Abstract
In order to select excellent strains with high CO2 fixation capability on a large scale, nine Spirulina species were cultivated in columnar photobioreactors with the addition of 10% CO2. The two species selected (208 and 220) were optimized for pH value, total dissolved inorganic carbon (DIC), and phosphorus content with intermittent CO2 addition in 4 m2 indoor raceway ponds. On the basis of biomass accumulation and CO2 fixation rate in the present study, the optimum pH, DIC, and phosphate concentration were 9.5, 0.1 mol L−1, and 200 mg L−1 for both strains, respectively. Lastly, the two strains selected were semi-continuously cultivated successfully for CO2 mitigation in 605 m2 raceway ponds aerated with food-grade CO2 purified from a coal chemical flue gas on a large scale. The daily average biomass dry weight of the two stains reached up to 18.7 and 13.2 g m−2 d−1, respectively, suggesting the two Spirulina strains can be utilized for mass production.
Keywords: CO2 mitigation, Spirulina sp., process optimization, large-scale cultivation, coal chemical flue gas
Introduction
Global warming caused by CO2 emissions due to human activities has become a significant environmental issue. It is reported that up to 7% of global CO2 emissions can be attributed to the anthropogenic emission of CO2 from coal-fired thermoelectric plants (Morais and Costa, 2007). With respect to CO2 sequestration, different technologies have been investigated, such as using amines or solid adsorbents (da Rosa et al., 2016; Sepulveda et al., 2019). Cardias et al. (2018) reported that the growth of Spirulina sp. and its CO2 fixation capacity increased significantly by separate addition or mixture of diethanolamine (DEA) and potassium carbonate (K2CO3). A promising strategy for enhancing CO2 sequestration in an environmentally friendly and sustainable manner was reported using small doses of sugars together with LED illumination during cultivation of Chlorella vulgaris in different sized photobioreactors (PBRs) (Fu et al., 2019).
Among the different strategies for mitigating CO2, biological CO2 mitigation through microalgae has recently received considerable attention due to their higher CO2 fixation capability and bioactive substances contained in their biomass (Wang et al., 2008; Yoo et al., 2010; Matsudo et al., 2012; Hancke et al., 2015; Duarte et al., 2017). Several studies have shown that microalgal growth can be improved by CO2 from the atmosphere or flue gases and they have better CO2 fixation abilities (10–50 times greater) than terrestrial plants (Chen et al., 2013; Yadav and Sen, 2017). For example, it was reported that Spirulina have ten times the CO2 fixation rate of land plants (Chen et al., 2013).
Spirulina is a filamentous and photosynthetic cyanobacterium that can grow in culture solutions with a pH of ~10 (Vonshak, 1997; Bao et al., 2012). Due to its high nutritional value and the presence of bioactive compounds, the alga is one of the most studied microalgae for commercial interests (Belay, 2008). Spirulina has been produced commercially in open raceway ponds on a large scale (Belay, 2002).
During cultivation, the cost of the carbon source should be considered, as it is the primary element that the cell requires. Using dissolved NaHCO3 (Costa et al., 2004), Na2CO3, both NaHCO3 and Na2CO3 (Binaghi et al., 2003), or CO2 as carbon sources, the alga can be cultivated in a small volumes or on a large scale for Spirulina biomass production (Rosa et al., 2011). The pH of the medium determines the solubility and availability of CO2 and nutrients and has an important effect on microalgal growth. Therefore, pH is one of the most critical environmental conditions in microalgal cultivation (Chen and Durbin, 1994). Phosphorus is also an important element for microalgal growth. Inorganic phosphate compounds, such as hydrogen phosphates (H2 and ), can be transformed into organic species via phosphorylation in microalgae (Razzak et al., 2017) and these organic species are valuable for cell growth.
To mitigate CO2, it is very important to have excellent microalgal strains. For example, it is a requirement to select alkali-tolerant microalgae to enhance algal growth by increasing the content of dissolved inorganic carbon (DIC) in the alkaline medium (Kuo et al., 2018). An efficient way to screen excellent algal strains was reported by Cheng et al. (2013), who showed that nuclear irradiation combined with CO2 domestication could improve biomass productivity and CO2 fixation of Chlorella species. However, work focused on process optimization, especially for the mass cultivation of Spirulina, and the simultaneous biological fixation of CO2 is limited.
The aim of this study was to select excellent strains with high CO2 fixation capabilities on a large scale. Firstly, nine Spirulina species were selected by injection of 10% CO2 under laboratory conditions. Then, the optimal conditions (pH value, total DIC, and phosphorus content) for biomass productivity were optimized for the selected strains with intermittent CO2 addition under pilot-scale production conditions (4 m2 indoor raceway ponds). Lastly, the two strains selected were semi-continuously successfully cultivated for CO2 mitigation while producing high biomass on a large scale in 605 m2 raceway ponds aerated with food-grade CO2 purified from a coal chemical flue gas (Cheng et al., 2018), suggesting the two Spirulina can be utilized for mass production.
Materials and Methods
Strain Selection in a Columnar Photobioreactor
Nine Spirulina species obtained from the Laboratory of Applied Microalgae Biology, Ocean University of China (LAMB, OUC) were screened for their growth characteristics and CO2 fixation capabilities and are listed in Table 1 along with their numbers. The nine strains were cultivated in 800 mL columnar photobioreactors with the addition of 10% CO2. The working volume was 650 mL and the initial biomass concentration was approximately (0.1 ± 0.02) g L−1. The modified Zarrouk medium (Zarrouk, 1966) consisted of the following (g L−1): Na2CO3, 13.61; NaHCO3, 4.03; NaNO3, 2.50; NaCl, 1.00; K2HPO4, 0.50; K2SO4, 1.00; MgSO47H2O, 0.20; CaCl2, 0.08; FeSO4 7H2O, 0.01; and Na2-EDTA, 0.08, with 2 mL trace element solution A5. The trace elements (A5) in the solution consisted of the following (g L−1): H3BO3, 2.86; MnCl24H2O, 1.80; (NH4)6Mo17O24, 0.02; ZnSO47H2O, 0.22; and CuSO45H2O, 0.08. The nine strains were cultured at a temperature of 28 ± 1°C, under LED illumination of 56–63 μmol photons m−2 s−1 in a 12:12 h dark-light (D:L) cycle. A total of 10% CO2 was injected into the columnar photobioreactors at a rate of 100 mL min−1 under illumination, whereas air was added to prevent algal cells from clustering when it was dark. The experiments were performed in triplicate and lasted 9 days.
Table 1.
Specific growth rate, biomass productivity, carbon content, and carbon dioxide fixation rate of nine Spirulina strains cultivated in a columnar photobioreactor with 10% CO2.
| Microalgal strain | Number | μ (d−1) | P (mg L−1 d−1) | C (% W W−1) | RCO2 (mg L−1 d−1) |
|---|---|---|---|---|---|
| Spirulina sp. | LAMB167 | 0.294 ± 0.011a | 154.93 ± 0.028d | 45.577 ± 0.111a | 258.80 ± 0.026c |
| Spirulina sp. | LAMB169 | 0.190 ± 0.002c | 34.78 ± 0.001f | 43.293 ± 0.424bc | 55.20 ± 0.001e |
| Spirulina platensis | LAMB171 | 0.326 ± 0.001a | 191.15 ± 0.005bc | 43.820 ± 0.361bc | 307.76 ± 0.006bc |
| Spirulina platensis | LAMB172 | 0.345 ± 0.002a | 209.11 ± 0.008ab | 43.400 ± 0.356bc | 332.88 ± 0.010b |
| Spirulina platensis | LAMB206 | 0.325 ± 0.004a | 177.04 ± 0.011cd | 42.590 ± 0.343c | 276.54 ± 0.011c |
| Spirulina platensis | LAMB207 | 0.345 ± 0.003a | 175.11 ± 0.010cd | 44.453 ± 0.199ab | 285.35 ± 0.008bc |
| Spirulina platensis | LAMB208 | 0.294 ± 0.000a | 180.30 ± 0.001c | 42.700 ± 0.496c | 282.31 ± 0.004bc |
| Spirulina sp. | LAMB220 | 0.365 ± 0.002a | 229.26 ± 0.007a | 41.110 ± 0.706d | 414.15 ± 0.032a |
| Spirulina sp. | LAMB221 | 0.274 ± 0.008b | 115.58 ± 0.016e | 39.940 ± 0.605d | 169.26 ± 0.014d |
Values in the same row with different lower-case letters are significantly different (P < 0.05). Values shown are means ± standard deviation.
Process Optimization in Indoor Raceway Ponds (4 m2)
Process optimization experiments with the two strains selected (208 and 220) were carried out in Inner Mongolia, China (38°18'−40°11'N, 106°41'−108°54'E). Strain 208 was selected due to its good helix pitch and longer trichome, which have a great influence on biomass harvesting efficiency (Cheng et al., 2018). It is also tolerant to temperatures up to 40°C (solution temperature), which is helpful for its large-scale cultivation in open raceway ponds. Strain 220 was chosen because of its high biomass productivity and CO2 fixation rate and its tolerance to higher CO2 concentrations. The two strains were cultivated in 4 m2 raceway ponds (average solution depth of 30 cm) with a working volume of 1.2 m3. To prevent exotic contamination, raceway ponds were built in a sun shed. To manipulate the solution pH and provide supplemental carbon, food-grade CO2 with a purity of 99.99% containing no heavy metals but trace other components (Cheng et al., 2018) was purified from a coal chemical flue gas (99% CO2) through a series of desulfurization processes, organosulfur hydrolysis, cooling dehumidification, adsorption, liquefaction, and distillation purification (Cheng et al., 2018) and intermittently injected into the culture solution through a fine tube at a rate of 1.5 L min−1. Aerated stones set in a line in the bottom of the raceway ponds were used to diffuse the CO2. A paddlewheel (motor power, 1.5 KW; rotational speed, 12 r min−1) was used to drive the culture solution to maintain the water velocity at 0.20 m s−1 from 7:00 a.m. to 7:00 p.m. during cultivation. Illumination was provided by natural sunlight. The culture medium used was an industrial formulation for Spirulina in Inner Mongolia. The medium consisted of the following (g L−1): NaNO3, 1.00; H3PO4, 0.20; NH4HCO3, 0.01; MgSO47H2O, 0.03; KCl, 0.5; FeSO4 7H2O, 0.01; and Na2-EDTA, 0.01, with 2 mL trace element solution A5. The trace elements solution (A5) was as described above (strain selection experiments). All experiments were undertaken in triplicate and the dry weight of each biomass was measured every day. During cultivation, the solution temperature, pH, and light intensity were measured five times every day at fixed times.
Cultures were grown at three different pH levels (9.5, 10.0, and 10.5 ± 0.05) to adjust the injection of CO2 for pH optimization experiments. For DIC optimization experiments, the total carbon concentrations of Na2CO3 and NaHCO3 were 0.06 (1.8 g/L Na2CO3, 3.6 g/L NaHCO3), 0.1 (3.0 g/L Na2CO3, 6.0 g/L NaHCO3), and 0.14 (4.2 g/L Na2CO3, 8.4 g/L NaHCO3) mol L−1, respectively, at pH 9.5 ± 0.05. For phosphate concentration optimization experiments, phosphate concentrations were set for 200, 225, and 250 mg L−1, respectively. The pH and DIC set were 9.5 and 0.1 mol L−1, respectively.
Large-Scale Cultivation in Open Raceway Ponds (605 m2)
Large scale cultivation of the two strains selected (208 and 220) were also undertaken in Inner Mongolia, China (38°18'−40°11'N, 106°41'−108°54'E). Cultivation was carried out in triplicate in 605 m2 open raceway ponds with a working volume of 193.6 m3 (110 m in length and 5.5 m in width, average solution depth of 32 cm) in a vinyl house. A paddlewheel (motor power, 1.5 KW; rotational speed, 36 r min−1) was used to drive the culture solution, keeping the water velocity at 0.20 m s−1 from 7:00 a.m. to 7:00 p.m. CO2 (99.99%) purified from an industrial CO2 flue gas (Cheng et al., 2018) was intermittently used to maintain the culture pH at 9.5–9.8. The above optimized process conditions (pH 9.5, DIC 0.1 mol L−1, phosphate concentration 200 mg L−1) were used to cultivate the two strains selected on a large scale. Illumination was also provided by natural sunlight. During cultivation, the solution temperature and light intensity were also measured five times every day at fixed times. The medium formation was the same as described for the process optimization experiments.
Analytical Methods
For biomass analysis, 50 mL samples were taken every day at a fixed time. The culture solution was filtered using GF/CTM (Whatman™) filters and washed three times with distilled water. Samples were subsequently dried in an air-dry oven at 60°C until a constant weight was obtained. The specific growth rate (μ, day−1) and the biomass productivity (PX) were calculated by the following equations (da Silva Vaz et al., 2016):
where Xt and X0 were the dry biomass concentrations (g L−1) at time t (day) and t0 (day), respectively.
The contents of HCO3− and in the culture solution were measured using a double-tracer technique according to Cheng et al. (2018). It needs to be pointed out that 0.1 mol L−1 HCL was used to titration instead of 0.1 mol L−1 H2SO4. All experiments were carried out twice and measured once at a fixed time every day during cultivation.
The CO2 fixation rate (R) of microalgae was calculated following the equation:
Carbon content in algal cells (Xcbm) was determined using an elemental analyzer (Vario EL III, Germany) (da Silva Vaz et al., 2016). MCO2 and MC were the molecular weights of carbon dioxide and carbon, respectively (Duarte et al., 2017).
Results and Discussion
Strain Selection
Nine Spirulina species were tested for their CO2 fixation capabilities by evaluating biomass productivity and CO2 fixation rate in 800 mL columnar photobioreactors under laboratory conditions. As shown in Table 1, the best biomass producers and CO2 fixation capabilities were found in the five strains, 171, 172, 207, 208, and 220. The highest CO2 fixation rate (414.15 mg L−1 d−1) was found in strain 220, which was also the most productive of all the strains tested. Although 208 strain was not the best for CO2 fixation rate, it was selected due to its good helix pitch and longer trichome (data not shown), which have a great influence on biomass harvesting efficiency (Cheng et al., 2018).
To improve the CO2 fixation rate, excellent algal strains need to be selected. Almomani et al. (2019) reported that mixed indigenous microalgae (MIMA, collected from a secondary basin of Doha South wastewater treatment plant) performed significantly better than a single Spirulina platensis (SP.PL) culture, especially with respect to growth and CO2 biofixation (Almomani et al., 2019). Badger and Price (1994) indicated that high CO2 levels could improve carbon fixation activity of the enzyme rubisco in microalgal cells, and rubisco facilitates the utilization of CO2, thus, increasing the biological fixation efficiency of CO2. Activities of some enzymes, such as rubisco and other enzymes related to CO2 biofixation, should be measured in future research, as metabolic activities of microalgae have a great influence on the rate of carbon uptake (Sydney et al., 2010).
Process Optimization of the Two Selected Strains Cultivated in Indoor Raceway Ponds (4 m2) pH Optimization
pH is one of the most critical environmental conditions in microalgal cultivation (Chen and Durbin, 1994). Different Spirulina strains have different optimum pH during cultivation. The optimum pH was 9.0 for a Spirulina sp. isolated from an oil polluted Xame pit, which showed the highest biomass concentration of 4.9 mg mL−1 on a dry weight basis (Ogbonda et al., 2007). An optimal culture pH of 9.5 for Spirulina platensis was reported by Chen et al. (2016), who indicated that maintaining a steady pH resulted in more efficient CO2 utilization and better cell growth than that obtained in a continuous CO2 feeding system. Similar to this research, the optimum culture pH was also 9.5 for both strains tested in this study, and the two strains achieved the highest CO2 fixation rate at this pH (Table 2). pH has an important impact on the distribution of DIC species in the culture medium, which strongly influences the growth of microalgae (Kuo et al., 2018).
Table 2.
Specific growth rate, biomass productivity, carbon content, and carbon dioxide fixation rate of two Spirulina strains cultivated in indoor raceway ponds (4 m2) at different pH values.
| pH | Strain | μ (d-1) | P (mg L−1 d−1) | C (% W W−1) | RCO2 (mg L−1 d−1) |
|---|---|---|---|---|---|
| 9.5 | 208 | 0.194 ± 0.004a | 29.20 ± 0.001a | 46.99 ± 0.856a | 50.30 ± 0.001a |
| 10.0 | 0.197 ± 0.002a | 24.80 ± 0.001b | 47.68 ± 0.686a | 43.35 ± 0.000b | |
| 10.5 | 0.164 ± 0.002b | 20.00 ± 0.000c | 45.99 ± 0.750a | 33.73 ± 0.001c | |
| 9.5 | 220 | 0.185 ± 0.190a | 26.40 ± 0.001a | 44.82 ± 1.237a | 43.36 ± 0.001a |
| 10.0 | 0.154 ± 0.005a | 21.27 ± 0.000b | 38.32 ± 0.601b | 29.87 ± 0.000b | |
| 10.5 | 0.154 ± 0.013a | 20.80 ± 0.001b | 37.93 ± 0.863b | 28.91 ± 0.001b |
Phosphate concentration was 200 mg L−1 and dissolved inorganic carbon (DIC) concentration was 0.1 mol L−1 in this experiment.
Values in the same row with different lower-case letters are significantly different (P < 0.05). Values shown are means ± standard deviation.
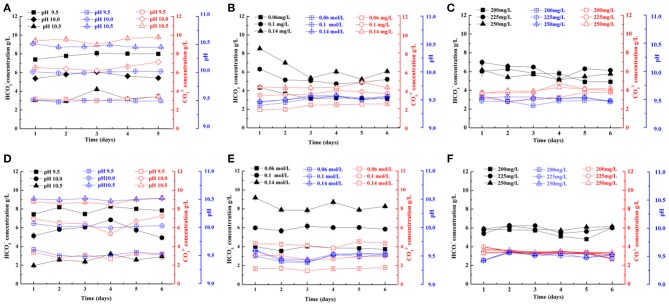
An increase in pH of the culture medium could inhibit algal growth (Nayak et al., 2013). Thus, it is very important to keep a steady pH of the culture medium during cultivation. The pH can be kept steady by intermittent CO2 supply due to the formation of carbonic acid as CO2 addition into the medium (Zeng et al., 2012). In this study, culture pH was manipulated by intermittent food-grade CO2 addition, purified from a coal chemical flue gas. Compared with the alkali salt, purified CO2 as a carbon source can improve the quality of Spirulina sp. and reduce the cost of cultivation; thus, it is both economical and profitable (Cheng et al., 2018). As shown in Figure 1, the culture pH was kept steady during cultivation for all three optimization experiments, suggesting it is necessary to keep pH stable by intermittent CO2 addition.
Figure 1.
and concentrations (g/L) of two Spirulina strains (208, A–C; 220, D–F) cultivated in indoor raceway ponds (4 m2) with different pH (A,D), total dissolved inorganic carbon (DIC) concentrations (B,E), and phosphoric acid concentrations (C,F).
DIC Optimization
The distribution of DIC species in the culture medium has influenced the growth of microalgae strongly (Kuo et al., 2018). Therefore, it is necessary to increase the availability of DIC in aqueous solution for enhancement of algal growth. DIC concentration in the culture medium can be increased by adding NaHCO3 (Nayak et al., 2018), which will increase in salinity due to Na+ accumulation. However, excessively high salinity inhibits the growth of microalgae (Pandit et al., 2017); thus, an optimal range of NaHCO3 should be controlled. As shown in Table 3, the CO2 fixation rates of both strains were significantly higher at 0.1 mol L−1 DIC than at other inorganic carbon concentrations, suggesting that this DIC concentration is optimal for both strains in this study.
Table 3.
Specific growth rate, biomass productivity, carbon content, and carbon dioxide fixation rate of two Spirulina strains cultivated in indoor raceway ponds (4 m2) with different dissolved inorganic carbon (DIC) concentrations.
| DIC (mol L−1) | Strains | μ (d−1) | P (mg L−1 d−1) | C (% W W−1) | RCO2 (mg L−1 d−1) |
|---|---|---|---|---|---|
| 0.06 | 208 | 0.133 ± 0.003b | 20.67 ± 0.000b | 47.33 ± 0.361b | 35.86 ± 0.000b |
| 0.1 | 0.161 ± 0.003a | 25.83 ± 0.001a | 48.83 ± 0.085a | 46.25 ± 0.002a | |
| 0.14 | 0.126 ± 0.006b | 20.05 ± 0.002b | 48.14 ± 0.424ab | 36.17 ± 0.003b | |
| 0.06 | 220 | 0.178 ± 0.002b | 37.54 ± 0.000b | 44.12 ± 0.297b | 60.73 ± 0.000c |
| 0.1 | 0.199 ± 0.004a | 44.75 ± 0.001a | 47.10 ± 0.693a | 77.27 ± 0.001a | |
| 0.14 | 0.190 ± 0.003a | 42.38 ± 0.001a | 43.30 ± 0.127b | 67.28 ± 0.002b |
Phosphate concentration was 200 mg L−1 and pH was 9.5 in this experiment.
Values in the same row with different lower-case letters are significantly different (P < 0.05). Values shown are means ± standard deviation.
The concentration of DIC in the culture medium can be increased by continuous or intermittent CO2 aeration, as has been demonstrated in many studies (Matsudo et al., 2012; Chen et al., 2016; Duarte et al., 2017; Qiu et al., 2017; Almomani et al., 2019). Bao et al. (2012) indicated that CO2 absorptivity has a positive correlation with pH value and a negative correlation with total carbon concentration. Similar to this study, the optimum total carbon concentration and pH ranges of Spirulina platensis were 0.03–0.09 mol L−1 and 9.7–10.0 in open raceway ponds, respectively.
Phosphate Concentration Optimization
Phosphorus is an important element for microalgal growth. In order to evaluate the effects of different phosphate concentrations on the growth and CO2 fixation rates of the two strains selected, a phosphorus concentration optimization experiment was performed in indoor raceway ponds (4 m2). As seen in Table 4, better biomass production and CO2 fixation capabilities were found at the lower phosphate concentrations (200 and 225 mg L−1) for both strains. There was a small difference in CO2 fixation rate between the two concentrations but this was not statistically significantly for both strains. Nitrogen and phosphorus consumption rates (mgL−1d−1) were evaluated for the phosphorus concentration optimization experiment. It was deduced that the phosphate concentrations set up in this study were not the limiting factors during cultivation due to the lower consumption rate of phosphate (data not shown). Therefore, the optimal phosphate concentration was 200 mg L−1 for both strains. Inorganic phosphate compounds such as hydrogen phosphates (H2 and ) can be transformed into organic species via phosphorylation in microalgae (Razzak et al., 2017), and these organic species are valuable for cell growth.
Table 4.
Specific growth rate, biomass productivity, carbon content, and carbon dioxide fixation rate of two Spirulina strains cultivated in indoor raceway ponds (4 m2) with different phosphate concentrations (mg L−1).
| Phosphate (mg L−1) | Strain | μ (d−1) | P (mg L−1 d−1) | C (% W W−1) | RCO2 (mg L−1 d−1) |
|---|---|---|---|---|---|
| 200 | 208 | 0.096 ± 0.004a | 19.33 ± 0.000ab | 47.56 ± 0.290a | 33.71 ± 0.616ab |
| 225 | 0.102 ± 0.002a | 20.33 ± 0.000a | 47.06 ± 0.106a | 35.08 ± 0.734a | |
| 250 | 0.090 ± 0.006a | 16.83 ± 0.001b | 48.21 ± 0.495a | 29.77 ± 2.389b | |
| 200 | 220 | 0.144 ± 0.006a | 22.17 ± 0.001a | 41.01 ± 1.011a | 33.35 ± 2.358a |
| 225 | 0.138 ± 0.017a | 20.46 ± 0.002a | 40.16 ± 0.332a | 30.12 ± 3.028ab | |
| 250 | 0.126 ± 0.005a | 18.28 ± 0.001a | 34.91 ± 0.750b | 23.41 ± 2.212b |
Dissolved inorganic content (DIC) concentration was 0.1 mol L−1 and pH was 9.5 in this experiment.
Values in the same row with different lower-case letters are significantly different (P < 0.05). Values shown are means ± standard deviation.
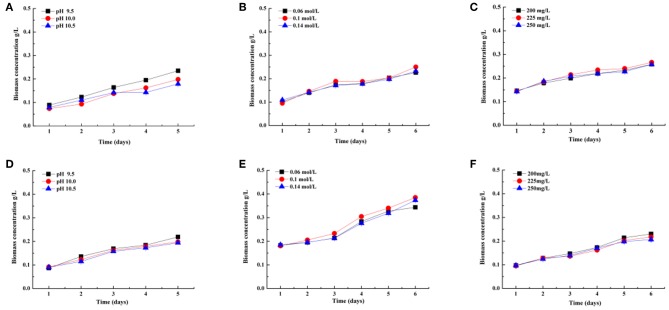
Figure 2 shows that biomass concentration (g L−1) of the two selected strains cultivated in indoor raceway ponds (4 m2) for process optimization experiments changed with the time of cultivation. The same conclusion can be drawn according to biomass concentration with the CO2 fixation rates. In other words, the optimal pH, DIC, and phosphate concentrations are 9.5, 0.1 mol L−1, and 200 mg L−1 on the basis of biomass accumulation in this study for both strains, respectively.
Figure 2.
Biomass concentration (g/L) of two Spirulina strains (208, A–C; 220, D–F) cultivated in indoor raceway ponds (4 m2) with different pH (A,D), total dissolved inorganic carbon (DIC) concentrations (B,E), and phosphoric acid concentrations (C,F).
The content of and in solutions was measured for process optimization experiments during cultivation. Cheng et al. (2018) reported that the and concentrations could be increased by aerating CO2 directly into the raceway pond. However, different to this report, the concentrations of and remained unchanged by intermittent CO2 addition and the contents of were higher than those of in most cases in this study (Figure 1). This may be because we maintained the culture pH steady during cultivation for all three optimization experiments. Maintaining a steady pH of the culture medium during cultivation is very important for improving algal growth. Furthermore, both dissolved CO2 and HCO3− can be utilized for algal growth, which are the dominant species as the pH was below 6.3 and ranged from 6.3 to 10 (Weiner, 2012). In this study, the pH ranged from 9.5 to 10.5, and the main species were and in the culture solution, which is beneficial for enhancement of algal growth.
In general, specific growth rates, biomass productivities, and carbon dioxide fixation rates of two strains found in indoor raceway ponds (4 m2) were much lower than those cultivated in the columnar photobioreactor. This may be mainly due to different cultivation conditions such as solution temperatures and light intensities. For Spirulina sp., 30°C is the optimum temperature for biomass production (Ogbonda et al., 2007). The average solution temperature during cultivation in the indoor raceway ponds ranged from 22 to 24°C, demonstrating that both strains grow in lower than the optimum temperatures. On the other hand, in indoor raceway cultivation, the algal cells were exposed to high light intensities most of the time, resulting in photoinhibition and photooxidation, and consequently slower growth (data not shown).
Cultivation of Two Spirulina Strains on a Large Scale
In order to evaluate whether two strains can be cultivated on a large scale for mass production, two strains were cultured under industrial conditions in open raceway ponds (605 m2) for 8 days. On the fourth day of cultivation, half the volume of the biomass was harvested to cater to industrial production. As shown in Figure 3, two strains were cultivated successfully in raceway ponds (605 m2) for industrial production and daily average biomass dry weight reached up to 18.7 (strain 208) and 13.2 g m−2 d −1 (strain 220), respectively (data not shown). Therefore, the two strains selected can be used for industrial production.
Figure 3.
The average sunlight intensity and solution temperature changes on a typical day (A) and biomass dry weight (B) of two Spirulina strains (208, 220) semi-continuously cultivated in open raceway ponds (605 m2).
Microalgal biomass production may be combined with direct biofixation of CO2 (about 1.8 kg of CO2 is needed for 1 kg of dry algal biomass). In other words, biomass production is directly proportional to CO2 fixation (Rodolfi et al., 2009). Different biomass compositions indicate different carbon metabolism in microalgae. The main destination of carbon is manifested by biomass production in microalgal cultivation, which gives important data regarding microalgal metabolism and might be considered in industrial applications (Sydney et al., 2010).
Biochemical composition of the algal biomass is another focus with respect to CO2 fixation by microalgae. Biochemical composition such as phycocyanin for Spirulina should be measured in future research due to the changes that can be seen by varying growth conditions, especially solution temperature and light intensities. Natural sunlight was used in our large-scale cultivation experiment. Using natural sunlight to culture cyanobacteria has some advantages, not only reducing production costs and reducing the burning of fossil fuels to generate electricity, but also mitigating CO2 emissions to the atmosphere.
The temperature during cultivation in the open raceway ponds on a large scale ranged from 32 to 37°C (Figure 3), which is above the optimum temperature for Spirulina, demonstrating both strains can grow outside their optimum temperatures. The tolerance to elevated temperatures of the strains we studied is an important factor for reducing flue gas released from coal chemical plant, which can be directly injected into open raceway ponds for CO2 fixation on a large scale.
Conclusion
The aim of this study was to investigate the influence of pH, total DIC concentration, and phosphorus content on the growth and CO2 assimilation efficiency of Spirulina cultured in open raceway ponds with intermittent CO2 addition on a large scale. CO2 and DIC (NaHCO3 and Na2CO3) were used as a carbon source and for pH control simultaneously. Relatively stable culture conditions were obtained in most of the runs except for solution temperature and light intensities, indicating that semi-continuous cultivation of two Spirulina strains in open raceway ponds on a large scale could be an efficient way for CO2 fixation to mitigate greenhouse effects while producing high biomass.
In conclusion, in the present study, the optimal DIC concentration, phosphate concentration, and pH conditions for biomass production were demonstrated for two Spirulina strains, which can be used to produce biomass and fix CO2 on a large scale. Overall, algal strain selection and process optimization of cultivation conditions may be a key area for future development (Duarte et al., 2017).
Data Availability Statement
The datasets generated for this study are available on request to the corresponding author.
Author Contributions
BZ, YL, JH, YZ, and KP conceived and designed the experiments and analyzed and interpreted the data. HS, QL, and GJ planned and performed various experiments. BZ and HS wrote the manuscript. All authors agreed on the final manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors thank the reviewers for their valuable comments. We thank International Science Editing (http://www.internationalscienceediting.com) for editing this manuscript.
Footnotes
Funding. This work was supported by the National Key Research and Development Program of China (2016YFB0601001).
References
- Almomani F., Ketife A. A., Judd S., Shurair M., Bhosale R. R., Znad H., et al. (2019). Impact of CO2 concentration and ambient conditions on microalgal growth and nutrient removal from wastewater by a photobioreactor. Sci. Total Environ. 662, 662–671. 10.1016/j.scitotenv.2019.01.144 [DOI] [PubMed] [Google Scholar]
- Badger M. R., Price G. D. (1994). The role of carbonic anhydrase in photosynthesis. Annu. Rev. Plant Phys. 45, 369–392. 10.1146/annurev.pp.45.060194.002101 [DOI] [Google Scholar]
- Bao Y. L., Liu M., Wu X., Cong W., Ning Z. X. (2012). In situ carbon supplementation in large-scale cultivations of Spirulina platensis in open raceway pond. Biotechnol. Bioprocess Eng. 17, 93–99. 10.1007/s12257-011-0319-9 [DOI] [Google Scholar]
- Belay A. (2002). The potential application of Spirulina (Arthrospira) as a nutritional and therapeutic supplement in health management. J. Am. Nutraceut. Assoc. 5, 27–48. [Google Scholar]
- Belay A. (2008). “Spirulina platensis (arthrospira): production and quality assurance,” in Spirulina in Human Nutrition and Health, eds Gershwin M. E, Belay A. (Portland, OR: Taylor & Francis, 2–23. [Google Scholar]
- Binaghi L., Del Borghi A., Lodi A., Converti A., Del Borghi M. (2003). Batch and fed-batch uptake of carbon dioxide by Spirulina platensis. Process. Biochem. 38, 1341–1346. 10.1016/S0032-9592(03)00003-7 [DOI] [Google Scholar]
- Cardias B. B., de Morais M. G., Costa J. A. V. (2018). CO2 conversion by the integration of biological and chemical methods: Spirulina sp. LEB 18 cultivation with diethanolamine and potassium carbonate addition. Bioresour. Technol. 267, 77–83. 10.1016/j.biortech.2018.07.031 [DOI] [PubMed] [Google Scholar]
- Chen C. Y., Durbin E. G. (1994). Effects of pH on the growth and carbon uptake of marine phytoplankton. Mar. Ecol. Prog. Ser. 109, 83–94. 10.3354/meps109083 [DOI] [Google Scholar]
- Chen C. Y., Kao P. C., Tan C. H, Show P. L., Cheah W. Y., Lee W. L., et al. (2016). Using an innovative pH-stat CO2 feeding strategy to enhance cell growth and C-phycocyanin production from Spirulina platensis. Biochem. Eng. J. 112,78–85. 10.1016/j.bej.2016.04.009 [DOI] [Google Scholar]
- Chen C. Y., Kao P. C., Tsai C. J., Lee D. J., Chang J. S. (2013). Engineering strategies for simultaneous enhancement of C-phycocyanin production and CO2 fixation with Spirulina platensis. Bioresour. Technol. 145, 307–312. 10.1016/j.biortech.2013.01.054 [DOI] [PubMed] [Google Scholar]
- Cheng J., Guo W. B., Ali K. A., Ye Q., Jin G. Y., Qiao Z. S. (2018). Promoting helix pitch and trichome length to improve biomass harvesting efficiency and carbon dioxide fixation rate by Spirulina sp. in 660 m2 raceway ponds under purified carbondioxide from a coal chemical flue gas. Bioresour. Technol. 261, 76–85. 10.1016/j.biortech.2018.04.017 [DOI] [PubMed] [Google Scholar]
- Cheng J., Huang Y, Feng J., Sun J., Zhou J. H., Cen K. F. (2013). Mutate chlorella sp. by nuclear irradiation to fix high concentrations of CO2. Bioresour. Technol. 136, 496–501. 10.1016/j.biortech.2013.03.072 [DOI] [PubMed] [Google Scholar]
- Costa J. A. V., Colla L. M., Filho P. F. D. (2004). Improving Spirulina platensis biomass yield using a fed-batch process. Bioresour. Technol. 92, 237–241. 10.1016/j.biortech.2003.09.013 [DOI] [PubMed] [Google Scholar]
- da Rosa G. M., Moraes L., de Souza M. D., Costa J. A. V. (2016). Spirulina cultivation with a CO2 absorbent: influence on growth parameters and macromolecule production. Bioresour. Technol. 200, 528–534. 10.1016/j.biortech.2015.10.025 [DOI] [PubMed] [Google Scholar]
- da Silva Vaz B., Costa J. A. V., de Morais M. G. (2016). CO2 biofixation by the cyanobacterium Spirulina sp. LEB 18 and the green alga chlorella fusca LEB 111 grown using gas effluents and solid residues of thermoelectric origin. Appl. Biochem. Biotechnol. 178, 418–429. 10.1007/s12010-015-1876-8 [DOI] [PubMed] [Google Scholar]
- Duarte J. H., de Morais E. G., Radmann E. M., Costa J. A. V. (2017). Biological CO2 mitigation from coal power plant by chlorella fusca and Spirulina sp. Bioresour. Technol. 234, 472–475. 10.1016/j.biortech.2017.03.066 [DOI] [PubMed] [Google Scholar]
- Fu W. Q., Gudmundsson S., Wichuk K., Palsson S., Palsson B. O., Salehi-Ashtiani K., et al. (2019). Sugar-stimulated CO2 sequestration by the green microalga chlorella vulgaris. Sci. Total Environ. 654, 275–283. 10.1016/j.scitotenv.2018.11.120 [DOI] [PubMed] [Google Scholar]
- Hancke K., Dalsgaard T., Sejr M. K., Markager S., Glud R. N. (2015). Phytoplankton productivity in an arctic fjord (West Greenland): estimating electron requirements for carbon fixation and oxygen production. PLoS ONE 10:e0133275. 10.1371/journal.pone.0133275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo C. M., Jian J. F., Sun Y. L., Lin T. H., Yang Y. C., Zhang W. X., et al. (2018). An efficient photobioreactors/raceway circulating system combined with alkaline-CO2 capturing medium for microalgal cultivation. Bioresour. Technol. 266, 398–406. 10.1016/j.biortech.2018.06.090 [DOI] [PubMed] [Google Scholar]
- Matsudo M. C., Bezerra R. P., Sato S., Converti A., de Carvalho J. C. M. (2012). Photosynthetic efficiency and rate of CO2 assimilation by arthrospira (Spirulina) platensis continuously cultivated in a tubular photobioreactor. Biotechnol. J. 7, 1412–1417. 10.1002/biot.201200177 [DOI] [PubMed] [Google Scholar]
- Morais M. G. D., Costa J. A. V. (2007). Biofixation of carbon dioxide by Spirulina sp. and Scenedesmus obliquus cultivated in a three-stage serial tubular photobioreactor. J. Biotechnol. 129, 439–445. 10.1016/j.jbiotec.2007.01.009 [DOI] [PubMed] [Google Scholar]
- Nayak M., Rath S. S., Thirunavoukkarasu M., Panda P. K., Mishra B. K., Mohanty R. C. (2013). Maximizing biomass productivity and CO2 biofixation of microalga, Scenedesmus sp. by using sodium hydroxide. J. Microbiol. Biotechnol. 23, 1260–1268. 10.4014/jmb.1302.02044 [DOI] [PubMed] [Google Scholar]
- Nayak M., Suh W. I., Lee B., Chang Y. K. (2018). Enhanced carbon utilization efficiency and FAME production of chlorella sp. HS2 through combined supplementation of bicarbonate and carbon dioxide. Energy.Convers. Manage. 156, 45–52. 10.1016/j.enconman.2017.11.002 [DOI] [Google Scholar]
- Ogbonda K. H., Aminigo R. E., Abu G. O. (2007). Influence of temperature and pH on biomass production and protein biosynthesis in a putative Spirulina sp. Bioresour. Technol. 98, 2207–2211. 10.1016/j.biortech.2006.08.028 [DOI] [PubMed] [Google Scholar]
- Pandit P. R., Fulekar M. H., Karuna M. S. L. (2017). Effect of salinity stress on growth, lipid productivity, fatty acid composition, and biodiesel properties in acutodesmus obliquus and chlorella vulgaris. Environ. Sci. Pollut. Res. Int. 24, 13437–13451. 10.1007/s11356-017-8875-y [DOI] [PubMed] [Google Scholar]
- Qiu R. H., Gao S., Lopez P. A., Ogden K. L. (2017). Effects of pH on cell growth, lipid production and CO2 addition of microalgae chlorella sorokiniana. Algal. Res. 28, 192–199. 10.1016/j.algal.2017.11.004 [DOI] [Google Scholar]
- Razzak S. A., Ali S. A. M., Hossain M. M., de Lasa H. (2017). Biological CO2 fixation with production of microalgae in wastewater - a review. Renew. Sust. Energy Rev. 76, 379–390. 10.1016/j.rser.2017.02.038 [DOI] [Google Scholar]
- Rodolfi L., Zittelli G. C., Bassi N., Padovani G., Biondi N., Bonini G., et al. (2009). Microalgae for oil: strain selection, induction of lipid synthesis and outdoor mass cultivation in a low-cost photobioreactor. Biotechnol. Bioeng. 102, 100–112. 10.1002/bit.22033 [DOI] [PubMed] [Google Scholar]
- Rosa A. P. C., Carvalho L. F., Goldbeck L., Costa J. A. V. (2011). Carbon dioxide fixation by microalgae cultivated in open bioreactors. Energy. Convers. Manage. 52, 3071–3073. 10.1016/j.enconman.2011.01.008 [DOI] [Google Scholar]
- Sepulveda C., Gómezb C., Bahraouic N. E. L., Acién G. (2019). Comparative evaluation of microalgae strains for CO2 capture purposes. J. CO2 Util. 30, 158–167. 10.1016/j.jcou.2019.02.004 [DOI] [Google Scholar]
- Sydney E. B., Sturm W., de Carvalho J. C., Thomaz-Soccol V., Larroche C., Pandey A., et al. (2010). Potential carbon dioxide fixation by industrially important microalgae. Bioresour. Technol. 101, 5892–5896. 10.1016/j.biortech.2010.02.088 [DOI] [PubMed] [Google Scholar]
- Vonshak A. (1997). “Spirulina: growth, physiology and biochemistry,” in Spirulina platensis (Arthrospira) Physiology, Cell-Biology and Biotechnology, ed Vonshak A. (London: Taylor & Francis, 43–66. [Google Scholar]
- Wang B., Li Y. Q., Wu N., Lan C. Q. (2008). CO2 bio-mitigation using microalgae. Appl. Microbiol. Biotechnol. 79, 707–718. 10.1007/s00253-008-1518-y [DOI] [PubMed] [Google Scholar]
- Weiner E. R. (2012). Applications of Environmental Aquatic Chemistry: A Practical Guide, 3rd Edn. Florida city, FL: CPC Press. [Google Scholar]
- Yadav G., Sen R. (2017). Microalgal green refinery concept for biosequestration of carbon dioxide vis-à-vis wastewater remediation and bioenergy production: recent technological advances in climate research. J. CO2 Util. 17, 188–206. 10.1016/j.jcou.2016.12.006 [DOI] [Google Scholar]
- Yoo C., Jun S. Y., Lee J. Y., Ahn C. Y., Oh H. M. (2010). Selection of microalgae for lipid production under high levels carbon dioxide. Bioresour. Technol. 101, S71–S74. 10.1016/j.biortech.2009.03.030 [DOI] [PubMed] [Google Scholar]
- Zarrouk C. J. (1966). Influence de Divers Facteurs Physiques et Chimiques sur la Croissance et la Photosynthèse de Spirulina Maxima (Setch. Et Garndner) Geitler. Faculte des Sciences, Universite de Paris, Paris. [Google Scholar]
- Zeng X. H., Danquah M. K., Zhang S. D., Zhang X., Wu M. Y., Chen X. D., et al. (2012). Autotrophic cultivation of Spirulina platensis for CO2 fixation and phycocyanin production. Chem. Eng. J. 183, 192–197. 10.1016/j.cej.2011.12.062 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated for this study are available on request to the corresponding author.