Abstract
Metformin beneficially impacts several aspects of metabolic syndrome including dysglycemia, obesity, and liver dysfunction, thus making it a widely used frontline treatment for early-stage type 2 diabetes, which is associated with these disorders. Several mechanisms of action for metformin have been proposed, including that it acts as an anti-inflammatory agent, possibly as a result of its impact on intestinal microbiota. In accord with this possibility, we observed herein that, in mice with diet-induced metabolic syndrome, metformin impacts the gut microbiota by preventing its encroachment upon the host, a feature of metabolic syndrome in mice and humans. However, the ability of metformin to beneficially impact metabolic syndrome in mice was not markedly altered by reduction or elimination of gut microbiota, achieved by the use of antibiotics or germfree mice. Although reducing or eliminating microbiota by itself suppressed diet-induced dysglycemia, other features of metabolic syndrome including obesity, hepatic steatosis, and low-grade inflammation remained suppressed by metformin in the presence or absence of gut microbiota. These results support a role for anti-inflammatory activity of metformin, irrespective of gut microbiota, in driving some of the beneficial impacts of this drug on metabolic syndrome.
Keywords: antibiotics, germ free, high-fat diet, metabolic syndrome, metformin, microbiota, steatosis
INTRODUCTION
The marked worldwide increased prevalence of type 2 diabetes (T2D) is thought to be driven, in large part, by increased prevalence of obesity (10). Obesity promotes an array of metabolic disorders collectively referred to as metabolic syndrome, whose features include hypertension, hyperlipidemia, and, most importantly, insulin resistance, which is the central and defining cause of T2D. Metformin (dimethyldiguanide), discovered in 1922 via investigating the ability of the herb Galega officinalis (goat’s rue) to lower blood glucose, is widely used as a frontline treatment for T2D, especially T2D associated with obesity, in part because of its ability to beneficially impact multiple parameters of metabolic syndrome, including obesity and liver dysfunction (1). Several mechanisms by which metformin alleviates metabolic syndrome have been proposed, including inhibition of mitochondrial function via respiratory chain complex I or glycerophosphate dehydrogenase, activation of 5′ AMP-activated protein kinase (AMPK), and amelioration of glucagon-induced cAMP. Moreover, several studies suggest a central role for gut microbiota in mediating metformin’s amelioration of metabolic syndrome.
The notion that metformin’s action is mediated by impacts on gut microbiota is supported by reports that, in both mice and humans, metformin alters microbiota composition to make it more resembling of microbiota of healthy hosts (13). Furthermore, transfer of microbiota from metformin-treated mice was observed to improve metabolic parameters in aged mice, suggesting that changes in microbiota play a functional role in its beneficial metabolic effects (26). A range of potential means by which select alterations in microbiota by metformin might ameliorate metabolic syndrome have been proposed, including impacting sensing of glucose in the small intestine (4), direct alteration of metabolism of ingested food so as to increase bacterial fermentation (11), and alteration of bile acids (15).
More generally, we hypothesize that metformin’s restoration of a healthy microbiota composition might reduce the microbiota’s capacity to induce host inflammation, which occurs in metabolic syndrome and is known to promote this disorder (6). Our observations that, in both mice and humans, dysglycemia is associated with reduced bacterial-epithelial distance, a state referred to as “microbiota encroachment,” suggests that alterations in microbiota composition and/or gene expression can influence microbiota localization, which may influence low-grade inflammation and, consequently, metabolic syndrome (8, 9). Hence, we hypothesized that metformin’s impact on microbiota composition might reduce microbiota encroachment, consequently ameliorating metabolic syndrome. In accord with this hypothesis, together with previous observations reporting that metformin results in beneficial impacts on gut microbiota (26, 29), we observed herein that metformin induced changes in microbiota composition that are associated with alleviation of microbiota encroachment, low-grade inflammation, and metabolic syndrome. However, in contrast to our hypothesis, we failed to demonstrate that the ability of metformin to alleviate metabolic syndrome required the presence of microbiota. In part, this observation may reflect that one of the central parameters of the metabolic syndrome that is treated by metformin, namely dysglycemia, is itself ameliorated by reducing or eliminating microbiota, thus obscuring metformin’s potential to improve glycemic control (5). Nonetheless, our results argue that metformin may ameliorate metabolic syndrome by reducing adiposity and/or low-grade inflammation per se rather than acting directly on gut microbiota.
EXPERIMENTAL PROCEDURES
Mice and High-Fat Diet Administration
Male, 4- to 6-wk-old C57BL/6 mice were purchased from Jackson Laboratory (Bar Harbor, ME) and maintained at Georgia State University (Atlanta, GA) under institutionally approved protocols (Institutional Animal Care and Use Committee no. A18006), housed five mice per cage with ALPHA-dri bedding (Shepherd Specialty Papers) and nestlets (Ancare), and fed ad libitum. Mice were fed standard grain-based chow (Purina Laboratory Diet 5001), which comprises relatively unrefined ingredients, and given 2 wk to allow for microbiota stabilization. Where indicated, mice were then administered a diet composed of 60% kcal from fat (Research Diet, D12492) for 8–12 wk. For germ-free (GF) experiments, we utilized an irradiated version of this diet (10–20 KGy) that achieves sterilization.
Metformin Administration
Metformin, 1,1-dimethyl biguanide hydrochloride (Sigma-Aldrich) was administered via intraperitoneal injection or orally concurrent with high-fat diet (HFD) exposure. For the intraperitoneal route, 100 μL of normal saline (NS; vehicle control) or NS containing an amount of metformin that resulted in 100 mg metformin/kg body wt, which equates to a 20-g mouse receiving 6 mg per day, was administered. For oral administration, metformin was added to drinking water at a concentration of 2 or 10 mg/mL, which was calculated to result in ingestion of 6 or 30 mg per day based on 3 mL of water consumption per day. These doses were selected as being typical from reading several papers (3, 27, 28). Although higher than those used in humans, they are approximately similar to those used in other studies in mice.
Microbiota Reduction
Antibiotics.
Mice were administered drinking water containing ampicillin (1.0 g/L) and neomycin (0.5 g/L) upon HFD feeding, and this was continued until mice were euthanized.
Germ-free mice.
Germ-free, wild-type C57BL/6 male mice were purchased from Taconic Inc. and maintained in IsoCages (Techniplast USA, West Chester, PA), using procedures which we have shown maintain a germfree state (31). To assure sterility of metformin, after dilution it was filtered through Nalgene Rapid-Flow Sterile Disposable Filter Units with polyethersulfone membrane of 0.2 μm units to protect against bacterial contamination. Similarly filtered water was administered to the control group. GF mice were fed autoclaved chow or a γ-irradiation (10–20 KGy) HFD. To verify that the HFD was truly sterilized by the irradiation, we measured levels of bacterial DNA in feces before and after feeding of this diet as previously described (31). After 8 wk of metformin treatment, mice were fasted overnight, removed from IsoCages, and immediately euthanized. Lastly, we note that the germfree experiment was done in parallel with another study focused on comparing chow-fed and HFD-fed mice and thus those mice served as control groups for metformin-treated mice (25).
Fasting Blood Glucose Measurement
Mice were placed in clean cages, fasted for 5 h or overnight as indicated, and blood glucose was measured by the Nova Max Glucose Meter and expressed in mg/dL.
Magnetic Resonance Imaging
Bruker MiniSpec MRI Body Composition Measurement was used for MRI fat percentage using open source MiniSpec software. Mice were weighed, placed into restrainer, and inserted into assay tube; data were collected for 2 min. Data are expressed as percentage of fat.
Bacteria Localization by Fluorescence in situ Hybridization Staining
Microbiota localization was performed as previously described (7, 31).
Microbiota Analysis by 16S rRNA Gene Sequencing
Microbiota composition was performed as previously described (7). Unprocessed sequencing data are deposited in the European Nucleotide Archive under accession number ERP117168.
RNA Isolation and Quantitative RT-PCR
Total RNA was extracted from transverse colonic tissues, liver, and adiposity using TRIzol (Invitrogen, Carlsbad, CA) according to the manufacturer’s protocol. Quantitative RT-PCR was performed using the iScript One-Step RT-PCR Kit with SYBR Green (Bio-Rad, Hercules, CA) in a CFX96 apparatus (Bio-Rad) with the primers listed in transcript levels quantified by normalization of each amplicon to housekeeping gene 36B4: 36B4, 5′-TCCAGGCTTTGGGCATCA-3′ and 5′CTTTATTCAGCTGCACATCACTCAGA-3′ from Invitrogen; CXCL1, 5′-TTGTGCGAAAAGAAGTGCAG-3′ and 5′-TACAAACACAGCCTCCCACA-3′ from Invitrogen; IL-6, 5′-GTGGCTAAGGACCAAGACCA-3′ and 5′-GGTTTGCCGAGTAGACCTCA-3′ from Invitrogen, and TNF-α, 5′-CGAGTGACAAGCCTGTAGCC-3′ and 5′-CATGCCGTTGGCCAGGA-3′from Invitrogen.
Quantitation of Microbiota by Quantitative PCR
Total DNA was isolated via QIAamp DNA Stool Mini Kit (Qiagen). DNA was then subjected to quantitative PCR using QuantiFast SYBR Green PCR kit (Bio-Rad) with universal 16S rRNA primers (515 F GTGCCAGCMGCCGCGGTAA and 806 R GGACTACHVGGGTWTCTAA) to quantitate total bacteria. Results are expressed as bacteria number per mg of stool, using a standard curve generated from dilutions of a purified culture of Escherichia coli. Proteobacteria analysis used the following primers purchased from Invitrogen: γ877F (5′-GCTAACGCATTAAGTRYCCCG and 5′ γ1066R GCCATGCRGCAAATGTCT-3′f), β979F (5′-AACGCGAAAAACCTTACCTACC and 5′-TGCCCTTTCGTAGCAACTAGTG-3′f), and ε940F (5′-TAGGCTTGACATTGATAGAATC and 5′-CTTACGAAGGCAGTCTCCTTA-3′).
Western Blot
Because of the high lipid content of livers from HFD-fed mice, samples were extracted using a high volume of lysis buffer (500 μL/100 mg), permitting easy separation of lipid and proteinaceous fractions, thus allowing protein quantification and SDS-PAGE immunoblotting to proceed using anti-phospho-AMPKα monoclonal and anti-AMPKα rabbit polyclonal antibodies from Cell Signaling at 1:200 dilutions.
Histology
Mouse colon, adipose tissue, and liver were fixed in 10% buffered formalin at room temperature before embedding in paraffin. Tissues were sectioned at 5-mm thickness and stained with hematoxylin-eosin. Images were collected by software OLYMPUS VS-ASW virtual slide system. Steatosis was graded 1–5 wherein a score of 1 was assigned for no discernable steatosis (relative to chow-fed mice), and scores of 2–5, on a 0.5 interval, were used to grade modest to severe steatosis.
ELISAs
Serum from overnight-fasted mice was assayed for macrophage chemotactic protein-1 (MCP-1) via a Duoset ELISA purchased from R&D Systems. Serum insulin was measured with an ELISA kit (cat. no. EZRMI-13K) purchased from Millipore, Inc. Fecal lipocalin-2 was measured as previously described.
Quantification and Statistical Analysis
Data were expressed as means ± SE. Statistical significance was analyzed by unpaired Student’s t test (GraphPad Prism 7). Differences between experimental groups were considered significant at P < 0.05.
RESULTS
Metformin’s Amelioration of Diet-Induced Metabolic Syndrome Is Associated with Beneficial Impacts on Gut Microbiota
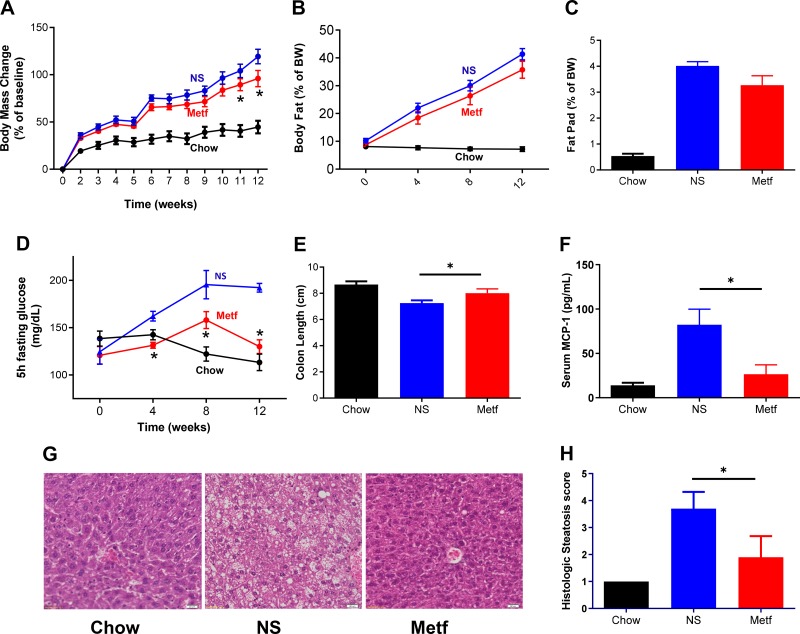
Although in humans metformin is generally administered orally, most studies of this drug in mice have relied upon systemic administration, typically intraperitoneal administration, in which relatively moderate doses of this drug are able to ameliorate various aspects of metabolic syndrome (17, 18). Hence, our initial approach was to examine the extent to which IP administration of metformin ameliorated metabolic syndrome and microbiota composition in mice fed an obesogenic Western-style HFD, which is high in saturated fats and low in fiber. Mice (C57BL/6 males) were maintained on standard grain-based rodent chow or, at 6 wk of age, administered an HFD ad libitum and then 2 wk later given daily injections of vehicle (normal saline) or metformin. Such metformin treatment ameliorated various other aspects of metabolic syndrome including obesity, as assessed by body weight and adiposity (Fig. 1, A–C and Supplemental Fig. S1; all Supplemental Material is available at https://doi.org/10.6084/m9.figshare.9794729.v1), dysglycemia (Fig. 1D), and hepatic steatosis (Fig. 1, G and H). Moreover, in accord with the general notion that metabolic syndrome is characterized by low-grade inflammation, which might be impacted by metformin (directly and/or via metformin’s impacts on gut microbiota), we observed that metformin ameliorated indices of HFD-induced low-grade inflammation, including colon shortening (Fig. 1E) and increases in levels of serum MCP-1 (Fig. 1F), which associates with diet-induced obesity (23, 31). Together, these results indicate that, in accord with previous studies, intraperitoneal administration of metformin ameliorated HFD-induced low-grade inflammation and metabolic syndrome.
Fig. 1.
Metformin (Metf) ameliorates high-fat diet (HFD)-induced metabolic syndrome. Male C57 BL/6 mice (6 wk old; n = 10 per condition) were maintained on a standard grain-based chow diet or administered a Western-style, low-fiber HFD for 12 wk. HFD-fed mice were injected intraperitoneally with normal saline (NS) or metformin (metf) daily from 2 wk post-diet exposure until being euthanized. A and B: weight and body composition were monitored. C: epididymal fat pad pass measured post-euthanasia. D: mice were fasted for 5 h every 4 wk, and blood glucose was measured. E: colon length measured post-euthanasia. F: serum macrophage chemotactic protein-1 (MCP-1) measured by ELISA following 8 wk HFD exposure and overnight fasting. G: representative hematoxylin-eosin-stained liver sections. H: histologic scoring of liver sections for extent lipid accumulation. Data are shown as means ± SE. *Statistical significance (P < 0.05) by Student’s t test for HFD/metformin vs. HFD/NS. BW, body weight; MCP-1, macrophage chemotactic protein-1.
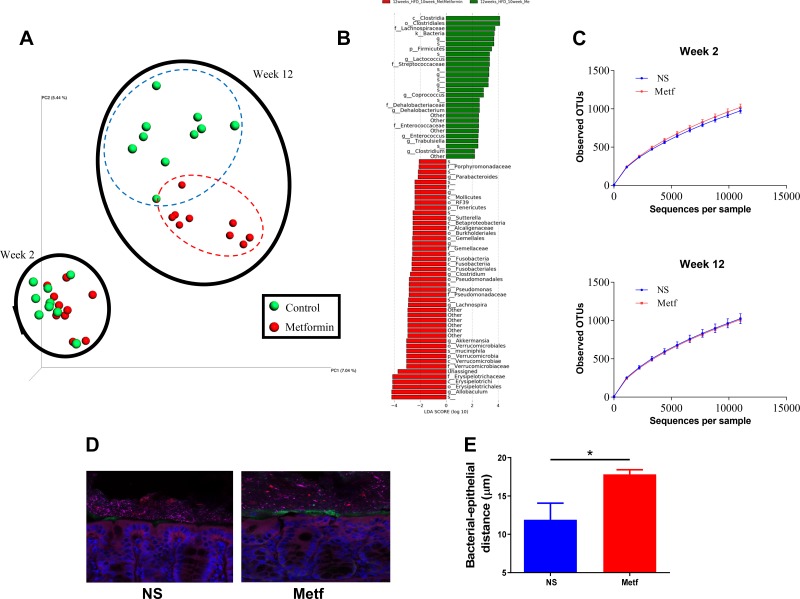
Next, we considered the extent to which metformin impacted gut microbiota composition, envisaging that metformin administered intraperitoneally might reach the intestinal lumen via bile trafficked by enterohepatic circulation and/or that metformin might indirectly impact the microbiota. Feces were collected from HFD-fed mice immediately before and 10 wk following intraperitoneal administration of vehicle or metformin. We utilized two cages of mice (n = 5 per cage) to reduce chances of observing differences that primarily reflected the tendency of mice to have microbiomes that resemble those of their cage-mates because of their coprophagic habits. Fecal microbiota composition was assessed via sequencing of 16S ribosomal RNA genes. Overall, untargeted assessment of microbiota composition was assessed by plotting of principal coordinates of Uni-Frac values. Prior to treatment, overall microbiota composition did not differ significantly between mice that had been assigned to the metformin and vehicle groups (Fig. 2A, wk 2). In contrast, at 10 wk following metformin treatment, there was a marked distinction in overall microbiota composition between metformin- and vehicle-treated mice (Fig. 2A, wk 12). The extent of this change was such that it could be visualized even amidst the large change in microbiota composition that resulted from prolonged exposure to an HFD. Such changes in microbiota composition reflected enrichment and depletion of a variety of taxa (Fig. 2B and Supplemental Fig. S2) without an impact on species richness (i.e., α-diversity) (Fig. 2C). One general consequence of alterations in the host-microbiota relationship in patients with diabetes and mice with metabolic syndrome is encroachment of gut microbiota into the inner mucus layer, which might result in such bacteria promoting low-grade inflammation (9). Moreover, metformin increases goblet cells, which increase mucus thickness (22). Hence, we measured by confocal microscopy the extent to which metformin impacted bacterial-epithelial distance. We observed that metformin treatment resulted in greater bacterial-epithelial distance (Fig. 2, D and E). Together, these results indicate that metformin treatment of HFD-fed mice resulted in changes in microbiota composition and localization that may reduce low-grade inflammation and are thus in accord with previous studies that suggest that the beneficial actions of this drug may be mediated, in part, by gut microbiota.
Fig. 2.
Metformin (Metf) impacts microbiota composition and reduces microbiota encroachment. Male C57 BL/6 mice (6 wk old; n = 10 per condition) were fed a high-fat diet (HFD) for 12 wk. HFD-fed mice were injected intraperitoneally with normal saline (NS) or metformin daily from 2 wk post-diet exposure until being euthanized. A: comparison of fecal microbiome composition via principal coordinate analysis of the UniFrac coordinates following wk 2 and wk 12; 0 and 10 wk post-metformin treatment. B: histogram shows linear discriminant analysis (LDA). C: rarefaction curves of number of observed operational taxonomic units (OTUs) based on sequence similarities for control and treatment group at wk 2 and wk 12. D: confocal microscopy analysis of microbiota localization; mucin 2 (green), actin (purple), bacteria (red), and DNA (blue). Scale bar = 20 μm. E: distance of closest bacteria to intestinal epithelial cells per condition over 5 high-powered fields per mouse. Pictures are representative of 10 biological replicates. Data are means ± SD; *P < 0.05 by Student’s t test.
Metformin Ameliorates Metabolic Syndrome in the Absence of a Microbiota
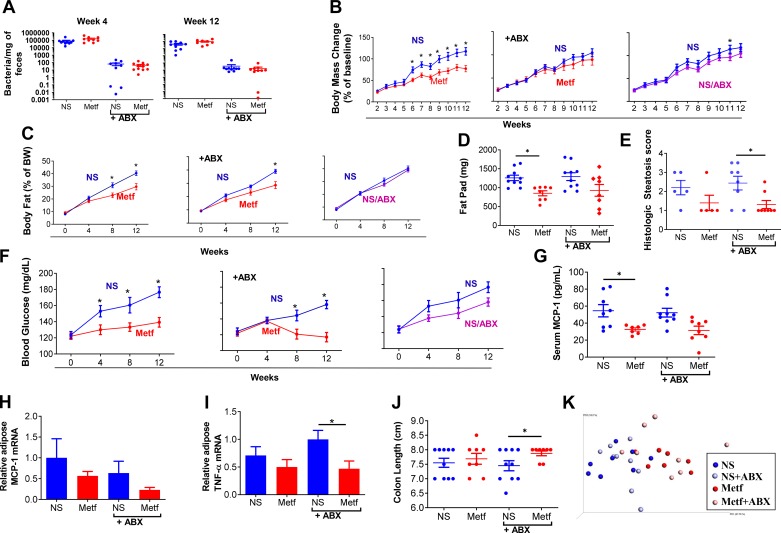
The above-described and previously reported (26, 29) impacts of metformin on gut microbiota can be envisaged to contribute to and/or reflect metformin’s ability to ameliorate metabolic syndrome. Hence, we next examined if reducing levels of gut microbiota via antibiotic treatment would impact the ability of this drug to ameliorate HFD-induced metabolic syndrome. Metformin or vehicle was administered to HFD-fed mice as described above or under conditions wherein vehicle- and metformin-treated mice were subjected to a mixture of antibiotics, namely ampicillin and neomycin, administered via their drinking water. As expected, antibiotics quickly resulted in a more than 3-log reduction in the total amount of bacteria per milligram of feces that was maintained, irrespective of metformin treatment, throughout the course of the 12-wk period of administration utilized (Fig. 3A). Although metformin did not reduce HFD-induced weight gain under conditions of antibiotic administration (Fig. 3B), this may have reflected that the weight gain itself was reduced by antibiotics. In any case, the extent to which metformin suppressed adiposity, as measured by MRI (Fig. 3C) or post-euthanasia fat pad mass (Fig. 3D), was not markedly impacted by antibiotic treatment, which itself had only a minor impact on increased adiposity, nor did the antibiotics prevent metformin from ameliorating hepatic steatosis (Fig. 3E and Supplemental Fig. S3). Moreover, whereas antibiotics moderately reduced fasting blood glucose in HFD-fed mice, particularly at later time points following HFD exposure, metformin still lowered this parameter, particularly at these later time points (Fig. 3F). Nor did antibiotic administration prevent metformin from ameliorating indices of low-grade inflammation that are known to result from exposure to HFD. Specifically, metformin reduced serum MCP-1 (Fig. 3G) and adipose levels of MCP-1 and TNFα mRNA (Fig. 3H and I), irrespective of antibiotic treatment. Additionally, although the extent to which metformin protected against colon shortening, which is an indicator of inflammation, was variable, it was significant under conditions of antibiotics (Fig. 3J). Lastly, use of Principal Coordinate Analysis to holistically consider all of these metabolic and inflammatory parameters (Fig. 3K) indicated that metformin-treated mice had similar overall metaboinflammatory phenotypes irrespective of antibiotic treatment. Thus, overall, use of antibiotics to markedly reduce gut bacterial loads did not support our original hypothesis that the ability of metformin to ameliorate metabolic syndrome was mediated by its actions on gut microbiota.
Fig. 3.
Amelioration of high-fat diet (HFD)-induced metabolic syndrome in antibiotic-treated mice. Male C57 BL/6 mice (6 wk old; n = 10 per condition) were fed an HFD for 12 wk. HFD-fed mice were injected intraperitoneally with normal saline (NS) or metformin (Metf) daily from 2 wk post-diet exposure until being euthanized, with or without antibiotics (ABX) in drinking water. A: PCR-based quantification of bacterial load adhered to feces on wk 4 and 12. B and C: body weight MRI and analyzing body composition every 4 wk. D: epididymal fat pad pass measured post-euthanasia. E: histologic scoring of section of liver. F: mice were fasted for 5 h every 4 wk, and blood glucose was measured. G: serum macrophage chemotactic protein-1 (MCP-1) measured by ELISA following 12 wk HFD exposure and overnight fasting. H and I: the mRNA was extracted from fat, and the expression level of MCP-1 and TNFα was analyzed by RT-PCR. J: colon length measured post-euthanasia. K: principal component analysis plot combining terminal parameters of weight, fat composition, fat pad mass, blood glucose, MCP-1, and colon length. Data are shown as means ± SE. *Statistical significance (P < 0.05) by Student’s t test for HFD/metformin vs. HFD/NS.
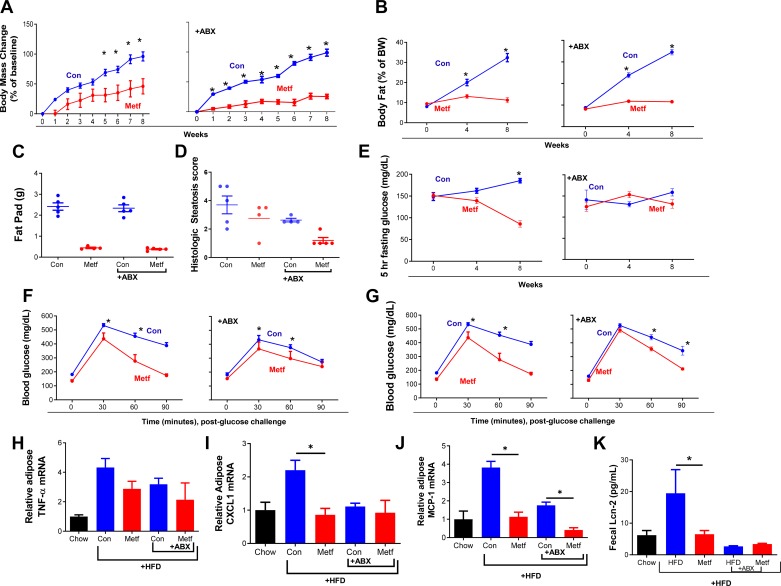
One caveat to the above-described studies is that administering metformin via the IP route may have reduced interaction of this drug with the intestinal mucosa and luminal microbiota. On the one hand, that intraperitoneal metformin altered gut microbiota, and that metformin administered intraperitoneally should reach the intestinal lumen via enterohepatic circulation, argues against the importance of this caveat. On the other hand, given that several studies, albeit largely association based, have implicated an important role for microbiota in mediating metformin’s impact, we sought to develop a means of orally delivering metformin that would ameliorate HFD-induced metabolic syndrome. Hence, we sought to develop an oral dosing regimen in which metformin markedly and reliably ameliorated HFD-induced metabolic syndrome. First, based on published work, we administered metformin in drinking water to deliver a daily dose of 6 mg·day−1·mouse−1 (300 mg/kg body wt) (12). This dose of metformin moderately reduced HFD-metabolic syndrome in Swiss-Webster mice (Supplemental Fig. S4) but had only slight impacts in C57BL/6 mice (Supplemental Fig. S5), in which the above studies and most work in this model has been performed. Hence, we selected a higher dose of oral metformin, 30 mg·day−1·mouse−1, which was well tolerated and clearly reduced many aspects of HFD-induced metabolic syndrome, including weight gain, increased adiposity, and hyperglycemia (Fig. 4). Antibiotics did not reduce such ability of metformin to reduce HFD-induced increases in weight gain, adiposity, and hepatic steatosis (Fig. 4, A–D and Supplemental Fig. S6). In accord with the notion that HFD-induced dysglycemia is itself mediated in part by microbiota, administration of antibiotics ameliorated, by themselves, HFD-induced dysglycemia as assessed by measure of fasting glucose, thus obscuring our ability to measure if metformin would prevent HFD-induced dysglycemia in the absence of microbiota (Fig. 4E). Nonetheless, under antibiotic treatment, metformin still improved glucose tolerance following 3 wk and 8 wk of HFD exposure (Fig. 4, F and G). Ability of metformin to reduce glucose levels was not, irrespective of antibiotics, associated with any adverse effects or general signs of ill health, which can itself lower blood glucose, nor did metformin result in any markers of inflammation that can occur from drug toxicity. Antibiotics did not appear to impact the extent to which metformin reduced low-grade inflammation, in that metformin treatment modestly but broadly reduced expression of a panel of indices of low-grade inflammation (Fig. 4, H–J). Overall, a similar pattern was seen in a distinct strain of mice (Swiss-Webster), in that whereas metformin only modestly reduced HFD-induced metabolic syndrome in these mice, antibiotics did not reduce that effect (Supplemental Fig. S4). Hence, analogous to the case for metformin administered by the intraperitoneal route, use of antibiotics to reduce levels of microbiota did not yield data to support the notion that microbiota play a key role in this drug’s ability to ameliorate metabolic syndrome.
Fig. 4.
Efficacy of oral metformin (Metf) amidst antibiotics. Male C57 BL/6 mice (6 wk old; n = 5 per condition) were maintained on a Western-style low-fiber high-fat diet (HFD) for 8 wk. HFD-fed mice were administered drinking water containing metformin or vehicle control (Con) from wk 1 of study, with or without antibiotics (ABX) in drinking water. A and B: body weightMRI and analyzing body composition. C: epididymal fat pad pass measured post-euthanasia. D: histologic scoring of section of liver. E: mice were fasted for 5 h every 4 wk, and glucose was measured. F and G: glucose measured 0, 30, 60, and 90 min after intraperitoneal injection of glucose at wk 3 and wk 8. H: mRNA extracted from adipose and expression level of macrophage chemotactic protein-1 (MCP)-1. I and J: CXCL1 and TNFα was measured by RT-PCR. K: fecal lipocalin-2 measured by ELISA at 8 wk timepoint. Data are shown as means ± SE. *Statistical significance (P < 0.05) by Student’s t test for HFD/metformin vs. HFD control.
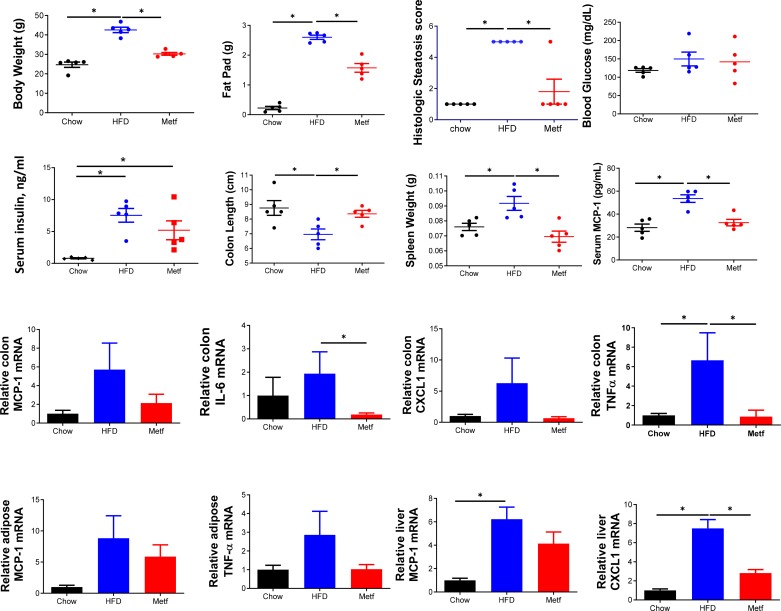
One potential explanation for the inability of antibiotics to suppress metformin’s amelioration of HFD-induced metabolic syndrome is that, despite broad suppression of total bacterial loads, perhaps select bacteria can bloom. We explored this possibility focusing on Proteobacteria, a phylum that we (Fig. 2) and others have observed contains much of the taxa enriched by metformin treatment (13, 26). We observed that whereas antibiotics suppressed increases in β-Proteobacteria, increases in γ- and ε-Proteobacteria were observed in metformin-treated mice amidst antibiotic treatment (Supplemental Fig. S7). Although it is difficult to discern if such changes contributed to metformin’s action in these conditions, this result serves as an example of the general limitation of investigating the role of microbiota via antibiotics. Indeed, antibiotics reduce but do not eliminate gut bacteria and can select for gut bacteria, such as those that populate inner mucus, that may have an outsize impact on low-grade inflammation and hence metabolic syndrome. Hence, we next used germfree mice as a means to examine the extent to which metformin might ameliorate HFD-induced metabolic syndrome in a host completely lacking gut bacteria. A technical limitation to germfree mice is that it is very difficult to track/monitor metabolic parameters without compromising the germfree state. Hence, groups of age-matched germ-free mice were split into three groups, visually assessed to be of similar body mass; and administered autoclaved grain-based chow, irradiated HFD (IR-HFD) (which we’ve shown does not compromise germfree status) or IR-HFD and oral metformin (filter-sterilized) via drinking water (31). In accord with the notion that microbiota may be more important for HFD-induced dysglycemia than obesity per se, under germfree conditions, IR-HFD still induced clear increases in weight and adiposity but induced only minimal dysglycemia (Fig. 5) in accord with our recent work (25). Although such minimal dysglycemia precluded the ability of metformin to correct it, metformin still clearly diminished the extent of adiposity induced by IR-HFD (Fig. 5 and Supplemental Fig. S5), albeit not to the extent observed in conventional mice (Fig. 4C). Metformin also continued to reduce hepatic steatosis in germfree mice (Fig. 5 and Supplemental Fig. S8D). Additionally, metformin-treated mice showed a trend , albeit not statistically significant, toward reduced insulin levels, which, together with the slight trend toward reduced glucose, was in accord with the notion that metformin’s ability to sensitize responsiveness to insulin remained intact. Metformin’s sensitization of insulin signaling is proposed to be related to its activation of AMPK. Hence, we analyzed metformin’s impact on levels of Phospho-AMPK in the livers of HFD-fed regular and GF mice. In accord with widely reported notions that metformin activates AMPK (14) and that AMPK activity is elevated in GF mice (2), we observed induction, albeit variable, of phospho-AMPK in conventional mice and elevated basal phospho-AMPK in GF mice (Supplemental Fig. S8C). We did not observe any additional induction of phospho-AMPK in metformin-treated GF mice. We view these results as being in accord with the notion that activation of phospho-AMPK might be important even in conventional mice but that additional actions of metformin drive its impact in GF mice. More generally, metformin continued to broadly ameliorate an array of indices of low-grade inflammation that were induced by IR-HFD. Specifically, metformin ameliorated colon shortening and reduced expression of MCP-1, IL-6, CXCL1, and TNFα in colon, liver, and adipose tissue (Fig. 4). Together, these results support the notion that metformin has anti-inflammatory activity, irrespective of gut microbiota, which may not be required for this drug to ameliorate metabolic syndrome.
Fig. 5.
Maintained efficacy of metformin (Metf) in high-fat diet (HFD)-fed germfree mice. Male C57 BL/6 mice (6 wk old; n = 5 per condition) were maintained on autoclaved chow or irradiated HFD (IR-HFD) for 8 wk while being administered drinking water containing metformin or vehicle. All parameters were measured following euthanasia. Body weight and epididymal fat pad. Histologic scoring of section of liver. Mice were fasted overnight, and blood glucose and insulin were measured. The colon length and spleen weight were also measured. Serum macrophage chemotactic protein-1 (MCP-1) was measured by ELISA and overnight fasting. The mRNA was extracted from the colon and expression level of MCP-1, IL-6, CXCL1 and TNFα was analyzed by RT-PCR. The mRNA was extracted from adipose and expression level of MCP-1 and TNFα was analyzed by RT-PCR. The mRNA was extracted from liver and expression level of MCP-1 and CXCL1 was analyzed by RT-PCR. The mRNA was extracted from adipose and expression level of MCP-1 and TNFα was analyzed by RT-PCR. Data are shown as means ± SE. *Statistical significance (P < 0.05) by Student’s t test for HFD/metformin vs. HFD control.
DISCUSSION
Metformin is among the world’s most widely prescribed drugs, largely because of its ability to effectively treat early-stage type 2 diabetes and, moreover, ameliorate many of the metabolic abnormalities, collectively referred to as metabolic syndrome, that are associated with dysglycemia (30). Limitations of metformin include limited potency and some gastrointestinal dysfunction, including nausea and diarrhea, in some patients. Hence, there has long been interest in understanding mechanisms by which metformin acts, as such knowledge might allow the design of better treatments for metabolic syndrome. Although an array of potential mechanisms that contribute to metformin’s action have been proposed, the extent to which such mechanisms are crucial contributors to this drug’s beneficial effects are less clear, nor have direct targets of metformin been convincingly defined.
Considering the recent extensive appreciation that gut microbiota contribute mightily to numerous aspects of host health, including the view promulgated by ourselves and others that microbiota dysbiosis drives low-grade inflammation that promotes metabolic syndrome (6), it is not surprising that multiple research groups hypothesized that metformin might impact gut microbiota in a manner that might contribute to metformin’s beneficial effects (20). In both rodents and humans, metformin’s beneficial effects were associated with alterations in the microbiome that supported this notion (18, 26, 29). However, since the conditions that metformin treats are themselves associated with alterations in gut microbiota composition, such results could also reflect metformin ameliorating metabolic syndrome, resulting in changes in gut microbiota. That the transplant of microbiota from metformin-treated hosts to mice not exposed to this drug conferred beneficial metabolic effects suggests that metformin-induced changes in microbiome are not purely a consequence of treatment benefits (26) but are also consistent with the possibility that such changes are both a cause and consequence of amelioration of metabolic syndrome. Our observation that metformin impacted the microbiota in a manner that reduced its encroachment into the mucus is in accord with this paradigm, but none of these findings address the extent to which the presence of a gut microbiota is necessary for metformin’s beneficial effects.
A central tenet in the microbiota field is that if the microbiota plays a crucial role in the process by which a particular intervention causes a phenotype, that intervention should not induce that phenotype in the absence of microbiota. For example, many interventions that cause colitis in conventional conditions are without effect in germ-free conditions (16). Another example of this tenet is our work showing that synthetic dietary emulsifiers that cause mucus thinning, low-grade inflammation, and metabolic syndrome in conventional conditions cause none of these in germ-free mice, indicating that these events are fully microbiota-dependent (7). Such appreciation of the importance of the microbiota in low-grade inflammation and metabolic syndrome led us to hypothesize that metformin would fail to impact metabolic syndrome in the absence of microbiota. However, our data did not support this hypothesis. Rather, neither antibiotic nor germ-free approaches prevented the ability of metformin to ameliorate metabolic syndrome. In particular, irrespective of whether microbiota were present, metformin attenuated HFD-induced low-grade inflammation, glucose intolerance, adiposity, and hepatic steatosis. Although many of the impacts of metformin in micro-ablated mice could be the result of reduced adiposity, the observation that it also prevented indices of low-grade inflammation in the gut are in accord with suggestions that metformin’s anti-inflammatory activity is central to its beneficial effects. Indeed, metformin is now recognized to have fairly broad anti-inflammatory effects (21), and our results herein are consistent with the possibility that such effects contribute to metformin’s protection against diet-induced metabolic syndrome.
A major caveat of our results is that one of the central features of metabolic syndrome, namely dysglycemia, was itself clearly reduced by reducing or eliminating microbiota, thus obscuring our ability to examine how metformin impacted it in these conditions. Another caveat of our findings is that effective doses of metformin in mice are higher than those used in humans, and therefore might possibly be mediated by distinct mechanisms. Indeed, although mice exhibited no behavioral, histopathologic, or other indicators of any ill effects from oral or intraperitoneal metformin, we cannot rule out the possibility that some type of subtle toxicity reduced adiposity, which led to the reductions in the metabolic/inflammatory parameters we measured. Lastly, we note that the extent to which metformin ameliorated HFD-induced adiposity was less in GF mice relative to antibiotic-treated mice, suggesting that metformin-induced changes in microbiota in antibiotic-treated mice might contribute to its efficacy therein. Such caveats notwithstanding, we submit that the most reasonable and simplest explanation for existing data and our results herein is a scenario wherein metformin’s ability to ameliorate metabolic syndrome results in alterations in gut microbiota that alleviate low-grade inflammation and subsequently further alleviate, and maintain alleviation of, metabolic syndrome. In support of this possibility, we note that other means of inducing dysglycemia, for example islet toxin streptozotocin, also induce microbiota dysbiosis and promote microbial breach of the gut barrier, and interventions that ameliorate such dysglycemia will reduce the associated dysbiosis (19, 24, 29).
The notion that the beneficial impact of metformin on gut microbiota might be indirect does not preclude the possibility that such effects are important to the efficacy of the drug in its real usage, wherein a microbiota is present. Indeed, amelioration of dysbiosis and microbiota encroachment may be critical to the lasting efficacy of this compound. By analogy, a weight loss intervention might facilitate the ability of a person to exercise, which is then critical for sustained efficacy of the intervention. Extending the analogy, transfer of exercise phenotype by itself may, like a fecal microbiota transplant, recapitulate many of the benefits of the original intervention. Thus, we do not question the conclusion of the array of studies that indicate that metformin ameliorates microbial dysbiosis and that such effects are important to the drug’s beneficial metabolic effects. We suggest that the gut microbiota is not germane to identifying the direct targets and primary mechanism of action of metformin.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants DK-099071 (to A. T. Gewirtz) and DK-083890 (to A. T. Gewirtz). B. Chassaing is supported by a Career Development Award from the Crohn's and Colitis Foundation of America.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
A.A. and A.T.G. conceived and designed research; A.A., J.Z., H.T., and B.C. performed experiments; A.A., J.Z., H.T., and B.C. analyzed data; A.A., B.C., and A.T.G. interpreted results of experiments; A.A., J.Z., and B.C. prepared figures; A.A., B.C., and A.T.G. drafted manuscript; J.Z., B.C., and A.T.G. edited and revised manuscript; A.A., J.Z., H.T., B.C., and A.T.G. approved final version of manuscript.
REFERENCES
- 1.An H, He L. Current understanding of metformin effect on the control of hyperglycemia in diabetes. J Endocrinol 228: R97–R106, 2016. doi: 10.1530/JOE-15-0447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bäckhed F, Manchester JK, Semenkovich CF, Gordon JI. Mechanisms underlying the resistance to diet-induced obesity in germ-free mice. Proc Natl Acad Sci USA 104: 979–984, 2007. doi: 10.1073/pnas.0605374104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bailey CJ, Flatt PR, Ewan C. Anorectic effect of metformin in lean and genetically obese hyperglycaemic (ob/ob) mice. Arch Int Pharmacodyn Ther 282: 233–239, 1986. [PubMed] [Google Scholar]
- 4.Bauer PV, Duca FA, Waise TMZ, Rasmussen BA, Abraham MA, Dranse HJ, Puri A, O’Brien CA, Lam TKT. Metformin alters upper small intestinal microbiota that impact a glucose-SGLT1-sensing glucoregulatory pathway. Cell Metab 27: 101–117.e5, 2018. doi: 10.1016/j.cmet.2017.09.019. [DOI] [PubMed] [Google Scholar]
- 5.Cani PD, Bibiloni R, Knauf C, Waget A, Neyrinck AM, Delzenne NM, Burcelin R. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes 57: 1470–1481, 2008. doi: 10.2337/db07-1403. [DOI] [PubMed] [Google Scholar]
- 6.Carvalho FA, Aitken JD, Vijay-Kumar M, Gewirtz AT. Toll-like receptor-gut microbiota interactions: perturb at your own risk! Annu Rev Physiol 74: 177–198, 2012. doi: 10.1146/annurev-physiol-020911-153330. [DOI] [PubMed] [Google Scholar]
- 7.Chassaing B, Koren O, Goodrich JK, Poole AC, Srinivasan S, Ley RE, Gewirtz AT. Dietary emulsifiers impact the mouse gut microbiota promoting colitis and metabolic syndrome. Nature 519: 92–96, 2015. [Erratum in: Nature 536: 238, 2016.] doi: 10.1038/nature14232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chassaing B, Ley RE, Gewirtz AT. Intestinal epithelial cell toll-like receptor 5 regulates the intestinal microbiota to prevent low-grade inflammation and metabolic syndrome in mice. Gastroenterology 147: 1363–1377.e17, 2014. doi: 10.1053/j.gastro.2014.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chassaing B, Raja SM, Lewis JD, Srinivasan S, Gewirtz AT. Colonic microbiota encroachment correlates with dysglycemia in humans. Cell Mol Gastroenterol Hepatol 4: 205–221, 2017. doi: 10.1016/j.jcmgh.2017.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chatterjee S, Khunti K, Davies MJ. Type 2 diabetes. Lancet 389: 2239–2251, 2017. doi: 10.1016/S0140-6736(17)30058-2. [DOI] [PubMed] [Google Scholar]
- 11.de la Cuesta-Zuluaga J, Mueller NT, Corrales-Agudelo V, Velásquez-Mejía EP, Carmona JA, Abad JM, Escobar JS. Metformin is associated with higher relative abundance of mucin-degrading Akkermansia muciniphila and several short-chain fatty acid-producing microbiota in the gut. Diabetes Care 40: 54–62, 2017. doi: 10.2337/dc16-1324. [DOI] [PubMed] [Google Scholar]
- 12.Duan Q, Song P, Ding Y, Zou MH. Activation of AMP-activated protein kinase by metformin ablates angiotensin II-induced endoplasmic reticulum stress and hypertension in mice in vivo. Br J Pharmacol 174: 2140–2151, 2017. doi: 10.1111/bph.13833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Forslund K, Hildebrand F, Nielsen T, Falony G, Le Chatelier E, Sunagawa S, Prifti E, Vieira-Silva S, Gudmundsdottir V, Pedersen HK, Arumugam M, Kristiansen K, Voigt AY, Vestergaard H, Hercog R, Costea PI, Kultima JR, Li J, Jørgensen T, Levenez F, Dore J, Nielsen HB, Brunak S, Raes J, Hansen T, Wang J, Ehrlich SD, Bork P, Pedersen O; MetaHIT consortium . Disentangling type 2 diabetes and metformin treatment signatures in the human gut microbiota. Nature 528: 262–266, 2015. [Erratum in: Nature 545: 116, 2017.] doi: 10.1038/nature15766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fryer LG, Parbu-Patel A, Carling D. The anti-diabetic drugs rosiglitazone and metformin stimulate AMP-activated protein kinase through distinct signaling pathways. J Biol Chem 277: 25226–25232, 2002. doi: 10.1074/jbc.M202489200. [DOI] [PubMed] [Google Scholar]
- 15.Guo GL, Xie W. Metformin action through the microbiome and bile acids. Nat Med 24: 1789–1790, 2018. doi: 10.1038/s41591-018-0273-6. [DOI] [PubMed] [Google Scholar]
- 16.Hansen AK, Hansen CH, Krych L, Nielsen DS. Impact of the gut microbiota on rodent models of human disease. World J Gastroenterol 20: 17727–17736, 2014. doi: 10.3748/wjg.v20.i47.17727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee H, Ko G. Effect of metformin on metabolic improvement and gut microbiota. Appl Environ Microbiol 80: 5935–5943, 2014. doi: 10.1128/AEM.01357-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee H, Lee Y, Kim J, An J, Lee S, Kong H, Song Y, Lee CK, Kim K. Modulation of the gut microbiota by metformin improves metabolic profiles in aged obese mice. Gut Microbes 9: 155–165, 2018. doi: 10.1080/19490976.2017.1405209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Patterson E, Marques TM, O’Sullivan O, Fitzgerald P, Fitzgerald GF, Cotter PD, Dinan TG, Cryan JF, Stanton C, Ross RP. Streptozotocin-induced type-1-diabetes disease onset in Sprague-Dawley rats is associated with an altered intestinal microbiota composition and decreased diversity. Microbiology 161: 182–193, 2015. doi: 10.1099/mic.0.082610-0. [DOI] [PubMed] [Google Scholar]
- 20.Rodriguez J, Hiel S, Delzenne NM. Metformin: old friend, new ways of action-implication of the gut microbiome? Curr Opin Clin Nutr Metab Care 21: 294–301, 2018. doi: 10.1097/MCO.0000000000000468. [DOI] [PubMed] [Google Scholar]
- 21.Schuiveling M, Vazirpanah N, Radstake TRDJ, Zimmermann M, Broen JCA. Metformin, a new era for an old drug in the treatment of immune mediated disease? Curr Drug Targets 19: 945–959, 2018. doi: 10.2174/1389450118666170613081730. [DOI] [PubMed] [Google Scholar]
- 22.Shin NR, Lee JC, Lee HY, Kim MS, Whon TW, Lee MS, Bae JW. An increase in the Akkermansia spp. population induced by metformin treatment improves glucose homeostasis in diet-induced obese mice. Gut 63: 727–735, 2014. doi: 10.1136/gutjnl-2012-303839. [DOI] [PubMed] [Google Scholar]
- 23.Tateya S, Tamori Y, Kawaguchi T, Kanda H, Kasuga M. An increase in the circulating concentration of monocyte chemoattractant protein-1 elicits systemic insulin resistance irrespective of adipose tissue inflammation in mice. Endocrinology 151: 971–979, 2010. doi: 10.1210/en.2009-0926. [DOI] [PubMed] [Google Scholar]
- 24.Thaiss CA, Levy M, Grosheva I, Zheng D, Soffer E, Blacher E, Braverman S, Tengeler AC, Barak O, Elazar M, Ben-Zeev R, Lehavi-Regev D, Katz MN, Pevsner-Fischer M, Gertler A, Halpern Z, Harmelin A, Aamar S, Serradas P, Grosfeld A, Shapiro H, Geiger B, Elinav E. Hyperglycemia drives intestinal barrier dysfunction and risk for enteric infection. Science 359: 1376–1383, 2018. doi: 10.1126/science.aar3318. [DOI] [PubMed] [Google Scholar]
- 25.Tran H, Bretin A, Adeshirlarijaney A, San Yeoh BS, Vijay-Kumar M, Zou J, Denning TL, Chassaing B, Gewirtz AT. “Western-diet”-induced adipose inflammation requires a complex gut microbiota. Cell Mol Gastroenterol Hepatol. In press. doi: 10.1016/j.jcmgh.2019.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu H, Esteve E, Tremaroli V, Khan MT, Caesar R, Mannerås-Holm L, Ståhlman M, Olsson LM, Serino M, Planas-Fèlix M, Xifra G, Mercader JM, Torrents D, Burcelin R, Ricart W, Perkins R, Fernàndez-Real JM, Bäckhed F. Metformin alters the gut microbiome of individuals with treatment-naive type 2 diabetes, contributing to the therapeutic effects of the drug. Nat Med 23: 850–858, 2017. doi: 10.1038/nm.4345. [DOI] [PubMed] [Google Scholar]
- 27.Zhang XR, Fu XJ, Zhu DS, Zhang CZ, Hou S, Li M, Yang XH. Salidroside-regulated lipid metabolism with down-regulation of miR-370 in type 2 diabetic mice. Eur J Pharmacol 779: 46–52, 2016. doi: 10.1016/j.ejphar.2016.03.011. [DOI] [PubMed] [Google Scholar]
- 28.Zhao W, Li A, Feng X, Hou T, Liu K, Liu B, Zhang N. Metformin and resveratrol ameliorate muscle insulin resistance through preventing lipolysis and inflammation in hypoxic adipose tissue. Cell Signal 28: 1401–1411, 2016. doi: 10.1016/j.cellsig.2016.06.018. [DOI] [PubMed] [Google Scholar]
- 29.Zhong MW, Liu SZ, Zhang GY, Zhang X, Liu T, Hu SY. Alterations in gut microbiota during remission and recurrence of diabetes after duodenal-jejunal bypass in rats. World J Gastroenterol 22: 6706–6715, 2016. doi: 10.3748/wjg.v22.i29.6706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhou J, Massey S, Story D, Li L. Metformin: an old drug with new applications. Int J Mol Sci 19: E2863, 2018. doi: 10.3390/ijms19102863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zou J, Chassaing B, Singh V, Pellizzon M, Ricci M, Fythe MD, Kumar MV, Gewirtz AT. Fiber-mediated nourishment of gut microbiota protects against diet-induced obesity by restoring IL-22-mediated colonic health. Cell Host Microbe 23: 41–53.e4, 2018. doi: 10.1016/j.chom.2017.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]