Abstract
Purpose
To compare the clinical outcome of patients with early-stage non-small cell lung cancer (NSCLC) who underwent either single-fraction (SF) or three-fraction (TF) stereotactic body radiation therapy (SBRT) at a single institution over 8 years.
Methods
Patients with peripherally located early-stage NSCLC who underwent SBRT between February 2007 and November 2015 were included in this study. SBRT was delivered without heterogeneity correction. Data were retrospectively reviewed and collected in an institutional review board-approved database. R software (version 3.3.2) was used for analyses.
Results
Of 159 total lung tumors, 65 lesions received 30 Gy (median 30 Gy) in 1 fraction, while the remaining 94 lesions received 48-60 Gy (median 60 Gy) in 3 fractions. Patients with a Karnofsky Performance Status (KPS) lower than 80 were more common in the SF-SBRT cohort (p=0.050). After a median follow-up of 22.2 months for SF-SBRT and 26.2 months for TF-SBRT cohorts (p=0.29), there was no statistically significant difference in overall survival (p=0.86), progression-free survival (p=0.95), local failure (p=0.95), nodal failure (p=0.91), and distant failure (p=0.49) at 24 months. At 1- and 2-year, overall survival rates were 86.1% and 63.2% for the SF-SBRT cohort, and 80.8% and 61.6% for the TF-SBRT cohort. At 1- and 2-year, local control rates were 95.1% and 87.8% for the SF-SBRT cohort, and 92.7% and 86.2% for the TF-SBRT cohort. Both regimens were well tolerated.
Conclusion
Despite more patients with poor performance status receiving SF-SBRT, SF- and TF-SBRT regimens showed no difference in clinical outcomes. SF-SBRT is now our standard approach.
Keywords: Lung tumor, NSCLC, SABR, SBRT, Patterns of failure
Micro Abstract
We compared the clinical outcome of single- and three-fraction stereotactic body radiation therapy schedules for early-stage non-small cell lung cancer (n=159). No statistical difference in clinical outcomes between these two regimens was observed, despite the single-fraction group that included more patients with worse performance status. This suggests a single-fraction regimen is a reasonable option.
Introduction
Surgery remains the standard treatment for early-stage non-small cell lung cancer (NSCLC) with a 5-year survival rate of 50-80%.1,2 Medically inoperable patients historically received either conventionally fractionated radiation therapy or stereotactic body radiation therapy (SBRT). In a recent trial, SBRT was shown to be better tolerated with improved disease control.3 Further studies of SBRT have demonstrated a local control rate of over 90% with OS and PFS rates comparable to surgery alone.4-7
Early-stage NSCLC can be categorized by its location relative to the proximal bronchial tree.8 Both single-fraction (SF) and three-fraction (TF) SBRT have shown to be reasonably well tolerated for peripherally located tumors (more than 2 cm away from the proximal bronchial tree) in prior studies, including Radiation Therapy Oncology Group (RTOG) 0915A.6,9-13 These regimens have been compared in a multi-institutional, phase II randomized study led by our institution, which has been reported in abstract form and showed equivalent outcomes and toxicities.12 Pending the final results of this prospective trial, and understanding that trial participation itself may impact outcome,14 we retrospectively investigated how these regimens performed in our clinical practice.
Methods
Patients
We retrospectively reviewed data from 155 patients who received definitive SF- and TF-SBRT for peripheral NSCLC between February 2007 and November 2015. Data were collected and maintained in an institutional review board-approved database at Roswell Park Cancer Institute (RPCI). Clinical trial patients who participated on RTOG 0915 were excluded from this analysis as the doses used on that study were different from the doses in this analysis. Patients treated at RPCI, on the multi-institutional randomized study I-124407, were randomized to the doses used in this analysis and thus included. All patients were screened for eligibility at the time of consult and offered trial enrollment if eligible; many patients, however, simply refused to be on study and were treated off-protocol at the discretion of the treating physician.
When treated off-protocol, a combination of factors (radiation oncologist preference, performance status, set-up and transportation issues) went into the decision making regarding number of fractions. In the initial period, single fraction was preferred for those with poor performance status, poor social support, poor access to transportation, and poor reproducibility of their treatment position in part due to other medical comorbidities. Over time as experience with single fraction increased, this became a more preferred approach of the treating radiation oncologists.
Staging and Operative Evaluation
Prior to SBRT, all patients had an initial consultation for history and physical examination, discussing treatment options and their risks and benefits. Computerized axial tomography (CT) and fluorodeoxyglucose-positron emission tomography (FDG-PET) scans were included in most cases at the time of the consultation. Endoscopic bronchoscopic ultrasound (EBUS) was routinely done as clinically indicated. All patients were presented at a multidisciplinary conference. Medical inoperability was determined by thoracic surgeons based factors such as poor pulmonary function including forced expiratory volume in a second less than 40% predicted, cardiovascular comorbidities including unstable angina, or poor performance status.
Treatment planning
The delivery method of SBRT at RPCI has been reported elsewhere.15 Normal tissue constraints were as per RTOG 0915 for SF-SBRT and RTOG 0236 for TF-SBRT.6,11 Three dimensional conformal radiation therapy with 11 non-coplanar fields was used in most cases. Intensity-modulated radiation therapy (IMRT) was used for 2 patients in the SF-SBRT and 3 patients in the TF-SBRT cohorts. Heterogeneity corrections were used only for patients treated with IMRT. Gross tumor volume (GTV) was outlined on each CT slice, and tumors were distinguished from adjacent structures using lung and soft tissue windows. Clinical target volume (CTV) was defined as equal to GTV. Internal target volume (ITV) was created using 4D CT. Planning target volume (PTV) was generated by the ITV with 0.5 cm margin added uniformly.
The prescription dose was delivered to the edge of the PTV. The prescription isodose surface was between 60% and 90% of the maximum dose. 99% of the PTV received greater than or equal to 90% of the prescription dose.
Follow up
Follow-up appointments were scheduled at 1.5, 3, and 6 months after the SBRT. Subsequent follow-ups occurred every 6 months for 2 years and annually thereafter. PET/CT at the 1-year follow-up and surveillance CT of the chest during other visits were performed to assess tumor response to SBRT.
Outcome assessment
Local failure was considered as tumor recurrence in the PTV or in the treated lobe of the lung. If a local lesion was PET-avid and no biopsy was done, it was classified as a local failure. If no PET scan was done and our radiologists reading the CT scan felt that a lesion may be even slightly concerning for malignancy, then we also classified it as a local failure. Nodal failure was considered as tumor recurrence in regional lymph nodes along the natural lymphatic drainage from the location of primary tumor. Distant failure was considered as tumor recurrence in uninvolved lobes or other organs.
We evaluated toxicity retrospectively from the clinical database using the Common Terminology Criteria for Adverse Events (version 4.0). Toxicities were categorized as either acute or late based upon whether they occurred within 30 days of the SBRT completion or afterwards. Patients with multiple toxicities were counted only once as having the toxicity with the highest grade. Only toxicities directly attributable to radiation treatment were included for analysis. Other adverse events that were considered to be caused by pre-existing respiratory comorbidities, such as chronic obstructive pulmonary disease exacerbation and pneumonia, were excluded.
Statistical methods
OS and PFS were assessed using the Kaplan-Meier method and log-rank tests. Local, nodal, and distant failures were evaluated by competing risks method and Gray’s tests. Potential prognostic factors were analyzed using Cox proportional hazards method. Categorical and continuous variables between two treatment cohorts were compared using Fisher’s exact test and Mann-Whitney U test, respectively.
All P values were two-sided. P values less than or equal to 0.05 were considered significant. R software version 3.3.2 (R Foundation for Statistical Computing, Vienna, Austria) was used for all the analyses.
Results
Baseline characteristics
A total of 155 patients with 159 early-stage NSCLC tumors were evaluated. Patient characteristics are described in Table 1. Of 159 tumors, 65 lesions received 30 Gy (median 30 Gy) in 1 fraction, while the remaining 94 lesions received 48-60 Gy (median 60 Gy) in 3 fractions. The SF-SBRT group proportionally had more patients with Karnofsky performance status (KPS) below 80 (p=0.05). Otherwise, characteristics were well balanced between the two treatment groups. Median age was 76.3 years old (IQR 70.5-82.3). The median smoking history in pack-years was 50 (IQR 40-75) and the median KPS was 80 (IQR 70-90). Therefore, patients were categorized by the cut off of 50 pack-years for smoking and 80 for KPS. Median size was 2.1 cm in diameter (IQR 1.5-3). The majority of lung tumors were stage 1A, clinical T1N0M0, with pathology of either adenocarcinoma or squamous carcinoma. Each treatment group had 21 patients who were medically operable but opted for SBRT (p=0.20). Malignancy was confirmed with biopsy in all patients. Each treatment group had 19 patients who were enrolled in the I-124407 clinical trial at RPCI (p=0.26).
Table 1.
Baseline characteristics
| 1 fraction (n=65) Number (%) |
3 fraction (n=94) Number (%) |
P value | |
|---|---|---|---|
| Age (years) | |||
| Median | 76.4 | 76.2 | 0.57 |
| IQR | 70.6-82.5 | 70.1-82.0 | |
| Gender | |||
| Female | 35 (54) | 47 (50) | 0.75 |
| Male | 30 (46) | 47 (50) | |
| Smoking (pack-year) | |||
| < 50 | 27 (42) | 30 (32) | 0.17 |
| ≥ 50 | 34 (52) | 62 (66) | |
| NA | 4 (6) | 2 (2) | |
| KPS | |||
| < 80 | 25 (38) | 22 (23) | 0.050 |
| ≥ 80 | 38 (58) | 69 (73) | |
| NA | 2 (3) | 3 (3) | |
| Stage | |||
| 1A | 54 (83) | 66 (70) | 0.16 |
| 1B | 10 (15) | 25 (27) | |
| 2A | 1 (2) | 1 (1) | |
| 2B | 0 (0) | 2 (2) | |
| T stage | |||
| T1 | 52 (80) | 71 (76) | 0.57 |
| T2 | 13 (20) | 23 (24) | |
| Histology | |||
| Adeno | 34 (52) | 35 (37) | 0.14 |
| Squamous | 25 (38) | 39 (41) | |
| Others | 1 (2) | 5 (5) | |
| N/A | 5 (8) | 15 (16) | |
| Size (cm) | |||
| Median | 2 | 2.2 | 0.67 |
| IQR | 1.5-3 | 1.5-3 | |
| Biopsy | |||
| Yes | 65 (100) | 94 (100) | 1 |
| No | 0 (0) | 0 (0) | |
| PET staging | |||
| Yes | 62 (95) | 92 (98) | 0.40 |
| No | 3 (5) | 2 (2) | |
| Medical operability | |||
| Inoperable | 44 (68) | 73 (78) | 0.20 |
| Operable | 21 (32) | 21 (22) | |
| Protocol | |||
| I-124407 | 19 (29) | 19 (20) | 0.26 |
| Not on protocol | 46 (71) | 75 (80) | (on protocol vs. not on protocol) |
| Follow up (months) | |||
| Median | 22.2 | 26.2 | 0.29 |
| IQR | 14.1-31.7 | 14.5-39.7 |
IQR: interquartile range; NA: not available; KPS: Karnofsky Performance Status; Adeno: adenocarcinoma; Squamous: squamous carcinoma; RTOG: Radiation Therapy Oncology Group; PET: positron emission tomography
Outcomes
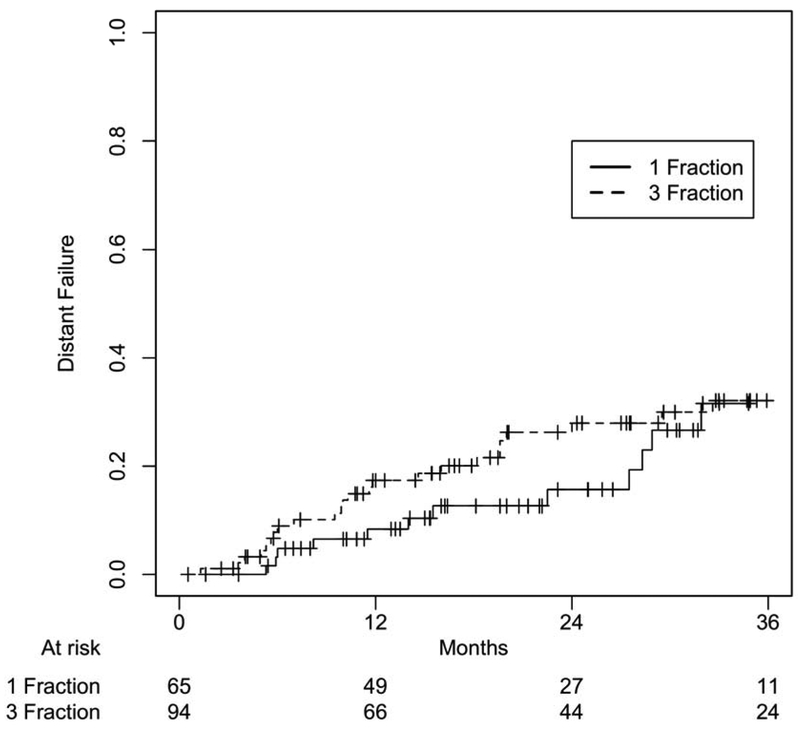
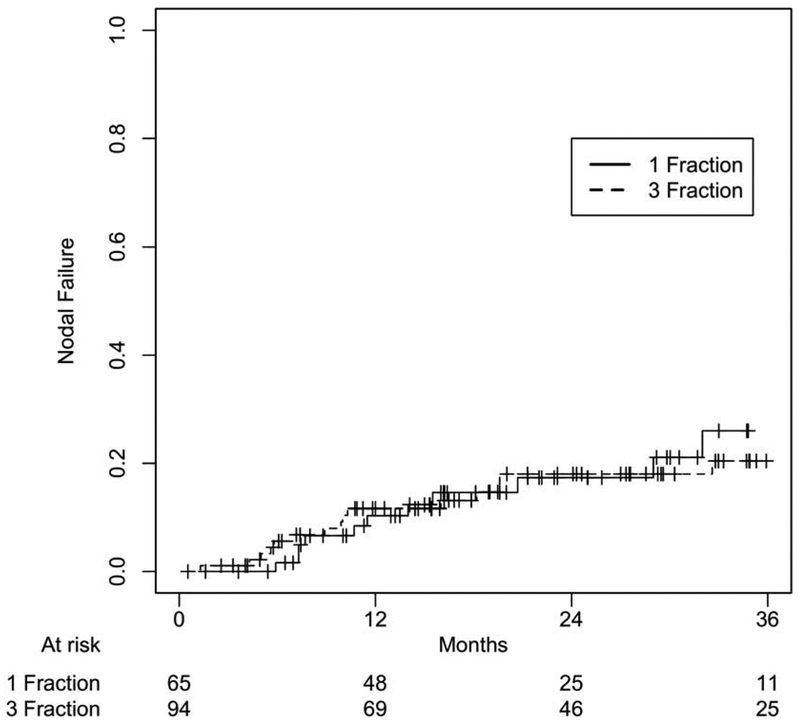
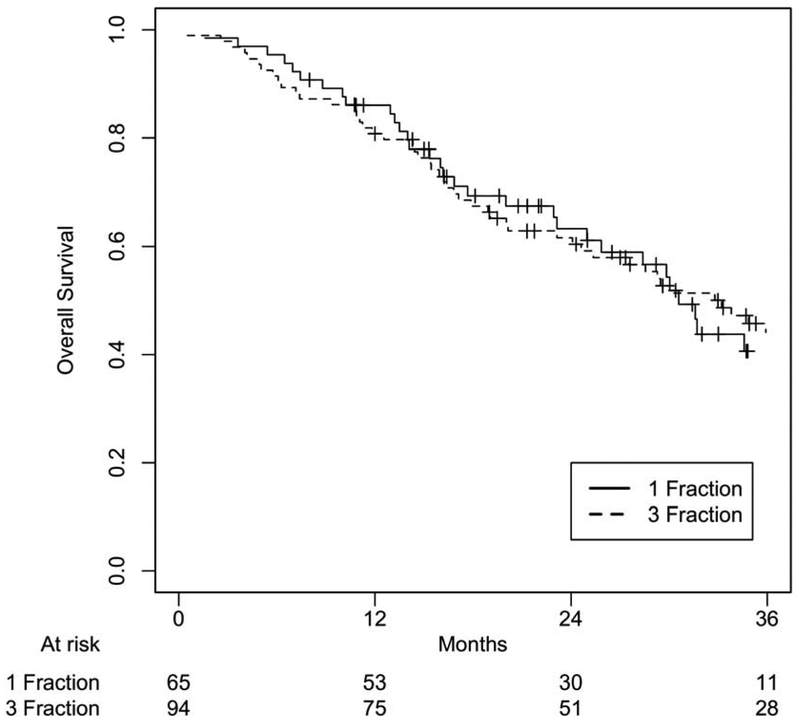
After a median follow-up of 22.2 months (IQR 14.1-31.7) for SF-SBRT and 26.2 months (IQR 14.5-39.7) for TF-SBRT groups (p=0.29), there was no statistically significant difference between these two cohorts in OS (p=0.86), PFS (p=0.95), local failure (p=0.95), nodal failure (p=0.91), and distant failure (p=0.49) at 24 months (Figure 1-4). 1- and 2-year OS rates were 86.1% and 63.2% for the SF-SBRT cohort, and 80.8% and 61.6% for the TF-SBRT cohort. 1- and 2-year local control rates were 95.1% and 87.8% for the SF-SBRT cohort, and 92.7% and 86.2% for the TF-SBRT cohort. 1- and 2-year distant control rates were 91.6% and 84.3% for the SF-SBRT cohort, and 82.6% and 72.0% for the TF-SBRT cohort.
Figure 1.
Overall Survival Based on Treatment Group. P value: 0.86.
Figure 4.
Distant Failure Based on Treatment Group. P value: 0.49.
Twenty-eight pneumonia and COPD exacerbation cases were excluded from analysis since they occurred at least 10 months after the radiation treatment and were considered to be consistent with pre-existing respiratory comorbidities. No grade 3+ pulmonary toxicity was reported within 6 months after the radiation treatment. No grade 3+ toxicity in the SF-SBRT group was reported, while only a single case of grade 3 pulmonary embolism in the TF-SBRT cohort. This case of pulmonary embolism was retrospectively re-reviewed. It was not excluded for analysis, because this patient did not have any pulmonary embolism prior to radiation therapy and a recent registry study showed that radiation therapy was associated with a higher risk for pulmonary embolism.16 Univariate analysis showed increasing tumor size as a continuous variable was associated with poor OS (HR 1.27, p=0.026), while male gender was associated with a higher risk for nodal (HR 3.11, p=0.0098) and distant failures (HR 1.99, p=0.045). Medical inoperability was not associated with poor OS (HR 1.29, p=0.26), tumor progression (HR 0.94, p=0.84), local failure (HR 0.83, p=0.68), nodal failure (HR 0.86, p=0.70), or distant failure (HR 0.79, p=0.50). Participation in a protocol was not associated with poor OS (HR 0.70, p=0.18), tumor progression (HR 0.97, p=0.93), local failure (HR 0.17, p=0.079), nodal failure (HR 1.23, p=0.63), or distant failure (HR 0.83, p=0.64).
Discussion
This retrospective review of 155 patients with peripheral early-stage NSCLC compares the clinical outcome of the SF- and TF-SBRT cohorts. To our knowledge, this is the largest comparison of these regimens reported in the literature. Despite the SF-SBRT cohort that included more patients with worse performance status, our SF- and TF-SBRT cohorts showed no significant difference in OS, PFS, local control, nodal control, distant control, or toxicity. This finding is consistent with the preliminary reports of our phase II randomized multi-institutional trial that showed equivalent OS (71% for single- and 61% for three-fraction regimens, P=0.44) and PFS (63% for single- and 51% for three-fraction regimens, P=0.99) at 2 years.12
The OS, PFS, and local control rates in the current SF-SBRT cohort were comparable to other studies.9,11-13,17,18 Outcomes of the TF-SBRT cohort was similarly comparable to prior studies.6,8,12,19-21 The distant failure rate at 1 year for our SF- SBRT cohort was 8.4%, which was lower than other studies of SF-SBRT, likely due to having smaller tumor lesions in our cohort.17,18 Larger tumor lesion has been shown to be associated with distant failure.22-24 The TF-SBRT cohort had a distant failure rate at 1 year of 17.4%, which is comparable to past studies.24-28 Both SF- and TF-SBRT regimens were well tolerated in our study, similar to those reported previously.6,8,9,11,13,20
In our study, decreasing tumor size was a favorable prognostic factor for overall survival. This finding is consistent with past studies.19,29-31 However, increasing tumor size was not associated with higher local, nodal, or distant failure rates in our study, which contrasts with those reported previously.22-24 This observation may be in part due to having a small sample size of those with T2 diseases in our analysis, only 20-24% of patients in each cohort. In our cohorts, medical inoperability was not associated with any worse clinical outcome. The Japan Clinical Oncology Group (JCOG) 0403 study similarly showed that OS and toxicities were comparable between medically operable and inoperable patients.33
In addition to the intrinsic limitations of retrospective reviews, the sample size of our patient cohorts was small and duration of follow up was short. Other variables were not collected in our database, such as cause of death, second-line treatments after the initial disease progression if offered, Charlson comorbidity index, GTV, frequency of other staging modalities such as EBUS, grade 2 toxicities, and routine pulmonary function test results.
Several prospective, multi-center clinical trials are currently awaiting publication of mature data to compare different SBRT regimens for early-stage NSCLC.11,12 In the interval, on the basis of the current analysis and the early data from I-124407, we have adopted SF-SBRT as our institutional standard.12
Conclusion
The SF- and TF-SBRT cohorts showed no significant difference in clinical outcomes. The similarity in clinical outcomes between these two schedules is notable, since patients with worse performance status tended to receive SF-SBRT. SF-SBRT is now our institutional standard regimen.
Figure 2.
Progression-Free Survival Based on Treatment Group. P value: 0.95.
Figure 3.
Nodal Failure Based on Treatment Group. P value: 0.91.
Clinical Practice Points.
Previous evidence showed that both SF- and TF-SBRT were well tolerated for early-stage medically inoperable peripheral NSCLC.
Despite the SF-SBRT cohort that included more patients with worse performance status, there was no statistical difference in clinical outcomes between both regimens.
The SF-SBRT regimen is a reasonable treatment option for early-stage peripheral NSCLC.
Acknowledgements
The authors would like to acknowledge Jennifer Meyer, PA for her efforts in collecting and maintaining clinical data.
Funding
This study was supported by an Alpha Omega Alpha Carolyn L. Kuckein Student Research Fellowship. This grant supported SJM for his 8 weeks of full-time, dedicated research during his academic year to participate in research at Roswell Park Cancer Institute as a student. This funding did not play a role in the conception and design of the study, the method of data collection and statistical analyses, the interpretation of data, nor the writing of this manuscript itself.
Abbreviation
- SF-SBRT
Single-fraction stereotactic body radiation therapy
- TF-SBRT
Three-fraction stereotactic body radiation therapy
Footnotes
Conflict of Interest
All authors declare that they have no competing interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.van Rens MT, de la Riviere AB, Elbers HR, et al. Prognostic assessment of 2,361 patients who underwent pulmonary resection for non-small cell lung cancer, stage I, II, and IIIA. Chest. 2000;117(2):374–379. [DOI] [PubMed] [Google Scholar]
- 2.Dominioni L, Imperatori A, Rovera F, et al. Stage I nonsmall cell lung carcinoma: analysis of survival and implications for screening. Cancer. 2000;89(11 Suppl):2334–2344. [DOI] [PubMed] [Google Scholar]
- 3.Nyman J, Hallqvist A, Lund JA, et al. SPACE - A randomized study of SBRT vs conventional fractionated radiotherapy in medically inoperable stage I NSCLC. Radiother Oncol. 2016;121(1):1–8. [DOI] [PubMed] [Google Scholar]
- 4.Onishi H, Shirato H, Nagata Y, et al. Hypofractionated stereotactic radiotherapy (HypoFXSRT) for stage I non-small cell lung cancer: updated results of 257 patients in a Japanese multi-institutional study. J Thorac Oncol. 2007;2(7 Suppl 3):S94–100. [DOI] [PubMed] [Google Scholar]
- 5.Chang JY, Senan S, Paul MA, et al. Stereotactic ablative radiotherapy versus lobectomy for operable stage I non-small-cell lung cancer: a pooled analysis of two randomised trials. Lancet Oncol. 2015;16(6):630–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Timmerman R, Paulus R, Galvin J, et al. Stereotactic body radiation therapy for inoperable early stage lung cancer. JAMA. 2010;303(11):1070–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang J, Yang F, Li B, et al. Which is the optimal biologically effective dose of stereotactic body radiotherapy for Stage I non-small-cell lung cancer? A meta-analysis. Int J Radiat Oncol Biol Phys. 2011;81(4):e305–316. [DOI] [PubMed] [Google Scholar]
- 8.Fakiris AJ, McGarry RC, Yiannoutsos CT, et al. Stereotactic body radiation therapy for early-stage non-small-cell lung carcinoma: four-year results of a prospective phase II study. Int J Radiat Oncol Biol Phys. 2009;75(3):677–682. [DOI] [PubMed] [Google Scholar]
- 9.Videtic GM, Stephans KL, Woody NM, et al. 30 Gy or 34 Gy? Comparing 2 single-fraction SBRT dose schedules for stage I medically inoperable non-small cell lung cancer. Int J Radiat Oncol Biol Phys. 2014;90(1):203–208. [DOI] [PubMed] [Google Scholar]
- 10.Hara R, Itami J, Kondo T, et al. Clinical outcomes of single-fraction stereotactic radiation therapy of lung tumors. Cancer. 2006;106(6):1347–1352. [DOI] [PubMed] [Google Scholar]
- 11.Videtic GM, Hu C, Singh AK, et al. NRG Oncology RTOG 0915 (NCCTG N0927): A randomized phase ll study comparing 2 stereotactic body radiation therapy (SBRT) schedules for medically inoperable patients with stage l peripheral non-small cell lung cancer. Int J Radiat Oncol Biol Phys. 2015;93(4):757–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Singh AK, Gomez J, Stephans KL, et al. A phase 2 randomized study of 2 stereotactic body radiation therapy regimens for medically inoperable patients with node-negative, peripheral non-small cell lung cancer. Int J Radiat Oncol Biol Phys. 2017;98(1):221–222. [Google Scholar]
- 13.Fritz P, Kraus HJ, Muhlnickel W, et al. Stereotactic, single-dose irradiation of stage I non-small cell lung cancer and lung metastases. Radiat Oncol. 2006;1:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Braunholtz DA, Edwards SJ, Lilford RJ. Are randomized clinical trials good for us (in the short term)? Evidence for a "trial effect". J Clin Epidemiol. 2001;54(3):217–224. [DOI] [PubMed] [Google Scholar]
- 15.Ma S, Syed YA, Rivers CI, et al. Comparison of single- and five-fraction schedules of stereotactic body radiation therapy for central lung tumors: a single institution experience. J Radiother Pract. 2017;16(2):148–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guy JB, Bertoletti L, Magne N, et al. Venous thromboembolism in radiation therapy cancer patients: Findings from the RIETE registry. Crit Rev Oncol Hematol. 2017;113:83–89. [DOI] [PubMed] [Google Scholar]
- 17.Le QT, Loo BW, Ho A, et al. Results of a phase I dose-escalation study using single-fraction stereotactic radiotherapy for lung tumors. J Thorac Oncol. 2006;1(8):802–809. [PubMed] [Google Scholar]
- 18.Hof H, Muenter M, Oetzel D, et al. Stereotactic single-dose radiotherapy (radiosurgery) of early stage nonsmall-cell lung cancer (NSCLC). Cancer. 2007;110(1):148–155. [DOI] [PubMed] [Google Scholar]
- 19.Bral S, Gevaert T, Linthout N, et al. Prospective, risk-adapted strategy of stereotactic body radiotherapy for early-stage non-small-cell lung cancer: results of a Phase II trial. IntJ Radiat Oncol Biol Phys. 2011;80(5):1343–1349. [DOI] [PubMed] [Google Scholar]
- 20.Samuels MA, Kandula S, Koru-Sengul T, et al. Stereotactic body radiotherapy in patients with stage I non-small-cell lung cancer aged 75 years and older: retrospective results from a multicenter consortium. Clin Lung Cancer. 2013;14(4):446–451. [DOI] [PubMed] [Google Scholar]
- 21.Nyman J, Johansson KA, Hulten U. Stereotactic hypofractionated radiotherapy for stage I non-small cell lung cancer--mature results for medically inoperable patients. Lung Cancer. 2006;51(1):97–103. [DOI] [PubMed] [Google Scholar]
- 22.Guckenberger M, Wulf J, Mueller G, et al. Dose-response relationship for image-guided stereotactic body radiotherapy of pulmonary tumors: relevance of 4D dose calculation. Int J Radiat Oncol Biol Phys. 2009;74(1):47–54. [DOI] [PubMed] [Google Scholar]
- 23.Baumann P, Nyman J, Lax I, et al. Factors important for efficacy of stereotactic body radiotherapy of medically inoperable stage I lung cancer. A retrospective analysis of patients treated in the Nordic countries. Acta Oncol. 2006;45(7):787–795. [DOI] [PubMed] [Google Scholar]
- 24.Baumann P, Nyman J, Hoyer M, et al. Outcome in a prospective phase II trial of medically inoperable stage I non-small-cell lung cancer patients treated with stereotactic body radiotherapy. J Clin Oncol. 2009;27(20):3290–3296. [DOI] [PubMed] [Google Scholar]
- 25.Stephans KL, Djemil T, Reddy CA, et al. A comparison of two stereotactic body radiation fractionation schedules for medically inoperable stage I non-small cell lung cancer: the Cleveland Clinic experience. J Thorac Oncol. 2009;4(8):976–982. [DOI] [PubMed] [Google Scholar]
- 26.Ricardi U, Filippi AR, Guarneri A, et al. Stereotactic body radiation therapy for early stage non-small cell lung cancer: results of a prospective trial. Lung Cancer. 2010;68(1):72–77. [DOI] [PubMed] [Google Scholar]
- 27.Bradley JD, El Naqa I, Drzymala RE, et al. Stereotactic body radiation therapy for early-stage non-small-cell lung cancer: the pattern of failure is distant. Int J Radiat Oncol Biol Phys. 2010;77(4):1146–1150. [DOI] [PubMed] [Google Scholar]
- 28.Andratschke N, Zimmermann F, Boehm E, et al. Stereotactic radiotherapy of histologically proven inoperable stage I non-small cell lung cancer: patterns of failure. Radiother Oncol. 2011;101(2):245–249. [DOI] [PubMed] [Google Scholar]
- 29.Milano MT, Zhang H, Usuki KY, et al. Definitive radiotherapy for stage I nonsmall cell lung cancer: a population-based study of survival. Cancer. 2012;118(22):5572–5579. [DOI] [PubMed] [Google Scholar]
- 30.Onimaru R, Fujino M, Yamazaki K, et al. Steep dose-response relationship for stage I non-small-cell lung cancer using hypofractionated high-dose irradiation by real-time tumor-tracking radiotherapy. Int J Radiat Oncol Biol Phys. 2008;70(2) :374–381. [DOI] [PubMed] [Google Scholar]
- 31.Beitler JJ, Badine EA, El-Sayah D, et al. Stereotactic body radiation therapy for nonmetastatic lung cancer: an analysis of 75 patients treated over 5 years. Int J Radiat Oncol Biol Phys. 2006;65(1):100–106. [DOI] [PubMed] [Google Scholar]
- 32.Spratt DE, Wu AJ, Adeseye V, et al. Recurrence Patterns and Second Primary Lung Cancers After Stereotactic Body Radiation Therapy for Early-Stage Non-Small-Cell Lung Cancer: Implications for Surveillance. Clin Lung Cancer. 2016;17(3):177–183 e172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nagata Y, Hiraoka M, Shibata T, et al. Prospective Trial of Stereotactic Body Radiation Therapy for Both Operable and Inoperable T1N0M0 Non-Small Cell Lung Cancer: Japan Clinical Oncology Group Study JCOG0403. Int J Radiat Oncol Biol Phys. 2015;93(5):989–996. [DOI] [PubMed] [Google Scholar]