Abstract
Metal nanoclusters (NCs) are ultra-small nanoparticles intermediate in size between small molecule complexes and nanoparticles. NCs with tunable surface functionality feature unique physical and chemical properties, however these properties are frequently compromised by the presence of undesired components such as excess ligands or mixtures of NCs. In a typical synthesis process, different NCs can be formed with varying numbers of metal atoms and/or ligands, and even NCs with the same number of metal atoms and ligands can have different spatial structures. The separation of pure NCs is important because different species have distinct optical and catalytic behavior. However, NCs can be difficult to purify or separate for a range of reasons. In this review we discuss established and emerging approaches for NC purification/separation, wih a focus on choosing the appropriate method depending on NC and application.
Keywords: Nanoclusters, Purification, Separation
Graphical Abstract
1. Introduction
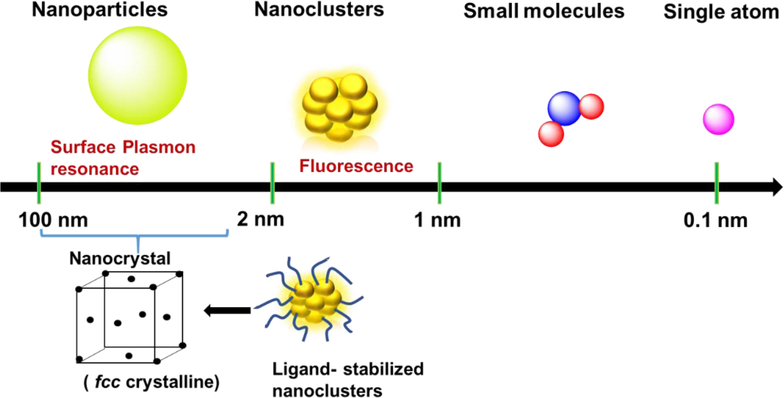
Nanoclusters (NCs) are ultra-small clusters smaller than ‘traditional’ nanoparticles (≥ ca.1.2 nm). NCs are fundamentally different from their larger derivatives (nanocrystals or crystalline nanoparticles (NPs)) where the optical properties are influenced by plasmon excitation, and are collective in contrast to the single-electron transitions found in NCs (Figure 1) [1, 2] [3]. The band structure of NCs becomes discontinuous and shows discrete energy levels [4] that generally lie between those of nanocrystals and small molecules. This band structure endows NCs with useful electronic, optical and chemical properties [5]. The small size of NC is important for catalysis because of the high ratio of the surface to interior atoms maximizes efficiency on a per-molecule basis [6]. NCs [7, 8] feature low toxicity [9–14], making them useful as green materials in areas including analysis, energy, biomedicines, and catalysts [10, 15–22]. Impurities in NCs, however, can significantly alter the properties of NCs. This challenge is exacerbated by challenges in removing unbound ligands, unreacted precursors, residual reducing reagents, and other NC/NP species [23].
Figure 1.
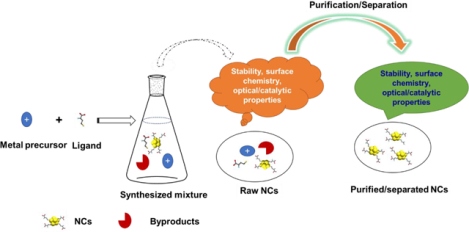
Metal NCs bridge the gap between nanoparticles and small molecule size and properties.
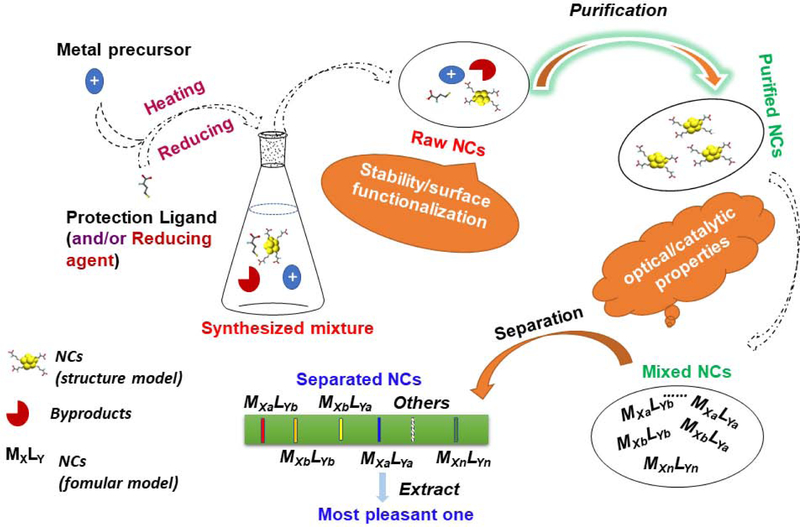
Multiple methods have been employed for the synthesis of NCs [24, 25]. The synthesized mixtures are often used directly without purification of the NCs [16, 18, 26–29]. In many biological application cases, surface functionalization is required to maximize the utility of NCs in complex media [30–32]. But in the presence of impurities such as unbound ligands the properties of these NCs can be compromised [33, 34]. Furthermore, the photonic properties of NCs are altered by the co-existence of various species of NCs after purification, an important consideration for imaging and photonic applications. Another example of the importance of isolating individual NC structures is provided by a study showing similar NCs with only one atom difference have significantly different catalytic activities [35]. Clearly, separation of NCs are needed for optimal behavior [36–39]. This review discusses two aspects crucial for the effective utilization of NCs (Figure 2): (1) purification of NCs from the unbound ligands and other components in the as-synthesized mixtures; (2) separation of mixtures of different species of NCs.
Figure 2.
Schematic illustration of a one-step synthesis of NCs and the challenges in employing as-made materials. MXLY indicates the mixture of NCs can be of different species because each parameter (atom number, ligand number, structure) can be varied: M, metal atom; L, ligand; X, the number of metal atoms; Y, the number of ligands.
2. Purification of NCs from the colloidal suspension
2.1. Polarity-based purification
2.1.1. Metal ion-induced precipitation
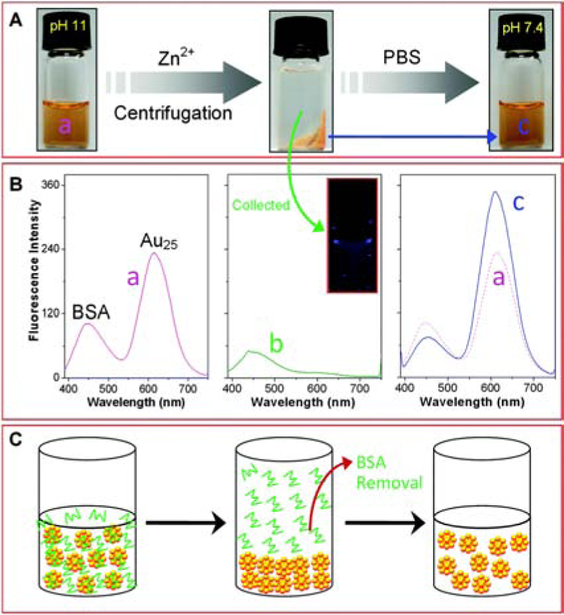
Free metal ions can bind to NCs through metallic bonding or electrostatic interaction with NC surface ligands. This association can cause precipitation of NCs [40–44]. As an example, Buan et al purified unbound bovine serum albumin (BSA) protein from BSA-protected AuNCs (BSA-AuNCs) with the assistance of Zn2+ (Figure 3) [45]. BSA-AuNCs were initially synthesized at pH 11 with OH− stably bound to the Au+ surface. Then, the fast addition of Zn2+ into BSA-AuNCs enabled the formation of zinc hydroxides leading to the precipitation of NCs. Meanwhile, BSA did not aggregate with the same concentration of Zn2+ under the same conditions (pH 11). The supernatant purified during precipitation of BSA-AuNCs only showed the emission of BSA, which indicated that NCs were successfully removed from the aqueous layer. Furthermore, the precipitated BSA-AuNCs were recovered by re-dissolving in phosphate-buffered saline (PBS) buffer at pH 7.4. The successful removal of the unbound BSA from BSA-AuNCs was confirmed by the fluorescence emission spectra of the starting materials and the purified product (Figure 3B). Both of the spectra showed two distinct emission peaks at 447 nm and 615 nm. However, the intensity ratio of the purified BSA-AuNCs (I447/I615) was decreased as 1:5 compared to 1:2 from the starting material, indicating unbound BSA was removed. While other effects can also change fluorescence propertis of NCs, [46] the effects of purification were also supported by more efficient detection of H2O2. It should be noted that using large amounts of foreign ions increases the post-purification time, which increases the oxidation tendency of NCs. Comparing to Zn2+ (5 mM), Ce3+ (ca. 50 μM) or Al3+ (ca. 75 μM) requires much less amount to induce the aggregation of some NCs through the interaction with the surface -OH [40, 47, 48]. The precipitation of NCs by using smaller amounts of ions might simplify the purification procedure.
Figure 3.
(A) Optical observations during the purification of BSA-AuNCs by Zn2+ induced precipitation method. (B) Fluorescence emission spectra of (a) the as-synthesized BSA-AuNCs, (b) the supernatant after precipitating BSA-AuNCs, and (c) the purified BSA-AuNCs after re-dispersing in PBS buffer. (C) Schematic illustration of the purification of BSA-AuNCs. Reproduced from Ref. [45] with permission from The Royal Society of Chemistry.
2.1.2. pH induced precipitation
NCs can be precipitated by protonation of their surfcae ligands [49][50]. In one example, BSA-AuNCs were purified by pH-induced precipitation under acidic conditions (Figure 4). The addition of acetic acid decreases the pH of the mixture of NCs to the isoelectric point of BSA (pI 4.8), The protonation process-induced partial conformational change of BSA led to the aggregation and precipitation of NCs. The process can then be reversed by adjusting the pH back to physiological pH. UV-Vis spectrum in Figure 4B indicates the precipitation-redissolution process. The characteristic absorption bands of BSA-AuNCs in the supernatant disappeared when BSA-AuNCs agglomerated after addition of acetic acid (curve a), but recovered by re-dissolving in alkaline medium (curve c). The TEM images also support that small BSA-AuNCs were recovered from acid-induced aggregates (Figure 4C) by the addition of NaOH (Figure 4D). This research group also used an acid precipitation method to purify small molecule functionalized NCs, including dihydrolipoic acid (DHLA) protected AgNCs (DHLA-AgNCs) and methionine protected AuNCs (Met-AuNCs) [51, 52]. As pH decreased, the protection ligands lost their charge repulsion, leading to the aggregation of NCs and the quenching of fluorescence that could be partially reversed in alkaline solutions. The researchers used NaBH4 as an etching agent to etch the large aggregates back to ultra-small NCs allowing full recovery of fluorescence.
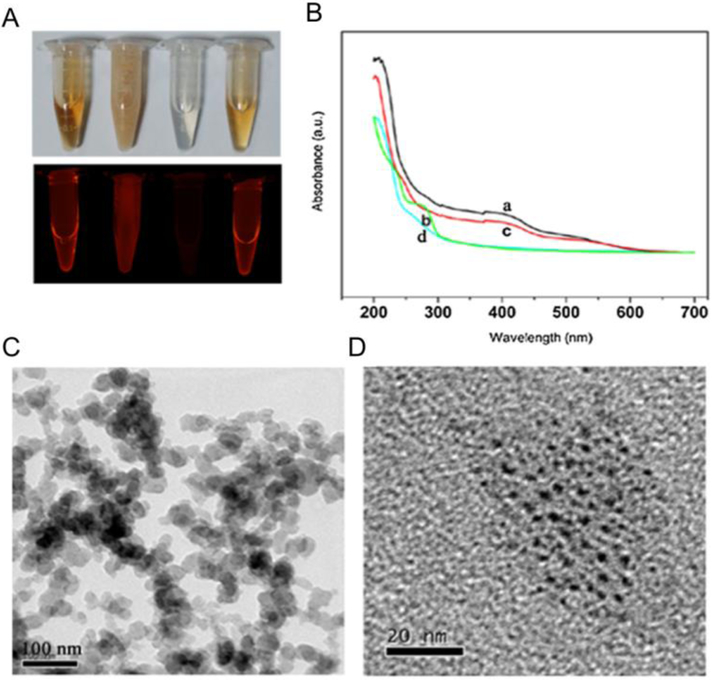
Figure 4.
Characterization of purified BSA-AuNCs. (A) Photographs of the corresponding solution (from left to right, BSA-AuNCs, the floccules under acidic conditions, the supernatant after removing the precipitate and the re-dispersed Au NCs under basic media) under visible-light (upper) and 365 nm UV-light irradiation (lower), (B) UV–vis absorption of (a) BSA–AuNCs, (b) the supernatant after removing the precipitate, (c) the re-dispersed AuNCs under basic media, and (d) BSA. (C) TEM image of precipitate. (D) TEM image of the re-dispersed AuNCs. Adapted with permission from ref. [50] Copyright 2017 Elsevier Ltd.
2.1.3. Solvent-induced precipitation
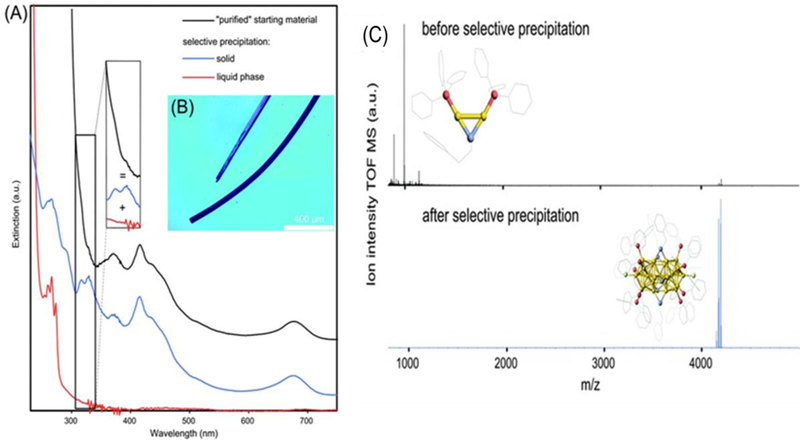
NCs can be precipitated based on solvent polarity [53]. Yin et al precipitated AuNCs with a 3:1 mixture of 2-propanol to water [10, 54]. The collected precipitate was resuspended in apure water to provide a stable suspension. In a similar approach, Galchenko et al used a selective precipitation method for purification of [Au25(TPP)10(PET)5X2]2+ NCs by using a mixture of methanol/water (Figure 5) [55]. For the starting material (NCs before purification), the UV-Vis spectrum (Figure 5A, black line) shows the overlapping spectrum of the impurity (Au2/Au3) (red line) and the purified product (Au25NCs) (blue line). The UV-Vis spectrum of the purified Au25NCs gives two new sharp transitions, with absorbance rising moderately < 300 nm. The successful purification was also confirmed by ESI-MS, shown in Fig. 5C. In the ESI-MS spectrum of the starting material, a strong peak was observed, which was assigned to the undesired impurity (Au2 complex). After purification, this intense peak disappeared and a new peak (purified Au25NCs) dominated the spectrum. For some water-soluble AuNCs, the fluorescence intensity decreased after the purification by irreversible aggregation [48] [56] [57–59]. This method avoids changes that can occur to the NC surface upon treatment with ions or changes in pH. Though the impurity was removed, the fluorescence behavior of [Au25(TPP)10(PET)5X2]2+ NCs was not mentioned before and after the selective precipitation by methanol/water, so assessing the need for further purification is challenging.
Figure 5.
(A) UV-Vis spectra of the “purified” [Au25(TPP)10(PET)5X2]2+ NC suspension and from its selectively separated compounds, y-offset for a better overview. The inset illustrates the broadening of Au25 NCs optical features by the presence of Au2/Au3 complexes. (B) Optical microscope image of Au25 NC crystals. (C) ESI-MS analysis of “purified” Au25 NC suspension and after selective purification. The inset shows the crystal structure of the main components (Au2 complex and Au25 NC). Reproduced from Ref. [55] with permission from The Royal Society of Chemistry.
2.1.4. Salt-induced precipitation
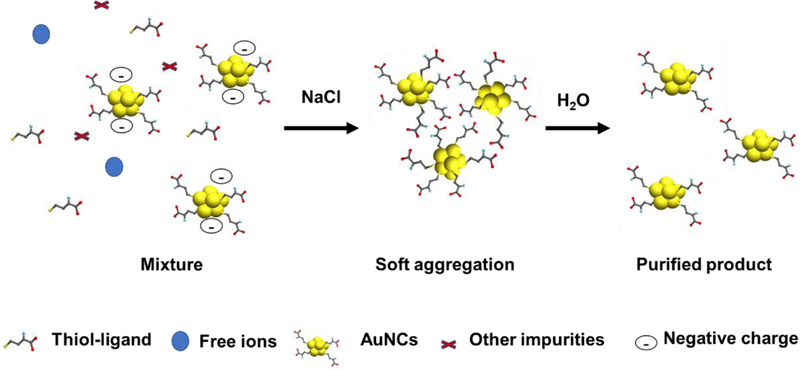
Salts (typically NaCl) can induce the precipitation of small molecules and nano materials, a strategy that has been used to purify thiol-modified DNA-labelled AuNPs [60]. Similarly, NCs protected by certain thiol ligands can be precipitated and hence separated from impurities using salt. In the presence of salt, the absolute value of the zeta potential for NCs gradually decreases, which reduces the repulsion between NCs. This results in the formation of “soft aggregates” that precipitate and can be separated by centrifugation. The effect of residual salt can be minimized by re-dissolving the precipitates in a large quantity of water (Figure 6), a rapid strategy that is useful for most conditions not requiring very low ionic strength. Sun et al generated 11-mercaptoundecanoic acid (11-MUA) protected AuNCs (11-MUA-AuNCs) in alkaline media [61]. These 11-MUA-AuNCs formed aggregates in the presence of a high concentration of NaCl and could be readily purified [62]. It should be noted that the neutralization of surface charge at high salt levels can alter NC structure and behavior [63]. Overall, salt precipitation protocols are cost-effective, fast, convenient, and can be customized to purify different NCs by selection of the appropriate solvent, salt or metal ions. However, NaCl is only one of several possible salts could be applied to affect the solubility of NCs for their purification.
Figure 6.
Purification of thiol-ligand modified AuNCs by the salt precipitation strategy.
2.2. Size based purification
2.2.1. Dialysis
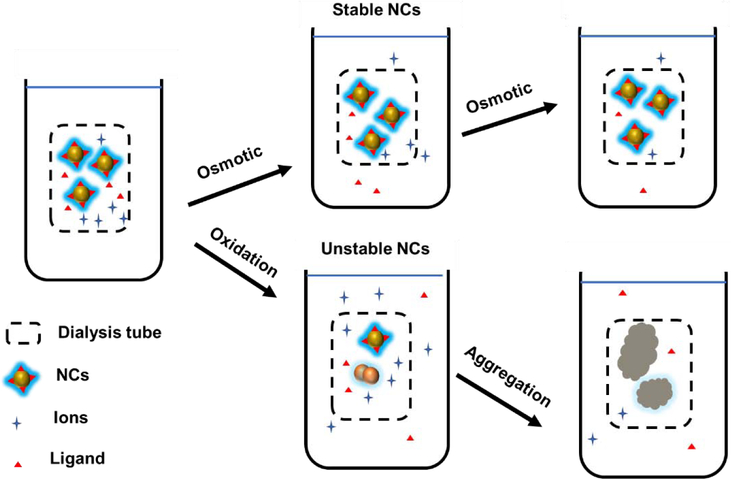
Dialysis employs a cost-effective semi-permeable membrane for size-dependent separation and is useful for the purification of NCs. For instance, a dialysis membrane (3.5 K molecular weight cutoff) was used to purify glutathione-protected AuNCs (GSH-AuNCs). [64] Small molecules such as GSH can cross the membrane while the relatively large GSH-AuNCs remain inside the membrane. After dialysis for 1 day, AuNCs were successfully separated, providing a bright yellow solution. While generally effective, dialysis procedures are time-consuming and NCs do not always survive the dialysis process due to oxidation (Figure 7). For instance, most CuNCs are not stable in water in the presence of oxygen [40, 65]. The copper species in these CuNCs were mostly copper (I) mixed with a small ratio of copper (0). These unstable copper NCs can undergo significant oxidation to Cu2+ after dialysis [66]. The loss of Cu2+ from the colloid of NCs according to chemical equilibrium changes the properties of CuNCs and further causes the NCs to aggregate [67–69]. There is also a concern for the purification of high molecular weight ligands. For instance, 14,000 molecular weight cut-off dialysis membrane was used for purification of polyvinylpyrrolidone (PVP) protected CuNCs. However, the average molecular weight of PVP is around 40 kDa, which is already larger than the pore size of the membrane [70]. In this case, the unbound PVP could not be easily purified away from the NCs. Overall, dialysis provides a cost-effective but time consuming method for the purification of stable NCs away from small molecule impurities.
Figure 7.
Purification of stable NCs by dialysis, showing challenges with unstable NCs.
2.2.2. Ultrafiltration
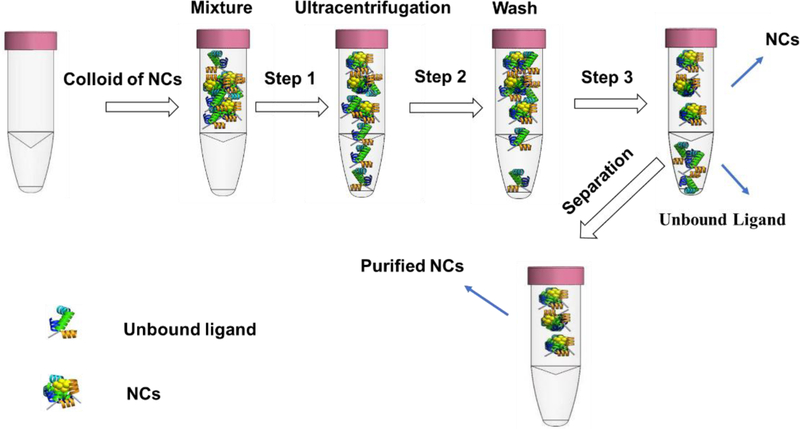
Ultrafiltration uses size-exclusion membranes to filter nanomaterials by the combination of an ultrafiltration tube and centrifugation [71]. For the purification, the NCs with higher molecular weight remains inside the filter, while the free ligands with low molecular weight pass through with solvent during centrifugation (Figure 8). In a recent study, Pramanik et al purified thioctic acid-protected AuNCs using a molecular weight cut-off (3 kDa) filter. The significant differences between the molecular weight of the AuNCs and thioctic acid enabled successful purification [72]. Another work used a 10 kDa cut-off filter to remove unbound peptides (Lys-Cys-Lys) from the peptide-protected AuNCs (K-AuNCs). Since the ultrafiltration relies on size differences between NCs and impurities, purification away from high molecular weight impurities (e.g. polymeric ligands) can be challenging.
Figure 8.
Scheme for purification of NCs by ultracentrifugation.
2.2.3. Size exclusion chromatography
Size exclusion chromatography (SEC) purifies analytes based on their size. The technique has been used for purification of proteins and organic molecules, but the use of this strategy to purify NCs is still limited [73]. Li et al used the G-75 Sephadex gel column for the purification of dansylated bovine serum albumin modified AuNCs (AuNC@dBSA) (Figure 9) [74]. Since the retention time for NCs in the column was shorter than the dansylated BSA (dBSA), AuNC@dBSA with red emission elute before the dBSA with green emission. After purification using an aqueous gel column packed with Sephadex G-75, the fluorescence intensity of AuNCs at 604 nm was enhanced, while the intensity at 465 nm decreased due to the removal of dBSA. The purified sample from this protocol improves the sensitivity of AuNC@dBSA to detect Hg2+ ion. This method can be extended for the purification of other NCs with different ligands. For instance, AuNCs@Keratin was purified with a column filled with Sephadex G25, [75] and Wang used a desalting column with cut-off 3 kDa for purification of the impurities from the colloid of Au22-CBz [76]. Size-exclusion chromatographic techniques provide a useful tool for purifying NCs from ligands as well as from each other.
Figure 9.
Fluorescence photographs of the G-75 Sephadex gel column purification procedure of AuNC@dBSA from dBSA under the 365 nm irradiation and the photos represent the elution at the indicated time periods. Reproduced from Ref. [74] with permission from The Royal Society of Chemistry.
The typical materials and equipment for size-exclusion methods including ultracentrifuges, dialysis, and chromatography are listed in Table 1. In combination with the purification time for dialysis (normally >24 hours), ultrafiltration (ca. 1 hour) and chromatography (ca. 3 hours), it can be concluded ultrafiltration and dialysis provide a most time-efficient and cost-effective process respectively. A range of purification processes in recent papers are summarized in Table 1. According to the table, most methods use size exclusion or polarity-based precipitation processes. However, the fluorescence properties of most NCs after purification are not mentioned compared to the starting materials, making assessment of the purity of the isolated NCs challenging.
Table 1.
NCs purification strategies
| NCs | Purification process | Mechanism | Time | Yield | Ref. |
|---|---|---|---|---|---|
| GSH-AuNCs | Dialysis | Size exclusion | Hours | Not available | [77] |
| Au-HHC NCs | Dialysis | Size exclusion | Hours | Not available | [11] |
| L-NIBC-AuNCs | Ultracentrifuge | Size exclusion | <1 h | Not available | [20] |
| PVP-CuNCs | Dialysis | Size exclusion | Hours | Not available | [70] |
| 64Cu-CuNCs | Ultracentrifuge | Size exclusion | <1 h | Purified with radio-iTLC (yield ≥95%) | [78] |
| Prot-NCs | Gel filtration and FPLC | Size exclusion | Hours | Not available | [79] |
| Au11(PPh3)8Cl2 | DCM/Hex solvent extraction | Polarity based extraction | <1 h | Yield (>60%) | [80] |
| GSH-AuNCs | Solvent induced precipitation (isopropanol). | Polarity based precipitation | <1 h | Not available | [81] |
FPLC: Fast protein liquid chromatography; HHC (H-CKRWWKWIRW-NH2) peptide; NIBC: N-isobutyryl-l-cysteine; Prot: CTPR proteins; GSH: Glutathione; PPh3: Triphenylphosphine; iTLC: radioactive thin layer chromatography.
The combination of different methods can be used for enhanced purification of NCs. For instance, papain-protected AuNCs (AuNCs@Papain) were purified by Yang et al with an Amicon Ultra-4 filter under centrifugation followed by dialysis (300 MWCO) to further purify AuNCs@Papain. Finally, the crude products were purified with a silica column chromatography using mixtures of methylene chloride and methanol as eluents [82].
3. Separation of different NC species
NCs with the same number of metal atoms can still vary from each other by number, type, and chirality of the ligands. NCs in these groups can show comparable physical properties such as size and polarity. Currently, the techniques for isolating different species of NCs are mainly based on chromatography (such as size, chirality, hydrophobicity) and electrophoresis.
3.1. Chromatography
3.1.1. Normal phase chromatography
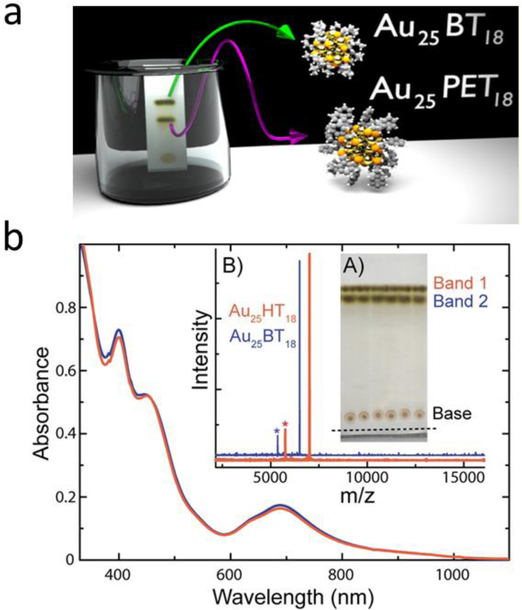
In normal phase chromatography, comparatively polar NCs bind strongly with stationary phases such as silica, while less polar NCs can be eluted away by non-polar mobile phases. Ghosh et al employed a normal phase thin-layer chromatography (TLC) strategy for separation of Au25PET18 (PET- phenylethanethiolate, C6H13S−) and Au25BT18 (BT- butanethiolate, C4H9S−) (Figure 10) based on differences in polarity and the affinity of different NCs towards a stationary phase. [83] The Au25PET18 were successfully separated from Au25BT18 even though the polarity difference between the two NCs was small. Two clear separated bands of Au25BT18 (less polar) and Au25PET18 (more polar) were observed on the TLC plate. Following extraction of the two bands of Au25BT18 and Au25PET18 from the TLC plate by dissolving in DCM, matrix-assisted laser desorption/ionization (MALDI) mass spectrum (MALDI-MS) confirmed the successful isolation of two NCs (Figure 10b, B). This method provides effective separation of species. However, such normal phase chromatography is best for hydrophobic NCs, but less useful for highly charged NCs.
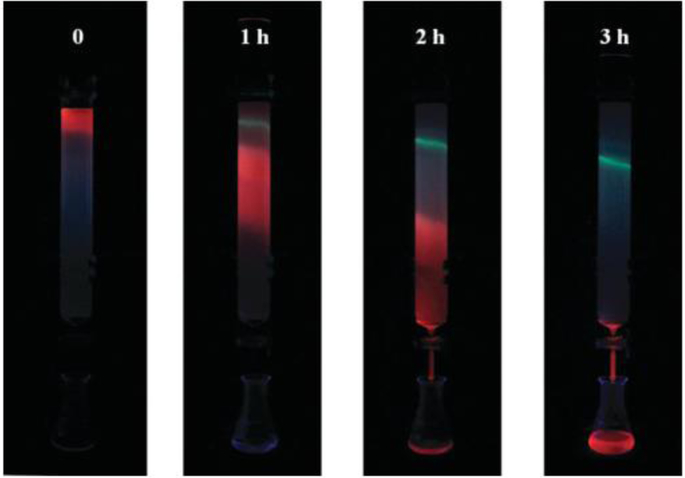
Figure 10.
(a) Scheme for the TLC plate used for the separation of NCs. (b) UV–Vis spectra of the TLC separated materials. (A) Photograph of the TLC plate used for cluster separation. Bands 1 and 2 are due to two separated clusters. The base shows the location where the mixture was spotted. The level of liquid is marked with a dashed line. (B) MALDI-MS data of TLC separated materials confirming that bands 1 (red trace) and 2 (blue trace) are pure Au25HT18 and Au25BT18, respectively. The fragmented product, Au21L14 is shown with an asterisk (*) in each trace. [83]. Copyright (2014) American Chemical Society.
3.1.2. Reversed-phase chromatography
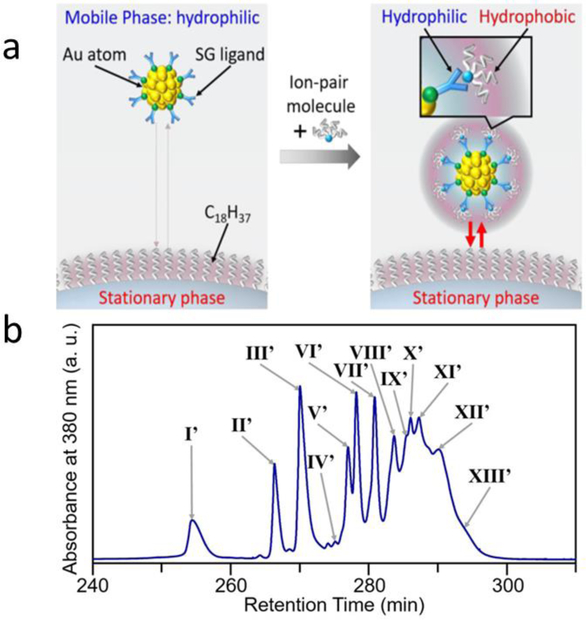
Reversed-phase (RP) chromatography employs a polar mobile phase, while the stationary phase is highly hydrophobic. As a result, hydrophobic molecules bind to the stationary phase and less hydrophobic/polar molecules pass through with the polar mobile phase. RP chromatography is an effective method for separating different water-soluble NCs. For instance, Niihori et al found that reversed-phase high-performance liquid chromatography (RP-HPLC) is efficient for separating thiolate (SR)-protected AuNCs (Aun(SR)m) of different sizes [84]. GSH-modified AuNCs are highly hydrophilic and were separated by increasing the hydrophobicity with an ion-pair reagent (Figure 11). The results show that ion pairs form at pH 8.8 between COO− group and [(C4H9)4N]+ ions on NCs, increasing the hydrophobicity of Aun(SR)m. At pH 8.8, both carboxylate groups are deprotonated and during elution small NCs eluted faster because of their lower hydrophobicity. The larger NCs with a greater number of COO− groups become more hydrophobic with ion pairing and eluted more slowly than smaller NCs. The NCs including Aun(S(PG))mNCs and Agn(SG)mNCs were successfully isolated by RP-HPLC. Figure 11b shows the typical resulting chromatogram for the separation of NCs at pH 8.8 in order. Peaks I′, II′, III′, V′, VI′, VII′, IX′, and XI′ are attributed to the separated NCs including Au10(SG)10, Au15(SG)13, Au18(SG)14, Au22(SG)16, Au25(SG)18, Au29(SG)20, Au33(SG)22, and Au39(SG)24.
Figure 11.
(a) Diagram showing the basic concept of RP-HPLC in the separation of hydrophilic Aun(SR)m clusters. (b) Chromatogram spectrum of each peak obtained during experiments at pH 8.8 using the cationic ion-pair reagent [(C4H9)4N]ClO4. Reprinted with permission from [84]. Copyright (2017) American Chemical Society.
Different species of NCs were separated by chromatography techniques based on different mechanisms such as their size, chirality, and polarity (Table 2). However, quantitative information has not been reported for these chromatographic techniques.
Table 2.
Separation of NCs by NP, RP, and other chromatography techniques
| Method | NCs | Water solubility | Separation mechanism | Evidence for separation (comparison) | Ref. |
|---|---|---|---|---|---|
| RP-HPLC | [Au24M(SR)18]c0 (M = Au, Pt, or Pd) | Soluble | Polarity | ESI-MS, UV-Vis | [85] |
| NP-HPLC | Cu5L6, Cu6L6, Cu7L6, Cu8L6, Cu9L6 (L= C10H16N3O6S) | Not Soluble | Sizes | ESI-MS, UV-Vis | [86] |
| Capillary-HPLC | Au130(ddt)50, Au137(ddt)56, Au144(ddt)60 | Not soluble | Sizes | ESI-TOF-MS | [87] |
| RP-HPLC | [Au9Ag12(SR)4(dppm)6X6]3+-C, [Au9Ag12(SR)4(dppm)6X6]3+-Ac | Soluble | Chirality | ESI-MS, CD | [88] |
| RP-HPLC | Aun(SG)m | Soluble | Sizes | Chromatogram, optical absorption | [84] |
ESI-TOF-MS: electrospray ionization time-of-flight mass spectrometry; CD: Circular dichroism spectroscopy
3.2. Electrophoresis
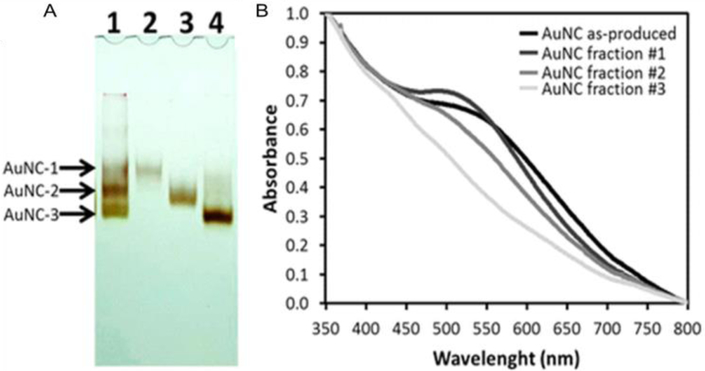
Electrophoresis is a technique to separate charged materials by using electrophoretic mobility. In a typical gel electrophoresis (GE) process, small NCs migrate quickly through the mesh of the gel, while larger NCs migrate more slowly, thereby allowing the separation of NCs of different sizes. Plascencia-Villa et al used polyacrylamide gel electrophoresis (PAGE) method for the separation of AuNCs with specific sizes, even though the difference between sizes was only ~0.5 nm (Figure 12). The normalized absorption spectra of the three different size NCs separated from electrophoresis shows different behaviors (Figure 12B). The starting materials showed a small broad absorbance at 500–600 nm. In contrast, the separated AuNCs corresponding to the upper band (AuNCs-1) showed absorption centered at 520 nm (typical SPR absorption for large AuNPs), while the other two fractions (middle and lower bands) did not indicate SPR (typical NC behavior). The difference of the UV-Vis spectra between AuNCs-1, AuNCs-2, and AuNCs-3 indicates they were successfully separated. [89] In another work, PAGE was employed to separate dithiothreitol, diacetylene, chiral dithiol, diglyme and biphenyldithiol protected NCs [90]. The as-formed products were clearly separated. All these NCs show high monodispersity with no aggregation after separation [91, 92].
Figure 12.
Size-dependent properties. (A) Polyacrylamide gel electrophoresis, to verify heterogeneity, separate, and purify AuNCs into fractions or subpopulations of specific size (line 1, original sample; line 2, fraction #1; line 3, fraction #2; line 4, fraction #3). (B) Absorbance spectroscopy of gold nanoclusters, as-produced mixture, and purified fractions. Adapted with permission from ref. [93] Copyright 2016 American Chemical Society.
Conclusion and outlook
NCs are emerging as highly useful materials in a wide range of fields. As applications emerge, effective purification of these NCs becomes critical. Polarity-based precipitation methods purify NCs with high efficiency, but the stability and hence properties of the NCs may be compromised. Size exclusion methods are useful but are efficient only when the size of NC and impurity is significantly different. For separation of different species of NCs, chromatography and gel electrophoresis techniques can isolate the NCs with only slightly different properties. But the yields for these strategies can vary. In summary, significant efforts should be directed towards the effective purification and separation process for NCs without destroying their original properties.
Highlights.
Different mechanisms for purification of nanoclusters are summarized on the basis of polarity and size.
Different techniques for separation of NCs are evaluated on the basis of chromatography and electrophoresis.
The insightful discussion on efficiency, yield and time for purification/separation are proposed.
The properties of NCs that support the success of the purification/separation protocol are stated.
ACKNOWLEDGMENT
The authors thank the China Scholarship Council (CSC) for the collaboration support.
Funding Sources
This work was supported by the NIH (AI134770 and EB022641).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
REFERENCES
- [1].Jin R Quantum sized, thiolate-protected gold nanoclusters. Nanoscale. 2010;2:343–62. [DOI] [PubMed] [Google Scholar]
- [2].Benjamin Foerster VAS, Emily A. Carter3, Carsten Sönnichsen, Stephan Link. Plasmon damping depends on the chemical nature of the nanoparticle interface. Science Advances 2019;5:eaav0704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Niihori Y, Hashimoto S, Koyama Y, Hossain S, Kurashige W, Negishi Y. Dynamic Behavior of Thiolate-Protected Gold–Silver 38-Atom Alloy Clusters in Solution. The Journal of Physical Chemistry C. 2019;123:13324–9. [Google Scholar]
- [4].Han Y, He DS, Liu Y, Xie S, Tsukuda T, Li ZY. Size and shape of nanoclusters: single-shot imaging approach. Small. 2012;8:2361–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Day PN, Pachter R, Nguyen KA, Jin R. Theoretical Prediction of Optical Absorption and Emission in Thiolated Gold Clusters. J Phys Chem A. 2019:in press. [DOI] [PubMed] [Google Scholar]
- [6].Zhao J, Ge L, Yuan H, Liu Y, Gui Y, Zhang B, et al. Heterogeneous gold catalysts for selective hydrogenation: from nanoparticles to atomically precise nanoclusters. Nanoscale. 2019;11:11429–36. [DOI] [PubMed] [Google Scholar]
- [7].Abbas MA, Kamat PV, Bang JH. Thiolated Gold Nanoclusters for Light Energy Conversion. ACS Energy Letters. 2018;3:840–54. [Google Scholar]
- [8].Alhilaly MJ, Huang RW, Naphade R, Alamer B, Hedhili MN, Emwas AH, et al. Assembly of Atomically Precise Silver Nanoclusters into Nanocluster-Based Frameworks. J Am Chem Soc. 2019;141:9585–92. [DOI] [PubMed] [Google Scholar]
- [9].Shi Y-e, Zhuang X, Cao L, Gou S, Xiong Y, Lai W-F, et al. Copper-Nanocluster-Based Transparent Ultraviolet-Shielding Polymer Films. ChemNanoMat. 2019;5:110–5. [Google Scholar]
- [10].Wang Q, Wang S, Hu X, Li F, Ling D. Controlled synthesis and assembly of ultra-small nanoclusters for biomedical applications. Biomater Sci. 2019;7:480–9. [DOI] [PubMed] [Google Scholar]
- [11].Pranantyo D, Liu P, Zhong W, Kang ET, Chan-Park MB. Antimicrobial Peptide-Reduced Gold Nanoclusters with Charge-Reversal Moieties for Bacterial Targeting and Imaging. Biomacromolecules. 2019:2922–33. [DOI] [PubMed] [Google Scholar]
- [12].Zhao H, Wen X, Li W, Li Y, Yin C. A copper-mediated on–off–on gold nanocluster for endogenous GSH sensing to drive cancer cell recognition. Journal of Materials Chemistry B. 2019;7:2169–76. [DOI] [PubMed] [Google Scholar]
- [13].Park S, Kim H, Lim SC, Lim K, Lee ES, Oh KT, et al. Gold nanocluster-loaded hybrid albumin nanoparticles with fluorescence-based optical visualization and photothermal conversion for tumor detection/ablation. J Control Release. 2019;304:7–18. [DOI] [PubMed] [Google Scholar]
- [14].Wu J, Li F, Hu X, Lu J, Sun X, Gao J, et al. Responsive Assembly of Silver Nanoclusters with a Biofilm Locally Amplified Bactericidal Effect to Enhance Treatments against Multi-Drug-Resistant Bacterial Infections. ACS Central Science. 2019:in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Chakraborty I, Pradeep T. Atomically Precise Clusters of Noble Metals: Emerging Link between Atoms and Nanoparticles. Chem Rev. 2017;117:8208–71. [DOI] [PubMed] [Google Scholar]
- [16].Kefayat A, Ghahremani F, Motaghi H, Amouheidari A. Ultra-small but ultra-effective: Folic acid-targeted gold nanoclusters for enhancement of intracranial glioma Tumors’ radiation therapy efficacy. Nanomedicine. 2019;16:173–84. [DOI] [PubMed] [Google Scholar]
- [17].Schira R, Rabilloud F. Localized Surface Plasmon Resonance in Free Silver Nanoclusters Agn, n = 20–147. The Journal of Physical Chemistry C. 2019;123:6205–12. [Google Scholar]
- [18].Xie Y, Liu Y, Yang J, Liu Y, Hu F, Zhu K, et al. Gold Nanoclusters for Targeting Methicillin-Resistant Staphylococcus aureus In Vivo. Angew Chem Int Ed Engl. 2018;57:3958–62. [DOI] [PubMed] [Google Scholar]
- [19].Prakash KT, Singh N, Venkatesh V. Synthesis of novel luminescent copper nanoclusters with substituent driven self-assembly and aggregation induced emission (AIE). Chem Commun. 2019;55:322–5. [DOI] [PubMed] [Google Scholar]
- [20].Gao G, Chen R, He M, Li J, Li J, Wang L, et al. Gold nanoclusters for Parkinson’s disease treatment. Biomaterials. 2019;194:36–46. [DOI] [PubMed] [Google Scholar]
- [21].Chao YC, Cheng KP, Lin CY, Chang YL, Ko YY, Hou TY, et al. Non-Toxic Gold Nanoclusters for Solution-Processed White Light-Emitting Diodes. Sci Rep. 2018;8:8860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Nair LV, Nair RV, Shenoy SJ, Thekkuveettil A, Jayasree RS. Blood brain barrier permeable gold nanocluster for targeted brain imaging and therapy: an in vitro and in vivo study. Journal of Materials Chemistry B. 2017;5:8314–21. [DOI] [PubMed] [Google Scholar]
- [23].Ojea-Jimenez I, Capomaccio R, Osorio I, Mehn D, Ceccone G, Hussain R, et al. Rational design of multi-functional gold nanoparticles with controlled biomolecule adsorption: a multi-method approach for in-depth characterization. Nanoscale. 2018;10:10173–81. [DOI] [PubMed] [Google Scholar]
- [24].Sokołowska K, Malola S, Lahtinen M, Saarnio V, Permi P, Koskinen K, et al. Towards Controlled Synthesis of Water-Soluble Gold Nanoclusters: Synthesis and Analysis. The Journal of Physical Chemistry C. 2019;123:2602–12. [Google Scholar]
- [25].Yu H, Rao B, Jiang W, Yang S, Zhu M. The photoluminescent metal nanoclusters with atomic precision. Coordination Chemistry Reviews. 2019;378:595–617. [Google Scholar]
- [26].Lei Y, Tang L, Xie Y, Xianyu Y, Zhang L, Wang P, et al. Gold nanoclusters-assisted delivery of NGF siRNA for effective treatment of pancreatic cancer. Nat Commun. 2017;8:15130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Zhou W, Zhu J, Teng Y, Du B, Han X, Dong S. Novel dual fluorescence temperature-sensitive chameleon DNA-templated silver nanocluster pair for intracellular thermometry. Nano Research. 2018;11:2012–23. [Google Scholar]
- [28].Yu Q, Gao P, Zhang KY, Tong X, Yang H, Liu S, et al. Luminescent gold nanocluster-based sensing platform for accurate H2S detection in vitro and in vivo with improved anti-interference. Light: Science & Applications. 2017;6:e17107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Xie Y, Xianyu Y, Wang N, Yan Z, Liu Y, Zhu K, et al. Functionalized Gold Nanoclusters Identify Highly Reactive Oxygen Species in Living Organisms. Advanced Functional Materials. 2018;28:1702026. [Google Scholar]
- [30].Pathak PK, Kumar A, Prasad BB. Functionalized nitrogen doped graphene quantum dots and bimetallic Au/Ag core-shell decorated imprinted polymer for electrochemical sensing of anticancerous hydroxyurea. Biosens Bioelectron. 2019;127:10–8. [DOI] [PubMed] [Google Scholar]
- [31].Zhang D, Li W, Ma Z, Han H. Improved ELISA for tumor marker detection using electro-readout-mode based on label triggered degradation of methylene blue. Biosens Bioelectron. 2019;126:800–5. [DOI] [PubMed] [Google Scholar]
- [32].Zhao L, Yin S, Ma Z. Ca2+-Triggered pH-Response Sodium Alginate Hydrogel Precipitation for Amplified Sandwich-Type Impedimetric Immunosensor of Tumor Marker. ACS Sens. 2019;4:450–5. [DOI] [PubMed] [Google Scholar]
- [33].Bhatt G, Mishra K, Ramanathan G, Bhattacharya S. Dielectrophoresis assisted impedance spectroscopy for detection of gold-conjugated amplified DNA samples. Sensors and Actuators B: Chemical. 2019;288:442–53. [Google Scholar]
- [34].Zhou X, Yang CT, Xu Q, Lou Z, Xu Z, Thierry B, et al. Gold Nanoparticle Probe-Assisted Antigen-Counting Chip Using SEM. ACS Appl Mater Interfaces. 2019;11:6769–76. [DOI] [PubMed] [Google Scholar]
- [35].Wang L, Shen K, Chen M, Zhu Y. One-core-atom loss in a gold nanocluster promotes hydroamination reaction of alkynes. Nanoscale. 2019;11:13767–72. [DOI] [PubMed] [Google Scholar]
- [36].Wei W, Lu Y, Chen W, Chen S. One-pot synthesis, photoluminescence, and electrocatalytic properties of subnanometer-sized copper clusters. J Am Chem Soc. 2011;133:2060–3. [DOI] [PubMed] [Google Scholar]
- [37].Chen T, Yao Q, Nasaruddin RR, Xie J. Electrospray Ionization Mass Spectrometry: A Powerful Platform for Noble-Metal Nanocluster Analysis. Angew Chem Int Ed Engl. 2019:in press. [DOI] [PubMed] [Google Scholar]
- [38].Li JJ, Guan ZJ, Lei Z, Hu F, Wang QM. Same Magic Number but Different Arrangement: Alkynyl-Protected Au25 with D3 Symmetry. Angew Chem Int Ed Engl. 2019;58:1083–7. [DOI] [PubMed] [Google Scholar]
- [39].Kang X, Zhu M. Tailoring the photoluminescence of atomically precise nanoclusters. Chem Soc Rev. 2019. [DOI] [PubMed] [Google Scholar]
- [40].Li D, Chen Z, Wan Z, Yang T, Wang H, Mei X. One-pot development of water soluble copper nanoclusters with red emission and aggregation induced fluorescence enhancement. RSC Advances. 2016;6:34090–5. [Google Scholar]
- [41].Basu S, Paul A, Chattopadhyay A. Zinc mediated crystalline assembly of gold nanoclusters for expedient hydrogen storage and sensing. Journal of Materials Chemistry A. 2016;4:1218–23. [Google Scholar]
- [42].Kuppan B, Maitra U. Instant room temperature synthesis of self-assembled emission-tunable gold nanoclusters: million-fold emission enhancement and fluorimetric detection of Zn2+. Nanoscale. 2017;9:15494–504. [DOI] [PubMed] [Google Scholar]
- [43].Li D, Zhao Y, Chen Z, Mei X, Qiu X. Enhancement of fluorescence brightness and stability of copper nanoclusters using Zn(2+) for ratio-metric sensing of S(2). Mater Sci Eng C Mater Biol Appl. 2017;78:653–7. [DOI] [PubMed] [Google Scholar]
- [44].Lu B, He Q, He Y, Chen X, Feng G, Liu S, et al. Dual-channel-coded microbeads for multiplexed detection of biomolecules using assembling of quantum dots and element coding nanoparticles. Anal Chim Acta. 2018;1024:153–60. [DOI] [PubMed] [Google Scholar]
- [45].Guan G, Zhang SY, Cai Y, Liu S, Bharathi MS, Low M, et al. Convenient purification of gold clusters by co-precipitation for improved sensing of hydrogen peroxide, mercury ions and pesticides. Chem Commun 2014;50:5703–5. [DOI] [PubMed] [Google Scholar]
- [46].Gayen C, Basu S, Goswami U, Paul A. Visible Light Excitation-Induced Luminescence from Gold Nanoclusters Following Surface Ligand Complexation with Zn2+ for Daylight Sensing and Cellular Imaging. Langmuir. 2019;35:9037–43. [DOI] [PubMed] [Google Scholar]
- [47].Li D, Wang G, Cheng L, Wang C, Mei X. Engineering the Self-Assembly Induced Emission of Copper Nanoclusters as 3D Nanomaterials with Mesoporous Sphere Structures by the Crosslinking of Ce3+. ACS Omega. 2018;3:14755–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Li D, Chen Z, Yang T, Wang H, Lu N, Mei X. Green synthesis of highly fluorescent AuNCs with red emission and their special sensing behavior for Al3+. RSC Advances. 2016;6:19182–9. [Google Scholar]
- [49].Vilg JV, Undeland I. pH-driven solubilization and isoelectric precipitation of proteins from the brown seaweed Saccharina latissima-effects of osmotic shock, water volume and temperature. J Appl Phycol. 2017;29:585–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Xiong H, Wang W, Liang J, Wen W, Zhang X, Wang S. A convenient purification method for metal nanoclusters based on pH-induced aggregation and cyclic regeneration and its applications in fluorescent pH sensors. Sensors and Actuators B: Chemical. 2017;239:988–92. [Google Scholar]
- [51].Xiong H, Zheng H, Wang W, Liang J, Wen W, Zhang X, et al. A convenient purification method for silver nanoclusters and its applications in fluorescent pH sensors for bacterial monitoring. Biosens Bioelectron. 2016;86:164–8. [DOI] [PubMed] [Google Scholar]
- [52].Deng HH, Zhang LN, He SB, Liu AL, Li GW, Lin XH, et al. Methionine-directed fabrication of gold nanoclusters with yellow fluorescent emission for Cu2+ sensing. Biosens Bioelectron. 2015;65:397–403. [DOI] [PubMed] [Google Scholar]
- [53].Sun J, Zhang J, Jin Y. 11-Mercaptoundecanoic acid directed one-pot synthesis of water-soluble fluorescent gold nanoclusters and their use as probes for sensitive and selective detection of Cr3+ and Cr6+. J Mater Chem C. 2013;1:138–43. [Google Scholar]
- [54].Yin PD Miao-miao, Chen Wen-qi, Xu Shi-ping, Yang Liyun, Jiang Feng-Lei, and Liu Yi. Thermodynamics and Mechanisms of the Interactions between Ultra-small Fluorescent Gold Nanoclusters and Human Serum Albumin, γ-Globulins and Transferrin: A Spectroscopic Approach. Langmuir. 2017; 33:5108–16. [DOI] [PubMed] [Google Scholar]
- [55].Galchenko M, Schuster R, Black A, Riedner M, Klinke C. Preparation of high-yield and ultra-pure Au25 nanoclusters: towards their implementation in real-world applications. Nanoscale. 2019;11:1988–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Liu J, Zhang QM, Feng Y, Zhou Z, Shih K. Solvent-Switching Gelation and Orange-Red Emission of Ultrasmall Copper Nanoclusters. Chemphyschem. 2016;17:225–31. [DOI] [PubMed] [Google Scholar]
- [57].Ling Y, Wu JJ, Gao ZF, Li NB, Luo HQ. Enhanced Emission of Polyethyleneimine-Coated Copper Nanoclusters and Their Solvent Effect. The Journal of Physical Chemistry C. 2015;119:27173–7. [Google Scholar]
- [58].Ai L, Liu Z, Zhou D, Liu J, Zou H, Wu Z, et al. Copper inter-nanoclusters distance-modulated chromism of self-assembly induced emission. Nanoscale. 2017;9:18845–54. [DOI] [PubMed] [Google Scholar]
- [59].Duan GX, Tian L, Wen JB, Li LY, Xie YP, Lu X. An atomically precise all-tert-butylethynide-protected Ag51 superatom nanocluster with color tunability. Nanoscale. 2018;10:18915–9. [DOI] [PubMed] [Google Scholar]
- [60].Christau S, Moeller T, Genzer J, Koehler R, von Klitzing R. Salt-Induced Aggregation of Negatively Charged Gold Nanoparticles Confined in a Polymer Brush Matrix. Macromolecules. 2017;50:7333–43. [Google Scholar]
- [61].Sun J, Yue Y, Wang P, He H, Jin Y. Facile and rapid synthesis of water-soluble fluorescent gold nanoclusters for sensitive and selective detection of Ag+. J Mater Chem C. 2013;1:908–13. [Google Scholar]
- [62].Hurst SJ, Lytton-Jean AK, Mirkin CA. Maximizing DNA loading on a range of gold nanoparticle sizes. Anal Chem. 2006;78:8313–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Li D, Chen Z, Yang T. Fluorescence enhancement of DHLA protected gold nanoclusters in the presence of salt. New Journal of Chemistry. 2016;40:3781–5. [Google Scholar]
- [64].Tang Y, Xu J, Xiong C, Xiao Y, Zhang X, Wang S. Enhanced electrochemiluminescence of gold nanoclusters via silver doping and their application for ultrasensitive detection of dopamine. Analyst. 2019:in press. [DOI] [PubMed] [Google Scholar]
- [65].Zhang P, Li D, Chen G, Mei X, Zhang J, Chen Z. Prepare tea polyphenols modified copper nanoclusters to promote the proliferation of MC3T3-E1 in high glucose microenvironment. New Journal of Chemistry. 2019:in press. [Google Scholar]
- [66].Li D, Wang G, Peng Y, Chen Z, Gao X, Cheng L, et al. Development of ratiometric sensing and white light-harvesting materials based on all-copper nanoclusters. Nanoscale Advances. 2019;1:1086–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Wang Z, Chen B, Susha AS, Wang W, Reckmeier CJ, Chen R, et al. All-Copper Nanocluster Based Down-Conversion White Light-Emitting Devices. Adv Sci. 2016;3:1600182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Rajamanikandan R, Ilanchelian M. Red emitting human serum albumin templated copper nanoclusters as effective candidates for highly specific biosensing of bilirubin. Materials Science and Engineering: C. 2019:in press. [DOI] [PubMed] [Google Scholar]
- [69].Yang J, Song N, Jia Q. Electrostatically controlled fluorometric assay for differently charged biotargets based on the use of silver/copper bimetallic nanoclusters modified with polyethyleneimine and graphene oxide. Mikrochim Acta. 2019;186:70. [DOI] [PubMed] [Google Scholar]
- [70].Li Y, Feng L, Yan W, Hussain I, Su L, Tan B. PVP-templated highly luminescent copper nanoclusters for sensing trinitrophenol and living cell imaging. Nanoscale. 2019:in press. [DOI] [PubMed] [Google Scholar]
- [71].Li P, Kumar A, Ma J, Kuang Y, Luo L, Sun X. Density gradient ultracentrifugation for colloidal nanostructures separation and investigation. Science Bulletin. 2018;63:645–62. [DOI] [PubMed] [Google Scholar]
- [72].Pramanik G, Humpolickova J, Valenta J, Kundu P, Bals S, Bour P, et al. Gold nanoclusters with bright near-infrared photoluminescence. Nanoscale. 2018;10:3792–8. [DOI] [PubMed] [Google Scholar]
- [73].Amartely H, Avraham O, Friedler A, Livnah O, Lebendiker M. Coupling Multi Angle Light Scattering to Ion Exchange chromatography (IEX-MALS) for protein characterization. Sci Rep. 2018;8:6907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Li HW, Ai K, Wu Y. Fluorescence visual gel-separation of dansylated BSA-protected gold-nanoclusters. Chem Commun 2011;47:9852–4. [DOI] [PubMed] [Google Scholar]
- [75].Wang J, Ma S, Ren J, Yang J, Qu Y, Ding D, et al. Fluorescence enhancement of cysteine-rich protein-templated gold nanoclusters using silver(I) ions and its sensing application for mercury(II). Sensors and Actuators B: Chemical. 2018;267:342–50. [Google Scholar]
- [76].Pyo K, Ly NH, Yoon SY, Shen Y, Choi SY, Lee SY, et al. Highly Luminescent Folate-Functionalized Au22 Nanoclusters for Bioimaging. Adv Healthc Mater. 2017;6:1700203. [DOI] [PubMed] [Google Scholar]
- [77].Wang L, Cao H-X, He Y-S, Pan C-G, Sun T-K, Zhang X-Y, et al. Facile preparation of amino-carbon dots/gold nanoclusters FRET ratiometric fluorescent probe for sensing of Pb2+/Cu2+. Sensors and Actuators B: Chemical. 2019;282:78–84. [Google Scholar]
- [78].Heo GS, Zhao Y, Sultan D, Zhang X, Detering L, Luehmann HP, et al. Assessment of Copper Nanoclusters for Accurate in Vivo Tumor Imaging and Potential for Translation. ACS Appl Mater Interfaces. 2019;11:19669–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Aires A, Llarena I, Moller M, Castro-Smirnov J, Cabanillas-Gonzalez J, Cortajarena AL. A Simple Approach to Design Proteins for the Sustainable Synthesis of Metal Nanoclusters. Angew Chem Int Ed Engl. 2019;58:6214–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Chen S, Ma H, Padelford JW, Qinchen W, Yu W, Wang S, et al. Near Infrared Electrochemiluminescence of Rod-Shape 25-Atom AuAg Nanoclusters That Is Hundreds-Fold Stronger Than That of Ru(bpy)3 Standard. J Am Chem Soc. 2019;141:9603–9. [DOI] [PubMed] [Google Scholar]
- [81].Liu Y, Dong P, Jiang Q, Wang F, Pang D-W, Liu X. Assembly-enhanced fluorescence from metal nanoclusters and quantum dots for highly sensitive biosensing. Sensors and Actuators B: Chemical. 2019;279:334–41. [Google Scholar]
- [82].Yang K, Wang S, Wang Y, Miao H, Yang X. Dual-channel probe of carbon dots cooperating with gold nanoclusters employed for assaying multiple targets. Biosens Bioelectron. 2017;91:566–73. [DOI] [PubMed] [Google Scholar]
- [83].Ghosh A, Hassinen J, Pulkkinen P, Tenhu H, Ras RH, Pradeep T. Simple and efficient separation of atomically precise noble metal clusters. Anal Chem. 2014;86:12185–90. [DOI] [PubMed] [Google Scholar]
- [84].Niihori Y, Kikuchi Y, Shima D, Uchida C, Sharma S, Hossain S, et al. Separation of Glutathionate-Protected Gold Clusters by Reversed-Phase Ion-Pair High-Performance Liquid Chromatography. Industrial & Engineering Chemistry Research. 2017;56:1029–35. [Google Scholar]
- [85].Niihori Y, Koyama Y, Watanabe S, Hashimoto S, Hossain S, Nair LV, et al. Atomic and Isomeric Separation of Thiolate-Protected Alloy Clusters. J Phys Chem Lett. 2018;9:4930–4. [DOI] [PubMed] [Google Scholar]
- [86].Baghdasaryan A, Grillo R, Roy Bhattacharya S, Sharma M, Reginato E, Theraulaz H, et al. Facile Synthesis, Size-Separation, Characterization, and Antimicrobial Properties of Thiolated Copper Clusters. ACS Applied Nano Materials. 2018;1:4258–67. [Google Scholar]
- [87].Black DM, Robles G, Bach SBH, Whetten RL. Gold Nanocluster Prospecting via Capillary Liquid Chromatography-Mass Spectrometry: Discovery of Three Quantized Gold Clusters in a Product Mixture of “2 nm Gold Nanoparticles”. Industrial & Engineering Chemistry Research. 2018;57:5378–84. [Google Scholar]
- [88].Jin S, Xu F, Du W, Kang X, C hen S, Zhang J, et al. Isomerism in Au-Ag Alloy Nanoclusters: Structure Determination and Enantioseparation of [Au9Ag12(SR)4(dppm)6X6](3). Inorg Chem. 2018;57:5114–9. [DOI] [PubMed] [Google Scholar]
- [89].Zhou M, Zeng C, Chen Y, Zhao S, Sfeir MY, Zhu M, et al. Evolution from the plasmon to exciton state in ligand-protected atomically precise gold nanoparticles. Nat Commun. 2016;7:13240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Sokolowska K, Hulkko E, Lehtovaara L, Lahtinen T. Dithiol-Induced Oligomerization of Thiol-Protected Gold Nanoclusters. J Phys Chem C Nanomater Interfaces. 2018;122:12524–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Waszkielewicz M, Olesiak-Banska J, Comby-Zerbino C, Bertorelle F, Dagany X, Bansal AK, et al. pH-Induced transformation of ligated Au25 to brighter Au23 nanoclusters. Nanoscale. 2018;10:11335–41. [DOI] [PubMed] [Google Scholar]
- [92].Gold Nanoparticles as Dual Functional Sensor to Detect E.coli DH5α as a Model for gram-negative bacteria.
- [93].Plascencia-Villa G, Demeler B, Whetten RL, Griffith WP, Alvarez M, Black DM, et al. Analytical Characterization of Size-Dependent Properties of Larger Aqueous Gold Nanoclusters. The Journal of Physical Chemistry C. 2016;120:8950–8. [DOI] [PMC free article] [PubMed] [Google Scholar]