Abstract
Macrophages are heterogenous cells of the innate immune system that can fluidly modulate their phenotype to respond to their local microenvironment. They are found throughout the renal compartments, where they contribute to homeostasis and function. However, renal injury activates molecular pathways that initially stimulate differentiation of macrophages into a proinflammatory M1 phenotype. Later in the course of healing, abundant apoptotic debris and anti-inflammatory cytokines induce the production of anti-inflammatory M2 macrophages, which contribute to tissue regeneration and repair. Thus, the dynamic balance of M1 and M2 populations may outline the burden of inflammation and process of tissue repair that define renal outcomes, which has been the impetus for therapeutic efforts targeting macrophages. This review will discuss the role of these phenotypes in the progression of chronic renal injury, potential pathogenic mechanisms, and the promise of macrophage-based therapeutic applications for chronic kidney disease.
Keywords: chronic kidney disease, fibrosis, inflammation, macrophages, tissue repair
INTRODUCTION
Inflammation is a prominent feature of chronic kidney disease (CKD) and plays a leading role in the progression of renal injury and increased cardiovascular mortality of these patients (42). Various effectors, including renal parenchymal release of damage-associated molecular patterns (40) (DAMPs), upregulation of proinflammatory transcription factors such as NF-κB (1) and subsequent proinflammatory cytokine pathways (44) [e.g., TNF-α and chemokine (C-C motif) ligand 2], and renal infiltration by immune cells (26), are responsible for the increased inflammation in CKD.
Macrophages are members of the innate immune system that arise from circulating monocytes or from populations of self-renewing resident tissue macrophages (17). Although they were initially characterized by their phagocytic activity (15), an equally important function of macrophages is their ability to regulate tissue homeostasis and promote immune tolerance (16). Macrophages change their phenotype in response to their local environment, leading to the development of a classification scheme used in vivo and in vitro, as described elsewhere (43). Briefly, macrophages are described as M1 or M2. Although this classification scheme may be overly simplistic (35), given that macrophage polarization is akin to a spectrum with M1 and M2 on opposite ends, the M1-M2 phenotype balance can provide valuable insights into the inflammatory status and pathophysiology of renal disease.
In the normal response to injury, damaged tissue releases DAMPs, leading to the differentiation of macrophages to the M1 phenotype, which is characterized by the release of proinflammatory cytokines and increased microbicidal and tumoricidal activity (24, 32, 39). Days to weeks after the initial injury, macrophages repolarize to the M2 phenotype, defined by release of anti-inflammatory mediators, growth factors, and proangiogenic cytokines to facilitate the healing process (24, 32, 39). Macrophages have gained attention in recent years for their role in the progression to CKD. In the kidney, M1 macrophages are considered deleterious, as they sustain the proinflammatory environment, leading to the progression of renal injury and development of fibrosis (19, 21, 26). In contrast, M2 macrophages are associated with renal repair and functional recovery (20, 32). This review will highlight mechanisms by which these cells may contribute to the pathogenesis of chronic renal diseases. Furthermore, we will briefly discuss the exciting potential of targeting macrophages as a therapeutic strategy for kidney disease.
MACROPHAGE POLARIZATION AND THE KIDNEY
Mounting evidence shows the capacity of renal macrophages to fluidly switch to either the proinflammatory or tissue-healing phenotype, depending on the microenvironment (27, 31, 39). The mechanisms underlying the switch from the M1 to M2 phenotype are multifactorial and not fully understood and are briefly discussed below.
Inflammatory Macrophages and Renal Injury
M1 macrophages act as first responders against pathogens and activate the immune system (43). However, their broad-reaching, rapid response comes at the cost of target specificity. Indeed, factors produced by M1 macrophages, such as TNF-α (46) and reactive oxygen species (3), may cause endothelial dysfunction (30) and death of healthy renal cells (51). If unchecked, persistent M1 infiltration and associated inflammation contribute to the decline of renal function (2, 11) and, ultimately, fibrosis (27, 36).
In the normal course of renal injury and repair, initiation of the healing response attenuates the release of DAMPs from the renal parenchyma. It is hypothesized that this downward trend in DAMP production may play a role in the shift to the M2 phenotype, as DAMPs are strong signals for maintaining M1 polarization (38). However, some renal recovery is required to initiate this response, as progressive injury leads to the sustained release of DAMPs and M1 polarization [as shown in CKD (6) and ischemia-reperfusion injury models (26)]. On the other hand, acute depletion of macrophages after renal injury, during the M1-predominated response, has been shown to be beneficial in immunologic-, toxin-, ischemia-, and hypertension-mediated renal injury models (8, 14, 21, 47). A recent study on novel mouse knockout of IL-1 receptor-associated kinase M, a macrophage-specific inhibitor of Toll-like receptor and IL-1 signaling, showed that this single knockout significantly increased M1 macrophages in the ischemia-reperfusion injured kidney and contributed to tubular loss, glomerulosclerosis, and interstitial fibrosis (26). Furthermore, IL-1 receptor-associated kinase M knockout prevented renal accumulation of M2 macrophages after acute injury, suggesting a role in the switch from the M1 to M2 phenotype (26). Together, these data suggest that M1 macrophages are active players in the pathogenesis of renal injury and may produce a vicious cycle, where damage leads to the release of DAMPs, which subsequently stimulate Toll-like receptors to further stimulate M1 polarization. Sufficient initial tissue healing to allow breakthrough of the M2 phenotype may thus be an important factor in recovery from acute injury versus progression to CKD.
Trophic Macrophages and Renal Repair
Macrophages have been traditionally seen as injurious mediators in the pathogenesis of kidney disease (22, 41). Indeed, previous studies have shown a protective effect of macrophage depletion acutely after renal injury in multiple models (9, 14, 21, 23, 45). However, other studies have shown that depletion of macrophages in the subacute stages of renal injury, during the peak of M2 infiltration, may exacerbate tubular damage and fibrosis, supporting the regenerative role of M2 macrophages (12, 27, 37). These disparate effects suggest that the timing for macrophage removal may be a crucial component of such strategies to boost renal repair.
Recent studies uncovering underlying mechanisms of M2-induced renal repair have identified a few promising candidates. One example is Wnt7b, a ligand of the Wnt pathway that drives the formation of the tubular epithelium during development. Wnt7b is reexpressed by macrophages after renal injury and signals through tubular cells to initiate cell proliferation and repair of the tubular basement membrane (28).
Fibrosis is a well-established hallmark of CKD, one that is hypothesized to be indicative of irreversible loss of function, as fibrotic tissue may physically obstruct microvascular proliferation and tubular regeneration (10). Mannose receptor C type 2 (MRC2), a fibrolytic cell surface receptor expressed on M2 macrophages, binds and internalizes collagen. MRC2-deficient mice developed worse fibrosis in the unilateral ureteral obstruction model (29). A recent study (26) has also supported the conclusion that M1 macrophages directly contribute to development of fibrosis by sustaining the renal inflammatory milieu and showed that when inflammation is suppressed, M2 macrophage infiltration does not associate with fibrosis, regardless of disease duration. Together, these findings suggest that the switch to M2 macrophages involves alteration of multiple pathways, leading to a trophic, anti-inflammatory effect on the renal parenchyma. Nevertheless, the net effect of M2 macrophages on fibrosis is still an area in need of research, as some studies have implicated chronic M2 infiltration in facilitating renal fibrogenesis where inflammation is severe (4, 22, 41).
T Cells and Macrophage Polarization
T cells of the adaptive immune system act as both controllers and effectors of macrophage function in CKD. T cells have been shown to increase macrophage recruitment into the kidney, which is a driving mechanism of injury in immune-mediated forms of kidney disease (18). T cells do not appear to directly influence M1/M2 polarization, as this differentiation has been shown to occur in recombination-activating gene-deficient mice (33). Conversely, M2 macrophages have been shown to induce regulatory T cell differentiation both in vitro and in vivo, resulting in reduced injury and fibrosis in an adriamycin-induced nephropathy model (5). The interplay between the adaptive immune system and macrophage polarization is a complex topic that warrants further study.
MACROPHAGE-BASED THERAPEUTICS FOR KIDNEY DISEASE: AN OPPORTUNITY
Myriad studies have shown both pathogenic and protective roles of macrophages in kidney disease of diverse etiology (7, 25, 27, 41), suggesting that macrophages may serve as targets to ameliorate renal injury via manipulation of their polarization.
Genetic Manipulation
Genetically engineered macrophages have been developed to modulate their effector function. Macrophages transduced with the gene for an IL-1 receptor antagonist reduced glomerular and tubular fibrosis in unilateral ureteral obstruction and anti-glomerular basement membrane glomerulonephritis models (52). Another approach is use of adenoviral transduction to induce expression of IL-4 or IL-10 (anti-inflammatory cytokines associated with M2 polarization) in bone marrow-derived macrophages (50). The macrophages were then transferred into kidneys of rats with nephrotoxic nephritis, where they homed to inflamed glomeruli, suppressed immune cell infiltration, and reduced the degree of glomerular injury (50). Finally, genetic manipulation of macrophages to induce expression of IκB, which inhibits nuclear translocation of NF-κB, reduced inflammatory IL-12, TNF-α, and nitric oxide signaling in classically activated M1 macrophages. This was associated with attenuated fibrosis, less crescent formation, and improved albuminuria in a nephrotoxic nephritis model (49).
A major advantage of genetic approaches is the induction of long-lasting changes in phenotype and cytokine expression in environments that would normally favor inflammatory polarization. Furthermore, natural chemotactic triggers cause macrophages to travel to areas of injured renal parenchyma, allowing the induced gene product to be secreted directly at the site of damage. However, major hurdles must be overcome before this therapeutic strategy is translated to clinical use; chief among these are ensuring the safety of viral transduction and reliably controlling the degree of gene suppression or induction.
Cytokine Manipulation
Administration of cytokines is a relatively straightforward method of modulating macrophage phenotype. Macrophages isolated from mouse spleens and exposed to IL-4 and/or IL-13 in vitro to induce the M2 phenotype were injected in an adriamycin-induced nephropathy model (48), resulting in reduced expression of proinflammatory cytokines and attenuated inflammatory markers in renal immune cells. Similar improvements have been observed after administration of M2 macrophages in models of ischemia-reperfusion injury and diabetic-induced renal injury (27). In tumor models, VEGF signaling through VEGF receptor type 1 has been shown to promote macrophage infiltration and differentiation into an angiogenic M2-like phenotype (34). Furthermore, our laboratory has recently found in a swine model of CKD (6) that renal therapy using a biopolymer-stabilized form of VEGF significantly shifts the phenotype of renal-infiltrating macrophages from indolamine 2,3-dioxygenase-expressing M1 to MRC1-expressing M2 without reducing the total number of macrophages (13). Interestingly, these M2 macrophages densely express VEGF and are associated with improved renal hemodynamics, microvascular integrity, and attenuation of renal fibrosis, suggesting that this phenotypic change after VEGF therapy may play a role in renal recovery in CKD (13). Future studies of macrophage depletion and reconstitution may be required to further establish whether a renal shift to the M2 phenotype is feasible, translatable, and functionally significant as a therapeutic strategy.
CONCLUSIONS
Regardless of etiology, renal parenchymal injury induces the release of chemotactic factors, renal infiltration of macrophages, and polarization to the M1 phenotype. M1 macrophages are characterized by the production of inflammatory cytokines and, if unopposed, may lead to tubular cell death, endothelial dysfunction, inflammation, and fibrosis while suppressing M2 repolarization. Thus, a sustained abundant M1 infiltration can contribute to the progression of renal injury (Fig. 1). Promising strategies to alter effector phenotype at the site of renal injury via genetic or cytokine manipulation of macrophages are being developed. However, current evidence warrants additional studies to better understand the specific roles and niches of macrophage phenotypes and their signaling pathways to unravel their translational application as therapeutic strategies to induce renal recovery.
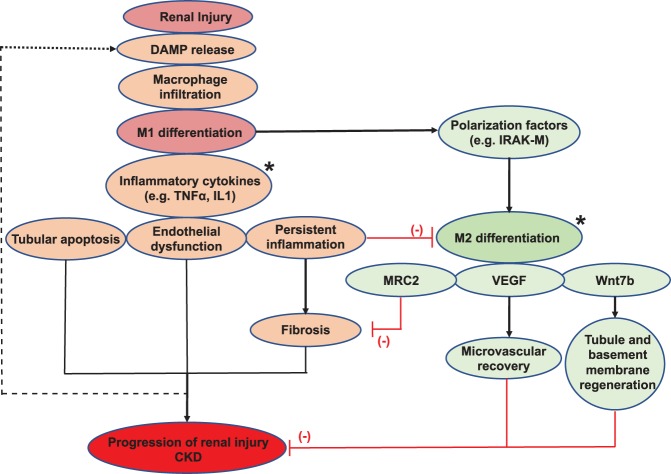
Fig. 1.
A schematic overview of macrophages in progression of renal injury. The initial renal damage leads to the release of chemotactic factors, inducing macrophage infiltration and subsequent differentiation to the M1 phenotype. M1 macrophages release multiple inflammatory cytokines, which drive tubular injury, endothelial dysfunction, and inflammation, leading to fibrosis and progression of renal injury toward chronic kidney disease (CKD) if left unchecked. In the normal course of healing, macrophages shift to the M2 phenotype and participate in the repair process through production of VEGF and Wnt7b (favoring microvascular recovery and parenchymal regeneration respectively, red lines) and surface expression of mannose receptor C type 2 (MRC2) to reduce the progression of fibrosis (red lines) and stimulate fibrolysis. M2 differentiation is favored by polarizing factors produced by M1 macrophages, such as IL-1 receptor-associated kinase M (IRAK-M), and inhibited by persistent inflammation favoring M1 polarization. Modulation of macrophage function or polarization state by genetic or cytokine approaches (*potential therapeutic targets) offers new potential therapeutic strategies for improving outcomes in kidney disease. DAMP, damage-associated molecular patterns.
GRANTS
This work was supported by National Institutes of Health Grants R01-HL-095638, P01-HL-51971, and P20-GM-104357 and American Heart Association Grants IPA3417016 and PRE34380274.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.E.E. and A.R.C. conceived and designed research; J.E.E. prepared figures; J.E.E. drafted manuscript; A.R.C. edited and revised manuscript; J.E.E. and A.R.C. approved final version of manuscript.
REFERENCES
- 1.Akchurin OM, Kaskel F. Update on inflammation in chronic kidney disease. Blood Purif 39: 84–92, 2015. doi: 10.1159/000368940. [DOI] [PubMed] [Google Scholar]
- 2.Amano MT, Castoldi A, Andrade-Oliveira V, Latancia MT, Terra FF, Correa-Costa M, Breda CNS, Felizardo RJF, Pereira WO, da Silva MB, Miyagi MYS, Aguiar CF, Hiyane MI, Silva JS, Moura IC, Camara NOS. The lack of PI3Kγ favors M1 macrophage polarization and does not prevent kidney diseases progression. Int Immunopharmacol 64: 151–161, 2018. doi: 10.1016/j.intimp.2018.08.020. [DOI] [PubMed] [Google Scholar]
- 3.Boyce NW, Tipping PG, Holdsworth SR. Glomerular macrophages produce reactive oxygen species in experimental glomerulonephritis. Kidney Int 35: 778–782, 1989. doi: 10.1038/ki.1989.52. [DOI] [PubMed] [Google Scholar]
- 4.Braga TT, Correa-Costa M, Guise YF, Castoldi A, de Oliveira CD, Hyane MI, Cenedeze MA, Teixeira SA, Muscara MN, Perez KR, Cuccovia IM, Pacheco-Silva A, Gonçalves GM, Camara NO. MyD88 signaling pathway is involved in renal fibrosis by favoring a TH2 immune response and activating alternative M2 macrophages. Mol Med 18: 1231–1239, 2012. doi: 10.2119/molmed.2012.00131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cao Q, Wang Y, Zheng D, Sun Y, Wang Y, Lee VW, Zheng G, Tan TK, Ince J, Alexander SI, Harris DC. IL-10/TGFβ-modified macrophages induce regulatory T cells and protect against adriamycin nephrosis. J Am Soc Nephrol 21: 933–942, 2010. doi: 10.1681/ASN.2009060592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chade AR, Williams ML, Engel J, Guise E, Harvey TW. A translational model of chronic kidney disease in swine. Am J Physiol Renal Physiol 315: F364–F373, 2018. doi: 10.1152/ajprenal.00063.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clauss S, Gross O, Kulkarni O, Avila-Ferrufino A, Radomska E, Segerer S, Eulberg D, Klussmann S, Anders HJ. Ccl2/Mcp-1 blockade reduces glomerular and interstitial macrophages but does not ameliorate renal pathology in collagen4A3-deficient mice with autosomal recessive Alport nephropathy. J Pathol 218: 40–47, 2009. doi: 10.1002/path.2505. [DOI] [PubMed] [Google Scholar]
- 8.D’Souza MJ, Oettinger CW, Shah A, Tipping PG, Huang XR, Milton GV. Macrophage depletion by albumin microencapsulated clodronate: attenuation of cytokine release in macrophage-dependent glomerulonephritis. Drug Dev Ind Pharm 25: 591–596, 1999. doi: 10.1081/DDC-100102213. [DOI] [PubMed] [Google Scholar]
- 9.Day YJ, Huang L, Ye H, Linden J, Okusa MD. Renal ischemia-reperfusion injury and adenosine 2A receptor-mediated tissue protection: role of macrophages. Am J Physiol Renal Physiol 288: F722–F731, 2005. doi: 10.1152/ajprenal.00378.2004. [DOI] [PubMed] [Google Scholar]
- 10.Duffield JS. Cellular and molecular mechanisms in kidney fibrosis. J Clin Invest 124: 2299–2306, 2014. doi: 10.1172/JCI72267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eirin A, Zhang X, Zhu XY, Tang H, Jordan KL, Grande JP, Dietz AB, Lerman A, Textor SC, Lerman LO. Renal vein cytokine release as an index of renal parenchymal inflammation in chronic experimental renal artery stenosis. Nephrol Dial Transplant 29: 274–282, 2014. doi: 10.1093/ndt/gft305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eirin A, Zhu XY, Li Z, Ebrahimi B, Zhang X, Tang H, Korsmo MJ, Chade AR, Grande JP, Ward CJ, Simari RD, Lerman A, Textor SC, Lerman LO. Endothelial outgrowth cells shift macrophage phenotype and improve kidney viability in swine renal artery stenosis. Arterioscler Thromb Vasc Biol 33: 1006–1013, 2013. doi: 10.1161/ATVBAHA.113.301164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Engel J, Williams E, Williams ML, Bidwell GL III, Chade AR. Targeted VEGF therapy induces long-term renal recovery in chronic kidney disease via macrophage polarization. Hypertension 74: 1113–1123, 2019. doi: 10.1161/HYPERTENSIONAHA.119.13469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ferenbach DA, Sheldrake TA, Dhaliwal K, Kipari TM, Marson LP, Kluth DC, Hughes J. Macrophage/monocyte depletion by clodronate, but not diphtheria toxin, improves renal ischemia/reperfusion injury in mice. Kidney Int 82: 928–933, 2012. doi: 10.1038/ki.2012.207. [DOI] [PubMed] [Google Scholar]
- 15.Gordon S. Elie Metchnikoff: father of natural immunity. Eur J Immunol 38: 3257–3264, 2008. doi: 10.1002/eji.200838855. [DOI] [PubMed] [Google Scholar]
- 16.Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat Rev Immunol 5: 953–964, 2005. doi: 10.1038/nri1733. [DOI] [PubMed] [Google Scholar]
- 17.Hashimoto D, Chow A, Noizat C, Teo P, Beasley MB, Leboeuf M, Becker CD, See P, Price J, Lucas D, Greter M, Mortha A, Boyer SW, Forsberg EC, Tanaka M, van Rooijen N, García-Sastre A, Stanley ER, Ginhoux F, Frenette PS, Merad M. Tissue-resident macrophages self-maintain locally throughout adult life with minimal contribution from circulating monocytes. Immunity 38: 792–804, 2013. doi: 10.1016/j.immuni.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang XR, Tipping PG, Shuo L, Holdsworth SR. Th1 responsiveness to nephritogenic antigens determines susceptibility to crescentic glomerulonephritis in mice. Kidney Int 51: 94–103, 1997. doi: 10.1038/ki.1997.12. [DOI] [PubMed] [Google Scholar]
- 19.Ikezumi Y, Suzuki T, Hayafuji S, Okubo S, Nikolic-Paterson DJ, Kawachi H, Shimizu F, Uchiyama M. The sialoadhesin (CD169) expressing a macrophage subset in human proliferative glomerulonephritis. Nephrol Dial Transplant 20: 2704–2713, 2005. doi: 10.1093/ndt/gfi105. [DOI] [PubMed] [Google Scholar]
- 20.Jetten N, Verbruggen S, Gijbels MJ, Post MJ, De Winther MP, Donners MM. Anti-inflammatory M2, but not pro-inflammatory M1 macrophages promote angiogenesis in vivo. Angiogenesis 17: 109–118, 2014. doi: 10.1007/s10456-013-9381-6. [DOI] [PubMed] [Google Scholar]
- 21.Jo SK, Sung SA, Cho WY, Go KJ, Kim HK. Macrophages contribute to the initiation of ischaemic acute renal failure in rats. Nephrol Dial Transplant 21: 1231–1239, 2006. doi: 10.1093/ndt/gfk047. [DOI] [PubMed] [Google Scholar]
- 22.Kim MG, Kim SC, Ko YS, Lee HY, Jo SK, Cho W. The role of M2 macrophages in the progression of chronic kidney disease following acute kidney injury. PLoS One 10: e0143961, 2015. doi: 10.1371/journal.pone.0143961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ko GJ, Boo CS, Jo SK, Cho WY, Kim HK. Macrophages contribute to the development of renal fibrosis following ischaemia/reperfusion-induced acute kidney injury. Nephrol Dial Transplant 23: 842–852, 2008. doi: 10.1093/ndt/gfm694. [DOI] [PubMed] [Google Scholar]
- 24.Kon V, Linton MF, Fazio S. Atherosclerosis in chronic kidney disease: the role of macrophages. Nat Rev Nephrol 7: 45–54, 2011. doi: 10.1038/nrneph.2010.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lan HY, Nikolic-Paterson DJ, Mu W, Atkins RC. Local macrophage proliferation in the progression of glomerular and tubulointerstitial injury in rat anti-GBM glomerulonephritis. Kidney Int 48: 753–760, 1995. doi: 10.1038/ki.1995.347. [DOI] [PubMed] [Google Scholar]
- 26.Lech M, Gröbmayr R, Ryu M, Lorenz G, Hartter I, Mulay SR, Susanti HE, Kobayashi KS, Flavell RA, Anders H-J. Macrophage phenotype controls long-term AKI outcomes—kidney regeneration versus atrophy. J Am Soc Nephrol 25: 292–304, 2014. doi: 10.1681/ASN.2013020152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee S, Huen S, Nishio H, Nishio S, Lee HK, Choi BS, Ruhrberg C, Cantley LG. Distinct macrophage phenotypes contribute to kidney injury and repair. J Am Soc Nephrol 22: 317–326, 2011. doi: 10.1681/ASN.2009060615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lin SL, Li B, Rao S, Yeo EJ, Hudson TE, Nowlin BT, Pei H, Chen L, Zheng JJ, Carroll TJ, Pollard JW, McMahon AP, Lang RA, Duffield JS. Macrophage Wnt7b is critical for kidney repair and regeneration. Proc Natl Acad Sci USA 107: 4194–4199, 2010. doi: 10.1073/pnas.0912228107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.López-Guisa JM, Cai X, Collins SJ, Yamaguchi I, Okamura DM, Bugge TH, Isacke CM, Emson CL, Turner SM, Shankland SJ, Eddy AA. Mannose receptor 2 attenuates renal fibrosis. J Am Soc Nephrol 23: 236–251, 2012. doi: 10.1681/ASN.2011030310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ma S, Zhu XY, Eirin A, Woollard JR, Jordan KL, Tang H, Lerman A, Lerman LO. Perirenal fat promotes renal arterial endothelial dysfunction in obese swine through tumor necrosis factor-α. J Urol 195: 1152–1159, 2016. doi: 10.1016/j.juro.2015.08.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mantovani A, Sozzani S, Locati M, Allavena P, Sica A. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol 23: 549–555, 2002. doi: 10.1016/S1471-4906(02)02302-5. [DOI] [PubMed] [Google Scholar]
- 32.Meng XM, Tang PMK, Li J, Lan HY. Macrophage phenotype in kidney injury and repair. Kidney Dis (Basel) 1: 138–146, 2015. doi: 10.1159/000431214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mills CD, Kincaid K, Alt JM, Heilman MJ, Hill AM. M-1/M-2 macrophages and the Th1/Th2 paradigm. J Immunol 164: 6166–6173, 2000. doi: 10.4049/jimmunol.1701141. [DOI] [PubMed] [Google Scholar]
- 34.Muramatsu M, Yamamoto S, Osawa T, Shibuya M. Vascular endothelial growth factor receptor-1 signaling promotes mobilization of macrophage lineage cells from bone marrow and stimulates solid tumor growth. Cancer Res 70: 8211–8221, 2010. doi: 10.1158/0008-5472.CAN-10-0202. [DOI] [PubMed] [Google Scholar]
- 35.Murray PJ, Allen JE, Biswas SK, Fisher EA, Gilroy DW, Goerdt S, Gordon S, Hamilton JA, Ivashkiv LB, Lawrence T, Locati M, Mantovani A, Martinez FO, Mege JL, Mosser DM, Natoli G, Saeij JP, Schultze JL, Shirey KA, Sica A, Suttles J, Udalova I, van Ginderachter JA, Vogel SN, Wynn TA. Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity 41: 14–20, 2014. doi: 10.1016/j.immuni.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nikolic-Paterson DJ, Wang S, Lan HY. Macrophages promote renal fibrosis through direct and indirect mechanisms. Kidney Int Suppl 4: 34–38, 2014. doi: 10.1038/kisup.2014.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nishida M, Okumura Y, Fujimoto S, Shiraishi I, Itoi T, Hamaoka K. Adoptive transfer of macrophages ameliorates renal fibrosis in mice. Biochem Biophys Res Commun 332: 11–16, 2005. doi: 10.1016/j.bbrc.2005.04.083. [DOI] [PubMed] [Google Scholar]
- 38.Peiseler M, Kubes P. Macrophages play an essential role in trauma-induced sterile inflammation and tissue repair. Eur J Trauma Emerg Surg 44: 335–349, 2018. doi: 10.1007/s00068-018-0956-1. [DOI] [PubMed] [Google Scholar]
- 39.Ricardo SD, van Goor H, Eddy AA. Macrophage diversity in renal injury and repair. J Clin Invest 118: 3522–3530, 2008. doi: 10.1172/JCI36150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rosin DL, Okusa MD. Dangers within: DAMP responses to damage and cell death in kidney disease. J Am Soc Nephrol 22: 416–425, 2011. doi: 10.1681/ASN.2010040430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Saito H, Tanaka T, Tanaka S, Higashijima Y, Yamaguchi J, Sugahara M, Ito M, Uchida L, Hasegawa S, Wakashima T, Fukui K, Nangaku M. Persistent expression of neutrophil gelatinase-associated lipocalin and M2 macrophage markers and chronic fibrosis after acute kidney injury. Physiol Rep 6: e13707, 2018. doi: 10.14814/phy2.13707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Saran R, Robinson B, Abbott KC, Agodoa LYC, Bhave N, Bragg-Gresham J, Balkrishnan R, Dietrich X, Eckard A, Eggers PW, Gaipov A, Gillen D, Gipson D, Hailpern SM, Hall YN, Han Y, He K, Herman W, Heung M, Hirth RA, Hutton D, Jacobsen SJ, Jin Y, Kalantar-Zadeh K, Kapke A, Kovesdy CP, Lavallee D, Leslie J, McCullough K, Modi Z, Molnar MZ, Montez-Rath M, Moradi H, Morgenstern H, Mukhopadhyay P, Nallamothu B, Nguyen DV, Norris KC, O’Hare AM, Obi Y, Park C, Pearson J, Pisoni R, Potukuchi PK, Rao P, Repeck K, Rhee CM, Schrager J, Schaubel DE, Selewski DT, Shaw SF, Shi JM, Shieu M, Sim JJ, Soohoo M, Steffick D, Streja E, Sumida K, Tamura MK, Tilea A, Tong L, Wang D, Wang M, Woodside KJ, Xin X, Yin M, You AS, Zhou H, Shahinian V. US Renal Data System 2017 annual data report: epidemiology of kidney disease in the United States. Am J Kidney Dis 71, Suppl 1: A7, 2018. [Erratum in Am J Kidney Dis 71: 501, 2018.] doi: 10.1053/j.ajkd.2018.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sica A, Mantovani A. Macrophage plasticity and polarization: in vivo veritas. J Clin Invest 122: 787–795, 2012. doi: 10.1172/JCI59643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stenvinkel P, Heimbürger O, Paultre F, Diczfalusy U, Wang T, Berglund L, Jogestrand T. Strong association between malnutrition, inflammation, and atherosclerosis in chronic renal failure. Kidney Int 55: 1899–1911, 1999. doi: 10.1046/j.1523-1755.1999.00422.x. [DOI] [PubMed] [Google Scholar]
- 45.Sung SA, Jo SK, Cho WY, Won NH, Kim HK. Reduction of renal fibrosis as a result of liposome encapsulated clodronate induced macrophage depletion after unilateral ureteral obstruction in rats. Nephron, Exp Nephrol 105: e1–e9, 2007. doi: 10.1159/000096859. [DOI] [PubMed] [Google Scholar]
- 46.Tipping PG, Leong TW, Holdsworth SR. Tumor necrosis factor production by glomerular macrophages in anti-glomerular basement membrane glomerulonephritis in rabbits. Lab Invest 65: 272–279, 1991. [PubMed] [Google Scholar]
- 47.Wang Y, Mahajan D, Tay YC, Bao S, Spicer T, Kairaitis L, Rangan GK, Harris DC. Partial depletion of macrophages by ED7 reduces renal injury in Adriamycin nephropathy. Nephrology (Carlton) 10: 470–477, 2005. doi: 10.1111/j.1440-1797.2005.00438.x. [DOI] [PubMed] [Google Scholar]
- 48.Wang Y, Wang YP, Zheng G, Lee VW, Ouyang L, Chang DH, Mahajan D, Coombs J, Wang YM, Alexander SI, Harris DC. Ex vivo programmed macrophages ameliorate experimental chronic inflammatory renal disease. Kidney Int 72: 290–299, 2007. doi: 10.1038/sj.ki.5002275. [DOI] [PubMed] [Google Scholar]
- 49.Wilson HM, Chettibi S, Jobin C, Walbaum D, Rees AJ, Kluth DC. Inhibition of macrophage nuclear factor-κB leads to a dominant anti-inflammatory phenotype that attenuates glomerular inflammation in vivo. Am J Pathol 167: 27–37, 2005. doi: 10.1016/S0002-9440(10)62950-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wilson HM, Stewart KN, Brown PA, Anegon I, Chettibi S, Rees AJ, Kluth DC. Bone-marrow-derived macrophages genetically modified to produce IL-10 reduce injury in experimental glomerulonephritis. Mol Ther 6: 710–717, 2002. doi: 10.1006/mthe.2002.0802. [DOI] [PubMed] [Google Scholar]
- 51.Yang N, Wu LL, Nikolic-Paterson DJ, Ng YY, Yang WC, Mu W, Gilbert RE, Cooper ME, Atkins RC, Lan HY. Local macrophage and myofibroblast proliferation in progressive renal injury in the rat remnant kidney. Nephrol Dial Transplant 13: 1967–1974, 1998. doi: 10.1093/ndt/13.8.1967. [DOI] [PubMed] [Google Scholar]
- 52.Yokoo T, Ohashi T, Utsunomiya Y, Kojima H, Imasawa T, Kogure T, Hisada Y, Okabe M, Eto Y, Kawamura T, Hosoya T. Prophylaxis of antibody-induced acute glomerulonephritis with genetically modified bone marrow-derived vehicle cells. Hum Gene Ther 10: 2673–2678, 1999. doi: 10.1089/10430349950016717. [DOI] [PubMed] [Google Scholar]