Abstract
Elevated allochthonous inputs of organic matter are increasingly recognized as a driver of ecosystem change in lakes, particularly when concurrent with eutrophication. Evaluation of lakes in a nutrient-color paradigm (i.e., based on total phosphorus and true color) enables a more robust approach to research and management. To assess temporal and spatial patterns in nutrient-color status for U.S. lakes and associated food web attributes, we analyzed the U.S. Environmental Protection Agency’s National Lakes Assessment (NLA) data. With 1000+ lakes sampled in 2007 and 2012 in a stratified random sampling design, the NLA enables rigorous assessment of lake condition across the continental U.S. We demonstrate that many U.S. lakes are simultaneously experiencing eutrophication and brownification to produce an abundance of “murky” lakes. Overall, “blue” lakes decreased by ~ 18% (46% of lakes in 2007 to 28% in 2012) while “murky” lakes increased by almost 12% (24% of lakes in 2007 to 35.4% in 2012). No statistical differences were observed in the proportions of “green” or “brown” lakes. Regionally, murky lakes significantly increased in the Northern Appalachian, Southern Plains, and Xeric ecoregions. Murky lakes exhibited the highest epilimnetic chlorophyll a concentrations, cyanobacterial densities, and microcystin concentrations. Total zooplankton biomass was also highest in murky lakes, primarily due to increased rotifer and copepod biomass. However, zooplankton : phytoplankton biomass ratios were low, suggesting reduced energy transfer to higher trophic levels. These results emphasize that many lakes in the U.S. are simultaneously “greening” and “browning”, with potentially negative consequences for water quality and food web structure.
Lakes are most often classified by their autotrophic status, or quantity of algal biomass, ranging from oligotrophic to hypereutrophic. This dominant classification scheme is likely driven by the global prevalence of eutrophication, or the “greening” of lakes, since the 1950s. Excessive algal biomass often results in reductions in water quality (Heisler et al. 2008; Smith and Schindler 2009) as well as food web stability (Jeppesen et al. 2000; Kuiper et al. 2015). Substantial efforts have therefore been made to reduce nutrient inputs to fresh waters (Jeppesen et al. 2005;Conley et al. 2009). However, eutrophication continues to be a systemic problem. A recent study indicated that the proportion of oligotrophic lakes in the continental U.S. decreased from 25% to 7% from 2007 to 2012, based on data from the U.S. Environmental Protection Agency’s aquatic resources survey (Stoddard et al. 2016). This trend was based on a comparison of lake total phosphorus (TP) concentrations, and the authors caution if TP concentrations continue to rise, oligotrophic lakes may soon be a rare feature on the North American landscape (Stoddard et al. 2016).
Many lakes in the northern hemisphere have also shown signs of “browning” due to the increased runoff of chromophoric (i.e., colored) dissolved organic matter (CDOM) (Solomon et al. 2015), and in some cases dissolved iron (Kritzberg and Ekström 2012), from the terrestrial watershed. The mechanisms driving brownification are debated, including changes in climate, hydrology, land use, and atmospheric sulfur deposition (Freeman et al. 2001; Monteith et al. 2007; Erlandsson et al. 2008; Haaland et al. 2010). Like eutrophication, as lakes increase in brown color, numerous complex changes occur to the physical and chemical environments that affect water quality and food web structure. For example, increased browning of oligotrophic lakes stimulates primary production through the addition of nutrients (Ask et al. 2012; Seekell et al. 2015; Solomon et al. 2015). However, excessive browning can reduce habitat quality for aquatic organisms, such as reductions in light for phytoplankton and visual predators, lower dissolved oxygen concentrations, and a shallowing of the mixing depth (Thrane et al. 2014; Solomon et al. 2015; Finstad et al. 2014). Lakes often shift from net autotrophic to net heterotrophic as increased inputs of organic matter resources stimulate increased bacterial decomposition, making them a source of CO2 to the atmosphere and contributor to global climate change (Cole et al. 1994; Larsen et al. 2011; Ask et al. 2012). In addition, lake “browning” is a human health concern as it increases the mobilization of heavy metals, such as mercury (Dittman et al. 2009), and the formation of disinfection byproducts during drinking water purification (Chow et al. 2007).
Given the possibility for lakes to “green,” “brown,” or both simultaneously, the nutrient-color paradigm represents a more appropriate classification scheme, relative to considering nutrient status alone (Williamson et al. 1999). The paradigm emphasizes both autotrophic and heterotrophic properties, identifying lakes as oligotrophic (blue), eutrophic (green), dystrophic (brown), or mixotrophic (murky) based on a lake’s total phosphorus concentration and true color. Nürnberg and Shaw (1998) and Webster et al. (2008) provide strong empirical support for the nutrient-color paradigm, noting that the effect of water color on chlorophyll a (Chl a) concentrations and Secchi depth is often independent of TP. However, these studies primarily focused on north-temperate lakes in North America. Here, we expand on this work to examine patterns in lake nutrient-color status across the conterminous U.S. (26°N–49°N) and the consequent effects on water quality and food web structure.
The fact that grazer communities often shift with algal production (Dodson et al. 2000; Jeppesen et al. 2000; Duggan et al. 2002; Pinto-Coelho et al. 2005) and that zooplankton taxa respond differentially to dissolved organic substances (Wehr et al. 1998; Cooke et al. 2015) suggests that grazer community structure likely differs with lake nutrient-color status. Some grazers specialize on only bacteria (Pourriot 1977) while others may be mechanically unable to eat bacteria but thrive on bacterivorous flagellates and ciliates (Arndt 1993). Grazers that readily consume both bacteria and algae may have a competitive advantage given the ability to switch between these two nutritional sources (Sanders et al. 1989; Berggren et al. 2014). In this study, we focus on zooplankton as they represent a central role in the aquatic food web, controlling algal abundance as well as serving as prey for higher trophic levels.
Data from the U.S. Environmental Protection Agency’s National Lake Assessment (NLA) are used to first examine the extent and magnitude of nutrient-color change in lakes between the 2007 and 2012 NLA surveys (U.S. EPA 2010, 2016). We then use data from the 2012 NLA survey to characterize differences in basal resources and zooplankton communities within the four lake types of the nutrient-color paradigm. Given the prevalence of eutrophication and brownification, we hypothesize that “blue” lakes will decrease in abundance while “green,” “brown,” and especially “murky” lakes will increase. These patterns are explored across the 48 conterminous U.S. as well as within specific ecoregions. We further hypothesize that basal pelagic resources will vary with lake nutrient-color status, and as a consequence, influence zooplankton biomass and community structure. Finally, we discuss the potential implications of changes in lake nutrient-color status for water quality and food web dynamics.
Methods
NLA dataset
The NLA is a synoptic sampling program of lakes, reservoirs, and ponds implemented across the conterminous U.S. on a 5-yr cycle. Approximately 1000 lakes are sampled in the summer (June–September) during each cycle. Lakes are selected from the National Hydrography Database (version 2; https://nhd.usgs.gov/) using a stratified randomized statistical design that stratifies based on aggregated Omernik level-III ecoregion and lake size. Natural and man-made lakes are treated equally in site selection, sampling protocols, and laboratory analyses’ methodology. From the statistical design, a total of 1028 lakes were sampled in the 2007 survey and 1038 lakes in the 2012 survey. Of these, 401 lakes were sampled in both assessment years. The 2007 NLA survey included lakes ≥4 ha in size while the 2012 NLA survey included lakes ≥1 ha. An extensive set of environmental variables was measured at each sampled lake, but we provide sampling details only for variables used in our analysis. All data are freely available online (https://www.epa.gov/national-aquatic-resource-surveys).
Field sample collections
Field sampling occurred throughout the summer, from June through September, with the average sample date ~ 2 weeks earlier in 2012 than in 2007 (i.e., average sampling date was July 17 in 2012 and July 31 in 2007; paired t-test p < 0.001). Each lake was sampled only once during each survey. Field crews use standardized sampling methods across all sites, with collections made during the morning to early afternoon. Biological and chemical samples used in this analysis were collected at a deep, open water location in each lake (i.e., ≥50 m in natural lakes and at a midpoint in reservoirs). At this location, water was collected from the photic zone of the lake (i.e., ≥50 m in lakes and at the midpoint of reservoirs) with a vertical, depth-integrated method, using a 2 m PVC tube with a 3.2 cm diameter fitted with a stopper and a valve (design by the Minnesota Pollution Control Agency;U.S. EPA 2012a, Section 5.5). Water was transferred from the integrated sampling device to a triple rinsed 4 L cubitainer. Once full, the cubitainer was inverted several times to gently mix the water sample, and then, nutrient, phytoplankton, and Chl a subsamples were taken. The cubitainer was then filled again following the same procedure to collect subsamples for general water chemistry parameters, including true color, pH, and acidneutralizing capacity.
Water quality data
Water samples for chemical analysis were placed on ice and shipped overnight to the Willamette Research Station in Corvallis, Oregon. All variables of interest were quantified at pre-specified levels of precision and accuracy, and methods were the same in both years (U.S. EPA 2009, 2012a). Detailed descriptions of all water quality analyses are found in the NLA 2012 Laboratory Operations Manual (Section 9, U.S. EPA 2012a). Briefly, (1) Chl a concentrations were measured on a fluorometer after extraction in 90% acetone, (2) nitrate-nitrite, ammonia, total nitrogen, and total phosphorus concentrations were measured with flow injection automated colorimetric analysis following sample digestion, (3) true color was estimated by visual comparison of filtered water samples to a calibrated glass color disk, (4) dissolved organic carbon (DOC) concentrations were measured using UV promoted persulfate oxidation to CO2 with infrared detection, (5) pH was measured with a ManSci PC-Titrate w/Titra-Sip autotitrator and Ross combination pH electrode, and (6) acid-neutralizing capacity was measured with automated acidimetric titration to pH < 3.5, with modified Gran plot analysis.
Phytoplankton data
After collection, phytoplankton samples were immediately preserved with Lugol’s and stored on ice in the dark until shipped to BSA Environmental Services in Beachwood, Ohio. Cells were identified, measured, and enumerated in Utermöhl sedimentation chambers under a compound microscope (U.S. EPA 2012b, Section 5.4). Phytoplankton abundance (cells mL−1) was estimated from the count data and the volume of water sampled. Cell dimension measurements in combination with phytoplankton abundance were then used to estimate the biovolume (μm3 mL−1) of each major taxonomic group.
During data processing, we removed phytoplankton orders constituting < 5% of any sample from the dataset. Of the remaining orders, genera contributing < 5% of any sample biovolume were removed. The subsequent biovolume data were converted to biomass (μg L−1 dry weight, to match zooplankton data), assuming a 20% conversion of μg L−1 wet weight to dry weight or an overall conversion factor of 10 mg wet weight : mg carbon (Strickland 1966). Phytoplankton biomass data were then used to compute zooplankton : phytoplankton biomass ratios in each lake. Only 2012 NLA phytoplankton data were used in our analysis because we were limited to using only 2012 NLA zooplankton data, as discussed below in “Zooplankton data”.
Given their potential negative effects on water quality and food web dynamics, particular attention was given to cyanobacterial densities as well as the concentration of microcystin across the four lake types. The latter was measured in unfiltered water samples using the Microtiter Plate Enzyme-Linked Immuno-Sorbent Assay (U.S. EPA 2012b, Section 3). Samples completed three freeze–thaw cycles to lyse cells before being processed using immunoassay kits purchased from Abraxis (Warminster, PA). Microcystin concentrations are considered potentially toxic to human health above 1 μg L−1 in finished drinking water and above 10 μg L−1 in recreational waters (U.S. EPA 2012b, Section 3).
Zooplankton data
We focused on zooplankton data collected during 2012 NLA because sampling protocols differed between 2007 and 2012. In 2012, field crews used a fine (50 μm) and a coarse (150 μm) Wisconsin mesh nets to collect zooplankton during the day. Both nets were towed vertically a total length of 5 m to sample the same volume in each lake. However, the number of tows varied with site depth (U.S. EPA 2012a, Section 5.5). All zooplankton samples were immediately narcotized and preserved with 95% ethanol in the field. Samples collected in Wisconsin were shipped to a state laboratory while all other samples were shipped to BSA Environmental Services. Both laboratories used standardized protocols to identify and enumerate zooplankton, which are detailed in the NLA Laboratory Operations Manual (U.S. EPA 2012b, Section 10.4).
Briefly, zooplankton were identified to the lowest level of taxonomic resolution, usually species. Identification, enumeration, and body measurements were performed under either a compound microscope (i.e., microzooplankton—rotifers and copepod nauplii) or a dissecting microscope (i.e., macrozooplankton) in a Sedgwick-Rafter cell or Ward Counting Wheel, respectively. For quality assurance among taxonomists and between laboratories, a random 10% of all samples were re-identified by independent taxonomists. Any differences among taxonomists were reconciled before the final zooplankton dataset was compiled. Zooplankton abundance was estimated from the number of individuals counted and the volume of water sampled. Zooplankton biomass was estimated based on published, standard length-width relationships (references listed in Section 10.5, U.S. EPA 2012b).
We noted that some zooplankton species were counted in both the 50 and 150 μm mesh samples. In this case, we averaged the results by sample location and taxon ID. For our analyses, we primarily focused on zooplankton biomass for the common members of the community. As with phytoplankton, we removed any zooplankton orders that constituted less than 5% of any sample. For the retained orders, we removed any genera that did not contribute at least 5% to at least one sample. These genus-level data were used for subsequent analysis, and aggregated to order-level as needed.
Lake nutrient-color status assignment
We categorized lakes following the methods of Nürnberg and Shaw (1998) and Webster et al. (2008). Lakes were classified as oligotrophic or “blue” if TP concentration ≥ 30 μg L−1 and true color ≤ 20 platinum cobalt units (PCU), eutrophic or “green” if TP > 30 μg L−1 and true color ≤ 20 PCU, dystrophic or “brown” if TP ≤ 30 μg L−1 and true color > 20 PCU, and mixotrophic or “murky” if TP > 30 μg L−1 and true color > 20 PCU.
The detection limits for total phosphorus were 3.9 and 2.9 μg L−1 in 2007 and 2012, respectively. All samples below this limit were flagged and removed from the NLA dataset by the EPA. Interestingly, no lakes were reported below detection limits in 2012 compared to 7% of samples in 2007 (Stoddard et al. 2016). True color was based on visual observations of water samples compared to a series of standards viewed through a color disk, with an estimated accuracy of 2.5 PCU (i.e., Hach Kit Model CO-1; EPA Method 110.2) (U.S. EPA 1987). Before analysis, water samples were passed through a 0.4 μm Nucleopore filter and/or centrifuged to remove any turbidity before color determination. While this method involves a subjective analysis, all color measurements were made by the same lab technician in the same laboratory in both 2007 and 2012.
We did not use DOC concentration as an estimate of true color as it was noted that many lakes with high DOC concentrations in the NLA dataset had comparatively low true color values. These systems tended to have high Chl a concentrations, suggesting large inputs of non-chromophoric, algal-derived organic carbon. In addition, some lakes with high true color values had relatively lower DOC concentrations, suggesting that increases in true color were associated with inorganic inputs, such as iron or manganese. Spectral data (e.g., a440) are not collected as part of the NLA sampling program, and therefore, could not be used as a proxy for true color.
Statistical analyses
All statistical analyses were performed in the R statistical environment (R Core Team 2017), with figures created using the “ggplot2” package (Wickham 2009). To assess potential shifts in lake nutrient-color status between 2007 and 2012, we first used the “spsurvey” package in R (Kincaid and Olsen 2016). We leveraged NLA’s stratified, randomized site selection process to develop statistically valid population estimates at continental and regional scales, using the entire datasets of lakes ≥ 4 ha in 2007 and 2012, equivalent to 1028 lakes in 2007 and 950 lakes in 2012. Population estimates were developed using a weighted Horvitz-Thompson estimation, with a local mean variance estimator to calculate 95% confidence intervals around the estimate for each assessment period (Lohr 1999; Stevens and Olsen 2003). We calculated change between the 2007 and 2012 populations at the national scale and within each of nine aggregate III ecoregions (Omernik 1987) using the change.analysis function in “spsurvey” (Kincaid and Olsen 2016).
We further analyzed shifts in lake nutrient-color status using the 401 lakes sampled in both 2007 and 2012, all of which were ≥ 4 ha in size. Unlike the dataset as a whole, these lakes were not purposefully selected to be representative of the entire population of lakes in the conterminous U.S. Nevertheless, we used them in our analysis to investigate changes in lake nutrient-color status within individual lakes over time. Each pair of points from each resampled lake was treated as a mathematical vector, calculating its magnitude and angle of direction. Using the “circular” package in R (Agostinelli and Lund 2017), we plotted the angles of direction on a rose diagram, calculated the mean angle of direction and bootstrapped the 95% confidence intervals with 999 permutations, and then performed a Rao Spacing Test to determine if the angles of direction were non-random. In addition, one-way ANOVAs were performed to assess potential differences in the angle of direction and magnitude of change between the four lake types. However, these ANOVA results should be assessed with caution given the differences in sample sizes: 204 blue lakes, 111 green lakes, 66 murky lakes, and 20 brown lakes (based on initial lake nutrient-color status in 2007).
We used the NLA 2012 dataset to investigate the effects of lake size and watershed characteristics on lake nutrient-color status as well as differences in food web structure across the four lake types. In total, there were 1013 lakes that fit our criteria for TP, color, phytoplankton, and zooplankton data. This set included 85 lakes 1–4 ha in surface area. Non-parametric tests were performed due to lack of normality and heteroscedasticity in the dataset. Given the overall number of statistical comparisons (i.e., 14 total Kruskal–Wallis tests described below), we adjusted alpha values using the Bonferroni correction.
Kruskal–Wallis tests in combination with the Dunn’s multiple comparison test, using the FSA package (Ogle 2017), were performed to determine whether lakes of varying nutrient-color status differed in the percentage of urban development, agriculture, forest cover, and wetlands within their watersheds as well as in total watershed area and lake area. To assess basal pelagic production, we used Kruskal–Wallis–Dunn tests to detect significant differences in Chl a concentrations, total phytoplankton biomass, the density of blue-green algae, and the concentration of microcystin toxin. To assess zooplankton production, we also used Kruskal–Wallis–Dunn tests to detect potential significant differences in zooplankton biomass with lake type, including comparisons of rotifer, copepod, cladoceran, and total biomass.
To visualize potential differences in zooplankton community structure associated with differences in lake nutrient-color status, we used non-metric multidimensional scaling (NMDS), both at the order- and genus-level. Permutational multivariate analysis of variance (PERMANOVA) was then used to test for significant differences in zooplankton community composition across lake nutrient-color status. Both the NMDS and PERMANOVA were run based on zooplankton density and biomass, using the “vegan” package in R (i.e., metaMDS and adonis functions, respectively) (Oksanen et al. 2017). The metaMDS automatically applies a square-root transformation and calculates Bray–Curtis dissimilarity distances.
Because many data points occurred near the intersection of the lake type benchmarks, we further investigated differences in zooplankton community biomass in “extreme” lakes within each nutrient-color lake type. Extreme was defined as follows: for blue lakes, values below first quartile for both TP (10 μg L−1) and color (7.5 PCU); for green lakes, below first quartile for color (11 PCU) and above third quartile for TP (124 μg L−1); for brown lakes, values above third quartile for color (44 PCU), and below first quartile for TP (15 μg L−1); and for murky lakes, values above third quartile for both TP (302 μg L−1) and color (38 PCU).
Results
Shifts in lake nutrient-color status: population estimates
At the continental-scale of the U.S., the proportion of blue lakes significantly decreased by 18%, from 45.7% of the population of lakes in 2007 to 27.7% of the population of lakes in 2012 (Table 1; Fig. 1). In contrast, the proportion of murky lakes increased by ~ 12%, from 23.5% in 2007 to 35.4% of the population in 2012 (Table 1; Fig. 1). There were no statistical differences in the proportions of brown or green lakes from 2007 to 2012 (Table 1; Fig. 1). Geographically, when the lakes were analyzed within each of the nine Omernik III ecological regions, we observed that: (1) in the Northern Appalachians, blue lakes decreased by 41.4%, brown lakes increased by 17.8%, and murky lakes increased by 26.8%, (2) in the Northern Plains, green lakes significantly increased by 18.9%, (3) in the Southern Plains, blue lakes significantly decreased by 25.6% and murky lakes increased by 29.8%, (4) in the Upper Midwest, blue lakes significantly decreased by 25%, and (5) in the Xeric region, green lakes significantly decreased by 30% while murky lakes increased by 34.2% (Table 1; Fig. 1). No significant changes were observed in the Coastal Plains, Southern Appalachians, Temperate Plains, or Western Mountains ecoregions (Table 1; Fig. 1). By 2012, murky lakes represented the most abundant lake type in the dataset (~ 35%), followed by blue lakes (~ 28%), green lakes (~ 27%), and brown lakes (~ 10%), based on 1013 lakes (i.e., includes lakes 1–4 ha).
Table 1.
Number of lakes classified in each lake nutrient-color category during the 2007 and 2012 NLA surveys (n = 1028 and 950 lakes, respectively), and in parentheses, the estimated percent of lakes on the landscape represented by the surveyed lakes (± standard error [SE]) within an ecoregion and at the national scale. These combined values were used in the population estimate analysis to determine shifts in lake nutrient-color status at the national and regional scale between 2007 and 2012. Ecoregions represent the nine aggregate III ecoregions designated by the EPA. No lakes were classified as “brown” in the Northern Plains in either 2007 or 2012. Only lakes > 4 ha were included in the analysis, which reduced the total number of lakes in the 2012 dataset.
| Blue |
Green |
Brown |
Murky |
|||||
|---|---|---|---|---|---|---|---|---|
| 2007 | 2012 | 2007 | 2012 | 2007 | 2012 | 2007 | 2012 | |
| Ecoregion | ||||||||
| Coastal plain | 20 (19.8±4.5) | 17 (18.7±5.4) | 37 (29.4±6.7) | 30 (23.9±5.8) | 5 (3.9±3.9) | 10 (5.3±5.3) | 39 (46.8±7.2) | 59 (52.1 ±6.9) |
| Northern Appalachian | 64 (75.1 ±4.9) | 36 (34.0±7.7) | 11 (8.7±2.3) | 6 (5.2±2.1) | 15 (14.1 ±3.9) | 39 (31.9±6.8) | 3 (2.13±1.2) | 9 (28.9±12.8) |
| Southern Appalachian | 76 (62.7±8.3) | 41 (48.2±9.4) | 32 (25.3±7.7) | 19 (28.1 ±7.0) | 1 (0.16±0.14) | 2 (5±3.1) | 11 (11.8±5.4) | 16 (18.7±5.2) |
| Northern Plains | 13 (7.8±5.1) | 7 (4.2±1.6) | 18 (16.5±5.4) | 21 (35.4±9.3) | – | – | 34 (75.8±7.6) | 43 (60.3±9.2) |
| Southern Plains | 38 (44.3±9.3) | 12 (18.7±10.5) | 50 (30.7±9.1) | 28 (25.4±11) | 3 (2.1 ±1.7) | 6 (3.2±2.1) | 37 (22.9±7.2) | 39 (52.7±15) |
| Temperate Plains | 28 (16±5.4) | 25 (14.6±5.8) | 76 (35.4±7.3) | 50 (28.7±6.5) | 2 (10.7±7.5) | 2 (0.15±0.1) | 32 (37.7±7.6) | 60 (56.5±8.8) |
| Upper Midwest | 93 (58.2±5.4) | 42 (33.1 ±7.7) | 20 (11.2±3.8) | 30 (19.8±5.5) | 15 (17.5±3.9) | 27 (31.7±9.1) | 17 (13.1 ±3.7) | 32 (15.4±3.6) |
| Western Mountains | 107 (58.7±8.4) | 74 (50.6±12.4) | 27 (18.4±6.9) | 34 (9.3±2.7) | 11 (12.2±4.7) | 11 (28.7±15.4) | 7 (10.6±6.3) | 31 (11.3±3.8) |
| Xeric | 38 (37.5±7.1) | 20 (30.4±9.3) | 36 (52.5±7.9) | 41 (22.5±5.4) | 2 (0.29±0.18) | 4 (3.2±1.7) | 10 (9.7±2.8) | 27 (43.9±10.2) |
| National | 477 (45.7±2.7) | 274 (27.7±3) | 307 (20.8±2.2) | 259 (20.6±2.4) | 54 (10.0±1.7) | 101 (16.4±3.2) | 190 (23.5±2.3) | 316 (35.4±3.6) |
Fig. 1.
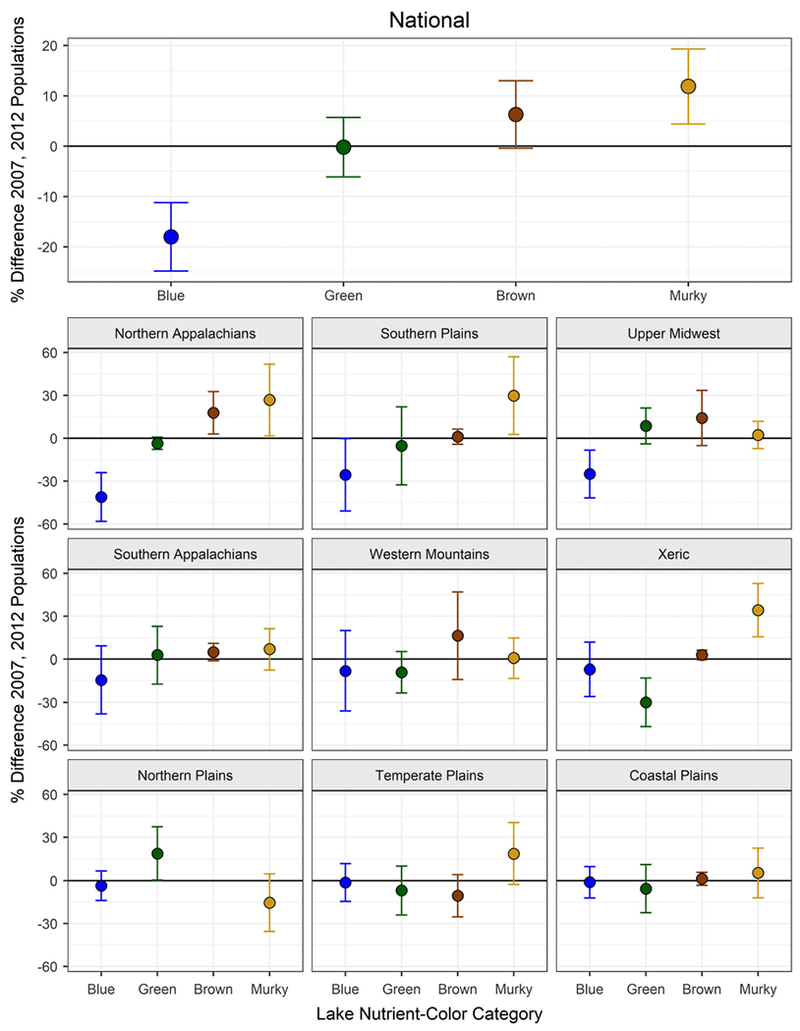
Change in nutrient-color status across the population of lakes ≥4 ha in the continental U.S. (national) and in nine aggregated Omernik level-III ecoregions (n = 1028 in 2007 and n = 950 in 2012). Each point represents the percent difference in the population within a nutrient-color category between 2007 and 2012. The population of lakes within a category can increase (above zero), remain the same, or decrease (below zero). The bars around the point represent the 95% confidence interval associated with that change. A change is statistically significant if the 95% confidence interval does not include zero.
Shifts in lake nutrient-color status: temporal trends in resampled lakes
Examining only the 401 resampled lakes, 64.8% remained in the same lake class and 35.2% changed class between 2007 and 2012 across the U.S. (Figs. 2a,b, 3). For the lakes that changed class, most shifted to green or murky status (Table 2). The mean angle of direction of change for all resampled lakes was 32.68° with a 95% confidence interval of 26.36°ߝ39.53° (Fig. 2c). For reference, an angle of 45° would indicate that a lake was increasing in equal proportions of TP and color. Thus, both total phosphorus and color increased in the majority of resampled lakes between 2007 and 2012, with proportionally greater increases in TP. The Rao spacing test confirmed that these shifts were significantly different from random (T-statistic = 204.47, p < 0.001). There were no significant differences in the angle of direction among the four lake classes (F = 2.013, df = 3, p = 0.11). However, there were significant differences in magnitude between lake classes (F =17.11, df = 3, p < 0.001), with murky lakes exhibiting the largest magnitudes of change (median = 59.54; range = 2–2364) and blue lakes exhibiting the smallest (median = 15.18; range = 1.3–166). The median magnitude of change for green lakes was 37.33 (range = 2.8–534) and 22.66 for brown lakes (range = 2.2–126). The large magnitude of change in murky lakes rarely led to a shift in nutrient-color status. Only 10 murky lakes displayed shifts: 8 to green, 2 to brown, and 0 to blue (Table 2).
Fig. 2.
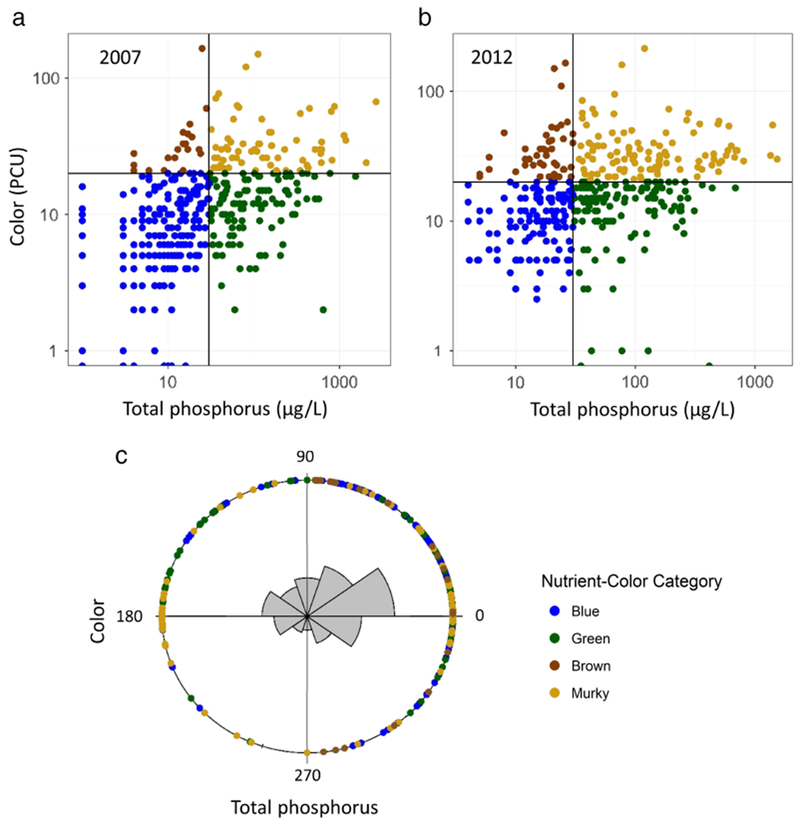
(a-c) Nutrient-color status of lakes in 2007 (a) and 2012 (b) and the direction of change between the two sampling years (c), based on the 401 resampled lakes in the NLA datasets. Panel C is a rose diagram of the angle of direction for the vector between each pair of points (i.e., a lake’s TP and color concentration in 2007 and 2012). Total phosphorus is on the x-axis and water color on the y-axis. The mean angle of direction was 32.68°, with a 95% confidence interval of 26.36°−39.53°, suggesting that most lakes increased in TP and color between the two NLA surveys. The colored dots around the circle depict the angle of direction for each individual lake. Colors represent a lake’s nutrient-color status in 2007. Note that fewer dots are located in the lower right quadrant, or toward blue (i.e., decreasing in TP and color).
Fig. 3.
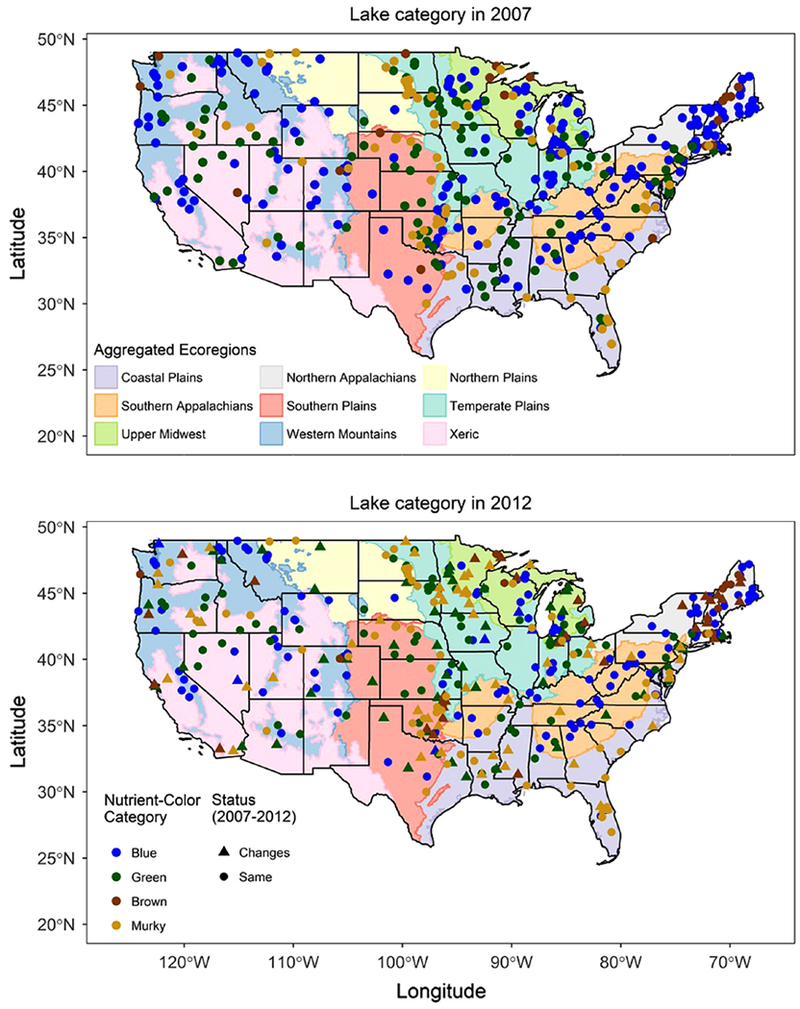
Location and nutrient-color status of the 401 lakes sampled in both the 2007 and 2012 NLA surveys overlaid on the nine Omerick III ecoregions across the U.S. nutrient-color status for all 401 lakes in 2007 are shown in the top panel; the bottom panel distinguishes lakes which changed nutrient-color status from those that remained the same.
Table 2.
Changes in true color and TP between the 2007 and 2012 NLA surveys for the 401 resampled lakes as well as information on lake size and watershed attributes. Number of lakes that changed lake class or remained in the same class are also provided.
| Number of lakes | Blue to blue |
Blue to brown |
Blue to green |
Blue to murky |
Brown to blue |
Brown to brown |
Brown to murky |
Green to blue |
Green to brown |
Green to green |
Green to murky |
Murky to brown |
Murky to green |
Murky to murky |
|
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 124 | 26 | 43 | 11 | 3 | 11 | 6 | 3 | 6 | 69 | 33 | 2 | 8 | 56 | ||
| Change in color (PCU) | Min | −13 | 2 | −8 | 6 | −16 | −2 | −97 | −1 | 5 | −20 | 3 | −2 | −19 | −121 |
| Mean | 3.79 | 18.85 | 3.16 | 19.36 | −11.33 | 32.91 | −10.5 | 2.67 | 15.83 | 2.63 | 15.76 | 9.5 | −10.13 | 6.3 | |
| Median | 4 | 17.5 | 5 | 15 | −15 | 11 | 1.5 | 1 | 15 | 4 | 12 | 9.5 | −9.5 | 5 | |
| Max | 16 | 38 | 14 | 44 | −3 | 126 | 52 | 8 | 35 | 18 | 46 | 21 | −2 | 174 | |
| Change in TP (μg L−1) | Min | −10 | −15 | 10 | 11 | 4 | 2 | 6 | −47 | −343.1 | −360 | −154 | −11 | −286 | −2364 |
| Mean | 7.52 | 7.31 | 35.33 | 47.18 | 9 | 5.27 | 33.83 | −33.67 | −74.63 | 2.81 | 35.03 | −6.5 | −29 | −60.66 | |
| Median | 8 | 9 | 29 | 27 | 11 | 4 | 25 | −33 | −23.5 | 13 | 36 | −6.5 | −4.5 | 5.5 | |
| Max | 20 | 21 | 111 | 166 | 12 | 16 | 104 | −21 | −11 | 262 | 534 | −2 | 92 | 780 | |
| Lake area (ha) | Min | 6.21 | 7.76 | 8.21 | 13.84 | 72.28 | 12.02 | 4.72 | 50.17 | 14.49 | 10.59 | 11.75 | 42.06 | 20.67 | 5.01 |
| Mean | 2997.53 | 372.84 | 939.98 | 107.04 | 127.9 | 87.65 | 94.97 | 2973.92 | 221.61 | 897.48 | 1688.41 | 72.76 | 1219.56 | 3729.29 | |
| Median | 90.04 | 82.11 | 195.8 | 84.03 | 127.9 | 43.16 | 49.5 | 88.5 | 112.27 | 170.24 | 125.94 | 72.76 | 29.23 | 48.33 | |
| Max | 125,497.42 | 4212.86 | 6559.39 | 533.12 | 183.52 | 282.88 | 382.94 | 8783.07 | 731.02 | 10,363.26 | 19,994.28 | 103.45 | 9530.15 | 167,489.61 | |
| % agriculture in watershed | Min | 0 | 0 | 0 | 0.21 | 0.29 | 0 | 0 | 2.39 | 0.05 | 0 | 0 | 0 | 1.25 | 0 |
| Mean | 10.27 | 8.66 | 23.59 | 19.5 | 31.43 | 0.74 | 0.79 | 29.44 | 10.87 | 30.33 | 32.75 | 3.32 | 34.98 | 26.53 | |
| Median | 2.38 | 1.84 | 12.92 | 12.24 | 31.43 | 0.04 | 0.38 | 28.63 | 5.55 | 21.68 | 30.35 | 3.32 | 39.1 | 19.38 | |
| Max | 64.62 | 70.08 | 74.41 | 67.6 | 62.57 | 4.4 | 3.4 | 57.31 | 27.4 | 87.97 | 82.8 | 6.63 | 62.86 | 81.78 | |
| % developed in watershed | Min | 0 | 0 | 0.45 | 0.08 | 0 | 0 | 0 | 6.81 | 2.27 | 0 | 0 | 2.39 | 0 | 0 |
| Mean | 12.14 | 6.84 | 7.46 | 12.34 | 3.15 | 2.55 | 0.8 | 23.69 | 9.84 | 8.79 | 8.87 | 3.32 | 6.48 | 7.34 | |
| Median | 4.61 | 3.83 | 6.04 | 10.06 | 3.15 | 1.08 | 0.61 | 11.47 | 5.47 | 3.71 | 4.41 | 3.32 | 4.18 | 4.13 | |
| Max | 88.96 | 36.69 | 59.21 | 38.13 | 6.29 | 10.3 | 2.16 | 52.78 | 35.23 | 91.04 | 72.71 | 4.25 | 21.07 | 59.1 |
Overall, 75% of the resampled lakes increased in TP concentrations, 73% increased in color, and 56% increased in both TP and color between 2007 and 2012 (Fig. 4). Changes in total phosphorus in blue lakes ranged from −15.0 μg L−1 to +166 μg L−1 between 2007 and 2012 whereas changes in color ranged from −13.0 PCU to +44.0 PCU (Table 2). These increases often tipped lakes from blue to green, brown, or murky (Table 2; Fig. 2a–c) and account for the decrease in blue lakes since 2007. In green lakes, changes in total phosphorus ranged from −360 μg L−1 to +534 μg L−1 whereas changes in color ranged from −20.0 PCU to +46.0 PCU between 2007 and 2012. Consequently, many lakes classified as green in 2007 became murky by 2012 (Table 2; Fig. 2c). Within brown lakes, total phosphorus only increased, ranging from +2.0 μg L−1 to +104.0 μg L−1, while changes in color ranged from −97.0 PCU to +126.0 PCU. Murky lakes had the widest range of change in total phosphorus, from −2364 μg L−1 to +780 μg L−1 (Table 2). Given the initially high TP concentrations for these lakes, the impact on lake classification was negligible. Changes in color in murky lakes ranged from −121 PCU to +174 PCU (Table 2).
Fig. 4.
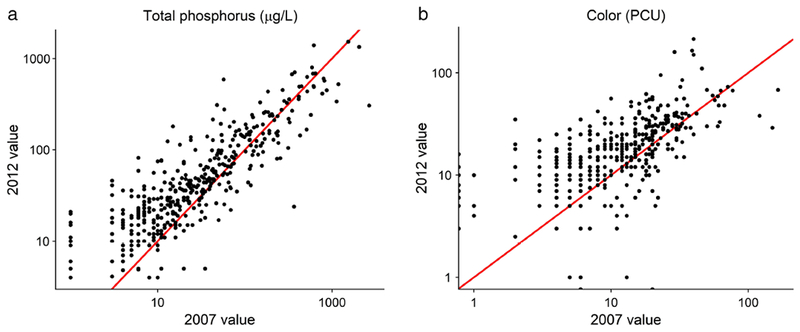
Comparison of total phosphorus concentrations (a) and true color (b) in the 401 lakes sampled during both the 2007 and 2012 NLA surveys plotted on a log scale. The red line represents the 1 : 1 ratio or zero change. Most lakes were observed to increase in total phosphorus and color between 2007 and 2012.
Land use/land cover, watershed size, and lake class
When examining percent land cover in the 2012 NLA dataset, blue and brown lakes had significantly more forest within their watersheds (KW chi-squared (χ2) = 157.87, df = 3, p < 0.0001) while green and murky lakes had a greater percentage of agriculture (KW χ2 = 85.663, df = 3, p < 0.0001) (Table 3). Brown and murky lakes had significantly more wetlands within their watersheds compared to blue and green lakes (KW χ2 = 55.527, df = 3, p < 0.0001) (Table 3). No significant differences in urban development were observed across the lake types (Table 3). In terms of total watershed area, blue and green lakes possessed significantly larger watersheds compared to brown and murky lakes in the 2012 NLA dataset (KW χ2 = 23.51, df = 3, p < 0.0001) (Table 3).
Table 3.
Attributes of 1013 lakes from the 2012 NLA survey which could be classified by lake class (i.e., reported total phosphorus concentration and water color).
| Blue (284 lakes) |
Brown (109 lakes) |
Green (273 lakes) |
Murky (347 lakes) |
|||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Attribute | Min | First quartile | Median | Third quartile | Max | Min | First quartile | Median | Third quartile | Max | Min | First quartile | Median | Third quartile | Max | Min | First quartile | Median | Third quartile | Max |
| Physical characteristics | ||||||||||||||||||||
| Latitude (decimal degrees) | 27.21 | 38.6 | 41.3 | 44.33 | 48.96 | 30.97 | 40.68 | 42.95 | 45.33 | 48.2 | 29.16 | 37.21 | 40.99 | 44.31 | 48.96 | 26.07 | 36.69 | 41.39 | 45.5 | 48.99 |
| Longitude (decimal degrees) | −123.28 | −111.61 | −89.94 | −83.29 | −67.7 | −124.23 | −94.36 | −84.6 | −71.81 | −67.2 | −124.05 | −109.86 | −96.84 | −88.72 | −70.66 | −123.93 | −101.58 | −96.37 | −87.47 | −70.89 |
| Area (ha) | 1.08 | 12.62 | 40.96 | 151.01 | 125,497 | 1.03 | 11.04 | 22.72 | 82.06 | 4212.86 | 1.16 | 12.92 | 38.09 | 211.43 | 46,299.7 | 1.1 | 9.35 | 22.44 | 71.35 | 167,490 |
| Elevation (masl) | 1.96 | 212.74 | 322.98 | 1259.52 | 3594.97 | 2.15 | 132.13 | 271.75 | 403.14 | 3032.18 | 0 | 198.02 | 365.04 | 912.18 | 3530.52 | −53.27 | 122.5 | 346.17 | 635.27 | 3105.21 |
| Temperature, average 0–2 m (°C) | 9.69 | 19.86 | 23.9 | 26.69 | 32.02 | 10.32 | 21.1 | 23.66 | 26.95 | 88.62 | 9.58 | 20.44 | 24.1 | 26.82 | 35.2 | 12.12 | 20.84 | 23.94 | 27.42 | 93.07 |
| Thermocline (m) | 0.25 | 3.55 | 5.4 | 7.45 | 34.25 | 0.25 | 2.25 | 3.34 | 4.57 | 15.33 | 0.25 | 2.23 | 4.25 | 5.66 | 19.4 | 0.12 | 0.73 | 1.73 | 3.15 | 14.33 |
| Secchi depth (m) | 0.55 | 2.1 | 3.27 | 4.83 | 27.95 | 0.39 | 1.38 | 1.9 | 2.71 | 5.6 | 0.1 | 0.55 | 0.98 | 1.75 | 16.4 | 0.02 | 0.35 | 0.65 | 1.23 | 28 |
| Chemical characteristics | ||||||||||||||||||||
| Dissolved oxygen, 2 m depth (mg L−1) | 1.49 | 7.57 | 8.42 | 9.3 | 14.5 | 1.55 | 7.17 | 7.93 | 8.62 | 10.6 | 1.25 | 7.13 | 8.06 | 9.21 | 23.5 | 0.3 | 6.25 | 7.67 | 9.21 | 31.8 |
| Dissolved organic carbon (mg L−1) | 0.4 | 2.03 | 3.11 | 4.82 | 28.1 | 2.05 | 4.36 | 6.14 | 8.31 | 36.1 | 0.23 | 3.69 | 5.4 | 7.57 | 31.2 | 0.58 | 6.34 | 10.06 | 16.43 | 515.81 |
| Color (PCU) | 0 | 7.5 | 11 | 15 | 20 | 21 | 25 | 30 | 44 | 165 | 0 | 11 | 15 | 18 | 20 | 21 | 25 | 30 | 38 | 724 |
| Total phosphorus (μg P L−1) | 4 | 10 | 16.5 | 24 | 30 | 5 | 15 | 20 | 25 | 30 | 31 | 45 | 65 | 124 | 1208 | 31 | 59 | 110 | 297.5 | 3636 |
| Total nitrogen (mg L−1) | 0.01 | 0.17 | 0.3 | 0.5 | 6.22 | 0.1 | 0.3 | 0.41 | 0.64 | 2.61 | 0.02 | 0.54 | 0.78 | 1.2 | 5.91 | 0.06 | 0.78 | 1.37 | 2.41 | 54 |
| Nitrogen : phosphorus (molar ratio) | 1.75 | 26.26 | 47.4 | 72.84 | 860.67 | 8.77 | 33.95 | 54.09 | 88.57 | 385.29 | 0.57 | 13.78 | 24.51 | 36.45 | 256.86 | 1.77 | 16.06 | 25.27 | 36.08 | 3736.61 |
| Dissolved inorganic nitrogen (mg N L−1) | 0 | 0.01 | 0.01 | 0.02 | 5.96 | 0.01 | 0.01 | 0.01 | 0.02 | 0.32 | 0 | 0.01 | 0.02 | 0.05 | 5.67 | 0 | 0.02 | 0.02 | 0.05 | 52.12 |
| DIN : TP ratio | 0 | 0.95 | 1.86 | 3.11 | 824.54 | 0.44 | 1.06 | 1.48 | 2.39 | 29.34 | 0.02 | 0.36 | 0.65 | 1.43 | 251.14 | 0.02 | 0.23 | 0.52 | 1.27 | 3606.79 |
| Calcium (mg L−1) | 0.12 | 3.26 | 13.41 | 28.24 | 287.4 | 0.55 | 2.4 | 4.83 | 17.9 | 55.31 | 1.1 | 15.6 | 29.4 | 46.7 | 594.9 | 0.61 | 8.92 | 22.02 | 44.58 | 324.9 |
| Chloride (mg L−1) | 0.04 | 0.82 | 3.88 | 13.68 | 1975.56 | 0.07 | 1 | 5.31 | 16.57 | 148 | 0.08 | 4.14 | 11.52 | 23.84 | 1996.31 | 0.08 | 3.92 | 12.6 | 31.24 | 18,012.7 |
| pH | 5.38 | 7.33 | 8.09 | 8.46 | 9.53 | 5.1 | 7 | 7.44 | 8.09 | 9.36 | 3.34 | 8.14 | 8.47 | 8.68 | 10.47 | 2.83 | 7.73 | 8.41 | 8.88 | 10.37 |
| Acid-neutralizing capacity (μg L−1) | 15.6 | 244.25 | 879.45 | 2239.75 | 12,529 | 24.1 | 158.2 | 324.1 | 1344 | 21,800 | −589.6 | 1151 | 2300 | 3321 | 29,112 | −3361.4 | 772.45 | 2283 | 3980.5 | 203,857 |
| Biological characteristics | ||||||||||||||||||||
| Chl a (μg L−1) | 0 | 1.51 | 2.81 | 5.19 | 41.84 | 0.87 | 2.83 | 4.68 | 7.76 | 99 | 0.33 | 5.12 | 15.5 | 35.45 | 373.33 | 0.22 | 9.28 | 24.48 | 62.8 | 764.64 |
| Cyanobacteria density (cells mL−1) | 0 | 301.31 | 2208.41 | 10,304.2 | 516,223 | 0 | 383.67 | 3234.58 | 14,556.4 | 759,826 | 0 | 1776.56 | 17,604.6 | 82,923.4 | 2,244,776 | 0 | 4168 | 34,213.3 | 190,111 | 8,757,487 |
| Microcystin concentration (μg L−1) | 0 | 0 | 0.07 | 0.11 | 14.85 | 0 | 0 | 0.07 | 0.11 | 3.83 | 0 | 0.06 | 0.1 | 0.17 | 42.17 | 0 | 0.08 | 0.13 | 0.41 | 66.69 |
| Phytoplankton biomass (μg dry weight L−1) | 0.33 | 61 | 156.9 | 365.74 | 5252.88 | 13.38 | 88.31 | 211.61 | 395.73 | 2154.19 | 0.41 | 193.8 | 579.83 | 1626.87 | 17,663.3 | 10.44 | 309.01 | 865.29 | 2718.87 | 61,842.5 |
| Zooplankton biomass (μg dry weight L−1) | 0.13 | 20.57 | 49.18 | 108.18 | 1579.85 | 0.74 | 21.33 | 54.66 | 119.21 | 578.73 | 0.18 | 40.16 | 88.37 | 256.24 | 3069.62 | 0.16 | 53.97 | 150.04 | 409.74 | 5448.9 |
| Zooplankton : Phytoplankton biomass ratio | 0 | 0.11 | 0.32 | 1.12 | 32.5 | 0.01 | 0.07 | 0.24 | 0.82 | 12.45 | 0 | 0.04 | 0.15 | 0.73 | 336.93 | 0 | 0.04 | 0.16 | 0.48 | 50.25 |
| Watershed characteristics | ||||||||||||||||||||
| Wateshed area (km2) | 0.04 | 2.31 | 8.28 | 49.45 | 604,527 | 0.16 | 2.13 | 11.28 | 39.46 | 1015.14 | 0.02 | 2.05 | 16.65 | 95.84 | 656,462 | 0.07 | 1.76 | 11.02 | 54.28 | 222,119 |
| % agricultural land use | 0 | 0 | 0.66 | 17.04 | 79.71 | 0 | 0 | 1.48 | 8.36 | 77.07 | 0 | 0.48 | 12.92 | 44.39 | 87.97 | 0 | 0.36 | 18.24 | 50.93 | 97.46 |
| % wetland cover | 0 | 0.01 | 0.77 | 5.13 | 67.96 | 0 | 1.64 | 5.91 | 14.05 | 60.74 | 0 | 0.16 | 0.99 | 4.5 | 63.8 | 0 | 0.34 | 2.93 | 9.45 | 94.1 |
| % urban development | 0 | 0.02 | 3.53 | 9.36 | 89.67 | 0 | 0.7 | 3.28 | 7.61 | 57.18 | 0 | 1.15 | 4.1 | 9.07 | 99.42 | 0 | 1.6 | 4.13 | 7.55 | 88.25 |
| % Forest cover | 0 | 16.42 | 50.89 | 69.71 | 97 | 0 | 29.99 | 57.08 | 78.39 | 96.81 | 0 | 1.09 | 14.32 | 46.02 | 96.81 | 0 | 0.32 | 8.47 | 42.75 | 94.6 |
| Road density (km km−2) | 0 | 0.99 | 1.65 | 2.61 | 16.89 | 0 | 0.95 | 1.56 | 2.31 | 6.08 | 0 | 1.05 | 1.66 | 2.47 | 18.26 | 0 | 1.11 | 1.52 | 2.17 | 10.12 |
Lake area and lake class
Lake area significantly differed with nutrient-color status (KW χ2 = 23.69, df = 3, p < 0.0001), with blue and green lakes significantly larger in surface area than brown and murky lakes. The median size of blue and green lakes was ~ 40 ha while that of brown and murky lakes was ~ 22 ha (Table 3). However, these results should be interpreted with caution. Frequency distributions of lake size revealed that most lakes were between 1 to 10 ha in surface area for all four lake classes. (In the 2007 NLA survey, most lakes were between 4 and 10 ha in size in each lake class.) Of the 85 lakes between 1 and 4 ha included in the 2012 NLA dataset, 21 were classified as blue (25%), 21 green (25%), 8 brown (9%), and 35 murky (41%). Thus, all four nutrient-color classes included lakes smaller in size, and the largest lake included the 2012 NLA dataset (~ 167,000 ha) was classified as murky (Table 2). Brown lakes displayed the narrowest range in lake area (i.e., 1–4213 ha, Table 3).
Basal pelagic resources
Based on the 2012 NLA survey, Chl a concentrations significantly differed among the lake types (KW χ2 = 361.21, df = 3, p < 0.0001), with higher concentrations in murky lakes (median = 24.48 μg L−1, range = 0.22–764.6 μg L−1) followed by green lakes (median = 13.27 μg L−1, range = 0.33–373.3 μg L−1) (Tables 3–4; Fig. 5a). Moreover, the density of cyanobacteria significantly differed among lake types (KW χ2 = 200, df = 3, p < 0.0001) and was highest in murky lakes (median = 34,210 cells mL−1, range = 0–8,757,000 cells mL−1) followed by green lakes (median = 14,460 cells mL−1, range = 0–2,245,000 cells mL−1) (Tables 3–4; Fig. 5b). The concentration of microcystin toxin was also significantly higher in murky lakes (median = 0.13 μg L−1, range = 0–66.7 μg L−1) followed by green lakes (median = 0.10 μg L−1, range = 0–42.2 μg L−1; KWχ2 = 100.16, df = 3, p < 0.0001) (Table 3). Blue and brown lakes did not significantly differ in Chl a concentrations, cyanobacterial density, or microcystin concentration (Tables 3–4; Fig. 5a,b). Despite increased phytoplankton and zooplankton biomass in murky lakes, the ratio of zooplankton to phytoplankton biomass was similar to that in green lakes and significantly lower than that in blue and brown lakes (KW χ2 =27.159, df = 3, p < 0.0001) (Table 3).
Table 4.
Results of Dunn tests comparing differences in Chl a concentrations, cyanobacteria density, and zooplankton biomass among lake nutrient-color classes. NS = not significant. Boxplots are shown in Fig. 5.
| Lake comparison | Chl a concentration | Cyanobacteria density | Rotifer biomass | Copepod biomass | Cladoceran biomass | Total zooplankton biomass |
|---|---|---|---|---|---|---|
| Blue–Brown | 0.001 | NS | NS | 0.006 | NS | NS |
| Blue–Green | <0.0001 | <0.0001 | <0.0001 | <0.0001 | NS | <0.0001 |
| Brown–Green | <0.0001 | <0.0001 | 0.006 | NS | NS | 0.0001 |
| Blue–Murky | <0.0001 | <0.0001 | <0.0001 | <0.0001 | NS | <0.0001 |
| Brown–Murky | <0.0001 | <0.0001 | <0.0001 | 0.0002 | NS | <0.0001 |
| Green–Murky | 0.0001 | 0.002 | 0.01 | 0.001 | NS | 0.001 |
Fig. 5.
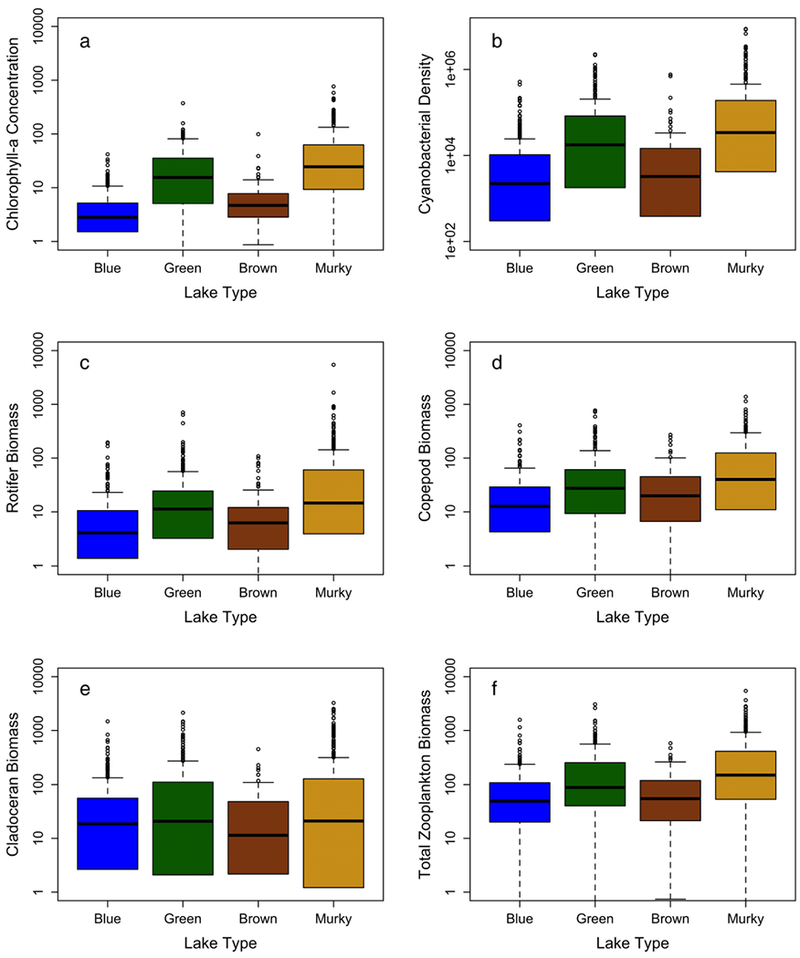
Boxplots of Chl a concentration (μg L−1) (a), cyanobacterial density (cells mL−1) (b), rotifer (c), copepod (d), cladoceran (e), and total zooplankton (f) biomass across the four lake types. Zooplankton biomass is in units of μg dry weight L−1. All plots are displayed on a log-scale with boxes color-coded by lake class, from blue to murky. The line in each box represents the median, the box represents the first and third quartiles, and the whiskers represent the lower and upper extremes. Outliers are shown as circles. Significance is listed in Table 4.
Zooplankton biomass and community structure
Total zooplankton biomass significantly differed with lake type in the 2012 NLA dataset (KW χ2 = 104.91, df = 3, p < 0.0001), with the highest biomass observed in murky lakes (median = 150.04 μg dry weight L−1, range = 0.16–5448.9 μg dry weight L−1) followed by green lakes (median = 88.37 μg dry weight L−1, range = 0.18–3069.62 μg dry weight L−1) and no difference in blue (median = 49.18 μg dry weight L−1, range = 0.13–1579.85 μg dry weight L−1) and brown lakes (median = 54.66 μg dry weight L−1, range = 0.74–578.73 μg dry weight L−1) (Table 4; Fig. 5f). Diplostraca, especially Daphnia spp., were the predominant contributors to zooplankton biomass in all four lake types (Fig. 6a,f; Supporting Information Table S1), and there were no differences in Diplostraca biomass across lake types (Fig. 5e; Table 4). Calanoids and cyclopoids constituted relatively equal proportions of biomass within each lake class (e.g., ~ 13% and 12%, respectively, in green lakes; ~ 13% and 12%, respectively, within murky lakes) (Fig. 6a-c;Supporting Information Table S1). However, copepod biomass significantly differed across lake types, with the highest copepod biomass observed in murky lakes, followed by green, blue, and brown lakes (Fig. 5d; Table 4).
Fig. 6.
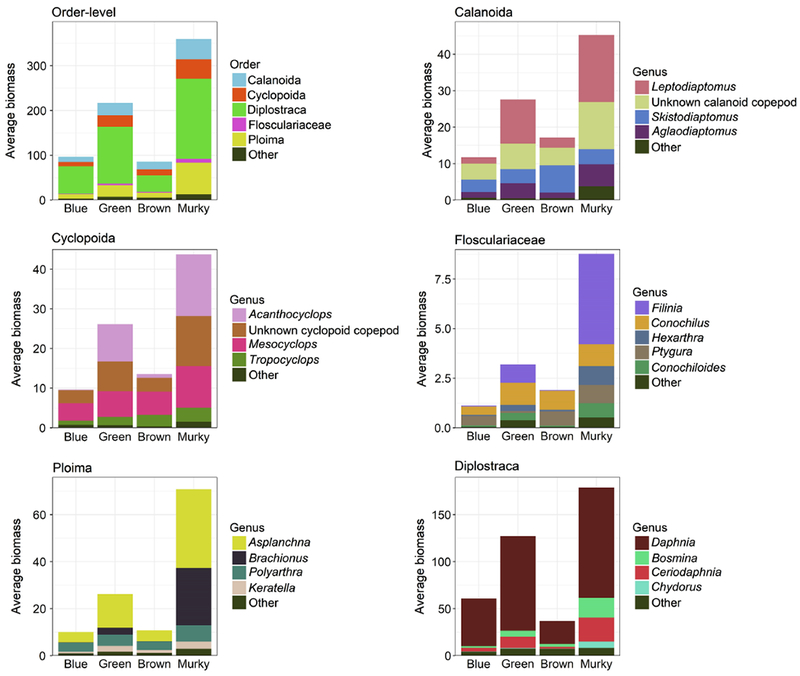
Average zooplankton biomass by order-level and genera-level across lake nutrient-color status, based on data from the 2012 NLA. Orders and genera contributing less than 5% of any sample were removed; order-level biomass is the sum total of all remaining genera within an order. Plot includes 1013 lakes total: 284 blue, 273 green, 109 brown, and 347 murky. Note differences in y-axis limits.
Although they represent a smaller proportion of the total biomass, the most notable differences in zooplankton among the lake types were observed within the rotifers (Fig. 6a-f). Rotifer biomass was significantly greater in murky lakes compared to the other lake types (Fig. 5c). The average compositional biomass of Ploima in murky lakes was nearly double that in blue lakes (i.e., ~ 20% vs. 10%, respectively) (Supporting Information Table S1). One-third of the Ploima biomass in murky lakes was comprised of Brachionus spp. compared to ~ 11% in green lakes and ~ 1% in blue and brown lakes (Fig. 6e). Asplanchna spp. also displayed higher biomass in murky lakes compared to the other lake types (Fig. 6e). Flosculariaceae contribute the least average biomass among the major zooplankton orders across all lake types but was again relatively higher in the murky lakes (Fig. 6a; Supporting Information Table S1). This related overwhelmingly to an increase in Filinia and Hexarthra biomass in murky lakes (Fig. 6d).
Despite these differences in rotifer and copepod biomass, multivariate approaches did not reveal significant differences in zooplankton community composition at the order-, genus-, or species-level related to lake nutrient-color status when all data points were included in the analyses (NMDS stress values ≥ 0.2; PERMANOVA R2 ≤ 0.05). When only the most extreme lakes were examined within each of the four lake types—for example, those murky lakes with TP and color values both in the upper quartiles and blue lakes with values in the lower quartiles—average zooplankton biomass more than doubled in murky lakes (Supporting Information Fig. S1), which are located primarily in the central U.S. (Supporting Information Fig. S2). Based on PERMANOVA, zooplankton communities in extreme murky lakes differed significantly from communities in extreme blue lakes (Pseudo-F = 10.91, df = 1, p = 0.001), with lake nutrient-color status accounting for nearly 18% of the observed variance (r2 = 0.176). Diplostraca continued to dominate the zooplankton biomass of murky lakes, with Daphnia spp. comprising the majority (60%) of Diplostraca present. In addition, an increase in Acanthocyclops proportion contributed to the higher cyclopoid biomass in extreme murky lakes. Zooplankton composition was nearly identical across all lakes when extreme lakes are excluded from each lake type (Supporting Information Fig. S3).
Discussion
Based on data from the EPA National Lakes Assessment, we demonstrate that an increasing proportion of lakes in the continental U.S. are simultaneously experiencing eutrophication (aka “greening”) and brownification (aka “browning”), such that murky lakes dominated the 2012 NLA survey. Once murky, our analysis further shows that it has not been common for lakes to shift back to simply brown or green. From a food web perspective, this shift in lakes toward murkiness promotes increased phytoplankton and zooplankton biomass. However, the low ratio of zooplankton to phytoplankton biomass suggests a reduction in energy transfer to higher trophic levels.
Shifting lake nutrient-color status
Between 2007 and 2012, there was a significant reduction in blue lakes and a significant increase in murky lakes in the continental U.S., particularly in the Northern Appalachian and Southern Plains ecoregions, based on population estimates from the NLA surveys. Moreover, analyzing the direction of change in the 401 resampled lakes revealed increases in both TP concentrations and color in over half of this subset of lakes. While our findings were similar from these two approaches, there were slight differences in the reported proportions of lakes changing nutrient-color status. This is likely due to two major factors. First, there were differences in sample sizes. The population analysis was developed from the full set of lakes ≥ 4 ha sampled in 2007 and 2012 (n = 1028 and 950, respectively) while the resampled lake analysis included only 401 lakes. Second, the approaches represent different aspects of change. The national analysis represents changes across the population of lakes ≥ 4 ha in the continental U.S. Due to NLA’s statistically representative site selection process, each sampled lake is weighted by its frequency of occurrence on the landscape, and this is used to develop statistically valid inferences from the sampled lakes to a target population of lakes in the continental U.S. The national change analysis compared the inferred 2007 and 2012 population-level results. In contrast, the 401 resampled lakes were not a representative subsample of the probability design. Rather, these lakes allowed us to examine changes in nutrient-color status within individual lakes and to start an initial exploration of the range of TP and color change experienced by lakes over time. While slightly different, these two analytical approaches demonstrated shifts in nutrient-color status both at the national scale of the population-level inferences, and within individual lakes.
Overall, these results suggest the continued presence of both lake greening and browning on a national scale. We cannot definitively say what is causing lakes to shift in nutrientcolor status (i.e., increase in TP and/or color), but land cover and land use patterns within a watershed often play a role. Current lake water quality reflects longer-term influences of a watershed. For example, the prevalence of agriculture within a lake basin is often linked to lake greening (Moss 2008; Schindler et al. 2016). This possibility is suggested in the NLA data, where green and murky lakes have a significantly higher percentage of agricultural land cover within their watersheds compared to blue and brown lakes. Increased runoff of nitrogen and phosphorus fertilizer from agricultural fields can dramatically stimulate phytoplankton growth, especially blooms of filamentous blue-green algae that can cover the surface of lakes (Heisler et al. 2008; Smith and Schindler 2009). Lake browning, on the other hand, is often associated with the prevalence of wetlands within a watershed (Larsen et al. 2011). Again, NLA data support these prior observations, with brown and murky lakes having a greater percentage of surrounding wetlands compared with blue and green lakes. Decomposition of organic matter is substantially reduced in wetlands due to a lack of dissolved oxygen. Consequently, wetlands often contribute increased inputs of CDOM to nearby lakes that stain water dark brown.
These observed patterns in land cover and use also coincide with regional patterns in lake nutrient-color status. The proportion of blue lakes significantly declined in ecoregions of the central U.S. but remained prominent at higher elevations in the Eastern and Western mountains. In contrast, green and murky lakes were prominent in the central and southeastern U.S. but occurred less frequently in mountainous regions. The central and southeastern U.S. are not only characterized by extensive agriculture but also include many types of wetland habitats (U.S. Fish and Wildlife Service 2011). Both have the potential to contribute to nutrient and organic matter loading (Dalzell et al. 2005; Solomon et al. 2015), leading to greener or murkier lakes. The brownification of blue lakes was especially evident in the northeastern U.S., which also has an abundance of wetland habitats—for example, 25% of the land area in Maine is categorized as wetland (US Fish and Wildlife Service 2011).
Changes in climate are also likely to influence shifts in lake nutrient-color status. A detailed, regional assessment of temperature and precipitation patterns in 2007 vs. 2012 for lakes in the NLA was beyond the scope of this study, but the importance of climate change must be acknowledged. Increased temperatures have been shown to increase soil DOM concentrations, and depending on hydrologic conditions, can lead to increased organic matter runoff to nearby inland waters (Freeman et al. 2001; Nguyen and Choi 2015). Moreover, increased precipitation, especially extreme events, is often linked to the brownification and eutrophication of lakes (Williamson et al. 2015; de Wit et al. 2016; Carpenter et al. 2018). In the present study, for example, the brownification of blue lakes in the northeastern U.S. may be facilitated by longer-term regional changes in climate; a 27% increase in precipitation and > 1.5°C increase in annual temperature has been observed here since 1901 (U.S. GCRP 2017). Nevertheless, the browning of lakes in this region may also be due to reductions in acid precipitation, which was occurring within the same timeframe (Monteith et al. 2007). Further analyses of shifting lake nutrient-color status with local and regional climate, as well as other atmospheric changes, are needed. Lakes in the NLA survey are sampled only once during the summer, but it is possible that lakes shift in nutrient-color status multiple times throughout a given year, or season.
Finally, brown and murky lakes were observed to be smaller in surface area than blue and green lakes. Lakes smaller in surface area tend to be shallower in depth, and therefore, have a smaller volume (Cael et al. 2017). Consequently, smaller lakes likely require less allochthonous inputs to cause major changes in lake TP and organic matter concentrations. Nevertheless, while statistically significant, we are not completely convinced that lake area is an important variable in determining lake nutrient-color status. Most lakes in all four of the lake classes were 1–10 ha in size, which is reflective of the global size distribution of lakes (Downing et al. 2006; Cael and Seekell 2016). Not surprisingly, many of the larger lakes in the 2012 NLA survey were classified as blue. However, the fact that there also were several large green lakes in the 2012 NLA survey highlights the effects of 100+ years of eutrophication across the continental U.S. The complex relationship between size, geographic local, climate, and lake nutrient-color status warrants further investigation.
Lake nutrient-color status and water quality
From a societal perspective, shifts in lake nutrient-color status can alter lakes in a manner that leads to nuisance or hazardous conditions. Some cyanobacteria, like Microcystis and Anabaena spp., produce toxins that can cause damage to the liver and nervous system as well as liver and colon cancer (Zanchett and Oliveira-Filho 2013; Wood 2016). In the present study, the highest recorded concentrations of the toxin microcystin occurred in murky lakes. Encouragingly, only 14 lakes in the 2012 NLA survey exceeded the 10 μg L−1 guidelines for recreation waters: 7 murky, 6 green, and 1 blue lake. However, ~ 14% of murky lakes and ~ 12% of green lakes exceeded the 1 μg L−1 provisional guideline set by the World Health Organization for finished drinking water. In contrast, < 1% of blue and 3% of brown lakes exceeded this limit. Both increased microcystin and cDOM concentrations in drinking water reservoirs pose technical challenges during drinking water purification (Hoeger et al. 2005; Chow et al. 2007).
Controlling the quantity and quality of allochthonous organic matter inputs from the watershed may be near impossible, particularly with respect to continued changes in global temperatures and precipitation patterns (Freeman et al. 2001; de Wit et al. 2016). However, attention to agricultural activity in the watershed may help stabilize or reverse changes in lake nutrient-color status, and thereby improve water quality. Yet a recent study by McCrackin et al. (2017) suggests that lakes are slow to, or may not, recover from eutrophication even after complete cessation of external nutrient loading. This is concordant with previous studies that show high variability in results of “reoligotrophication” efforts (Jeppesen et al. 2005). Resistance to recovery may be related to ongoing internal loading of phosphorus from lake sediments caused by anoxic conditions in the hypolimnion. The latter is often exacerbated by lake greening and browning (Solomon et al. 2015; Williamson et al. 2015; McCrackin et al. 2017).
Pelagic basal resources across lake types
Phytoplankton biomass increased across the lake types from blue to murky, coinciding with increases in TP concentration and/or water color. Increased phosphorus inputs have long been known to stimulate primary production. However, recent studies have demonstrated that organic matter inputs can also fuel increased algal growth, particularly in nutrientlimited systems with low DOC concentrations (Klug 2002; Cooke et al. 2015; Seekell et al. 2015; Williamson et al. 2015; Fergus et al. 2016). This increase in primary production is believed to be associated with nutrient additions and UV protection acquired from increasing organic matter inputs.
As lakes continue to darken in brown color, primary production has been shown to decrease due to the reduction in light for photosynthesis (Ask et al. 2012; Thrane et al. 2014; Solomon et al. 2015). Here, surprisingly, the increased color of murky lakes did not appear to have an inhibitory effect on epilimnetic phytoplankton, as they displayed the highest phytoplankton biomass. The shallowing of the mixed layer with increased color may enable photosynthetic cells access to light in the surface waters despite an overall reduction in light penetration through the water column (Solomon et al. 2015). Moreover, a relatively large proportion of algal biomass in murky lakes was composed of cyanobacteria, which often create mats across the surface of lakes (Heisler et al. 2008; Smith and Schindler 2009). Increased nutrient loading associated with organic matter inputs, in combination with inorganic phosphorus loads, may further fuel primary production in murky lakes compared to the other lake types.
In terms of bioavailability, however, the proportion of inedible phytoplankton has been shown to increase with lake trophy (Watson and Kalff 1981; Jeppesen et al. 2000; Hessen et al. 2003; Heathcote et al. 2016). Here, densities of blue-green algae and concentrations of microcystin toxin were high in green, and especially, murky lakes, which may deter zooplankton feeding (Gliwicz and Lampert 1990; Leflaive and Ten-Hage 2007). This inference is supported by the low zooplankton : phytoplankton biomass ratio observed in green and murky lakes. Low zooplankton to phytoplankton biomass ratios suggest a reduction in energy transfer efficiency through the food web. According to Downing et al. (2001), cyanobacteria account for an average of 60% of phytoplankton biomass at TP concentrations above 80–90 μg L−1, and Jeppesen et al. (2000) found that herbivorous zooplankton consume 50% of algal biomass per day in lakes of low trophy (<50 μg PL−1) but only 16–19% at higher trophy (200–400 μg P L−1). Compared to other algal taxa, cyanobacteria have lower concentrations of key fatty acids, such as DHA and EPA (Taipale et al. 2016). Thus, while phytoplankton biomass was high in green and murky lakes, a relatively large portion may represent a non-viable or less nutritious resource to higher trophic levels.
Zooplankton community structure across lake types
As predicted, blue and brown lakes had significantly lower total zooplankton biomass compared to green and murky lakes, which coincided with differences in phytoplankton biomass across the lake types. Murky lakes displayed the highest zooplankton biomass, primarily due to an increase in rotifer and copepod biomass. Typically, eutrophic systems are cited as having high rotifer biomass (Pace 1986); however, here we show that mixotrophic “murky” systems support even greater rotifer biomass. Predatory copepods, both calanoid and cyclopoid, may in turn benefit from the increase in rotifer biomass (Brandl 2005).
The rotifers Asplanchna, Filinia, Hexartha, and Brachionus spp. were particularly abundant in murky lakes. While Asplanchna can be omnivorous and predatory, these other rotifers are known to be at least partially and potentially entirely bacterivorous (Starkweather et al. 1979; Sanders et al. 1989; Ooms-Wilms et al. 1995). Some macrozooplankton can also consume bacteria, and in some cases, a combined algalbacteria diet enhances growth rates (e.g., Daphnia, Freese and Martin-Creuzburg 2013). Although we do not have estimates of bacterial abundance or biomass for the NLA lakes, bacterial densities generally increase with increasing food resources, including increased primary production and organic matter concentrations (Cole et al. 1988; Wetzel 2001). Zooplankton in murky lakes may therefore have the option to feed on an abundance of both algae and/or bacteria (Jones 1992; Moore et al. 2004).
Despite high zooplankton biomass, zooplankton to phytoplankton biomass ratios were lower in green and murky lakes compared to blue and brown lakes. We further investigated crustacean (i.e., copepods and cladocerans) vs. rotifer biomass to determine whether increased rotifers in green and murky lakes could explain the lower zooplankton : phytoplankton biomass ratios. However, median rotifer to phytoplankton biomass ratios were similar in all lake types (~ 0.02–0.03) while median crustacean zooplankton to phytoplankton biomass ratios were higher in blue (~ 0.24) and brown (~ 0.17) lakes compared to green and murky lakes (~ 0.08). These results provide further supporting evidence that the low zooplankton : phytoplankton biomass ratios in green and murky lakes are related to increases in inedible algae, reducing energy transfer to higher trophic levels.
Fish and lake nutrient-color status
It is possible that low zooplankton to phytoplankton biomass ratios in green and murky lakes are due to increased fish predation (Jeppesen et al. 2000; Hessen et al. 2003). Unfortunately, the NLA dataset does not provide information on fish communities. In general, fish biomass increases with lake productivity, with a shift to more planktivores compared with piscivores (Jeppesen et al. 2000). However, the combination of organic matter and nutrient inputs may reduce fish biomass. Murky lakes, in particular, may provide less usable habitat for fish given lower light and dissolved oxygen levels, particularly at deeper depths (Stasko et al. 2012). Karlsson et al. (2015) noted a decrease in fish biomass with increasing lake DOC concentration, which they equated with an increase in lake color. Similarly, Craig et al. (2017) recently reported that bluegill from humic lakes were smaller in body size and had reduced fecundity compared to those in clear lakes. Taipale et al. (2016) noted that perch from lakes with high phosphorus and dissolved organic carbon concentrations generally had smaller body sizes, which correlated with lower concentrations of nutritional EPA and DHA fatty acids. Thus, despite the increase in basal resources in murky lakes, the quality of food for higher trophic levels may be substantially less, leading to slower growth rates and poorer nutritional quality in fish. From a lake management perspective, this presents a challenge for using trophic cascades to biologically control algal blooms (Carpenter et al. 2001).
Potential caveats
We acknowledge that these results are based on one time point for each lake collected during the summer. As mentioned above, it is possible that lakes exhibit seasonal shifts in lake nutrient-color status as nutrient and organic matter inputs fluctuate throughout the year. Interannual fluctuations in weather (e.g., drought) are also likely influential, and caution must be applied here when interpreting long-term trends based on two time points collected 5 yr apart. Further exploration of seasonal and interannual patterns in lake nutrientcolor status is indeed warranted, and suitable data may be available in long-term lake monitoring programs. We encourage future research in this area as management strategies to improve or maintain ecosystem health will likely differ depending on lake nutrient-color status.
In addition, zooplankton samples were only collected during the day and may therefore underestimate the density and biomass of large cladocerans and copepods exhibiting diel vertical migration. This is particularly true for lakes deeper than 5 m where essentially only the surface waters were sampled (US EPA 2012b). Large zooplankton are especially likely to avoid the surface waters of blue lakes during daylight in avoidance of damaging UV radiation (Leech and Williamson 2001) and visually feeding predators. Seasonal differences in zooplankton community structure may also have influenced our results given that the NLA sampling occurred from June through September. Future studies related to the consequences of lake nutrient-color changes need to consider the full spectrum of zooplankton as well as effects on seasonal shifts in community composition and structure.
Conclusions
Murky lakes are increasing nationwide in the continental U.S., which presents many challenges for lake management. Our results indicate that water quality and lake food webs within each of the four lake types function differently. If these differences are not taken into account, it will be difficult to make realistic predictions as to how lakes will respond to future perturbations, such as climate warming and other anthropogenic impacts. Rather than focusing separately on eutrophication or brownification, more research is needed to understand how the combined “greening” and “browning” of lakes affects ecological processes within murky systems and to develop potential strategies that reverse their nutrient-color status.
Supplementary Material
Acknowledgments
The 2007 and 2012 NLA data were a result of the collective efforts of dedicated field crews, laboratory staff, data management and quality control staff, analysts, and many others from EPA, states, tribes, federal agencies, universities, and other organizations. Please contact nars-hq@epa.gov with any questions. This study was graciously supported by professional development funds from Longwood University to D. M. L. David Peck assisted with the clarification of water quality analyses. Suggestions from Steve Katz, Bryan Brown, and two anonymous reviewers improved the manuscript.
Footnotes
Additional Supporting Information may be found in the online version of this article.
Conflict of Interest
None declared.
References
- Agostinelli C, and Lund U. 2017. R package ‘circular’: Circular statistics (version 0.4-93). https://r-forge.r-project.org/projects/circular/ [Google Scholar]
- Arndt H 1993. Rotifers as predators on components of the microbial web (bacteria, heterotrophic flagellates, ciliates)—A review. Hydrobiologia 255-256: 231–246. doi: 10.1007/BF00025844 [DOI] [Google Scholar]
- Ask J, Karlsson J, and Jansson M. 2012. Net ecosystem production in clear-water and brown-water lakes. Global Biogeochem. Cycles 26: 7. doi: 10.1029/2010GB003951 [DOI] [Google Scholar]
- Berggren M, Ziegler SE, St-Gelais NF, Beisner BE, and del Giorgio PA. 2014. Contrasting patterns of allochthony among three major groups of crustacean zooplankton in boreal and temperate lakes. Ecology 95: 1947–1959. doi: 10.1890/13-0615.1 [DOI] [PubMed] [Google Scholar]
- Brandl Z 2005. Freshwater copepods and rotifers: Predators and their prey. Hydrobiologia 546: 475–489. doi: 10.1007/s10750-005-4290-3 [DOI] [Google Scholar]
- Cael BB, and Seekell DA. 2016. The size-distribution of Earth’s lakes. Sci. Rep. 6: 633. doi: 10.1038/srep29633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cael BB, Heathcote AJ, and Seekell DA. 2017. The volume and mean depth of Earth’s lakes. Geophys. Res. Lett. 44: 209–218. [Google Scholar]
- Carpenter SR, and others. 2001. Trophic cascades, nutrients, and lake productivity: Whole-lake experiments. Ecol. Monogr. 71: 163–186. doi: 10.1890/0012-9615(2001)071[0163:TCNALP]2.0.CO;2 [DOI] [Google Scholar]
- Carpenter SR, Booth EG, and Kucharik CJ. 2017. Extreme precipitation and phosphorus loads from two agricultural watersheds. Limnol. Oceanogr. 3: 1221–1233. doi: 10.1002/lno.10767 [DOI] [Google Scholar]
- Chow AT, Dahlgren RA, andHarrison JA. 2007. Watershed sources of disinfection byproduct precursors in the Sacramento and San Joaquin rivers, California. Environ. Sci. Technol. 41: 7645–7652. doi: 10.1021/es070621t [DOI] [PubMed] [Google Scholar]
- Cole JJ, Findlay S, and Pace ML. 1988. Bacterial production in fresh and saltwater ecosystems: A cross-system overview. Mar. Ecol. Prog. Ser. 43: 1–10. [Google Scholar]
- Cole JJ, Caraco NF, Kling GW, and Kratz TK. 1994. Carbon dioxide supersaturation in the surface waters of lakes. Science 265: 1568–1570. [DOI] [PubMed] [Google Scholar]
- Conley DJ, Paerl HW, Howarth RW, Boesch DF, Seitzinger SP, Havens KE, Lancelot C, and Likens GE. 2009. Controlling eutrophication: Nitrogen and phosphorus. Science 323: 1014–1015. doi: 10.1126/science.1167755 [DOI] [PubMed] [Google Scholar]
- Cooke SL, and others. 2015. Direct and indirect effects of additions of chromophoric dissolved organic matter on zooplankton during large-scale mesocosm experiments in an oligotrophic lake. Freshw. Biol. 60: 2362–2378. doi: 10.1111/fwb.12663 [DOI] [Google Scholar]
- Craig N, Jones SE, Weidel BC, and Solomon CT. 2017. Life history constraints explain negative relationship between fish productivity and dissolved organic carbon in lakes. Ecol. Evol. 7: 6201–6209. doi: 10.1002/ece3.3108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalzell BJ, Filley TR, and Harbor JM. 2005. Flood pulse influences on terrestrial organic matter export from an agricultural watershed. J. Geophys. Res. 110: G02011. doi: 10.1029/2005JG000043 [DOI] [Google Scholar]
- de Wit HA, and others. 2016. Current browning of surface waters will be further promoted by wetter climate. Environ. Sci. Technol. 3: 430–435. doi: 10.1021/acs.estlett.6b00396 [DOI] [Google Scholar]
- Dittman JA, Shanley JB, Driscoll CT, Aiken GR, Chalmers AT, and Towse JE. 2009. Ultraviolet absorbance as a proxy for total dissolved mercury in streams. Environ. Pollut. 157: 1953–1956. doi: 10.1016/j.envpol.2009.01.031 [DOI] [PubMed] [Google Scholar]
- Dodson SI, Arnott SE, and Cottingham KL. 2000. The relationship in lake communities between primary productivity and species richness. Ecology 81: 2662–2679. doi: 10.1890/0012-9658(2000)081[2662:TRILCB]2.0.CO;2 [DOI] [Google Scholar]
- Downing JA, Watson SB, and McCauley E. 2001. Predicting cyanobacteria dominance in lakes. Can. J. Fish. Aquat. Sci. 58: 1905–1908. [Google Scholar]
- Downing JA, and others. 2006. The global abundance and size distribution of lakes, ponds, and impoundments. Limnol. Oceanogr 51: 2388–2397. [Google Scholar]
- Duggan IC, Green JD, and Shiel RJ. 2002. Distribution of rotifer assemblages in North Island, Zealand, lakes: New Relationships to environmental and historical factors. Freshw. Biol. 47: 195–206. doi: 10.1046/j.1365-2427.2002.00742.x [DOI] [Google Scholar]
- Erlandsson M, Buffam I, Folster J, Laudon H, Temnerud J, Weyhenmeyer GA, and Bishop K. 2008. Thirty-five years of synchrony in the organic matter concentrations of Swedish rivers explained by variation in flow and sulphate. Glob. Change Biol. 14: 1191–1198. [Google Scholar]
- Fergus CE, Finley AO, Soranno PA, and Wagner T. 2016. Spatial variation in nutrient and water color effects on lake chlorophyll at macroscales. PLoS One 11: 1–20. doi: 10.1371/journal.pone.0164592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finstad AG, Helland IP, Ugedal O, Hesthagen T, and Hessen DO. 2014. Unimodal response of fish yield to dissolved organic carbon. Ecology Letters 17: 36–43. [DOI] [PubMed] [Google Scholar]
- Freeman C, Evans CD, Monteith DT, Reynolds B, and Fenner N. 2001. Export of organic carbon from peat soils. Nature 412: 785–785. [DOI] [PubMed] [Google Scholar]
- Freese HM, and Martin-Creuzburg D. 2013. Food quality of mixed bacteria-algae diets for Daphnia magna. Hydrobiologia 715: 63–76. doi: 10.1007/s10750-012-1375-7 [DOI] [Google Scholar]
- Gliwicz ZM, and Lampert W. 1990. Food thresholds in Daphnia species in the absence and presence of blue-green filaments. Ecology 71: 691–702. doi: 10.2307/1940323 [DOI] [Google Scholar]
- Haaland S, Hongve D, Laudon H, Riise G, and Vogt RD. 2010. Quantifying the drivers of the increasing colored organic matter in boreal surface waters. Environ. Sci. Technol 44: 2975–2980. [DOI] [PubMed] [Google Scholar]
- Heathcote AJ, Filstrup CT, Kendall D, and Downing JA. 2016. Biomass pyramids in lake plankton: Influence of cyanobacteria size and abundance. Inland Waters 6: 250–257. [Google Scholar]
- Heisler J, and others. 2008. Eutrophication and harmful algal blooms: A scientific consensus. Harmful Algae 8: 3–13. doi: 10.1016/j.hal.2008.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hessen DO, Faafeng BA, and Brettum P. 2003. Autotroph: herbivore biomass ratios;carbon deficits judged from plankton data. Hydrobiologia 491: 167–175. [Google Scholar]
- Hoeger SJ, Hitzfeld BC, and Dietrich DR. 2005. Occurrence and elimination of cyanobacterial toxins in drinking water treatment plants. Toxicol. Appl. Pharm. 203: 231–242. [DOI] [PubMed] [Google Scholar]
- Jeppesen E, Peder Jensen J, Sondergaard M, Lauridsen T, and Landkildehus F. 2000. Trophic structure, species richness and biodiversity in Danish lakes: Changes along a phosphorus gradient. Freshw. Biol. 45: 201–218. doi: 10.1046/j.1365-2427.2000.00675.x [DOI] [Google Scholar]
- Jeppesen E, and others. 2005. Lake responses to reduced nutrient loading: An analysis of contemporary long-term data from 35 case studies. Freshw. Biol. 50: 1747–1771. doi: 10.1111/j.1365-2427.2005.01415.x [DOI] [Google Scholar]
- Jones RI 1992. The influence of humic substances on lacustrine planktonic food chains. Hydrobiologia 229: 73–91. doi: 10.1007/BF00006992 [DOI] [Google Scholar]
- Karlsson J, Bergstrom AK, Bystrom P, Gudasz C, Rodriguez P, and Hein C. 2015. Terrestrial organic matter input suppresses biomass production in lake ecosystems. Ecology 96: 2870–2876. doi: 10.1890/15-0515.1 [DOI] [PubMed] [Google Scholar]
- Kincaid TM, and Olsen AR. 2016. spsurvey: Spatial survey design and analysis R package version 3.3. R Foundation for Statistical Computing. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klug JL 2002. Positive and negative effects of allochthonous dissolved organic matter and inorganic nutrients on phytoplankton growth. Can. J. Fish. Aquat. Sci. 59: 85–95. doi: 10.1139/f01-194 [DOI] [Google Scholar]
- Kritzberg ES, and Ekström SM. 2012. Increasing iron concentrations in surface waters—A factor behind brownification. Biogeosciences 9: 1465–1478. doi: 10.5194/bg-9-1465-2012 [DOI] [Google Scholar]
- Kuiper JJ, van Altena C, de Ruiter PC, van Gerven LPA, Janse JH, and Mooij WM. 2015. Food-web stability signals critical transitions in temperate shallow lakes. Nature Communications 6: ncomms8727. doi: 10.1038/ncomms8727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen S, Andersen T, and Hessen DO. 2011. The pCO2 in boreal lakes: Organic carbon as a universal predictor? Global Biogeochem. Cycles 25: GB2012. doi: 10.1029/2010GB003864 [DOI] [Google Scholar]
- Leech DM, and Williamson CE. 2001. In situ exposure to ultraviolet radiation alters the depth distribution of Daphnia. Limnol. Oceanogr. 46: 416–420. doi: 10.4319/lo.2001.46.2.0416 [DOI] [Google Scholar]
- Leflaive J, and Ten-Hage L. 2007. Algal and cyanobacterial secondary metabolites in freshwaters: A comparison of allelopathic compounds and toxins. Freshw. Biol. 52: 199–214. doi: 10.1111/j.1365-2427.2006.01689.x [DOI] [Google Scholar]
- Lohr SL 1999. Sampling: Design and analysis. Pacific Grove, CA: Duxbury Press. [Google Scholar]
- McCrackin ML, Jones HP, Jones PC, and Moreno-Mateos D. 2017. Recovery of lakes and coastal marine ecosystems from eutrophication: A global meta-analysis. Limnol. Oceanogr. 62: 507–518. doi: 10.1002/lno.10441 [DOI] [Google Scholar]
- Moore JC, and others. 2004. Detritus, trophic dynamics and biodiversity. Ecol. Lett. 7: 584–600. doi: 10.1111/j.1461-0248.2004.00606.x [DOI] [Google Scholar]
- Monteith DT, and others. 2007. Dissolved organic carbon trends resulting from changes in atmospheric deposition chemistry. Nature 450: 537–541. [DOI] [PubMed] [Google Scholar]
- Moss B 2008. Water pollution by agriculture. Phil. Trans. R. Soc. Lond. B Biol. Sci. 363: 659–666. doi: 10.1098/rstb.2007.2176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen HVM, and Choi JH. 2015. Changes in the dissolved organic matter leaching from soil under severe temperature and N-deposition. Environ. Monit. Assess. 187: 1–15. doi: 10.1007/s10661-015-4549-8 [DOI] [PubMed] [Google Scholar]
- Nürnberg GK, and Shaw M. 1998. Productivity of clear and humic lakes: Nutrients, phytoplankton, bacteria. Hydrobiologia 382: 97–112. doi: 10.1023/A:1003445406964 [DOI] [Google Scholar]
- Ogle DH 2017. FSA: Fisheries stock analysis. R package version 0.8.10. R Foundation for Statistical Computing. [Google Scholar]
- Oksanen J, and others. 2017. vegan: Community Ecology Package. R Foundation for Statistical Computing. [Google Scholar]
- Omernik JM 1987. Ecoregions of the conterminous United States. Ann. Assoc. Amer. Geogr. 77: 118–125. doi: 10.1111/j.1467-8306.1987.tb00149.x [DOI] [Google Scholar]
- Ooms-Wilms AL, Postema G, and Gulati RD. 1995. Evaluation of bacterivory of rotifera based on measurements of in situ ingestion of fluorescent particles, including some comparisons with cladocera. J. Plankton Res. 17: 1057–1077. doi: 10.1093/plankt/17.5.1057 [DOI] [Google Scholar]
- Pace ML 1986. An empirical analysis of zooplankton community size structure across lake trophic gradients. Limnol. Oceanogr. 31: 45–55. doi: 10.4319/lo.1986.31.1.0045 [DOI] [Google Scholar]
- Pinto-Coelho R, Pinel-Alloul B, Methot G, and Havens KE. 2005. Crustacean zooplankton in lakes and reservoirs of temperate and tropical regions: Variation with trophic status. Can. J. Fish. Aquat. Sci. 62: 348–361. doi: 10.1139/F04-178 [DOI] [Google Scholar]
- Pourriot R 1977. Food and feeding habits of Rotifera. Arch.Hydrobiol.-Beih. Ergebn. Limnol. 8: 243–260. [Google Scholar]
- R Core Team. 2017. R: A language and environment for statistical computing. R Foundation for Statistical Computing. [Google Scholar]
- Sanders RW, Porter KG, Bennett SJ, and DeBiase AE. 1989. Seasonal patterns of bacterivory by flagellates, ciliates, rotifers, and cladocerans in a freshwater planktonic community. Limnol. Oceanogr. 34: 673–687. doi: 10.4319/lo.1989.34.4.0673 [DOI] [Google Scholar]
- Schindler DW, Carpenter SR, Chapra SC, Hecky RE, and Orihel DM. 2016. Reducing phosphorus to curb lake eutrophication is a success. Environ. Sci. Technol. 50: 8923–8929. doi: 10.1021/acs.est.6b02204 [DOI] [PubMed] [Google Scholar]
- Seekell DA, Lapierre J-F, Ask J, Bergstrom A-K, Deininger A, Rodriguez P, and Karlsson J. 2015. The influence of dissolved organic carbon on primary production in northern lakes. Limnol. Oceanogr. 60: 1276–1285. doi: 10.1002/lno.10096 [DOI] [Google Scholar]
- Smith VH, and Schindler DW. 2009. Eutrophication science: where do we go from here? Trends Ecol. Evol. 24: 201–207. [DOI] [PubMed] [Google Scholar]
- Solomon CT, and others. 2015. Ecosystem consequences of changing inputs of terrestrial dissolved organic matter to lakes: Current knowledge and future challenges. Ecosystems 18: 376–389. doi: 10.1007/s10021-015-9848-y [DOI] [Google Scholar]
- Starkweather PL, Gilbert JJ, and Frost TM. 1979. Bacterial feeding by the rotifer Brachionus calyciflorus: Clearance and ingestion rates, behavior and population dynamics. Oecologia 44: 26–30. doi: 10.1007/BF00346392 [DOI] [PubMed] [Google Scholar]
- Stasko AD, Gunn JM, and Johnston TA. 2012. Role of ambient light in structuring north-temperate fish communities: Potential effects of increasing dissolved organic carbon concentration with a changing climate. Environ. Rev. 20: 173–190. doi: 10.1139/a2012-010 [DOI] [Google Scholar]
- Stevens DL, and Olsen AR. 2003. Variance estimation for spatially balanced samples of environmental resources. Environmetrics 14: 593–610. [Google Scholar]
- Stoddard JL, Van Sickle J, Herlihy AT, Brahney J, Paulsen S, Peck DV, Mitchell R, and Pollard AI. 2016. Continental-scale increase in lake and stream phosphorus: Are oligotrophic systems disappearing in the United States? Environ. Sci. Technol. 50: 3409–3415. doi: 10.1021/acs.est.5b05950 [DOI] [PubMed] [Google Scholar]
- Strickland JDH 1966. Measuring the Production of Marine Phytoplankton Fisheries Research Board of Canada Bulletin, no. 122. Pacific Oceanographic Group, Nanaimo, B.C. [Google Scholar]
- Taipale SJ, Vuorio K, Strandberg U, Kahilainen KK, Järvinen M, Hiltunen M, Peltomaa E, and Kankaala P. 2016. Lake eutrophication and brownification downgrade availability and transfer of essential fatty acids for human consumption. Environ. Int. 96: 156–166. doi: 10.1016/j.envint.2016.08.018 [DOI] [PubMed] [Google Scholar]
- Thrane J-E, Hessen DO, and Andersen T. 2014. The absorption of light in lakes: Negative impact of dissolved organic carbon on primary productivity. Ecosystems 17: 1040–1052. [Google Scholar]
- U.S. GCRP. 2017. Climate science special report: Fourth National Climate Assessment, p 470 In Wuebbles DJ, Fahey DW, Hibbard KA, Dokken DJ, Stewart BC, and Maycock TK [eds.]. U.S. Global Change Research Program, v. I Washington, DC: US GCRP. doi: 10.7930/J0J964J6 [DOI] [Google Scholar]
- U.S. EPA. 1987. Handbook of Methods for Acid Deposition Studies: Laboratory Analyses for Surface Water Chemistry. EPA/600/4-87/026. U.S. Environmental Protection Agency, Office of Research and Development. [Google Scholar]
- U.S. EPA. 2009. Survey of the Nation’s Lakes: Integrated Quality Assurance Project Plan. EPA/841-B-07-003. U.S. Environmental Protection Agency, Office of Water and Office of Research and Development. [Google Scholar]
- U.S. EPA. 2010. National Lakes Assessment 2007. U.S. Environmental Protection Agency. [Google Scholar]
- U.S. EPA. 2012a. 2012 National Lakes Assessment. Field Operations Manual. EPA 841-B-11-003. U.S. Environmental Protection Agency. [Google Scholar]
- U.S. EPA. 2012b. 2012 National Lakes Assessment. Laboratory Operations Manual. EPA-841-B-11-004. U.S. Environmental Protection Agency. [Google Scholar]
- U.S. EPA. 2016. National Lakes Assessment 2012. U.S. Environmental Protection Agency. [Google Scholar]
- U.S. Fish and Wildlife Service. 2011. National Wetlands Inventory. US Department of Interior, Fish and Wildlife Service, Available from http://www.fws.gov/wetlands.
- Watson S, and Kalff J. 1981. Relationships between nanno-plankton and lake trophic status. Can. J. Fish. Aquat. Sci. 38: 960–967. doi: 10.1139/f81-129 [DOI] [Google Scholar]
- Webster KE, and others. 2008. An empirical evaluation of the nutrient-color paradigm for lakes. Limnol. Oceanogr. 53: 1137–1148. doi: 10.4319/lo.2008.53.3.1137 [DOI] [Google Scholar]
- Wehr JD, Holen DA, MacDonald MM, and Lonergan SP. 1998. Effects of different organic carbon sources on a freshwater plankton community. Can. J. Fish. Aquat. Sci. 55: 2150–2160. doi: 10.1139/cjfas-55-9-2150 [DOI] [Google Scholar]
- Wetzel RG 2001. Limnology: Lake and river ecosystems, 3rd Edition Academic Press. [Google Scholar]
- Wickham H 2009. Ggplot2: Elegant graphics for data analysis. Springer-Verlag. [Google Scholar]
- Williamson CE, Morris DP, Pace ML, and Olson OG. 1999. Dissolved organic carbon and nutrients as regulators of lake ecosystems: Resurrection of a more integrated paradigm. Limnol. Oceanogr. 44: 795–803. doi: 10.4319/lo.1999.44.3_part_2.0795 [DOI] [Google Scholar]
- Williamson CE, Overholt EP, Pilla RM, Leach TH, Brentrup JA, Knoll LB, Mette EM, and Moeller RE. 2015. Ecological consequences of long-term browning in lakes. Sci. Rep. 5: srep18666. doi: 10.1038/srep18666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood R 2016. Acute animal and human poisonings from cyanotoxin exposure—A review of the literature. Environ. Int. 91: 276–282. doi: 10.1016/j.envint.2016.02.026 [DOI] [PubMed] [Google Scholar]
- Zanchett G, and Oliveira-Filho EC. 2013. Cyanobacteria and cyanotoxins: From impacts on aquatic ecosystems andhuman health to anticarcinogenic effects. Toxins 5: 1896–1917. doi: 10.3390/toxins5101896 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


