Abstract
Skeletal muscle handles ~80–90% of the insulin-induced glucose uptake. In skeletal muscle, insulin binding to its cell surface receptor triggers redistribution of intracellular glucose transporter GLUT4 protein to the cell surface, enabling facilitated glucose uptake. In adipocytes, the eight-protein exocyst complex is an indispensable constituent in insulin-induced glucose uptake, as it is responsible for the targeted trafficking and plasma membrane-delivery of GLUT4. However, the role of the exocyst in skeletal muscle glucose uptake has never been investigated. Here we demonstrate that the exocyst is a necessary factor in insulin-induced glucose uptake in skeletal muscle cells as well. The exocyst complex colocalizes with GLUT4 storage vesicles in L6-GLUT4myc myoblasts at a basal state and associates with these vesicles during their translocation to the plasma membrane after insulin signaling. Moreover, we show that the exocyst inhibitor endosidin-2 and a heterozygous knockout of Exoc5 in skeletal myoblast cells both lead to impaired GLUT4 trafficking to the plasma membrane and hinder glucose uptake in response to an insulin stimulus. Our research is the first to establish that the exocyst complex regulates insulin-induced GLUT4 exocytosis and glucose metabolism in muscle cells. A deeper knowledge of the role of the exocyst complex in skeletal muscle tissue may help our understanding of insulin resistance in type 2 diabetes.
Keywords: exocyst, GLUT4, skeletal muscle
INTRODUCTION
Skeletal muscle is crucial for whole body glucose homeostasis, as it is responsible for ~80–90% of the insulin-stimulated glucose uptake (7). When circulating insulin binds to its cell surface receptor, a cascade of intracellular signaling events ultimately leads to the exocytosis of glucose transporter type 4 (GLUT4) to the plasma membrane (28), thus increasing glucose uptake in adipose and muscle tissue. While the molecular mechanism of targeted intracellular GLUT4 trafficking has been extensively studied in cultured adipocytes (23, 48), less is known about the factors regulating GLUT4 trafficking in skeletal muscle cells. This is especially relevant for a better understanding of the pathophysiology of diabetes and insulin resistance, where insulin-induced GLUT4 trafficking and, consequently, glucose transport are impaired. Deciphering the molecular mechanisms of insulin-dependent GLUT4 delivery and glucose uptake will further our knowledge of how skeletal muscle contributes to glucose homeostasis.
Conserved from yeast to humans, the exocyst complex is important for the targeted delivery and docking of cytoplasmic vesicles to specific sites on the plasma membrane (39). The exocyst protein complex consists of eight subunits, EXOC1 (traditionally named Sec3), EXOC2 (Sec5), EXOC3 (Sec6), EXOC4 (Sec8), EXOC5 (Sec10), EXOC6 (Sec15), EXOC7 (Exo70), and EXOC8 (Exo84). Recent work in mammalian epithelial cells revealed that the exocyst holocomplex forms upon the association of two tetrameric subcomplexes that can independently bind to the plasma membrane (1). Proper vesicle docking is dependent on the assembly of these subcomplexes (1). EXOC5, a member of the vesicle-proximal subcomplex 2 (SC2) acts as a bridge between the EXOC6 subunit, which binds to a small Rab GTPase on the exocytic vesicle, and the rest of the exocyst (21). Exoc5 silencing in cell culture and zebrafish models causes partial protein degradation of several other exocyst members (16, 54), suggesting that downregulation of EXOC5 alone can trigger destabilization of the exocyst complex and decreases its activity.
In earlier studies using cultured 3T3-L1 adipocytes, the exocyst was shown as a key regulator of insulin-stimulated GLUT4 exocytosis. In 2003, Inoue et al. (23) described that the activation of the TC10 small GTPase, in response to insulin, recruited the assembly of the exocyst at the plasma membrane. They also showed, if mutant exocyst members were overexpressed, GLUT4 storage vesicles (GSVs) failed to fuse to the plasma membrane (23). In 2005, Ewart et al. (15) reported that the overexpression of exocyst EXOC3 or EXOC4 in 3T3-L1 cells increased the exocytosis of GSVs in response to insulin. More recently, it was shown that insulin signaling in 3T3-L1 cells induced RalA activation at the GSVs, which was also necessary for exocyst-mediated GSV exocytosis (10). While in cultured 3T3-L1 adipocytes, it has been well established that the exocyst complex is essential for the docking of insulin-induced GSVs; this has never been tested in any other cell type nor in an animal model. Given the high degree of conservation of the mechanisms regulating insulin-induced glucose uptake between adipocytes and muscle (5, 6, 25, 38), we hypothesized that the exocyst also takes a central role in skeletal muscle glucose metabolism as well. Although it has been revealed that exocyst genes are highly expressed in muscle, almost no studies have been published investigating exocyst functions in this tissue (47). Using a skeletal myoblast cell culture model, we investigated the effects of modulated exocyst activity on GLUT4 trafficking and glucose uptake in response to insulin.
MATERIALS AND METHODS
Antibodies.
Primary antibodies used in this study are as follows: Akt1 (Santa Cruz Biotechnology, catalog no. sc-5298, RRID: AB_626658); β-actin (Cell Signaling Technology, catalog no. 4970, RRID: AB_2223172) (34); cytochrome-c oxidase IV family (Cell Signaling Technology, catalog no. 4850, RRID: AB_2085424) (53); dystrophin (Proteintech Group, catalog no. 12715-1-AP, RRID: AB_10640422) (32); ERK1/2 (Santa Cruz Biotechnology, catalog no. sc-514302, RRID: AB_2571739); EXOC1 (Proteintech Group, catalog no. 11690-1-AP, RRID: AB_2231500) (35); EXOC5 (Proteintech Group, catalog no. 17593-1-AP, RRID: AB_2101582, and Santa Cruz Biotechnology, SC-514802) (17); Exo70 (EXOC7) (Santa Cruz Biotechnology, catalog no. sc-365825, RRID: AB_10843358) (20); glyceraldehyde-3-phosphate dehydrogenase (Proteintech Group, catalog no. 60004-1-Ig, RRID: AB_2107436) (31); Myc-tag (Proteintech Group, catalog no. 60003-2-Ig, RRID: AB_2734122) (29); phospho-AKT (Proteintech Group, catalog no. 66444-1-IG, RRID: AB_2782958); phospho-ERK1/2 (Santa Cruz Biotechnology, catalog no. sc-136521, RRID: AB_10856869); sarcomeric myosin heavy chain (DSHB, catalog no. MF 20, RRID: AB_2147781) (4); sodium/potassium-transporting ATPase subunit-α1 (Na-+K+-ATPase) (Santa Cruz Biotechnology, catalog no. sc-48345, RRID: AB_626712, Proteintech Group, catalog no. 14418-1-AP, RRID: AB_2227873) (19, 50); solute carrier family 2 member 1 (Santa Cruz Biotechnology, catalog no. sc-377228, Proteintech Group, catalog no. 21829-1-AP, RRID: AB_10837075); and solute carrier family 2 member 4 (Santa Cruz Biotechnology, catalog no. sc-53566, RRID: AB_629533, Proteintech Group, catalog no. 21048-1-AP) (42). Secondary antibodies are as follows: IRDye 800 conjugated goat anti-rabbit IgG (LI-COR Biosciences, catalog no. 926-32211, RRID: AB_621843); IRDye 800CW goat anti-mouse IgG (LI-COR Biosciences, catalog no. 926-32210, RRID: AB_621842); IRDy(R) 680RD goat anti-rabbit IgG (LI-COR Biosciences, catalog no. 925-68071, RRID: AB_2721181); IRDye 680RD donkey anti-mouse IgG (LI-COR Biosciences, catalog no. 926-68072, RRID: AB_1095362); DyLight 488 anti-mouse IgG (Vector Laboratories, catalog no. DI-2488, RRID: AB_2307439); DyLight 594 anti-rabbit IgG (Vector Laboratories, catalog no. DI-1594, RRID: AB_2336413); DyLight 488 anti-rabbit IgG (Vector Laboratories, catalog no. DI-1488, RRID: AB_2336402); DyLight 594 anti-mouse IgG (Vector Laboratories, catalog no. DL-2594).
Cell Culture.
Rat L6 myoblasts stably expressing GLUT4 with an exo-facial myc-epitope (L6-GLUT4myc) (49) were purchased from Kerafast Inc. (RRID: CVCL_0P25). Cells were verified to be mycoplasma free using a PCR-based Mycoplasma Test Kit (PromoKine, catalog no. PK-CA91-1024). L6-GLUT4myc myoblasts (between passage numbers 9–25) were cultured in complete minimum essential medium-α (MEM-α) with nucleosides supplemented with 10% (vol/vol) fetal bovine serum (FBS) (Atlanta Biologicals, catalog no. S11150), and 1% antibiotic-antimycotic solution (vol/vol) (100 U/mL of penicillin and 100 μg/mL of streptomycin) (Corning, catalog no. 30-004-CL).
The stock solution of endosidin-2 (ES2) (Cayman Chemical, catalog no. 21888) was 30 mg/mL in DMSO. For inhibition experiments, cells were treated with 100 μM ES2 simultaneously with insulin in Krebs-Ringer buffer with 0.1% BSA for 30 min. Untreated controls were exposed to equal amounts of DMSO.
Differentiation.
L6-GLUT4myc myoblast was differentiated following the recommendation of the distributor. Wild-type (WT) L6-GLUT4myc and ΔExoc5 cells were treated at the same time under the same conditions. Briefly, the myoblasts were plated into six-well plates at a density of 5 × 105 cells/well. The cells were allowed to grow to ~90% confluence in complete medium and then were exchanged for differentiation medium (MEM-α with 2% horse serum, and 1% antimycotic-antibiotic solution). Cells were incubated for 7 days under these conditions to enable their differentiation into myotubes, although ΔExoc5 cells were differentiated up to 14 days. The differentiation medium was refreshed once every 2 days.
Cell fractionation.
L6 myoblasts were fractionated using the Minute Plasma Membrane Protein Isolation and Cell Fractionation Kit (Invent Biotechnologies, catalog no. SM-005). Cells were cultured to 80–90% confluence in complete medium and serum-starved in MEM-α with 0.5% FBS for 3 h. Cells were then stimulated with 0 nM or 100 nM insulin in Krebs Ringer buffer with 0.1% BSA for 30 min during ES2 experiments.
Protein analysis and Western blotting.
L6-GLUT4myc cells were cultured to 80–90% confluence in complete medium, and proteins were extracted using radioimmunoprecipitation assay buffer supplemented with phosphatase inhibitors (Sigma-Aldrich, catalog no. P5726) and protease inhibitors (Sigma-Aldrich, catalog no. P8340). Protein concentrations were determined by Bradford assay. Proteins were separated by SDS-PAGE in 4–15% precast polyacrylamide gels (Bio-Rad, catalog no. 4561086) and transferred onto a polyvinylidene fluoride membrane. The membrane was blocked with 5% BSA or 5% milk in phosphate buffer with 0.2% Tween 20 (PBST) for 1 h. The membrane was incubated with primary antibodies diluted 1:1,000 in 5% BSA in PBST at 4°C overnight. Following washes, the blots were incubated with secondary antibodies diluted 1:15,000 in 5% BSA in PBST with 0.02% SDS for 45 min. Blots were scanned with a LI-COR Odyssey Clx scanner, and densitometry was performed using the Image Studio software (LI-COR Biosciences). Individual protein levels were normalized to either sodium/potassium-transporting ATPase subunit-α1 in plasma membrane fractions, or GAPDH in whole cell lysates. Differences in individual protein levels between any two samples were evaluated using Student’s t tests in Graphpad Prism software (Graphpad Software).
Generation of ΔExoc5 L6-GLUT4myc myoblasts.
We used a CRISPR/Cas9 genome editing approach to generate Exoc5 knockout cells, using Genecopoeia’s CRISPR/Cas9 and rat Exoc5-targeting sgRNA constructs, along with a custom-made donor plasmid containing a green fluorescent protein (GFP) reporter and a puromycin resistance cassette flanked by rat Exoc5-specific homology arms. This strategy enabled for the selection of knockout cells that have undergone homology-based repair following a double-strand DNA break mediated by Cas9 within exon 2 of the Exoc5 gene. L6-GLUT4myc cells were cotransfected at 70–80% confluence with Exoc5 -targeting sgRNA (RCP250338, GeneCopoeia), Cas9 (CP-C9NU-01-B, GeneCopoeia), and the donor clone (DC-RTN250338-D01-B, GeneCopoeia) plasmids for 12 h using the Mirus TransIT-X2 transfection reagent. Cells carrying the donor clone sequence were selected for using 2 µg/mL puromycin for 7 days. Single-cell clones were isolated by using fluorescence-activated cells sorting (FACS) for GFP-positive cells.
Glucose analog uptake assays.
Glucose uptake was measured using either a Glucose Colorimetric Assay Kit (Biovision, catalog no. K676-100), utilizing 2-deoxy-d-glucose (2DG) or a fluorescent glucose analog 2-(N-(7-nitrobenz-2-oxa-1,3-diazol-4-yl)amino)-2-deoxyglucose (2-NBDG) (Cayman Chemical Company, catalog no. 11046).
For 2DG uptake, the L6-GLUT4myc myoblasts were plated in six-well plates at a density of 5 × 105 cells/well. Cells were allowed to attach overnight and then were serum-starved in MEM-α with 0.5% FBS for 3 h. The cells were stimulated with 0 nM or 100 nM human recombinant insulin Krebs Ringer buffer (130 mM NaCl, 5 mM KCl, 1.3 mM CaCl2, 1.3 mM MgSO4, and 25 mM HEPES) with 0.1% BSA for 30 min at 37°C. Then 2DG was added to a final concentration of 100 µM. After 5 min, the 2DG was removed, and cells were washed with cold PBS. Cells were then detached with 0.25% Trypsin EDTA and centrifuged at 500 g for 5 min. The 2DG metabolite, 2DG6P, was measured in the lysates with the Glucose Colorimetric Assay kit following the manufacturer’s protocol. 2DG6P values were normalized to total protein, as determined by Bradford assay.
For 2-NBDG assays, L6-GLUT4myc myoblasts were plated, serum starved, and then stimulated, as described for the 2DG uptake assay. Following stimulation, cells were incubated with 50 µM 2-NBDG for 5 min, washed, and then lifted by trypsinization and resuspended in PBS. The mean fluorescence from 30,000 single cell events was measured using a BD Accuri C6 flow cytometer. Dead cells were excluded from analysis using propidium iodide. Relative 2DG and 2NBDG uptake was analyzed using Graphpad Prism software. Differences in glucose uptake levels between any two samples were evaluated using Student’s t tests.
Quantitative PCR.
To measure gene expression levels, real-time quantitative PCR (qPCR) was performed as previously described, using the 2^(−∆∆Ct) approach to calculate fold changes of expression (17, 18). Briefly, RNA from 80 to 90% confluent WT L6-GLUT4myc and heterozygous ΔExoc5 knockout (ΔExoc5) myoblasts was collected using the RNeasy Mini kit (Qiagen). The iScript reverse transcriptase (Bio-Rad) was used to generate cDNA from extracted RNA samples. qPCR was performed on a CFX96 Real Time System (Bio-Rad), using the iTaq Universal SYBR Green Supermix (Bio-Rad), as recommended by the manufacturer. To assess gene expression, the following primers were used: rat Exoc5 forward: 5′-TCCACACGAGACGTTCAATG-3′, reverse: 5′-AGCATGTGAAGCGGAGAC-3′ (IDT, PrimeTime qPCR, no. Rn.PT.58.9297907), and rat Ppia forward: 5′-CCATTATGGCGTGTGAAGTC-3′, reverse: 5′-GCAGACAAAGTTCCAAAGACAG-3′ (IDT, PrimeTime qPCR, no. Rn.PT.39a.22214830), as a housekeeping gene. Expression data were analyzed using Graphpad Prism software. Differences in gene expression levels between any two samples were evaluated using Student’s t tests.
Immunofluorescence.
Immunofluorescent staining of L6-GLUT4mycp myoblasts was performed as previously described (40). Briefly, cells were washed thrice with PBS, fixed with 4% paraformaldehyde, then permeabilized with 0.1% Triton-X 100 in PBS. Following blocking with 0.1% BSA in PBS, samples were incubated in primary antibodies targeting the exocyst [anti-EXOC1 (Proteintech Group), anti-EXOC5 (Proteintech Group), or anti-EXOC7 (Santa Cruz Biotechnology)] and primary antibodies targeting GLUT4myc [anti-myc (Proteintech Group) or anti-solute carrier family 2 member 4 (Santa Cruz Biotechnology)] at 4°C overnight. After washing, the cells were incubated with DyLight secondary antibodies (DyLight 488 anti-rabbit IgG and DyLight 594 anti-mouse, Vector Laboratories) at a 1:1,000 dilution at room temperature for 1 h. Nuclei were stained with DAPI for 5 min. All samples were mounted with Fluoromount-G mounting medium (Thermo Fisher Scientific) and then dried overnight. For imaging purposes, the cells were viewed with a Leica SP8 confocal microscope, using a ×63 objective. Treated and control cells were collected and analyzed at the same time under the same conditions. Using the ImageJ software’s JACoP plugin, we calculated the Pearson’s correlation coefficient to quantify colocalization of GLUT4myc with the exocyst subunits. Pearson R coefficient 0 signifies no correlation trend, +1 implies complete cocorrelation, and −1 indicates an inverse correlation.
Proximity ligation amplification.
L6-GLUT4myc myoblasts were grown on chamber slides to 70–80% confluence. The cells were then serum-starved in MEM-α with 0.5% FBS for 3 h, then stimulated with 0 nM or 100 nM human recombinant insulin in Krebs Ringer buffer with 0.1% BSA (pH 7.4) for 30 min at 37°C. Following treatment, the cells were washed with ice-cold PBS, fixed in 4% paraformaldehyde, permeabilized with 0.01% Triton-X 100 in PBS, and blocked using a blocking buffer of 1% BSA in PBS for 1 h at room temperature. Then cells were incubated with primary antibodies (rabbit anti-GLUT4, Proteintech, with either mouse anti-EXOC5, Santa Cruz Biotechnology, or mouse anti-EXOC7, Santa Cruz Biotechnology) at 4°C overnight. After washing, the secondary antibody conjugate mixture (Duolink in situ PLA probe anti-goat plus and Duolink in situ PLA probe anti-mouse minus) was added, followed by the addition of the ligation mixture. Following ligation, the signals were amplified with fluorescently labeled oligonucleotide detection probes (Duolink In Situ Detection Reagents Red, Sigma), and samples were mounted using the Duolink In Situ Mounting Medium with DAPI and detected by confocal microscopy. Treated and control cells were collected at the same time under the same conditions.
Cell surface GLUT4Myc analysis.
GLUT4myc on the plasma membrane was measured using methods adapted from Wang et al. (49). Briefly, myoblasts were plated at a cell density of 3 × 105 cells/well into eight-well chamber slides and allowed to attach overnight. For analysis of myotubes, myoblasts were then allowed to differentiate for 7 days in differentiation medium. Cells were serum starved in MEM-α with 0.5% FBS for 3 h, and then stimulated with 0 nM or 100 nM insulin in Krebs Ringer buffer with 0.1% BSA for 30 min in the presence of ES2 or an equivalent volume of DMSO. Following fixation with 4% paraformaldehyde for 10 min at room temperature, the paraformaldehyde was neutralized with 5% BSA in PBS for 10 min. The cells were then blocked with 5% BSA in PBS for 30 min, and surface myc was labeled with primary antibody (Proteintech Group, catalog no. 60003-2-Ig, RRID: AB_2734122) diluted 1:100 in 5% BSA in PBS. Following a wash with PBS, and permeabilization in 0.1% Triton X-100 for 10 min, the cells were rinsed and incubated with an anti-β-actin primary antibody (Cell Signaling Technology, catalog no. 4970, RRID: AB_2223172) diluted 1:100. Cells were subsequently washed with PBS and incubated with secondary antibodies (LI-COR Biosciences, catalog no. 926-32211, RRID: AB_621843; LI-COR Biosciences, catalog no. 926-68072, RRID: AB_1095362) diluted 1:1,000 for 30 min. Slides were rinsed with PBS and drained before scanning with Odyssey Clx scanner (LI-COR Biosciences). Densitometry was performed using the Image Studio software (LI-COR Biosciences). Background fluorescence was determined by measuring cells exposed to secondary antibodies only. For quantification of levels of GLUT4myc on the plasma membrane, myc signal was normalized to β-actin signal to adjust for cell number.
Measuring oxygen consumption rate and extracellular acidification using Seahorse technology.
Oxygen consumption rate (OCR) and extracellular acidification rate (ECAR), reflecting oxidative phosphorylation and glycolysis, respectively, were measured on the Seahorse Bioscience XFe96 Analyzer (Agilent Technologies). For the extracellular flux assays, cells were plated at a density of 2.5 × 104 cells per well in Seahorse XF96 cell culture microplates (Agilent Technologies) 2 h before the assay. The glycolytic rate assay was performed in XF base media without phenol red containing 5 mM HEPES, 25 mM glucose, 1 mM sodium pyruvate, and 2 mM l-glutamine. Cells were rinsed with assay medium before incubation for 1 h at 37°C in a non-CO2 incubator. Following incubation, measurements were taken for 60 min, followed by stimulation with insulin (1 µg/mL) or vehicle. Baseline OCR and ECAR measurements of the insulin or vehicle-treated cells were obtained for 60 min before injection of specific metabolic inhibitors. The following inhibitors were added to each well: rotenone/antimycin A (Rot/AA) (0.5 µM each) and 2DG (50 mM). Results were analyzed using WAVE version 2.6.0.31 (Agilent Technologies) and GraphPad Prism (GraphPad Software). The data are presented as glycolytic proton efflux rate, which is defined as the number of protons derived from glycolysis and exported by cells into the assay medium over time without the mitochondrial contribution to acidification.
Statistical methods.
Experiments were repeated at least three times. Graphs show mean ± SD, unless otherwise indicated. Statistical comparisons between the subgroups were assessed by one-way analysis of variance (ANOVA). In all other cases, Student’s t test was performed to measure P values and determine whether there was a significant difference between two groups. P values of < 0.05 were considered statistically significant. For the Seahorse analysis, outlier detection was performed using the interquartile method, and outliers were excluded from the analysis. Area under the curve (positive incremental) values were calculated based on the trapezoid model, relative to the baseline, using the GraphPad Prism software (GraphPad, Inc.) (2).
RESULTS
Insulin increases exocyst and GLUT4 enrichment in the plasma membrane fraction of L6-GLUT4myc myoblasts.
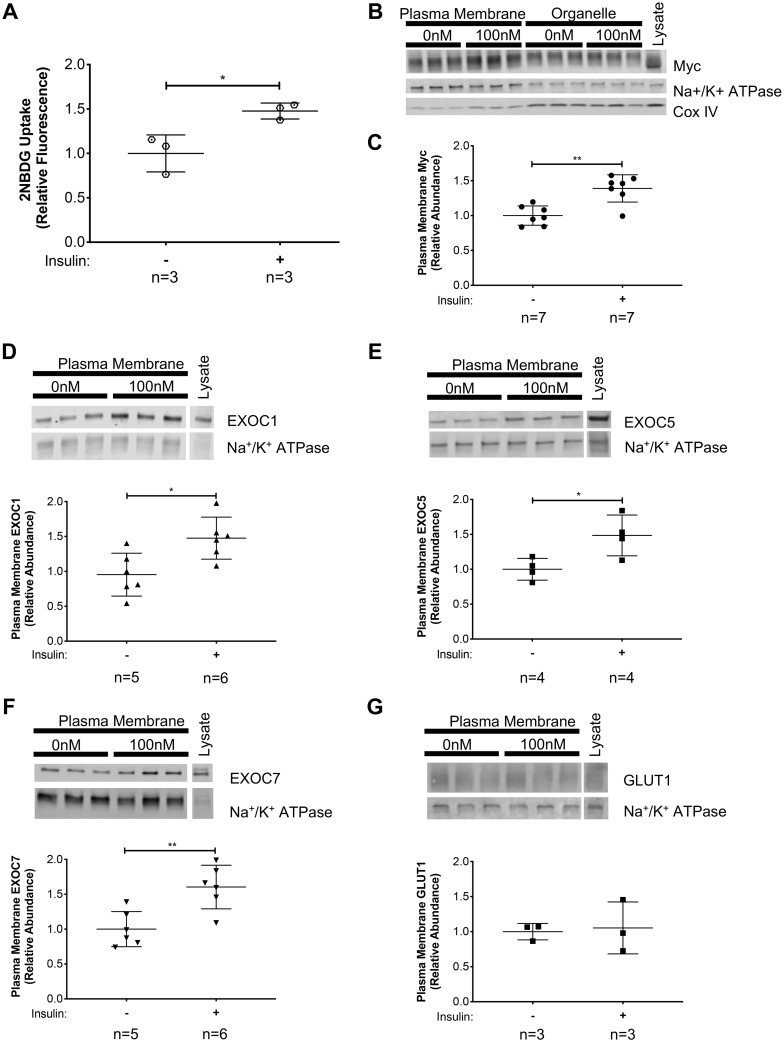
To determine whether the exocyst complex is an essential part of the insulin-induced GLUT4 membrane trafficking machinery in skeletal muscle cells, we used L6-GLUT4myc rat myoblasts as our model. These myoblasts stably overexpress a functional chimera of rat GLUT4 with a human myc-tag on the first exo-facial loop (GLUT4myc) (49). Previous studies have shown that L6-GLUT4myc myoblasts incorporate GLUT4myc into the plasma membrane in response to insulin, consequently increasing glucose uptake (37). First, we confirmed that insulin-induction in myoblasts indeed triggered a significant increase in the uptake of the fluorescent glucose analog 2-NBDG compared with uninduced controls (Fig. 1A). Following subcellular fractionation, enrichment of plasma membrane or organelle-specific proteins in the fractions collected was confirmed by evaluating levels of the membrane-associated protein Na+-K+-ATPase, and the mitochondrium-specific CoxIV (Fig. 1B). To determine plasma membrane enrichment of target proteins upon insulin signal, the target protein levels were normalized to the plasma membrane-specific loading control, Na+-K+-ATPase. Subcellular fractionation of L6-GLUT4myc myoblasts revealed that, in response to insulin, exocyst subunits EXOC1, EXOC5, and EXOC7 became enriched in the plasma membrane fraction, similarly to the myc-tagged GLUT4 transporter (Fig. 1, C–F). The basal glucose transporter GLUT1 did not show such enrichment in the plasma membrane fraction of L6 GLUT4myc myoblasts in response to insulin treatment (Fig. 1G). These data show that, in skeletal myoblasts, the exocyst is recruited to the plasma membrane in response to insulin, along with the glucose transporter GLUT4.
Fig. 1.
Exocyst components and glucose transporter type 4 (GLUT4) are translocated to the plasma membrane of skeletal myoblasts following insulin stimulation. A: insulin-stimulated glucose uptake in L6-GLUT4myc myoblasts was verified using the fluorescent glucose analog, 2-(N-(7-nitrobenz-2-oxa-1,3-diazol-4-yl)amino)-2-deoxyglucose (2-NBDG). B–G: L6-GLUT4myc myoblasts were stimulated with 100 nM insulin for 10 min. Plasma membrane proteins were collected via subcellular fractionation and analyzed by Western blotting. B: representative images of Western analyses of subcellular fractionations. Analysis is shown of protein enrichment in subcellular fractions, where GLUT4myc distribution in subcellular fractions was detected, along with the plasma membrane protein Na+-K+-ATPase and the mitochondrial protein CoxIV. C: quantification of plasma membrane GLUT4myc, as detected by a myc-tag-specific antibody, normalized to measured levels of the membrane protein Na+-K+-ATPase. D–G: quantification of EXOC1, EXOC5, and EXOC7, and GLUT1 protein levels, respectively, in the plasma membrane fractions of insulin-stimulated myoblast cells. Signal intensities for myc, EXOC1, EXOC5, EXOC7, and GLUT1 were normalized to plasma membrane-specific Na+-K+-ATPase levels. Images and graphs presented are representative of data from 3 independent experiments. Values are means ± SD in relative units (vs. untreated cells); n, no. of replicates. *P < 0.05 and **P < 0.01, compared with untreated control.
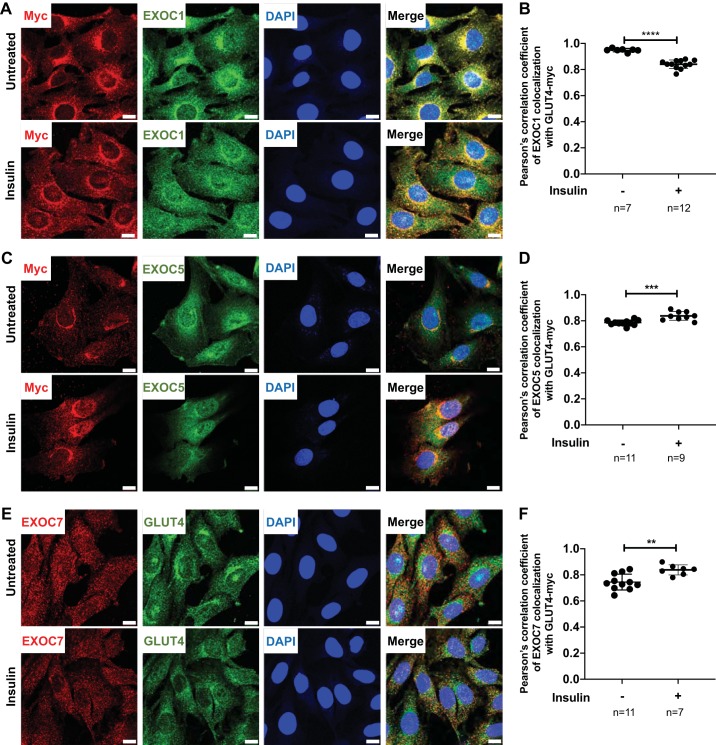
Subcellular colocalization of EXOC5 and EXOC7 with GLUT4 increases after insulin stimulation.
We evaluated the subcellular localization of the exocyst subunits EXOC1, EXOC5, and EXOC7 in relation to GLUT4. L6-GLUT4myc myoblasts were analyzed under basal and insulin-induced conditions using immunofluorescent staining (Fig. 2). Fluorescent immunostaining showed that the exocyst members EXOC1 (Fig. 2A), EXOC5 (Fig. 2C), and EXOC7 (Fig. 2E) colocalize with GLUT4 in the perinuclear region of the L6-GLUT4myc myoblasts, suggesting that some subunits of the exocyst complex may be preassembled on GSVs at a basal state (Fig. 2, A, C, and E, top panels). After 30 min of insulin induction, we observed increased colocalization of GLUT4myc and the exocyst components EXOC5 and EXOC7 throughout the cytoplasm and cell membrane, supported by our calculations of the Pearson’s correlation coefficients (Fig. 2, C–F). Overall colocalization of EXOC1 with GLUT4myc is slightly reduced upon insulin treatment, as indicated by the Pearson’s colocalization correlation analysis (Fig. 2B), with the site of colocalization between EXOC1 and GLUT4myc shifting from the perinuclear region to the myoblast plasma membrane (Fig. 2A, bottom panels).
Fig. 2.
Exocyst subunit EXOC5 colocalizes with glucose transporter type 4 (GLUT4) in skeletal myoblasts under basal and insulin-stimulated conditions. A, C, and E: confocal images of untreated (top panels) and insulin-induced (bottom panels) L6-GLUT4myc rat skeletal myoblasts immunostained for exocyst subunits (using anti-EXOC1, EXOC5, or EXOC7 antibodies) and GLUT4myc [using anti-myc (A and C) or anti-GLUT4 (E) antibodies]. Nuclei were stained with DAPI (blue). Scale bars: 10 µm. B, D, and F: GLUT4myc and exocyst subunit colocalization expressed as average coefficients of correlation (Pearson’s). Data presented are representative of 3 independent preparations. Values are means ± SD in relative units (vs. untreated cells); n, no. of images analyzed. **P < 0.01, ***P < 0.005, and ****P < 0.001 compared with untreated control.
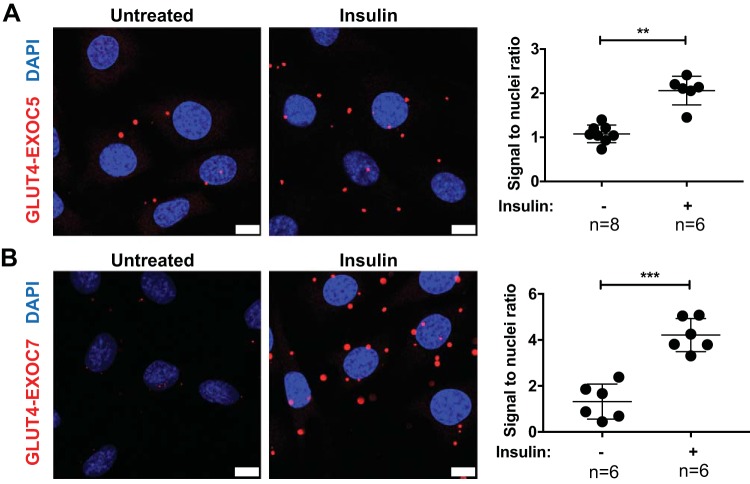
Supporting the above observations, we measured association between GLUT4myc and the exocyst subunits EXOC5 and EXOC7 using a proximity ligation assay approach (Fig. 3). The proximity ligation assay uses antibody-DNA conjugates to target the proteins of interest. Binding of the conjugates to their respective targets (i.e., the primary antibodies recognizing the exocyst subunit and a cytoplasmic domain of GLUT4myc) results in the assembly of a circular DNA molecule. Rolling circle amplification creates numerous copies of this DNA sequence that can be detected using fluorescently labeled oligonucleotides and microscopy. This enables the high-resolution detection of protein colocalization in situ (45). Protein colocalization was quantified in untreated and insulin-induced L6 GLUT4myc myoblasts by counting PLA signal-to-nuclei ratios using ImageJ. Insulin treatment of these skeletal myoblasts resulted in a significant increase of EXOC5 and GLUT4myc association, as indicated by an increased signal-to-nuclei ratio (Fig. 3A). Similarly, insulin induction resulted in an elevated ratio of proximal GLUT4myc and EXOC7 molecules (Fig. 3B). Our data suggests that, in skeletal myoblasts, the exocyst is in close proximity with GSVs in a basal state, and insulin induction triggers an elevated association of the exocyst complex subunits and GSVs.
Fig. 3.
Exocyst subunits show an increased association with glucose transporter type 4 (GLUT4) storage vesicles in response to insulin in myoblasts. Colocalization of exocyst subunits EXOC5 and EXOC7 with GLUT4 vesicles was demonstrated using the proximity ligation assay, where immunofluorescent signal (red dots) indicates the close proximity between the target proteins. Confocal microscopy images and quantification of a proximity ligation assay interrogating EXOC5 and GLUT4 proximity (A) and EXOC7 and GLUT4 association (B) in untreated and insulin-induced L6 GLUT4myc myoblasts are shown. Nuclei were stained with DAPI, and results were quantified by counting the number of red signals per nuclei, with a minimum of 5 samples (at least 100 cells total) counted for each assay. Scale bar = 10 µm. Values are means ± SD in relative units (vs. wild-type L6 GLUT4myc cells); n, no. of images analyzed. **P < 0.01. ***P < 0.005.
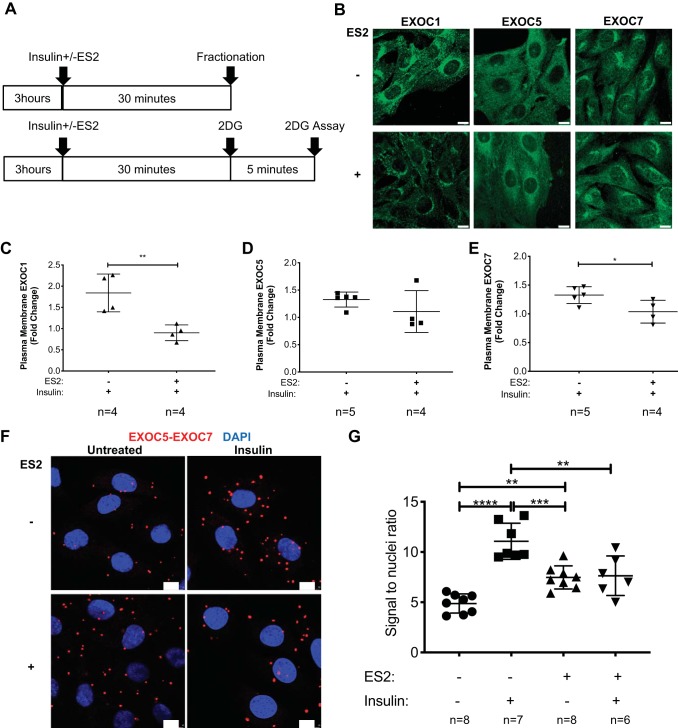
The exocyst inhibitor ES2 hinders exocyst complex assembly and membrane delivery in response to insulin.
Discovered among a set of 2,016 chemicals by using a plant-based, high-throughput method for detecting compounds that affect vesicular trafficking, ES2 binds specifically to EXOC7 (or Exo70 in the traditional nomenclature) and has been shown to inhibit exocyst function in Arabidopsis and HeLa cells (51). First, we used immunofluorescent staining to determine the impact of ES2 treatment on the intracellular localization of exocyst subunits EXOC1, EXOC5, and EXOC7. While EXOC1 and EXOC5 appear to shift toward perinuclear localization upon ES2 treatment, immunofluorescent staining of EXOC7 did not reveal such changes (Fig. 4B). Of note, while ES2 treatment of HeLa cervical epitheloid carcinoma cells led to an accumulation of the tagged GFP-rEXO70 subunit in intracellular compartments (51), we did not observe similar changes in response to ES2 for any of the exocyst subunits evaluated. We utilized subcellular fractionation to assess if ES2 prevented the recruitment of the exocyst complex to the plasma membrane in response to insulin stimulus. We found that 30 min of ES2 treatment did not significantly impact basal exocyst membrane association, but diminished the insulin-induced plasma membrane enrichment of EXOC1 and EXOC7 (Fig. 4, C–E). While ES2 treatment appears to decrease insulin-induced EXOC5 membrane levels as well, the difference was not statistically significant. Furthermore, proximity ligation assays revealed that, in response to insulin, exocyst subunits EXOC5 and EXOC7 showed a significantly elevated association, indicative of exocyst complex assembly. ES2 treatment alone resulted in a significant increase in exocyst subunit assembly, but this effect was smaller than that of insulin alone. Furthermore, in the presence of ES2, insulin treatment failed to trigger further exocyst assembly (Fig. 4, F and G). These observations suggest that, in skeletal myoblasts, insulin triggers the assembly of the exocyst trafficking complex, and the exocyst inhibitor ES2 hinders exocyst assembly and recruitment to the plasma membrane in response to insulin.
Fig. 4.
Endosidin 2 (ES2) attenuates plasma membrane enrichment of exocyst subunits and decreases subunit assembly rates after insulin stimulation. A: graphical interpretation of the experimental timeline. L6 GLUT4myc myoblasts were stimulated with 100 nM insulin and ES2 for 30 min and were either collected for subcellular fractionation (top) or treated with 2-deoxy-d-glucose (2DG) to evaluate glucose uptake (bottom). B: immunofluorescent staining of exocyst subunits EXOC1, EXOC5, and EXOC7 in control (top row) and ES2-treated (bottom row) L6 GLUT4myc myoblasts. Scale bar = 10 µm. Images presented are representative of 3 independent experiments. C–E: quantification of the change in plasma membrane protein levels of exocyst subunits EXOC1, EXOC5, and EXOC7 in response to insulin in the presence or absence of exocyst inhibitor ES2. Data, representative of 3 independent experiments, are presented as means ± SD in relative units; n, no. of replicates. *P < 0.05 and **P < 0.01, compared with insulin treatment in the absence of ES2. F and G: confocal microscopic images and quantification of a proximity ligation assay evaluating EXOC5 and EXOC7 subunit proximity in L6 GLUT4myc myoblasts following insulin treatment, in the presence or absence of ES2 exocyst inhibitor. Data, representative of 3 independent preparations, are presented as means ± SD; n, no. of images analyzed. **P < 0.01. ***P < 0.0005. ****P < 0.0001.
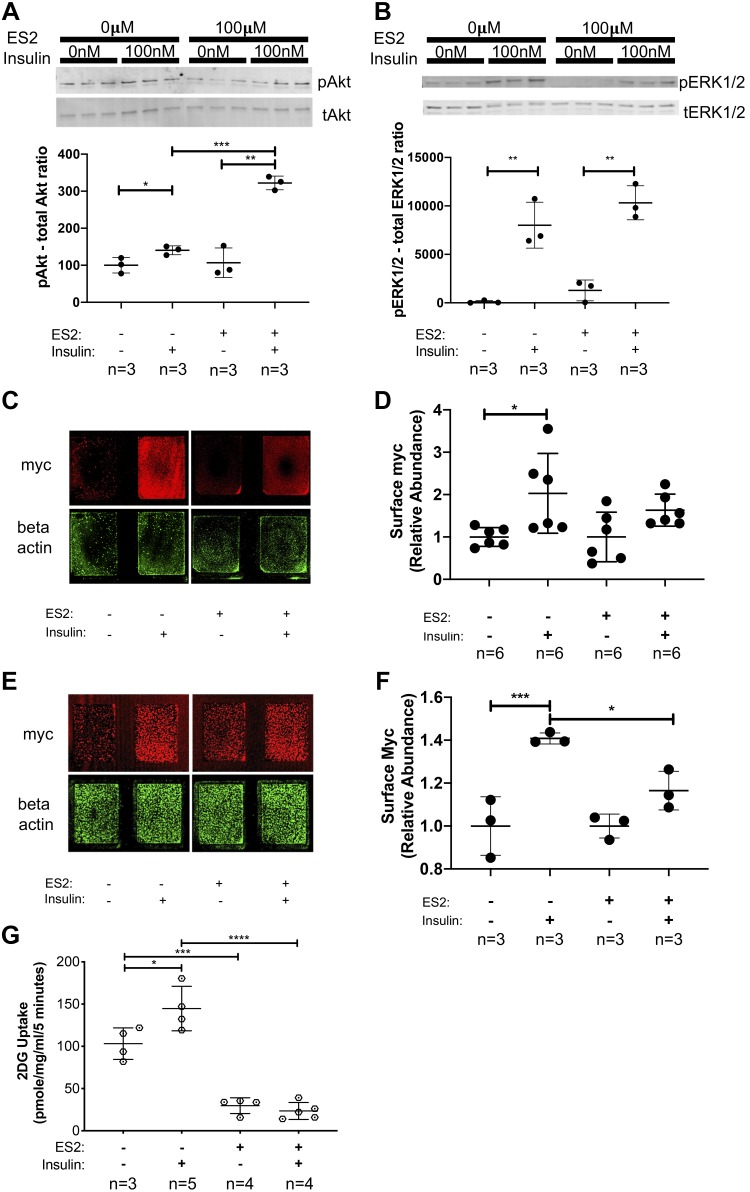
The exocyst inhibitor ES2 impairs GLUT4myc membrane delivery and glucose uptake.
To determine how the inhibition of the exocyst trafficking complex affects insulin signaling, GLUT4myc membrane delivery, and subsequent glucose uptake, we treated L6-GLUT4myc myoblasts with insulin in the presence or absence of exocyst inhibitor ES2. Insulin treatment in the presence of ES2 resulted in a significant increase in Akt and ERK1/2 phosphorylation (Fig. 5, A and B), suggesting that ES2 does not impair insulin signaling pathway activation. Moreover, treatment with ES2 further increases Akt phosphorylation levels in response to insulin (Fig. 5A). To measure levels of GLUT4myc at the cell surface, we modified a method from Wang et al. (49), by using an immunostaining method on cells grown on chamber slides followed by detection with a high-resolution fluorescent scanner. We found that, while insulin alone induced a significant increase in cell surface myc in WT L6 GLUT4myc myoblasts, ES2 treatment hindered cell surface delivery of GLUT4myc in response to insulin (Fig. 5, C and D). Similarly, when we quantified surface GLUT4myc levels in differentiated WT L6 GLUT4myc myotubes, we observed that, in the presence of the exocyst inhibitor ES2, insulin treatment did not trigger a significant increase in cell surface GLUT4myc levels (Fig. 5, E and F). Supporting these findings, insulin did not increase 2DG uptake in L6-GLUT4myc myoblasts that were cotreated with the exocyst inhibitor ES2 for 30 min (Fig. 5G). Together, these data suggest that, in skeletal muscle cells, the exocyst inhibitor ES2 hinders exocyst assembly and recruitment to the plasma membrane, thereby disrupting GLUT4 vesicle docking and membrane incorporation, which leads to an overall decreased glucose uptake.
Fig. 5.
Endosidin 2 (ES2) treatment hinders insulin-induced cell surface delivery of GLUT4myc and subsequent glucose uptake. A and B: analysis of insulin signaling pathway activation in the presence of exocyst inhibitor ES2. Phosphoprotein levels of both pAkt and pERK1/2 targets were normalized to total protein levels of Akt and Erk1/2, respectively. Data, representative of 3 independent experiments, are presented as means ± SD in relative units; n, no. of replicates. *P < 0.05. **P < 0.01. ***P < 0.005. C: representative immunofluorescent staining of cell surface GLUT4myc in L6 GLUT4myc myoblasts treated with insulin in the presence or absence of ES2. Signal intensities of entire surface of the chamberslide wells were measured with a fluorescent scanner, and surface GLUT4myc levels were normalized to β-actin levels measured after a subsequent permeabilization and immunostaining of the samples. D: quantification of relative cell surface GLUT4myc levels in myoblasts treated with insulin in the presence or absence of ES2. Data are means ± SD in arbitrary units relative to untreated control; n, no. of replicates. *P < 0.05. E: representative staining of cell surface GLUT4myc in differentiated L6 GLUT4myc myotubes treated with insulin in the presence or absence of ES2. Signal intensities of surface GLUT4myc were normalized to β-actin levels as described above. F: quantification of relative cell surface GLUT4myc levels in differentiated myotubes treated with insulin in the presence or absence of ES2. Data are means ± SD in arbitrary units relative to untreated control; n, no. of replicates. *P < 0.05. ***P < 0.005. G: measurement of 2-deoxy-d-glucose (2DG) uptake in skeletal myoblasts in response to insulin in the presence or absence of ES2. Data are means ± SD; n, no. of replicates. *P < 0.05. ***P < 0.005. ****P < 0.001. Images and data presented are representative of 3 independent preparations.
Heterozygous Exoc5 knockout in myoblasts impairs GLUT4myc membrane delivery.
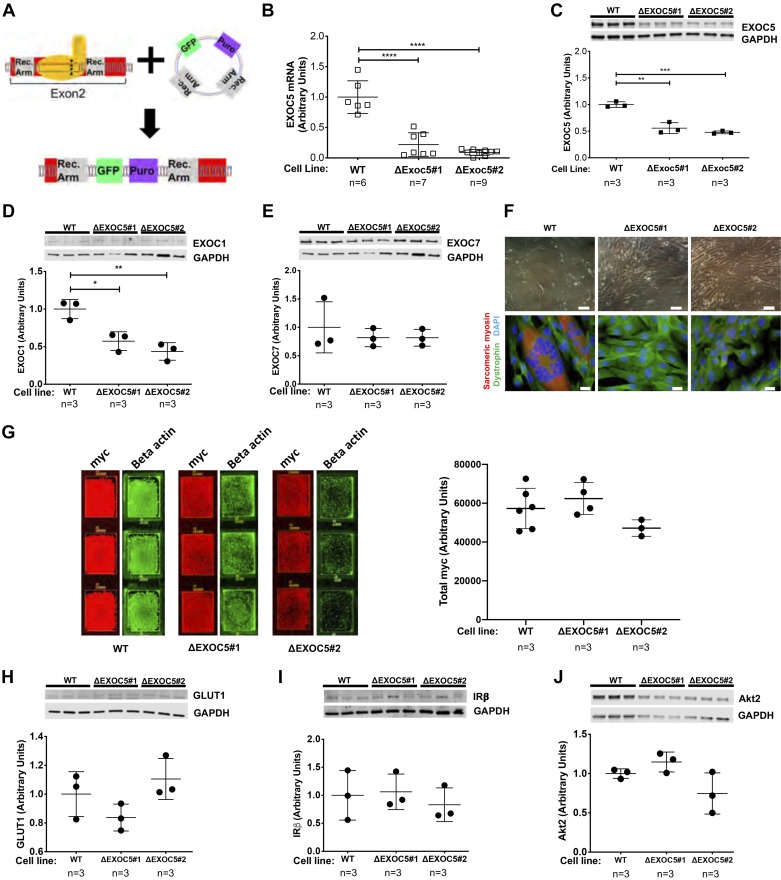
To determine whether a genetic knockout of Exoc5 affects GLUT4 exocytosis in skeletal myoblasts, we generated an exocyst-deficient cell culture model. To do this, we used CRISPR-Cas9 methodology with sgRNA targeting the second exon of Exoc5. Homology-directed repair enabled us to insert a puromycin resistance cassette and a GFP-reporter gene into exon 2 of Exoc5, thereby disrupting the gene while allowing for clonal selection (Fig. 6A). Following establishment of clonal cell lines with FACS, PCR-based genotyping and sequencing revealed heterozygous Exoc5 knockout in all clones isolated. Quantitative real-time PCR confirmed an 80–90% decrease in expression of Exoc5 mRNA in two selected heterozygous knockout clones (Fig. 6B). However, through Western blot analysis, we observed a modest decrease (~50%) in EXOC5 protein in these same ∆Exoc5 clones (Fig. 6C). In addition to the significant decrease in EXOC5, EXOC1 protein levels were decreased as well (Fig. 6D), but EXOC7 levels were not different (Fig. 6E). This agrees with previous reports showing that RNAi-mediated silencing of Exoc5 affects protein levels of other exocyst components. In addition, myoblast cells with this heterozygous knockout of Exoc5 showed defects in differentiation. When WT and ΔExoc5 L6-GLUT4myc cells were induced to differentiate, ΔExoc5 clones showed significant impairment in myotube formation, as shown by light microscopy following 14 days of differentiation (Fig. 6F, top row). This was later confirmed by the near complete lack of sarcomeric myosin-positive myotubes formed after 7 days of differentiation by ΔExoc5 cells (Fig. 6F, bottom row). These results not only suggest that the exocyst function is required for the proper differentiation of skeletal myoblasts into myotubes, but led us to use undifferentiated myoblasts for our further experiments.
Fig. 6.
Heterozygous knockout of EXOC5 decreases EXOC1 subunit expression, impairs myotube differentiation, but does not affect expression of insulin signaling components. A: our approach to gene editing using CRISPR/Cas9 mediated homologous recombination resulted in the insertion of a green fluorescent protein (GFP) reporter and a puromycin resistance cassette into exon 2 of the EXOC5 gene. B: quantification of EXOC5 mRNA levels in ΔExoc5 L6-GLUT4myc clonal myoblasts using quantitative real-time PCR. C–E: quantification of exocyst subunit EXOC1, EXOC5, and EXOC7 protein levels in ΔExoc5 and wild-type (WT) L6-GLUT4myc myoblasts using Western blot analysis. F: assessment of differentiation ability in ΔExoc5 and WT L6-GLUT4myc myoblasts using light microscopy and immunofluorescent staining. Myotube formation was indicated by the formation of multinucleated, sarcomeric myosin-positive (red) structures. Dystrophin (green) and DAPI (blue) were used to visualize the cell membrane and nucleus, respectively. Double scale bars = 100 µm; single scale bars = 10 µm. G: representative immunofluorescent staining and quantification of total GLUT4myc levels in permeabilized L6 GLUT4myc myoblasts. Signal intensities of entire surface of the chamberslide wells were measured with a fluorescent scanner, and total GLUT4myc levels were normalized to β-actin levels measured. H–J: quantification of GLUT1, insulin receptor β-subunit (IRβ), and Akt2 protein levels, respectively, in ΔExoc5 and WT L6-GLUT4myc myoblasts using Western blot analysis. Images and data are representative of 3 independent experiments. Values are means ± SD in relative units (vs. WT L6-GLUT4myc cells); n, no. of replicates. *P < 0.05. **P < 0.01. ***P < 0.005. ****P < 0.001.
Next, we investigated how heterozygous Exoc5 knockout affects insulin signaling and subsequent GLUT4myc membrane delivery in skeletal myoblasts. We quantified the baseline expression levels of GLUT1, GLUT4, insulin signaling pathway components, as well as signaling pathway activation and GLUT4myc membrane delivery in response to insulin in L6 GLUT4myc WT and ΔExoc5 clones. Baseline protein levels of GLUT4myc, GLUT1, insulin receptor β-subunit and Akt2 was not altered by EXOC5 disruption (Fig. 6, G–J), demonstrating that elements of the insulin signaling pathway remained intact in the ΔExoc5 clones.
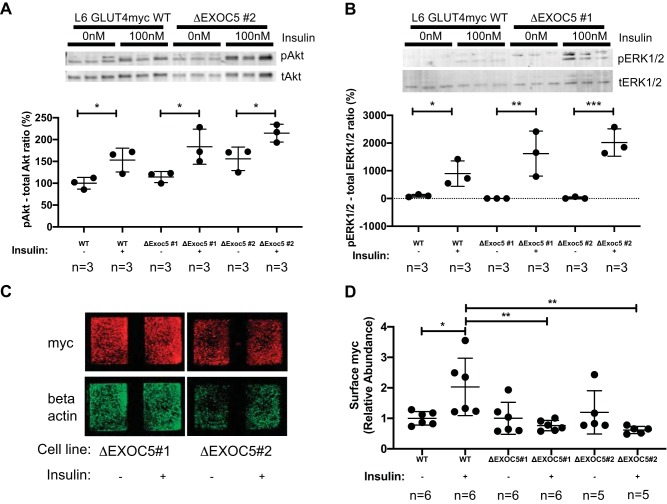
Furthermore, when we evaluated cellular response to insulin treatment in WT and ΔExoc5 myoblast clones, we found that the insulin pathway activation remained intact, as demonstrated by an increase in Akt and Erk1/2 phosphorylation in response to insulin treatment (Fig. 7, A and B). We quantified cell surface GLUT4myc levels of the ΔExoc5 clones upon insulin induction, as described earlier. Our data demonstrates that disrupted EXOC5 expression in skeletal myoblasts leads to a significantly decreased membrane delivery of the GLUT4myc transporter in response to insulin (Fig. 7, C and D).
Fig. 7.
Heterozygous knockout of EXOC5 results in decreased cell surface delivery of GLUT4myc in response to insulin. A and B: analysis of insulin signaling pathway activation in ΔExoc5 clones in response to insulin-representative Western blots and quantification. Levels of both pAkt and pERK1/2 targets were normalized to total protein levels of Akt and Erk1/2, respectively. C: representative immunofluorescent staining of cell surface GLUT4myc in ΔExoc5 L6 GLUT4myc myoblasts treated with insulin. Signal intensities of surface GLUT4myc detected by an anti-myc antibody were normalized to β-actin levels measured after a subsequent permeabilization and immunostaining of the samples. D: quantification of relative cell surface GLUT4myc levels in wild-type (WT) and ΔExoc5 L6 GLUT4myc myoblasts treated with insulin. Images and data are representative of 3 independent experiments. Values are means ± SD in relative units (vs. WT L6-GLUT4myc cells); n, no. of replicates. *P < 0.05. **P < 0.01. ***P < 0.005.
Heterozygous Exoc5 knockout impairs insulin-induced glucose uptake in L6-GLUT4myc skeletal myoblasts and disrupts cellular metabolism.
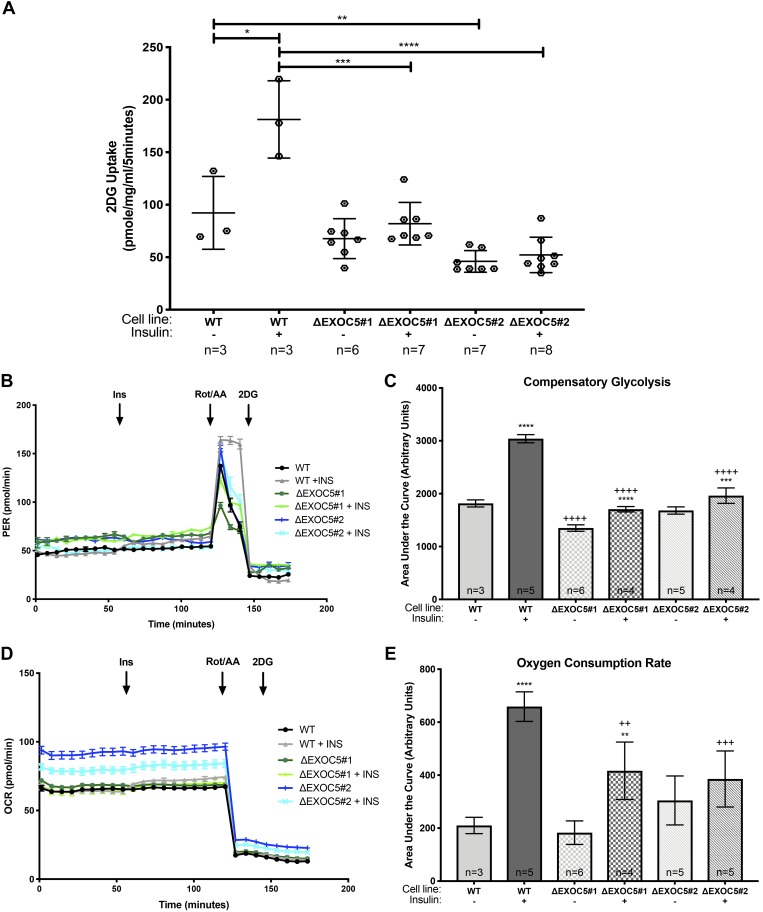
We then investigated how this heterozygous Exoc5 knockout affects glucose uptake in skeletal myoblasts and measured changes in intracellular levels of the glucose analog 2DG after insulin induction in undifferentiated WT myoblasts and ΔExoc5 clones. None of the ΔExoc5 L6-GLUT4myc clones studied showed an increase in 2DG uptake in response to insulin, supporting a role for EXOC5 in insulin-induced GLUT4 exocytosis in skeletal muscle (Fig. 8A). Next, we determined how the glycolytic and mitochondrial functions were affected by impaired glucose uptake in response to insulin in WT L6 GLUT4myc myoblasts and ΔExoc5 clones by using a Seahorse XFe96 extracellular flux analyzer. The Seahorse XF analyzer simultaneously assessed the glycolytic rate measured as ECAR and mitochondrial function measured as the OCR. We found that basal glycolysis was similar in untreated WT and ΔExoc5 L6-GLUT4myc clones, (Fig. 8B), suggesting that, during a steady state of metabolism, lacking energy demand, Exoc5 knockout in muscle cells did not impact glycolysis. During compensatory glycolysis induced by blocking mitochondrial electron transfer via Rot/AA treatment, we found a significantly higher compensatory glycolysis level in all insulin-treated cells compared with their respective untreated controls (Fig. 8C). Untreated ΔExoc5 L6-GLUT4myc clones showed a compensatory glycolytic capacity like control WT L6-GLUT4myc myoblasts, but insulin treatment failed to induce an increase in compensatory glycolytic capacity as high as that of the WT L6-GLUT4myc myoblasts (Fig. 8, B and C). Basal mitochondrial function was similar for all untreated cell lines, while the insulin-induced OCR in WT L6-GLUT4myc myoblasts was higher than in ΔExoc5 L6-GLUT4myc clones (Fig. 8, D and E), indicating an increased mitochondrial function in the WT L6-GLUT4myc myoblasts in the presence of the hormone. Insulin induction led to an increase in mitochondrial oxidation rate in ΔExoc5 L6-GLUT4myc cells as well, but this increase was significantly lower than that of the insulin-treated WT L6-GLUT4myc myoblasts (Fig. 8E). These findings indicated a dysregulation of glycolysis and an altered metabolic response to insulin treatment in exocyst-deficient skeletal myoblasts.
Fig. 8.
Heterozygous knockout of EXOC5 impairs glucose uptake and blunts insulin’s effect on cellular metabolism in skeletal myoblasts. A: measurement of 2-deoxy-d-glucose (2DG) uptake following insulin stimulus in ΔExoc5 and wild-type (WT) L6-GLUT4myc myoblasts. B–E: analysis of glycolytic rate in response to insulin using a Seahorse XF Analyzer. B: quantification of proton efflux rate (PER) as an indicator of cellular glycolytic function. Insulin was injected after 60 min, followed by the inhibition of the electron transport chain with rotenone/antimycin A (Rot/AA) and the glycolytic inhibitor 2DG. C: quantification of PER associated with compensatory glycolysis following Rot/AA addition in ΔExoc5 and WT L6-GLUT4myc myoblasts in the presence or absence of insulin. D and E: measurement of oxygen consumption rates (OCR) as an indicator of mitochondrial function in ΔExoc5 and WT L6-GLUT4myc myoblasts in the presence or absence of insulin. Values are means ± SD in relative units (vs. untreated L6-GLUT4myc cells) from 3 independent experiments; n, no. of replicates. *P < 0.05, **P < 0.01, ***P < 0.005, and ****P < 0.001, compared with untreated. ++P < 0.01, +++P < 0.005, and ++++P < 0.001, compared with similarly treated WT cells.
DISCUSSION
Previous studies have demonstrated that the exocyst complex is necessary for the docking of GSVs to the plasma membrane, but so far this has only been studied in cultured adipocytes (23). In 3T3-L1 cells, EXOC7 is recruited to the plasma membrane by the GTPase TC10 in response to insulin signaling (23). TC10 is key for stabilizing the exocyst complex and GSVs at lipid rafts following insulin stimulation (24). EXOC7 binding is followed by EXOC3 and EXOC4 recruitment to the plasma membrane (23), and the overexpression of EXOC3 and EXOC4 increases insulin-induced glucose uptake in adipocytes (15). Components of the GLUT4 trafficking mechanism show a high degree of conservation in major metabolic tissues, including adipose tissue and skeletal muscle (8, 12, 26, 27, 36, 41). Yet it was not known if the exocyst serves as a regulator of insulin-induced GLUT4 exocytosis in skeletal muscle cells, even though skeletal muscle is responsible for the majority of glucose uptake in response to insulin (13). To investigate if the exocyst also regulates GLUT4 trafficking, glucose uptake, and glucose homeostasis in skeletal muscle, we analyzed the insulin response in vitro in a skeletal myoblast culture.
This study is the first to demonstrate that the exocyst trafficking complex is necessary for insulin-induced glucose uptake in skeletal muscle cells. We show that exocyst subunits, EXOC1, EXOC5, and EXOC7, colocalize in the perinuclear region with GSVs in a basal, unstimulated state, and that the subunits associate with GLUT4-containing vesicles during their insulin-induced translocation to the plasma membrane. Our data suggest that the exocyst possibly has a role in GSV translocation to the myoblast plasma membrane, as exocyst subunits show an increased association with GSVs upon insulin signal. It is likely that, similar to adipocytes (10), small GTPases known to interact with exocyst subunits upon insulin induction, such as RalA, coordinate the exocyst complex, GSVs, and translocation machinery in skeletal muscle cells as well.
After leaving their storage depots and approaching the plasma membrane, new sets of signaling molecules target GSVs to specific regions of the plasma membrane. In adipocytes, insulin signaling leads to the activation of the small GTPase TC10 and its association with EXOC7 (44), which directly binds to the plasma membrane through its interaction with phosphatidylinositol 4,5-bisphosphate (22). According to our data, the relative protein levels of exocyst subunits EXOC1, EXOC5, and EXOC7 are increased in the plasma membrane fraction in response to insulin treatment in L6-GLUT4myc myoblasts. This enrichment is paralleled by the increase in GLUT4myc in the plasma membrane fraction. Furthermore, we demonstrate that insulin triggers an increase in exocyst subunit association, suggesting holocomplex assembly. Our results are the first to indicate that the exocyst complex is triggered to assemble and is also recruited to the skeletal muscle cell’s membrane in response to insulin. Furthermore, this induced assembly of the exocyst complex coincides with GLUT4 vesicle delivery.
We also showed that treatment with the exocyst inhibitor, ES2, significantly impaired membrane recruitment of the EXOC1, EXOC5, and EXOC7 exocyst components in response to insulin. ES2 was initially identified through a plant-based screen of small chemical inhibitors as a compound that impacts cell membrane trafficking (51). It targets exocyst member EXOC7 not only in plants, but also in mammalian cells and is the first known small molecule inhibitor of the exocyst complex (51). This previous work showed an intracellular compartmental accumulation of GFP-rExo70, a GFP-tagged version of EXOC7 in HeLa cells. However, we found that ES2 treatment does not result in a similar subcellular accumulation of EXOC7 or exocyst subunits EXOC1 and EXOC5 in myoblasts. This may be due to an altered functionality or response of the GFP-tagged EXOC7 to ES2 treatment. In mammalian cell culture, GFP-tagging the subunits of exocyst SC2 other than EXOC7 (i.e., EXOC5, EXOC6, and EXOC8) has proven challenging, as the tagged subunits are often incapable of complex formation with the endogenous subunits and thus disrupt exocyst function (1). ES2 treatment alone did significantly increase exocyst subunit assembly, compared with baseline. However, this increase was less than the effect of insulin induction. Since ES2 binds to the EXOC7 subunit, this suggests ES2 may cause a conformation shift in EXOC7 that encourages interaction with other subunit members, but prevents exocyst docking or release activity.
Insulin treatment in the presence of ES2 successfully triggered signaling pathway activation, suggesting that the inhibitor does not impair insulin signaling. ES2 treatment of both L6-GLUT4myc myoblasts and differentiated myotubes diminished the membrane delivery of the insulin-responsive GLUT4 transporter and impaired cellular glucose uptake in myoblasts. It is, therefore, likely that, in skeletal muscle cells, the assembly of the exocyst holocomplex at the plasma membrane is necessary for GLUT4 vesicle docking and exocytosis. In addition, ES2 treatment appears to impact basal glucose uptake as well, since ES2 alone decreased basal 2-DG uptake in myoblasts. This may be due to either an effect of ES2 on GLUT1 membrane trafficking, or the involvement of the exocyst complex in GLUT1 recycling. Recent work in Caenorhabditis elegans demonstrated a role for the exocyst subunit EXOC5 in the endosomal recycling of clathrin-independent endocytic pathway cargos, such as GLUT1 (9).
A previous study of the exocyst complex demonstrated that Exoc5 silencing leads to protein degradation of other subunits (54). Heterozygous Exoc5 knockout in L6-GLUT4myc myoblasts resulted in lower protein levels of exocyst subunit EXOC1 and an overall decrease in exocyst activity. This also led to a failure of insulin to induce glucose uptake and was supported by our assessment of cellular metabolism. Using a Seahorse Extracellular Flux Analyzer, we were able to measure changes in glycolysis and mitochondrial activity. We found that, in the presence of insulin, OCRs were higher in all cell lines compared with untreated controls. Of note, compensatory glycolysis in the absence of insulin was significantly lower in one of the ΔExoc5 L6-GLUT4myc clones, which would suggest a decreased glucose flux, independent of insulin action. In addition, compensatory glycolysis was significantly reduced in insulin-treated ΔExoc5 L6-GLUT4myc myoblasts compared with insulin-treated WT cells. These data suggest that the metabolic action of insulin signaling is blunted in the exocyst-deficient skeletal muscle cells, due to the significant decrease in GLUT4-mediated glucose uptake.
Our work thus demonstrates that the EXOC1, EXOC5, and EXOC7 subunits of the octameric exocyst complex not only colocalize with GLUT4myc storage vesicles in skeletal myoblasts, but, in response to insulin, these subunits are recruited to the plasma membrane. Furthermore, disrupted exocyst complex activity due to either general inhibition by ES2 or decreased subunit expression in ΔExoc5 L6-GLUT4myc myoblasts impairs insulin-stimulated GLUT4myc membrane delivery and subsequent glucose uptake. Details of the mechanisms regulating exocyst-mediated GLUT4 delivery downstream of insulin signaling in skeletal muscle remain to be investigated.
Small G proteins are likely to be key upstream effectors of the exocyst regarding site-specific targeting of GSVs in skeletal muscle cells (52). In adipocytes, Rab10 and RalA activate the exocyst complex and are necessary for glucose uptake, and RalA mediates the connection between the exocyst complex and GSVs (10, 43). However, the Rab proteins that are necessary for GLUT4 exocytosis in adipose tissue are different for those that are necessary in skeletal muscle (11, 30, 46). In addition to insulin signaling, contraction-induced signal pathways also use small GTPases to stimulate GLUT4 exocytosis (30). Therefore, how these GTPases might differentially regulate exocyst activity in skeletal muscle during insulin and contraction-induced GLUT4 exocytosis warrants further studies.
Insulin resistance in skeletal muscle is a principle component in the pathophysiology of type 2 diabetes mellitus (14). Treatment of type 2 diabetes mellitus aims at lowering hyperglycemia, and insulin-dependent and -independent approaches that improve peripheral glucose disposal are sought after. It has been proposed that GLUT4-associated proteins could be potential therapeutic targets for diabetes, because they would bypass the need for proper levels of insulin and insulin receptor signaling (33). Transgenic modulation of GLUT4 expression significantly impacts glucose homeostasis, and GLUT4 overexpression in high-fat-diet-fed mice improves insulin sensitivity (3). Therefore, directly increasing exocyst-mediated GLUT4 translocation to the plasma membrane of major metabolic tissues could provide a mechanism to overcome insulin resistance in diabetic and prediabetic patients. Further studies to elucidate the exocyst-mediated GLUT4 trafficking and glucose uptake in skeletal muscle may provide the opportunity to develop a novel therapeutic approach.
GRANTS
This work was supported in part by National Institutes of Health funding (P20-GM-113134 to N. Polgar, P20-GM-103456-06A1-8293 and R01-DK-117308 awarded to B. Fogelgren, and B. A. Fujimoto was supported by 3T32-HL-115505-05S2 awarded to Ralph Shohet, Director of the Center for Cardiovascular Research, Principal Investigator on the T32 grant) and by funds from Bioscience Research Infrastructure Development for Grant Enhancement and Success (G12MD007601 awarded to B. Fogelgren).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
B.A.F., B.F., and N.P. conceived and designed research; B.A.F., M.Y., L.C., A.P.S.P., and N.P. performed experiments; B.A.F., M.Y., L.C., A.P.S.P., M.J.C., and N.P. analyzed data; B.A.F., A.P.S.P., M.J.C., B.F., and N.P. interpreted results of experiments; B.A.F. and N.P. prepared figures; B.A.F. and N.P. drafted manuscript; B.A.F., M.J.C., B.F., and N.P. edited and revised manuscript; B.A.F., M.Y., L.C., A.P.S.P., M.J.C., B.F., and N.P. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. Mariana Gerschenson of the Diabetes Research Center of the University of Hawaii for expert advice using the Seahorse XFe96 Extracellular Flux Analyzer. We also express appreciation to the Microscopy, Imaging and Flow Cytometry Core at the University of Hawaii Cancer Center for assistance and advice with flow cytometry; The Health Sciences Microscopy and Imaging Core for help with confocal microscopy; and the Molecular and Cellular Immunology Core for excellence in cell sorting services.
REFERENCES
- 1.Ahmed SM, Nishida-Fukuda H, Li Y, McDonald WH, Gradinaru CC, Macara IG. Exocyst dynamics during vesicle tethering and fusion. Nat Commun 9: 5140, 2018. [Erratum in Nat Commun 10: 326, 2019.] doi: 10.1038/s41467-018-07467-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allison DB, Paultre F, Maggio C, Mezzitis N, Pi-Sunyer FX. The use of areas under curves in diabetes research. Diabetes Care 18: 245–250, 1995. doi: 10.2337/diacare.18.2.245. [DOI] [PubMed] [Google Scholar]
- 3.Atkinson BJ, Griesel BA, King CD, Josey MA, Olson AL. Moderate GLUT4 overexpression improves insulin sensitivity and fasting triglyceridemia in high-fat diet-fed transgenic mice. Diabetes 62: 2249–2258, 2013. doi: 10.2337/db12-1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bader D, Masaki T, Fischman DA. Immunochemical analysis of myosin heavy chain during avian myogenesis in vivo and in vitro. J Cell Biol 95: 763–770, 1982. doi: 10.1083/jcb.95.3.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bailyes EM, Navé BT, Soos MA, Orr SR, Hayward AC, Siddle K. Insulin receptor/IGF-I receptor hybrids are widely distributed in mammalian tissues: quantification of individual receptor species by selective immunoprecipitation and immunoblotting. Biochem J 327: 209–215, 1997. doi: 10.1042/bj3270209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boucher J, Kleinridders A, Kahn CR. Insulin receptor signaling in normal and insulin-resistant states. Cold Spring Harb Perspect Biol 6: a009191, 2014. doi: 10.1101/cshperspect.a009191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brüning JC, Michael MD, Winnay JN, Hayashi T, Hörsch D, Accili D, Goodyear LJ, Kahn CR. A muscle-specific insulin receptor knockout exhibits features of the metabolic syndrome of NIDDM without altering glucose tolerance. Mol Cell 2: 559–569, 1998. doi: 10.1016/S1097-2765(00)80155-0. [DOI] [PubMed] [Google Scholar]
- 8.Cheatham B, Volchuk A, Kahn CR, Wang L, Rhodes CJ, Klip A. Insulin-stimulated translocation of GLUT4 glucose transporters requires SNARE-complex proteins. Proc Natl Acad Sci USA 93: 15169–15173, 1996. doi: 10.1073/pnas.93.26.15169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen S, Li L, Li J, Liu B, Zhu X, Zheng L, Zhang R, Xu T. SEC-10 and RAB-10 coordinate basolateral recycling of clathrin-independent cargo through endosomal tubules in Caenorhabditis elegans. Proc Natl Acad Sci USA 111: 15432–15437, 2014. doi: 10.1073/pnas.1408327111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen XW, Leto D, Chiang SH, Wang Q, Saltiel AR. Activation of RalA is required for insulin-stimulated Glut4 trafficking to the plasma membrane via the exocyst and the motor protein Myo1c. Dev Cell 13: 391–404, 2007. doi: 10.1016/j.devcel.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 11.Chen Y, Wang Y, Zhang J, Deng Y, Jiang L, Song E, Wu XS, Hammer JA, Xu T, Lippincott-Schwartz J. Rab10 and myosin-Va mediate insulin-stimulated GLUT4 storage vesicle translocation in adipocytes. J Cell Biol 198: 545–560, 2012. doi: 10.1083/jcb.201111091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cong LN, Chen H, Li Y, Zhou L, McGibbon MA, Taylor SI, Quon MJ. Physiological role of Akt in insulin-stimulated translocation of GLUT4 in transfected rat adipose cells. Mol Endocrinol 11: 1881–1890, 1997. doi: 10.1210/mend.11.13.0027. [DOI] [PubMed] [Google Scholar]
- 13.DeFronzo RA, Jacot E, Jequier E, Maeder E, Wahren J, Felber JP. The effect of insulin on the disposal of intravenous glucose. Results from indirect calorimetry and hepatic and femoral venous catheterization. Diabetes 30: 1000–1007, 1981. doi: 10.2337/diab.30.12.1000. [DOI] [PubMed] [Google Scholar]
- 14.DeFronzo RA, Tripathy D. Skeletal muscle insulin resistance is the primary defect in type 2 diabetes. Diabetes Care 32, Suppl 2: S157–S163, 2009. doi: 10.2337/dc09-S302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ewart MA, Clarke M, Kane S, Chamberlain LH, Gould GW. Evidence for a role of the exocyst in insulin-stimulated Glut4 trafficking in 3T3-L1 adipocytes. J Biol Chem 280: 3812–3816, 2005. doi: 10.1074/jbc.M409928200. [DOI] [PubMed] [Google Scholar]
- 16.Fogelgren B, Lin SY, Zuo X, Jaffe KM, Park KM, Reichert RJ, Bell PD, Burdine RD, Lipschutz JH. The exocyst protein Sec10 interacts with polycystin-2 and knockdown causes PKD-phenotypes. PLoS Genet 7: e1001361, 2011. doi: 10.1371/journal.pgen.1001361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fogelgren B, Polgar N, Lui VH, Lee AJ, Tamashiro KK, Napoli JA, Walton CB, Zuo X, Lipschutz JH. Urothelial defects from targeted inactivation of exocyst Sec10 in mice cause ureteropelvic junction obstructions. PLoS One 10: e0129346, 2015. doi: 10.1371/journal.pone.0129346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fogelgren B, Yang S, Sharp IC, Huckstep OJ, Ma W, Somponpun SJ, Carlson EC, Uyehara CFT, Lozanoff S. Deficiency in Six2 during prenatal development is associated with reduced nephron number, chronic renal failure, and hypertension in Br/+ adult mice. Am J Physiol Renal Physiol 296: F1166–F1178, 2009. doi: 10.1152/ajprenal.90550.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Freestone D, Cater MA, Ackland ML, Paterson D, Howard DL, de Jonge MD, Michalczyk A. Copper and lactational hormones influence the CTR1 copper transporter in PMC42-LA mammary epithelial cell culture models. J Nutr Biochem 25: 377–387, 2014. doi: 10.1016/j.jnutbio.2013.11.011. [DOI] [PubMed] [Google Scholar]
- 20.Germain MA, Chatel-Chaix L, Gagné B, Bonneil É, Thibault P, Pradezynski F, de Chassey B, Meyniel-Schicklin L, Lotteau V, Baril M, Lamarre D. Elucidating novel hepatitis C virus-host interactions using combined mass spectrometry and functional genomics approaches. Mol Cell Proteomics 13: 184–203, 2014. doi: 10.1074/mcp.M113.030155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guo W, Roth D, Walch-Solimena C, Novick P. The exocyst is an effector for Sec4p, targeting secretory vesicles to sites of exocytosis. EMBO J 18: 1071–1080, 1999. doi: 10.1093/emboj/18.4.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.He B, Xi F, Zhang X, Zhang J, Guo W. Exo70 interacts with phospholipids and mediates the targeting of the exocyst to the plasma membrane. EMBO J 26: 4053–4065, 2007. doi: 10.1038/sj.emboj.7601834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Inoue M, Chang L, Hwang J, Chiang SH, Saltiel AR. The exocyst complex is required for targeting of Glut4 to the plasma membrane by insulin. Nature 422: 629–633, 2003. doi: 10.1038/nature01533. [DOI] [PubMed] [Google Scholar]
- 24.Inoue M, Chiang SH, Chang L, Chen XW, Saltiel AR. Compartmentalization of the exocyst complex in lipid rafts controls Glut4 vesicle tethering. Mol Biol Cell 17: 2303–2311, 2006. doi: 10.1091/mbc.e06-01-0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.James DE, Brown R, Navarro J, Pilch PF. Insulin-regulatable tissues express a unique insulin-sensitive glucose transport protein. Nature 333: 183–185, 1988. doi: 10.1038/333183a0. [DOI] [PubMed] [Google Scholar]
- 26.Krook A, Roth RA, Jiang XJ, Zierath JR, Wallberg-Henriksson H. Insulin-stimulated Akt kinase activity is reduced in skeletal muscle from NIDDM subjects. Diabetes 47: 1281–1286, 1998. doi: 10.2337/diab.47.8.1281. [DOI] [PubMed] [Google Scholar]
- 27.Kruszynska YT, Worrall DS, Ofrecio J, Frias JP, Macaraeg G, Olefsky JM. Fatty acid-induced insulin resistance: decreased muscle PI3K activation but unchanged Akt phosphorylation. J Clin Endocrinol Metab 87: 226–234, 2002. doi: 10.1210/jcem.87.1.8187. [DOI] [PubMed] [Google Scholar]
- 28.Leto D, Saltiel AR. Regulation of glucose transport by insulin: traffic control of GLUT4. Nat Rev Mol Cell Biol 13: 383–396, 2012. doi: 10.1038/nrm3351. [DOI] [PubMed] [Google Scholar]
- 29.Li J, Zhang S, Hu Q, Zhang K, Jin J, Zheng X, Yin Z, Wang X. The NKD1/Rac1 feedback loop regulates the invasion and migration ability of hepatocarcinoma cells. Sci Rep 6: 26971, 2016. doi: 10.1038/srep26971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li Z, Yue Y, Hu F, Zhang C, Ma X, Li N, Qiu L, Fu M, Chen L, Yao Z, Bilan PJ, Klip A, Niu W. Electrical pulse stimulation induces GLUT4 glucose transporter translocation in C2C12 myotubes that depends on Rab8A, Rab13 and Rab14. Am J Physiol Endocrinol Metab 314: E478–E493, 2018. doi: 10.1152/ajpendo.00103.2017. [DOI] [PubMed] [Google Scholar]
- 31.Liu X, Feng R, Du L. The role of enoyl-CoA hydratase short chain 1 and peroxiredoxin 3 in PP2-induced apoptosis in human breast cancer MCF-7 cells. FEBS Lett 584: 3185–3192, 2010. doi: 10.1016/j.febslet.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 32.Lu IL, Chen C, Tung CY, Chen HH, Pan JP, Chang CH, Cheng JS, Chen YA, Wang CH, Huang CW, Kang YN, Chang HY, Li LL, Chang KP, Shih YH, Lin CH, Kwan SY, Tsai JW. Identification of genes associated with cortical malformation using a transposon-mediated somatic mutagenesis screen in mice. Nat Commun 9: 2498, 2018. doi: 10.1038/s41467-018-04880-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Michael LF, Wu Z, Cheatham RB, Puigserver P, Adelmant G, Lehman JJ, Kelly DP, Spiegelman BM. Restoration of insulin-sensitive glucose transporter (GLUT4) gene expression in muscle cells by the transcriptional coactivator PGC-1. Proc Natl Acad Sci USA 98: 3820–3825, 2001. doi: 10.1073/pnas.061035098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Möller K, Sigurbjornsdottir S, Arnthorsson AO, Pogenberg V, Dilshat R, Fock V, Brynjolfsdottir SH, Bindesboll C, Bessadottir M, Ogmundsdottir HM, Simonsen A, Larue L, Wilmanns M, Thorsson V, Steingrimsson E, Ogmundsdottir MH. MITF has a central role in regulating starvation-induced autophagy in melanoma. Sci Rep 9: 1055, 2019. doi: 10.1038/s41598-018-37522-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mukerji J, Olivieri KC, Misra V, Agopian KA, Gabuzda D. Proteomic analysis of HIV-1 Nef cellular binding partners reveals a role for exocyst complex proteins in mediating enhancement of intercellular nanotube formation. Retrovirology 9: 33, 2012. doi: 10.1186/1742-4690-9-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nelson VL, Jiang YP, Dickman KG, Ballou LM, Lin RZ. Adipose tissue insulin resistance due to loss of PI3K p110α leads to decreased energy expenditure and obesity. Am J Physiol Endocrinol Metab 306: E1205–E1216, 2014. doi: 10.1152/ajpendo.00625.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Niu W, Huang C, Nawaz Z, Levy M, Somwar R, Li D, Bilan PJ, Klip A. Maturation of the regulation of GLUT4 activity by p38 MAPK during L6 cell myogenesis. J Biol Chem 278: 17953–17962, 2003. doi: 10.1074/jbc.M211136200. [DOI] [PubMed] [Google Scholar]
- 38.Piper RC, Hess LJ, James DE. Differential sorting of two glucose transporters expressed in insulin-sensitive cells. Am J Physiol Cell Physiol 260: C570–C580, 1991. doi: 10.1152/ajpcell.1991.260.3.C570. [DOI] [PubMed] [Google Scholar]
- 39.Polgar N, Fogelgren B. Regulation of cell polarity by exocyst-mediated trafficking. Cold Spring Harb Perspect Biol 10: a031401, 2018. doi: 10.1101/cshperspect.a031401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Polgar N, Lee AJ, Lui VH, Napoli JA, Fogelgren B. The exocyst gene Sec10 regulates renal epithelial monolayer homeostasis and apoptotic sensitivity. Am J Physiol Cell Physiol 309: C190–C201, 2015. doi: 10.1152/ajpcell.00011.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Randhawa VK, Bilan PJ, Khayat ZA, Daneman N, Liu Z, Ramlal T, Volchuk A, Peng XR, Coppola T, Regazzi R, Trimble WS, Klip A. VAMP2, but not VAMP3/cellubrevin, mediates insulin-dependent incorporation of GLUT4 into the plasma membrane of L6 myoblasts. Mol Biol Cell 11: 2403–2417, 2000. doi: 10.1091/mbc.11.7.2403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rebello CJ, Greenway FL, Lau FH, Lin Y, Stephens JM, Johnson WD, Coulter AA. Naringenin promotes thermogenic gene expression in human white adipose tissue. Obesity (Silver Spring) 27: 103–111, 2019. doi: 10.1002/oby.22352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sano H, Peck GR, Blachon S, Lienhard GE. A potential link between insulin signaling and GLUT4 translocation: Association of Rab10-GTP with the exocyst subunit Exoc6/6b. Biochem Biophys Res Commun 465: 601–605, 2015. doi: 10.1016/j.bbrc.2015.08.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Satoh S, Nishimura H, Clark AE, Kozka IJ, Vannucci SJ, Simpson IA, Quon MJ, Cushman SW, Holman GD. Use of bismannose photolabel to elucidate insulin-regulated GLUT4 subcellular trafficking kinetics in rat adipose cells. Evidence that exocytosis is a critical site of hormone action. J Biol Chem 268: 17820–17829, 1993. [PubMed] [Google Scholar]
- 45.Söderberg O, Leuchowius KJ, Gullberg M, Jarvius M, Weibrecht I, Larsson LG, Landegren U. Characterizing proteins and their interactions in cells and tissues using the in situ proximity ligation assay. Methods 45: 227–232, 2008. doi: 10.1016/j.ymeth.2008.06.014. [DOI] [PubMed] [Google Scholar]
- 46.Sun Y, Bilan PJ, Liu Z, Klip A. Rab8A and Rab13 are activated by insulin and regulate GLUT4 translocation in muscle cells. Proc Natl Acad Sci USA 107: 19909–19914, 2010. doi: 10.1073/pnas.1009523107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ting AE, Hazuka CD, Hsu SC, Kirk MD, Bean AJ, Scheller RH. rSec6 and rSec8, mammalian homologs of yeast proteins essential for secretion. Proc Natl Acad Sci USA 92: 9613–9617, 1995. doi: 10.1073/pnas.92.21.9613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Uhm M, Bazuine M, Zhao P, Chiang SH, Xiong T, Karunanithi S, Chang L, Saltiel AR. Phosphorylation of the exocyst protein Exo84 by TBK1 promotes insulin-stimulated GLUT4 trafficking. Sci Signal 10: eaah5085, 2017. doi: 10.1126/scisignal.aah5085. [DOI] [PubMed] [Google Scholar]
- 49.Wang Q, Khayat Z, Kishi K, Ebina Y, Klip A. GLUT4 translocation by insulin in intact muscle cells: detection by a fast and quantitative assay. FEBS Lett 427: 193–197, 1998. doi: 10.1016/S0014-5793(98)00423-2. [DOI] [PubMed] [Google Scholar]
- 50.Yan X, Xun M, Dou X, Wu L, Zhang F, Zheng J. Activation of Na+-K+-ATPase with DRm217 attenuates oxidative stress-induced myocardial cell injury via closing Na+-K+-ATPase/Src/Ros amplifier. Apoptosis 22: 531–543, 2017. doi: 10.1007/s10495-016-1342-2. [DOI] [PubMed] [Google Scholar]
- 51.Zhang C, Brown MQ, van de Ven W, Zhang ZM, Wu B, Young MC, Synek L, Borchardt D, Harrison R, Pan S, Luo N, Huang YMM, Ghang YJ, Ung N, Li R, Isley J, Morikis D, Song J, Guo W, Hooley RJ, Chang CEA, Yang Z, Zarsky V, Muday GK, Hicks GR, Raikhel NV. Endosidin2 targets conserved exocyst complex subunit EXO70 to inhibit exocytosis. Proc Natl Acad Sci USA 113: E41–E50, 2016. doi: 10.1073/pnas.1521248112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang XM, Ellis S, Sriratana A, Mitchell CA, Rowe T. Sec15 is an effector for the Rab11 GTPase in mammalian cells. J Biol Chem 279: 43027–43034, 2004. doi: 10.1074/jbc.M402264200. [DOI] [PubMed] [Google Scholar]
- 53.Zhu Z, Yue Q, Xie J, Zhang S, He W, Bai S, Tian S, Zhang Y, Xiong M, Sun Z, Huang C, Li Y, Zheng K, Ye L. Rapamycin-mediated mTOR inhibition impairs silencing of sex chromosomes and the pachytene piRNA pathway in the mouse testis. Aging (Albany NY) 11: 185–208, 2019. doi: 10.18632/aging.101740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zuo X, Guo W, Lipschutz JH. The exocyst protein Sec10 is necessary for primary ciliogenesis and cystogenesis in vitro. Mol Biol Cell 20: 2522–2529, 2009. doi: 10.1091/mbc.e08-07-0772. [DOI] [PMC free article] [PubMed] [Google Scholar]