Abstract
Extracellular matrix (ECM) mimicking hydrogel scaffolds have greatly improved the physiological relevance of in vitro assays, but introduce another dimension that creates variability in cell related readouts when compared to traditional 2D cells-on-plastic assays. We have developed a synthetic poly(ethylene glycol) (PEG) based ECM mimicking hydrogel and tested it against two gold standard animal-based naturally derived hydrogel scaffolds in MCF7 cell response. We have used the percent coefficient of variation (CV) as a metric to evaluate the reproducibility of said responses. Results indicated that PEG hydrogels performed similarly to naturally derived gold standards, and variance was similar in basic characterization assays, such as viability and cell adherence. PEG based hydrogels had lower CV values in estrogen receptor driven responses to several doses of estrogen in both estrogen receptor transactivation and estrogen induced proliferation.
Keywords: ECM, hydrogel, PEG, collagen, Matrigel, variance
Graphical Abstract
Introduction
The majority of breast cancers are estrogen receptor positive (ER+), and their development and progression is regulated by ER.1 ER is activated by various estrogens and estrogen mimicking compounds. Thus, it is essential to develop relevant culture methods to study ER-binding events and their effects on cancer progression.2,3
ER studies traditionally rely on 2D plastic dish cell culture assays, animal models that are expensive and ethically questionable, and/or extensive data mining from patient or population surveys. Conventional 2D assays are ideal for mechanistic studies due to their simplicity and affordability, but are often of limited value when predicting the effects of chemicals due to the lack of tumor microenvironment (TME) components (e.g., relevant stiffness and adhesion promoting cues).4–6 Cellular and non-cellular components from the TME have been shown to play a critical role in cancer development and progression, and have further been found to affect ER signaling and transactivation.7–12 To counter the lack of TME constituents, many cell-based screening assays utilize extracellular matrix (ECM) mimicking hydrogel scaffolds to increase their relevance.13–17 However, the choice of scaffold can dramatically influence assay variability and cell behavior.
ECM-mimicking materials are generally sorted into two categories of hydrogels: naturally and synthetically derived.18 Models of breast cancer that include naturally derived ECM hydrogels often include the gold standard, Matrigel, used to mimic the basement membrane of the mammary duct,19–22 and/or collagen I, the primary fibrous component immediately surrounding the mammary duct.23–25 While both of these hydrogels provide a more relevant microenvironment for in vitro models (as compared to 2D-on-plastic models), they each have shortcomings. For example, chemical characterizations of Matrigel have revealed that there are thousands of different compounds in the hydrogel, and each lot of Matrigel varies in composition.26–29 Collagen I (col I) is also derived from an animal source, so its components also vary batch-to-batch. In addition to batch-to-batch variability, the same lot of col I generates different scaffold structures depending on the neutralization pH, polymerization time, and polymerization temperature. These variables result in dramatic differences in col I fiber bundle thickness and length, ultimately affecting cellular behavior.30 Additionally, naturally derived hydrogels may contain estrogenic compounds, which can affect cellular responses to ER agonists.
Synthetically derived hydrogels are typically modular in design and the category includes several different types of polymers: poly(ethylene glycol) (PEG), hyaluronan, alginate, acrylate, dextran, and variously modified agarose monomers.13,31–34 Synthetically derived hydrogels have successfully been used for cell culture,35 and have been shown to generate well defined matrices,36 with highly tunable properties.37 Particularly, PEG derived hydrogels can be modified to include various adhesion promoting peptides, small molecule sequestration moieties, and cleavable and non-cleavable cross-linkers.33,38 Additionally, PEG hydrogels can be tailored to maintain specific stiffness or undergo reactions to weaken or stiffen the hydrogel, which can also help unravel related cellular responses.39 Despite their high versatility, PEG hydrogels have been historically less utilized because of their synthetic origin. However, synthetic hydrogels can help provide insight on cell response to ECM conditions (e.g., substrate stiffness, adhesion cues and pore size), due to the fine control they allow of architecture and mechanical properties.32,38–43 We hypothesize that a synthetically defined ECM in an ER+ breast cancer model will exhibit reduced variability in response to ER ligand 17β-estradiol (E2), when compared to models constructed with naturally derived ECMs.
To our knowledge, no direct comparison of naturally derived and synthetically derived ER+ breast cancer models has been conducted. To compare the effects of synthetically and naturally derived materials on cellular responses to ER agonists, we compared two PEG hydrogel compositions to naturally derived hydrogels, namely a col I matrix, and a col I matrix mixed with Matrigel. We chose these hydrogels because they represent some of the most commonly used hydrogels in in vitro models. For this investigation, we used a highly utilized and validated cell line: MVLN. This cell line was derived via stable transfection of MCF7 to stably express ER response element in a fusion protein with luciferin (herein, we will refer to MVLN as MCF7).44 In this cell type, ER transactivation correlates linearly with luciferase expression, as reported by us and others.45–47 We used this feature to quantify ER transactivation and multiplex assays currently utilized in breast cancer studies to compare responses to estrogen in cell number, cell viability, cell proliferation, and ER transactivation. Additionally, we evaluated batch-to-batch effects of Matrigel on estrogen induced ER transactivation by comparing three different lots of reduced growth factor Matrigel. Herein, we discuss the performance of PEG hydrogels and compare them to commonly used naturally derived hydrogels for each of the ER+ breast cancer assays, including cell adhesion and performance in ER transactivation studies. Our PEG hydrogels showed a similar performance and suitability for cell culture experiments, as well as lower variance in estrogen-driven response assays. We believe these findings could be widely applicable across the tissue-engineering field due to the multitude of choices of ECM mimicking hydrogels.
Materials and Methods
Cell Culture
MCF7 cells stably transfected with a luciferase construct just before the estrogen response element (ERE) were used in this manuscript.46 The construct reports ER agonist binding via luminescent signal, in a linear fashion.45–47 Maintenance of the MCF7 cell line was carried out in DMEM base media with 10% fetal bovine serum (FBS) (Gibco) and 1% penicillin/streptomycin (P/S) (Gibco). Cells were passaged every 3 days, or when cell confluence reached 80%. Prior to all experiments, MCF7s were cultured in in estrogen deprived media containing phenol red free DMEM (Thermo Fisher) supplemented with 10% charcoal dextran stripped FBS, 1% penicillin/streptomycin and 1% L-Glutamine (Gibco). Before conducting experiments with 17-β estradiol (E2) treatment, MCF7 cells were cultured in phenol-red free DMEM and 1% P/S without serum (referred to as serum free media) to reduce the basal response to vehicle and avoid blocking adhesion-promoting peptide activity with non-specific binding of serum.
8-Arm Star PEG-Norbornene Functionalization
The protocol for norbornene functionalization of 20kD 8-arm star poly(ethylene glycol) monomer (JenKem USA) is outlined in Schwartz et al.38 Briefly, 10g PEG-monomer, 4.8mL pyridine (Sigma-Aldrich), and 0.72g 4-(dimethylamino) pyridine (DMAP) (Aldrich), were dissolved in 120mL dichloromethane (DCM) (Fluka) in an oven dried round bottom flask equipped with a stir bar. 12.6g N,N’-Dicyclohexylcarbodiimide (DCC) (Fluka) was dissolved in 90mL DCM in a separate oven dried round bottom flask. Both flasks were purged with anhydrous nitrogen for 1 h. 15mL Norbornene acid (5-norbornene-2-carboxylic acid, Sigma-Aldrich) was added to the DCC containing flask. Contents of the PEG flask were cannula transferred to the DCC containing flask while stirring at room temperature, and the reaction continued stirring over night. The salt precipitate was separated from the solution through a medium fritted filter. The solution was then precipitated in cold 7% hexanes/diethyl ether (Sigma-Aldrich) solution and the solid product was air dried on filter paper. H1NMR was analyzed for reaction yield and was calculated at 97% functionalization.
PEG Pre-Cursor Solution Formulation
PEG hydrogel scaffold was prepared at 50mg/mL PEG monomer, 60% cross-linked, and contained 0, 2, 4, 6 or 8mM Cyclic RGDS adhesion peptide (6, and 8 mM are referred to as PEG6 and PEG8 in this manuscript), 8mM being the maximum concentration allowed by un-crosslinked PEG arms. Calculated quantities of each component of the PEG hydrogel (Table 1) were mixed in 1.5mL Eppendorf vials via vortex for at least 30 seconds: norbornene-functionalized 8-arm PEG-star monomer (JenKem USA), MMP cleavable cross-linker (KCGGPQGIAGQGCK-NH2, GenScript), Cyclic RGDS (GenScript), 1% (%w/v) Irgacure 2959 (Sigma-Aldrich) in 1x phosphate buffer solution (PBS), and 1x PBS. PEG hydrogel pre-cursor solutions were then added to μ-plates.
Table 1.
Composition of the hydrogel scaffolds used in this paper.
| PEG 6 | 41.7μL PEG-norbornene (300mg/mL), 22.5μL Cross linker peptide (133.5mM Thiol), 98.1μL CycRGDS (15.3mM), 46.0μL 1% Irgacure 2959, 41.7μL PBS Total Solution Volume: 250μL |
| PEG 8 | 41.7μL PEG-norbornene (300mg/mL), 22.5μL Cross linker peptide (133.5mM Thiol), 130.8μL CycRGDS (15.3mM), 46.0μL 1% Irgacure 2959, 9.0μL PBS Total Solution Volume: 250μL |
| Col I | 143.9μL Rat Tail Collagen I (8.34mg/mL), 7.2μL 10x PBS, 8.5μL NaOH (0.5M), 140.4μL PBS Total Solution Volume: 300μL |
| Col I + Mat | 143.9μL Rat Tail Collagen I (8.34mg/mL), 7.2μL 10x PBS, 8.5μL NaOH (0.5M), 72.2μL PBS, 68.2μL Matrigel (8.8mg/mL) Total Solution Volume: 300μL |
| Matrigel | Undiluted growth factor reduced Matrigel (3 different lots) |
PEG Hydrogel Scaffold Formation
The inner wells in a 96 μ-plate (Ibidi) were coated with 0.01% Poly(L-lysine) (Sigma-Aldrich) solution for 5 min, and then aspirated and left to dry completely. After vortexing the PEG pre-cursor solution, 8.0μL of the solution was pipetted into the bottom of each inner well. The plate was then exposed to ~10mW/cm2 UV light (source) for 2 min to crosslink the hydrogel and click the adhesion promoting peptide to the crosslink-free hydrogel arms. The PEG hydrogel scaffolds were then allowed to hydrate in PBS for 2 days, with a PBS change after 24 h.
Naturally Derived Hydrogel Scaffold Formation
Poly(ethyleneimine) (PEI) and glutaraldehyde (GA) are used to crosslink col I to the PEI/GA coated surface. The inner wells of a 96 μ-plate (Ibidi) were coated with 10μL of 0.2% PEI for 10 min and aspirated. Then, 10μL of 0.04% GA was added to the wells, allowed to react with the PEI coating for 30 min, and aspirated. Each well was then washed three times for 5 min with deionized water to remove excess GA.
Naturally derived hydrogel solutions were made by mixing 10xPBS, 0.5M NaOH for neutralization, and col I (+/− Matrigel) (Table 1). Final solution compositions for naturally derived hydrogels were: 4mg/ml Col I hydrogels and 4 mg/ml col I + 2mg/ml Matrigel hydrogels. Hydrogels containing col I were incubated on ice for 20–25 min to allow for uniform fiber formation, as seen in Sung et al.30 Immediately following the washes, 8.0μL aliquots of the hydrogel solutions were pipetted to the inner wells of the plate. Matrigel (growth factor reduced) was used undiluted after thawing for 30 minutes and using chilled tips. Hydrogels were allowed to polymerize at room temperature for 10 min, and then covered with serum free culture media and transferred to a sterile incubator at 37°C for at least 1 h prior to cell seeding.
Cell Seeding
After culturing MCF7 cells for 48 h in phenol-red free media with charcoal-stripped serum, cells were trypsinized from the culture flask with phenol-red free trypsin/EDTA (0.25mM). Cells were centrifuged, resuspended, and seeded at a concentration of 22,500 cells per well in 33μL of serum free media, then incubated at 37°C for 24 h. Culture media was then aspirated from the wells and replaced with the appropriate E2 and vehicle (0.1% ethanol) treatments.
17B-estradiol (E2) Estrogen Receptor Alpha (ER) Transactivation
ER transactivation was measured for MCF7s dosed with E2 (0.001 nM, 0.1nM, 1nM, and 10 nM) on each hydrogel subtype. These concentrations have been reported previously by us and others, and are in the physiological range for both healthy individuals and cancer patients.45,48–50 Cells were treated with E2 at different doses or vehicle control 24 h after seeding. MCF7 cells were cultured in the vehicle or treatment media for 48 h, exposed to 1mM beetle luciferin (Promega) in serum-free media and luminescence was immediately quantified on a Chemidoc (Biorad). Luminescence is linear to ER transactivation. After luminescence quantification, wells were washed once with serum-free media. For each hydrogel and E2 dose, data was fit to a nonlinear regression curve to find the EC50 (the concentration that induces a 50% response of the maximal ER transactivation). At least three independent experiments were performed, with three technical replicates each.
Proliferation Assay
Wells were filled with serum-free media containing 1mM Click-it EDU Plus (Thermo Fisher) Component A and incubated for 2 h. Then, cells were fixed in 4% paraformaldehyde for 15 min. After cell fixation, Click-it EDU Plus standard protocol for staining was followed. Plates were washed in PBS twice a day for a two-week period in order to lower the background from non-specifically absorbed Click-it EDU Plus stain. We were unable to test proliferation on cells seeded on the Matrigel lots due to the spheroid-like nature of cell growth, hence, it was not possible to obtain reliable percentages of Click-It EDU Plus or Hoechst signal.
Image Analysis: Viability
We analyzed cell viability,i.e.;3 wells per material and E2 dose. Images were taken of cells stained with ethidium bromide homodimer and Hoechst. Ethidium bromide homodimer and Hoechst images were quantified in a region of interest using a maxima finder in an automated image-processing tool: JEXperiment.51,52 The percentage of viable cells was calculated by subtracting the number of ethidium bromide homodimer positive nuclei from total the number of Hoechst positive nuclei, then divided by the number of total Hoechst positive nuclei.
Image Analysis: Click-it EDU Plus
We imaged and analyzed for estrogen-induced proliferation. Images in the Click-it EDU Plus channel (Alexa Flour 647) that exhibited high background were discarded. This procedure was done for two independent experiments, with 5 total technical replicates (individual wells) represented in the data set. Click-it EDU Plus positive nuclei and Hoechst stained nuclei were quantified in a region of interest using a maxima finder in JEXperiment. Click-it EDU Plus-positive nuclei number was divided by the number of Hoechst-positive nuclei to obtain the percentage of proliferative cells.
Percent Coefficient of Variation Calculations
With the aim of quantifying inter-assay variability, we used a normalized measure of standard deviation of the average of experimental replicates: percent coefficient of variation (CV). CV values were calculated as the standard deviation of the mean divided by the mean. CVs were calculated across all experimental replicates (i.e.; mean of technical replicates) for each material and E2 condition for each assay and reported in line graphs.
Statistical Analysis
Line fittings, CV calculations and statistical analysis were performed in Graphpad Prism 7. We pooled technical replicates and report the average and standard deviation. A ROUT’s test (1%) was used to determine outliers between technical replicates, and no outliers were detected. Normality was challenged by a Shapiro-Wilk normality test. Normally distributed data was subjected to one-way ANOVA with a multiple comparisons’ correction via Tukey post-hoc test. If the values failed the normality test, we used a Holm-Sidak post-hoc test. A two-way ANOVA was used to compare multiple groups to determine differences between material and E2 treatment with a multiple comparisons’ correction with a Tukey test. ANOVA results are reported as
| (1) |
where df = degrees of freedom.
Results
Hydrogel Composition and Experimental Setup
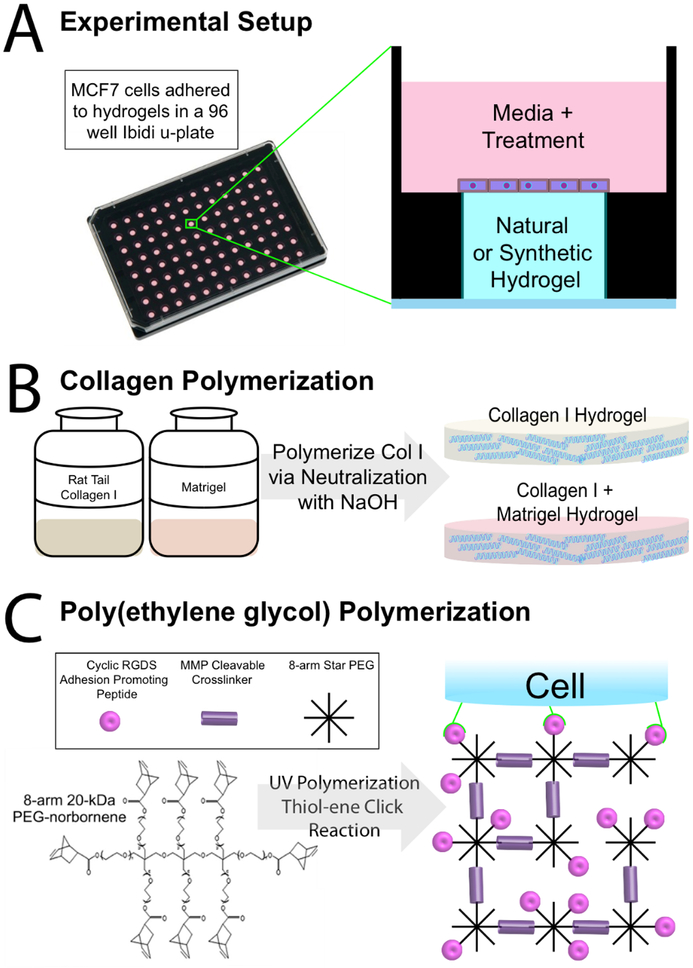
We developed synthetic hydrogel scaffolds from 8-arm star PEG hydrogel monomers functionalized with norbornene. These hydrogels are modular in construction and can incorporate any desired concentration of PEG monomer, cross-linker, and cell adhesion promoting peptides via a Michael-type addition reaction between an alkene and thiol group (Figure 1C).38,53 Our specific formulas included 50mg/mL PEG monomer, 60% cross-linked with an MMP cleavable cross-linker. We chose 60% crosslinking due to its limited swelling and more suitable resulting interfaces for cell culture (Figure S6).
Figure 1.
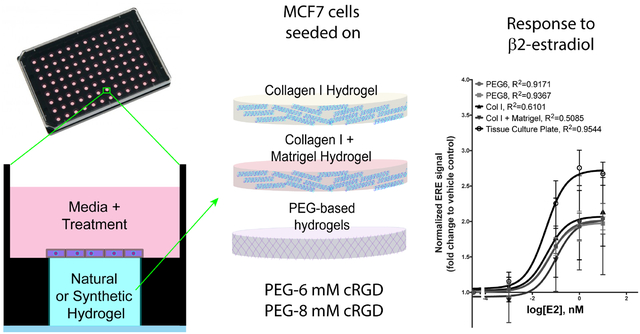
Experimental and Hydrogel Overview A. Side-view schematic of 2D cells on hydrogel culture in Ibidi 96 μ-plate, which are optimal for flat topography of hydrogels allowing for facile optical analysis. ER+ breast cancer tissue models on each of the different hydrogels are subject to 4 concentrations of E2 (17-β estradiol) or vehicle. B. Col I and col I + Matrigel are polymerized to a 4mg/mL col I concentration and a 4mg/mL col I + 2mg/mL Matrigel concentration. C. Poly (ethylene glycol) (PEG) (50mg/mL) hydrogel made by UV crosslinking 60% of arms of 8-arm star PEG monomers with an MMP cleavable cross-linker, and incorporating 6mM and 8mM concentrations of CycRGDS adhesion-promoting peptide (PEG6 and PEG8, respectively).
To identify PEG scaffolds suitable for MCF7 cell culture, we functionalized with concentrations ranging from 0–8mM of adhesion peptide (Figure S1). Particularly, we used a CRGDS peptide sequence in a cyclic conformation (CycRGDS), which contains RGD, a sequence found in several ECM fibers and promotes high rates of cell adhesion.54,55 To validate and compare our PEG hydrogels to commonly used naturally derived hydrogels, we prepared various hydrogel compositions in a 96 μ-plate (Figure 1A). Col I and Matrigel served as our naturally derived hydrogels, and two PEG based ECM compositions served as our synthetically derived hydrogels (Figure 1B). Col I and Matrigel were chosen due to their popularity for biological studies and high rates of cell adhesion (as measured via cell density after seeding).
We evaluated MCF7 cell adhesion and phenotype on top of flat hydrogels made from the different formulations of PEG hydrogel scaffolds with different concentrations of adhesion promoting peptides. From the screened conditions, PEG hydrogel scaffolds that incorporate 6mM and 8mM CycRGDS presented the highest cell density. Selected PEG hydrogel scaffolds will be referred to as PEG6 and PEG8 (6mM and 8mM of CycRGDS, respectively), for the remainder of this text.
Interestingly, while in other hydrogels cells adopted a regular monolayer conformation, in all batches of Matrigel they tended to form aggregates (see Figure S5). This effect of Matrigel has been thoroughly leveraged in the literature to produce spheroids of various cell types. While these aggregates are alive and functional (Figure S5), the cell conformation made it impossible to estimate cell nuclei counts to as we reported for all other conditions. Therefore, we decided to exclude the Matrigel-only condition from our viability and adhesion assays.
After natural and synthetic hydrogels compositions were chosen, we began screening for the effects of various concentrations of E2 (17-β estradiol) on cells on each type of hydrogel.
Cell Viability on Hydrogel Scaffolds
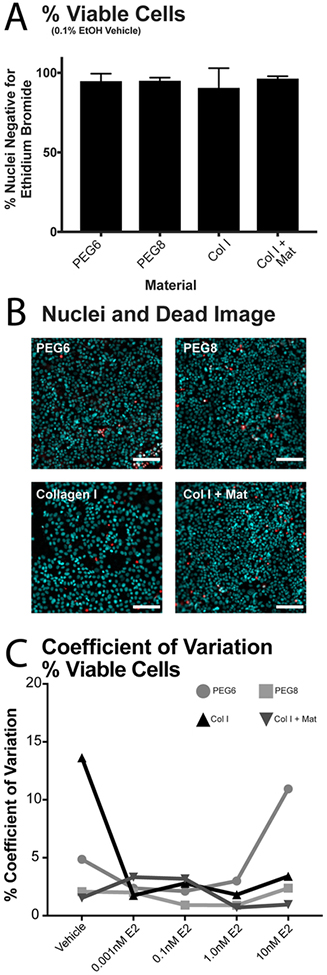
Using our selected hydrogels (PEG6, PEG8, col I and col I + mat), we performed viability experiments on all our conditions, and we compared the effects of each type of hydrogel on the viability of MCF7 cells in serum-free media (to ensure high cell viability during estrogen deprivation prior to E2 dose response experiments performed later in this article). We calculated cell viability by dividing the number of nuclei negative for ethidium bromide homodimer (a marker for dead cells) by the total number of nuclei. Representative results (vehicle control) are shown in Figure 2A, whereas the rest of the conditions can be found in Supplementary Figure 2. Results indicate that cells have at least 90% average viability with no difference in means by one-way ANOVA (F(3,8)=0.4, p=0.7) (Figure 2A). Additionally, we verified cell viability in serum free conditions for all E2 doses (Figure S2), and found no significant difference in cell viability between serum-free and serum-containing media (Figure S3). Representative images of cell viability are shown in Figure 2B.
Figure 2.
Cell viability on different hydrogel scaffolds. A. Quantification of cell viability as percentage of nuclei negative for Ethidium Bromide Homodimer. No significant differences in viability were found between all four hydrogels (vehicle) via one-way ANOVA (F(3,8)=0.4, p=0.7). Representative data from one experimental replicate under serum free vehicle control. B. Representative images of MCF7 cells on hydrogel scaffolds in serum free vehicle control media. Staining: Hoechst (cyan) and Ethidium Bromide Homodimer (red). Scale bar is 250μm. C. Coefficient of variation (CV) of viability data calculated for each hydrogel scaffold at 5 doses of E2.
Next, we performed an E2 dose response experiment, and treated MCF7 cells on our scaffolds of choice to several concentrations of E2 (0.001, 0.1, 1 and 10 nM). The first readout tested in the dose response experiment was viability in response to E2. We adapted analytical methods from the United States Food and Drug Administration (FDA) used to assess inter-assay variation.56,57 The inter-assay CV was calculated by dividing the standard deviation of the means by the mean of the means. We calculated the percent coefficient of variance (CV) for the % viable cells on each hydrogel scaffold under each E2 dosage. The CV of viability across all E2 doses on the hydrogel scaffolds remained flat and under 5% except for the extreme ends of E2 dosing (2 CV values spiked to between 10–15%). (Figure 2C)
Cell Density on Hydrogel Scaffolds
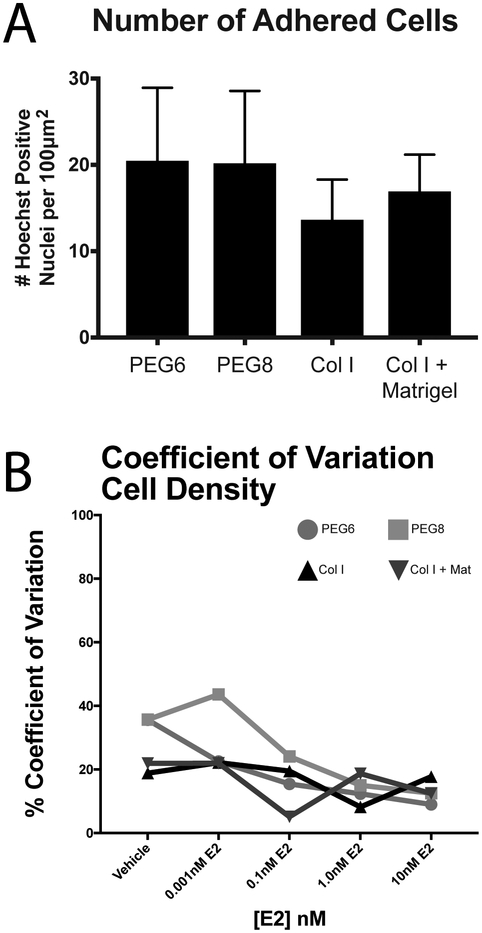
As we discussed before, cell adhesion is crucial for cell viability and function.58,59 Therefore, we compared cell adhesion on our PEG hydrogels to the subset of naturally derived hydrogels (Figure 3A), measured by quantifying the total number of cells adhered to the hydrogel scaffold (via nuclei staining). PEG6 and PEG8 were an average of 19.6 and 19.3 cells adhered per 100μm2, respectively. Cellular adhesion was comparable in col I with an average of 13.1 cells/100μm2; and col I + Matrigel with an average of 16.2 cells/100μm2. The differences were not statistically significant between all hydrogel scaffolds (F(3,44)=2.7, p=0.06). These results indicate that PEG promotes cell adhesion similarly to commonly used naturally derived hydrogels.
Figure 3.
Cell density is not affected by material choice. A. Number of adhered cells under vehicle treatment. Trend is representative across all E2 treatments. One-way ANOVA results in no statistical difference between means (F(3,44)=2.7, p=0.06). B. Calculated CV values for number of adhered cells on each hydrogel in each dose of E2. N= 4 independent experiments, with 3 technical replicates each.
To learn more about inter-assay reproducibility of our experiments in PEG versus naturally derived scaffolds, we plotted the inter-assay CV of both the number of adhered cells in Figure 3B. CV values for the number of adhered cells decreased over E2 doses on all four hydrogel scaffolds, with PEG hydrogels starting with higher values of CV at 35%, but decreased to similar values of CV compared to the col I containing hydrogel scaffold at higher doses of E2, between 10–20%.
Estrogen Receptor Alpha (ER) Transactivation on Hydrogel Scaffolds
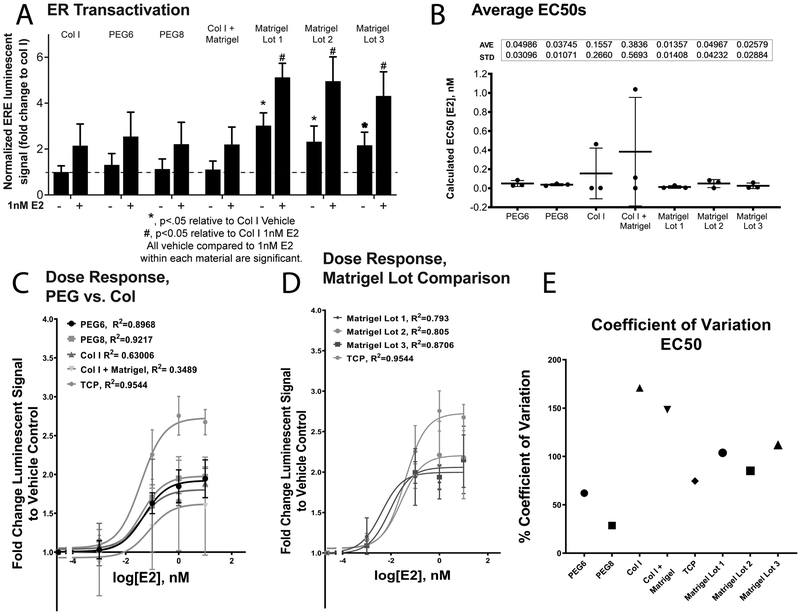
Finally, we studied the effects of E2 on MCF7 cells seeded on PEG by directly measuring ER transactivation, i.e., ER binding to estrogen response elements (ERE) of ER-driven genes. We used col I as a control, since this hydrogel is a gold standard hydrogel scaffold in the organotypic model field. We also studied three different lots of growth factor reduced Matrigel in the ER transactivation study because Matrigel is also commonly used, with differences in lot concentration overlooked. All hydrogels were exposed to a 1nM E2 or vehicle control for 48 h. Then, we measured ER transactivation (Figure 4A). All lots of Matrigel exhibited higher ER transactivation in vehicle and estrogen treated conditions when compared to the vehicle and estrogen treated conditions of collagen I. Baseline ER transactivation (vehicle control) was 3.0, 2.3, and 2.2 times higher in Matrigel lots 1, 2, and 3, as compared to col 1. Interestingly, MCF7 cells on col I + matrigel hydrogel did not show the same increase in ER transactivation as Matrigel alone hydrogels. Particularly, the baseline response (vehicle control) and 1nM E2 response were not significantly different. PEG6 and PEG8 did not show any differences when compared to col I.
Figure 4.
Fold change of ER transactivation A. ERE luminescent signal was normalized within assays and expressed as fold change increase to col I vehicle. Data illustrates the differences in baseline ER transactivation on each of the materials. All lots of Matrigel show higher baseline ER transactivation than col I containing hydrogels or PEG hydrogels. Vehicle and 1nM E2 (saturation dose) were compared. B. EC50s are calculated from ER transactivation dose response curves. Individual data points represent each experimental replicate. One-way ANOVA with Holm-Sidak post-hoc test did not disprove null hypothesis (F(6,14)=0.9, p=0.5). A & B represent average ±SD. C. Dose response (n=3 independent experimental replicates) on each of the PEG hydrogel scaffolds, col I, and col I + Mat. Standard 2D tissue culture plate data is represented on each graph for comparison (TCP). D. Dose-response curves from three Matrigel lots. Standard 2D tissue culture plate data is represented on each graph for comparison. E. CV of average EC50s on each material type.
For a more comprehensive view on the hydrogel’s effect on ER transactivation, four concentrations of E2 (0.001, 0.1, 1.0, and 10nM E2) were tested on each hydrogel scaffold (Figure S4). These concentrations were chosen due to their physiological relevance, and have been reported in previous papers.45,48 Specifically, studies show that E2 levels around 1μM are pharmacological levels, whereas 1 nM-1pM are typically physiological levels in pre- and post-menopausal women, as well as cancer patients. Therefore, the concentrations represent different physiological levels of E2.50 Estrogen-induced ER transactivation, represented as normalized dose response curves (Figure 4C&D), was not significantly different between the different hydrogels, where on each condition the fold-change relative to vehicle was approximately equal to 2.
Averages of EC50s (i.e. the concentration at which ER transactivation is at half the maximal response) were calculated for each independent experimental and then compared among different hydrogels. EC50s of the different hydrogels were not significantly different from each other when compared with a one-way ANOVA (F(6,14)=0.9, p=0.5). The average EC50s range between 0.009 nM (PEG6) and 0.1 nM (Matrigel 3). (Figure 4B).
We then calculated the CV of EC50s reported in Figure 4B to illustrate the inter-assay reproducibility of these experiments. To do this, we calculated the EC50 in each assay and averaged between assays, finally dividing by the standard deviation of the mean. This calculation provided a readout of inter-assay variation.
The CV values of the average EC50 on col I and col I + mat (171% and 148%) were at least 2.75 times as high as the CV values of the PEG hydrogel scaffolds (62% for PEG6 and 28% for PEG8). Unexpectedly, Matrigel alone resulted in CV values closer to PEG hydrogels than the collagen I containing hydrogels. However, Matrigel lots 1 & 3 CV values were twice as high as PEG based hydrogel scaffolds. CV values for Matrigel alone were 104%, 85% and 112%, respectively.
Proliferation on Hydrogel Scaffolds
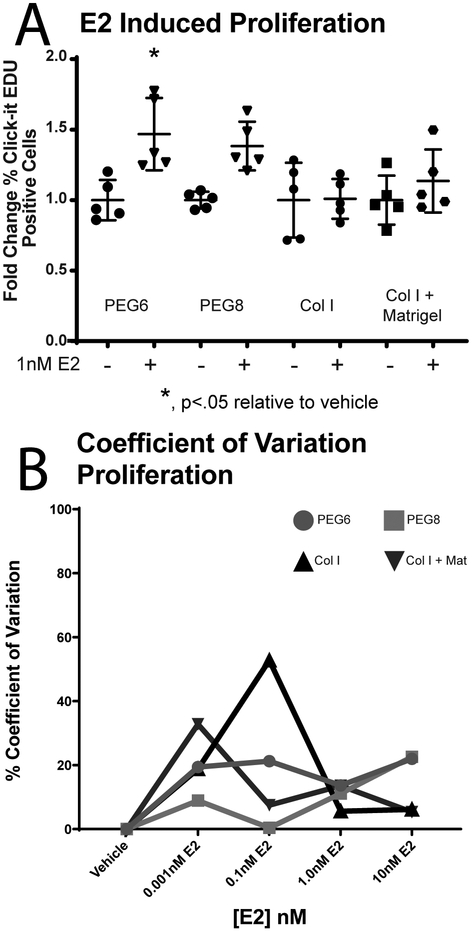
In ER+ breast cancer cells, ER transactivation stimulates proliferation.2 The Click-It Plus EDU assay40,60 (i.e., indicating number of proliferating cells) was normalized to total nuclei count to test cell proliferation on each of the PEG and naturally derived hydrogel materials (Figure 5a, complete data for all E2 concentrations in Figure S4). PEG showed an increase in fold change of proliferation with a 1.5 fold change on PEG6 and a 1.4 (non-significant) fold change PEG8. Non-significant 1.0 fold and 1.1 fold changes occurred on the collagen containing hydrogels. A two-way ANOVA performed on data from fold change resulted in a significant interaction (F(3,34)=2.9,p=0.05) between materials, with a significant difference between materials (F(3,34)=2.9, p=0.05) and E2 treatment (F(7,32)=5.134,p=0.0005).
Figure 5.
Proliferation of MCF7s on hydrogel scaffolds. A. PEG6 resulted in a significant 1.5 fold change (normal to col I veh) in proliferation between vehicle and 1nM E2 treatment. No significant fold change on PEG8 (1.4), col I (1.0) or col I + mat (1.1). Individual data points represent one technical replicate from two independent experiments. B. CV inter-experimental values of proliferation fold-change normal to col I vehicle. N=5 replicates in two independent experiments.
Proliferation normalized to col I fold change values were used to calculate inter-assay CV values in the same manner as cellular adherence (Figure 5B). Col I consistently had the highest CV values, peaking at 46.8%, and the other three hydrogel scaffolds did not exceed 33%. CV values were lowest for PEG 6 and PEG 8 at higher concentrations of E2, but the higher concentration of E2 did not lower CV values for collagen containing hydrogels.
Discussion
In vitro models have the potential to bridge gaps between fundamental studies in biology, toxicology, tissue engineering, drug discovery, and clinically relevant studies.10,16 It is known that cells on tissue culture plates show dysregulated proliferation, behavior and response to toxicants as compared to in vivo conditions (e.g., cell proliferation in tissue culture plates can happen as quickly as every 18 hours, which is not representative of in vivo behavior).45,61,62 The use of hydrogel scaffolds (lower stiffness material than tissue culture plates) for in vitro assays has demonstrated cell functions closer to in vivo conditions, such as slower cell proliferation and different drug/toxicant sensitivity.45,63,64 Therefore, ECM inclusion (i.e., hydrogel scaffolds) in in vitro models raises the physiological relevance of the biology, but an important question remains in the field: what type of ECM hydrogel mimic should be used in in vitro models? Most often, naturally derived hydrogels from animal sources are used because they are considered to recapitulate the tissue microenvironment, however, their chemical makeup is inconsistent between batches. PEG hydrogels, and other synthetically derived hydrogels, have been increasingly used in in vitro models due to their controllable chemical composition and the ability to modify their functional groups to elicit specific biological responses. However, synthetic hydrogels have not gained widespread use in the in vitro model field because they are not thought to recapitulate the function of the tissue microenvironment in its entirety. In this study, we sought to identify which hydrogel scaffolds would lower variance in endpoint quantification. For this, we chose to study an ER+ tumorigenic cell line, MCF7, transfected with a luciferase reporter for the estrogen response element, allowing us to multiplex ER+ breast cancer related assays with measurement of estrogen receptor transactivation. To evaluate the use of PEG hydrogels in estrogen-driven breast cancer research, we evaluated responses critical to the development and progression of ER+ breast cancer, ER transactivation and proliferation. We compared PEG hydrogels to naturally derived hydrogels to study whether the hydrogels influenced variance in quantification of typical model characterizations such as viability and cell adherence.
Our results indicate that the hydrogel scaffolds have no significant effect on the MCF7 cellular adherence indicating that each hydrogel scaffold is an acceptable candidate for in vitro models. Col I and Matrigel are commonly used ECM hydrogels and are known to support cell adherence. PEG, after functionalization with RGD-containing adhesion protein peptides has been shown to promote adhesion comparable to prior studies.55,65 We observed comparable cell adherence on PEG hydrogel scaffolds versus the col I containing hydrogels, countering intuition that naturally derived col I containing hydrogels would promote higher adherence. This observation, paired with data in Supp. Fig. 1, highlights the utility of the simple PEG hydrogel functionalization that allows researchers to tailor hydrogels to elicit cell adherence in varying ranges of cell number.
By observing results from the live and dead stain assay as a reading of final percent cell viability we saw no statistically significant difference in percentage of viable cells from material to material, indicating that PEG performed comparably in cell viability and adherence to naturally derived hydrogels.
Calculating CV values gives us insight into the reproducibility of the results among different experiments. Specifically, we calculated CV percentage as a normalized measure of standard deviation of the average of all experimental replicates, therefore producing only one resulting statistical value per assay.66 The results are represented graphically as one point per condition. At higher doses of E2, CV values are similar between our PEG hydrogels and the selection of naturally derived hydrogels, indicating that results are equally reproducible on each of the hydrogel scaffolds. The decrease in CV values as E2 dose increases, in both viability assays and cell adherence is likely due to MCF7 cells’ need for estrogen to survive, and not necessarily the choice of material.
We analyzed the results from ER transactivation in a similar manner to cell adherence and viability, first by directly comparing the results of the assay between each hydrogel scaffold, and then comparing the CV of the averaged results. Interestingly, the batch-to-batch comparison of ER transactivation resulted in a higher amount of ER transactivation in vehicle and 1nM E2 dose when compared to both col I containing hydrogel scaffolds and PEG hydrogel scaffolds, indicating the presence of ER binding compounds. We did not see an increase in ER transactivation on col I + mat hydrogel scaffold, when compared to col I. This is likely due to the dilution of the Matrigel from original stock concentration. Additionally, the larger pore size reported in the literature for col I + mat hydrogel scaffold could allow for ER binding compounds to be washed out of the hydrogel more easily compared to Matrigel only hydrogel scaffolds.67–69 We did not see a difference in ER transactivation in baseline (vehicle) or fold change response to 1nM E2 between any of the col I containing hydrogel scaffolds or PEG hydrogel scaffolds.
In addition to performing statistical comparisons among hydrogels for ERE activity, we also calculated the coefficient of variance between the EC50s of each independent experiment, to assess inter-experiment variance. We expected to see higher coefficients of variance in the Matrigel only data, as its variable batch-to-batch chemical composition is typically referenced as a source of variance in data. However, with the coefficients of variation being highest in col I containing hydrogels, we propose that the chemical composition is not the modulator of variable response, but that it is the variance in structural architecture that modulates cellular response, ultimately causing variance in readouts.30 Our ER transactivation data highlights the higher variance in col I containing hydrogels, but not in Matrigel only hydrogels, leaving us to hypothesize that the variability in fiber formation from the same lot of col I could be more of an influence on cellular response than variations in composition alone.30 These results suggest that full control over hydrogel composition and structure is key to data reproducibility, and point toward the use of PEG hydrogels for these assays.
An important marker in ER+ breast cancer is increased cell proliferation.2 Proliferation data collected on each of the hydrogels indicates a significant increase in fold change on PEG6, as well as a non-statistically significant increase in proliferation on PEG8. However, the proliferation data gathered on col I containing hydrogels were not significant between a saturated dose of E2 and vehicle. The no-fold change in data on the col I containing hydrogel scaffolds can be attributed to the variance in proliferative response to E2 on col I containing hydrogels. The CV values for proliferation on the col I containing hydrogels is considerably higher (at least 2-fold) for 1nM E2 dose, and generally higher in CV values for all doses of E2. We could also attribute the lack of E2 induced proliferation on the col I containing hydrogels to the concentration of the col I fibers and associated stiffness of the substrate. Many cells-on-gel E2 induced proliferation studies of MCF7 and other breast cancer cell lines have been conducted, but do not look at the effects of higher col I fiber concentration, most times only looking at increased stiffness of the hydrogels with an additive hydrogel substrate – keeping the col I fiber concentration constant. In general, E2 induced proliferation studies are difficult to conduct, as there is a tight balance between length of treatment and culture and cell death in in vitro models. e.g., the nuclear counts of all E2 concentration treatments, we do not see a significant change in cell number after 2 days of E2 treatment. The significant increase in proliferation on PEG6, even after 48 h of E2 treatment, and smaller associated CV values highlights the advantage that synthetically derived hydrogels (and more specifically PEG) can provide a more consistent hydrogel architecture, and therefore more reproducible results.
Conclusion
We have assessed the response of MCF7 cells to E2 on our PEG hydrogels and compared the results to commonly used naturally derived hydrogels, in 5 assays generally studied in ER+ breast cancer studies. However, we saw no difference in CV values for both viability assay and cell adherence at higher values of E2, suggesting that E2 presence was more important than hydrogel scaffold choice for these types of assays, and therefore presenting the use of PEG hydrogels as an alternative to naturally derived hydrogels. We found that fold change in proliferation was significantly higher on PEG6, a synthetically derived hydrogel scaffold. We have also shown that CV values among different experiments were generally higher for col I containing hydrogels in both ER transactivation and proliferation assays, assays that test for the response to ER binding compounds in vitro. The higher CV values on col I containing hydrogel scaffolds for ER transactivation and proliferation assays, assays that are extremely informative for ER+ breast cancer studies, suggest that a synthetically derived hydrogel is a good alternative for short term and high throughput studies. However, various applications for in vitro models could benefit from the lower variance reported in assays from the synthetic hydrogel models. For example, chemical toxicity testing and drug testing throughput could be increased on synthetically derived hydrogels if the number of replicates could be reduced due to the higher reproducibility of results.
While CV values were generally lower for synthetically derived hydrogels, we cannot conclude that synthetically derived hydrogels are the ultimate answer for increasing reproducibility in assays. We predict that naturally derived hydrogels will continue to have an appropriately large role in the in vitro model field due to their ability to sustain cell viability. Overall, our results indicate that either naturally or synthetically derived hydrogels are appropriate ECM mimics in organotypic models.
Supplementary Material
Figure S1: Cell Adherence to PEG hydrogel scaffolds with varying concentrations of CycRGD adhesion promoting peptide
Figure S2: Viability serum free media for all E2 doses
Figure S3: Viability in Serum-containing media versus Serum Free Media for the different hydrogel conditions used
Figure S4: Fold change proliferation data for all E2 doses
Figure S5: Phenotype of MCF7 cells on Matrigel
Figure S6: PEG hydrogel swelling optimization
Acknowledgements
The authors of this article would like to thank Gustav Heiden for chemical synthesis work that supported this project, Jose Ayuso and Jordan Ciciliano for edits and suggestions. We would also like to thank Professor Linda Schuler for her never-ending insights and encouragement in the number of meetings that shaped this document.
Funding Sources
We would especially like to thank EPA STAR Grant #83573701 for funding this research. Additional funding was provided by University of Wisconsin Carbone Cancer Center: Cancer Center Support Grant, NIH NCI P30 CA014520. Funding was also provided by: NIH NIEHS T32 ES007015-39, K99ES028744, NIH NCI R01CA186134, and NIH R01HL093282-01A1.
Footnotes
Conflicts of Interest
David J. Beebe holds equity in Bellbrook Labs LLC, Tasso Inc., Salus Discovery LLC, Lynx Biosciences Inc., Stacks to the Future LLC, Turba LLC, and Onexio Biosystems LLC. David J. Beebe is a consultant for Abbott Laboratories.
Brian P. Johnson holds equity in Onexio Biosystems LLC.
William L. Murphy is a Co-Founder and shareholder in Stem Pharm, Inc.
Supporting information statement
Supporting information includes 4 pages and 6 figures:
Sources
- (1).American Cancer Society. Breast Cancer, Facts & Figures 2017–2018. Breast Cancer Facts Fig. 2017. [Google Scholar]
- (2).Morgan MM; Johnson BP; Livingston MK; Schuler LA; Alarid ET; Sung KE; Beebe DJ Personalized in Vitro Cancer Models to Predict Therapeutic Response: Challenges and a Framework for Improvement. Pharmacol. Ther 2016, 165, 79–92. 10.1016/J.PHARMTHERA.2016.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Ali S; Coombes RC Estrogen Receptor Alpha in Human Breast Cancer: Occurrence and Significance. J. Mammary Gland Biol. Neoplasia 2000, 5 (3), 271–281. 10.1023/A:1009594727358. [DOI] [PubMed] [Google Scholar]
- (4).Imamura Y; Mukohara T; Shimono Y; Funakoshi Y; Chayahara N; Toyoda M; Kiyota N; Takao S; Kono S; Nakatsura T; et al. Comparison of 2D- and 3D-Culture Models as Drug-Testing Platforms in Breast Cancer. Oncol. Rep 2015, 33 (4), 1837–1843. 10.3892/or.2015.3767. [DOI] [PubMed] [Google Scholar]
- (5).Tasdemir N; Bossart EA; Li Z; Zhu L; Sikora MJ; Levine KM; Jacobsen BM; Tseng GC; Davidson NE; Oesterreich S Comprehensive Phenotypic Characterization of Human Invasive Lobular Carcinoma Cell Lines in 2D and 3D Cultures. Cancer Res. 2018, canres.1416.2018. 10.1158/0008-5472.CAN-18-1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Nguyen D-HT; Stapleton SC; Yang MT; Cha SS; Choi CK; Galie PA; Chen CS Biomimetic Model to Reconstitute Angiogenic Sprouting Morphogenesis in Vitro. Proc. Natl. Acad. Sci. U. S. A 2013, 110 (17), 6712–6717. 10.1073/pnas.1221526110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Lu P; Weaver VM; Werb Z The Extracellular Matrix: A Dynamic Niche in Cancer Progression. J. Cell Biol. 2012, 196 (4), 395–406. 10.1083/jcb.201102147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Frantz C; Stewart KM; Weaver VM The Extracellular Matrix at a Glance. J. Cell Sci. 2010, 123 (Pt 24), 4195–4200. 10.1242/jcs.023820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Kass L; Erler JT; Dembo M; Weaver VM Mammary Epithelial Cell: Influence of Extracellular Matrix Composition and Organization during Development and Tumorigenesis. Int. J. Biochem. Cell Biol. 2007, 39 (11), 1987–1994. 10.1016/j.biocel.2007.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Johnson KR; Leight JL; Weaver VM Demystifying the Effects of a Three-Dimensional Microenvironment in Tissue Morphogenesis. Methods Cell Biol. 2007, 83, 547–583. 10.1016/S0091-679X(07)83023-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Hansen K R and Bissell, J. Tissue Architecture and Breast Cancer: The Role of Extracellular Matrix and Steroid Hormones. Endocr. Relat. Cancer 2000, 7 (2), 95–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Weigelt B; Bissell MJ Unraveling the Microenvironmental Influences on the Normal Mammary Gland and Breast Cancer. Semin. Cancer Biol. 2008, 18 (5), 311–321. 10.1016/j.semcancer.2008.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Miroshnikova YA; Jorgens DM; Spirio L; Auer M; Sarang-Sieminski AL; Weaver VM Engineering Strategies to Recapitulate Epithelial Morphogenesis within Synthetic Three-Dimensional Extracellular Matrix with Tunable Mechanical Properties. Phys. Biol 2011, 8 (2), 026013 10.1088/1478-3975/8/2/026013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Lo AT; Mori H; Mott J; Bissell MJ Constructing Three-Dimensional Models to Study Mammary Gland Branching Morphogenesis and Functional Differentiation. J. Mammary Gland Biol. Neoplasia 2012, 17 (2), 103–110. 10.1007/s10911-012-9251-7. [DOI] [PubMed] [Google Scholar]
- (15).Grafton MMG; Wang L; Vidi P-A; Leary J; Lelièvre SA; Petersen OW; Bissell MJ; Hutmacher DW; Yu H; Davidson NE; et al. Breast On-a-Chip: Mimicry of the Channeling System of the Breast for Development of Theranostics. Integr. Biol 2011, 3 (4), 451 10.1039/c0ib00132e. [DOI] [PubMed] [Google Scholar]
- (16).Weigelt B; Ghajar CM; Bissell MJ The Need for Complex 3D Culture Models to Unravel Novel Pathways and Identify Accurate Biomarkers in Breast Cancer. Adv. Drug Deliv. Rev 2014, 69–70(1) W, 42–51. 10.1016/j.addr.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Mabry KM; Schroeder ME; Payne SZ; Anseth KS Three-Dimensional High-Throughput Cell Encapsulation Platform to Study Changes in Cell-Matrix Interactions. ACS Appl. Mater. Interfaces 2016, 8 (34), 21914–21922. 10.1021/acsami.5b11359. [DOI] [PubMed] [Google Scholar]
- (18).Deming TJ; Klok H-A; Armes SP; Becker ML; Champion JA; Chen EY-X; Heilshorn SC; van Hest JCM; Irvine DJ; Johnson JA; et al. Polymers at the Interface with Biology. Biomacromolecules 2018, 19 (8), 3151–3162. 10.1021/acs.biomac.8b01029. [DOI] [PubMed] [Google Scholar]
- (19).Shaw KRM; Wrobel CN; Brugge JS Use of Three-Dimensional Basement Membrane Cultures to Model Oncogene-Induced Changes in Mammary Epithelial Morphogenesis. J. Mammary Gland Biol. Neoplasia 2004, 9 (4), 297–310. 10.1007/s10911-004-1402-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Benton G; Kleinman HK; George J; Arnaoutova I Multiple Uses of Basement Membrane-like Matrix (BME/Matrigel) in Vitro and in Vivo with Cancer Cells. Int. J. Cancer 2011, 128 (8), 1751–1757. 10.1002/ijc.25781. [DOI] [PubMed] [Google Scholar]
- (21).Leblond CP; Inoue S Structure, Composition, and Assembly of Basement Membrane. Am. J. Anat 1989, 185 (4), 367–390. 10.1002/aja.1001850403. [DOI] [PubMed] [Google Scholar]
- (22).Soofi SS; Last JA; Liliensiek SJ; Nealey PF; Murphy CJ The Elastic Modulus of Matrigel as Determined by Atomic Force Microscopy. J. Struct. Biol 2009, 167 (3), 216–219. 10.1016/j.jsb.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Barcus CE; Keely PJ; Eliceiri KW; Schuler LA Stiff Collagen Matrices Increase Tumorigenic Prolactin Signaling in Breast Cancer Cells. J. Biol. Chem 2013, 288 (18), 12722–12732. 10.1074/jbc.M112.447631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Barcus CE; Holt EC; Keely PJ; Eliceiri KW; Schuler LA Dense Collagen-I Matrices Enhance Pro-Tumorigenic Estrogen-Prolactin Crosstalk in MCF-7 and T47D Breast Cancer Cells. PLoS One 2015, 10 (1), e0116891 10.1371/journal.pone.0116891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Gentleman E; Lay AN; Dickerson DA; Nauman EA; Livesay GA; Dee KC Mechanical Characterization of Collagen Fibers and Scaffolds for Tissue Engineering. Biomaterials 2003, 24 (21), 3805–3813. 10.1016/S0142-9612(03)00206-0. [DOI] [PubMed] [Google Scholar]
- (26).Vukicevic S; Kleinman HK; Luyten FP; Roberts AB; Roche NS; Reddi AH Identification of Multiple Active Growth Factors in Basement Membrane Matrigel Suggests Caution in Interpretation of Cellular Activity Related to Extracellular Matrix Components. Exp. Cell Res. 1992, 202 (1), 1–8. [DOI] [PubMed] [Google Scholar]
- (27).Hughes CS; Postovit LM; Lajoie GA Matrigel: A Complex Protein Mixture Required for Optimal Growth of Cell Culture. Proteomics 2010, 10 (9), 1886–1890. 10.1002/pmic.200900758. [DOI] [PubMed] [Google Scholar]
- (28).Kleinman HK; Martin GR Matrigel: Basement Membrane Matrix with Biological Activity. Semin. Cancer Biol. 2005, 15 (5), 378–386. 10.1016/j.semcancer.2005.05.004. [DOI] [PubMed] [Google Scholar]
- (29).Hughes CS; Radan L; Betts D; Postovit LM; Lajoie GA Proteomic Analysis of Extracellular Matrices Used in Stem Cell Culture. Proteomics 2011, 11 (20), 3983–3991. 10.1002/pmic.201100030. [DOI] [PubMed] [Google Scholar]
- (30).Sung KE; Su G; Pehlke C; Trier SM; Eliceiri KW; Keely PJ; Friedl A; Beebe DJ Control of 3-Dimensional Collagen Matrix Polymerization for Reproducible Human Mammary Fibroblast Cell Culture in Microfluidic Devices. Biomaterials 2009, 30 (27), 4833–4841. 10.1016/j.biomaterials.2009.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Lutolf MP; Hubbell JA Synthetic Biomaterials as Instructive Extracellular Microenvironments for Morphogenesis in Tissue Engineering. Nat. Biotechnol 2005, 23 (1), 47–55. 10.1038/nbt1055. [DOI] [PubMed] [Google Scholar]
- (32).Deming TJ Synthetic Polypeptides for Biomedical Applications. Prog. Polym. Sci 2007, 32 (8–9), 858–875. 10.1016/j.progpolymsci.2007.05.010. [DOI] [Google Scholar]
- (33).Serban MA; Prestwich GD Modular Extracellular Matrices: Solutions for the Puzzle. Methods 2008, 45 (1), 93–98. 10.1016/j.ymeth.2008.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Hoffman AS Hydrogels for Biomedical Applications. Adv. Drug Deliv. Rev 2012, 64, 18–23. 10.1016/j.addr.2012.09.010. [DOI] [PubMed] [Google Scholar]
- (35).Almany L; Seliktar D Biosynthetic Hydrogel Scaffolds Made from Fibrinogen and Polyethylene Glycol for 3D Cell Cultures. Biomaterials 2005, 26 (15), 2467–2477. 10.1016/J.BIOMATERIALS.2004.06.047. [DOI] [PubMed] [Google Scholar]
- (36).Gjorevski N; Sachs N; Manfrin A; Giger S; Bragina ME; Ordóñez-Morán P; Clevers H; Lutolf MP Designer Matrices for Intestinal Stem Cell and Organoid Culture. Nature 2016, 539 (7630), 560–564. 10.1038/nature20168. [DOI] [PubMed] [Google Scholar]
- (37).Raeber GP; Lutolf MP; Hubbell JA Molecularly Engineered PEG Hydrogels: A Novel Model System for Proteolytically Mediated Cell Migration. Biophys. J 2005, 89 (2), 1374–1388. 10.1529/BIOPHYSJ.104.050682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Schwartz MP; Fairbanks BD; Rogers RE; Rangarajan R; Zaman MH; Anseth KS A Synthetic Strategy for Mimicking the Extracellular Matrix Provides New Insight about Tumor Cell Migration. Integr. Biol. (Camb). 2010, 2 (1), 32–40. 10.1039/b912438a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Hansen TD; Koepsel JT; Le NN; Nguyen EH; Zorn S; Parlato M; Loveland SG; Schwartz MP; Murphy WL Biomaterial Arrays with Defined Adhesion Ligand Densities and Matrix Stiffness Identify Distinct Phenotypes for Tumorigenic and Non-Tumorigenic Human Mesenchymal Cell Types. Biomater. Sci 2014, 2 (5), 745–756. 10.1039/C3BM60278H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Singh SP; Schwartz MP; Tokuda EY; Luo Y; Rogers RE; Fujita M; Ahn NG; Anseth KS A Synthetic Modular Approach for Modeling the Role of the 3D Microenvironment in Tumor Progression. Sci. Rep 2016, 5 (1), 17814 10.1038/srep17814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Fairbanks BD; Schwartz MP; Halevi AE; Nuttelman CR; Bowman CN; Anseth KS A Versatile Synthetic Extracellular Matrix Mimic via Thiol-Norbornene Photopolymerization. Adv. Mater 2009, 21 (48), 5005–5010. 10.1002/adma.200901808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).Zhou W; Stukel JM; Cebull HL; Willits RK Tuning the Mechanical Properties of Poly(Ethylene Glycol) Microgel-Based Scaffolds to Increase 3D Schwann Cell Proliferation. Macromol. Biosci 2016, 16 (4), 535–544. 10.1002/mabi.201500336. [DOI] [PubMed] [Google Scholar]
- (43).Nguyen EH; Zanotelli MR; Schwartz MP; Murphy WL Differential Effects of Cell Adhesion, Modulus and VEGFR-2 Inhibition on Capillary Network Formation in Synthetic Hydrogel Arrays. Biomaterials 2014, 35 (7), 2149–2161. 10.1016/J.BIOMATERIALS.2013.11.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (44).Pons M; Gagne D; Nicolas JC; Mehtali M A New Cellular Model of Response to Estrogens: A Bioluminescent Test to Characterize (Anti) Estrogen Molecules. Biotechniques 1990, 9 (4), 450–459. [PubMed] [Google Scholar]
- (45).Morgan MM; Livingston MK; Warrick JW; Stanek EM; Alarid ET; Beebe DJ; Johnson BP Mammary Fibroblasts Reduce Apoptosis and Speed Estrogen-Induced Hyperplasia in an Organotypic MCF7-Derived Duct Model. Sci. Rep 2018, 8 (1), 7139 10.1038/s41598-018-25461-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (46).Demirpence E; Duchesne MJ; Badia E; Gagne D; Pons M MVLN Cells: A Bioluminescent MCE-7-Derived Cell Line to Study the Modulation of Estrogenic Activity. J. Steroid Biochem. Mol. Biol 1993, 46 (3), 355–364. [DOI] [PubMed] [Google Scholar]
- (47).Lee H; Lee J; Choi K; Kim K-T Comparative Analysis of Endocrine Disrupting Effects of Major Phthalates in Employed Two Cell Lines (MVLN and H295R) and Embryonic Zebrafish Assay. Environ. Res 2019, 172, 319–325. 10.1016/J.ENVRES.2019.02.033. [DOI] [PubMed] [Google Scholar]
- (48).Le Bail J-C; Champavier Y; Chulia A-J; Habrioux G Effects of Phytoestrogens on Aromatase, 3β and 17β-Hydroxysteroid Dehydrogenase Activities and Human Breast Cancer Cells. Life Sci. 2000, 66 (14), 1281–1291. 10.1016/S0024-3205(00)00435-5. [DOI] [PubMed] [Google Scholar]
- (49).Teoh H; Leung SWS; Man RYK Short-Term Exposure to Physiological Levels of 17β-Estradiol Enhances Endothelium-Independent Relaxation in Porcine Coronary Artery. Cardiovasc. Res 1999, 42 (1), 224–231. 10.1016/S0008-6363(98)00265-X. [DOI] [PubMed] [Google Scholar]
- (50).Russo J; Russo IH The Role of Estrogen in the Initiation of Breast Cancer. J. Steroid Biochem. Mol. Biol 2006, 102 (1–5), 89–96. 10.1016/j.jsbmb.2006.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (51).Warrick JW. JEX. https://github.com/jaywarrick/JEX.
- (52).Warrick JW; Timm A; Swick A; Yin J Tools for Single-Cell Kinetic Analysis of Virus-Host Interactions. PLoS One 2016, 11 (1), e0145081 10.1371/journal.pone.0145081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (53).Fairbanks BD; Schwartz MP; Halevi AE; Nuttelman CR; Bowman CN; Anseth KS A Versatile Synthetic Extracellular Matrix Mimic via Thiol-Norbornene Photopolymerization. Adv. Mater 2009, 21 (48), 5005–5010. 10.1002/adma.200901808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (54).Enemchukwu NO; Cruz-Acuña R; Bongiorno T; Johnson CT; García JR; Sulchek T; García AJ Synthetic Matrices Reveal Contributions of ECM Biophysical and Biochemical Properties to Epithelial Morphogenesis. J. Cell Biol. 2016, 212 (1), 113–124. 10.1083/jcb.201506055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (55).Weiss MS; Bernabé BP; Shikanov A; Bluver DA; Mui MD; Shin S; Broadbelt LJ; Shea LD The Impact of Adhesion Peptides within Hydrogels on the Phenotype and Signaling of Normal and Cancerous Mammary Epithelial Cells. Biomaterials 2012, 33 (13), 3548–3559. 10.1016/j.biomaterials.2012.01.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (56).Tiwari G; Tiwari R Bioanalytical Method Validation: An Updated Review. Pharm. Methods 2010, 1 (1), 25–38. 10.4103/2229-4708.72226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (57).Hanneman SK; Cox CD; Green KE; Kang D-H Estimating Intra-and Inter-Assay Variability in Salivary Cortisol. 10.1177/1099800411404061. [DOI] [PubMed]
- (58).Reddig PJ; Juliano RL Clinging to Life: Cell to Matrix Adhesion and Cell Survival. Cancer Metastasis Rev. 2005, 24 (3), 425–439. 10.1007/s10555-005-5134-3. [DOI] [PubMed] [Google Scholar]
- (59).Khalili A; Ahmad M; Khalili AA; Ahmad MR A Review of Cell Adhesion Studies for Biomedical and Biological Applications. Int. J. Mol. Sci 2015, 16 (8), 18149–18184. 10.3390/ijms160818149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (60).Nguyen EH; Zanotelli MR; Schwartz MP; Murphy WL Differential Effects of Cell Adhesion, Modulus and VEGFR-2 Inhibition on Capillary Network Formation in Synthetic Hydrogel Arrays. Biomaterials 2014, 35 (7), 2149–2161. 10.1016/j.biomaterials.2013.11.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (61).Leipzig ND; Shoichet MS The Effect of Substrate Stiffness on Adult Neural Stem Cell Behavior. Biomaterials 2009, 30 (36), 6867–6878. 10.1016/J.BIOMATERIALS.2009.09.002. [DOI] [PubMed] [Google Scholar]
- (62).Shain KH; Dalton WS Cell Adhesion Is a Key Determinant in de Novo Multidrug Resistance (MDR): New Targets for the Prevention of Acquired MDR. Mol. Cancer Ther. 2001, 1 (1), 69–78. [PubMed] [Google Scholar]
- (63).Yeung T; Georges PC; Flanagan LA; Marg B; Ortiz M; Funaki M; Zahir N; Ming W; Weaver V; Janmey PA Effects of Substrate Stiffness on Cell Morphology, Cytoskeletal Structure, and Adhesion. Cell Motil. Cytoskeleton 2005, 60 (1), 24–34. 10.1002/cm.20041. [DOI] [PubMed] [Google Scholar]
- (64).Discher DE; Janmey P; Wang Y-L Tissue Cells Feel and Respond to the Stiffness of Their Substrate. Science 2005, 310 (5751), 1139–1143. 10.1126/science.1116995. [DOI] [PubMed] [Google Scholar]
- (65).Hansen TD; Koepsel JT; Le NN; Nguyen EH; Zorn S; Parlato M; Loveland SG; Schwartz MP; Murphy WL Biomaterial Arrays with Defined Adhesion Ligand Densities and Matrix Stiffness Identify Distinct Phenotypes for Tumorigenic and Non-Tumorigenic Human Mesenchymal Cell Types. Biomater. Sci 2014, 2 (5), 745 10.1039/c3bm60278h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (66).Harmon-Jones E; Beer JS Methods in Social Neuroscience; Guilford Press, 2009. [Google Scholar]
- (67).Lieleg O; Ribbeck K Biological Hydrogels as Selective Diffusion Barriers. Trends Cell Biol. 2011, 21 (9), 543–551. 10.1016/j.tcb.2011.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (68).Anguiano M; Castilla C; Maška M; Ederra C; Peláez R; Morales X; Muñoz-Arrieta G; Mujika M; Kozubek M; Muñoz-Barrutia A; et al. Characterization of Three-Dimensional Cancer Cell Migration in Mixed Collagen-Matrigel Scaffolds Using Microfluidics and Image Analysis. PLoS One 2017, 12 (2), e0171417 10.1371/journal.pone.0171417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (69).Zaman MH; Trapani LM; Sieminski AL; MacKellar D; Gong H; Kamm RD; Wells A; Lauffenburger DA; Matsudaira P; Matsudaira P Migration of Tumor Cells in 3D Matrices Is Governed by Matrix Stiffness along with Cell-Matrix Adhesion and Proteolysis. Proc. Natl. Acad. Sci 2006, 103 (29), 10889–10894. 10.1073/pnas.0604460103. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1: Cell Adherence to PEG hydrogel scaffolds with varying concentrations of CycRGD adhesion promoting peptide
Figure S2: Viability serum free media for all E2 doses
Figure S3: Viability in Serum-containing media versus Serum Free Media for the different hydrogel conditions used
Figure S4: Fold change proliferation data for all E2 doses
Figure S5: Phenotype of MCF7 cells on Matrigel
Figure S6: PEG hydrogel swelling optimization