2017) 4661–4672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Cosme MM, Lister AL, Van Der Kraak G, Inhibition of spawning in zebrafish (Danio rerio): Adverse outcome pathways of quinacrine and ethinylestradiol. Gen Comp Endocrinol 219 (2015) 89–101. [DOI] [PubMed] [Google Scholar]
- [24].De Wit M, Keil D, van der Ven K, Vandamme S, Witters E, De Coen W, An integrated transcriptomic and proteomic approach characterizing estrogenic and metabolic effects of 17 α-ethinylestradiol in zebrafish (Danio rerio). General and comparative endocrinology. 167 (2010) 190–201. [DOI] [PubMed] [Google Scholar]
- [25].Denslow ND, Chow MC, Kroll KJ, Green L, Vitellogenin as a biomarker of exposure for estrogen or estrogen mimics. Ecotoxicology. 8 (1999) 385–398. [Google Scholar]
- [26].Depiereux S, De Meulder B, Bareke E, Berger F, Le Gac F, Depiereux E, et al. , Adaptation of a bioinformatics microarray analysis workflow for a toxicogenomic study in rainbow trout. PloS one. 10 (2015) e0128598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Depiereux S, Le Gac F, De Meulder B, Pierre M, Helaers R, Guiguen Y, et al. , Meta-analysis of microarray data of rainbow trout fry gonad differentiation modulated by ethynylestradiol. PloS one. 10 (2015) e0135799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Depiereux S, Liagre M, Danis L, De Meulder B, Depiereux E, Segner H, et al. , Intersex occurrence in rainbow trout (Oncorhynchus mykiss) male fry chronically exposed to ethynylestradiol. PLoS One. 9 (2014) e98531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Dix DJ, Houck KA, Martin MT, Richard AM, Setzer RW, Kavlock RJ, The ToxCast program for prioritizing toxicity testing of environmental chemicals. Toxicol Sci 95 (2007) 5–12. [DOI] [PubMed] [Google Scholar]
- [30].Dobbins LL, Brain RA, Brooks BW, Comparison of the sensitivities of common in vitro and in vivo assays of estrogenic activity: application of chemical toxicity distributions. Environ Toxicol Chem 27 (2008) 2608–2616. [DOI] [PubMed] [Google Scholar]
- [31].Dominguez GA, Quattro JM, Denslow ND, Kroll KJ, Prucha MS, Porak WF, et al. , Identification and transcriptional modulation of the largemouth bass, Micropterus salmoides, vitellogenin receptor during oocyte development by insulin and sex steroids. Biol Reprod 87 (2012) 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Doyle MA, Bosker T, Martyniuk CJ, Maclatchy DL, Munkittrick KR, The effects of 17-alpha-ethinylestradiol (EE2) on molecular signaling cascades in mummichog (Fundulus heteroclitus). Aquat Toxicol 134–135 (2013) 34–46. [DOI] [PubMed] [Google Scholar]
- [33].Dreier DA, Denslow ND, Martyniuk CJ, Computational analysis of the ToxCast estrogen receptor agonist assays to predict vitellogenin induction by chemicals in male fish. Environmental toxicology and pharmacology. 53 (2017) 177–183. [DOI] [PubMed] [Google Scholar]
- [34].Eidem JK, Kleivdal H, Kroll K, Denslow N, van Aerle R, Tyler C, et al. , Development and validation of a direct homologous quantitative sandwich ELISA for fathead minnow (Pimephales promelas) vitellogenin. Aquat Toxicol 78 (2006) 202–206. [DOI] [PubMed] [Google Scholar]
- [35].Ellison CM, Piechota P, Madden JC, Enoch SJ, Cronin MT, Adverse outcome pathway (AOP) informed modeling of aquatic toxicology: QSARs, read-across, and interspecies verification of modes of action. Environmental science & technology. 50 (2016) 3995–4007. [DOI] [PubMed] [Google Scholar]
- [36].Feswick A, Isaacs M, Biales A, Flick RW, Bencic DC, Wang RL, et al. , How consistent are we? Interlaboratory comparison study in fathead minnows using the model estrogen 17alpha-ethinylestradiol to develop recommendations for environmental transcriptomics. Environ Toxicol Chem (2017). [DOI] [PMC free article] [PubMed]
- [37].Feswick A, Loughery JR, Isaacs MA, Munkittrick KR, Martyniuk CJ, Molecular initiating events of the intersex phenotype: Low-dose exposure to 17alpha-ethinylestradiol rapidly regulates molecular networks associated with gonad differentiation in the adult fathead minnow testis. Aquat Toxicol 181 (2016) 46–56. [DOI] [PubMed] [Google Scholar]
- [38].Feswick A, Munkittrick KR, Martyniuk CJ, Estrogen-responsive gene networks in the teleost liver: What are the key molecular indicators? Environmental toxicology and pharmacology. 56 (2017) 366–374. [DOI] [PubMed] [Google Scholar]
- [39].Finne E, Cooper G, Koop B, Hylland K, Tollefsen K, Toxicogenomic responses in rainbow trout (Oncorhynchus mykiss) hepatocytes exposed to model chemicals and a synthetic mixture. Aquatic toxicology. 81 (2007) 293–303. [DOI] [PubMed] [Google Scholar]
- [40].Folmar LC, Denslow ND, Rao V, Chow M, Crain DA, Enblom J, et al. , Vitellogenin induction and reduced serum testosterone concentrations in feral male carp (Cyprinus carpio) captured near a major metropolitan sewage treatment plant. Environmental Health Perspectives. 104 (1996) 1096–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Gao J, Zhang Y, Zhang T, Yang Y, Yuan C, Jia J, et al. , Responses of gonadal transcriptome and physiological analysis following exposure to 17α-ethynylestradiol in adult rare minnow Gobiocypris rarus. Ecotoxicology and environmental safety. 141 (2017) 209–215. [DOI] [PubMed] [Google Scholar]
- [42].Garcia-Reyero N, Kroll KJ, Liu L, Orlando EF, Watanabe KH, Sepulveda MS, et al. , Gene expression responses in male fathead minnows exposed to binary mixtures of an estrogen and antiestrogen. BMC Genomics. 10 (2009) 308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Genovese G, Regueira M, Piazza Y, Towle DW, Maggese MC, Lo Nostro F, Time-course recovery of estrogen-responsive genes of a cichlid fish exposed to waterborne octylphenol. Aquat Toxicol 114–115 (2012) 1–13. [DOI] [PubMed] [Google Scholar]
- [44].Glasauer SM, Neuhauss SC, Whole-genome duplication in teleost fishes and its evolutionary consequences. Molecular genetics and genomics. 289 (2014) 1045–1060. [DOI] [PubMed] [Google Scholar]
- [45].Gunnarsson L, Kristiansson E, Forlin L, Nerman O, Larsson DG, Sensitive and robust gene expression changes in fish exposed to estrogen--a microarray approach. BMC Genomics. 8 (2007) 149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Harding LB, Schultz IR, Goetz GW, Luckenbach JA, Young G, Goetz FW, et al. , High-throughput sequencing and pathway analysis reveal alteration of the pituitary transcriptome by 17α-ethynylestradiol (EE2) in female coho salmon, Oncorhynchus kisutch. Aquatic toxicology. 142 (2013) 146–163. [DOI] [PubMed] [Google Scholar]
- [47].Hawkins MB, Thornton JW, Crews D, Skipper JK, Dotte A, Thomas P, Identification of a third distinct estrogen receptor and reclassification of estrogen receptors in teleosts. Proc Natl Acad Sci U S A. 97 (2000) 10751–10756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Hirakawa I, Miyagawa S, Katsu Y, Kagami Y, Tatarazako N, Kobayashi T, et al. , Gene expression profiles in the testis associated with testis–ova in adult Japanese medaka (Oryziaslatipes) exposed to 17α-ethinylestradiol. Chemosphere 87 (2012) 668–674. [DOI] [PubMed] [Google Scholar]
- [49].Hook SE, Skillman AD, Gopalan B, Small JA, Schultz IR, Gene expression profiles in rainbow trout, Onchorynchus mykiss, exposed to a simple chemical mixture. Toxicological sciences. 102 (2007) 42–60. [DOI] [PubMed] [Google Scholar]
- [50].Hsu H-H, Lin L-Y, Tseng Y-C, Horng J-L, Hwang P-P, A new model for fish ion regulation: identification of ionocytes in freshwater-and seawater-acclimated medaka (Oryzias latipes). Cell and tissue research. 357 (2014) 225–243. [DOI] [PubMed] [Google Scholar]
- [51].Huang R, Sakamuru S, Martin MT, Reif DM, Judson RS, Houck KA, et al. , Profiling of the Tox21 10K compound library for agonists and antagonists of the estrogen receptor alpha signaling pathway. Sci Rep 4 (2014) 5664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Huff M, da Silveira WA, Carnevali O, Renaud L, Hardiman G, Systems analysis of the liver transcriptome in adult male zebrafish exposed to the plasticizer (2-ethylhexyl) phthalate (DEHP). Scientific reports. 8 (2018) 2118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Hultman MT, Song Y, Tollefsen KE, 17alpha-Ethinylestradiol (EE2) effect on global gene expression in primary rainbow trout (Oncorhynchus mykiss) hepatocytes. Aquat Toxicol 169 (2015) 90–104. [DOI] [PubMed] [Google Scholar]
- [54].Iguchi T, Watanabe H, Katsu Y, Mizutani T, Miyagawa S, Suzuki A, et al. , Developmental toxicity of estrogenic chemicals on rodents and other species. Congenital anomalies 42 (2002) 94–105. [DOI] [PubMed] [Google Scholar]
- [55].Jackson LM, Felgenhauer BE, Klerks PL, Feminization, altered gonadal development, and liver damage in least killifish (Heterandria formosa) exposed to sublethal concentrations of 17alpha-ethinylestradiol. Ecotoxicol Environ Saf 170 (2019) 331–337. [DOI] [PubMed] [Google Scholar]
- [56].Jastrow A, Gordon DA, Auger KM, Punska EC, Arcaro KF, Keteles K, et al. , Tools to minimize interlaboratory variability in vitellogenin gene expression monitoring programs. Environ Toxicol Chem 36 (2017) 3102–3107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Jing J, Wu J, Liu W, Xiong S, Ma W, Zhang J, et al. , Sex-biased miRNAs in gonad and their potential roles for testis development in yellow catfish. PLoS One. 9 (2014) e107946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Jobling S, Nolan M, Tyler CR, Brighty G, Sumpter JP, Widespread sexual disruption in wild fish. Environmental science & technology. 32 (1998) 2498–2506. [Google Scholar]
- [59].Jobling S, Sumpter J, Detergent components in sewage effluent are weakly oestrogenic to fish: an in vitro study using rainbow trout (Oncorhynchus mykiss) hepatocytes. Aquatic toxicology. 27 (1993) 361–372. [Google Scholar]
- [60].Katsiadaki I, Williams TD, Ball JS, Bean TP, Sanders MB, Wu H, et al. , Hepatic transcriptomic and metabolomic responses in the Stickleback (Gasterosteus aculeatus) exposed to ethinyl-estradiol. Aquatic toxicology. 97 (2010) 174–187. [DOI] [PubMed] [Google Scholar]
- [61].Kazeto Y, Goto-Kazeto R, Thomas P, Trant JM, Molecular characterization of three forms of putative membrane-bound progestin receptors and their tissue-distribution in channel catfish, Ictalurus punctatus. J Mol Endocrinol 34 (2005) 781–791. [DOI] [PubMed] [Google Scholar]
- [62].Kelder J, Azevedo R, Pang Y, de Vlieg J, Dong J, Thomas P, Comparison between steroid binding to membrane progesterone receptor alpha (mPRalpha) and to nuclear progesterone receptor: correlation with physicochemical properties assessed by comparative molecular field analysis and identification of mPRalpha-specific agonists. Steroids 75 (2010) 314–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Kidd KA, Blanchfield PJ, Mills KH, Palace VP, Evans RE, Lazorchak JM, et al. , Collapse of a fish population after exposure to a synthetic estrogen. Proceedings of the National Academy of Sciences. 104 (2007) 8897–8901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Knapen D, Angrish MM, Fortin MC, Katsiadaki I, Leonard M, Margiotta-Casaluci L, et al. , Adverse outcome pathway networks I: Development and applications. Environ Toxicol Chem 37 (2018) 1723–1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Kong HJ, Lee IK, Kim J, Kim WJ, Kim HS, Cho WS, et al. , RNA-Seq-based transcriptome analysis of Korean rose bitterling (Rhodeus uyekii) exposed to synthetic estrogen 17-alpha-ethinylestradiol (EE2). Mar Genomics 24 Pt 3 (2015) 233–236. [DOI] [PubMed] [Google Scholar]
- [66].Lalone CA, Villeneuve DL, Burgoon LD, Russom CL, Helgen HW, Berninger JP, et al. , Molecular target sequence similarity as a basis for species extrapolation to assess the ecological risk of chemicals with known modes of action. Aquat Toxicol 144–145 (2013) 141–154. [DOI] [PubMed] [Google Scholar]
- [67].LaLone CA, Villeneuve DL, Lyons D, Helgen HW, Robinson SL, Swintek JA, et al. , Editor’s Highlight: Sequence Alignment to Predict Across Species Susceptibility (SeqAPASS): A Web-Based Tool for Addressing the Challenges of Cross-Species Extrapolation of Chemical Toxicity. Toxicol Sci 153 (2016) 228–245. [DOI] [PubMed] [Google Scholar]
- [68].Larkin P, Folmar LC, Hemmer MJ, Poston AJ, Lee HS, Denslow ND, Array technology as a tool to monitor exposure of fish to xenoestrogens. Mar Environ Res 54 (2002) 395–399. [DOI] [PubMed] [Google Scholar]
- [69].Lyssimachou A, Arukwe A, Alteration of brain and interrenal StAR protein, P450scc, and Cyp11beta mRNA levels in atlantic salmon after nominal waterborne exposure to the synthetic pharmaceutical estrogen ethynylestradiol. J Toxicol Environ Health A 70 (2007) 606–613. [DOI] [PubMed] [Google Scholar]
- [70].Maclatchy DL, Vanderkraak GJ, The phytoestrogen β-sitosterol alters the reproductive endocrine status of goldfish. Toxicology and applied pharmacology 134 (1995) 305–312. [DOI] [PubMed] [Google Scholar]
- [71].Mangiamele LA, Gomez JR, Curtis NJ, Thompson RR, GPER/GPR30, a membrane estrogen receptor, is expressed in the brain and retina of a social fish (Carassius auratus) and colocalizes with isotocin. J Comp Neurol 525 (2017) 252–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Mansouri K, Abdelaziz A, Rybacka A, Roncaglioni A, Tropsha A, Varnek A, et al. , CERAPP: Collaborative Estrogen Receptor Activity Prediction Project. Environ Health Perspect 124 (2016) 1023–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Martyniuk CJ, Are we closer to the vision? A proposed framework for incorporating omics into environmental assessments. Environmental toxicology and pharmacology 59 (2018) 87–93. [DOI] [PubMed] [Google Scholar]
- [74].Martyniuk CJ, Gerrie ER, Popesku JT, Ekker M, Trudeau VL, Microarray analysis in the zebrafish (Danio rerio) liver and telencephalon after exposure to low concentration of 17alpha-ethinylestradiol. Aquat Toxicol 84 (2007) 38–49. [DOI] [PubMed] [Google Scholar]
- [75].Martyniuk CJ, Xiong H, Crump K, Chiu S, Sardana R, Nadler A, et al. , Gene expression profiling in the neuroendocrine brain of male goldfish (Carassius auratus) exposed to 17alpha-ethinylestradiol. Physiol Genomics 27 (2006) 328–336. [DOI] [PubMed] [Google Scholar]
- [76].Matthews J, Celius T, Halgren R, Zacharewski T, Differential estrogen receptor binding of estrogenic substances: a species comparison. The Journal of steroid biochemistry and molecular biology. 74 (2000) 223–234. [DOI] [PubMed] [Google Scholar]
- [77].Matthiessen P, Wheeler JR, Weltje L, A review of the evidence for endocrine disrupting effects of current-use chemicals on wildlife populations. Critical reviews in toxicology. 48 (2018) 195–216. [DOI] [PubMed] [Google Scholar]
- [78].Mav D, Shah RR, Howard BE, Auerbach SS, Bushel PR, Collins JB, et al. , A hybrid gene selection approach to create the S1500+ targeted gene sets for use in high-throughput transcriptomics. PLoS One. 13 (2018) e0191105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Miller HD, Clark BW, Hinton DE, Whitehead A, Martin S, Kwok KW, et al. , Anchoring ethinylestradiol induced gene expression changes with testicular morphology and reproductive function in the medaka. PLoS One. 7 (2012) e52479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Moens LN, Van der Ven K, Van Remortel P, Del-Favero J, De Coen WM, Expression profiling of endocrine-disrupting compounds using a customized Cyprinus carpio cDNA microarray. Toxicological Sciences. 93 (2006) 298–310. [DOI] [PubMed] [Google Scholar]
- [81].Moens LN, van der Ven K, Van Remortel P, Del-Favero J, De Coen WM, Gene expression analysis of estrogenic compounds in the liver of common carp (Cyprinus carpio) using a custom cDNA microarray. Journal of biochemical and molecular toxicology. 21 (2007) 299–311. [DOI] [PubMed] [Google Scholar]
- [82].Munkittrick K, Portt C, Kraak GVD, Smith I, Rokosh D, Impact of bleached kraft mill effluent on population characteristics, liver MFO activity, and serum steroid levels of a Lake Superior white sucker (Catostomus commersoni) population. Canadian Journal of Fisheries and Aquatic Sciences. 48 (1991) 1371–1380. [Google Scholar]
- [83].Munkittrick KR, McMaster ME, McCarthy LH, Servos M, Van Der Kraak G, An overview of recent studies on the potential of pulp-mill effluents to alter reproductive parameters in fish. Journal of Toxicology and Environmental Health, Part B Critical Reviews. 1 (1998) 347–371. [DOI] [PubMed] [Google Scholar]
- [84].Nelson ER, Habibi HR, Estrogen receptor function and regulation in fish and other vertebrates. Gen Comp Endocrinol 192 (2013) 15–24. [DOI] [PubMed] [Google Scholar]
- [85].Pang Y, Thomas P, Involvement of estradiol-17β and its membrane receptor, G protein coupled receptor 30 (GPR30) in regulation of oocyte maturation in zebrafish, Danio rario. General and comparative endocrinology. 161 (2009) 58–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Pang Y, Thomas P, Role of G protein-coupled estrogen receptor 1, GPER, in inhibition of oocyte maturation by endogenous estrogens in zebrafish. Dev Biol 342 (2010) 194–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Porseryd T, Caspillo NR, Volkova K, Elabbas L, Källman T, Dinnétz P, et al. , Testis transcriptome alterations in zebrafish (Danio rerio) with reduced fertility due to developmental exposure to 17α-ethinyl estradiol. General and comparative endocrinology. 262 (2018) 44–58. [DOI] [PubMed] [Google Scholar]
- [88].Porseryd T, Volkova K, Reyhanian Caspillo N, Källman T, Dinnetz P, Porsh Hällström I, Persistent effects of developmental exposure to 17α-ethinylestradiol on the zebrafish (Danio rerio) brain transcriptome and behavior. Frontiers in behavioral neuroscience. 11 (2017) 69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Prokkola JM, Katsiadaki I, Sebire M, Elphinstone-Davis J, Pausio S, Nikinmaa M, et al. , Microarray analysis of di-n-butyl phthalate and 17alpha ethinyl-oestradiol responses in three-spined stickleback testes reveals novel candidate genes for endocrine disruption. Ecotoxicol Environ Saf 124 (2016) 96–104. [DOI] [PubMed] [Google Scholar]
- [90].Purdom C, Hardiman P, Bye V, Eno N, Tyler C, Sumpter J, Estrogenic effects of effluents from sewage treatment works. Chemistry and Ecology 8 (1994) 275–285. [Google Scholar]
- [91].Renaud L, Agarwal N, Richards DJ, Falcinelli S, Hazard ES, Carnevali O, et al. , Transcriptomic analysis of short-term 17α-ethynylestradiol exposure in two Californian sentinel fish species sardine (Sardinops sagax) and mackerel (Scomber japonicus). Environmental pollution. 244 (2019) 926–937. [DOI] [PubMed] [Google Scholar]
- [92].Rose E, Flanagan SP, Jones AG, The effects of synthetic estrogen exposure on the sexually dimorphic liver transcriptome of the sex-role-reversed Gulf pipefish. PloS one. 10 (2015) e0139401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Rotroff DM, Martin MT, Dix DJ, Filer DL, Houck KA, Knudsen TB, et al. , Predictive endocrine testing in the 21st century using in vitro assays of estrogen receptor signaling responses. Environ Sci Technol 48 (2014) 8706–8716. [DOI] [PubMed] [Google Scholar]
- [94].Saaristo M, Johnstone CP, Xu K, Allinson M, Wong BBM, The endocrine disruptor, 17alpha-ethinyl estradiol, alters male mate choice in a freshwater fish. Aquat Toxicol 208 (2019) 118–125. [DOI] [PubMed] [Google Scholar]
- [95].Saaristo M, McLennan A, Johnstone CP, Clarke BO, Wong BB, Impacts of the antidepressant fluoxetine on the anti-predator behaviours of wild guppies (Poecilia reticulata). Aquatic toxicology. 183 (2017) 38–45. [DOI] [PubMed] [Google Scholar]
- [96].Saaristo M, Wong BB, Mincarelli L, Craig A, Johnstone CP, Allinson M, et al. , Characterisation of the transcriptome of male and female wild-type guppy brains with RNA-Seq and consequences of exposure to the pharmaceutical pollutant, 17α-ethinyl estradiol. Aquatic toxicology. 186 (2017) 28–39. [DOI] [PubMed] [Google Scholar]
- [97].Sandström O, Neuman E, Karås P, Effects of a bleached pulp mill effluent on growth and gonad function in Baltic coastal fish. Water Science and Technology. 20 (1988) 107–118. [Google Scholar]
- [98].Santos EM, Paull GC, Van Look KJ, Workman VL, Holt WV, van Aerle R, et al. , Gonadal transcriptome responses and physiological consequences of exposure to oestrogen in breeding zebrafish (Danio rerio). Aquat Toxicol 83 (2007) 134–142. [DOI] [PubMed] [Google Scholar]
- [99].Schiller V, Wichmann A, Kriehuber R, Schaefers C, Fischer R, Fenske M, Transcriptome alterations in zebrafish embryos after exposure to environmental estrogens and anti-androgens can reveal endocrine disruption. Reproductive Toxicology. 42 (2013) 210–223. [DOI] [PubMed] [Google Scholar]
- [100].Selman K, Wallace RA, Oocyte growth in the sheepshead minnow: Uptake of exogenous proteins by vitellogenic oocytes. Tissue and Cell. 14 (1982) 555–571. [DOI] [PubMed] [Google Scholar]
- [101].Skillman AD, Nagler JJ, Hook SE, Small JA, Schultz IR, Dynamics of 17alpha-ethynylestradiol exposure in rainbow trout (Oncorhynchus mykiss): absorption, tissue distribution, and hepatic gene expression pattern. Environ Toxicol Chem 25 (2006) 2997–3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Smith L, Lavelle C, Silva-Sanchez C, Denslow N, Sabo-Attwood T, Early phosphoproteomic changes for adverse outcome pathway development in the fathead minnow (Pimephales promelas) brain. Scientific reports. 8 (2018) 10212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Soimasuo M, Karels A, Leppänen H, Santti R, Oikari A, Biomarker responses in whitefish (Coregonus lavaretus L. sl) experimentally exposed in a large lake receiving effluents from pulp and paper industry. Archives of environmental contamination and toxicology. 34 (1998) 69–80. [DOI] [PubMed] [Google Scholar]
- [104].Sridevi P, Chaitanya RK, Prathibha Y, Balakrishna SL, Dutta-Gupta A, Senthilkumaran B, Early exposure of 17alpha-ethynylestradiol and diethylstilbestrol induces morphological changes and alters ovarian steroidogenic pathway enzyme gene expression in catfish, Clarias gariepinus. Environ Toxicol 30 (2015) 439–451. [DOI] [PubMed] [Google Scholar]
- [105].Sumpter JP, Jobling S, Vitellogenesis as a biomarker for estrogenic contamination of the aquatic environment. Environmental health perspectives. 103 (1995) 173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Thomas P, Rapid steroid hormone actions initiated at the cell surface and the receptors that mediate them with an emphasis on recent progress in fish models. General and comparative endocrinology. 175 (2012) 367–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Tice RR, Austin CP, Kavlock RJ, Bucher JR, Improving the human hazard characterization of chemicals: a Tox21 update. Environ Health Perspect. 121 (2013) 756–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Tollefsen KE, Scholz S, Cronin MT, Edwards SW, de Knecht J, Crofton K, et al. , Applying adverse outcome pathways (AOPs) to support integrated approaches to testing and assessment (IATA). Regulatory Toxicology and Pharmacology. 70 (2014) 629–640. [DOI] [PubMed] [Google Scholar]
- [109].Tran TKA, Yu RMK, Islam R, Nguyen THT, Bui TLH, Kong RYC, et al. , The utility of vitellogenin as a biomarker of estrogenic endocrine disrupting chemicals in molluscs. Environ Pollut 248 (2019) 1067–1078. [DOI] [PubMed] [Google Scholar]
- [110].Tyler C, Jobling S, Sumpter J, Endocrine disruption in wildlife: a critical review of the evidence. Critical reviews in toxicology. 28 (1998) 319–361. [DOI] [PubMed] [Google Scholar]
- [111].Tyler C, Routledge E, Oestrogenic effects in fish in English rivers with evidence of their causation. Pure and Applied Chemistry. 70 (1998) 1795–1804. [Google Scholar]
- [112].Van Der Kraak G, Munkittrick K, McMaster M, Portt C, Chang J, Exposure to bleached kraft pulp mill effluent disrupts the pituitary-gonadal axis of white sucker at multiple sites. Toxicology and Applied Pharmacology. 115 (1992) 224–233. [DOI] [PubMed] [Google Scholar]
- [113].Vera-Chang MN, St-Jacques AD, Gagne R, Martyniuk CJ, Yauk CL, Moon TW, et al. , Transgenerational hypocortisolism and behavioral disruption are induced by the antidepressant fluoxetine in male zebrafish Danio rerio. Proc Natl Acad Sci U S A (2018). [DOI] [PMC free article] [PubMed]
- [114].Villeneuve D, Volz DC, Embry MR, Ankley GT, Belanger SE, Leonard M, et al. , Investigating alternatives to the fish early-life stage test: a strategy for discovering and annotating adverse outcome pathways for early fish development. Environ Toxicol Chem. 33 (2014) 158–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Villeneuve DL, Angrish MM, Fortin MC, Katsiadaki I, Leonard M, Margiotta-Casaluci L, et al. , Adverse outcome pathway networks II: network analytics. Environmental toxicology and chemistry. 37 (2018) 1734–1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Wang RL, Bencic D, Biales A, Flick R, Lazorchak J, Villeneuve D, et al. , Discovery and validation of gene classifiers for endocrine-disrupting chemicals in zebrafish (danio rerio). BMC Genomics 13 (2012) 358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Wang RL, Bencic D, Biales A, Lattier D, Kostich M, Villeneuve D, et al. , DNA Microarray-based ecotoxicological biomarker discovery in a small fish model species. Environmental Toxicology and Chemistry: An International Journal. 27 (2008) 664–675. [DOI] [PubMed] [Google Scholar]
- [118].Wang RL, Biales A, Bencic D, Lattier D, Kostich M, Villeneuve D, et al. , DNA microarray application in ecotoxicology: experimental design, microarray scanning, and factors affecting transcriptional profiles in a small fish species. Environmental Toxicology and Chemistry: An International Journal. 27 (2008) 652–663. [DOI] [PubMed] [Google Scholar]
- [119].Webster TMU, Williams TD, Katsiadaki I, Lange A, Lewis C, Shears JA, et al. , Hepatic transcriptional responses to copper in the three-spined stickleback are affected by their pollution exposure history. Aquatic toxicology. 184 (2017) 26–36. [DOI] [PubMed] [Google Scholar]
- [120].Yadetie F, Zhang X, Hanna EM, Aranguren-Abadía L, Eide M, Blaser N, et al. , RNA-Seq analysis of transcriptome responses in Atlantic cod (Gadus morhua) precision-cut liver slices exposed to benzo [a] pyrene and 17α-ethynylestradiol. Aquatic toxicology. 201 (2018) 174–186. [DOI] [PubMed] [Google Scholar]
- [121].Young BJ, Lopez GC, Cristos DS, Crespo DC, Somoza GM, Carriquiriborde P, Intersex and liver alterations induced by long-term sublethal exposure to 17alpha-ethinylestradiol in adult male Cnesterodon decemmaculatus (Pisces: Poeciliidae). Environ Toxicol Chem 36 (2017) 1738–1745. [DOI] [PubMed] [Google Scholar]
- [122].Zacharewski TR, Berhane K, Gillesby BE, Burnison BK, Detection of estrogen-and dioxin-like activity in pulp and paper mill black liquor and effluent using in vitro recombinant receptor/reporter gene assays. Environmental science & technology. 29 (1995) 2140–2146. [DOI] [PubMed] [Google Scholar]
- [123].Zhang Z, Wang J, Gao M, Li X, Cheng Y, Zhang X, et al. , New methods for purification of Paralichthys olivaceus lipovitellin and immunoassay-based detection of vitellogenin. Ecotoxicol Environ Saf 180 (2019) 624–631. [DOI] [PubMed] [Google Scholar]
- [124].Zhu Y, Rice CD, Pang Y, Pace M, Thomas P, Cloning, expression, and characterization of a membrane progestin receptor and evidence it is an intermediary in meiotic maturation of fish oocytes. Proceedings of the National Academy of Sciences. 100 (2003) 2231–2236. [DOI] [PMC free article] [PubMed] [Google Scholar]