Abstract
Non-alcoholic steatohepatitis (NASH) is a progressive form of non-alcoholic fatty liver disease (NAFLD) that may lead to liver cirrhosis or hepatocellular carcinoma. Here, we examined the diagnostic utility of tri-antennary tri-sialylated mono-fucosylated glycan of alpha-1 antitrypsin (AAT-A3F), a non-invasive glycobiomarker identified in a previous study of NASH diagnosis. This study included 131 biopsy-proven Japanese patients with NAFLD. We evaluated the utility of AAT-A3F in NASH diagnosis, and conducted genetic analysis to analyse the mechanism of AAT-A3F elevation in NASH. Serum AAT-A3F concentrations were significantly higher in NASH patients than in NAFL patients, and in patients with fibrosis, lobular inflammation, and ballooning. Hepatic FUT6 gene expression was significantly higher in NASH than in NAFL. IL-6 expression levels were significantly higher in NASH than in NAFL and showed a positive correlation with FUT6 expression levels. The serum-AAT-A3F levels strongly correlated with hepatic FUT6 expression levels. AAT-A3F levels increased with fibrosis, pathological inflammation, and ballooning in patients with NAFLD and may be useful for non-invasive diagnosis of NASH from the early stages of fibrosis.
Subject terms: Diagnostic markers, Hepatology
Introduction
Non-alcoholic fatty liver disease (NAFLD) is a hepatic manifestation of metabolic syndrome that is frequently associated with obesity, diabetes mellitus, and dyslipidaemia, without a history of significant alcohol consumption1. NAFLD is sub-divided into non-alcoholic fatty liver (NAFL; a non-progressive form of NAFLD) and non-alcoholic steatohepatitis (NASH; a progressive form that can lead to cirrhosis and the development of hepatocellular carcinoma [HCC]2–4).
To date, liver biopsy remains the gold standard for NAFLD diagnosis and staging5. However, liver biopsy is an invasive procedure that may lead to undesirable complications6,7 and sampling bias. Several non-invasive biomarkers and scoring systems have been reported8, but no established diagnostic tests or biomarkers are available at present. Thus, identifying and validating novel non-invasive biomarkers would be valuable.
Glycosylation is one of the most common post-translational modifications occurring in proteins. Glycosylation can affect the biological activities of proteins, their transport towards the cell surface, and stabilization of their functional conformations9. Glycans on human glycoproteins are mainly composed of only 10 kinds of monosaccharides: glucose, galactose, mannose, N-acetylglucosamine, N-acetylgalactosamine, fucose, N-acetylneuraminic acid (a type of sialic acid), xylose, glucuronic acid, and iduronic acid. However, the order of sugars on glycans and the mode of linkage between each sugar result in high diversity of glycan structures. Glycans are the determinants of some clinically used cancer biomarkers such as CA19-9, CA125, and AFP-L3. Regarding liver diseases, glycosylation changes on immunoglobulins10, haptoglobin11, and alpha-1 antitrypsin12 were reported for patients with liver cirrhosis. Recently, mac-2-binding protein glycosylation isomer (M2BPGi) was reported as a novel fibrosis biomarker13 and has shown utility in NAFLD14. As represented by these molecules, glycosylation changes reflecting liver disease severity are now receiving increasing attention.
Previously, we determined the N-glycomic profile of focused serum glycoproteins from patients with NAFL or NASH by focused protein glycomic analysis (FPG), and successfully discovered several NASH biomarker candidates. Among them, tri-antennary tri-sialylated mono-fucosylated glycan of alpha-1 antitrypsin (AAT-A3F) showed the most dynamic change. In addition, a simplified method named immunoprecipitation glycomics (IPG), which employs affinity bead purification and matrix-assisted laser desorption/ionization–time-of-flight mass spectrometry (MALDI–TOF MS), was developed for the rapid determination of AAT-A3F15. The simplified method enabled us to quantitatively measure AAT-A3F in a high-throughput manner.
In this study, we investigated the diagnostic utility of AAT-A3F identified in our previous exploratory study on NASH biomarkers.
Results
Characteristics of the NAFLD patients
A total of 131 NAFLD patients (57 with NAFL, 74 with NASH) were enrolled in this study. Patients were divided into NAFL and NASH groups based on the pathological appearance of their liver biopsy specimens. The NASH group had a higher proportion of females than the NAFL group. Age, AST, HbA1c, FBS, and HOMA-IR were significantly higher in the NASH group than in the NAFL group. In contrast, albumin and LDL-C were significantly lower in the NASH group than in the NAFL group. BMI, Plt, ALT, AFP, and TG were not significantly different between the NAFL and NASH groups. Serum cCK18 and M2BPGi levels were significantly higher in the NASH group than in the NAFL group. Both fibrosis-prediction equations (FIB-4 index and APRI) were significantly higher in the NASH group than in the NAFL group. As shown in Table 1, the NAFLD activity scores (NAS) in the NAFL group for steatosis were 0–3 (n = 0, 23, 22, and 12, respectively); those for lobular inflammation were 0–3 (n = 24, 30, 3, and 0, respectively); and those for hepatocyte ballooning were 0–2 (n = 54, 2, and 1, respectively). The NAS (NAFLD activity score) in NASH group for steatosis were 0–3 (n = 0, 23, 30, and 21, respectively); those for lobular inflammation were 0–3 (n = 0, 38, 31, and 5, respectively); and those for hepatocyte ballooning were 0–2 (n = 1, 46, and 27, respectively). The Brunt classifications of the fibrosis stage in the NAFL group were 0–4 (n = 30, 27, 0, 0, and 0, respectively), while those in the NASH group were 0–4 (n = 2, 37, 10, 23, and 2, respectively).
Table 1.
Clinical, serological, and pathological characteristics of the NAFLD patients.
| NAFL | NASH | P value | |
|---|---|---|---|
| N | 57 | 74 | |
| Male (%) | 68.4 | 43.2 | <0.01 |
| Age (Yr) | 48 ± 15 | 55 ± 15 | <0.01 |
| BMI (kg/m2) | 28 ± 4 | 30 ± 6 | NS |
| Plt (104/μL) | 22 ± 6 | 20 ± 7 | NS |
| Albumin (g/dL) | 4.4 ± 0.3 | 4.2 ± 0.4 | <0.01 |
| T-bil (mg/dL) | 0.8 ± 0.4 | 0.8 ± 0.3 | NS |
| AST (IU/L) | 50 ± 24 | 67 ± 38 | <0.01 |
| ALT (IU/L) | 93 ± 67 | 86 ± 64 | NS |
| ChE (U/L) | 379 ± 92 | 328 ± 121 | NS |
| γ-GTP (IU/L) | 97 ± 113 | 82 ± 59 | NS |
| AFP (ng/mL) | 2.4 ± 1.8 | 3.5 ± 2.7 | NS |
| TG (mg/dL) | 174 ± 83 | 158 ± 79 | NS |
| LDL-C (mg/dL) | 131 ± 29 | 114 ± 39 | <0.05 |
| HDL-C (mg/dL) | 50 ± 15 | 45 ± 16 | NS |
| HbA1c (%) | 5.9 ± 0.7 | 6.2 ± 1.8 | <0.001 |
| FBS (mg/dL) | 106 ± 26 | 123 ± 45 | <0.05 |
| IRI (μIU/L) | 20.0 ± 30.5 | 14.1 ± 11.1 | NS |
| HOMA-IR | 5.7 ± 9.3 | 4.5 ± 4.4 | <0.05 |
| cCK18 (U/L) | 572 ± 359 | 927 ± 698 | <0.01 |
| M2BPGi (COI) | 0.73 ± 0.66 | 1.24 ± 0.95 | <0.001 |
| FIB4 index | 1.55 ± 1.4 | 2.31 ± 1.53 | <0.001 |
| APRI | 0.60 ± 0.34 | 0.94 ± 0.69 | <0.001 |
| Pathological findings | |||
| Steatosis (0/1/2/3) | 0/23/22/12 | 0/23/30/21 | |
| Lobular inflammation (0/1/2/3) | 24/30/3/0 | 0/38/31/5 | |
| Ballooning (0/1/2) | 54/2/1 | 1/46/27 | |
| Fibrosis (0/1/2/3/4) | 30/27/0/0/0 | 2/37/10/23/2 | |
Data are presented as mean ± SD. P values correspond to the comparison between NAFL and NASH groups. Mann Whitney U tests for continuous factors and Fisher’s exact test for categorical factors were used. Abbreviations: NAFL, non-alcoholic fatty liver disease; NASH, non-alcoholic steatohepatitis.
AAT and AAT-A3F concentrations in serum from NAFLD patients
Serum AAT concentrations were not significantly different between the NAFL and NASH groups (0.87 ± 0.17 mg/mL in the NAFL group, 0.93 ± 0.23 mg/mL in the NASH group; Fig. 1a). However, serum AAT-A3F concentrations were significantly higher in the NASH group (7.63 ± 4.04 μM) than in the NAFL group (14.40 ± 14.08 μM; P < 0.001; Fig. 1b).
Figure 1.
Serum AAT and AAT-A3F concentrations in patients with NAFLD. The vertical axis represents AAT levels in mg/mL and AAT-A3F levels in μM, and the horizontal axis represents the patient groups. Data are shown as mean ± SD. (A) The AAT levels were 0.87 ± 0.17 mg/mL for the NAFL group, and 0.93 ± 0.23 mg/mL for the NASH group (n.s.). (B) The AAT-A3F levels were 7.63 ± 4.04 μM for the NAFL group and 14.4 ± 14.08 μM for the NASH group (P < 0.001).
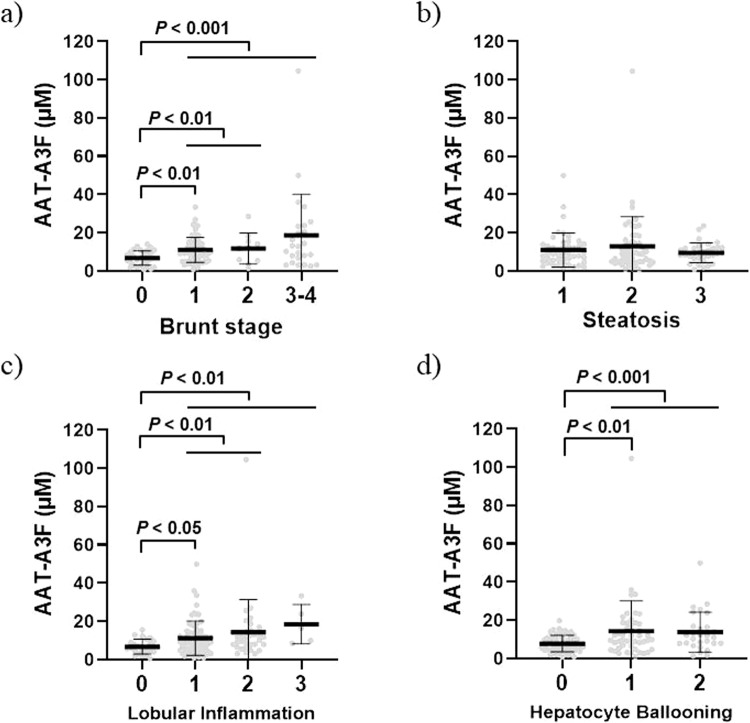
With respect to the Brunt stage, the serum AAT-A3F levels significantly increased with fibrosis. Serum AAT-A3F levels at stage F0 was 6.75 ± 3.73 μM, that at stage F1 was 11.00 ± 6.58 μM, that at stage F2 was 11.66 ± 8.11 μM, and that at stage F3/4 (with advanced fibrosis) was 18.55 ± 21.53 μM (P < 0.01; Fig. 2a). Furthermore, the relationship between serum AAT-A3F concentration and pathological activity was analysed according to the NAS classification. Serum AAT-A3F levels were not associated with steatosis (S1, 11.06 ± 8.92 μM; S2, 12.98 ± 15.46 μM; S3, 9.60 ± 5.14 μM; Fig. 2b), but significantly increased with lobular inflammation (LI0, 6.74 ± 3.88 μM; LI1, 11.15 ± 9.02 μM; LI2, 14.37 ± 17.03 μM; LI3, 18.45 ± 10.31 μM; Fig. 2c) and hepatocyte ballooning (B0, 7.80 ± 4.36 μM; B1, 14.29 ± 15.74 μM; B2, 13.77 ± 10.38 μM; Fig. 2d).
Figure 2.
AAT-A3F levels according to the pathological score classification. The vertical axis represents AAT-A3F levels in μM, and the horizontal axis represents the Brunt stage and NAS (based on steatosis, lobular inflammation, and hepatocyte ballooning). Data are shown as mean ± SD. (a) The AAT-A3F levels were 6.75 ± 3.73 μM for stage 0, 11.00 ± 6.58 μM for stage 1, 11.66 ± 8.11 μM for stage 2, and 18.55 ± 21.53 μM for stages 3 and 4. (b) The AAT-A3F levels were 11.06 ± 8.92 μM for steatosis 1, 12.98 ± 15.46 μM for steatosis 2, and 9.60 ± 5.14 μM for steatosis 3. (c) The AAT-A3F levels were 6.74 ± 3.88 μM for lobular inflammation 0, 11.15 ± 9.02 μM for lobular inflammation 1, 14.37 ± 17.03 μM for lobular inflammation 2, and 18.45 ± 10.31 μM for lobular inflammation 3. (d) The AAT-A3F levels were 7.80 ± 4.36 μM for hepatocyte ballooning 0, 14.29 ± 15.74 μM for hepatocyte ballooning 1, and 13.77 ± 10.38 μM for hepatocyte ballooning 2.
Correlation between serum AAT-A3F concentrations and clinicopathological parameters
We analysed correlations between serum AAT-A3F levels and clinicopathological parameters. According to the clinical data, the AAT-A3F levels showed a significant negative correlation with albumin (r = −0.252, P < 0.001), ChE (r = −0.284, P < 0.001), and TG (r = −0.113, P < 0.05), and a significant positive correlation with M2BPGi (r = 0.415, P < 0.001), and FIB-4 index (r = 0.268, P < 0.01). The pathological data revealed a significant positive correlation between the AAT-A3F levels and lobular inflammation (r = 0.314, P < 0.01), hepatocyte ballooning (r = 0.285, P < 0.01), and fibrosis (r = 0.292, P < 0.001), as shown in Table 2.
Table 2.
Correlation between AAT-A3F and clinicopathological parameters.
| r | P value | |
|---|---|---|
| Age (Yr) | 0.092 | NS |
| BMI (kg/m2) | 0.066 | NS |
| Plt (104/μL) | −0.172 | NS |
| Albumin (g/dL) | −0.252 | <0.001 |
| T-bil (mg/dL) | 0.071 | NS |
| AST (IU/L) | 0.196 | NS |
| ALT (IU/L) | −0.019 | NS |
| ChE (U/L) | −0.284 | <0.001 |
| γ-GTP (IU/L) | 0.165 | NS |
| AFP (ng/mL) | 0.188 | NS |
| TG (mg/dL) | −0.113 | <0.05 |
| LDL-C (mg/dL) | 0.087 | NS |
| HDL-C (mg/dL) | −0.136 | NS |
| HbA1c (%) | 0.035 | NS |
| FBS (mg/dL) | 0.009 | NS |
| IRI (μIU/L) | 0.178 | NS |
| HOMA-IR | 0.130 | NS |
| cCK18 (U/L) | 0.121 | NS |
| M2BPGi (COI) | 0.415 | <0.001 |
| FIB4 index | 0.268 | <0.01 |
| APRI | 0.236 | NS |
| Pathological findings | ||
| Steatosis (0/1/2/3) | −0.012 | NS |
| Lobular inflammation (0/1/2/3) | 0.314 | <0.01 |
| Ballooning (0/1/2) | 0.285 | <0.01 |
| Fibrosis (0/1/2/3/4) | 0.292 | <0.001 |
The relationship between AAT-A3F and clinicopathological parameters was analysed using Spearman’s R correlations.
Diagnostic performance of AAT-A3F and other biomarkers for predicting NASH
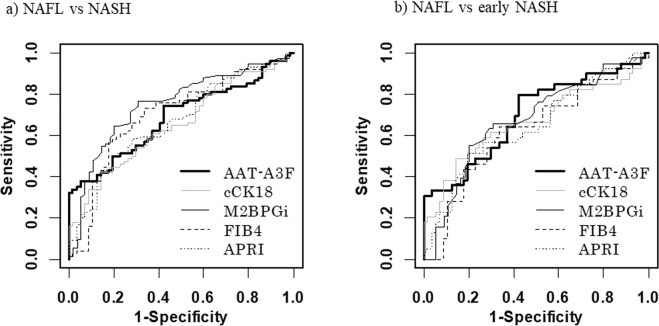
Using ROC analysis, the cut-off value of serum AAT-A3F for NASH diagnosis was set to 14.1 μM. The area under the ROC curve (AUROC) of AAT-A3F was 0.687, and the sensitivity, specificity, PPV, NPV of AAT-A3F were 38%, 95%, 90%, and 54%, respectively. The cut-off value of other markers for NASH diagnosis was set to 977 for cCK18, 0.69 for M2BPGi, 1.44 for FIB4 index, and 0.86 for APRI. The AUROC values of NASH diagnosis were 0.655 for cCK18, 0.749 for M2BPGi, 0.700 for the FIB4 index, and 0.672 for the APRI (Table 3, Fig. 3a).
Table 3.
Diagnostic performance of AAT-A3F and other biomarkers for predicting NASH.
| AUC | Cut-off | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | |
|---|---|---|---|---|---|---|
| AAT-A3F | 0.687 | 14.1 | 38 | 95 | 90 | 54 |
| cCK18 | 0.655 | 977 | 42 | 86 | 79 | 53 |
| M2BPGi | 0.749 | 0.69 | 77 | 69 | 77 | 69 |
| FIB4 index | 0.700 | 1.44 | 73 | 67 | 74 | 66 |
| APRI | 0.672 | 0.86 | 50 | 81 | 77 | 55 |
Abbreviations: AUC, area under the curve; NPV, negative predictive value; PPV, positive predictive value; NASH: non-alcoholic steatohepatitis
Figure 3.
Diagnostic performance of AAT-A3F and other biomarkers for predicting NASH and early NASH. (a) ROC curves for diagnosing NASH. (b) ROC curves for diagnosing early NASH.
The cut-off value of serum AAT-A3F for diagnosis of early NASH (Brunt stages 0–1), was set to 7.9 μM in the ROC analysis. The AUROC of AAT-A3F was 0.696, and the sensitivity, specificity, PPV, NPV of AAT-A3F were 79%, 58%, 56%, and 80%, respectively. The cut-off value of other markers for early NASH diagnosis was set to 977 for cCK18, 0.79 for M2BPGi, 1.44 for FIB4 index, and 0.86 for APRI, similar to NASH diagnosis. The AUROC values of early NASH were 0.665 for cCK18, 0.673 for M2BPGi, 0.624 for the FIB4 index, and 0.648 for the APRI (Table 4, Fig. 3b).
Table 4.
Diagnostic performance of AAT-A3F and other biomarkers for predicting early NASH (Brunt stage S0-1).
| AUC | Cut-off | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | |
|---|---|---|---|---|---|---|
| AAT-A3F | 0.696 | 7.9 | 79 | 58 | 56 | 80 |
| cCK18 | 0.665 | 977 | 49 | 86 | 70 | 71 |
| M2BPGi | 0.673 | 0.79 | 55 | 80 | 66 | 72 |
| FIB4 index | 0.624 | 1.44 | 64 | 67 | 57 | 73 |
| APRI | 0.648 | 0.86 | 51 | 81 | 65 | 71 |
Abbreviations: AUC, area under the curve; NPV, negative predictive value; PPV, positive predictive value; NASH: non-alcoholic steatohepatitis.
Gene expression analysis
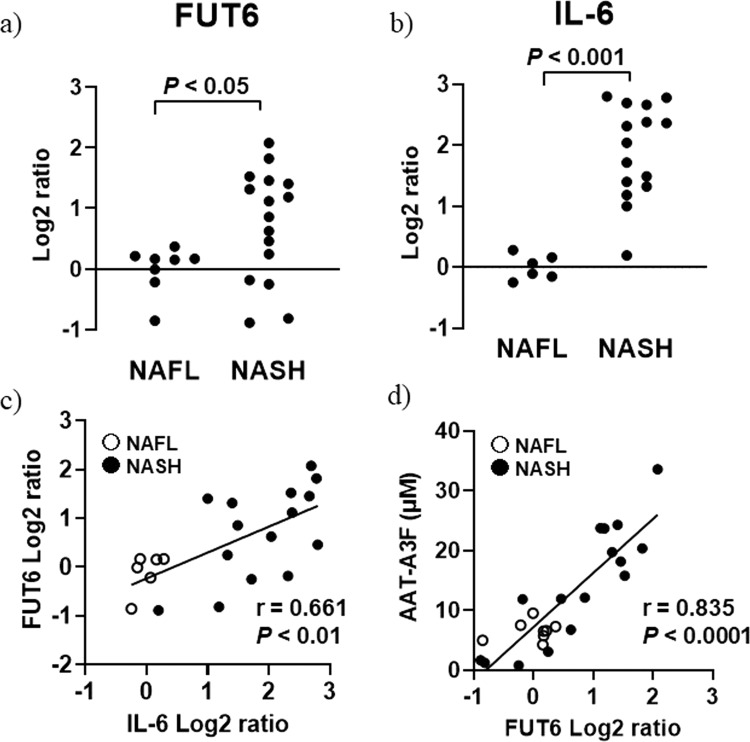
Hepatic FUT6 gene expression levels were significantly higher in the NASH group than in the NAFL group (0 ± 0.384 in the NAFL group, 0.747 ± 0.910 in the NASH group (log2 ratio), P < 0.05) (Fig. 4a). IL-6 expression levels were significantly higher in NASH than in NAFL (0 ± 0.202 in the NAFL group, 1.891 ± 0.777 in the NASH group (log2 ratio), P < 0.001) (Fig. 4b). A significant positive correlation was observed between the expression levels of FUT6 and IL-6 (r = 0.661, P < 0.01) (Fig. 4c). The serum levels of AAT-A3F strongly correlated with hepatic FUT6 expression levels (r = 0.835, P < 0.0001) (Fig. 4d).
Figure 4.
Gene expression analysis. (a) The vertical axis represents the FUT6 expression level (log2 scale), and the horizontal axis represents the patient groups. (b) The vertical axis represents the IL-6 expression level (log2 scale), and the horizontal axis represents the patient groups. (c) The vertical axis represents the FUT6 expression level (log2 scale), and the horizontal axis represents IL-6 expression level (log2 scale). A scatter diagram of FUT6 and IL-6 expression is shown. (d) The vertical axis represents the AAT-A3F expression level in μM, and the horizontal axis represents the FUT6 expression level (log2 scale). A scatter diagram of AAT-A3F and FUT6 expression is shown.
Discussion
In patients with NAFLD, NAFL shows a non-progressive clinical course, whereas NASH is a serious disease with a high risk of both overall and liver-related morbidity and mortality2–4. Therefore, differentiating between NAFL and NASH is extremely important in the clinical management of NAFLD patients. Although liver biopsy is still considered a gold standard for differentiating between NASH and NAFL5, a non-invasive and reliable diagnostic biomarker is required.
Here, we validated the diagnostic utility of AAT-A3F identified in our previous study15. Serum AAT levels were not different between patients with NASH and NAFL, but AAT-A3F was significantly elevated in patients with NASH versus patients with NAFL. In addition, AAT-A3F levels increased significantly not only with hepatic fibrosis, but also with intrahepatic inflammation and hepatocyte ballooning. AAT is a major, liver-derived circulating protein that functions as a natural inhibitor of various serine proteases and is a component of the acute-phase response16. During inflammation, the liver synthesises acute phase proteins (APPs), including AAT, which are thought to contribute to the inflammatory response, but also play a central role in limiting local and systemic inflammation17. APPs play a dual role in inflammation, and AAT can induce the production of both pro-inflammatory interleukin 1 (IL-1) and anti-inflammatory IL-1 receptor antagonist (IL-1Ra) in peripheral blood mononuclear cells. Other studies have shown that AAT inhibits neutrophil superoxide production18, prevents hepatocyte apoptosis19, and functions as an endogenous inhibitor of pro-inflammatory cytokine production in whole blood20. Thus, AAT is thought to be related to intrahepatic inflammation.
To clarify the mechanism underlying AAT-A3F elevation in NASH, we evaluated gene expression levels in liver biopsy tissues. The expression level of the FUT6 gene, whose protein product is responsible for outer arm fucosylation of glycoproteins secreted from the liver21, was significantly higher in NASH than in NAFL. It was previously reported that FUT6 expression was positively regulated by IL-622, and hence, we determined the hepatic expression level of IL-6. IL-6 expression level was significantly higher in NASH than in NAFL, as previously described23. A positive and significant correlation between the expression levels of IL-6 and FUT6 was observed, indicating that inflammatory response accompanying IL-6 production in NASH liver can lead to FUT6 upregulation. The serum level of AAT-A3F was strongly correlated with the hepatic FUT6 expression level. Therefore, we conclude that hepatic inflammation caused the elevation in FUT6 expression, resulting in an increase in outer arm fucosylation of AAT produced in the liver.
In this study, the AUROC for NASH diagnosis of AAT-A3F was 0.687, which was higher than cCK18. The apoptosis marker cCK18 was reported to be useful for distinguishing between healthy subjects and those with NAFL, and further between patients with NAFL versus those with NASH24. In this study, the diagnostic utility of AAT-A3F was found to be higher than that of cCK18 in our cohort. The AUROC for NASH diagnosis of AAT-A3F was lower than that of M2BPGi and FIB-4 index, however, the AUROC values of AAT-A3F for diagnosing early NASH was higher than that of biomarkers tested in this study. Furthermore, while the cut-off values of cCK18, M2BPGi, FIB-4 index, and APRI for diagnosing NASH and early NASH were very close, the cut-off values in AAT-A3F were nearly doubled. This suggests that AAT-A3F can stratify NASH by the degree of fibrosis. These results may perhaps be because AAT-A3F also reflects liver inflammation and fibrosis. Although liver fibrosis was considered the most important factor for predicting the prognosis of NASH25, the mortality rate is reported to increase since mild fibrosis26,27. In addition, lobular inflammation has been reported as a risk factor for the progression of liver fibrosis, compared to fatty liver alone28. In general, the prognosis of patients with NAFLD is often lower than that of the general population29; therefore, it is necessary to intervene at an early stage with heathier diet and exercise. Identifying biomarkers that indicate lobular inflammation and hepatocyte ballooning as well as fibrosis such as AAT-A3F can lead to early diagnosis and treatment of NASH, which helps reduce mortality.
In the previous study, we comprehensively analysed N-glycans of serum glycoproteins in NAFLD patients using the FPG method and found that AAT-A3F could be a useful biomarker for NASH diagnosis. FPG is a useful method for biomarker discovery, but it is not suitable for examinations of many samples, because it requires many steps. Therefore, we have developed a simplified IPG method suitable for the measurement of a large number of samples. Using the IPG method, we verified the usefulness of AAT-A3F in this study. Because the IPG method can be used for any proteins, this method will be applicable to the analysis of glycobiomarkers related to various diseases.
The primary limitation of our study was that the number of patients included was relatively small, and our NAFLD patients were enrolled from a regional liver disease hospital. Therefore, the potential for referral bias cannot be ruled out in our study. Thus, our findings for liver biopsy-proven NAFLD might not represent patients with NAFLD in the general population. Therefore, larger multicentre studies are required to validate our results. The secondary limitation is that we used liver biopsy as the gold standard for assessing the utility of AAT-A3F. This technique has serious limitations including those related to sampling errors and pathological assessment. Therefore, liver biopsy specimens from various facilities were also centrally evaluated by a hepatopathologist in this study. The third limitation is the small sample size that was subjected to gene expression analyses. Although in a multi-centre study, the number of the liver tissue storage are limited, we analysed all possible cases.
In conclusion, this multicentre study revealed that AAT-A3F was especially increased in not only fibrosis, but also pathological inflammation and hepatocyte ballooning in patients with NAFLD, and AAT-A3F was considered useful for non-invasive diagnosis of early NASH. Furthermore, it was beneficial for the diagnosis of NASH by combining AAT-A3F with fibrosis markers such as M2BPGi and FIB4 index depending of progression stage of NASH (Fig. 5).
Figure 5.
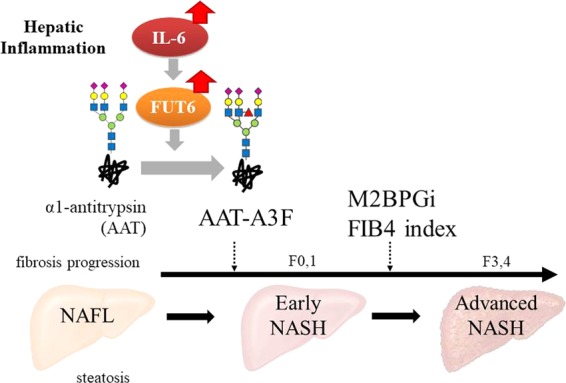
Application of AAT-A3F to clinical diagnosis of NASH. Summarize the findings of this study and show the usefulness of AAT-A3F in NASH diagnosis with illustrations.
Methods
Study populations
In this study, we included 131 biopsy-proven NAFLD patients from the Hokkaido University Hospital and other affiliated hospitals belonging to NORTE study group. These patients were examined between 2007 and 2017 and underwent a percutaneous liver needle biopsy. Biopsied liver samples were embedded in paraffin blocks following standard procedures and stained with haematoxylin and eosin, Masson’s trichrome stain, and Gitter stain. All biopsy specimens were evaluated by a hepatopathologist blinded to the clinical data. Samples were investigated and quantified based on NAFLD-activity scoring30 for steatosis (0–3), lobular inflammation (0–3), and hepatocyte ballooning (0–2). NASH was diagnosed when steatosis, ballooning, lobular inflammation and hepatocyte ballooning of any grade were all present at the same time31,32. Individual fibrosis parameters were scored based on the fibrosis stage of the Brunt classification33. Early NASH was defined as NASH with no or mild fibrosis (Brunt stages S0–1). Advanced fibrosis was classified as a Brunt stage of 3–4 (S3–4).
The exclusion criteria for this study included daily alcohol consumption (>30 g for men or >20 g for women) and another hepatic disease such as hepatitis B, hepatitis C, hepatocellular carcinoma, autoimmune hepatitis, primary biliary cholangitis, primary sclerosing cholangitis, hemochromatosis, α1-antitrypsin deficiency, Wilson’s disease, or congestive liver disease, according to our previous study15. The study protocol complied with the ethical guidelines of the Declaration of Helsinki and was approved by the Institutional Review Board of Hokkaido University Hospital and each participating hospital. Written informed consent to participate in this study was obtained from each patient. This study was registered in the UMIN Clinical Trials Registry as UMIN000025644.
Collection of clinical data
Clinical data including gender and age were obtained for each patient at the time of liver biopsy. Anthropometric variables (body height and weight) were measured in the standing position, and the body mass index (BMI) was calculated as the weight divided by height in meters squared. Serum biochemical variables (platelet count [Plt], albumin, total bilirubin, aspartate aminotransferase [AST], alanine aminotransferase [ALT], γ‐glutamyltransferase, cholinesterase [ChE], α-fetoprotein [AFP], triglyceride [TG], low-density lipoprotein LDL-cholesterol [LDL‐C], high-density lipoprotein cholesterol, haemoglobin A1c [HbA1c], fasting blood sugar [FBS], and immunoreactive insulin) were measured with a conventional automated analyser. Insulin resistance was evaluated based on the homeostasis model assessment as an index of insulin resistance (HOMA-IR) value using the following equation: HOMA-IR value = fasting insulin (µU/mL) × fasting glucose (mg/dL)/405. Caspase-cleaved cytokeratin 18 (cCK18) and M2BPGi were measured as markers of hepatocellular apoptosis and liver fibrosis, respectively. The formula used for predicting liver fibrosis from non-invasively obtained data was as follows: fibrosis 4 (FIB4) index = age × AST (IU/L) × Plt ( × 109/L)−1 × √ALT (IU/L)−1, as reported previously34. The AST-to-platelet ratio index [APRI] was calculated as AST (IU/L) × AST (upper limit of normal [IU/L])−1 × Plt (×109/L)−1 35.
Measurement of tri-sialylated mono-fucosylated tri-antennary glycan (A3F) bound to alpha-1 antitrypsin (AAT)
Serum concentrations of AAT-A3F were determined as previously described14. Briefly, AAT was purified from 50 μL of serum using commercially available immune-affinity beads (Alpha-1 Antitrypsin Select, GE Healthcare). The serum AAT concentration was determined by ELISA (Human Serpin A1 DuoSet ELISA, R&D Systems, Minneapolis, MN) with a standard AAT protein (Sigma-Aldrich, A9024). After tryptic digestion of AAT, N-glycans were released with N-glycosidase F. Released glycans were purified and derivatised with Nα-((aminooxy)acetyl) tryptophanylarginine methyl ester using a glycoblotting procedure36 and analysed with an Ultraflex II TOF/TOF mass spectrometer. The amount of A3F was quantified by the area ratio using ethyl-esterified A3 (3.8 pmol) as an external glycan. Serum concentrations of AAT-A3F were calculated by dividing the amount of A3F by AAT concentrations of each patient.
Real-time quantitative PCR analysis
We evaluated gene expression levels in liver tissues from 8 patients with NAFL and 16 patients with NASH. Total RNA was extracted from biopsied liver samples using the AllPrep DNA/RNA/Protein Mini Kit (Qiagen, Venlo, Netherlands). The quantity and quality of the isolated RNA were determined using an Agilent 2100 Bioanalyzer with the RNA 6000 Nano Kit (Agilent Technologies). Total RNA (500 ng) was reverse transcribed using PrimeScript RT Master Mix (Perfect Real Time) (Takara Bio, Shiga, Japan). Real-time quantitative PCR was performed using the Applied Biosystems 7500 Real-Time PCR System. Gene-specific primers were purchased from Takara Bio. Gene expression values were normalised to that of the reference gene, peptidylprolyl isomerase A, and calculated based on the ΔΔCt method37.
Statistical analysis
Statistical analysis was conducted using R software (version 3.5.1). Results are presented as the mean ± standard deviation (SD) for continuous data, and as numbers (percentages) for categorical data. Statistical analysis included descriptive statistics, analysis of variance, Mann–Whitney U test, Fischer’s exact test, and Spearman R correlations. All P values were two-tailed, and the threshold of statistical significance was set at P < 0.05. The diagnostic performance of the markers was assessed by analysing receiver operating characteristic (ROC) curves. The probabilities of true positives (sensitivity), true negatives (specificity), positive-predictive values (PPVs), and negative-predictive values (NPVs) were determined for selected cut-off values, and the AUROC was calculated for each index. Cut-off points were determined based on the optimum sum of the sensitivity and specificity.
Acknowledgements
The authors would like to thank all patients and their families as well as the investigators and staff of the participating institutions in the NORTE study group. This work was partly supported by a grant entitled, “The Matching Program for Innovations in Future Drug Discovery and Medical Care,” from the Ministry of Education, Culture, Science, and Technology, Japan, and Japan Agency for Medical Research and Development (ID 19fk0210018h0003).
Author contributions
K.O. practiced this study and wrote the manuscript. T.K., M.H., and K.H. measured AAT-A3F and performed statistical analysis. J.F., H.H., and Y.S. developed a measurement system for AAT-A3F. A.N., K.S., N.K., M.O., M.U., M.N., T.S., G.S., K.M., M.B., K.F., K.T., T.K., M.O., T.H., K.S., S.T., I.T. and T.M. accumulated cases. T.M. performed pathological evaluation. N.S. designed this study. K.O., T.K. and J.F. contributed equally to the work. ALL authors approved the final version of the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Koji Ogawa, Takashi Kobayashi and Jun-ichi Furukawa.
References
- 1.Ratziu V, et al. A position statement on NAFLD/NASH based on the EASL 2009 special conference. J. Hepatol. 2010;53:372–384. doi: 10.1016/j.jhep.2010.04.008. [DOI] [PubMed] [Google Scholar]
- 2.Younossi ZM, et al. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73–84. doi: 10.1002/hep.28431. [DOI] [PubMed] [Google Scholar]
- 3.Sayiner M, et al. Epidemiology of Nonalcoholic Fatty Liver Disease and Nonalcoholic Steatohepatitis in the United States and the Rest of the World. Clin. Liver Dis. 2016;20:205–214. doi: 10.1016/j.cld.2015.10.001. [DOI] [PubMed] [Google Scholar]
- 4.Rafiq N, et al. Long-term follow-up of patients with nonalcoholic fatty liver. Clin. Gastroenterol. Hepatol. 2009;7:234–238. doi: 10.1016/j.cgh.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 5.Angulo P. Nonalcoholic fatty liver disease. N. Engl. J. Med. 2002;346:1221–1231. doi: 10.1056/NEJMra011775. [DOI] [PubMed] [Google Scholar]
- 6.Cadranel JF, Rufat P, Degos F. Practices of liver biopsy in France: results of a prospective nationwide survey. For the Group of Epidemiology of the French Association for the Study of the Liver (AFEF) Hepatology. 2000;32:477–481. doi: 10.1053/jhep.2000.16602. [DOI] [PubMed] [Google Scholar]
- 7.Froehlich F, Lamy O, Fried M, Gonvers JJ. Practice and complications of liver biopsy. Results of a nationwide survey in Switzerland. Dig. Dis. Sci. 1993;38:1480–1484. doi: 10.1007/BF01308607. [DOI] [PubMed] [Google Scholar]
- 8.Wieckowska A, Feldstein AE. Diagnosis of nonalcoholic fatty liver disease: invasive versus noninvasive. Semin. Liver Dis. 2008;28:386–395. doi: 10.1055/s-0028-1091983. [DOI] [PubMed] [Google Scholar]
- 9.Velan B, et al. N-glycosylation of human acetylcholinesterase: effects on activity, stability and biosynthesis. Biochem. J. 1993;296:649–656. doi: 10.1042/bj2960649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Klein A, Michalski JC, Morelle W. Modifications of human total serum N-glycome during liver fibrosis-cirrhosis, is it all about immunoglobulins? Proteomics Clin. Appl. 2010;4:372–378. doi: 10.1002/prca.200900151. [DOI] [PubMed] [Google Scholar]
- 11.Zhu J, et al. Analysis of serum haptoglobin fucosylation in hepatocellular carcinoma and liver cirrhosis of different etiologies. J. Proteome. Res. 2014;13:2986–3997. doi: 10.1021/pr500128t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Comunale MA, et al. Linkage specific fucosylation of alpha-1-antitrypsin in liver cirrhosis and cancer patients: implications for a biomarker of hepatocellular carcinoma. PLoS One. 2010;5:e12419. doi: 10.1371/journal.pone.0012419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kuno A, et al. A serum “sweet-doughnut” protein facilitates fibrosis evaluation and therapy assessment in patients with viral hepatitis. Sci. Rep. 2013;3:1065. doi: 10.1038/srep01065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kamada Y, et al. Use of Mac-2 binding protein as a biomarker for nonalcoholic fatty liver disease diagnosis. Hepatol. Commun. 2017;1:780–791. doi: 10.1002/hep4.1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kobayashi Takashi, Ogawa Koji, Furukawa Jun-ichi, Hanamatsu Hisatoshi, Hato Megumi, Yoshinaga Tomoyo, Morikawa Kenichi, Suda Goki, Sho Takuya, Nakai Masato, Higashino Kenichi, Numata Yoshito, Shinohara Yasuro, Sakamoto Naoya. Quantifying Protein-Specific N-Glycome Profiles by Focused Protein and Immunoprecipitation Glycomics. Journal of Proteome Research. 2019;18(8):3133–3141. doi: 10.1021/acs.jproteome.9b00232. [DOI] [PubMed] [Google Scholar]
- 16.Greene CM, et al. α1-Antitrypsin deficiency. Nat. Rev. Dis. Primers. 2016;2:16051. doi: 10.1038/nrdp.2016.51. [DOI] [PubMed] [Google Scholar]
- 17.Tilg H, Vannier E, Vachino G, Dinarello CA, Mier JW. Antiinflammatory properties of hepatic acute phase proteins: preferential induction of interleukin 1 (IL-1) receptor antagonist over IL-1 beta synthesis by human peripheral blood mononuclear cells. J. Exp. Med. 1993;178:1629–1636. doi: 10.1084/jem.178.5.1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bucurenci N, Blake DR, Chidwick K, Winyard PG. Inhibition of neutrophil superoxide production by human plasma alpha 1-antitrypsin. FEBS Lett. 1992;300:21–24. doi: 10.1016/0014-5793(92)80156-B. [DOI] [PubMed] [Google Scholar]
- 19.Van Molle W, Libert C, Fiers W, Brouckaert P. Alpha 1-acid glycoprotein and alpha 1-antitrypsin inhibit TNF-induced but not anti-Fas-induced apoptosis of hepatocytes in mice. J. Immunol. 1997;159:3555–3564. [PubMed] [Google Scholar]
- 20.Pott GB, Chan ED, Dinarello CA, Shapiro L. Alpha-1-antitrypsin is an endogenous inhibitor of proinflammatory cytokine production in whole blood. J. Leukoc. Biol. 2009;85:886–895. doi: 10.1189/jlb.0208145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brinkman-Van der Linden EC, et al. A missense mutation in the FUT6 gene results in total absence of alpha3-fucosylation of human alpha1-acid glycoprotein. J. Biol. Chem. 1996;271:14492–14495. doi: 10.1074/jbc.271.24.14492. [DOI] [PubMed] [Google Scholar]
- 22.Bassagañas S, et al. Inflammatory cytokines regulate the expression of glycosyltransferases involved in the biosynthesis of tumor-associated sialylated glycans in pancreatic cancer cell lines. Cytokine. 2015;75:197–206. doi: 10.1016/j.cyto.2015.04.006. [DOI] [PubMed] [Google Scholar]
- 23.Bertola A, et al. Hepatic expression patterns of inflammatory and immune response genes associated with obesity and NASH in morbidly obese patients. PLoS One. 2010;5:e13577. doi: 10.1371/journal.pone.0013577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Feldstein AE, et al. Cytokeratin-18 fragment levels as noninvasive biomarkers for nonalcoholic steatohepatitis: a multicenter validation study. Hepatology. 2009;50:1072–1078. doi: 10.1002/hep.23050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hagström H, et al. Fibrosis stage but not NASH predicts mortality and time to development of severe liver disease in biopsy-proven NAFLD. J. Hepatol. 2017;67:1265–1273. doi: 10.1016/j.jhep.2017.07.027. [DOI] [PubMed] [Google Scholar]
- 26.Dulai PS, et al. Increased risk of mortality by fibrosis stage in nonalcoholic fatty liver disease: Systematic review and meta-analysis. Hepatology. 2017;65:1557–1565. doi: 10.1002/hep.29085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Angulo P, et al. Liver Fibrosis, but No Other Histologic Features, Is Associated With Long-term Outcomes of Patients With Nonalcoholic Fatty Liver Disease. Gastroenterology. 2015;149:389–397. doi: 10.1053/j.gastro.2015.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pais R, et al. A systematic review of follow-up biopsies reveals disease progression in patients with non-alcoholic fatty liver. J. Hepatol. 2013;59:550–556. doi: 10.1016/j.jhep.2013.04.027. [DOI] [PubMed] [Google Scholar]
- 29.Adams LA, et al. The natural history of nonalcoholic fatty liver disease: a population-based cohort study. Gastroenterology. 2005;129:113–121. doi: 10.1053/j.gastro.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 30.Kleiner DE, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313–1321. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 31.Kleiner DE, et al. Nonalcoholic Fatty Liver Disease: Pathologic Patterns and Biopsy Evaluation in Clinical Research. Semin. Liver Dis. 2012;32:3–13. doi: 10.1055/s-0032-1306421. [DOI] [PubMed] [Google Scholar]
- 32.Bedossa P, et al. Histopathological algorithm and scoring system for evaluation of liver lesions in morbidly obese patients. Hepatology. 2012;56:1751–1759. doi: 10.1002/hep.25889. [DOI] [PubMed] [Google Scholar]
- 33.Brunt EM, Janney CG, Di Bisceglie AM, Neuschwander-Tetri BA, Bacon BR. Nonalcoholic steatohepatitis: a proposal for grading and staging the histological lesions. Am. J. Gastroenterol. 1999;94:2467–2474. doi: 10.1111/j.1572-0241.1999.01377.x. [DOI] [PubMed] [Google Scholar]
- 34.Shah AG, et al. Comparison of noninvasive markers of fibrosis in patients with nonalcoholic fatty liver disease. Clin. Gastroenterol. Hepatol. 2009;7:1104–1112. doi: 10.1016/j.cgh.2009.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wai CT, et al. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology. 2003;38:518–526. doi: 10.1053/jhep.2003.50346. [DOI] [PubMed] [Google Scholar]
- 36.Fujitani N, et al. Total cellular glycomics allows characterizing cells and streamlining the discovery process for cellular biomarkers. Proc. Natl. Acad. Sci USA. 2013;110:2105–2110. doi: 10.1073/pnas.1214233110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]