Abstract
Phyllanthus niruri L. is a widespread tropical plant which is used in Ayurvedic system for liver and kidney ailments. The present study aims at specifying the most active hepatoprotective extract of P. niruri and applying a bio-guided protocol to identify the active compounds responsible for this effect. P. niruri aerial parts were extracted separately with water, 50%, 70% and 80% ethanol. The cytoprotective activity of the extracts was evaluated against CCl4-induced hepatotoxicity in clone-9 and Hepg2 cells. Bioassay-guided fractionation of the aqueous extract (AE) was accomplished for the isolation of the active compounds. Antioxidant activity was assessed using DPPH (1, 1-diphenyl-2-picrylhydrazyl) radical scavenging method and ferric reducing antioxidant power (FRAP). The in vivo hepatoprotective activity of AE was evaluated in CCl4-induced hepatotoxicity in rats at different doses after determination of its LD50. Pretreatment of clone-9 and Hepg2 with different concentrations of AE (1, 0.1, 0.01 mg/ml) had significantly reduced the levels of alanine aminotransferase (ALT) and aspartate aminotransferase (AST) against CCl4 injures, and restored the activity of the natural antioxidants; glutathione (GSH) and superoxide dismutase (SOD) towards normalization. Fractionation of AE gave four fractions (I-IV). Fractions I, II, and IV showed a significant in vitro hepatoprotective activity. Purification of I, II and IV yielded seven compounds; corilagin C1, isocorilagin C2, brevifolin C3, quercetin C4, kaempferol rhamnoside C5, gallic acid C6, and brevifolin carboxylic acid C7. Compounds C1, C2, C5, and C7 showed the highest (p< 0.001) hepatoprotective potency, while C3, C4, and C6 exhibited a moderate (p< 0.001) activity. The AE exhibited strong antioxidant DPPH (IC50 11.6 ± 2 μg/ml) and FRAP (79.352 ± 2.88 mM Ferrous equivalents) activity. In vivo administration of AE in rats (25, 50, 100 and 200 mg/kg) caused normalization of AST, ALT, alkaline phosphatase (ALP), lactate dehydrogenase (LDH), total cholesterol (TC), triglycyrides (TG), total bilirubin (TB), glucose, total proteins (TP), urea and creatinine levels which were elevated by CCl4. AE also decreased TNF-α, NF-KB, IL-6, IL-8, IL10 and COX-2 expression, and significantly antagonizes the effect of CCl4 on the antioxidant enzymes SOD, catalase (CAT), glutathione reductase (GR), and glutathione peroxidase (GSP). The histopathological study also supported the hepatoprotective effect of AE. P. niruri isolates exhibited a potent hepatoprotective activity against CCl4-induced hepatotoxicity in clone-9 and Hepg2 cell lines through reduction of lipid peroxidation and maintaining glutathione in its reduced form. This is attributable to their phenolic nature and hence antioxidative potential.
Introduction
Liver injury, caused by viruses, drugs and chemicals, is a significant toxicological problem [1–3]. The damage is associated with metabolic and synthetic dysfunctions which can lead to fatal complications [4]. CCl4-induced acute liver injury is the best characterized system of xenobiotic-induced hepatotoxicity and a common screening model for evaluation of the hepatoprotective potential of drugs [5]. The pathogenesis of the damage is multivariate [6] involving propagation of a chain of free radicals, leading to lipid peroxidation and destruction of cellular membranes, followed by triggering the inflammatory response of the body [7, 8]. In spite of the fact that advances in understanding of the liver damage molecular mechanisms are achieved, there are still limited effective hepatoprotective interventions. Thus, herbal alternatives drew much attention as a safe solution for this problem.
The Phyllanthus genus contains over 600 species distributed throughout the tropical and subtropical regions of the world. The plants of genus Phyllantus have long been used to treat liver diseases [9]. A wide number of experimental studies have demonstrated the hepatoprotective potential of Phyllantus plants in in vitro and in vivo systems [10–12].
P. niruri has a good reputation in herbal medicine systems such as Indian Ayurveda, Traditional Chinese Medicine and Indonesian Jamu for over 2000 years. P. niruri has been used as a remedy for many ailments such as dyspepsia, influenza, diuretics, vaginitis, hyperglycaemia, jaundice and removing kidney stones [13]. P. niruri is named in Spanish as Chanca Piedra, this means stone breaker, as it was used as an excellent remedy for gallstones and kidney stones elimination [14]. It is named Quebra Pedra in Brazilian herbal medicine, where it is considered an effective remedy for urinary and bladder disorders as well as hepatic disorders and hyperglycemia. P. niruri is used as a remedy for asthma, bronchitis, coughs in India, for this reason it is named Pitirishi or Budhatri [13]. P. niruri was specifically tested for its hepatoprotective [15–17], antioxidant [18–20], antihyperuricemic [21] and lipid lowering activities [22]. Its actions were evaluated on various organs including liver, kidneys and testes [23].
This study aims at optimizing a method for extraction of P. niruri based on evaluation of the in vitro hepatoprotective activity on rat liver normal cell line (clone-9) and human liver hepatoma cells (Hepg2). In addition, this study focuses on applying a bioassay guided fractionation of the active extract to identify the active fraction and then the active isolate, and finally to confirm the hepatoprotective activity of the active extract via evaluation of its in vivo hepatoprotective activity against CCl4-induced hepatotoxicity in rats.
Materials and methods
General
Silica gel 60 (70–230 mesh ASTM; Fluka, Steinheim, Germany), Sephadex LH 20 (Pharmacia, Stockholm, Sweden) and Diaion HP-20 AG (75–150 μm, Mitsubishi Chemical Industries Co. Ltd) were used for column chromatography. Thin-layer chromatography (TLC) was performed on silica gel GF254 precoated plates (Fluka, Steinheim, Germany). The used solvent systems were S1 [methylene chloride-methanol-formic acid (9.5:0.5:0.2 v/v/v)], S2 [methylene chloride-methanol-formic acid (8.5:1.5:0.2 v/v/v)] and S3 [methylene chloride-methanol-formic acid (7:3:0.2 v/v/v)]. Bruker NMR was used for 1H-NMR (400 MHz) and 13C-NMR (100 MHz) measurements. The NMR spectra were recorded in deuterated dimethyl sulfoxide (DMSO-d6) and chemical shifts were given in δ (ppm) relative to TMS as internal standard. CCl4, Ellman's reagent and thiobarbituric acid (TBA) were purchased from Sigma Chemical Co., St Louis, MO, USA. n-Butanol, dipotassium hydrogen phosphate, potassium dihydrogen phosphate and trichloroacetic acid were purchased from El-Gomhoreya Chemical Co, Cairo, Egypt. All other chemicals were of highest grade commercially available materials.
Plant material
The aerial parts of Phyllanthus niruri L. were obtained from HCA products Sdn Bhd (922996-T), Blok N1,UPM MTDC Technology Center, Universiti Putra Malaysia 43400 Serdang Selangor, spring 2016. The aerial parts were harvested in the morning and put inside plastic containers during the collecting period then placed in oven at a control temperature of 40–50°C. The drying process took 2–3 hours to ensure that the raw material was dry and no substance of humidity. The dried aerial parts were powdered and placed inside a glass container. The plant was kindly identified in the Forest Research institute, Malaysia. Plant samples were also authenticated by Prof. Dr. Wafaa Amer, Department of Botany, Faculty of Science, Cairo University, Giza, Egypt.
Preparation of the aqueous extract
The aqueous extract (AE, DA001NW) was obtained from HCA products Sdn Bhd, where the dried powdered aerial parts (40 kg) of P. niruri were boiled with 200 liters of RO water (water purified with reverse osmosis) for 2h and 30 min. The extract was concentrated in a rotary evaporator for 3 hours at 60°C to 20 liters. The extract was then dried in a spray dryer by heating for 6h and 30 min at a temperature of 120°C and yielded 8 kg powdered extract. The (AE) was tested for potentially toxic or harmful compounds (toxic metals, adulterants, adventitious toxin, foreign materials and residual pesticides) by HCA products Sdn Bhd and that no such compounds were identified in the analysis.
Preparation of the crude ethanol extracts
The powdered dried P. niruri aerial parts (50 g powder for each extract) were separately extracted using 50%, 70% and 80% ethanol. The liquid-material ratio was 90:1 in a three-stage procedure (each in a ratio of 30:1), using ultrasonic bath at 60°C for 30 minutes each time. The extracts were concentrated separately using rotary evaporator under reduced pressure (at 40°C) to yield solid residues weighing 5.35, 4.14 and 2.42 g of 50%, 70% and 80% ethanol extracts, respectively.
In vitro hepatoprotective activity
Cell culture
Rat liver normal cell line (clone-9) and human liver hepatoma cell line (Hepg2) (VACSERA, Giza, Egypt) were maintained in the tissue culture facility, Faculty of Pharmacy, Ain Shams University, Cairo, Egypt. Cells were grown in Dulbecco’s modified Eagle’s medium (DMEM).
Cells were maintained at 37°C in a 5% CO2 atmosphere with 95% humidity. The stock culture was grown in 25 cm2 culture flasks and all experiments were carried out in 96 microtitreplates. Cell culture reagents were obtained from Lonza (Basel, Switzerland) [24].
CCl4 induced toxicity in Clone-9 and Hepg2 cell lines
The protective activities of the samples were examined in vitro at three concentrations (1, 0.1, 0.01 mg/mL). Cultured clone-9 and Hepg2 monolayer cells were incubated with the different concentrations of samples. At 1 h after incubation, CCl4 was added at a final concentration of 40 mM (in 0.05% DMSO). Negative control incubations were treated only with phosphate-buffered saline for 1 h and then exposed for CCl4. Positive control incubations were incubated with Silymarin for 1 h before exposure to CCl4. All incubations continued for 24 h. Then, media and cell lysates were collected and stored at -20°C until analysis. Activities of alanine aminotransferase (ALT) and aspartate aminotransferase (AST) were assessed in the supernatant media. Glutathione (GSH) content and superoxide dismutase (SOD) activity were evaluated in the cell lysates using commercial kits (Biodiagnostics, Cairo, Egypt) according to the manufacturer’s instructions.
Fractionation of the aqueous extract
Four hundred grams of the AE were suspended in 600 ml distilled water and fractionated over Diaion HP 20 column (60 cm L × 5 cm D, 500g) using 100% H2O, 50% MeOH in H2O, 100% MeOH, and (CH3)2CO yielding four fractions I-IV, respectively. The solvent in each case was evaporated under reduced pressure at 40°C to yield 160.47, 100.43, 8.20, and 1.56 g, respectively.
Isolation from the AE bioactive fractions (Fr I, II and IV)
Fr-I (10 g) was subjected to fractionation over a silica gel column (2 x 25 cm, 150 g). Gradient elution was performed using methanol-ethyl acetate mixtures. Fractions (100 ml, each) were collected and monitored by TLC using solvent system (S1). Sub-fraction (20% methanol in ethyl acetate) was chromatographed over a sephadex LH 20 column. The elution was carried out using 100% methanol to give two pure compounds C1 (white amorphous powder, 322.5 mg) and C2 (white amorphous powder, 278 mg).
Fr-II (20 g) was subjected to fractionation over a silica gel column (3.5 x 25 cm, 300 g). Gradient elution was performed using n-hexane-ethyl acetate mixtures. The polarity was increased by 1% every 100 ml till 100% ethyl acetate then ethyl acetate-methanol mixtures were used for elution by increasing polarity by 1% every 100 ml till 25% methanol. Fractions (100 ml, each) were collected and monitored by TLC using solvent system (S1). Sub-fractions (50% and 70% ethyl acetate in n-hexane) were separetly chromatographed over a sephadex LH 20 columns using (100% and 80% methanol, respectively) to yield pure compounds C3 (white amorphous powder, 435.4 mg) and C4 (yellow amorphous powder, 387.3 mg). Sub-fraction (5–10% methanol in ethyl acetate) was chromatographed over a sephadex LH 20 column. The elution carried out using 90% methanol to give compounds C5 (yellow amorphous powder, 110.2 mg) and C6 (white amorphous powder, 405.1 mg).
A weighed amount (1 g) of Fr-IV was subjected to fractionation over a silica gel column (1.5 x 20, 50g). Gradient elution was performed using methanol-dichloromethane mixtures. Fractions (100 ml, each) were collected and monitored by TLC using solvent systems (S2-S3). Sub-fraction (30–40% methanol in dichloromethane) was chromatographed over a silica gel column. The elution was carried out using dichloromethane–methanol (9.8:0.2 v/v). Similar fractions, showing pure spots, were pooled together to yield one pure compound C7 (yellow amorphous powder, 264.8 mg).
Evaluation of antioxidant activity
DPPH radical scavenging method
DPPH free radical scavenging activity of the aqueous plant extract was carried out using the method described by Shimada et al. [25]. Briefly, 0.1mM solution of DPPH• in methanol was prepared. Then, 1 ml of this solution was added to 3 ml of extract solution at different conc. (25–75μg/ ml). The mixture was shaken vigorously and allowed to stand at room temperature for 30 min. Then the absorbance was measured at 517 nm in Asys microplate reader. Lower absorbance of the reaction mixture indicated a higher free radical scavenging activity.
DPPH scavenging effect (%) = 100 − [((A0-A1)/A0) × 100]
Ferric Reducing Antioxidant Power Assay (FRAP)
A slightly modified method of Benzei and Strain [26] was carried out to estimate the ferric reducing ability of the AE. Briefly, 10 μL of samples (AE and gallic acid) were mixed with 190 μL of reaction mixture (152 μL FRAP acetate buffer, pH 3.6, 19 μL ferric tripyridyl triazine (Fe III TPTZ) and 19 μL FeCl3). The absorbance was measured immediately at 594 nm in kinetic mode for 60 minutes at 37°C. To test the reproducibility of the assays, the antioxidant activities were measured three times. FRAP values (mM Fe (II)) were calculated using a linear regression equation for standard ferrous sulphate.
In vivo experiments
Animals and experimental protocol
Male Wistar rats (200–250 g) were supplied by the Animal Breeding Laboratory, Helwan, Egypt. Animals were housed in an air-conditioned atmosphere (22±2°C, 12 h light–dark cycle). They were provided with rodent chow and water ad libitum. The investigation was performed in agreement with the Animal Research: Reporting of in vivo Experiments (ARRIVE) rules, created by the National Center for the Replacement, Refinement and Reduction of Animals in Research (NC3Rs). The investigation was approved by the Ethics Committee for Animal Experimentation of Faculty of Pharmacy, Cairo University (Permit Number: MP 2162).
LD50
LD50 was determined using acute toxic class method as per OECD (2001) (Organization for Economic Co-operation and Development, Guideline-423, adopted on 17th December, 2001). Based on a previous pilot study in our laboratories, a limit test was performed. Animals were fasted overnight and the P. niruri was administered orally using gastric feeding needle at a dose of 5000 mg/kg (10 ml/kg dosing volume).
Hepatoprotective activity
To determine the least effective hepatoprotective dose, a dose-response study was conducted. Animals were divided into six groups of 6 rats each. Group I: control that received water via oral injection followed by intraperitoneal (IP) injection of corn oil after 4 h. Group II: CCl4 hepatotoxicity group which was injected once with 1 mL/kg of CCl4-corn oil 50% mixture. Groups III-VI: pretreated by oral bolus injection of AE (25, 50, 100 and 200 mg/kg, respectively), then given CCl4 IP injection after 4 h of the pretreatment. Rats were monitored twice a day to avoid any potential distress to animals. Signs of severe distress, abnormal behavior, writhing or convulsions were taken as indicators for immediate sacrifice of animals. However, none of such signs were observed with the doses used in the current study. Euthanasia was performed at 24 h after CCl4 challenge. At the end of the experiment, the rats were sacrificed under sodium pentobarbitone anaesthesia according to the guidelines for euthanasia in the Guide for the Care and Use of Laboratory Animals (2011). Histopathological findings together with AST and ALT levels were used to determine the least effective hepatoprotective dose to be used for further investigations.
Tissue and serum samples preparation
Blood samples were obtained from the retro-orbital plexus and allowed to clot. This was followed by centrifugation (3000 rpm for 10 min) to separate the serum. It was stored till needed at -80°C. Animals were then sacrificed and liver tissues were dissected and homogenized to produce a 20% homogenate.
Biochemical analyses
Activities of serum ALT, AST and alkaline phosphatase (ALP), serum levels of total bilirubin (TB), total proteins (TP), triglycerides (TG), total cholesterol (TC), creatinine, glucose, and urea were colorimetrically determined using kits (Spectrum Diagnostics, Cairo, Egypt). Lactate dehydrogenase (LDH) leakage was kinetically estimated using a commercial kit (BioSystems, Barcelona, Spain).
Thiobarbituric acid reactive substances (TBARS) level was measured as malondialdehyde (MDA) was estimated to determine the lipid peroxidation [27]. The reaction mixture composed of 0.5 ml homogenate, 2.5 ml 20% trichloroacetic acid (TCA), and 1.0 ml 0.6% thiobarbituric acid (TBA) was heated for 20 min then cooled and 4 ml n-butanol was added. The absorbance was measured at 535 nm. MDA level was expressed as nmol of MDA/g wet tissue.
To determine reduced glutathione (GSH), the mixture (0.5 mL homogenate+ 0.5 ml 10% TCA) was centrifuged for 10 min at 3000 rpm. The resulting supernatant (0.2 mL) was added to (1.7 mL phosphate buffer + 0.1 mL Ellman's reagent) then the absorbance was recorded within 5 min at 412 nm [28]. The results were expressed as μmol of GSH/g wet tissue.
The antioxidant enzymes catalase (CAT), glutathione reductase (GR), superoxide dismutase (SOD), and glutathione peroxidase (GSP) levels were determined using kits (Biodiagnostics, Cairo, Egypt). A fluorometric assay was used to determine levels of ROS, such as O2−∙, ∙OH, and H2O2. Nonfluorescent DCFDA was oxidized to the highly fluorescent 2',7'-dichlorofluorescin (DCF) in the presence of esterases and ROS, including lipid peroxides [29]. For the assay, 50 μM DCFDA was added to liver homogenates for 250 μL of final volume. Changes in fluorescence intensity were measured on a fluorescence plate reader, GENios (Tecan Instrument, Salzburg, Austria), with excitation and emission wavelengths set at 485 and 530 nm, respectively.
Total NO content was estimated spectrophotometrically in the liver homogenate by measuring absorbance of the formed chromophoric azo derivative [30]. For protein precipitation, the homogenate was incubated for 48 h with absolute ethanol. Then the supernatant was incubated with vanadium trichloride. This was followed by the addition of Griess reagent. The mixture was incubated for 30 minutes at 37°C and absorbance was measured at 540 nm. Results were expressed as μmol/g wet tissue.
Immunohistochemical assays
Liver sections (4 μm) were cut, fixed (65°C oven for 1 hr) and placed in 60 mL of triology working solution (Cell Marque, CA-USA. Cat# 920p-06). Sections were autoclaved and immersed in tris buffered saline. Three drops of rabbit polyclonal anti-rat cyclooxygenase-2 antibody (Thermoscientific, COX-2 Cat#RB-9072-R7) and/or rabbit polyclonal antibody to rat inducible nitric oxide synthase (Thermoscientific, iNOS Cat#RB-9242-R7) were applied then the slides were incubated. Biotinylated secondary antibody was added and incubated with the enzyme conjugate. Diaminobenzidine chromogen was added and rinsed. Light microscope examination after counterstaining was performed.
Protein level of inflammatory Cytokines (using ELISA)
Liver tissues from each group were homogenized in potassium phosphate buffer. The homogenates were centrifuged at 4000 xg for 10 min at 4°C. The supernatant was used for determination of the inflammatory cytokines. The levels of tumor necrosis factor-α (TNF-α), IL6, IL8, IL10 and NF-kB in tissue homogenates were determined by enzyme-linked immunosorbent assay (ELISA) using a rat immunoassay kit (RayBiotech, Norcross, USA) according to the recommendations of the manufacturer using a Microtiter plate reader capable of reading at 450 nm (Sunrise, Austria).
Histopathological study
Liver specimens from the two lobes were taken and fixed in 10% formalin then embedded in paraffin. Sections (4 μm thickness) were cut and stained with hematoxylin and eosin then examined under light microscopy.
Statistical analysis
Results are presented as mean ± SD. Statistical analyses were carried out using One-way ANOVA followed by Tukey–Kramer as a post hoc test to conduct multiple comparisons for assessment of response variation among different groups. Differences between means were considered significant at p<0.05. The analyses were performed using GraphPad Instat software, version 3.
Results
In vitro hepatoprotective guided isolation of the major constituents
The results indicated that CCl4 induced toxicity in clone-9 cell line Table 1 and Table 2, and Hepg2 cell line Table 3 and Table 4, caused significant elevation of AST, ALT, GSH, and SOD levels. The toxicity induced by CCl4 in the clone-9 and Hepg2 cells was significantly (p< 0.001) recovered by treatment with P. niruri extracts. AE in all tested doses (0.01–1 mg/mL) significantly prevented CCl4 induced elevation of AST, ALT, GSH, and SOD levels. Its effect on these enzymes levels at 1 mg/ml approached that of the standard silymarin at the same dose (Table 1 and Table 3). It is worth to note that 50, 70 and 80% ethanol extracts of P. niruri had no significant amelioration to CCl4 toxicity.
Table 1. Protective effect of different extracts and fractions of P. niruri on CCl4 induced toxicity in clone-9 cell line.
| Group | Dose mg/ml |
AST U/mL |
ALT U/mL |
GSH mg/dL |
SOD U/mL |
|---|---|---|---|---|---|
| Control | 48.31±12.11 | 30.48±8.69 | 19.55±11.10 | 13.95±2.04 | |
| CCl4 | 40mM | 124.06±6.84 | 73.22±5.12 | 66.09±3.57 | 38.87±7.33 |
| Silymarin | 1 | 50.01±3.25* | 30.96±5.60* | 21.26±8.00* | 14.16±6.32 |
| 0.1 | 61.33±1.22* | 36.87±2.31* | 26.77±2.21* | 20.39±5.00 | |
| 0.01 | 77.02±1.02* | 40.52±0.69* | 33.28±1.65* | 21.44±3.33 | |
| AE | 1 | 81.03±5.62* | 44.25±6.34* | 33.57±7.26* | 22.98±4.06 |
| 0.1 | 82.11±2.36* | 48.25±2.22* | 36.28±1.26* | 24.56±6.12 | |
| 0.01 | 89.43±6.25* | 53.36±6.00* | 36.89±4.53* | 28.39±8.31 | |
| Fr-I | 1 | 78.63±9.12* | 42.54±3.25* | 35.16±5.11* | 23.15±4.12 |
| 0.1 | 79.66±5.32* | 44.36±4.65* | 36.87±2.26* | 25.01±3.72 | |
| 0.01 | 80.40±6.24* | 45.03±5.08* | 38.13±4.03* | 26.54±2.29 | |
| Fr-II | 1 | 77.12±1.63* | 40.98±5.31* | 30.95±4.06* | 19.72±6.32 |
| 0.1 | 77.73±1.26* | 40.99±3.26* | 29.84±6.28* | 20.01±4.23 | |
| 0.01 | 78.01±1.01* | 41.22±8.36* | 30.15±9.14 | 20.98±4.69 | |
| Fr-III | 1 | 99.86±9.11 | 60.52±5.59 | 66.35±4.53 | 46.38±6.66 |
| 0.1 | 105.12±4.69 | 60.96±11.23 | 66.53±9.30 | 48.03±7.96 | |
| 0.01 | 112.25±10.32 | 62.53±8.32 | 70.05±9.33 | 50.36±10.23 | |
| Fr-IV | 1 | 60.68±4.35* | 41.35±8.32* | 43.52±6.87* | 18.67±7.62 |
| 0.1 | 73.54±8.99* | 41.80±12.36* | 43.62±8.89* | 26.54±8.71 | |
| 0.01 | 80.65±4.69* | 44.63±8.79* | 44.87±7.65* | 29.56±4.09 | |
| 50% EtOH extract | 1 | 89.43±11.12 | 53.28±0.97 | 42.36±8.02 | 29.65±9.32 |
| 0.1 | 95.87±2.36 | 64.32±11.30 | 47.99±4.33 | 33.57±2.19 | |
| 0.01 | 111.52±5.62 | 69.25±6.55 | 61.23±7.32 | 35.26±2.22 | |
| 70% EtOH extract | 1 | 90.03±10.36 | 50.27±3.02 | 49.26±15.03 | 28.63±7.64 |
| 0.1 | 115.23±2.36 | 65.23±5.66 | 55.32±10.23 | 30.28±7.36 | |
| 0.01 | 118.23±3.65 | 69.52±4.86 | 61.53±8.01 | 32.21±6.86 | |
| 80% EtOH extract | 1 | 76.25±8.66 | 38.15±6.47 | 37.99±6.52 | 20.36±4.65 |
| 0.1 | 80.95±6.57 | 42.65±9.35 | 43.21±6.23 | 25.36±4.69 | |
| 0.01 | 98.63±7.32 | 50.36±9.82 | 54.21±2.35 | 31.57±6.04 |
Values are mean±S.D. of three independent experiments carried out in triplicates.
*: Significance level at p< 0.001, compared to control and CCl4 groups; AE: aqueous extract.
Table 2. Protective effect of the isolated compounds from P. niruri on CCl4 induced toxicity in clone-9 cell line.
| Group | Dose mg/ml |
AST U/mL |
ALT U/mL |
GSH mg/dL |
SOD U/mL |
|---|---|---|---|---|---|
| Control | 62.35±5.32 | 34.63±0.36 | 36.75±3.58 | 22.97±5.16 | |
| CCl4 | 40mM | 136.11±4.69 | 87.62±8.95 | 70.12±5.66 | 62.38±5.92 |
| Silymarin | 1 | 66.25±3.69* | 36.52±11.56* | 36.98±12.35* | 25.59±4.21 |
| 0.1 | 73.54±0.36* | 40.58±13.51* | 39.33±15.32* | 28.28±6.21 | |
| 0.01 | 81.23±1.98* | 48.62±13.25* | 43.22±11.18* | 33.26±6.23 | |
| C1 | 1 | 64.52±5.6* | 35.68±17.23* | 39.87±16.22* | 23.01±3.36 |
| 0.1 | 76.58±6.58* | 43.56±20.00* | 43.68±18.30* | 26.71±5.06 | |
| 0.01 | 82.59±6.08* | 50.06±16.33* | 49.62±16.35* | 29.63±7.31 | |
|
C2 |
1 | 69.52±2.06* | 40.25±18.62* | 40.32±10.62* | 27.59±5.12 |
| 0.1 | 83.26±7.12* | 46.52±16.52* | 49.61±13.49* | 31.26±4.26 | |
| 0.01 | 91.03±2.03* | 50.68±19.24* | 53.22±16.15* | 33.68±1.15 | |
|
C3 |
1 | 95.24±2.33* | 58.21±22.30* | 49.55±17.98* | 34.52±2.05 |
| 0.1 | 100.25±3.02* | 65.31±22.37* | 54.29±22.21 | 39.62±5.21 | |
| 0.01 | 111.03±5.03* | 70.36±24.03* | 58.09±30.04* | 43.34±6.04 | |
|
C4 |
1 | 106.25±5.03* | 54.32±22.05* | 50.62±19.32* | 40.68±6.07 |
| 0.1 | 112.87±1.03* | 55.06±23.28* | 50.01±25.03* | 41.11±0.36 | |
| 0.01 | 120.71±1.06* | 56.87±16.24* | 57.62±14.21* | 50.28±3.68 | |
|
C5 |
1 | 70.36±6.24* | 41.62±19.22* | 39.99±20.17* | 27.35±5.17 |
| 0.1 | 75.68±5.01* | 43.05±11.56* | 40.98±18.42* | 28.95±1.03 | |
| 0.01 | 81.24±2.04* | 50.32±10.57* | 44.68±17.62* | 30.39±9.24 | |
|
C6 |
1 | 99.04±0.28* | 52.16±9.08* | 49.27±16.27* | 40.16±1.19 |
| 0.1 | 99.02±2.06* | 51.97±11.67* | 47.62±24.26* | 41.03±5.16 | |
| 0.01 | 106.52±5.17* | 55.67±19.07* | 56.27±18.09 | 41.36±2.58 | |
|
C7 |
1 | 69.51±8.31* | 43.28±19.20* | 38.62±22.51* | 26.24±1.16 |
| 0.1 | 81.62±5.07* | 49.51±9.27* | 43.26±15.20* | 30.25±7.16 | |
| 0.01 | 97.57±1.67* | 58.62±16.27* | 48.62±11.09* | 37.82±2.08 |
Values are mean±S.D. of three independent experiments carried out in triplicates.
*: Significance level at p< 0.001, compared to control and CCl4 groups; C1: corilagin; C2: isocorilagin; C3: brevifolin; C4: quercetin; C5: kaempferol rhamnoside; C6: gallic acid; C7: brevifolin carboxylic acid
Table 3. Protective effect of different extracts and fractions of P. niruri on CCl4 induced toxicity in Hepg2 cell line.
| Group | Dose | AST | ALT | GSH | SOD |
|---|---|---|---|---|---|
| (mg/mL) | (U/mL) | (U/mL) | (mg/dL) | (U/mL) | |
| Control | 51.32±6.28 | 28.80±3.85 | 21.45±15.45 | 18.55±3.00 | |
| CCl4 | 40 mM | 116.55±9.61 | 63.09±7.61 | 48.12±3.30 | 43.30±5.50 |
| Silymarin | 1 | 55.11±2.03* | 28.58±3.54* | 22.44±8.93* | 21.15±1.93* |
| 0.1 | 59.46±0.67* | 30.07±4.58* | 24.01±2.25* | 23.24±1.74* | |
| 0.01 | 67.49±1.77* | 34.63±4.47* | 27.39±2.57* | 26.10±2.19* | |
| AE | 1 | 76.93±7.57* | 42.25±4.05* | 31.92±2.03* | 28.27±3.98* |
| 0.1 | 77.32±3.70* | 40.51±5.06* | 31.58±6.12* | 29.44±2.92* | |
| 0.01 | 79.95±3.80* | 41.88±5.85* | 32.65±7.02* | 30.45±3.01* | |
| Fr-I | 1 | 73.82±3.12* | 38.47±4.99* | 30.10±2.18* | 28.22±2.68* |
| 0.1 | 76.35±1.54* | 38.95±5.90* | 30.92±2.91* | 29.65±2.37* | |
| 0.01 | 78.88±2.48* | 40.68±5.02* | 32.06±7.32* | 30.39±2.66* | |
| Fr-II | 1 | 64.70±96.97* | 35.83±4.82* | 26.92±9.92* | 23.62±3.52 |
| 0.1 | 65.06±3.40* | 34.23±4.60* | 26.61±1.82* | 24.69±2.53 | |
| 0.01 | 67.48±3.50* | 35.49±4.24* | 27.59±2.65 | 25.62±2.62 | |
| Fr-III | 1 | 104.69±8.95 | 56.82±6.70 | 43.26±3.35 | 38.81±5.03 |
| 0.1 | 105.15±4.37 | 54.76±7.26 | 42.86±5.86 | 40.21±3.80 | |
| 0.01 | 108.25±4.49 | 56.37±7.37 | 44.13±3.92 | 41.40±3.91 | |
| Fr-IV | 1 | 57.79±11.54* | 34.66±3.71* | 34.66±2.71* | 19.84±4.61* |
| 0.1 | 64.35±3.84* | 34.09±4.78* | 26.38±2.34* | 24.29±2.63* | |
| 0.01 | 79.17±7.46* | 43.32±5.71* | 32.80±4.89* | 29.17±4.00* | |
| 50% EtOH extract | 1 | 87.56±4.50 | 46.03±5.73 | 35.80±9.41 | 33.25±3.39 |
| 0.1 | 93.43±5.23 | 49.33±6.36 | 38.26±3.15 | 35.36±3.73 | |
| 0.01 | 101.13±3.49 | 52.31±6.04 | 41.13±4.86 | 38.87±3.48 | |
| 70% EtOH extract | 1 | 100.90±3.63 | 52.27±6.77 | 41.06±4.70 | 38.74±3.51 |
| 0.1 | 101.59±2.49 | 52.04±7.07 | 41.19±3.57 | 39.33±3.26 | |
| 0.01 | 105.724±1.72 | 53.72±7.54 | 42.76±7.49 | 41.17±3.20 | |
| 80% EtOH extract | 1 | 81.021±4.32 | 42.67±5.26 | 33.15±2.13 | 30.73±3.18 |
| 0.1 | 86.65±5.02 | 45.84±5.72 | 35.50±8.81 | 32.75±3.51 | |
| 0.01 | 94.04±3.35 | 48.70±6.13 | 38.27±2.37 | 36.12±3.26 |
Values are mean±S.D. of three independent experiments carried out in triplicates.
*: Significance level at p< 0.001, compared to control and CCl4 groups; AE: aqueous extract.
Table 4. Protective effect of the isolated compounds from P. niruri on CCl4 induced toxicity in Hepg2 cell line.
| Group | Dose | AST | ALT | GSH | SOD |
|---|---|---|---|---|---|
| (mg/mL) | (U/mL) | (U/mL) | (mg/dL) | (U/mL) | |
| Control | 51.32±6.28 | 28.80±3.85 | 21.45±15.45 | 18.55±3.00 | |
| CCl4 | 116.55±9.61 | 63.09±7.61 | 48.12±3.30 | 43.30±5.50 | |
| Silymarin | 1 | 55.11±2.03* | 28.58±7.54* | 22.44±8.93* | 21.15±1.93* |
| 0.1 | 59.46±0.67* | 30.07±8.58* | 24.01±11.25* | 23.24±1.74* | |
| 0.01 | 67.49±1.77* | 34.63±6.47* | 27.39±9.57* | 26.10±2.19* | |
| C1 | 1 | 56.08±3.66* | 29.87±13.07* | 23.03±8.44* | 21.09±2.38* |
| 0.1 | 65.72±3.49* | 34.59±14.04* | 26.88±12.04* | 24.94±2.56* | |
| 0.01 | 73.77±3.69* | 38.73±15.56* | 30.15±14.83* | 28.04±2.83* | |
| C2 | 1 | 62.28±3.98* | 33.13±13.22* | 25.56±9.52* | 23.44±2.62* |
| 0.1 | 72.85±2.79* | 37.82±14.54* | 29.67±11.97* | 27.93±2.57* | |
| 0.01 | 78.94±5.37* | 42.16±12.03* | 32.45±12.84* | 29.63±3.41* | |
| C3 | 1 | 73.28±4.11* | 38.69±14.91* | 30.01±14.44* | 27.74±2.93* |
| 0.1 | 78.63±4.77* | 41.70±12.22* | 32.255±12.03* | 29.66±3.24* | |
| 0.01 | 85.66±3.18* | 44.42±18.32* | 34.87±12.41* | 32.86±3.00* | |
| C4 | 1 | 85.45±3.32* | 44.38±18.08* | 34.80±12.26* | 32.74±3.03* |
| 0.1 | 86.08±2.27* | 44.17±15.26* | 34.92±10.05* | 33.28±2.80* | |
| 0.01 | 89.86±1.57* | 45.71±16.42* | 36.36±13.80* | 34.96±2.74* | |
| C5 | 1 | 59.75±3.81* | 31.78±13.55* | 24.53±9.68* | 22.49±2.51* |
| 0.1 | 70.09±2.79* | 36.44±11.59* | 28.56±11.97* | 26.84±2.50* | |
| 0.01 | 76.07±5.21* | 40.64±8.11* | 31.27±12.88* | 28.54±3.29* | |
| C6 | 1 | 80.60±3.22* | 41.91±14.72* | 32.84±12.55* | 30.86±2.88* |
| 0.1 | 81.22±2.20* | 41.71±15.87* | 32.96±12.32* | 31.38±2.65* | |
| 0.01 | 84.88±1.53* | 43.20±18.94* | 34.345±13.02* | 33.01±2.59* | |
| C7 | 1 | 58.48±3.65* | 31.07±13.77* | 23.10±15.31* | 22.04±2.44* |
| 0.1 | 68.48±3.49* | 35.97±14.99* | 27.99±13.04* | 26.03±2.63* | |
| 0.01 | 76.53±3.69* | 40.11±11.51* | 31.26±12.83* | 29.13±2.90* |
Values are mean±S.D. of three independent experiments carried out in triplicates.
*: Significance level at p< 0.001, compared to control and CCl4 groups; C1: corilagin; C2: isocorilagin; C3: brevifolin; C4: quercetin; C5: kaempferol rhamnoside; C6: gallic acid; C7: brevifolin carboxylic acid.
Fractionation of AE on Diaion HP20 yielded four subfractions I-IV, on testing their in vitro hepatorotective activity on clone-9 and Hepg2 cell lines, Fr I, II, and IV (0.01–1 mg/ml) significantly (p< 0.001) reduced CCl4 induced elevation of AST, ALT, and GSH. Fr I and IV also caused significant (p< 0.001) reduction in SOD levels at all tested doses. Fr-IV was the most active fraction in reduction of all toxicity parameters. Treatment of Hepg2 cells with 1 mg/ml Fr-IV reduced AST, ALT, GSH, and SOD levels to 57.79 ± 11.54, 34.66 ± 3.71, 34.66 ± 2.71, and 19.84 ± 4.61 respectively. It reduced SOD more than the reduction caused by silymarin (21.15 ± 1.93) at a dose of 1mg/mL.
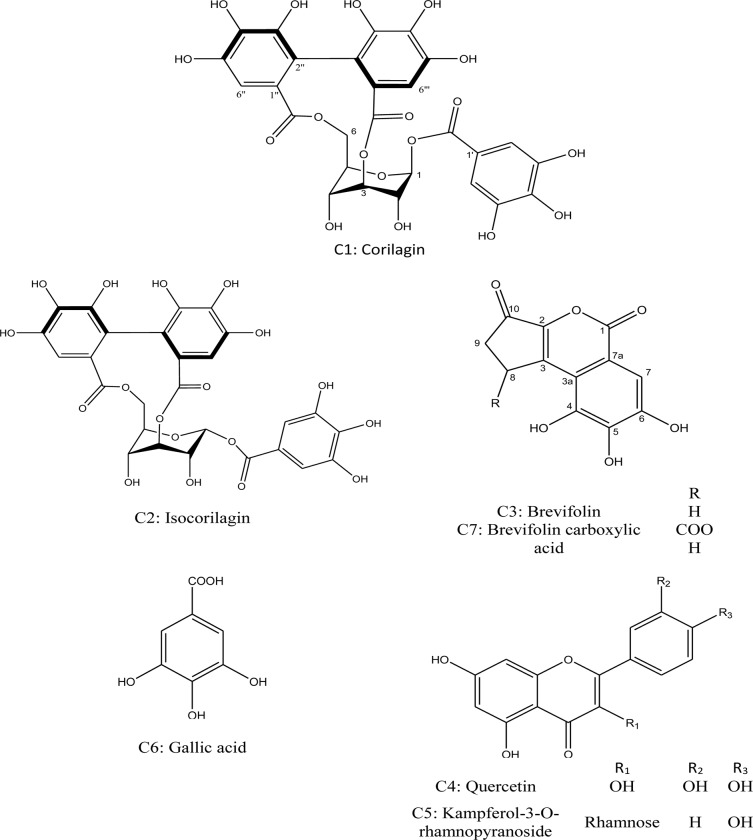
Purification of fractions I, II and IV yielded seven compounds corilagin (β-1-O-galloyl-3, 6-(R)-hexahydroxydiphenoyl-D-glucose) C1, isocorilagin (α-1-O-galloyl-3, 6-(R)-hexahydroxydiphenoyl-D-glucose) C2 (Sprenger et al., 2016), brevifolin C3 [31], quercetin C4, kaempferol rhamnoside C5 [32], gallic acid C6 and brevifolin carboxylic acid C7 [33]. C1-7 structures were established on the basis of physicochemical properties and spectral analysis (1H, 13C-NMR, COSY, HSQC, and HMBC). 1H and 13C-NMR are shown in S1 and S2 Tables and the structures of the compounds are presented in Fig 1.
Fig 1. Chemical structures of the compounds isolated from P. niruri.
Surprisingly, all isolated compounds (C1-C7) at different tested doses 0.01–1 mg/ml significantly (p< 0.001) reduce CCl4 induced elevation of AST, ALT. They also restore the activity of SOD and GSH in clone-9 and Hepg2 cell lines. Corilagin C1, isocorilagin C2, kaempferol rhamnoside C5, and brevifolin carboxylic acid C7 demonstrated strong hepatoprotective activity, whereas, brevifolin C3, quercetin C4 and gallic acid C6 showed moderate activity.
Antioxidant activity
DPPH radical scavenging method
The AE showed a better antioxidant potential when compared to standard ascorbic acid. IC 50 obtained values were 11.6 ± 2 and 12± 3.5 μg/ml. for AE and ascorbic acid respectively. It means that AE, at a higher concentration, captured more free radicals formed by DPPH resulting in increasing IC 50 value.
Ferric Reducing Antioxidant Power Assay (FRAP)
Tested AE and gallic acid showed FRAP values of 79.352 ± 2.88 and 62.85±4.08 mM Ferrous equivalents, respectively. Results showed that the AE has a higher FRAP value than gallic acid; indicating more electron donating capacity of AE in comparison to the standard gallic acid.
In vivo hepatoprotective activity of AE
LD50 study
No mortality was observed after oral administration of AE up to 5000 mg/kg, hence, the extract is considered GHS category 5 or unclassified with LD50 cut off greater than 5000 mg/kg. Also, no food aversion or adverse behavioral changes was observed in treated rats.
Dose-response and histopathological findings
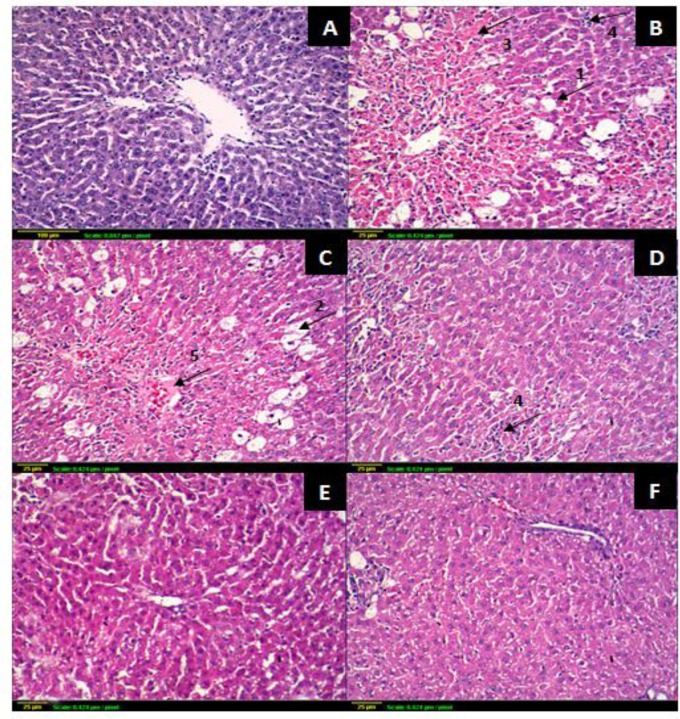
To determine the least effective hepatoprotective dose of AE, AST and ALT levels were determined after CCl4 challenge in rats pretreated with AE. Histopathological findings are presented in Table 5 and Fig 2. A 2.5 and 6 fold increase of AST and ALT levels, respectively, in CCl4 group compared to the control group was observed. The 25 and 50 mg kg-1day-1 doses were unable to produce significant reversal of these enzymes. However, a significant decrease was observed starting from a dose of the 100 mg kg-1day-1.
Table 5. Effect of P. niruri AE different doses on AST and ALT levels after rat acute CCl4 intoxication.
| Group | AST (U/L) | ALT (U/L) |
|---|---|---|
| Control | 134.21±33.49*** | 40.19±10.11*** |
| CCl4 (1 mL/kg) | 327.19±28.04 | 247.65±17.59 |
| CCl4+ AE (25 mg/kg) | 320.63±45.51 | 233.7±20.38 |
| CCl4+ AE (50 mg/kg) | 314.91±32.87 | 228.15±14.93 |
| CCl4+ AE (100 mg/kg) | 252.98±5.71** | 183.43±47.1** |
| CCl4+ AE (200 mg/kg) | 231.93±34.67*** | 176.25±38.1** |
Values are mean± SD.
*: p<0.05
**: p<0.01
***: p<0.001 compared to CCl4 group. AE: aqueous extract.
Fig 2. Effect of P. niruri AE on rats liver histopathology after acute CCl4 intoxication.
(A) control portal area and surrounding hepatocytes; (B) CCl4; (C) CCl4 + 25 mg AE; (D) CCl4+ 50 mg AE; (E) CCl4+ 100 mg AE; (F) CCl4+200 mg AE. (x100), n = 6. (1): severe ballooning degeneration; (2): fatty changes; (3): necrosis; (4): inflammatory cells infiltration; (5): marked congestion.
The histological observations shown in Fig 2 supported these results where a dose-dependent restoration of cellular integrity was confirmed. The CCl4 group showed a marked destruction of the hepatic architecture, accompanied with severe fatty changes, severe ballooning degeneration, necrosis, and inflammatory cells infiltration. A dose of 25 mg kg-1day-1 of AE with CCl4 causes a similar damage with a marked congestion, while a dose of 50 mg kg-1day-1 of AE demonstrates a lesser damage with a marked inflammation. Administration of AE (100 & 200 mg kg-1day-1) showed a marked improvement with restoration of cellular architecture. Thus, the least dose of AE that showed hepatic architecture protection is 100 mg kg-1day-1, therefore it was used for all the other investigations.
Hepatotoxicity and oxidative stress markers
Effect of AE (100 mg kg-1day-1, p.o.) on hepatic toxicity indices, oxidative stress markers, and antioxidant enzymes in rats subjected to acute CCl4 intoxication are summarized in Table 6.
Table 6. Effect of P. niruri AE (100 mg kg-1day-1) on hepatic toxicity indices, oxidative stress markers, inflammatory markers and antioxidant enzymes after rats acute CCl4 intoxication.
| Control | CCl4 | CCl4+ AE | ||
|---|---|---|---|---|
| H.T.I. | LDH (U/L) | 173.58±64.56*** | 408.16±40.99 | 258.44±58.38*** |
| ALP (U/L) | 153.8±31.27** | 239.4±47.4 | 176.8±29.44* | |
| TB (mg/dl) | 0.24±0.0077* | 0.26±0.0061 | 0.25±0.01* | |
| TP (g/dl) | 7.02±1.36* | 4.88±0.73 | 6.11±1.23* | |
| TC (mg/dl) | 47.42±8.56* | 77.55±28.54 | 45.35±10.75* | |
| TG (mg/dl) | 40.66±15.56** | 87±34.75 | 33.7±7.44** | |
| Glucose (mg/dl) | 190.64±40.74 | 153.75±12.12 | 190.26±42.45* | |
| Urea (mg/dl) | 29.08±10.12* | 39.57±2.86 | 29.51±5.5* | |
| Creatinine (mg/dl) | 2.45±0.27*** | 4.09±0.21 | 3.75±0.15* | |
| O.S.M. | MDA (nmol/g) | 35.17±3.31*** | 60.11±8.09 | 38.03±2.88*** |
| GSH (μmol/g) | 3.53±0.92** | 1.997±0.49 | 3.54±0.83** | |
| ROS (Flu/min/mg protein) | 5.2±0.25** | 7.8±0.31 | 5.7±0.33** | |
| I.M. | NF-kB (ng/mg protein) | 15.5 ± 0.15 | 32.5 ± 0.23*** | 24.3 ± 0.18** |
| IL-6 (ng/mg protein) | 45.3 ± 3.19* | 11.8 ± 4.21 | 49 ± 4.65* | |
| IL-8 (ng/mg protein) | 55 ± 11.42 | 98 ± 15.32 *** | 65 ± 8.25 | |
| IL-10 (ng/mg protein) | 35 ± 8.52 | 66 ± 6.5*** | 51 ± 9.32** | |
| TNF (ng/mg protein) | 21 ±8.1* | 32 ± 3.6 | 23 ± 2.9* | |
| A.O.E. | SOD (U/mg wet tissue) | 68.75±10.46*** | 217.42±81.34 | 106.25±34.69** |
| CAT (U/mg wet tissue) | 160.97±18.43* | 180.71±8.46 | 163.43±9.06* | |
| GSP (U/mg wet tissue) | 17.66±3.05 | 14.73±3 | 20.29±6.68* | |
| GR (U/mg wet tissue) | 15.71±1.27* | 12.4±2.39 | 15.68±1.3* |
Values are mean ± SD. n = 6.
*: p<0.05
**: p<0.01
***: p<0.001 compared to CCl4 group. AE: aqueous extract; A.O.E: antioxidant enzymes; H.T.I: hepatic toxicity indices; I.M.: inflammatory markers; O.S.M: oxidative stress markers.
After the CCl4 challenge, significant changes were observed in the hepatic toxicity indices, there was an obvious LDH leakage evident through the 2.5 fold increase in its serum levels compared to the control group. Serum levels of ALP, TB, TC, TG, urea and creatinine significantly increased, too. Pretreatment of animals with AE significantly (p<0.05–0.01) reduced levels of theses hepatotoxicity markers compared to CCl4 group. Also a marked depletion of serum TP, accompanied by hypoglycemia was observed in CCl4 group. Although no statistically significant changes in glucose levels in the pretreated rats were recorded, the differences are easily observed. AE increased TP levels, however this increase did not reach that of the control. Glucose levels were normalized by administration of AE.
CCl4 increases MDA levels two folds of the control group and caused GSH depletion accompanied with 1.5 fold increase of ROS. These changes in the oxidative stress markers were successively restored by AE pretreatment. Pretreatment with AE decreased the MDA level to reach 38.03 ± 2.88 nmol/g, instead of being 60.11 ± 8.09 nmol/g in CCl4 group, this value is similar to some extent to that of the control group (35.17 ± 3.31 nmol/g). The extract was able to restore the GSH depletion caused by CCl4 to 3.54 ± 0.83 μmol/g which is equal to its level in the control group. Moreover, the ROS were diminished in AE treated group to show no significant difference when compared with the control group.
As regards the antioxidant enzymes activities, SOD and CAT levels, increased by the CCl4 administration and approached normal levels by AE pretreatment. On the other hand, GSP and GR activities were reduced by CCl4 challenge and boosted significantly by AE pre-administration. GSP and GR activities reach 20.29 ± 6.68 U/mg wet tissue and 15.68 ± 1.3 U/mg wet tissue respectively in the group pretreated with the extract. These values are higher than or even equal to their activities in the control group.
Inflammatory markers
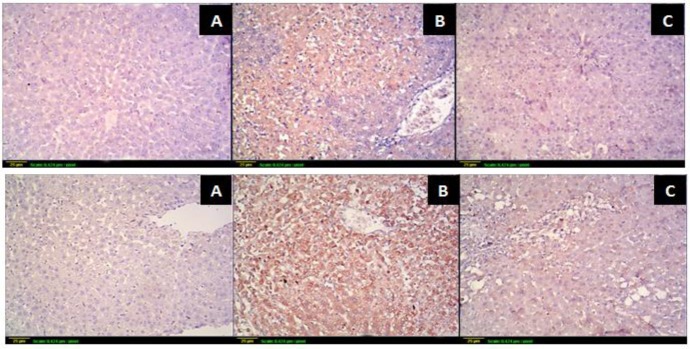
Studying AE effect on inflammatory markers revealed potent activity. The immunohistochemical study revealed increase in hepatic COX-2 and iNOS expression (Figs 3 and 4) after CCl4 administration. CCl4 caused extensive expression appeared as intense brown color. Combination of CCl4 and AE (100 mg kg-1day-1, p.o.) caused the extent of expression to decrease markedly denoted by less brown color. In other words, AE significantly attenuated the increase of COX-2 and iNOS caused by CCl4. In addition, CCl4 challenge was accompanied by excessive production of the pro-inflammatory mediators NO and TNF-α. The NO elevation caused by CCl4 was attenuated by AE pretreatment and its level approached that of the control. Nevertheless, the higher amounts of TNF-α, NF-KB, IL-6, IL-8 and IL10, revealed by ELISA technique in the CCl4 group, were decreased by AE administration.
Fig 3. Immunohistochemical effect of P. niruri AE (100 mg kg-1day-1) on COX-2 and iNOS expression.
Expression of COX-2 (Higher panel) and iNOS (lower pannel) by immunohistochemical staining (x100); (A): control; (B) CCl4; (C) CCl4+ 100 mg kg-1 day-1 P. niruri AE.
Fig 4. Effect of P. niruri AE (100 mg kg-1day-1) on COX-2 and iNOS.
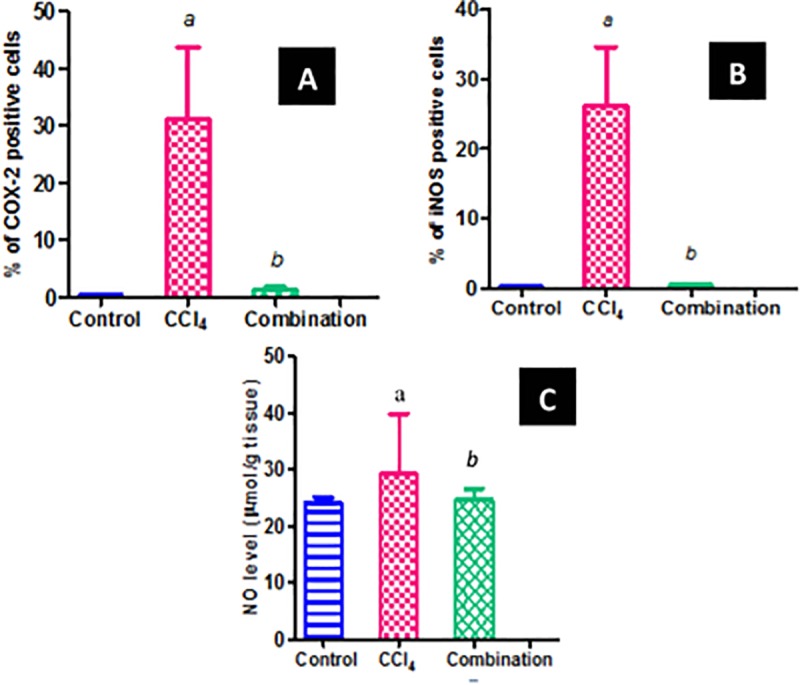
(A) % of COX-2; (B) % of iNOS positive cells as measured by image analysis; (C) NO levels; a: significantly different from control group; b: significantly different from CCl4 group.
Discussion
The plant-derived natural products have long been sources of drugs and medicinal agents. Their large diversity makes them very important in drug discovery, leading to the identification of novel molecules that have various physiological and pathological effects.
Hepatic diseases are a group of diseases which could be effectively prevented or treated by natural products. They constitute a serious global concern, having fatal complications which can end in death. Accordingly, finding a safe, effective, protective and several-mechanistic drug can provide an optimum solution against these complications.
In this study, the hepatoprotective effect of P. niruri was investigated using CCl4 induced hepatotoxicity in the clone-9 and Hepg2 cell lines.
In vitro cytotoxicity and hepatoprotective potential of plants using Hepg2 are important for primary screening, as it is an ideal model for studying in vitro xenobiotic metabolism and liver toxicity [34]. CCl4 caused the production of reactive oxygen species (ROS) in a time-dependent manner which is usually accompanied by lipid peroxidation which reaches its maximum after 24h of incubation. Thus, clone-9 and Hepg2 cells were incubated for 24 h with CCl4 for studying the hepatoprotective effect of AE against CCl4-induced toxicity [34].
The released leakage enzymes-AST and ALT indicate the extent of cellular damage, this release was observed at 24 h exposure to CCl4. The hepatotoxin also induces membrane damage and oxidative injury which leads to a significant decrease in SOD activity and GSH content in cells. Treatment of the cells with different concentrations of AE caused a significant restoration of the altered parameters towards the normal. Possibly the mechanism underlying AE potent hepatoprotective effect is its ability to maintain glutathione in the reduced state by virtue of its antioxidative powers and inhibit lipid peroxidation.
Fractionation of AE on Diaion HP20 yielded four subfractions I-IV, on testing their in vitro hepatoprotective activity on clone-9 and Hepg2 cell lines, only I, II and IV showed significant activity through reducing the levels of ALT, AST, the activity of SOD and GSH content.
Purification of fraction I yielded corilagin (β-1-O-galloyl-3,6-(R)-hexahydroxydiphenoyl-D-glucose) C1 and isocorilagin (α-1-O-galloyl-3,6-(R)-hexahydroxydiphenoyl-D-glucose) C2, fraction II yielded brevifolin C3, quercetin C4, kaempferol rhamnoside C5, gallic acid C6, and fraction IV yielded brevifolin carboxylic acid C7.
1H- NMR spectral data (DMSO-d6) of compound C1 showed a β glucose moiety assigned as 6.22 (d, J = 7.5 Hz, H-1), 3.89 (d, J = 7.5 Hz, H-2), 4.61 (br.s, H-3), 4.28 (br.s, H-4), 4.37 (t, J = 8.1 Hz, H-5), 4.24 (dd, J = 10.8, 7.7 Hz, H-6a), 3.97 (dd, J = 10.8, 10.2 Hz, H-6b), singlet at 7.03 ppm indicating the galloyl protons and two singlets appeared as 6.51 and 6.58 ppm assigned to the hexahydroxydiphenoyl protons.13C-NMR spectral data also showed the characteristic signals of the β glucose, galloyl moiety and the two hexahydroxydiphenoyl moieties. The HMBC correlations indicated that C1 could be identified as corilagin. Compound C2 was identical to C1 in every respect except the doublet of the anomeric H at J = 2.2 Hz, indicating the α-configuration, thus compound C2 was identified as isocorilagin [35].
The 1H-NMR spectra (DMSO-d6) of C7 showed two aliphatic protons at δH 2.98 (2H, dd) and 4.42 (1H, dd) assigned to the methylene Hs at CH2-9 and the methine H at CH-8, respectively, in addition to a singlet from the aromatic proton at δ 7.29 ppm assigned to H-7.
The 13C NMR spectrum of C7 exhibited 13 individual C signals, among which the most two upfield resonances at δ 37.8 and 42.8 ppm were attributed to the aliphatic methylenic C-9 and the methinic C-8, while the three most downfield signals at δ 161.5, 195.4 and 173.9 ppm were assigned to the carbonyl C-1, C-10, and COOH, respectively. This compound was identified as brevifolin carboxylic acid. The 1H-NMR and 13C-NMR spectra (DMSO-d6) of C3 is similar to C7 except for the absence of the signal at δC 173.9 (COOH) with the subsequent upfield shift of C-9 and C-8 which appeared at δC 33.5 and 24.3 ppm, respectively. Thus, compound C3 was identified as brevifolin.
Few reports were traced on the hepatoprotective and antioxidant activities of some of the proposed isolated compounds. Quercetin C4 produced a significant hepatoprotection against country made liquor and paracetamol challenge models [36]. Gallic acid C6 significantly reverses the increased levels of liver marker enzymes, TNF-α, lipid peroxidation levels, and the depleted antioxidant status in paracetamol-challenged mice [37]. Corilagin C1 possesses antioxidant, hepatoprotective activities via scavenging action for O2·− and peroxyl radicals and also inhibited ROS production from leukocytes stimulated by phorbol-12-myristate acetate [38]. It significantly reduced galactosamine (GalN) and lipopolysaccharide (LPS) induced hepatotoxicity. It reduced ALT, AST, and decreased radical formation and lipid peroxidation in GalN/LPS treatment model [38]. Nothing was traced on isocorilagin C2, brevifolin C3, kaempferol rhamnoside C5, and brevifolin carboxylic acid C7.
No data was traced on the hepatoprotective activity of the isolated compounds against CCl4-induced hepatotoxicity on the clone-9 and Hepg2 cell lines. Hence, the authors were encouraged to test the isolates on CCl4-induced hepatotoxicity in the clone-9 and Hepg2 cell lines. Compounds C1-C7 at different tested doses reduced CCl4 induced elevation of AST, ALT, and restored the activity of SOD and GSH in the clone-9 and Hepg2 cell lines. Corilagin C1, isocorilagin C2, kaempferol rhamnoside C5, and brevifolin carboxylic acid C7 demonstrated strong hepatoprotective activity. Whereas, brevifolin C3, quercetin C4, gallic acid C6 showed moderate activity.
AE was chosen for the in vivo testing as the plant used in traditional medicine in Malaysia and other countries as a herbal tea and we needed to put a scientific basis for this traditional use. The present in vivo study presents the hepatoprotective actions of AE in an acute CCl4-induced hepatic injury model in rats. Liver injury induced by CCl4 is a common model for hepatoprotective effect screening, where a single exposure can rapidly lead to severe hepatic steatosis and necrosis. The 1-day model was implemented because CCl4 has the peak of its deleterious toxic effects at 24 h post-injection, then values were slowly normalized [39–41]. Thus, a drug like P. niruri, which can reverse CCl4 damage during its peak, would be a perfect candidate for treating liver diseases. In addition, the fact that the LD50 has not been yet reached by a dose of 17g/kg proves that the drug is highly safe, as according to Horn and Rhiouani [42, 43] who reported that plant extracts with LD50 values higher than 2–3 g/kg are considered nontoxic.
CCl4 causes drastic increases in serum levels of AST, ALT, ALP, LDH, TC, TG, total bilirubin, urea and creatinine. These effects can be attributed to the hepatocellular damage it causes during the course of its metabolism [44, 45]. This was evident in the histopathology slides where CCl4 caused a complete destruction of the cellular architecture. Pretreatment of rats with AE reduced these alterations successively. It is a remarkable fact that TC and TG were decreased by AE to levels below the control group, suggesting an antihyperlipidemic effect, a property presented in a previous study [22].
The observed effects of CCl4 on serum parameters as TC, TG, TP and glucose gain supported by previous studies [46]. Cholesterol efflux capacity is strongly associated with liver disease mortality [47]. The observed restoration of normal levels of TC and TG indicates compensated liver function supported by administration of AE. Also, this lipid lowering activity might be explained based on the findings of Khanna et al.[48] who reported that Phyllanthus niruri inhibits hepatic cholesterol biosynthesis, increases fecal bile acids excretion and enhances plasma lecithin.
The obtained data indicated that CCl4 intoxication was associated with hypoglycemia. This is consistent with the reported toxicity of the CCl4 to hepatocytes as it causes an energy deficit and a decline in glycogen content that ultimately leads to hypoglycemia [49]. Therefore, it can be concluded that the known anti-apoptotic properties of the Phyllanthus species [50] are involved in protecting against hepatocyte damage by CCl4. Also, the ability of the plant extract to mitigate CCl4-induced decline in serum TP highlights the ability of Phyllanthus niruri to improve the synthetic function of the liver. This is in line with previous reports [51].
Lipid peroxidation is perceived as one of the principal steps of CCl4-induced liver damage. Therefore, the antioxidant activity is a very important property in hepatoprotection. In this study, there was an increase in MDA with concomitant decrease in GSH, GSP, and GR after CCl4 challenge. However, their levels in the pretreated group were comparable to those of the control group, indicating that AE has a significant free radical scavenging antioxidant activity. MDA has long been used as a biomarker of oxidative stress [52, 53], and the increase of MDA reflects enhanced lipid peroxidation and tissue injury. GSH on the other hand, represents the non-enzymatic part of the host antioxidant defense mechanism, whereas SOD, CAT, GSP, and GR constitute the enzymatic part. GSH can effectively scavenge free radicals, where it is oxidized by GSP into glutathione disulfide which can be reduced back to GSH by GR with the consumption of NADPH [54]. GSH can react also with different electrophiles, xenobiotics and physiological metabolites to form mercapturates, which are catalyzed by other antioxidant enzymes. SOD mainly catalyzes the conversion of the highly reactive superoxide anion to O2 and to the less reactive species H2O2, which can be then destroyed by CAT and GSP [55].
As seen from the present study, these enzymes are functionally and structurally impaired by the overload of free radicals resulting in their dysfunction during hepatotoxicity [56]. However, SOD and CAT activities were increased by CCl4, in contrast to GSP and GR. Several studies have found a decreased antioxidant enzymes expression following CCl4 administration [57, 58], others, including the current study, found increased levels after CCl4 challenge [59–61]. One probable cause of this increase was suggested to be the high free radical challenge that the enzymes encounter, leading to their overexpression [62]. Also, impairment of the bile flow result in the accumulation of toxic bile salts within the hepatocytes, with resultant injury caused by their detergent action. Also, bile salts can cause mitochondrial dysfunction by interfering with electron transport and hence H2O2 and superoxide formation. This causes SOD and CAT overexpression [63]. Another study pointed out that SOD expression increases in response to high nitrate levels [64].
Although liver injury caused firstly from the metabolism of CCl4 to the trichloromethyl radical and the resulted oxidative stress chain, secondary damage occurs from inflammatory processes that are initiated by Kupffer cells activation [65]. Activated Kupffer cells release some pro-inflammatory mediators that activate other cells in the liver (endothelial cells, stellate cells and hepatocytes), inducing chemokines expression which attract and activate circulating inflammatory cells [66]. One of these pro-inflammatory mediators is TNF-α [67]. The obtained results showed a marked increase in its levels in the CCl4 group, whereas P.niruri suppressed its expression. TNF- α stimulates the release of other cytokines from Kuppfer cells, in addition to induction of phagocyte oxidative metabolism and nitric oxide production [68].
NO, in turn, can exacerbate oxidative stress by forming peroxynitrite [69]. NO has pleiotropic effects. It is well documented that NO plays an important role in liver injury [70, 71], where the hepatocytes produce large quantities of NO [72] due to tissue damage and inflammation caused by a variety of xenobiotics as CCl4. Accordingly, data presented by various studies [73, 74] suggests a potential role of NO as an important mediator of CCl4-induced hepatotoxicity. The present results are in accordance with the above mentioned reports. High total NO levels were observed in the CCl4 group, which slowly approached normal levels by AE pretreatment.
The heightened inflammatory response was also evident by increased expression of COX-2 and iNOS. Cyclooxygenases are predominantly involved in inflammatory responses as they are the rate-limiting enzymes in prostaglandin (PG) biosynthesis from arachidonic acid. COX-2 is the inducible isoform that is responsible for PGs increased production in response to proinflammatory stimuli [75]. Thus, COX-2 expression inhibition is thought to be the most important target for assessing anti-inflammatory activities as it is specific to the inflamed tissue [76]. P. niruri resulted in decreased COX-2 expression.
Several reports have evidenced that overproduction of iNOS occurs in rats liver with CCl4-induced acute liver injury [73, 77] acting as a mediator in its pathogenesis [78]. Increased expression levels in the CCl4 group were confirmed by immunohistochemical staining which revealed inhibition of iNOS by AE.
Although Lee et al. [79] have previously reviewed the hepatoprotective potential of the hexane fraction and isolated compounds from P. niruri, they recommended further cheminformatics, toxicological and mechanistic studies in order to aid the progress of the plant to clinical trial studies. In the present work, the bioactive metabolites were isolated from the aqueous extract and further highlighted the anti-inflammatory properties of P. niruir, as evidenced by inhibition of COX-2, TNF-α, NF-KB, IL-6, IL-8 and IL10 as a possible mechanism of hepatoprotection in addition to the known antioxidant activities. The observed hepatoprotective effects of P. niruri AE may be due to the polyphenolic compounds. Further studies are needed in order to explore possible synergistic effects of different compounds present in the plant as well as their molecular mechanism working against hepatic damage.
Conclusion
In conclusion, the obtained experimental data strongly support the view that P. niruri can be used as a promising hepatoprotective agent. Its activity can be attributed to its potent antioxidant and anti-inflammatory actions of its phenolic constituents. Reaching these positive findings seen in this article concerning the promising hepatoprotective activities of the aqueous extract and its constituents, it is recommended that preclinical studies on the herbal tea of the plant should be initiated.
Supporting information
(DOCX)
(DOCX)
(DOCX)
(XLSX)
Acknowledgments
This research was made possible as part of Ministry of Agriculture Malaysia initiative under the New Key Economic Areas Entry Point Project on High Value Herbals awarded to Natural Wellness Biotech (M) Sdn Bhd. The authors express their gratitude and appreciation for the trust and opportunity given. The authors extend their gratitude to Cairo University staff for their collaboration and excellent teamwork.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The authors received no specific funding for this work. Natural Wellness Biotech organization only provided support in the form of research materials but did not have any additional role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.De Abajo FJ, Montero D, Madurga M, Garcia Rodriguez LA. Acute and clinically relevant drug-induced liver injury: a population based case-control study. Br J Clin Pharmacol. 2004;58(1):71–80. Epub 2004/06/23. 10.1111/j.1365-2125.2004.02133.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hoek JB, Pastorino JG. Ethanol, oxidative stress, and cytokine-induced liver cell injury. Alcohol. 2002;27(1):63–8. 10.1016/s0741-8329(02)00215-x [DOI] [PubMed] [Google Scholar]
- 3.Jaeschke H, Gores GJ, Cederbaum AI, Hinson JA, Pessayre D, Lemasters JJ. Mechanisms of hepatotoxicity. Toxicol Sci. 2002;65(2):166–76. Epub 2002/01/29. 10.1093/toxsci/65.2.166 . [DOI] [PubMed] [Google Scholar]
- 4.Orhan DD, Orhan N, Ergun E, Ergun F. Hepatoprotective effect of Vitis vinifera L. leaves on carbon tetrachloride-induced acute liver damage in rats. Journal of Ethnopharmacology. 2007;112(1):145–51. 10.1016/j.jep.2007.02.013 [DOI] [PubMed] [Google Scholar]
- 5.Jain NK, Lodhi S, Jain A, Nahata A, Singhai AK. Effects of Phyllanthus acidus (L.) Skeels fruit on carbon tetrachloride-induced acute oxidative damage in livers of rats and mice. Zhong Xi Yi Jie He Xue Bao. 2011;9(1):49–56. Epub 2011/01/14. 10.3736/jcim20110109 . [DOI] [PubMed] [Google Scholar]
- 6.McGregor D, Lang M. Carbon tetrachloride: Genetic effects and other modes of action. Mutation Research/Reviews in Genetic Toxicology. 1996;366(3):181–95. [DOI] [PubMed] [Google Scholar]
- 7.Edwards MJ, Keller BJ, Kauffman FC, Thurman RG. The involvement of Kupffer cells in carbon tetrachloride toxicity. Toxicol Appl Pharmacol. 1993;119(2):275–9. Epub 1993/04/01. 10.1006/taap.1993.1069 . [DOI] [PubMed] [Google Scholar]
- 8.Recknagel RO, Glende EA Jr, Dolak JA, Waller RL. Mechanisms of carbon tetrachloride toxicity. Pharmacology & Therapeutics. 1989;43(1):139–54. [DOI] [PubMed] [Google Scholar]
- 9.Calixto JB, Santos AR, Cechinel Filho V, Yunes RA. A review of the plants of the genus Phyllanthus: their chemistry, pharmacology, and therapeutic potential. Med Res Rev. 1998;18(4):225–58. Epub 1998/07/17. . [DOI] [PubMed] [Google Scholar]
- 10.Pramyothin P, Ngamtin C, Poungshompoo S, Chaichantipyuth C. Hepatoprotective activity of Phyllanthus amarus Schum. et. Thonn. extract in ethanol treated rats: in vitro and in vivo studies. J Ethnopharmacol. 2007;114(2):169–73. Epub 2007/09/18. 10.1016/j.jep.2007.07.037 . [DOI] [PubMed] [Google Scholar]
- 11.Khatoon S, Rai V, Rawat AK, Mehrotra S. Comparative pharmacognostic studies of three Phyllanthus species. J Ethnopharmacol. 2006;104(1–2):79–86. Epub 2005/10/21. 10.1016/j.jep.2005.08.048 . [DOI] [PubMed] [Google Scholar]
- 12.Asha VV, Akhila S, Wills PJ, Subramoniam A. Further studies on the antihepatotoxic activity of Phyllanthus maderaspatensis Linn. J Ethnopharmacol. 2004;92(1):67–70. Epub 2004/04/22. 10.1016/j.jep.2004.02.005 . [DOI] [PubMed] [Google Scholar]
- 13.Bagalkotkar G, Sagineedu SR, Saad MS, Stanslas J. Phytochemicals from Phyllanthus niruri Linn. and their pharmacological properties: a review. The Journal of pharmacy and pharmacology. 2006;58(12):1559–70. Epub 2007/03/03. 10.1211/jpp.58.12.0001 . [DOI] [PubMed] [Google Scholar]
- 14.Bilal C. Healing in Urology: Clinical Guidebook to Herbal and Alternative Therapies: World Scientific; 2016. [Google Scholar]
- 15.Hiraganahalli BD, Chinampudur VC, Dethe S, Mundkinajeddu D, Pandre MK, Balachandran J, et al. Hepatoprotective and antioxidant activity of standardized herbal extracts. Pharmacogn Mag. 2012;8(30):116–23. Epub 2012/06/16. 10.4103/0973-1296.96553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tatiya AU, Surana SJ, Sutar MP, Gamit NH. Hepatoprotective effect of poly herbal formulation against various hepatotoxic agents in rats. Pharmacognosy Res. 2012;4(1):50–6. Epub 2012/01/10. 10.4103/0974-8490.91040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Syamasundar KV, Singh B, Thakur RS, Husain A, Kiso Y, Hikino H. Antihepatotoxic principles of Phyllanthus niruri herbs. J Ethnopharmacol. 1985;14(1):41–4. Epub 1985/09/01. 10.1016/0378-8741(85)90026-1 . [DOI] [PubMed] [Google Scholar]
- 18.Sabir SM, Rocha JBT. Water-extractable phytochemicals from Phyllanthus niruri exhibit distinct in vitro antioxidant and in vivo hepatoprotective activity against paracetamol-induced liver damage in mice. Food Chemistry. 2008;111(4):845–51. 10.1016/j.foodchem.2008.04.060 [DOI] [Google Scholar]
- 19.Bhattacharjee R, Sil PC. Protein isolate from the herb, Phyllanthus niruri L. (Euphorbiaceae), plays hepatoprotective role against carbon tetrachloride induced liver damage via its antioxidant properties. Food Chem Toxicol. 2007;45(5):817–26. Epub 2006/12/19. 10.1016/j.fct.2006.10.029 . [DOI] [PubMed] [Google Scholar]
- 20.Harish R, Shivanandappa T. Antioxidant activity and hepatoprotective potential of Phyllanthus niruri. Food Chemistry. 2006;95(2):180–5. 10.1016/j.foodchem.2004.11.049 [DOI] [Google Scholar]
- 21.Murugaiyah V, Chan K-L. Mechanisms of antihyperuricemic effect of Phyllanthus niruri and its lignan constituents. Journal of Ethnopharmacology. 2009;124(2):233–9. 10.1016/j.jep.2009.04.026 [DOI] [PubMed] [Google Scholar]
- 22.Khanna AK, Rizvi F, Chander R. Lipid lowering activity of Phyllanthus niruri in hyperlipemic rats. Journal of Ethnopharmacology. 2002;82(1):19–22. 10.1016/s0378-8741(02)00136-8 [DOI] [PubMed] [Google Scholar]
- 23.Manjrekar AP, Jisha V, Bag PP, Adhikary B, Pai MM, Hegde A, et al. Effect of Phyllanthus niruri Linn. treatment on liver, kidney and testes in CCl4 induced hepatotoxic rats. Indian J Exp Biol. 2008;46(7):514–20. Epub 2008/09/24. . [PubMed] [Google Scholar]
- 24.Pareek A, Godavarthi A, Issarani R, Nagori BP. Antioxidant and hepatoprotective activity of Fagonia schweinfurthii (Hadidi) Hadidi extract in carbon tetrachloride induced hepatotoxicity in HepG2 cell line and rats. Journal of ethnopharmacology. 2013;150(3):973–81. 10.1016/j.jep.2013.09.048 [DOI] [PubMed] [Google Scholar]
- 25.Shimada K, Fujikawa K, Yahara K, Nakamura T. Antioxidative properties of xanthan on the autoxidation of soybean oil in cyclodextrin emulsion. Journal of agricultural and food chemistry. 1992;40(6):945–8. [Google Scholar]
- 26.Benzie IF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Analytical biochemistry. 1996;239(1):70–6. 10.1006/abio.1996.0292 [DOI] [PubMed] [Google Scholar]
- 27.Mihara M, Uchiyama M. Determination of malonaldehyde precursor in tissues by thiobarbituric acid test. Anal Biochem. 1978;86(1):271–8. Epub 1978/05/01. 0003-2697(78)90342-1 [pii]. 10.1016/0003-2697(78)90342-1 . [DOI] [PubMed] [Google Scholar]
- 28.Ellman GL. Tissue sulfhydryl groups. Archives of Biochemistry and Biophysics. 1959;82(1):70–7. 10.1016/0003-9861(59)90090-6 [DOI] [PubMed] [Google Scholar]
- 29.LeBel CP, Ischiropoulos H, Bondy SC. Evaluation of the probe 2', 7'-dichlorofluorescin as an indicator of reactive oxygen species formation and oxidative stress. Chemical research in toxicology. 1992;5(2):227–31. 10.1021/tx00026a012 [DOI] [PubMed] [Google Scholar]
- 30.Miranda KM, Espey MG, Wink DA. A Rapid, Simple Spectrophotometric Method for Simultaneous Detection of Nitrate and Nitrite. Nitric Oxide. 2001;5(1):62–71. 10.1006/niox.2000.0319 [DOI] [PubMed] [Google Scholar]
- 31.Nawwar MA, Hussein SA, Merfort I. NMR spectral analysis of polyphenols from Punica granatum. Phytochemistry. 1994;36(3):793–8. [Google Scholar]
- 32.Habib-ur-Rehman, Yasin KA, Choudhary MA, Khaliq N, Atta-ur-Rahman, Choudhary MI, et al. Studies on the chemical constituents of Phyllanthus emblica. Natural Product Research. 2007;21(9):775–81. 10.1080/14786410601124664 [DOI] [PubMed] [Google Scholar]
- 33.Zhang L-Z, Guo Y, Tu G, Guo W, Miao F. Studies on chemical constituents of Phyllanthus urinaria L. Zhongguo Zhong yao za zhi = Zhongguo zhongyao zazhi = China journal of Chinese materia medica. 2000;25(10):615–7. [PubMed] [Google Scholar]
- 34.Krithika R, Mohankumar R, Verma RJ, Shrivastav PS, Mohamad IL, Gunasekaran P, et al. Isolation, characterization and antioxidative effect of phyllanthin against CCl4-induced toxicity in HepG2 cell line. Chemico-biological interactions. 2009;181(3):351–8. Epub 2009/07/07. 10.1016/j.cbi.2009.06.014 . [DOI] [PubMed] [Google Scholar]
- 35.Sprenger RF, Thomasi SS, Ferreira AG, Cass QB, Batista Junior JM. Solution-state conformations of natural products from chiroptical spectroscopy: the case of isocorilagin. Organic & biomolecular chemistry. 2016;14(13):3369–75. Epub 2016/03/08. 10.1039/c6ob00049e . [DOI] [PubMed] [Google Scholar]
- 36.Gulati RK, Agarwal S, Agrawal SS. Hepatoprotective studies on Phyllanthus emblica Linn. and quercetin. Indian journal of experimental biology. 1995;33(4):261–8. Epub 1995/04/01. . [PubMed] [Google Scholar]
- 37.Rasool MK, Sabina EP, Ramya SR, Preety P, Patel S, Mandal N, et al. Hepatoprotective and antioxidant effects of gallic acid in paracetamol-induced liver damage in mice. The Journal of pharmacy and pharmacology. 2010;62(5):638–43. Epub 2010/07/09. 10.1211/jpp.62.05.0012 . [DOI] [PubMed] [Google Scholar]
- 38.Kinoshita S, Inoue Y, Nakama S, Ichiba T, Aniya Y. Antioxidant and hepatoprotective actions of medicinal herb, Terminalia catappa L. from Okinawa Island and its tannin corilagin. Phytomedicine: international journal of phytotherapy and phytopharmacology. 2007;14(11):755–62. Epub 2007/02/13. 10.1016/j.phymed.2006.12.012 . [DOI] [PubMed] [Google Scholar]
- 39.Janakat S, Al-Merie H. Optimization of the dose and route of injection, and characterisation of the time course of carbon tetrachloride-induced hepatotoxicity in the rat. Journal of Pharmacological and Toxicological Methods. 2002;48(1):41–4. 10.1016/S1056-8719(03)00019-4 [DOI] [PubMed] [Google Scholar]
- 40.Smejkalova J, Simek J, Rouchal J, Dvorackova I. The time course of biochemical and histological changes following carbon tetrachloride-induced liver damage in rats of both sexes. Physiol Bohemoslov. 1985;34(6):494–501. Epub 1985/01/01. . [PubMed] [Google Scholar]
- 41.Rees KR, Sinha KP. Blood enzymes in liver injury. J Pathol Bacteriol. 1960;80:297–307. Epub 1960/10/01. . [PubMed] [Google Scholar]
- 42.Rhiouani H, Settaf A, Lyoussi B, Cherrah Y, Lacaille-Dubois MA, Hassar M. Effects of saponins from Herniaria glabra on blood pressure and renal function in spontaneously hypertensive rats. Therapie. 1999;54(6):735–9. Epub 2000/03/10. . [PubMed] [Google Scholar]
- 43.Horn HJ. Simplified LD50 (or ED50) Calculations. Biometrics. 1956;12(3):311–22. [Google Scholar]
- 44.Amacher DE. Serum Transaminase Elevations as Indicators of Hepatic Injury Following the Administration of Drugs. Regulatory Toxicology and Pharmacology. 1998;27(2):119–30. 10.1006/rtph.1998.1201 [DOI] [PubMed] [Google Scholar]
- 45.Sturgill MG, Lambert GH. Xenobiotic-induced hepatotoxicity: mechanisms of liver injury and methods of monitoring hepatic function. Clin Chem. 1997;43(8 Pt 2):1512–26. Epub 1997/08/01. . [PubMed] [Google Scholar]
- 46.Breikaa RM, Algandaby MM, El-Demerdash E, Abdel-Naim AB. Biochanin A protects against acute carbon tetrachloride-induced hepatotoxicity in rats. Bioscience, biotechnology, and biochemistry. 2013;77(5):909–16. 10.1271/bbb.120675 [DOI] [PubMed] [Google Scholar]
- 47.Säemann MD, Poglitsch M, Kopecky C, Haidinger M, Hörl WH, Weichhart T. The versatility of HDL: a crucial anti‐inflammatory regulator. European journal of clinical investigation. 2010;40(12):1131–43. 10.1111/j.1365-2362.2010.02361.x [DOI] [PubMed] [Google Scholar]
- 48.Khanna A, Rizvi F, Chander R. Lipid lowering activity of Phyllanthus niruri in hyperlipemic rats. Journal of ethnopharmacology. 2002;82(1):19–22. 10.1016/s0378-8741(02)00136-8 [DOI] [PubMed] [Google Scholar]
- 49.Mehendale H, Roth R, Gandolfi AJ, Klaunig J, Lemasters J, Curtis L. Novel mechanisms in chemically induced hepatotoxicity. The FASEB journal. 1994;8(15):1285–95. 10.1096/fasebj.8.15.8001741 [DOI] [PubMed] [Google Scholar]
- 50.Kalekar SA, Munshi RP, Thatte UM. Do plants mediate their anti-diabetic effects through anti-oxidant and anti-apoptotic actions? an in vitro assay of 3 Indian medicinal plants. BMC complementary and alternative medicine. 2013;13(1):257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tewari D, Mocan A, Parvanov ED, Sah AN, Nabavi SM, Huminiecki L, et al. Ethnopharmacological approaches for therapy of jaundice: Part II. Highly used plant species from Acanthaceae, Euphorbiaceae, Asteraceae, Combretaceae, and Fabaceae families. Front Pharmacol. 2017;8:519 10.3389/fphar.2017.00519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lykkesfeldt J. Malondialdehyde as biomarker of oxidative damage to lipids caused by smoking. Clinica Chimica Acta. 2007;380:50–8. [DOI] [PubMed] [Google Scholar]
- 53.Matés JM, Pérez-Gómez C, De Castro IN. Antioxidant enzymes and human diseases. Clinical Biochemistry. 1999;32(8):595–603. 10.1016/s0009-9120(99)00075-2 [DOI] [PubMed] [Google Scholar]
- 54.Fang Y-Z, Yang S, Wu G. Free radicals, antioxidants, and nutrition. Nutrition. 2002;18(10):872–9. 10.1016/s0899-9007(02)00916-4 [DOI] [PubMed] [Google Scholar]
- 55.Fridovich I. Superoxide and superoxide dismutases. Free Radical Biology and Medicine. 1993;15(5):472. [Google Scholar]
- 56.Zhu R, Wang Y, Zhang L, Guo Q. Oxidative stress and liver disease. Hepatol Res. 2012. Epub 2012/04/12. 10.1111/j.1872-034X.2012.00996.x . [DOI] [PubMed] [Google Scholar]
- 57.Ramkumar KM, Rajesh R, Anuradha CV. Food restriction attenuates blood lipid peroxidation in carbon tetrachloride–intoxicated rats. Nutrition. 2003;19(4):358–62. 10.1016/s0899-9007(02)00961-9 [DOI] [PubMed] [Google Scholar]
- 58.Hsu C-T. Ultrastructural changes in liver damage induced by carbon tetrachloride in spontaneously hypertensive rats and Wistar–Kyoto rats. Journal of the Autonomic Nervous System. 1998;70(1–2):79–83. 10.1016/s0165-1838(98)00035-6 [DOI] [PubMed] [Google Scholar]
- 59.Smyth R, Munday MR, York MJ, Clarke CJ, Dare T, Turton JA. Dose response and time course studies on superoxide dismutase as a urinary biomarker of carbon tetrachloride-induced hepatic injury in the Hanover Wistar rat. Int J Exp Pathol. 2009;90(5):500–11. Epub 2009/09/22. IEP666 [pii] 10.1111/j.1365-2613.2009.00666.x . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Popovic M, Janicijevic-Hudomal S, Kaurinovic B, Rasic J, Trivic S. Effects of various drugs on alcohol-induced oxidative stress in the liver. Molecules. 2008;13(9):2249–59. Epub 2008/10/03. 13092249 [pii]. 10.3390/molecules13092249 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Di Simplicio P, Mannervik B. Enzymes involved in glutathione metabolism in rat liver and blood after carbon tetrachloride intoxication. Toxicology Letters. 1983;18(3):285–9. 10.1016/0378-4274(83)90108-x [DOI] [PubMed] [Google Scholar]
- 62.Yoko A, Akira N. Oxidative stress-induced activation of microsomal glutathione S-transferase in isolated rat liver. Biochemical Pharmacology. 1993;45(1):37–42. 10.1016/0006-2952(93)90374-6 [DOI] [PubMed] [Google Scholar]
- 63.Ismail NA, Okasha SH, Dhawan A, Abdel-Rahman AO, Shaker OG, Sadik NA. Antioxidant enzyme activities in hepatic tissue from children with chronic cholestatic liver disease. Saudi J Gastroenterol. 2010;16(2):90–4. Epub 2010/03/27. 10.4103/1319-3767.61234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Beyer W, Imlay J, Fridovich I. Superoxide Dismutases In: Waldo EC, Kivie M, editors. Progress in Nucleic Acid Research and Molecular Biology. Volume 40: Academic Press; 1991. p. 221–53. 10.1016/s0079-6603(08)60843-0 [DOI] [PubMed] [Google Scholar]
- 65.Ishiyama H, Ogino K, Hobara T. Role of Kupffer cells in rat liver injury induced by diethyldithiocarbamate. Eur J Pharmacol. 1995;292(2):135–41. Epub 1995/01/13. 10.1016/0926-6917(95)90005-5 . [DOI] [PubMed] [Google Scholar]
- 66.Colten HR. Tissue-specific regulation of inflammation. J Appl Physiol. 1992;72(1):1–7. Epub 1992/01/01. 10.1152/jappl.1992.72.1.1 . [DOI] [PubMed] [Google Scholar]
- 67.DeCicco LA, Rikans LE, Tutor CG, Hornbrook KR. Serum and liver concentrations of tumor necrosis factor-α and interleukin-1β following administration of carbon tetrachloride to male rats. Toxicology Letters. 1998;98:115–21. 10.1016/s0378-4274(98)00110-6 [DOI] [PubMed] [Google Scholar]
- 68.Lowenstein CJ, Snyder SH. Nitric oxide, a novel biologic messenger. Cell. 1992;70(5):705–7. Epub 1992/09/04. 10.1016/0092-8674(92)90301-r . [DOI] [PubMed] [Google Scholar]
- 69.Morio LA, Chiu H, Sprowles KA, Zhou P, Heck DE, Gordon MK, et al. Distinct roles of tumor necrosis factor-alpha and nitric oxide in acute liver injury induced by carbon tetrachloride in mice. Toxicol Appl Pharmacol. 2001;172(1):44–51. Epub 2001/03/27. 10.1006/taap.2000.9133 . [DOI] [PubMed] [Google Scholar]
- 70.Clemens MG. Nitric oxide in liver injury. Hepatology. 1999;30(1):1–5. Epub 1999/07/01. S0270913999003018 [pii] 10.1002/hep.510300148 . [DOI] [PubMed] [Google Scholar]
- 71.Rockey DC, Chung JJ. Regulation of inducible nitric oxide synthase and nitric oxide during hepatic injury and fibrogenesis. Am J Physiol. 1997;273(1 Pt 1):G124–30. Epub 1997/07/01. 10.1152/ajpgi.1997.273.1.G124 . [DOI] [PubMed] [Google Scholar]
- 72.Vos TA, Van Goor H, Tuyt L, De Jager-Krikken A, Leuvenink R, Kuipers F, et al. Expression of inducible nitric oxide synthase in endotoxemic rat hepatocytes is dependent on the cellular glutathione status. Hepatology. 1999;29(2):421–6. Epub 1999/01/27. S0270913999000634 [pii] 10.1002/hep.510290231 . [DOI] [PubMed] [Google Scholar]
- 73.Al-Shabanah OA, Alam K, Nagi MN, Al-Rikabi AC, Al-Bekairi AM. Protective effect of aminoguanidine, a nitric oxide synthase inhibitor, against carbon tetrachloride induced hepatotoxicity in mice. Life Sci. 2000;66(3):265–70. Epub 2000/02/09. S0024320599005895 [pii]. 10.1016/s0024-3205(99)00589-5 . [DOI] [PubMed] [Google Scholar]
- 74.Chamulitrat W, Jordan SJ, Mason RP. Nitric oxide production during endotoxic shock in carbon tetrachloride-treated rats. Mol Pharmacol. 1994;46(2):391–7. Epub 1994/08/01. . [PubMed] [Google Scholar]
- 75.Smith WL, Garavito RM, DeWitt DL. Prostaglandin endoperoxide H synthases (cyclooxygenases)-1 and -2. J Biol Chem. 1996;271(52):33157–60. Epub 1996/12/27. 10.1074/jbc.271.52.33157 . [DOI] [PubMed] [Google Scholar]
- 76.Hu K-Q. Cyclooxygenase 2 (COX2)-prostanoid pathway and liver diseases. Prostaglandins, Leukotrienes and Essential Fatty Acids. 2003;69(5):329–37. 10.1016/j.plefa.2003.07.001 [DOI] [PubMed] [Google Scholar]
- 77.Zhu W, Fung PC. The roles played by crucial free radicals like lipid free radicals, nitric oxide, and enzymes NOS and NADPH in CCl(4)-induced acute liver injury of mice. Free Radic Biol Med. 2000;29(9):870–80. Epub 2000/11/07. S0891-5849(00)00396-8 [pii]. 10.1016/s0891-5849(00)00396-8 . [DOI] [PubMed] [Google Scholar]
- 78.Gardner CR, Heck DE, Yang CS, Thomas PE, Zhang XJ, DeGeorge GL, et al. Role of nitric oxide in acetaminophen-induced hepatotoxicity in the rat. Hepatology. 1998;27(3):748–54. Epub 1998/03/21. S0270913998001104 [pii] 10.1002/hep.510270316 . [DOI] [PubMed] [Google Scholar]
- 79.Lee NY, Khoo WK, Adnan MA, Mahalingam TP, Fernandez AR, Jeevaratnam K. The pharmacological potential of Phyllanthus niruri. Journal of pharmacy and pharmacology. 2016;68(8):953–69. 10.1111/jphp.12565 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
(DOCX)
(XLSX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.