Abstract
Background
Polyetheretherketone (PEEK) is a popular material for posterior lumbar interbody fusion (PLIF) cages, although osseointegration remains limited. To optimize PEEK cage characteristics, titanium (Ti) and calcium phosphate (CaP) nanocoatings have been developed with proven mechanical safety. This multicenter randomized controlled trial compared the clinical and radiological outcome parameters of nanocoated and uncoated PEEK cages, up to 1 year after surgery.
Methods
Standard open PLIF surgery was performed on 127 patients, randomized in 3 groups: Ti-nanocoated (n = 44), CaP-nanocoated (n = 46), and uncoated PEEK cages (n = 37). Clinical assessments up to 1 year after surgery included visual analogue scales (VASs), Oswestry Disability Index (ODI), and 36-Item Short Form Survey (SF-36). Primary radiological outcome parameters were implant stability and fusion status, assessed by x-ray and computed tomography (CT) scans. Patients, surgeons, and postsurgery analysts were blinded.
Results
PLIF surgery with all cage types resulted in significant improvements of clinical outcome parameters, exceeding the minimum clinically important differences. No significant differences in VAS, ODI, or SF-36 scores were found among the 3 groups. One year after the surgery, 65.6% of patients with uncoated PEEK cages achieved definite fusion. Significantly more patients with nanocoated PEEK cages achieved definite fusion: 93.9% for Ti nanocoating (P = .0034) and 88.0% for CaP nanocoating (P = .032). No significant differences in fusion were found between the nanocoated cage types (P = .4318).
Conclusions
The similar clinical outcome improvements after 1 year suggest that nanocoated PEEK cages have the same safety and efficacy as the clinically accepted uncoated PEEK cages. Furthermore, nanocoated PEEK cages achieved a better fusion rate than uncoated PEEK cages at the 1-year follow-up. A 5-year follow-up study is warranted to revisit the findings.
Clinical Relevance
The safety, efficacy, and enhanced osseointegration of nanocoated PEEK cages were demonstrated. Osseointegration is a significant predictor of positive long-term clinical outcomes and improved implant longevity, implying a clinical added value of nanocoatings. Enhanced osseointegration becomes even more important in minimally invasive spine surgery and in patients at risk for incomplete fusion.
Keywords: PLIF, fusion cages, PEEK, nanocoating, titanium, calcium phosphate
INTRODUCTION
Lumbar interbody fusion surgery is an accepted treatment for degenerative spinal disorders, for example, chronic low back pain due to degenerative intervertebral disc disease and/or facet joint arthrosis.1,2 One of the most popular lumbar interbody fusion surgeries is the posterior lumbar interbody fusion (PLIF).3–6 It involves removing the intervertebral disc and the placement of a cage within the intervertebral space. The cage preserves the height of the intervertebral space and allows the posterior decompression of neural elements, restoration of the anterior column weight-bearing function, and the correction of the degenerative deformities, thereby stabilizing the painful motion segments. To improve biological fusion, the cages are filled with bone graft that can be harvested in the iliac crest, or collected from the resected spinous processes, lamina, and facet joints.7
Two of the most popular materials for fusion cages are titanium (Ti and its alloys) and polyetheretherketone (PEEK).8 Ti alloys are sufficiently strong under physiological loads and are biocompatible.8 Furthermore, Ti can achieve good osseointegration, especially with the right surface topography.8,9 Ti however has a high radiodensity, increasing the difficulty to assess osseointegration in radiological imaging.8 Ti also has a much higher elastic modulus than bone, leading to more subsidence and impact failure.8,10 PEEK on the other hand is an inert biocompatible polymer with a relatively low elastic modulus, similar to that of bone, hence reducing subsidence and implant failure.8,11,12 PEEK is also a radiolucent material, permitting easy assessment of radiological imaging.8,11 Due to PEEK's inert and hydrophobic nature, osseointegration however remains limited.8,11 Consequently, fibrous layers often appear at the PEEK-bone surface.13,14 Osseointegration is desirable, as several studies indicate that postoperative bony fusion is a significant predictor of positive long-term clinical outcomes and improved implant longevity.8,15,16
Given its interesting material properties, PEEK may be an attractive platform upon which to tailor new biomaterials.11 There indeed is a surging research interest to enhance the osseointegration of PEEK cages by applying surface treatments and/or coatings.8,11,15,17–20 Various types of bioactive coatings have been developed to improve upon the limited osseointegration of PEEK, for example, calcium phosphate (CaP),13,17 hydroxyapatite (HA),8,11 carbon coatings,21 and Ti.8,11,15,18,19
The biological impact of all these coatings has been thoroughly investigated. Especially for PEEK cages with Ti plasma-sprayed coatings, there is extensive literature available describing cell culture and animal experiments.11,15,18,19 These experiments demonstrate that the Ti plasma-sprayed coatings result in a better cell adhesion and osseointegration, confirming the theoretical added value of these Ti coatings.18,19
However, the mechanical safety of the coatings, that is, resistance against abrasion and the overall strength of the coating-implant interface, is important as well. When the coating is worn off, there is no longer a positive effect on the osseointegration.13 Furthermore, Ti particles are shown to cause inflammatory reactions, negatively impacting implant stability and causing pseudarthrosis.13 At present, there is no conclusive literature on what amount of wear can be tolerated by human bodies. From a clinical point of view, it is therefore recommended to keep wear to a minimum.13 Recent research has however demonstrated that the wear of Ti plasma-sprayed coatings is above a limit derived from US Food and Drug Administration (FDA) guidance documents, raising questions on the long-term stability of PEEK implants with Ti plasma-sprayed coatings.13,22
In an attempt to optimize coating characteristics for PEEK cages, PEEK cages with a Ti or CaP nanocoating have been developed.13,17 The thickness of the nanocoating is orders of magnitude smaller than the typical plasma-sprayed coatings. Kienle et al13 showed that these nanocoated cages have slightly more wear than uncoated cages, albeit significantly less than Ti plasma-sprayed coatings and well below the limit derived from FDA guidelines. An animal study performed by Meers et al17 demonstrated that the Ti nanocoating has a beneficial effect on the osseointegration, while preserving the radiolucency and elasticity of the PEEK cages.
Despite the abundance of scientific research on coated PEEK implants, only a limited number of clinical trials have been performed, indicating the necessity of more clinical trials to establish these implants in clinical practice.15 We wished to focus on cages for which biological impact and mechanical safety had been extensively investigated. Therefore, we used the aforementioned Ti-nanocoated and CaP-nanocoated PEEK cages.13,17
The aim of this 3-arm multicenter randomized controlled study was primarily to investigate the clinical outcome improvements of the nanocoated PEEK cages compared with those of the normal uncoated PEEK cages, to ascertain the safety and efficacy of the nanocoated PEEK cages. Additionally, the study examined the radiological outcome to assess the osseointegration or fusion rate of the nanocoated PEEK cages versus the uncoated PEEK cages. The comparisons were made until 1 year after the surgical procedures.
MATERIALS AND METHODS
The 3-arm multicenter randomized controlled trial was approved by the local ethics committee and was registered at AZ Maria Middelares (Gent, Belgium, protocol number: PMCFU Ortho1301_BE_PLIF Rev 2.2). Patients received full information and provided written informed consent prior to inclusion in the study.
Patients
Three clinical centers participated in the study: Regionaal Ziekenhuis Sint-Trudo (Sint-Truiden, Belgium), AZ Maria Middelares (Gent, Belgium), and AZ Delta (Roeselare, Belgium). Patients attending the surgical consultations of these clinical centers were evaluated for inclusion in the trial. Patients were recruited between August 2013 and October 2014.
Inclusion criteria were as follows: patients, aged between 18 and 75 years, with chronic mechanical low back pain with or without pain radiation to the knee (> 6 months). These patients had to be refractory to pharmacological and nonsurgical conservative treatment and were scheduled for stabilization and decompression via PLIF surgery with supplemental posterior fixation. The back pain could be attributed to degenerative disc disease, spondylolisthesis (maximum grade 1), or spinal canal stenosis, and the pathology should have been restricted to 1 level.
The exclusion criteria were as follows: patients requiring surgical treatment at more than 1 level or with previous fusion surgery at the affected levels. Furthermore, patients having an active malignancy or having chemotherapy for any kind of malignancy in the past year were excluded, as were patients with an active local or systemic infection or a systemic disease (including HIV, AIDS, and hepatitis). Proven osteoporosis where unilateral pedicle screw fixation may lead to the fracture of the endplate was an additional exclusion criterion, as was the use of postoperative stimulation or a history of recent drug or alcohol abuse. Finally, patients participating in another research project were excluded as well.
The diagnosis was made based on the medical history, a physical and neurological examination, and medical imaging.
PLIF Cages
Three types of cages were used: a PEEK cage with Ti nanocoating (group A – TSC), an uncoated PEEK cage (group B – reCreo), and a PEEK cage with CaP nanocoating (group C – osteoCon) (Orthobion GmbH, Konstanz, Germany). The properties of all 3 cages are summarized in Table 1.13 All cages have the same dimensions and angulation. The thickness of the Ti nanocoating is approximately 270 nm, whereas the CaP nanocoating is less than 100 nm thick.
Table 1.
Overview of PLIF cage specifications used in the study. PEEK cages with 2 coatings were tested: Ti nanocoating and CaP nanocoating. Uncoated PEEK cages were used as control group.
|
Uncoated |
Ti Nanocoating |
CaP Nanocoating |
|
| Size (L × W × H), mm | 25 × 11 × 10 | 25 × 11 × 10 | 25 × 11 × 10 |
| Angulation, ° | 4 | 4 | 4 |
| Coating material | None | Ti | CaP |
| Coating thickness, nm | NA | ± 270 | < 100 |
| Coating method | NA | Sputter coating | Dip coating |
| Manufacturer | Orthobion GmbH | ||
| Commercial name | reCreo | TSC | osteoCon |
Abbreviations: CaP, calcium phosphate; NA, not available; PEEK, polyetheretherketone; PLIF, posterior lumbar interbody fusion; Ti, titanium.
Treatment
The PLIF procedures were performed by 3 experienced orthopedic surgeons in 3 clinical centers, using a standardized surgical technique. The PLIF technique was identical for all 3 types of cages in this study.
The PLIF implant consisted of 2 identical PEEK cages (with or without nanocoating) with tantalum (Ta) pins as marker (in a variety of sizes). The intervertebral space was implanted with the 2 interbody cages prepared with local autograft, originating from the resected spinous process, lamina, and facet joints. The remaining interbody space surrounding the implant was also filled with local autograft. No allografts or artificial bone grafts were used to ensure a standard PLIF procedure. The fusion was further supported by a pedicle screw fixation system (Orthobion GmbH).
Randomization and Blinding
A computer-generated randomization schedule determined the type of cage for every patient. The implants were delivered to the clinical centers only with an indication of group A, B, or C. The patients were blinded to their cage type and treatment. In theory, surgeons were blinded as well. Nonetheless, they might have denoted color differences between the different cage types, that is, dark gray for the Ti nanocoating, whereas the uncoated PEEK cages and CaP nanocoated cages are off-white. Finally, everyone involved in postsurgery analysis was blinded as well, for example, the independent spine radiologist assessing the radiological outcome or the statistician performing the statistical analysis.
Outcome Measures
The primary outcome parameters in this study were both clinical and radiological outcome parameters.
To assess the clinical outcome, spinal surgeries rely on health-related quality-of-life questionnaires.23 In this study, the primary clinical outcome parameters were the scores from 3 commonly used patient-reported questionnaires: visual analogue scales (VAS) for back and leg pain (10-point scale ranging from 0: no pain to 10: worst imaginable pain), the Oswestry Disability Index (ODI), and the 36-Item Short Form Survey (SF-36).23,24 Patients were asked to report VAS back and leg pain and the ODI preoperatively, and at 3, 6, and 12 months after the surgery. The SF-36 was filled out preoperatively and 12 months after the surgery.
The primary radiological outcome was the implant stability and fusion status, assessed by x-ray and computed tomography (CT) scans. To assess possible cage migration, standing anteroposterior and lateral radiographs were performed preoperatively, and at 3, 6, and 12 months after the surgery. Research has shown that thin-section helical CT scans have a superior sensitivity to detect pseudarthrosis,25,26 and that it is recommended to use CT imaging with fine-cut axial and multiplanar reconstruction views to assess fusion status.27 Furthermore, thin-section helical scans minimize the area necessary to assess bony fusion, dramatically reducing the estimated cancer risk for patients.28 Therefore, patients underwent helical CT scans of the operated spine level 6 and 12 months after surgery. Ultrathin axial slices were reconstructed with combined iterative and filtered back projection techniques using both bone and soft tissue algorithms. Images were then interactively reconstructed, and a subset of data were reviewed in oblique sagittal and coronal planes perpendicular to the cages by an experienced independent spine radiologist, blinded to the cage types. Fusion of the operated segment was scored using a grading scale based on established evaluation criteria (Table 2).29,30 Examples of 3 of the 4 fusion grades are illustrated in Figures 1a–c.
Table 2.
Classification criteria to assess bony fusion on computed tomography scans.
|
Grade |
Classification |
Description |
| 1 | Definite fusion | Presence of 2 or more bridging bony trabeculae passing from one vertebral endplate to the other in both the sagittal and coronal planes |
| 2 | Probable fusion | At least 1 bridging bony trabecula passing from one vertebral endplate to the other in the sagittal or coronal plane, but not grade 1 |
| 3 | Probable pseudarthrosis | Absence of clear bridging bony trabeculae passing from one vertebral endplate to the other, but close bone approximation (less than 2 mm apart) |
| 4 | Definite pseudarthrosis | Clear separation of bone from both segments (more than 2 mm apart) |
Figure 1.
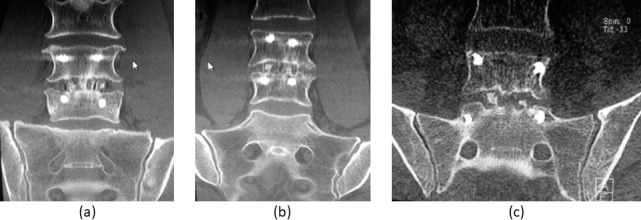
Graphical illustration of the bony fusion classification on computed tomography scans. Grade 1 fusion is classified as definite fusion (a), grade 2 represents probable fusion (b), and grade 3 shows probable pseudarthrosis (c).
Secondary outcome focused on the potential side effects and complications. For each patient, the perioperatively blood loss (mL), operation time, and hospital stay duration were recorded. A neurological examination was performed to assess function and potential side effects.
Statistical Methods
A sample size calculation was carried out before the study. The underlying assumption of the initial power analysis was that the evolution of the clinical outcome parameters would not be different among the 3 groups, 1 year after the study. The desired power was 95% (type II error = 0.05) and the type I error = 0.05. We conservatively set the effect size equal to 0.2, that is, differences between the different groups are considered significant if they are larger than 0.2 times the standard deviation (SD). The sample size was calculated based on a repeated measures analysis of variance (ANOVA) test for 3 groups and 4 repeated measures moments (before surgery, 3, 6, and 12 months after surgery). The correlation among the repeated measures was estimated to be 0.5. The resulting sample size was n = 69 or 23 patients per treatment group.
Patients' basic characteristics were compared using the χ2 test and the 1-way ANOVA test. When comparing the outcome means of the 3 cage types at a given moment in time, 1-way ANOVA tests were used. We performed paired samples t tests to assess the outcome evolution between 2 moments in time for a given treatment group. Finally, we analyzed if the outcome evolution over time was different for the 3 treatment groups, by using repeated measures ANOVA tests. The Pillai trace test was applied in the repeated measures ANOVA tests, because of its robustness for deviations from the test assumptions.31
All statistical analyses have been done by an independent statistician blinded to the study protocol. The power calculations were performed with G*Power 3.1.32 Hypothesis testing on proportions was calculated with Statistica (Statistica 7.1, StatSoft, Tulsa, Oklahoma). SPSS (IBM SPSS Statistics for Windows, Version 22.0, Armonk, New York: IBM Corp) was used for all other statistical analyses, for example, patient characteristics, and hypothesis testing on means. Data are reported as mean and SD, unless otherwise stated. Differences are considered statistically significant if P < .05.
RESULTS
Patient Population
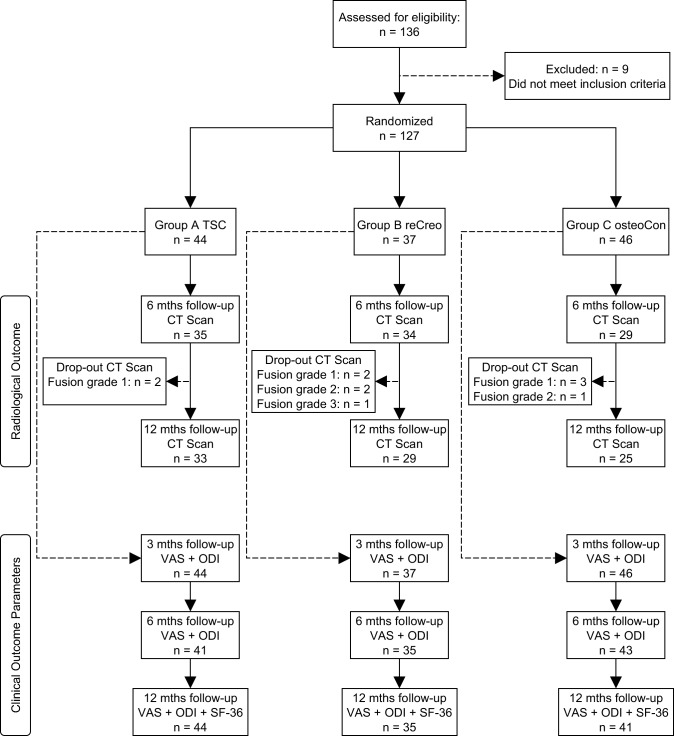
A total number of 136 patients were assessed for eligibility. Nine patients did not meet the inclusion criteria, and 127 patients were thus included in the study. Forty-four patients were randomized into group A (TSC, PEEK cages with Ti nanocoating), 37 patients into group B (reCreo, controls: uncoated PEEK cages), and 46 patients into group C (osteoCon, PEEK cages with CaP nanocoating).
The baseline characteristics of the patients, for example, gender, age, and the operated levels, are summarized in Table 3. Gender and age were comparable among the 3 treatment groups. Furthermore, the number of patients at each operated level did not differ among the 3 treatment groups. The most common operated levels were L4-L5 (50 out of 127 patients) and L5-S1 (64 out of 127 patients). Further analysis showed that the average age did not differ between male (50.25 y [9.48]) and female patients (50.76 y [9.87]) (independent samples t test; P = .767), or between operated levels (1-way ANOVA; P = .100). There was also no statistically significant relation between gender and the position of the operated levels (χ2 test; P = .626).
Table 3.
Patient baseline characteristics.
|
Total |
Group A (TSC, Ti) |
Group B (reCreo, Control) |
Group C (osteoCon, CaP) |
P |
|
| N patients | 127 | 44 | 37 | 46 | |
| Gender, male/female | 61/66 | 17/27 | 21/16 | 23/23 | .252a |
| Age, mean (SD), y | 50.51 (9.65) | 49.98 (9.73) | 51.46 (8.39) | 50.26 (10.63) | .773b |
| Operated levels | .834a | ||||
| T6-T7 | 3 | 1 | 1 | 1 | |
| L1-L2 | 1 | 0 | 0 | 1 | |
| L2-L3 | 1 | 0 | 1 | 0 | |
| L3-L4 | 8 | 4 | 2 | 2 | |
| L4-L5 | 50 | 18 | 13 | 19 | |
| L5-S1 | 64 | 21 | 20 | 23 |
Abbreviations: CaP, calcium phosphate; Ti, titanium.
χ2 test.
One-way analysis of variance.
The study flow diagram is schematically represented in Figure 2. All radiological and clinical outcome parameters were assessed preoperatively for each patient. All patients were asked to return for a follow-up CT scan after 6 and 12 months. However, some patients could not be convinced to participate when the clinical outcome was satisfactory, considering the risks associated with radiation exposure.28,33–35 At 6 months after the surgery, we performed CT scans of 35 patients in group A, 34 patients in group B, and 29 patients in group C. In group A, 2 patients with fusion grade 1 after 6 months did not want to return for a CT scan 12 months after the surgery. Furthermore, in group B, 2 patients with fusion grade 1, 2 patients with fusion grade 2, and 1 patient with fusion grade 3 after 6 months chose not to participate in the follow-up CT scan after 12 months. In group C, 3 patients with fusion grade 1 and 1 patient with fusion grade 2 decided not to receive another CT scan, 12 months after the surgery. All patients who dropped out cited the unnecessary radiation exposure, while the clinical outcome was deemed satisfactory.
Figure 2.
Schematic representation of the study flow diagram.
We also asked all patients to fill out the questionnaires to determine the clinical outcome parameters: VAS back and leg pain, ODI, and SF-36. All patients participated in the 3-month follow-up (VAS and ODI). After 6 months, 3 patients in group A and C and 2 patients in group B did not report their clinical outcome parameters. At the 12-month follow-up (VAS, ODI, and SF-36), all patients in group A participated (including the 3 drop-outs after 6 months). There were no patients in group B who dropped out at the 12-month follow-up compared with the 6-month follow-up. In group C, 2 more patients decided not to fill out the health-related quality-of-life questionnaires at the 12-month follow-up.
Clinical Outcome Parameters
The VAS scores for back pain (average painful moment) are summarized in Table 4. Furthermore, Figure 3 depicts the means (and the 95% confidence interval) of the VAS score for back pain (average painful moment) for the 3 groups. It is clear that there was a significant improvement in back pain intensity between the preoperative and the last follow-up measurements for each group. At each of the 4 reporting moments, there was no statistically significant difference among the 3 groups. Moreover, a repeated measures ANOVA test showed that there was no time × type interaction (Pillai trace; P = .478), indicating that the 3 groups showed the same VAS score evolution in time. Similar results were found for VAS scores of leg and arm pain (least, average, and most painful moment).
Table 4.
Mean visual analogue scale score of back pain (average painful moment), per treatment group at 4 moments in time (before the surgery and at 3, 6, and 12 months after the surgery).
|
Group A (TSC, Ti), Score (SD) |
Group B (reCreo, Control), Score (SD) |
Group C (osteoCon, CaP), Score (SD) |
P
Between Groups |
|
| Preoperative | 7.48 (1.40) | 7.48 (1.51) | 7.31 (1.73) | .839a |
| 3 mo | 3.60 (2.44) | 3.88 (2.41) | 3.60 (2.18) | .846a |
| 6 mo | 4.14 (2.72) | 4.50 (2.57) | 3.50 (2.47) | .225a |
| 12 mo | 4.20 (2.85) | 3.79 (2.59) | 3.19 (2.57) | .234a |
| P 12 mo vs preoperative | .000b,* | .000b,* | .000b,* |
Abbreviations: CaP, calcium phosphate; SD, standard deviation; Ti, titanium.
One-way analysis of variance.
Paired samples t test.
Statistically significant difference (P < .05).
Figure 3.
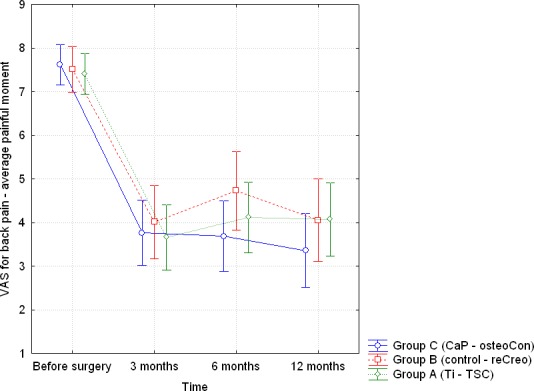
Visual analogue scale score for back pain (average painful moment): depiction of the mean scores and the 95% confidence interval for the 3 groups at each of the 4 reporting moments.
Table 5 shows the mean values for the ODI at each reporting moment for the 3 cage types. The ODI improved significantly over time for each cage type (paired samples t test; P = .000). Again, there were no statistically significant differences in ODI values among the 3 groups at each measurement moment. Furthermore, there was no time × type interaction (Pillai trace; P = .647). This indicates that the 3 study groups showed the same ODI evolution in time.
Table 5.
Mean Oswestry Disability Index score per treatment group at different time points.
|
Group A (TSC, Ti), Score (SD) |
Group B (reCreo, Control), Score (SD) |
Group C (osteoCon, CaP), Score (SD) |
P
Between Groups |
|
| Preoperative | 43.86 (14.05) | 45.78 (16.40) | 44.22 (14.44) | .831a |
| 3 mo | 29.30 (21.33) | 29.49 (17.38) | 24.72 (15.62) | .387a |
| 6 mo | 25.32 (20.30) | 27.91 (19.70) | 22.88 (18.24) | .529a |
| 12 mo | 27.34 (21.60) | 24.29 (16.88) | 20.15 (17.58) | .220a |
| P 12 mo vs preoperative | .000b,* | .000b,* | .000b,* |
Abbreviations: CaP, calcium phosphate; SD, standard deviation; Ti, titanium.
One-way analysis of variance.
Paired samples t test.
Statistically significant difference (P < .05).
Table 6 illustrates the evolution of the physical functioning and bodily pain, both included in the SF-36 survey. Physical functioning and bodily pain improved significantly over time in the 3 treatment groups. There were no statistically significant differences among the 3 groups at each moment in time. Once again, the Pillai trace showed no significant interaction for the time × type test: P = .453 (SF-36 physical functioning) and P = .848 (SF-36 bodily pain).
Table 6.
Mean 36-Item Short Form Survey scores per treatment group at different moments in time.
|
Group A (TSC, Ti), Score (SD) |
Group B (reCreo, Control), Score (SD) |
Group C (osteoCon, CaP), Score (SD) |
P
Between Groups |
|
| Physical functioning, preoperative | 44.55 (18.86) | 40.41 (22.53) | 50.54 (21.71) | .090a |
| Physical functioning, 12 mo | 63.57 (27.97) | 63.97 (23.89) | 67.84 (23.68) | .694a |
| P 12 mo vs preoperative | .000b,* | .000b,* | .001b,* | |
| Bodily pain, preoperative | 27.27 (15.77) | 29.65 (12.59) | 29.24 (19.41) | .777a |
| Bodily pain, 12 mo | 56.45 (28.32) | 54.94 (24.70) | 55.93 (27.45) | .971a |
| P 12 mo vs preoperative | .000b,* | .000b,* | .000b,* |
Abbreviations: CaP, calcium phosphate; SD, standard deviation; Ti, titanium.
One-way analysis of variance.
Paired samples t test.
Statistically significant difference (P < .05).
Radiological Outcome
The radiological outcome of the study was determined by an independent spine radiologist. The fusion grades for each treatment group at the 6-month follow-up and the 12-month follow-up are summarized in Tables 7 and 8.
Table 7.
Summary of the fusion grades per treatment group, as identified on computed tomography scans performed 6 months after the surgery. Both the absolute and relative number of patients with a particular fusion grade are reported for each group. Fusion grade 1 is definite fusion, fusion grade 2 is probable fusion, and fusion grade 3 is probable pseudarthrosis.
|
Fusion Grade |
Group A (TSC, Ti) |
Group B (reCreo, Control) |
Group C (osteoCon, CaP) |
P, A vs B |
P, B vs C |
P, A vs C |
| 1 | ||||||
| n | 27 | 10 | 23 | |||
| % | 77.1 | 29.4 | 79.3 | .0001a,* | .0001a,* | .8329b |
| 2 | ||||||
| n | 6 | 21 | 5 | |||
| % | 17.1 | 61.8 | 17.2 | |||
| 3 | ||||||
| n | 2 | 3 | 1 | |||
| % | 5.7 | 8.8 | 3.4 | |||
Abbreviations: CaP, calcium phosphate; Ti, titanium.
One-sided percentage test.
Two-sided percentage test.
Statistically significant difference (P < .05).
Table 8.
Summary of the fusion grades per treatment group, as identified on computed tomography scans performed 12 months after the surgery. Both the absolute and relative number of patients with a particular fusion grade are reported for each group. Fusion grade 1 is definite fusion, fusion grade 2 is probable fusion, and fusion grade 3 is probable pseudarthrosis.
|
Fusion Grade |
Group A (TSC, Ti) |
Group B (reCreo, Control) |
Group C (osteoCon, CaP) |
P, A vs B |
P, B vs C |
P, A vs C |
| 1 | ||||||
| n | 31 | 19 | 22 | |||
| % | 93.9 | 65.5 | 88.0 | .0034a,* | .0320a,* | .4318b |
| 2 | ||||||
| n | 1 | 10 | 3 | |||
| % | 3.0 | 34.5 | 12.0 | |||
| 3 | ||||||
| n | 1 | 0 | 0 | |||
| % | 3.0 | 0.0 | 0.0 | |||
Abbreviations: CaP, calcium phosphate; Ti, titanium.
One-sided percentage test.
Two-sided percentage test.
Statistically significant difference (P < .05).
At the 6-month follow-up CT scan, 77.1% of the patients in group A (PEEK cages with Ti nanocoating) had fusion grade 1 (Table 7). In group C (PEEK cages with CaP nanocoating), 79.3% of the patients achieved definite fusion. On the other hand, only 29.4% of the patients in the control group B (uncoated PEEK cages) had fusion grade 1. Because we hypothesized that the fusion rate would be better in the cages with nanocoating, we used a 1-sided percentage test to assess the statistical differences between group A and B and group B and C. From Table 7, it is clear that both group A (Ti nanocoating) and group C (CaP nanocoating) had a larger relative number of patients with fusion grade 1 than group B (uncoated PEEK control cages) (1-sided percentage test; P = .0001). Because there was no hypothesis that 1 type of nanocoating had a better radiological outcome than the other, a 2-sided percentage test was used to compare groups A and C. No statistically significant differences in fusion grade 1 were noted between the 2 different nanocoating types (2-sided percentage test; P = .8329).
In Table 8, we summarize the fusion grades for each cage type at the 12-month follow-up. One year after the surgery, 93.9% of the patients in group A (Ti nanocoating) achieved definite fusion. In group C (CaP nanocoating), 88.0% of the patients were classified as fusion grade 1. In the control group B, 65.6% of the patients had definite fusion. Again, significantly more patients achieved definite fusion, when implanted with PEEK cages having either type of nanocoating (Ti or CaP) compared with patients with uncoated PEEK cages. There were also no statistically significant differences in the relative number of patients with fusion grade 1 between the 2 groups with a nanocoating (2-sided percentage test; P = .4318).
Side Effects and Complications
No cage migration was observed in the complete study population. Blood loss and the hospital stay duration were comparable between groups. No infections were reported.
DISCUSSION
This randomized controlled study investigated the potential clinical and radiological benefits of PEEK cages with a nanocoating (Ti or CaP) compared with the standard uncoated PEEK cages. One year after the surgery, all cages had a statistically significant improvement of the clinical outcome parameters. However, there were no statistically significant differences in clinical outcome parameters between the 3 treatment groups. On the other hand, significantly more patients achieved definite fusion when implanted with nanocoated (Ti or CaP) PEEK cages compared with uncoated PEEK cages.
A total of 127 patients were included in the study, significantly more than the a priori sample size calculation of 23 patients per group, as determined in the statistical methods. We decided to include more patients to anticipate the possible attrition rate during a clinical trial. The attrition rate was the highest for the radiological examinations. Patients were informed of the increased cancer risk due to CT scan radiation.28,33–35 By only visualizing the operated levels, we were able to limit the radiation exposure to approximately 20% compared with the exposure of a full lumbar spine scan, effectively reducing the estimated cancer risks as much as possible.28 Although we asked all patients to return for follow-up CT scans and informed them of our precautionary measures to reduce the cancer risk, a significant number of patients decided not to participate and reduce their exposure to radiation. At every radiological or clinical follow-up, at least 25 patients per group were examined, exceeding the a priori sample size of 23 patients per group. A post hoc calculation of the sample size accuracy was performed to ensure the statistical power of our results. The calculation was based on the number of patients and the VAS scores for leg and back pain at an average painful moment. The data showed that there were 4 repeated measures moments and 3 treatment groups. The average correlation between the dependent variables was 0.41. Fixing the type I error at 0.05, we obtained that the statistical power was equal to 0.81. In other words, the likelihood that this PLIF randomized controlled trial will detect an effect, when there is truly an effect is a satisfactory 81%.
As we wanted to focus on the difference between the cage material types, we decided to keep the surgical technique as standard as possible. Therefore, all patients received the standard open PLIF surgery and no minimally invasive spine surgery (MISS) was performed. Additionally, we did not use allografts or artificial bone grafts, to eliminate potential bias with regards to the fusion rate.
Our study findings clearly demonstrate that PLIF surgery with all 3 cage types (uncoated PEEK cage, PEEK cage with Ti nanocoating, and PEEK cage with CaP nanocoating) had a statistically significant effect on the clinical outcome parameters (VAS, ODI, and SF-36). From Table 4, it is clear that the average VAS score for back pain decreased from 7.5 on a 10-point scale to an average score between 3.2 and 4.2 (depending on the cage type). As the minimum clinically important difference (MCID) for back pain is 1.2, the 3.3- to 4.3-point reduction in back pain demonstrates the clinically significant pain reduction of the PLIF procedure for all 3 types of cages.23 Furthermore, the ODI decreased at least with a score of 17 points (Table 5). This again exceeds the MCID of 12.8, as proposed by Copay et al.23 Finally, the physical and mental functioning of the patients improved significantly for all patients in the 3 study groups. No statistically significant differences in VAS, ODI, or SF-36 scores were found between the 3 groups, indicating that the 3 cage types had a similar improvement of clinical outcome parameters in the year following the PLIF surgery. Our results suggest that the nanocoated PEEK cages have the same safety and efficacy as the clinically accepted uncoated PEEK cages, 1 year after the surgery.
When investigating the radiological outcome of the study, it is immediately clear that the nanocoated PEEK cages achieved significantly more definite fusion compared with the uncoated PEEK cages. Only 65.5% of the patients who received uncoated PEEK cages were identified with definite fusion, 12 months after the surgery. Definite fusion already appeared after 6 months in 77% to 79% of the patients with nanocoated PEEK cages. After 1 year, over 90% of the patients with nanocoated PEEK cages had definite fusion, indicating that better and faster osseointegration arises when using a nanocoating. No statistical differences in fusion were observed between Ti- and CaP-nanocoated PEEK cages.
Several studies suggest that successful osseointegration is correlated with improved implant stability and positive long-term clinical outcomes.8,15,16 Positive effects of osseointegration on the clinical outcome can be noticed between 1 and 2 years after the surgery.36,37 The different osseointegration pathways indeed cause a gradual increase in elastic modulus and hardness that can take multiple years to complete.38,39 So although enhanced osseointegration might already be visible on the radiological images 1 year after the surgery, the clinical outcome could still be unchanged as the mechanical properties of the fusion are not yet fully completed. Additionally, pseudarthrosis might already be present 1 year after the surgery, but patients might not yet experience negative clinical effects. The negative clinical effects of pseudarthrosis typically appear between 1 and 2 years after the initial surgery, sometimes necessitating a cage extraction and a new implant. Research has shown that although pseudarthrosis can be asymptomatic on a short-term follow-up, it might lead to reoperations up to 10 years after the initial surgery.16,40 Therefore, it is not surprising that no statistical differences in clinical outcome are found among the 3 study groups 1 year after the surgery, despite the enhanced osseointegration of the 2 nanocoated PEEK cages. Nonetheless, the expectation is that future 5-year follow-up studies should be able to discern the long-term clinical benefits or disadvantages.15 The enhanced osseointegration has additional clinical relevance because of recent evolutions in PLIF surgery. Open spine surgical techniques are increasingly interchanged for MISSs.41–43 The benefits of MISSs are reduced blood loss, shorter length of hospital stays, smaller portals, and a reduced stripping of muscles.41–44 MISS is also associated with lower rates of complications, for example, surgical site infections.43 These advantages of MISS should allow a speedier recovery of patients, as well as a significant pain reduction. On the other hand, one cannot harvest and use local autograft to the same extent in MISS as in open PLIF surgeries. Local autograft is often added around the cages, as it contributes to the fusion. Therefore, the enhanced osseointegration of nanocoated PEEK cages becomes even more important in MISS, as the fusion contribution of local autograft is reduced. Finally, enhanced osseointegration should also benefit patients at risk for incomplete fusion, for example, smokers, and elderly or osteoporotic patients. There may also be clinical added value in multilevel fusion procedures or cage revision procedures.
Our study corroborates several animal and cell culture experiments described in the literature. There indeed is an abundance of research that demonstrated the enhanced osseointegration of Ti coatings,11,15,18,19 and CaP coatings.17,45 In contrast, there are only a limited number of clinical studies that explored the use of coated PEEK cages.15 To the best of our knowledge, there are no clinical studies that explored the use of PEEK cages with a CaP coating in PLIF. There are however clinical studies available that used Ti-coated PEEK cages in spinal interbody fusion surgery. In a systematic review by Assem et al,15 only 7 clinical studies were included. The review demonstrated the safety and efficacy of PEEK implants with a Ti coating because these cages had similar clinical outcome parameters and fusion rates as uncoated PEEK cages at an early follow-up of 1 year.15 Only 2 studies reported improved fusion rates, albeit not statistically significant.15 No follow-up reports on the long-term benefits and complications were available for the Ti-coated PEEK cages. It is noteworthy that these studies most frequently used a Ti plasma-sprayed coating. As mentioned in the introduction, the claim of mechanical safety for these coatings is not warranted.13 Repetitive impacts to the cages result in abrasion of the coating. The abrasion not only reduces the enhanced osseointegration of the coating, but the debris is also shown to cause inflammatory reactions, calling into question the long-term stability of the cages.13 The nanocoated PEEK cages used in this randomized controlled trial kept wear to a minimum because the thickness was significantly smaller than that of the plasma-sprayed coatings.13 An animal study by Meers et al17 showed that the Ti-nanocoated PEEK cages had a beneficial effect on the osseointegration. Our clinical study confirms these findings, given the statistically significant improvement in radiological outcome of the nanocoated PEEK cages.
A limitation of the study is the relatively short follow-up period of 1 year. As previously mentioned, 1 year is too short to assess the long-term benefits of enhanced osseointegration or the increased reoperation rate due to pseudarthrosis. Future research attention will therefore be devoted to a 5-year follow-up of this randomized controlled trial to investigate the hypothesis that the enhanced osseointegration of nanocoated PEEK cages also leads to increased clinical benefits. Potentially, statistically significant differences might arise between the Ti-nanocoated and CaP-nanocoated PEEK cages. The nanocoating thickness and material might indeed influence the clinical and radiological outcome. More research on these influences is needed.
In conclusion, we performed a randomized controlled trial to compare the 1-year radiological and clinical outcome parameters of 3 cage types: PEEK cages with a Ti nanocoating, PEEK cages with a CaP nanocoating, and uncoated PEEK cages. All 3 cage types had a similar improvement in clinical outcome parameters after 1 year, indicating that the nanocoated PEEK cages have a similar safety and efficacy as the standard PEEK cages. Furthermore, nanocoated PEEK cages were shown to have a better fusion rate than uncoated PEEK cages. No statistically significant differences were found between the Ti and CaP nanocoating. The results of this study are clinically relevant as enhanced osseointegration is a significant predictor of positive long-term clinical outcomes and improved implant longevity. Moreover, enhanced osseointegration becomes even more important in MISS, which is gaining traction in clinical practice. Furthermore, patients at risk for incomplete fusion might benefit from enhanced osseointegration of nanocoated PEEK cages. The findings of this study will be revisited in a 5-year follow-up study of the randomized controlled trial.
Acknowledgments
The authors thank Frederik Soetaert, PhD, for his valuable feedback on the manuscript and the data analysis.
REFERENCES
- 1.Mobbs RJ, Phan K, Malham G, Seex K, Rao PJ. Lumbar interbody fusion: techniques, indications and comparison of interbody fusion options including PLIF, TLIF, MI-TLIF, OLIF/ATP, LLIF and ALIF. J Spine Surg. 2015;1(1):2–18. doi: 10.3978/j.issn.2414-469X.2015.10.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barrett-Tuck R, Del Monaco D, Block JE. One and two level posterior lumbar interbody fusion (PLIF) using an expandable, stand-alone, interbody fusion device: a VariLift® case series. J Spine Surg. 2017;3(1):9–15. doi: 10.21037/jss.2017.02.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cloward RB. The treatment of ruptured lumbar intervertebral discs by vertebral body fusion. I. Indications, operative technique, after care. J Neurosurg. 1953;10(2):154–168. doi: 10.3171/jns.1953.10.2.0154. [DOI] [PubMed] [Google Scholar]
- 4.Brantigan JW, Steffee AD, Lewis ML, Quinn LM, Persenaire JM. Lumbar interbody fusion using the Brantigan I/F cage for posterior lumbar interbody fusion and the variable pedicle screw placement system: two-year results from a Food and Drug Administration investigational device exemption clinical trial. Spine (Phila Pa 1976) 2000;25(11):1437–1446. doi: 10.1097/00007632-200006010-00017. [DOI] [PubMed] [Google Scholar]
- 5.Steffee AD, Sitkowski D. Posterior lumbar interbody fusion and plates. Clin Orthop. 1988;227:99–102. [PubMed] [Google Scholar]
- 6.Yoshihara H, Yoneoka D. National trends in the surgical treatment for lumbar degenerative disc disease: United States, 2000 to 2009. Spine J. 2015;15(2):265–271. doi: 10.1016/j.spinee.2014.09.026. [DOI] [PubMed] [Google Scholar]
- 7.Jones CI, III, Khalil JG, Fischgrund J. Lumbar interbody cages. In: Steinmetz MP, Benzel EC, editors. Benzel's Spine Surgery: Techniques, Complication Avoidance, and Management 4th ed. Philadelphia, PA: Elsevier;; 2017. pp. 696–701. [Google Scholar]
- 8.Rao PJ, Pelletier MH, Walsh WR, Mobbs RJ. Spine interbody implants: material selection and modification, functionalization and bioactivation of surfaces to improve osseointegration. Orthop Surg. 2014;6(2):81–89. doi: 10.1111/os.12098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Olivares-Navarrete R, Gittens RA, Schneider JM, et al. Osteoblasts exhibit a more differentiated phenotype and increased bone morphogenetic protein production on titanium alloy substrates than on poly-ether-ether-ketone. Spine J. 2012;12(3):265–272. doi: 10.1016/j.spinee.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Seaman S, Kerezoudis P, Bydon M, Torner JC, Hitchon PW. Titanium vs. polyetheretherketone (PEEK) interbody fusion: meta-analysis and review of the literature. J Clin Neurosci. 2017;44:23–29. doi: 10.1016/j.jocn.2017.06.062. [DOI] [PubMed] [Google Scholar]
- 11.Kurtz SM, Devine JN. PEEK biomaterials in trauma, orthopedic, and spinal implants. Biomaterials. 2007;28(32):4845–4869. doi: 10.1016/j.biomaterials.2007.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Benezech J, Garlenq B, Larroque G. Flexible stabilisation of the degenerative lumbar spine using PEEK rods. Adv Orthop. 2016;2016:7369409. doi: 10.1155/2016/7369409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kienle A, Krieger A, Willems K, Wilke HJ. Resistance of coated polyetheretherketone lumbar interbody fusion cages against abrasion under simulated impaction into the disc space. J Appl Biomater Funct Mater. 2018;2018:2280800018782854. doi: 10.1177/2280800018782854. [DOI] [PubMed] [Google Scholar]
- 14.Mahjoubi H, Buck E, Manimunda P, et al. Surface phosphonation enhances hydroxyapatite coating adhesion on polyetheretherketone and its osseointegration potential. Acta Biomater. 2017;47:149–158. doi: 10.1016/j.actbio.2016.10.004. [DOI] [PubMed] [Google Scholar]
- 15.Assem Y, Mobbs RJ, Pelletier MH, Phan K, Walsh WR. Radiological and clinical outcomes of novel Ti/PEEK combined spinal fusion cages: a systematic review and preclinical evaluation. Eur Spine J. 2017;26(3):593–605. doi: 10.1007/s00586-015-4353-8. [DOI] [PubMed] [Google Scholar]
- 16.Kornblum MB, Fischgrund JS, Herkowitz HN, Abraham DA, Berkower DL, Ditkoff JS. Degenerative lumbar spondylolisthesis with spinal stenosis: a prospective long-term study comparing fusion and pseudarthrosis. Spine. 2004;29(7):726–733. doi: 10.1097/01.brs.0000119398.22620.92. [DOI] [PubMed] [Google Scholar]
- 17.Meers CM, Verleye GB, Smeets D, et al. Fine grained osseointegrative coating improves biocompatibility of PEEK in heterotopic sheep model. Int J Spine Surg. 2015;9:35. doi: 10.14444/2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Walsh WR, Bertollo N, Christou C, Schaffner D, Mobbs RJ. Plasma-sprayed titanium coating to polyetheretherketone improves the bone-implant interface. Spine J. 2015;15(5):1041–1049. doi: 10.1016/j.spinee.2014.12.018. [DOI] [PubMed] [Google Scholar]
- 19.Yoon BJV, Xavier F, Walker BR, Grinberg S, Cammisa FP, Abjornson C. Optimizing surface characteristics for cell adhesion and proliferation on titanium plasma spray coatings on polyetheretherketone. Spine J. 2016;16(10):1238–1243. doi: 10.1016/j.spinee.2016.05.017. [DOI] [PubMed] [Google Scholar]
- 20.Vogel D, Dempwolf H, Baumann A, Bader R. Characterization of thick titanium plasma spray coatings on PEEK materials used for medical implants and the influence on the mechanical properties. J Mech Behav Biomed Mater. 2018;77:600–608. doi: 10.1016/j.jmbbm.2017.09.027. [DOI] [PubMed] [Google Scholar]
- 21.Awaja F, Cools P, Lohberger B, Nikiforov AY, Speranza G, Morent R. Functionalized, biocompatible, and impermeable nanoscale coatings for PEEK. Mater Sci Eng C Mater Biol Appl. 2017;76:865–870. doi: 10.1016/j.msec.2017.03.153. [DOI] [PubMed] [Google Scholar]
- 22.Torstrick FB, Klosterhoff BS, Westerlund LE, et al. Impaction durability of porous polyether-ether-ketone (PEEK) and titanium-coated PEEK interbody fusion devices. Spine J. 2018;18(5):857–865. doi: 10.1016/j.spinee.2018.01.003. [DOI] [PubMed] [Google Scholar]
- 23.Copay AG, Glassman SD, Subach BR, Berven S, Schuler TC, Carreon LY. Minimum clinically important difference in lumbar spine surgery patients: a choice of methods using the Oswestry Disability Index, Medical Outcomes Study questionnaire Short Form 36, and Pain Scales. Spine J. 2008;8(6):968–974. doi: 10.1016/j.spinee.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 24.Kersten RFMR, van Gaalen SM, de Gast A, Öner FC. Polyetheretherketone (PEEK) cages in cervical applications: a systematic review. Spine J. 2015;15(6):1446–1460. doi: 10.1016/j.spinee.2013.08.030. [DOI] [PubMed] [Google Scholar]
- 25.Peters MJM, Bastiaenen CHG, Brans BT, Weijers RE, Willems PC. The diagnostic accuracy of imaging modalities to detect pseudarthrosis after spinal fusion: a systematic review and meta-analysis of the literature. Skeletal Radiol. 2019;48(10):1499–1510. doi: 10.1007/s00256-019-03181-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Resnick DK, Choudhri TF, Dailey AT, et al. Guidelines for the performance of fusion procedures for degenerative disease of the lumbar spine. Part 4: radiographic assessment of fusion. J Neurosurg Spine. 2005;2(6):653–657. doi: 10.3171/spi.2005.2.6.0653. [DOI] [PubMed] [Google Scholar]
- 27.Choudhri TF, Mummaneni PV, Dhall SS, et al. Guideline update for the performance of fusion procedures for degenerative disease of the lumbar spine. Part 4: radiographic assessment of fusion status. J Neurosurg Spine. 2014;21(1):23–30. doi: 10.3171/2014.4.SPINE14267. [DOI] [PubMed] [Google Scholar]
- 28.Richards PJ, George J, Metelko M, Brown M. Spine computed tomography doses and cancer induction. Spine (Phila Pa 1976) 2010;35(4):430–433. doi: 10.1097/BRS.0b013e3181cdde47. [DOI] [PubMed] [Google Scholar]
- 29.Shah RR, Mohammed S, Saifuddin A, Taylor BA. Comparison of plain radiographs with CT scan to evaluate interbody fusion following the use of titanium interbody cages and transpedicular instrumentation. Eur Spine J. 2003;12(4):378–385. doi: 10.1007/s00586-002-0517-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Humadi A, Freeman BJ, Moore RJ, et al. A comparison of radiostereometric analysis and computed tomography for the assessment of lumbar spinal fusion in a sheep model. Evid Based Spine Care J. 2013;4(2):78–89. doi: 10.1055/s-0033-1357359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Olson CL. Comparative robustness of six tests in multivariate analysis of variance. J Am Stat Assoc. 1974;69(348):894–908. [Google Scholar]
- 32.Faul F, Erdfelder E, Lang AG, Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39(2):175–191. doi: 10.3758/bf03193146. [DOI] [PubMed] [Google Scholar]
- 33.Brenner DJ, Hall EJ. Computed tomography - an increasing source of radiation exposure. N Engl J Med. 2007;357:2277–2284. doi: 10.1056/NEJMra072149. [DOI] [PubMed] [Google Scholar]
- 34.Berrington de González A, Mahesh M, Kim KP, et al. Projected cancer risks from computed tomographic scans performed in the United States in 2007. Arch Intern Med. 2009;169(22):2071–2077. doi: 10.1001/archinternmed.2009.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smith-Bindman R, Lipson J, Marcus R, et al. Radiation dose associated with common computed tomography examinations and the associated lifetime attributable risk of cancer. Arch Intern Med. 2009;169(22):2078–2086. doi: 10.1001/archinternmed.2009.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Noshchenko A, Lindley EM, Burger EL, Cain CM, Patel VV. What is the clinical relevance of radiographic nonunion after single-level lumbar interbody arthrodesis in degenerative disc disease? A meta-analysis of the YODA Project Database. Spine (Phila Pa 1976) 2016;41(1):9–17. doi: 10.1097/BRS.0000000000001113. [DOI] [PubMed] [Google Scholar]
- 37.Park Y, Ha JW, Lee YT. The effect of a radiographic solid fusion on clinical outcomes after minimally invasive transforaminal lumbar interbody fusion. Spine J. 2011;11(3):205–212. doi: 10.1016/j.spinee.2011.01.023. [DOI] [PubMed] [Google Scholar]
- 38.Baldassarri M, Bonfante E, Suzuki M, et al. Mechanical properties of human bone surrounding plateau root form implants retrieved after 0.3-24 years of function. J Biomed Mater Res B Appl Biomater. 2012;100(7) doi: 10.1002/jbm.b.32786. 2015:2021. [DOI] [PubMed] [Google Scholar]
- 39.Coelho PG, Jimbo R. Osseointegration of metallic devices: current trends based on implant hardware design. Arch Biochem Biophys. 2014;561:99–108. doi: 10.1016/j.abb.2014.06.033. [DOI] [PubMed] [Google Scholar]
- 40.Chun DS, Baker KC, Hsu WK. Lumbar pseudarthrosis: a review of current diagnosis and treatment. Neurosurg Focus. 2015;39(4):E10. doi: 10.3171/2015.7.FOCUS15292. [DOI] [PubMed] [Google Scholar]
- 41.Goldstein CL, Macwan K, Sundararajan K, Rampersaud YR. Comparative outcomes of minimally invasive surgery for posterior lumbar fusion: a systematic review. Clin Orthop Relat Res. 2014;472(6):1727–1737. doi: 10.1007/s11999-014-3465-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Goldstein CL, Macwan K, Sundararajan K, Rampersaud YR. Perioperative outcomes and adverse events of minimally invasive versus open posterior lumbar fusion: meta-analysis and systematic review. J Neurosurg Spine. 2016;24(3):416–427. doi: 10.3171/2015.2.SPINE14973. [DOI] [PubMed] [Google Scholar]
- 43.Vazan M, Gempt J, Meyer B, Buchmann N, Ryang YM. Minimally invasive transforaminal lumbar interbody fusion versus open transforaminal lumbar interbody fusion: a technical description and review of the literature. Acta Neurochir (Wien) 2017;159(6):1137–1146. doi: 10.1007/s00701-017-3078-3. [DOI] [PubMed] [Google Scholar]
- 44.Imada AO, Huynh TR, Drazin D. Minimally invasive versus open laminectomy/discectomy, transforaminal lumbar, and posterior lumbar interbody fusions: a systematic review. Cureus. 2017;9(7):e1488. doi: 10.7759/cureus.1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Surmenev RA, Surmeneva MA, Ivanova AA. Significance of calcium phosphate coatings for the enhancement of new bone osteogenesis - a review. Acta Biomater. 2014;10(2):557–579. doi: 10.1016/j.actbio.2013.10.036. [DOI] [PubMed] [Google Scholar]