Abstract
Gonorrhea is a sexually transmitted infection with 87 million new cases per year globally. Increasing antibiotic resistance has severely limited treatment options. A mechanism that Neisseria gonorrhoeae uses to evade complement attack is binding of the complement inhibitor C4b-binding protein (C4BP). We screened 107 porin B1a (PorB1a) and 83 PorB1b clinical isolates randomly selected from a Swedish strain collection over the last 10 years and noted that 96/107 (89.7%) PorB1a and 16/83 (19.3%) PorB1b bound C4BP; C4BP binding substantially correlated with the ability to evade complement-dependent killing (r = 0.78). We designed 2 chimeric proteins that fused C4BP domains to the backbone of IgG or IgM (C4BP-IgG; C4BP-IgM) with the aim of enhancing complement activation and killing of gonococci. Both proteins bound gonococci (KD C4BP-IgM = 2.4 nM; KD C4BP-IgG 980.7 nM), but only hexameric C4BP-IgM efficiently outcompeted heptameric C4BP from the bacterial surface, resulting in enhanced complement deposition and bacterial killing. Furthermore, C4BP-IgM substantially attenuated the duration and burden of colonization of 2 C4BP-binding gonococcal isolates but not a non–C4BP-binding strain in a mouse vaginal colonization model using human factor H/C4BP–transgenic mice. Our preclinical data present C4BP-IgM as an adjunct to conventional antimicrobials for the treatment of gonorrhea.
Keywords: Infectious disease, Therapeutics
Keywords: Bacterial infections, Complement, Immunotherapy
A chimeric protein that comprises a complement inhibitor fragment fused to IgM Fc has been developed as an immunotherapeutic against gonorrhea.
Introduction
Gonorrhea is a sexually transmitted infection caused by Neisseria gonorrhoeae that infects both men and women. N. gonorrhoeae can establish infections in the urogenital tract, rectum, and pharynx; is associated with high morbidity and socioeconomic consequences; and remains a public health problem worldwide (1). Complications from untreated gonococcal infections include ectopic pregnancy, infertility in women, and increased risk of HIV infection. Gonorrhea can also be transmitted from mother to neonate and cause blindness or life-threatening disseminated infection (2). Gonococci have become resistant to almost every conventional antibiotic currently in clinical use, and we might be entering an era of untreatable gonorrhea (3–6). Therefore, the need for new treatment options has become a pressing issue.
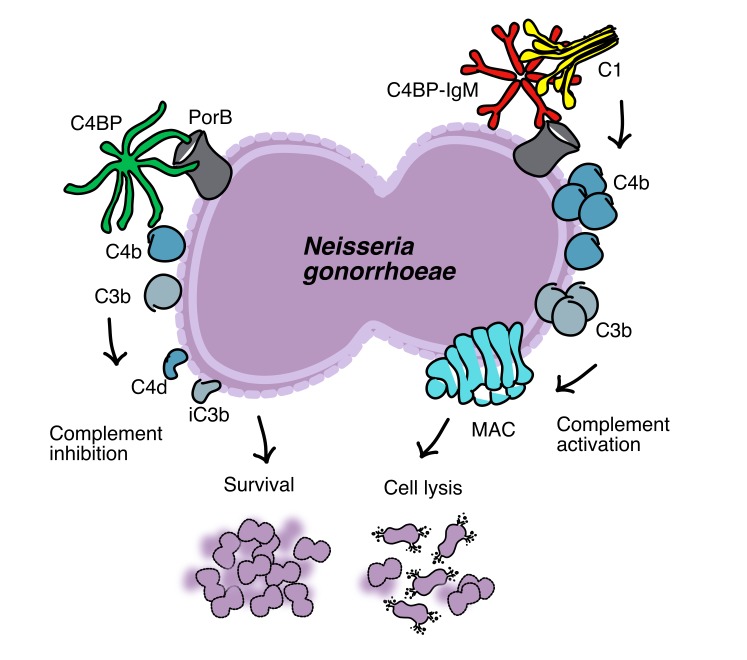
An emerging approach to control microbial infections is to target bacterial virulence mechanisms (7, 8). Pathogens have evolved various strategies to escape the innate immune response, including killing by the complement system (9, 10). The complement pathway represents one of the most ancient innate immune systems that has been conserved through evolution, which protects the host against infections. Invading pathogens activate complement either because of differences in surface composition that are recognized by the host as foreign or “non-self” (alternative and lectin pathways) or through antibody binding (classical pathway). This leads to the initiation of activation; sequential proteolytic cleavage results in the formation of central C3 convertases and opsonization of the target with iC3b, which leads to phagocytosis, release of proinflammatory anaphylatoxins (C5a, C3a) that attract white blood cells, and finally formation of a lytic membrane attack complex (MAC) that directly kills gram-negative pathogens (11). To protect the body from unwanted complement activation and damage, the complement system is tightly regulated. C4b-binding protein (C4BP) is one of the major soluble complement inhibitors, which blocks complement cascade at the level of C3 convertases (9, 12).
Several pathogens have developed strategies to escape from complement-mediated killing by recruiting complement inhibitors such as C4BP to their surface, resulting in decreased activation of the complement cascade, favoring bacterial survival (13–16). The exclusively human pathogen N. gonorrhoeae binds C4BP through its major outer membrane protein, porin B (PorB) (17), which dampens classical pathway activation and mediates resistance to complement. PorB is an approximately 34- to 37-kDa transmembrane protein that is essential for survival of the organism and functions as a selective anion channel (18). PorB proteins are encoded by 2 mutually exclusive alleles of porB; based on the PorB molecule expressed, gonococcal strains may be classified as either PorB1a or PorB1b (previously referred to as Por1A and Por1B, respectively). We previously documented that C4BP binds both PorB isoforms with a small preference for PorB1a (8 strains out of 11 tested) versus PorB1b (8 strains out of 18 tested) (17). PorB1a strains are frequently associated with disseminated infection, while PorB1b strains usually cause local infections of the genital tract (19). Surveillance data suggest that PorB1a strains are less prevalent than PorB1b strains (20–22).
Because of the global threat of antimicrobial-resistant gonorrhea, we targeted the ability of gonococci to bind C4BP by developing a potentially novel antimicrobial fusion protein, which combines the domains of C4BP that are required for binding to gonococci with the Fc domain of IgG and IgM to promote MAC-mediated killing of the pathogen. Because complement-inhibitory activity of C4BP requires the third CCP domain (23), these fusion proteins should not inhibit complement, while they should preserve the ability to deposit on bacteria. Previous work by our groups have shown that a chimeric protein that comprises the microbial binding domains of another complement inhibitor, factor H (FH), fused to Fc is efficacious in vitro and in vivo against Streptococcus pyogenes (24), N. gonorrhoeae (7), N. meningitidis (25), and Haemophilus influenzae (26) and provided the rationale for targeting N. gonorrhoeae–C4BP interactions.
Results
C4BP binds N. gonorrhoeae and protects the bacterium from serum-mediated killing.
Prior work has shown that selected strains of N. gonorrhoeae bind human C4BP (17). We supported the previous results using 6 laboratory strains of N. gonorrhoeae (C4BP-binding N. gonorrhoeae 15253, FA1090, 1291, and MS11 and the non–C4BP-binding N. gonorrhoeae F62 and 252) either with purified, fluorescently labeled C4BP or with 10% of normal human serum (NHS) as a source of C4BP (Figure 1, A and B). All C4BP-binding gonococcal strains survived in NHS (Supplemental Figure 1A; supplemental material available online with this article; https://doi.org/10.1172/jci.insight.131886DS1), suggesting a role for C4BP in protecting bacteria from complement-mediated lysis. However, some C4BP nonbinders may possess other serum resistance mechanisms, such as FH recruitment (for example, strain 252; Supplemental Figure 1D). Of note, when gonococci were incubated with heat-inactivated human serum (HI NHS), C4BP binding decreased, suggesting that the protein binds not only to PorB, but possibly also to complement C3/C4 fragments deposited on the bacterial surface after complement activation, because C4BP does not lose binding capacity and activity at 56°C (Supplemental Figure 1, B and C, and ref. 27).
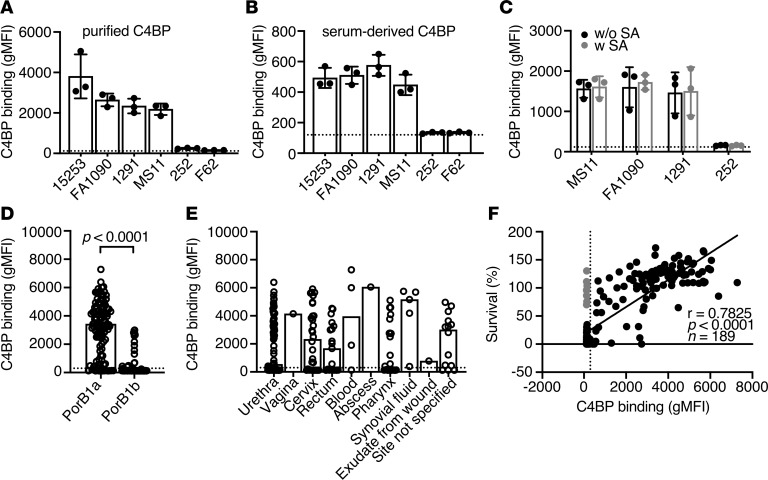
Figure 1. C4BP binds to N. gonorrhoeae.
(A and B) Binding of C4BP from normal human serum (NHS; 10%) or purified Alexa Fluor 647–labeled C4BP (20 μg/mL) to 6 laboratory strains of N. gonorrhoeae. (C) Binding of Alexa Fluor 647–labeled C4BP to 4 laboratory strains of N. gonorrhoeae in the presence (with SA) or in the absence (without SA) of sialylation. (D and E) Binding of Alexa Fluor 647–labeled C4BP (20 μg/mL) to 190 clinical isolates of N. gonorrhoeae. The isolates are grouped according to their expressed subclass of PorB and anatomical site of isolation. In D, P value was calculated by Mann-Whitney U test (P < 0.0001). (F) Spearman’s correlation analysis of survival of gonococcal clinical isolates in 10% NHS versus their C4BP binding (r = 0.7825; P < 0.0001; n = 189). Serum-resistant isolates incapable of binding C4BP are highlighted in gray. In A, B, and C bars display mean ± SD, with circles indicating independent repeats. Dotted line refers to gMFI average value in the absence of protein. In D, E, and F dotted line refers to the cutoff for positivity (300 gMFI) calculated as gMFI mean value + 3 SD of unspecific background of signal obtained for strain 252 that does not bind C4BP; bars display median and circles correspond to the mean for each sample from 2 independent experiments performed in duplicate.
Some serum-sensitive N. gonorrhoeae strains become serum resistant in vivo because of sialylation (28), which leads to binding of FH that similarly to C4BP blocks the complement cascade at the level of C3. Here we show that sialylation of gonococci does not affect C4BP binding (Figure 1C) while it increases FH deposition on the surface of the pathogens, resulting in increased survival, a phenomenon that was expected (Supplemental Figure 1, D and E).
We measured C4BP binding to 190 recently isolated gonococcal clinical strains — 107 PorB1a and 83 PorB1b isolates — using flow cytometry (Supplemental Table 2). Laboratory strains 15253 and 252 were used as positive and negative controls, respectively, for C4BP binding. The cutoff for positivity was fixed at geometrical mean fluorescence intensity (gMFI) + 3 SD of unspecific background of signal obtained for strain 252 that does not bind C4BP. Ninety-six (89.7%) of 107 PorB1a isolates bound C4BP, while only 16 (19.3%) of 83 PorB1b strains bound C4BP (Figure 1D). Similar to previously published data, the majority of gonococci binding C4BP belong to the PorB1a subclass (17). Furthermore, no association was found between C4BP binding and anatomical site of isolation (Figure 1E).
Serum resistance of clinical isolates was thereafter tested in the presence of 10% NHS, which reflects complement activity in midcycle cervical secretions (29). A strong correlation was observed between survival of gonococci and their ability to bind C4BP (Spearman’s analysis r = 0.7825, P < 0.0001, and n = 189; Figure 1F). Interestingly, only 8 of 106 serum-resistant isolates did not bind C4BP (shown as gray dots in Figure 1F); 4 of these strains (strains 26, 107, 120, 185) bound FH (Supplemental Figure 1F). Similarly, the laboratory strain 252, which does not bind C4BP, survived in NHS (Supplemental Figure 1A) and bound FH. The mechanism(s) by which gonococcal strains 53, 112, 167, and 188 resist serum killing in the absence of C4BP or FH binding remains unclear.
Expression of C4BP-IgG and C4BP-IgM.
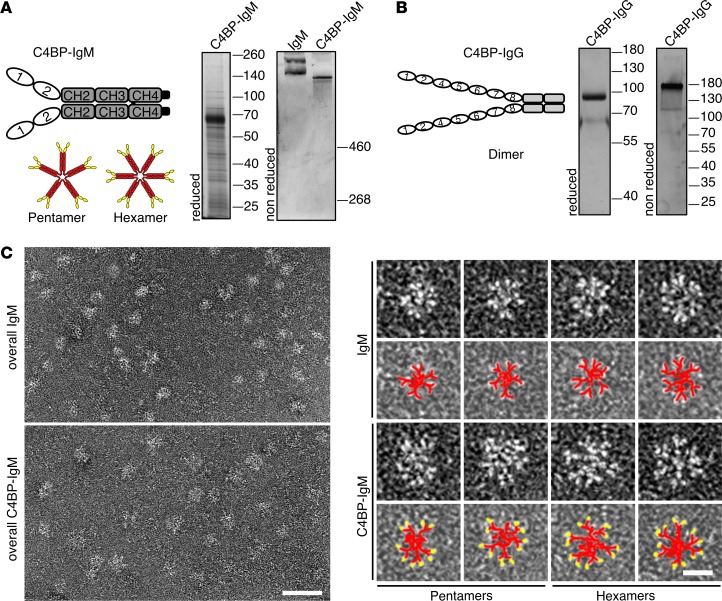
To promote complement-mediated elimination of serum-resistant N. gonorrhoeae strains, taking advantage of their ability to bind C4BP, we created the 2 fusion proteins, C4BPΔCCP3-IgG and C4BP(CCP1/2)-IgM, by subcloning C4BP domains on the backbone of a constant portion of IgG or IgM (Figure 2, A and B). C4BP-IgG and C4BP-IgM constructs were prepared in the expression vectors pClaire and pTorsten, respectively. Both fusion proteins were expressed recombinantly in eukaryotic cells and purified using affinity chromatography. Purity and size of the obtained proteins were verified by SDS-PAGE using silver staining (Figure 2, A and B). The reduced monomer of C4BP-IgG is about 75 kDa, which assembles as a dimer of 150 kDa. C4BP-IgM has a molecular mass of approximately 52 kDa when reduced, whereas if unreduced it appears as a multimer with double bands larger than 500 kDa, similar to the double bands of human control IgM. Because C4BP-IgM is a fusion with IgM CH2-CH4, which commonly assembles in multimers, we analyzed it by electron microscopy. Similar to human IgM, C4BP-IgM exists as either pentamers or hexamers; the latter was the prevalent form and constituted approximately 80% of recombinantly expressed C4BP-IgM (Figure 2C).
Figure 2. C4BP-IgM and C4BP-IgG fusion proteins.
(A and B) Schematic representation and silver-stained SDS-PAGE of C4BP-IgM (A) and C4BP-IgG (B). (C) Scanning electron microscopy of C4BP-IgM and IgM. Left: Overviews of IgM and C4BP-IgM. Scale bar: 50 nm. Right: High-magnification examples of pentamers and hexamers of IgM and C4BP-IgM. Scale bar: 25 nm. Under each high-magnification particle there is a representation in pseudocolors (red, IgM; yellow, C4BP).
Only C4BP-IgM outcompetes C4BP binding to bacteria.
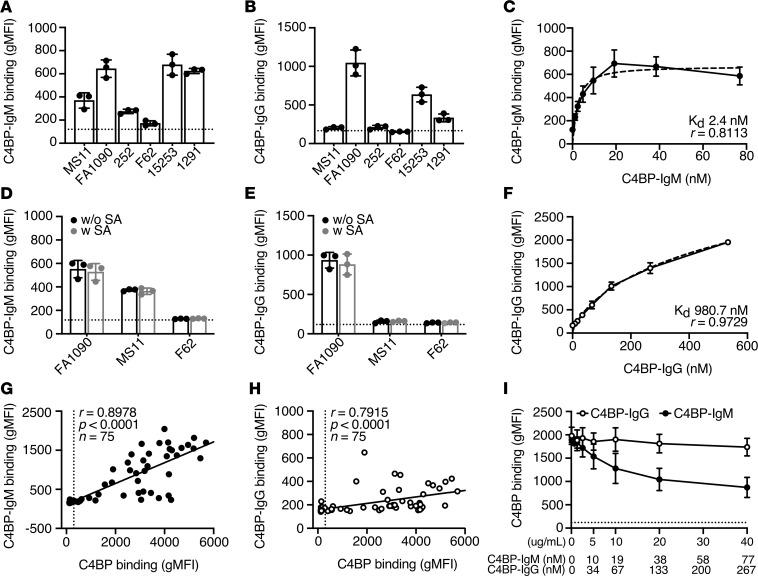
Both C4BP-IgG and C4BP-IgM fusion proteins contain CCP1-2 domains of C4BP, which are required for the binding to gonococcal PorB. Using flow cytometry, we showed that both fusion proteins bound the laboratory strains that also bound native C4BP, with the exception of MS11, which bound C4BP-IgM but not C4BP-IgG (Figure 3, A and B). Of note, sialylation (SA) of bacteria, which occurs in vivo and did not affect native C4BP binding, also did not affect C4BP-IgG or C4BP-IgM binding to the bacteria (Figure 3, D and E). For gonococcal strain FA1090 we then estimated the binding affinity for the 2 fusion proteins, showing that C4BP-IgM has higher affinity than C4BP-IgG (KD 2.4 nM vs. 980.7 nM) (Figure 3, C and F). This is likely because of the fact that the multimeric rearrangement of C4BP-IgM results in up to 12 binding sites compared with 2 of the dimeric C4BP-IgG. Analysis of binding to a selection of gonococcal clinical isolates (n = 75) using flow cytometry showed a strong correlation between binding of C4BP and that of fusion proteins (Spearman’s correlation analysis: C4BP-IgM r = 0.8978, P < 0.0001; C4BP-IgG r = 0.7915, P < 0.0001; Figure 3, G and H).
Figure 3. C4BP-IgM and C4BP-IgG fusion proteins bind to N. gonorrhoeae.
(A and B) Binding of C4BP-IgM (A) and C4BP-IgG (B) (20 μg/mL) to 6 laboratory strains of N. gonorrhoeae. Bars display mean ± SD, with circles indicating independent repeats. Dotted line refers to gMFI average value in the absence of protein. Binding curves of C4BP-IgM (C) and C4BP-IgG (F) to N. gonorrhoeae FA1090. Dotted lines refer to nonlinear fitting of binding curves using multiple site binding model in GraphPad. Values for estimated KD and r2 are reported in each graph. Binding of C4BP-IgM (D) and C4BP-IgG (E) (20 μg/mL) to 3 laboratory strains of N. gonorrhoeae with or without sialic acid (SA). Bars display mean ± SD, with circles indicating individual replicates. Dotted line refers to gMFI average value in the absence of protein. Spearman’s correlation analysis of Alexa Fluor 488–labeled C4BP-IgM (G) or C4BP-IgG (H) binding versus Alexa Fluor 647–labeled C4BP binding of gonococcal clinical isolates (r = 0.8978, P < 0.0001; r = 0.7915, P < 0.0001; respectively. n = 75). Vertical dotted line refers to the cutoff for positivity for C4BP binding. (Ι) Competition between Alexa Fluor 647–labeled C4BP (5 μg/mL in NHS 2.5% + OmCI 23 μg/mL) and C4BP fusion proteins (0–40 μg/mL; 1:2 dilutions) for the binding to N. gonorrhoeae FA1090. Horizontal dotted line indicates the baseline value for gMFI measured in the absence of Alexa Fluor 647–labeled C4BP. In graphs C, F, and I, each dot represents the mean ± SD of 3 independently performed experiments.
We next investigated the competition between native C4BP and C4BP fusion proteins at the gonococcal surface. Bacteria were mixed simultaneously with human serum supplemented with fluorescently labeled C4BP and increasing concentrations of either C4BP-IgM or C4BP-IgG, and the ability to recruit C4BP was thereafter evaluated with flow cytometry. As shown in Figure 3I, C4BP-IgM but not C4BP-IgG efficiently inhibited binding of Alexa Fluor 647–labeled C4BP to gonococci in a dose-dependent fashion, indicating a direct competition between the 2 proteins. These data indicate that the fusion proteins and C4BP may share common binding sites on the surface of bacteria. Inhibition of C4BP binding by C4BP-IgM was incomplete, possibly because some C4BP remained bound to C3/C4 fragments deposited on bacterial surface. In contrast, dimeric C4BP-IgG could not compete with the heptameric C4BP, likely because of lower avidity binding to the target bacterium. Therefore, we selected C4BP-IgM for subsequent experiments.
C4BP-IgM triggers complement activation.
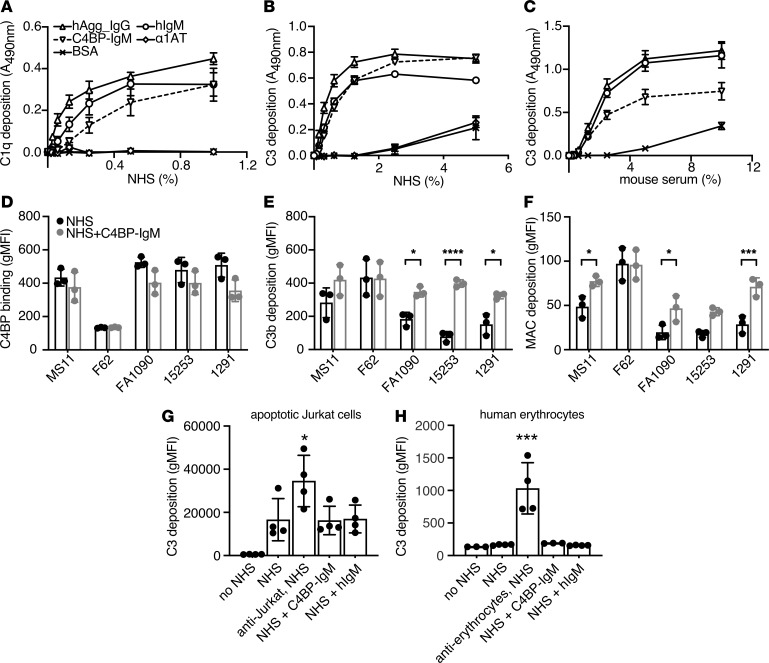
C4BP-IgM immobilized on microtiter wells efficiently induced complement C1q and C3b deposition from human serum in a dose-dependent manner and to a similar extent as positive controls IgM and aggregated IgG, indicating that the fusion protein efficiently activated the classical pathway of the complement system in vitro. Neither bovine serum albumin (BSA) nor α-1-antitrypsin (α1AT), which were used as negative controls, mediated complement deposition (Figure 4, A and B). Furthermore, C3b deposition was also observed using mouse serum (Figure 4C), showing that C4BP-IgM also activated murine complement, an important consideration for subsequent testing of the fusion protein in mice.
Figure 4. C4BP-IgM promotes complement activation selectively on the bacterial surface.
(A) C1q deposition on plates coated with C4BP-IgM fusion protein, human aggregated IgG (hAgg_IgG), human IgM (hIgM), α1AT, or BSA. (B and C) C3 deposition from either human serum (NHS) or mouse serum on C4BP-IgM fusion protein or hAgg_IgG, hIgM, α1AT, or BSA immobilized on plate. Each dot represents mean ± SEM of 3 independently performed experiments. (D–F) C4BP binding and complement deposition on the surface of 5 laboratory strains of N. gonorrhoeae using 10% NHS with or without C4BP-IgM 20 μg/mL. Bars display mean ± SD, with circles indicating independent repeats. P values were performed by 2-way ANOVA with Sidak’s multiple-comparisons test. (G and H) C3 complement deposition on the surface of apoptotic Jurkat cells or human erythrocytes. Each bar represents the mean ± SD of 4 independently performed experiments. Differences were analyzed using 1-way ANOVA with Dunnett’s multiple-comparisons test. *P < 0.05, ***P < 0.005, and ****P < 0.0001 as indicated, or compared with NHS.
To determine whether C4BP-IgM also enhanced complement deposition on intact bacteria, we incubated 5 laboratory strains of N. gonorrhoeae with 10% NHS with or without 20 μg/mL C4BP-IgM and measured surface deposition of complement proteins by flow cytometry. Of note, the concentration of serum used here is 4-fold higher than that used in competition assays (Figure 3I); therefore, inhibition of C4BP binding was not as evident (Figure 4D). Despite only modest inhibition of C4BP binding to gonococci, C4BP-IgM significantly enhanced deposition of both C3b fragments (Figure 4E) and MAC complexes (Figure 4F) on gonococci compared with NHS alone. Furthermore, we examined the effect of C4BP-IgM on complement deposition on human cells. Apoptotic Jurkat cells and human erythrocytes were incubated with 5% NHS in the presence or in the absence of 20 μg/mL C4BP-IgM, and C3 deposition was detected by flow cytometry. Similar to the irrelevant negative control protein hIgM, C4BP-IgM did not enhance complement deposition either on human dying cells or on human erythrocytes, whereas the anti-Jurkat and anti-erythrocyte antibodies used as positive controls efficiently sensitized the cells to C3 deposition (Figure 4, G and H).
C4BP-IgM enhances complement-mediated killing of gonococci.
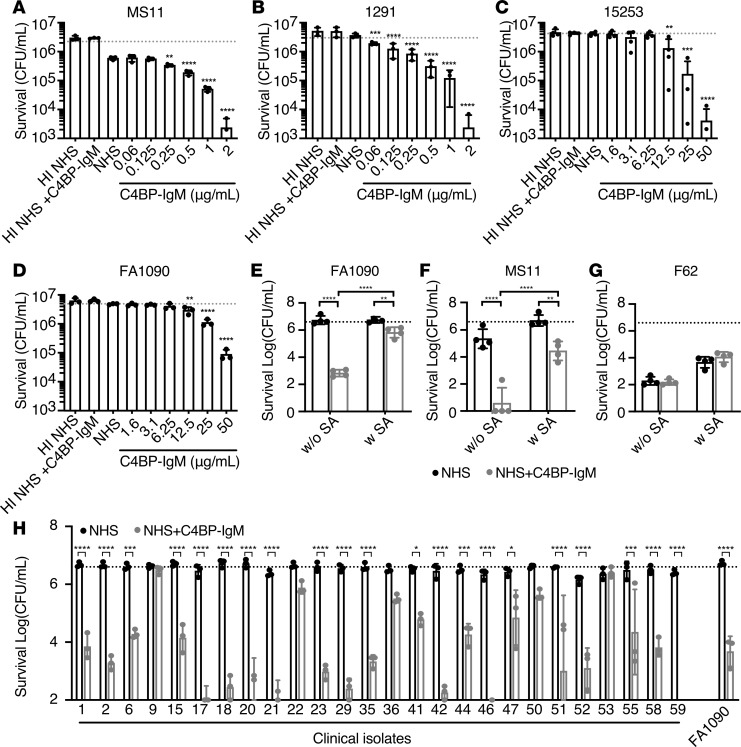
We then determined whether increased complement deposition on bacteria, mediated by C4BP-IgM, promoted complement-dependent killing of N. gonorrhoeae by human serum. C4BP-IgM enhanced in a dose-dependent manner killing of all 4 laboratory strains (15253, FA1090, MS11, and 1291) (Figure 5, A–D). Of note, MS11 and 1291 strains were incubated with 5% NHS because these strains were intrinsically more sensitive to complement than 15253 and FA1090; the latter 2 strains were tested using 20% NHS. Accordingly, killing of MS11 and 1291 occurred in the presence of only 0.25 μg/mL of C4BP-IgM, while a higher concentration (12.5 μg/mL) was required to obtain killing of 15253 and FA1090 over baseline survival in NHS alone. Of note, 20 μg/mL C4BP-IgM promoted significant killing of bacteria even when they were sialylated (FA1090 and MS11) but no effect for F62, which did not bind the protein (Figure 5, E–G). However, sialylation decreased the ability of C4BP-IgM to kill bacteria, at least in part because sialic acid increases FH recruitment to the surface of bacteria (Figure 5, E and F, and Supplemental Figure 1, D and E). Indeed, C4BP-IgM binding to gonococci did not affect FH recruitment to the bacterial surface both in the presence and in the absence of lipooligosaccharide sialylation (LOS) (Supplemental Figure 1, G and H). We also evaluated the efficacy of C4BP-IgM against 26 recent clinical quinolone-resistant isolates of N. gonorrhoeae that all bound C4BP (Supplemental Table 2). All but 2 of the isolates (isolates 9 and 53, the latter an FH binder) were efficiently killed by 20 μg/mL of C4BP-IgM (>1 log10 reduction in CFU/mL) (Figure 5H). These results show efficacy of C4BP-IgM against a majority of current N. gonorrhoeae clinical isolates that bound C4BP. Certain strains that possess additional mechanisms to evade the complement-mediated attack resisted killing by C4BP-IgM.
Figure 5. C4BP-IgM promotes complement-mediated killing of N. gonorrhoeae.
(A–D) Complement-dependent bactericidal activity of C4BP-IgM on 4 laboratory strains of N. gonorrhoeae. Strains MS11 (A) and 1291 (B) were incubated with 5% NHS, while strains 15253 (C) and FA1090 (D) were tested with 20% NHS. Heat-inactivated NHS (HI NHS) was used as a negative control. One-way ANOVA with Dunnett’s multiple-comparisons test was performed considering NHS as reference. (E–G) Complement-mediated bactericidal activity of 20 μg/mL C4BP-IgM in 10% NHS on FA1090, MS11 and F62 in the presence (w SA) or in the absence (w/o SA) of LOS. P values were calculated by 2-way ANOVA with Tukey’s multiple comparison of log10 (CFU/mL). (H) Complement-mediated bactericidal activity of 20 μg/mL C4BP-IgM on 26 clinical isolates of N. gonorrhoeae in the presence of 10% NHS. P values were performed by 2-way ANOVA with Sidak’s multiple-comparisons test. In all graphs horizontal dotted line refers to the starting amount of bacteria used in the assay, and each bar represents the mean ± SD of at least 3 independently performed experiments. *P < 0.05, **P < 0.01, ***P < 0.005, and ****P < 0.0001 as indicated, or compared with NHS.
C4BP-IgM enhances clearance of infection in a mouse model of gonorrhea.
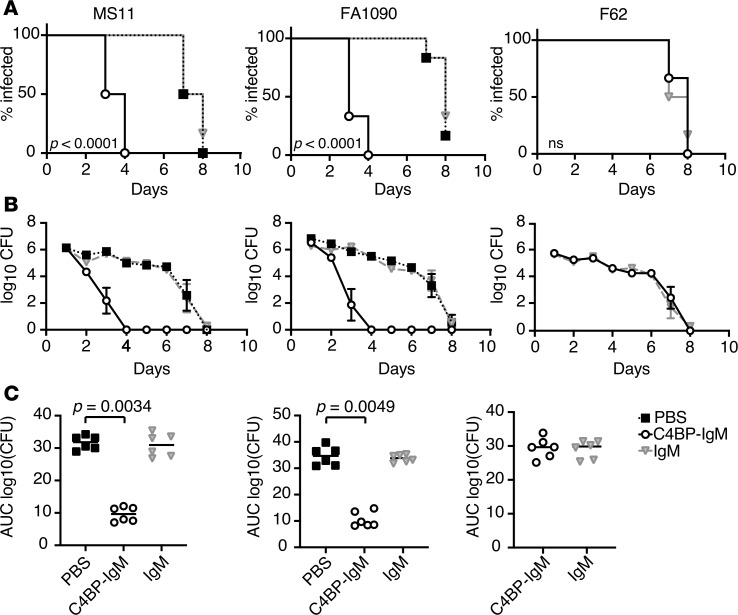
We next evaluated the efficacy of C4BP-IgM against N. gonorrhoeae MS11, FA1090, and F62 in a mouse vaginal colonization model. Gonococci bind to FH and C4BP in a human-specific manner (30, 31). Thus, complement activation on gonococci in wild-type mice is unimpeded because mouse FH and C4BP do not bind to N. gonorrhoeae, which would overestimate the efficacy of C4BP-IgM. We therefore used FH/C4BP–Tg mice to provide a more rigorous platform to test the efficacy of C4BP-IgM in the context of a more “humanized” complement system. The selected strains differ in their ability to bind C4BP and to resist serum-mediated killing in vitro: MS11 and FA1090 are C4BP binders and serum resistant (in the unsialylated state), whereas F62 does not bind C4BP and is serum sensitive. A purified plasma IgM from a patient with myeloma was used as a negative control and did not bind to or promote killing of N. gonorrhoeae laboratory strains (Supplemental Figure 2, A and B). Treatment of mice infected with either MS11 or FA1090 (6 animals per group) with 5 μg/day of C4BP-IgM intravaginally daily significantly reduced the duration of infection (P < 0.0001 versus control groups) in both cases, clearing the bacteria from 50% and 67% of the animals at day 3 for MS11 and FA1090 infection, respectively (Figure 6A). Moreover, C4BP-IgM treatment of mice infected with either N. gonorrhoeae MS11 or FA1090 had a drastically reduced bacterial burden over time with complete clearance of bacteria on day 4 (Figure 6B). Comparison of the AUC as a measure of cumulative burden of infection over time revealed that C4BP-IgM treatment significantly reduced the bacterial loads in both groups of mice infected by N. gonorrhoeae MS11 or FA1090 compared with untreated controls (P = 0.0034 and P = 0.0049, respectively; Figure 6C). Infection with strain F62, which does not bind C4BP, was unaffected by C4BP-IgM treatment. In parallel, the myeloma IgM did not affect the course of infection in all groups, indicating that the antibacterial efficacy was related to bacteria-bound C4BP-IgM.
Figure 6. Treatment with C4BP-IgM decreases infection with N. gonorrhoeae strains MS11 and FA1090 in the mouse vaginal colonization model.
Tg human FH/C4BP mice in a BALB/c background treated with Premarin (conjugated estrogens) (n = 6 per group) were challenged with 4.1 × 107 CFU/10 μL for strain MS11, 3.6 × 107 CFU/10 μL for strain FA1090, and 3.2 × 107 CFU/10 μL for strain F62, all at day 0. Mice were then treated intravaginally daily with 5 μg/d of C4BP-IgM (solid black line with opened circles), 5 μg/d of “nonspecific” IgM (solid gray line with triangles) or 10 μL PBS (vehicle control; dotted line with black squares) from day 1 to day 8. Vaginas were swabbed daily to enumerate CFU. (A) Kaplan-Meier analysis of time to clearance of infection. Group comparison was done using log-rank (Mantel-Cox) test. Significance for MS11 and FA1090 were set at 0.0167 (Bonferroni’s correction for 3 groups). (B) Log10 CFU versus time (days). Symbols indicate mean values ± SEM. (C) Bacterial burdens consolidated over time (AUC of log10 CFU analysis) for the 3 groups. Median values are shown for each group. For strains MS11 and FA1090 treatment groups were compared using the nonparametric Kruskal-Wallis equality of populations rank test. The χ2 with ties (2 degrees of freedom) for MS11 and FA1090 were 11.56 (P = 0.0004) and 11.42 (P = 0.0005), respectively. Pairwise comparisons across groups were made with Dunn’s post hoc test (P = 0.0034, and P = 0.0049 C4BP-IgM treatment vs. PBS, for MS11 and FA1090, respectively). AUC comparisons across the 2 treatment groups with strain F62 was made with the 2-sample Wilcoxon’s rank-sum (Mann-Whitney) test.
Discussion
This study identifies C4BP-IgM as a potentially novel antimicrobial fusion protein against N. gonorrhoeae. We tested 190 clinical gonococcal isolates cultured in Sweden in the last 10 years, and we observed that 96/107 (89.7%) PorB1a and 16/83 (19.3%) PorB1b bound C4BP. Binding of C4BP is a strategy used by several strains of N. gonorrhoeae to evade complement, which may provide them a survival advantage in their human host. We have targeted this bacterial immune evasion mechanism by creating a chimeric protein composed of C4BP CCP1-2 and Fc of IgM, which binds to bacteria and promotes complement activation on the microbial surface. We showed that C4BP-IgM fusion protein outcompeted C4BP and efficiently promoted MAC-mediated killing of gonococci. Finally, we demonstrated efficacy of C4BP-IgM in clearing infection in the gonococcal mouse vaginal colonization model.
Gonorrhea is a sexually transmitted infection caused by the obligate human pathogen N. gonorrhoeae. In 2016, the WHO reported 87 million cases occurring worldwide (2, 32). Current treatment of gonococcal infections is based on dual therapy with third-generation cephalosporin (for example, ceftriaxone) in combination with azithromycin. However, this treatment is already ineffective in some patients (5, 33). Considering that untreatable gonorrhea cases might soon become a reality, identifying new prevention and treatment options is crucial. In addition to new antibiotics or a novel combination of the currently used ones, new therapies have been suggested to prevent recurrence of the infection: IL-12 to promote Th1-driven adaptive immune response (34), vaginal lactobacillus strains to counteract gonococcal growth (35), and fatty acid derivatives for a fast anti-gonococcal activity targeting membrane disruption (36). Here we present a potentially novel therapeutic approach that targets a virulence mechanism of bacteria — its ability to bind the complement inhibitor C4BP.
Data obtained from China and Europe approximately a decade ago suggest that about 30% and 70% of gonococcal isolates express PorB1a and PorB1b, respectively (20, 21). Based on our data and previous studies (17), if we assume that 90% of PorB1a isolates and about 20% of PorB1b isolates bind C4BP, we expect C4BP-IgM to provide about 45% strain coverage.
Initially, we created 2 chimeric proteins combining α chain domains of C4BP with 2 types of immunoglobulins, IgG and IgM. In C4BP-IgG, the full length of C4BP α chain was used with the intent to target different pathogens, which possibly bind various domains of C4BP. In contrast, when IgM portion was used, we considered only the first 2 domains of C4BP essential for C4BP binding to N. gonorrhoeae because the IgM structure alone is already relatively large. Only C4BP-IgM, but not the C4BP-IgG fusion protein, could compete with the multimeric C4BP for binding to the bacterial surface, even though the inhibition was not complete. We previously documented that the monomeric α chain of C4BP did not bind to PorB, indicating a requirement for the polymeric form of C4BP to stably interact with PorB (17). Therefore, C4BP-IgM was prioritized for further preclinical studies. However, it is worth noting that both C4BP fusion proteins could kill gonococci in vitro, although the IgM derivative was more effective on a molar basis (Supplemental Figure 2C). C4BP-IgM proved effective in the mouse vaginal colonization model using human FH/C4BP–transgenic mice — these mice provide gonococci with human FH and C4BP and raise the threshold for complement-dependent killing by immunotherapeutics as would be encountered in humans. Treatment with C4BP-IgM markedly reduced the duration of infection and bacterial load in animals challenged with the 2 C4BP-binding gonococcal strains, but not the non–C4BP-binding strain, showing the selectivity of action of C4BP-IgM on gonococci able to bind C4BP and not because of nonspecific activation of complement in solution.
Interestingly, we documented that binding of C4BP was not affected by sialylation of bacteria, the latter commonly happening after bacterial colonization in vivo and resulting in FH recruitment and protection against complement attack. Likewise, C4BP-IgM binding was not affected by the presence of LOS sialic acid; although bactericidal activity of the chimeric protein was reduced, it was still effective in killing the sialylated organisms (Figure 5, E and F), suggesting that the major activity promoted by the protein is to induce a potent complement activation on the surface of bacteria that counteracts the presence of complement inhibitors. A similar strategy was successfully demonstrated in a recent study showing the effectiveness of a chimeric anti-gonococcal antibody that recognizes LOS epitope and was modified to enhance complement activation through a single amino acid mutation in Fc (37). Besides, previous studies hampering FH recruitment by gonococci using 2 types of FH-IgG chimeric proteins showed resolution of gonococcal colonization in the same mouse model used here (7, 38). Thus, both strategies may be useful adjunctives to antimicrobials. In addition, combination of the 2 C4BP-IgM and FH-IgG chimeric proteins may represent a rational strategy to target simultaneously a broad range of strains, which might be able to bind either C4BP or FH or both proteins. C4BP-IgM targets PorB, FH domains 18–20 fused to IgG Fc bind sialylated LOS and PorB (39, 40), and FH domains 6 and 7 fused to Fc target gonococcal NspA (41). Combination treatment directed against distinct molecules that serve important immune evasion functions (complement inhibition) or are essential for gonococcal viability (e.g., PorB) will make the development of resistance unlikely. It is important to note that PorB is essential for gonococcal viability and is constitutively expressed on the surface of gonococci (42, 43). PorB “escape mutants” that may develop following treatment with FH-IgG or C4BP-IgM are likely to lack the ability to bind to these complement inhibitors and incur a considerable fitness cost; inability to evade complement would enable the host to rapidly clear such mutants that resist these immunotherapeutics.
The use of fusion proteins as treatment might have some advantages. First, the chimeric proteins may reduce the use of antibiotics and development of antibiotic resistance. Excessive antibiotic use is also associated with destruction of resident commensal microflora. Indeed, using C4BP-IgM as a treatment, in contrast to antibiotic therapy, would selectively target pathogens that have developed the ability to escape complement attack by binding C4BP. Therefore, endogenous microflora, such as lactobacilli, which are not able to recruit the complement inhibitors, would not be affected (44). Moreover, C4BP-IgM lacks CCP3 domain, which is responsible for binding to C4b fragments deposited on the cell surface after complement activation; therefore, C4BP-IgM deposition on C4b-decorated cells will be also excluded. Additionally, C4BP-IgM is not recruited by apoptotic cells because it lacks the β chain of C4BP that is associated with protein S, which in turn recognizes the exposed phosphatidylserine molecules on dying cells (45). Accordingly, C4BP-IgM did not deposit complement on human erythrocytes or apoptotic cells.
In conclusion, we have created a potentially novel therapeutic to kill gonococci by targeting a key immune evasion mechanism. C4BP-IgM fusion protein represents an innovative strategy to trigger complement-mediated elimination of gram-negative pathogens, with a goal to improve the treatment and combat antimicrobial resistance. Besides, the study of the efficacy of this new class of antibacterial proteins can be expanded to a larger spectrum of pathogens able to recruit the complement inhibitor C4BP on their surface, such as Neisseria meningitidis (46), Haemophilus influenzae (47), Bordetella pertussis (48), Moraxella catarrhalis (49), and Candida albicans (50). Notably, the ability to bind complement inhibitors has been exploited by microbes through evolution. The use of such chimeric proteins to selectively activate complement on pathogens might offer a novel therapeutic avenue against multidrug-resistant bacterial infections.
Methods
Bacterial strains.
Strains 15253 (51), FA1090 (52), 1291 (53), F62 (54), MS11 (55), and 252 (39) have all been described previously, and Supplemental Table 1 summarizes their characteristics. Genotypic/phenotypic characteristics of clinical N. gonorrhoeae isolates cultured in 2010–2018 in Sweden (but patients infected in >20 additional countries) and obtained from the WHO Collaborating Centre for Gonorrhoea and other sexually transmitted infections, Department of Laboratory Medicine, Örebro University Hospital, Örebro, Sweden, are listed in Supplemental Table 2. All N. gonorrhoeae isolates were stored frozen at –80°C in trypticase soy broth containing 20% glycerol.
Proteins and antibodies.
Chocolate agar plates were prepared from gonococcal agar base (Difco, 228950) following the published procedure (56). 5′-cytidinemonophospho-N-acetylneuraminic acid (CMP-Neu5Ac) from MilliporeSigma (C8271) was used as the source of sialic acid for gonococci by spreading 500 μL of the compound (25 μg/mL in sterile H2O) on the plate at least 30 minutes before inoculating bacteria.
Human C4BP was purified from human citrate plasma by affinity chromatography using anti-C4BP antibody MK104 (57). Plasma purified C4BP and recombinant C4BP-IgM and C4BP-IgG fusion proteins were fluorescently labeled using Alexa Fluor 647 (A30009) and Alexa Fluor 488 (A10235) microscale labeling kits, respectively, from Molecular Probes. For flow cytometry assays, bacteria were stained with either carboxyfluorescein succinimidyl ester (CFSE) obtained from Fluka (catalog 21888) or CellTrace calcein violet from Thermo Fisher Scientific (catalog C34858) and resuspended in Hanks balanced salt solution containing 0.15 mM CaCl2 and 1 mM MgCl2 (HBSS++) from Gibco, Thermo Fisher Scientific (catalog 14025092). Binding of C4BP from NHS was detected with anti-C4BP antibody MK67 (specific for CCP4 domain of the α chain of C4BP) (57). Antibodies used for detection of C4BP-IgM and C4BP-IgG were anti-IgM Alexa Fluor 647 from MilliporeSigma (SAB4600436) and anti-hIgG Alexa Fluor 488 from Invitrogen, Thermo Fisher Scientific (A11013), respectively. To detect biotinylated FH purified from NHS in-house, streptavidin Alexa Fluor 647 conjugate was used (Thermo Fisher Scientific, S32357). Antibodies used for detection of complement deposition were as follows: in plate: polyclonal anti-human C1q (Dako, A0136); monoclonal anti-human C3d (Quidel, A207), followed by HRP swine anti-rabbit (Dako, P0399); and FITC-conjugated goat against mouse C3 (ICN, 55500); and on the surface of bacteria: polyclonal anti-human C3d (Dako, A0063) and monoclonal anti-human neoepitope C9 (Hycult Biotech, HM2167-IA), followed by Alexa Fluor 647 goat anti-mouse (Thermo Fisher Scientific, A21235). Polyclonal human IgM from Nordic Biosite (OAMA04116) was tested for binding to N. gonorrhoeae. Irrelevant IgM from human myeloma plasma (Athens Research and Technology, 16-16-090713-M) was used as a negative control in selected in vitro and in vivo experiments.
NHS.
NHS was obtained from whole blood collected from 11 normal healthy adult volunteers who provided written consent according to the recommendations of the local ethical committee in Lund, Sweden (permit 2017/582), and the Declaration of Helsinki (58). After clotting of whole blood at 25°C for 30 minutes, NHS from each donor was separated by centrifugation at 1500 g for 20 minutes at 4°C, pooled, divided into aliquots, and stored at –80°C. Human serum inactivated by heat at 56°C for 30 minutes (HI NHS) was used as a negative control for complement activation.
Expression and purification of C4BP-IgM and C4BP-IgG fusion proteins in CHO cells.
DNA sequences coding for CCP1–2 domains of C4BP and for the CH2–CH4 constant portion of IgM were subcloned in frame with signal peptide sequence into the expression vector pClaire. For C4BP-IgG fusion protein, the construct was prepared by subcloning of entire sequence of an α chain of C4BP lacking CCP3 domain into the expression vector pTorsten; both plasmids were provided by Brad Spiller (Cardiff University, Cardiff, United Kingdom). Chimeric proteins were stably expressed in adherent CHO cells (Life Technologies, Thermo Fisher Scientific) cultured in serum-free OptiMEM Glutamax at 37°C in 5% CO2, collected every second day, and replaced for 1 day with complete medium supplemented with 100 μg/mL hygromycin to maintain the selection of transfected clones. C4BP-IgM was purified from cell culture supernatants by affinity chromatography using anti-C4BP antibody MK104 coupled to Affi-Gel 10 (Bio-Rad) as described previously (23). C4BP-IgG was purified using protein A column (GE Healthcare). Bound proteins were eluted using 3 M guanidium chloride and glycine pH 2.7, respectively. Protein eluate was dialyzed against PBS at 4°C, and molecular mass and purity were evaluated by silver staining of proteins separated by SDS-PAGE.
Electron microscopy.
The multimeric form of C4BP-IgM molecules was analyzed by negative staining and electron microscopy as described previously (59). Human IgM was tested in parallel. Solutions of 1 μM of proteins were prepared in PBS. Aliquots of 5 μL were adsorbed to carbon-coated grids for 1 minute, then washed with 2 drops of water, and then stained with 2 drops of uranyl formate (SPI Supplies). Glow discharge at low pressure in air was used to render the grids hydrophilic. The grids were analyzed using a Jeol JEM 1230 electron microscope operated at a 60-kV accelerating voltage. Images were recorded with a Gatan Multiscan 791 CCD camera.
Complement activation assay.
Microtiter plates (MaxiSorp BreakApart; Nunc) were coated with C4BP-IgM (10 μg/mL), human IgM (10 μg/mL), or aggregated human IgG (5 μg/mL; Kiovig, Baxalta) as positive controls, and α1AT (10 μg/mL) or BSA (1%) as negative controls, all diluted in PBS. The plates were incubated overnight at 4°C and washed 4 times with washing buffer (50 mM Tris [pH 8], 150 mM NaCl, 0.1% Tween 20) and blocked with 1% BSA in PBS for 1 hour at 37°C. Increasing concentrations of NHS diluted in GVB++ (5 mM veronal buffer [pH 7.3], 140 mM NaCl, 0.1% gelatin, 1 mM MgCl2, and 5 mM CaCl2) were incubated for 45 or 20 minutes at 37°C to detect C1q or C3d deposition, respectively. Complement deposition was detected with anti-C1q or anti-C3d, both followed by swine anti-rabbit antibody, all diluted in the blocking buffer. Color was developed using OPD tablets (Kem-En-Tec Diagnostics), and signal was measured at OD 490 (Cary 50 MPR microplate reader; Varian).
Flow cytometry assays for binding of C4BP-IgM/C4BP-IgG and complement proteins to bacteria.
Bacteria were cultured on a chocolate agar plate overnight and subcultured on new plates for at least 5 hours with or without CMP-Neu5Ac, the latter in case of sialylated gonococci. Harvested bacteria were then diluted to OD 2 in HBSS++ and stained with 10 μM CFSE or 1 mM calcein violet. Binding of C4BP and C4BP-IgM or C4BP-IgG to bacteria was measured by flow cytometry after incubation at 37°C for 30 minutes with the purified Alexa Fluor 647– or Alexa Fluor 488–labeled proteins, respectively, diluted in HBSS++. To verify the stability of C4BP after heat inactivation of NHS (HI NHS), purified C4BP was treated at 56°C for 30 minutes (C4BP at 56°C) and then tested as above. In some experiments, binding to unlabeled proteins was measured using anti-C4BP MK67 followed by Alexa Fluor 647–conjugated anti-mouse, Alexa Fluor 647–conjugated anti-hIgM, or Alexa Fluor 488–conjugated anti-hIgG, all diluted in HBSS++. For competition assays, increasing concentrations of C4BP fusion proteins were added to 2.5% NHS supplemented with 5 μg/mL of Alexa Fluor 647–labeled C4BP and with OmCI (23 μg/mL) used as a C5 inhibitor preventing cell lysis (60) in GVB++ followed by incubation at 37°C for 30 minutes with bacteria. In parallel experiments, complement activation on bacteria was induced by incubation of gonococci with 10% NHS diluted in GVB++ with or without C4BP-IgM (20 μg/mL). Serum incubation time was 15 minutes for C9 (MAC formation) and 30 minutes for C3d, the latter in the presence of OmCI (23 μg/mL), both reactions at 37°C. After incubation, deposited complement proteins on the surface of gonococci were detected by flow cytometry using the specific antibodies listed above. In additional experiments, FH binding was measured by incubation of calcein violet–stained bacteria with biotinylated FH purified from NHS in-house and followed by streptavidin Alexa Fluor 647 conjugate. To explore the competition between FH and C4BP-IgM for the binding to the bacterial surface, selected strains (30, 35, 36, and FA1090) were incubated with both biotinylated FH and unlabeled C4BP-IgM, and binding of the proteins was measured using streptavidin Alexa Fluor 488 conjugate and Alexa Fluor 647–conjugated anti-IgM antibody. For analysis, bacteria were identified as CFSE- or calcein violet–positive events, and gMFI was calculated with FlowJo software.
Complement deposition on human erythrocytes or apoptotic cells.
Red blood cells were isolated from whole blood of healthy volunteers collected in tubes containing lepirudin (Refludan 50, Celgene). Apoptosis of Jurkat cells was induced by incubation with 0.5 μM of staurosporine for 14 hours at 37°C and 5% CO2. Erythrocytes and apoptotic Jurkat cells were then incubated with NHS 5% diluted in GVB++ in the absence or in the presence of 20 μg/mL C4BP-IgM. Human IgM (20 μg/mL) from myeloma plasma was used as an irrelevant protein. Rabbit anti-erythrocyte antibody (Abcam, 197770) and rabbit anti-Jurkat antibody (produced in-house, ref. 61), both diluted in HBSS++ to 25 or 50 μg/mL, respectively, were used to sensitize the cells before incubation with serum and were used as positive controls. C3 deposited on human cells was detected by flow cytometry using the FITC-labeled anti-C3c antibody (Dako, F0201). Apoptotic cells were gated on a population positive for APC-annexin V (ImmunoTools, 31490016).
Serum bactericidal assay.
Bactericidal assays with NHS in the presence of C4BP-IgM fusion protein were performed similarly to those described previously (38). Briefly, bacteria were cultured on a chocolate agar plate overnight and subcultured on new plates for at least 5 hours with or without CMP-Neu5Ac, the latter in case of sialylated gonococci. Approximately 2 × 105 CFU of harvested N. gonorrhoeae in GVB++ were incubated with 5%, 10%, or 20% human complement (NHS diluted in GVB++) in the presence or the absence of C4BP-IgM or C4BP-IgG (concentration indicated for each experiment) diluted in GVB++ in a final volume of 100 μL. Aliquots of 25 μL reaction mixtures were collected at the beginning of the assay (t0) and plated onto chocolate agar in duplicate in 10–3 dilution. After incubation at 37°C for 30 minutes (t30), another aliquot of 25 μL was collected, diluted in PBS, and plated onto chocolate agar in duplicate (10–1 or 10–2 or 10–3). Survival was calculated as the number of viable CFU per milliliter at t30, while CFU per milliliter at t0 is referred to as the starting number of bacteria in each graph. In some cases, survival was calculated as percentage of the number of viable colonies at t30 relative to t0. Serum-resistant strains were defined as having survival greater than 50%.
Mouse vaginal colonization model.
Female Tg BALB/c mice that expressed human FH and human C4BP (15), 6–8 weeks of age, in the diestrus phase of the estrous cycle were started on treatment (that day) with 0.1 mg Premarin (Pfizer; conjugated estrogens) in 200 μL of water given s.c. on each of 3 days, −2, 0, and +2 (2 days before, the day of, and 2 days after inoculation), to prolong the estrus phase of the reproductive cycle and promote susceptibility to N. gonorrhoeae infection. Antibiotics (vancomycin, colistin, neomycin, trimethoprim, and streptomycin) ineffective against N. gonorrhoeae were also used to reduce competitive microflora. Mice were infected on day 0 with strain MS11, FA1090, or F62 (inoculum specified for each experiment). Mice were treated daily with 5 μg of either C4BP-IgM or human irrelevant IgM intravaginally from day 1 until the conclusion of the experiment. In the experiments with FA1090 and MS11, we also included a group of mice that were given a corresponding volume of PBS (vehicle controls). Bacterial load was determined by counting CFU growth from the vaginal swab collected daily.
Statistics.
Statistical analyses were performed using GraphPad Prism v8.0. Mann-Whitney U test was used to analyze the difference in C4BP binding between subgroups PorB1a and PorB1b. Spearman’s correlation analysis was performed for comparison of C4BP binding versus serum survival or versus C4BP fusion protein binding. Two-way ANOVA with Sidak’s multiple-comparisons or Tukey’s multiple-comparisons test and 1-way ANOVA with Dunnett’s multiple-comparisons test were used to examine the differences between experimental results in complement deposition and serum killing assays. Significant differences are indicated with asterisks: *P < 0.05, **P < 0.01, ***P < 0.005, and ****P < 0.0001. For animal experiments, clearance of gonococcal infection over time was analyzed with Kaplan-Meier curves, and groups were compared using log-rank (Mantel-Cox) test. Burden of bacteria, expressed as log10 CFU over time, was evaluated as mean ± SEM; the cumulative CFU as AUC over time was analyzed as mean of AUC[log10 (CFU)]/mouse, and groups were compared using the nonparametric Kruskal-Wallis rank test.
Study approval.
Healthy adult volunteers provided written informed consent according to the recommendations of the local ethical committee in Lund, Sweden (permit 2017/582), and the Declaration of Helsinki (58). Use of animals in this study was performed in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals by the NIH (National Academies Press, 2011). The protocol was approved by the Institutional Animal Care and Use Committee at the University of Massachusetts Medical School.
Author contributions
SB, JS, KM, DE, KR, MU, SR, and AMB designed research studies. SB, JS, KM, DE, SG, BZ, MM, and SJ conducted experiments. SB, JS, KR, SR, and AMB analyzed data. SJ and MU provided strains and data for the clinical isolates. SB, SR, and AMB wrote the manuscript.
Supplementary Material
Acknowledgments
We thank Nancy Nowak and Rosane DeOliveira for technical assistance. SR was supported by National Institutes of Health grants AI119327, AI114790, AI132296, AI147930 and AI136007. This study was supported by grants from the Swedish Research Foundation (2018-02392), The Söderberg Foundation, The Österlund Foundation, the Royal Physiographic Society of Lund, and Sten K. Johnsons Foundation.
Version 1. 10/29/2019
In-Press Preview
Version 2. 12/05/2019
Electronic publication
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
Copyright: © 2019, American Society for Clinical Investigation.
Reference information: JCI Insight. 2019;4(23):e131886.https://doi.org/10.1172/jci.insight.131886.
Contributor Information
Serena Bettoni, Email: serena.bettoni@med.lu.se.
Jutamas Shaughnessy, Email: jutamas.shaughnessy@umassmed.edu.
Karolina Maziarz, Email: karolina.maziarz@med.lu.se.
David Ermert, Email: david.ermert@med.lu.se.
Sunita Gulati, Email: sunita.gulati@umassmed.edu.
Bo Zheng, Email: Bo.Zheng@umassmed.edu.
Matthias Mörgelin, Email: matthias@colzyx.com.
Susanne Jacobsson, Email: susanne.jacobsson@regionorebrolan.se.
Kristian Riesbeck, Email: kristian.riesbeck@med.lu.se.
Magnus Unemo, Email: magnus.unemo@regionorebrolan.se.
Sanjay Ram, Email: sanjay.ram@umassmed.edu.
Anna M. Blom, Email: anna.blom@med.lu.se.
References
- 1.Unemo M, et al. Sexually transmitted infections: challenges ahead. Lancet Infect Dis. 2017;17(8):e235–e279. doi: 10.1016/S1473-3099(17)30310-9. [DOI] [PubMed] [Google Scholar]
- 2.Quillin SJ, Seifert HS. Neisseria gonorrhoeae host adaptation and pathogenesis. Nat Rev Microbiol. 2018;16(4):226–240. doi: 10.1038/nrmicro.2017.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Unemo M, Shafer WM. Antibiotic resistance in Neisseria gonorrhoeae: origin, evolution, and lessons learned for the future. Ann N Y Acad Sci. 2011;1230:E19–E28. doi: 10.1111/j.1749-6632.2011.06215.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ohnishi M, et al. Is Neisseria gonorrhoeae initiating a future era of untreatable gonorrhea?: detailed characterization of the first strain with high-level resistance to ceftriaxone. Antimicrob Agents Chemother. 2011;55(7):3538–3545. doi: 10.1128/AAC.00325-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Unemo M, Golparian D, Syversen G, Vestrheim DF, Moi H. Two cases of verified clinical failures using internationally recommended first-line cefixime for gonorrhoea treatment, Norway, 2010. Euro Surveill. 2010;15(47):19721. doi: 10.2807/ese.15.47.19721-en. [DOI] [PubMed] [Google Scholar]
- 6.Alirol E, et al. Multidrug-resistant gonorrhea: A research and development roadmap to discover new medicines. PLoS Med. 2017;14(7):e1002366. doi: 10.1371/journal.pmed.1002366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shaughnessy J, et al. A novel factor H-Fc chimeric immunotherapeutic molecule against Neisseria gonorrhoeae. J Immunol. 2016;196(4):1732–1740. doi: 10.4049/jimmunol.1500292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mühlen S, Dersch P. Anti-virulence strategies to target bacterial infections. Curr Top Microbiol Immunol. 2016;398:147–183. doi: 10.1007/82_2015_490. [DOI] [PubMed] [Google Scholar]
- 9.Ermert D, Blom AM. C4b-binding protein: the good, the bad and the deadly. Novel functions of an old friend. Immunol Lett. 2016;169:82–92. doi: 10.1016/j.imlet.2015.11.014. [DOI] [PubMed] [Google Scholar]
- 10.Hovingh ES, van den Broek B, Jongerius I. Hijacking complement regulatory proteins for bacterial immune evasion. Front Microbiol. 2016;7:2004. doi: 10.3389/fmicb.2016.02004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ricklin D, Hajishengallis G, Yang K, Lambris JD. Complement: a key system for immune surveillance and homeostasis. Nat Immunol. 2010;11(9):785–797. doi: 10.1038/ni.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blom AM, Villoutreix BO, Dahlbäck B. Functions of human complement inhibitor C4b-binding protein in relation to its structure. Arch Immunol Ther Exp (Warsz) 2004;52(2):83–95. [PubMed] [Google Scholar]
- 13.Rooijakkers SH, van Strijp JA. Bacterial complement evasion. Mol Immunol. 2007;44(1-3):23–32. doi: 10.1016/j.molimm.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 14.Blom AM, Hallström T, Riesbeck K. Complement evasion strategies of pathogens-acquisition of inhibitors and beyond. Mol Immunol. 2009;46(14):2808–2817. doi: 10.1016/j.molimm.2009.04.025. [DOI] [PubMed] [Google Scholar]
- 15.Ermert D, et al. Virulence of group A streptococci is enhanced by human complement inhibitors. PLoS Pathog. 2015;11(7):e1005043. doi: 10.1371/journal.ppat.1005043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Laabei M, Ermert D. Catch me if you can: Streptococcus pyogenes complement evasion strategies. J Innate Immun. 2019;11(1):3–12. doi: 10.1159/000492944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ram S, et al. Binding of C4b-binding protein to porin: a molecular mechanism of serum resistance of Neisseria gonorrhoeae. J Exp Med. 2001;193(3):281–295. doi: 10.1084/jem.193.3.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Massari P, Ram S, Macleod H, Wetzler LM. The role of porins in neisserial pathogenesis and immunity. Trends Microbiol. 2003;11(2):87–93. doi: 10.1016/S0966-842X(02)00037-9. [DOI] [PubMed] [Google Scholar]
- 19.O’Brien JP, Goldenberg DL, Rice PA. Disseminated gonococcal infection: a prospective analysis of 49 patients and a review of pathophysiology and immune mechanisms. Medicine (Baltimore) 1983;62(6):395–406. doi: 10.1097/00005792-198311000-00005. [DOI] [PubMed] [Google Scholar]
- 20.Sun A, et al. Predominant porB1A and porB1B genotypes and correlation of gene mutations with drug resistance in Neisseria gonorrhoeae isolates in Eastern China. BMC Infect Dis. 2010;10:323. doi: 10.1186/1471-2334-10-323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Unemo M. Neisseria gonorrhoeae 2009. http://www.regionorebrolan.se/Files-sv/USO/Kliniker_enheter/Laboratoriemedicin/Mikro/Dokument/Neisseria/GC2009arsrapport.pdf Updated May 12, 2010. Accessed November 7, 2019.
- 22.Liao M, et al. Clusters of circulating Neisseria gonorrhoeae strains and association with antimicrobial resistance in Shanghai. J Antimicrob Chemother. 2008;61(3):478–487. doi: 10.1093/jac/dkm544. [DOI] [PubMed] [Google Scholar]
- 23.Blom AM, Kask L, Dahlbäck B. Structural requirements for the complement regulatory activities of C4BP. J Biol Chem. 2001;276(29):27136–27144. doi: 10.1074/jbc.M102445200. [DOI] [PubMed] [Google Scholar]
- 24.Blom AM, et al. Factor H-IgG chimeric proteins as a therapeutic approach against the gram-positive bacterial pathogen Streptococcus pyogenes. J Immunol. 2017;199(11):3828–3839. doi: 10.4049/jimmunol.1700426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shaughnessy J, et al. Fusion protein comprising factor H domains 6 and 7 and human IgG1 Fc as an antibacterial immunotherapeutic. Clin Vaccine Immunol. 2014;21(10):1452–1459. doi: 10.1128/CVI.00444-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wong SM, Shaughnessy J, Ram S, Akerley BJ. Defining the Binding region in Factor H to develop a therapeutic factor h-fc fusion protein against non-typeable Haemophilus influenzae. Front Cell Infect Microbiol. 2016;6:40. doi: 10.3389/fcimb.2016.00040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kask L, Villoutreix BO, Steen M, Ramesh B, Dahlbäck B, Blom AM. Structural stability and heat-induced conformational change of 2 complement inhibitors: C4b-binding protein and factor H. Protein Sci. 2004;13(5):1356–1364. doi: 10.1110/ps.03516504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gulati S, et al. Utilizing CMP-Sialic acid analogs to unravel Neisseria gonorrhoeae lipooligosaccharide-mediated complement resistance and design novel therapeutics. PLoS Pathog. 2015;11(12):e1005290. doi: 10.1371/journal.ppat.1005290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Price RJ, Boettcher B. The presence of complement in human cervical mucus and its possible relevance to infertility in women with complement-dependent sperm-immobilizing antibodies. Fertil Steril. 1979;32(1):61–66. doi: 10.1016/S0015-0282(16)44117-8. [DOI] [PubMed] [Google Scholar]
- 30.Ngampasutadol J, et al. Human C4b-binding protein selectively interacts with Neisseria gonorrhoeae and results in species-specific infection. Proc Natl Acad Sci USA. 2005;102(47):17142–17147. doi: 10.1073/pnas.0506471102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ngampasutadol J, et al. Human factor H interacts selectively with Neisseria gonorrhoeae and results in species-specific complement evasion. J Immunol. 2008;180(5):3426–3435. doi: 10.4049/jimmunol.180.5.3426. [DOI] [PubMed] [Google Scholar]
- 32. World Health Organization. More than 1 million new curable sexually transmitted infections every day. http://www.who.int/news-room/detail/06-06-2019-more-than-1-million-new-curable-sexually-transmitted-infections-every-day Updated June 6, 2019. Accessed November 7, 2019.
- 33.Chisholm SA, et al. An outbreak of high-level azithromycin resistant Neisseria gonorrhoeae in England. Sex Transm Infect. 2016;92(5):365–367. doi: 10.1136/sextrans-2015-052312. [DOI] [PubMed] [Google Scholar]
- 34.Liu Y, et al. Intravaginal administration of interleukin 12 during genital gonococcal infection in mice induces immunity to heterologous strains of Neisseria gonorrhoeae. mSphere. 2018;3(1):e00421-17. doi: 10.1128/mSphere.00421-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Foschi C, Salvo M, Cevenini R, Parolin C, Vitali B, Marangoni A. Vaginal lactobacilli reduce Neisseria gonorrhoeae viability through multiple strategies: an in vitro study. Front Cell Infect Microbiol. 2017;7:502. doi: 10.3389/fcimb.2017.00502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Churchward CP, Alany RG, Kirk RS, Walker AJ, Snyder LAS. Prevention of ophthalmia neonatorum caused by Neisseria gonorrhoeae using a fatty acid-based formulation. MBio. 2017;8(4):e00534-17. doi: 10.1128/mBio.00534-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gulati S, et al. Complement alone drives efficacy of a chimeric antigonococcal monoclonal antibody. PLoS Biol. 2019;17(6):e3000323. doi: 10.1371/journal.pbio.3000323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shaughnessy J, et al. Human Factor H domains 6 and 7 fused to IgG1 Fc are immunotherapeutic against Neisseria gonorrhoeae. J Immunol. 2018;201(9):2700–2709. doi: 10.4049/jimmunol.1701666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ram S, McQuillen DP, Gulati S, Elkins C, Pangburn MK, Rice PA. Binding of complement factor H to loop 5 of porin protein 1A: a molecular mechanism of serum resistance of nonsialylated Neisseria gonorrhoeae. J Exp Med. 1998;188(4):671–680. doi: 10.1084/jem.188.4.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Madico G, Ngampasutadol J, Gulati S, Vogel U, Rice PA, Ram S. Factor H binding and function in sialylated pathogenic neisseriae is influenced by gonococcal, but not meningococcal, porin. J Immunol. 2007;178(7):4489–4497. doi: 10.4049/jimmunol.178.7.4489. [DOI] [PubMed] [Google Scholar]
- 41.Lewis LA, Rice PA, Ram S. Role of gonococcal neisserial surface protein a (nspa) in serum resistance and comparison of its Factor H binding properties with those of its meningococcal counterpart. Infect Immun. 2019;87(2):e00658-18. doi: 10.1128/IAI.00658-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zak K, Diaz JL, Jackson D, Heckels JE. Antigenic variation during infection with Neisseria gonorrhoeae: detection of antibodies to surface proteins in sera of patients with gonorrhea. J Infect Dis. 1984;149(2):166–174. doi: 10.1093/infdis/149.2.166. [DOI] [PubMed] [Google Scholar]
- 43.Unemo M, Olcén P, Albert J, Fredlund H. Comparison of serologic and genetic porB-based typing of Neisseria gonorrhoeae: consequences for future characterization. J Clin Microbiol. 2003;41(9):4141–4147. doi: 10.1128/JCM.41.9.4141-4147.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nissilä E, et al. Genotypic and phenotypic diversity of Lactobacillus rhamnosus clinical isolates, their comparison with strain GG and their recognition by complement system. PLoS ONE. 2017;12(5):e0176739. doi: 10.1371/journal.pone.0176739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Webb JH, Blom AM, Dahlbäck B. Vitamin K-dependent protein S localizing complement regulator C4b-binding protein to the surface of apoptotic cells. J Immunol. 2002;169(5):2580–2586. doi: 10.4049/jimmunol.169.5.2580. [DOI] [PubMed] [Google Scholar]
- 46.Jarva H, Ram S, Vogel U, Blom AM, Meri S. Binding of the complement inhibitor C4bp to serogroup B Neisseria meningitidis. J Immunol. 2005;174(10):6299–6307. doi: 10.4049/jimmunol.174.10.6299. [DOI] [PubMed] [Google Scholar]
- 47.Hallström T, Blom AM, Zipfel PF, Riesbeck K. Nontypeable Haemophilus influenzae protein E binds vitronectin and is important for serum resistance. J Immunol. 2009;183(4):2593–2601. doi: 10.4049/jimmunol.0803226. [DOI] [PubMed] [Google Scholar]
- 48.Berggård K, Lindahl G, Dahlbäck B, Blom AM. Bordetella pertussis binds to human C4b-binding protein (C4BP) at a site similar to that used by the natural ligand C4b. Eur J Immunol. 2001;31(9):2771–2780. doi: 10.1002/1521-4141(200109)31:9<2771::AID-IMMU2771>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 49.Nordström T, Blom AM, Forsgren A, Riesbeck K. The emerging pathogen Moraxella catarrhalis interacts with complement inhibitor C4b binding protein through ubiquitous surface proteins A1 and A2. J Immunol. 2004;173(7):4598–4606. doi: 10.4049/jimmunol.173.7.4598. [DOI] [PubMed] [Google Scholar]
- 50.Meri T, Blom AM, Hartmann A, Lenk D, Meri S, Zipfel PF. The hyphal and yeast forms of Candida albicans bind the complement regulator C4b-binding protein. Infect Immun. 2004;72(11):6633–6641. doi: 10.1128/IAI.72.11.6633-6641.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mandrell RE, Griffiss JM, Macher BA. Lipooligosaccharides (LOS) of Neisseria gonorrhoeae and Neisseria meningitidis have components that are immunochemically similar to precursors of human blood group antigens. Carbohydrate sequence specificity of the mouse monoclonal antibodies that recognize crossreacting antigens on LOS and human erythrocytes. J Exp Med. 1988;168(1):107–126. doi: 10.1084/jem.168.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hitchcock PJ, Hayes SF, Mayer LW, Shafer WM, Tessier SL. Analyses of gonococcal H8 antigen. Surface location, inter- and intrastrain electrophoretic heterogeneity, and unusual 2-dimensional electrophoretic characteristics. J Exp Med. 1985;162(6):2017–2034. doi: 10.1084/jem.162.6.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dudas KC, Apicella MA. Selection and immunochemical analysis of lipooligosaccharide mutants of Neisseria gonorrhoeae. Infect Immun. 1988;56(2):499–504. doi: 10.1128/iai.56.2.499-504.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shafer WM, Joiner K, Guymon LF, Cohen MS, Sparling PF. Serum sensitivity of Neisseria gonorrhoeae: the role of lipopolysaccharide. J Infect Dis. 1984;149(2):175–183. doi: 10.1093/infdis/149.2.175. [DOI] [PubMed] [Google Scholar]
- 55.Schneider H, Griffiss JM, Boslego JW, Hitchcock PJ, Zahos KM, Apicella MA. Expression of paragloboside-like lipooligosaccharides may be a necessary component of gonococcal pathogenesis in men. J Exp Med. 1991;174(6):1601–1605. doi: 10.1084/jem.174.6.1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.McQuillen DP, Gulati S, Rice PA. Complement-mediated bacterial killing assays. Meth Enzymol. 1994;236:137–147. doi: 10.1016/0076-6879(94)36013-8. [DOI] [PubMed] [Google Scholar]
- 57.Härdig Y, Hillarp A, Dahlbäck B. The amino-terminal module of the C4b-binding protein alpha-chain is crucial for C4b binding and factor I-cofactor function. Biochem J. 1997;323(Pt 2):469–475. doi: 10.1042/bj3230469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.World Medical Association. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191–2194. doi: 10.1001/jama.2013.281053. [DOI] [PubMed] [Google Scholar]
- 59.Sjöberg AP, Manderson GA, Mörgelin M, Day AJ, Heinegård D, Blom AM. Short leucine-rich glycoproteins of the extracellular matrix display diverse patterns of complement interaction and activation. Mol Immunol. 2009;46(5):830–839. doi: 10.1016/j.molimm.2008.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nunn MA, et al. Complement inhibitor of C5 activation from the soft tick Ornithodoros moubata. J Immunol. 2005;174(4):2084–2091. doi: 10.4049/jimmunol.174.4.2084. [DOI] [PubMed] [Google Scholar]
- 61.Escudero-Esparza A, Kalchishkova N, Kurbasic E, Jiang WG, Blom AM. The novel complement inhibitor human CUB and Sushi multiple domains 1 (CSMD1) protein promotes factor I-mediated degradation of C4b and C3b and inhibits the membrane attack complex assembly. FASEB J. 2013;27(12):5083–5093. doi: 10.1096/fj.13-230706. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.