Abstract
Prostate cancer (PCa) is one of the leading causes of cancer deaths in men. In this cancer, the stem cell transcription factor SOX2 increases during tumor progression, especially as the cancer progresses to the highly aggressive neuroendocrine-like phenotype. Other studies have shown that knockdown of RB1 and TP53 increases the expression of neuroendocrine markers, decreases the sensitivity to enzalutamide, and increases the expression of SOX2. Importantly, knockdown of SOX2 in the context of RB1 and TP53 depletion restored sensitivity to enzalutamide and reduced the expression of neuroendocrine markers. In this study, we examined whether elevating SOX2 is not only necessary, but also sufficient on its own to promote the expression of neuroendocrine markers and confer enzalutamide resistance. For this purpose, we engineered LNCaP cells for inducible overexpression of SOX2 (i-SOX2-LNCaP). As shown previously for other tumor cell types, inducible elevation of SOX2 in i-SOX2-LNCaP inhibited cell proliferation. SOX2 elevation also increased the expression of several neuroendocrine markers, including several neuropeptides and synaptophysin. However, SOX2 elevation did not decrease the sensitivity of i-SOX2-LNCaP cells to enzalutamide, which indicates that elevating SOX2 on its own is not sufficient to confer enzalutamide resistance. Furthermore, knocking down SOX2 in C4–2B cells, a derivative of LNCaP cells which is far less sensitive to enzalutamide and which expresses much higher levels of SOX2 than LNCaP cells, did not alter the growth response to this anti-androgen. Thus, our studies indicate that NE marker expression can increase independently of the sensitivity to enzalutamide.
Keywords: SOX2, prostate tumor cells, neuroendocrine, enzalutamide, PSA, notch signaling
Introduction
Over 180,000 men are diagnosed each year with prostate cancer (PCa) in the United States. After lung cancer, PCa is the second highest cause of cancer deaths in men in the United States with over 29,000 men expected to die from PCa in 2019 (Siegel, Miller, & Jemal, 2019). Improved screening technologies have increased early clinical detection, and the death rate of PCa has declined over the past 20 years. Although low risk patients with localized PCa can be effectively treated with surgery and radiation, intermediate and high risk patients often relapse after initial therapy. Androgen deprivation therapy (ADT) is the standard of care for advanced PCa, but the benefit of ADT is not durable. Most patients with advanced PCa become unresponsive to androgen ablation with a median time of 2 to 3 years (Chen, Sawyers, & Scher, 2008; Knudsen and Penning 2010), and the cancer recurs as an aggressive form known as castration-resistant prostate cancer (CRPC). Second-line therapies, such as enzalutamide, abiraterone acetate, and chemotherapy (docetaxel), prolong patient survival, but these treatments are not curative and are accompanied by significant adverse effects. Patients who no longer respond to second-line therapies eventually progress to highly aggressive metastatic CRPC with a median survival of under one year.
Several recent studies have implicated the stem cell transcription factor SOX2 in the progression and poor prognosis of patients with metastatic CRPC. Analysis of clinical samples demonstrated that SOX2 expression is low during the early stages of PCa, but increases significantly as Gleason scores increase (Russo et al., 2016; Kregel et al., 2013; Jia et al., 2011). In addition, SOX2 is expressed in the majority of lymph node positive PCa specimens (Kregel et al., 2013, Esposito et al., 2015) and is expressed in a majority of metastatic sites, even in cases where SOX2 is not readily detected in the primary prostatic adenocarcinoma specimen (Yu et al., 2014). Hence, increased SOX2 expression in advanced PCa is strongly associated with poor survival (Russo et al., 2016; Kregel et al., 2013; Jia et al., 2011). Relevant to the studies described in this report, SOX2 expression is particularly high in neuroendocrine (NE) like cells, which are found in highly aggressive PCa (Russo et al., 2016; Mu et al., 2017; Ku et al., 2017; Parimi et al., 2014; Terry & Beltran, 2014; Grigore, Ben-Jacob, & Farach-Carson, 2016). Although NE-like cells are largely quiescent in early stage PCa, these cells have been shown to influence other cells in the tumor (Bonkhoff et al., 1991; Bonkhoff, Stein, & Remberger, 1995). In this regard, quiescent, early stage NE-like cells are believed to release growth factors and peptide hormones (e.g. bombesin and neurotensin) that stimulate growth of surrounding cells (DaSilva et al., 2013, Alonzeau et al., 2013). Other studies indicate that NE-like cells not only influence their immediate neighbors, but can also have systemic effects on the entire tumor by promoting the castration-resistant phenotype of advanced PCa (Jin et al., 2004).
Although SOX2 expression in PCa was reported nearly 20 years ago (Sattler et al., 2000), two recent studies provide compelling evidence that SOX2 warrants careful study in this cancer. These studies demonstrate that the combined loss of both RB1 and TP53, which occurs in more aggressive forms of human PCa, leads to resistance to the anti-androgen enzalutamide and to large increases in SOX2 expression (Mu et al., 2017;Ku et al., 2017). One of these studies examined how knocking down both RB1 and TP53 in the enzalutamide-sensitive PCa cell line LNCaP affects cell behavior (Mu et al., 2017). They observed three prominent effects: 1) substantial increases in SOX2; 2) tumors formed by these cells exhibited significant resistance to enzalutamide compared to parental LNCaP cells; and 3) increases in NE markers, due to reprogramming of LNCaP cells. Importantly, knocking down SOX2 by shRNA in RB1/T53-depleted LNCaP cells substantially reversed the resistance to enzalutamide in vivo. Equally important, knocking down SOX2 reversed increases in expression of NE markers.
The knockdown of SOX2 in the RB1/TP53-depleted LNCaP cells demonstrates that SOX2 is necessary for induction of NE markers and resistance to enzalutamide in this context. However, these studies did not address whether elevated SOX2 is sufficient for the induction of NE markers and resistance to enzalutamide. In this report, we directly address whether SOX2 is sufficient to upregulate the expression of NE markers by using LNCaP cells engineered for inducible elevation of SOX2. As observed previously in seven other tumor cell lines, including DU145 prostate tumor cells (Cox et al., 2012; Wuebben et al., 2016), elevating SOX2 inhibited the growth of LNCaP cells. Importantly, elevating SOX2 led to increases in the expression of several NE markers, such as synaptophysin (SYP). In contrast, elevating SOX2 did not reduce the growth inhibitory effects of enzalutamide or its ability to decrease expression of the androgen receptor target gene prostate specific antigen (PSA). Moreover, knocking down SOX2 in C4–2B cells did not alter the growth response to enzalutamide. C4–2B cells are a derivative of LNCaP cells that are less sensitive to enzalutamide and express much higher levels of SOX2 than LNCaP cells, Thus, elevating SOX2 in LNCaP cells appears to be necessary and sufficient to elevate expression of NE genes, but it is not sufficient alone to block the inhibitory effects of enzalutamide.
Materials and Methods
Cell Culture
Early passage LNCaP cells (Ishimaru et al., 2002) were obtained from Ming-Fong Lin (University of Nebraska Medical Center) and cultured in Dulbecco’s Modified Eagle’s Medium supplemented with 10% fetal bovine serum. C4–2B prostate cells were also cultured in Dulbecco’s Modified Eagle’s Medium supplemented with 10% fetal bovine serum (Thalmann et al., 1994). Doxycycline (Dox, Clontech, Mountain View, CA) was suspended in phosphate-buffered saline. To induce Flag-tagged SOX2 in engineered LNCaP cells, Dox was added to the culture media at the concentrations indicated. Enzalutamide (MDV3100, Selleckchem, Houston, TX) and RO4929097 (Selleckchem, Houston, TX) were suspended in DMSO and used at the indicated concentrations. MTT assays were used to assess relative cell growth in triplicate samples, as described previously (Cox et al., 2012; Wuebben et al., 2016). To assess the effects on elevated SOX2 on the clonal growth of engineered LNCaP cells, the cells were plated on tissue culture plastic coated with poly-lysine. For these studies, tissue culture plastic was coated overnight with poly-D-lysine (Sigma, St. Louis MO) resuspended in sterile water at 20 μg/ml. Clonal growth of cells was determined by an investigator who was unaware of sample designation. Statistical significance was determined using a two-tailed students t-test with a significance threshold of p=.05.
Cell Engineering for SOX2 overexpression
LNCaP cells were engineered for Dox-inducible SOX2 expression as described previously (Cox et al., 2012; Wuebben et al., 2016). In brief, two lentiviral vectors were used to transduce LNCaP cells. One vector, pLVX-tetO-(fs)SOX2, expresses SOX2 from a Dox-inducible promoter. The second vector expresses the reverse tet-transactivator pLVX-Tet-On® Advanced (modified from a CMV promoter to a PGK promoter) (Cox et al., 2012). Exogenous SOX2 is expressed when Dox binds to the reverse tet-transactivator. Transduced LNCaP cells were selected in culture media containing 5 μg/ml puromycin (P8833, Sigma-Aldrich, St. Louis, MO) for 2 days followed by treatment with 600 μg/ml G418 Sulfate (#631308, Clontech, Mountain View, CA) for 4 days and then 7 additional days at 400 μg/ml to generate i-SOX2-LNCaP cells.
Engineering cells for knockdown of SOX2.
C4–2B cells were engineered for Dox-inducible knockdown of SOX2, as described previously (Wuebben et al., 2016). C4–2B cells were transduced using a TRIPZ lentiviral vector obtained from Open Biosystems (GE Dharmacon, Lafayette, CO). This TRIPZ vector results in resistance to puromycin as well as constitutive expression of a reverse tet transactivator that drives the expression of the shRNA and red fluorescent protein (RFP) in the presence of Dox. I-KD(SOX2)-C4–2B cells were selected with puromycin, as described above.
RNA Isolation and cDNA synthesis
RNA was isolated using the RNeasy kit (Qiagen, Germantown, MD). In brief, cells were lysed in the provided RLT buffer and spun through Qiashredder columns (Qiagen, Germantown, MD) according to manufacturer’s protocols. Cells were eluted from the column in RNase-free water. cDNA synthesis was performed using the High Fidelity 1st Strand cDNA synthesis kit (Agilent Technologies, La Jolla, CA). Synthesis was performed for 1 hour at 42°C after which the reaction was terminated for 15 minutes at 70°C. The primers used in RT-qPCR to measure relative mRNA levels are provided in Table 1. qPCR amplification and quantification was performed on a Bio Rad CFX96 Real time PCR detection system (Bio Rad, Hercules, CA) and detected using RT2 SYBR Green qPCR mastermix (Qiagen, Germantown, MD). Transcripts for each gene (control and Dox-treated) were measured in triplicate. Expression of each gene was normalized to a GAPDH loading control. Relative expression of transcripts was determined using the calculation 2ΔCT, where ΔCT is the average change in cycle threshold between triplicate control or Dox-treated samples. Statistical significance was determined using a two-tailed students t-test with a significance threshold of p=.05.
Table 1:
Primer sequences used for RT-qPCR analyses.
| Name | Forward Primer Sequence 5’−3’ | Reverse Primer Sequence 5’−3’ |
|---|---|---|
| GAPDH | ACAGCGACACCCACTCCTCC | GAGGTCCACCACCCTGTTGC |
| PSA | CAGTCTGCGGCGGTGTT | GCAAGATCACGCTTTTGTTCCT |
| NKX3.1 | CAGATAAGACCCCAAGTGCC | CAGAGCCAGAGCCAGAGG |
| TMPRSS2 | GTCCCCACTGTCTACGAGGT | CAGACGACGGGGTTGGAAG |
| BRN2 | CGGCGGATCAAACTGGGATTT | TTGCGCTGCGATCTTGTCTAT |
| NTS | TGCTTTAGATGGCTTTAGCTTGG | TTCCTGGATTAACTCCCAGTGT |
| GRP | AAAGAGCACAGGGGAGTCTTC | TCCTTTGCTTCTATGAGACCCA |
| CALCA | TCTAAGCGGTGCGGTAATCTG | CAGTTTGGGGGAACGTGTGA |
| HES1 | CCTGTCATCCCCGTCTACAC | CACATGGAGTCCGCCGTAA |
| HEY1 | ATCTGCTAAGCTAGAAAAAGCCG | GTGCGCGTCAAAGTAACCT |
| YAP | ACGTTCATCTGGGACAGCAT | GTTGGGAGATGGCAAAGACA |
Western Blotting
Whole cell protein was extracted using RIPA buffer (ThermoFisher, Rockford, IL). RIPA buffer was supplemented with protease and phosphatase inhibitors as previously described (Cox et al., 2012; Wuebben et al., 2016). Western blot analyses were performed as previously described (Cox et al., 2012; Wuebben et al., 2016). Nuclear and cytoplasmic protein extracts were generated using the NE-PER fractionation kit (ThermoFisher, Rockford, IL) according to the manufacturer’s protocol with additional inhibitors as described previously (Cox et al., 2012; Wuebben et al., 2016). The following antibodies were used for western blotting: SOX2 (#3579, Cell Signaling Technology, Danvers, MA, 1:1,000), SYP (#5461, Cell Signaling Technology, Danvers, MA, 1:1,000), AR (#3202, Cell Signaling Technology, Danvers, MA, 1:1,000), Hes1 (#11988, Cell Signaling Technology, Danvers, MA, 1:1,000), YAP (#14074, Cell Signaling Technology, Danvers, MA, 1:1,000), PSA (#5365, Cell Signaling Technology, Danvers, MA, 1:1,000), HDAC1 (#34586, Cell Signaling Technology, Danvers, MA, 1:1,000), and β-Tubulin (#2146, Cell Signaling Technology, Danvers, MA, 1:1,000). HDAC1 and β-Tubulin were used as protein loading controls. All antibodies were detected with an anti-rabbit-IgG-AP secondary antibody (A3687, Sigma-Aldrich, 1:5,000) as described previously (Cox et al., 2012; Wuebben et al., 2016). Changes in protein expression were estimated as described previously (Nowling et al., 2000).
Results
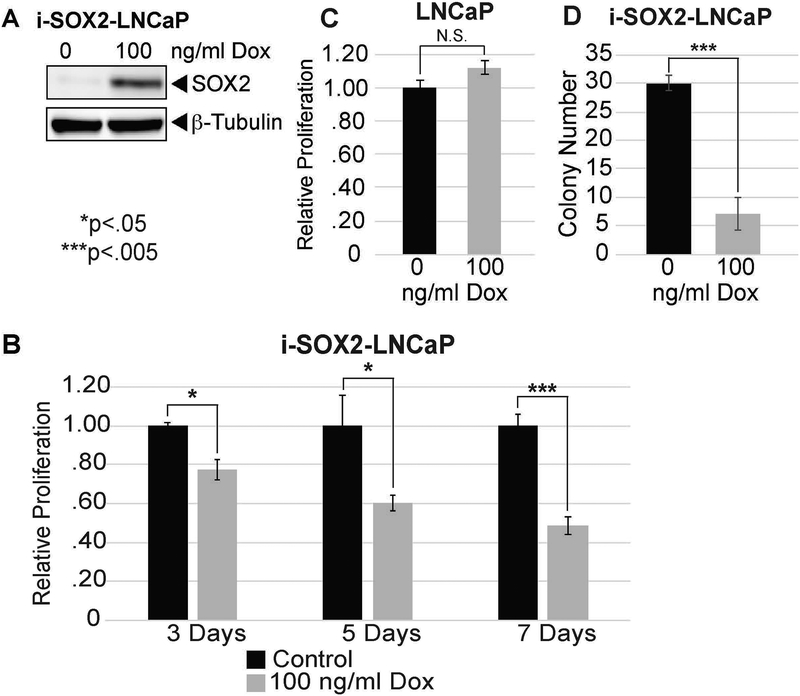
To determine whether elevation of SOX2 is sufficient to induce NE markers and reduce sensitivity to enzalutamide in prostate tumor cells without depletion of RB1 and TP53, we engineered early passage, androgen-dependent LNCaP cells for inducible overexpression of SOX2. These cells were generated by transducing LNCaP cells with two lentiviral vectors, which have been used previously to elevate the expression of SOX2 in response to doxycycline (Dox) (Cox et al., 2012; Wuebben et al., 2016). We refer to these cells as i-SOX2-LNCaP cells. Although LNCaP cells express low levels of SOX2 at the RNA level, which can be measured by RT-PCR (data not shown), they do not express sufficient SOX2 to be detected at the protein level by western blot analysis (Fig 1A). When Dox was added to the culture medium of i-SOX2-LNCaP cells, the expression of SOX2 was elevated (Fig 1A). We also determined that elevating SOX2 in i-SOX2-LNCaP cells inhibited cell growth. For these studies, we used Dox at the concentration that maximally reduced growth over a period of 4 days (data not shown). Elevating SOX2 led to growth inhibition, as measured by MTT assay, as early as day 3 and growth inhibition was increased further at days 5 and 7 (Fig 1B). This effect of SOX2 is not due to the effect of Dox itself on the growth of these cells, because the growth of the parental (unengineered) LNCaP cells was not inhibited by Dox at the concentration used (Fig 1C). Moreover, elevating SOX2 reduced the number of colonies formed when i-SOX2-LNCaP cells were plated at low density (Fig. 1D).
Figure 1. SOX2 elevation inhibits the proliferation of androgen-dependent LNCaP cells.
A. Western blot analysis of SOX2 overexpression in i-SOX2-LNCaP whole cell extracts following growth with or without Dox. HDAC1 was used as a protein loading control. B. Cell proliferation of i-SOX2-LNCaP cells was determined by MTT assay following growth at 3, 5, and 7 days in the absence and presence of Dox at the indicated concentration. Relative proliferation was normalized to the control at each time-point. C. MTT analysis of parental (unengineered) LNCaP cells after 4 days growth in the presence of Dox (100 ng/ml). D. Clonal growth of i-SOX2-LNCaP cells in the presence and absence of Dox (100 ng/ml) after 8 days in culture. Twenty random fields were scored for each treatment. Error bars represent standard deviation; statistical significance was determined by a 2-tailed student’s t-test (N.S. not significant,*p<.05,**p<.01,***p<.005).
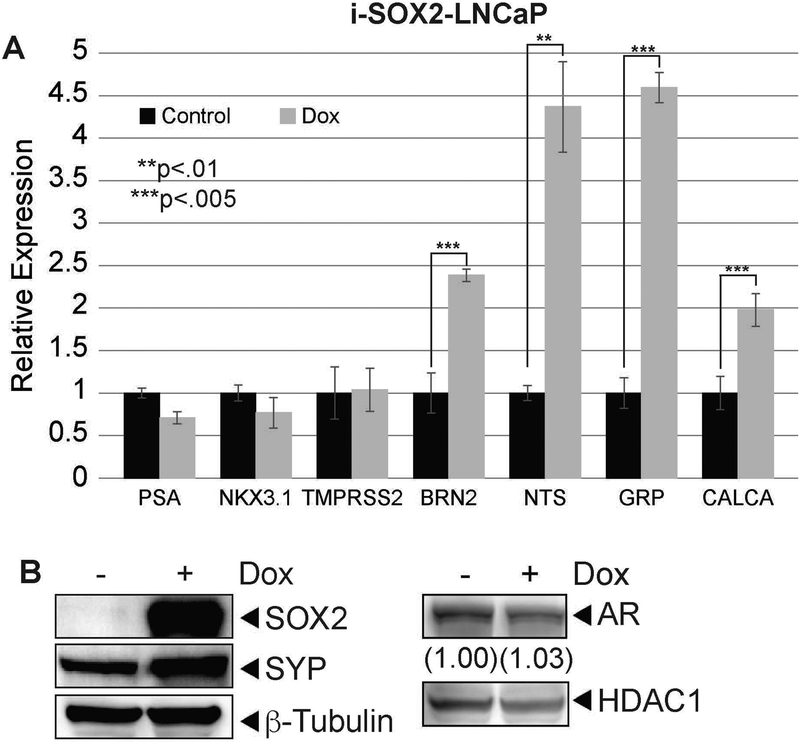
To examine how elevation of SOX2 affects the NE properties of i-SOX2-LNCaP cells, we initially monitored the expression of the androgen receptor (AR), several AR response genes, and several NE markers. When SOX2 was elevated, there was little or no change in the expression of AR and three AR response genes (PSA, NKX3.1, and TMPRSS2) (Fig 2A). In contrast, the expression of four NE markers (BRN2, NTS, GRP, and CALCA) increased significantly at the RNA level (Fig 2A). The expression of neurotensin (NTS), bombesin/gastrin-releasing peptide (GRP), and calcitonin-related gene peptide (CALCA) are particularly interesting, because they have been linked to the adverse effects of PCa NE-like cells (DaSilva et al., 2013; Aljameeli, Thakkar, & Shah, 2017; Ishimaru et al., 2002; Sabbisetti et al, 2005). To extend these findings, we examined the expression of another well-characterized NE marker, SYP, at the protein level. Unlike the expression of AR, which exhibited little or no change at the protein level, expression of SYP increased ~4-fold when SOX2 was elevated (Fig 2B). Together, these studies demonstrate that elevation of SOX2 is sufficient to upregulate expression of NE markers, without significantly altering AR signaling.
Figure 2. Elevating SOX2 is sufficient to induce neuroendocrine plasticity in i-SOX2-LNCaP cells.
A. RT-qPCR analysis of AR target genes (PSA, NKX3.1, TMPRSS2) and neuroendocrine markers (BRN2, NTS, GRP, CALCA) in i-SOX2-LNCaP cells after 48 hrs growth in the presence or absence of Dox (100 ng/ml). B. Western blot analysis of SYP and AR expression in i-SOX2-LNCaP whole cell extracts grown in the presence and absence of Dox (100 ng/ml) for 48 hrs. HDAC1 and β-tubulin were used as protein loading controls. Error bars represent standard deviation; statistical significance was determined by a 2-tailed student’s t-test (*p<.05,**p<.01,***p<.005).
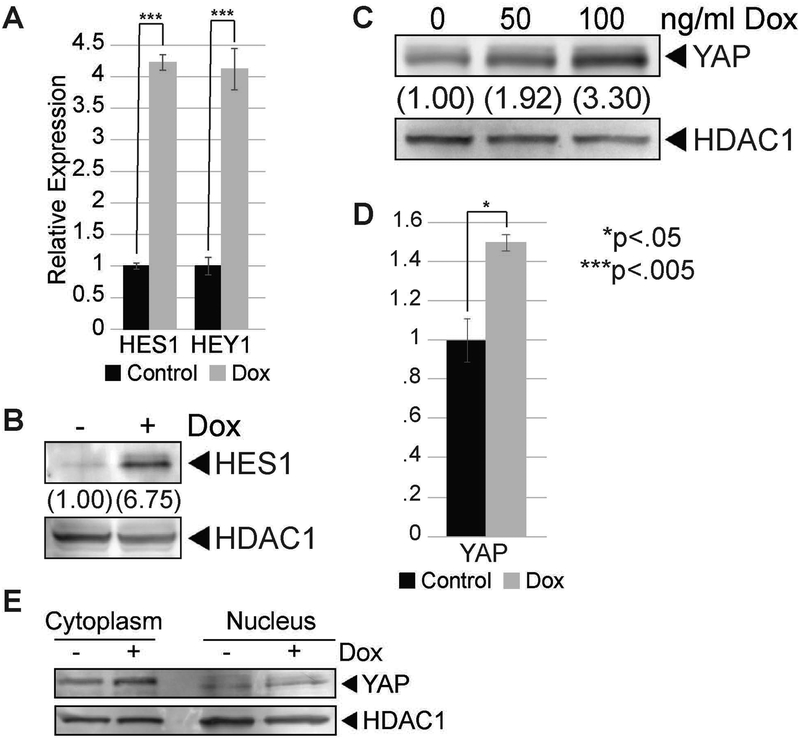
We also examined how elevation of SOX2 in i-SOX2-LNCaP cells affects the expression of other genes associated with NE-markers and the pathogenesis of PCa. Notch signaling has been associated with the NE phenotype of several cancers (Yao et al., 2018; Meder et al., 2016). Interestingly, when SOX2 was elevated in i-SOX2-LNCaP cells, we observed an increase in the expression of two well-characterized, downstream targets of Notch signaling. At the RNA level, we observed increases in the expression of both Hes1 and Hey1 (Fig 3A). In addition, we observed increases in the expression of Hes1 at the protein level (Fig 3B). We also examined the expression of the Yes-associated protein (YAP), because its expression has been reported to be increased by SOX2 in osteosarcoma cells (Basu-Roy et al., 2014). Equally important, YAP has been linked to poor prognosis for PCa, and elevating YAP reduces the dependence of PCa cells on androgens (Zhang et al, 2015). When SOX2 was elevated in i-SOX2-LNCaP cells, we observed an increase in the expression of YAP at the protein level (Fig 3C), but only a modest increase at the RNA level (Fig 3D). The increase in YAP was initially surprising given that it is associated with increased growth of PCa cells (Zhang et al., 2015), and the growth of i-SOX2-LNCaP cells is inhibited when SOX2 is elevated (Fig 1B). However, analysis of the subcellular localization of YAP indicated that the increase in YAP is due primarily to increased levels in the cytoplasm (Fig 3E), not in the nucleus where it would increase the transcription of growth-promoting genes. Thus, increases in YAP expression are unlikely to be responsible for increases in the expression of neuroendocrine genes when SOX2 is elevated. However, this does not rule out a possible role for YAP in the expression of neuroendocrine genes in some other context. Collectively, our studies indicate that elevation of SOX2 in i-SOX2-LNCaP cells increases the expression of both NE markers and several genes associated with other important signaling pathways.
Figure 3. SOX2 elevation modulates Notch and Hippo signaling in i-SOX2-LNCaP cells.
A. RT-qPCR analysis of HES1 and HEY1 in i-SOX2-LNCaP cells after 48 hrs growth in the presence or absence of Dox (100 ng/ml). B. Western blot analysis of HES1 expression in i-SOX2-LNCaP whole cell extracts after 48 hrs growth in the presence or absence of Dox (50 ng/ml). C. Western blot analysis of YAP expression in i-SOX2-LNCaP whole cell extracts after 48 hrs treatment with and without Dox dose (100 ng/ml). D. RT-qPCR analysis of YAP expression in i-SOX2-LNCaP cells after 48 hrs Dox (100 ng/ml) treatment. E. Western blot analysis of YAP protein localization in i-SOX2-LNCaP nuclear and cytoplasmic protein extracts after 48 hrs treatment with or without Dox (100 ng/ml). Error bars represent standard deviation; statistical significance was determined by a 2-tailed student’s t-test (*p<.05,***p<.005).
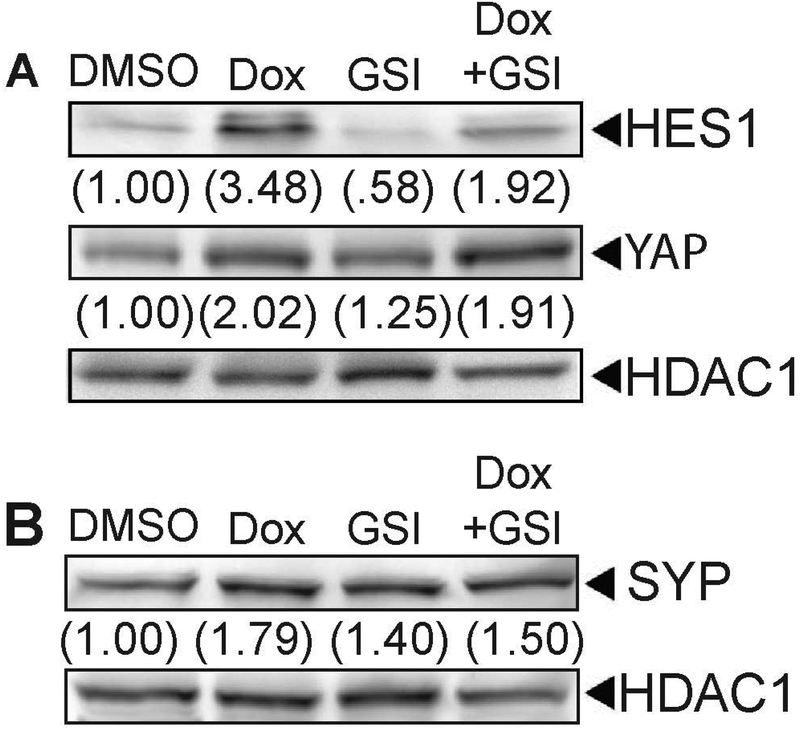
Currently, it is unclear how elevating SOX2 increases the expression of NE markers. Although there are reports linking Notch signaling to expression of NE genes, there are conflicting reports regarding Notch signaling and expression of NE markers (Yao et al., 2018; Meder et al., 2016). To determine whether the increase in Notch signaling when SOX2 is elevated contributes to increases in NE marker expression, we tested whether blocking the increase in Notch signaling with the use of a γ-secretase inhibitor (GSI) would alter the expression of SYP. As expected, GSI decreased the expression of Hes1 at the protein level (Fig 4 A). In contrast, GSI treatment had no effect on the expression of YAP (Fig 4A) or the expression of SYP (Fig 4B). Thus, it appears that the increase in the expression of NE markers is unlikely to result from the upregulation of Notch signaling when SOX2 is elevated in i-SOX2-LNCaP cells.
Figure 4. Increased YAP and Synaptophysin expression is independent of Notch elevation by SOX2 overexpression.
A. Western blot analysis of HES1 and YAP in i-SOX2-LNCaP whole cell extracts after 48 hrs growth in the presence or absence of Dox (100 ng/ml) and 10 μM RO4929097 (GSI). B. Western blot analysis of SYP expression in i-SOX2-LNCaP whole cell extracts after 48 hrs growth in the presence or absence of Dox and RO4929097. HDAC1 was used as a protein loading control.
We also considered the possibility that increases in NE-markers when SOX2 is elevated are the result of growth inhibition given that NE-like cells exhibit little or no growth during the early stages of PCa (Bonkhoff, Stein, & Remberger, 1995). To test the connection between growth inhibition and expression of NE markers, we treated i-SOX2-LNCaP cells with enzalutamide, which is a clinically used AR inhibitor that inhibits the growth of LNCaP cells (Mu et al., 2017). As expected, enzalutamide inhibited the growth of i-SOX2-LNCaP cells (Fig 5A) and the expression of the AR target gene PSA, but had little or no effect on the expression of AR at the concentrations used (Fig 5B). Importantly, enzalutamide had little effect on the expression of SYP at any concentration tested (Fig 5C). Although there appeared to be a small increase in SYP (50% or less), this was far smaller than the increase in SYP when SOX2 was elevated (~4-fold, Fig 2B). Hence, the increase in NE markers does not appear to be a consequence of growth inhibition when SOX2 is elevated in i-SOX2-LNCaP cells.
Figure 5. Increased Synaptophysin expression is not a consequence of growth inhibition.
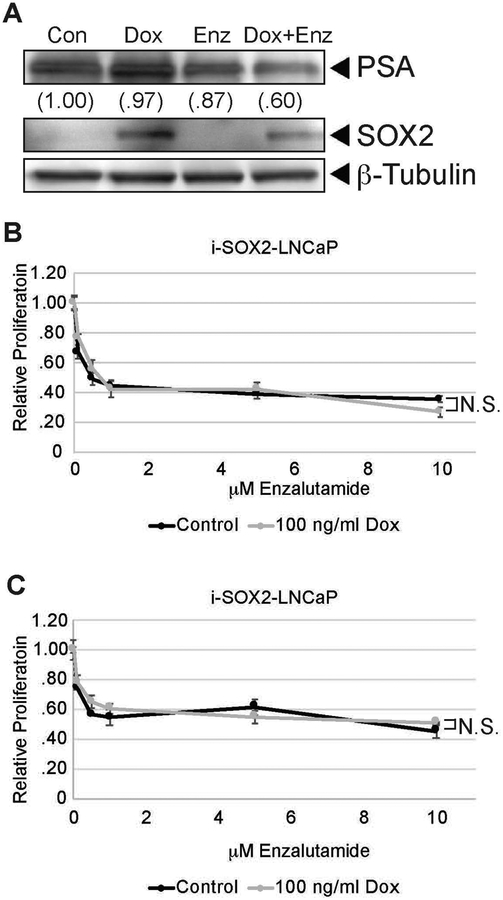
A. Growth of i-SOX2-LNCaP cells after 4 days in the presence of enzalutamide at the indicated concentrations. B. Western blot analysis of AR and PSA in i-SOX2-LNCaP whole cell extracts after 48 hrs growth in the presence of enzalutamide at the indicated concentrations. C. Western blot analysis of SYP expression in i-SOX2-LNCaP whole cell extracts after 48 hrs growth in the presence of enzalutamide at the indicated concentrations. The relative values of SYP expression at each enzalutamide concentration are provide in parentheses. HDAC1 was used as a protein loading control in panels (B) and (C). Error bars represent standard deviation; statistical significance was determined by a 2-tailed student’s t-test (*p<.05).
Importantly, we examined whether altering the expression of SOX2 influenced the ability of enzalutamide to inhibit the growth of prostate tumor cells. As noted earlier, knocking down RB1 and TP53 in LNCaP cells led to a large increase in SOX2 and a loss of growth inhibition by enzalutamide (Mu et al., 2017). Here, we initially examined whether elevating SOX2 without knocking down RB1 and TP53 would block the responses of i-SOX2-LNCaP cells to enzalutamide. We determined that elevating SOX2 failed to block the ability of enzalutamide to reduce the expression of PSA (Fig 6A). More specifically, the reduction in PSA expression in the presence of elevated SOX2 and enzalutamide (Fig 6A) is similar to the reduction in PSA expression when treated with enzalutamide on its own (Fig. 5B). Moreover, elevating SOX2 had no significant effect on growth inhibition by enzalutamide at any concentration of enzalutamide tested after 4 days (Fig 6B) and 8 days of SOX2 elevation (Fig. 6C). Together, these findings indicate that although SOX2 elevation is sufficient to increase the expression of NE markers in LNCaP cells, it is not sufficient to confer resistance to enzalutamide.
Figure 6. SOX2 elevation does not confer enzalutamide resistance to i-SOX2-LNCaP cells.
A. Western blot analysis of PSA and SOX2 in i-SOX2-LNCaP whole cell extracts treated for 48 hrs in the presence or absence of Dox (100 ng/ml) and enzalutamide (5 μM). β-Tubulin was used as a protein loading control. B. Growth of i-SOX2-LNCaP cells after 4 days in the presence or absence of Dox (100 ng/ml) and the indicated concentrations of enzalutamide. To correct for growth inhibition of elevated SOX2 in the absence of enzalutamide, the growth of the cells in the presence and absence of Dox were each set to 1. This growth study was performed three additional times at 5 μM enzalutamide and 100 ng/ml of Dox, and similar results were obtained in each case. C. Growth of i-SOX2-LNCaP cells after 8 days in the presence or absence of Dox (100 ng/ml) and 6 days of enzalutamide at the concentrations indicated. To correct for the inhibition of growth by elevated SOX2 in the absence of enzalutamide, the growth of the cells in the presence and absence of Dox were each set to 1. NS: not statistically significant.
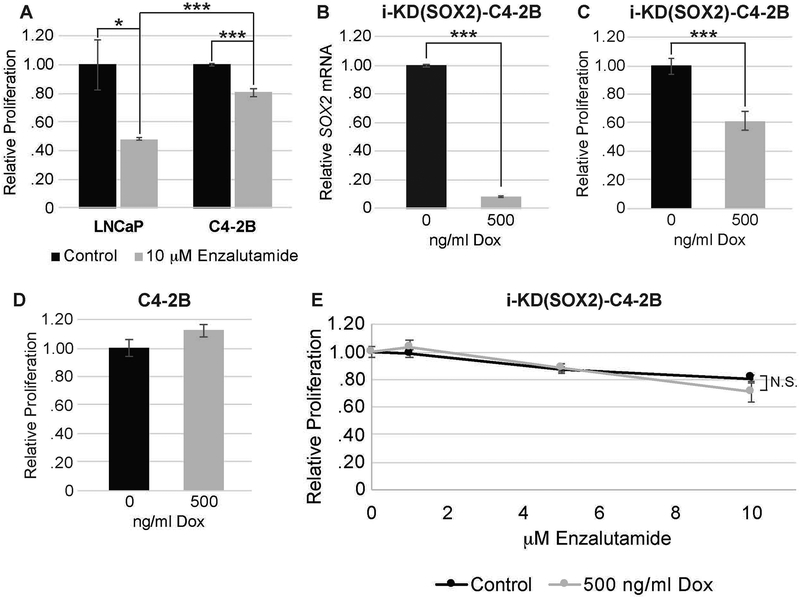
To further investigate the role of SOX2 in the responses of prostate tumor cells to enzalutamide, we examined whether knocking down SOX2 would affect the response to enzalutamide. For this purpose, we engineered C4–2B cells for knockdown of SOX2 by transducing the cells with a lentiviral vector that encodes a Dox-inducible SOX2 shRNA, which we refer to as i-KD(SOX2)-C4–2B. The SOX2 shRNA used in this study was used previously to knockdown SOX2 in pancreatic tumor cells (Wuebben et al., 2016). C4–2B cells were selected for this study because they are androgen receptor positive and express higher levels of SOX2 than LNCaP cells (>30-fold higher at the RNA level – data not shown). In addition, C4–2B cells are significantly less sensitive to enzalutamide than LNCaP cells (Fig. 7A). When i-KD(SOX2)-C4–2B cells were treated with Dox (500 ng/ml), we observed a strong knockdown of SOX2 mRNA (Fig. 7B). At this concentration of Dox, we also observed ~40% reduction in growth after 4 days (Fig. 7C). In contrast, the growth of parental C4–2B cells was not inhibited at this concentration of Dox (Fig. 7D). Importantly, knocking down SOX2 did not increase sensitivity of C4–2B cells to enzalutamide (Fig. 7E). Thus, it appears that the levels of SOX2 are not the prime determinant of the response of prostate tumor cells to enzalutamide.
Figure 7: SOX2 depletion does not alter the responses of i-KD(SOX2)-C4-2B cells to enzalutamide.
A. Responses of i-SOX2-LNCaP and i-SOX2-C4-2B cells to enzalutamide (10 μM) after 4 days of treatment. The growth of the control cells was set to 1. B. RT-qPCR analysis of SOX2 expression in i-KD(SOX2)-C4–2B cells after 48 hrs growth in the presence or absence of 500 ng/ml Dox. C. Growth of i-KD(SOX2)-C4–2B cells after 4 days in the presence or absence of 500 ng/ml Dox. D. Growth of parental C4–2B after 4 days in the presence or absence of 500 ng/ml Dox. E. Growth of i-KD(SOX2)-C4–2B cells after 4 days in the presence or absence of Dox (500 ng/ml) and the indicated concentrations of enzalutamide. To correct for inhibition of growth by SOX2 knockdown in the absence of enzalutamide, the growth of the cells in the presence and absence of Dox were each set to 1. This experiment was repeated at 250 ng/ml of Dox and same range of enzalutamide concentrations, and similar results were obtained. Error bars represent standard deviation; statistical significance was determined by a 2-tailed student’s t-test (***p<.005), NS: not statistically significant.
Discussion:
Previous studies demonstrated that SOX2 increases during PCa progression and its expression is correlated with negative outcomes (Russo et al., 2016; Kregel et al., 2013; Jia et al., 2011). Moreover, SOX2 has been implicated in the castration-resistant phenotype of PCa. Importantly, a recent study has shown that increased SOX2 is critical for enzalutamide resistance and a NE phenotype in the context of RB1 and TP53 depletion (Mu et al., 2017). This study demonstrated that loss of RB1 and TP53 in LNCaP increased the expression of NE markers and conferred resistance to the anti-androgen enzalutamide. Significantly, loss of RB1 and TP53 also led to a large increase in SOX2 mRNA, and knocking down SOX2 in this context reversed NE marker expression and restored enzalutamide sensitivity in vivo (Mu et al., 2017). While these studies demonstrated that SOX2 is necessary for NE phenotypic plasticity and drug resistance in this context, they did not address whether elevating SOX2 on its own in PCa cells is sufficient to induce the expression of NE markers and confer resistance to enzalutamide. In this study, we demonstrate that elevating SOX2 from a Dox-inducible promoter is sufficient to increase the expression of NE markers in LNCaP cells. Specifically, when SOX2 was elevated, mRNA expression of BRN2, NTS, GRP, and CALCA was increased significantly. We also observed increased protein expression of SYP upon elevation of SOX2. Interestingly, SOX2 overexpression did not significantly alter the expression of AR or several AR target genes. The elevated expression of NE genes, despite sustained AR signaling, suggests that elevating SOX2 in LNCaP induces lineage plasticity rather than transdifferentiation into NE-like cells.
In this study, we also examined the effects of elevating and knocking down SOX2 on the responses of PCa cells to enzalutamide. Previous studies have implicated SOX2 in the resistance to drugs used clinically. In the case of breast tumor cells, knocking down SOX2 increased the sensitivity to paclitaxel (Mukherjee et al., 2017). Conversely, elevating SOX2 in pancreatic ductal adenocarcinoma (PDAC) cells reduced the growth inhibitory effects of clinically used AKT and MEK inhibitors (Wuebben et al., 2016). Moreover, increasing SOX2 in PC3 PCa cells enhanced resistance to paclitaxel through the upregulation of PI3K/AKT signaling (Li et al., 2014). Importantly, as discussed above, knocking down SOX2 reverses the androgen-independent phenotype observed upon loss of RB1 and TP53, demonstrating a causal role for elevated SOX2 in conferring androgen-independence in this context. In contrast, our studies demonstrate that elevating SOX2 in i-SOX2-LNCaP cells, which express wild-type RB1 and TP53, does not block the growth inhibitory effects of enzalutamide. Furthermore, we show that knocking down SOX2 in C4–2B cells, which also express wild-type RB1 and TP53, does not alter the sensitivity to enzalutamide. Thus, our studies indicate that neither elevating nor knocking down SOX2 acutely alters the sensitivity of prostate tumor cells to enzalutamide.
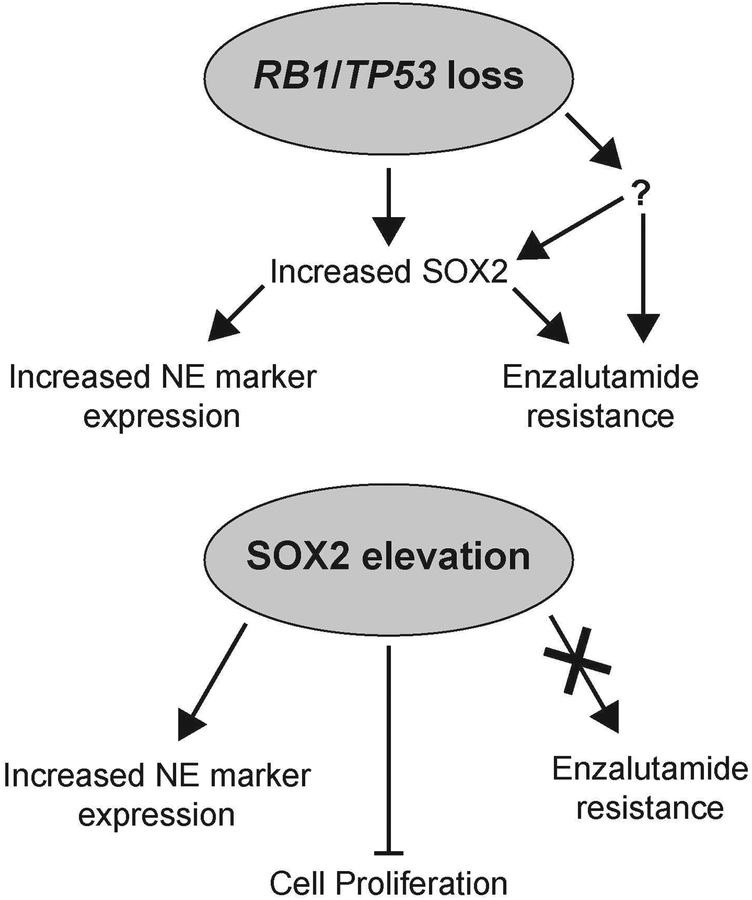
Our finding that elevating and knocking down SOX2 on its own is not sufficient to alter the response to enzalutamide suggests that the loss of RB1 and TP53 confer antiandrogen resistance, as discussed above, through other mechanisms in addition to SOX2 elevation (Fig 7). Intriguingly, RB1 has been shown previously to repress SOX2 expression and oppose reprogramming to induced pluripotent stem cells (iPSCs) (Kareta et al., 2015). Therefore, it is possible that one of the critical functions of RB1 loss in promoting NE plasticity and castration resistance is to elevate SOX2 expression. In the future, characterizing the additional mechanisms through which loss of RB1 and TP53 confer androgen independence may identify therapeutic targets that restore sensitivity to second-line antiandrogens.
The finding that SOX2 elevation in LNCaP cells increases NE marker expression without affecting sensitivity to enzalutamide is highly significant, because many studies use the expression of NE markers, such as synaptophysin, to indicate an androgen-independent phenotype. However, our studies demonstrate that the elevation of NE markers can occur independently of enzalutamide resistance. Importantly, the increased expression of neuroendocrine markers does not appear to be due to the growth inhibitory effects of elevated SOX2, because i-SOX2-LNCaP cells treated with enzalutamide exhibited growth inhibition, yet did not display increased expression of the NE marker SYP.
Our studies also demonstrate that inducible elevation of SOX2 in i-SOX2-LNCaP cells leads to growth inhibition. We have shown previously that elevating SOX2 with an inducible promoter in medulloblastoma, glioblastoma, and PDAC inhibits cell proliferation (Cox et al., 2012; Wuebben et al., 2016). In agreement with our findings, other studies have implicated elevated SOX2 in the quiescence of both normal cells during development and tumor cells (reviewed in Metz & Rizzino, 2019). In both lung cancer and medulloblastoma, quiescent tumor cells have been shown to express elevated levels of SOX2 (Malladi et al., 2016; Vanner et al., 2014). Our findings are also supported by the observation that during development, cells with elevated SOX2 expression in the central nervous system and endoderm-derived organs are less likely to display proliferative markers in comparison to cells with decreased SOX2 within the same organ (Hagey & Muhr, 2014; Hagey et al., 2018). Importantly, knockdown and overexpression of SOX2 in endoderm-derived tissues demonstrated that high levels of SOX2 restrict cell proliferation during development (Hagey et al., 2018). Collectively, these findings indicate that elevating SOX2 above basal levels inhibits proliferation of tumor cells as well as cells during normal development.
In conclusion, we demonstrate that elevated SOX2 is sufficient to increase NE marker expression, but is not sufficient to confer resistance to enzalutamide. Going forward, it will be important to characterize the mechanisms by which SOX2 inhibits the proliferation of tumor cells, because our studies have shown that this occurs in multiple human tumor types. Additionally, it will be important to identify mechanisms that cooperate with SOX2 elevation to induce NE plasticity and castration resistance in order to help identify therapeutic targets that are able to restore sensitivity to the anti-androgen enzalutamide.
Figure 8: Models for the action of SOX2 on its own and in the context of RB1/TP53 deficiency in prostate cancer.
Acknowledgments:
Ming-Fong Lin is thanked for his gift of early passage LNCaP cells. Heather Rizzino is thanked for editorial assistance.
Funding: This work was supported in part by a grant from the National Institute of General Medical Sciences (GM106397), a grant from the Nebraska Department of Health (2020–47), and funds from Fred & Pamela Buffett Cancer Center. Core facilities of the Fred & Pamela Buffett Cancer Center are supported by a Cancer Center Support grant from the National Cancer Institute, P30 CA036727.
Data Availability: The data that support the findings of this study are available from the corresponding author upon reasonable requests.
Footnotes
Conflict of Interests: The authors declare that they have no conflict of interests.
References:
- Aljameeli A, Thakkar A, & Shah G (2017). Calcitonin receptor increases invasion of prostate cancer cells by recruiting zonula occludens-1 and promoting PKA-mediated TJ disassembly. Cellular Signalling, 36, 1–13. [DOI] [PubMed] [Google Scholar]
- Alonzeau J, Alexandre D, Jeandel L, Courel M, Hautot C, Yamani FE, … Chartrel N (2013). The neuropeptide 26RFa is expressed in human prostate cancer and stimulates the neuroendocrine differentiation and the migration of androgen-independent prostate cancer cells. European Journal of Cancer, 49(2), 511–519. [DOI] [PubMed] [Google Scholar]
- Basu-Roy U, Bayin NS, Rattanakorn K, Han E, Placantonakis DG, Mansukhani A, and Basilico C (2014). Sox2 antagonizes the Hippo pathway to maintain stemness in cancer cells. Nature Communications. 6:6411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonkhoff H, Stein U, Remberger K (1995). Endocrine-paracrine cell types in the prostate and prostatic adenocarcinoma are postmitotic cells. Human Pathology, 26(2), 167–170. [DOI] [PubMed] [Google Scholar]
- Bonkhoff H, Wernert N, Dhom G, & Remberger K (1991). Relation of endocrine-paracrine cells to cell proliferation in normal, hyperplastic, and neoplastic human prostate. The Prostate, 19(2), 91–98. [DOI] [PubMed] [Google Scholar]
- Chen Y, Sawyers CL, & Scher HI (2008). Targeting the androgen receptor pathway in prostate cancer. Current Opinion in Pharmacology, 8(4), 440–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox JL, Wilder PJ, Desler M, & Rizzino A (2012). Elevating SOX2 Levels Deleteriously Affects the Growth of Medulloblastoma and Glioblastoma Cells. PLoS ONE, 7(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasilva JO, Amorino GP, Casarez EV, Pemberton B, & Parsons SJ (2012). Neuroendocrine-derived peptides promote prostate cancer cell survival through activation of IGF-1R signaling. The Prostate, 73(8), 801–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito S, Russo MV, Airoldi I, Tupone MG, Sorrentino C, Barbarito G, … Carlo ED (2015). SNAI2/Slug gene is silenced in prostate cancer and regulates neuroendocrine differentiation, metastasis-suppressor and pluripotency gene expression. Oncotarget, 6(19). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigore AD, Ben-Jacob E, & Farach-Carson MC (2015). Prostate Cancer and Neuroendocrine Differentiation: More Neuronal, Less Endocrine? Frontiers in Oncology, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagey DW, & Muhr J (2014). Sox2 Acts in a Dose-Dependent Fashion to Regulate Proliferation of Cortical Progenitors. Cell Reports, 9(5), 1908–1920. [DOI] [PubMed] [Google Scholar]
- Hagey DW, Klum S, Kurtsdotter I, Zaouter C, Topcic D, Andersson O, … Muhr J (2018). SOX2 regulates common and specific stem cell features in the CNS and endoderm derived organs. PLOS Genetics, 14(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igawa T, Lin FF, Lee MS, Karan D, Batra SK, & Lin MF (2002). Establishment and characterization of androgen-independent human prostate cancer LNCaP cell model. Prostate. 50(4), 222–235. [DOI] [PubMed] [Google Scholar]
- Ishimaru H, Kageyama Y, Hayashi T, Nemoto T, Eishi Y, & Kihara K (2002). Expression of Matrix Metalloproteinase-9 and Bombesin/Gastrin-Releasing Peptide in Human Prostate Cancers and their Lymph Node Metastases. Acta Oncologica, 41(3), 289–296. [DOI] [PubMed] [Google Scholar]
- Jia X, Li X, Xu Y, Zhang S, Mou W, Liu Y, … Li N (2011). SOX2 promotes tumorigenesis and increases the anti-apoptotic property of human prostate cancer cell. Journal of Molecular Cell Biology, 3(4), 230–238. [DOI] [PubMed] [Google Scholar]
- Jiang N, Ke B, Hjort-Jensen K, Iglesias-Gato D, Wang Z, Chang P, … Niu Y (2017). YAP1 regulates prostate cancer stem cell-like characteristics to promote castration resistant growth. Oncotarget, 8(70). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin RJ, Wang Y, Masumori N, Ishii K, Tsukamoto T, Shappell SB, … Matusik RJ (2004). NE-10 Neuroendocrine Cancer Promotes the LNCaP Xenograft Growth in Castrated Mice. Cancer Research, 64(15), 5489–5495. [DOI] [PubMed] [Google Scholar]
- Kareta MS, Gorges LL, Hafeez S, Benayoun BA, Marro S, Zmoos AF, … Wernig M (2015). Inhibition of pluripotency networks by the Rb tumor suppressor restricts reprogramming and tumorigenesis. Cell Stem Cell, 16(1), 39–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudsen KE, & Penning TM (2010). Partners in crime: Deregulation of AR activity and androgen synthesis in prostate cancer. Trends in Endocrinology & Metabolism, 21(5), 315–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kregel S, Kiriluk KJ, Rosen AM, Cai Y, Reyes EE, Otto KB, … Griend DJ (2013). Sox2 Is an Androgen Receptor-Repressed Gene That Promotes Castration-Resistant Prostate Cancer. PLoS ONE, 8(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ku SY, Rosario S, Wang Y, Mu P, Seshadri M, Goodrich ZW, … Goodrich DW (2017). Rb1 and Trp53 cooperate to suppress prostate cancer lineage plasticity, metastasis, and antiandrogen resistance. Science, 355(6320), 78–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D, Zhao L, Zheng X, Lin P, Lin F, Li Y, … Yu X (2014). Sox2 is involved in paclitaxel resistance of the prostate cancer cell line PC-3 via the PI3K/Akt pathway. Molecular Medicine Reports, 10(6), 3169–3176. [DOI] [PubMed] [Google Scholar]
- Malladi S, Macalinao DG, Jin X, He L, Basnet H, Zou Y, … Massagué J (2016). Metastatic Latency and Immune Evasion through Autocrine Inhibition of WNT. Cell, 165(1), 45–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meder L, König K, Ozretić L, Schultheis AM, Ueckeroth R, Ade CP, … Buettner R (2016). NOTCH, ASCL1, p53, and RB alterations define an alternative pathway driving neuroendocrine and small cell lung carcinomas. International Journal of Cancer. 138(4), 927–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metz EP, & Rizzino A SOX2 dosage: A critical determinant in the functions of Sox2 in both normal and tumor cells. (2019). Journal of Cellular Physiology 234(11):19298–19306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mu P, Zhang Z, Benelli M, Karthaus WR, Hoover E, Chen C, … Sawyers CL (2017). SOX2 promotes lineage plasticity and antiandrogen resistance in TP53- and RB1-deficient prostate cancer. Science, 355(6320), 84–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee P, Gupta A, Chattopadhyay D, & Chatterji U (2017). Modulation of SOX2 expression delineates an end-point for paclitaxel-effectiveness in breast cancer stem cells. Scientific Reports. 7(1), 9170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowling TK, Johnson LR, Wiebe MS, & Rizzino A (2000). Identification of the transactivation domain of the transcription factor Sox-2 and an associated co-activator. Journal of Biological Chemistry. 275(6),3810–3818. [DOI] [PubMed] [Google Scholar]
- Parimi V, Goyal R, Poropatich K, Yang XJ (2014). Neuroendocrine differentiation of prostate cancer: a review. American Journal of Clinical and Experimental Urology 4,273–285. [PMC free article] [PubMed] [Google Scholar]
- Russo MV, Esposito S, Tupone MG, Manzoli L, Airoldi I, Pompa P, … Carlo ED (2015). SOX2 boosts major tumor progression genes in prostate cancer and is a functional biomarker of lymph node metastasis. Oncotarget, 7(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabbisetti VS, Chirugupati S, Thomas S, Vaidya KS, Reardon D, Chiriva-Internati M, … Shah GV (2005). Calcitonin increases invasiveness of prostate cancer cells: Role for cyclic AMP-dependent protein kinase A in calcitonin action. International Journal of Cancer, 117(4), 551–560. [DOI] [PubMed] [Google Scholar]
- Siegel RL, Miller KD, & Jemal A (2019). Cancer statistics, 2019. CA: A Cancer Journal for Clinicians, 69(1), 7–34. [DOI] [PubMed] [Google Scholar]
- Thalmann GN, Anezinis PE, Chang SM, Zhau HE, Kim EE, Hopwood VL, … Chung LWL (1994). Androgen-independent Cancer Progression and Bone Metastasis in the LNCaP Model of Human Prostate Cancer. Cancer Research, 54(10), 2577–2581. [PubMed] [Google Scholar]
- Vanner R, Remke M, Gallo M, Selvadurai H, Coutinho F, Lee L, … Dirks P (2014). Quiescent Sox2 Cells Drive Hierarchical Growth and Relapse in Sonic Hedgehog Subgroup Medulloblastoma. Cancer Cell, 26(1), 33–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wuebben EL, Wilder PJ, Cox JL, Grunkemeyer JA, Caffrey T, Hollingsworth MA, & Rizzino A (2016). SOX2 functions as a molecular rheostat to control the growth, tumorigenicity and drug responses of pancreatic ductal adenocarcinoma cells. Oncotarget, 7(23). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao E, Lin C, Wu Q, Zhang K, Song H, & Chuang PT (2018) Notch Signaling Controls Transdifferentiation of Pulmonary Neuroendocrine Cells in Response to Lung Injury. Stem Cells. 36(3), 377–391. [DOI] [PubMed] [Google Scholar]
- Yu X, Cates JM, Morrissey C, You C, Grabowska MM, Zhang J, … Matusik RJ (2014). SOX2 expression in the developing, adult, as well as, diseased prostate. Prostate Cancer and Prostatic Diseases, 17(4), 301–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Yang S, Chen X, Stauffer S, Yu F, Lele SM, … Dong J (2015). The Hippo Pathway Effector YAP Regulates Motility, Invasion, and Castration-Resistant Growth of Prostate Cancer Cells. Molecular and Cellular Biology, 35(8), 1350–1362. [DOI] [PMC free article] [PubMed] [Google Scholar]