Abstract
We aimed to determine if prematurity and lower birth weight are associated with poorer lung function in a non-western developed setting with less marked confounding by socioeconomic position. Using multivariable linear regression in Hong Kong’s “Children of 1997” birth cohort, adjusted associations of prematurity and birth weight with forced expiratory volume in 1 second (FEV1), forced vital capacity (FVC), and forced expiratory flow at 25–75% of the pulmonary volume (FEF25–75%) at ~17.5 years were assessed. Associations for birth weight were stronger in boys for FEV1 (boys: 0.31 L, 95% confidence interval (CI) 0.24 to 0.38, girls: 0.18 L, 95% CI 0.12 to 0.25), FVC (boys: 0.36 L, 95% CI 0.27 to 0.44, girls: 0.22 L, 95% CI 0.15 to 0.28) and FEF25–75% (boys: 0.35 L, 95% CI 0.21 to 0.49, girls: 0.22 L, 95% CI 0.09 to 0.34) adjusted for age, socioeconomic position and infant and maternal characteristics. Similarly adjusted, preterm birth (compared to full-term birth) was associated with lower FEV1/FVC and FEF25–75%. Thus, associations of lower birth weight, especially in boys, and prematurity with poorer lung function at 17.5 years were found. Identifying underlying mechanism might contribute to the improvement of pulmonary health and the prevention of adult respiratory illness.
Subject terms: Risk factors, Epidemiology
Introduction
The developmental origins of health and diseases (DOHaD) hypothesis emphasizes the role of poorer early growth, particularly in the first 1000 days, in non-communicable diseases (NCDs)1. Observationally poorer intrauterine experiences, proxied by birth weight or premature birth, are adversely associated with many aspects of adult health, including poorer lung function2–6. Lung function contributes both directly to chronic diseases via lung diseases and indirectly as a cardiovascular-related risk factor7,8, with potentially a causal role of forced expiratory volume9.
The DOHaD hypothesis has major implications for the care of mothers and babies. The DOHaD hypothesis largely rests on observational evidence from Western settings, which can be a very effective guide to action, for example as regards the harms of smoking10. On the other hand, there have been occasions when observational evidence has not been such a reliable guide, for example as regards the effects of hormone replacement therapy or vitamins11,12, likely because of residual confounding. Observational studies of birth attributes are open to confounding by maternal smoking, maternal overweight and lower socioeconomic position, which are also associated with worse birth outcomes and many health-conditions, including poorer lung function13–16. Birth weight also depends on gestational age which may not always be accurate, particularly in studies from before the routine use of ultrasound dating scans, when prematurity may be related to lower lung function17–20. The DOHaD hypothesis that early life is the critical period for respiratory health also does not consider evolutionary biology life history trade-offs, where early survival to reproductive age could trade-off against adult health including respiratory function. Lung function tracks throughout life21. Observationally, birth weight is inversely associated with indicators of restrictive lung function (forced vital capacity (FVC)) but evidence is weaker for the association of indicators of obstructive patterns (lower FEV1/FVC) in adults5. Previous studies have shown airway obstruction (lower FEV1, FEV1/FVC and forced expiratory flow between 25% and 75% of the pulmonary volume (FEF25–75%)) in extreme preterm births while fewer studies have focused on lung function in late preterm births6,19,22–26.
Evidence from twin studies, which are less open to confounding by family socio-economic position, is limited, but suggests lower birth weight is associated with poorer lung function, i.e. lower expiry in the first second of forced expiration (FEV1) and FVC27–29. More generally, Mendelian randomization studies of birth weight are difficult to interpret because of confounding by infant and/or maternal genetics30,31, but a study of monozygotic twins suggested little effect of birth weight on ischemic heart disease32. However, randomized controlled trials concerning the long-term effects of interventions targeting birth weight on lung function, are difficult and expensive to implement, and few such trials exist33,34. To our knowledge no Mendelian randomization studies have assessed whether birth weight is a causal factor for lung function, despite the relation between lung function and cardiovascular disease7–9. Hong Kong is a developed non-Western city, with social infrastructure and economic development similar to Western countries, where few women smoke, maternal overweight is less common than elsewhere35, and gestational age and birth weight are less confounded by socio-economic position36. Assessing the role of birth weight in lung function in this setting is less open to confounding possibly because of the less evident association of parental height with socioeconomic position in this population mainly formed by migrants from southern China in the mid-20th century37. In this unique non-Western setting we examined the relations of birth weight and prematurity with lung function at ~17.5 years in a Hong Kong Chinese birth cohort: “Children of 1997”. We also considered whether the associations varied by sex, because of the shorter life expectancy in men than women.
Methods
Ethics statement
Ethical approval for the study, including comprehensive health related analyses, was obtained from the Institutional Review Board of the University of Hong Kong/Hospital Authority Hong Kong West Cluster (HKU/HA HKW IRB). Informed written consent was obtained from the parents/guardians, or from the participant if 18 years or older, before participation in the Biobank Clinical Follow-up. All methods were performed in accordance with the relevant guidelines and regulations.
Data source
The current study utilizes a population-representative Chinese birth cohort from Hong Kong, “Children of 1997”, covering 88% of all births between April 1 and May 31, 1997, as described elsewhere38. At the infant’s first visit for free vaccinations and postnatal preventive care at all Maternal and Child Health Centers (MCHCs) in Hong Kong, 8237 mother-infant pairs were enrolled in an 18-month study to examine the association of environmental smoking with infant health39. Maternal characteristics (materal age at delivery and maternal smoking), family characteristics (education and residency status) and infant characteristics (birth weight, gestational age, parity and sex) were parent-or-career reported using a self-administered questionnaire in Chinese. Socioeconomic characteristics, such as parental occupation, household size and monthly household income were also reported. The study was resurrected as a birth cohort in 2005, with record linkage to Maternal and Child Health Center (MCHC) clinical records of growth and development including weight from birth to 5 years, and height from 3 months (with 96% success matching, n = 7999). In 2008–2012, active follow-up via three postal and/or telephone surveys was conducted. In 2013–2016, a Biobank clinical follow-up was conducted including anthropometrics, and a health check. Lung function was measured by spirometry (SpiroBank G Spirometer with WinspiroPRO software) and cleaned according to the American Thoracic Society/European Respiratory Society (ATS/ERS) criteria40. All lung function measurements were performed in a standing position with normal breath at rest before each test. Forceful slow inhalation and quick exhalation were performed not more than six times for at least three acceptable blows according to the ATS/ERS criteria, and the flow-volume curves with data were recorded. Any blow with a curve that did not resemble the ATS/ERS criteria predicted graph was considered as unacceptable and discarded. The spirometric curves with the maximum sum of FVC and FEV1 were selected.
Exposures
Given the birth weight in our population representative cohort is lower than the WHO standard, the WHO standard is not suitable for measuring the body size at birth in our study, hence we considered birth weight as internal sex- and gestational age-specific z-scores (standard deviation score) for singletons in this population-representative birth cohort41,42. Gestational age was calculated from the interval between expected and actual date of delivery. Preterm birth was defined as birth before 37 completed gestational weeks. Using the most commonly used cutoffs (10th and 90th percentile in singletons), we categorized sex- and gestational age-specific z-score for birth weight into small-for-gestational age (SGA), appropriate-for-gestational age (AGA) and large-for-gestational age (LGA)43.
Outcomes
The outcomes were lung function as FVC, FEV1, FEF25–75% and FEV1/FVC, measured at ~17.5 years. Z-scores of these spirometric indices were based on the Global Lung Function Initiative (GLI) reference 44, which has specific age-, height- and sex- equations for South East Asians based on data from Hong Kong, southwest China, Taiwan and Thailand (collected in 1996–2002) and the Hong Kong reference 45.
Statistical analysis
Baseline characteristics of the participants included and excluded were compared using Cohen’s w and Cohen’s d effect size, for categorical variables and continuous variables, where <0.1 and <0.2, respectively, indicate small differences between groups46. Analysis of variance (ANOVA) was used to compare birth weight, birth weight z-score and gestational age by potential confounders. Locally weighted scatterplot smoothing (LOWESS) curves were used to visualize the associations of birth weight and prematurity with lung function. Multivariable linear regression was used to examine adjusted associations of exposures and outcomes. Differences by sex were assessed from the significance of the relevant interaction term adjusted for confounding interactions. Potential confounders considered, i.e., common causes of birth weight or gestational age and lung function at ~17.5 years, included birth order, maternal birthplace, maternal smoking, maternal age at delivery, and parental socioeconomic position (including household income, highest parental occupation at recruitment and highest parental occupation). Potential mediators, such as breastfeeding, age of puberty, respiratory infections, weight and smoking, which might mediate the effect of birth weight on lung function but not cause birth weight were not included as confounders to avoid over adjustment47. To illustrate the potential effect of height, we present 2 models with different adjustment. Model 1 adjusted for confounders. Model 2 additionally adjusted for height in the sensitivity analysis using lung function in original units (Table S2). We only included singletons.
To predict missing values for gestational age (0.48% missing) and potential confounders (0.48% to 12.6% missing), multiple imputation based on additive regression and predictive mean matching was used. The regression model for multiple imputation incorporated exposures, outcomes and potential confounders, and interaction terms. To account for the probability of exclusion, inverse probability weighting was used to minimize possible selection bias induced by participants without valid lung function measurements. The inverse probability weights were calculated based on a logistic regression model with predictor variables including the exposures and the measured potential confounders after multiple imputation48. The single estimated β coefficients (mean difference) and 95% CI were summarized from 10 imputed datasets using Rubin’s rules and the inverse probability weights. Statistical analyses were conducted using R version 3.3.1 (R Foundation, Vienna, Austria).
Results
As of January, 2017, 29 of the original 8327 cohort participants had permanently withdrawn and were excluded. Of the remaining 8298 participants, 6850 were considered potentially contactable for the Biobank clinical follow-up in 2013–2016. Of these, 3460 attended and completed the lung function test. Lung function curves for 415 participants failed the lung function acceptibility criteria and were excluded, leaving 3030 with valid lung function. Among these 3030, mean birth weight was 3156 grams for girls and 3234 grams for boys. Gestational age was 39.0 weeks on average, 136 (4.5%) were preterm with an average gestational age of 34.8 weeks, of whom 99 (72.8%) were born after 34 gestational weeks. Birth weight and gestational age in the participants with and without valid lung function data did not differ (Table 1). They also had similar maternal and socioeconomic characteristics (Table 1).
Table 1.
Comparison of baseline characteristics for those with and without spirometry at ~17.5 years in Hong Kong’s “Children of 1997” birth cohort.
| Participants with spirometric data (N = 3033) | Participants without spirometric data (N = 5265) | Cohen’s w | |||
|---|---|---|---|---|---|
| N | % | N | % | ||
| Sex | 0.03 | ||||
| Female | 1475 | 49.0 | 2426 | 46.4 | |
| Male | 1522 | 51.0 | 2803 | 53.6 | |
| Sex and gestational age adjusted birth weight Z-score | 0.02 | ||||
| ≤−2 SD | 48 | 1.6 | 85 | 1.7 | |
| −2 SD–1SD | 390 | 13.1 | 699 | 13.7 | |
| −1 SD −+1 SD | 2100 | 70.4 | 3512 | 69.1 | |
| +1 SD −+2 SD | 361 | 12.1 | 629 | 12.4 | |
| >2 SD | 83 | 2.8 | 159 | 3.1 | |
| Birth Order | <0.01 | ||||
| 1 | 1403 | 47.5 | 2391 | 47.7 | |
| 2 | 1225 | 41.5 | 2065 | 41.2 | |
| 3 or above | 323 | 10.9 | 556 | 11.1 | |
| Preterm birth | 0.03 | ||||
| Preterm | 136 | 4.5 | 297 | 5.7 | |
| Full-term | 2883 | 95.5 | 4878 | 94.2 | |
| Maternal age at delivery, years | 0.07 | ||||
| ≤24 | 294 | 9.7 | 714 | 14.0 | |
| 25–29 | 944 | 31.2 | 1568 | 30.9 | |
| 30–34 | 1176 | 38.9 | 1908 | 37.7 | |
| ≥35 | 607 | 20.1 | 873 | 17.2 | |
| Mother’s birthplace | 0.04 | ||||
| Hong Kong | 1769 | 58.7 | 3018 | 62.6 | |
| Mainland China | 1247 | 41.3 | 1805 | 37.4 | |
| Maternal smoking | 0.04 | ||||
| No | 2839 | 96.2 | 4722 | 94.2 | |
| Yes | 112 | 3.8 | 291 | 5.8 | |
| Household income | 0.04 | ||||
| 1st quintile | 496 | 18.2 | 955 | 21.2 | |
| 2nd quantile | 552 | 20.2 | 932 | 20.7 | |
| 3rd quantile | 545 | 20.0 | 884 | 19.6 | |
| 4th quantile | 552 | 20.3 | 867 | 19.3 | |
| 5th quantile | 575 | 21.1 | 865 | 19.2 | |
| Highest parental education | 0.04 | ||||
| Grade 9 or below | 861 | 28.5 | 1606 | 31.6 | |
| Grade 10–11 | 1296 | 42.9 | 2151 | 42.4 | |
| Grade 12 or above | 863 | 28.6 | 1320 | 26.0 | |
| Highest parental occupation | 0.05 | ||||
| I (professional) | 711 | 26.8 | 1003 | 22.7 | |
| II (managerial) | 392 | 14.8 | 730 | 16.5 | |
| IIINM (non-manual skilled) | 762 | 28.8 | 1284 | 29.1 | |
| IIIM (manual skilled) | 444 | 16.8 | 771 | 17.5 | |
| VI (semiskilled) | 256 | 9.7 | 470 | 10.7 | |
| V (unskilled) | 85 | 3.2 | 154 | 3.5 | |
Table 2 shows birth weight was positively associated with birth order, but was not clearly associated with maternal age or socioeconomic position. Lower gestational age was associated with higher parental education and older maternal age at delivery, but was not clearly associated with birth order, maternal smoking, household income or highest parental occupation. Those with native-born mothers had lower birth weight and gestational age than those whose mothers were born elsewhere.
Table 2.
Baseline characteristics by gestational age, birth weight, and birth weight z-score adjusted for sex and gestational age in Hong Kong’s “Children of 1997” Birth Cohort.
| Characteristics | Gestational age (week) | Birth weight (kg) | Birth weight z-score adjusted for sex and gestational age | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | % | mean | SD | P | mean | SD | P | mean | SD | P | |
| Sex | |||||||||||
| Female | 1488 | 49.0 | 39.1 | 1.53 | <0.001 | 3.16 | 0.42 | <0.001 | −0.01 | 0.99 | 0.796 |
| Male | 1546 | 51.0 | 38.9 | 1.67 | 3.23 | 0.45 | 0.00 | 0.97 | |||
| Birth order | |||||||||||
| 1 | 1403 | 47.5 | 39.1 | 1.60 | 0.012 | 3.16 | 0.42 | <0.001 | −0.12 | 0.96 | <0.001 |
| 2 | 1225 | 41.5 | 38.9 | 1.62 | 3.21 | 0.45 | 0.07 | 0.98 | |||
| 3 or above | 323 | 11.0 | 39.0 | 1.50 | 3.29 | 0.43 | 0.27 | 0.98 | |||
| Maternal age at birth, years | |||||||||||
| ≤24 | 294 | 9.7 | 39.3 | 1.69 | <0.001 | 3.17 | 0.40 | 0.399 | −0.16 | 0.88 | 0.004 |
| 25–29 | 944 | 31.2 | 39.1 | 1.60 | 3.21 | 0.42 | −0.01 | 0.97 | |||
| 30–34 | 1176 | 38.9 | 39.0 | 1.54 | 3.19 | 0.44 | −0.01 | 0.97 | |||
| ≥35 | 607 | 20.0 | 38.6 | 1.67 | 3.18 | 0.47 | 0.08 | 1.05 | |||
| Maternal smoking | |||||||||||
| Smoker | 112 | 3.8 | 39.1 | 1.81 | 0.385 | 3.16 | 0.41 | 0.422 | −0.10 | 0.96 | 0.234 |
| Non-smoker | 2839 | 96.2 | 39.0 | 1.60 | 3.20 | 0.44 | 0.00 | 0.98 | |||
| Maternal birthplace | |||||||||||
| Hong Kong | 1769 | 58.7 | 38.8 | 1.59 | <0.001 | 3.16 | 0.44 | <0.001 | −0.06 | 0.99 | <0.001 |
| Mainland China and elsewhere | 1247 | 41.3 | 39.2 | 1.61 | 3.25 | 0.43 | 0.07 | 0.97 | |||
| Household income (Quantile) | |||||||||||
| 1 | 496 | 18.2 | 39.2 | 1.64 | <0.001 | 3.23 | 0.42 | 0.093 | 0.03 | 0.97 | 0.469 |
| 2 | 552 | 20.3 | 38.9 | 1.71 | 3.20 | 0.47 | 0.03 | 1.03 | |||
| 3 | 545 | 20.0 | 39.0 | 1.54 | 3.20 | 0.42 | −0.01 | 0.96 | |||
| 4 | 552 | 20.3 | 38.9 | 1.52 | 3.17 | 0.43 | −0.06 | 1.01 | |||
| 5 | 575 | 21.1 | 38.8 | 1.57 | 3.17 | 0.43 | −0.04 | 0.94 | |||
| Highest parental education at recruitment | |||||||||||
| Grade 9 or below | 861 | 28.5 | 39.1 | 1.72 | <0.001 | 3.23 | 0.44 | 0.062 | 0.05 | 0.98 | 0.082 |
| Grade 10–11 | 1296 | 42.9 | 39.0 | 1.59 | 3.19 | 0.44 | −0.04 | 1.00 | |||
| Grade 12 or above | 863 | 28.6 | 38.8 | 1.50 | 3.18 | 0.43 | −0.00 | 0.96 | |||
| Highest parental occupation | |||||||||||
| I (professional) | 711 | 26.8 | 38.9 | 1.55 | <0.001 | 3.18 | 0.45 | 0.037 | −0.05 | 0.94 | 0.012 |
| II (managerial) | 392 | 14.8 | 38.7 | 1.64 | 3.20 | 0.45 | 0.08 | 0.99 | |||
| IIINM (non-manual skilled) | 762 | 28.8 | 39.0 | 1.58 | 3.17 | 0.43 | −0.09 | 1.00 | |||
| IIIM (manual skilled) | 444 | 16.8 | 39.2 | 1.62 | 3.24 | 0.43 | 0.06 | 1.04 | |||
| VI (semiskilled) | 256 | 9.7 | 39.0 | 1.91 | 3.21 | 0.46 | 0.03 | 1.04 | |||
| V (unskilled) | 85 | 3.2 | 39.2 | 1.60 | 3.28 | 0.44 | 0.18 | 1.04 | |||
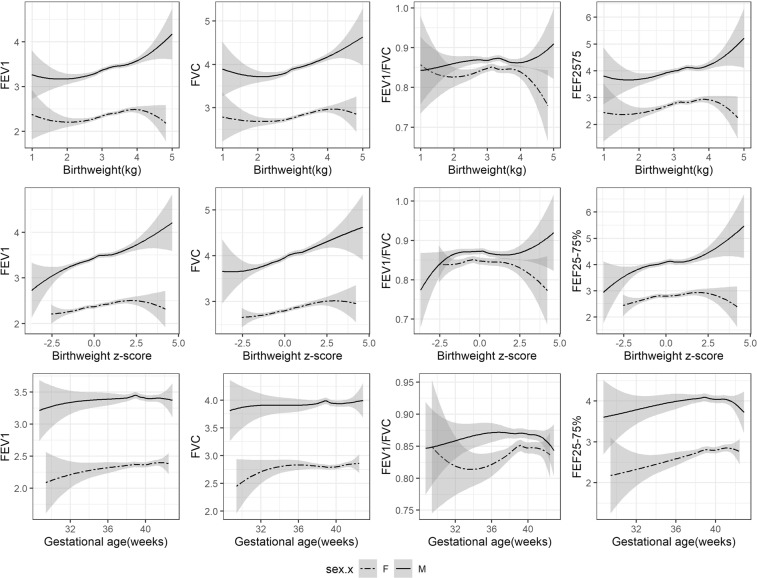
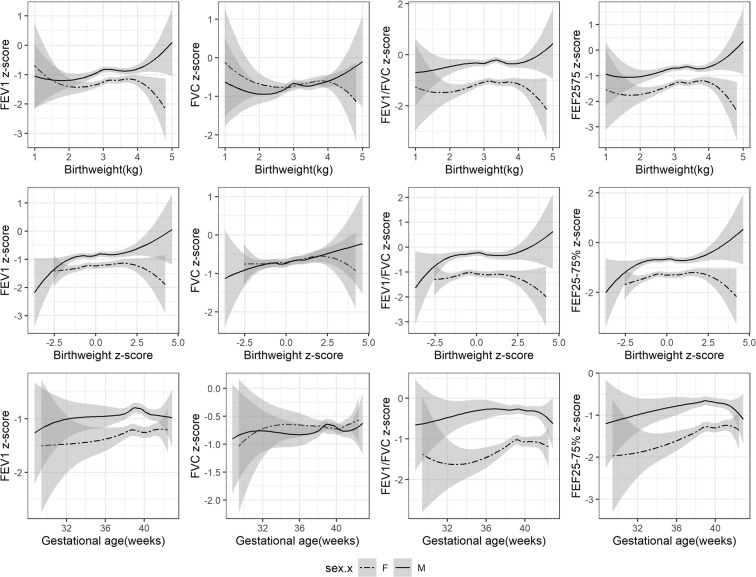
Generally positive linear associations of birth weight in the normal range with FEV1, FVC and FEF25–75% were found in boys and girls using LOWESS curves (Fig. 1), but not with FEV1/FVC ratio. Gestational age of less than 39 week was positively associated with FEV1/FVC ratio and FEF25–75%, but associations with FEV1 and FVC was less clear. The slope of birth weight for gestational age z-score with FEV1 and FVC was slightly steeper in boys than girls, with a significant sex-interaction (Table S1). Similar associations were seen for LOWESS curves of lung function using z-scores, but no clear difference by sex was seen for the slopes (Fig. 2). The associations of birth weight with FEV1 (p-value for interaction 0.02) and FVC (p-value for interaction 0.01) differed by sex, with stronger positive associations in boys than girls, adjusted for age, socioeconomic position and maternal and infant characteristics (Table 3). Birth weight was similarly positively associated with FEF25–75% in boys and girls. Birth weight was not associated with FEV1/FVC ratio. Similarly, LGA (compared to AGA) was associated with higher FEV1, FVC and FEF25–75% and SGA was associated with lower FEV1, FVC and FEF25–75% but not with FEV1/FVC ratio. These associations were more evident in boys. This pattern remained after additionally adjusting for height, with the estimate slightly attenuated towards the null (Table S2). A consistent pattern of associations was found in the sex-specific analysis of lung function z-scores based on the sex-, age- and height specific equations from the GLI references (Table 4). The sex difference was not clear in analysis of z-scores (Table S1), although the positive associations of birth weight with FEV1 and FVC appeared stronger in boys than girls (Table 4).
Figure 1.
LOWESS curves showing association of birth weight, gestational age with lung function in original units by sex in Hong Kong’s “Children of 1997” Birth Cohort. Abbreviations: FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; FEF25–75%, forced expiratory flow at 25–75% of the pulmonary volume. Birth weight z-score refers to internal sex- and gestational age-specific z-scores.
Figure 2.
LOWESS curves showing association of birth weight, gestational ages with lung function in z-scores by sex in Hong Kong’s “Children of 1997” Birth Cohort. Abbreviations: FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; FEF25–75%, forced expiratory flow at 25–75% of the pulmonary volume. Birth weight z-scores refers to internal sex- and gestational age-specific z-scores. Lung function z-scores refers to age, height and sex specific z-scores based on the Global Lung Function Initiative (GLI) references.
Table 3.
Associations of birth weight and gestational age with lung function in original units at ~17.5 years by sex in Hong Kong’s “Children of 1997” birth cohort (After inverse probability weighting and multiple imputation).
| Sex | Exposures | FEV1, L | FVC, L | FEV1/FVC | FEF25–75%, L/s | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Crude | Model 1a | Crude | Model 1a | Crude | Model 1a | Crude | Model 1a | ||||||||||
| β | 95%CI | β | 95%CI | β | 95%CI | β | 95%CI | β | 95%CI | β | 95%CI | β | 95%CI | β | 95%CI | ||
| Girls | Gestational age, w | 0.02* | 0.00 to 0.03 | 0.02* | 0.00 to 0.03 | 0.01 | −0.01 to 0.02 | 0.01 | −0.01 to 0.02 | 0.00* | 0.00 to 0.01 | 0.00* | 0.00 to 0.01 | 0.05* | 0.02 to 0.07 | 0.05* | 0.02 to 0.07 |
| Preterm | |||||||||||||||||
| Full term birth | Ref | — | Ref | — | Ref | — | Ref | — | Ref | — | Ref | — | Ref | — | Ref | — | |
| Preterm birth | −0.09 | −0.20 to 0.02 | −0.09 | −0.21 to 0.02 | 0.00 | −0.11 to 0.12 | 0.00 | −0.12 to 0.11 | −0.03* | −0.06 to −0.01 | −0.03* | −0.06 to −0.01 | −0.31* | −0.53 to −0.09 | −0.31* | −0.53 to −0.09 | |
| Birth weight, kg # | 0.17* | 0.11 to 0.22 | 0.18* | 0.12 to 0.25 | 0.18* | 0.12 to 0.23 | 0.22* | 0.15 to 0.28 | 0.01 | −0.01 to 0.02 | 0.00 | −0.01 to 0.01 | 0.26* | 0.15 to 0.36 | 0.22* | 0.09 to 0.34 | |
| Birth weight z-score b # | 0.07* | 0.05 to 0.09 | 0.07* | 0.05 to 0.09 | 0.08* | 0.06 to 0.11 | 0.08* | 0.06 to 0.11 | 0.00 | −0.01 to 0.02 | 0.00 | −0.01 to 0.01 | 0.09* | 0.04 to 0.13 | 0.08* | 0.04 to 0.13 | |
| Size for gestational age | |||||||||||||||||
| AGA | Ref | — | Ref | — | Ref | — | Ref | — | Ref | — | Ref | — | Ref | — | Ref | — | |
| LGA | 0.14* | 0.06 to 0.21 | 0.14* | 0.06 to 0.21 | 0.18* | 0.11 to 0.26 | 0.18* | 0.11 to 0.26 | −0.01 | −0.02 to 0.01 | −0.01 | −0.02 to 0.01 | 0.17* | 0.02 to 0.31 | 0.16* | 0.01 to 0.31 | |
| SGA# | −0.11* | −0.19 to −0.03 | −0.09* | −0.17 to −0.01 | −0.11* | −0.19 to −0.03 | −0.09* | −0.18 to −0.01 | −0.01 | −0.02 to 0.01 | 0.00 | −0.01 to 0.01 | −0.20* | −0.35 to −0.04 | −0.18* | −0.34 to −0.02 | |
| Boys | Gestational age, w | 0.00 | −0.01 to 0.02 | 0.00 | −0.01 to 0.02 | 0.00 | −0.02 to 0.02 | 0.00 | −0.02 to 0.02 | 0.00 | 0.00 to 0.00 | 0.00 | 0.00 to 0.00 | 0.02 | −0.01 to 0.05 | 0.01 | −0.02 to 0.04 |
| Preterm | |||||||||||||||||
| Full term birth | Ref | — | Ref | — | Ref | — | Ref | — | Ref | — | Ref | — | Ref | — | Ref | — | |
| Preterm birth | −0.03 | −0.14 to 0.08 | −0.04 | −0.15 to 0.08 | 0.00 | −0.11 to 0.12 | 0.01 | −0.12 to 0.14 | −0.01* | −0.03 to 0.00 | −0.01* | −0.03 to 0.00 | −0.23* | −0.45 to −0.01 | −0.23* | −0.45 to −0.01 | |
| Birth weight, kg # | 0.22* | 0.16 to 0.28 | 0.31* | 0.24 to 0.38 | 0.25* | 0.18 to 0.32 | 0.36* | 0.27 to 0.44 | 0.00 | −0.01 to 0.01 | 0.00 | −0.01 to 0.01 | 0.28* | 0.16 to 0.39 | 0.35* | 0.21 to 0.49 | |
| Birth weight z-score b # | 0.12* | 0.09 to 0.15 | 0.12* | 0.09 to 0.14 | 0.14 | 0.11 to 0.17 | 0.14* | 0.11 to 0.17 | 0.00 | 0.00 to 0.00 | 0.00 | 0.00 to 0.00 | 0.13* | 0.08 to 0.19 | 0.13* | 0.07 to 0.18 | |
| Size for gestational age | |||||||||||||||||
| AGA | Ref | — | Ref | — | Ref | — | Ref | — | Ref | — | Ref | — | Ref | — | Ref | — | |
| LGA | 0.21* | 0.12 to 0.30 | 0.20* | 0.11 to 0.30 | 0.22* | 0.12 to 0.32 | 0.22* | 0.11 to 0.32 | 0.00 | −0.01 to 0.02 | 0.00 | −0.01 to 0.02 | 0.30* | 0.12 to 0.47 | 0.28* | 0.11 to 0.45 | |
| SGA# | −0.24* | −0.34 to −0.14 | −0.24* | −0.34 to −0.14 | −0.28* | −0.40 to −0.16 | −0.28* | −0.39 to −0.16 | 0.00 | −0.02 to 0.02 | 0.00 | −0.02 to 0.02 | −0.22* | −0.42 to −0.03 | −0.21* | −0.40 to −0.01 | |
Abbreviations: SGA, small-for-gestational age; AGA, appropriate-for-gestational age; LGA, large-for-gestational age; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; FEF25–75%, forced expiratory flow at 25–75% of the pulmonary volume.
aModel 1: Adjusted for age, birth order, maternal age at birth, maternal smoking, maternal birthplace, and parental social-economic positions (SEP, including household income, the highest parental occupation at recruitment and the highest parental occupation).
bApart from the adjustment in footnote a, adjusted for gestational age.
cFor average gestational age (39 weeks), the mean for birthweight of girls and boys are 3377 grams and 3433 grams respectively, and the SD for birthweight of girls and boys are 374 grams and 421 grams respectively.
*Statistically significant association with lung function at the 0.05 level.
#Statistically significant interaction with sex on FEV1 and FVC.
Table 4.
Associations of birth weight and gestational age with lung function in z-scores at ~17.5 years in Hong Kong’s “Children of 1997” birth cohort (After inverse probability weighting and multiple imputation).
| Sex | Exposures | FEV1, z-score | FVC, z-score | FEV1/FVC, z-score | FEF25–75%, z-scores | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Crude | Model 1a | Crude | Model 1a | Crude | Model 1a | Crude | Model 1a | ||||||||||
| β | 95%CI | β | 95%CI | β | 95%CI | β | 95%CI | β | 95%CI | β | 95%CI | β | 95%CI | β | 95%CI | ||
| Both | Gestational age, w | 0.01 | −0.02 to 0.03 | 0.01 | −0.02 to 0.03 | 0.00 | −0.02 to 0.03 | 0.00 | −0.02 to 0.03 | 0.01 | −0.02 to 0.04 | 0.02 | −0.01 to 0.05 | 0.02* | 0.00 to 0.05 | 0.02* | 0.00 to 0.05 |
| Preterm | |||||||||||||||||
| Full term birth | Ref | — | Ref | — | Ref | — | Ref | — | Ref | — | Ref | — | Ref | — | Ref | — | |
| Preterm birth | −0.07 | −0.25 to 0.12 | −0.07 | −0.26 to 0.11 | 0.08 | −0.10 to 0.26 | 0.07 | −0.10 to 0.25 | −0.26* | −0.48 to 0.04 | −0.26* | −0.48 to −0.04 | −0.26* | −0.46 to 0.05 | −0.26* | −0.46 to −0.06 | |
| Birth weight, kg b | 0.20* | 0.11 to 0.29 | 0.26* | 0.15 to 0.37 | 0.12* | 0.03 to 0.21 | 0.18* | 0.07 to 0.28 | 0.19* | 0.08 to 0.30 | 0.22 | −0.09 to 0.36 | 0.26* | 0.16 to 0.37 | 0.31* | 0.19 to 0.43 | |
| Birth weight z-score c | 0.07* | 0.03 to 0.12 | 0.07* | 0.03 to 0.12 | 0.07* | 0.03 to 0.11 | 0.07* | 0.03 to 0.11 | 0.03 | 0.02 to 0.08 | 0.03 | −0.02 to 0.08 | 0.07* | 0.03 to 0.12 | 0.07* | 0.02 to 0.12 | |
| Size for gestational age | |||||||||||||||||
| AGA | Ref | — | Ref | — | Ref | — | Ref | — | Ref | — | Ref | — | Ref | — | Ref | — | |
| LGA | 0.12 | −0.02 to 0.25 | 0.12 | −0.02 to 0.26 | 0.13* | 0.00 to 0.27 | 0.14* | 0.00 to 0.27 | 0.04 | −0.13 to 0.20 | 0.04 | −0.13 to 0.20 | 0.15* | 0.00 to 0.30 | 0.15* | 0.00 to 0.30 | |
| SGA# | −0.19* | −0.34 to −0.05 | −0.19* | −0.34 to −0.04 | −0.14 | −0.29 to 0.01 | −0.13 | −0.28 to −0.01 | −0.11 | −0.29 to 0.07 | 0.10 | −0.28 to 0.08 | −0.20* | −0.36 to −0.03 | −0.19* | −0.36 to −0.02 | |
| Girls | Gestational age, w | 0.03 | −0.01 to 0.07 | 0.03* | 0.00 to 0.07 | 0.01 | −0.03 to 0.04 | 0.01 | −0.03 to 0.04 | 0.05* | 0.01 to 0.10 | 0.06* | 0.02 to 0.11 | 0.06* | 0.02 to 0.10 | 0.07* | 0.02 to 0.11 |
| Preterm | |||||||||||||||||
| Full term birth | Ref | — | Ref | — | Ref | — | Ref | — | Ref | — | Ref | — | Ref | — | Ref | — | |
| Preterm birth | −0.21 | −0.51 to 0.08 | −0.23 | −0.53 to 0.06 | 0.05 | −0.22 to 0.33 | 0.03 | −0.25 to 0.31 | −0.48* | −0.82 to −0.14 | −0.47* | −0.82 to −0.12 | −0.31* | −0.53 to −0.09 | −0.31* | −0.53 to −0.09 | |
| Birth weight, kg b | 0.17* | 0.03 to 0.31 | 0.17* | 0.03 to 0.31 | 0.11 | −0.02 to 0.25 | 0.11 | −0.02 to 0.25 | 0.14 | −0.02 to 0.31 | 0.14 | −0.02 to 0.31 | 0.25* | 0.10 to 0.41 | 0.25* | 0.10 to 0.41 | |
| Birth weight z-score c | 0.06* | 0.00 to 0.12 | 0.06* | 0.00 to 0.12 | 0.06* | 0.00 to 0.12 | 0.06* | 0.00 to 0.12 | 0.02 | −0.05 to 0.09 | 0.02 | −0.06 to 0.09 | 0.07* | 0.01 to 0.14 | 0.07* | 0.00 to 0.14 | |
| Size for gestational age | |||||||||||||||||
| AGA | Ref | — | Ref | — | Ref | — | Ref | — | Ref | — | Ref | — | Ref | — | Ref | — | |
| LGA | 0.12 | −0.08 to 0.31 | 0.11 | 0.09 to 0.31 | 0.19* | 0.00 to 0.38 | 0.18 | −0.01 to 0.37 | −0.04 | −0.27 to 0.19 | −0.05 | −0.28 to 0.19 | 0.12 | −0.02 to 0.34 | 0.12 | −0.10 to 0.34 | |
| SGA# | −0.14 | −0.35 to 0.06 | −0.12 | −0.33 to 0.09 | −0.07 | −0.27 to 0.13 | −0.06 | −0.25 to 0.14 | −0.12 | −0.36 to 0.13 | −0.10 | −0.35 to 0.14 | −0.22 | −0.44 to 0.01 | −0.20 | −0.43 to 0.03 | |
| Boys | Gestational age, w | 0.00 | −0.03 to 0.04 | 0.00 | −0.03 to 0.03 | 0.00 | −0.03 to 0.03 | 0.00 | −0.04 to 0.03 | 0.01 | −0.03 to 0.05 | 0.01 | −0.03 to 0.05 | 0.02 | −0.02 to 0.05 | 0.01 | −0.02 to 0.05 |
| Preterm | |||||||||||||||||
| Full term birth | Ref | — | Ref | — | Ref | — | Ref | — | Ref | — | Ref | — | Ref | — | Ref | — | |
| Preterm birth | −0.03 | −0.25 to 0.20 | −0.03 | −0.26 to 0.20 | 0.10 | −0.14 to 0.33 | 0.09 | −0.15 to 0.32 | −0.23 | −0.50 to 0.04 | −0.22 | −0.50 to 0.05 | −0.23* | −0.49 to 0.02 | −0.24* | −0.49 to −0.02 | |
| Birth weight, kg b | 0.16* | 0.04 to 0.28 | 0.22* | 0.07 to 0.36 | 0.13* | 0.01 to 0.26 | 0.20* | 0.05 to 0.35 | 0.08 | −0.06 to 0.22 | 0.09 | −0.08 to 0.27 | 0.17* | 0.04 to 0.30 | 0.20* | 0.03 to 0.36 | |
| Birth weight z-score c | 0.08* | 0.03 to 0.14 | 0.08* | 0.02 to 0.13 | 0.08* | 0.02 to 0.14 | 0.07* | 0.02 to 0.13 | 0.03 | −0.03 to 0.10 | 0.03 | −0.04 to 0.10 | 0.07* | 0.01 to 0.13 | 0.06* | 0.00 to 0.12 | |
| Size for gestational age | |||||||||||||||||
| AGA | Ref | — | Ref | — | Ref | — | Ref | — | Ref | — | Ref | — | Ref | — | Ref | — | |
| LGA | 0.12 | −0.06 to 0.30 | 0.10 | −0.08 to 0.29 | 0.08 | −0.11 to 0.27 | 0.08 | −0.11 to 0.27 | 0.11 | −0.10 to 0.33 | 0.10 | −0.12 to 0.32 | 0.18 | −0.03 to 0.38 | 0.16 | −0.04 to 0.36 | |
| SGA | −0.21* | −0.41 to −0.01 | −0.20* | −0.40 to 0.00 | −0.21 | −0.43 to −0.01 | −0.20 | −0.42 to 0.02 | −0.03 | −0.27 to 0.22 | −0.02 | −0.27 to 0.22 | −0.12 | −0.35 to 0.11 | −0.11 | −0.34 to −0.12 | |
Abbreviations: SGA, small-for-gestational age; AGA, appropriate-for-gestational age; LGA, large-for-gestational age; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; FEF25–75%, forced expiratory flow at 25–75% of the pulmonary volume.
aAdjusted for birth order, maternal age at birth, maternal smoking, maternal birthplace, and parental social-economic positions (SEP, including household income, the highest parental occupation at recruitment and the highest parental occupation).
bApart from the adjustment in footnote a, adjusted for gestational age.
The associations of gestational age and preterm birth with lung function did not differ by sex, with no clear association of gestational age with lung function (Table S1). Preterm birth (compared to full-term birth) was associated with lower FEV1/FVC ratio and FEF25–75% (Table 5). This pattern remained after additionally adjusting for height, with similar effect size (Table S2). Consistent results were found after converting lung function into z-scores (Table S4).
Table 5.
Associations of birth weight and gestational age with lung function in original units at ~17.5 years in Hong Kong’s “Children of 1997” birth cohort (After inverse probability weighting and multiple imputation).
| Exposures | FEV1, L | FVC, L | FEV1/FVC | FEF25–75%, L/s | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Crude | Adjusteda | Crude | Adjusteda | Crude | Adjusteda | Crude | Adjusteda | |||||||||
| β | 95%CI | β | 95%CI | β | 95%CI | β | 95%CI | β | 95%CI | β | 95%CI | β | 95%CI | β | 95%CI | |
| Gestational age, w | −0.01 | −0.03 to 0.00 | 0.01 | 0.00 to 0.02 | −0.02 | −0.03 to 0.00 | 0.01 | −0.01 to 0.02 | 0.00 | 0.00 to 0.00 | 0.00 | 0.00 to 0.00 | 0.00 | −0.02 to 0.03 | 0.03* | 0.01 to 0.05 |
| Preterm | ||||||||||||||||
| Full term birth | Ref | — | Ref | — | Ref | — | Ref | — | Ref | — | Ref | — | Ref | — | Ref | — |
| Preterm birth | 0.03 | −0.08 to 0.15 | −0.06 | −0.14 to 0.02 | 0.11 | −0.02 to 0.24 | 0.01 | −0.08 to 0.10 | −0.02* | −0.03 to 0.00 | −0.02* | −0.04 to −0.01 | −0.15* | −0.34 to 0.03 | −0.26* | −0.42 to −0.11 |
| Birth weight, kg # | 0.30* | 0.24 to 0.35 | 0.26* | 0.21 to 0.30 | 0.33* | 0.26 to 0.39 | 0.30 | 0.25 to 0.35 | 0.01 | 0.00 to 0.01 | 0.00 | −0.01 to 0.01 | 0.39* | 0.30 to 0.48 | 0.30* | 0.20 to 0.39 |
| Birth weight z-score b # | 0.10* | 0.07 to 0.13 | 0.10* | 0.08 to 0.11 | 0.12* | 0.09 to 0.14 | 0.11 | 0.09 to 0.13 | 0.00 | 0.00 to 0.00 | 0.00 | 0.00 to 0.00 | 0.11* | 0.07 to 0.15 | 0.11* | 0.07 to 0.14 |
| Size for gestational age | ||||||||||||||||
| AGA | Ref | — | Ref | — | Ref | — | Ref | — | Ref | — | Ref | — | Ref | — | Ref | — |
| LGA | 0.17* | 0.09 to 0.26 | 0.18* | 0.12 to 0.24 | 0.20* | 0.10 to 0.29 | 0.21* | 0.14 to 0.27 | 0.00 | −0.01 to 0.01 | 0.00 | −0.01 to 0.01 | 0.23* | 0.09 to 0.37 | 0.23* | 0.12 to 0.35 |
| SGA# | −0.22* | −0.31 to −0.13 | −0.17* | −0.23 to 0.10 | −0.24* | −0.35 to −0.14 | −0.19* | −0.26 to −0.11 | 0.00 | −0.02 to 0.01 | 0.00 | −0.01 to 0.01 | −0.27* | −0.41 to −0.12 | −0.20* | −0.32 to −0.08 |
Abbreviations: SGA, small-for-gestational age; AGA, appropriate-for-gestational age; LGA, large-for-gestational age; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; FEF25–75%, forced expiratory flow at 25–75% of the pulmonary volume.
aAdjusted for sex and confounders in Model 1: age, birth order, maternal age at birth, maternal smoking, maternal birthplace, and parental social-economic positions (SEP, including household income, the highest parental occupation at recruitment and the highest parental occupation).
bApart from the adjustment in footnote a, adjusted for gestational age.
cFor average gestational age (39 weeks), the mean for birthweight of girls and boys are 3377 grams and 3433 grams respectively, and the SD for birthweight of girls and boys are 374 grams and 421 grams respectively.
*Statistically significant association with lung function at the 0.05 level.
#Statistically significant interaction with sex on FEV1 and FVC.
Discussion
In this developed non-Western setting with little confounding by socioeconomic position, inverse associations of birth weight with lung function, assessed by FEV1, FVC and FEF25–75% at ~17.5 years were evident, particularly in boys. These findings are consistent with previous meta-analysis of observational studies from Western settings4,5 but add by showing boys to be more sensitive to birth weight. Given lung function z-scores are sex-specific, sex-differences in the associations of birth weight with lung function z-scores was attenuated. Similar to previous findings4, these associations remained after adjusting for height, suggesting that the association of birth weight with lung function is independent of height. Inconsistent association of birth weight with FEV1/FVC were observed in several previous studies5, while our study showed no association. Prematurity was inversely associated lower FEV1/FVC and FEF25–75% and our previous study in this cohort showed preterm infants more prone to asthma49, these findings suggests impaired airway development in preterm births, which is consistent with other findings in late adolescence19,20,22.
In animal experiments, sheep and lambs with intrauterine growth restriction (IUGR) have structurally and functionally impaired lung development, suggesting that insults which cause IUGR may detrimentally affect the lungs resulting in persistent alternation in structure and function in later life50–53. Discrepancies also exist in the concentrations of surfactant proteins between IUGR and AGA human infants54,55. Alternatively, lower birth weight may represent less muscle mass even in adulthood which might reduce forced expiratory airflow56,57. Extremely premature newborns are born with fewer enlarged alveoli with thicker alveolar walls and hence their immature lung cannot function normally (with reduced effective gas exchange surface area) at birth, which may result in long-term lung functional abnormalities in later life58. Late preterm births are born at the late saccular stage of lung development when the lung volume and surface area is developing rapidly. Delivery during this period may also result in a less mature lung at birth and dysregulate alveolar development. Infants with immature lungs and poor airway function may be more susceptible to respiratory diseases after birth, which are also linked to worse lung function in late adolescence and early adulthood17,18,59. Other exposures that might lead to preterm birth, such as adverse maternal nutritional status, hypertensive disorders and overweight during pregnancy, might also affect airway development in utero60. Finally, poorer maternal lung function, for whatever reason, could result in both poorer birth outcomes and poorer offspring lung function. Apart from the negative exposure causing preterm birth, preterm births are more likely to receive neonatal intensive care, among which some interventions such as mechanical ventilation and oxygen therapy were associated with adverse respiratory health, abnormal lung growth and development61.
Stronger associations of birth weight with poor lung function in boys than girls have been observed before62,63 Boys are more vulnerable to respiratory diseases than girls64,65, but why this should affect particularly lower birth weight boys is unclear. Alternatively, androgens may adversely affect surfactant production coupled with the later start of pulmonary surfactant production in male fetuses66, which might make lighter boys more vulnerable. Finally lower birth weight may reduce muscle mass more in men than women56,67, possibly with corresponding effects on respiratory function via the respiratory muscles. Mechanistic studies are needed to distinguish between these possibilities so as to identify the best interventions to protect lung function in lower birth weight boys.
Although our study was conducted in a non-western setting with less obvious social patterning of birth weight and prematurity, several limitations exist. First, selection bias may exist, however, those with and without lung function had similar baseline characteristics (Table 1). Additionally, inverse probability weighting should help to recover the original sample based on measured covariates, thereby minimizing potential bias. Second, observational studies are open to unmeasured confounding, such as by air pollution68. Nevertheless, key confounders including maternal smoking and socioeconomic position were adjusted for in this study and hence our associations are unlikely to be explained by socioeconomic position or related attributes. Third, poorly reported gestational age without information from ultrasound scans might result in misclassification, which is likely to be small and non-differential because the information was reported by the main caregivers shortly after birth when gestational age looms large. Fourth, we did not assess potential mediators, such as diet and physical activity, and some potential parental confounders, such as maternal nutrition status. Fifth, the internal sex and gestational age specific z-scores for birth weight in the extremely preterm children (≤28 weeks) might be less accurate due to the small sample size of the very preterm births. Replication in other settings with birth weight and gestational age little confounded by socioeconomic factors, or re-examination using Mendelian Randomization accounting for maternal genetics is warranted to confirm the causal effects.
Conclusions
In a population with minimal confounding by socioeconomic position, birth weight was inversely associated with FEV1, FVC and FEF25–75%, particularly among boys, indicating lower birth weight may reduce lung function mainly in the airway capacity at 17.5 years. In contrast, prematurity was associated with lower FEV1/FVC and FEF25–75%, indicating preterm birth may impair airway development, which suggests increasing vulnerability to obstructive lung diseases. As such, our study suggests that both lower birth weight and prematurity may have long-lasting effects on lung function, which may be particularly detrimental for men.
Supplementary information
Acknowledgements
We thank the late Dr. Connie O for coordinating the project and all the fieldwork for the initial study in 1997–98. This work is a sub-study of the “Children of 1997” birth cohort which was initially supported by the Health Care and Promotion Fund, Health and Welfare Bureau, Government of the Hong Kong Special Administrative Region [HCPF Grant # 216106] and re-established in 2005 funded by the Health and Health Services Research Fund [HHSRF Grant # 03040771, 05060671, 07080751, 07080841] and the Research Fund for the Control of Infectious Diseases in Hong Kong (RFCID Grant # 04050172, 06060592), Government of the Hong Kong SAR. The birth cohort has also received funding from the University Research Committee Strategic Research Theme of Public Health Granted Research, The University of Hong Kong. The most recent follow-up was partly funded by the WYNG Foundation.
Author contributions
Ms. Baoting He contributed to the formulation of analysis-plan, data analysis and drafted the manuscript. Dr. C. Mary Schooling is the guarantor of the content of the manuscript, including the data analysis plan, and supervising the drafting of the manuscript. Dr. Gabriel M. Leung, Dr. Man Ki Kwok, Dr. Shiu Lun Au Yeung, Dr. Shi Lin Lin, Dr. June Yue Yan Leung, Dr. Lai Ling Hui and Dr. Albert M Li contributed to interpretation of the data and the review of the analysis-plan and manuscript preparation. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Data availability
Data are available upon reasonable request from the “Children of 1997” data access committee: aprmay97@hku.hk.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
is available for this paper at 10.1038/s41598-019-56086-7.
References
- 1.Kotecha S. Lung growth: implications for the newborn infant. Archives of Disease in Childhood-Fetal and Neonatal Edition. 2000;82:F69–F74. doi: 10.1136/fn.82.1.F69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Balte P, et al. Relationship between birth weight, maternal smoking during pregnancy and childhood and adolescent lung function: A path analysis. Respiratory medicine. 2016;121:13–20. doi: 10.1016/j.rmed.2016.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kotecha SJ, Watkins WJ, Henderson AJ, Kotecha S. The effect of birth weight on lung spirometry in white, school-aged children and adolescents born at term: a longitudinal population based observational cohort study. The Journal of pediatrics. 2015;166:1163–1167. doi: 10.1016/j.jpeds.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 4.Lawlor DA, Ebrahim S, Davey Smith G. Association of birth weight with adult lung function: findings from the British Women’s Heart and Health Study and a meta-analysis. Thorax. 2005;60:851–858. doi: 10.1136/thx.2005.042408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saad NJ, Patel J, Burney P. & Minelli, C. Birth Weight and Lung Function in Adulthood: A Systematic Review and Meta-analysis. Annals of the American Thoracic. Society. 2017;14:994–1004. doi: 10.1513/AnnalsATS.201609-746SR. [DOI] [PubMed] [Google Scholar]
- 6.Vollsaeter M, et al. Adult respiratory outcomes of extreme preterm birth. A regional cohort study. Annals of the American Thoracic Society. 2015;12:313–322. doi: 10.1513/AnnalsATS.201406-285OC. [DOI] [PubMed] [Google Scholar]
- 7.Pan J, et al. The association of pulmonary function with carotid atherosclerosis in older Chinese: Guangzhou Biobank Cohort Study-CVD Subcohort. Atherosclerosis. 2015;243:469–476. doi: 10.1016/j.atherosclerosis.2015.09.036. [DOI] [PubMed] [Google Scholar]
- 8.Lee HM, Liu MA, Barrett-Connor E, Wong ND. Association of lung function with coronary heart disease and cardiovascular disease outcomes in elderly: the Rancho Bernardo study. Respiratory medicine. 2014;108:1779–1785. doi: 10.1016/j.rmed.2014.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Au Yeung SL, Borges MC, Lawlor DA. Association of Genetic Instrumental Variables for Lung Function on Coronary Artery Disease Risk: A 2-Sample Mendelian Randomization Study. Circulation. Genomic and precision medicine. 2018;11:e001952. doi: 10.1161/CIRCGEN.117.001952. [DOI] [PubMed] [Google Scholar]
- 10.Alberg AJ, Shopland DR, Cummings KM. The 2014 Surgeon General’s Report: Commemorating the 50th Anniversary of the 1964 Report of the Advisory Committee to the US Surgeon General and Updating the Evidence on the Health Consequences of Cigarette Smoking. American Journal of Epidemiology. 2014;179:403–412. doi: 10.1093/aje/kwt335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lawlor DA, Davey Smith G, Kundu D, Bruckdorfer KR, Ebrahim S. Those confounded vitamins: what can we learn from the differences between observational versus randomised trial evidence? Lancet. 2004;363:1724–1727. doi: 10.1016/S0140-6736(04)16260-0. [DOI] [PubMed] [Google Scholar]
- 12.Petitti D. Commentary: hormone replacement therapy and coronary heart disease: four lessons. Int J Epidemiol. 2004;33:461–463. doi: 10.1093/ije/dyh192. [DOI] [PubMed] [Google Scholar]
- 13.Parker JD, Schoendorf KC, Kiely JL. Associations between measures of socioeconomic status and low birth weight, small for gestational age, and premature delivery in the United States. Annals of epidemiology. 1994;4:271–278. doi: 10.1016/1047-2797(94)90082-5. [DOI] [PubMed] [Google Scholar]
- 14.Ong Thida, Schechter Michael, Yang Jing, Peng Limin, Emerson Julia, Gibson Ronald L., Morgan Wayne, Rosenfeld Margaret. Socioeconomic Status, Smoke Exposure, and Health Outcomes in Young Children With Cystic Fibrosis. Pediatrics. 2017;139(2):e20162730. doi: 10.1542/peds.2016-2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leermakers ET, et al. Maternal weight, gestational weight gain and preschool wheezing: the Generation R Study. Eur Respir J. 2013;42:1234–1243. doi: 10.1183/09031936.00148212. [DOI] [PubMed] [Google Scholar]
- 16.Cnattingius S, et al. Maternal obesity and risk of preterm delivery. Jama. 2013;309:2362–2370. doi: 10.1001/jama.2013.6295. [DOI] [PubMed] [Google Scholar]
- 17.Gough A, et al. Impaired lung function and health status in adult survivors of bronchopulmonary dysplasia. European Respiratory Journal. 2014;43:808–816. doi: 10.1183/09031936.00039513. [DOI] [PubMed] [Google Scholar]
- 18.Gross SJ, Iannuzzi DM, Kveselis DA, Anbar RD. Effect of preterm birth on pulmonary function at school age: a prospective controlled study. The Journal of pediatrics. 1998;133:188–192. doi: 10.1016/S0022-3476(98)70219-7. [DOI] [PubMed] [Google Scholar]
- 19.Kotecha SJ, et al. Effect of preterm birth on later FEV1: a systematic review and meta-analysis. Thorax. 2013;68:760–766. doi: 10.1136/thoraxjnl-2012-203079. [DOI] [PubMed] [Google Scholar]
- 20.Kotecha SJ, et al. Effect of late preterm birth on longitudinal lung spirometry in school age children and adolescents. Thorax. 2012;67:54–61. doi: 10.1136/thoraxjnl-2011-200329. [DOI] [PubMed] [Google Scholar]
- 21.Bush A. Lung Development and Aging. Annals of the American Thoracic Society. 2016;13:S438–S446. doi: 10.1513/AnnalsATS.201602-112AW. [DOI] [PubMed] [Google Scholar]
- 22.Thunqvist P., Gustafsson P. M., Schultz E. S., Bellander T., Berggren-Brostro m E., Norman M., Wickman M., Melen E., Hallberg J. Lung Function at 8 and 16 Years After Moderate-to-Late Preterm Birth: A Prospective Cohort Study. PEDIATRICS. 2016;137(4):e20152056–e20152056. doi: 10.1542/peds.2015-2056. [DOI] [PubMed] [Google Scholar]
- 23.den Dekker HT, et al. Early growth characteristics and the risk of reduced lung function and asthma: A meta-analysis of 25,000 children. J Allergy Clin Immunol. 2016;137:1026–1035. doi: 10.1016/j.jaci.2015.08.050. [DOI] [PubMed] [Google Scholar]
- 24.Simpson SJ, et al. Altered lung structure and function in mid-childhood survivors of very preterm birth. Thorax. 2017;72:702–711. doi: 10.1136/thoraxjnl-2016-208985. [DOI] [PubMed] [Google Scholar]
- 25.Thunqvist P, et al. Lung function after extremely preterm birth-A population-based cohort study (EXPRESS) Pediatric pulmonology. 2018;53:64–72. doi: 10.1002/ppul.23919. [DOI] [PubMed] [Google Scholar]
- 26.Hadchouel A, et al. Association between asthma and lung function in adolescents born very preterm: results of the EPIPAGE cohort study. Thorax. 2018;73:1174–1176. doi: 10.1136/thoraxjnl-2017-211115. [DOI] [PubMed] [Google Scholar]
- 27.Ortqvist AK, et al. Fetal Growth and Childhood Lung Function in the Swedish Twin Study on Prediction and Prevention of Asthma. Annals of the American Thoracic. Society. 2017;14:1147–1153. doi: 10.1513/AnnalsATS.201611-908OC. [DOI] [PubMed] [Google Scholar]
- 28.Nikolajev K, Koskela H, Korppi M. Birth weight and adult lung function: a within-pair analysis of twins followed up from birth. World journal of pediatrics: WJP. 2008;4:222–226. doi: 10.1007/s12519-008-0041-7. [DOI] [PubMed] [Google Scholar]
- 29.Nikolajev K, Heinonen K, Hakulinen A, Lansimies E. Effects of intrauterine growth retardation and prematurity on spirometric flow values and lung volumes at school age in twin pairs. Pediatric pulmonology. 1998;25:367–370. doi: 10.1002/(SICI)1099-0496(199806)25:6<367::AID-PPUL2>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 30.Au Yeung SL, Lin SL, Li AM, Schooling CM. Birth weight and risk of ischemic heart disease: A Mendelian randomization study. Sci Rep. 2016;6:38420. doi: 10.1038/srep38420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zanetti D, et al. Birthweight, Type 2 Diabetes Mellitus, and Cardiovascular Disease: Addressing the Barker Hypothesis With Mendelian Randomization. Circulation. Genomic and precision medicine. 2018;11:e002054. doi: 10.1161/CIRCGEN.117.002054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oberg S, Cnattingius S, Sandin S, Lichtenstein P, Iliadou AN. Birth weight predicts risk of cardiovascular disease within dizygotic but not monozygotic twin pairs: a large population-based co-twin-control study. Circulation. 2011;123:2792–2798. doi: 10.1161/CIRCULATIONAHA.110.987339. [DOI] [PubMed] [Google Scholar]
- 33.Devakumar D, et al. Effects of antenatal multiple micronutrient supplementation on lung function in mid-childhood: follow-up of a double-blind randomised controlled trial in Nepal. European Respiratory Journal. 2015;45:1566–1575. doi: 10.1183/09031936.00188914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nikièma L, et al. Effectiveness of facility-based personalized maternal nutrition counseling in improving child growth and morbidity up to 18 months: A cluster-randomized controlled trial in rural Burkina Faso. PLOS ONE. 2017;12:e0177839. doi: 10.1371/journal.pone.0177839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Leung TY, et al. Trends in maternal obesity and associated risks of adverse pregnancy outcomes in a population of Chinese women. BJOG: an international journal of obstetrics and gynaecology. 2008;115:1529–1537. doi: 10.1111/j.1471-0528.2008.01931.x. [DOI] [PubMed] [Google Scholar]
- 36.Leung JY, Leung GM, Schooling CM. Socioeconomic disparities in preterm birth and birth weight in a non-Western developed setting: evidence from Hong Kong’s ‘Children of 1997’ birth cohort. Journal of epidemiology and community health. 2016;70:1074–1081. doi: 10.1136/jech-2015-206668. [DOI] [PubMed] [Google Scholar]
- 37.Schooling CM, Leung GM. A socio-biological explanation for social disparities in non-communicable chronic diseases: the product of history? Journal of epidemiology and community health. 2010;64:941–949. doi: 10.1136/jech.2008.086553. [DOI] [PubMed] [Google Scholar]
- 38.Schooling CM, Hui LL, Ho LM, Lam T-H, Leung GM. Cohort profile:‘children of 1997’: a Hong Kong Chinese birth cohort. International journal of epidemiology. 2011;41:611–620. doi: 10.1093/ije/dyq243. [DOI] [PubMed] [Google Scholar]
- 39.Lam TH, Leung GM, Ho LM. The effects of environmental tobacco smoke on health services utilization in the first eighteen months of life. Pediatrics. 2001;107:E91. doi: 10.1542/peds.107.6.e91. [DOI] [PubMed] [Google Scholar]
- 40.Miller MR, et al. Standardisation of spirometry. Eur Respir J. 2005;26:319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 41.Hui LL, et al. Are universal standards for optimal infant growth appropriate? Evidence from a Hong Kong Chinese birth cohort. 2008;93:561–565. doi: 10.1136/adc.2007.119826. [DOI] [PubMed] [Google Scholar]
- 42.Fenton TR, Kim JH. A systematic review and meta-analysis to revise the Fenton growth chart for preterm infants. BMC Pediatrics. 2013;13:59. doi: 10.1186/1471-2431-13-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zeve D, Regelmann MO, Holzman IR, Rapaport R. Small at Birth, but How Small? The Definition of SGA Revisited. Hormone research in paediatrics. 2016;86:357–360. doi: 10.1159/000449275. [DOI] [PubMed] [Google Scholar]
- 44.Quanjer PH, et al. Multi-ethnic reference values for spirometry for the 3-95-yr age range: the global lung function 2012 equations. Eur Respir J. 2012;40:1324–1343. doi: 10.1183/09031936.00080312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ip MS, Karlberg EM, Karlberg JP, Luk KD, Leong JC. Lung function reference values in Chinese children and adolescents in Hong Kong. I. Spirometric values and comparison with other populations. American journal of respiratory and critical care medicine. 2000;162:424–429. doi: 10.1164/ajrccm.162.2.9905057. [DOI] [PubMed] [Google Scholar]
- 46.Cohen, J. Statistical Power Analysis for the Behavioral Sciences, (Taylor & Francis, 2013).
- 47.Schisterman EF, Cole SR, Platt RW. Overadjustment bias and unnecessary adjustment in epidemiologic studies. Epidemiology (Cambridge, Mass.) 2009;20:488–495. doi: 10.1097/EDE.0b013e3181a819a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Seaman SR, White IR, Copas AJ, Li L. Combining multiple imputation and inverse-probability weighting. Biometrics. 2012;68:129–137. doi: 10.1111/j.1541-0420.2011.01666.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Leung JY, Lam HS, Leung GM, Schooling CM. Gestational Age, Birthweight for Gestational Age, and Childhood Hospitalisations for Asthma and Other Wheezing Disorders. Paediatric and perinatal epidemiology. 2016;30:149–159. doi: 10.1111/ppe.12273. [DOI] [PubMed] [Google Scholar]
- 50.Orgeig S, Crittenden TA, Marchant C, McMillen IC, Morrison JL. Intrauterine growth restriction delays surfactant protein maturation in the sheep fetus. American journal of physiology. Lung cellular and molecular physiology. 2010;298:L575–583. doi: 10.1152/ajplung.00226.2009. [DOI] [PubMed] [Google Scholar]
- 51.Briana DD, Malamitsi-Puchner A. Small for gestational age birth weight: impact on lung structure and function. Paediatric respiratory reviews. 2013;14:256–262. doi: 10.1016/j.prrv.2012.10.001. [DOI] [PubMed] [Google Scholar]
- 52.Joyce BJ, et al. Compromised respiratory function in postnatal lambs after placental insufficiency and intrauterine growth restriction. Pediatric research. 2001;50:641–649. doi: 10.1203/00006450-200111000-00018. [DOI] [PubMed] [Google Scholar]
- 53.Rozance PJ, et al. Intrauterine growth restriction decreases pulmonary alveolar and vessel growth and causes pulmonary artery endothelial cell dysfunction in vitro in fetal sheep. American journal of physiology. Lung cellular and molecular physiology. 2011;301:L860–871. doi: 10.1152/ajplung.00197.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Briana DD, et al. Clara cell protein in full-term pregnancies: the influence of intrauterine growth restriction. Pediatric pulmonology. 2010;45:1186–1191. doi: 10.1002/ppul.21305. [DOI] [PubMed] [Google Scholar]
- 55.Briana DD, et al. The effect of intrauterine growth restriction on circulating surfactant protein D concentrations in the perinatal period. Reproductive sciences (Thousand Oaks, Calif.) 2010;17:653–658. doi: 10.1177/1933719110366165. [DOI] [PubMed] [Google Scholar]
- 56.Yliharsila H, et al. Birth size, adult body composition and muscle strength in later life. International journal of obesity (2005) 2007;31:1392–1399. doi: 10.1038/sj.ijo.0803612. [DOI] [PubMed] [Google Scholar]
- 57.Rossi A, et al. Body composition and pulmonary function in the elderly: a 7-year longitudinal study. International journal of obesity (2005) 2008;32:1423–1430. doi: 10.1038/ijo.2008.103. [DOI] [PubMed] [Google Scholar]
- 58.Colin AA, McEvoy C, Castile RG. Respiratory morbidity and lung function in preterm infants of 32 to 36 weeks’ gestational age. Pediatrics. 2010;126:115–128. doi: 10.1542/peds.2009-1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Stern DA, Morgan WJ, Wright AL, Guerra S, Martinez FD. Poor airway function in early infancy and lung function by age 22 years: a non-selective longitudinal cohort study. Lancet (London, England) 2007;370:758–764. doi: 10.1016/S0140-6736(07)61379-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zugna D, et al. Maternal complications in pregnancy and wheezing in early childhood: a pooled analysis of 14 birth cohorts. Int J Epidemiol. 2015;44:199–208. doi: 10.1093/ije/dyu260. [DOI] [PubMed] [Google Scholar]
- 61.Kotecha SJ, Dunstan FD, Kotecha S. Long term respiratory outcomes of late preterm-born infants. Seminars in fetal & neonatal medicine. 2012;17:77–81. doi: 10.1016/j.siny.2012.01.004. [DOI] [PubMed] [Google Scholar]
- 62.Ubilla C, Bustos P, Amigo H, Oyarzun M, Rona RJ. Nutritional status, especially body mass index, from birth to adulthood and lung function in young adulthood. Annals of human biology. 2008;35:322–333. doi: 10.1080/03014460801978937. [DOI] [PubMed] [Google Scholar]
- 63.Suresh S, Mamun AA, O’Callaghan M, Sly PD. The impact of birth weight on peak lung function in young adults. Chest. 2012;142:1603–1610. doi: 10.1378/chest.11-2976. [DOI] [PubMed] [Google Scholar]
- 64.Debley JS, Redding GJ, Critchlow CW. Impact of adolescence and gender on asthma hospitalization: a population-based birth cohort study. Pediatric pulmonology. 2004;38:443–450. doi: 10.1002/ppul.20108. [DOI] [PubMed] [Google Scholar]
- 65.Jensen-Fangel S, et al. Gender differences in hospitalization rates for respiratory tract infections in Danish youth. Scandinavian journal of infectious diseases. 2004;36:31–36. doi: 10.1080/00365540310017618. [DOI] [PubMed] [Google Scholar]
- 66.Seaborn T, Simard M, Provost PR, Piedboeuf B, Tremblay Y. Sex hormone metabolism in lung development and maturation. Trends in endocrinology and metabolism: TEM. 2010;21:729–738. doi: 10.1016/j.tem.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 67.Costa Junior D, et al. Influence of Body Composition on Lung Function and Respiratory Muscle Strength in Children With Obesity. Journal of clinical medicine research. 2016;8:105–110. doi: 10.14740/jocmr2382w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Huang JV, Leung GM, Schooling CM. The association of air pollution with birthweight and gestational age: evidence from Hong Kong’s ‘Children of 1997’ birth cohort. Journal of public health (Oxford, England) 2017;39:476–484. doi: 10.1093/pubmed/fdw068. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available upon reasonable request from the “Children of 1997” data access committee: aprmay97@hku.hk.