Abstract
The primeval function of the mammalian hippocampus (HPC) remains uncertain. Implicated in learning and memory, spatial navigation, and neuropsychological disorders, evolutionary theory suggests that the HPC evolved from a primeval chemosensory epithelium. Deficits in sensing of internal body status ('interoception') in patients with HPC lesions argue that internal sensing may be conserved in higher vertebrates. We studied the expression patterns in mouse brain of 250 endocrine receptors that respond to blood-borne ligands. Key findings are (i) the proportions and levels of endocrine receptor expression in the HPC are significantly higher than in all other comparable brain regions. (ii) Surprisingly, the distribution of endocrine receptor expression within mouse HPC was found to be highly structured: receptors signaling 'challenge' are segregated in dentate gyrus (DG), whereas those signaling 'sufficiency' are principally found in cornu ammonis (CA) regions. Selective expression of endocrine receptors in the HPC argues that interoception remains a core feature of hippocampal function. Further, we report that ligands of DG receptors predominantly inhibit both synaptic potentiation and neurogenesis, whereas CA receptor ligands conversely promote both synaptic potentiation and neurogenesis. These findings suggest that the hippocampus acts as an integrator of body status, extending its role in context-dependent memory encoding from 'where' and 'when' to 'how I feel'. Implications for anxiety and depression are discussed.
Introduction
Current thinking predominantly attributes to the hippocampus (HPC) a pivotal role in learning and memory, in spatial navigation, and in anxiety, stress, and depression. However, the central function of the HPC in both memory and neuropsychological disorders may be consistent with an underlying role in internal sensing (interoception). Previous studies have implicated cortical regions, limbic brain, and thalamus, as well as the hypothalamus and brainstem regions, among others, in interoception [1]. The HPC (and adjoining amygdala) is a prominent contender–in addition to his profound learning and memory deficits following HPC surgery to alleviate severe recurrent epilepsy [2], the famous patient H.M. was unable to sense internal states such as hunger [3]. Similar observations have been made in rodents with selective HPC lesions [4–6].
A role for the HPC in internal sensing is consistent with evolutionary theory that the HPC (and olfactory system) arose from a chemosensory epithelium, but with the closing of the brain ventricles during evolution the hippocampus retained the capacity to sense the internal milieu of the body [7–9]. It is of note that the 'rostral migratory stream' in neonatal mice directly connects the HPC and the chemosensing olfactory system [10], consistent with a common developmental origin. In addition, a key characteristic of traditional sensory epithelia such as the olfactory system and retina in many vertebrate species is that neurogenesis continues into adulthood [11,12], and neurogenesis is also prominent in adult hippocampus, principally underlying the dentate gyrus (DG) (reviewed in [13].
Internal sensing is a key modulator of behavior. Hunger and thirst are induced by deficiencies in nutrient and water, respectively, and elicit clear adaptive motivations and behaviors. Other diverse internal states, ranging from salt deficiency to hormonal status to inflammation/infection, exert powerful effects on multiple aspects of brain function, centrally including adaptive behavior as well as learning and memory, but the target brain region(s) and receptors remain poorly defined.
The anatomy of the mammalian HPC is consistent with an internal sensory role. The hippocampal formation lies at the interface (limbus, 'fringe') between the lower brain and the mass of the cerebral cortex. In terms of blood supply, the HPC is perhaps the most highly irrigated of all brain regions, and is also flanked by the central and lateral ventricles with the choroid plexus [14]. In cross-section, the formation is divided into CA regions CA1 and CA3 (with a short intervening structure, CA2), and the DG. There may be a further functionally distinct region, the dentate hilus, but this is less secure. Gene expression surveys largely confirm this anatomy [15,16]. Some have introduced additional subdivisions both within the DG–CA circuit [17] and along the length of the hippocampus [18]. However, for simplicity we retain the conventional subdivisions CA1–CA3 and DG.
To address the physiological role of the HPC we previously employed differential hybridization [19], candidate gene screening [20], and gene-trapping [21] to identify genes selectively expressed in HPC. This revealed that the mouse HPC expresses several endocrine receptors and signaling molecules, potentially indicating a role of the HPC in internal sensing of body physiology [9]. The aim of the present study was therefore to test rigorously the hypothesis that the hippocampus is involved in interoception through systematic analysis of the expression patterns of endocrine receptors across mouse brain, including subregions of the HPC.
Specifically, we sought to answer two central questions. (i) Does the mouse HPC express a greater diversity and/or level of endocrine receptors than other brain regions such as the cortex and the cerebellum? (ii) If a greater level of expression is found, are these receptors expressed uniformly across the HPC, or are different receptors differently distributed in the different subdivisions of the HPC?–and can the pattern of expression tell us anything about the function of the HPC? We report that the HPC is the principal brain site of endocrine receptor expression and, perhaps surprisingly, this analysis revealed a highly segregated distribution of receptor expression in mouse hippocampus.
Methods
Endocrine receptors
A list was assembled of receptor molecules in mice and humans that respond to endocrine (blood-borne) ligands. We elected to study 250 receptors, a number chosen to minimize the risk that a small number of atypical receptors or experimental artifacts might bias the overall picture, weighed against the labor-intensive constraints of manually analyzing a larger number of receptors. To assemble the list, the GeneCards database (www.genecards.org) was searched at random for genes/gene products containing 'receptor'. A preliminary list (>>250 receptor candidates) was manually filtered to exclude (i) non-receptor entries (e.g., receptor downstream kinase, etc.), (ii) evident receptors for neurotransmitter and non-diffusible cell–cell interaction molecules, and (iii) receptors not listed in the primary database consulted (Allan Brain Atlas) as well as receptors whose expression profiles were classified as failing quality control. Although principally cell-surface molecules, the final list includes intracellular receptors with an endocrine role (e.g., nuclear receptors). This generated a list of 253 endocrine receptors (Table A in S1 Appendix; the molecular functions of specific groups of receptors are discussed in Box 1).
Box 1. Observations on specific receptors
Estrogen receptors
Estrogen receptors (ERs) include the classical ERs ESR1 (ERα), ESR2 (ERβ), the estrogen-related (ESRR) receptors, as well as the membrane estrogen receptor GPR30/GPER. Perplexingly, no significant expression of the major receptors (ESR1/ESR2) was detected in mouse hippocampus, and there was also no expression of GPR30 (data: ABA); this was confirmed by inspection of HippoSeq (not presented) and we consider this finding to be reliable, further confirmed by a report that ER protein reactivity assessed by immunohistochemistry is largely absent from the principal neurons of the rat HPC [70]. The absence of ESR1 and ESR2 is challenging given multiple reports of estrogen-responsive biological changes in the HPC (e.g., [71,72]), including our own work [73]. Although our survey revealed prominent expression of ESRRG in HPC, E2 is not known activate this receptor; cholesterol (but not E2) is reported to activate the related receptor, ESRRA [74], and it is likely that there are other endogenous ligands for ESRRG, but E2 seems unlikely.
Despite the absence of ESR1 and ESR2, ligand radiolabeling studies confirmed E2 binding specifically to rat CA regions [75]. The identity of the specific receptor responsible is not known, but this could reflect E2 binding to the androgen receptor, AR, that is well expressed in CA regions. E2 binds with high affinity to AR (Kd = ~0.2 nM) but does not normally drive transcription activation unless in the presence of coactivators such as ARA70, SRC1, or β-catenin [76]). Both SRC1 and β-catenin are well expressed throughout the mouse hippocampus (data: ABA). Furthermore, E2 is rapidly metabolized in vivo to estrone (E1) by HSD17B enzymes (that are well expressed in HPC–data: ABA) and thence to estrone sulfate (E1S) by sulfotransferases: E1S is reported to activate AR in the 10 nM range [77], and also has a 20-fold longer half-life than E2. Overall, some of the biological activities ascribed to E2 in the HPC might be explained by AR targeting by E2 or its metabolites.
Of note, the gating enzyme, CYP7B1, that blocks alternative ER/AR activation by metabolizing androstanediol/androstenediol and DHEA (see [78]), is most highly expressed in DG [79], thereby blocking the action of alternative physiological estrogens and androgens in DG, further restricting their action to CA regions.
Fibroblast growth factors (FGFs), Klotho (KL), and lactase-phlorizin hydrolase (LCT)
FGFs comprise two groups of ligand that have distinct biological functions. The first group consists of membrane-bound ligands that mediate cell–cell interactions. The second group, the endocrine FGFs (principally FGF19, FGF21, and FGF23), lack the membrane-attachment motif, are released into the extracellular milieu, and enter the bloodstream, where they mediate systemic endocrine effects (reviewed in [80,81]). In the present analysis we report selective expression of FGF receptors FGFR1, FGFR3, and KL in CA1 regions of the HPC. We note also that ABA reports selective hippocampal expression (No. 2 in the top 100 HPC genes [22]) of lactase (LCT), also known as lactase-phlorizin hydrolase, with selective expression in CA1. Hippocampal expression might be surprising given the prominent role of LCT in human tolerance to lactose ingestion, but lactose metabolism may be incidental: there are indications that LCT plays a signaling role. LCT is conserved in fish and Xenopus, where lactose is not known (although the enzyme is also active against plant-derived laminaribiose). It is also a membrane-bound polypeptide. LCT harbors 3–4 glycosidase domains, but the first two display no enzymatic activity, and are therefore likely to have a different function [82]. There are two LCT homologs in mammals, lactase/Klotho-like (LCTL) and Klotho (KL). Both appear to have, like LCT, a C-terminal transmembrane region. KL has a single glycosidase domain whereas LCT and LCTL have 3–4. Importantly, both KL and LCTL are coreceptors for endocrine-type FGFs [83]. LCT has not yet been tested but, in view of strong homologies with both LCTL and KL, it is inferred that LCT is also a coreceptor for endocrine FGFs, which would be consistent with joint expression of FGF1R, FGF3R, KL, and LCT in HPC. It is not yet known whether these differ in their selectivity for different types of FGFs.
Somatostatin (SST) receptors
SST was first described as a hypothalamic peptide that governs pituitary hormone secretion, and thus contributes to regulation of the HPA axis [84]. Of the five SST receptors (SSTR1–5), most attention has focused on SSTR2 and SSTR4. Both bind SST and the related molecule corticostatin. Although HPC expression of SSTR2 was not detected in ABA (Table B in S1 Appendix), HippoSeq reports selective expression in DG, in contrast to selective expression of SSTR4 in CA regions–confirming earlier literature that SSTR2 and SSTR4 have non-overlapping patterns of expression, with SSTR2 being expressed in DG and SSTR4 in CA regions [85]. The two receptors play different (and perhaps converse) roles, as revealed by agonist and knockout experiments [85–87]. In the framework reported here SSTR4 may be classified as a 'sufficiency' receptor whereas SSTR2 may be classified as 'challenge' on the basis of its effects on stress responses [88]. Further research will be necessary to unravel whether this might partly reflect receptor binding to different physiological ligands, with the added complexity that SST/corticostatin are themselves expressed in some brain neurons (e.g., [84]), and could thus have dual endocrine/neurotransmitter functions.
Glucocorticoid receptors
Although the mineralocorticoid receptor (MR/NR3C2) is widely held to be a receptor for aldosterone (ALDO), that regulates salt and ion balance, MR in brain principally responds to the stress hormone cortisol (in human)/corticosterone (in rodents) ('CORT'). This is because receptor specificity is governed by enzymatic 'gating' (e.g., [78,89]): the classical sites for ALDO actions (e.g., kidney and colon) express high levels of 11BHSD enzymes that rapidly metabolize CORT into inert metabolites. By contrast, ALDO is resistant to enzymatic gating, and becomes the principal ligand for kidney MR. Therefore, in brain regions such as the HPC, that lack discernable 11BHSD expression, CORT becomes the principal ligand for MR; indeed, the affinity of MR for CORT is higher than for ALDO, and circulating levels of CORT exceed those of ALDO; hippocampal MR is thus the principal brain receptor for adrenal glucocorticoid stress hormones in mouse [90].
The situation in human is slightly different because, unlike mouse, rat, and marmoset [91], where expression of MR is targeted to DG, human MR expression is more broadly across both CA and DG regions [92]. However, unlike mouse, human hippocampus expresses a gating enzyme (HSD11B1L/HSD11B3) in CA regions (but not in DG; data: ABA)–a brain-enriched enzyme that converts CORT to inactive cortisone [93]. Although the kinetic parameters of the enzyme have not yet been studied in detail, the action of MR could also be restricted to DG in human, mirroring the situation in mouse, but by a different mechanism.
Quantification of mouse brain endocrine receptor expression data
Primary analysis relied on the Allen Brain Atlas (ABA; http://mouse.brain-map.org/), a publicly available repository of in situ hybridization gene expression data across mouse brain [22] made available by the Allen Institute for Brain Science established by Paul G. Allen. To retrieve expression patterns we entered search terms (e.g., Gene1) into http://mouse.brain-map.org/search/show, sagittal sections were selected in all cases when these were available. The 'expression' option and the target brain region (typically mid-brain including the hippocampus) were selected, a screenshot was taken; data for all 253 receptors were recorded at the same magnification and intensity in a repository of image files. To quantitate expression levels ImageJ [23,24] was employed. Using default settings, and a standard image size, representative brain regions (HPC; cortex, CX; and cerebellum, CB) were selected using a cursor box of constant size and analyzed using the 'measure' function of ImageJ (the olfactory bulb could not be systematically analyzed because this structure can be lost during dissection, and the small relative size of the mouse hypothalamus precludes analysis at the resolution afforded by ABA). In each case the 'Mean' function was used instead of the integrated density function 'IntDen' because, at constant image size, the relative values are the same. The same technique was used for hippocampal subregions, but the cursor box was manually fitted to the separate regions (CA1, CA2, CA3, DG). The 'Mean' function in these cases represents relative (total) expression of the target gene within the region measured. These analyses generate a digital intensity reading on a scale of 0 to 255. The program accommodates different colors as follows: black, 0.00; red, 85/255 (0.333); yellow, 170/255 (0.666); white, 255/255 (1.000), mirroring the output of the ABA. Because region selection is to some extent subjective, subregion expression analysis was performed by two independent researchers; in cases of disparity consensus was reached following reanalysis of the primary data. Values were then normalized–a biologically realistic data transformation because (i) the signal for each target depends on the hybridization properties of the specific probe employed, (ii) the biological effects of a given receptor will vary across a wide range depending on ligand concentration, ligand affinity, and downstream signal transduction, and (iii) for a given gene, the inter-regional pattern (ratio) of expression across the brain/hippocampus (unlike absolute values) is likely to be independent of the specific probe/hybridization parameters. For normalization, the highest expression value was selected (100%) and expression in other regions was expressed as a percentage of maximum. Inter-region expression ratios in whole brain were calculated from the un-normalized expression data. Primary data for receptor gene expression across the brain are given in Table B in S1 Appendix, and for hippocampal subregions in Table C in S1 Appendix.
Heat mapping and statistical analysis
All analyses focused on genes that were expressed in at least one of the selected brain regions (98 genes in Fig 1, and 86 genes in Fig 2), and were conducted in the R programming environment, version 3.3.3 [25]. Heatmaps were generated using heatmap.2 in the gplots library for R. Note that heatmap.2 provides dendrograms to aid visualization of relationships among components of the heatmap but provides no statistics to indicate support for the presented dendrograms versus alternative, competing dendrograms. Therefore, we strongly caution against overinterpretation of the dendrograms presented.
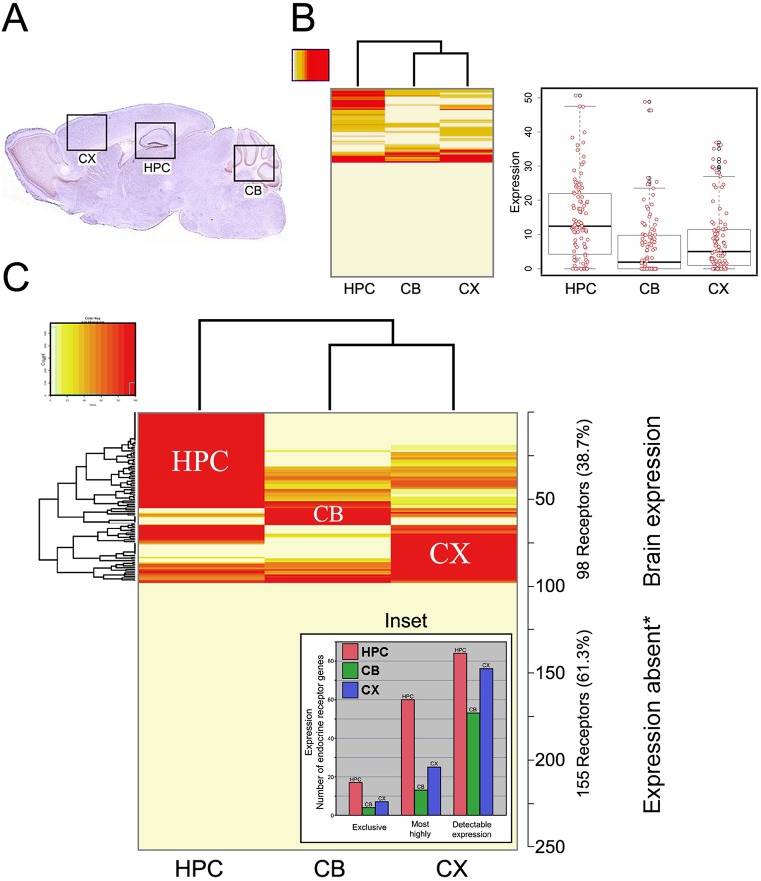
Fig 1. Endocrine receptor gene expression in mouse brain and enrichment in the hippocampus (HPC).
More than one third of all endocrine receptors were detectably expressed in brain, where they are likely to modulate brain function and cognition. Expression was restricted to specific brain regions: other than hippocampus (HPC), cerebellum (CB), and cortex (CX), there was little evidence for specific gene expression in other comparable regions (~4%; see text). (A) Mouse brain section highlighting the three regions studied in detail: HPC, CB, and CX. (B) (Left) Heatmap of 'raw' (unnormalized expression data, see Methods) for HPC versus CB and CX. (Right) Scatterplots of unnormalized expression levels; horizontal lines are medians and quartiles showing that the mean expression level of all receptors in HPC is significantly higher than in either CB or CX. (C) Normalized (maximum expression level = 100%) gene expression data. On three counts, the HPC (red), versus CB (green) and CX (blue), is the major site of expression of endocrine receptors (253 receptors examined) as further evidenced by the inset showing (i) exclusive expression in HPC, (ii) most prominent expression in HPC, (iii) overall number of receptors expressed. *Receptors showing no detectable expression or low-level/punctate/irreproducible expression are classified as expression absent. Note that the dendrograms (generated by heatmap.2), depicted in A and B, are not supported by statistical analysis versus alternative, competing dendrograms. Genes that are expressed exclusively in HPC, CB, or CB were not distributed among these three regions with equal probability, and ‘exclusive genes’ were expressed most often in HPC; the same result emerges when considering genes that are expressed most prominently in one brain region. Thus, the HPC expresses both a greater number and level of endocrine receptor genes than any other brain region analyzed.
Fig 2. Subregional representation of 86 receptors expressed in mouse hippocampus (HPC).

Data are normalized to the maximum expression level. *The data indicate that some receptors are somewhat restricted in their expression pattern to one subregion, whereas others are expressed in combinations of regions. Note: the depicted dendrograms (generated by heatmap.2) are not supported by statistical analysis versus alternative, competing dendrograms. There were significant positive correlations between CA2 and CA3, and significant negative correlations between CA1 and DG (Table D in S1 Appendix).
To test whether gene expression profiles differ across brain regions (HPC, CX, and CB) we measured the correlation in gene expression among brain regions. To this end, we analyzed normalized gene expression (see above) because variation in probe affinity may generate spurious correlations. We calculated the correlation using arcsine square root transformed values of normalized gene expression, and used case-bootstrapping to generate 95% confidence intervals (R package ‘boot’ [26,27]; bootstrapped 10 000 replicates).
Wilcoxon signed rank tests and paired t tests were used to determine whether non-normalized gene expression differed among brain regions (Wilcoxon tests to compare HPC, CX and CB; paired t tests to compare CA1, CA2, CA3, and DG). We used Chi-square goodness of fit tests to determine whether genes that are exclusively (or alternatively, predominantly) expressed in HPC, CB, or CX are distributed equally among these regions. We used a series of three binomial tests to determine whether the numbers of genes expressed differed among HPC, CB and CX. Pairwise correlation analysis is given in Table D in S1 Appendix.
Informative genes
For the majority of receptor genes the biological function of the receptor and/or the identity of the ligand(s) remains unknown. For further analysis we therefore selected an 'informative' subset of 32 genes where information is available concerning the biological role (or inferred role) of the ligand/receptor pair. This subset included receptors for known diffusible hormones (e.g., estrogen, glucocorticoids, progesterone), for cytokines (e.g., interleukins, interferons, tumor necrosis factor), and growth factors (e.g., fibroblast growth factor). The list of informative genes is presented in Table E in S1 Appendix.
Inter-region expression ratios in hippocampus; statistical analysis
Normalized expression data were used to test whether gene expression ratios among hippocampus regions differed between group A versus B genes (for an explanation of groups A and B see Results and Discussion). The mean expression data for CA (CA1–3) and DG were calculated and then log-transformed (1 or 2 was added to all values prior to log-transformation to account for zeros; the outcome was the same in both cases). Pairwise DG/CA expression ratios (Δ) were calculated from Δ = log(DG) − log(CA) {therefore, Δ = log(DG/CA)}. Welch’s t test was employed to assess statistical significance of pairwise differences in ratios (i.e., Δ) for informative (group A, challenge; and group B, sufficiency) genes. The same approach was employed for HippoSeq data (below).
However, because the distribution of Δ may violate the assumptions of t-tests, we additionally used a permutation test to confirm conclusions from the t-test. The permutation test has two stages. First, average Δ was calculated for each group of A and B genes, and the difference between these averages was calculated. This value represents the observed difference in average Δ between groups A and B. Second, (i) Δ values were randomized among groups A and B, (ii) average Δ of these randomized data was calculated for group A and B genes, and (iii) the difference between these average Δ values between groups A and B was calculated. We repeated this second stage 10 000 times to generate a null distribution, against which we compared the observed difference in average Δ between group A and B genes to yield the P value reported here.
We used Dunn–Sidak corrected critical P values to assess significance when making multiple comparisons (Pcrit = 0.0169 and 0.00851 for three and six comparisons, respectively).
Cross-validation of expression data
To validate data from the Allan Brain Atlas we consulted HippoSeq (https://hipposeq.janelia.org) [28], a database of gene expression data. HippoSeq is based on transgenic tagging of subregions of mouse HPC, brain microdissection, fluorescence cell-sorting retrieval of target HPC CA pyramidal cell/dentate neuronal populations, and deep sequencing of mRNA populations. A revised input format (kind courtesy of Cembrowski et al.) allowed query of multiple genes, generating a table of absolute readcounts (FPKM, fragments per kb of transcript per million mapped reads). Cross-comparison to ABA established a lower limit (null expression) where 4 FPKM equated to an undetectable hybridization signal (not presented). Parallels and differences between the ABA and HippoSeq studies are summarized in Table F in S1 Appendix.
Because ABA is more robust than HippoSeq in terms of the number of animals studied (a small number of unrepresentative animals would be less likely to affect conclusions based on ABA rather than on HippoSeq, Table F in S1 Appendix), and because an in situ hybridization pattern (ABA, particularly if confirmed by identical patterns generated in other mouse strains or species) may be more immune to bias than an automatically generated value (HippoSeq), ABA was preferred over HippoSeq for our primary analysis, although both are reported where appropriate.
Analysis of receptor function
PubMed was searched for the name of each individual receptor in conjunction with 'synaptic potentiation' OR 'synaptic plasticity' OR 'long-term potentiation' OR 'LTP' OR 'neurogenesis'. Relevant publications were manually tabulated for ligand effects on both parameters and are listed in Table H in S1 Appendix. Intergroup pairwise comparisons of effects (inhibition versus stimulation) of literature-recorded ligands on LTP and neurogenesis employed both Student's unpaired t test and chi-square test.
Results
A representative list of 253 endocrine receptors was compiled (neurotransmitter receptors and cell–cell interaction molecules were excluded; Table A in S1 Appendix). In situ hybridization patterns were extracted from the Allen Mouse Brain Atlas (ABA); these were manually scanned and quantified (Methods). Where appropriate, values were normalized and inter-region ratios calculated.
Brain distribution of endocrine receptor expression
We report that, of all endocrine receptors, 98/253 (38.7%) were detectably expressed in brain. This argues that, in addition to regulating body physiology including growth, development, reproduction, and homeostasis, etc., a major proportion of endocrine receptors may directly regulate brain function and cognition.
We also report that endocrine receptor expression in mouse brain is principally limited to specific brain regions. Only a small number of genes were expressed in major areas such as the olfactory bulb (OLF), thalamus, pons/medulla, pallidum, or striatum (4.3%; see below). This focused our attention on HPC, cortex (CX), and cerebellum (CB). Hypothalamus could not be examined (Methods and Discussion).
Regarding our first question–the proportion of endocrine receptors expressed in mouse HPC–we report that 86 of 253 (34.0%) endocrine receptors are expressed in HPC, a higher number than in either CB (53) or CX (76). Importantly, the level of expression was highest in HPC. Of all receptors with detectable expression in brain (n = 98), 61.3% were most prominently expressed in the principal neuronal layers (pyramidal and granule cells) of the HPC (versus 9.1% in CB and 25.5% in CX). Indeed, 17.3% of brain-expressed endocrine receptors were exclusively expressed in HPC (compared to 4.1% and 7.1% that were exclusively expressed in CB and CX, respectively). Fig 1 presents heatmaps of the normalized and un-normalized expression data for these three brain regions, and the inset gives numerical values for exclusivity, most prominent, and detectable expression.
Non-normalized gene expression differed significantly in all pairwise comparisons among HPC, CB, and CX. The HPC expressed these genes at significantly higher levels than either CX or CB (Wilcoxon signed rank test; vs CX: V = 3467, P = 3.266e−06; vs CB: V = 3527, P = 1.398e−08), and CX expressed genes at higher levels then CB (V = 2208.5, P = 0.009944). All comparisons remained significant after accounting for multiple comparisons. Overall, the probability of detectable gene expression was significantly higher for HPC than CB (binomial test, P = 0.0101), but did not differ for remaining comparisons (binomial tests; HPC and CX: P = 0.58; CX and CB: P = 0.052); these results remain unchanged after accounting for multiple comparisons.
Although we were unable to systematically screen for expression in OLF (Methods), a small number of genes from our selection were expressed in OLF (Ednrb, Epor, Ccr3, Crhr1, Nrp1, and Nmbr) of which only Ccr3 and Nmbr appeared to be specific for OLF. Remaining genes were expressed in striatum and/or pallidum (Acvrl1, Nfgr, Rarb) or in pons/medulla (Adipor2, Esrrg). No other brain regions stood out with other than trace expression in this survey (small foci of low-level expression, not presented); in total, these represent 4.3% of all the endocrine receptors studied, a far lower proportion than in either HPC, CB, or CX.
We conclude that, based on 253 receptors, there is significantly greater endocrine receptor gene expression in HPC than in either CB or CX, or in any other comparable brain region analyzed (noting that hypothalamus could not be studied; Discussion).
Distribution across hippocampal subregions
With regard to our second question–the pattern of expression within the HPC–all the receptors studied with detectable HPC expression (n = 86; Fig 1) identified mRNA within the cell bodies of the principal excitatory neurons (pyramidal cells, DG neurons) of the HPC. However, the expression patterns of the assembled genes were non-randomly distributed across subregions–although some were detectably expressed in all subregions, many were expressed only in restricted regions of the HPC. Fig 2 presents the distribution (heatmap) of receptor expression across the different regions of the mouse HPC. To address correlations between HPC subregions, we performed pairwise correlation analysis (Table D in S1 Appendix). Normalized gene expression was significantly negatively correlated between DG and CA1, and positively correlated between CA2 and CA3. All remaining combinations of CA1, CA2, CA3, and DG provided no evidence of correlated gene expression (Table D in S1 Appendix).
To validate the subregional distributions in mouse HPC, we compared ABA in situ hybridization data against a second database, HippoSeq (Methods; this database only addresses HPC expression). Although there were some discordances, the HippoSeq database supported the overall subregional expression patterns detected by in situ hybridization (Table F in S1 Appendix).
Distribution of receptors with established roles: Subregion–function correlations reveal a challenge–sufficiency axis
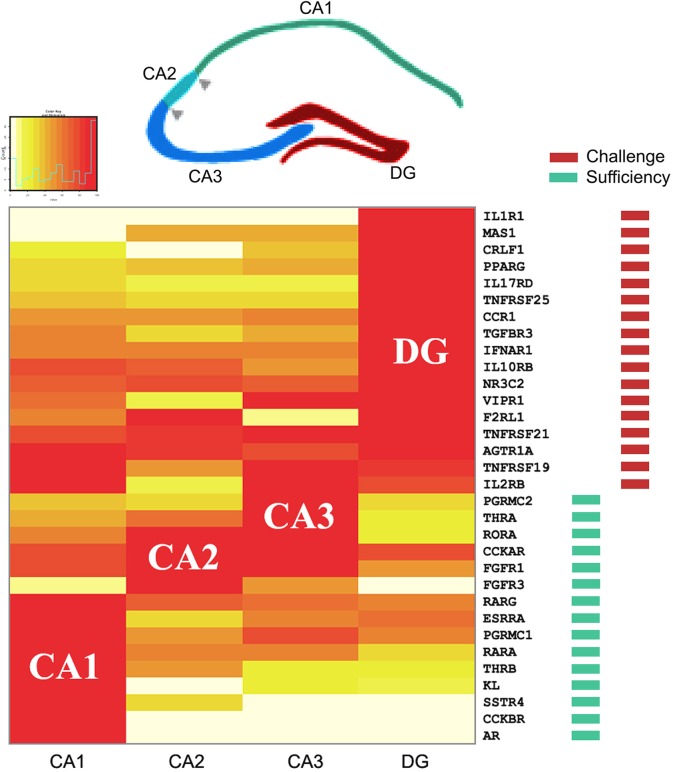
For the majority of the receptors studied here the biological 'meaning' is unknown, either because the receptor ligand is unknown or because the physiological role of the ligand(s) has not been established. To illustrate, the first and last genes in our list, Acvr1 and Vmnr234, respectively encode activin A receptor type 1 and a vomeronasal-like receptor. Ligands for ACVR1 include both inhibins and activins, that inhibit and activate diverse physiological processes and, moreover, have opposing functions; the primary in vivo ligand for ACVR1 in the CNS remains unknown. For VMNR234, the ligand is also unknown. Given this uncertainty we examined receptors from an 'informative' list (n = 32) where the function of the ligand is known (or inferred): these include angiotensins, cytokines, fibroblast growth factor (FGF), interleukins/interferons, prostaglandins, retinoids, steroid hormones (androgens, estrogens, glucocorticoids and mineralocorticoids), tumor growth factor (TGF), and tumor necrosis factor (TNF) (Methods and Table E in S1 Appendix). This revealed a gradient of expression across the HPC, where some receptors were principally expressed in DG regions, and others were principally expressed in CA regions (Fig 3).
Fig 3. Expression of 'informative' endocrine receptors in subregions of the mouse hippocampus (HPC).
(Above) Principal neuroatomical subdivisions of the rodent HPC (adapted from the model of [15]). (Below) Informative (see main text) receptors sorted according to regional expression (heatmap, normalized data) with CA1 and DG at the two extremes (Methods) showing expression clustering of receptor types in different regions (e.g., 'sufficiency'–FGF receptors FGFR1, FGFR3, and KL in CA regions; and 'challenge'–interleukin and TNF receptors IL1R1, IL17RD, IL10RB, IL2RB, TNFRSRF 25, TNFRSF21, TNFRSF19 in DG).
Receptor categorization by function
To understand this pattern we sought a unifying principle that might underpin and explain the gradient of receptor expression. It became apparent that receptor function differed according to location within the HPC. Receptors reflecting stress of various types (e.g., receptors for inflammatory cytokines and glucocorticoids) provided a clue because their expression was clustered in DG. Conversely, it was noted that receptors responding to growth-promoting ligands (e.g., growth factors and sex steroids) were principally localized in CA regions. On this basis it was possible to classify each ligand/receptor pair into two groups.
Because one group of receptor ligands (designated 'group A') signal loss of homeostasis and/or physiological stress of various types (these ligands include angiotensins–blood pressure fall; glucocorticoids–stress hormones; cytokines, interferons, and TNF–immune challenge), we describe these here as denoting 'challenge', whereas a second group of ligands ('group B') conversely includes growth-promoting hormones and factors (e.g., androgens, estrogens, fibroblast growth factor, retinoids), which we term here 'sufficiency' (more detailed listing and discussion of receptor function is presented in Box 1 and Table H in S1 Appendix). Although this classification is fully open to debate and refinement, we believe that it provides a potential interpretation of the observed gradient of expression.
As shown in Fig 3, there was unexpected clustering of group A ('challenge') receptor expression in DG, whereas group B ('sufficiency') receptors were predominantly expressed in CA regions.
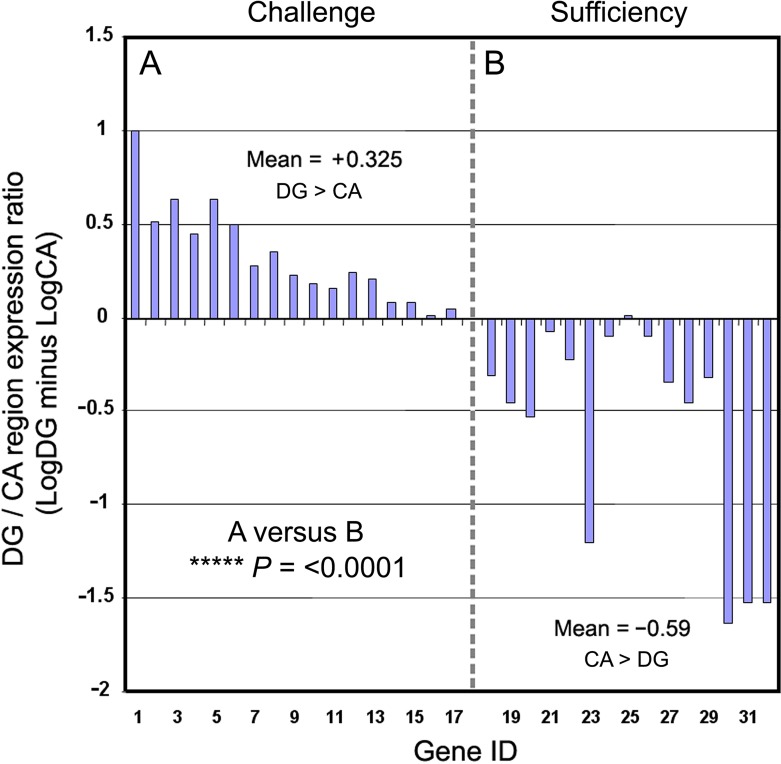
To address the statistical significance of the patterning of group A versus group B observation we calculated the ratios between different hippocampal subregions (mean of CA regions versus DG) by conversion to log10 values and subtraction (Methods) and plotted the results for the two groups A and B (Fig 4). The ratio of gene expression in CA to DG differed significantly between group A and B genes (Welch’s t-test, t = 4.22, df = 27.69, P = 0.00024). Permutation tests confirmed these findings. The analysis was then repeated for the HippoSeq data; this also achieved significance for CA regions versus DG (P = 0.0061; Table F in S1 Appendix).
Fig 4. Ratios of CA versus DG expression for informative receptors.
(A) Group A (DG/challenge). (B) Group B (CA/sufficiency). Individual genes are ordered as in Fig 3. The differential DG versus CA pattern of expression was highly significant.
We conclude that the expression pattern is highly structured within mouse HPC, and that group A receptors ('challenge') are preferentially expressed in DG, and group B receptors ('sufficiency') are selectively expressed in CA regions (Fig 3).
Further receptors confirm the generality of the axis
To test whether the axis extends to other endocrine receptors, we examined the expression pattern (in both ABA and HippoSeq) of other informative receptors (that were not on our original list) whose ligand is known and that are expressed in brain. We identified seven such receptors. All were expressed in mouse HPC (although some were only expressed at low levels, Table G in S1 Appendix). Challenge receptors [interleukin 6 receptor, growth hormone secretagogue receptor (ghrelin receptor), opioid growth factor receptor, irisin receptor, somatostatin receptor, leptin receptor, and glucagon-like peptide 1 receptor] were all expressed at higher levels in DG than in CA regions, whereas sufficiency receptors were expressed at highest level in CA regions (glucagon-like peptide 1 receptor) or were expressed at similar levels in CA and DG (leptin receptor) (Table G in S1 Appendix), confirming (7/7) that the DG versus CA differential ratio extends to other receptors, reinforcing the generality of our findings.
HPC receptors are functional: Synaptic potentiation and neurogenesis
We addressed whether the informative receptors are functional in vivo and in vitro by literature searching regarding two output measures: synaptic potentiation (long-term potentiation, LTP) and neurogenesis. The evidence argues that these endocrine receptors are fully functional and modulate both LTP and neurogenesis.
Synaptic potentiation
Although not all receptors have been studied in the literature, there was evidence that DG ligands predominantly inhibit local LTP, whereas CA ligands promote LTP. For example, DG ligands IL-1, IL-2, IFN-α, IFN-γ, TGF-β, and TNF-α all inhibit LTP in rodent hippocampus [29–36]. By contrast, CA1 ligands such as cholecystokinin (CCK), different types of FGF, and somatostatin (SST) are reported to enhance hippocampal LTP [37–40]. Thyroid hormone deficiency is associated with pronounced deficits in synaptic plasticity (e.g., [41–43]). Caution is urged, however, because some ligands may have distinct (even converse) effects on CA1 versus DG LTP, perhaps pointing to functional differences in the receptors expressed in different hippocampal regions. Nonetheless, based on the published literature, a clear pattern emerges in which challenge ligands (DG) predominantly impair LTP, whereas sufficiency ligands (CA) promote LTP (Fig 5 and Table H in S1 Appendix).
Fig 5. Differential effects of receptor activation on long-term potentiation (LTP) and neurogenesis.
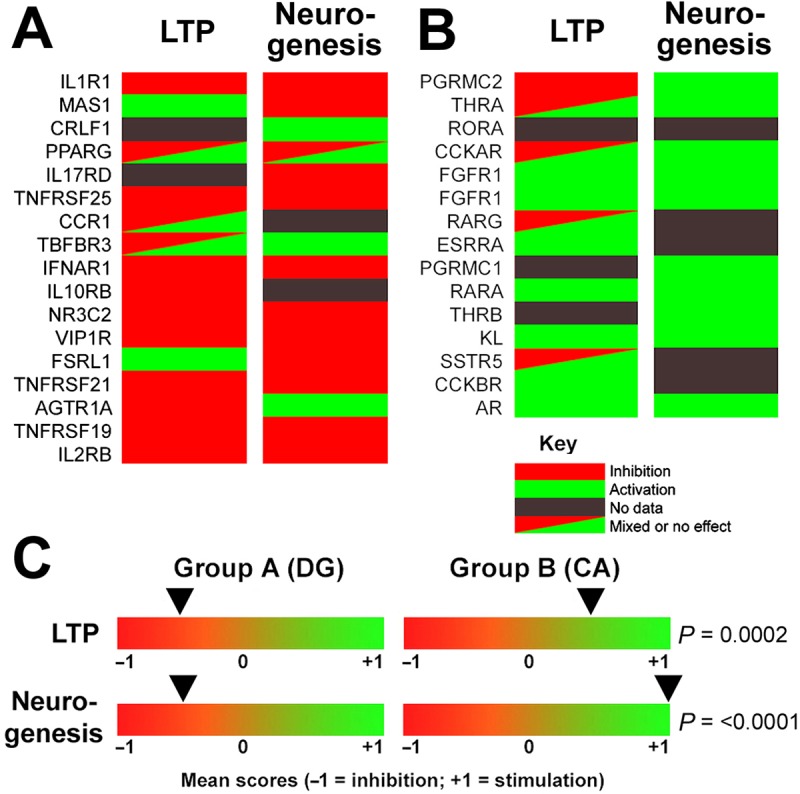
(A) Group A (DG/challenge). (B) Group B (CA/sufficiency). Individual genes are ordered as in Figs 3 and 4. (C) Mean scores for the two groups, demonstrating that group A receptors tend to suppress both LTP and neurogenesis, whereas group B receptors tend to promote both parameters. The differential patterns of stimulation/inhibition of LTP and neurogenesis were highly significant between the two groups.
Neurogenesis
The literature also records differential effects of DG and CA ligands. Group A (DG/challenge) ligands such as glucocorticoids, interleukins, interferons, and TNF-α are reported to inhibit neurogenesis (e.g., [44–50]) whereas group B (CA/sufficiency) ligands such as estrogen, progesterone, and FGF stimulate neurogenesis (e.g., [51–55]. There are some discordances, particularly when comparing long- and short-term effects (for example for glucocorticoids, reviewed in [47]). However, agents targeting DG predominantly suppress neurogenesis, whereas those targeting CA regions increase neurogenesis (Fig 5 and Table H in S1 Appendix).
Because of the small number of samples, differences between each group (DG/CA)/parameter (LTP/neurogenesis) and a random distribution were not uniformly significant (range P = 0.005–0.114 for four comparisons and two statistical tests). By contrast, intergroup comparisons revealed that the differences between groups A and B regarding LTP and neurogenesis were consistently highly significant (LTP, t test, P = 0.0001; chi-square test, P = 0.0028; neurogenesis, t test, P = 0.0002; chi-square test, P = 0.006) confirming that the patterns are indeed different.
In conclusion, ligand effects on both LTP and neurogenesis confirm that these hippocampal receptors are functional. Moreover, they indicate that the challenge/sufficiency axis extends to receptor function, wherein DG/challenge receptors predominantly inhibit both neurogenesis and synaptic plasticity, whereas CA/sufficiency ligands principally promote both parameters.
Discussion
This work confirms and extends prior suggestions that the HPC is involved in internal sensing, as reflected here by greater expression of endocrine receptors than in any other brain region, including CX and CB.
With regard to our first question (how many receptors), we report that 86 of 253 (34%) endocrine receptor genes are expressed in mouse HPC, and 17/98 (17.3%) are exclusively expressed in HPC, values markedly higher than for any other brain region. This accords with our previous data, based on small sample size, that 37% (21–59%, 95% CI) of mouse genes are expressed in HPC, a selection that predominantly encodes endocrine receptors and signaling molecules [21]. Aside from CX and CB, only low-level expression of these receptors was observed in other comparable brain regions (e.g., OLF, thalamus, pons/medulla, pallidum, or striatum; hypothalamus was not studied); these represent ca 4% of all receptors studied. However, we do not exclude the possibility that some receptors are expressed in other brain regions at levels below the limit of detection of in situ hybridization.
Thus, of all major brain regions in mouse, endocrine receptor genes are most prominently expressed in HPC, attesting that the present-day HPC is likely to play a role in sensing and responding to internal blood-borne (endocrine) markers of body physiology, arguing that the sensory function (interoception) attributed to the primeval hippocampus [7–9] has been retained to this day.
Our analysis has focused largely on hormonal ligands and has not addressed whether the HPC can directly sense levels of low molecular weight ligands (e.g., minerals, pH, CO2, etc.) because much less is known about their receptors. For example, NHE4 (SLC9A4), that is activated by hypertonicity, is well expressed in HPC (Allen Brain Atlas), but its exact function is unknown. It could mediate direct sensing of metabolites, although this remains speculative. It is likely that, with evolution, the mouse HPC now responds principally to peripheral hormones that act as proxies for metabolite levels. For example, aldosterone, a salt regulatory hormone, targets glucocorticoid receptors in the HPC.
Regarding our second question (patterning within the HPC), we report a highly significant non-random distribution of receptor expression across different HPC subregions of mouse HPC. Receptors whose biological function is known or may be inferred ('informative' genes, n = 32) were expressed in a highly structured pattern within the formation. Ligands signaling different aspects of challenge (termed here group A: stress, infection, inflammation, blood pressure fall) were principally found to target receptors expressed in DG, whereas ligands signaling aspects of sufficiency (group B: androgens, endocrine FGF, estrogens, progestins, retinoic acid, thyroid hormones) instead principally target the CA regions, with a mean 8.33-fold difference in the DG versus CA expression ratio (P < 0.0001).
Although the validity of this distinction remains open to debate (see Results for the underlying rationale), for the purposes of discussion we term this a 'challenge/sufficiency' axis. The highly ordered (DG vs CA) segregation of receptor expression in mouse brain raises the question of the function of this segregation (see below).
We also report that the challenge/sufficiency axis accurately mirrors the effects of DG versus CA ligands on physiological functions within the HPC. With few exceptions, DG/challenge receptors inhibit, whereas CA/sufficiency ligands promote, both neurogenesis and synaptic potentiation.
The contrasting effects on synaptic potentiation suggest that the hippocampus might act as an integrator of positive and negative information. Given the paradigmatic hippocampal circuit: cortex → DG → CA3 → CA1 → cortex, the output of the hippocampus is likely to represent the summation of ligand effects on DG and CA regions. The recorded modulation of synaptic potentiation (and thus of overall neurotransmission through the HPC) by endocrine receptor ligands leads us to speculate that the ancestral function of LTP may have been to indicate relevant physiological states worthy of encoding in memory traces, ranging from no LTP (highly adverse context) to potent LTP (highly beneficial context).
A key question concerns whether the challenge/sufficiency axis is reiterated in primates. Preliminary inspection of the microarray-based Allan Human Brain Atlas (http://human.brain-map.org/) fully confirms selective endocrine receptor expression in human HPC, consistent with internal sensing deficits in HPC-ablated patient H.M. [3], but the human data (from elderly individuals) are not strictly comparable to the analyzed data from young mice (and are therefore not presented). It is possible that DG/CA patterning may be less well conserved in human, but we note that strict conservation of this patterning across vertebrates is unlikely because, for example, birds and reptiles lack a morphological dentate gyrus (e.g., [56]). Indeed, there is no a priori reason why physical segregation of challenge versus sufficiency signaling should be necessary. We suspect that mouse brain may be a special (but informative) case–analysis of this species has pointed, for the first time, to differential HPC receptor localization according to function, providing a new and unexpected perspective on hippocampal function.
Although comprehensive in situ receptor expression data in human are so far lacking, there is firm evidence that a functional challenge/sufficiency axis also operates. The human HPC is at the heart of anxiety [57,58], as well as of stress responses and depression. Extensive review would be out of place, but we note that clinical administration of 'challenge' ligands (DG in mouse) such as IL-1α, IL-2, IFN-α, IFN-β, and TNF-α produces malaise and sickness behavior [59–64], that has been suggested to be akin to anxiety/depression, whereas 'sufficiency' ligands (CA regions in mouse) such as androgens, IGF-1, and thyroid hormone have converse positive effects (e.g., [65–67]), all of which target HPC receptors, indicating that the axis is also functional in human. Systematic inventory of clinical data on challenge/sufficiency ligands will be necessary to confirm this contention.
Nonetheless, we observe an accurate correlation between ligands targeting CA regions and antidepressant/anxiolytic benefits, and the converse for DG ligands. This parallels effects on neurogenesis, where CA ligands predominantly promote neurogenesis in the HPC whereas DG ligands inhibit neurogenesis. This is of special note given that stimulation of HPC neurogenesis has been directly linked to antidepressant action and has been used for new antidepressant drug screening (e.g., [68,69]); differential receptor localization may provide novel indicators for the development of new antidepressants/anxiolytics.
In sum, the selective expression of endocrine receptors in mouse HPC, further highlighted by challenge–sufficiency patterning of endocrine receptor expression, argues that internal sensing remains a core function of the HPC. This accords with evolutionary theory that the HPC arose from a chemosensory epithelium [7–9], and argues that the present-day HPC in particular has retained the ability to monitor the internal milieu of the body. Interoception mediated by the hippocampus may thus provide a new dimension to context-dependent memory encoding, extending from 'where' and 'when' to 'how I feel'.
It will be vital to test these concepts in mice genetically engineered to express designer receptors only in DG versus CA regions, and to study the effect of ligand administration on physiology, behavior, and memory. It would also be very informative to study cross-species conservation of expression in larger mammals (rabbit, sheep, non-human primates) where the relative contribution of the hypothalamus (that was too small to be analyzed) could be examined in detail. Moreover, in addition to looking forwards (from mouse to primates), it would be highly illuminating (i) to examine in detail the trajectories of endocrine receptor expression during early development, and (ii) to address the expression profiles of homologs of these genes in other representatives of the vertebrate lineage including birds, reptiles, and fish. One promising line of investigation will be to dissect memory processes in the earliest organisms that encode associations between different internal and external stimuli. Addressing the earliest precedents, and the traces these have left in extant species, will be a fertile territory for new insights into the operation of the human brain.
Supporting information
Supplementary tables A–H.
(PDF)
Acknowledgments
We thank the Allen Brain Institute (Seattle, WA, USA) and Janelia (Ashburn, VA, USA) for making their data publicly available, and to whom we express our deep appreciation. S.S. thanks the Carnegie Trust for the Universities of Scotland for a vacation scholarship. We also thank an anonymous reviewer for suggesting further genes for study. All data needed to evaluate the conclusions in the paper are presented in the paper and/or the supplementary materials online.
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Craig AD. How do you feel? Interoception: the sense of the physiological condition of the body. Nat Rev Neurosci. 2002;3, 655–666. 10.1038/nrn894 [DOI] [PubMed] [Google Scholar]
- 2.Scoville WB and Milner B. Loss of recent memory after bilateral hippocampal lesions. J Neurol Neurosurg Psychiat. 1957;20, 11–21. 10.1136/jnnp.20.1.11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hebben N, Corkin S, Eichenbaum H, Shedlack K. Diminished ability to interpret and report internal states after bilateral medial temporal resection: case H.M. Behav Neurosci. 1985;99, 1031–1039. 10.1037//0735-7044.99.6.1031 [DOI] [PubMed] [Google Scholar]
- 4.Davidson TL and Jarrard LE. A role for hippocampus in the utilization of hunger signals. Behav Neural Biol. 1993;59, 167–171. 10.1016/0163-1047(93)90925-8 [DOI] [PubMed] [Google Scholar]
- 5.Clifton PG, Vickers SP, Somerville EM. Little and often: ingestive behaviour patterns following hippocampal lesions in rats. Behav Neurosci. 1998;112, 502–511. 10.1037//0735-7044.112.3.502 [DOI] [PubMed] [Google Scholar]
- 6.Davidson TL, Kanoski SE, Chan K, Clegg DJ, Benoit SC, Jarrard LE. Hippocampal lesions impair retention of discriminative responding based on energy state cues. Behav Neurosci. 2010;124, 97–105. 10.1037/a0018402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Riss W, Halpern M, Scalia F. Anatomical aspects of the evolution of the limbic and olfactory systems and their potential significance for behaviour. Ann NY Acad Sci. 1969;159, 1096–1111. 10.1111/j.1749-6632.1969.tb13000.x [DOI] [PubMed] [Google Scholar]
- 8.Nieuwenhuys R, Ten Donkelaar HJ, Nicholson C. The Central Nervous System of Vertebrates. Berlin: Springer; 1997. [Google Scholar]
- 9.Lathe R. Hormones and the hippocampus. J Endocrinol. 2001;169, 205–231. 10.1677/joe.0.1690205 [DOI] [PubMed] [Google Scholar]
- 10.Altman J. Autoradiographic and histological studies of postnatal neurogenesis. IV. Cell proliferation and migration in the anterior forebrain, with special reference to persisting neurogenesis in the olfactory bulb. J Comp Neurol. 1969;137, 433–457. 10.1002/cne.901370404 [DOI] [PubMed] [Google Scholar]
- 11.Lledo PM and Valley M. Adult olfactory bulb neurogenesis. Cold Spring Harb Perspect Biol. 2016;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wilken MS and Reh TA. Retinal regeneration in birds and mice. Curr Opin Genet Dev. 2016;40, 57–64. 10.1016/j.gde.2016.05.028 [DOI] [PubMed] [Google Scholar]
- 13.Ming GL and Song H. Adult neurogenesis in the mammalian brain: significant answers and significant questions. Neuron. 2011;70, 687–702. 10.1016/j.neuron.2011.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Duvernoy H, Cattin F, Risold P-Y. The Human Hippocampus: Functional Anatomy, Vascularization and Serial Sections with MRI. Heidelberg: Springer; 2013. [Google Scholar]
- 15.Lein ES, Zhao X, Gage FH. Defining a molecular atlas of the hippocampus using DNA microarrays and high-throughput in situ hybridization. J Neurosci. 2004;24, 3879–3889. 10.1523/JNEUROSCI.4710-03.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bienkowski MS, Bowman I, Song MY, Gou L, Ard T, Cotter K et al. Integration of gene expression and brain-wide connectivity reveals the multiscale organization of mouse hippocampal networks. Nat Neurosci. 2018;21, 1628–1643. 10.1038/s41593-018-0241-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thompson CL, Pathak SD, Jeromin A, Ng LL, MacPherson CR, Mortrud MT et al. Genomic anatomy of the hippocampus. Neuron. 2008;60, 1010–1021. 10.1016/j.neuron.2008.12.008 [DOI] [PubMed] [Google Scholar]
- 18.Thompson CL, Ng L, Menon V, Martinez S, Lee CK, Glattfelder K et al. A high-resolution spatiotemporal atlas of gene expression of the developing mouse brain. Neuron. 2014;83, 309–323. 10.1016/j.neuron.2014.05.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stapleton G, Steel M, Richardson M, Mason JO, Rose KA, Morris RG et al. A novel cytochrome P450 expressed primarily in brain. J Biol Chem. 1995;270, 29739–29745. 10.1074/jbc.270.50.29739 [DOI] [PubMed] [Google Scholar]
- 20.Davies BJ, Pickard BS, Steel M, Morris RG, Lathe R. Serine proteases in rodent hippocampus. J Biol Chem. 1998;273, 23004–22011. 10.1074/jbc.273.36.23004 [DOI] [PubMed] [Google Scholar]
- 21.Steel M, Moss J, Clark KA, Kearns IR, Davies CH, Morris RG et al. Gene-trapping to identify and analyze genes expressed in the mouse hippocampus. Hippocampus. 1998;8, 444–457. [DOI] [PubMed] [Google Scholar]
- 22.Lein ES, Hawrylycz MJ, Ao N, Ayres M, Bensinger A, Bernard A et al. Genome-wide atlas of gene expression in the adult mouse brain. Nature. 2007;445, 168–176. 10.1038/nature05453 [DOI] [PubMed] [Google Scholar]
- 23.Rueden CT, Schindelin J, Hiner MC, DeZonia BE, Walter AE, Arena ET et al. ImageJ2: ImageJ for the next generation of scientific image data. BMC Bioinformatics. 2017;18, 529 10.1186/s12859-017-1934-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9, 671–675. 10.1038/nmeth.2089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.R Core Team. R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing; 2019. [Google Scholar]
- 26.Canty A and Ripley B. Boot: bootstrap R (S-Plus) functions. R package version 1 3–20. 2017. [Google Scholar]
- 27.Davison AC and Hinkley DV. Bootstrap Methods and Their Applications. Cambridge: Cambridge University Press; 1997. [Google Scholar]
- 28.Cembrowski MS, Wang L, Sugino K, Shields BC, Spruston N. Hipposeq: a comprehensive RNA-seq database of gene expression in hippocampal principal neurons. Elife. 2016;5, e14997 10.7554/eLife.14997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tancredi V, Zona C, Velotti F, Eusebi F, Santoni A. Interleukin-2 suppresses established long-term potentiation and inhibits its induction in the rat hippocampus. Brain Res. 1990;525, 149–151. 10.1016/0006-8993(90)91331-a [DOI] [PubMed] [Google Scholar]
- 30.Katsuki H, Nakai S, Hirai Y, Akaji K, Kiso Y, Satoh M. Interleukin-1 beta inhibits long-term potentiation in the CA3 region of mouse hippocampal slices. Eur J Pharmacol. 1990;181, 323–326. 10.1016/0014-2999(90)90099-r [DOI] [PubMed] [Google Scholar]
- 31.Tancredi V, D'Arcangelo G, Grassi F, Tarroni P, Palmieri G, Santoni A et al. Tumor necrosis factor alters synaptic transmission in rat hippocampal slices. Neurosci Lett. 1992;146, 176–178. 10.1016/0304-3940(92)90071-e [DOI] [PubMed] [Google Scholar]
- 32.Bellinger FP, Madamba S, Siggins GR. Interleukin 1 beta inhibits synaptic strength and long-term potentiation in the rat CA1 hippocampus. Brain Res. 1993;628, 227–234. 10.1016/0006-8993(93)90959-q [DOI] [PubMed] [Google Scholar]
- 33.Coogan A and O'Connor JJ. Inhibition of NMDA receptor-mediated synaptic transmission in the rat dentate gyrus in vitro by IL-1 beta. Neuroreport. 1997;8, 2107–2110. 10.1097/00001756-199707070-00004 [DOI] [PubMed] [Google Scholar]
- 34.Ikegaya Y, Saito H, Torii K, Nishiyama N. Activin selectively abolishes hippocampal long-term potentiation induced by weak tetanic stimulation in vivo. Jpn J Pharmacol. 1997;75, 87–89. 10.1254/jjp.75.87 [DOI] [PubMed] [Google Scholar]
- 35.Mendoza-Fernandez V, Andrew RD, Barajas-Lopez C. Interferon-alpha inhibits long-term potentiation and unmasks a long-term depression in the rat hippocampus. Brain Res. 2000;885, 14–24. 10.1016/s0006-8993(00)02877-8 [DOI] [PubMed] [Google Scholar]
- 36.Griffin R, Nally R, Nolan Y, McCartney Y, Linden J, Lynch MA. The age-related attenuation in long-term potentiation is associated with microglial activation. J Neurochem. 2006;99, 1263–1272. 10.1111/j.1471-4159.2006.04165.x [DOI] [PubMed] [Google Scholar]
- 37.Ishiyama J, Saito H, Abe K. Epidermal growth factor and basic fibroblast growth factor promote the generation of long-term potentiation in the dentate gyrus of anaesthetized rats. Neurosci Res. 1991;12, 403–411. 10.1016/0168-0102(91)90071-6 [DOI] [PubMed] [Google Scholar]
- 38.Matsuoka N, Kaneko S, Satoh M. Somatostatin augments long-term potentiation of the mossy fiber-CA3 system in guinea-pig hippocampal slices. Brain Res. 1991;553, 188–194. 10.1016/0006-8993(91)90823-e [DOI] [PubMed] [Google Scholar]
- 39.Hisajima H, Saito H, Abe K, Nishiyama N. Effects of acidic fibroblast growth factor on hippocampal long-term potentiation in fasted rats. J Neurosci Res. 1992;31, 549–553. 10.1002/jnr.490310319 [DOI] [PubMed] [Google Scholar]
- 40.Yasui M and Kawasaki K. CCKB-receptor activation augments the long-term potentiation in guinea pig hippocampal slices. Jpn J Pharmacol. 1995;68, 441–447. 10.1254/jjp.68.441 [DOI] [PubMed] [Google Scholar]
- 41.Vara H, Munoz-Cuevas J, Colino A. Age-dependent alterations of long-term synaptic plasticity in thyroid-deficient rats. Hippocampus. 2003;13, 816–825. 10.1002/hipo.10132 [DOI] [PubMed] [Google Scholar]
- 42.Dong J, Yin H, Liu W, Wang P, Jiang Y, Chen J. Congenital iodine deficiency and hypothyroidism impair LTP and decrease C-fos and C-jun expression in rat hippocampus. Neurotoxicology. 2005;26, 417–426. 10.1016/j.neuro.2005.03.003 [DOI] [PubMed] [Google Scholar]
- 43.Taskin E, Artis AS, Bitiktas S, Dolu N, Liman N, Suer C. Experimentally induced hyperthyroidism disrupts hippocampal long-term potentiation in adult rats. Neuroendocrinology. 2011;94, 218–227. 10.1159/000328513 [DOI] [PubMed] [Google Scholar]
- 44.Iosif RE, Ekdahl CT, Ahlenius H, Pronk CJ, Bonde S, Kokaia Z et al. Tumor necrosis factor receptor 1 is a negative regulator of progenitor proliferation in adult hippocampal neurogenesis. J Neurosci. 2006;26, 9703–9712. 10.1523/JNEUROSCI.2723-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Erta M, Quintana A, Hidalgo J. Interleukin-6, a major cytokine in the central nervous system. Int J Biol Sci. 2012;8, 1254–1266. 10.7150/ijbs.4679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vallieres L, Campbell IL, Gage FH, Sawchenko PE. Reduced hippocampal neurogenesis in adult transgenic mice with chronic astrocytic production of interleukin-6. J Neurosci. 2002;22, 486–492. 10.1523/JNEUROSCI.22-02-00486.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Saaltink DJ and Vreugdenhil E. Stress, glucocorticoid receptors, and adult neurogenesis: a balance between excitation and inhibition? Cell Mol Life Sci. 2014;71, 2499–2515. 10.1007/s00018-014-1568-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Odaka H, Adachi N, Numakawa T. Impact of glucocorticoid on neurogenesis. Neural Regen Res. 2017;12, 1028–1035. 10.4103/1673-5374.211174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Borsini A, Alboni S, Horowitz MA, Tojo LM, Cannazza G, Su KP et al. Rescue of IL-1beta-induced reduction of human neurogenesis by omega-3 fatty acids and antidepressants. Brain Behav Immun. 2017;65, 230–238. 10.1016/j.bbi.2017.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Borsini A, Cattaneo A, Malpighi C, Thuret S, Harrison NA, Zunszain PA et al. Interferon-alpha reduces human hippocampal neurogenesis and increases apoptosis via activation of distinct STAT1-dependent mechanisms. Int J Neuropsychopharmacol. 2018;21, 187–200. 10.1093/ijnp/pyx083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yoshimura S, Takagi Y, Harada J, Teramoto T, Thomas SS, Waeber C et al. FGF-2 regulation of neurogenesis in adult hippocampus after brain injury. Proc Natl Acad Sci U S A. 2001;98, 5874–5879. 10.1073/pnas.101034998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fowler CD, Liu Y, Wang Z. Estrogen and adult neurogenesis in the amygdala and hypothalamus. Brain Res Rev. 2008;57, 342–351. 10.1016/j.brainresrev.2007.06.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Suzuki S, Gerhold LM, Bottner M, Rau SW, Dela CC, Yang E et al. Estradiol enhances neurogenesis following ischemic stroke through estrogen receptors alpha and beta. J Comp Neurol. 2007;500, 1064–1075. 10.1002/cne.21240 [DOI] [PubMed] [Google Scholar]
- 54.Chan M, Chow C, Hamson DK, Lieblich SE, Galea LA. Effects of chronic oestradiol, progesterone and medroxyprogesterone acetate on hippocampal neurogenesis and adrenal mass in adult female rats. J Neuroendocrinol. 2014;26, 386–399. 10.1111/jne.12159 [DOI] [PubMed] [Google Scholar]
- 55.Kang W and Hebert JM. FGF signaling is necessary for neurogenesis in young mice and sufficient to reverse its decline in old mice. J Neurosci. 2015;35, 10217–10223. 10.1523/JNEUROSCI.1469-15.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bingman VP and Muzio RN. Reflections on the structural-functional evolution of the hippocampus: what is the big deal about a dentate gyrus? Brain Behav Evol. 2017;90, 53–61. 10.1159/000475592 [DOI] [PubMed] [Google Scholar]
- 57.Gray JA. The Neuropsychology of Anxiety: An Enquiry into the Functions of the Septo-Hippocampal System. Oxford: Oxford University Press; 1982. [Google Scholar]
- 58.Gray JA and McNaughton N. The Neuropsychology of Anxiety: An Enquiry into the Functions of the Septo-Hippocampal System. Oxford: Oxford University Press; 2000. [Google Scholar]
- 59.Smith JW, Urba WJ, Curti BD, Elwood LJ, Steis RG, Janik JE et al. The toxic and hematologic effects of interleukin-1 alpha administered in a phase I trial to patients with advanced malignancies. J Clin Oncol. 1992;10, 1141–1152. 10.1200/JCO.1992.10.7.1141 [DOI] [PubMed] [Google Scholar]
- 60.Capuron L, Ravaud A, Gualde N, Bosmans E, Dantzer R, Maes M et al. Association between immune activation and early depressive symptoms in cancer patients treated with interleukin-2-based therapy. Psychoneuroendocrinology. 2001;26, 797–808. 10.1016/s0306-4530(01)00030-0 [DOI] [PubMed] [Google Scholar]
- 61.Valentine AD, Meyers CA, Kling MA, Richelson E, Hauser P. Mood and cognitive side effects of interferon-alpha therapy. Semin Oncol. 1998;25, 39–47. [PubMed] [Google Scholar]
- 62.Raison CL, Demetrashvili M, Capuron L, Miller AH. Neuropsychiatric adverse effects of interferon-alpha: recognition and management. CNS Drugs. 2005;19, 105–123. 10.2165/00023210-200519020-00002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Exton MS, Baase J, Pithan V, Goebel MU, Limmroth V, Schedlowski M. Neuropsychological performance and mood states following acute interferon-beta-1b administration in healthy males. Neuropsychobiology. 2002;45, 199–204. 10.1159/000063671 [DOI] [PubMed] [Google Scholar]
- 64.Creaven PJ, Plager JE, Dupere S, Huben RP, Takita H, Mittelman A et al. Phase I clinical trial of recombinant human tumor necrosis factor. Cancer Chemother Pharmacol. 1987;20, 137–144. 10.1007/bf00253968 [DOI] [PubMed] [Google Scholar]
- 65.Zarrouf FA, Artz S, Griffith J, Sirbu C, Kommor M. Testosterone and depression: systematic review and meta-analysis. J Psychiatr Pract. 2009;15, 289–305. 10.1097/01.pra.0000358315.88931.fc [DOI] [PubMed] [Google Scholar]
- 66.Gruber AJ and Pope HG Jr. Psychiatric and medical effects of anabolic-androgenic steroid use in women. Psychother Psychosom. 2000;69, 19–26. 10.1159/000012362 [DOI] [PubMed] [Google Scholar]
- 67.Bauer M, Hellweg R, Graf KJ, Baumgartner A. Treatment of refractory depression with high-dose thyroxine. Neuropsychopharmacology. 1998;18, 444–455. 10.1016/S0893-133X(97)00181-4 [DOI] [PubMed] [Google Scholar]
- 68.Hanson ND, Owens MJ, Nemeroff CB. Depression, antidepressants, and neurogenesis: a critical reappraisal. Neuropsychopharmacology. 2011;36, 2589–2602. 10.1038/npp.2011.220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yan HC, Cao X, Gao TM, Zhu XH. Promoting adult hippocampal neurogenesis: a novel strategy for antidepressant drug screening. Curr Med Chem. 2011;18, 4359–4367. 10.2174/092986711797200471 [DOI] [PubMed] [Google Scholar]
- 70.Weiland NG, Orikasa C, Hayashi S, McEwen BS. Distribution and hormone regulation of estrogen receptor immunoreactive cells in the hippocampus of male and female rats. J Comp Neurol. 1997;388, 603–612. [DOI] [PubMed] [Google Scholar]
- 71.Walf AA and Frye CA. A review and update of mechanisms of estrogen in the hippocampus and amygdala for anxiety and depression behavior. Neuropsychopharmacology. 2006;31, 1097–1111. 10.1038/sj.npp.1301067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bean LA, Ianov L, Foster TC. Estrogen receptors, the hippocampus, and memory. Neuroscientist. 2014;20, 534–545. 10.1177/1073858413519865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sugiyama N, Andersson S, Lathe R, Fan X, Alonso-Magdalena P, Schwend T et al. Spatiotemporal dynamics of the expression of estrogen receptors in the postnatal mouse brain. Mol Psychiatry. 2009;14, 223–32, 117. 10.1038/mp.2008.118 [DOI] [PubMed] [Google Scholar]
- 74.Wei W, Schwaid AG, Wang X, Wang X, Chen S, Chu Q et al. Ligand activation of ERRalpha by cholesterol mediates statin and bisphosphonate effects. Cell Metab. 2016;23, 479–491. 10.1016/j.cmet.2015.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Shughrue PJ and Merchenthaler I. Evidence for novel estrogen binding sites in the rat hippocampus. Neuroscience. 2000;99, 605–612. 10.1016/s0306-4522(00)00242-6 [DOI] [PubMed] [Google Scholar]
- 76.Thin TH, Kim E, Yeh S, Sampson ER, Chen YT, Collins LL et al. Mutations in the helix 3 region of the androgen receptor abrogate ARA70 promotion of 17beta-estradiol-induced androgen receptor transactivation. J Biol Chem. 2002;277, 36499–36508. 10.1074/jbc.M202824200 [DOI] [PubMed] [Google Scholar]
- 77.Bjerregaard-Olesen C, Ghisari M, Kjeldsen LS, Wielsoe M, Bonefeld-Jorgensen EC. Estrone sulfate and dehydroepiandrosterone sulfate: transactivation of the estrogen and androgen receptor. Steroids. 2016;105, 50–58. 10.1016/j.steroids.2015.11.009 [DOI] [PubMed] [Google Scholar]
- 78.Lathe R and Kotelevtsev Y. Steroid signaling: ligand-binding promiscuity, molecular symmetry, and the need for gating. Steroids. 2014;82, 14–22. 10.1016/j.steroids.2014.01.002 [DOI] [PubMed] [Google Scholar]
- 79.Rose K, Allan A, Gauldie S, Stapleton G, Dobbie L, Dott K et al. Neurosteroid hydroxylase CYP7B: vivid reporter activity in dentate gyrus of gene-targeted mice and abolition of a widespread pathway of steroid and oxysterol hydroxylation. J Biol Chem. 2001;276, 23937–23944. 10.1074/jbc.M011564200 [DOI] [PubMed] [Google Scholar]
- 80.Beenken A and Mohammadi M. The structural biology of the FGF19 subfamily. Adv Exp Med Biol. 2012;728, 1–24. 10.1007/978-1-4614-0887-1_1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Itoh N, Ohta H, Konishi M. Endocrine FGFs: evolution, physiology, pathophysiology, and pharmacotherapy. Front Endocrinol 2015;6, 154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jost B, Duluc I, Richardson M, Lathe R, Freund JN. Functional diversity and interactions between the repeat domains of rat intestinal lactase. Biochem J. 1997;327, 95–103. 10.1042/bj3270095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Fon TK, Bookout AL, Ding X, Kurosu H, John GB, Wang L et al. Research resource: comprehensive expression atlas of the fibroblast growth factor system in adult mouse. Mol Endocrinol. 2010;24, 2050–2064. 10.1210/me.2010-0142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Eigler T and Ben-Shlomo A. Somatostatin system: molecular mechanisms regulating anterior pituitary hormones. J Mol Endocrinol. 2014;53, R1–19. 10.1530/JME-14-0034 [DOI] [PubMed] [Google Scholar]
- 85.Prévot TD, Gastambide F, Viollet C, Henkous N, Martel G, Epelbaum J et al. Roles of hippocampal somatostatin receptor subtypes in stress response and emotionality. Neuropsychopharmacology. 2017;42, 1647–1656. 10.1038/npp.2016.281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gastambide F, Viollet C, Lepousez G, Epelbaum J, Guillou JL. Hippocampal SSTR4 somatostatin receptors control the selection of memory strategies. Psychopharmacology (Berl). 2009;202, 153–163. [DOI] [PubMed] [Google Scholar]
- 87.Gastambide F, Lepousez G, Viollet C, Loudes C, Epelbaum J, Guillou JL. Cooperation between hippocampal somatostatin receptor subtypes 4 and 2: functional relevance in interactive memory systems. Hippocampus. 2010;20, 745–757. 10.1002/hipo.20680 [DOI] [PubMed] [Google Scholar]
- 88.Prévot TD, Viollet C, Epelbaum J, Dominguez G, Beracochea D, Guillou JL. Sst2-receptor gene deletion exacerbates chronic stress-induced deficits: consequences for emotional and cognitive ageing. Prog Neuropsychopharmacol Biol Psychiatry. 2018;86, 390–400. 10.1016/j.pnpbp.2018.01.022 [DOI] [PubMed] [Google Scholar]
- 89.Chapman K, Holmes M, Seckl J. 11beta-hydroxysteroid dehydrogenases: intracellular gate-keepers of tissue glucocorticoid action. Physiol Rev. 2013;93, 1139–1206. 10.1152/physrev.00020.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.De Kloet ER, Reul JM, Sutanto W. Corticosteroids and the brain. J Steroid Biochem Mol Biol. 1990;37, 387–394. 10.1016/0960-0760(90)90489-8 [DOI] [PubMed] [Google Scholar]
- 91.Pryce CR, Feldon J, Fuchs E, Knuesel I, Oertle T, Sengstag C et al. Postnatal ontogeny of hippocampal expression of the mineralocorticoid and glucocorticoid receptors in the common marmoset monkey. Eur J Neurosci. 2005;21, 1521–1535. 10.1111/j.1460-9568.2005.04003.x [DOI] [PubMed] [Google Scholar]
- 92.Seckl JR, Dickson KL, Yates C, Fink G. Distribution of glucocorticoid and mineralocorticoid receptor messenger RNA expression in human postmortem hippocampus. Brain Res. 1991;561, 332–337. 10.1016/0006-8993(91)91612-5 [DOI] [PubMed] [Google Scholar]
- 93.Huang C, Wan B, Gao B, Hexige S, Yu L. Isolation and characterization of novel human short-chain dehydrogenase/reductase SCDR10B which is highly expressed in the brain and acts as hydroxysteroid dehydrogenase. Acta Biochim Pol. 2009;56, 279–289. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary tables A–H.
(PDF)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.