Abstract
Purpose
Anterior gradient 3 (AGR3) belongs to human anterior gradient (AGR) family. The function of AGR3 on cancer remains unknown. This research aimed to investigate if AGR3 had prognostic values in invasive ductal carcinoma (IDC) of breast cancer and could promote tumor progression.
Materials and Methods
AGR3 expression was detected in breast benign lesions, ductal carcinoma in situ and IDC by immunohistochemistry analysis. AGR3’s correlations with clinicopathological features and prognosis of IDC patients were analyzed. By cell function experiments, collagen gel droplet-embedded culture drug sensitivity test and cytotoxic analysis, AGR3’s impacts on proliferation, invasion ability, and chemotherapeutic drug sensitivity of breast cancer cells were also detected.
Results
AGR3 was up-regulated in luminal subtype of histological grade I-II of IDC patients and positively correlated with high risks of recurrence and distant metastasis. AGR3 high expression could lead to bone or liver metastasis and predict poor prognosis of luminal B. In cell lines, AGR3 could promote proliferation and invasion ability of breast cancer cells which were consistent with clinical analysis. Besides, AGR3 could indicate poor prognosis of breast cancer patients treated with taxane but a favorable prognosis with 5-fluoropyrimidines. And breast cancer cells with AGR3 high expression were resistant to taxane but sensitive to 5-fluoropyrimidines.
Conclusion
AGR3 might be a potential prognostic indicator in luminal B subtype of IDC patients of histological grade I-II. And patients with AGR3 high expression should be treated with chemotherapy regimens consisting of 5-fluoropyrimidines but no taxane.
Keywords: Anterior gradient 3, Cancer, Prognosis
Introduction
Breast cancer is one of the most common cancers in women and has been a major cause for cancer death of women [1]. In clinical work, breast cancer is classified into four molecule subtypes mainly based on status of estrogen receptor (ER), progesterone receptor (PR), Ki67, and human epidermal growth factor receptor 2 (HER2), consisting of luminal A, luminal B, HER2-overexpressing and triple-negative breast cancer (TNBC) [2]. Luminal A and B are ER and PR positive whereas the other two are negative. Among of them, luminal subtype accounts for approximately 70% of all breast cancer patients, TNBC accounts for about 19% and HER2-overexpressing accounts for the rest [2-4]. The presence of ER means that tumor may respond to endocrine therapy taking ER signaling pathways as targets. So endocrine therapy has been a major method to treat luminal patients, luminal A is much more sensitive to this method than luminal B [3] and patients with luminal A need no other treatment such as extra chemotherapy [4]. In luminal B, additional chemotherapy combined with endocrine therapy is very important. But the resistance of chemotherapy in this subtype is common, so recurrence rate is much higher than luminal A and new molecular targets should be developed to improve outcome of patients [5].
The proteins of human anterior gradient (AGR) family have three members, respectively named AGR1, AGR2, and AGR3. In higher vertebrates, AGR1 is lost due to decreased regeneration ability, but AGR2 and AGR3 still express [6]. However, physiological functions of AGR2 and AGR3 in vertebrates are poorly understood. In tumors such as breast [7-10], ovarian [11], prostate [12], esophageal [13], lung [14], and pancreas cancers [15], AGR2 has been acknowledged as an oncogene and a biomarker indicating poor prognosis of patients. Comparing to definite role of AGR2, AGR3’s impacts on cancers are still unclear. Reports about AGR3 in cancers are few and contradictory.
In our clinicopathologic study, we performed immunohistochemistry (IHC) analysis to detect AGR3 expression in 51 cases of breast benign lesions, 62 cases of ductal carcinoma in situ (DCIS) and 336 cases of invasive ductal carcinoma (IDC). Our samples were randomly selected and covered all molecular subtypes and all histological grades. We confirmed that AGR3 mainly expressed in the IDC patients of luminal subtype of histological grade Ⅰ-Ⅱ and could predict poor prognosis of luminal B. AGR3 highly expressed IDC patients tend to occur bone metastasis and liver metastasis. In breast cancer cell lines, we found that AGR3 could promote proliferation and invasion ability of cancer cells which proved that AGR3 could accelerate progression of breast cancer from the perspective of cell functions. Besides, AGR3 high expression indicated a poor prognosis of breast cancer patients treated with taxane but a favorable prognosis treated with 5-fluoropyrimidines. Performing collagen gel dropletembedded culture drug sensitivity test (CD-DST) and cytotoxic analysis to achieve more results, we found that breast cancer cells with AGR3 high expression were resistant to taxane but sensitive to 5-fluoropyrimidines. All of these results above had never been reported before.
Materials and Methods
1. Patient selection and clinical information
Paraffin-embedded specimens were randomly selected from 51 cases of breast benign lesions, 62 cases of DCIS and 336 cases of IDC diagnosed between 2004 and 2009. All specimens came from the Department of Breast Cancer Pathology and Research Laboratory, Tianjin Medical University Cancer Institute and Hospital (Tianjin, China). All diagnosis of histopathology was confirmed according to World Health Organization (WHO) criteria. The median age of IDC patients was 52 years old which ranged from 28 to 89. None of them had received any oncotherapy before operation. Three hundred thirty-six cases of IDC patients included 240 cases of luminal subtype (71.4%), 28 cases of HER2-overexpressing subtype (8.3%), 62 cases of triple-negative subtype (18.5%), and six cases without information about molecular subtype (1.8%).
After operation, the chemotherapy regimens that IDC patients received included 118 cases of TE/TA (taxane+epirubicin/taxane+doxorubicin) regimen, 111 cases of CEF (cyclophosphamide, epirubicin, and 5-fluoropyrimidines)/CAF (cyclophosphamide, doxorubicin, and 5-fluoropyrimidines) regimen, 78 cases of CMF (cyclophosphamide, methotrexate, and 5-fluoropyrimidines) regimen, nine cases of platinum-based regimen and seven cases just treated with taxane.
2. Patients’ prognostic information
All IDC patients were followed up with 5-146 months. In this period, 14 cases (4.2%) developed tumor recurrence, 58 cases (17.3%) developed distant metastasis, and 25 cases (7.4%) died for cancer. And 56 cases developed cancer progression within 5 years.
In addition, 58 cases of patients with distant metastasis included 43 cases of bone metastasis, 17 cases of lung metastasis, 13 cases of liver metastasis, eight cases of brain metastasis, and 19 cases with multiple organic metastases.
3. Immunohistochemical staining
AGR3 expression was detected by IHC. All procedures were performed in Benchmark XT (Roche, Basel, Switzerland). Antigen retrieval was performed in citrate buffer at 121°C for 2 minutes 15 seconds. After retrieval, sections were steeped in 3% H2O2 buffer for 25 minutes and in 10% goat serum buffer for 25 minutes. Then, the sections were steeped in primary antibody against AGR3 (1:100, HPA053942, Sigma, St. Louis, MO) at 4°C for one night. Next, sections were steeped in anti-rabbit secondary antibody for 20 minutes and in horseradish peroxidase–conjugated streptavidin also for 20 minutes. Finally, sections were incubated with 3,3′-diaminobenzidinetetra hydrochloride which was peroxidase substrate.
4. Evaluation of staining
All stained sections were reviewed independently by two pathologists under light microscopy. Evaluation of AGR3 depended on staining intensity and area. Intensity was graded like this: 0 (–) no staining, 1 (+) weak staining, 2 (++) moderate staining, and 3 (+++) strong staining. Then patients were categorized into two groups: group 1 (the grade of intensity was 2-3 and the staining area of tumor cells was 75%-100%) and group 2 (the grade of intensity was 1 or the staining area of tumor cells was 0%-74%). Additionally, group1 was defined as AGR3 high expression and group 2 was defined as AGR3 low expression.
Evaluation of ER and PR was based on the 2010 American Society of Clinical Oncology/College of American Pathologists (ASCO/CAP) guideline. Sections were regarded as positive cases if the staining area of tumor cells with nuclear staining was more than 1%.
5. Cell culture and reagents
MDA-MB-231 and T47D cell lines were bought from American Type Culture Collection (Manassas, VA) which had been checked by DNA profiling. MDA-MB-231 were cultured with Dulbecco's modified Eagle's medium and T47D were cultured with 1640 medium which were mixed with 10% fetal bovine serum (FBS). Both two cell lines were incubated in a 5% CO2 incubator at 37°C.
6. Construction of lentiviral vector expressed with full length of AGR3
Full length of human AGR3 gene (GenBank accession No. NM_176813) was amplified in T47D cell line by polymerase chain reaction (PCR). The PCR primers were as follows: forward, 5′-GAGAGCTAGCGCCACCATGATGCTACACTCAGCT-3′ and reverse, 5′-TCTCGGATCCTTAAGCGTAATCTGGAACATCGTATGGGTACATTAGCTCTGACTGAATAAGTCTTAATGCTTT-3′. The production of PCR contained full length of AGR3 gene, 3×Flag label and HA label. Then the production was cloned into pCDH-CMVMCS-EF1-Puro lentiviral vector. The sequences of the inserts were checked by gene sequencing technique. Lentiviral plasmid, packing plasmids ΔR and pVSVg were transfected into HEK-293T cells to make lentiviruses.
Then, MDA-MB-231 cells were infected by lentiviruses and screened by puromycin. After verification by western blot, stable clones were named as 3×Flag-AGR3-HA/MDA-MB-231.
7. Construction of lentiviral vector knocking down AGR3
Two different AGR3 specific RNA interference sequences were applied (siAGR3#1-#2). The sequences were as follows: siAGR3#1, 5′-gccttcacttcaaagaagtca-3′; siAGR3#2, 5′-gatgacatcacttgggtacaa-3′. Lentiviral plasmid, packing plasmids ΔR and pVSVg were transfected into HEK-293T cells to make lentiviruses. T47D cells were infected by lentiviruses and verified by western blot. Cells knocking down AGR3 successfully were named as siAGR3/T47D.
8. Western blot
Western blot was performed to detect AGR3 expression of breast cancer tissues and cells. The protein was lysed by lysis buffer (sodium dodecyl sulfate) on ice. After separating with sodium dodecyl sulfate polyacrylamide gel electrophoresis, protein was electro-transferred onto nitrocellulose membranes. The nitrocellulose membranes were incubated by primary antibodies. The primary antibodies contained AGR3 (ab201464, Abcam, Cambridge, UK), Flag (AF519–1, Beyotime, Shanghai, China), and β-actin (SC-47778, Santa Cruz Biotechnology, Santa Cruz, CA). Then the nitrocellulose membranes were treated with secondary antibodies.
9. Cell ATP/viability assay and sulforhodamine B assay
In cell ATP/viability assay, the cell lines were incubated in 24-well plates. Every type of cell line had six replicates. After 5 days, ATP level was measured by Cell Titer-Glo luminescent cell viability assay kit (catalog number G7571, Promega, Madison, WI). In sulforhodamine B (SRB) assay, cells were also incubated in 24-well plates for 5 days. After that, cells were fixed with 10% trichloroacetic acid and then stained with SRB (0.4%) buffer in 1% acetic acid. Lastly, added Tris-base (10 mM) into the plate to dissolve SRB and measured absorbance at 564 nm.
10. Migration assay
Migration assays were performed on cell lines using 24-well transwell chambers (Corning, New York, NY). Cells with 200 μL serum-free medium were added into the upper chambers and 600 μL medium containing 5% FBS were added into the lower chambers. The cells were incubated at 37°C for 16 hours. Then, scraped the cells at upper layer of polyethylene membrane and stained the cells at lower layer with Giemsa solution. Lastly, taken photograph under light microscopy and quantified the cell number in randomly selected fields. All experiments were performed in triplicates.
11. Invasion assay
Invasion assays were also performed on cell lines using 24-well transwell chambers (Corning). But the upper chambers were coated with prediluted extracellular matrix. Then cells with medium containing 0.5% FBS were added into the upper chambers and 600 μL medium containing 10% FBS were added into the lower chambers. After incubating at 37°C for 24 hours, the invading cells were stained, counted and photographed under light microscopy as the procedure of migration assay. All experiments were performed in triplicates.
12. Cytotoxic analysis
To measure the cytotoxic effects of 5-fluoropyrimidine (5-FU) and taxane, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assays were performed on cell lines. Briefly, cells were seeded at 24-well plates. After incubation to allow cells adhesion, cells were exposed to various concentrations of taxane or 5-FU (MDA-MB-231, taxane: 15 to 5×104 ng/mL, 5-FU: 1 to 1.5×104 μg/mL; T47D, taxane: 2.5 to 2.5×104 ng/mL, 5-FU: 0.1 to 5×103 μg/mL) with 500 μL/well medium for 48 hours. Besides, drug-free medium was added to the control and blank wells. After exposure was finished, 500 μL MTT (5 mg/mL) were added to each well. After 4-hour incubation, MTT solution was removed and 500 μL dimethyl sulfoxide (Sigma) were added into the wells to dissolve the insoluble formazan crystals. After dissolution, 150 μL supernatant of every well was removed into the 96-well plates and the absorbance of the dye was measured at a wave length of 570 nm. All experiments were performed in triplicates and calculated the IC50 value.
13. Wound healing assay
To measure the migration ability of the cell lines, wound healing assay was performed. The cells were seeded at 6-well plates. Then, a scratch was performed with a 10 μL pipette tip. After removing cell debris by phosphate-buffered saline, cells were incubated in a medium without FBS at 37℃. The migration distances were measured at 0, 6, 12, 18, and 24 hours. The cell-free area of the wounds was estimate under a microscope. All assays were performed in triplicates.
14. Statistical analyses
Using SPSS ver. 17.0 software package (SPSS Inc., Chicago, IL) to perform statistical analysis. Group comparisons were calculated by Mann-Whitney U test, Kruskal-Wallis test, ANOVA test, and chi-square test. Two variables’ correlation was calculated by Spearman’s rank correlation test. Using Kaplan-Meier method to calculated patients’ overall survival (OS) survival and progression-free survival (PFS) time. p-values were computed by log-rank test. The relevant factors for patients’ prognosis were identified by Cox proportional hazards regression model. In vitro experiments, using two-tailed Student’s t test to detect if the difference between two groups had statistical meaning or not. In all statistical analyses, two-sided p < 0.05 was regarded as statistical significance.
15. Ethical statement
All information about patients in this study were obtained with informed consent. And this study was approved by the Ethic Committee of Tianjin Medical University Cancer Institute and Hospital. The approval number of ethic committee was bc2017019.
Results
1. AGR3 expression increased with the development of breast tumor malignancy
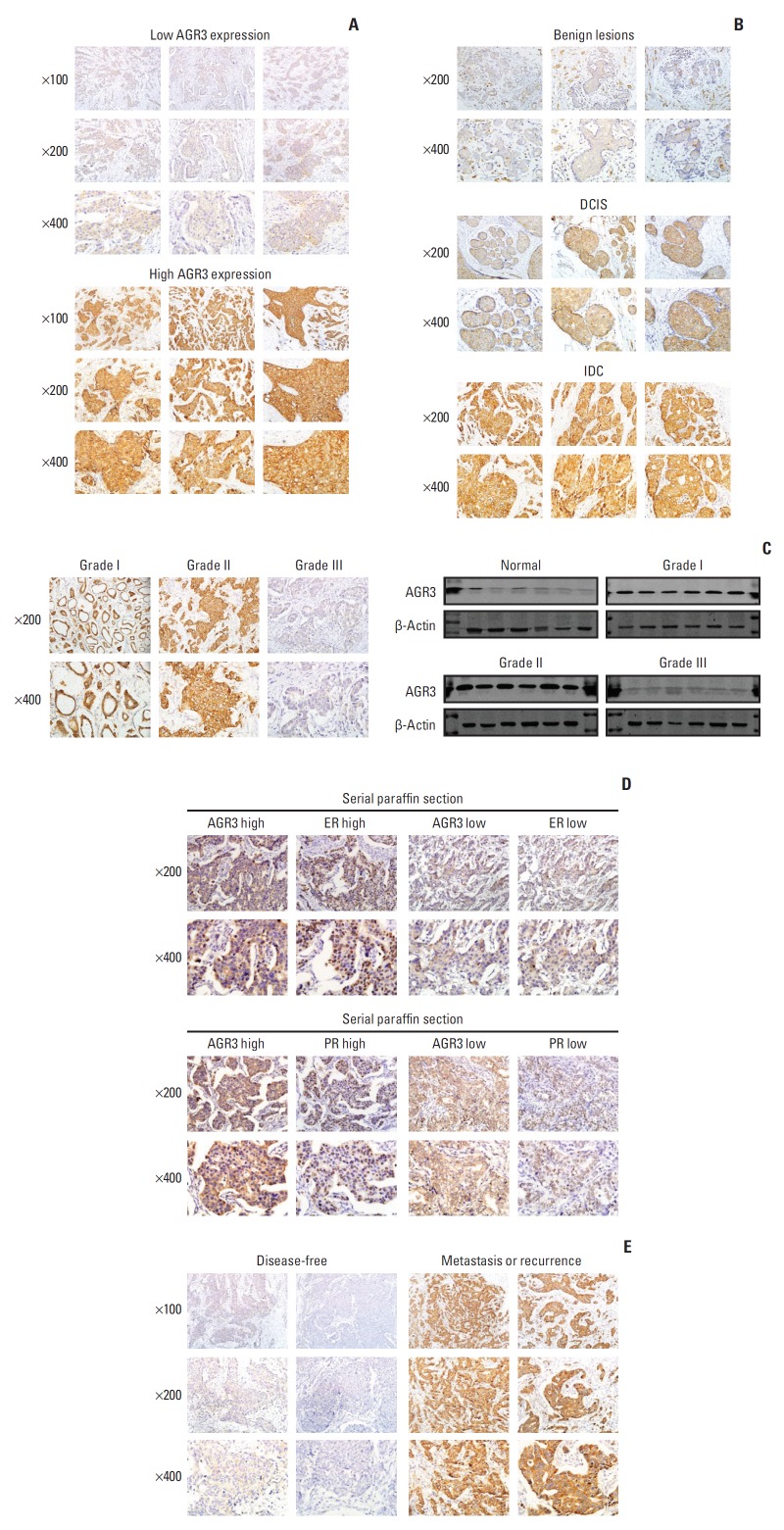
We detected AGR3 expression at proteomic level by IHC in 51 cases of breast benign lesion tissues, 62 cases of DCIS tissues and 336 cases of IDC tissues. The immunohistochemical staining of AGR3 was assessed and representative images of staining were shown in Fig. 1A. We found that AGR3 mainly located in cytoplasm of epithelial cells of mammary gland ducts in breast tissues. From benign lesions to DCIS and to IDC, AGR3 expression gradually evaluated (Fig. 1B). 7.8% (4/51) of benign lesions, 27.4% (17/62) of DCIS and 38.4% (129/336) of IDC specimens showed high expression of AGR3 (rs=0.195, p < 0.001) (S1 Table). This indicated that AGR3 expression increased with the development of breast tumor malignancy.
Fig. 1.
Anterior gradient 3 (AGR3) expression increased with the development of breast tumor malignancy and was negatively correlated with histological grade but positively correlated with estrogen receptor (ER)/progesterone receptor (PR) status. (A) Representative immunohistochemistry (IHC) images of AGR3 low expression group (upper) and AGR3 high expression group (lower). (B) AGR3 expression increased with progression of malignancy degree of lesions. AGR3 expression of benign lesions was low, ductal carcinoma in situ (DCIS) was higher and IDC was the highest. (C) Representative IHC images of AGR3 expression in different histological grades of IDC (left panel). Western blot analysis of AGR3 expression in the frozen breast tumor specimens consisting of normal tissues, grade I, grade II, and grade III. Every type of tissues had 6 cases. β-actin was used as a loading control (right panel). (D) The expression of AGR3, ER, and PR were detected by IHC in serial paraffin sections. The upper panel was representative IHC images of AGR3 and ER. The lower was AGR3 and PR. (E) Representative IHC images of AGR3 expression in disease-free group and recurrence or metastasis group.
2. AGR3 mainly expressed in luminal subtype of IDC patients of histological grade Ⅰ-Ⅱ
In IDC, we analyzed the relationship between AGR3 expression and clinicopathological features of patients. We found that high expression of AGR3 was associated with high ER positivity (rs=0.336, p < 0.001), high PR positivity (rs=0.225, p < 0.001), high risk of recurrence or metastasis (rs=0.179, p=0.001), and low histological grade (rs=–0.160, p=0.004) (Table 1). The relationships between AGR3 expression and patients’ age (rs=–0.099, p=0.069), tumor size (rs= 0.041, p=0.457), lymph node metastasis (rs=–0.007, p=0.901), distant metastasis (rs=0.077, p=0.161), HER2 status (rs=–0.098, p=0.074), and Ki-67 status (rs=–0.009, p=0.874) were not obvious (Table 1).
Table 1.
AGR3 expression and pathological features of IDC patients
| Pathological feature | No. | AGR3 expression, n (%) |
rs | p-value | |
|---|---|---|---|---|---|
| Low | High | ||||
| Age (yr) | |||||
| < 50 | 156 | 88 (56.4) | 68 (43.6) | –0.099 | 0.069 |
| ≥ 50 | 180 | 119 (66.1) | 61 (33.9) | ||
| Histological gradea) | |||||
| Ⅰ | 14 | 7 (50.0) | 7 (50.0) | –0.160 | 0.004** |
| Ⅱ | 252 | 147 (58.3) | 105 (41.7) | ||
| Ⅲ | 59 | 46 (78.0) | 13 (22.0) | ||
| Histological gradea) | |||||
| Ⅰ-Ⅱ | 266 | 154 (57.9) | 112 (42.1) | –0.159 | 0.004** |
| Ⅲ | 59 | 46 (78.0) | 13 (22.0) | ||
| Tumor size (cm)a) | |||||
| < 2 | 79 | 51 (64.6) | 28 (35.4) | 0.041 | 0.457 |
| 2-5 | 231 | 141 (61.0) | 90 (39.0) | ||
| > 5 | 23 | 13 (56.5) | 10 (43.5) | ||
| Lymph node metastases | |||||
| 0 | 122 | 75 (61.5) | 47 (38.5) | –0.007 | 0.901 |
| 1-3 | 100 | 62 (62.0) | 38 (38.0) | ||
| 4-9 | 52 | 30 (57.7) | 22 (42.3) | ||
| > 9 | 62 | 40 (64.5) | 22 (35.5) | ||
| Distant metastasis | |||||
| No | 278 | 176 (63.3) | 102 (36.7) | 0.077 | 0.161 |
| Yes | 58 | 31 (53.4) | 27 (46.6) | ||
| ER statusa) | |||||
| Negative | 123 | 102 (82.9) | 21 (17.1) | 0.336 | < 0.001 |
| Positive | 212 | 104 (49.1) | 108 (50.9) | ||
| PR statusa) | |||||
| Negative | 121 | 92 (76.0) | 29 (24.0) | 0.225 | < 0.001 |
| Positive | 214 | 114 (53.3) | 100 (46.7) | ||
| HER2 statusa) | |||||
| – to + | 265 | 157 (59.2) | 108 (40.8) | –0.098 | 0.074 |
| ++ to +++ | 69 | 49 (71.0) | 20 (29.0) | ||
| Ki-67 statusa) | |||||
| Negative | 96 | 59 (61.5) | 37 (38.5) | –0.009 | 0.874 |
| Positive | 234 | 146 (62.4) | 88 (37.6) | ||
| Recurrence or distant metastasisa) | |||||
| No | 261 | 173 (66.3) | 88 (33.7) | 0.179 | 0.001 |
| Yes | 69 | 31 (44.9) | 38 (55.1) | ||
p-value was calculated by Spearman’s rank correlation test. IDC, invasive ductal carcinoma; AGR3, anterior gradient 3; ER, estrogen receptor; PR, progesterone receptor; HER2, human epidermal growth factor receptor 2.
p < 0.01.
Some missing data.
Fifty percent (7/14) of grade Ⅰ, 41.7% (105/252) of grade Ⅱ, and 22.0% (13/59) of grade Ⅲ showed high expression of AGR3 (rs=–0.160, p=0.004) (Table 1). Taking grade Ⅰ and Ⅱ as a group of low grade and grade Ⅲ as a group of high grade, we found that 42.1% (112/266) of grade Ⅰ-Ⅱ showed high expression of AGR3 and 22.0% (13/59) of grade Ⅲ showed high expression of AGR3. The difference also had statistical meaning (rs=–0.159, p=0.004) (Table 1). In addition, western blot analysis were performed in fresh tissues of IDC of different grades (6 cases of normal breast tissues, 6 cases of grade Ⅰ, 6 cases of grade Ⅱ, and 6 cases of grade Ⅲ). Results also showed that AGR3 expression in grade Ⅰ and Ⅱ was significantly higher than grade Ⅲ (Fig. 1C).
All results above indicated that AGR3 mainly expressed in IDC patients of histological grade Ⅰ-Ⅱ. In grade Ⅰ-Ⅱ, the relationship between AGR3 expression and clinicopathological features were also analyzed. High expression of AGR3 was also associated with high ER positivity (rs=0.333, p < 0.001), high PR positivity (rs=0.224, p < 0.001) and high rate of recurrence or metastasis (rs=0.220, p < 0.001) (S2 Table). The correlations between AGR3 expression and patients’ age (rs= –0.099, p=0.105), tumor size (rs=0.035, p=0.647), lymph node metastasis (rs=–0.029, p=0.756), distant metastasis (rs=0.089, p=0.149), HER2 status (rs=–0.113, p=0.066), and Ki-67 status (rs=0.014, p=0.823) were also not obvious (S2 Table). But in grade Ⅲ, all clinicopathological features had no correlation with AGR3 expression (S3 Table).
Then, we detected AGR3, ER, and PR expression in serial paraffin sections by IHC. The results also showed that AGR3 expression was positively correlated with ER and PR status. Representative images of staining were shown in Fig. 1D. Based on the important roles of ER and PR in molecular subtypes [2], further analysis was performed to confirm the relationship between AGR3 expression and molecular subtypes. We found that AGR3 expression in luminal subtype was much higher than non-luminal subtype in IDC. Forty-five point six percent (31/68) of luminal A, 47.7% (82/172) of luminal B, 10.7% (3/28) of HER2-overexpressing, and 14.5% (9/62) of TNBC showed high expression of AGR3 (rs= –0.249, p < 0.001) (Table 2).
Table 2.
Relationship between AGR3 expression and molecular subtypes in IDC
| Molecular subtype | No. | AGR3 expression, n (%) |
rs | p-value | |
|---|---|---|---|---|---|
| Low | High | ||||
| Luminal A | 68 | 37 (54.4) | 31 (45.6) | –0.249 | 0.000*** |
| Luminal B | 172 | 90 (52.3) | 82 (47.7) | ||
| HER2-overexpressing | 28 | 25 (89.3) | 3 (10.7) | ||
| TNBC | 62 | 53 (85.5) | 9 (14.5) | ||
p-value (luminal A vs. luminal B)=0.770, p-value (HER2-overexpressing vs. TNBC)=0.876, p-value (luminal A and luminal B vs. HER2-overexpressing and TNBC)=0.000; p-value was calculated by Spearman’s rank correlation test. AGR3, anterior gradient 3; IDC, invasive ductal carcinoma; HER2, human epidermal growth factor receptor 2; TNBC, triple-negative breast cancer.
p < 0.001.
Moreover, in IDC patients of grade Ⅰ-Ⅱ, AGR3 expression in luminal subtype was also much higher than non-luminal subtype. Forty-seven point four percent (27/57) of luminal A, 50.3% (72/143) of luminal B, 5.3% (1/19) of HER2-overexpressing, and 19.5% (8/41) of TNBC showed high expression of AGR3 (rs=–0.218, p < 0.001) (S4 Table). But grade Ⅲ had no such relationship (rs=–0.241, p=0.136) (S5 Table). All of these results above indicated that AGR3 mainly expressed in luminal subtype of IDC patients of histological grade Ⅰ-Ⅱ.
3. High expression of AGR3 was a promoting factor for breast cancer patients to develop recurrence or distant metastasis
Table 1 showed that AGR3 expression was positively correlated with high risk of recurrence or metastasis. The representative images of IHC were shown in Fig. 1E. In order to get more accurate results, similar analysis was completed in different molecular subtypes. Additionally, we also analyzed the relationship between AGR3 expression and risk of recurrence or metastasis within 5 years.
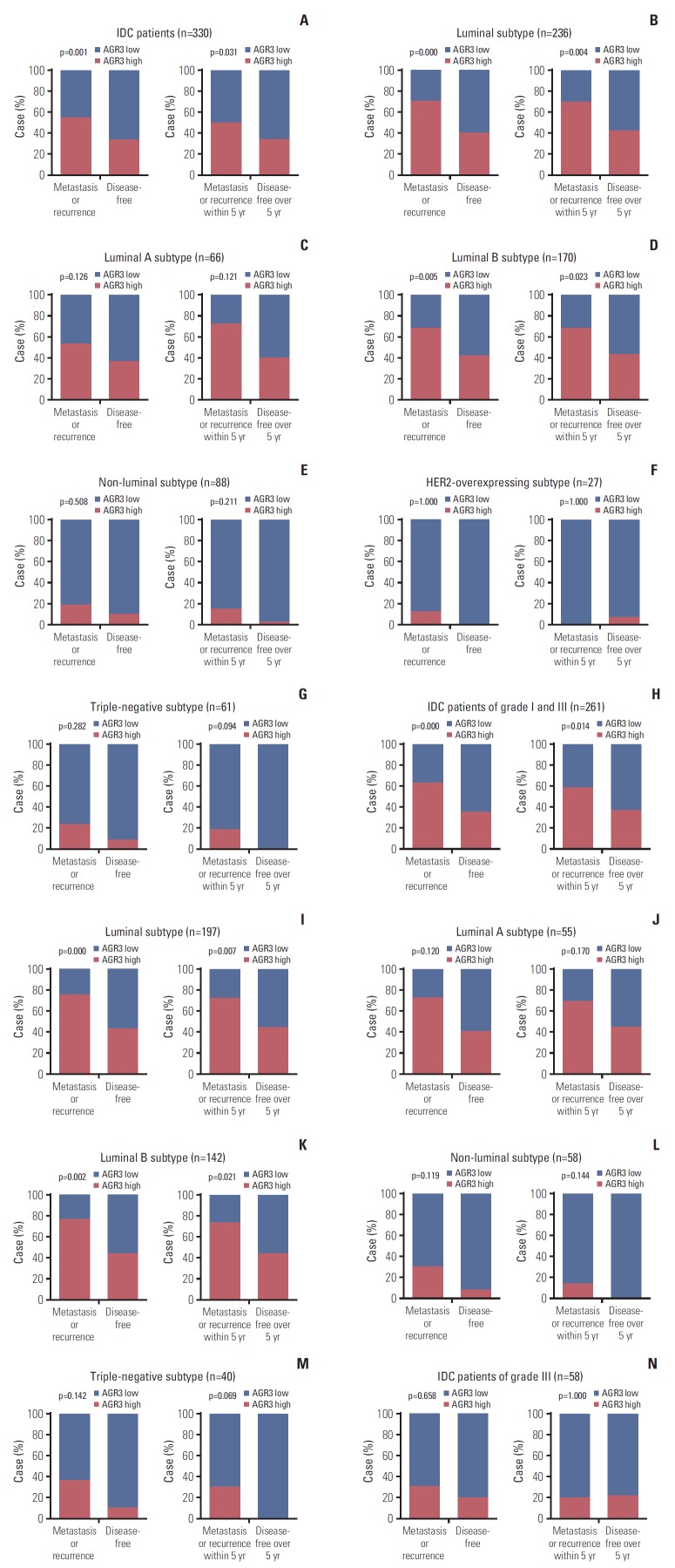
In IDC, AGR3 expression of patients occurring recurrence or metastasis was much higher than disease-free patients (p=0.001); AGR3 expression of patients occurring recurrence or metastasis within 5 years was also higher than patients disease-free over 5 years (p=0.031) (Fig. 2A). Then, in luminal and luminal B subtype of IDC, AGR3 expression of patients occurring recurrence or distant metastasis was higher than disease-free patients (luminal, p < 0.001; luminal B, p=0.005); AGR3 expression of patients occurring recurrence or distant metastasis within 5 years was also higher than patients disease-free over 5 years (luminal, p=0.004; luminal B, p=0.023) (Fig. 2B and D). But in luminal A and non-luminal subtype including HER2-overexpressing and TNBC, AGR3 expression had no relationship with recurrence or metastasis (Fig. 2C and E-G).
Fig. 2.
High expression of anterior gradient 3 (AGR3) was associated with high risk of recurrence or metastasis in luminal B subtype of invasive ductal carcinoma (IDC) patients of grade I-II. Compared the proportion of AGR3 highly expressed patients in the group developing metastasis or recurrence (MR group) and the group disease-free (DF group). Besides, compared the proportion of AGR3 highly expressed patients in the group developing metastasis or recurrence within 5 years (MR 5 years group) and the group disease-free over 5 years (DF 5 years group). The proportion of every group was as follows. (A) MR, 55.1%; DF, 33.7%; MR 5 years, 50.0%; DF 5 years, 33.9%. (B) Luminal of MR, 70.8%; luminal of DF, 40.9%; luminal of MR 5 years, 69.4%; luminal of DF 5 years, 42.2%. (C) Luminal A of MR, 56.9%; luminal A of DF, 37.7%; luminal A of MR 5 years, 72.7%; luminal A of DF 5 years, 40.0%. (D) Luminal B of MR, 68.6%; luminal B of DF, 42.2%; luminal B of MR 5 years, 68.0%; luminal B of DF 5 years, 43.0%. (E) Non-luminal of MR, 19.0%; non-luminal of DF, 10.4%; non-luminal of MR 5 years, 15.0%; non-luminal of DF 5 years, 2.6%. (F) Human epidermal growth factor receptor 2 (HER2)-overexpressing of MR, 13.0%; HER2-overexpressing of DF, 0%; HER2-overexpressing of MR 5 years, 0%; HER2-overexpressing of DF 5 years, 7.7%. (G) Triple-negative breast cancer (TNBC) of MR, 23.5%; TNBC of DF, 9.1%; TNBC of MR 5 years, 18.7%; TNBC of DF 5 years, 0%. (H) Grade Ⅰ-Ⅱ of MR, 64.0%; grade Ⅰ-Ⅱ of DF, 36.5%; grade Ⅰ-Ⅱ of MR 5 years, 58.5%; grade Ⅰ-Ⅱ of DF 5 years, 37.0%. (I) Luminal of grade Ⅰ-Ⅱ in MR, 75.68%; luminal of grade Ⅰ-Ⅱ in DF, 43.13%; luminal of grade Ⅰ-Ⅱ in MR 5 years, 72.41%; luminal of grade Ⅰ-Ⅱ in DF 5 years, 44.44%. (J) Luminal A of grade Ⅰ-Ⅱ in MR, 72.72%; luminal A of grade Ⅰ-Ⅱ in DF, 40.91%; luminal A of grade Ⅰ-Ⅱ in MR 5 years, 70.0%; luminal A of grade Ⅰ-Ⅱ in DF 5 years, 44.8%. (K) Luminal B of grade Ⅰ-Ⅱ in MR, 76.9%; luminal B of grade Ⅰ-Ⅱ in DF, 43.9%; luminal B of grade Ⅰ-Ⅱ in MR 5 years, 73.7%; luminal B of grade Ⅰ-Ⅱ in DF 5 years, 44.3%. (L) Non-luminal of grade Ⅰ-Ⅱ in MR, 30.8%; non-luminal of grade Ⅰ-Ⅱ in DF, 8.9%; non-luminal of grade Ⅰ-Ⅱ in MR 5 years, 17.0%; non-luminal of grade Ⅰ-Ⅱ in DF 5 years, 0%. (M) TNBC of grade Ⅰ-Ⅱ in MR, 36.4%; TNBC of grade Ⅰ-Ⅱ in DF, 10.3%; TNBC of grade Ⅰ-Ⅱ in MR 5 years, 30.0%; TNBC of grade Ⅰ-Ⅱ in DF 5 years, 0%. (N) Grade Ⅲ of MR, 30.8%; grade Ⅲ of DF, 20.0%; grade Ⅲ of MR 5 years, 20.7%; grade Ⅲ of DF 5 years, 22.2%.
In IDC of grade Ⅰ-Ⅱ, we got similar results. AGR3 expression of patients occurring recurrence or distant metastasis was higher than disease-free patients (p < 0.001); AGR3 expression of patients occurring recurrence or distant metastasis within 5 years was also higher than patients disease-free over 5 years (p=0.014) (Fig. 2H). Besides, the relationship between AGR3 expression and recurrence or metastasis still existed in luminal and luminal B subtype (Fig. 2I and K), but didn’t exist in luminal A, non-luminal and TNBC (Fig. 2J, L, and M). The number of HER2-overexpressing specimens was too few, so the investigation could not be performed. And in IDC of grade Ⅲ, AGR3 expression also had no relationship with recurrence or metastasis (Fig. 2N). All results above reminded that high expression of AGR3 indicated high risk of recurrence or metastasis in luminal B subtype of IDC patients of grade Ⅰ-Ⅱ.
In addition, we investigated the relationship between AGR3 expression and metastatic organs such as bone, lung, liver, and brain which were common metastatic organs of breast cancer patients. We found that AGR3 expression was correlated with bone metastasis (total IDC patients: rs=0.119, p=0.029; grade Ⅰ-Ⅱ patients: rs=0.216, p=0.031) and liver metastasis (total IDC patients: rs=0.127, p=0.020; grade Ⅰ-Ⅱ patients: rs=0.135, p=0.027) in IDC of grade Ⅰ and Ⅱ (Table 3). But there was no correlation between AGR3 expression and lung metastasis (total IDC patients: rs=–0.015, p=0.788; grade Ⅰ-Ⅱ patients: rs=0.009, p=1.0) or brain metastasis (total IDC patients: rs=–0.003, p=0.958; grade Ⅰ-Ⅱ patients: rs=0.020, p=1.0) (Table 3). In IDC of grade Ⅲ, relationships between AGR3 expression and these four metastatic organs were not obvious (S6 Table). These results indicated that high expression of AGR3 could lead to high risk of bone and liver metastasis.
Table 3.
Relationship between AGR3 expression and metastatic organs in IDC patients of grade Ⅰ-Ⅱ
| Metastatic organs | No. (IDC) | AGR3 expression of IDC |
rs | p-value | No. (grade Ⅰ-Ⅱ) | AGR3 expression of grade Ⅰ-Ⅱ |
rs | p-value | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Low | High | Low | High | |||||||
| Bone metastasis | ||||||||||
| No | 293 | 187 (63.8) | 106 (36.2) | 0.119 | 0.029* | 234 | 154 (65.8) | 80 (34.2) | 0.216 | 0.031* |
| Yes | 43 | 20 (46.5) | 23 (53.5) | 32 | 14 (43.8) | 18 (56.3) | ||||
| Lung metastasis | ||||||||||
| No | 319 | 196 (61.4) | 123 (38.6) | –0.015 | 0.788 | 257 | 149 (58.0) | 108 (42.0) | 0.009 | 1.000 |
| Yes | 17 | 11 (64.7) | 6 (35.3) | 9 | 5 (55.6) | 4 (44.4) | ||||
| Liver metastasis | ||||||||||
| No | 323 | 203 (62.8) | 120 (37.2) | 0.127 | 0.020* | 257 | 152 (59.1) | 105 (40.9) | 0.135 | 0.027* |
| Yes | 13 | 4 (30.8) | 9 (69.2) | 9 | 2 (22.2) | 7 (77.8) | ||||
| Brain metastasis | ||||||||||
| No | 328 | 202 (61.6) | 126 (38.4) | –0.003 | 0.958 | 262 | 152 (58.0) | 110 (42.0) | 0.020 | 1.000 |
| Yes | 8 | 5 (62.5) | 3 (37.5) | 4 | 2 (50.0) | 2 (50.0) | ||||
p-value was calculated by Spearman’s Rank-Correlation test. AGR3, anterior gradient 3; IDC, invasive ductal carcinoma.
p < 0.05.
4. AGR3 was an independent prognostic factor in luminal B patients of grade Ⅰ-Ⅱ
Considering relationship between AGR3 expression and high risk of recurrence or metastasis. Kaplan-Meier analysis was performed in 330 cases of IDC with complete follow-up data to explore the value of AGR3 in prognosis.
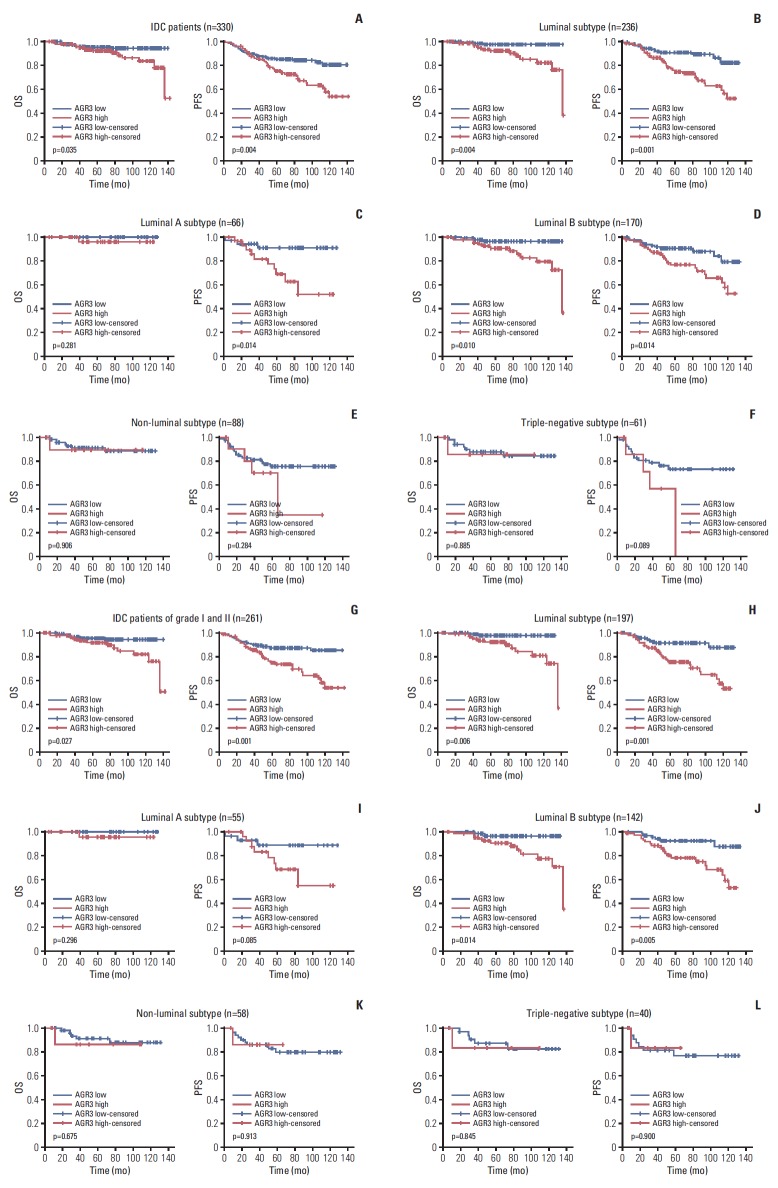
In IDC, both OS and PFS of AGR3 high expression group were significantly shorter than AGR3 low expression group (OS, p=0.035; PFS, p=0.004) (Fig. 3A). With further analysis in different molecular subtypes, we found that OS and PFS of AGR3 high expression group shortened in luminal subtype (OS, p=0.004; PFS, p=0.001) (Fig. 3B). Separately, in luminal A, AGR3 high expression could lead to shorter PFS (p=0.014) but had no influence on OS (Fig. 3C); in luminal B, both OS and PFS significantly shorten if AGR3 highly expressed (OS, p=0.010; PFS, p=0.014) (Fig. 3D). Whereas, in nonluminal and TNBC subtype, AGR3 expression had no influence on either OS or PFS (Fig. 3E and F). Number of HER2-overexpressing patients was too few, so Kaplan-Meier analysis could not be performed.
Fig. 3.
High expression of anterior gradient 3 (AGR3) was associated with poor prognosis in luminal B subtype of invasive ductal carcinoma (IDC) patients of grade I-II. (A) Overall survival (OS) and progression-free survival (PFS) curves of 330 cases of IDC patients were shown, respectively. (B) OS and PFS of 236 luminal patients were shown, respectively. (C) OS and PFS of 66 luminal A patients were shown, respectively. (D) OS and PFS of 170 luminal B patients were shown, respectively. (E) OS and PFS of 88 non-luminal patients were shown, respectively. (F) OS and PFS of 61 triple-negative breast cancer (TNBC) patients were shown, respectively. (G) OS and PFS curves of 261 IDC patients of grade Ⅰ-Ⅱ were shown, respectively. (H) OS and PFS of 197 luminal patients of grade Ⅰ-Ⅱ were shown, respectively. (I) OS and PFS of 55 luminal A patients of grade Ⅰ-Ⅱ were shown, respectively. (J) OS and PFS of 142 luminal B patients of grade Ⅰ-Ⅱ were shown, respectively. (K) OS and PFS of 58 non-luminal patients of grade Ⅰ-Ⅱ were shown, respectively. (L) OS and PFS of 40 TNBC patients of grade Ⅰ-Ⅱ were shown, respectively.
In IDC of grade Ⅰ-Ⅱ, we got similar results. OS and PFS also significantly shortened when AGR3 highly expressed (OS, p=0.027; PFS, p=0.001) (Fig. 3G). And AGR3’s impacts on OS and PFS still existed in luminal and luminal B subtype (Fig. 3H and J), but didn’t exist in luminal A, non-luminal or TNBC (Fig. 3I, K and L). Kaplan-Meier analysis still could not be performed in HER2-overexpressing subtype of grade Ⅰ-Ⅱ due to the small number of specimens.
Results above reminded that high expression of AGR3 indicated poor prognosis in luminal B subtype of IDC patients of grade Ⅰ-Ⅱ. In addition, in multivariate Cox regression analysis, we found that AGR3 expression was an independent predictor for both OS and PFS (Table 4).
Table 4.
Univariate and multivariate analysis for OS and PFS in IDC patients of grade Ⅰ-Ⅱ
| Variable | OS (univariate) |
OS (multivariate) |
PFS (univariate) |
PFS (multivariate) |
||||
|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | p-value | HR (95% CI) | p-value | HR (95% CI) | p-value | HR (95% CI) | p-value | |
| Age | 0.911 (0.385-2.156) | 0.832 | 1.071 (0.430-2.664) | - | 0.676 (0.386-1.187) | 0.173 | 0.759 (0.422-1.367) | - |
| Tumor size | 1.624 (0.740-3.565) | 0.227 | 1.017 (0.456-2.270) | - | 1.852 (1.096-3.130) | 0.021 | 1.139 (0.656-1.976) | - |
| Lymph node metastasis | 1.680 (1.149-2.455) | 0.007 | 1.757 (1.173-2.630) | 0.006 | 1.651 (1.294-2.106) | 0.000 | 1.679 (1.303-2.164) | < 0.001 |
| ER status | 0.766 (0.316-1.857) | 0.556 | 0.436 (0.147-1.295) | - | 0.739 (0.417-1.309) | 0.300 | 0.559 (0.282-1.109) | - |
| PR status | 0.907 (0.366-2.251) | 0.834 | 0.780 (0.277-2.197) | - | 0.645 (0.366-1.137) | 0.129 | 0.498 (0.261-0.950) | - |
| AGR3 expression | 2.695 (1.082-6.713) | 0.033 | 4.161 (1.406-12.312) | 0.010 | 2.531 (1.421-4.511) | 0.002 | 3.856 (1.953-7.613) | < 0.001 |
OS, overall survival; PFS, progression-free survival; IDC, invasive ductal carcinoma; HR, hazard ratio; CI, confidence interval; ER, estrogen receptor; PR, progesterone receptor; AGR3, anterior gradient 3.
5. AGR3 affected proliferation and invasion ability of breast cancer cells
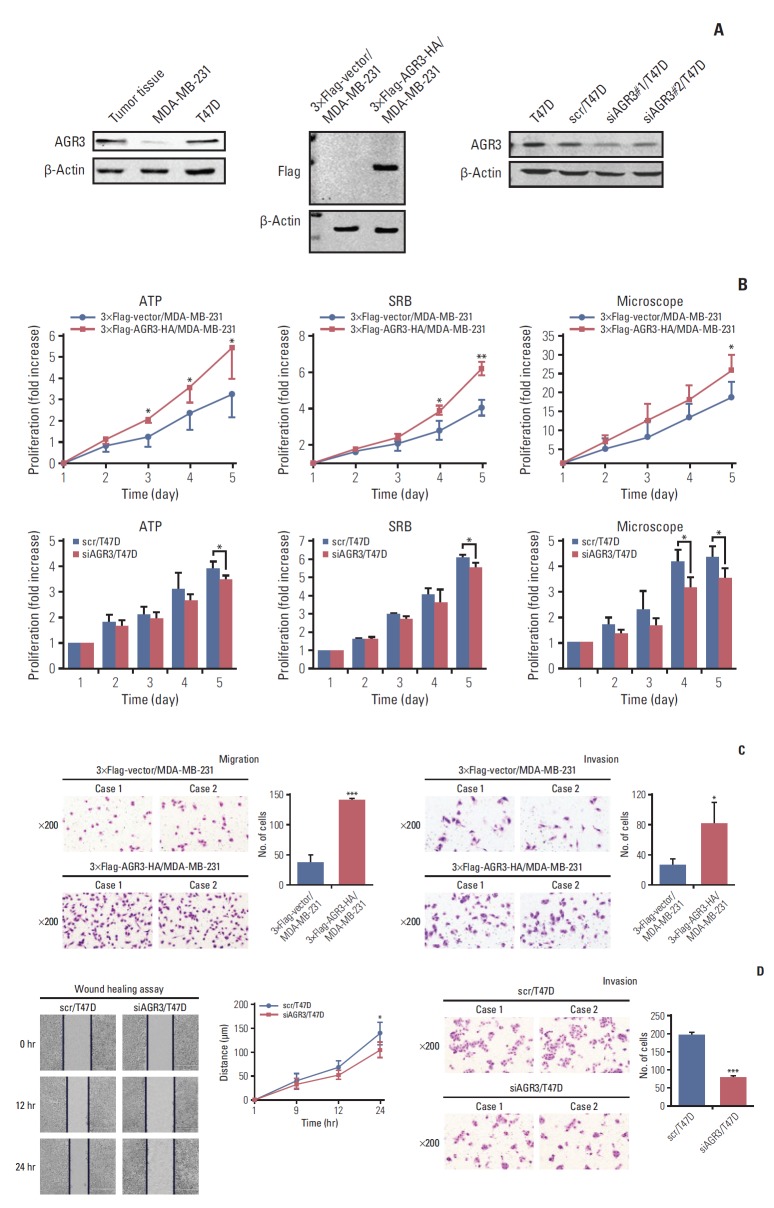
After confirming the promoting role of AGR3 in clinical specimens, we detected AGR3 expression in breast cancer cell lines by western blot. In western blot analysis, we took fresh tissues with AGR3 high expression as positive control. We found that AGR3 highly expressed in luminal cell line T47D, but low expressed in TNBC cell line MDA-MB-231 (Fig. 4A). We overexpressed full length of AGR3 in MDA-MB-231 and knocked down AGR3 in T47D (named as 3× Flag-AGR3-HA/MDA-MB-231 and siAGR3/T47D, respectively) to make sure the role of AGR3 in vitro. The overexpression and interference effects were detected by western blot (Fig. 4A). The interference sequence of siAGR3#1/T47D was used in vitro studies.
Fig. 4.
Anterior gradient 3 (AGR3) promoted proliferation and invasion ability of breast cancer cells. (A) Western blot analysis of AGR3 expression in MDA-MB-231 and T47D cells (patient tissues with AGR3 high expression as positive control) (left panel). AGR3 expression was detected by primary flag antibodies in 3×Flag-vector/MDA-MB-231 and 3×Flag-AGR3-HA/MDA-MB-231 cells (middle panel). AGR3 expression was detected by primary AGR3 antibodies in T47D, scr/T47D and siAGR3/T47D cells (right panel). (B) Proliferation ability was detected by ATP and sulforhodamine B (SRB) assays in 3×Flag-vector/MDA-MB-231 and 3×Flag-AGR3-HA/MDA-MB-231 cells, respectively (upper panel in left and middle). Proliferation assay was repeated by using Nikon ECLIPSE Ti microscope to count cell number in 3×Flag-vector/MDA-MB-231 and 3×Flag-AGR3-HA/MDA-MB-231 cells (upper panel in right). Proliferation ability was also detected by ATP and SRB assays in scr/T47D and siAGR3/T47D cells, respectively (lower panel in left and middle). Proliferation assay was also repeated by using Nikon ECLIPSE Ti microscope to count cell numbers in scr/T47D and siAGR3/T47D cells (lower panel in right). Bars are mean±SD. All experiments were performed 3 times independently (*p < 0.05, **p < 0.01). (C) Migration ability was detected by Matrigel Boyden chamber assays in 3×Flag-vector/MDA-MB-231 and 3×Flag-AGR3-HA/MDA-MB-231 cells (upper panel). Migration ability was detected by wound healing assays in scr/T47D and siAGR3/T47D cells (lower panel). Bars are mean±SD. All experiments were performed 3 times independently (*p < 0.05, ***p < 0.001). (D) Invasion ability was detected by Matrigel Boyden chamber assays in 3×Flag-vector/MDA-MB-231 and 3×Flag-AGR3-HA/MDA-MB-231 cells (upper panel). Invasion ability was detected by Matrigel Boyden chamber assays in scr/T47D and siAGR3/T47D cells (lower panel). Bars are mean±SD. All experiments were performed 3 times independently (*p < 0.05, ***p < 0.001).
In ATP and SRB assays, proliferation ability of 3×Flag-AGR3-HA/MDA-MB-231 significantly increased comparing to 3×Flag-vector/MDA-MB-231; proliferation ability of siAGR3/T47D significantly reduced comparing to scr/T47D (Fig. 4B). Besides, we performed proliferation assays by using Nikon ECLIPSE Ti microscope to count cell numbers. The results were the same as ATP and SRB assays (Fig. 4B).
In Matrigel Boyden chamber assays and wound healing assays, migration and invasion ability of 3×Flag-AGR3-HA/MDA-MB-231 significantly increased comparing to 3×Flag-vector/MDA-MB-231; migration and invasion ability of siAGR3/T47D significantly reduced comparing to scr/T47D (Fig. 4C and D). All results of cellular functional experiments were consistent with results we got from clinical samples and indicated that AGR3 might improve the development of breast cancer by promoting tumor cells’ proliferation and invasion ability.
6. AGR3 high expression indicated a poor prognosis of breast cancer patients treated with taxane but a favorable prognosis treated with 5-fluoropyrimidines
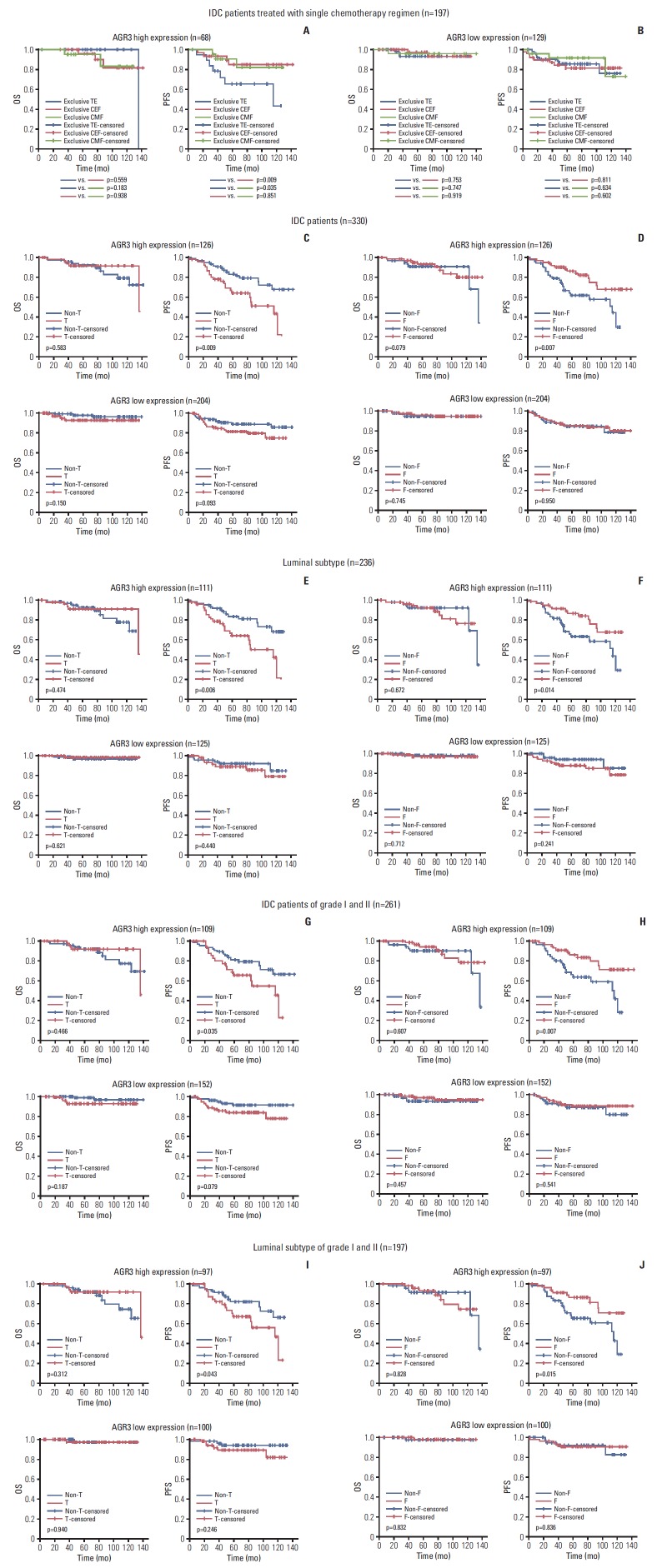
From AGR3 highly expressed IDC specimens, we choose 19 cases only receiving TE/TA chemotherapy regimen, 29 cases only receiving CEF/CAF chemotherapy regimen and 20 cases only receiving CMF chemotherapy regimen. These regimens were commonly used in the treatment of breast cancer patients. By performing Kaplan-Meier analysis, we found that PFS of TE/TA group were significantly shorter than CEF/CAF and CMF group (TE/TA vs. CEF/CAF, p=0.009; TE/TA vs. CMF, p=0.035). But PFS had no significant difference between CEF/CAF and CMF group (Fig. 5A). In addition, from AGR3 low expressed IDC specimens, we choose 57 cases only receiving TE/TA chemotherapy regimen, 48 cases only receiving CEF/CAF chemotherapy regimen and 24 cases only receiving CMF chemotherapy regimen. Kaplan-Meier analysis showed that both OS and PFS had no correlation with chemotherapy regimen in AGR3 low expressed IDC (Fig. 5B).
Fig. 5.
Anterior gradient 3 (AGR3)’s indication role for therapeutic response of taxane and 5-fluoropyrimidines in luminal patients of grade I-II. (A) Overall survival (OS) and progression-free survival (PFS) curves of AGR3 highly expressed invasive ductal carcinoma (IDC) patients with different chemotherapy regimens were shown, respectively. TE, taxane+epirubicin; CEF, cyclophosphamide, epirubicin and 5-fluoropyrimidines; CMF, cyclophosphamide, methotrexate and 5-fluoropyrimidines. (B) OS and PFS curves of AGR3 low expressed IDC patients with different chemotherapy regimens were shown, respectively. (C) OS and PFS curves of AGR3 highly expressed IDC patients with taxane treatment were shown, respectively (upper panel). OS and PFS curves of AGR3 low expressed IDC patients with taxane treatment were shown, respectively (lower panel). T, taxane. (D) OS and PFS curves of AGR3 highly expressed IDC patients with 5-fluoropyrimidines treatment were shown, respectively (upper panel). OS and PFS curves of AGR3 low expressed IDC patients with 5-fluoropyrimidines treatment were shown, respectively (lower panel). F, 5-fluoropyrimidine. (E) OS and PFS curves of AGR3 highly expressed luminal patients with taxane treatment were shown, respectively (upper panel). OS and PFS curves of AGR3 low expressed luminal patients with taxane treatment were shown, respectively (lower panel). (F) OS and PFS curves of AGR3 highly expressed luminal patients with 5-fluoropyrimidines treatment were shown, respectively (upper panel). OS and PFS curves of AGR3 low expressed luminal patients with 5-fluoropyrimidines treatment were shown, respectively (lower panel). (G) OS and PFS curves of AGR3 highly expressed IDC patients of grade Ⅰ-Ⅱ with taxane treatment were shown, respectively (upper panel). OS and PFS curves of AGR3 low expressed IDC patients of grade Ⅰ-Ⅱ with taxane treatment were shown, respectively (lower panel). (H) OS and PFS curves of AGR3 highly expressed IDC patients of grade Ⅰ-Ⅱ with 5-fluoropyrimidines treatment were shown, respectively (upper panel). OS and PFS curves of AGR3 low expressed IDC patients of grade Ⅰ-Ⅱ with 5-fluoropyrimidines treatment were shown, respectively (lower panel). (I) OS and PFS curves of AGR3 highly expressed luminal patients of grade Ⅰ-Ⅱ with taxane treatment were shown, respectively (upper panel). OS and PFS curves of AGR3 low expressed luminal patients of grade Ⅰ-Ⅱ with taxane treatment were shown, respectively (lower panel). (J) OS and PFS curves of AGR3 highly expressed luminal patients of grade Ⅰ-Ⅱ with 5-fluoropyrimidines treatment were shown, respectively (upper panel). OS and PFS curves of AGR3 low expressed luminal patients of grade Ⅰ-Ⅱ with 5-fluoropyrimidines treatment were shown, respectively (lower panel).
In AGR3 highly expressed IDC patients, CEF/CAF and CMF chemotherapy regimens could lead better prognosis than TE/TA chemotherapy regimen. We speculated that AGR3 might change the sensitivity of one drug in these regimens. We divided all specimens into ten groups based on whether chemotherapy regimen consisted of taxane, epirubicin/doxorubicin, cyclophosphamide, methotrexate or 5-fluoropyrimidines. These groups were respectively named as T/non-T group, E/non-E group, C/non-C group, M/non-M group and F/non-F group. We wanted to know whether presence or absence of one particular drug would change the prognosis of AGR3 highly expressed IDC patients by comparing “X” group to “non-X” group.
We found that, in AGR3 highly expressed IDC patients, PFS of T group was shorter than non-T group (p=0.009) (Fig. 5C) and PFS of F group was longer than non-F group (p=0.007) (Fig. 5D). But when comparing other groups, epirubicin/doxorubicin, cyclophosphamide, and methotrexate had no impact on both OS and PFS (S7A, S7B and S7C Fig.). In AGR3 low expressed IDC patients, OS and PFS also had no difference during any comparison.
Then, more analysis was performed in different molecular subtypes. In luminal subtype of AGR3 highly expressed IDC patients, PFS of T group was also shorter than non-T group (p=0.006) (Fig. 5E) and PFS of F group was also longer than non-F group (p=0.014) (Fig. 5F). But in non-luminal subtype, both OS and PFS had no correlation with taxane and 5-fluoropyrimidine (S8A and S8B Fig.). And in AGR3 low expressed IDC patients, no matter luminal subtype or non-luminal subtype had no such relationship (Fig. 5E, 5F, S8A and S8B Fig.).
Besides, in IDC patients of grade Ⅰ-Ⅱ, PFS of T group was also shorter than non-T group (p=0.035) (Fig. 5G) and PFS of F group was also longer than non-F group (p=0.007) (Fig. 5H) when AGR3 highly expressed. And the impact of taxane and 5-fluoropyrimidine on PFS also existed in luminal subtype (Fig. 5I and J) but not in non-luminal subtype (S8C and S8D Fig.). In addition, grade Ⅲ patients had no such relationship (S8E and S8F Fig.). All of these results above indicated that AGR3 high expression indicated a poor prognosis of breast cancer patients treated with taxane but a favorable prognosis treated with 5-fluoropyrimidines.
7. Breast cancer cells with AGR3 high expression were resistant to taxane but sensitive to 5-fluoropyrimidines
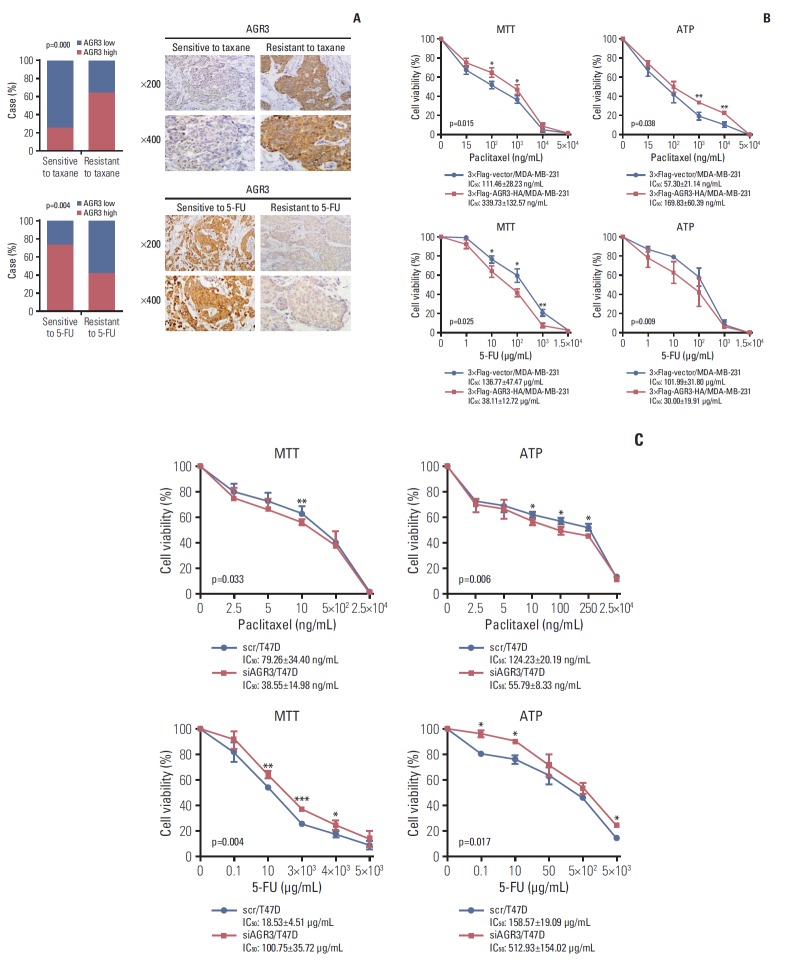
CD-DST assay had been widely applied in clinical work to test sensitivities of chemotherapy drugs. Based on the results of CD-DST, we chose other 161 cases of IDC specimens consisting of 44 cases sensitive to taxane, 39 cases resistant to taxane, 42 cases sensitive to 5-fluoropyrimidines and 36 cases resistant to 5-fluoropyrimidines. Then, we performed IHC to detect AGR3 expression in these specimens. We found that AGR3 expression of patients sensitive to taxane was much lower than patients resistant to taxane (p < 0.001) (Fig. 6A) but AGR3 expression of patients sensitive to 5-fluoropyrimidines was much higher than patients resistant to 5-fluoropyrimidines (p=0.004) (Fig. 6A).
Fig. 6.
Breast cancer cells with anterior gradient 3 (AGR3) high expression were resistant to taxane but sensitive to 5-fluoropyrimidines (5-FU). (A) Compared the proportion of AGR3 highly expressed patients in groups with different drug sensitivities. Taxane sensitive group was 25% and resistant group was 64.1% (p=0.000, upper panel in left). Upper panel in right was representative immunohistochemistry (IHC) images of AGR3 expression in taxane sensitive and resistant group. 5-FU sensitive group was 73.3% and resistant group was 42.1% (p=0.004, lower panel in left). Lower panel in right were representative IHC images of AGR3 expression in 5-FU sensitive and resistant group. (B) With the treatment of taxane, cell viability of 3×Flag-AGR3-HA/MDA-MB-231 cells was much higher than 3×Flag-vector/MDA-MB-231 cells (*p < 0.05, ** p < 0.01, upper panel). Taxane IC50 values of 3×Flag-AGR3-HA/MDA-MB-231 cells in MTT and ATP assay were much higher than 3×Flag-vector/MDA-MB-231 cells (MTT, p=0.015; ATP, p=0.038, upper panel). With the treatment of 5-FU, cell viability of 3×Flag-AGR3-HA/MDA-MB-231 cells was much lower than 3×Flag-vector/MDA-MB-231 cells (*p < 0.05, **p < 0.01, lower panel). 5-FU IC50 value of 3×Flag-AGR3-HA/MDA-MB-231 cells in MTT and ATP assay were much lower than 3×Flag-vector/MDA-MB-231 cells (MTT, p=0.025; ATP, p=0.009, lower panel). (C) With the treatment of taxane, cell viability of siAGR3/T47D cells was much lower than scr/T47D cells (*p < 0.05, ** p < 0.01, upper panel). Taxane IC50 values of siAGR3/T47D cells in MTT and ATP assay were much lower than scr/T47D cells (MTT, p=0.033; ATP, p=0.006, upper panel). With the treatment of 5-FU, cell viability of siAGR3/T47D cells was much higher than scr/T47D cells (*p < 0.05, **p < 0.01, ***p < 0.001, lower panel). 5-FU IC50 value of siAGR3/T47D cells in MTT and ATP assay were much higher than scr/T47D cells (MTT, p=0.004; ATP, p=0.017, lower panel).
In breast cancer cell lines, 3×Flag-AGR3-HA/MDA-MB-231 and siAGR3/T47D were performed MTT and ATP assay to measure cell viability with various concentrations of taxane and 5-fluoropyrimidines. Moreover, the IC50 values of taxane and 5-fluoropyrimidines in different cells were also detected. 3×Flag-vector/MDA-MB-231 and 3×Flag-AGR3-HA/MDA-MB-231 cells were treated with 15 to 5×104 ng/mL of taxane and 1-1.5×104 μg/mL of 5-fluoropyrimidines for 48 hours. Both MTT and ATP assay showed that 3×Flag-AGR3-HA/MDA-MB-231 cells exhibited more resistant to taxane but more sensitive to 5-fluoropyrimidines than 3×Flag-vector/MDA-MB-231 cells (Fig. 6B). Scr/T47D and siAGR3/T47D cells were treated with 2.5 to 2.5×104 ng/mL of taxane and 0.1-5×103 μg/mL of 5-fluoropyrimidines for 48 hours. Both MTT and ATP assay showed that siAGR3/T47D cells exhibited more sensitive to taxane but more resistant to 5-fluoropyrimidines than scr/T47D cells (Fig. 6C).
All of these results above indicated that breast cancer cells with AGR3 high expression were resistant to taxane but sensitive to 5-fluoropyrimidines. These results explained why AGR3 high expression could indicate a poor prognosis of breast cancer patients treated with taxane but a favorable prognosis treated with 5-fluoropyrimidines.
Discussion
AGR3’s impact on cancers is still unclear. Reports about AGR3 in cancers are few and contradictory. In tumors, AGR3 protein was firstly identified in the membrane of breast cancer cell lines by proteomic screening [16]. But later, Loader’s group confirmed that AGR3 overexpressed in the cytoplasm of breast cancer cells [8]. And Garczyk et al. [17] found that AGR3’s mRNA significantly increased in breast cancer tissues of histological grade Ⅰ-Ⅱ, but not in grade Ⅲ and AGR3 played a role of indicating poor prognosis in grade Ⅰ-Ⅱ which were consistent with our results. But they didn’t perform more study about the correlation between AGR3 and molecular subtypes. They also found that combining expression of AGR3 and AGR2 in serum sample of breast cancer patients could diagnosed early breast cancer [17]. Besides, it had been demonstrated that AGR3 also overexpressed in ovarian [18] and prostate [19] cancer. In ovarian cancer, Gray et al. [20] found that AGR3 was up-regulated by a hormone (ER)-independent mechanism and could mediate resistance to cisplatin. In prostate cancer, Vaarala et al. [21] found that AGR3 highly elevated in prostate cancer tissues and Bu et al. [22] found that both AGR2 and AGR3 genes were regulated by androgen and estrogen. Moreover, androgen receptor-binding sites in the promoter region of AGR2 and AGR3 were identified in the report of Bu et al. [22]. In these studies, researchers considered that AGR3 could promote cancer development and regarded AGR3 as an indicator of poor prognosis of cancer patients. However, some studies took opposite opinions. In breast cancer, Obacz et al. [23] found that AGR3 was associated with differentiation level, weak proliferation and favorable prognosis of patients. In ovarian cancer, AGR3 expression in serous borderline ovarian tumors and low-grade serous ovarian carcinomas was much higher than high-grade serous ovarian carcinomas and AGR3 was associated with longer patient survival [24].
The role of AGR3 remained uncertain and researchers even got opposite results in the same kind of tumor tissues. We thought it was due to the limit of sample size. With larger scale of samples, our study confirmed that AGR3 expression significantly increased with the development of breast tumor malignancy and had close relationships with molecular subtypes and histological grades. AGR3 mainly expressed in luminal subtype of IDC patients of histological grade Ⅰ-Ⅱ. Most importantly, in luminal B subtype, high expression of AGR3 was correlated with high risk of recurrence and metastasis and could predict poor prognosis of patients. Therefore, AGR3 might be a potential prognostic indicator of luminal B subtype in which patients were more likely to occur therapeutic resistance and poor outcome than luminal A [5]. As for why AGR3 was also overexpressed in luminal A but couldn’t indicate prognosis of this subtype, we considered that it was due to the excellent sensitivity to endocrine therapy in luminal A. And we considered that AGR3’s overexpression in luminal subtype of IDC was due to the transcription activating function of ERα. Welboren et al. [25] had verified several ERα-binding sites existing on the promoter regions of AGR2 and AGR3. But the accurate mechanism needed more proofs. Besides, through further analysis, we found that the majority of luminal patients were grade Ⅰ-Ⅱ (83.33%) but only 16.67% of luminal patients were grade Ⅲ. This result probably explained why AGR3 mainly expressed in grade Ⅰ-Ⅱ rather than grade Ⅲ.
Besides, we firstly found that taxane lead to worse outcome but 5-fluoropyrimidine lead to favorable outcome in AGR3 highly expressed IDC. Then, by performing CD-DST and cytotoxic analysis, we demonstrated that breast cancer cells with AGR3 high expression were resistant to taxane but sensitive to 5-fluoropyrimidines. As we known, in clinic therapy, luminal B patients were routinely treated with additional chemotherapy on the basis of endocrine therapy [5]. These indicated that luminal B patients with AGR3 high expression should be treated with chemotherapy regimens consisting of 5-fluoropyrimidines but not taxane.
As we known, taxane could cure cancer patients depending on its antimitotic characteristic. But ER stress had been identified as another mechanism of taxane cytotoxic effects. The ER stress mediated by taxane could induce apoptosis of cancer cells [26]. In this process, unfolded protein response (UPR) signaling pathway could weaken ER stress to maintain ER homeostasis and help cells survive [27]. Researchers had demonstrated that AGR2 could bind GRP78 which was a central trigger of UPR. The interaction of AGR2 and GRP78 would activate UPR signaling pathway to attenuate cell death induced by ER stress [28]. As homologous protein, AGR2 and AGR3 gene shared 71% sequence identity and lay adjacent to one another at chromosomal position 7p21 [22-24]. Therefore, in tumor cells, we speculated that AGR3 might also interact with GRP78 to activate UPR signaling pathway. Then, the UPR would atte-nuates ER stress mediated by taxane so that tumor cells could survive from the taxane treatment. Besides, Kim et al. [29] found that ER stress induced 5-fluoropyrimidines resistance in human colon cancer cells and Yun et al. [30] found that down-regulation of GRP78 could lead to enhanced sensitivity of cancer cells to cytotoxic effect of 5-fluoropyrimidines [30]. Therefore, we considered that AGR3 could promote sensitivity of breast cancer cells to 5-fluoropyrimidines also due to the interaction with GRP78. All of these speculations need a large number of experiments to prove.
Migration and invasion ability of tumor cells played important roles in the development of cancer. We reported for the first time that AGR3 could promote the proliferation and invasion ability of tumor cells which might be the biological basis of AGR3’s prompting role in cancer.
In conclusion, our research confirmed that, in luminal B subtype of IDC patients of histological grade Ⅰ-Ⅱ, AGR3 high expression indicated poor prognosis and patients should be treated with chemotherapy regimens consisting of 5-fluoropyrimidines but not taxane. AGR3 could promote tumor progression by improving tumor cells’ proliferation ability, invasion ability, and chemotherapeutic drug resistance. AGR3 might be a potential prognostic indicator for classifying prognosis and therapy regimens in this population. But all of these conclusions need more experiments and analysis to confirm.
Acknowledgments
This work was supported by National Scientific Foundation of China (81572851) from Dr. Yongjie Ma and National Scientific Foundation of China (81672636) from Dr. Feng Gu. And our clinical trial registration number was bc2017019.
Footnotes
Conflict of interest relevant to this article was not reported.
Electronic Supplementary Material
Supplementary materials are available at Cancer Research and Treatment website (https://www.e-crt.org).
AGR3 expression in different types of breast tumors
AGR3 expression and pathological features in IDC of grade Ⅰ-Ⅱ
AGR3 expression and pathological features in IDC patients of grade Ⅲ
Relationship between AGR3 and molecular subtypes in IDC of grade Ⅰ-Ⅱ
Relationship between AGR3 and molecular subtypes in IDC of grade Ⅲ
Relationship between AGR3 and metastatic organs in IDC of grade Ⅲ
Anterior gradient 3 (AGR3) had no indication function for therapeutic response of doxorubicin, cyclophosphamide and methotrexate in breast cancer patients. (A) Overall survival (OS) and progression-free survival (PFS) curves of AGR3 highly expressed invasive ductal carcinoma (IDC) patients with epirubicin or doxorubicin treatment were shown, respectively (upper panel). OS and PFS curves of AGR3 low expressed IDC patients with epirubicin or doxorubicin treatment were shown, respectively (lower panel). (B) OS and PFS curves of AGR3 highly expressed IDC patients with cyclophosphamide treatment were shown, respectively (upper panel). OS and PFS curves of AGR3 low expressed IDC patients with cyclophosphamide treatment were shown, respectively (lower panel). (C) OS and PFS curves of AGR3 highly expressed IDC patients with methotrexate treatment were shown, respectively (upper panel). OS and PFS curves of AGR3 low expressed IDC patients with methotrexate treatment were shown, respectively (lower panel).
Anterior gradient 3 (AGR3) had no indication function for therapeutic response of taxane and 5-fluoropyrimidines in non-luminal patients or grade Ⅲ patients. (A) Overall survival (OS) and progression-free survival (PFS) curves of AGR3 highly expressed non-luminal patients with taxane treatment were shown, respectively (upper panel). OS and PFS curves of AGR3 low expressed non-luminal patients with taxane treatment were shown, respectively (lower panel). (B) OS and PFS curves of AGR3 highly expressed non-luminal patients with 5-fluoropyrimidines treatment were shown, respectively (upper panel). OS and PFS curves of AGR3 low expressed non-luminal patients with 5-fluoropyrimidines treatment were shown, respectively (lower panel). (C) OS and PFS curves of AGR3 highly expressed non-luminal patients of grade Ⅰ-Ⅱ with taxane treatment were shown, respectively (upper panel). OS and PFS curves of AGR3 low expressed non-luminal patients of grade Ⅰ-Ⅱ with taxane treatment were shown, respectively (lower panel). (D) OS and PFS curves of AGR3 highly expressed non-luminal patients of grade Ⅰ-Ⅱ with 5-fluoropyrimidines treatment were shown, respectively (upper panel). OS and PFS curves of AGR3 low expressed non-luminal patients of grade Ⅰ-Ⅱ with 5-fluoropyrimidines treatment were shown, respectively (lower panel). (E) OS and PFS curves of AGR3 highly expressed IDC patients of grade Ⅲ with taxane treatment were shown, respectively (upper panel). OS and PFS curves of AGR3 low expressed IDC patients of grade Ⅲ with taxane treatment were shown, respectively (lower panel). (F) OS and PFS curves of AGR3 highly expressed IDC patients of grade Ⅲ with 5-fluoropyrimidines treatment were shown, respectively (upper panel). OS and PFS curves of AGR3 low expressed IDC patients of grade Ⅲwith 5-fluoropyrimidines treatment were shown, respectively (lower panel).
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Mendes TF, Kluskens LD, Rodrigues LR. Triple negative breast cancer: nanosolutions for a big challenge. Adv Sci (Weinh) 2015;2:1500053. doi: 10.1002/advs.201500053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abdel-Hafiz HA. Epigenetic mechanisms of tamoxifen resistance in luminal breast cancer. Diseases. 2017;5:E16. doi: 10.3390/diseases5030016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hart CD, Sanna G, Siclari O, Biganzoli L, Di Leo A. Defining optimal duration and predicting benefit from chemotherapy in patients with luminal-like subtypes. Breast. 2015;24 Suppl 2:S136–42. doi: 10.1016/j.breast.2015.07.033. [DOI] [PubMed] [Google Scholar]
- 5.Tran B, Bedard PL. Luminal-B breast cancer and novel therapeutic targets. Breast Cancer Res. 2011;13:221. doi: 10.1186/bcr2904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ivanova AS, Tereshina MB, Ermakova GV, Belousov VV, Zaraisky AG. Agr genes, missing in amniotes, are involved in the body appendages regeneration in frog tadpoles. Sci Rep. 2013;3:1279. doi: 10.1038/srep01279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thompson DA, Weigel RJ. hAG-2, the human homologue of the Xenopus laevis cement gland gene XAG-2, is coexpressed with estrogen receptor in breast cancer cell lines. Biochem Biophys Res Commun. 1998;251:111–6. doi: 10.1006/bbrc.1998.9440. [DOI] [PubMed] [Google Scholar]
- 8.Fletcher GC, Patel S, Tyson K, Adam PJ, Schenker M, Loader JA, et al. hAG-2 and hAG-3, human homologues of genes involved in differentiation, are associated with oestrogen receptor-positive breast tumours and interact with metastasis gene C4.4a and dystroglycan. Br J Cancer. 2003;88:579–85. doi: 10.1038/sj.bjc.6600740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wilson CL, Sims AH, Howell A, Miller CJ, Clarke RB. Effects of oestrogen on gene expression in epithelium and stroma of normal human breast tissue. Endocr Relat Cancer. 2006;13:617–28. doi: 10.1677/erc.1.01165. [DOI] [PubMed] [Google Scholar]
- 10.Hrstka R, Nenutil R, Fourtouna A, Maslon MM, Naughton C, Langdon S, et al. The pro-metastatic protein anterior gradient-2 predicts poor prognosis in tamoxifen-treated breast cancers. Oncogene. 2010;29:4838–47. doi: 10.1038/onc.2010.228. [DOI] [PubMed] [Google Scholar]
- 11.Park K, Chung YJ, So H, Kim K, Park J, Oh M, et al. AGR2, a mucinous ovarian cancer marker, promotes cell proliferation and migration. Exp Mol Med. 2011;43:91–100. doi: 10.3858/emm.2011.43.2.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang JS, Gong A, Cheville JC, Smith DI, Young CY. AGR2, an androgen-inducible secretory protein overexpressed in prostate cancer. Genes Chromosomes Cancer. 2005;43:249–59. doi: 10.1002/gcc.20188. [DOI] [PubMed] [Google Scholar]
- 13.Pohler E, Craig AL, Cotton J, Lawrie L, Dillon JF, Ross P, et al. The Barrett’s antigen anterior gradient-2 silences the p53 transcriptional response to DNA damage. Mol Cell Proteomics. 2004;3:534–47. doi: 10.1074/mcp.M300089-MCP200. [DOI] [PubMed] [Google Scholar]
- 14.Fritzsche FR, Dahl E, Dankof A, Burkhardt M, Pahl S, Petersen I, et al. Expression of AGR2 in non small cell lung cancer. Histol Histopathol. 2007;22:703–8. doi: 10.14670/HH-22.703. [DOI] [PubMed] [Google Scholar]
- 15.Ramachandran V, Arumugam T, Wang H, Logsdon CD. Anterior gradient 2 is expressed and secreted during the development of pancreatic cancer and promotes cancer cell survival. Cancer Res. 2008;68:7811–8. doi: 10.1158/0008-5472.CAN-08-1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Adam PJ, Boyd R, Tyson KL, Fletcher GC, Stamps A, Hudson L, et al. Comprehensive proteomic analysis of breast cancer cell membranes reveals unique proteins with potential roles in clinical cancer. J Biol Chem. 2003;278:6482–9. doi: 10.1074/jbc.M210184200. [DOI] [PubMed] [Google Scholar]
- 17.Garczyk S, von Stillfried S, Antonopoulos W, Hartmann A, Schrauder MG, Fasching PA, et al. AGR3 in breast cancer: prognostic impact and suitable serum-based biomarker for early cancer detection. PLoS One. 2015;10:e0122106. doi: 10.1371/journal.pone.0122106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li H, Narahara H. 15-Deoxy-Δ(12,14)-prostaglandin J(2) induces growth inhibition, cell cycle arrest and apoptosis in human endometrial cancer cell lines. Int J Mol Med. 2013;31:778–88. doi: 10.3892/ijmm.2013.1268. [DOI] [PubMed] [Google Scholar]
- 19.Pascal LE, Vencio RZ, Page LS, Liebeskind ES, Shadle CP, Troisch P, et al. Gene expression relationship between prostate cancer cells of Gleason 3, 4 and normal epithelial cells as revealed by cell type-specific transcriptomes. BMC Cancer. 2009;9:452. doi: 10.1186/1471-2407-9-452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gray TA, MacLaine NJ, Michie CO, Bouchalova P, Murray E, Howie J, et al. Anterior Gradient-3: a novel biomarker for ovarian cancer that mediates cisplatin resistance in xenograft models. J Immunol Methods. 2012;378:20–32. doi: 10.1016/j.jim.2012.01.013. [DOI] [PubMed] [Google Scholar]
- 21.Vaarala MH, Hirvikoski P, Kauppila S, Paavonen TK. Identification of androgen-regulated genes in human prostate. Mol Med Rep. 2012;6:466–72. doi: 10.3892/mmr.2012.956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bu H, Schweiger MR, Manke T, Wunderlich A, Timmermann B, Kerick M, et al. Anterior gradient 2 and 3: two prototype androgen-responsive genes transcriptionally upregulated by androgens and by oestrogens in prostate cancer cells. FEBS J. 2013;280:1249–66. doi: 10.1111/febs.12118. [DOI] [PubMed] [Google Scholar]
- 23.Obacz J, Brychtova V, Podhorec J, Fabian P, Dobes P, Vojtesek B, et al. Anterior gradient protein 3 is associated with less aggressive tumors and better outcome of breast cancer patients. Onco Targets Ther. 2015;8:1523–32. doi: 10.2147/OTT.S82235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.King ER, Tung CS, Tsang YT, Zu Z, Lok GT, Deavers MT, et al. The anterior gradient homolog 3 (AGR3) gene is associated with differentiation and survival in ovarian cancer. Am J Surg Pathol. 2011;35:904–12. doi: 10.1097/PAS.0b013e318212ae22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Welboren WJ, van Driel MA, Janssen-Megens EM, van Heeringen SJ, Sweep FC, Span PN, et al. ChIP-Seq of ERalpha and RNA polymerase II defines genes differentially responding to ligands. EMBO J. 2009;28:1418–28. doi: 10.1038/emboj.2009.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mhaidat NM, Thorne R, Zhang XD, Hersey P. Involvement of endoplasmic reticulum stress in Docetaxel-induced JNK-dependent apoptosis of human melanoma. Apoptosis. 2008;13:1505–12. doi: 10.1007/s10495-008-0276-8. [DOI] [PubMed] [Google Scholar]
- 27.McGrath EP, Logue SE, Mnich K, Deegan S, Jager R, Gorman AM, et al. The unfolded protein response in breast cancer. Cancers (Basel) 2018;10:E344. doi: 10.3390/cancers10100344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ryu J, Park SG, Lee PY, Cho S, Lee DH, Kim GH, et al. Dimerization of pro-oncogenic protein Anterior Gradient 2 is required for the interaction with BiP/GRP78. Biochem Biophys Res Commun. 2013;430:610–5. doi: 10.1016/j.bbrc.2012.11.105. [DOI] [PubMed] [Google Scholar]
- 29.Kim JK, Kang KA, Piao MJ, Ryu YS, Han X, Fernando PM, et al. Endoplasmic reticulum stress induces 5-fluorouracil resistance in human colon cancer cells. Environ Toxicol Pharmacol. 2016;44:128–33. doi: 10.1016/j.etap.2016.05.005. [DOI] [PubMed] [Google Scholar]
- 30.Yun S, Han YS, Lee JH, Kim S, Lee SH. Enhanced susceptibility to 5-fluorouracil in human colon cancer cells by silencing of GRP78. Anticancer Res. 2017;37:2975–84. doi: 10.21873/anticanres.11651. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
AGR3 expression in different types of breast tumors
AGR3 expression and pathological features in IDC of grade Ⅰ-Ⅱ
AGR3 expression and pathological features in IDC patients of grade Ⅲ
Relationship between AGR3 and molecular subtypes in IDC of grade Ⅰ-Ⅱ
Relationship between AGR3 and molecular subtypes in IDC of grade Ⅲ
Relationship between AGR3 and metastatic organs in IDC of grade Ⅲ
Anterior gradient 3 (AGR3) had no indication function for therapeutic response of doxorubicin, cyclophosphamide and methotrexate in breast cancer patients. (A) Overall survival (OS) and progression-free survival (PFS) curves of AGR3 highly expressed invasive ductal carcinoma (IDC) patients with epirubicin or doxorubicin treatment were shown, respectively (upper panel). OS and PFS curves of AGR3 low expressed IDC patients with epirubicin or doxorubicin treatment were shown, respectively (lower panel). (B) OS and PFS curves of AGR3 highly expressed IDC patients with cyclophosphamide treatment were shown, respectively (upper panel). OS and PFS curves of AGR3 low expressed IDC patients with cyclophosphamide treatment were shown, respectively (lower panel). (C) OS and PFS curves of AGR3 highly expressed IDC patients with methotrexate treatment were shown, respectively (upper panel). OS and PFS curves of AGR3 low expressed IDC patients with methotrexate treatment were shown, respectively (lower panel).
Anterior gradient 3 (AGR3) had no indication function for therapeutic response of taxane and 5-fluoropyrimidines in non-luminal patients or grade Ⅲ patients. (A) Overall survival (OS) and progression-free survival (PFS) curves of AGR3 highly expressed non-luminal patients with taxane treatment were shown, respectively (upper panel). OS and PFS curves of AGR3 low expressed non-luminal patients with taxane treatment were shown, respectively (lower panel). (B) OS and PFS curves of AGR3 highly expressed non-luminal patients with 5-fluoropyrimidines treatment were shown, respectively (upper panel). OS and PFS curves of AGR3 low expressed non-luminal patients with 5-fluoropyrimidines treatment were shown, respectively (lower panel). (C) OS and PFS curves of AGR3 highly expressed non-luminal patients of grade Ⅰ-Ⅱ with taxane treatment were shown, respectively (upper panel). OS and PFS curves of AGR3 low expressed non-luminal patients of grade Ⅰ-Ⅱ with taxane treatment were shown, respectively (lower panel). (D) OS and PFS curves of AGR3 highly expressed non-luminal patients of grade Ⅰ-Ⅱ with 5-fluoropyrimidines treatment were shown, respectively (upper panel). OS and PFS curves of AGR3 low expressed non-luminal patients of grade Ⅰ-Ⅱ with 5-fluoropyrimidines treatment were shown, respectively (lower panel). (E) OS and PFS curves of AGR3 highly expressed IDC patients of grade Ⅲ with taxane treatment were shown, respectively (upper panel). OS and PFS curves of AGR3 low expressed IDC patients of grade Ⅲ with taxane treatment were shown, respectively (lower panel). (F) OS and PFS curves of AGR3 highly expressed IDC patients of grade Ⅲ with 5-fluoropyrimidines treatment were shown, respectively (upper panel). OS and PFS curves of AGR3 low expressed IDC patients of grade Ⅲwith 5-fluoropyrimidines treatment were shown, respectively (lower panel).