Keywords: Bacteroidetes, biomarker, Firmicutes, healthy, intestine, lactobacillus reuteri, metabolites, microbiota, newborn, probiotic
Abstract
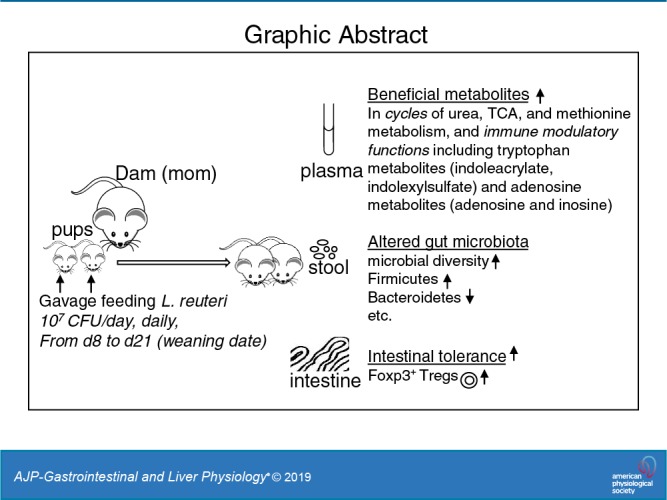
Early administration of Lactobacillus reuteri DSM 17938 (LR) prevents necrotizing enterocolitis and inhibits regulatory T-cell (Treg)-deficiency-associated autoimmunity in mice. In humans, LR reduces crying time in breastfed infants with colic, modifies severity in infants with acute diarrheal illnesses, and improves pain in children with functional bowel disorders. In healthy breastfed newborns with evolving microbial colonization, it is unclear if early administration of LR can modulate gut microbiota and their metabolites in such a way as to promote homeostasis. We gavaged LR (107 colony-forming units/day, daily) to C57BL/6J mice at age of day 8 for 2 wk. Both male and female mice were investigated in these experiments. We found that feeding LR did not affect clinical phenotype or inflammatory biomarkers in plasma and stool, but LR increased the proportion of Foxp3+ regulatory T cells (Tregs) in the intestine. LR also increased bacterial diversity and the relative abundance of p_Firmicutes, f_Lachnospiraceae, f_Ruminococcaceae, and genera Clostridium and Candidatus arthromitus, while decreasing the relative abundance of p_Bacteriodetes, f_Bacteroidaceae, f_Verrucomicrobiaceae, and genera Bacteroides, Ruminococcus, Akkermansia, and Sutterella. Finally, LR exerted a major impact on the plasma metabolome, upregulating amino acid metabolites formed via the urea, tricarboxylic acid, and methionine cycles and increasing tryptophan metabolism. In conclusion, early oral administration of LR to healthy breastfed mice led to microbial and metabolic changes which could be beneficial to general health.
NEW & NOTEWORTHY Oral administration of Lactobacillus reuteri DSM 17938 (LR) to healthy breastfed mice promotes intestinal immune tolerance and is linked to proliferation of beneficial gut microbiota. LR upregulates plasma metabolites that are involved in the urea cycle, the TCA cycle, methionine methylation, and the polyamine pathway. Herein, we show that LR given to newborn mice specifically increases levels of tryptophan metabolites and the purine nucleoside adenosine that are known to enhance tolerance to inflammatory stimuli.
INTRODUCTION
The American Academy of Pediatrics has recommended that infants be exclusively breastfed for the first 6 mo of life. By 2018, there has been a marked increase in the numbers of breastfed babies, with 47% being exclusively breastfed at 3 mo and 25% being exclusively breastfed at 6 mo, almost reaching Healthy People 2020-established goals in the United States (9).
Lactobacillus reuteri DSM 17938 (LR) is a probiotic originally isolated from a Peruvian mother’s breast milk (52). This probiotic has been shown to prevent necrotizing enterocolitis in stressed newborn animals by inhibiting the Toll-like receptor 4-mediated NF-κB pathway, facilitating the induction of immune-modulating regulatory T cells (Tregs), and lowering proinflammatory effector-memory T cells in the intestinal mucosa (34, 35). LR is also under investigation for the prevention of necrotizing enterocolitis in premature infants (38, 43). In humans, LR has been shown to reduce the severity of acute infant diarrhea (17, 48) and to decrease crying time in infants with colic (49). A previous study of LR transcriptomes during different phases of the growth cycle suggested that this LR strain is predicted to produce health-promoting factors, including vitamins (important for nutrition), exopolysaccharide (for immune stimulation), and enzymes (related to amino acid metabolism) (45). However, detailed investigations of the relation of LR to bioactive metabolites in the healthy breastfed host have not been undertaken, to our knowledge. In this study we observed marked changes in both the gut microbiota and plasma metabolome after oral feeding LR to breastfed mice. In addition, we examined T cells connected to immune tolerance (Tregs) in the intestine and mesenteric lymph nodes. Our observations provide novel insights into the mechanisms by which LR may improve health.
MATERIALS AND METHODS
Mice
Wild-type (WT) C57BL/6J mice (6–8 wk-old) were purchased from Jackson Laboratories and allowed to acclimatize for 2 wk before experimentation. All mice were housed in the specific pathogen-free animal facility at the University of Texas Health Science Center at Houston. This study was carried out in accordance with the recommendations of the Guide for the Care and Use of Laboratory Animals and the Institutional Animal Care and Use Committee. The protocol was approved by the Institutional Animal Care and Use Committee (protocol no. AWC-14-056 and 17-0045).
LR Preparation and Treatment of Mice
Human breast milk-derived LR was provided by BioGaia (Stockholm, Sweden). LR was prepared as described previously (35). The main species of bacteria used in probiotic formulations are lactobacilli (such as LR, a gram-positive and anaerobic bacteria, residing in the upper human gastrointestinal tract) and bifidobacteria, which are classified as anaerobic bacteria and therefore require an oxygen-free environment for growth (and reside primarily in the lower gastrointestinal tract). Briefly, LR was anaerobically cultured in an optimal deMan-Rogosa-Sharpe (MRS) medium at 37°C for 24 h and then plated in MRS agar at specific serial dilutions and grown anaerobically at 37°C for 48–72 h to count colonies for generating the standard curve of bacterial colony-forming units (CFU) per milliliter grown on MRS agar versus absorbance at 600 nm at known concentrations. Quantitative analysis of bacteria in the culture media on colony-forming units per milliliters then was calculated by comparing absorbance at 600 nm of cultures by using the standard curve.
Newborn mice were generated by breeding of C57BL/6J female to male mice and were remained with their dams. Both male and female newborn mice were investigated in these experiments. Each newborn mouse was given by gavage freshly grown LR in cultured MRS media (107 CFU/day, 100 µL) daily, starting from 8 days of age (d8) to d21 before weaning (WTL; n = 8 mice); control each newborn mouse was given by gavage an identical volume of MRS media, daily (WTC; n = 8 mice). Mice were euthanized on d22 to collect blood and cecal contents. The plasma and cecal contents were stored immediately at −80°C freezer for further plasma and stool metabolomics analysis and stool microbiota analysis. We collected mouse ileum, colon, and mesenteric lymph nodes and isolated immune cells for analysis of T-cell subsets. We also collected tissues including liver, lung, ileum, and colon for histological analysis.
Histological Evaluation
Liver, lung, ileum, and colon collected from each mouse were fixed and processed by the Cellular and Molecular Morphology Core Laboratory (the Texas Medical Center Digestive Diseases Center, Houston, TX) and stained with hematoxylin and eosin for histological evaluation.
Assays for Inflammatory Biomarkers
Plasma cytokine assays.
Plasma cytokine levels of IFN-γ and IL-4 were assessed using a mouse multispot proinflammatory panel kit from Meso Scale Discovery, according to the manufacturer’s protocol.
Fecal calprotectin and CXCL1/KC assays.
Stool extracts were prepared by using the extraction buffer containing 0.1 M Tris, 0.15 M NaCl, 1.0 M urea, 10 mM CaCl2, 0.1 M citric acid monohydrate, 5 g/l BSA, and 0.25 mM thimerosal (pH 8.0). Then, 4.9 ml of extraction buffer were added to 100-mg sample (giving a dilution factor of 50, assuming the density of feces to be 1 g/ml). The minimal 50 mg of stool for each sample were used, and the amount of extraction buffer was adapted proportional to the amount of stool sample. Samples were vortexed until no large particles were present. Part of the homogenate was transferred into a fresh tube and centrifuged for 5 min at ≥3,000 g. The stool extract supernatants were collected and stored at −80°C until used in the assays. Mouse fecal calprotectin (cat. no. MBS2602300; MyBioSource) and mouse CXCL1/KC (R&D Systems) were examined by using ELISA, according to manufacturers’ protocols, respectively. Final levels of fecal calprotectin and CXCL1/KC are expressed as picograms per grams stool weight.
In Vitro Tissue Preparation and Staining Cells for Flow Cytometry Analysis
Single cell suspensions from the terminal small intestine, colon, and mesenteric lymph node were obtained by gently fragmenting and filtering the tissues through 40-µm cell strainers (BD Bioscience, San Jose, CA) into RPMI 1640 (Sigma, St. Louis, MO) complete medium containing collagenase V (0.1 mg/ml) from Clostridium histolyticum (Sigma). MACS buffer consisting of phosphate-buffered saline, 0.5% bovine serum albumin (Hyclone GE Life Science, Logan, UT), and 2 mM EDTA (Lonza, Bethesda, MD) was used for washing the cells. Finally, cells were resuspended in MACS buffer for surface and intracellular staining using specific antibodies including CD19 (6D5) conjugated with Alexa Fluor 700 (AF700), CD3 (17A2) conjugated with cyanine dye 7 (Cy7; PE-Cy7), CD4 (GK1.5) conjugated with peridinin-chlorophyl proteins (PerCP/Cy5.5), CD8a (53–6.7) conjugated with phycoerythrin, Foxp3 (FJK-16s) conjugated with allophycocyanin, and CD44 (IM7) conjugated with FITC from Biolegend (San Diego, CA). All samples were analyzed with Gallios Flow Cytometer (Beckman-Coulter) and processed with FlowJo (TreeStar, Ashland, OR).
Determining the Population Structure of the Microbiota in C57BL/6 Mice
Sequencing and bioinformatics were performed by the Louisiana State University School of Medicine Microbial Genomics Resource Group (http://metagenomics.lsuhsc.edu). Genomic DNA extraction from cecal contents was performed using QIAamp Fast DNA Stool Mini Kit (Qiagen, Germantown, MD), and bacterial profiling by amplification and sequencing of the V4 region of the 16S rRNA gene was performed as previously described (5). The 16S ribosomal DNA hypervariable regions V4 were PCR amplified using primers V4F GTGCCAGCMGCCGCGGTAA and V4R GGACTACHVGGGTWTCTAAT with Illumina adaptors and molecular barcodes to produce amplicons. Samples were sequenced on an Illumina MiSeq (Illumina, San Diego, CA) using V4 sequencing kit. The forward and reverse read files were processed, and the original filtered reads were mapped to the operational taxonomic units (OTUs) at 97% identity through the UPARSE pipeline (drive5, Tiburon, CA) (16). Relative abundance of each OTU was examined at phylum, class, order, family, genus, and species levels. Bacterial α- and β-diversity metrics, as well as taxonomic community assessments, were produced using QIIME 1.8 (open source, http://qiime.org/) (6).
Plasma and Stool Global Metabolomic Analysis
Plasma and stool metabolites of WTC and WTL were submitted, processed, and measured by Metabolon (https://www.metabolon.com/) (21). A total of 688 compounds of known identity (named biochemicals) in plasma, and 766 named biochemicals in stool were detected by a nontargeted metabolomic analysis platform including ultra performance liquid chromatography-tandem mass spectrometer and gas chromatography-mass spectrometry, respectively. The global metabolomics profile data including fold change of WTL/WTC was reported by Metabolon.
Statistical Analysis
Group comparisons of microbiota α diversity were measured using Shannon diversity and evenness; β diversity was measured by principle coordinates analysis, and the relative abundance of bacteria and the differences of metabolites were analyzed by t test (2 group comparison). The frequency of immune cells in intestinal mucosa one-way ANOVA corrected for multiple comparisons with Tukey posttests by using Prism 4.0 (GraphPad Software, San Diego, CA). Data are presented as means ± SE. The Spearman’s rank correlation coefficient determined between metabolites and microbiota and between metabolites themselves was calculated for integrative analysis. P < 0.05 were considered significant.
We applied principle components analysis to test the clustering of the whole metabolome of each sample from each testing group by the R package stats. We measured the difference of individual biochemical metabolite from the testing groups by fold change and the significance of differences by one-way ANOVA. The up- and downregulated metabolites were defined as those with 1.2 or 0.8-fold changes and P < 0.05, respectively. The data processing and analyzing were run by using an in-house developed program the Analytical Toolkits for Integrative Omics (ATiOmics): Metabolomics and Microbiomics (https://xiangjut.shinyapps.io/atiomics).
RESULTS
LR Feeding Does Not Affect Clinical Phenotype but Boosts Treg Cells in the Intestinal Mucosa
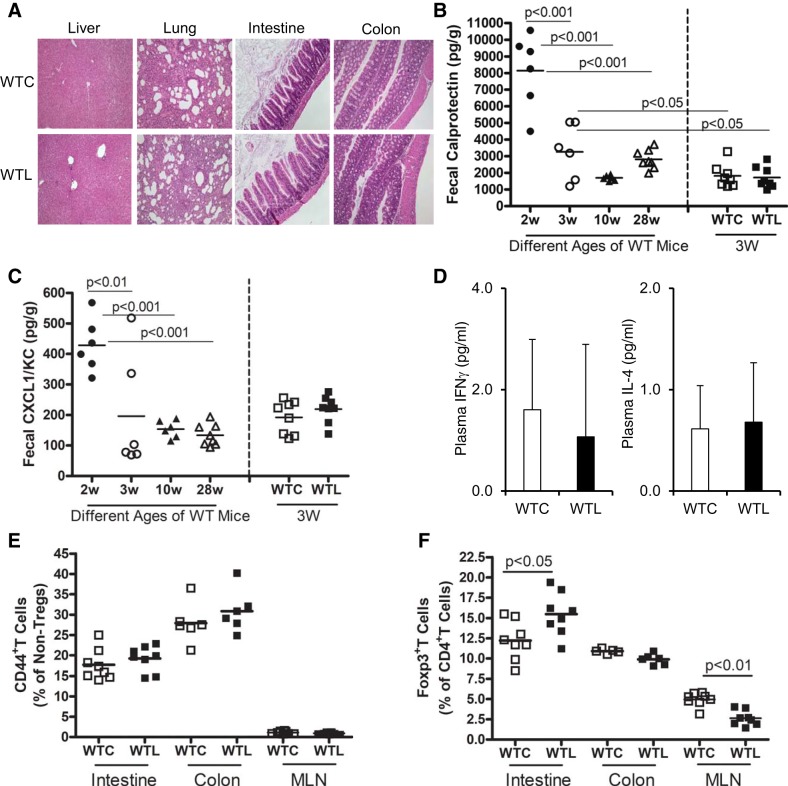
We observed that there were no effects of LR administration on growth, survival, or other manifestations of general health in normal mouse pups fed on their dams during the period of study. Histological observation of important organs including liver, lung, small intestine, and colon demonstrated normal phenotype (Fig. 1A). To determine if LR feeding of normal mice would affect inflammatory biomarkers, we measured the levels of calprotectin, an antimicrobial peptide produced by gut neutrophils (Fig. 1B) and the chemokine CXCL1/KC (Fig. 1C) in cecal contents from mice comparing WTL with WTC mice. We also measured the levels of fecal calprotectin and CXCL1/KC of normal mice without gavage exposure at different ages. Results indicated that at 2 wk of age (d15) mice had higher levels of both calprotectin and CXCL1/KC than those seen at 3 wk of age (d22) or in adulthood (10 and 28 wk). Oral gavage feeding of LR (WTL) in healthy breastfed mice did not change the level of these inflammatory biomarkers compared with those mice gavaged with MRS media (WT). However, in WT mice with gavage-feeding of placebo or probiotic (WTC or WTL), fecal calprotectin levels were mildly decreased compared with WT mice without any feeding intervention at the same age (3 wk; P < 0.05; Fig. 1B).
Fig. 1.
Effect of Lactobacillus reuteri (LR) on inflammatory biomarkers in healthy breastfed mice. A: normal histology representatives of the liver, lung, small intestine, and colon of wild-type control (WTC) vs. wild-type LR fed (WTL). B: levels of fecal calprotectin assessed by ELISA. C: levels of fecal chemokine CXCL1/KC assessed by ELISA; w, week. D: plasma levels of IFNγ and IL-4 assessed by ELISA. E: percentage of CD44+ T cells of nonregulatory T-cells (Tregs) analyzed by flow cytometry. MLN, mesenteric lymph nodes. F: percentage of Foxp3+ T cells (Tregs) in CD4+ T-cell populations analyzed by flow cytometry. Both male and female mice were investigated in these experiments. A total of 16 mice (n = 8 in WTC and n = 8 in WTL) were employed for the analysis to compare the groups between WTL and WTC. In addition, for the different ages of WT mice, n = 6 in 2 wk, n = 6 in 3 wk, n = 6 in 10 wk, and n = 7 in 28 wk of age (B and C). P < 0.05 indicates significance, determined by two-tailed, one-way ANOVA corrected for multiple comparisons with Tukey posttests for B and C; nonpaired Student’s t test for 2 group comparisons.
Concentrations of inflammatory cytokines IFN-γ (a TH1 cytokine) and IL-4 (a TH2 cytokine) in plasma were very low, and there were no difference between WTL and WTC mice (Fig. 1D). In addition, LR had no effect on CD44+T cells in the intestine, colon, or mesenteric lymph nodes of healthy breastfed mice, indicating that LR did not affect inflammation-related T-effector cells (Fig. 1E). Our previous study showed that feeding of LR to newborn breastfed rats significantly increased the percentage of Tregs in the intestine compared with formula-fed newborn rats at very early ages (32). Herein, we examined the effect of oral feeding of LR on B cells and T cells in the intestinal mucosa, defining the cell populations using cell markers analyzed by flow cytometry (Supplemental Fig. S1A; all Supplemental Figures are available at https://doi.org/10.6084/m9.figshare.9684875.v1). We observed that oral feeding of LR to healthy breastfed mice had no effect on the proportion of CD19+ B cells in lymphocytes in the intestinal mucosa of mice (Supplemental Fig. S1Bi), but LR feeding reduced the proportion of CD3+ T cells in lymphocytes in the ileum (Supplemental Fig. S1Bii). Among the T-cell populations, LR had no effects on CD4+T helper cells but reduced the percentage of CD8+ cytotoxic T cells in the ileum (Supplemental Fig. S1, Biii and Biv). We found that there were no effects of LR on T cells and B cells in the colon of mice (Supplemental Fig. S1, Bii–Biv). Importantly, LR increased the proportion of regulatory T cells (Foxp3+Tregs as a percentage of total CD4+T cells) in the small intestine of normal breastfed mice. Concurrently, LR feeding reduced the proportion of Foxp3+Tregs in CD4+T cells in the mesenteric lymph nodes, which may indicate that LR promotes Treg-trafficking from mesenteric lymph nodes to small intestine (Fig. 1F).
Oral Feeding LR to Healthy Breastfed Mice Changes Gut Microbiota
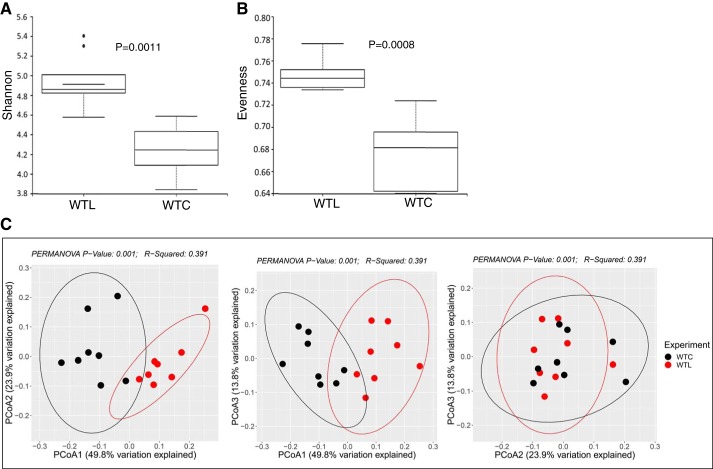
Because LR has been linked to beneficial effects on mucosal immunity, we examined the gut microbial community affected by oral feeding LR to healthy breastfed pups. We found that LR feeding led to important changes in the gut microbiota, as indicated by an increase in alpha diversity (Fig. 2, A and B) measured by Shannon diversity index (P = 0.0011; Fig. 2A) and by microbial evenness measurement (P = 0.0008; Fig. 2B) in WTL mice. LR feeding also shifted the microbial community composition, as shown by beta diversity, measured using principle coordinates analysis in WTL mice compared with WTC mice (permutational multivariate ANOVA, P = 0.001; Fig. 2C).
Fig. 2.
Effect of Lactobacillus reuteri (LR) on α diversity and β diversity of gut microbiota. A: Shannon α diversity: wild-type LR fed (WTL) vs. wild-type control (WTC), P = 0.0011. B: evenness of α diversity: WTL vs. WTC, P = 0.0008. C: 2-dimensional principle coordinates analysis (PCoA) plot of clusters of WTC vs. WTL: P1 vs. P2 (left), P1 vs. P3 (middle), and P2 vs. P3 (right). WTC: black dots; WTL: red dots. WTL vs. WTC: P = 0.001. PERMANOVA, permutational multivariate ANOVA.
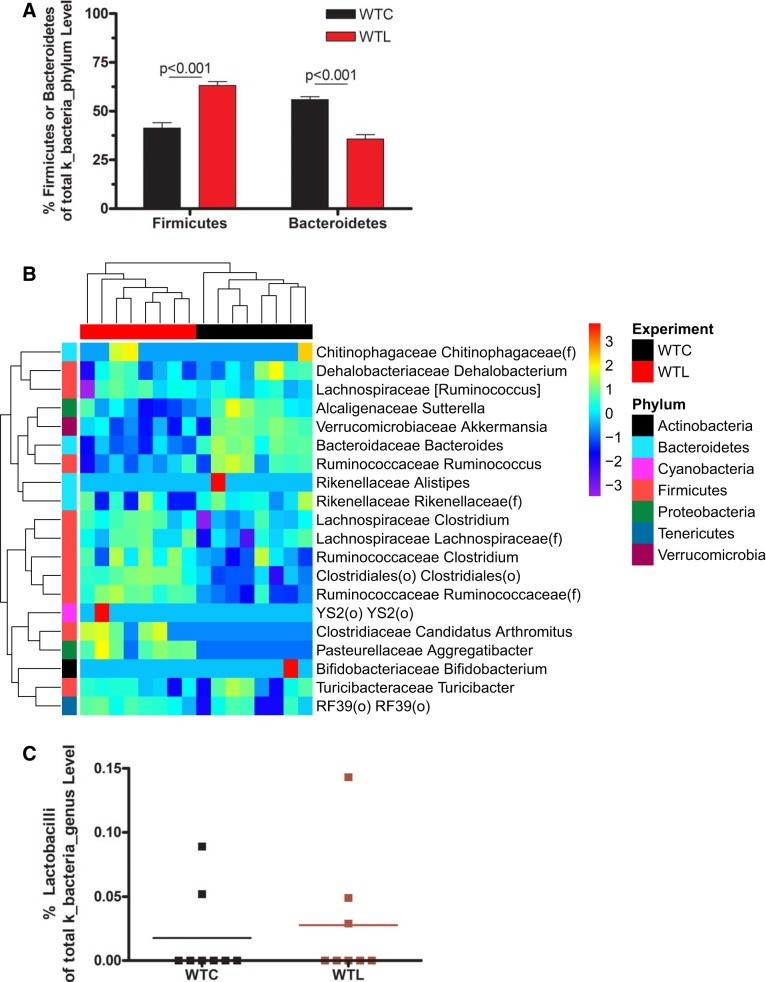
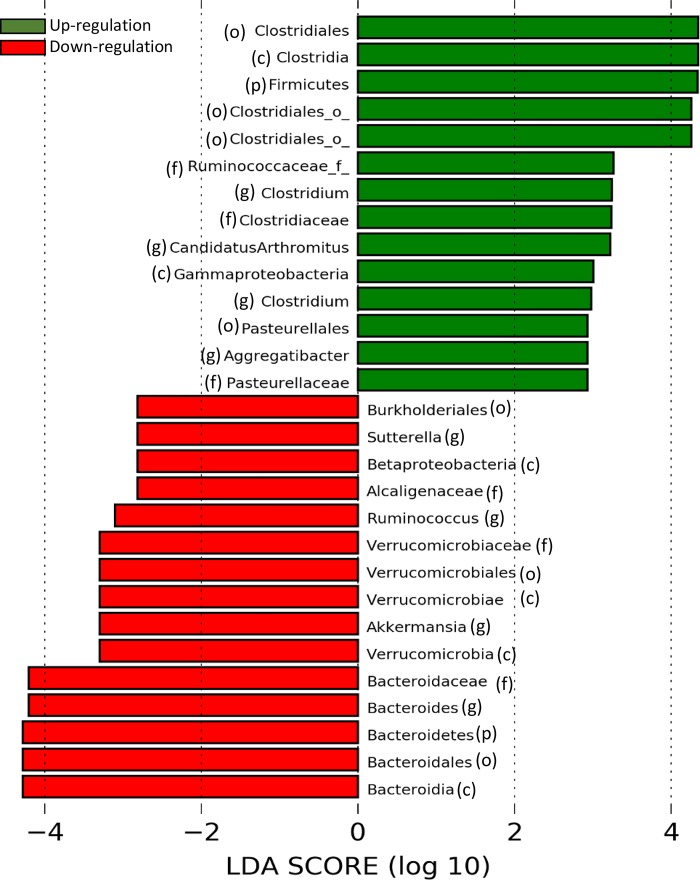
The core microbiota of healthy breastfed mice were composed of taxa belonging to the phylum Firmicutes (~50%) (including genera Clostridium and Ruminococcus) and to the phylum Bacteroidetes (~45%) (including genera Bacteroides, Rikenellaceae, and S24-7). The relative abundance of specific bacteria was calculated based on the OTUs of the bacteria normalized to identified total OTU counts by 16S rDNA sequencing in the sample (Supplemental Table S1; see https://doi.org/10.6084/m9.figshare.9684911.v1). In response to LR feeding, the relative abundance of Firmicutes increased, whereas Bacteroidetes was decreased (P < 0.001) at the phylum level (Fig. 3A and Supplemental Table S1). Heat-map (Fig. 3B), linear discriminant analysis (Fig. 4), and cladogram analysis of the gut microbiota (Supplemental Fig. S2A) additionally showed upregulation of o_Clostridiales, f_Lachnospiraceae, f_Ruminococcaceae, and g_Clostridium and g_Candidatus arthromitus. The relative abundance of downregulated taxa included o_Bacteroidales, o_Verrucomicrobiales, f_Bacteroidaceae, f_Verrucomicrobiaceae, and g_Bacteroides, g_Ruminococcus, g_Akkermansia, and g_Sutterella. We noted that LR feeding also differentially changed Proteobacteria, with an upregulation of g_Pasteurellaceae aggregatibacter (Gamma proteobacteria) and a downregulation of g_Alcaligenaceae sutterrella (Beta proteobacteria) (Figs. 3B and 4). The relative abundance of lactobacilli in the cecal sample was low (<0.3% of total bacterial OTUs at genus level) with only two to three out of eight samples in each group detectable, which was not changed by LR feeding (Fig. 3C). Random forest analysis showed that discriminant species defining the most significant difference between groups of pups with and without LR feeding were o_Clostridiales, g_Bacteroides, f_Ruminococcaceae, and g_Akkemansia (Supplemental Fig. S2B).
Fig. 3.
Effect of Lactobacillus reuteri (LR) on the relative abundance of bacterial community in healthy breastfed mice. A: relative abundance of Firmicutes and Bacteroidetes at phylum level. B: heatmap of bacteria for the top 20 genus from random forest importance to compare with wild-type control (WTC) vs. wild-type LR fed (WTL). C: relative abundance of Lactobacilli at genus level.
Fig. 4.
Calculated linear discriminant analysis (LDA) scores to evaluate the up- or downregulation of bacteria by oral feeding Lactobacillus reuteri (LR) to healthy breastfed mice. The right bars are green indicating upregulation by LR; the left bars are red indicating downregulation by LR. Bacterial classification in parenthesis: p, phylum level; c, class level; o, order level; f, family level; and g, genus level.
Gavage Feeding of LR to Healthy Breastfed Mice Changes the Plasma Metabolomic Profile
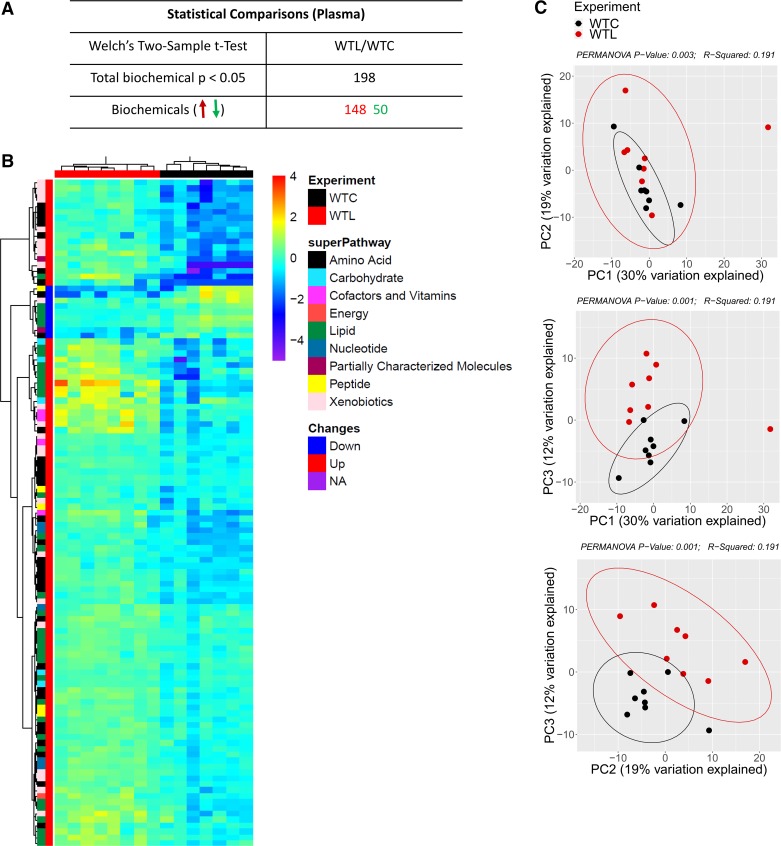
Altogether, there were 198 out of 688 identified biochemicals altered by LR, with 148 upregulated and 50 downregulated (P < 0.05) in plasma compared with media control (Fig. 5A). The heatmap of metabolites from individual mice showed distinct signatures of the metabolomics profile comparing WTC and WTL mice. LR feeding produced the most significant changes in levels of amino acid, lipids, cofactors, vitamins, and xenobiotics (Fig. 5B). Permutational multivariate ANOVA analysis indicated a significant difference in the global metabolome between groups WTC and WTL (P = 0.001), as demonstrated by principal component analysis (Fig. 5C).
Fig. 5.
Effect of Lactobacillus reuteri (LR) on plasma metabolomics profile in healthy breastfed mice. A: counts of biochemical (metabolites) up- or downregulated by LR oral feeding. Red indicates upregulation and the green indicates downregulation. B: heatmap of plasma metabolites in each individual mouse classified as groups of wild-type control (WTC) (black) and wild-type LR fed (WTL) (red). C: 2-dimensional principle components analysis (PCA) plot of metabolome clusters of WTC (black dots) and WTL (red dots). Each dot represents 1 sample. PC1 vs. PC2 (top), PC1 vs. PC3 (middle), and PC2 vs. PC3 (bottom); P = 0.001. PERMANOVA, permutational multivariate ANOVA.
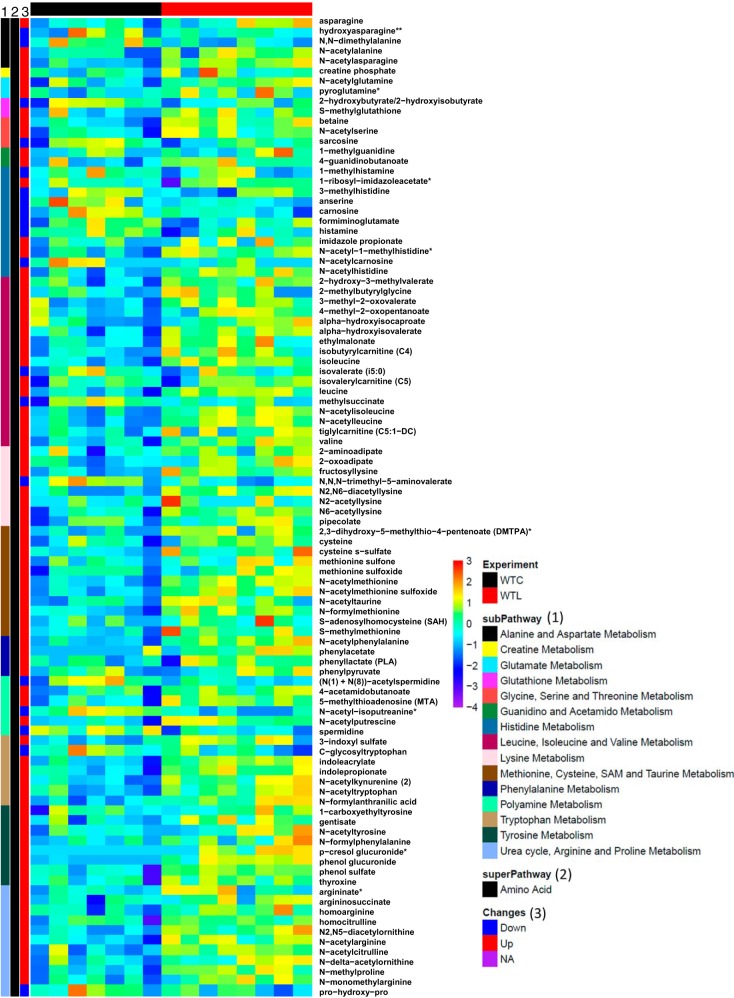
The Effects of LR on Amino Acid Levels in Healthy Breastfed Mice
The heatmap in Fig. 6 shows the changes of amino acid by LR.
Fig. 6.
Heatmap of altered metabolites of amino acid pathway compared the groups of wild-type control (WTC) vs. wild-type Lactobacillus reuteri fed (WTL).
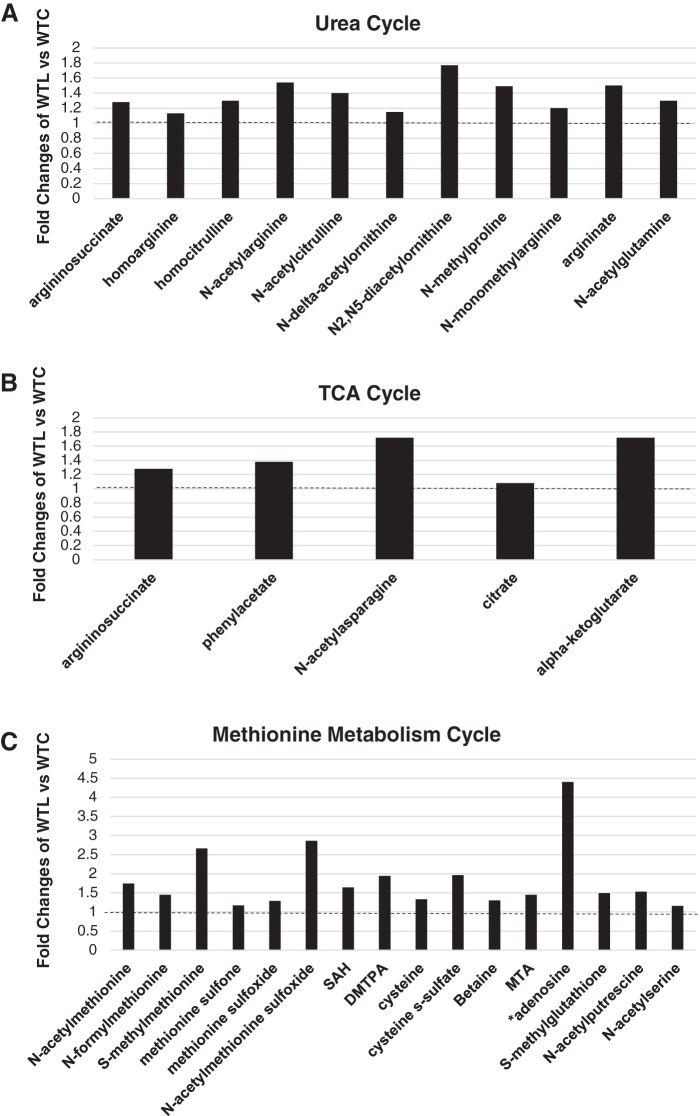
Upregulated Amino Acid Metabolites Are Involved in the Urea Cycle
The upregulated metabolites associated with LR feeding were mainly involved in the urea cycle and metabolism of arginine. Bacterial modified ornithine (N-delta-acetylornithine and N2,N5-diacetylornithine), arginine (N-acetylarginine, homoargine, and N-monomethylarginine), and citrulline (N-acetylcitrulline, homocitrulline) were significantly upregulated by LR. Argininosuccinate, an intermediate of the urea cycle and intestinal arginine synthesis, was also upregulated by LR; this metabolite is interconnected with the tricarboxylic acid (TCA) cycle and can generate fumarate. We observed that most amino acids that were upregulated by LR were acetylated (most likely by gut microbiota), including N-acetyl-alanine, N-acetyl-serine, N-acetyl-lysine, N-acetyl-cysteine, N-acetyl-leucine, N-acetyl-isoleucine, and N-actyl-tyrosine. All these amino acids participate in TCA cycle and can be converted to pyruvate and/or acetyl-CoA. LR treatment increased levels of N-acetyltyrosine and phenylacetate, which participates in TCA to form fumarate. LR also upregulated asparagine and N-acetyl-asparagine (Fig. 6 and Fig. 7, A and B).
Fig. 7.
The upregulated metabolites in amino acid pathways by Lactobacillus reuteri (LR) in healthy breastfed mice. A: metabolites that are significantly upregulated by LR in urea cycle. B: metabolites that are significantly upregulated by LR in tricarboxylic acid (TCA) cycle. C: metabolites that are significantly upregulated in methionine metabolism cycle. DMTPA, 2,3-dihydroxy-5-methylthio-4-pentenoate; MTA, 5-methylthio-adenosine; SAH, S-adenosyl-homocysteine. Data represent the average fold changes (>1, dotted lines) of wild-type LR fed (WTL) compared with wild-type control (WTC) with P < 0.05 between groups analyzed by Metabolon; n = 8 mice per group.
Upregulated Amino Acid Metabolites Are Involved in the Physiological Metabolism of Methionine
LR increased several methionine derivatives (N-acetyl-methionine, N-acetyl-methionine sulfoxide, N-formyl-methionine, and S-methyl-methionine). Other upregulated metabolites involved in the methionine pathway were betaine, 5-methylthio-adenosine, and S-adenosyl-homocysteine. The latter is metabolized to homocysteine, then cysteine (N-acetyl-cysteine, the major antioxidant in liver), and finally taurine (N-acetyl-taurine), with cysteine used for the synthesis glutathione (S-methyl-glutathione; Figs. 6 and 7C).
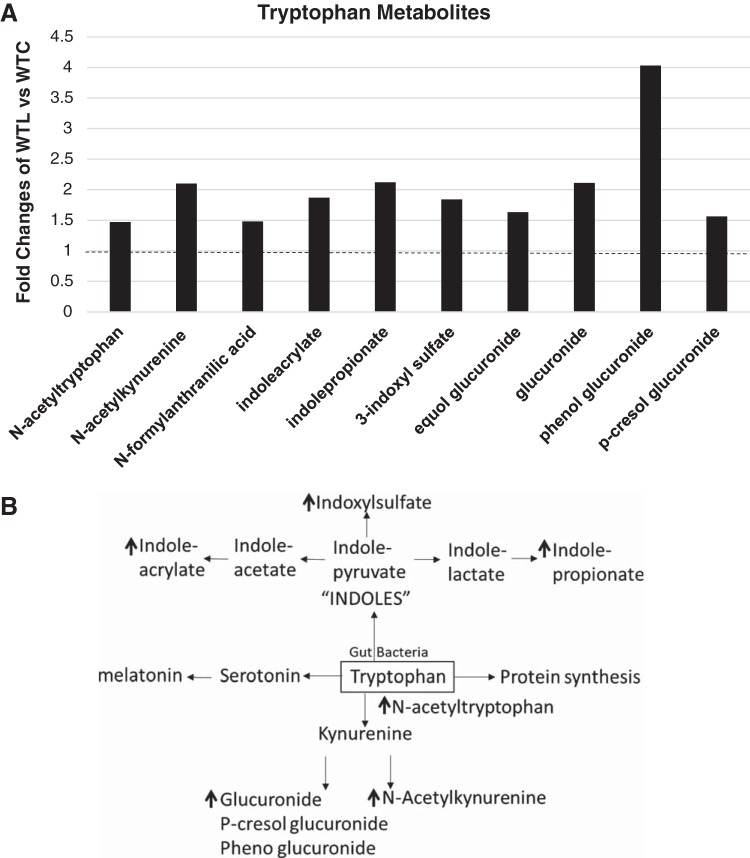
Microbiome-Modified Metabolites of Tryptophan Are Increased By LR
In addition to participating in protein synthesis, tryptophan can produce 1) serotonin and melatonin; 2) kynurenine, N-acetylkynurenine, anthronilic acid, and glucuronide; and 3) “indoles” such as indolepyruvate, indolelactate, indolepropionate, indoleacetate, indoleacrylate and indoxylsulfate (Fig. 8). Orally feeding LR to healthy breastfed mice significantly upregulated N-acetyltryptophan, N-acetylkynurenine, indolepropionate, indoleacrylate, and indoxylsulfate (Fig. 8).
Fig. 8.
Upregulated tryptophan metabolites in amino acid pathways by Lactobacillus reuteri (LR) in healthy breastfed mice. A: metabolites that are significantly upregulated by LR in urea cycle. Data represent the average fold changes (>1, dotted line) of wild-type LR fed (WTL) compared with wild-type control (WTC) with P < 0.05 between groups analyzed by Metabolon; n = 8 mice per group. B: tryptophan metabolism. Upregulated metabolites by LR are indicated by arrows.
LR Feeding Alters Several Nucleotide, Lipid, and Vitamin Levels in Healthy Breastfed Mice
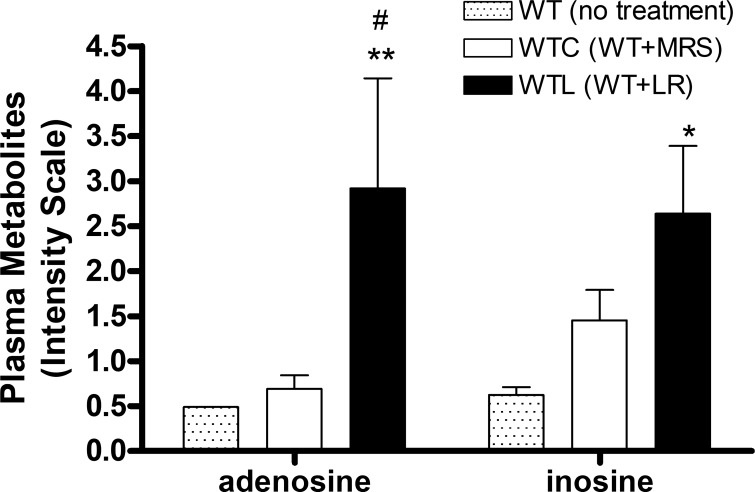
Plasma adenosine level showed a more than fourfold increase in mice that were fed with LR, compared with mice fed with control media (Table 1 and Fig. 9). LR also increased plasma cAMP level. There were no increases in adenosine metabolites such as inosine, xanthine, and hypoxanthine in plasma of healthy breastfed mice after orally feeding of LR compared with feeding MRS medium control; however, inosine increased in LR feeding group compared with that in WT mice without feeding (Fig. 9). Oral feeding of LR differentially changed the plasma levels of metabolites participating in fatty acid oxidation. LR upregulated short and medium chains of acryl carnitine and downregulated long chain, unsaturated and hydroxyl acryl carnitine. LR also increased carnitine, deoxycarnitine, and mevalonate (Table 1). Finally, LR increased vitamin levels, including ascorbate and its products (including threonate) and vitamin B6 metabolic products (pyridoxamine and pyridoxal) (Table 1).
Table 1.
Up- or downregulation of metabolites (nucleotide, lipid, and vitamin) in plasma by LR in healthy breastfed mice
| Super Pathway/Subpathway/Biochemical Name | Fold Changes of WTL vs. WTC |
|---|---|
| Nucleotide | |
| Purine metabolism, adenine containing | |
| Adenosine | 4.4 |
| Adenosine-3′,5′-cyclic monophosphate (cAMP) | 1.4 |
| Lipid | |
| Acyl carnitine, short chain | |
| Acetylcarnitine (C2) | 1.3 |
| Isocaproylcarnitine | 1.8 |
| Acyl carnitine, medium chain | |
| Hexanoylcarnitine (C6) | 5.9 |
| Octanoylcarnitine (C8) | 2.8 |
| Laurylcarnitine (C12) | 0.7 |
| Acyl carnitine, long-chain saturated | |
| Myristoylcarnitine (C14) | 0.7 |
| Pentadecanoylcarnitine (C15) | 0.8 |
| Palmitoylcarnitine (C16) | 0.8 |
| Acyl carnitine, monounsaturated | |
| Oleoylcarnitine (C18:1) | 0.8 |
| Eicosenoylcarnitine (C20:1) | 0.7 |
| Acyl carnitine, polyunsaturated | |
| Linoleoylcarnitine (C18:2) | 0.8 |
| Dihomo-linoleoylcarnitine (C20:2) | 0.8 |
| Arachidonoylcarnitine (C20:4) | 0.7 |
| Dihomo-linolenoylcarnitine (C20:3n3 or 6) | 0.7 |
| Acyl xarnitine, hydroxy | |
| 3-Hydroxypalmitoylcarnitine | 0.6 |
| 3-Hydroxyoleoylcarnitine | 0.7 |
| Carnitine metabolism | |
| Deoxycarnitine | 1.3 |
| Carnitine | 1.2 |
| Mevalonate metabolism | |
| Mevalonate | 1.9 |
| Vitamin | |
| Ascorbate and aldarate metabolism | |
| Threonate | 1.6 |
| Oxalate (ethanedioate) | 1.8 |
| Vitamin B6 metabolism | |
| Pyridoxamine | 2.2 |
| Pyridoxal | 2.5 |
LR, Lactobacillus reuteri. Fold changes of wild-type LR fed (WTL) vs. wild-type control (WTC): >1 upregulation; <1 downregulation, P < 0.05.
Fig. 9.
Plasma adenosine and inosine detected by metabolomics analysis. The comparisons of 3 groups are as indicated. MRS, deMan-Rogosa-Sharpe. #P < 0.05, wild-type by Lactobacillus reuteri (LR) fed (WTL) vs. wild-type control (WTC). *P < 0.05, **P < 0.01, WTL vs. WT no treatment; n = 8 mice per group.
DISCUSSION
LR is a potent regulator of the immune system, a “shaper” of the gut microbiota, and a modulator of plasma and fecal metabolomic profile, each potentially contributing to improve early life gut inflammation (21). Even though LR can be viewed as a normal commensal of the human and rodent intestine, its daily oral administration at probiotic doses resulted in major changes to the microbiota and metabolites. Most of the changes observed in the current study are novel and would be predicted to be beneficial to the host. Our observations may help to explain some of the benefits of LR in newborns, including protection against lipopolysaccharide-induced inflammation (33), necrotizing enterocolitis (34), and x-linked immunodeficiency disease (21).
Microbial Effects of LR
The core microbiota are composed of species belonging to the phyla Firmicutes and Bacteriodetes, and these phyla constitute over 90% of the known phylogenetic categories found in the healthy breastfed mouse gut, which is similar to the core microbiota in human babies (42, 50). Gavage feeding LR to healthy breastfed mice increased the relative abundance of o_Clostridiales, f_Lachnospiraceae, g_Clostridium, and g_Candidatus arthromitus, while decreasing the relative abundance of g_Bacteroides, g_Sutterella, g_Akkermansia, and g_Ruminococcus.
In recent years, members of Lachnospiraceae, a family of the order Clostridiales, have been found to degrade complex polysaccharides, yielding short-chain fatty acids such as acetate, butyrate, and propionate, which can be used for energy by the host (2). Previous study has suggested that segmented filamentous bacteria (SFB), intestinal symbionts of a variety of vertebrates, are affiliated with the family Clostridiaceae, in which SFB from different animal hosts are members of a distinct clade, designated Candidatus arthromitus (3, 39). SFB typically form filaments that are solidly anchored in gut epithelial cells, especially in the ileum (28). In mice, SFB play a key role in the postnatal maturation of gut innate and adaptive immune functions, and notably, they can induce a strong IgA response and the recruitment and activation of lamina propria CD4+ T cells and intraepithelial CD8+ T lymphocytes (30, 46). Mouse-specific Candidatus arthromitus was shown not only to induce a strong TH17 response but also to foster TH1, TH2, and Treg responses in C3H/HeN mice (18). In our current study, we also observed that LR feeding produced an increase in the percentage of Treg cells in the intestine of healthy breastfed mice. The above evidence indicates that oral feeding of LR to healthy breastfed mice increases the abundance of beneficial bacteria.
Bacteroides that were downregulated by LR have been related to diseases such as diabetes and necrotizing enterocolitis. Mice with streptozotocin-induced type 1 diabetes developed an increase in the relative abundance of Bacteroides (12). A recent report from a human prospective study indicated that Bacteroides were associated with the incidence and severity of necrotizing enterocolitis (22). Sutterella, a member of the proteobacteria, has been frequently associated with human diseases such as autism (53), Down syndrome (1), and inflammatory bowel disease (27, 51). Recent studies of three different species of Sutterella showed that they are widely prevalent commensals with mild proinflammatory characteristics in the human gastrointestinal tract. They do not contribute significantly to the disrupted epithelial homeostasis associated with microbiota dysbiosis or an increase of Proteobacteria (23). Akkermansia is a mucin-degrading gram-negative microorganism, which inversely correlates with the onset of inflammation related to obesity and insulin resistance (14, 15). However, increased Akkermansia was observed in autoimmune diseases such as multiple sclerosis (26). Recent studied showed that increased Akkermansia is linked with multiple sclerosis-associated changes in spore-forming bacteria and proinflammatory T lymphocytes (8). In addition, indextran sulfate sodium-induced colitis, the numbers of Lactobacilli were decreased while there were increased numbers of Akkermansia and intestinal sulfate-reducing bacteria Desulfovibrio (19). The rates of sulphidogenesis have been associated with ulcerative colitis and correlated with reduced mucosal thickness (44). It is unclear whether Akkermansia has any sulfate-reducing capacity. In healthy breastfed mice, we observed that LR reduces the relative abundance of Akkermansia but LR has no effects on clinical phenotype in mice and in contrast promotes intestinal immune tolerance such as increases in Tregs. The interaction between LR and Akkermansia in healthy breastfed mice and in disease models requires further study. Ruminococcus (which was downregulated by LR) has been associated with allergy in infants, the susceptibility of inflammatory bowel disease, and type I diabetes in children (11, 31, 41). Our current study therefore indicates that LR beneficially affect the gut microbiota in healthy breastfed mice.
Effects of LR on Plasma Metabolites
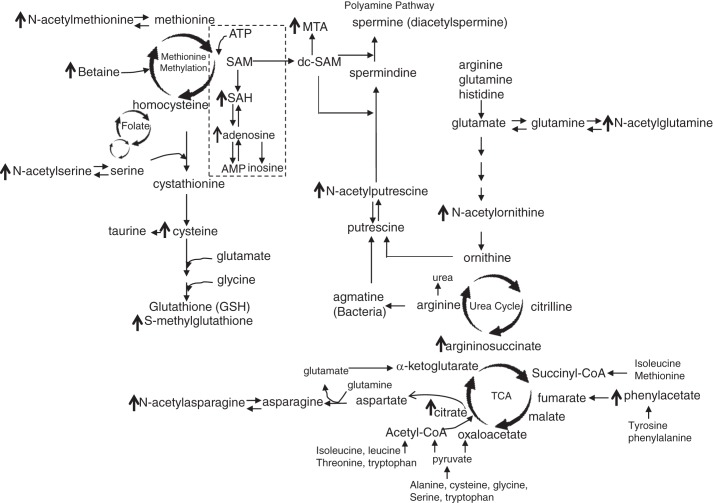
The gut microbiota operates in a coordinated manner with the host via metabolites, which can activate cell signaling pathways. We were able to observe that LR significantly changed metabolomic profiles in plasma of healthy breastfed mice. A number of upregulated metabolites participated in amino acid metabolism and in networks linking the urea cycle, TCA cycle, methionine cycle, polyamine pathway, and tryptophan signaling (Fig. 10). LR also was associated with several potentially beneficial modifications of lipids, nucleotides and vitamins.
Fig. 10.
Summarized connections among the amino acid pathway affected by Lactobacillus reuteri (LR)in healthy breastfed mice. Arrows indicate amino acid metabolites upregulated by LR in plasma. Three major circles affected by LR demonstrated as urea cycle, tricarboxylic acid (TCA) cycle, and methionine methylation cycle. Changed metabolites also related to polyamine pathway. SAM, S-adenosylmethionine; MTA, 5-methylthio-adenosine; SAH, S-adenosyl-homocysteine.
Amino Acid Pathways
LR upregulated N-acetylornithine, which is an intermediary in the pathway for microbial citrulline and arginine synthesis from dietary glutamine and glutamate in the intestine (55). In addition, intestinal epithelial cells from most mammals (including mice and humans) can generate citrulline from glutamate, glutamine, and proline, with citrulline being converted into arginine in extra-hepatic tissues (e.g., primarily the small intestine in neonates and the kidneys in adults). When serum levels of citrulline are increased, endogenous production of arginine is coordinately increased (13), facilitating better muscle protein synthesis in humans (4). To our knowledge, animal cells do not synthesize N-acetylornithine from other amino acids. In summary, because glutamine and arginine are essential to gut mucosal integrity and restitution (10, 25), gut bacterial synthesis of N-acetylornithine may have a major beneficial role in newborn nutrition and may be related to a healthy gut barrier.
Ornithine and arginine can generate polyamines (e.g., spermine) in the gut by the polyamine-synthetic pathway. The LR-upregulated metabolite N-acetyl-putrescine is a precursor of spermine. We found that diacetylspermine was elevated in LR-fed cecal contents. Spermine is involved in many distinct cellular functions including modulation of gene transcription and translation, signal transduction, receptor-ligand interactions, cell proliferation, and cell migration. Spermine also plays an important role in gastrointestinal mucosal healing and has potential clinical applications for patients with mucosal injury-associated disorders (29).
The urea cycle, which is very active metabolically in hepatocytes and to a lesser extent in enterocytes, is physiologically linked to the TCA cycle via the intermediary oxaloacetate (47). LR may promote the formation of phenylacetate from tyrosine, evidenced by increases in N-acetyltyrosine and phenylacetate after feeding LR. Phenylacetate via conversion to fumarate is able to enter the TCA cycle, promoting the formation of aspartate (37), consistent with our finding of LR-associated increased levels of N-acetylasparagine and guanidinosuccinate.
Another important observation was that LR promoted an increase in a group of antioxidants in the intestine, including N-acetylcysteine, N-acetyltaurine, and N-acetylserine. These amino acid derivatives are related to microbial amino acid metabolism and N-acetylcysteine to glutathione synthesis. N-aceytlcysteine is widely recognized the major hepatic antioxidant (40) and is now known to regulate protein synthesis in intestinal epithelial cells (57). N-aceytlcysteine reduces inflammation, alleviates oxidative stress, improves energy status, and ameliorates tissue damage in the intestine induced by lipopolysaccharide in piglets (24). Interestingly, orally feeding LR to breastfed newborn mice was associated with enhanced methionine metabolism (36, 56). Well-known metabolites of methionine metabolism such as S-adenosylmethionine (SAM) and S-adenosyl homocysteine, which yield the nucleoside adenosine.
We have shown that adenosine and its metabolite inosine have anti-inflammatory functions, associated with inhibition of TH1, TH2, and TH17 differentiation by T cells. Our previous studies in Treg-deficiency autoimmune disease model demonstrated a novel mechanism of LR action, mediated by the interaction of adenosine/inosine with adenosine receptor A2A to reduce multiorgan inflammation and prolong the survival of Treg-deficient mice (20, 21). In addition to generation of adenosine, SAM can be transformed into decarboxylated-SAM (dc-SAM), which can participate in polyamine synthesis and methionine pathway by formation of 5′-methylthioadenosine.
Finally, LR-upregulated N-acetyltryptophan and tryptophan metabolites have been linked to higher levels of anti-inflammatory indoles. Previous studies showed that indoleacrylic acid promotes intestinal epithelial barrier function and mitigates inflammatory responses with the potential for therapeutic benefit (54).
Lipid and Vitamin Metabolism
Orally feeding LR to healthy breastfed mice also decreased carnitine-conjugated fatty acids, with an increase in short- and medium-chain fatty acyl carnitines and decrease in long-chain acyl carnitines. Acyl-carnitine participates in fatty acid β-oxidation to produce acetyl-CoA, further to TCA cycle to generate energy. The differential effects of LR on acyl-carnitine in healthy mice may impact the balance of acetyl-CoA production. Additionally, lactic acid bacteria produce B-group vitamins, owing to their inherent B-group vitamin-encoding genes including riboflavin (B2); 6-hydroxymethyl-7,8-dihydropterin pyrophosphate; tetrahydrofolate-polyglutamate; chorismite; para-aminobenzoic acid (B11 folate); and cobalamin (B12) biosynthetic genes (7). We did not observe an upregulation of all B-group vitamins, but we did find evidence for increased vitamin B6 and ascorbate metabolism. These modulations by LR may be due to LR by itself or other gut microbiota modulated by LR, a topic worthy of further study.
In summary, we have shown a number of potentially beneficial effects of LR DSM 17938 in healthy breastfed mice. LR promotes immune tolerance in the intestine and is linked to proliferation of beneficial gut microbiota. LR upregulates plasma metabolites that play important roles in the urea cycle, the TCA cycle, methionine methylation, and the polyamine pathway. LR enhances the levels of tryptophan metabolites, including indoles and adenosine, which are known to enhance tolerance to inflammatory stimuli. Our findings provide an insight into the mechanisms by which gut bacteria and their metabolic products may be able to influence growth, metabolism, and bowel function in newborn hosts. The findings indicate that this probiotic strain affects the gut microbiota and the plasma metabolome in healthy breastfed mice.
Additional studies are required to confirm that such changes are beneficial to the host. The changed metabolites by this specific probiotic will be tested in protecting intestinal barrier function, in their anti-inflammatory activities locally and systemically, and in modulating immune cell responses (inducing tolerogenic dendritic cells and proinflammatory T cells). We will use in vitro and in vivo mouse models, including newborn mice with cow-milk-based formula-related gut inflammation or mice with diseases such as immune deficiency-induced autoimmunity (FoxP3 deletion), as well as other disease models involving in gut microbial dysbiosis including inflammatory bowel disease models.
GRANTS
The probiotic Lactobacillus reuteri DSM 17938 was obtained as a gift from BioGaia. These studies were supported by National Center for Complementary and Integrative Health Grant R01-AT-007083 (to J. M. Rhoads and Y. Liu), and, in part, by National Institute of Allergy and Infectious Diseases Grant R03-AI-117442 (to Y. Liu) and National Institute of Diabetes and Digestive and Kidney Diseases Grant DK-56338 (to J. M. Rhoads), which funds the Texas Medical Center Digestive Diseases Center. We acknowledge support of BioGaia in other research projects, but this project was not supported by BioGaia.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Y.L. and J.M.R. conceived and designed research; Y.L., B.H., T.K.H., J.F., S.P., M.L., and J.C. performed experiments; Y.L., X.T., B.H., T.K.H., C.M.T., E.B., J.F., S.P., M.L., and J.C. analyzed data; Y.L., X.T., C.M.T., E.B., D.Q.T., S.R., G.W., and J.M.R. interpreted results of experiments; Y.L., X.T., and J.F. prepared figures; Y.L. and X.T. drafted manuscript; C.M.T., D.Q.T., S.R., G.W., and J.M.R. edited and revised manuscript; Y.L., X.T., B.H., T.K.H., C.M.T., E.B., J.F., S.P., M.L., J.C., D.Q.T., S.R., G.W., and J.M.R. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Drs. Douglas G. Burrin (Pediatric Nutrition and Gastroenterology, Baylor College of Medicine), Lenard M. Lichtenberger (Integrative Biology and Pharmacology, The University of Texas Health Science Center at Houston), and Robert Britton (Molecular Virology and Microbiology, Baylor College of Medicine) for assistance with the gut microbiota and metabolomics data interpretation. We also thank Dr. Eamonn Connolly (BioGaia, Stockholm, Sweden) for providing Lactobacillus reuteri DSM 17938.
REFERENCES
- 1.Biagi E, Candela M, Centanni M, Consolandi C, Rampelli S, Turroni S, Severgnini M, Peano C, Ghezzo A, Scurti M, Salvioli S, Franceschi C, Brigidi P. Gut microbiome in Down syndrome. PLoS One 9: e112023, 2014. doi: 10.1371/journal.pone.0112023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Biddle A, Stewart L, Blanchard J, Leschine S. Untangling the genetic basis of fibrolytic specialization by Lachnospiraceae and Ruminococcaceae in diverse gut communities. Diversity (Basel) 5: 627–640, 2013. doi: 10.3390/d5030627. [DOI] [Google Scholar]
- 3.Bolotin A, de Wouters T, Schnupf P, Bouchier C, Loux V, Rhimi M, Jamet A, Dervyn R, Boudebbouze S, Blottière HM, Sorokin A, Snel J, Cerf-Bensussan N, Gaboriau-Routhiau V, van de Guchte M, Maguin E. Genome sequence of “Candidatus Arthromitus” sp. Strain SFB-mouse-NL, a commensal bacterium with a key role in postnatal maturation of gut immune functions. Genome Announc 2: e00705-14, 2014. doi: 10.1128/genomeA.00705-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Breuillard C, Cynober L, Moinard C. Citrulline and nitrogen homeostasis: an overview. Amino Acids 47: 685–691, 2015. doi: 10.1007/s00726-015-1932-2. [DOI] [PubMed] [Google Scholar]
- 5.Bruce-Keller AJ, Salbaum JM, Luo M, Blanchard E IV, Taylor CM, Welsh DA, Berthoud HR. Obese-type gut microbiota induce neurobehavioral changes in the absence of obesity. Biol Psychiatry 77: 607–615, 2015. doi: 10.1016/j.biopsych.2014.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, Fierer N, Peña AG, Goodrich JK, Gordon JI, Huttley GA, Kelley ST, Knights D, Koenig JE, Ley RE, Lozupone CA, McDonald D, Muegge BD, Pirrung M, Reeder J, Sevinsky JR, Turnbaugh PJ, Walters WA, Widmann J, Yatsunenko T, Zaneveld J, Knight R. QIIME allows analysis of high-throughput community sequencing data. Nat Methods 7: 335–336, 2010. doi: 10.1038/nmeth.f.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Capozzi V, Russo P, Dueñas MT, López P, Spano G. Lactic acid bacteria producing B-group vitamins: a great potential for functional cereals products. Appl Microbiol Biotechnol 96: 1383–1394, 2012. doi: 10.1007/s00253-012-4440-2. [DOI] [PubMed] [Google Scholar]
- 8.Cekanaviciute E, Pröbstel AK, Thomann A, Runia TF, Casaccia P, Katz Sand I, Crabtree E, Singh S, Morrissey J, Barba P, Gomez R, Knight R, Mazmanian S, Graves J, Cree BA, Zamvil SS, Baranzini SE. Multiple sclerosis-associated changes in the composition and immune functions of spore-forming bacteria. mSystems 3: 3e00083-18, 2018. doi: 10.1128/mSystems.00083-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention (CDC) Breastfeeding Report Card https://www.cdc.gov/breastfeeding/data/reportcard.htm. [2018].
- 10.Chapman JC, Liu Y, Zhu L, Rhoads JM. Arginine and citrulline protect intestinal cell monolayer tight junctions from hypoxia-induced injury in piglets. Pediatr Res 72: 576–582, 2012. doi: 10.1038/pr.2012.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chua HH, Chou HC, Tung YL, Chiang BL, Liao CC, Liu HH, Ni YH. Intestinal dysbiosis featuring abundance of Ruminococcus gnavus associates with allergic diseases in infants. Gastroenterology 154: 154–167, 2018. doi: 10.1053/j.gastro.2017.09.006. [DOI] [PubMed] [Google Scholar]
- 12.Costa FR, Françozo MC, de Oliveira GG, Ignacio A, Castoldi A, Zamboni DS, Ramos SG, Câmara NO, de Zoete MR, Palm NW, Flavell RA, Silva JS, Carlos D. Gut microbiota translocation to the pancreatic lymph nodes triggers NOD2 activation and contributes to T1D onset. J Exp Med 213: 1223–1239, 2016. doi: 10.1084/jem.20150744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dai ZL, Li XL, Xi PB, Zhang J, Wu G, Zhu WY. Regulatory role for l-arginine in the utilization of amino acids by pig small-intestinal bacteria. Amino Acids 43: 233–244, 2012. doi: 10.1007/s00726-011-1067-z. [DOI] [PubMed] [Google Scholar]
- 14.Derrien M, Belzer C, de Vos WM. Akkermansia muciniphila and its role in regulating host functions. Microb Pathog 106: 171–181, 2017. doi: 10.1016/j.micpath.2016.02.005. [DOI] [PubMed] [Google Scholar]
- 15.Derrien M, Vaughan EE, Plugge CM, de Vos WM. Akkermansia muciniphila gen. nov., sp. nov., a human intestinal mucin-degrading bacterium. Int J Syst Evol Microbiol 54: 1469–1476, 2004. doi: 10.1099/ijs.0.02873-0. [DOI] [PubMed] [Google Scholar]
- 16.Edgar RC. UPARSE: highly accurate OTU sequences from microbial amplicon reads. Nat Methods 10: 996–998, 2013. doi: 10.1038/nmeth.2604. [DOI] [PubMed] [Google Scholar]
- 17.Francavilla R, Lionetti E, Castellaneta S, Ciruzzi F, Indrio F, Masciale A, Fontana C, La Rosa MM, Cavallo L, Francavilla A. Randomised clinical trial: Lactobacillus reuteri DSM 17938 vs. placebo in children with acute diarrhoea–a double-blind study. Aliment Pharmacol Ther 36: 363–369, 2012. doi: 10.1111/j.1365-2036.2012.05180.x. [DOI] [PubMed] [Google Scholar]
- 18.Gaboriau-Routhiau V, Rakotobe S, Lécuyer E, Mulder I, Lan A, Bridonneau C, Rochet V, Pisi A, De Paepe M, Brandi G, Eberl G, Snel J, Kelly D, Cerf-Bensussan N. The key role of segmented filamentous bacteria in the coordinated maturation of gut helper T cell responses. Immunity 31: 677–689, 2009. doi: 10.1016/j.immuni.2009.08.020. [DOI] [PubMed] [Google Scholar]
- 19.Håkansson Å, Tormo-Badia N, Baridi A, Xu J, Molin G, Hagslätt ML, Karlsson C, Jeppsson B, Cilio CM, Ahrné S. Immunological alteration and changes of gut microbiota after dextran sulfate sodium (DSS) administration in mice. Clin Exp Med 15: 107–120, 2015. doi: 10.1007/s10238-013-0270-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.He B, Hoang TK, Tran DQ, Rhoads JM, Liu Y. Adenosine A2A receptor deletion blocks the beneficial effects of Lactobacillus reuteri in regulatory T-deficient scurfy mice. Front Immunol 8: 1680, 2017. doi: 10.3389/fimmu.2017.01680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.He B, Hoang TK, Wang T, Ferris M, Taylor CM, Tian X, Luo M, Tran DQ, Zhou J, Tatevian N, Luo F, Molina JG, Blackburn MR, Gomez TH, Roos S, Rhoads JM, Liu Y. Resetting microbiota by Lactobacillus reuteri inhibits T reg deficiency-induced autoimmunity via adenosine A2A receptors. J Exp Med 214: 107–123, 2017. doi: 10.1084/jem.20160961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heida FH, van Zoonen AG, Hulscher JB, Te Kiefte BJ, Wessels R, Kooi EM, Bos AF, Harmsen HJ, de Goffau MC. A necrotizing enterocolitis-associated gut microbiota is present in the meconium: results of a prospective study. Clin Infect Dis 62: 863–870, 2016. doi: 10.1093/cid/ciw016. [DOI] [PubMed] [Google Scholar]
- 23.Hiippala K, Kainulainen V, Kalliomäki M, Arkkila P, Satokari R. Mucosal prevalence and interactions with the epithelium indicate commensalism of Sutterella spp. Front Microbiol 7: 1706, 2016. doi: 10.3389/fmicb.2016.01706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hou Y, Wang L, Yi D, Wu G. N-acetylcysteine and intestinal health: a focus on its mechanism of action. Front Biosci 20: 872–891, 2015. doi: 10.2741/4342. [DOI] [PubMed] [Google Scholar]
- 25.Jacobi SK, Odle J. Nutritional factors influencing intestinal health of the neonate. Adv Nutr 3: 687–696, 2012. doi: 10.3945/an.112.002683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jangi S, Gandhi R, Cox LM, Li N, von Glehn F, Yan R, Patel B, Mazzola MA, Liu S, Glanz BL, Cook S, Tankou S, Stuart F, Melo K, Nejad P, Smith K, Topçuolu BD, Holden J, Kivisäkk P, Chitnis T, De Jager PL, Quintana FJ, Gerber GK, Bry L, Weiner HL. Alterations of the human gut microbiome in multiple sclerosis. Nat Commun 7: 12015, 2016. doi: 10.1038/ncomms12015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Javurek AB, Spollen WG, Johnson SA, Bivens NJ, Bromert KH, Givan SA, Rosenfeld CS. Effects of exposure to bisphenol A and ethinyl estradiol on the gut microbiota of parents and their offspring in a rodent model. Gut Microbes 7: 471–485, 2016. doi: 10.1080/19490976.2016.1234657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Klaasen HL, Koopman JP, Poelma FG, Beynen AC. Intestinal, segmented, filamentous bacteria. FEMS Microbiol Rev 8: 165–180, 1992. doi: 10.1111/j.1574-6968.1992.tb04986.x. [DOI] [PubMed] [Google Scholar]
- 29.Konturek PC, Brzozowski T, Konturek SJ, Szlachcic A, Hahn EG. Polyamines and epidermal growth factor in the recovery of gastric mucosa from stress-induced gastric lesions. J Clin Gastroenterol 27, Suppl 1: S97–S104, 1998. doi: 10.1097/00004836-199800001-00016. [DOI] [PubMed] [Google Scholar]
- 30.Lécuyer E, Rakotobe S, Lengliné-Garnier H, Lebreton C, Picard M, Juste C, Fritzen R, Eberl G, McCoy KD, Macpherson AJ, Reynaud CA, Cerf-Bensussan N, Gaboriau-Routhiau V. Segmented filamentous bacterium uses secondary and tertiary lymphoid tissues to induce gut IgA and specific T helper 17 cell responses. Immunity 40: 608–620, 2014. doi: 10.1016/j.immuni.2014.03.009. [DOI] [PubMed] [Google Scholar]
- 31.Leiva-Gea I, Sánchez-Alcoholado L, Martín-Tejedor B, Castellano-Castillo D, Moreno-Indias I, Urda-Cardona A, Tinahones FJ, Fernández-García JC, Queipo-Ortuño MI. Gut microbiota differs in composition and functionality between children with type 1 diabetes and MODY2 and healthy control subjects: a case-control study. Diabetes Care 41: 2385–2395, 2018. doi: 10.2337/dc18-0253. [DOI] [PubMed] [Google Scholar]
- 32.Liu Y, Fatheree NY, Dingle BM, Tran DQ, Rhoads JM. Lactobacillus reuteri DSM 17938 changes the frequency of Foxp3+ regulatory T cells in the intestine and mesenteric lymph node in experimental necrotizing enterocolitis. PLoS One 8: e56547, 2013. doi: 10.1371/journal.pone.0056547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu Y, Fatheree NY, Mangalat N, Rhoads JM. Human-derived probiotic Lactobacillus reuteri strains differentially reduce intestinal inflammation. Am J Physiol Gastrointest Liver Physiol 299: G1087–G1096, 2010. doi: 10.1152/ajpgi.00124.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu Y, Fatheree NY, Mangalat N, Rhoads JM. Lactobacillus reuteri strains reduce incidence and severity of experimental necrotizing enterocolitis via modulation of TLR4 and NF-κB signaling in the intestine. Am J Physiol Gastrointest Liver Physiol 302: G608–G617, 2012. doi: 10.1152/ajpgi.00266.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu Y, Tran DQ, Fatheree NY, Marc Rhoads J. Lactobacillus reuteri DSM 17938 differentially modulates effector memory T cells and Foxp3+ regulatory T cells in a mouse model of necrotizing enterocolitis. Am J Physiol Gastrointest Liver Physiol 307: G177–G186, 2014. doi: 10.1152/ajpgi.00038.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Locasale JW. Serine, glycine and one-carbon units: cancer metabolism in full circle. Nat Rev Cancer 13: 572–583, 2013. doi: 10.1038/nrc3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Natelson S, Tseng HY, Sherwin JE. On the biosynthesis of guanidinosuccinate. Clin Chem 24: 2108–2114, 1978. [PubMed] [Google Scholar]
- 38.Oncel MY, Sari FN, Arayici S, Guzoglu N, Erdeve O, Uras N, Oguz SS, Dilmen U. Lactobacillus Reuteri for the prevention of necrotising enterocolitis in very low birthweight infants: a randomised controlled trial. Arch Dis Child Fetal Neonatal Ed 99: F110–F115, 2014. doi: 10.1136/archdischild-2013-304745. [DOI] [PubMed] [Google Scholar]
- 39.Pamp SJ, Harrington ED, Quake SR, Relman DA, Blainey PC. Single-cell sequencing provides clues about the host interactions of segmented filamentous bacteria (SFB). Genome Res 22: 1107–1119, 2012. doi: 10.1101/gr.131482.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pei Y, Liu H, Yang Y, Yang Y, Jiao Y, Tay FR, Chen J. Biological activities and potential oral applications of N-acetylcysteine: progress and prospects. Oxid Med Cell Longev 2018: 2835787, 2018. doi: 10.1155/2018/2835787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pigneur B, Lepage P, Mondot S, Schmitz J, Goulet O, Doré J, Ruemmele FM. Mucosal healing and bacterial composition in response to enteral nutrition vs. steroid-based induction therapy—a randomised prospective clinical trial in children with Crohn’s disease. J Crohn’s Colitis 13: 846–855, 2019. doi: 10.1093/ecco-jcc/jjy207. [DOI] [PubMed] [Google Scholar]
- 42.Qin J, Li R, Raes J, Arumugam M, Burgdorf KS, Manichanh C, Nielsen T, Pons N, Levenez F, Yamada T, Mende DR, Li J, Xu J, Li S, Li D, Cao J, Wang B, Liang H, Zheng H, Xie Y, Tap J, Lepage P, Bertalan M, Batto JM, Hansen T, Le Paslier D, Linneberg A, Nielsen HB, Pelletier E, Renault P, Sicheritz-Ponten T, Turner K, Zhu H, Yu C, , et al.; MetaHIT Consortium . A human gut microbial gene catalogue established by metagenomic sequencing. Nature 464: 59–65, 2010. doi: 10.1038/nature08821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rojas MA, Lozano JM, Rojas MX, Rodriguez VA, Rondon MA, Bastidas JA, Perez LA, Rojas C, Ovalle O, Garcia-Harker JE, Tamayo ME, Ruiz GC, Ballesteros A, Archila MM, Arevalo M. Prophylactic probiotics to prevent death and nosocomial infection in preterm infants. Pediatrics 130: e1113–e1120, 2012. doi: 10.1542/peds.2011-3584. [DOI] [PubMed] [Google Scholar]
- 44.Rowan FE, Docherty NG, Coffey JC, O’Connell PR. Sulphate-reducing bacteria and hydrogen sulphide in the aetiology of ulcerative colitis. Br J Surg 96: 151–158, 2009. doi: 10.1002/bjs.6454. [DOI] [PubMed] [Google Scholar]
- 45.Saulnier DM, Santos F, Roos S, Mistretta TA, Spinler JK, Molenaar D, Teusink B, Versalovic J. Exploring metabolic pathway reconstruction and genome-wide expression profiling in Lactobacillus reuteri to define functional probiotic features. PLoS One 6: e18783, 2011. doi: 10.1371/journal.pone.0018783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schnupf P, Gaboriau-Routhiau V, Cerf-Bensussan N. Host interactions with segmented filamentous bacteria: an unusual trade-off that drives the post-natal maturation of the gut immune system. Semin Immunol 25: 342–351, 2013. doi: 10.1016/j.smim.2013.09.001. [DOI] [PubMed] [Google Scholar]
- 47.Shambaugh GE., 3rd Urea biosynthesis I. The urea cycle and relationships to the citric acid cycle. Am J Clin Nutr 30: 2083–2087, 1977. doi: 10.1093/ajcn/30.12.2083. [DOI] [PubMed] [Google Scholar]
- 48.Shornikova AV, Casas IA, Isolauri E, Mykkänen H, Vesikari T. Lactobacillus reuteri as a therapeutic agent in acute diarrhea in young children. J Pediatr Gastroenterol Nutr 24: 399–404, 1997. doi: 10.1097/00005176-199704000-00008. [DOI] [PubMed] [Google Scholar]
- 49.Sung V, Collett S, de Gooyer T, Hiscock H, Tang M, Wake M. Probiotics to prevent or treat excessive infant crying: systematic review and meta-analysis. JAMA Pediatr 167: 1150–1157, 2013. doi: 10.1001/jamapediatrics.2013.2572. [DOI] [PubMed] [Google Scholar]
- 50.Tap J, Mondot S, Levenez F, Pelletier E, Caron C, Furet JP, Ugarte E, Muñoz-Tamayo R, Paslier DL, Nalin R, Dore J, Leclerc M. Towards the human intestinal microbiota phylogenetic core. Environ Microbiol 11: 2574–2584, 2009. doi: 10.1111/j.1462-2920.2009.01982.x. [DOI] [PubMed] [Google Scholar]
- 51.Tyler AD, Knox N, Kabakchiev B, Milgrom R, Kirsch R, Cohen Z, McLeod RS, Guttman DS, Krause DO, Silverberg MS. Characterization of the gut-associated microbiome in inflammatory pouch complications following ileal pouch-anal anastomosis. PLoS One 8: e66934, 2013. doi: 10.1371/journal.pone.0066934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Walter J, Britton RA, Roos S. Host-microbial symbiosis in the vertebrate gastrointestinal tract and the Lactobacillus reuteri paradigm. Proc Natl Acad Sci USA 108, Suppl 1: 4645–4652, 2011. doi: 10.1073/pnas.1000099107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Williams BL, Hornig M, Parekh T, Lipkin WI. Application of novel PCR-based methods for detection, quantitation, and phylogenetic characterization of Sutterella species in intestinal biopsy samples from children with autism and gastrointestinal disturbances. MBio 3: e00261-11, 2012. doi: 10.1128/mBio.00261-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wlodarska M, Luo C, Kolde R, d’Hennezel E, Annand JW, Heim CE, Krastel P, Schmitt EK, Omar AS, Creasey EA, Garner AL, Mohammadi S, O’Connell DJ, Abubucker S, Arthur TD, Franzosa EA, Huttenhower C, Murphy LO, Haiser HJ, Vlamakis H, Porter JA, Xavier RJ. Indoleacrylic acid produced by commensal Peptostreptococcus species suppresses inflammation. Cell Host Microbe 22: 25–37.e6, 2017. doi: 10.1016/j.chom.2017.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wu G. Amino Acids: Biochemistry and Nutrition. Boca Raton, FL: CRC, 2013. [Google Scholar]
- 56.Yang M, Vousden KH. Serine and one-carbon metabolism in cancer. Nat Rev Cancer 16: 650–662, 2016. doi: 10.1038/nrc.2016.81. [DOI] [PubMed] [Google Scholar]
- 57.Yi D, Hou Y, Wang L, Long M, Hu S, Mei H, Yan L, Hu CA, Wu G. N-acetylcysteine stimulates protein synthesis in enterocytes independently of glutathione synthesis. Amino Acids 48: 523–533, 2016. doi: 10.1007/s00726-015-2105-z. [DOI] [PubMed] [Google Scholar]