Abstract
BACKGROUND
Rifaximin has been shown to reduce the incidence of hepatic encephalopathy and other complications in patients with cirrhosis. However, few studies have investigated the effect of rifaximin in cirrhotic patients with refractory ascites.
AIM
To evaluate the effects of rifaximin in the treatment of refractory ascites and to preliminarily explore its possible mechanism.
METHODS
A total of 75 cirrhotic patients with refractory ascites were enrolled in the study (50 in a rifaximin and 25 in a control group). Patients in the rifaximin group were divided into two subgroups according to the presence of spontaneous bacterial peritonitis and treatment with or without other antibiotics (19 patients treated with rifaximin and 31 patients treated with rifaximin plus intravenous antibiotics). All patients received conventional treatment for refractory ascites, while patients in the rifaximin group received oral rifaximin-α 200 mg four times daily for at least 2 wk. The ascites grade, fasting weight, liver and kidney function, and inflammatory factors in the plasma were evaluated before and after treatment. In addition, the gut microbiota was determined by metagenomics sequencing to analyse the changes in the characteristics of the gut microbiota before and after rifaximin treatment. The patients were followed for 6 mo.
RESULTS
Compared with the control group, the fasting weight of patients significantly decreased and the ascites significantly subsided after treatment with rifaximin (P = 0.011 and 0.009, respectively). The 6-mo survival rate of patients in the rifaximin group was significantly higher than that in the control group (P = 0.048). The concentration of interferon-inducible protein 10 decreased significantly in the rifaximin group compared with that in the control group (P = 0.024). The abundance of Roseburia, Haemophilus, and Prevotella was significantly reduced after rifaximin treatment, while the abundance of Lachnospiraceae_noname, Subdoligranulum, and Dorea decreased and the abundance of Coprobacillus increased after treatment with rifaximin plus intravenous antibiotics. The gene expression of virulence factors was significantly reduced after treatment in both subgroups treated with rifaximin or rifaximin plus intravenous antibiotics.
CONCLUSION
Rifaximin mitigates ascites and improves survival of cirrhotic patients with refractory ascites. A possible mechanism is that rifaximin regulates the structure and function of intestinal bacteria, thus improving the systemic inflammatory state.
Keywords: Rifaximin, Cirrhosis, Refractory ascites, Inflammatory factors, Gut microbiota, Metagenomics sequencing
Core tip: This study showed that the unabsorbed antibiotics, rifaximin, mitigates ascites and improves the survival of cirrhotic patients with refractory ascites and the possible mechanism is that rifaximin regulates the structure and function of intestinal bacteria, thus improving the systemic inflammatory state.
INTRODUCTION
Patients with refractory ascites have a poor prognosis, with a 6-mo survival rate of 50%[1]. Current management methods include limited intake of sodium, adequate diuretics plus albumin, large-volume paracentesis, and transjugular intrahepatic portosystemic shunt. Liver transplantation (LT) is the only effective strategy to cure cirrhosis and its complications. However, most patients cannot receive LT because of its high cost, the shortage of the donated liver, and many other factors. The role of the gut-liver axis in the occurrence and development of complications of cirrhosis has aroused great attention. Microbiota, dysbiosis, and bacterial translocation (BT) have been shown to be involved in the pathogenesis of cirrhosis. Bacteria and their products are introduced into blood via the intestines and then increase the blood levels of endotoxin and inflammatory factors, which, in turn, accelerate liver fibrosis and stimulate the production of vasodilator substances. These events cause reduced systemic vascular resistance and an activated sympathetic nervous system and renin-angiotensin-aldosterone system (RAAS), eventually leading to hyperdynamic circulation, which plays an important role in the pathogenesis of refractory ascites[2-6].
Rifaximin, a non-absorbable rifamycin derivate, exhibits a high antibiotic activity against both aerobic and anaerobic Gram-positive and -negative micro-organisms[7,8]. Furthermore, rifaximin is hardly absorbed by the intestines because of its pyridine ring added to rifamycin; thus, high concentrations are present in the intestines, making it difficult to induce drug-resistant strains[8,9]. Previous studies have shown that rifaximin treatment can reduce the concentrations of interleukin (IL)-6, tumour necrosis factor alpha (TNF-α), and endotoxin in blood, thus improving systemic haemodynamics and decreasing the hepatic venous pressure gradient in patients with cirrhosis[10-12]. Rifaximin has been demonstrated to exert positive effects in the prevention and treatment of hepatic encephalopathy (HE), and to prevent the development of esophagogastric and gastric variceal bleeding, spontaneous bacterial peritonitis (SBP), and hepatorenal syndrome (HRS)[10,13]. Hence, our study evaluated the clinical efficacy of rifaximin and its effects on intestinal flora characteristics and the systemic inflammatory state in cirrhotic patients with refractory ascites.
MATERIALS AND METHODS
Ethical considerations
The study protocol conformed to the Declaration of Helsinki and was approved by the Biomedical Research Ethics Committee of Peking University First Hospital (No. 2017[1367]). All participants provided written informed consent.
Patients and study design
A total of 143 cirrhotic patients with refractory ascites admitted to the Beijing You An Hospital Affiliated to Capital Medical University were screened between November 2016 and May 2018 and, eventually, 75 patients were enrolled in this real-world study. All the patients received routine treatment for refractory ascites according to Chinese guidelines on the management of ascites and complications in cirrhosis in 2017[14]: (1) Bed rest, limited sodium intake (salt intake < 6 g/d), maintenance of the balance of water, electrolytes, and acid-base; (2) Alcohol quitting or antiviral treatment; (3) Intravenous infusion with reduced glutathione 1.2 g/d dissolved in 100 mL of 0.9% sodium chloride injection; (4) Oral furosemide 80 mg/d + spironolactone 160 mg/d; (5) Intravenous infusion with human albumin (Switzerland, CSL Behring) 10-20 g, once per day; and (6) Corresponding antibiotics for patients who met the diagnostic criteria for SBP. Additionally, patients in the rifaximin group received oral rifaximin α (Italy, Alpha Wassermann) 200 mg, four times/d for 3 wk to 4 wk. A 6-mo follow-up was performed after treatment to evaluate the survival once per month.
The primary endpoints were clinical efficacies, including changes in the fasting weight, ascites regression, and survival rate, while the secondary endpoints were changes in liver and kidney function. Ascites regression was evaluated according to the change in the grade of ascites according to the Chinese guidelines on the management of ascites and complications in cirrhosis in 2017[14,15]: Markedly effective: Change from grade 3 to grade 1 or from grade 3/2 to none; effective: Change from grade 3 to grade 2 or from grade 2 to grade 1; invalid: Increase or no change after the treatment.
Inclusion and exclusion criteria
The inclusion criteria were as follows: (1) Age ranging 18-80 years; (2) Gender was not limited; (3) Diagnosed with cirrhosis based on cirrhosis imaging findings or endoscopic findings of oesophageal varices; and (4) In line with a cirrhotic refractory ascites diagnosis according to Chinese guidelines on the management of ascites and complications in cirrhosis in 2017[14]: (1) Lack of a response (defined as a mean fasting weight loss < 0.8 kg over 4 d) to 1-wk treatment with a diuretic (furosemide 80 mg/d or spironolactone 160 mg/d) and a diuretic plus antibiotic (SBP), or the reappearance of grade 2/3 ascites within 4 wk of therapy with large-volume paracentesis combined with a human serum albumin level of 10-20 g/d; or (2) The appearance of diuretic-related complications such as HE, serum creatinine > 2.0 mg/dL, serum sodium < 125 mmol/L, or serum potassium > 6.0 mmol/L. The exclusion criteria were as follows: (1) HE grades 2-4 according to the West Haven diagnostic criteria[16]; (2) Upper gastrointestinal bleeding within 1 wk before enrolment; (3) Combined with other gut diseases; (4) Continued abuse of alcohol and/or symptoms of withdrawal; (5) Combined with invasive tumours; and (6) Combined with other serious diseases.
Clinical and laboratory data
The data concerning demographics, clinical history, aetiology and complications of cirrhosis, and comorbidities were collected. The fasting weight (kg) was recorded. The grades of ascites were defined as follows according to the patient's symptoms, signs, and colour Doppler ultrasound: Grade 1: No abdominal distension, shifting dullness (-), ascites (detected by ultrasound) located in multiple gaps with a depth < 3 cm; grade 2: Moderate symmetric abdominal distension, shifting dullness (±), and a depth of 3-10 cm; and grade 3: Significant abdominal distension, or even abdominal distension leading to umbilical hernia, shifting dullness (+), and a depth of > 10 cm[14,15].
Laboratory indicators were collected on day 0 (d0) and day 15 (d15) and included the WBC, HGB, PLT, ALT, AST, TBil, Alb, BUN, Scr, serum K+, Na+, prothrombin activity, and INR. The MDRD formula was used to calculate the estimate glomerular filtration rate (eGFR).
The diagnostic criteria for SBP and acute kidney injury (AKI) were as follows[14,15]: Patients with one or more of the following symptoms, signs, or laboratory abnormalities could be diagnosed with SBP: (1) Abdominal pain, abdominal tenderness or rebound tenderness, and an increase in abdominal muscular tension; (2) Systemic inflammatory response syndrome: Fever or normothermia, chills, tachycardia, and tachypnoea; (3) Deterioration of liver function without an underlying cause; (4) Sudden absence of a response to diuretics or renal failure; (5) Polymorphonuclear cell count in ascites ≥ 0.25 × 109/L; (6) Positive ascites bacterial culture; (7) Serum procalcitonin concentration > 0.5 ng/mL; and (8) Exclusion of infection in other sites. AKI was diagnosed in case of patients with acute renal failure, manifesting as an acute and significant decrease in GFR and Scr > 133 μmol/L.
Measurement of inflammation and endotoxin markers
The concentrations of IL-6, IL-8, TNF-α, monocyte chemoattractant protein-1, interferon-inducible protein 10 (IP-10), and lipopolysaccharide-binding protein (LBP) were determined with Luminex (Magnetic Luminex Assay; R&D Systems Europe, Ltd., Abingdon OX14 3NB, United Kingdom) in fasting venous plasma samples, which were collected into 10-mL EDTA tubes on d0 and d15 and were quickly separated and then stored in a freezer at -80 °C after centrifugation at 3000 r/min for 15 min.
Analysis of composition of gut microbiota
Fresh faeces were collected in the rifaximin group at d0 and d15 and were stored in a freezer at -80 °C within 2 h. The DNA of the faecal flora was analysed by metagenomics sequencing (Illumina HiSeq), and the gene structure was predicted with Meta Gene Mark, followed by bioinformatics analysis.
Statistical analysis
The statistical review of this study was performed by biomedical statisticians. The Student’s t-test (including independent sample and paired sample), χ2 test, Fisher’s exact test, Mann-Whitney U test, and PERMANOVA test were selected according to different types of variables. The cumulative incidence was used to describe the rate of death and competing risk model (Gray’s test) was used to test the difference of survival rates between the two groups. Data processing was performed using BMI SPSS 22.0, GraphPad Prism 6, and Stata 14.0 software. The biological information of intestinal microbiota was analysed with R Statistical software. P values < 0.05 (two-tail) were considered statistically significant.
RESULTS
Patient characteristics
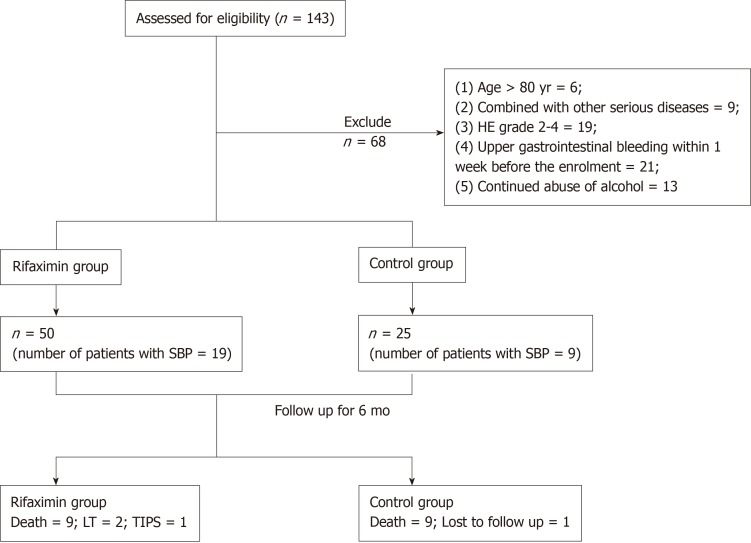
One hundred and forty-three patients were assessed for eligibility, and 75 patients who fulfilled the inclusion criteria were finally enrolled in the study (Figure 1). No significant differences were found in the clinical data, including age, gender distribution, aetiology, Child-Pugh grade, model for end-stage liver disease (MELD) score, ascites grade, dosage of diuretic, and proportion of patients with SBP and AKI between the rifaximin group (n = 50; 19 patients treated with rifaximin and 31 patients treated with rifaximin plus intravenous antibiotics therapy) and the control group (n = 25) (Table 1).
Figure 1.
Flow chart for patient selection. SBP: Spontaneous bacterial peritonitis; TIPS: Transjugular intrahepatic portosystemic shunt; LT: Liver transplantation.
Table 1.
Baseline characteristics of patients, n (%)
| Characteristic | Rifaximin group (n = 50) | Control group (n = 25) | P value |
| Age | 54.60 ± 9.05 | 59.04 ± 10.01 | 0.057 |
| Sex (male/female) | 42/8 | 18/7 | 0.221 |
| Etiology | |||
| HBV | 14 (28) | 9 (36) | 0.479 |
| HCV | 3 (6) | 0 (0) | 0.546 |
| Alcohol | 22 (44) | 7 (28) | 0.180 |
| HBV/alcohol | 7 (14) | 3 (12) | 1.000 |
| NASH | 1 (2) | 3 (12) | 0.105 |
| AIH | 1 (2) | 0 (0) | 1.000 |
| PBC | 0 (0) | 1 (4) | 0.333 |
| Cryptogenic | 2 (4) | 2 (8) | 0.597 |
| Child-Pugh grade (B/C) | 21/29 | 16/9 | 0.072 |
| MELD score | 10 (6-13) | 8 (6-10) | 0.094 |
| Furosemide (mg/d) | 80 (40-80) | 80 (40-80) | 0.246 |
| Spironolactone (mg/d) | 160 (80-160) | 160 (100-160) | 0.315 |
| Ascites (grade 1/2) | 38/12 | 20/5 | 0.978 |
| AKI | 3 (6) | 2 (8) | 1.000 |
| SBP | 19 (38) | 9 (36) | 0.866 |
| Hypertension | 7 (14) | 5 (20) | 0.738 |
| Diabetes mellitus | 19 (38) | 9 (36) | 0.866 |
| Biochemistry | |||
| ALT (U/L) | 24.85 (13.75-42.58) | 20.50 (16.40-34.95) | 0.673 |
| Alb (g/L) | 28.40 ± 5.30 | 27.68 ± 3.79 | 0.526 |
| TBil (μmol/L) | 43.80 (25.88-93.78) | 24.70 (18.75-55.55) | 0.039 |
| Scr (μmol/L) | 70.20 (57.00-93.53) | 64.20 (53.10-74.35) | 0.256 |
| Urea (mmol/L) | 7.17 ± 4.70 | 6.68 ± 3.32 | 0.641 |
| eGFR (mL/min 1.73 m2) | 95.49 (76.17-114.81) | 98.80 (86.80-109.37) | 0.897 |
| Coagulation | |||
| PTA (%) | 54.00 (45.00-68.50) | 64.00 (56.50-74.50) | 0.030 |
| INR | 1.53 ± 0.39 | 1.37 ± 0.26 | 0.074 |
| Routine blood test | |||
| WBC (109/L) | 4.38 (3.41-6.04) | 3.87 (3.26-6.78) | 0.955 |
| HGB (g/L) | 101.22 ± 25.91 | 98.36 ± 23.03 | 0.642 |
| PLT (109/L) | 77 (54-102) | 101 (56-172) | 0.069 |
Values are given as the mean ± SD if they follow a normal distribution, otherwise given as medians (interquartile range). HBV: Hepatitis B virus; HCV: Hepatitis C virus; NASH: Nonalcoholic steatohepatitis; AIH: Autoimmune hepatitis; PBC: Primary biliary cirrhosis; AKI: Acute kidney injury; SBP: Spontaneous bacterial peritonitis; eGFR: Estimate glomerular filtration rate; PTA: Prothrombin activity; MELD: Model for end-stage liver disease.
Rifaximin reduces the body weight significantly and mitigates ascites in cirrhotic patients with refractory ascites
After the 2-wk treatment, the weight of the patients in the rifaximin group (median: 70.00 kg vs 69.00 kg) decreased more significantly than that in the control group (median: 70.00 kg vs 69.00 kg) (P = 0.011). The ascites subsided significantly in the rifaximin group (18 markedly effective, 21 effective, and 11 invalid) compared with that in the control group (9 markedly effective, 3 effective, and 13 invalid) (P = 0.009).
Rifaximin treatment improves the 6-month survival in cirrhotic patients with refractory ascites
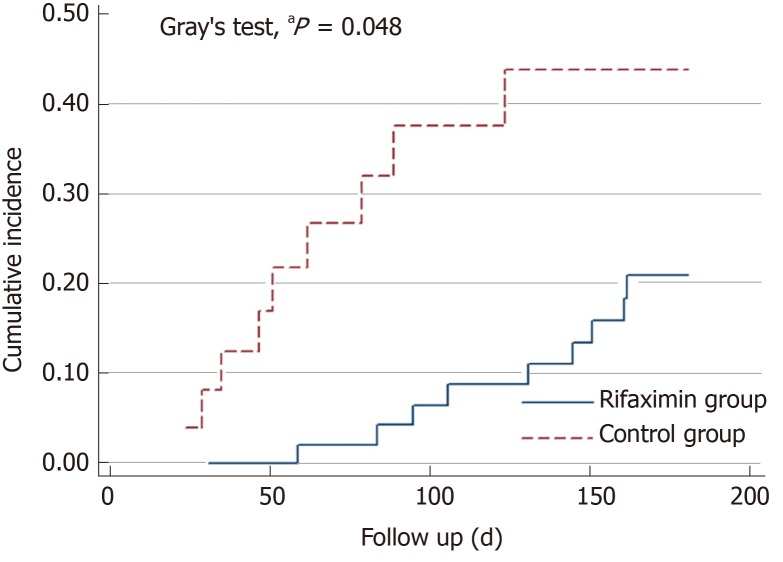
Nine patients in the rifaximin group died within 6 mo, two patients underwent LT, and one underwent transjugular intrahepatic portosystemic shunt during the follow-up. However, nine patients died within 6 mo and one patient was lost to follow-up in the control group (Figure 1). The cumulative survival rate at 6 mo was significantly higher in the rifaximin group than in the control group (subdistribution hazard ratio = 2.53, 95%CI: 1.01-6.38, P = 0.048) (Figure 2).
Figure 2.
Gray’s test showed that the cumulative survival was significantly higher in the rifaximin group than in the control group. aP < 0.05 vs control group.
Two weeks of treatment with rifaximin has no significant effect on liver and kidney function in cirrhotic patients with refractory ascites
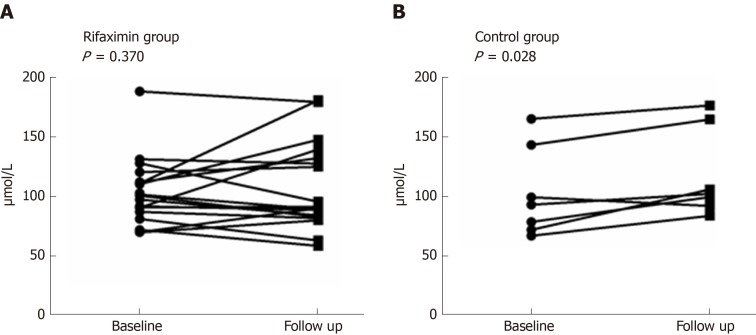
After 2 wk of treatment, the Child-Pugh scores of patients decreased significantly in both the rifaximin and control groups (P < 0.001 and 0.015, respectively), while no significant change was noted in the MELD scores (P = 0.202 and 0.189, respectively). No significant difference was noted in the Child-Pugh and MELD scores between the rifaximin and control groups (P = 0.666 and 0.688, respectively). The concentrations of Scr in the rifaximin and control groups were significantly increased after treatment (P = 0.002 and < 0.001, respectively), but no significant difference was found in the change of Scr between the groups (P = 0.258) (Table 2). Analysis of patients with abnormal renal function at baseline (eGFR < 90 mL/min 1.73 m2) (20 patients in the rifaximin group and 7 patients in the control group) showed that the concentration of Scr in the rifaximin group was slightly lower than that before treatment (P = 0.370), but there was a significant increase in the control group (P = 0.028); no significant difference was noted in the change of Scr between the two groups (P = 0.268) (Figure 3).
Table 2.
Changes in clinical data of patients after treatment in the two groups
|
Rifaximin group (n = 50) |
Control group (n = 25) |
P value |
|||||
| d0 | d15 | mean delta | d0 | d15 | Mean delta | ||
| Weight (kg) | 70.00 (60.00-78.75) | 69.00 (58.00-74.00) | -3.30 | 62.00 (55.00-71.25) | 59.5 (54.88-59.5) | -1.20 | 0.011 |
| Cr (μmol/L) | 70.20 (57.00-93.53) | 78.25 (63.10-92.68) | 11.82 | 9 (8.00-11.00) | 73.0 (63.35-95.65) | 13.54 | 0.777 |
| Urea (mmol/L) | 7.17±4.70 | 9.07±6.71 | 1.90 | 8 (5.50-10.00) | 7.63±3.31 | 0.95 | 0.258 |
| eGFR (mL/min 1.73 m2) | 95.49 (76.17-114.81) | 93.11 (71.92-108.00) | -5.20 | 98.80 (86.80-109.37) | 92.0 (61.59-99.54) | -9.68 | 0.136 |
| Child-Pugh score | 10 (8.00-11.25) | 9 (8.00-10.25) | -0.90 | 9 (8.00-11.00) | 8 (7.50-10.50) | -0.76 | 0.666 |
| MELD score | 10 (6.00-13.00) | 11.5 (6.75-15.25) | 0.60 | 8 (5.50-10.00) | 8 (6.50-10.00) | 0.92 | 0.688 |
Delta was defined as the value after treatment minus the value before treatment. eGFR: Estimate glomerular filtration rate; MELD: Model for end-stage liver disease.
Figure 3.
Changes in the concentration of Scr after treatment in patients with renal dysfunction. A: A downtrend in the rifaximin group; B: An uptrend in the control group.
Rifaximin improves the systemic inflammatory state of cirrhotic patients with refractory ascites
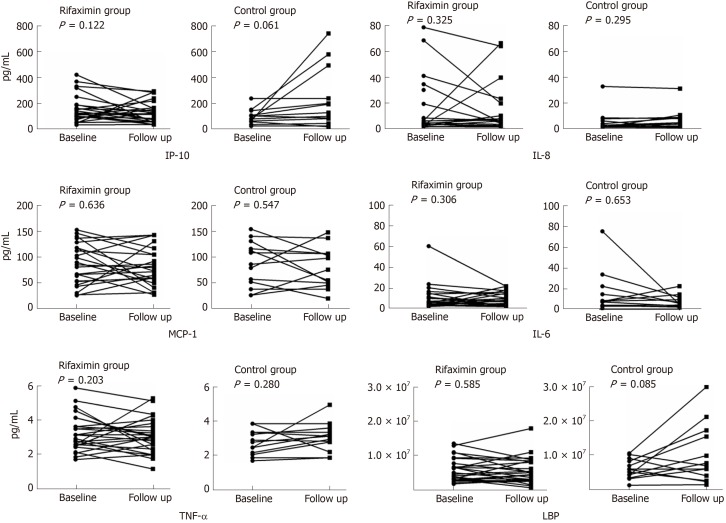
Finally, 39 patients (26 in the rifaximin group and 13 in the control group) were tested for the markers of inflammation and endotoxin before and after treatment. The concentration of IP-10 decreased significantly in the rifaximin group (187.83 ± 180.08 at baseline vs 150.35 ± 126.22 at follow-up) compared with that in the control group (98.53 ± 57.02 at baseline vs 228.25 ± 232.65 at follow-up) (P = 0.02). Changes in the concentrations of IL-6, IL-8, TNF-α, monocyte chemoattractant protein-1, and LBP were also analysed, and no differences were observed in these markers after treatment (Table 3). However, Figure 4 shows that the concentrations of TNF-α and LBP in patients treated with rifaximin decreased after treatment but increased in the control group.
Table 3.
Changes in the cytokine and lipopolysaccharide-binding protein levels in patients after treatment in the two groups
|
Rifaximin group (n = 26) |
Control group (n = 13) |
P value | |||
| d0 | d15 | d0 | d15 | ||
| IL-6 (pg/mL) | 29.73 ± 101.82 | 8. 93 ± 6.13 | 27.05 ± 45.70 | 18.75 ± 39.11 | 0.644 |
| IL-8 (pg/mL) | 45.12 ± 91.71 | 117.47 ± 359.77 | 31.51 ± 90.82 | 42.92 ± 128.15 | 0.557 |
| TNF-α (pg/mL) | 3.62 ± 2.22 | 3.23 ± 1.31 | 2.79 ± 0.72 | 3.07 ± 0.83 | 0.356 |
| MCP-1 (pg/mL) | 93.32 ± 49.00 | 98.41 ± 61.21 | 86.71 ± 44.14 | 79.61 ± 39.87 | 0.481 |
| IP-10 (pg/mL) | 187.83 ± 180.08 | 150.35 ± 126.22 | 98.53 ± 57.02 | 228.25 ± 232.65 | 0.024 |
| LBP (pg/mL) | 6140682.77 ± 3478004.74 | 5828915.42 ± 3761441.65 | 5784964.46 ± 2841716.75 | 9792449.77 ± 8492517.57 | 0.071 |
The data are given as the mean ± SD. Interleukin-6, interleukin-8, tumour necrosis factor alpha, and monocyte chemoattractant protein-1 suggest an inflammatory response; interferon-inducible protein 10 activates inflammatory cells and probably affects hepatic stellate cells and cirrhosis; and lipopolysaccharide-binding protein reflects the degree of the inflammatory response caused by endotoxin. IL-6: Interleukin-6; IL-8: Interleukin-8; TNF-α: Tumour necrosis factor alpha; MCP-1: Monocyte chemoattractant protein-1; LBP: Lipopolysaccharide-binding protein. IP-10: Interferon-inducible protein 10.
Figure 4.
Changes in inflammatory factors and lipopolysaccharide-binding protein after treatment in the two groups. The concentrations of interferon-inducible protein 10, tumour necrosis factor alpha, and lipopolysaccharide-binding protein showed a downtrend in the rifaximin group but uptrend in the control group, and interferon-inducible protein 10 decreased significantly in the rifaximin compared with the control group (Table 3). IL-6: Interleukin-6; IL-8: Interleukin-8; TNF-α: Tumour necrosis factor alpha; MCP-1: Monocyte chemoattractant protein-1; LBP: Lipopolysaccharide-binding protein; IP-10: Interferon-inducible protein 10.
Rifaximin affects the characteristics and function of gut microbiota in cirrhotic patients with refractory ascites
A total of 40 stool samples from 20 patients (12 patients treated with rifaximin and 8 patients treated with rifaximin plus intravenous antibiotics) were subjected to bioinformatics analysis after metagenomics sequencing. The diversity Shannon index was calculated using function diversity, the differences before and after treatment were tested using the Wilcoxon test, and the beta diversity was analysed using the non-metric multidimensional scale. Lefse analysis and the Wilcoxon signed-rank test were applied to analyse the changes in the gut microbiota after treatment. The analysis of virulence was performed on all predicted genes using the Virulence Factors Database.
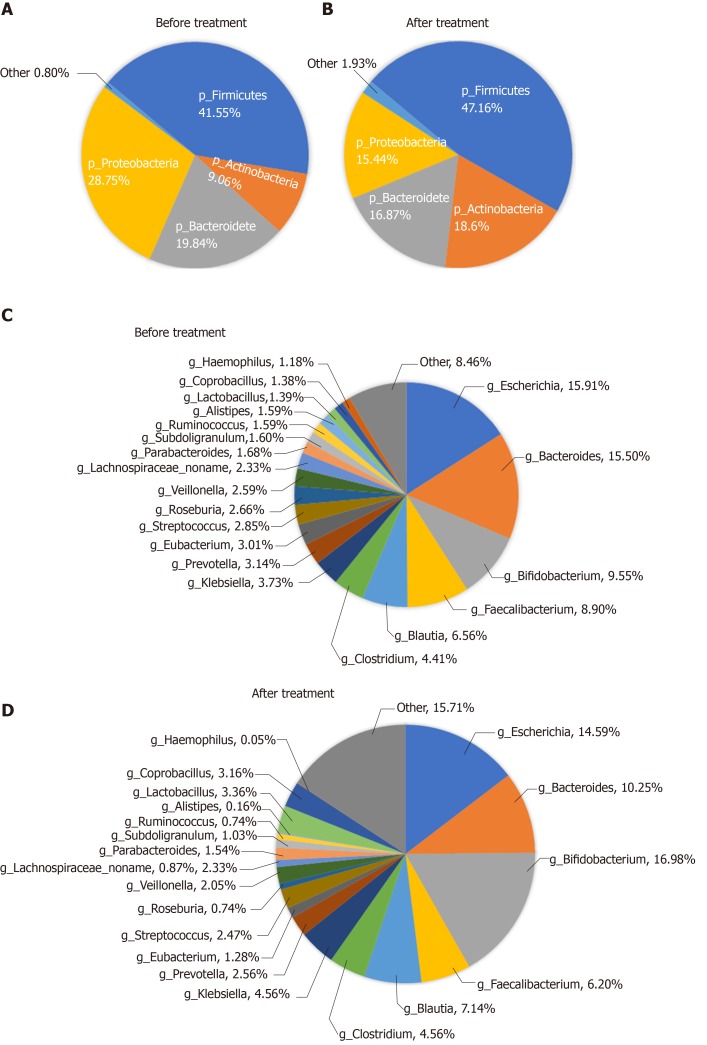
Analysis of composition of gut microbiota: The bacteria in the stools of patients before and after treatment were mainly classified into Firmicutes, Proteobacteria, Bacteroidetes, and Actinobacteria at the phylum level, of which the most dominant phylum was Firmicutes (41.55% before vs 47.16% after treatment). At the genus level, they were mainly classified into Escherichia, Bacteroides, Bifidobacterium, and Faecalibacterium (Figure 5).
Figure 5.
Abundance of the microbiota before and after treatment at the phylum and genus levels. A and B: Phylum level; C and D: Genus level.
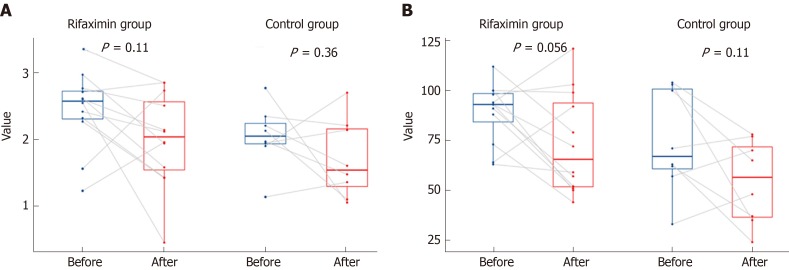
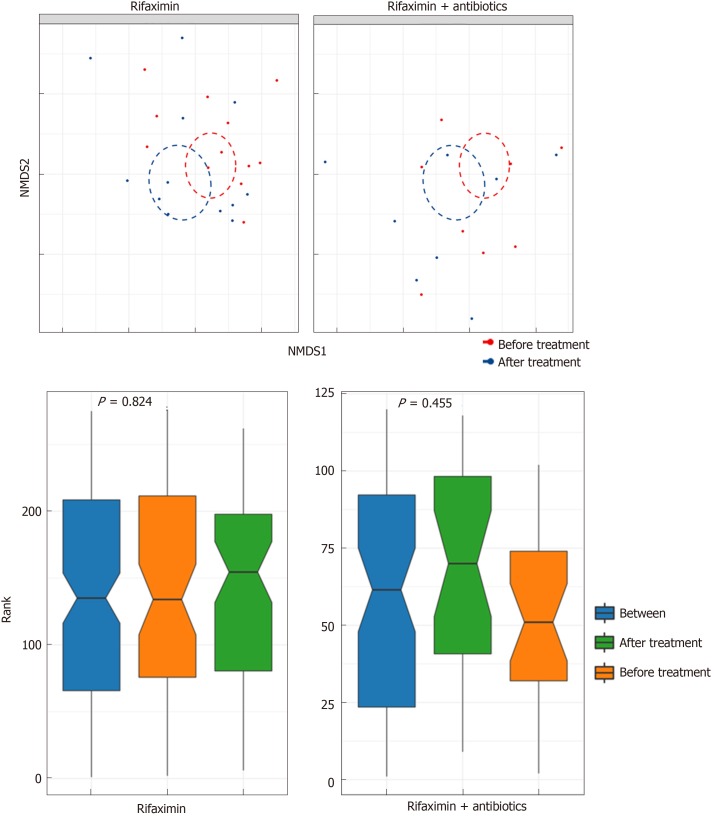
Analysis of diversity: The Shannon index (P = 0.11 and 0.36), richness (P = 0.055 and 0.11, respectively), and beta diversity (P = 0.824 and 0.455, respectively) of faecal microbiota did not change significantly after treatment with rifaximin and rifaximin plus intravenous antibiotics (Figures 6 and 7).
Figure 6.
Changes in the Shannon index and Richness in patients of the two groups before and after treatment. A: Changes in the Shannon index in patients of the two groups before and after treatment (no significant decrease); B: Changes in the Richness in patients of the two groups before and after treatment: (no significant decrease).
Figure 7.
Change in the beta diversity of patients in the two groups before and after treatment.
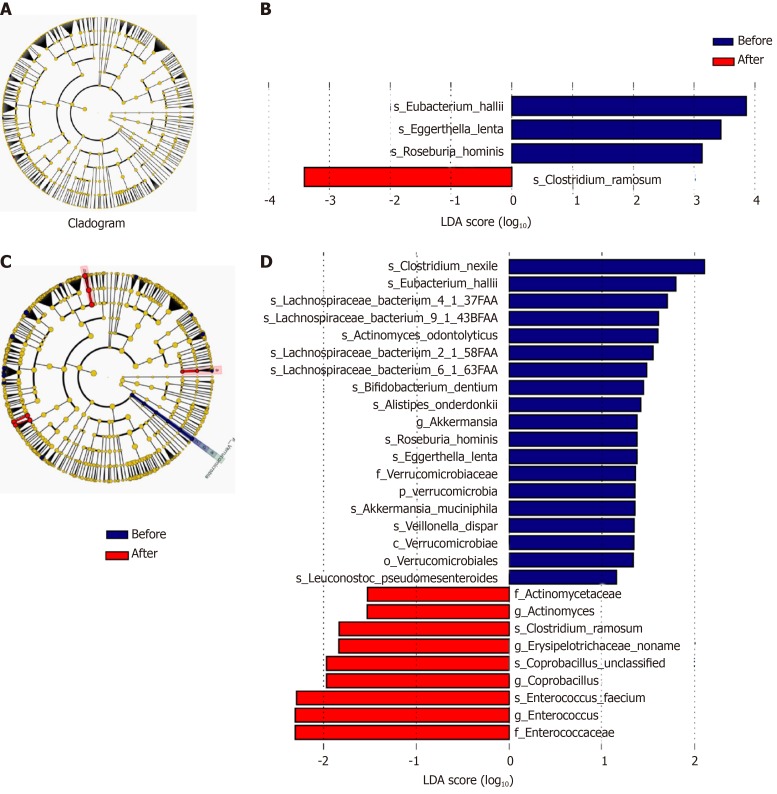
Analysis of different microbiota in faecal samples: Lefse analysis showed that, at the phylum, class, order, family, and genus levels, no significant change was found in the faecal flora in patients treated with rifaximin. However, in patients treated with rifaximin plus intravenous antibiotics, the abundance of Verrucomicrobia, Verrucomicrobiae, Verrucomicrobiales, Verrucomicrobiaceae, and Akkermansia significantly decreased, and the abundance of Actinomycetaceae, Enterococcaceae, Actinomyces, Enterococcus, and Coprobacillus significantly increased. At the species level, the abundance of Clostridium_ramosum was significantly increased and that of Eggerthella_lenta, Roseburia_hominis, and Eubacterium_hallii significantly decreased after treatment with rifaximin. However, Clostrium_nexile, Eubacterium_hallii, Lachnospiraceae-bacterium4_1_37FAA, Lachnospiraceae-bacterium9_1_43BFAA, Actinomyces-odontolyticus, Lachnospiraceae-bacterium2_1_58FAA, Lachnospiraceae-bacterium6_1_63FAA, Bifidobacterium-Dentium, Alistipes-Onderdonkii, Roseburia_hominis, Escherichia coli, Veillonella_dispar, and Leuconostoc_pseudomesenteroides decreased significantly and the abundance of Clostridium_ramosum, Enterococcus_faecium, and Coprobacillus_unclassified was significantly increased after treatment with rifaximin plus intravenous antibiotics (Figure 8).
Figure 8.
Cladogram and Linear discriminant analysis score of Lefse analysis of patients in the subgroup of rifaximin and rifaximin plus intravenous antibiotics. A and B: Subgroup of rifaximin; C and D: Rifaximin plus intravenous antibiotics. The taxonomic levels are shown in the cladograms, represented by rings with kingdoms at the innermost ring and genera at the outermost ring, the small circles at each classification level represent different classifications at that level, and the diameters represent the relative abundance. Yellow represents no significant difference while blue and red represent significantly different microbiota before and after treatment, respectively. The Linear discriminant analysis score shows significant differences at > 2.0; red and blue represent before and after treatment, respectively, and the length represents the Linear discriminant analysis score, which is the degree of significant difference. LDA: Linear discriminant analysis.
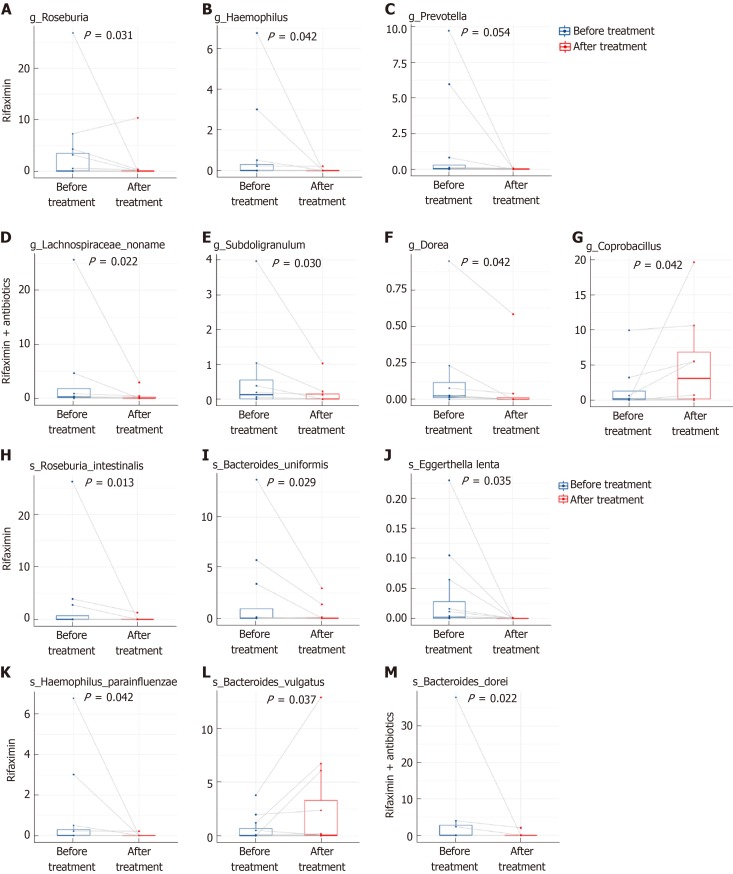
Wilcoxon test showed that, at the genus level, the abundance of Roseburia, Haemophilus, and Prevotella was significantly reduced after treatment with rifaximin, while the abundance of Lachnospiraceae_noname, Subdoligranulum, and Dorea decreased and the abundance of Coprobacillus increased significantly after treatment with rifaximin plus intravenous antibiotics (Figure 9). At the species level, the abundance of Roseburia_intestinalis, Bacteroides_uniformis, Eggerthella_lenta, and Haemophilus_parainfluenzae was significantly reduced, and the relative abundance of Bacteroidetes_vulgatus was significantly increased after treatment with rifaximin. Additionally, the abundance of Bacteroides_dorei was significantly reduced after treatment with rifaximin plus intravenous antibiotics (Figure 9). The abundance of Bifidobacterium and Lactobacillus in the intestinal flora, as well as the abundance of Faecalibacterium_prausnitzii, decreased after both treatments, although the changes were not significant (Table 4).
Figure 9.
Abundance of microbiota with significant changes at the genus and species levels. A-G: Abundance of microbiota with significant changes at the genus level; H-M: Abundance of microbiota with significant changes at the species level.
Table 4.
Changes in the abundance of beneficial bacteria after treatment
| Classification |
Rifaximin |
Rifaximin + antibiotics |
||||
| Before | After | P value | Before | After | P value | |
| g_Lactobacillus | 1.924 | 3.903 | 0.791 | 0.581 | 2.535 | 0.547 |
| g_Bifidobacterium | 12.567 | 21.949 | 0.301 | 5.036 | 9.524 | 0.641 |
| s_Faecalibacterium_prausnitzii | 10.448 | 8.193 | 0.910 | 6.590 | 3.206 | 0.148 |
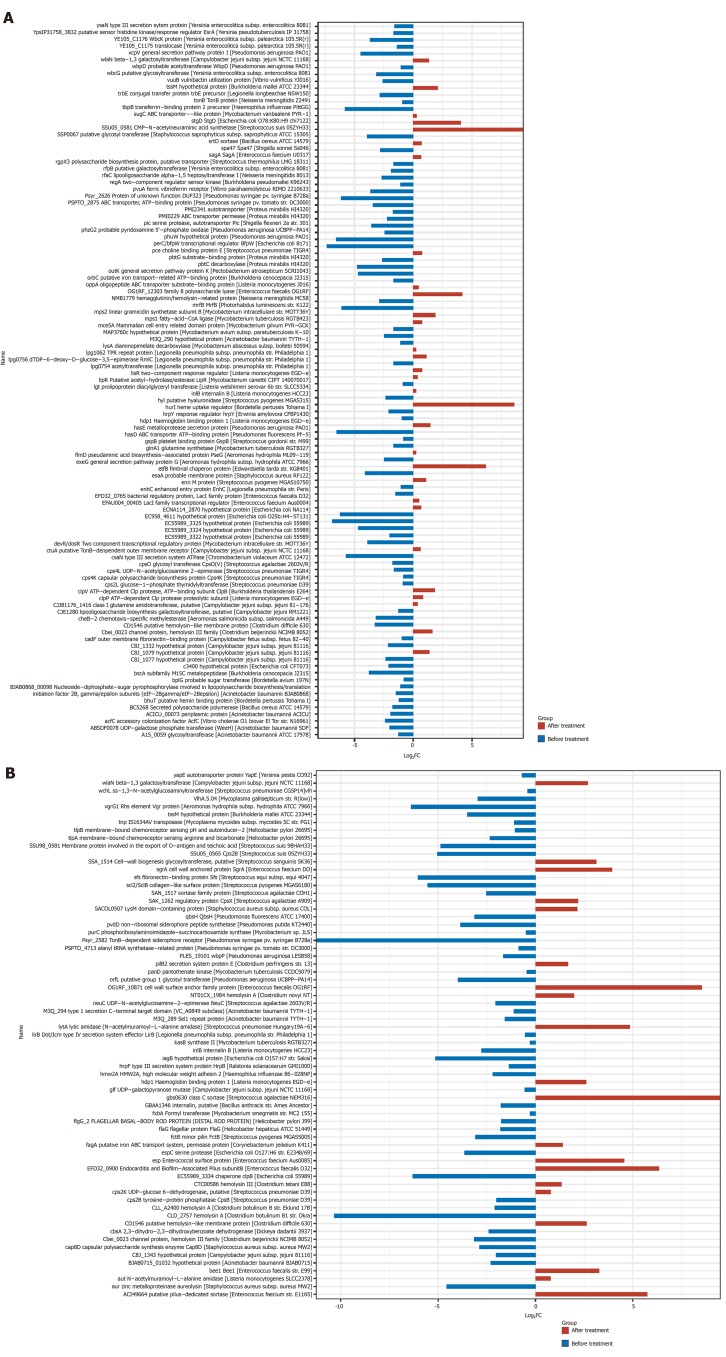
Analysis of bacterial virulence: The obtained non-redundant reference gene set was compared with the Virulence Factors Database using DIAMOND (v0.7.9.58), and virulence analysis was performed on all predicted genes. The results are shown in Figure 10. The total expression of virulence factor genes of the intestinal flora in both groups was significantly reduced after treatment.
Figure 10.
Expression of virulence factor genes before and after treatment with rifaximin and rifaximin plus intravenous antibiotics. A: Treatment with rifaximin; B: Treatment with rifaximin plus intravenous antibiotics.
The ratio of virulence factor gene expression after treatment and before treatment is taken as log2 (fold change), which is calculated as log2 (mean value after treatment/mean value before treatment), and log2 (fold change) < 0 indicated that the virulence factor gene expression was decreased significantly after treatment.
DISCUSSION
The gut microbiota is an important component of the intestinal micro-environment that is involved in the metabolism of various substances, the composition of the mucosal barrier, and the development and maturation of the immune system[17]. There is increasing evidence that a significant difference exists in the gut microbiota between patients with cirrhosis and healthy people[18,19]. Based on the assumption that bacterial overgrowth and translocation cause the systemic inflammatory state, which aggravates the dysfunction of stellate cells and hyperdynamic circulation in patients with cirrhosis, this study suggests that rifaximin can be used to interfere with the process above and improve the haemodynamics by targeting the gut microbiota.
Our study showed that treatment with rifaximin mitigated ascites and improved the survival in cirrhotic patients with refractory ascites. However, 2-wk treatment with rifaximin revealed no significant effect on liver and kidney function. This study also analysed the changes in the concentrations of IL-6, TNF-α, and other inflammatory factors, as well as LBP, in the plasma of cirrhotic patients with refractory ascites. No significant change was noted in the inflammatory factors other than IP-10 after treatment with rifaximin, although the concentrations of TNF-α and LBP showed a declining trend, which is consistent with the results of a previous randomized controlled trial[20]. Previous studies have shown that rifaximin inhibits activation of the NF-κB signalling transduction pathway and downregulates the expression of cytokines and chemokines[21,22]. Studies have also shown that rifaximin can reduce the concentrations of IL-6, TNF-α, and endotoxin in patients with decompensated cirrhosis, thereby exercising the effects of reducing portal pressure and improving haemodynamics[11,12,23]. However, these studies and their follow-up did not set up a controlled trial. Although a case-control study showed that long-term treatment with rifaximin reduced the incidence of esophagogastric and gastric variceal bleeding, SBP, and HRS in patients with decompensated alcoholic cirrhosis and improved their survival rate[13], rifaximin treatment in patients had already been confirmed to be effective before enrolment; thus, there may exist a selection bias. A randomized, double-blind, controlled trial revealed that 4 wk of treatment with rifaximin did not affect the hepatic venous pressure gradient, systemic haemodynamics, GFR, or levels of vasoactive hormones and had no impact on the inflammatory state and only minor effects on BT and intestinal bacterial composition in stable, decompensated cirrhosis[20,24]. Considering the mechanism of action of rifaximin, it may exert effects on patients with poor liver function and severely disordered haemodynamics; hence, a randomized controlled trial performed on a subgroup analysis of patients with Child-Pugh C liver function and elevated LBP at baseline still showed no significant difference[20,24]. Our study performed subgroup analysis in patients with lower eGFR than normal at baseline, and the results suggest a trend of decline in the levels of Scr after treatment with rifaximin but a trend of elevation in patients in the control group, suggesting that rifaximin can show advantages in patients with severely disordered haemodynamics and a high inflammatory state. Regrettably, this study did not assess the effect of long-term treatment with rifaximin on these indicators and did not collect haemodynamic-related data. Thus, further research is needed to assess the effect of long-term treatment with rifaximin on the prognosis of cirrhotic patients with severely disordered haemodynamics and a high inflammatory state.
Studies on irritable bowel syndrome (IBS) have found that rifaximin can inhibit the growth of Escherichia coli, Enterococcus faecalis, Staphylococcus aureus, Klebsiella, Enterobacterium, and other non-Enterobacterium-Gram-negative enterobacteria in vitro[25] and increase the abundance of Lactobacilli and reduce the number of Bacillus filiformis[26]. Thus, rifaximin is considered to inhibit the growth of pathogenic bacteria without affecting the normal composition of gut microbiota. In recent years, rifaximin has been increasingly studied in the prevention and treatment of cirrhosis complications such as SBP and HRS, but the effect of rifaximin in patients with decompensated cirrhosis remains controversial. Studies have shown that rifaximin has minor effects on bacterial composition, inflammation, and BT in stable, decompensated cirrhosis[20,24]. Other studies have shown no significant microbial change besides a modest decrease in Veillonellaceae and Streptococcus, and an increase in Eubacteriaceae was observed after rifaximin treatment in patients with HE[27-29]. Considering that rifaximin reduces endotoxaemia but has no significant effect on intestinal flora, it is preferred that rifaximin is more likely to exert pharmacological effects through the following pathways: (1) Altering bacterial function and virulence; (2) Improving intestinal barrier function; and (3) Inducing resistance to some flora[28,30]. Studies have confirmed that rifaximin inhibits the adhesion and translocation of bacteria, reduces the virulence of bacteria, and regulates the metabolism of gut microbiota[27,31-33]. Using metagenomic sequencing, this study evaluated the changes in the intestinal flora in cirrhotic patients with refractory ascites after treatment with rifaximin and rifaximin plus intravenous antibiotics. Previously, it was generally believed that the diversity of gut microbiota was decreased in disease conditions, particularly in patients with intestinal diseases, and increased after the disease was relieved[34]. Our study suggested a decline in the richness of gut microbiota in cirrhotic patients with refractory ascites after treatment with rifaximin, probably related to the premise of treatment with antibiotics in this study. In this study, patients with refractory ascites and severely disordered haemodynamics were selected as the research subjects, and the changes in the gut microbiota after treatment with rifaximin are not consistent with the results of previous studies with rifaximin in the complications of cirrhosis such as HE and ascites.
We compared our results with the differences between the structure of gut microbiota in cirrhotic patients and healthy people reported by Qin et al[19]: (1) At the genus level, an increase in the abundance of Haemophilus and Prevotella and a decrease in the abundance of Roseburia, Subdoligranulum, and Dorea were found in patients with cirrhosis by Qin et al[19]; we found a decrease in the abundance of Haemophilus, Prevotella, and Roseburia after treatment with rifaximin but a decrease in Subdoligranulum and Dorea after treatment with rifaximin plus intravenous antibiotics in cirrhotic patients with refractory ascites; and (2) At the species level, a decrease in the abundance of Haemophilus_parainfluenzae, Roseburia_intestinalis, and Bacteroides_uniformis was found in patients with cirrhosis by Qin et al[19] as well as in cirrhotic patients with refractory ascites after treatment with rifaximin in our study.
Studies have shown an increase in the concentration of Bifidobacterium in the stool of patients with inflammatory bowel disease after rifaximin treatment[35,36]. The abundance of Lactobacilli in faecal samples increased one month after treatment with rifaximin in patients with different gastrointestinal diseases such as IBS, inflammatory bowel disease and diverticulosis, but the composition of the gut microbiota did not show any significant change[37]. Another study suggested that the abundance of Faecalibacterium_prausnitzii is increased at the end of rifaximin treatment in non-constipation IBS, and the composition of the gut microbiota did not change significantly[38]. Bifidobacterium has an intestinal and systemic anti-inflammatory effect[39]; butyrate production by Faecalibacterium_prausnitzii was involved in the regulation of proliferation, apoptosis, and differentiation of gastrointestinal epithelial cells, exercising an immunomodulatory effect[40,41]; Lactobacillus has anti-inflammatory, immunomodulatory, anti-oxidative, anti-bacterial, and anti-viral properties[30,42]. Our results suggest that the abundance of Bifidobacterium and Lactobacillus in the gut microbiota is increased after treatment with rifaximin and rifaximin plus intravenous antibiotics in cirrhotic patients with refractory ascites, while the abundance of Faecalibacterium_prausnitzii had a slight decrease.
In conclusion, this study evaluated the effects of rifaximin in cirrhotic patients with refractory ascites with regard to clinical efficacy, plasma inflammatory factors, and changes in the gut microbiota. We concluded that rifaximin may affect the structure and function of the gut microbiota and improve the systemic inflammatory response, thereby improving the clinical symptoms and survival rate in cirrhotic patients with refractory ascites. Studies with a large sample size are still needed to verify our conclusions, and the role of these flora remains to be further explored, since the changes in the gut microbiota in cirrhotic patients with refractory ascites remain unavailable.
ARTICLE HIGHLIGHTS
Research background
Patients with refractory ascites have a poor prognosis and there is no effective treatment except for liver transplantation. Rifaximin has been shown to reduce the incidence of hepatic encephalopathy and other complications in patients with cirrhosis. However, few studies have investigated the effect of rifaximin in cirrhotic patients with refractory ascites. And the mechanism of rifaximin in cirrhotic patients with refractory ascites remains unclear.
Research motivation
Previous studies have shown that rifaximin treatment can reduce the concentrations of interleukin-6, tumour necrosis factor alpha, and endotoxin in blood, thus improving systemic haemodynamics and decreasing the hepatic venous pressure gradient in patients with cirrhosis. Rifaximin has been demonstrated to exert positive effects in the prevention and treatment of hepatic encephalopathy and to prevent the development of esophagogastric and gastric variceal bleeding, spontaneous bacterial peritonitis, and hepatorenal syndrome. These provided ideas for the study and treatment in cirrhotic patients with refractory ascites.
Research objectives
The role of the gut-liver axis in the occurrence and development of complications of cirrhosis has aroused great attention. Microbiota dysbiosis and bacterial translocation have been shown to be involved in the progression of cirrhosis. Bacteria and their products are introduced into blood via the intestines and then increase the blood levels of endotoxin and inflammatory factors, which in turn accelerate liver fibration and stimulate the production of vasodilator substances. These events cause reduced systemic vascular resistance and an activated sympathetic nervous system and renin-angiotensin-aldosterone system, eventually leading to hyperdynamic circulation, which plays an important role in the pathogenesis of refractory ascites. Therefore,improving the gut microenvironment may benefit cirrhotic patients with refractory ascites. We conducted this study to explore the effects of rifaximin in cirrhotic patients with refractory ascites.
Research methods
All patients received conventional treatment for refractory ascites, while patients in the rifaximin group received oral rifaximin-α 200 mg four times daily for at least 2 wk. The ascites grade, fasting weight, liver and kidney function, and the inflammatory factors in the plasma were evaluated before and after treatment. In addition, the gut microbiota was determined by metagenomics sequencing (Illumina HiSeq) to analyse the changes in the characteristics of the gut microbiota before and after rifaximin treatment. The patients were followed for 6 mo. This study evaluated the effects of rifaximin in cirrhotic patients with refractory ascites with regard to clinical efficacy, laboratory indicators, inflammatory factors, and intestinal microbiota. The concentrations of interleukin-6, interleukin-8, tumour necrosis factor alpha, monocyte chemoattractant protein-1, interferon-inducible protein 10, and lipopolysaccharide-binding protein were determined with Luminex (Magnetic Luminex Assay; R&D Systems Europe, Ltd., Abingdon OX14 3NB, United Kingdom), which was highly reliable. Intestinal microbiota can be annotated to the level of species and the study of the intestinal microbiota can be deepened to the level of genes and functions using metagenomic sequencing, compared to 16S rDNA sequencing.
Research results
Compared with the control group, the fasting weight of patients decreased and the ascites significantly subsided after treatment with rifaximin. The 6-mo survival rate of patients in the rifaximin group was significantly higher than that in the control group. The concentration of interferon-inducible protein 10 decreased significantly in the rifaximin group compared with that in the control group. The abundance of Roseburia, Haemophilus, and Prevotella was significantly reduced after rifaximin treatment, while the abundance of Lachnospiraceae_noname, Subdoligranulum, and Dorea decreased and the abundance of Coprobacillus increased after treatment with rifaximin plus intravenous antibiotics. The gene expression of virulence factors was significantly reduced after treatment in both subgroups treated with rifaximin or rifaximin plus intravenous antibiotics. These findings provide new ideas for study in cirrhotic patients with refractory ascites-targeting the gut microbiota. The functions of these changed intestinal bacteria remain to be explored in the future.
Research conclusions
This study evaluated the clinical efficacy of rifaximin and its effects on intestinal flora characteristics and the systemic inflammatory state in cirrhotic patients with refractory ascites, targeting the gut microbiota. We concluded that rifaximin mitigates ascites and improves survival of cirrhotic patients with refractory ascites, and a possible mechanism is that rifaximin regulates the structure and functions of intestinal bacteria, thus improving the systemic inflammatory state. These provide new ideas for clinical dealing with cirrhotic patients with refractory ascites-targeting the gut microbiota.
Research perspectives
Further research is needed to assess the effect of long-term treatment with rifaximin on the prognosis of cirrhotic patients with severely disordered haemodynamics and a high inflammatory state. Randomized controlled studies with a large sample size are still needed to verify our conclusions, and the role of these floras remains to be further explored, since the changes in the gut microbiota in cirrhotic patients with refractory ascites remain unavailable.
ACKNOWLEDGEMENTS
The authors would like to acknowledge the QuantiHealth Co., Ltd. for metagenomics sequencing.
Footnotes
Institutional review board statement: The study protocol conformed to the Declaration of Helsinki and was approved by the Biomedical Research Ethics Committee of Peking University First Hospital.
Informed consent statement: All participants provided written informed consent for using samples and materials prior to study enrollment.
Conflict-of-interest statement: All authors declare no conflict of interest. Additionally, they have substantially contributed to this current study and approved the content of the manuscript.
STROBE statement: The authors have read the STROBE Statement-checklist of items, and the manuscript was prepared and revised according to the STROBE Statement-checklist of item.
Manuscript source: Unsolicited manuscript
Corresponding Author's Membership in Professional Societies: Liver Diseases Society of Beijing Medical Association (Fellow), Member of ERCP Youth Study Group of Gastroenterology Branch of Beijing Medical Association (Fellow), Fatty Alcoholic Liver Diseases Group of the Liver Diseases Society of Beijing Medical Association (Fellow), Medical Hepatology Group of the Liver Diseases Society of Beijing Medical Association (Fellow), and Gastroenterology Branch of Beijing Medical Association (Fellow).
Peer-review started: October 18, 2019
First decision: November 22, 2019
Article in press: December 21, 2019
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kamimura K, Ridola L S-Editor: Tang JZ L-Editor: Wang TQ E-Editor: Ma YJ
Contributor Information
Xin-Yue Lv, Department of Gastroenterology and Hepatology, Beijing You An Hospital, Capital Medical University, Beijing 100069, China.
Hui-Guo Ding, Department of Gastroenterology and Hepatology, Beijing You An Hospital, Capital Medical University, Beijing 100069, China.
Jun-Fu Zheng, Department of Gastroenterology and Hepatology, Beijing You An Hospital, Capital Medical University, Beijing 100069, China.
Chun-Lei Fan, Department of Gastroenterology and Hepatology, Beijing You An Hospital, Capital Medical University, Beijing 100069, China.
Lei Li, Department of Gastroenterology and Hepatology, Beijing You An Hospital, Capital Medical University, Beijing 100069, China. m13699119545@163.com.
Data sharing statement
No additional data are available.
References
- 1.Biecker E. Diagnosis and therapy of ascites in liver cirrhosis. World J Gastroenterol. 2011;17:1237–1248. doi: 10.3748/wjg.v17.i10.1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Betrapally NS, Gillevet PM, Bajaj JS. Gut microbiome and liver disease. Transl Res. 2017;179:49–59. doi: 10.1016/j.trsl.2016.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wiest R, Lawson M, Geuking M. Pathological bacterial translocation in liver cirrhosis. J Hepatol. 2014;60:197–209. doi: 10.1016/j.jhep.2013.07.044. [DOI] [PubMed] [Google Scholar]
- 4.Møller S, Hobolth L, Winkler C, Bendtsen F, Christensen E. Determinants of the hyperdynamic circulation and central hypovolaemia in cirrhosis. Gut. 2011;60:1254–1259. doi: 10.1136/gut.2010.235473. [DOI] [PubMed] [Google Scholar]
- 5.Villanueva C, Albillos A, Genescà J, Abraldes JG, Calleja JL, Aracil C, Bañares R, Morillas R, Poca M, Peñas B, Augustin S, Garcia-Pagan JC, Pavel O, Bosch J. Development of hyperdynamic circulation and response to β-blockers in compensated cirrhosis with portal hypertension. Hepatology. 2016;63:197–206. doi: 10.1002/hep.28264. [DOI] [PubMed] [Google Scholar]
- 6.Piano S, Tonon M, Angeli P. Management of ascites and hepatorenal syndrome. Hepatol Int. 2018;12:122–134. doi: 10.1007/s12072-017-9815-0. [DOI] [PubMed] [Google Scholar]
- 7.Hoover WW, Gerlach EH, Hoban DJ, Eliopoulos GM, Pfaller MA, Jones RN. Antimicrobial activity and spectrum of rifaximin, a new topical rifamycin derivative. Diagn Microbiol Infect Dis. 1993;16:111–118. doi: 10.1016/0732-8893(93)90004-q. [DOI] [PubMed] [Google Scholar]
- 8.Gillis JC, Brogden RN. Rifaximin. A review of its antibacterial activity, pharmacokinetic properties and therapeutic potential in conditions mediated by gastrointestinal bacteria. Drugs. 1995;49:467–484. doi: 10.2165/00003495-199549030-00009. [DOI] [PubMed] [Google Scholar]
- 9.Descombe JJ, Dubourg D, Picard M, Palazzini E. Pharmacokinetic study of rifaximin after oral administration in healthy volunteers. Int J Clin Pharmacol Res. 1994;14:51–56. [PubMed] [Google Scholar]
- 10.Ponziani FR, Gerardi V, Pecere S, D'Aversa F, Lopetuso L, Zocco MA, Pompili M, Gasbarrini A. Effect of rifaximin on gut microbiota composition in advanced liver disease and its complications. World J Gastroenterol. 2015;21:12322–12333. doi: 10.3748/wjg.v21.i43.12322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kalambokis GN, Mouzaki A, Rodi M, Pappas K, Fotopoulos A, Xourgia X, Tsianos EV. Rifaximin improves systemic hemodynamics and renal function in patients with alcohol-related cirrhosis and ascites. Clin Gastroenterol Hepatol. 2012;10:815–818. doi: 10.1016/j.cgh.2012.02.025. [DOI] [PubMed] [Google Scholar]
- 12.Vlachogiannakos J, Saveriadis AS, Viazis N, Theodoropoulos I, Foudoulis K, Manolakopoulos S, Raptis S, Karamanolis DG. Intestinal decontamination improves liver haemodynamics in patients with alcohol-related decompensated cirrhosis. Aliment Pharmacol Ther. 2009;29:992–999. doi: 10.1111/j.1365-2036.2009.03958.x. [DOI] [PubMed] [Google Scholar]
- 13.Vlachogiannakos J, Viazis N, Vasianopoulou P, Vafiadis I, Karamanolis DG, Ladas SD. Long-term administration of rifaximin improves the prognosis of patients with decompensated alcoholic cirrhosis. J Gastroenterol Hepatol. 2013;28:450–455. doi: 10.1111/jgh.12070. [DOI] [PubMed] [Google Scholar]
- 14.Chinese Society of Hepatology, Chinese Medical Association. Guidelines on the management of ascites and complications in cirrhosis. Zhonghua Gan Zang Bing Za Zhi. 2017;25:664–677. doi: 10.3760/cma.j.issn.1007-3418.2017.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chinese Society of Hepatology, Chinese Medical Association. Xu X, Duan Z, Ding H, Li W, Jia J, Wei L, Linghu E, Zhuang H. Chinese guidelines on the management of ascites and its related complications in cirrhosis. Hepatol Int. 2019;13:1–21. doi: 10.1007/s12072-018-09923-2. [DOI] [PubMed] [Google Scholar]
- 16.Blei AT, Córdoba J Practice Parameters Committee of the American College of Gastroenterology. Hepatic Encephalopathy. Am J Gastroenterol. 2001;96:1968–1976. doi: 10.1111/j.1572-0241.2001.03964.x. [DOI] [PubMed] [Google Scholar]
- 17.Larsson E, Tremaroli V, Lee YS, Koren O, Nookaew I, Fricker A, Nielsen J, Ley RE, Bäckhed F. Analysis of gut microbial regulation of host gene expression along the length of the gut and regulation of gut microbial ecology through MyD88. Gut. 2012;61:1124–1131. doi: 10.1136/gutjnl-2011-301104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen Y, Yang F, Lu H, Wang B, Chen Y, Lei D, Wang Y, Zhu B, Li L. Characterization of fecal microbial communities in patients with liver cirrhosis. Hepatology. 2011;54:562–572. doi: 10.1002/hep.24423. [DOI] [PubMed] [Google Scholar]
- 19.Qin N, Yang F, Li A, Prifti E, Chen Y, Shao L, Guo J, Le Chatelier E, Yao J, Wu L, Zhou J, Ni S, Liu L, Pons N, Batto JM, Kennedy SP, Leonard P, Yuan C, Ding W, Chen Y, Hu X, Zheng B, Qian G, Xu W, Ehrlich SD, Zheng S, Li L. Alterations of the human gut microbiome in liver cirrhosis. Nature. 2014;513:59–64. doi: 10.1038/nature13568. [DOI] [PubMed] [Google Scholar]
- 20.Kimer N, Pedersen JS, Tavenier J, Christensen JE, Busk TM, Hobolth L, Krag A, Al-Soud WA, Mortensen MS, Sørensen SJ, Møller S, Bendtsen F members of the CoRif study group. Rifaximin has minor effects on bacterial composition, inflammation, and bacterial translocation in cirrhosis: A randomized trial. J Gastroenterol Hepatol. 2018;33:307–314. doi: 10.1111/jgh.13852. [DOI] [PubMed] [Google Scholar]
- 21.Mencarelli A, Renga B, Palladino G, Claudio D, Ricci P, Distrutti E, Barbanti M, Baldelli F, Fiorucci S. Inhibition of NF-κB by a PXR-dependent pathway mediates counter-regulatory activities of rifaximin on innate immunity in intestinal epithelial cells. Eur J Pharmacol. 2011;668:317–324. doi: 10.1016/j.ejphar.2011.06.058. [DOI] [PubMed] [Google Scholar]
- 22.Yang L, Liu B, Zheng J, Huang J, Zhao Q, Liu J, Su Z, Wang M, Cui Z, Wang T, Zhang W, Li Q, Lu H. Rifaximin Alters Intestinal Microbiota and Prevents Progression of Ankylosing Spondylitis in Mice. Front Cell Infect Microbiol. 2019;9:44. doi: 10.3389/fcimb.2019.00044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kalambokis GN, Tsianos EV. Rifaximin reduces endotoxemia and improves liver function and disease severity in patients with decompensated cirrhosis. Hepatology. 2012;55:655–656. doi: 10.1002/hep.24751. [DOI] [PubMed] [Google Scholar]
- 24.Kimer N, Pedersen JS, Busk TM, Gluud LL, Hobolth L, Krag A, Møller S, Bendtsen F Copenhagen Rifaximin (CoRif) Study Group. Rifaximin has no effect on hemodynamics in decompensated cirrhosis: A randomized, double-blind, placebo-controlled trial. Hepatology. 2017;65:592–603. doi: 10.1002/hep.28898. [DOI] [PubMed] [Google Scholar]
- 25.Pistiki A, Galani I, Pyleris E, Barbatzas C, Pimentel M, Giamarellos-Bourboulis EJ. In vitro activity of rifaximin against isolates from patients with small intestinal bacterial overgrowth. Int J Antimicrob Agents. 2014;43:236–241. doi: 10.1016/j.ijantimicag.2013.12.008. [DOI] [PubMed] [Google Scholar]
- 26.Gao J, Gillilland MG, 3rd, Owyang C. Rifaximin, gut microbes and mucosal inflammation: unraveling a complex relationship. Gut Microbes. 2014;5:571–575. doi: 10.4161/gmic.32130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bajaj JS, Heuman DM, Sanyal AJ, Hylemon PB, Sterling RK, Stravitz RT, Fuchs M, Ridlon JM, Daita K, Monteith P, Noble NA, White MB, Fisher A, Sikaroodi M, Rangwala H, Gillevet PM. Modulation of the metabiome by rifaximin in patients with cirrhosis and minimal hepatic encephalopathy. PLoS One. 2013;8:e60042. doi: 10.1371/journal.pone.0060042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kaji K, Takaya H, Saikawa S, Furukawa M, Sato S, Kawaratani H, Kitade M, Moriya K, Namisaki T, Akahane T, Mitoro A, Yoshiji H. Rifaximin ameliorates hepatic encephalopathy and endotoxemia without affecting the gut microbiome diversity. World J Gastroenterol. 2017;23:8355–8366. doi: 10.3748/wjg.v23.i47.8355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schulz C, Schütte K, Vilchez-Vargas R, Vasapolli R, Malfertheiner P. Long-Term Effect of Rifaximin with and without Lactulose on the Active Bacterial Assemblages in the Proximal Small Bowel and Faeces in Patients with Minimal Hepatic Encephalopathy. Dig Dis. 2019;37:161–169. doi: 10.1159/000494216. [DOI] [PubMed] [Google Scholar]
- 30.Ponziani FR, Zocco MA, D'Aversa F, Pompili M, Gasbarrini A. Eubiotic properties of rifaximin: Disruption of the traditional concepts in gut microbiota modulation. World J Gastroenterol. 2017;23:4491–4499. doi: 10.3748/wjg.v23.i25.4491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jiang ZD, Ke S, Dupont HL. Rifaximin-induced alteration of virulence of diarrhoea-producing Escherichia coli and Shigella sonnei. Int J Antimicrob Agents. 2010;35:278–281. doi: 10.1016/j.ijantimicag.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 32.Brown EL, Xue Q, Jiang ZD, Xu Y, Dupont HL. Pretreatment of epithelial cells with rifaximin alters bacterial attachment and internalization profiles. Antimicrob Agents Chemother. 2010;54:388–396. doi: 10.1128/AAC.00691-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schrodt C, McHugh EE, Gawinowicz MA, Dupont HL, Brown EL. Rifaximin-mediated changes to the epithelial cell proteome: 2-D gel analysis. PLoS One. 2013;8:e68550. doi: 10.1371/journal.pone.0068550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Matsuoka K, Kanai T. The gut microbiota and inflammatory bowel disease. Semin Immunopathol. 2015;37:47–55. doi: 10.1007/s00281-014-0454-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brigidi P, Swennen E, Rizzello F, Bozzolasco M, Matteuzzi D. Effects of rifaximin administration on the intestinal microbiota in patients with ulcerative colitis. J Chemother. 2002;14:290–295. doi: 10.1179/joc.2002.14.3.290. [DOI] [PubMed] [Google Scholar]
- 36.Maccaferri S, Vitali B, Klinder A, Kolida S, Ndagijimana M, Laghi L, Calanni F, Brigidi P, Gibson GR, Costabile A. Rifaximin modulates the colonic microbiota of patients with Crohn's disease: an in vitro approach using a continuous culture colonic model system. J Antimicrob Chemother. 2010;65:2556–2565. doi: 10.1093/jac/dkq345. [DOI] [PubMed] [Google Scholar]
- 37.Ponziani FR, Scaldaferri F, Petito V, Paroni Sterbini F, Pecere S, Lopetuso LR, Palladini A, Gerardi V, Masucci L, Pompili M, Cammarota G, Sanguinetti M, Gasbarrini A. The Role of Antibiotics in Gut Microbiota Modulation: The Eubiotic Effects of Rifaximin. Dig Dis. 2016;34:269–278. doi: 10.1159/000443361. [DOI] [PubMed] [Google Scholar]
- 38.Soldi S, Vasileiadis S, Uggeri F, Campanale M, Morelli L, Fogli MV, Calanni F, Grimaldi M, Gasbarrini A. Modulation of the gut microbiota composition by rifaximin in non-constipated irritable bowel syndrome patients: a molecular approach. Clin Exp Gastroenterol. 2015;8:309–325. doi: 10.2147/CEG.S89999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Selinger CP, Bell A, Cairns A, Lockett M, Sebastian S, Haslam N. Probiotic VSL#3 prevents antibiotic-associated diarrhoea in a double-blind, randomized, placebo-controlled clinical trial. J Hosp Infect. 2013;84:159–165. doi: 10.1016/j.jhin.2013.02.019. [DOI] [PubMed] [Google Scholar]
- 40.Flint HJ, Scott KP, Duncan SH, Louis P, Forano E. Microbial degradation of complex carbohydrates in the gut. Gut Microbes. 2012;3:289–306. doi: 10.4161/gmic.19897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Plöger S, Stumpff F, Penner GB, Schulzke JD, Gäbel G, Martens H, Shen Z, Günzel D, Aschenbach JR. Microbial butyrate and its role for barrier function in the gastrointestinal tract. Ann N Y Acad Sci. 2012;1258:52–59. doi: 10.1111/j.1749-6632.2012.06553.x. [DOI] [PubMed] [Google Scholar]
- 42.Di Cerbo A, Palmieri B, Aponte M, Morales-Medina JC, Iannitti T. Mechanisms and therapeutic effectiveness of lactobacilli. J Clin Pathol. 2016;69:187–203. doi: 10.1136/jclinpath-2015-202976. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No additional data are available.