Abstract
Sinus node dysfunction (SND) is often associated with atrial fibrillation (AF). Amiodarone is the most frequently used agent for maintaining sinus rhythm in patients with AF, but it impairs the sinoatrial node (SAN) function in one-third of AF patients. This study aims to gain mechanistic insights into the effects of the antiarrhythmic agents in the setting of AF-induced SND. We have adapted a human SAN model to characterize the SND conditions by incorporating experimental data on AF-induced electrical remodelling, and then integrated actions of drugs into the modified model to assess their efficacy. Reductions in pacing rate upon the implementation of AF-induced electrical remodelling associated with SND agreed with the clinical observations. And the simulated results showed the reduced funny current (If) in these remodelled targets mainly contributed to the heart rate reduction. Computational drug treatment simulations predicted a further reduction in heart rate during amiodarone administration, indicating that the reduction was the result of actions of amiodarone on INa, IKur, ICaL, ICaT, If and beta-adrenergic receptors. However, the heart rate was increased in the presence of disopyramide. We concluded that disopyramide may be a desirable choice in reversing the AF-induced SND phenotype.
Subject terms: Atrial fibrillation, Atrial fibrillation
Introduction
Sinus node dysfunction (SND) is associated with abnormal sinoatrial node (SAN) impulse formation resulting in sinus bradycardia. As the elderly population continues to increase, SND is becoming an increasingly common medical condition in patients with atrial fibrillation (AF)1. AF and SND often coexist and interact in clinical practice, but the causal link between these two arrhythmias remains unclear2,3. AF itself may alter the function of the normal SAN or promote pre-existing SND. AF is believed to shut down the normal function of the SAN by long-term overdrive suppression of its activity4. AF-induced SND was evidenced by slowed intrinsic heart rate, which was gradually reversed after the termination of AF5–7. The intrinsic heart rate was jointly regulated by the voltage (cyclic activation and deactivation of membrane ion channels) and calcium clocks (rhythmic spontaneous sarcoplasmic reticulum calcium release)8. In the pacing-induced canine model of AF, changes in membrane ion channels and calcium handling proteins imply the impact of the voltage and calcium clocks. Alterations in hyperpolarization-activated cyclic nucleotide-gated channels HCN4, slow delayed-rectifier α-subunit KvLQT1, L-type/T-type calcium current subunit Cav1.2/Cav3.16 and calcium handling proteins9 were observed in SAN cells, suggesting that AF may lead to SAN remodeling and thereby SND.
It was shown in large-scale clinical studies that amiodarone is the most effective drug for maintaining sinus rhythm in patients with AF10–13. However, amiodarone impairs SAN function in one-third of AF patients14. Touboul et al. showed that amiodarone has no effects on sinus cycle length (CL) in pacing-induced AF patients15, whereas Hoffmann et al. showed that it depresses SAN function in some patients with sick sinus syndrome16, suggesting that amiodarone cannot be used safely in all patients with the tachy-brady syndrome. Furthermore, Mun et al. demonstrated that unresponsiveness of SAN to sympathetic stimulation could be a mechanism of the SND induced by amiodarone17. Thus, ionic mechanisms underlying amiodarone-induced bradycardia under the AF-induced SND condition remain unclear.
Mathematical models of cardiac cells are widely considered as potentially important tools for safety pharmacology evaluation18–20. Rodriguez et al.21, Bottino et al.22, Mirams et al.23 and Davies et al.24 used ion channel data acquired from routine high-throughput screens to infer results during compound development. Pharmaceutical companies, the Comprehensive in Vitro Proarrythmia Assay (CiPA), the United States Food and Drug Administration (FDA) and the Cardiac Safety Research Consortium raised in silico drug assays to provide integrative, high-throughput, cost-effective and efficient solutions25. Prediction of arrhythmogenicity was improved by considering the effects of drugs on multiple ion channels, the therapeutic plasma drug concentrations and the use of biophysically detailed mathematical models of cardiac electrical activity26.
In the present work, based on a biophysically detailed mathematical model of human SAN cells27, we developed a human SND model by considering AF-induced electrical remodelling, which includes changes in membrane ionic currents and calcium handling. The ionic mechanisms of SND in patients with AF were investigated by assessing the role of each remodelled target in regulating SAN automaticity. The amiodarone effects were integrated into the SND cell model by adapting the ion channel conductivities to the dose-dependent inhibition of the currents. The impacts of amiodarone on voltage clock and autonomic regulation of SAN cells were investigated by assessing the roles of membrane ionic currents, calcium handling and sympathetic and/or parasympathetic stimulation, respectively. Furthermore, some antiarrhythmic agents (i.e., disopyramide, quinidine and digoxin) used for improving sinoatrial node function in the setting of AF-induced SND were assessed.
Methods
This work was conducted based on a mathematical model and didn’t involve any experimental animals or human participants.
Modelling AF-induced electrical remodelling underlying SND
The human SAN cell model developed by Fabbri et al.27 was used as the base model for single-cell simulations in this study since the model was based on and validated using electrophysiological data from isolated human SAN pacemaker cells. In the cellular model, an ordinary differential equation was used to describe the transmembrane potential V:
| 1 |
where t is the time, Cm (57 pF) is the capacitance across the cell membrane, and Iion is the total ionic current across the membrane.
| 2 |
The precise behavior of the individual channels was based on a wide range of human cell electrophysiological data, and details can be found in the study conducted by Fabbri et al.27
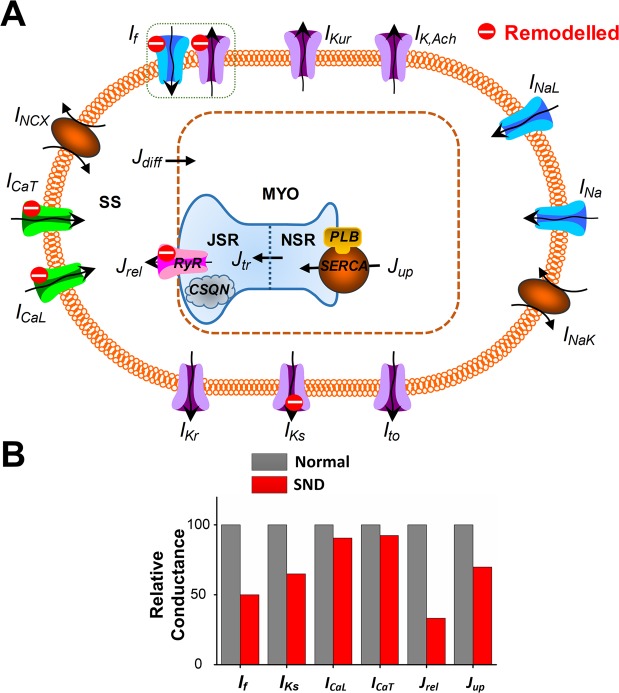
Under the SND conditions, AF-induced electrical remodelling included voltage clock-associated ionic currents and calcium clock-associated calcium handling. The ionic current formulations were modified based on data from Yeh et al., who investigated the voltage clock of single SAN cells from dogs that underwent atrial tachypacing and measured SAN transcript expressions for If –associated subunits, IK-related subunits and ICa subunits6. Alterations in calcium handling properties were derived from experimental data of Joung et al., who evaluated the calcium clock of single SAN cells in pacing-induced AF dogs, and determined expression of RyR, SERCA and phospholamban (PLB)9. In the Fabbri et al. model, If, ICaL, ICaT, IKs, Jrel and Jup were decreased to 50%, 90%, 92%, 65%, 33% and 71%, respectively, for describing the AF-induced SND condition (Fig. 1A,B and Supplementary Table 1).
Figure 1.
Illustration of atrial fibrillation (AF)-induced remodelling processes and electrophysiological properties and calcium dynamics in the human sinoatrial node (SAN) cell model. (A) Schematic presentation of the cell model. Formulations for AF-induced remodelled currents and fluxes (red) were based directly on experimental data of the canine model of pacing-induced AF. The model includes four compartments: bulk myoplasm (myo), junctional sarcoplasmic reticulum (JSR), network sarcoplasmic reticulum (NSR), and subspace (SS). Currents into the subspace: funny current (If; representing both sodium and potassium components), L-type calcium current (ICaL), T-type calcium current (ICaT), fast sodium current (INa), transient outward potassium current (Ito), rapid delayed rectifier potassium current (IKr), slow delayed rectifier potassium current (IKs), ultrarapid delayed rectifier potassium current (IKur), acetylcholine-sensitive muscarinic potassium current (IKACh), sodium-calcium exchange current (INCX) and sodium-potassium pump current (INaK). Ionic fluxes: calcium flux (Jrel) through ryanodine receptor (RyR), NSR to JSR calcium translocation (Jtr), calcium uptake (Jup) into NSR via SERCA/phospholamban (PLB) and diffusion calcium flux from subspace to myoplasm (Jdiff). AF-induced targets: If, ICaT, ICaL, Jrel R and Jup. (B) Relative conductance between normal and SND conditions.
Modelling effects of drugs on SND
In the present study, we focused on investigating the effects of amiodarone on SAN automaticity. The effects of amiodarone were incorporated into the cellular model by modifying ionic currents, involving beta-adrenergic receptor and membrane targets. For the block effects of amiodarone on the beta-adrenergic receptor, we decreased the effects of ISO stimulation by 15.2%28. For the effects of amiodarone on membrane targets, we integrated the block effects of amiodarone on funny current (If)29, sodium currents (INa)30, calcium currents (ICaL28 and ICaT31), potassium currents (Ito32, IKr28, IKs33, IKur34 and IKACh35), sodium/calcium exchange current INCX36, and sodium/potassium pump current INaK.37 Block of ionic currents provoked by amiodarone was simulated by including the fraction of unblocked channels in their formulations, estimated using the standard sigmoid dose-response curve parametrized using the half-maximal inhibitory concentration (IC50) and Hill coefficient (nH)26. Within the framework of pore block theory, the maximal conductance gi of an ionic current type i was modified in a concentration-dependent manner, such that
| 3 |
where gcontrol,i represents the maximal conductance of the i current channel in drug-free conditions and D is the drug concentration. According to clinical data, the therapeutic concentration range of amiodarone, disopyramide, quinidine and digoxin, respectively, was 0.77~3.88 μM38–43, 6~15 µM44, 4~17 μM45 and 0.64~2.56 nM46. Therefore, D for amiodarone, disopyramide, quinidine and digoxin was set to be 1.55 μM, 10 µM, 4 μM and 1 nM, respectively. In addition, the concentration-dependent effects of drugs were also investigated at the low, middle, and high concentrations (Supplementary Table 2). The IC50 and nH values describing the effects of drugs on ionic currents were listed in the Supplementary Tables 3–6.
Autonomic modulation
According to the method of Fabbri et al.27, autonomic regulation of SAN cells was studied by simulating the effects of acetylcholine (ACh, 10 nM) and isoprenaline (ISO, 1.0 μM) stimulations. Changes in If, ICaL, IKs, IKACh, INaK and Jup due to ACh and ISO stimulations were listed in the Supplementary Table 7.
Multicellular 1D simulations
A 1D SAN-atrial model, which consists of 30 SAN cells and 60 atrial cells, was developed to simulate electrical waves under the AF-induced SND condition in the presence of drugs. In the 1D cable model, the mathematical model of the human atrial myocyte developed in our previous study47–52 was used, and AF was simulated by introducing electrical remodelling based on experimental data53–62 (listed in the Supplementary Table 8). The membrane potential is described by:
| 4 |
where V(i) is the membrane potential of the ith cell, t is time, Iion is the sum of the transmembrane ionic currents, Cm is the total membrane capacitance and Ggap is the gap-junction coupling, which is given by:
| 5 |
The heterogeneity of the SAN was implemented in the model following the strategies of Zhang et al.63 and Garny et al.37 The method uses the parameters of the central and the peripheral cell to determine the characteristics of transitional cells. A scaling factor Fcell is calculated by:
| 6 |
with the location i of cells (i=1 central; i=30 peripheral). For 60 atrial cells, Cm = 1 pF and Ggap = 400 nS/pF.
Simulation protocol and data analysis
To quantitatively assess the sensitivity of action potential (AP) and calcium features to parameters affected by electrical remodelling/drug actions, we changed values of parameters associated with each target between 100% to x%. x was set to be the minimal value to generate AP. Maximum diastolic potential (MDP), diastolic depolarization rate (DDR100), maximum voltage of AP (OS), maximum rate of rise of membrane potential (dV/dt)max, maximum value of intracellular calcium concentration (Caimax), minimum value of intracellular calcium concentration (Caimin) and heart rate were used to quantify electrophysiological characteristics of SAN myocytes. And electrophysiological features of atrial cells were quantified by measuring MDP, OS, (dV/dt)max, Caimax, Caimin and AP duration at 90% repolarization (APD90). An explicit Euler method for solving the ordinary differential Eq. (4) with a time step of 0.00001 s was used. Simulations were run until steady-state was reached after 100 s (~10000000 times). For 1D simulations, it took about 5 h using the Intel Core I5–4210m Processor (3 M Cache, up to 3.20 GHz) to compute 100 s.
Results
Effects of AF-induced electrical remodeling on electrophysiological properties in SND
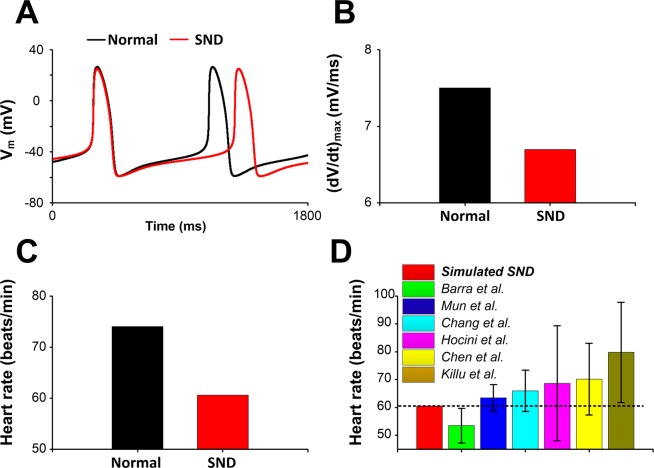
Single-cell simulations were performed under normal and SND conditions and effects of AF-induced electrical remodeling on AP were investigated (Fig. 2). Note that due to AF-induced electrical remodelling, the SND AP had a decrease in OS from 26.4 mV to 24.9 mV, a more gradual transition from phase 4 to phase 0 (DDR100, from 56.7 mV/s to 46.4 mV/s) (Fig. 2A) and a small (dV/dt)max (Fig. 2B). Compared with the normal condition, the heart rate in the SND case decreased from 74 to 60 beats/min (Fig. 2C), which was consistent with clinical findings17,64–68 (Fig. 2D).
Figure 2.
Action potentials (Vm) and the heart rate of the human sinoatrial node (SAN) cell. (A,B) Comparison of action potentials (Vm) and maximum rate of rise of membrane potential ((dV/dt)max) between normal and SND conditions. (C) AF-induced electrophysiological remodelling slowed the heart rate. (D) Simulated results are compared with the clinical findings (Barra et al.64, Mun et al.17, Chang et al.65, Hocini et al.66, Chen et al.67 and Killu et al.68) in patients with SND after catheter ablation of AF.
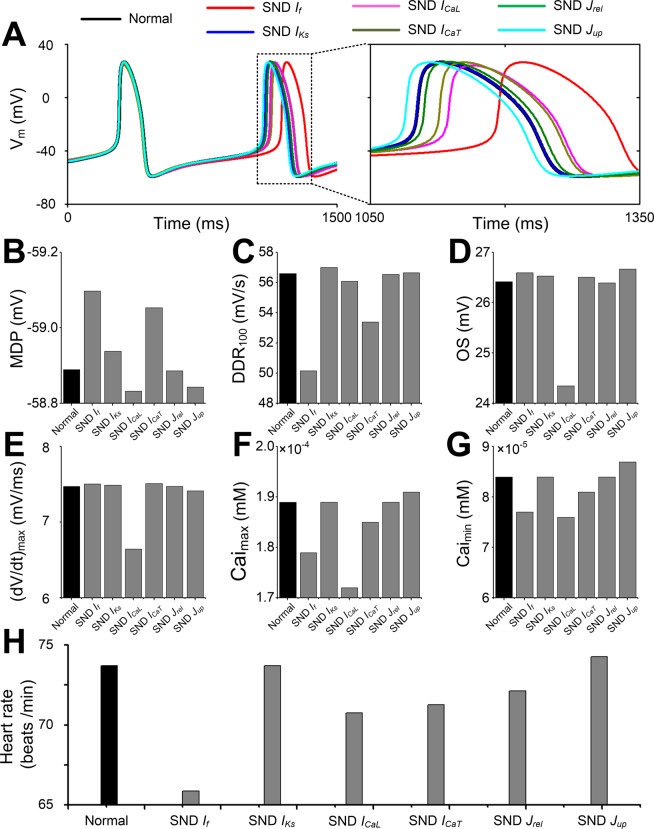
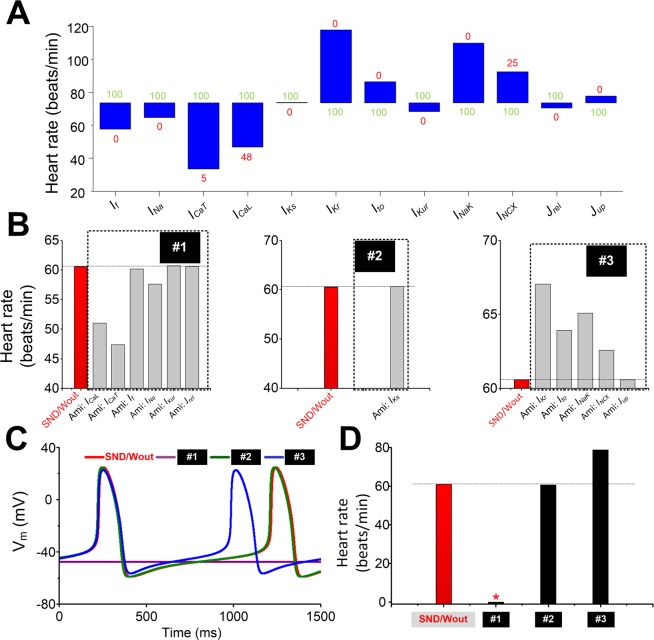
To investigate the role of each remodeled ionic current/flux under the SND condition, a series of control simulations with individual ionic remodeling were performed. AP (Fig. 3A), MDP (Fig. 3B), DDR100 (Fig. 3C), OS (Fig. 3D), (dV/dt)max (Fig. 3E), Caimax (Fig. 3F), Caimin (Fig. 3G) and heart rate (Fig. 3H) of remodeled SAN cells were compared with AP features in normal cells. A more detailed analysis of the AP features reveals that changes in MDP and Caimin were almost negligible for all remodelled conditions. DDR100 and Caimax showed a substantial decrease for the SND If condition, whereas OS, (dV/dt)max and Caimax showed a reduction for the SND ICaL condition. Of note, a significant reduction in heart rate (from 74 to 65.8 beats/min) was only observed in the SND If condition, but not under other remodeled conditions.
Figure 3.
Effects of individual remodelling targets under sinus node dysfunction (SND) on action potential (AP) and the heart rate. (A) Action potential, (B) MDP, (C) DDR100, (D) OS, (E) (dV/dt)max, (F) Caimax, (G) Caimax and (H) heart rate between normal (black) and remodelled sinoatrial node cells. Six remodelled cellular components (funny current, If; slow delayed rectifier potassium current, IKs; L-type calcium current, ICaL; T-type calcium current, ICaT, calcium flux through ryanodine receptor (Jrel) and SERCA (Jup)), are shown, respectively.
Effects of amiodarone on ionic currents/fluxes of the human sinus node
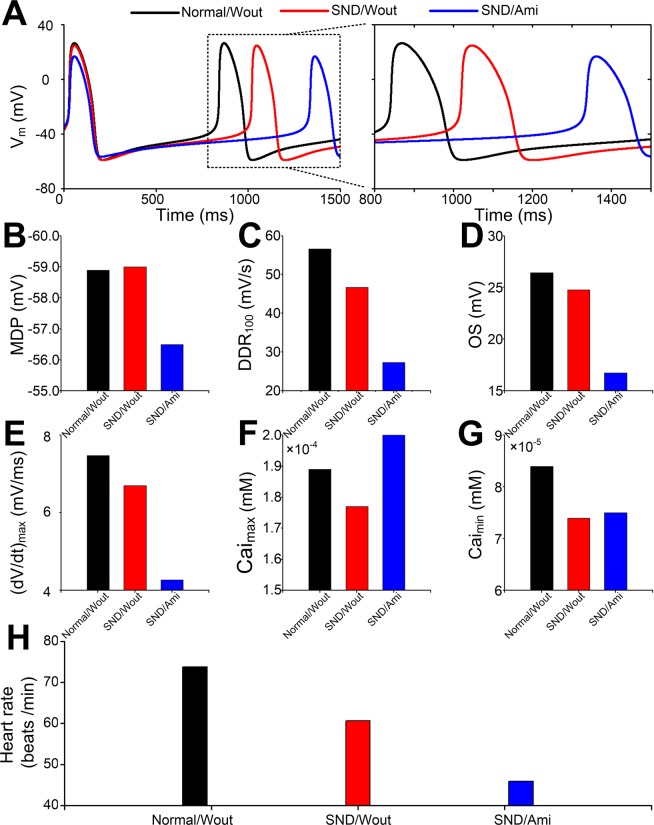
To investigate the effects of amiodarone on SAN function in patients with AF, we simulated APs (Fig. 4A) and computed heart rate using the SND model by including the actions of amiodarone on ionic currents. In the presence of amiodarone, MDP was increased from -58.99 mV for the SND condition to -56.50 mV (Fig. 4B), DDR100, OS and (dV/dt)max were reduced (Fig. 4C–E), and Caimax and Caimin were slightly increased (Fig. 4F,G). The model also predicted a decrease in the heart rate (Fig. 4H).
Figure 4.
Effects of amiodarone on action potential (AP) and the heart rate under the sinus node dysfunction (SND) condition. (A) Action potential, (B) MDP, (C) DDR100, (D) OS, (E) (dV/dt)max, (F) Caimax, (G) Caimax and (H) heart rate between normal without amiodarone (Normal/Wout), SND without amiodarone (SND/Wout) and SND with amiodarone (SND/Ami) cells.
To quantitatively assess the sensitivity of pacing rate to each target (ICaL, ICaT, If, INa, IKur, IKs, IKr, Ito, INaK, INCX, Jrel or Jup) of AF-induced remodelling and amiodarone actions, we carried out experiments by reducing each ionic current/flux from 100% to x % (the minimal value to generate AP). As is shown in Fig. 5A, a reduction in heart rate in blocking ICaL, ICaT, If, INa, IKur, or Jrel condition, an increase in heart rate in the case of inhibiting IKr, Ito, INaK, INCX or Jup, and no significant changes (i.e., changes in heart rate are no more than one beat) in heart rate under blocking IKs condition are observed (Supplementary Table 9). Based on the role of each ionic current/flux modulated by amiodarone in regulating heart rate, ionic currents were grouped into three categories: #1 (ICaL, ICaT, If, INa, IKur and Jrel), #2 (IKs) and #3 (IKr, Ito, INaK, INCX and Jup). Figure 5B showed changes in heart rate when each ionic current/flux was blocked only in the presence of 1.55 µM amiodarone. The combined effect of amiodarone on AP was further investigated in each of the groups (Fig. 5C), and the respective heart rate was 0, 60.63 and 78.81 beats/min, respectively (Fig. 5D). Thus, opposing effects were present. However, the combined actions of targets in the #1 far outweighed the effects of amiodarone on membrane targets in the #3.
Figure 5.
Effects of amiodarone on ionic currents/fluxes and consequent changes in heart rate. (A) Changes in heart rate when each ion current is reduced from 100% (green number) to x% (red number) in SAN cells. (B) Heart rate of sinus node dysfunction (SND) cells in the presence of amiodarone action on each target only. Inhibitions of ICaL, ICaT, If, INa, IKur and Jrel cause reductions in the heart rate, inhibitions of IKs have no effect on the heart rate, and inhibitions of IKr, Ito, If, INaK, INCX and Jup lead to increases in the heart rate. Group one (#1): ICaL, ICaT, If, INa, IKur and Jrel; group two (#2): IKs; and group three (#3): IKr, Ito, If, INaK, INCX and Jup. (C-D) Action potentials (APs) and the heart rate of SND cells in the presence of AMI action on targets in the group #1, #2 and #3. The Red star indicates heart arrest.
Effects of amiodarone on autonomic modulation of the human sinus node
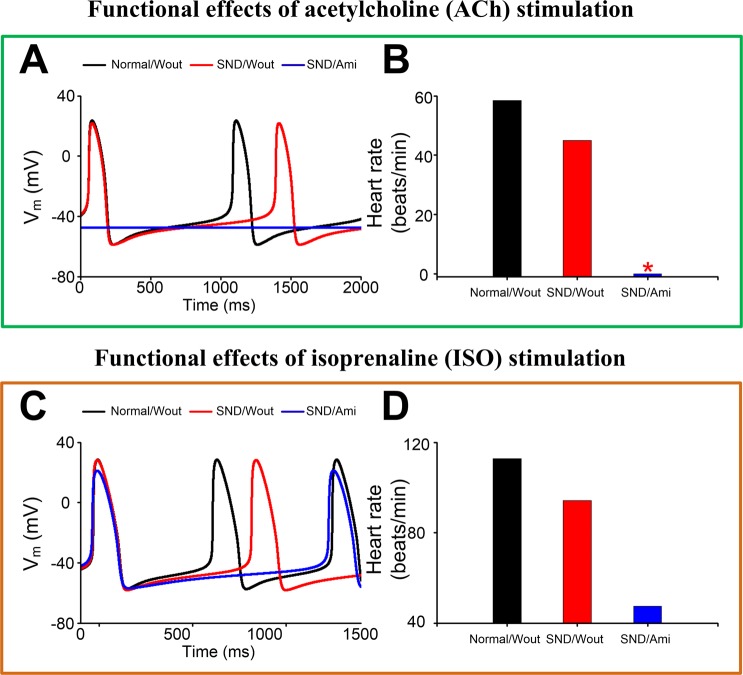
To investigate the effects of amiodarone on the autonomic modulation of human SAN in patients with AF, we simulated APs of SAN cells with ACh and ISO stimulation under the normal without amiodarone (Normal/Wout), SND/Wout and SND with amiodarone (SND/Ami) conditions. After the administration of ACh, the heart rate for Normal/Wout and SND/Wout cells was 58.40 and 45.03 beats/min, respectively. The amiodarone actions further reduced the heart rate and caused heart arrest under the SND/Ami condition (Fig. 6A,B). After the administration of ISO, the heart rate for Normal/Wout and SND/Wout cells was 112.72 and 94.23 beats/min, respectively. With the addition of amiodarone on top of ISO, the heart rate was reduced to 47.47 beats/min under the SND/Ami condition (Fig. 6C,D).
Figure 6.
Functional effects of acetylcholine (ACh) and isoprenaline (ISO) stimulation. Effects of ACh on membrane potential (A, Vm) and the heart rate (B) in the normal without amiodarone (Normal/Wout), SND/Wout and SND with amiodarone (SND/Ami) conditions. Effects of ISO on membrane potential (C, Vm) and the heart rate (D).
Effects of disopyramide, quinidine and digoxin on the human sinus node
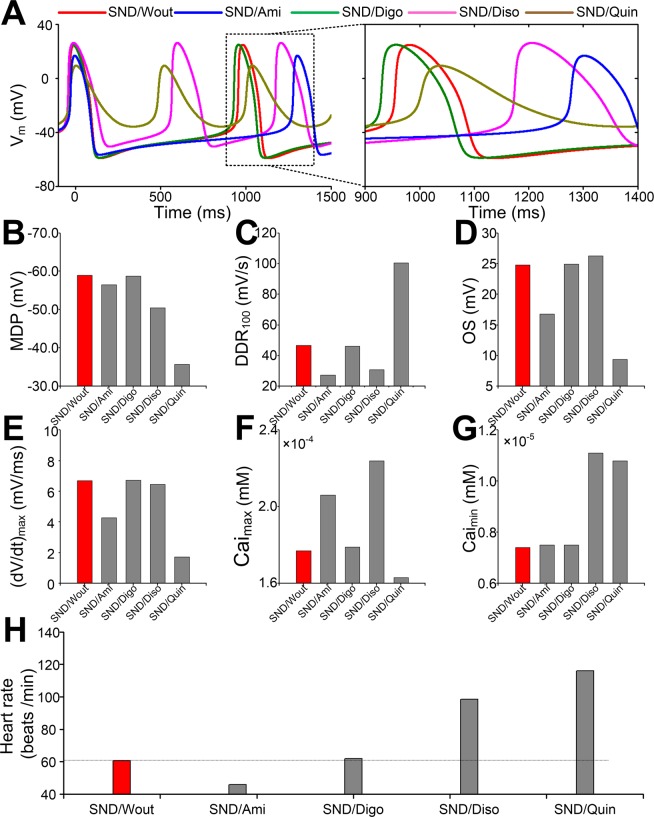
To examine whether there are antiarrhythmic drugs that have efficacy for the treatment of AF-induced SND, we investigated the effects of amiodarone (Ami), digoxin (Digo), disopyramide (Diso) and quinidine (Quin) on SAN function (Fig. 7). Compared with the drug-free SND condition (SND/Wout), there was an increase in cycle length in the presence of amiodarone, but a reduction in cycle length was observed in the presence of digoxin, quinidine and disopyramide (Fig. 7A). In the presence of disopyramide (SND/Diso), MDP, OS, Caimax and Caimin were slightly increased, whereas (dV/dt)max had almost no changes and DDR100 was slightly reduced. In the presence of quinidine (SND/Quin), MDP, DDR100 and Caimin were increased, whereas OS, (dV/dt)max and Caimax were significantly reduced. In the presence of digoxin (SND/Digo), all biomarkers had almost no changes (Fig. 7B–G). The heart rate was reduced from 60.62 to 45.94 beats/min in the presence of amiodarone, whereas the heart rate was increased from 60.62 to 61.79, 98.60 and 116.03 beats/min, respectively, in the presence of digoxin, disopyramide and quinidine (Fig. 7H).
Figure 7.
Effects of amiodarone (Ami), digoxin (Digo), disopyramide (Diso) and quinidine (Quin) on action potential (AP) and the heart rate in sinus node dysfunction (SND). (A) AP, (B) MDP, (C) DDR100, (D) OS, (E) (dV/dt)max, (F) Caimax, (G) Caimax and (H) heart rate under the SND condition (SND/Wout) are compared with SND in the presence of amiodarone (SND/Ami), digoxin (SND/Digo), disopyramide (SND/Diso) and quinidine (SND/Quin), respectively.
The concentration-dependent effects of these drugs were also investigated at the low, middle, and high concentrations. Compared with the drug-free SND condition (SND/Wout), heart arrest was observed in the presence of amiodarone (SND/Ami(High)) or quinidine (SND/Quin(High)) at the high concentration, an increase in heart rate was predicted in the presence of disopyramide, and no significant changes in heart rate were shown in the presence of digoxin (Supplementary Fig. 1).
Further simulations were conducted to examine whether these antiarrhythmic drugs have efficacy for improving the SAN function using a 1D SAN-atrial model. Compared with the normal condition (Supplementary Fig. 2A), the number of electrical waves within 5 s reduced from 7 to 5 beats under the SND condition without any drugs (Supplementary Fig. 2B). In the presence of drugs, the number of electrical waves under the SND condition showed an increase in the presence of disopyramide (Supplementary Fig. 2C), no significant changes in the presence of digoxin (Supplementary Fig. 2D), a reduction in the presence of amiodarone (Supplementary Fig. 2E) and SAN arrest in the presence of quinidine (Supplementary Fig. 2F).
Discussion
In the present study, we formulated a human AP model of AF-induced SND and investigated the impact of amiodarone on human SAN function. We further assessed whether other drugs (i.e., disopyramide, quinidine and digoxin) could reverse the AF-induced SND phenotype. The major findings of this study are as follows: (1) the AF-induced SND can be mainly attributed to down-regulation of If; (2) the effects of amiodarone lead to a lower DDR100 and more prolonged diastolic depolarization phase, resulting in a slower pacemaking rate and contributing to the impact of amiodarone on human SAN function; (3) the bradycardiac effects of amiodarone are likely to be amplified by vagal nerve activity (simulated addition of ACh to the SND cells with amiodarone causes SAN arrest); and (4) our model predicted an increase in pacemaking rate in the presence of disopyramide. Together, these data point to voltage-clock dysfunction underlying SND and provide evidence substantiating the impact of amiodarone on the function of the SAN.
The leading causes of bradycardia under the AF-induced SND are electrophysiological remodelling related to the voltage clock of the human SAN. Previous experiments on canine SAN cells have demonstrated that AF-induced remodelling of ion channels, particularly for the “pacemaker” subunit If, may contribute to the clinically significant association between SND and AF6. Our simulated effects of remodelled If are concordant with this experimental findings33. In additional to remodelled If, changes in IKs, ICaL and ICaT were observed in SAN cells of the pacing-induced AF canine model69. We assumed that a similar ionic remodelling may also occur in human AF-induced SND. The combined effect of the electrical remodelling slowed down the heart rate significantly. The reduction in heart rate was mainly the result of a lower DDR100 arising from the downregulation of inward currents (including If, ICaL and ICaT). And remodelled IKs has a negligible effect on DDR100 and the pacemaking rate. These findings agree with previous work showing the strong contributions of If, ICaL and ICaT to DDR100 and the pacing rate27. The sensitivity analysis highlighted the strong impact of ICaL on heart rate. 52% block of ICaL is able to lead to heart arrest, whereas 95% block of ICaT results in hart arrest and a slow heart rate is obtained with 100% block of If (Fig. 5A). The illustrative investigation of the effects of changes in maximal conductances of ionic current suggests that ICaL plays a role in pacemaking, which is consistent with the sensitivity analysis by Fabbri et al.27. Under the SND condition, ICaL, ICaT and If were decreased to 90%, 92% and 50%, respectively (Fig. 1B). Remodelled ICaL and ICaT caused a reduction in heart rate from 74 to 70.7 and 71.2 beats/min, respectively, whereas remodelled If led to a slower heart rate from 74 to 65.8 beats/min. Therefore, the reduced If under the SND condition mainly contributed to the heart rate reduction.
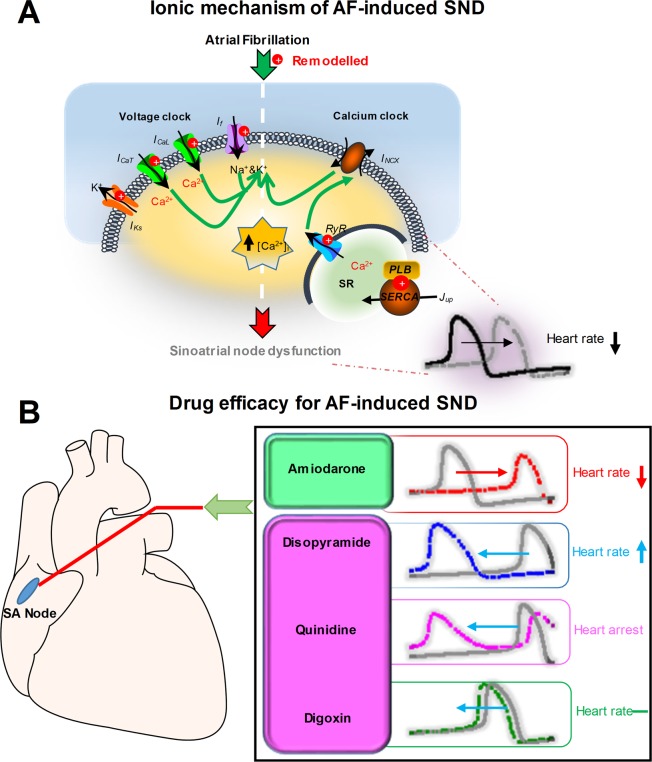
Previous experiments have shown that AF-induced SND is also associated with calcium handling abnormalities. Here, we also investigated the effects of calcium handling properties on SAN automaticity. AF-induced changes in calcium handling (the downregulation of Jrel and Jup) in our human SAN model are similar to experimental data from a canine model of pacing-induced AF9. Downregulation of Jrel could decrease the pacemaking rate (from 73.7 to 72.1 beats/min) which is consistent with experimental observations9, whereas remodelled Jup could increase heart rate (from 73.7 to 74.2 beats/min) which is a good agreement with previous modelling study27. However, changes in the pacemaking rate due to calcium handling abnormalities were almost negligible. Altogether, our simulation results indicate that voltage- clock malfunction might be the mechanism underlying AF-induced SND (Fig. 8A) and our SND mathematical model for human SAN cells can be useful in the design of experiments and the development of drugs.
Figure 8.
Ionic mechanisms of atrial fibrillation (AF)-induced sinoatrial node dysfunction (SND) and drug efficacy for AF-induced SND. (A) Atrial fibrillation-induced electrical remodelling includes If, ICaL, ICaT, IKs, Jrel and Jup. If, ICaL and ICaT in combination may be responsible for the diastolic depolarization, the so-called “voltage clock”. Diastolic spontaneous calcium release via RyR increases the calcium concentration ([Ca2+]i) and activates sodium-calcium exchanger. The inward INCX contributes to the diastolic depolarization, the so-called “calcium clock”. Therefore, AF-induced electrical remodelling impairs both the voltage clock and the calcium clock, resulting in the heart rate reduction and SND. (B) Under the AF-induced SND condition, the action of amiodarone impairs sinoatrial node function, leading to a reduction in the sinus rate, whereas disopyramide may improve sinoatrial node function and increase the sinus rate.
Amiodarone is the most frequently used agent to reverse AP shortening (Supplementary Fig. 3) in patients with AF, which is partly resulted from electrical remodelling70 (Supplementary Table 10). However, computational drug treatment simulations predicted a dramatical reduction in the pacemaking rate, indicating the impact of amiodarone on the SAN function. These findings agree with the previous work showing amiodarone-induced bradycardia in AF patients71. These changes in the pacemaking rate may be attributable to the effects of amiodarone on multiple ion channels and beta-adrenergic receptors. Inhibition of INa, IKur, ICaL, ICaT, If, Jrel and beta-adrenergic receptors leads to a slower heart rate, whereas the block of IKr, Ito, IKACh, INaK, INCX and Jup causes a higher heart rate. Thus, opposing effects were present. However, deceleration effects of amiodarone far outweighed its acceleration effects, leading to a slow pacemaking rate. In addition, the slow heart rate since the actions of amiodarone on ICaL in the current study in accordance with the work of Nattel et al.72, suggesting that changes in SAN function can be attributable to amiodarone’s calcium channel-blocking properties and account for the adverse consequence of amiodarone.
Our study substantiates the notion that beta-adrenergic blocking effects of amiodarone may explain the unresponsiveness of SAN to sympathetic stimulation in AF patients17. Reports of AF patients suggest that amiodarone causes SND, which results in reduced P-wave amplitude at baseline and during ISO infusion. The anti-adrenergic effects of amiodarone showed a reduction of receptor density in the cellular membrane, suggesting that amiodarone leads to the unresponsiveness of SAN to ISO stimulation73. Moreover, the overall pacing rate acceleration because of the administration of 1 µM ISO was the result of a balance between opposing contributors. Previous modelling studies have demonstrated that in five targets (If, ICaL, INaK, IKs and Jup) ISO-induced changes occur in If and ICaL, leading to a faster heart rate27. However, amiodarone has block effects on both If and ICaL. And our simulated results show that the SAN pacing rate decreases in the presence of amiodarone. These studies and our results suggest that amiodarone causes bradycardia by partly inhibiting If, ICaL and beta-adrenergic receptors in the human SAN. Thus, our results suggest that amiodarone cannot be used safely in patients that have SND associated with AF. We further investigated effects of quinidine, disopyramide and digoxin on the function of the SAN under the AF-induced SND condition and simulated results demonstrated that disopyramide was necessary to considerably increase the heart rate (Fig. 8B).
Several limitations specific to this study are addressed here. Firstly, the electrophysiological representation of AF-induced remodelling in the human SND model is based on data from previous canine models of AF6,9, however, because of the lack of experimental data on humans. Special attention must be paid to the differences between canine and human sinoatrial node74. Secondly, the blocking effects of drugs on ionic currents were modelled using the total plasma concentrations of drugs, nH and IC50 in the present study. However, drug efficacy needs to be related to free drug concentrations, not the total plasma concentrations75. Special attention should be paid to explain our simulated results, and free drug concentrations of drugs in plasma should be used to further assess the efficacy of drugs in the treatment of AF-induced SND. In addition, the large variability in IC50 was observed for most of the drugs, including amiodarone76. In a previous study, differential responses of ventricular and atrial ion channels to antiarrhythmic drugs were observed77. In the present study, IC50 values are chosen based on experimental data from atrial cells (where data are available) and large ventricular cells (where atrial data are not available). Thirdly, the uncertainty analysis is important for a more precise evaluation of the safety of antiarrhythmic drugs25,78. Therefore, our models and methodology should be improved, and statistics and treatment of uncertainties should be considered78–80 and further investigated. Fourthly, the block effects of amiodarone on beta-adrenergic receptors were modelled with the same percentage decrease in the effects of beta-adrenergic receptor stimulation on targets. The strict linearity is unlikely and the modelling approach should be improved based on experimental data. Fifthly, simulated results demonstrated that inhibition of IKr increases heart rate, but experimental studies showed that dofetilide (an IKr blocker) depolarized the maximum diastolic potentials, reduced the slope of the pacemaker potential and then abolished spontaneously firing action potentials in the nodal cells, suggesting IKr blockers slowed spontaneous activity81,82. The IKr model of Fabbri et al. model should be modified and verified based on these experimental data. Finally, the coupling between calcium clock and voltage clock is limited in the Fabbri et al. model, but the coupled-clock mechanism was established in two mathematical models by Kharche et al.83, and Maltsev and Lakatta84. Therefore, the Fabbri et al. model should be improved and calcium clock under the AF-induced SND condition should be further investigated.
In this study, we presented a simulation study investigating the effects of the antiarrhythmic agents in the setting of AF-induced SND. On the basis of the simulations, it can be concluded that amiodarone may impair the function of the SAN by slowing the heart rate whereas it has an antiarrhythmic effect on AF by prolonging AP. Additionally, disopyramide can reverse the AF-induced SND phenotype by increasing the heart rate. Therefore, disopyramide may be a desirable choice under the AF-induced SND condition.
Supplementary information
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 61901192) (J.B.),the National Key R&D Program of China (No.2019YFC0120100 and 2019YFC0121900) (J.B. and Y.L.) and the Science and Technology Planning Project of Guangdong Province (No.2015B020214004 and No.2015B020233010) (Y.L.).
Author contributions
J.B. conceived and designed the study. J.B. conducted the experiments. J.B., Y.L. and H.Z. drafted the manuscript, interpreted the data, and reviewed, revised and approved the final version of this manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Jieyun Bai, Email: bai_jieyun@126.com.
Henggui Zhang, Email: henggui.zhang@manchester.ac.uk.
Supplementary information
is available for this paper at 10.1038/s41598-019-57246-5.
References
- 1.Lamas GA, et al. The mode selection trial (MOST) in sinus node dysfunction: design, rationale, and baseline characteristics of the first 1000 patients. American heart journal. 2000;140:541–551. doi: 10.1067/mhj.2000.109652. [DOI] [PubMed] [Google Scholar]
- 2.Monfredi O, Boyett M. Sick sinus syndrome and atrial fibrillation in older persons—a view from the sinoatrial nodal myocyte. Journal of molecular and cellular cardiology. 2015;83:88–100. doi: 10.1016/j.yjmcc.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 3.Zhao J, Liu T, Li G. Relationship Between Two Arrhythmias: Sinus Node Dysfunction and Atrial Fibrillation. Archives of Medical Research. 2014;45:351–355. doi: 10.1016/j.arcmed.2014.04.005. [DOI] [PubMed] [Google Scholar]
- 4.Hadian D, Zipes DP, Olgin JE, Miller JM. Short‐term rapid atrial pacing produces electrical remodeling of sinus node function in Humans. Journal of cardiovascular electrophysiology. 2002;13:584–586. doi: 10.1046/j.1540-8167.2002.00584.x. [DOI] [PubMed] [Google Scholar]
- 5.Fedorov VV, et al. Complex interactions between the sinoatrial node and atrium during reentrant arrhythmias in the canine heart. Circulation. 2010;122:782–789. doi: 10.1161/CIRCULATIONAHA.109.935288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yeh Y-H, et al. Funny current downregulation and sinus node dysfunction associated with atrial tachyarrhythmia: a molecular basis for tachycardia-bradycardia syndrome. Circulation. 2009;119:1576–1585. doi: 10.1161/CIRCULATIONAHA.108.789677. [DOI] [PubMed] [Google Scholar]
- 7.John RM, Kumar S. Sinus node and atrial arrhythmias. Circulation. 2016;133:1892–1900. doi: 10.1161/CIRCULATIONAHA.116.018011. [DOI] [PubMed] [Google Scholar]
- 8.Joung B, Chen P-S, Lin S-F. The role of the calcium and the voltage clocks in sinoatrial node dysfunction. Yonsei medical journal. 2011;52:211–219. doi: 10.3349/ymj.2011.52.2.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Joung B, et al. Mechanisms of sinoatrial node dysfunction in a canine model of pacing-induced atrial fibrillation. Heart Rhythm. 2010;7:88–95. doi: 10.1016/j.hrthm.2009.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shinagawa K, Shiroshita-Takeshita A, Schram G, Nattel S. Effects of antiarrhythmic drugs on fibrillation in the remodeled atrium: insights into the mechanism of the superior efficacy of amiodarone. Circulation. 2003;107:1440–1446. doi: 10.1161/01.CIR.0000055316.35552.74. [DOI] [PubMed] [Google Scholar]
- 11.Roy D, et al. Amiodarone to prevent recurrence of atrial fibrillation. New England Journal of Medicine. 2000;342:913–920. doi: 10.1056/NEJM200003303421302. [DOI] [PubMed] [Google Scholar]
- 12.Kodama I, Kamiya K, Toyama J. Amiodarone: ionic and cellular mechanisms of action of the most promising class III agent. American Journal of Cardiology. 1999;84:20–28. doi: 10.1016/S0002-9149(99)00698-0. [DOI] [PubMed] [Google Scholar]
- 13.Kondratyeva D, Afanasiev S, Popov S, Batalov R. Amiodarone Modulation of Intracellular Transport of Calcium Ions in Cardiomyocites. Pharmacology & Pharmacy. 2012;3:307–315. doi: 10.4236/pp.2012.33041. [DOI] [Google Scholar]
- 14.Essebag V, Hadjis T, Platt RW, Abrahamowicz M, Pilote L. Effect of amiodarone dose on the risk of permanent pacemaker insertion. Pacing and clinical electrophysiology. 2004;27:1519–1525. doi: 10.1111/j.1540-8159.2004.00670.x. [DOI] [PubMed] [Google Scholar]
- 15.Touboul P, Atallah G, Gressard A, Kirkorian G. Effects of amiodarone on sinus node in man. Heart. 1979;42:573–578. doi: 10.1136/hrt.42.5.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoffmann A, Jost M, Burckhardt D, Kappenberger L. Effect of amiodarone on sinus node function in patients with sick sinus syndrome. Clinical cardiology. 1987;10:451–452. doi: 10.1002/clc.4960100808. [DOI] [PubMed] [Google Scholar]
- 17.Mun H-S, et al. Chronic amiodarone therapy impairs the function of the superior sinoatrial node in patients with atrial fibrillation. Circulation Journal. 2013;77:2255–2263. doi: 10.1253/circj.CJ-12-1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li Z, et al. Assessment of an In Silico Mechanistic Model for Proarrhythmia Risk Prediction Under the Ci PA Initiative. Clinical Pharmacology & Therapeutics. 2018;105:466–475. doi: 10.1002/cpt.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jie-Yun B, Kuan-Quan W, Heng-Gui Z. Potential pathogenesis discovery of arrhythmia based on cardiac electrophysiological models: research progress. Progress in Biochemistry and Biophysics. 2016;43:128–140. [Google Scholar]
- 20.Bai, J. et al. In silico investigation mechanisms underlying atrial fibrillation due to impaired Pitx2. PLoS computational biologyIn Press (2019). [DOI] [PMC free article] [PubMed]
- 21.Passini E, et al. Human in silico drug trials demonstrate higher accuracy than animal models in predicting clinical pro-arrhythmic cardiotoxicity. Frontiers in physiology. 2017;8:668. doi: 10.3389/fphys.2017.00668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bottino D, et al. Preclinical cardiac safety assessment of pharmaceutical compounds using an integrated systems-based computer model of the heart. Progress in biophysics and molecular biology. 2006;90:414–443. doi: 10.1016/j.pbiomolbio.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 23.Beattie KA, et al. Evaluation of an in silico cardiac safety assay: using ion channel screening data to predict QT interval changes in the rabbit ventricular wedge. Journal of pharmacological and toxicological methods. 2013;68:88–96. doi: 10.1016/j.vascn.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Davies M, et al. An in silico canine cardiac midmyocardial action potential duration model as a tool for early drug safety assessment. American Journal of Physiology-Heart and Circulatory Physiology. 2012;302:H1466–1480. doi: 10.1152/ajpheart.00808.2011. [DOI] [PubMed] [Google Scholar]
- 25.Fermini B, et al. A new perspective in the field of cardiac safety testing through the comprehensive in vitro proarrhythmia assay paradigm. Journal of biomolecular screening. 2016;21:1–11. doi: 10.1177/1087057115594589. [DOI] [PubMed] [Google Scholar]
- 26.Romero L, et al. In Silico QT and APD Prolongation Assay for Early Screening of Drug-Induced Proarrhythmic Risk. Journal of chemical information and modeling. 2018;58:867–878. doi: 10.1021/acs.jcim.7b00440. [DOI] [PubMed] [Google Scholar]
- 27.Fabbri A, Fantini M, Wilders R, Severi S. Computational analysis of the human sinus node action potential: model development and effects of mutations. The Journal of physiology. 2017;595:2365–2396. doi: 10.1113/JP273259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Heijman, J., Heusch, G. & Dobrev, D. Pleiotropic effects of antiarrhythmic agents: dronedarone in the treatment of atrial fibrillation. Clinical Medicine Insights: Cardiology, 7, CMC. S8445 (2013). [DOI] [PMC free article] [PubMed]
- 29.Fan X, et al. Novel electropharmacological activity of amiodarone on human HCN channels heterologously expressed in the Xenopus oocytes. European journal of pharmacology. 2011;669:15–23. doi: 10.1016/j.ejphar.2011.07.039. [DOI] [PubMed] [Google Scholar]
- 30.Lalevée N, Barrère‐lemaire S, Gautier P, Nargeot J, Richard S. Effects of Amiodarone and Dronedarone on Voltage‐Dependent Sodium Current in Human Cardiomyocytes. Journal of cardiovascular electrophysiology. 2003;14:885–890. doi: 10.1046/j.1540-8167.2003.03064.x. [DOI] [PubMed] [Google Scholar]
- 31.Yamashita N, et al. Short-and long-term amiodarone treatments regulate Cav3. 2 low-voltage-activated T-type Ca2+ channel through distinct mechanisms. Molecular pharmacology. 2006;69:1684–1691. doi: 10.1124/mol.105.021253. [DOI] [PubMed] [Google Scholar]
- 32.Varró A, Virág L, Papp JG. Comparison of the chronic and acute effects of amiodarone on the calcium and potassium currents in rabbit isolated cardiac myocytes. British journal of pharmacology. 1996;117:1181–1186. doi: 10.1111/j.1476-5381.1996.tb16713.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kamiya K, et al. Short-and long-term effects of amiodarone on the two components of cardiac delayed rectifier K+ current. Circulation. 2001;103:1317–1324. doi: 10.1161/01.CIR.103.9.1317. [DOI] [PubMed] [Google Scholar]
- 34.Kobayashi S, et al. Inhibitory effect of bepridil on hKv1. 5 channel current: comparison with amiodarone and E-4031. European journal of pharmacology. 2001;430:149–157. doi: 10.1016/S0014-2999(01)01381-4. [DOI] [PubMed] [Google Scholar]
- 35.Watanabe Y, Hara Y, Tamagawa M, Nakaya H. Inhibitory effect of amiodarone on the muscarinic acetylcholine receptor-operated potassium current in guinea pig atrial cells. Journal of Pharmacology and Experimental Therapeutics. 1996;279:617–624. [PubMed] [Google Scholar]
- 36.Watanabe Y, Kimura J. Inhibitory effect of amiodarone on Na+/Ca2+ exchange current in guinea‐pig cardiac myocytes. British journal of pharmacology. 2000;131:80–84. doi: 10.1038/sj.bjp.0703527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gray DF, et al. Amiodarone inhibits the Na+-K+ pump in rabbit cardiac myocytes after acute and chronic treatment. Journal of Pharmacology and Experimental Therapeutics. 1998;284:75–82. [PubMed] [Google Scholar]
- 38.Podrid PJ. Amiodarone: reevaluation of an old drug. Annals of internal medicine. 1995;122:689–700. doi: 10.7326/0003-4819-122-9-199505010-00008. [DOI] [PubMed] [Google Scholar]
- 39.Sauro SC, DeCarolis DD, Pierpont GL, Gornick CC. Comparison of plasma concentrations for two amiodarone products. Annals of Pharmacotherapy. 2002;36:1682–1685. doi: 10.1345/aph.1A403. [DOI] [PubMed] [Google Scholar]
- 40.Loewe A, et al. In-silico assessment of the dynamic effects of amiodarone and dronedarone on human atrial patho-electrophysiology. Europace. 2014;16:iv30–iv38. doi: 10.1093/europace/euu230. [DOI] [PubMed] [Google Scholar]
- 41.PRITCHETT EL. Evolution and revolution in drug labeling: regulation of antiarrhythmic drugs by the Food and Drug Administration 1962–1996. Pacing and clinical electrophysiology. 1998;21:1457–1469. doi: 10.1111/j.1540-8159.1998.tb00218.x. [DOI] [PubMed] [Google Scholar]
- 42.Vassallo P, Trohman RG. Prescribing amiodarone: an evidence-based review of clinical indications. Jama. 2007;298:1312–1322. doi: 10.1001/jama.298.11.1312. [DOI] [PubMed] [Google Scholar]
- 43.Redfern W, et al. Relationships between preclinical cardiac electrophysiology, clinical QT interval prolongation and torsade de pointes for a broad range of drugs: evidence for a provisional safety margin in drug development. Cardiovascular research. 2003;58:32–45. doi: 10.1016/S0008-6363(02)00846-5. [DOI] [PubMed] [Google Scholar]
- 44.Koch-Weser J. Disopyramide. New England Journal of Medicine. 1979;300:957–962. doi: 10.1056/NEJM197904263001705. [DOI] [PubMed] [Google Scholar]
- 45.Yan M, et al. Stereoselective blockage of quinidine and quinine in the hERG channel and the effect of their rescue potency on drug-induced hERG trafficking defect. International journal of molecular sciences. 2016;17:1648. doi: 10.3390/ijms17101648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Goldberger ZD, Goldberger AL. Therapeutic ranges of serum digoxin concentrations in patients with heart failure. American Journal of Cardiology. 2012;109:1818–1821. doi: 10.1016/j.amjcard.2012.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bai J, Gladding PA, Stiles MK, Fedorov VV, Zhao J. Ionic and cellular mechanisms underlying TBX5/PITX2 insufficiency-induced atrial fibrillation: Insights from mathematical models of human atrial cells. Scientific reports. 2018;8:15642. doi: 10.1038/s41598-018-33958-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bai J, Wang K, Li Q, Yuan Y, Zhang H. Pro-arrhythmogenic effects of CACNA1C G1911R mutation in human ventricular tachycardia: insights from cardiac multi-scale models. Scientific reports. 2016;6:31262. doi: 10.1038/srep31262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bai J, et al. Computational cardiac modeling reveals mechanisms of ventricular arrhythmogenesis in long QT syndrome type 8: CACNA1C R858H mutation linked to ventricular fibrillation. Frontiers in physiology. 2017;8:771. doi: 10.3389/fphys.2017.00771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bai J, Yin R, Wang K, Zhang H. Mechanisms underlying the emergence of post-acidosis arrhythmia at the tissue level: A theoretical study. Frontiers in physiology. 2017;8:195. doi: 10.3389/fphys.2017.00195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liu H, Bai J, Wang K, Li Q, Yuan Y. Simulation study of ventricular arrhythmia in post acidosis. Prog. Biochem. Biophys. 2016;43:716–724. [Google Scholar]
- 52.Bai J, Lu Y, Lo AC, Zhao J, Zhang H. Proarrhythmia in the p. Met207Val PITX2c-linked familial atrial fibrillation-insights from modelling. Frontiers in Physiology. 2019;10:1314. doi: 10.3389/fphys.2019.01314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sossalla S, et al. Altered Na+ currents in atrial fibrillation: effects of ranolazine on arrhythmias and contractility in human atrial myocardium. Journal of the American College of Cardiology. 2010;55:2330–2342. doi: 10.1016/j.jacc.2009.12.055. [DOI] [PubMed] [Google Scholar]
- 54.Caballero R, et al. In humans, chronic atrial fibrillation decreases the transient outward current and ultrarapid component of the delayed rectifier current differentially on each atria and increases the slow component of the delayed rectifier current in both. Journal of the American College of Cardiology. 2010;55:2346–2354. doi: 10.1016/j.jacc.2010.02.028. [DOI] [PubMed] [Google Scholar]
- 55.Dobrev D, Ravens U. Remodeling of cardiomyocyte ion channels in human atrial fibrillation. Basic research in cardiology. 2003;98:137–148. doi: 10.1007/s00395-003-0409-8. [DOI] [PubMed] [Google Scholar]
- 56.Li G, Nattel S. Properties of human atrial ICa at physiological temperatures and relevance to action potential. American Journal of Physiology-Heart and Circulatory Physiology. 1997;272:H227–H235. doi: 10.1152/ajpheart.1997.272.1.H227. [DOI] [PubMed] [Google Scholar]
- 57.Christ T, et al. L-type Ca2+ current downregulation in chronic human atrial fibrillation is associated with increased activity of protein phosphatases. Circulation. 2004;110:2651–2657. doi: 10.1161/01.CIR.0000145659.80212.6A. [DOI] [PubMed] [Google Scholar]
- 58.Van Wagoner DR, et al. Atrial L-type Ca2+ currents and human atrial fibrillation. Circulation research. 1999;85:428–436. doi: 10.1161/01.RES.85.5.428. [DOI] [PubMed] [Google Scholar]
- 59.Schotten U, et al. Atrial fibrillation-induced atrial contractile dysfunction: a tachycardiomyopathy of a different sort. Cardiovascular research. 2002;53:192–201. doi: 10.1016/S0008-6363(01)00453-9. [DOI] [PubMed] [Google Scholar]
- 60.Wang J, et al. Regional expression of sodium pump subunits isoforms and Na+-Ca++ exchanger in the human heart. The Journal of clinical investigation. 1996;98:1650–1658. doi: 10.1172/JCI118960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Voigt N, et al. Cellular and molecular mechanisms of atrial arrhythmogenesis in patients with paroxysmal atrial fibrillation. Circulation. 2014;129:145–156. doi: 10.1161/CIRCULATIONAHA.113.006641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bokník P, et al. Regional expression of phospholamban in the human heart. Cardiovascular research. 1999;43:67–76. doi: 10.1016/S0008-6363(99)00053-X. [DOI] [PubMed] [Google Scholar]
- 63.Zhang H, et al. Mathematical models of action potentials in the periphery and center of the rabbit sinoatrial node. American Journal of Physiology-Heart and Circulatory Physiology. 2000;279:H397–H421. doi: 10.1152/ajpheart.2000.279.1.H397. [DOI] [PubMed] [Google Scholar]
- 64.Barra, S. et al. Acute and sub-acute sinus node dysfunction following pulmonary vein isolation: a case series. European Heart Journal-Case Reports (2018). [DOI] [PMC free article] [PubMed]
- 65.Chang H-Y, et al. Sinus node dysfunction in atrial fibrillation patients: the evidence of regional atrial substrate remodelling. Europace. 2012;15:205–211. doi: 10.1093/europace/eus219. [DOI] [PubMed] [Google Scholar]
- 66.Hocini M, et al. Reverse remodeling of sinus node function after catheter ablation of atrial fibrillation in patients with prolonged sinus pauses. Circulation. 2003;108:1172–1175. doi: 10.1161/01.CIR.0000090685.13169.07. [DOI] [PubMed] [Google Scholar]
- 67.CHEN YW, et al. Pacing or Ablation: Which Is Better for Paroxysmal Atrial Fibrillation‐Related Tachycardia‐Bradycardia Syndrome? Pacing and Clinical Electrophysiology. 2014;37:403–411. doi: 10.1111/pace.12340. [DOI] [PubMed] [Google Scholar]
- 68.Killu AM, et al. Acute sinus node dysfunction after atrial ablation: incidence, risk factors, and management. Pacing and Clinical Electrophysiology. 2016;39:1116–1125. doi: 10.1111/pace.12934. [DOI] [PubMed] [Google Scholar]
- 69.Sanchez-Chapula JA. Mechanism of transient outward K+ channel block by disopyramide. Journal of Pharmacology and Experimental Therapeutics. 1999;290:515–523. [PubMed] [Google Scholar]
- 70.Grandi E, et al. Human atrial action potential and Ca2+ model: sinus rhythm and chronic atrial fibrillation. Circulation research. 2011;109:1055–1066. doi: 10.1161/CIRCRESAHA.111.253955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ovsyshcher IE, Barold SS. Drug induced bradycardia: To pace or not to pace? Pacing and clinical electrophysiology. 2004;27:1144–1147. doi: 10.1111/j.1540-8159.2004.00597.x. [DOI] [PubMed] [Google Scholar]
- 72.Nattel S, Talajic M, Quantz M, DeRoode M. Frequency-dependent effects of amiodarone on atrioventricular nodal function and slow-channel action potentials: evidence for calcium channel-blocking activity. Circulation. 1987;76:442–449. doi: 10.1161/01.CIR.76.2.442. [DOI] [PubMed] [Google Scholar]
- 73.Celestino D, Medei E, Moro S, Elizari MV, Sicouri S. Acute in vitro effects of dronedarone, an iodine-free derivative, and amiodarone, on the rabbit sinoatrial node automaticity: a comparative study. Journal of cardiovascular pharmacology and therapeutics. 2007;12:248–257. doi: 10.1177/1074248407303225. [DOI] [PubMed] [Google Scholar]
- 74.Kalyanasundaram A, Li N, Hansen B, Zhao J, Fedorov V. Canine and human sinoatrial node: differences and similarities in the structure, function, molecular profiles, and arrhythmia. Journal of veterinary cardiology. 2019;22:2–19. doi: 10.1016/j.jvc.2018.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gonzalez D, Schmidt S, Derendorf H. Importance of relating efficacy measures to unbound drug concentrations for anti-infective agents. Clinical microbiology reviews. 2013;26:274–288. doi: 10.1128/CMR.00092-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kramer J, et al. MICE models: superior to the HERG model in predicting Torsade de Pointes. Scientific reports. 2013;3:2100. doi: 10.1038/srep02100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cheng H, Cannell MB, Hancox JC. Differential responses of rabbit ventricular and atrial transient outward current (Ito) to the Ito modulator NS5806. Physiological reports. 2017;5:e13172. doi: 10.14814/phy2.13172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ni, H., Morotti, S. & Grandi, E. A heart for diversity: simulating variability in cardiac arrhythmia research. Frontiers in physiology, 9 (2018). [DOI] [PMC free article] [PubMed]
- 79.Lee Y-S, Liu OZ, Sobie EA. Decoding myocardial Ca2+ signals across multiple spatial scales: a role for sensitivity analysis. Journal of molecular and cellular cardiology. 2013;58:92–99. doi: 10.1016/j.yjmcc.2012.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lee Y-S, Liu OZ, Hwang HS, Knollmann BC, Sobie EA. Parameter sensitivity analysis of stochastic models provides insights into cardiac calcium sparks. Biophysical journal. 2013;104:1142–1150. doi: 10.1016/j.bpj.2012.12.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lei M, Brown HF. Two components of the delayed rectifier potassium current, IK, in rabbit sino‐atrial node cells. Experimental physiology. 1996;81:725–741. doi: 10.1113/expphysiol.1996.sp003972. [DOI] [PubMed] [Google Scholar]
- 82.Tohse N, Kanno M. Effects of dofetilide on membrane currents in sinoatrial node cells of rabbit. The Japanese Journal of Pharmacology. 1995;69:303–309. doi: 10.1254/jjp.69.303. [DOI] [PubMed] [Google Scholar]
- 83.Kharche S, Yu J, Lei M, Zhang H. A mathematical model of action potentials of mouse sinoatrial node cells with molecular bases. American Journal of Physiology-Heart and circulatory physiology. 2011;301:H945–963. doi: 10.1152/ajpheart.00143.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Maltsev VA, Lakatta EG. Synergism of coupled subsarcolemmal Ca2 clocks and sarcolemmal voltage clocks confers robust and flexible pacemaker function in a novel pacemaker cell model. Am J Physiol Heart Circ Physiol. 2009;296:H561–H562. doi: 10.1152/ajpheart.01118.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.