Abstract
This systematic review evaluated the effectiveness of strategies to identify and manage patients with familial risk of breast, ovarian, colorectal and prostate cancer in primary care to improve clinical outcomes. MEDLINE, EMBASE, CINAHL and Cochrane library were searched from January 1980 to October 2017. We included randomised controlled trials (RCT) and non-randomised studies of interventions (NRSI). Primary outcomes were cancer incidence, cancer-related clinical outcomes or the identification of cancer predisposition; secondary outcomes were the appropriateness of referral, uptake of preventive strategies and cognitive and psychological effect. From 11,842 abstracts, 111 full texts were reviewed and three eligible studies (nine articles) identified. Two were cluster RCTs and one NRSI; all used risk assessment software. No studies identified our primary outcomes, with no consistent outcome across the three studies. In one RCT, intervention improved the proportion of genetic referrals meeting referral guidelines for breast cancer (OR 4.5, 95% CI 1.6 to 13.1). In the other RCT, there was no difference in screening adherence between the intervention and control group. However, there was borderline increased risk perception (OR 1.89, 95% CI 0.99 to 3.59) in the subgroup that under-estimated their colon cancer risk. In the NRSI, there was no change in psychological distress in patients at increased familial breast cancer risk, but population risk patients had reduced anxiety after intervention (state anxiety mean change − 3, 95% CI − 5 to − 2). Future studies should have better-defined comparator groups and longer follow-up and assess outcomes using validated tools.
Electronic supplementary material
The online version of this article (10.1007/s12687-019-00419-6) contains supplementary material, which is available to authorized users.
Keywords: Primary health care, Genetic predisposition to disease, Breast neoplasm, Ovarian neoplasms, Colorectal neoplasms, Prostatic neoplasms
Introduction
Familial cancer risk increases an individual’s life time chance of developing cancer and at an earlier age of onset (Kerber and O'Brien 2005; Paluch-Shimon et al. 2016; Qureshi et al. 2009). A Swedish Cancer Registry study found that cancers with the highest familial proportions (proportion of cases with affected parents/siblings) were prostate, breast and colorectal cancer (Hemminki et al. 2008). As well as being the most common cancers worldwide, they are associated with the commonest cancer-related gene mutations (Qureshi et al. 2007; World Cancer Research Fund). For instance, BRCA1 mutations increase the risk of breast, ovarian and prostate cancer, whilst DNA mismatch repair gene mutations are associated with Lynch Syndrome (Qureshi et al. 2007).
Familial cancers are usually divided into three categories. For example, the English National Institute for Health and Care Excellence (NICE) categorised breast cancer risk into at or near population (< 17% lifetime risk), moderate (17% to 29%) and high risk (> 30%) (NICE 2017). A 2005 California population survey reported the prevalence of strong and moderate familial cancer risk to be 5% and 7% for breast, 1% and 5% for colorectal and prostate cancer. This risk stratification was based on the proximity of affected relatives and age at cancer diagnosis (Scheuner et al. 2010).
As illustrated above, the definition of familial cancer risk varies in different countries and guidelines. Nevertheless, high risk generally indicates the probability of single gene disorder with Mendelian inheritance (Duffy et al. 2013; Qureshi et al. 2007; Scheuner et al. 2010). Conversely, moderate risk may be due to combinations of multiple low penetrance gene mutations with or without shared environmental or behavioural risk factors (Qureshi et al. 2007).
Preventive measures such as surveillance, prophylactic surgery or chemoprevention can reduce cancer incidence and mortality for patients with familial cancer risk (Carbine et al. 2018; Cuzick et al. 2013; Domchek et al. 2010; Duffy et al. 2013). A Cochrane review found that bilateral risk reducing mastectomy decreased breast cancer incidence and death, particularly in women with BRCA1/2 mutations (Carbine et al. 2018). The FH01 study estimated that annual mammogram for women aged 40–49 with moderate familial breast cancer risk (defined as at least 3% risk for this age group) reduced breast cancer mortality by 40% (Duffy et al. 2013). In a 15-year controlled trial, colonoscopy screening at three-year intervals reduced the colorectal cancer rate by 62% and overall mortality by 65% in families with Lynch Syndrome (Järvinen et al. 2000).
For at-risk patients to benefit from these preventive measures, primary care providers play a crucial role. To assess familial cancer risk, primary care providers need to collect a family history; the English NICE guideline suggests using family history tools to collect comprehensive family histories (NICE 2017). Clinical decision support systems can then be used to translate this information into risk strata with evidence-based recommendation on appropriate management, e.g., referral to genetic services for those at high familial risk or reassurance of patients at near population risk (NICE 2017; Paluch-Shimon et al. 2016; U.S. Preventive Services Task Force 2015).
However, it is still unclear if familial cancer risk assessment and management in primary care improve clinically relevant outcome, such as cancer morbidity and mortality. Previous systematic reviews focused on the impact of multifactorial cancer risk assessment tools, the validity of family history tools, specialist risk assessment services and familial breast cancer only (Cleophat et al. 2018; Hilgart et al. 2012; Qureshi et al. 2009; Walker et al. 2015).
The current systematic review focused on the effectiveness of primary care interventions to identify and manage patients at familial cancer risk, to improve clinical outcomes for breast, ovarian, prostate and colorectal cancers. This will help policy makers decide which familial cancer risk assessment interventions are worth adopting and help researchers identify the gaps in evidence.
Methods
The Cochrane Collaboration’s guidance on review of interventions and the PRISMA-P checklist were followed (Higgins and Green 2011; Shamseer et al. 2015). The protocol was registered on PROSPERO in December 2017 (PROSPERO 2017).
Literature search
Databases searched were MEDLINE, EMBASE, CINAHL and Cochrane library. Aligned with the introduction of familial cancer clinics in the late 1980s, the search period was from 1 Jan 1980 to 4 October 2017 (Hilgart et al. 2012). We used controlled vocabulary and free text terms based on the concepts of ‘cancer: breast, ovarian, colorectal and prostate’, ‘familial/hereditary cancer’ and ‘primary health care’.
With the Zetoc database, we also searched the table of contents within the last 5 years for Journal of Community Genetics, European Journal of Human Genetics, Genetics in Medicine and Public Health Genomics. Other searches included clinical trial registries (U.S. National Institutes of Health (www.clinicaltrials.gov), ISRCTN registry, WHO International Clinical Trials Registry Platform), The Networked Digital Library of Theses and Dissertations, the conference proceedings within the last 5 years for European Society of Human Genetics Conference and American College of Medical Genetics and Genomics annual meetings and the reference list of included studies (see supplementary material 1 for full details of the search strategy)
Study selection
Two authors screened the titles and abstracts (SL and MP/BD) and full texts (SL and MP) independently. Discrepancies were resolved with a third author (NQ). Authors of studies were contacted where clarification were required.
Studies were eligible if published in English and evaluated an intervention that identified and managed patients at risk of familial breast, ovarian, colorectal or prostate cancer. Data must have been presented separately for each cancer type, except breast and ovarian cancer, as BRCA1/2-associated breast and ovarian cancer is a recognised hereditary cancer syndrome (Petrucelli et al. 2010). Randomised controlled trials (RCT) and non-randomised studies for intervention (NRSI) were eligible. Reviews, genetic epidemiology studies with no clinical intervention, stand-alone guidelines, case reports, editorials, qualitative studies, abstracts and studies with no comparator arm were excluded.
Participants included were adults aged > 18 with no previous history of cancer or known cancer genetic mutation. The intervention must have been based in primary care or non-specialist community health service and care managed by primary care providers. We defined primary care providers as health professionals who delivered care to undifferentiated patients as the first contact point in the community. This could be a general practitioner (family doctor or family physician), internal medicine physician or obstetrician/gynaecologist practising in the community (Qureshi et al. 2007).
The primary outcomes were cancer incidence; cancer-related morbidity, mortality and survival; and identification of cancer predisposition (increased familial risk) as defined by study authors. Secondary outcomes were appropriateness of specialist referrals (as defined by study authors), uptake of preventive strategies and cognitive and psychological effect measured with validated tools.
Data extraction and analysis
Data on study characteristics and pre-specified outcomes were extracted by two reviewers independently (SL and BD/JL) using standardised forms and discrepancies resolved with a third author (NQ). Where there were multiple publications from the same study, the data were grouped together and treated as a single study (Higgins and Green 2011).
Quality assessment
Two authors reviewed the risk of bias for the included studies independently (SL and NQ/SW) with discrepancies resolved by a third author (SW/NQ). The Cochrane Collaboration Risk of Bias tool was used for RCT, and the ROBINS-I tool was used for NRSI (Higgins et al. 2011; Sterne et al. 2016). The GRADE approach was used to rate the certainty of evidence for the included outcomes (Schünemann et al. 2013).
Results
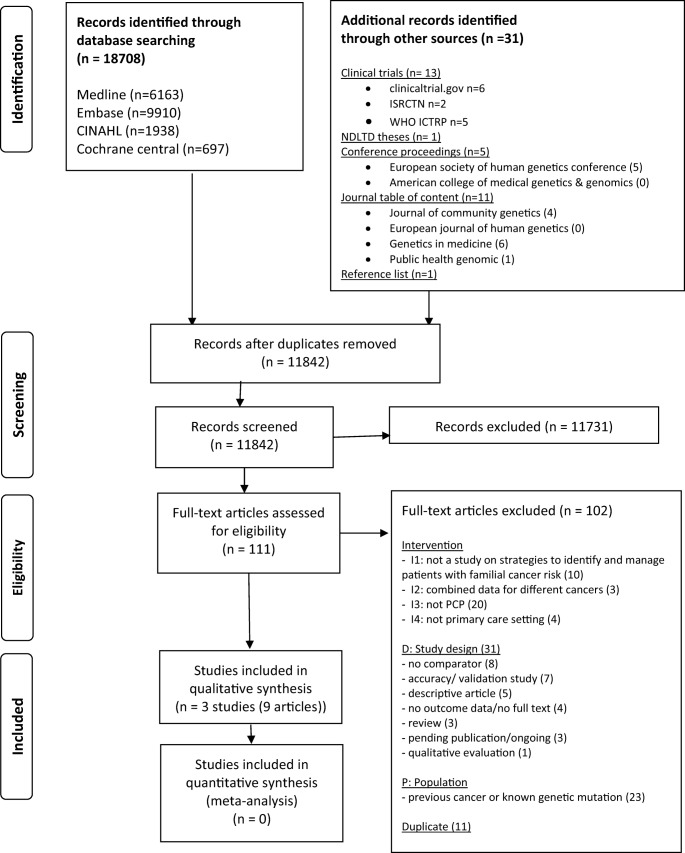
From the initial 11,842 titles and abstracts, we screened 111 full texts for eligibility (Fig. 1). Three studies comprising nine articles were included (Emery et al. 2007; Family Healthware Trial (O’Neil et al. 2009; Acheson et al. 2010; Rubinstein et al. 2011a, b; Ruffin et al. 2011; Wang et al. 2012, 2015); Van Erkelens et al. 2017). Only four outcomes were identified. No studies reported the same outcomes. Three further studies were identified that are ongoing or awaiting publication (ISRCTN 2014; Naicker et al. 2013; Voils 2017). Supplementary material 2 presents the table of excluded studies with reasons for exclusion.
Fig. 1.
PRISMA flow diagram of study selection
Due to the limited number of included studies with varying study designs and study interventions, meta-analysis was not feasible. The outcomes were presented as a narrative summary. See supplementary material 3 for further details.
Included studies
Table 1 summarised the characteristics of the three included studies. Of these, two were cluster RCTs (Emery et al. 2007; Family Healthware Trial) and one NRSI (uncontrolled before and after study) (Van Erkelens et al. 2017). Two studies were based in Europe and one in the USA. Two studies evaluated interventions for breast, ovarian and colorectal cancer, and one study for breast cancer only. Follow-up duration ranged from 2 weeks to 12 months, with a median follow-up time of 6 months. The average age of patients ranged from 51 to 56. Patients were predominantly white, female and college educated.
Table 1.
Summary description of included studies
| Author, year | Study design | Country, setting | Participants | Intervention | Comparator | Outcomes |
|---|---|---|---|---|---|---|
| Emery et al. 2007 | Cluster RCT | UK, primary care | Patients expressing concern about cancer family history | Lead clinician attended educational session and given access to software that conducts familial risk assessment to inform genetic referrals. | Lead clinician attended educational session and mailed familial cancer guidelines. |
1. Proportion of GP referrals consistent with guidelines 2. Proportion of GP referrals assessed to be at increased risk by genetic clinic |
|
Family Healthware Trial 1. O’Neill et al. 2009 2. Acheson et al. 2010 3. Rubinstein et al. 2011a 4. Rubinstein et al. 2011b 5. Ruffin et al. 2011 6. Wang et al. 2012 7. Wang et al. 2015 |
Cluster RCT | USA, primary care | Existing patient list or patients with upcoming appointments | Patient received personalised familial risk assessment and prevention messages generated by software. | Patient received standard prevention messages about screening and healthy lifestyle choices. |
1. Adherence to cancer screening 2. Cognitive: Patient risk perception |
| Van Erkelens et al. 2017 | NRSI: uncontrolled before after study | The Netherlands, population BC screening programme | Women attending population BC screening | Patient completed FBC risk assessment and received risk status and advice online. | Same patients 2 weeks after initial FBC risk assessment. | Psychological: Patient anxiety and depression (STAI and HADS) |
BC, breast cancer; FBC, familial breast cancer; GP, general practitioner; HADS, Hospital Anxiety Depression Scale; NRSI, non-randomised study of intervention; RCT, randomised controlled trial; STAI, State-Trait Anxiety Inventory
All three studies used a bespoke software for familial cancer risk assessment: a clinician pedigree drawing tool based on patient completed family history questionnaire (Emery et al. 2007), a patient facing familial risk assessment tool online or via telephone interview (Family Healthware Trial) and a patient online self-test (Van Erkelens et al. 2017). All three subsequently generated a risk-based action plan: one informed general practitioners who needed genetic referral (Emery et al. 2007), another provided personalised familial risk assessment outcome and prevention plan for patients and all types of primary care providers (Family Healthware Trial), and the final study advised patients with increased risk to consult their primary care providers (unspecified health care professionals) (Van Erkelens et al. 2017).
Two studies used a proactive approach by screening all patients with an upcoming appointment with their primary care provider (Family Healthware Trial) or attending population-based breast cancer screening (Van Erkelens et al. 2017). One study employed a reactive approach and only conducted a familial risk assessment when approached by patients concerned about their cancer family history (Emery et al. 2007).
Primary outcome
No studies identified the review’s primary outcome (cancer incidence, cancer-related morbidity, mortality, survival, or identification of cancer predisposition). Although the Family Healthware Impact Trial reported the characteristics of patients with interim cancer diagnosis during the 6-month follow-up period (five intervention and two control patients reported a new breast cancer diagnosis; 17 intervention and 10 control patients reported ‘other’ cancer; none reported colon or ovarian cancer diagnosis), the authors excluded these patients from the analyses of screening adherence as it was not clear whether the tests or consultations were performed for screening or diagnostic purposes during the intervention period (Rubinstein et al. 2011a).
Secondary outcome
None of the three studies reported the same outcomes. The four secondary outcomes reported were the appropriateness of specialist referrals, uptake of preventive strategies, patients’ self-reported risk perception and patients’ self-reported anxiety and depression. Details of each outcome were described below. Using the GRADE approach, these outcomes had low to very low certainty of evidence (Table 2). This is driven by weakness in the study design, leading to the risk of bias (see the Risk of bias section).
Table 2.
GRADE evidence profile
| Outcome/cancer | Effect | Number of participants (studies) | GRADE criteria | Certainty in evidence | ||||
|---|---|---|---|---|---|---|---|---|
| Risk of bias | Inconsistency | Indirectness | Imprecision | Publication bias | ||||
| I. Appropriateness of specialist referral: general practitioners’ referral letter (Emery et al. 2007)* | ||||||||
| Breast |
Proportions meeting referral guidelines OR 4.5 (1.6 to 13.1) |
45 practices, 167 patients (1 cluster RCT) | Present | Not applicable | Not serious | Absent | Not applicable |
⊕⊕○○a Low |
|
Proportions confirmed at increased risk at genetic clinic OR 1.4 (0.6 to 3.5) |
45 practices, 111 patients (1 cluster RCT) | Present | Not applicable | Not serious | Present | Not applicable |
⊕○○○b Very low |
|
| Colorectal |
Proportions meeting referral guidelines OR 6.5 (0.5 to 83.7) |
45 practices, 101 patients (1 cluster RCT) | Present | Not applicable | Not serious | Present | Not applicable |
⊕○○○c Very low |
|
Proportions confirmed at increased risk at genetic clinic OR 0.2 (0.1 to 0.8) |
45 practices, 74 patients (1 cluster RCT) | Present | Not applicable | Not serious | Absent | Not applicable |
⊕○○○d Very low |
|
| II. Uptake of preventive strategies: improvement in proportion of patients adherent to risk-based screening (Rubinstein et al. 2011a)** | ||||||||
| Breast | Mammography 9% (intervention) vs 7% (control) improvement, p = 0.82 | 41 practices, 2063 patients (1 cluster RCT) | Present | Not applicable | Absent | Present | Not applicable |
⊕○○○e Very low |
| Colorectal | Colon cancer screening 8% vs 7% improvement, p = 0.95 | 41 practices, 2016 patients (1 cluster RCT) | Present | Not applicable | Absent | Present | Not applicable |
⊕○○○f Very low |
| III. Psychological: patients’ anxiety and distress (Van Erkelens et al. 2017)*** | ||||||||
| Breast |
State anxiety (STAI) immediately after self-test Increased risk − 2 (− 6 to 2) Population risk − 2 (− 2 to − 1) |
186 patients (1 uncontrolled before after study) | Present | Not applicable | Absent | Present | Not applicable |
⊕○○○g Very low |
| Breast |
State anxiety (STAI) 2 weeks after self-test Increased risk 3 (− 5 to 10) Population risk − 3 (− 5 to − 2) |
186 patients (1 uncontrolled before after study) | Present | Not applicable | Absent | Present | Not applicable |
⊕○○○h Very low |
| Breast |
Trait anxiety (STAI) 2 weeks after self-test Increased risk 0 (− 3 to 4) Population risk − 1 (− 2 to − 1) |
186 patients (1 uncontrolled before after study) | Present | Not applicable | Absent | Present | Not applicable |
⊕○○○i Very low |
| Breast |
Hospital anxiety and depression score (HADS) 2 weeks after self-test Increased risk 1 (− 3 to 6) Population risk − 0 (− 1 to 0) |
186 patients (1 uncontrolled before after study) | Present | Not applicable | Absent | Present | Not applicable |
⊕○○○j Very low |
*Effects are adjusted odds ratio (95% confidence intervals) unless otherwise specified
**Effects are difference in screening adherence pre- and post-intervention period, p value for comparison between study arms, adjusting for practice clustering, risk, and baseline adherence
***Effects are mean change from baseline (95% confidence intervals) unless otherwise specified
aDowngraded by 1 for high risk of bias (allocation concealment, blinding, responder bias), downgraded by 1 as unable to assess inconsistency and publication bias
bDowngraded by 2 for high risk of bias (allocation concealment, blinding, incomplete outcome (participant non-attendance), responder bias) and imprecision (confidence interval crossing one), downgraded by 1 as unable to assess inconsistency and publication bias
cDowngraded by 2 for high risk of bias (allocation concealment, blinding, responder bias) and imprecision (wide confidence interval), downgraded by 1 as unable to assess inconsistency and publication bias
dDowngraded by 2 for high risk of bias (allocation concealment, blinding, incomplete outcome (participant non-attendance), responder bias), downgraded by 1 as unable to assess inconsistency and publication bias
e, f,Downgraded by 2 for high risk of bias (randomisation, allocation concealment, blinding, incomplete outcome, selective reporting) and imprecision (no sample size and confidence interval crosses zero); downgraded by 1 as unable to assess inconsistency and publication bias
g, h, i, jDowngraded by 2 for critical risk of bias (non-randomised studies of intervention, confounding, missing data) and imprecision (no sample size calculation), downgraded by 1 as unable to assess inconsistency and publication bias
I. Appropriateness of specialist referrals
Emery et al.’s cluster RCT showed that the use of a risk assessment and decision support software resulted in significantly higher proportion of general practitioners’ referral letters meeting the referral guidelines for breast cancer (93% intervention vs 73% control, OR 4.5, 95% CI 1.6 to 13.1) but not for colorectal cancer (99% vs 92%, OR 6.5, 95% CI 0.5 to 83.7) (2007).
After specialist review at the genetic clinic, the proportion of general practitioners’ referrals that were confirmed as increased risk was similar for intervention and control for breast cancer (77% vs 70%, OR 1.4, 95% CI 0.6 to 3.5). In contrast, for colorectal cancer, the proportion assessed to be at increased risk by the specialist was lower in the intervention arm (56% vs 85%, OR 0.2, 95% CI 0.1 to 0.8) (Emery et al. 2007).
II. Uptake of preventive strategies
The Family Healthware cluster RCT found that 6 months post-intervention, there was no significant difference in improved adherence between the intervention and control arm for risk-based mammography (improvement in adherence, 9% intervention vs 7% control, p = 0.82) and colorectal cancer screening (8% vs 7%, p = 0.95). This was also the case for the subgroup of patients who were not adherent at baseline. During the intervention period, there was no difference between study arm in the number of women receiving CA-125 blood test and transvaginal ultrasound for ovarian cancer risk (supplementary material 3) (Rubinstein et al. 2011a).
III. Cognitive effect: patients’ risk perception
The Family Healthware Trial did not report this outcome for all patients. However, in the subgroup of patients who under-estimated their risk, more of the intervention patients’ risk perception became consistent with their risk status at 6 months for colorectal cancer, although this was of borderline significance (17% vs 10%, OR 1.89, 95% CI 0.99 to 3.59). This was not observed for breast or ovarian cancer (Rubinstein et al. 2011a).
IV. Psychological effect: patients’ anxiety and depression
Van Erkelens et al.’s NRSI used the State-Trait Anxiety Inventory (STAI) and Hospital Anxiety and Depression Scale (HADS). The analysis of the total study population was not presented. Subgroup analysis by risk status was provided: women told to be at population risk for breast cancer had reduced anxiety immediately after self-risk assessment (mean change of state anxiety − 2, 95% CI − 2 to − 1) and at 2 weeks (− 3, 95% CI − 5 to − 2). The HADS score remained unchanged at 2 weeks. For women at increased breast cancer risk, there was no consistent change in anxiety and depression (Table 2). The mean score for STAI and HADS were below the levels of clinical significance and similar to those of the general population (supplementary material 3) (2017).
Risk of bias
All three included studies were at high risk of bias (Table 3). For Emery et al.’s cluster RCT, allocation concealment and blinding of participants and clinicians were not possible. The patient’s non-attendance at the genetic clinic was 28% (45/162) for intervention and 38% (32/84) for control, contributing to attrition bias. Responder bias was evident from the 74% (125/170) practices that declined to participate. The author commented that this recruitment rate is consistent with similar primary care trials and that practices that were interested in genetic medicine were more likely to participate (2007).
Table 3.
Risk of bias table
| RCT (Cochrane risk of bias tool) | Random sequence generation | Allocation concealment | Blinding of participants and personnel | Blinding of outcome assessment | Incomplete outcome data | Selective reporting | Other bias | Overall bias |
|---|---|---|---|---|---|---|---|---|
| Emery et al. 2007 | + | – | – | + | – | + | ? | – |
| Family Healthware Trial | ? | ? | – | – | – | ? | – | – |
| NRSI (ROBINS-I risk of bias tool) | Confounding | Participants selection | Classification of intervention | Deviation from intended interventions | Missing data | Measurement of outcomes | Selection of reported result | Overall bias |
| Van Erkelens et al. 2017 | Critical | Serious | Moderate | Low | Serious | Moderate | Moderate | Critical |
+, low risk; ?, unclear risk; −, high risk
The Family Healthware Trial had no description of the random sequence generation or allocation concealment. From the published study design, there appeared to be no blinding. The participant recruitment rate was low (18%) with high attrition: 20% intervention (542/2650) and 20% control (324/1598) participants withdrew from consent to follow-up. Results for the change in risk perception were only reported for the subgroup who underestimated their risk. The selection of participants who were free of comorbidities led to healthy volunteer bias. The lengthy baseline questionnaire may have altered the behaviour in the control group, reducing the intervention effect.
In Van Erkelen’s NRSI, there was no control of the confounders such as age and sociodemographic factors. Finally, 35% (101/287) of patients at baseline were lost to follow-up (2017).
Excluded studies: patients with a personal history of cancer
Two studies were excluded for having participants with a personal history of cancer but met other eligibility criteria: one cluster RCT and one before-after study (supplementary material 4) (Wilson et al. 2005; Wilson et al. 2006; Orlando et al. 2011; Orlando et al. 2013; Wu et al. 2013; Orlando et al. 2014; Orlando et al. 2016). Overall, there were 4 (22/588) to 8% (23/282) of participants with a personal history of cancer. Similar to the main review, the secondary outcomes reported were the appropriateness of referrals and uptake of preventive strategies. However, the findings were different from the main review: intervention had no impact on the appropriateness of genetic referrals (Wilson et al. 2005; Wilson et al. 2006), but there was improved preventive uptake of surveillance (breast magnetic resonance imaging) and gynaecology assessment for ovarian cancer screening (supplementary material 3) (Orlando et al. 2016).
Discussion
Main findings
This is a comprehensive systematic review on the long-term clinical impact of primary care assessment and management of patients with familial breast, ovarian, prostate and colorectal cancer risk. Our review spanned the past 37 years and identified three studies. None of these studies assessed the review’s primary outcome: cancer incidence, morbidity, mortality, survival or identification of cancer predisposition. The follow-up period (2 weeks to 12 months) would have been too short to identify the primary outcomes. For instance, a large community cohort study estimated that a period of 5 years is required for 1000 colorectal cancer cases to be identified from a sample size of 500,000 recruits (UK Biobank 2007).
The secondary outcomes predominantly evaluated short-term outcomes of process and psychological measures; these evidences were of limited quality due to weakness in the study design. The strongest evidence emerged from a cluster RCT, demonstrating improved appropriateness of general practitioners’ genetic referral letters for patients at familial breast cancer risk. However, this still had a low GRADE level of certainty (Emery et al. 2007).
Comparison with previous systematic review
To our knowledge, no systematic review has evaluated the clinical impact of familial cancer risk assessment and management by non-specialist primary care providers in primary care settings. The previous four reviews covered broader areas of multifactorial cancer risk assessment tools, the validity and nature of cancer family history tools and familial breast cancer risk assessment by genetic services (Cleophat et al. 2018; Hilgart et al. 2012; Qureshi et al. 2009; Walker et al. 2015). All of these reviews shared some similar findings to the current review.
Walker et al. reviewed RCTs that evaluated the impact of cancer risk assessment tools in primary care. They identified 11 trials compared with three trials in our review, as we focused on familial cancer risk assessment, limited the types of cancer to those known to have a genetic component, grouped papers from the same study as a single trial and included only outcomes measured with validated tools. Despite focusing on familial cancer, our review findings were consistent with Walker et al.’s, specifically, there is limited evidence available on the effectiveness of cancer risk assessment on the uptake of screening and risk assessment does not increase psychological distress (2015).
Two reviews identified between 18 to 29 cancer family history tools used in primary care; a third of the tools provided risk stratification and action plan for patients or clinicians (Cleophat et al. 2018; Qureshi et al. 2009). Compared with structured genetic interviews, Qureshi et al. found that the tools demonstrated a 75–100% agreement of risk stratification (2009). In Cleophat et al.’s review, the validation methods and results were inconsistent. There was no formal evaluation of clinical utility but similar to our review, Cleophat et al. suggested potential benefits: improved quality of genetic referrals, increased compliance with cancer screening and no increase in psychological distress (2018).
Finally, both our review and Hilgart et al.’s Cochrane review suggested that familial cancer risk assessment may improve the accuracy of patients’ risk perception and anxiety, even though the Cochrane review only included familial breast cancer services delivered by genetic specialists (2012).
Strength of the review and included studies
The strength of this systematic review is the robust search strategy and focused eligibility criteria. Restricting the evidence to the highest level of experimental study design but recognising the paucity of literature in this field, we expanded the inclusion criteria beyond RCT to NRSI. Two independent reviewers conducted the eligibility screen, data extraction and risk of bias assessment. To help interpret the results, we conducted a rigorous assessment of the evidence quality using established methods from Cochrane and GRADE (Higgins et al. 2011; Schünemann et al. 2013; Sterne et al. 2016).
Two of three included studies employed cluster RCT design, which is suitable for studies in primary care where cross-contamination of participants in the same primary care practice can dilute the effect of the intervention (Emery et al. 2007; Family Healthware Trial). Included studies also used validated measures for psychological outcomes: in Van Erkelen’s study, the impact of familial cancer risk assessment on patient psychological outcomes was measured using STAI and HADS (2017).
Weakness of the review and included studies
Due to the low number of included studies with variable study designs and interventions, a quantitative synthesis was not feasible. The study design requirement of an intervention study and a comparator group increased the review’s robustness but limited the number of included studies. Further, the risk of bias was high across all studies; hence, the results need to be interpreted with caution.
Studies that combined data for patients with and without previous cancer history were excluded. As the aim of the review was to identify the impact of intervention on cancer mortality and morbidity, it was decided that participants with cancer history would not be included. Similarly, studies that combined outcome data for different cancers that could not be disentangled were excluded.
It was difficult to have a true comparator that reflected current usual care. In Emery et al.’s RCT, the lead clinician in both the intervention and control arm received an education session on cancer genetics, although continuing medical education could be considered as part of usual practice (2007). In the Family Healthware Trial, the control arm had a lengthy baseline survey, which may have had an intervention effect (Rubinstein et al. 2011a). Finally, studies predominantly included white educated females, limiting the findings’ generalisability to the wider population.
Implication for future research
More studies are needed in primary care settings where the majority of health consultations take place (NHS England 2013). Current studies are not generalizable to the wider population; in particular, future studies need better representation from deprived and ethnic minority groups. Future studies should also incorporate robust comparator groups and use validated outcome measures. Current studies often do not state the participants’ age range or personal history of cancer in the eligibility criteria, necessitating correspondence with the author. We suggest future studies should also make these inclusion criteria clearer.
Clinical trials with longer follow-up will allow for evaluation of clinical impact such as cancer-related outcome, but with relatively low prevalence of cancers with inherited predisposition, this would require studies with large sample sizes. Although classified as lower level of evidence, prospective cohort studies with robust design and longer follow-up may provide good quality clinical outcome data.
It has been 30 years since the introduction of familial cancer clinics, and since then there has been great advances in preventive management of familial cancer risk. We still need large well design studies to help us determine if systematic familial cancer risk assessment should be introduced as a routine case-finding approach in primary care.
Electronic supplementary material
(DOCX 87 kb)
Acknowledgements
We would like to thank Jeanette Eldridge, senior research librarian at the University of Nottingham for her help with the literature search strategy and Hannah Carpenter, PhD student at the Primary Care Division, University of Nottingham for reviewing the protocol.
Funding
SL and MP are National Institute for Health Research (NIHR) funded Academic Clinical Fellows.
Compliance with ethical standards
Conflict of interest
Nadeem Qureshi is a member of the NICE Guideline Development Group for Familial Breast Cancer and the Advisory Board for Journal of Community Genetics. Siang Ing Lee, Mitesh Patel, Brittany Dutton, Stephen Weng and Jocelyn Luveta declare no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Acheson Louise S, Wang Catharine, Zyzanski Stephen J, Lynn Audrey, Ruffin Mack T, Gramling Robert, Rubinstein Wendy S, O'Neill Suzanne M, Nease Donald E. Family history and perceptions about risk and prevention for chronic diseases in primary care: A report from the Family Healthware™ Impact Trial. Genetics in Medicine. 2010;12(4):212–218. doi: 10.1097/GIM.0b013e3181d56ae6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbine NE, Lostumbo L, Wallace J, Ko H (2018) Risk-reducing mastectomy for the prevention of primary breast cancer. Cochrane Database Syst Rev (4):CD002748. 10.1002/14651858.CD002748.pub4 [DOI] [PMC free article] [PubMed]
- Cleophat JE, Nabi H, Pelletier S, Bouchard K, Dorval M. What characterizes cancer family history collection tools? A critical literature review. Curr Oncol. 2018;25(4):e335–e350. doi: 10.3747/co.25.4042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuzick J, Sestak I, Bonanni B, Costantino JP, Cummings S, DeCensi A, Dowsett M, Forbes JF, Ford L, LaCroix A, Mershon J, Mitlak BH, Powles T, Veronesi U, Vogel V, Wickerham DL, SERM Chemoprevention of Breast Cancer Overview Group Selective oestrogen receptor modulators in prevention of breast cancer: an updated meta-analysis of individual participant data. Lancet. 2013;381(9880):1827–1834. doi: 10.1016/S0140-6736(13)60140-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domchek SM, Friebel TM, Singer CF, Evans DG, Lynch HT, Isaacs C, Garber JE, Neuhausen SL, Matloff E, Eeles R, Pichert G, van t'veer L, Tung N, Weitzel JN, Couch FJ, Rubinstein WS, Ganz PA, Daly MB, Olopade OI, Tomlinson G, Schildkraut J, Blum JL, Rebbeck TR. Association of risk-reducing surgery in BRCA1 or BRCA2 mutation carriers with cancer risk and mortality. JAMA. 2010;304(9):967–975. doi: 10.1001/jama.2010.1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy SW, Mackay J, Thomas S, Anderson E, Chen THH, Ellis I, Evans G, Fielder H, Fox R, Gui G, Macmillan D, Moss S, Rogers C, Sibbering M, Wallis M, Warren R, Watson E, Whynes D, Allgood P, Caunt J. Evaluation of mammographic surveillance services in women aged 40-49 years with a moderate family history of breast cancer: a single-arm cohort study. Health Technol Assess. 2013;17(11):vii–xiv, 1-95. doi: 10.3310/hta17110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emery J, Morris H, Goodchild R, Fanshawe T, Prevost AT, Bobrow M, Kinmonth AL. The GRAIDS Trial: a cluster randomised controlled trial of computer decision support for the management of familial cancer risk in primary care. Br J Cancer. 2007;97(4):486–493. doi: 10.1038/sj.bjc.6603897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemminki K, Sundquist J, Bermejo JL. How common is familial cancer? Ann Oncol. 2008;19(1):163–167. doi: 10.1093/annonc/mdm414. [DOI] [PubMed] [Google Scholar]
- Higgins JPT, Green S. (eds). (2011) Cochrane handbook for systematic reviews of interventions version 5.1.0. The Cochrane Collaboration
- Higgins JPT, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilgart JS, Coles B, Iredale R (2012) Cancer genetic risk assessment for individuals at risk of familial breast cancer. Cochrane Database of Syst Rev (2):CD003721. 10.1002/14651858.CD003721.pub3 [DOI] [PMC free article] [PubMed]
- International Standard Randomised Controlled Trials Number (ISRCTN) Registry. (2014) Proactive familial breast cancer risk assessment in primary care (phase 2). http://www.isrctn.com/ISRCTN16117197. Accessed 10th October 2018
- Järvinen HJ, Aarnio M, Mustonen H, Aktan–Collan K, Aaltonen LA, Peltomäki P, Chapelle ADL, Mecklin J–P. Controlled 15-year trial on screening for colorectal cancer in families with hereditary nonpolyposis colorectal cancer. Gastroenterology. 2000;118(5):829–834. doi: 10.1016/S0016-5085(00)70168-5. [DOI] [PubMed] [Google Scholar]
- Kerber RA, O'Brien E. A cohort study of cancer risk in relation to family histories of cancer in the Utah population database. Cancer. 2005;103(9):1906–1915. doi: 10.1002/cncr.20989. [DOI] [PubMed] [Google Scholar]
- Naicker S, Meiser B, Goodwin A, et al. Which tests is best? A randomised controlled trial to evaluate the use of familial phenotype to risk appropriately screen for colorectal cancer in the general population. Psycho-Oncology. 2013;22:27. [Google Scholar]
- National Institute for Health and Care Excellence (NICE). (2017) Familial breast cancer: classification care and managing breast cancer and related risks in people with a family history of breast cancer [CG164]. NICE, London [PubMed]
- NHS England. (2013) Transforming primary care in London: general practice a call to action. NHS England, London. https://www.england.nhs.uk/london/wp-content/uploads/sites/8/2013/11/Call-Action-ACCESSIBLE.pdf Accessed 16th October 2018
- O'Neill Suzanne M., Rubinstein Wendy S., Wang Catharine, Yoon Paula W., Acheson Louise S., Rothrock Nan, Starzyk Erin J., Beaumont Jennifer L., Galliher James M., Ruffin Mack T. Familial Risk for Common Diseases in Primary Care. American Journal of Preventive Medicine. 2009;36(6):506–514. doi: 10.1016/j.amepre.2009.03.002. [DOI] [PubMed] [Google Scholar]
- Orlando LA, Hauser ER, Christianson C, Powell KP, Buchanan AH, Chesnut B, Agbaje AB, Henrich VC, Ginsburg G (2011) Protocol for implementation of family health history collection and decision support into primary care using a computerized family health history system. BMC Health Serv Res 11(264). 10.1186/1472-6963-11-264 [DOI] [PMC free article] [PubMed]
- Orlando LA, Henrich VC, Hauser ER, Wilson C, Ginsburg GS. Genomedical connection. The genomic medicine model: an integrated approach to implementation of family health history in primary care. Per Med. 2013;10(3):295–306. doi: 10.2217/pme.13.20. [DOI] [PubMed] [Google Scholar]
- Orlando LA, Wu RR, Beadles C, Himmel T, Buchanan AH, Powell KP, Hauser ER, Henrich VC, Ginsburg GS. Implementing family health history risk stratification in primary care: impact of guideline criteria on populations and resource demand. Am J Med Genet C Semin Med Genet. 2014;166C(1):24–33. doi: 10.1002/ajmg.c.31388. [DOI] [PubMed] [Google Scholar]
- Orlando LA, Wu RR, Myers RA, Buchanan AH, Henrich VC, Hauser ER, Ginsburg GS. Clinical utility of a Web-enabled risk-assessment and clinical decision support program. Genet Med. 2016;18(10):1020–1028. doi: 10.1038/gim.2015.210. [DOI] [PubMed] [Google Scholar]
- Paluch-Shimon S, Cardoso F, Sessa C, Balmana J, Cardoso MJ, Gilbert F, Senkus E, ESMO Guidelines Committee Prevention and screening in BRCA mutation carriers and other breast/ovarian hereditary cancer syndromes: ESMO Clinical Practice Guidelines for cancer prevention and screening. Ann Oncol. 2016;27(suppl 5):v103–v110. doi: 10.1093/annonc/mdw327. [DOI] [PubMed] [Google Scholar]
- Petrucelli N, Daly MB, Feldman GL. Hereditary breast and ovarian cancer due to mutations in BRCA1 and BRCA2. Genet Med. 2010;12(5):245–259. doi: 10.1097/GIM.0b013e3181d38f2f. [DOI] [PubMed] [Google Scholar]
- Preventive Services Task Force US (2015) Risk assessment, genetic counseling, and genetic testing for BRCA-related cancer in women: recommendation statement. Am Fam Physician 91(2) [PubMed]
- PROSPERO (2017) International prospective register of systematic review. https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=83418 Accessed 26th September 2017
- Qureshi N, Wilson B, Santaguida P et al (2007) Collection and use of cancer family history in primary care. Evidence Report/Technology Assessment No. 159. AHRQ Publication No. 08-E001. Agency for Healthcare Research and Quality, Rockville, MD
- Qureshi N, Carroll JC, Wilson B, Santaguida P, Allanson J, Brouwers M, Raina P. The current state of cancer family history collection tools in primary care: a systematic review. Genet Med. 2009;11(7):495–506. doi: 10.1097/GIM.0b013e3181a7e8e0. [DOI] [PubMed] [Google Scholar]
- Rubinstein Wendy S., Acheson Louise S., OʼNeill Suzanne M., Ruffin Mack T., Wang Catharine, Beaumont Jennifer L., Rothrock Nan. Clinical utility of family history for cancer screening and referral in primary care: A report from the Family Healthware Impact Trial. Genetics in Medicine. 2011;13(11):956–965. doi: 10.1097/GIM.0b013e3182241d88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinstein WS, O'Neill SM, Rothrock N, Starzyk EJ, Beaumont JL, Acheson LS, Wang C, Gramling R, Galliher JM, Ruffin MT. Components of family history associated with women's disease perceptions for cancer: a report from the Family HealthwareTM Impact Trial. Genet Med. 2011;13(1):52–62. doi: 10.1097/GIM.0b013e3181fbe485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruffin MT, Nease DE, Jr, Sen A, et al. Effect of preventive messages tailored to family history on health behaviors: the Family Healthware Impact Trial. Ann Fam Med. 2011;9(1):3–11. doi: 10.1370/afm.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheuner MT, McNeel TS, Freedman AN. Population prevalence of familial cancer and common hereditary cancer syndromes. The 2005 California Health Interview Survey. Genet Med. 2010;12(11):726–735. doi: 10.1097/GIM.0b013e3181f30e9e. [DOI] [PubMed] [Google Scholar]
- Schünemann H, Brożek J, Guyatt G, Oxman A. (eds) (2013) GRADE handbook for grading quality of evidence and strength of recommendations. The GRADE Working Group, 2013
- Shamseer L, Moher D, Clarke M, Ghersi D, Liberati A, Petticrew M, Shekelle P, Stewart LA, the PRISMA-P Group Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ. 2015;349:g7647. doi: 10.1136/bmj.g7647. [DOI] [PubMed] [Google Scholar]
- Sterne JA, Hernán MA, Reeves BC, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. doi: 10.1136/bmj.i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UK Biobank. (2007) Protocol for a large-scale prospective epidemiological resource. http://www.ukbiobank.ac.uk/wp-content/uploads/2011/11/UK-Biobank-Protocol.pdf Accessed 10th October 2018
- Van Erkelens A, Sie AS, Manders P, et al. Online self-test identifies women at high familial breast cancer risk in population-based breast cancer screening without inducing anxiety or distress. Eur J Cancer. 2017;78:45–52. doi: 10.1016/j.ejca.2017.03.014. [DOI] [PubMed] [Google Scholar]
- Voils C (2017) Impact of family history and decision support on high-risk cancer screening. ClinicalTrials.gov. https://clinicaltrials.gov/show/NCT02247336 Accessed 10th October 2018
- Walker JG, Licqurish S, Chiang PPC, Pirotta M, Emery JD. Cancer risk assessment tools in primary care: a systematic review of randomized controlled trials. Ann Fam Med. 2015;13(5):480–489. doi: 10.1370/afm.1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Sen A, MTt R, et al. Family history assessment. impact on disease risk perceptions Am J Prev Med. 2012;43(4):392–398. doi: 10.1016/j.amepre.2012.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Sen A, Plegue M, Ruffin MT, IV, O'Neill SM, Rubinstein WS, Acheson LS, Yoon PW, Valdez R, Irizarry-de la Cruz M, Khoury MJ, Jorgensen C, Scheuner MT, Rubinstein WS, O'Neill SM, Rothrock N, Beaumont JL, Khan S, Ali D, Ruffin MT, Nease D, Acheson LS, Zyzanski SJ, Wiesner GL, Werner J, Pace WD, Galliher JM, Brandt E, Wang C, Gramling R, Starzyk EJ. Impact of family history assessment on communication with family members and health care providers: a report from the Family Healthware Impact Trial (FHITr) Prev Med. 2015;77:28–34. doi: 10.1016/j.ypmed.2015.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson BJ, Torrance N, Mollison J, et al. Improving the referral process for familial breast cancer genetic counselling: findings of three randomised controlled trials of two interventions. Health Technology Assessment (Winchester, England) 2005;9(3):iii–iv, 1-12. doi: 10.3310/hta9030. [DOI] [PubMed] [Google Scholar]
- Wilson BJ, Torrance N, Mollison J, Watson MS, Douglas A, Miedzybrodzka Z, Gordon R, Wordsworth S, Campbell M, Haites N, Grant A. Cluster randomized trial of a multifaceted primary care decision-support intervention for inherited breast cancer risk. Fam Pract. 2006;23(5):537–544. doi: 10.1093/fampra/cml026. [DOI] [PubMed] [Google Scholar]
- World Cancer Research Fund. Worldwide cancer data: global cancer statistics for the most common cancers. https://www.wcrf.org/dietandcancer/cancer-trends/worldwide-cancer-data Accessed 23rd October 2018
- Wu RR, Orlando LA, Himmel TL, Buchanan AH, Powell KP, Hauser ER, Agbaje AB, Henrich VC, Ginsburg GS (2013) Patient and primary care provider experience using a family health history collection, risk stratification, and clinical decision support tool: a type 2 hybrid controlled implementation-effectiveness trial. BMC Fam Pract 14(111). 10.1186/1471-2296-14-111 [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 87 kb)