Abstract
A major challenge in the delivery of cargo (genes and/or drugs) to cells using nanostructured vehicles is the ability to safely penetrate plasma membranes by escaping the endosome before degradation, later releasing the payload into the cytoplasm or organelle of interest. Lipids are a class of bio-compatible molecules that self-assemble into a variety of liquid crystalline constructs. Most of these materials can be used to encapsulate drugs, proteins, and nucleic acids to deliver them safely into various cell types. Lipid phases offer a plethora of structures capable of forming complexes with biomolecules, most notably nucleic acids. The physichochemical characteristics of the lipid molecular building blocks, one might say the lipid primary structure, dictates how they collectively interact to assemble into various secondary structures. These include bilayers, lamellar stacks of bilayers, two-dimensional (2D) hexagonal arrays of lipid tubes, and even 3D cubic constructs. The liquid crystalline materials can be present in the form of aqueous suspensions, bulk materials or confined to a film configuration depending on the intended application (e.g. bolus vs surface-based delivery). This work compiles recent findings of different lipid-based liquid crystalline constructs both in films and particles for gene and drug delivery applications. We explore how lipid primary and secondary structures endow liquid crystalline materials with the ability to carry biomolecular cargo and interact with cells.
Keywords: lipid-based liquid crystals, lipid films, lipid particles, small molecules, drug delivery, gene delivery
1. Introduction
The amphiphilic character of lipids, i.e. molecules composed by a hydrophilic -polar- head and at least one hydrophobic -non polar- tail, helps to explain much of their biological function in vivo: lipids are the primary building blocks of cell membranes. In addition, amphiphilicity in lipids explains their polymorphism i.e. their ability to self- assemble in aqueous solutions into liquid crystalline materials of various geometries. While the plasma membrane adopts a bilayer form in the cell, other lipid structures such as inter-bilayer contacts (stalks), 2D hexagonal lipid tubes, and cubic structures are also prevalent across many natural membranes; yet their role remains elusive [1–3].
Lipid polymorphism is particularly relevant when designing lipid-based lyotropic liquid crystals (LLCs) for biomedical applications such as drug and gene delivery. Most LLCs are lyotropic (i.e. resulting from the self-assembly of amphiphilic lipid molecules present in water or in a humid environment) [4]. LLCs can exist in bulk form, thin films or as particles suspended in solution. The lamellar (i.e. composed by layers) [5], inverted [6] and normal [7] hexagonal, as well as cubic [8] LLCs phases have all been applied for the delivery nucleic acids into live cells and animals (Fig. 1).
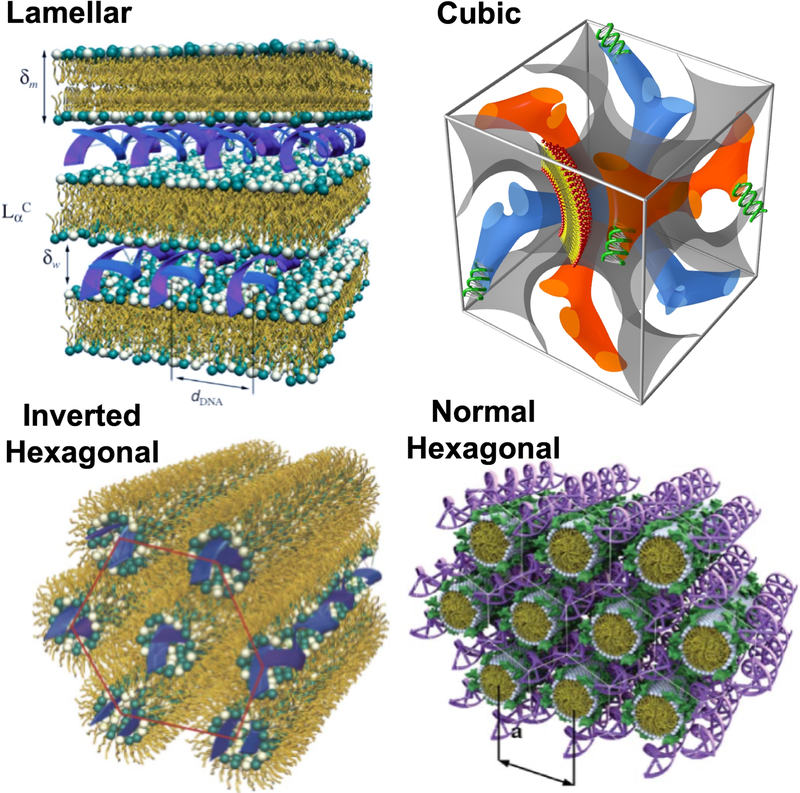
Figure 1.
Schematic representation of the lamellar, cubic, and hexagonal complexes for delivery of nucleic acids. [9]. Reprinted by permission of the publisher (Taylor & Francis Ltd)
The design and engineering of lamellar and non-lamellar LLC structures represent a hallmark of nanomedicine due to the fact that drug/gene encapsulation and transport are dictated by the nanostructure of the carrier. For example, lamellar lipid films, including supported lipid bilayers and hybrid lipid-polymer membranes are able to encapsulate hydrophobic drugs. Their release profile is controlled by one-dimensional permeability of drugs across membranes as well as the number of layers. Permeability is passive but can be enhanced by the introduction of interfacial defects (i.e. domain formation) or by increasing membrane fluidity [10–12]. Other controlled release methods rely on activating phase transformations, e.g. a lamellar or 2D hexagonal structure supported on a substrate can transform into a 3D-cubic lipid film [13]. In this case, the release is activated by increasing the number of spatiotemporal pathways for the drug to diffuse out of the materials. In bulk, LLCs are randomly oriented throughout the test tube. However, the introduction of a substrate and the formation of a thin film orients LLCs along a preferential order at micron scales. This is important when considering drug leak-out via phase transformations. For instance, if we consider an hydrophilic drug, a 3D cubic phase allows for 3D diffusion whereas an inverted hexagonal phase with tubes laying flat on a substrate only provides a single one-dimensional leak-out pathway. Likewise, lamellar lipid particles such as liposomes and non-lamellar lipid particles such as 2D-hexagonal phases and cubosomes (lipid-based 3D bicontinuous cubic particles) enable the release of high concentrations of cargo into cells [8,14–16] through efficient drug leak-out mechanisms that include passive permeation and/or endosomal membrane fusion. An illustration of LLC structures prepared as films on a substrate and as suspended particles in solution is shown in Table 1.
Table 1.
Lamellar and non-lamellar lipid-based films and particles for delivery of small molecules. Top left: supported 1D-lamellar siRNA complex. Reprinted by permission of the publisher (John Wiley and Sons) [13]. Top right: liposome-siRNA complex. Bottom left: supported 3D bicontinuous cubic lipid film loaded with siRNA. Reprinted by permission of the publisher (John Wiley and Sons) [13]. Bottom right: cubosome-siRNA complex. Reprinted with permission from [19]. Copyright 2018 American Chemical Society.
Accelerating the design of better therapeutic agents mandates elucidating the spatiotemporal distribution of LLC structures within cells, and their transport and cargo release mechanisms. This includes interactions with the extra and intracellular environment, cell macromolecules (e.g. transmembrane proteins) and organelles (e.g. endosomes) [28]. In this review article, we highlight lipid-based small molecule release prompted by phase transformation, as well as the importance of harnessing mesoscale structure in order to improve the performance of cargo delivery into cells. A major research goal on lipid complexes is to find the correlation between the different LLC structures and small molecule delivery efficiency. The purpose of this review is to highlight, categorise, and contrast various lamellar and non-lamellar LLC-based particulate and supported nanostructures as carriers of small molecules into the eukaryotic cytosol in order to inform the design of next-generation LLC-based nanocarriers.
1.1. Delivery of small molecules
Achieving efficient delivery of small molecules in ways that are specific to cell type and non-toxic is one of the major challenges in the design of nanomedicines. Encapsulation of drugs into a carrier avoids various sources of toxicity and prolongs the circulation time of drugs in the bloodstream in order to reach their target of interest before clearance. Since nanocarrier chemistry is rather modular, one can even modify the particles with specific cell-targeting units. This has however proven to be more challenging than anticipated [29]. In fact, relying on physical properties such as size and shape [30–32] can result in equally efficient (or better) strategies to improve performance.
Internalisation of materials in the cytosol requires overcoming at least two biological barriers: 1) crossing the semi-permeable cell membrane, and 2) escaping the endosome in a timely manner before the endosome-lysosome degradation pathway [33]. Membrane fusion and plasma membrane permeation are two different processes that both cost energy. Membrane fusion refers to the event of two different lipid bilayers merging into a single continuous bilayer. Membrane crossing depends on membrane permeability and physicochemical characteristics of the crossing molecules and it can occur via passive and active diffusion and/or membrane fusion, among others. It is noteworthy that membrane fusion is a well-established mechanism to mediate intracellular trafficking of molecules [34–36]. While membrane-fusion events are frequent in nature (e.g. during exocytosis, synaptic junctions, and endocytosis), crossing the plasma membrane is a much more selective process. Plasma membranes act as specific barriers against certain species such as ions [37,38], requiring protein ion pumps and carriers for instance. The majority of nanoparticles cross the plasma membrane via endocytosis. Unfortunately, the fate of most of these materials is often trapping and degradation inside the endosome. Endosomal escape is thus a crucial step that materials much overcome for efficient delivery of cargo to cells [39–41]. In this review article, we focus on a vastly unexplored physicochemical characteristic of drug carriers beyond composition, surface, size and shape: their internal nanostructure (Fig. 2). In particular, we discuss the process of delivery of nucleic acids for gene silencing (siRNA) and hydrophobic small molecules (paclitaxel) using different lamellar and non-lamellar LLC films and particles. Lyotropic liquid crystals are promising materials to study the effects of structure on cell internalisation.
Figure 2.
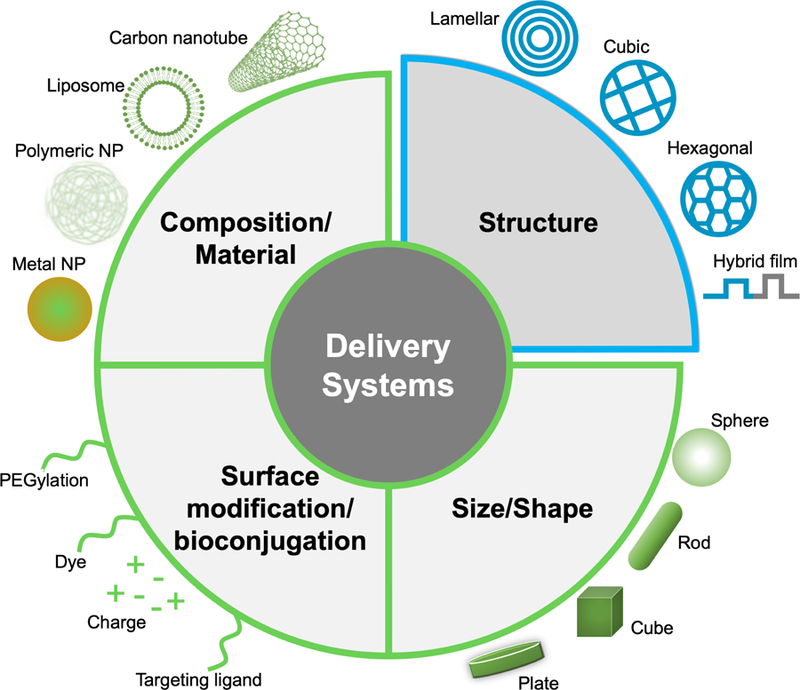
Schematic representation of engineered physicochemical properties of drug delivery systems. Typically, size, shape and surface of nanoparticles (NPs) have concentrated the attention for the design of novel nanomedicines. The internal structure of particles and/or films is now known to play a pivotal role in the process internalisation of small molecules by cells.
1.1.1. Nucleic acids
The delivery of nucleic acids into the cytosol has become a growing field in biomedicine [7,20,22,25,33,40,42–49]. siRNA is a class of double stranded short (ribo)nucleic acids (21–23 nucleotides long) capable of achieving gene knockdown (i.e. silenced or decreased gene expression) in eukaryotic cells. The clinical potential of siRNA in diseases such as cancer, neurodegenerative disorders and viral infectious diseases has been amply shown [50]. DNA depends upon entering the nucleus to initiate the transfection process. In contrast, siRNA does not require entering the nucleus: once it enters the cytosol, it is incorporated into a protein complex that finds and destroys target messenger RNA, which further propagates gene silencing [51,52]. Naked siRNA has shown silencing efficiency but is prone to enzymatic degradation and its negative charge prevents it to easily enter the cytosol. The design of non-cytotoxic carriers that deliver siRNA in a localised manner [47] and provide further specificity to gene silencing has become a critical challenge for the development of better therapies. Various lipid-nucleic acid complexes have been developed in order to address some of these issues and in this paper we describe a few examples [8,13,19,20,40,41,49,53,54].
1.1.2. Hydrophobic drugs
The hydrophobicity of some therapeutic molecules stand as a significant challenge to viable drug therapies. For instance, Gram-negative bacteria are inherently resistant to many hydrophobic antibiotics including fusidic acid (0.52 KDa), erythromycin (0.73 KDa), novobiocin (0.61 kDa) and rifampin (0.82 kDa) due to the permeability barrier of their outer membrane [55]. In addition, many anticancer drugs such as paclitaxel (0.85 kDa) and rapamycin (0.91 kDa) are highly hydrophobic and readily crystallise. Consequently, their application depends on either chemical modifications to increase their solubility or the use of carriers [56]. The internalisation of hydrophobic therapeutic small molecules into the cytoplasm is another significant challenge that can be addressed by using nanocarriers [57]. With super hydrophobic molecules such as paclitaxel, crystallisation is not easily hindered, even upon inclusion in a drug carrier [58]. Lipid-based structures have been explored in order to prevent drug crystallisation and improve drug internalisation with minor toxic effects [59,60].
2. Lipid ftlms: lamellar and non-lamellar liquid crystal phases for drug/gene delivery
Lipids can be stabilised in the form of adsorbed thin films, adopting a variety of structures that are very amenable to drug/gene incorporation and release.
2.1. Lipid-only films
Typical phospholipid bilayers are around 10 nm thick with an interbilayer spacing (i.e. thickness of water layer) of around 2 nm [4,61,62]. Depending on surface type, lipid monolayer alkyl tails point towards the surface (i.e. hydrophobic) or towards air (i.e. hydrophilic), serving as the initial interface onto which bilayers and multiple layers of bilayers can be deposited to build hierarchic mesostructures (Fig. 3). The films can be formed by direct spreading (i.e. depositing organic lipid solutions onto substrates by drop casting or spin coating) or layer-by-layer assembly (ie. depositing layers one by one where layering is formed by electrostatic interactions) [11,63–65]. Variations within methods allow to control film thickness and interlayer incorporation of molecules. Drop cast methods yield films of a few microns thick whereas spin coated lipid films can be as thin as 200 nm. Supported lipid layers (Fig. 3) [61,62] tend to preserve the fluidity or rigidity of the bulk lipid phase. Gel-phases where alkyl chains are all trans, liquid crystalline phases with fluid tails, and liquid ordered phases have all been observed in supported systems [66], providing a convenient model to study membrane behaviour [67]. The advantage of having a supported system is the control of the bilayer orientation. Multiple lipid layers have then the ability to mimic the structure and organisation of a variety of dynamic molecular assemblies found in nature such as multilamellar bodies in lungs [68]. The plasma membrane consists of a single bilayer, but other cell membranes (e.g. those in the endoplasmic reticulum, around axons in nerve cells [69], mitochondrial membranes) are multilayered.
Figure 3.
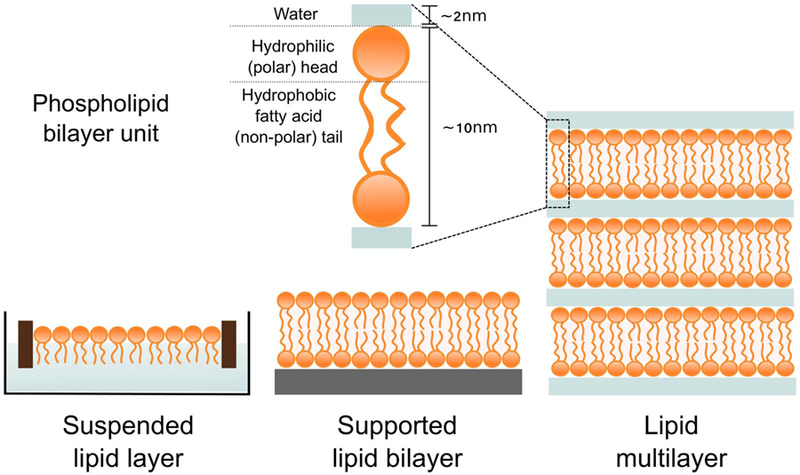
Schematic representation of different lipid layers. A phospholipid bilayer unit represents the building block of the lipid membrane.
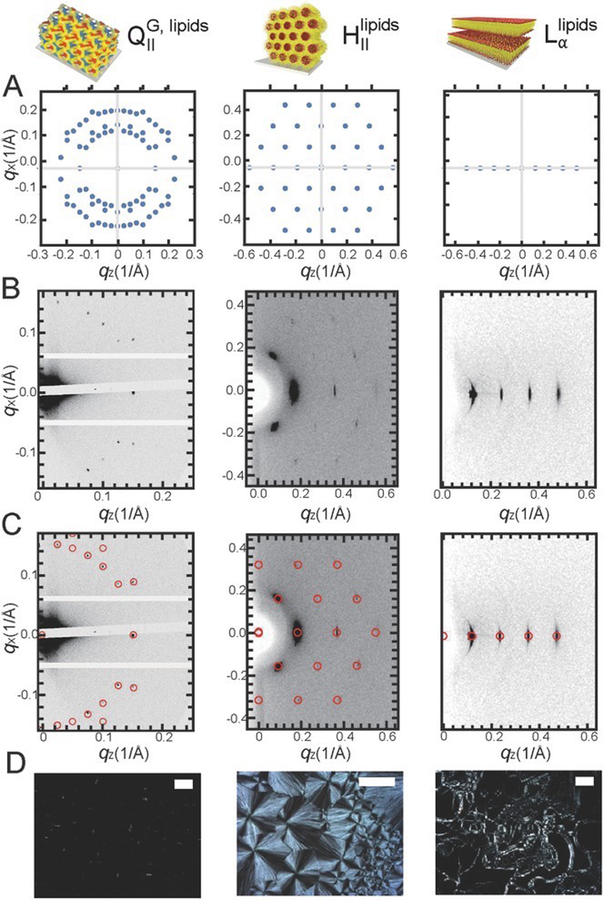
Lipid film structures can be characterised with X-ray and microscopy techniques. For instance, anisotropic lamellar or hexagonal phases exhibit birefringence with unique optical textures that enable distinguishing between existing phases. Conversely, cubic phases are isotropic and not birefringent, appearing dark under polarised light [11]. X-ray under grazing incidence (GI-SAXS) combined with polarised microscopy is a very powerful approach to elucidate lipid film nanostructure, and orientation with respect to the substrate. Rich polymorphism can be achieved by a rational choice of lipid composition and ambient conditions (Fig. 4). 3D bicontinuous cubic, 2D hexagonal, and multilamellar phases can clearly be identified using polarised microscopy and GI-SAXS. It is worth noting that the samples exist in the form of thin films where the nanostructure is completely uniform and oriented at a single preferred orientation giving rise to single-crystal like diffraction spots.
Figure 4.
A) Reciprocal space patterns of different space groups simulated by NANOCELL. B) 2D GISWAXD patterns for selfassembled lipid films of a bicontinuous cubic gyroid (QG), an inverted hexagonal (HII), and a lamellar (Lα) structure from left to right. Films have highly ordered structures as indicated by spot patterns instead of rings. C) NANOCELL simulation overlaid onto the GISWAXD data. The fact that simulated patterns perfectly match with actual data proves that lipid self-assembled films have well-organised structures. D) Polarisation light microscopy images of lipid films with distinct textures. Optically isotropic cubic phases appear as a black background under the polariser while an inverted hexagonal phase shows focal conical texture and a lamellar phase (Lα) represents oily streaks with uniaxial texture. A nanostructure of each film is illustrated at the top of the figure. Reprinted by permission of the publisher (John Wiley and Sons) [13].
Given their uniform orientation and anisotropic optical properties as a function of humidity and temperature, lamellar and non-lamellar lipid films may be utilised for two main purposes: as hosts to sense and report molecular/analyte binding and also as platforms to deliver small molecules in situ. In fact, the lipid films described in Fig. 4 were successfully employed as a platform for surface-based siRNA internalisation and gene knockdown in cells [13]. 3D bicontinuous structures are particularly interesting. These structures comprise a continuous lipid bilayer where the middle of the bilayer conforms into a periodic minimal surface with negative Gaussian curvature [70]. The bilayer is in contact with unconnected networks of water channels. Such structure yields high internal surface area per volume (400 m2 g−1) [71], which enables high loading capacity and/or fast release of drug molecules or genes. In this case, layer- by-layer methods are not amenable and 3D bicontinuous cubic films are produced by either drop casting or spin coating. Monoolein and phytantriol lipid molecules are well known for their ability to form cubic phases in both bulk and supported thin films [11,72–77]. In addition to X-ray scattering, inverse bicontinuous cubic phase films have been directly visualised using atomic force microscopy (AFM) in fluid [76].
2.2. Lipid-drug/gene films
Surface-based delivery of drugs and genes allows spatial control and sustained release with locally high concentration and enhanced cell contact, which is particularly attractive against localised diseases. Lipids have the capacity to display a rich phase behaviour when supported onto surfaces (Fig. 4) [12,19,77–79], but their use has been mostly reserved as cell membrane models. Lipid films are able to incorporate large amounts of small molecules (hydrophobic and/or hydrophobic) and phase transformations between different nanostructures can be tuned by lipid composition, temperature and humidity. This responsive and often reversible phase behaviour has extraordinary potential as a means to synthesise triggerable and controlled drug delivery platforms [11]. Unlike most polymeric systems in which cargo delivery relies on degradation or swelling, the delivery of small molecules by surface–based lipid layers can be activated by phase transitions. Most of the existing films used in substrate-mediated gene delivery are assembled in a layer-by-layer fashion [11,63] where the hydrophobic small molecules are incorporated within the layers that become part of the LLC structure. Kang and coworkers [13] previously investigated the potential of surface–based lipid films to deliver siRNA to HeLa cells in vitro and elucidated the mechanisms by which the film’s LLC phase controlled the siRNA delivery efficiency. Substrate-mediated cellular delivery was based on soft adsorbed lipid materials with highly ordered and oriented nanoscale structures. These supported films were highly responsive to environmental conditions, enabling siRNA silencing via designed activation of the phase transition in supported lipid films as a function of humidity and temperature. Starting from drier up to more humid conditions, resulted in a range of structures covering 2D-inverted hexagonal, 1D-lamellar, up to 3D-bicontinuous cubic gyroid, respectively.
The corresponding phase diagram [13] indicates that lamellar phases can be found in both dry and wet films as well as in bulk solution with and without siRNA. The only-hexagonal phase appeared in dry conditions with and without siRNA. Finally, the cubic phase was identified only in wet films and in bulk solutions with and without siRNA. These phase transformations were reversible and stable for multiple cycles of wetting and drying with a transition time scale of a few minutes. Scattering data indicated that some degree of order was lost in the lipid-nucleic acid complexes in comparison with the lipid-only structures: siRNA was indeed included in the lipid structure. Moreover, the encapsulation efficiency of the lipid films was beyond 97%. As mentioned above, switching from a 2D hexagonal or a 1D lamellar to a 3D in bicontinuous cubic phases is a strategy capable of increasing the number of pathways for gene leak out (Fig. 5). The resultant lipid-siRNA supported films successfully enabled knock-down of specific genes (around 50% efficiency), resulting in the 3D bicontinuous cubic phase having the higher gene silencing efficiency. This surpassed that of lamellar systems in this study as well as previously performed experiments of surface-based DNA delivery [79–81].
Figure 5.
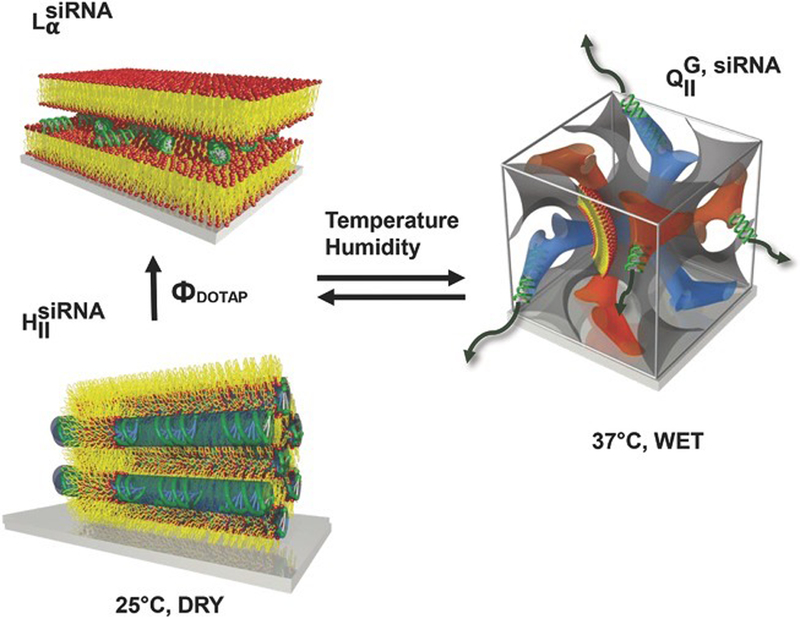
Schematic of different nanostructures as oriented onto solid substrates as lipid composition and environmental conditions change. This highlights the potential of using lipid film transformations for actuated surface-based cellular delivery. The diffusion of biomolecules is enhanced from 1D in lamellar phases to 3D in bicontinuous cubic phases by substrate hydration at 37 °C. The phase transitions are reversible demonstrating the equilibrium nature of these systems. Reprinted by permission of the publisher (John Wiley and Sons) [13].
Lee and coworkers [12] reported temperature-sensitive lipid-based layered films supported on thin, bioresorbable implants. In this work, the authors designed a uniform, multilayered coating of a thermally triggerable biological lipid membrane embedded with one or more drug molecules such as doxorubicin and dextran, made with biocompatible materials in order to retain the molecules for months in vitro, and rapidly release them upon heating at usual fever temperatures (41 43 °C). Two coexisting phases of the lipid membranes were observed: liquid ordered with rigid tails, and liquid disordered with tails that can undergo rapid conformational changes.
The liquid ordered phase tended to form a columnar lamellar phase [66]. Small molecules were sandwiched between the lipid layers. In this design, thermal actuation induced a transition to the liquid disordered phase allowing diffusion of the drugs out of the system in a highly controlled manner. Scattering data confirmed the liquid ordered/disordered phase transition at 41.5 °C. In addition, the release profile of the small molecules showed that drug loading and release rate also depended on molecular weight and lipid membrane charge density. Therefore, proper design of thermosensitive lipid membranes that enhances the interactions with the drugs (e.g. enhancing diffusivity) represents another approach to localised deliver of small molecules in a precisely controlled manner.
2.3. Hybrid lipid-polymer membranes
Transport through lipid membranes is ubiquitous in nature. Membrane proteins such as ion channels and aquaporins mediate transport of ions and water, respectively. However, the membrane itself contains mechanisms that modulate and enhance passive transport. As described above in the work of Lee et al. [12], coexistence between multilayered lipid membranes with domains of liquid disordered alkyl chains and liquid ordered states leads to enhanced permeation of drugs through membranes. Inspired by this observation we explored the formation of membrane domains and associated permeability by hybridising a lipid bilayer with an amphiphilic block-copolymer [10, 11,17].
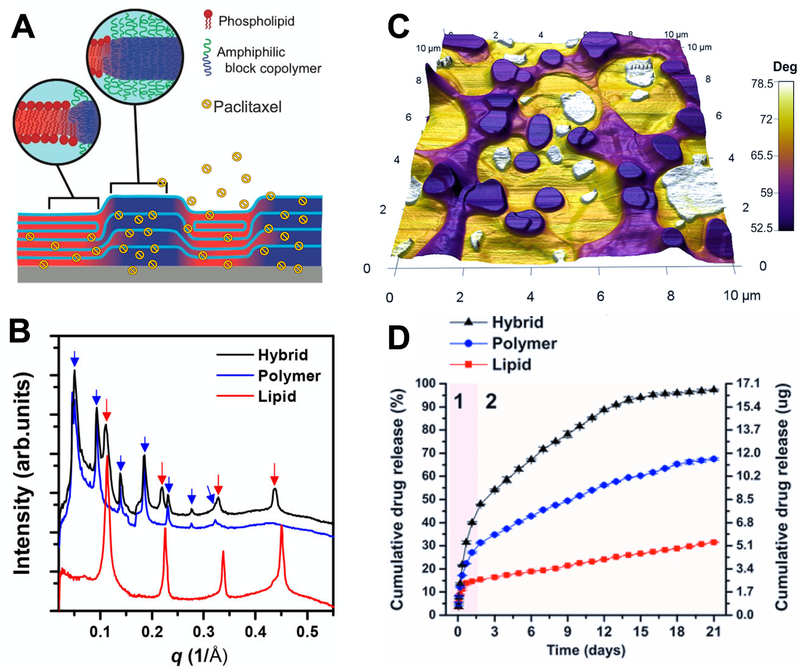
Similar to lipids, amphiphilic block co-polymers (BCP) have the ability to self-assemble in water into spherical, lamellar, and cylindrical morphologies. When hybridised in a single membrane, saturated phospholipids and polymers partition into lipid-rich and BCP-rich domains in the membrane plane [82,83]. Recently, the Leal group showed, for the first time, that lipid-rich and the BCP-rich domains align in complete registry across films several microns thick when such hybrid membranes are stacked [10] (Fig. 6A). This columnar organisation of domains makes this system phase-separated in three dimensions. The domains can be rather large yielding two distinct lamellar diffraction patterns in X-ray scattering with a lipid-rich repeat spacing of d = 5.6 nm and BCP-rich, d = 12.4 nm (Fig. 6B). The interfacial region between domains is rather wide as well, and the domains display different mechanical properties as it can be observed by AFM in phase mode (Fig. 6C), consistent with the presence of polymer-rich and lipid-rich areas.
Figure 6.
A) Schematic illustration of hybrid films for paclitaxel incorporation and delivery: lipid (red) and polymer (blue) domains. B) Radial average intensity versus q profiles of hybrid, lipid and polymer films.
C) AFM phase contrast image overlaid onto 3D height (topography) image of hybrid films. Note the colour contrast between different regions, indicative of the presence of different domains. D) Cumulative release profiles of paclitaxel from the hybrid films. Reprinted (adapted) with permission from [10]. Copyright 2017 American Chemical Society.
Depending on the nature of the drug molecule, it may accumulate in specific domains. For instance, Kowal and coworkers [84] found that membrane proteins are preferentially found in the more fluid regions of a hybrid-polymer monolayer. They reported that tuning the composition of the polymer-lipid mixtures favoured successful insertion of membrane proteins with desirable spatial distributions (i.e. in polymer-rich or/and lipid-rich regions). In our work, we found that paclitaxel is preferentially located at the interface between polymer-rich and lipid-rich domains [11,17]. Interestingly, the 3D phase separated domains enhanced the permeation of hydrophobic paclitaxel molecules (Fig. 6D). Through a combination of transport studies, solid-state NMR, and GI-SAXS, this synergistic effect was attributed to lipid and BCP hydrophobic mismatch and alteration of local packing of lipid alkyl chains due to the presence of BCPs. In other words, domain interfaces serve as pathways for enhanced permeation [10,11,17]. In addition to enhanced permeation, it was found that paclitaxel crystallisation within the lipid-BCP hybrid membranes is significantly suppressed compared to the case of paclitaxel crystallisation embedded in lipid-only or polymer-only membranes. This fact is biomedically crucial, and we argue that it can be generalised towards various drug delivery systems. Purposeful engineering of either phase-separated domains within membranes, or assembly of composite liquid crystal membranes could be a route to suppress hydrophobic drug crystallisation and increase permeation rates.
3. Lipid particles: lamellar and non-lamellar liquid crystal phases for drug/gene delivery
In the previous sections, we discussed examples of bulk and thin film LLCs with and without cargo adopting a rich variety of structures. The carriers must be stable suspensions of nanoparticles with sizes in the range of 50–100 nm for the majority of gene or drug delivery applications. LLC nanoparticles are prepared in a variety of ways depending on the type of LLC, the drug or gene that needs to be encapsulated, and the required size. The most popular LLC nanoparticle is the liposome which derives from the bulk lamellar phase. Liposomes have been used for the encapsulation and release of numerous drugs and many liposomal formulations are currently on the market [85]. The focus of this paper is to highlight the importance of LLC nanostructures in terms of their ability to deliver cargo to cells. Hence, we will not survey the vast usage of liposomal applications and will narrow the discussion to the particular case of liposomes used for nucleic acid delivery where a relevant structural transformation exists. Most bulk LLCs have been formulated in a nanoparticulate form. Liposomes obtained from the lamellar phase, hexasomes stemming from the hexagonal phase, and cubosomes (Fig. 7) originating from the 3D bicontinuous cubic phase exemplify LLCs reported in various gene and drug delivery applications [86].
Figure 7.
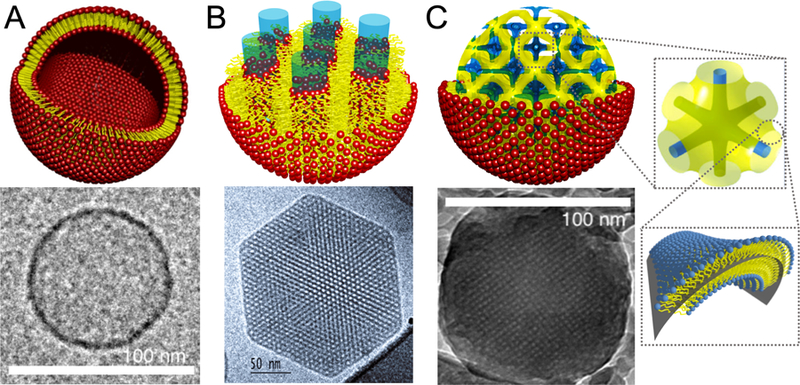
Structure of three different lipid particles and their respective Cryo-EM images. A) Schematic of a liposome and the correspondent Cryo-EM image indicating a size of ca 100 nm in diameter. B) Hexosome representattion and the correspondent Cryo-EM image of a ca 200 nm particle. The Cryo-EM data is reprinted with permission from [86]. Copyright 2005 American Chemical Society. C) Cubosome schematics having a primitive bicontinuous cubic structure enclosed by a single lipid leaflet, where yellow represents the midplane of the lipid bilayer and blue the water channels. A single unit cell is also represented to the right of the cubosome schematic. The Cryo-EM shows a ca 100 nm cubosome. Reprinted with permission from [19]. Copyright 2018 American Chemical Society.
3.1. Liposomes and lipoplexes
Liposomes are traditionally prepared by either sonication of a lamellar phase in bulk or extrusion, or both [87,88]. The result is a closed spherical lipid membrane with an aqueous interior with controllable diameters of around 50–500 nm which are not thermodynamically favoured but are temporally stable for long periods of time before fusion and aggregation back into a bulk lamellar phase. More recently, microfluidic approaches based on nanoemulsion synthesis have proven to be a useful method to achieve very small and monodisperse liposomes, even when loaded with cargo [49]. As means to increase shelf-life stability as well as circulation time in blood, most liposomal formulations nowadays have a lipid component that is covalentely linked to a polyethylene glycol unit at the headgroup (PEG-lipid) [27,89]. Because of the structural resemblance between liposomes and cell membranes, they are widely used as model systems [90] in addition to drug and gene delivery. Owing to their LC nature, liposomes are responsive to external stimuli such as humidity, temperature, pressure, or even ultrasound [21].
Unlike most small drug systems, upon addition of DNA or even very short siRNA molecules, strong electrostatic and entropic (counterion release) interactions lead to a transformation from a single membrane vesicle into a multilayered structure (Fig. 9 C). These particles are designated as lipoplexes, referring to the interaction between cationic liposomes with nucleic acids. Such layered structure is composed of lipid bilayers separated by bulk water where a single layer of nucleic acid is located, constituting a lipid-nucleic acid ”sandwiched” lamellar structure. When the nucleic acid is long enough, DNA organises itself as in the smectic phase with a LC director orthogonal to that of the lipid lamellar phase [5,9,18,23,25,26]. Reviews of lipoplexes for gene delivery can be found in [22,91]. Lipoplexes (overall positive charge) exhibit significantly higher transfection efficiency than other carriers (e.g. anionic liposomes) [9]. The development of lipoplexes with low cytotoxicity is highly important, which arises in lipoplexes by virtue of the cationic lipid component of the complex [48]. The synthesis of degradable cationic lipids that contain a disulfide bond spacer between the headgroup and the lipophilic tails is a clever strategy to cleave the lipoplexes in the reducing environment of the cytosol and thus decrease cationic lipid toxicity [25].
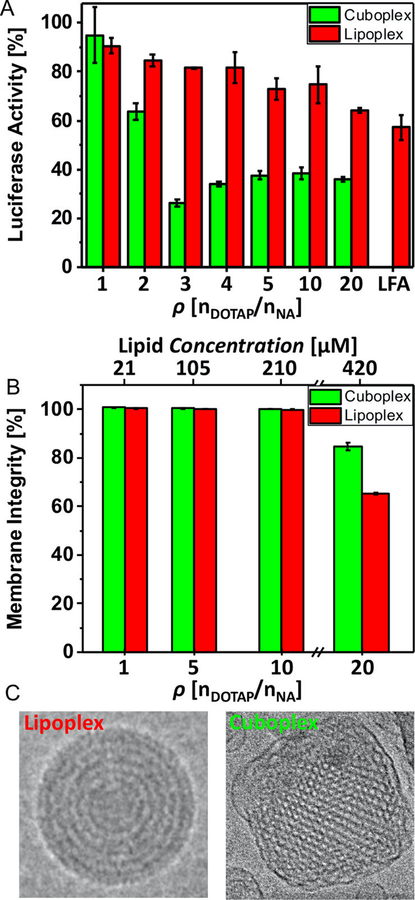
Figure 9.
Gene silencing cuboplexes. A) Luciferase gene knockdown in HeLa-Luc cells of cuboplexes (green bar) and lipoplexes (red bar) at different charge ratios. For cuboplexes, the maximum knockdown efficiency was of 73.6% versus 35.8% from lipoplexes. B) Membrane integrity test of HeLa-Luc cells when incubated with cuboplexes and lipoplexes. C) Representative Cryo-EM images of a lipoplex and a cuboplex. Reprinted with permission from [19]. Copyright 2018 American Chemical Society.
3.2. Cubosomes and cuboplexes
As mentioned above, the lipid bicontinuous cubic phase can be expressed as an infinite series of periodic minimal surfaces (gyroid, diamond, or primitive), which divide the space into two separated water channels (see Fig. 1 for a schematic representation of the gyroid). Traditionally, the gyroid phase is present at low water content while the diamond phase occurs at high water content [92]. The Leal lab pioneered the stabilisation of highly ordered 3D bicontinuous of any desired space group in excess water by the incorporation of cationic and PEGylated lipids [93,94]. In addition, unit cell dimensions can be modulated from 15 to 70 nm by controlling lipid additive composition and processing conditions. The cubic phase possess several structural advantages –including membrane elasticity with fusogenic properties, i.e. the ability to facilitate membranes fusion- that make them attractive for gene and drug delivery. In addition, cubic phases occur in biological membranes across all biological kingdoms, in particular, in membranous processes related to pathophysiological conditions. An exhaustive review with examples of cubic phases in biological membranes can be found in [3].
Analogously to liposomes, cubosomes are nanoscale suspensions derived by a bulk cubic phase (Fig. 7). Firstly identified during fat digestion [95] and later described with respect to their physicochemical properties [96], cubosomes have received enormous attention in the past years [97,98]. They can be synthesised in various forms but, unlike liposomes, their stabilisation in aqueous solutions traditionally requires high temperature homogenisation of their bulk phase in the presence of a stabiliser (pluronic polymers) [86]. This procedure is problematic when cubosomes are intended to serve as carriers for easily degradable biomolecules. A paramount breakthrough in cubosome preparation was the discovery that i) a judicious choice of lipids matching a region in the bulk-phase diagram can be found where the cubic phase is prevalent and ii) that the incorporation of a PEGylated lipid allows the formation of cubosomes in exactly the same way as liposomes are produced. Ultrachilled mild sonication leads to the stabilisation of highly ordered nanoparticles with 3D bicontinuous internal structure which is very amenable to the incorporation of sensitive biomolecules such as siRNA [20]. Most importantly, the system is perfectly biocompatible since the stabiliser is no longer a toxic pluronic polymer. Instead, the same PEGylated lipid used in clinically viable stealth liposomes suffices to provide self-life (and blood circulation) stability. Other studies have suggested milder bottom-up preparation routes, instead of homogenisation or sonication of the bulk-phase. Bottom-up approaches utilise volatile solvents to solubilise the lipids that are then added drop-wise into bulk water. Owing to the low solubility of lipids in water, nanoprecipitates are formed and their size can be controlled by volatile solvent/water mixing rate. Upon volatile solvent extraction, the nanoprecipitates develop as cubosomes suspended in water [99]. However, the process gives rise to polydisperse and large size cubosomes due to the bulk-scale nature of mixing volatile solvents and water.
Recently, the Leal lab devised the first microfluidic synthesis of cubosomes which uses the principles of nanoprecipitation but at the scale of the miniaturised volumes contained in microfluidic chaotic mixers. The result is a strikingly uniform, well-ordered solution of cubosomes with record small sizes of around 75 nm [19]. Upon complete removal of the volatile solvent, siRNA was successful incorporated within the water channels of the cubosomes. Cryo-EM images of cubosomes do not permit observation of siRNA, but modifying siRNA with a small gold nanoparticle is a strategy to increase contrast, thus revealing loaded nucleic acids within the cubosome water channels (Fig. 8). To the best of our knowledge this is the first direct observation of a cubosome successfully loaded with a nucleic acid, prompting the Leal lab to give cubosomes incorporated with siRNA the name of cuboplexes. Indeed, the encapsulation of nucleic acids into cubic phases, which are rather rigid and rod-like, is not straightforward. DNA tends to pack end-to-end and become incorporated with molecules as short as 11 base-pairs into cubosomes, leading to the transformation of a cubic phase into a hexagonal structure [24]. For the case of siRNA, packing end-to-end, is not ideal and even though typically molecules have between 20 and 30 base-pairs, their inclusion in cubic phases of different symmetry groups is possible [8,13,16,19,20,22,48]. This is achievable under the constraint of keeping the amount of cationic lipid significantly lower compared to the cubic-phase forming lipid. Increasing the cationic charge density of the cubic phase while keeping the composition of cationic lipids low is attainable by using lipids that carry multiple charges in the headgroup. Leal and coworkers [16] reported the synthesis of bicontinuous cubic phases using pentavalent cationic lipids. As expected, the incorporation of siRNA within small cubosomes is also very sensitive to the route of assembly. For example, as described above, microfluidic synthesis of liposomes and lipoplexes containing siRNA was successfully employed to produce small and monodisperse nanoparticles [49]. In this case, siRNA was uploaded upstream at the aqueous phase inlet of the microfluidics device. Interestingly, cuboplexes could not be produced in the same manner. Cubosome formation hinges on the ability of particles in the nanoemulsion to fuse during flow. The presence of siRNA during this process hinders fusion and the resultant cubosomes are significantly less ordered. For this reason, siRNA encapsulation downstream after complete evaporation of the volatile solvent is preferred. Sensitivity to processing conditions in cubosome and cuboplex formation is to be expected. Analogous to liposomes, these LLCs nanoparticles are not equilibrium forms, and exist as kinetically trapped systems.
Figure 8.
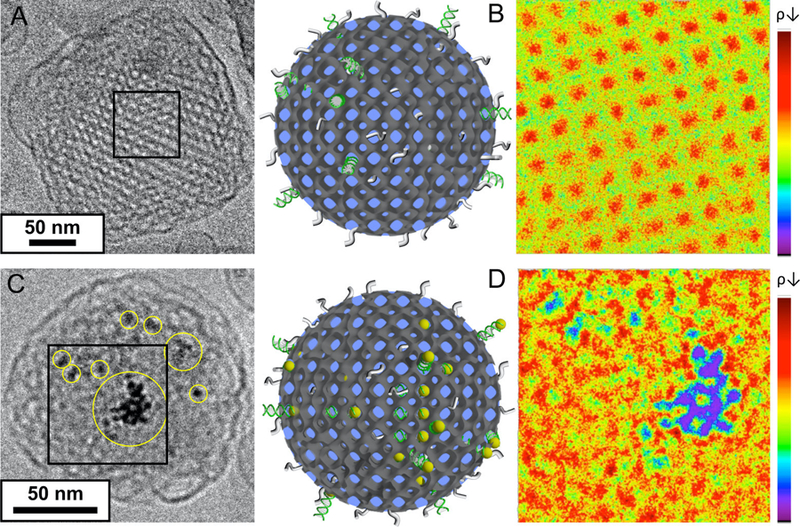
siRNA loading. A) and C) Cryo-EM images of cubosomes having A) siRNA and C) siRNA–gold nanoparticles conjugates. siRNA–gold nanoparticles conjugates provide much higher contrast to locate siRNA molecules within the cubosomes. B) and D) 2D electron density maps of selected boxes (70 × 70 nm) shown in A) and C) (red for low and blue for high electron density). B) The well-ordered lipid membrane (green colour) is forming a square lattice aligned in the [100] direction. The red colour in the map represents less electron dense regions, the water channels. On the other hand, D) shows additional blue and purple regions (high electron density) corresponding to gold nanoparticles locations and hence the approximate siRNA location. This is a direct proof of siRNA incorporation into a cubosome. Schematics of cuboplexes are also shown in the middle of the figure. Reprinted with permission from [19]. Copyright 2018 American Chemical Society.
Cuboplexes exhibit remarkable siRNA delivery efficiency and specific gene knockdown compared to conventional lipoplexes (Fig. 9) at low membrane charge density σM, demonstrating that high delivery efficiency is not controlled by membrane charge density. This is relevant to biomedical applications because cationic lipids are usually the source of toxicity in lipid nanoparticles. As an example, gene expression was significantly decreased when cuboplexes were used to encapsulate an siRNA sequence targeting luciferase. The amount of siRNA in lipoplexes and cuboplexes is comparable, thus the effect is not related to higher siRNA concentration. We are currently investigating the potential mechanisms associated to these results, but we have indications suggesting that the main process of cellular uptake is endocytosis [13]. The use of endocytosis inhibitors allude to the idea that cubic phases possess higher endosomolytic properties (i.e. enhanced capability of escaping the endosome) than LLCs in the lamellar structure.
3.3. Hexagonal phase
Bulk 2D hexagonal structures both with and without embedded nucleic acids have been extensively studied. Aqueous suspensions of the 2D hexagonal phase, i.e. hexosomes (Fig. 7), have been reported [86] but their use as nanocarriers [100] has received much less attention, particularly for nucleic acid delivery. Bulk 2D hexagonal phases can be either of the normal type (i.e. lipid tubes with fatty interiors assembly into a 2D hexagonal lattice with DNA [7,101]), or of the inverse type (i.e. the tubes are aqueous on the inside having DNA inserted and fatty alkyl chains point outwards [6,9]). The stabilisation of the normal hexagonal phase in lipids is only possible for a small selection of molecules with rather large headgroups. As a result, most reported lipid hexagonal phases (with and without cargo) are of the inverse type. This is also the case for hexosomes. Even when nanoscale hexosomes loaded with DNA or siRNA have not been directly reported, the bulk inverted hexagonal phase has been utilised to deliver siRNA in very dilute conditions [102] and DNA [103] to cells. It is postulated that under these conditions particles in solution resemble the structure of the bulk phase, however large and polydisperse. The internalisation of the hexagonal phase in the cytosol appears to be independent of charge density σM, and exhibits high transfection efficiency [9] and gene silencing [102]. It has been suggested that hexagonal phases rely on direct fusion with the plasma membrane to release their payload, instead of endocytosis [103,104]. This suggests a distinctly new mechanism of action for these lipid-nucleic acid complexes. Since the hexagonal phases tend to form tubular symmetric structures (i.e. pore-like structures), it would be possible for these structures to rapidly fuse with cellular membranes through pore formation even before the endocytosis process occurs. The latter could explain the cytotoxic effects observed of hexagonal phases [102] compromising the integrity of the cell membrane. Nevertheless, one may take advantage of the ultra fusogenic nature of the hexagonal phase if its onset is triggered after another less toxic LLC structure is internalised via endocytosis. For instance, Leal and coworkers [24] found that temperature modulation allows controlling the length of linearly stacked DNA, inducing reversible structural transitions from inverse hexagonal phases to cubic phases. Therefore, hexagonal phases could either be temporarily induced, or a combination of phases could be used to modulate the internalisation into the cytosol without compromising cytotoxicity.
3.4. Summary of LLC structures and their interaction with cells
Intracellular delivery of molecular therapeutics is a cell-dependent process. In this sense, outcomes on matter-cell interactions in the past years have influenced the rational design of nanocarriers. Chan and coworkers [105] illustrate the three generations of nanomedicine engineering, highlighting the nano-bio interaction as a critical aspect of successful therapeutic internalisation. The first generation consisted in basic nanomaterials to address drug delivery and safety issues, the second generation improved surface modification (bioconjugation), and the third generation consisted in ”smart” environment-responsive nanomaterials. We would like to expand this generation of materials for therapeutic internalisation into the cytosol by including materials whose internal structure plays a pivotal role in the cell-carrier interaction and hence internalisation.
Lipid-based lyotropic liquid crystals are increasingly pertinent as potentially safe delivery systems and the main cell internalisation mechanism is endocytosis. Depending on physicochemical properties the endocytosis pathway is different [36,106] and we argue that different nanostructures may activate specific endocytosis pathways too [13]. Figure 10 depicts a schematic representation of the different proposed mechanisms for the interaction of each LLC structure, loaded with a nucleic acid, with cells. Lipoplexes which derive from the bulk lamellar phase have optimal performance at a narrow range of membrane charge density σM. The membrane charge density σM is a predictive parameter of delivery efficiency [104].
Figure 10.
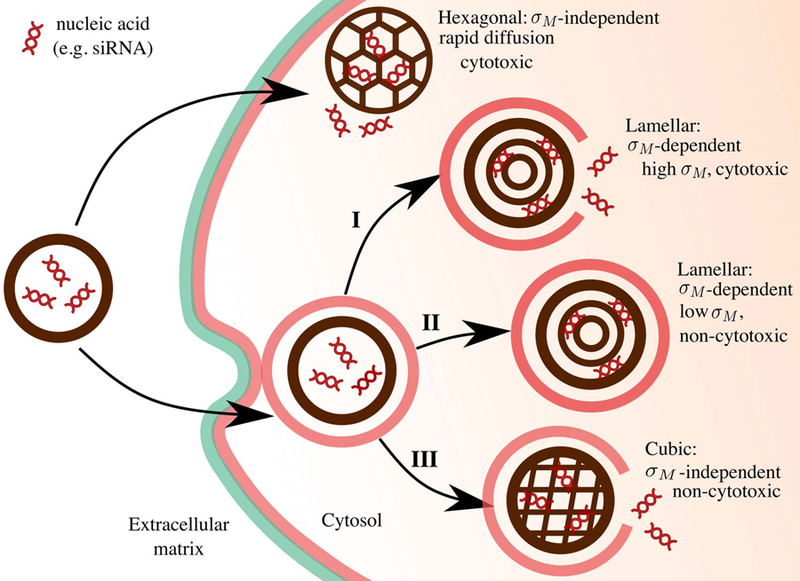
A schematic representation of the cellular pathway and transfection mechanisms of different lipid-nucleic acid complexes. Due to its pore-like nature, the hexagonal phase, fuses directly with the cell membrane, crossing it rapidly avoiding endocytosis, but leading to cytotoxic effects. On the other hand, both, the lamellar and the cubic phases, enter the cell mostly via endocytosis. Lamellar phase toxicity depends on membrane charge density (regime I and II) while the cubic phase endocytosis does not depend on membrane charge density and does not present cytotoxic effects (regime III). Adapted with permission [18]. Copyright 2016 Elsevier.
The Bell curve [104] for lamellar and non-lamellar lipid particles categorises LLC structures into three regimes. In the regime I, at high σM lipoplexes readily fuse with the anionic endosomal membranes, facilitating the release of the complexes into the cell cytoplasm. However, high cationic lipid content imposes critical cytoxicity. In regime II σM is low, there are no toxicity effects but the electrostatic power of the lipoplex to fuse and burst out of the endosomal membranes is too weak, resulting in lipoplex degradation upon the endosome-lysosome pathway. In the regime III, σM is just large enough to fuse with and escape the endosome but not too large to prevent complex dissociation upon cell entry or raise cytoxicity. Hexagonal phases seem to directly interfere with the plasma membrane to release their payload without using endocytosis, resulting often times in undesirable toxicity. Cuboplexes, which stem from bulk bicontinuous cubic phases, are synthesised with very low cationic lipid content. In this case, cuboplexes (and cubosomes) fuse with endosomal membranes to release their payload in a σM -independent manner. Due to the negative Gaussian curvature, Helfrich membrane elasticity theory [70] predicts that the cost of fusing a 3D bicontinuous cubic bilayer with an endosomal membrane is considerably lower compared to a parent lipoplex (i.e. positive Gaussian curvature).
4. Outlook
The objectives of this review on lipid-based liquid crystalline structures are: 1) to collect and categorise recent findings in lamellar and non-lamellar lipid films and particles for the delivery of small molecules, 2) to display some beneficial and adverse effects of using lipid crystal phases as nanocarriers, and 3) to lead to the design of optimal lipid phases for drug and gene delivery that take into account material structure. First, the delivery of small molecules and nucleic acids into the cells requires the use of nanocarriers to lower drug/gene degradation and off-target toxicity, and to enhance accumulation at the required cite. In this paper, we focus on how physical aspects of drug and gene carriers are powerful modulators of their interactions with cells. Many studies focus on nanoparticle size. Here we expand on a new physical parameter which is the effect of nanostructure of lipid-based nanoparticles (liposomes and non-liposomal formulations) in their ability to efficiently release their payload. The amphiphilic nature of lipid molecules and their self-assembly capabilities provide a plethora of liquid crystalline phases that may be exploited for the design of novel nanocarriers. Both, lamellar and non-lamellar phases (layers, hexagonal and cubic phases) can be prepared as supported films as well as as particles in solution. Supported lipid phases allow flexibility in the design of lamellarity and localised cargo release. On the other hand, lipid particles allow improved delivery efficiency and lower cytotoxity. Finally, we hope for future research to focus on better understanding the mechanisms of endosomal escape, interactions between lipid-based liquid crystalline phases with different small molecules (hydrophobic, hydrophilic, amphiphilic) and substrates (for substrate-mediated films), and moreover, on taking advantage of the variety of phases found in lipid-based liquid crystals for the development of safe and smart drugs that can be easily modulated to internalise cargo into the cytosol in a spatiotemporally controlled manner. In this regard, an open question and challenge for future generations lingers: can lipid-based lyotropic liquid crystals enter the cytosol in a non-toxic, endocytic-independent manner? This could benefit the delivery of cargo to promising cell types that do not tolerate endocytosis such as certain types of cells of the immune system. For the promise of intelligent nanocarriers to be fulfilled, matter-cell interactions must be greatly investigated taking into account not only composition, surface modification, targeting, and size/shape but also structure at the nano and meso-scale.
Acknowledgments
Funding
This work was supported by the Office of Naval Research under Grant N000141612886 and N000141812087; the National Science Foundation under Grant DMR-1554435; and the National Institutes of Health under Grant 1DP2EB024377–01.
References
- [1].Verkleij AJ. Lipidic intramembranous particles. Biochimica et Biophysica Acta (BBA) - Reviews on Biomembranes. 1984;779(1):43–63. [DOI] [PubMed] [Google Scholar]
- [2].Yorke M, Dickson D. Lamellar to tubular conformational changes in the endoplasmic reticulum of the retinal pigment epithelium of the newt, notophthalmus viridescens. Cell Tissue Res. 1985;241(3):629–637. [DOI] [PubMed] [Google Scholar]
- [3].Almsherqi ZA, Landh T, Kohlwein SD, et al. Chapter 6 cubic membranes In: International review of cell and molecular biology. Vol. 274 Elsevier; 2009. p. 275–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Evans DF, Wennerström H. The colloidal domain: Where physics, chemistry, biology, and technology meet. Wiley-Vch New York; 1999. [Google Scholar]
- [5].Radler JO. Structure of DNA-cationic liposome complexes: Dna intercalation in multilamellar membranes in distinct interhelical packing regimes. Science. 1997; 275(5301):810–814. [DOI] [PubMed] [Google Scholar]
- [6].Koltover I. An inverted hexagonal phase of cationic liposome-DNA complexes related to DNA release and delivery. Science. 1998;281(5373):78–81. [DOI] [PubMed] [Google Scholar]
- [7].Ewert KK, Evans HM, Zidovska A, et al. A columnar phase of dendritic lipid-based cationic liposome-dna complexes for gene delivery: Hexagonally ordered cylindrical micelles embedded in a DNA honeycomb lattice. J Am Chem Soc. 2006;128(12):3998–4006. [DOI] [PubMed] [Google Scholar]
- [8].Leal C, Bouxsein NF, Ewert KK, et al. Highly efficient gene silencing activity of siRNA embedded in a nanostructured gyroid cubic lipid matrix. J Am Chem Soc. 2010; 132(47):16841–16847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Safinya C, Ewert K, Leal C. Cationic liposome–nucleic acid complexes: Liquid crystal phases with applications in gene therapy. Liq Cryst. 2011;38(11–12):1715–1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Kang M, Lee B, Leal C. Three-dimensional microphase separation and synergistic permeability in stacked lipid–polymer hybrid membranes. Chem Mater. 2017; 29(21):9120–9132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Kang M, Tuteja M, Centrone A, et al. Nanostructured lipid-based films for substrate- mediated applications in biotechnology. Adv Funct Mater. 2018;28(9):1704356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Lee CH, Kim H, Harburg DV, et al. Biological lipid membranes for on-demand, wireless drug delivery from thin, bioresorbable electronic implants. NPG Asia Mater. 2015; 7(11):e227–e227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Kang M, Leal C. Soft nanostructured films for actuated surface-based siRNA delivery. Adv Funct Mater. 2016;26(31):5610–5620. [Google Scholar]
- [14].Clogston J, Caffrey M. Controlling release from the lipidic cubic phase. amino acids, peptides, proteins and nucleic acids. J Controlled Release. 2005;107(1):97–111. [DOI] [PubMed] [Google Scholar]
- [15].Santel A, Aleku M, Keil O, et al. RNA interference in the mouse vascular endothelium by systemic administration of siRNA-lipoplexes for cancer therapy. Gene Ther. 2006; 13(18):1360–1370. [DOI] [PubMed] [Google Scholar]
- [16].Leal C, Ewert KK, Shirazi RS, et al. Nanogyroids incorporating multivalent lipids: Enhanced membrane charge density and pore forming ability for gene silencing. Langmuir. 2011;27(12):7691–7697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Tuteja M, Kang M, Leal C, et al. Nanoscale partitioning of paclitaxel in hybrid lipid– polymer membranes. Analyst. 2018;143(16):3808–3813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Kang M, Kim H, Leal C. Self-organization of nucleic acids in lipid constructs. Curr Opin Colloid Interface Sci. 2016;26:58–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Kim H, Sung J, Chang Y, et al. Microfluidics synthesis of gene silencing cubosomes. ACS Nano. 2018;12(9):9196–9205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Kim H, Leal C. Cuboplexes: Topologically active siRNA delivery. ACS Nano. 2015; 9(10):10214–10226. [DOI] [PubMed] [Google Scholar]
- [21].Kang M, Huang G, Leal C. Role of lipid polymorphism in acoustically sensitive liposomes. Soft Matter. 2014;10(44):8846–8854. [DOI] [PubMed] [Google Scholar]
- [22].Safinya CR, Ewert KK, Majzoub RN, et al. Cationic liposome–nucleic acid complexes for gene delivery and gene silencing. New J Chem. 2014;38(11):5164–5172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Shirazi RS, Ewert KK, Silva BFB, et al. Structural evolution of environmentally responsive cationic liposome–DNA complexes with a reducible lipid linker. Langmuir. 2012;28(28):10495–10503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Leal C, Ewert KK, Bouxsein NF, et al. Stacking of short DNA induces the gyroid cubic-to-inverted hexagonal phase transition in lipid–DNA complexes. Soft Matter. 2013; 9(3):795–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Shirazi RS, Ewert KK, Leal C, et al. Synthesis and characterization of degradable multivalent cationic lipids with disulfide-bond spacers for gene delivery. Biochimica et Biophysica Acta (BBA) - Biomembranes. 2011;1808(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Bouxsein NF, Leal C, McAllister CS, et al. Two-dimensional packing of short DNA with nonpairing overhangs in cationic liposome–DNA complexes: From Onsager nematics to columnar nematics with finite-length columns. J Am Chem Soc. 2011;133(19):7585–7595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Leal C, Rgnvaldsson S, Fossheim S, et al. Dynamic and structural aspects of PEGylated liposomes monitored by NMR. J Colloid Interface Sci. 2008;325(2):485–493. [DOI] [PubMed] [Google Scholar]
- [28].Luo J, Hhn M, Reinhard S, et al. IL4-receptor-targeted dual antitumoral apoptotic peptide—siRNA conjugate lipoplexes. Adv Funct Mater. 2019;:1900697.
- [29].Torchilin VP. Multifunctional nanocarriers. Adv Drug Delivery Rev. 2012;64:302–315. [DOI] [PubMed] [Google Scholar]
- [30].Davis ME, Chen ZG, Shin DM. Nanoparticle therapeutics: An emerging treatment modality for cancer In: Nanoscience and technology. Co-Published with Macmillan Publishers Ltd, UK; 2009. p. 239–250. [Google Scholar]
- [31].Tang L, Yang X, Yin Q, et al. Investigating the optimal size of anticancer nanomedicine. Proceedings of the National Academy of Sciences. 2014;111(43):15344–15349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Zhang YS, Zhang YN, Zhang W. Cancer-on-a-chip systems at the frontier of nanomedicine. Drug discovery today. 2017;22(9):1392–1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Sahay G, Querbes W, Alabi C, et al. Efficiency of siRNA delivery by lipid nanoparticles is limited by endocytic recycling. Nat Biotechnol. 2013;31(7):653–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Jahn R, Lang T, Südhof TC. Membrane fusion. Cell. 2003;112(4):519–533. [DOI] [PubMed] [Google Scholar]
- [35].Chernomordik LV, Kozlov MM. Membrane hemifusion: crossing a chasm in two leaps. Cell. 2005;123(3):375–382. [DOI] [PubMed] [Google Scholar]
- [36].Doherty GJ, McMahon HT. Mechanisms of endocytosis. Annu Rev Biochem. 2009; 78:857–902. [DOI] [PubMed] [Google Scholar]
- [37].Scatchard G. The effect of dielectric constant difference on hyperfiltration of salt solutions. J Phys Chem. 1964;68(5):1056–1061. [Google Scholar]
- [38].Parsegian A. Energy of an ion crossing a low dielectric membrane: solutions to four relevant electrostatic problems. Nature. 1969;221(5183):844–846. [DOI] [PubMed] [Google Scholar]
- [39].Zabner J, Fasbender AJ, Moninger T, et al. Cellular and molecular barriers to gene transfer by a cationic lipid. J Biol Chem. 1995;270(32):18997–19007. [DOI] [PubMed] [Google Scholar]
- [40].Wasungu L, Hoekstra D. Cationic lipids, lipoplexes and intracellular delivery of genes. J Controlled Release. 2006;116(2):255–264. [DOI] [PubMed] [Google Scholar]
- [41].Zuhorn IS, Bakowsky U, Polushkin E, et al. Nonbilayer phase of lipoplex–membrane mixture determines endosomal escape of genetic cargo and transfection efficiency. Mol Ther. 2005;11(5):801–810. [DOI] [PubMed] [Google Scholar]
- [42].Castanotto D, Rossi JJ. The promises and pitfalls of RNA-interference-based therapeutics. Nature. 2009;457(7228):426–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Reischl D, Zimmer A. Drug delivery of siRNA therapeutics: Potentials and limits of nanosystems. Nanomed Nanotechnol Biol Med. 2009;5(1):8–20. [DOI] [PubMed] [Google Scholar]
- [44].Nguyen DN, Green JJ, Chan JM, et al. Polymeric materials for gene delivery and DNA vaccination. Adv Mater. 2009;21(8):847–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Pichon C, Billiet L, Midoux P. Chemical vectors for gene delivery: Uptake and intracellular trafficking. Curr Opin Biotechnol. 2010;21(5):640–645. [DOI] [PubMed] [Google Scholar]
- [46].Zhang P, Wagner E. History of polymeric gene delivery systems In: Topics in current chemistry collections. Springer International Publishing; 2017. p. 1–39. [DOI] [PubMed] [Google Scholar]
- [47].Whitehead KA, Langer R, Anderson DG. Knocking down barriers: Advances in siRNA delivery. Nat Rev Drug Discov. 2009;8(2):129–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Chan CL, Ewert KK, Majzoub RN, et al. Optimizing cationic and neutral lipids for efficient gene delivery at high serum content. J Gene Med. 2014;16(3–4):84–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Belliveau NM, Huft J, Lin PJ, et al. Microfluidic synthesis of highly potent limit-size lipid nanoparticles for in vivo delivery of siRNA. Molecular Therapy - Nucleic Acids. 2012;1:e37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Devi GR. siRNA-based approaches in cancer therapy. Cancer Gene Ther. 2006; 13(9):819–829. [DOI] [PubMed] [Google Scholar]
- [51].Elbashir SM, Harborth J, Lendeckel W, et al. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature. 2001;411(6836):494–498. [DOI] [PubMed] [Google Scholar]
- [52].Chernousova S, Epple M. Live-cell imaging to compare the transfection and gene silencing efficiency of calcium phosphate nanoparticles and a liposomal transfection agent. Gene Ther. 2017;24(5):282–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Ewert KK, Zidovska A, Ahmad A, et al. Cationic liposome–nucleic acid complexes for gene delivery and silencing: Pathways and mechanisms for plasmid DNA and siRNA In: Topics in current chemistry. Springer Berlin Heidelberg; 2010. p. 191–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Semple SC, Akinc A, Chen J, et al. Rational design of cationic lipids for siRNA delivery. Nat Biotechnol. 2010;28(2):172–176. [DOI] [PubMed] [Google Scholar]
- [55].Savage PB. Multidrug-resistant bacteria: Overcoming antibiotic permeability barriers of gram-negative bacteria. Ann Med. 2001;33(3):167–171. [DOI] [PubMed] [Google Scholar]
- [56].Pourjavadi A, Amin SS, Hosseini SH. Delivery of hydrophobic anticancer drugs by hydrophobically modified alginate based magnetic nanocarrier. Ind Eng Chem Res. 2018; 57(3):822–832. [Google Scholar]
- [57].Sokolova V, Epple M. Inorganic nanoparticles as carriers of nucleic acids into cells. Angew Chem Int Ed. 2008;47(8):1382–1395. [DOI] [PubMed] [Google Scholar]
- [58].Bernsdorff C, Reszka R, Winter R. Interaction of the anticancer agent taxol TM (paclitaxel) with phospholipid bilayers. Journal of Biomedical Materials Research: An Official Journal of The Society for Biomaterials, The Japanese Society for Biomaterials, and The Australian Society for Biomaterials. 1999;46(2):141–149. [DOI] [PubMed] [Google Scholar]
- [59].Wu J, Liu Q, Lee RJ. A folate receptor-targeted liposomal formulation for paclitaxel. Int J Pharm. 2006;316(1–2):148–153. [DOI] [PubMed] [Google Scholar]
- [60].Koudelka, Turnek J. Liposomal paclitaxel formulations. J Controlled Release. 2012; 163(3):322–334. [DOI] [PubMed] [Google Scholar]
- [61].Rand RP. Interacting phospholipid bilayers: Measured forces and induced structural changes. Annu Rev Biophys Bioeng. 1981;10(1):277–314. [DOI] [PubMed] [Google Scholar]
- [62].Johnson S, Bayerl T, McDermott D, et al. Structure of an adsorbed dimyristoylphosphatidylcholine bilayer measured with specular reflection of neutrons. Biophys J. 1991;59(2):289–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Mennicke U, Salditt T. Preparation of solid-supported lipid bilayers by spin-coating. Langmuir. 2002;18(21):8172–8177. [Google Scholar]
- [64].Gupta G, Iyer S, Leasure K, et al. Stable and fluid multilayer phospholipid–silica thin films: Mimicking active multi-lamellar biological assemblies. ACS Nano. 2013;7(6):5300–5307. [DOI] [PubMed] [Google Scholar]
- [65].Heath GR, Li M, Polignano IL, et al. Layer-by-layer assembly of supported lipid bilayer poly-l-lysine multilayers. Biomacromolecules. 2015;17(1):324–335. [DOI] [PubMed] [Google Scholar]
- [66].Tayebi L, Ma Y, Vashaee D, et al. Long-range interlayer alignment of intralayer domains in stacked lipid bilayers. Nature Mater. 2012;11(12):1074–1080. [DOI] [PubMed] [Google Scholar]
- [67].Lihan M, Tajkhorshid E. Investigating cholesterol dynamics and interactions with the dopamine transporter using a membrane mimetic model. Biophys J. 2018;114(3):275a. [Google Scholar]
- [68].Steer D, Leung SSW, Meiselman H, et al. Structure of lung-mimetic multilamellar bodies with lipid compositions relevant in pneumonia. Langmuir. 2018;34(25):7561–7574. [DOI] [PubMed] [Google Scholar]
- [69].Widder K, Trger J, Kerth A, et al. Interaction of myelin basic protein with myelin-like lipid monolayers at air–water interface. Langmuir. 2018;34(21):6095–6108. [DOI] [PubMed] [Google Scholar]
- [70].Helfrich W. Elastic properties of lipid bilayers: Theory and possible experiments. Zeitschrift für Naturforschung C. 1973;28(11–12):693–703. [DOI] [PubMed] [Google Scholar]
- [71].Ericsson B, Eriksson P, Löfroth J, et al. Polymeric drugs and drug delivery systems In: ACS Symp. Ser. 469; American Chemical Society Washington, DC; 1991. p. 251–265. [Google Scholar]
- [72].Richardson SJ, Burton MR, Staniec PA, et al. Aligned platinum nanowire networks from surface-oriented lipid cubic phase templates. Nanoscale. 2016;8(5):2850–2856. [DOI] [PubMed] [Google Scholar]
- [73].Akbar S, Elliott JM, Rittman M, et al. Facile production of ordered 3D platinum nanowire networks with “Single diamond” bicontinuous cubic morphology. Adv Mater. 2012;25(8):1160–1164. [DOI] [PubMed] [Google Scholar]
- [74].Squires AM, Akbar S, Tousley ME, et al. Experimental evidence for proposed transformation pathway from the inverse hexagonal to inverse diamond cubic phase from oriented lipid samples. Langmuir. 2015;31(28):7707–7711. [DOI] [PubMed] [Google Scholar]
- [75].Richardson SJ, Staniec PA, Newby GE, et al. Predicting the orientation of lipid cubic phase films. Langmuir. 2014;30(45):13510–13515. [DOI] [PubMed] [Google Scholar]
- [76].Rittman M, Frischherz M, Burgmann F, et al. Direct visualisation of lipid bilayer cubic phases using atomic force microscopy. Soft Matter. 2010;6(17):4058. [Google Scholar]
- [77].Rittman M, Amenitsch H, Rappolt M, et al. Control and analysis of oriented thin films of lipid inverse bicontinuous cubic phases using grazing incidence small-angle x-ray scattering. Langmuir. 2013;29(31):9874–9880. [DOI] [PubMed] [Google Scholar]
- [78].Yamauchi F, Koyamatsu Y, Kato K, et al. Layer-by-layer assembly of cationic lipid and plasmid DNA onto gold surface for stent-assisted gene transfer. Biomaterials. 2006; 27(18):3497–3504. [DOI] [PubMed] [Google Scholar]
- [79].Perry SL, Neumann SG, Neumann T, et al. Challenges in nucleic acid-lipid films for transfection. AIChE J. 2013;59(9):3203–3213. [Google Scholar]
- [80].Neumann T, Gajria S, Tirrell M, et al. Reversible structural switching of a DNA-DDAB film. J Am Chem Soc. 2009;131(10):3440–3441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Neumann T, Gajria S, Bouxsein NF, et al. Structural responses of DNA-DDAB films to varying hydration and temperature. J Am Chem Soc. 2010;132(20):7025–7037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Gettel DL, Sanborn J, Patel MA, et al. Mixing, diffusion, and percolation in binary supported membranes containing mixtures of lipids and amphiphilic block copolymers. J Am Chem Soc. 2014;136(29):10186–10189. [DOI] [PubMed] [Google Scholar]
- [83].Chen D, Santore MM. Hybrid copolymer–phospholipid vesicles: Phase separation resembling mixed phospholipid lamellae, but with mechanical stability and control. Soft Matter. 2015;11(13):2617–2626. [DOI] [PubMed] [Google Scholar]
- [84].Kowal J, Wu D, Mikhalevich V, et al. Hybrid polymer–lipid films as platforms for directed membrane protein insertion. Langmuir. 2015;31(17):4868–4877. [DOI] [PubMed] [Google Scholar]
- [85].Torchilin VP. Recent advances with liposomes as pharmaceutical carriers. Nat Rev Drug Discov. 2005;4(2):145–160. [DOI] [PubMed] [Google Scholar]
- [86].Barauskas J, Johnsson M, Tiberg F. Self-assembled lipid superstructures: beyond vesicles and liposomes. Nano Lett. 2005;5(8):1615–1619. [DOI] [PubMed] [Google Scholar]
- [87].Patil YP, Jadhav S. Novel methods for liposome preparation. Chem Phys Lipids. 2014; 177:8–18. [DOI] [PubMed] [Google Scholar]
- [88].Nele V, Holme MN, Kauscher U, et al. Effect of formulation method, lipid composition, and PEGylation on vesicle lamellarity: A small-angle neutron scattering study. Langmuir. 2019;35(18):6064–6074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Allen TM. Stealth liposomes. Vol. 20 CRC Press; 2018. [Google Scholar]
- [90].Bangham AD, Hill MW, Miller NGA. Preparation and use of liposomes as models of biological membranes In: Methods in membrane biology. Springer US; 1974. p. 1–68. [Google Scholar]
- [91].Safinya CR, Deek J, Beck R, et al. Liquid crystal assemblies in biologically inspired systems. Liq Cryst. 2013;40(12):1748–1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Larsson K. Two cubic phases in monoolein–water system. Nature. 1983;304(5927):664–664. [Google Scholar]
- [93].Kim H, Song Z, Leal C. Super-swelled lyotropic single crystals. Proc Natl Acad Sci USA. 2017;114(41):10834–10839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Leung SSW, Leal C. The stabilization of primitive bicontinuous cubic phases with tunable swelling over a wide composition range. Soft Matter. 2019;15(6):1269–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Patton J, Carey M. Watching fat digestion. Science. 1979;204(4389):145–148. [DOI] [PubMed] [Google Scholar]
- [96].Larsson K. Cubic lipid-water phases: Structures and biomembrane aspects. J Phys Chem. 1989;93(21):7304–7314. [Google Scholar]
- [97].Barriga HMG, Holme MN, Stevens MM. Cubosomes: The next generation of smart lipid nanoparticles? Angew Chem Int Ed. 2018;58(10):2958–2978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Karami Z, Hamidi M. Cubosomes: Remarkable drug delivery potential. Drug Discovery Today. 2016;21(5):789–801. [DOI] [PubMed] [Google Scholar]
- [99].Martiel I, Sagalowicz L, Handschin S, et al. Facile dispersion and control of internal structure in lyotropic liquid crystalline particles by auxiliary solvent evaporation. Langmuir. 2014;30(48):14452–14459. [DOI] [PubMed] [Google Scholar]
- [100].Rodrigues L, Kyriakos K, Schneider F, et al. Characterization of lipid-based hexosomes as versatile vaccine carriers. Mol Pharmaceutics. 2016;13(11):3945–3954. [DOI] [PubMed] [Google Scholar]
- [101].Leal C, Wads L, Olofsson G, et al. The hydration of a DNA-Amphiphile complex. J Phys Chem B. 2004;108(9):3044–3050. [Google Scholar]
- [102].Bouxsein NF, McAllister CS, Ewert KK, et al. Structure and gene silencing activities of monovalent and pentavalent cationic lipid vectors complexed with siRNA . Biochemistry (Mosc). 2007;46(16):4785–4792. [DOI] [PubMed] [Google Scholar]
- [103].Lin AJ, Slack NL, Ahmad A, et al. Three-dimensional imaging of lipid gene-carriers: Membrane charge density controls universal transfection behavior in lamellar cationic liposome-DNA complexes. Biophys J. 2003;84(5):3307–3316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Ahmad A, Evans HM, Ewert K, et al. New multivalent cationic lipids reveal bell curve for transfection efficiency versus membrane charge density: Lipid-dna complexes for gene delivery. J Gene Med. 2005;7(6):739–748. [DOI] [PubMed] [Google Scholar]
- [105].Albanese A, Tang PS, Chan WC. The effect of nanoparticle size, shape, and surface chemistry on biological systems. Annu Rev Biomed Eng. 2012;14:1–16. [DOI] [PubMed] [Google Scholar]
- [106].Sahay G, Alakhova DY, Kabanov AV. Endocytosis of nanomedicines. J Controlled Release. 2010;145(3):182–195. [DOI] [PMC free article] [PubMed] [Google Scholar]