Abstract
Transient receptor potential vanilloid family member 4 (TRPV4) transcript and protein expression increased in the urinary bladder and lumbosacral dorsal root ganglia of transgenic mice with chronic urothelial overexpression of nerve growth factor (NGF-OE). We evaluated the functional role of TRPV4 in bladder function with open-outlet cystometry, void spot assays, and natural voiding (Urovoid) assays with the TRPV4 antagonist HC-067047 (1 μM) or vehicle in NGF-OE and littermate wild-type (WT) mice. Blockade of TRPV4 at the level of the urinary bladder significantly (P ≤ 0.01) increased the intercontraction interval (2.2-fold) and void volume (2.6-fold) and decreased nonvoiding contractions (3.0-fold) in NGF-OE mice, with lesser effects (1.3-fold increase in the intercontraction interval and 1.3-fold increase in the void volume) in WT mice. Similar effects of TRPV4 blockade on bladder function in NGF-OE mice were demonstrated with natural voiding assays. Intravesical administration of HC-067047 (1 µM) significantly (P ≤ 0.01) reduced pelvic sensitivity in NGF-OE mice but was without effect in littermate WT mice. Blockade of urinary bladder TRPV4 or intravesical infusion of brefeldin A significantly (P ≤ 0.01) reduced (2-fold) luminal ATP release from the urinary bladder in NGF-OE and littermate WT mice. The results of the present study suggest that TRPV4 contributes to luminal ATP release from the urinary bladder and increased voiding frequency and pelvic sensitivity in NGF-OE mice.
Keywords: ATP, bladder overactivity, cystometry, natural voiding, pelvic pain
INTRODUCTION
Transient receptor potential (TRP) channel families are a superfamily of nonspecific cation channels that are generally permeable to Ca2+, Na+, and K+ and may act as sensors of stretch and/or chemical irritation in the lower urinary tract (22, 46). Specifically, TRP vanilloid family member 4 (TRPV4) expression has been demonstrated in basal and intermediate urothelial cells of mice and rats (19–21, 24, 29). TRPV4 is also expressed in the detrusor muscle, but TRPV4 expression is ~20- to 36-fold greater in urothelial than detrusor cells (16). Measurements of ionic currents and Ca2+ events induced by agonists (4α-phorbol 12,13-didecanoate and GSK1016790A) or stretch have demonstrated functional expression of TRPV4 in urothelial cells, detrusor cells (19–21, 24), and platelet-derived growth factor receptor-α-positive lamina propria cells (36), but functional evidence is lacking in sensory neurons (2). Contributions of TRPV4 to normal bladder function and bladder dysfunction after cyclophosphamide (CYP)-induced bladder inflammation have also been demonstrated (21).
TRPV4 channels are strong candidates as mechanosensors in the urinary bladder (46). TRPV4 agonists have been shown to increase reflex bladder contraction amplitude during conscious cystometry, decrease bladder capacity, and induce overactivity in vivo (1, 9, 48, 67). In contrast, TRPV4 antagonists counteract these effects by increasing bladder capacity and decreasing micturition frequency in mice and rats with CYP-induced cystitis (21). TRPV4 knockout (KO) mice exhibit altered voiding functions, including an increased intermicturition interval, decreased voiding frequency, increased frequency of nonvoiding contractions (NVCs), and increased total urine volume output (19, 24). TRPV4 KO mice are more resistant to CYP-induced cystitis, with the expected increases in voiding frequency and decreases in void volume (VV) being less pronounced (19, 20).
In the urinary bladder, TRPV4 may mediate sensory transduction by stretch-induced ATP release from urothelial cells. Agonist- and stretch-induced activation of TRPV4 correlates with ATP release from urothelial cells (24, 40, 50). This ATP release is significantly greater in urothelial cells from wild-type (WT) than TRPV4 KO mice (24, 40, 50). TRPV4 antagonist application significantly reduces the increase in Ca2+ as well as ATP release (24, 50). The proximity of other cells and tissues in the urinary bladder (e.g., muscle cells, urothelial cells, interstitial cells, and afferent nerves) that express purinergic receptors may suggest a functional role for TRPV4 in purinergic signaling and bladder sensory transduction (1, 24, 50, 71).
We (29) have previously demonstrated increased TRPV4 transcript and protein expression in the urothelium, suburothelium, suburothelial nerve plexus, and lumbosacral dorsal root ganglia (DRG) of mice with chronic urothelial overexpression of nerve growth factor (NGF-OE), consistent with previous studies demonstrating that TRP channel function and modulation may be dependent on target tissue expression of growth factors (e.g., NGF and glial-derived neurotrophic factor) (18, 37, 43, 60). Interestingly, NGF-OE mice display opposing phenotypic changes in bladder function, including reduced bladder capacity, increased frequency and amplitude of voiding, and increased referred somatic pelvic hypersensitivity (59), compared with TRPV4 KO mice (24). The role of NGF in bladder sensory function and inflammation-induced referred hyperalgesia has been well documented (33, 34, 38, 69). In NGF-OE and littermate WT mice, the present study addressed the contribution of TRPV4 channels to 1) urinary bladder function, 2) somatic (i.e., pelvic) sensitivity, and 3) luminal ATP release during the filling phase of the urinary bladder.
MATERIALS AND METHODS
Animals
Experiments involving animal research.
The experiments in the Vizzard laboratory were performed in accordance with institutional and national guidelines and regulations. The University of Vermont (UVM) Institutional Animal Care and Use Committee (IACUC) approved all experimental protocols involving animal use. Animal care was under the supervision of the UVM Office of Animal Care Management in accordance with the Association for Assessment and Accreditation of Laboratory Animal Care and National Institutes of Health guidelines. All efforts were made to minimize the potential for animal pain, stress, or distress.
NGF-OE mice.
As previously described, NGF-OE transgenic mice were generated at Roche Palo Alto (material transfer agreement with Roche Palo Alto and Dr. Debra Cockayne) in collaboration with Dr. Henry Sun at New York University Medical School (26, 28, 59). Mice were bred locally at the Larner College of Medicine at the UVM. Animal genotype was confirmed by Southern and/or PCR analyses; all mice had the inbred genetic C57BL/6J background and were derived from F15–F17 generations maintained through a hemizygous backcross strategy with C57BL/6 WT mice. The UVM IACUC approved all experimental protocols involving animals (IACUC nos. 08-085 and 13-030). The UVM Office of Animal Care Management oversaw all animal use in accordance with Association for Assessment and Accreditation of Laboratory Animal Care and National Institutes of Health guidelines. All efforts were made to minimize the potential for animal pain, stress, or distress. Separate cohorts of female littermate WT and NGF-OE mice were used in the following experiments. Although both male and female NGF-OE mice exhibit the previously described phenotype (27, 59), we used female mice because of the female predominance of interstitial cystitis/bladder pain syndrome (IC/BPS) (17, 51, 58). As previously described (28–30, 59), NGF-OE mice used in the present study exhibited significantly (P ≤ 0.001) increased NGF transcript and protein expression in the urothelium, with no changes in the detrusor (data not shown). For all experiments described below, analyses were performed in a blinded manner: genotype/experimental groups were decoded after completion of data analyses.
Conscious, open-outlet, continuous-fill cystometry.
Mice were anesthetized with isoflurane (3–4%), a lower midline abdominal incision was made, and polyethylene tubing (PE-10, Clay Adams, Parsippany, NJ) was inserted into the bladder dome and secured with a nylon purse-string suture (6-0) (31, 59). The end of the tubing was heat flared, but the catheter did not extend into the bladder body or neck, nor was it associated with inflammation or altered cystometric function (31, 59). The distal end of the tubing was sealed, tunneled subcutaneously, and externalized at the back of the neck (31, 59). Abdominal and neck incisions were closed with nylon sutures (6-0). Mice received postoperative analgesic (carprofen, 5.0 mg/kg sc, once a day for 2 days) and allowed to recover from survival surgery for 72 h before cystometry.
For cystometry in conscious mice, an unrestrained animal was placed in a Plexiglas cage with a wire bottom. Before the start of the recording, the bladder was emptied and the catheter was connected via a T tube to a pressure transducer (model PT300, Grass Instrument, West Warwick, RI) and microinjection pump (model 22, Harvard Apparatus, South Natick, MA). A small animal cystometry laboratory station (MED Associates, Fairfax, VT) was used for urodynamic measurements (31, 59). Saline solution was infused at room temperature into the bladder at a rate of 25 µL/min to elicit repetitive bladder contractions. At least six reproducible micturition cycles were recorded after the initial stabilization period of 25–30 min (31, 59). The following cystometric parameters were recorded in each animal: baseline pressure (pressure at the beginning of bladder filling), threshold pressure (bladder pressure immediately before micturition), peak micturition pressure, intercontraction interval (ICI; time between micturition events), infused volume, VV, bladder compliance (in µL/cmH2O), and presence of NVCs (31, 59). NVCs were defined as rhythmic intravesical pressure increases 5 cmH2O above baseline, during the filling phase, without the release of fluid from the urethra. Mice had a residual volume of <10 μL. Bladder compliance (in µL/cmH2O) was calculated as the ratio of the infused volume to the pressure difference between the postvoid resting pressure and threshold pressure for inducing a micturition contraction (75). At the conclusion of the experiment, mice were euthanized (5% isoflurane plus thoracotomy).
Conscious cystometry and effects of a TRPV4 receptor antagonist, a potent and selective TRPV4 antagonist, on bladder function in NGF-OE mice.
The effects of HC-067047, a potent and selective TRPV4 antagonist (21), on urinary bladder function in littermate WT and NGF-OE mice were assessed using conscious, open-outlet cystometry with continuous intravesical instillation of saline (31, 47, 59). Two groups of mice were evaluated: WT and NGF-OE mice were infused intravesically with vehicle (0.9% saline) and HC-067047 (1 µM, n = 8). For intravesical administration of HC-067047, mice were anesthetized with 2% isoflurane, and HC-067047 (<0.2 mL) was injected through the bladder catheter. Animals were maintained under anesthesia to prevent expulsion of HC-067047 via a voiding reflex. HC-067047 remained in the bladder for 30 min, at which time the drug was drained, the bladder was washed with saline, and animals were allowed to recover from anesthesia for 20 min before experimentation. These experiments were performed in the same mice before and after treatment with HC-067047. To summarize, the experimental design involves a one-time, intravesical infusion of HC-067047 (1 µM), with cystometric data collection ~75 min after infusion. At the conclusion of the experiment, the animal was euthanized (5% isoflurane plus thoracotomy). Experiments were conducted at similar times of the day to avoid the possibility that circadian variations were responsible for changes in bladder capacity measurements. Data were compared by ANOVA.
In control experiments in WT and NGF-OE mice (n = 6 each), we used conscious cystometry to evaluate the effects of intravesical instillation of the TRPV4 agonist GSK1016790A (30 or 100 nM) on bladder function, as described above. We then evaluated if intravesical instillation of the TRPV4 antagonist HC-067047 (1 µM) could reverse the effects. These experiments were also conducted in TRPV4−/− mice (n = 6) bred locally at the Larner College of Medicine at the UVM (K. S. Thorneloe, GSK, Collegeville, PA).
Exclusion criteria.
No mice were removed from the present study due to adverse events (e.g., significant postoperative event, lethargy, pain, or distress) (31, 59). Behaviors (e.g., grooming and defecation) rendered bladder pressure measurements during these events unusable.
Urovoid noninvasive evaluation of conscious voiding behavior in WT and NGF-OE mice with and without TRPV4 blockade.
We used the Urovoid system (Med Associates), a noninvasive means of measuring voiding function, to complement our conscious cystometry experiments. The Urovoid system is designed to quantify conscious urinary voiding behavior (frequency and VV) in unrestrained conscious mice for prolonged periods of time (48–72 h) without the need for surgery or catheter implantation. A mouse was singly housed in one cage for 48–72 h with ad libitum access to food and water. Urine was collected below the cage on a balance, and urine weight was recorded over time. The grated cage floor is necessary to allow urine and feces to pass through for accurate measurements, and no animal remained in this cage for >72 h. In preparation for Urovoid experiments, mice were first acclimated to the 12:12-h light-dark cycle, identical to that in the central animal care facility, and the Urovoid chamber for 1 h in the morning on each of 2 consecutive days before data accumulation. Time (in h) under these conditions acts as a zeitgeber (ZT): ZT0 refers to the beginning of daylight in a cycle and ZT12 is the beginning of night, under experimental conditions of 12 h in light and 12 h in darkness (12:12 LD). After the completion of these measurements, animals were returned to their home cages for future experimentation. Using this system, we were able to assess, in real time, voiding activity in WT and NGF-OE mice with and without intravesical infusion of the TRPV4 antagonist HC-067047 (1 µM) via a transurethral catheter. For these experiments, a transurethral bladder catheterization method was used to avoid abdominal and neck incisions and exteriorization of tubing from the neck incision when research personnel would have no contact with mice for extended time periods. A transurethral catheter (PE-10, Clay Adams), lubricated with paraffin oil, was gently inserted into the bladder through the urethra. The catheter was advanced 1.2–1.5 cm into the bladder and adjusted so that the end of the catheter did not contact the bladder wall. For intravesical administration of HC-067047, mice were anesthetized with 2% isoflurane, and HC-067047 (<0.2 mL) was injected through the bladder catheter; animals were maintained under anesthesia to prevent expulsion of HC-067047 via a voiding reflex. HC-067047 remained in the bladder for 30 min, the drug was drained, the bladder was washed with saline, and animals were allowed to recover from anesthesia for 20 min and then placed in the Urovoid metabolic chamber. Data were analyzed offline using the Urovoid voiding frequency analysis system (Med Associates). Data were compared by repeated-measures ANOVA.
Urination patterns.
Using a void spot assay (25), we evaluated the urination patterns in WT and NGF-OE mice with and without intravesical infusion of the TRPV4 antagonist HC-067047 (1 µM) via a transurethral catheter. For intravesical administration of HC-067047, mice were anesthetized with 2% isoflurane, and HC-067047 (<0.2 mL) was injected through the bladder catheter (single instillation); animals were maintained under anesthesia to prevent expulsion of HC-067047 via a voiding reflex. HC-067047 remained in the bladder for 30 min, the drug was drained, the bladder was washed with saline, and animals were allowed to recover from anesthesia for 20 min and then placed individually in cages lined with Whatman grade 3 filter paper for 2 h under continuous monitoring (25, 63). After 2 h, the filter paper was examined under ultraviolet light to determine the presence and size of urine spots, and the images were scanned (ChemiDoc Imaging System, Bio-Rad Laboratories, Hercules, CA). The area (in cm2) of urine spots was determined: large (0.2−10 cm2) (10, 25) and small (<0.2 cm2) (10, 25) spots were quantified. Mice were habituated to cages for 1 h in the morning (between 9 and 11 AM) on each of 2 consecutive days before data accumulation (25, 63). Data were compared by ANOVA.
Mechanical sensitivity testing.
Referred (secondary) hyperalgesia was measured by testing the frequency of withdrawal responses to the application of calibrated von Frey monofilaments to the abdomen (12, 59) region overlying the urinary bladder, with TRPV4 antagonist delivered intravesically via a transurethral catheter. Four separate groups (n = 10 each) of mice were evaluated: WT mice with vehicle, NGF-OE mice with vehicle, WT mice with HC-067047 (1 µM), and NGF-OE mice with HC-067047 (1 µM). A transurethral bladder catheterization method was used to avoid the need for an abdominal incision. HC-067047 (<1 mL) remained in the bladder for 30 min, at which time the drug was drained, the bladder was washed with saline, the catheter was removed, and animals were allowed to recover from anesthesia for 20 min before experimentation. Mechanical sensitivity was assessed using von Frey monofilaments (Stoelting, Wood Dale, IL) with forces of 0.1–4 g applied to the pelvic region (12, 59). All mice were first habituated in a clear acrylic testing chamber for 20 min/day for 4 days with a fan to generate ambient noise. On the day of testing, mice were placed in the acrylic testing chamber on top of a metal mesh floor (IITC Life Science, Woodland Hills, CA) and habituated again for 10 min before application of von Frey filaments in an up-and-down motion for 1–3 s with a minimum interstimulus interval of 2 min (12, 59). Stimulation was confined to the lower abdominal area overlying the urinary bladder. The following behaviors were considered positive responses to pelvic region stimulation: sharp retraction of the abdomen, jumping, or immediate licking or scratching of the pelvic area (12, 59). Separate cohorts of mice were used for cystometry, conscious voiding evaluation, and somatic sensitivity testing. Data were compared by ANOVA.
ATP release.
NGF-OE and littermate WT mice (n = 4–8) were anesthetized with 2% isoflurane and euthanized by decapitation. The abdominal cavity was opened by a midline incision, and the urinary bladder, urethra, and ureters were excised and transferred to ice-cold dissection solution. The ureters were isolated, dissected, and ligated with nylon suture adjacent to the bladder wall. The urinary bladder, urethra, and ureters were transferred to a recording chamber with oxygenated (95% O2-5% CO2) physiological saline solution (PSS; 0.9%) circulating at 37°C and cannulated through the urethra with a 22-gauge blunt needle. The cannula was attached to a remote-controlled syringe pump and a pressure transducer to monitor intravesical pressure. Vehicle or drugs were administered at a rate of 25 µL/min intravesically up to 25 cmH2O for the filling phase and then manually emptied via a three-way stopcock. There was a 10-min rest between the emptying phase and the start of the next filling phase. After 1 h of vehicle or drug administration, the instillate was collected from two separate, but consecutive, emptying cycles and immediately flash frozen until ATP analysis (32). Upon collection, instillate samples also received adenylyl-imidodiphosphate (200 µM) to limit ATP hydrolysis that may result from a compromised barrier. As previously described (32), ATP was quantified using the ATP bioluminescence assay kit (Sigma-Aldrich, St. Louis, MO) according to the manufacturer’s instructions except that the recommended concentration of reagents and samples was reduced by half. ATP was measured using a plate reader (model H4, BioTek, Winooski, VT) within the UVM Advanced Genome Technologies Core. The bioluminescence emitted by the sample was plotted against the calibration curve to determine the final concentration (in pmol/mL) of ATP. Calibration curves were run with each assay using the drugs being evaluated in the present study (Supplemental Figure S1; see https://doi.org/10.6084/m9.figshare.9794759) to account for potential interference. Statistical analyses were performed on the ATP concentration (in pmol/mL) of the instillate before or after drug administration by Student’s paired t test for all group means.
Materials
A working concentration of 1 μM was selected for HC-067047 (Bachem, Torrance, CA) based on previous publications (19, 20, 48) and pilot studies. Brefeldin A (BFA; R&D Systems) was reconstituted to 50 mM in DMSO (99.5%) and stored at −20°C. Stock solutions were diluted to a working concentration (10 µM) with PSS to remain consistent with previous studies demonstrating decreased ATP release from the urothelium (44, 64). 10Panx (R&D Systems) was reconstituted to 1 mg/mL in PSS and stored at −20°C. The IC50 value of 10Panx for the inhibition of pannexin-1 currents in overexpressed pannexin-1 human embryonic kidney cells is 52 ± 12 μM (56). We used 10Panx at a working concentration of 50 μM, because intravesical 10Panx near the IC50 concentration decreased voiding frequency (68). Pyridoxalphosphate-6-azophenyl-2′,4′-disulfonic acid tetrasodium salt (PPADS; R&D Systems) was reconstituted to 100 mM in distilled water and stored protected from light at −20°C to prevent photodecomposition. A working concentration of 300 μM was selected for PPADS, because a previous study (70) reported decreased distension-evoked afferent nerve discharge that was not observed at lower concentrations (76). GSK1016790 was purchased from Sigma and used at a working concentration of 100 nM based on previous studies (36, 42, 48).
Statistical Analyses
All values are means ± SE. Outliers were identified using the extreme studentized deviate test on GraphPad Prism (v. 6.07, La Jolla, CA) and excluded from further analysis. Comparisons among experimental groups were made using ANOVA, repeated-measures ANOVA, and paired or upaired t tests where appropriate. When the F test statistic exceeded the critical value at α = 0.05, Sidak’s multiple-comparisons test was used to compare group means.
RESULTS
Cystometry Showed That TRPV4 Receptor Blockade With Intravesical Infusion of HC-067047 Increased ICI and VV in Conscious NGF-OE and Littermate WT Mice
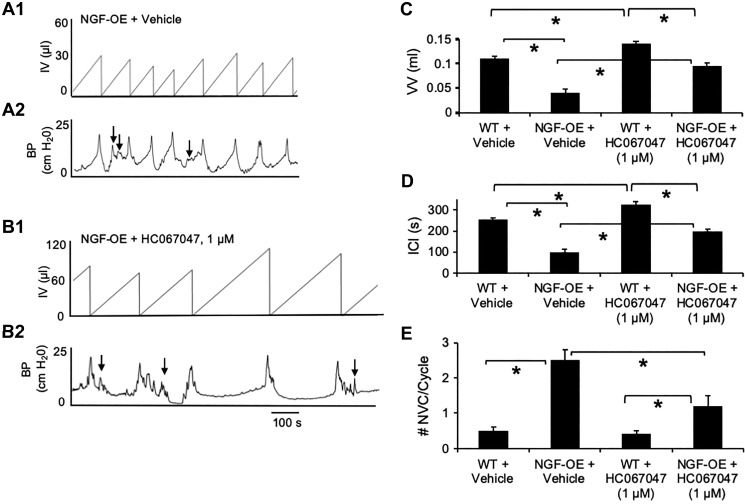
Conscious cystometry was performed in freely moving NGF-OE and littermate WT mice before intravesical infusion of a potent and selective TRPV4 antagonist (HC-067047, 1 µM) to establish baseline voiding frequency, ICI, and VV (Fig. 1, A–D). As previously demonstrated (30, 59), NGF-OE mice exhibited decreased VV (3.2-fold) and ICI (3.1-fold) compared with littermate WT mice (Fig. 1, A, C, and D). After intravesical infusion of the TRPV4 antagonist HC-067047 (1 µM), the same NGF-OE mice exhibited significantly (P ≤ 0.01) increased ICI (2.2-fold) and VV (2.6-fold) (Fig. 1, B–D). Intravesical infusion of vehicle into NGF-OE mice did not affect the cystometric parameters evaluated (Fig. 1, C and D). In littermate WT mice, intravesical instillation of HC-067047 (1 µM) also significantly (P ≤ 0.01) increased VV (1.3-fold) and ICI (1.3-fold) compared with WT mice infused with vehicle (Fig. 1, C and D), but to a lesser extent than in NGF-OE mice. NVCs (increases in baseline pressure with an amplitude of ≥5 cmH2O without expulsion of urine during the filling phase) were frequently observed in NGF-OE mice (Fig. 1, A2 and E). Consistent with previous studies, NGF-OE mice exhibited a significant (P ≤ 0.01) increase (5-fold) in the number of NVCs per voiding cycle compared with littermate WT mice (Fig. 1E). Intravesical infusion of HC-067047 (1 µM) reduced (3.1-fold) the number of NVCs in NGF-OE mice (Fig. 1, B2 and E). Intravesical infusion of vehicle into NGF-OE mice was without effect on NVCs (Fig. 1E). Intravesical infusion of vehicle or HC-067047 (1 µM) into littermate WT mice did not affect NVCs (Fig. 1E). All changes in urinary bladder function with intravesical instillation of HC-067047 (1 µM) persisted for the duration of the data collection period (~75 min). Intravesical pressure (baseline, threshold, and maximum) was not significantly affected after HC-067047 (1 µM) instillation in NGF-OE or WT mice (Table 1). Urinary bladder compliance was significantly (P ≤ 0.01) reduced in NGF-OE mice compared with WT mice (Table 1). Intravesical infusion of HC-067047 (1 µM) significantly (P ≤ 0.01) increased bladder compliance in NGF-OE mice but was without effect on WT mice (Table 1).
Fig. 1.
Intravesical infusion of the transient receptor potential vanilloid family member 4 (TRPV4) antagonist HC-067047 reduces voiding frequency and nonvoiding contractions (NVCs) in transgenic mice with chronic urothelial overexpression of nerve growth factor (NGF-OE), as demonstrated using continuous-fill, open-outlet, conscious cystometry. A and B: representative bladder function recordings from the same NGF-OE mouse before and after intravesical instillation of the TRPV4 antagonist HC-067047 (1 µM). A1 and A2: before HC-067047 instillation, NGF-OE mice exhibited increased voiding frequency and an increased number of NVCs during the filling phase (arrows). B1 and B2: after HC-067047 instillation, NGF-OE mice exhibited a reduction in voiding frequency [i.e., increased duration of intercontraction interval (ICI) and increased infused volume (IV) before micturition event]. BP, bladder pressure. C–E: summary histograms of void volume (VV), ICI, and NVCs measured from bladder function testing by conscious cystometry in wild-type (WT) and NGF-OE mice before and after intravesical instillation of HC-067047 (1 µM). NGF-OE mice exhibited significantly (*P ≤ 0.01) reduced VV (C) and ICI (D) compared with littermate WT mice. Intravesical instillation of HC-067047 (1 µM) significantly (*P ≤ 0.01) increased VV (C) and ICI (D) in NGF-OE and littermate WT mice. NGF-OE mice exhibited a significantly (*P ≤ 0.01) greater number of NVCs per voiding cycle than littermate WT mice (E). Intravesical infusion of HC-067047 (1 µM) significantly (*P ≤ 0.01) reduced the number of NVCs during the filling phase of the cycle compared with vehicle-treated NGF-OE mice (E). The number of NVCs was still significantly (*P ≤ 0.01) greater in NGF-OE mice treated with HC-067047 than in littermate WT mice treated with HC-067047 (E). Intravesical infusion of HC-067047 was without effect in littermate WT mice (E). Values are means ± SE; n = 8 for each group.
Table 1.
Effects of intravesical infusion of HC-067047 in WT and NGF-OE mice on bladder pressure and compliance
| Bladder Pressure, cmH2O |
||||
|---|---|---|---|---|
| Experimental Groups | Baseline | Threshold | Maximum | Bladder Compliance, µL/cmH2O |
| WT + vehicle | 18.4 ± 2.3 | 22.3 ± 1.7 | 32.4 ± 1.4 | 28.8 ± 3.2 |
| NGF-OE + vehicle | 19.1 ± 1.5 | 21.5 ± 1.2 | 31.5 ± 1.8 | 13.3 ± 5.6* |
| WT + HC-067047 (1 µM) | 18.3 ± 1.4 | 22.4 ± 1.6 | 30.3 ± 1.6 | 33.8 ± 4.8 |
| NGF-OE + HC-067047 (1 µM) | 20.7 ± 1.2 | 23.2 ± 1.4 | 30.1 ± 1.5 | 30.7 ± 3.4* |
Values are means ± SE; n = 8 for each group. Bladder pressure (baseline, threshold, and maximum) and bladder compliance were measured using conscious cystometry in littermate wild-type (WT; n = 8) and transgenic mice with chronic urothelial overexpression of nerve growth factor (NGF-OE; n = 8) before and after intravesical instillation of HC-067047 (1 µM). NGF-OE and littermate WT mice exhibited similar bladder pressures. Intravesical instillation of HC-067047 (1 µM) was without effect on any bladder pressure measured in littermate WT or NGF-OE mice. Urinary bladder compliance was significantly reduced in NGF-OE mice compared with WT mice. Intravesical infusion of HC-067047 (1 µM) significantly increased bladder compliance in NGF-OE mice but was without effect on bladder compliance in WT mice.
P ≤ 0.01.
In control experiments with WT (n = 6) and NGF-OE (n = 6) mice, intravesical instillation of the TRPV4 agonist GSK1016790A (100 nM) significantly (P ≤ 0.01) decreased ICI (increased voiding frequency) and VV (Table 2). These effects of GSK1016790A on voiding frequency in WT mice are consistent with previously published studies (16, 48, 67). The magnitude (2.0-fold decrease) of the effect was comparable between WT and NGF-OE mice (Table 2). No effects on bladder pressure (baseline, threshold, or maximum) were observed with GSK1016790A (100 nM; data not shown). The increased voiding frequency was reversed in WT and NGF-OE mice with intravesical instillation of the TRPV4 antagonist HC-067047 (1 µM). No effects on VV or ICI were observed when a lower concentration (30 nM) of GSK1016790A was infused. Although higher concentrations of GSK1016790A and HC-067047 have been used for different routes of administration (14, 21) or in different species (16), robust changes in bladder function were observed when lower concentrations were delivered intravesically in mice in the present study as well as in previous studies (36, 42, 48). Thus, lower concentrations were used to minimize off-target effects. To confirm specificity of the TRPV4 agonist and antagonist used in the present study, TRPV4−/− mice were infused intravesically with GSK1016790A (100 nM) and then with HC-067047 (1 µM). As previously demonstrated in TRPV4−/− mice (21) and TRPV4−/− rats (16), no effects on VV or ICI were observed in TRPV4−/− mice infused with GSK1016790A or HC-067047 (Table 2). TRPV4−/− mice exhibited significantly (P ≤ 0.01) increased VV and ICI compared with NGF-OE and WT mice, consistent with prior studies (21, 24) (Table 2). The absence of functional effects of a TRPV4 agonist or antagonist in TRPV4−/− mice is consistent with the absence of TRPV4 protein expression in urinary bladders from TRPV4−/− mice (29).
Table 2.
Effects of GSK1016790A on voiding frequency and VV are reversed by HC-067047 in WT and NGF-OE mice
| Experimental Groups | VV, µL | ICI, s |
|---|---|---|
| WT + vehicle | 122.4 ± 10.3 | 262.4 ± 8.5 |
| WT + GSK1016790A (100 nM) | 59.4 ± 9.5* | 125.4 ± 4.7* |
| WT + GSK1016790A + HC-067047 (1 µM) | 112.5 ± 8.7* | 253.6 ± 4.5* |
| NGF-OE + vehicle | 52.2 ± 6.2 | 97.6 ± 10.2 |
| NGF-OE + GSK1016790A (100 nM) | 24.4 ± 5.6* | 48.5. ± 9.4* |
| NGF-OE + GSK1016790A + HC-067047 (1 µM) | 45.5 ± 3.5* | 101.7 ± 11.8* |
| TRPV4−/− + vehicle | 215.8 ± 18.9* | 381.7 ± 35.0* |
| TRPV4−/− + GSK1016790A (100 nM) | 219.2 ± 17.8* | 390.4 ± 22.6* |
| TRPV4−/− + GSK1016790A + HC-067047 (1 µM) | 224.1 ± 11.2* | 393.3 ± 33.2* |
Values are means ± SE; n = 6 for each group. In control, conscious cystometry experiments with continuous infusion and an open outlet, effects of intravesical instillation of the transient receptor potential vanilloid family member 4 (TRPV4) agonist GSK1016790A (100 nM) were evaluated in wild-type (WT; n = 6) and transgenic mice with chronic urothelial overexpression of nerve growth factor (NGF-OE; n = 6) followed by intravesical instillation of the TRPV4 antagonist HC-067047 (1 µM). GSK1016790A (100 nM) significantly decreased intercontraction interval [ICI (voiding frequency)] and void volume (VV) in WT and NGF-OE mice compared with vehicle treatment. Increased voiding frequency and reduced VV were reversed in WT and NGF-OE mice with intravesical instillation of HC-067047 (1 µM). Intravesical infusion of GSK1016790A (100 nM) followed by intravesical infusion of HC-067047 (1 µM) significantly increased VV and ICI compared with GSK1016790A alone. Effects of intravesical instillation of GSK1016790A (100 nM) followed by intravesical instillation of HC-067047 (1 µM) were also evaluated in TRPV4−/−mice (n = 6). GSK1016790A (100 nM) had no effect on ICI or VV in TRPV4−/− mice compared with vehicle. Subsequent intravesical infusion of HC-067047 (1 µM) was also without effect on VV and ICI compared with GSK1016790A alone or vehicle. VV and ICI were significantly greater for vehicle-, TRPV4 agonist-, or TRPV4 antagonist-treated TRPV4−/− mice than for WT or NGF-OE mice with vehicle or drug treatment.
P ≤ 0.01.
Void Spot Assays Demonstrate Increased Void Area in NGF-OE Mice Treated With the TRPV4 Antagonist HC-067047
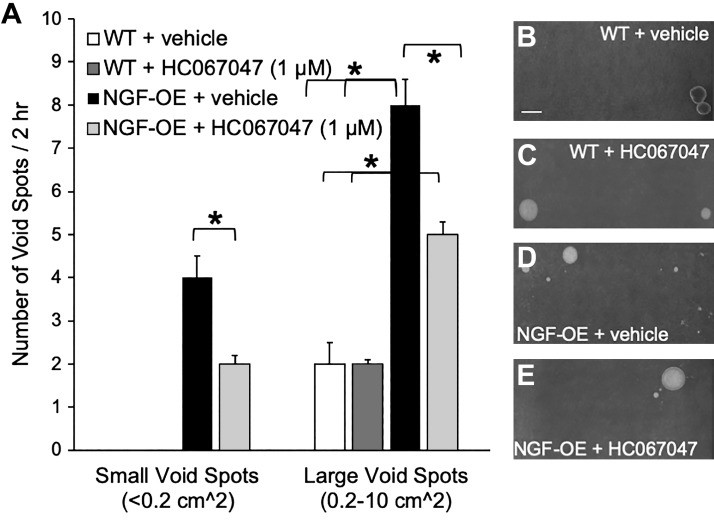
Individual NGF-OE and littermate WT mice with or without intravesical instillation of HC-067047 or vehicle were placed for 2 h in separate cages lined with filter paper to absorb urine expelled with micturition events. WT mice intravesically infused with vehicle or the TRPV4 receptor antagonist HC-067047 exhibited a comparable number (2 spots/2 h) of large (0.2−10 cm2) void spots (Fig. 2, A–C). NGF-OE mice treated intravesically with vehicle exhibited a greater number of small (<0.2 cm2) void spots (3.9 ± 1.2 spots/2 h) and larger void spots (7.7 ± 1.4 spots/2 h) than WT mice treated with vehicle (Fig. 2, A and D). NGF-OE mice intravesically infused with HC-067047 exhibited a significantly (P ≤ 0.01) reduced number of small (<0.2 cm2) and large (0.2−10 cm2) void spots (Fig. 2, A and E).
Fig. 2.
A: summary histogram of the number and area of urine spots on filter paper over a 2-h period from transgenic mice with chronic urothelial overexpression of nerve growth factor (NGF-OE) and littermate wild-type (WT) mice of both sexes with intravesical instillation of vehicle (saline, 0.9%) or the transient receptor potential vanilloid family member 4 (TRPV4) receptor antagonist HC-067047 (1 µM). NGF-OE mice with intravesical instillation of saline (vehicle) excreted a combination of small (≤0.2 cm2) and larger (0.2−10 cm2) void spots. In contrast, littermate WT mice treated with vehicle only excreted larger-area void spots. After NGF-OE mice received intravesical HC-067047 (1 µM), the number of small and larger area void spots was significantly (*P ≤ 0.01) reduced. After WT mice received intravesical HC-067047 (1 µM), the number and area of larger void spots were unchanged compared with WT mice that received intravesical saline. More large area void spots were excreted by NGF-OE mice treated with vehicle or HC-067047 (*P ≤ 0.01) than by WT mice treated with vehicle or HC-067047. Values are means ± SE; n = 6–8 for each group. B–E: representative images of urine spots on filter paper over a 2-h period visualized with ultraviolet light from WT mice + vehicle (B), WT mice + HC-067047 (C), NGF-OE mice + vehicle (D), and NGF-OE mice + HC-067047 (E). Scale bar = 3 cm.
Urovoid Assessment Shows That TRPV4 Receptor Blockade With Intravesically Infused HC-067047 Increased ICI and Void Mass in Conscious NGF-OE and Littermate WT Mice
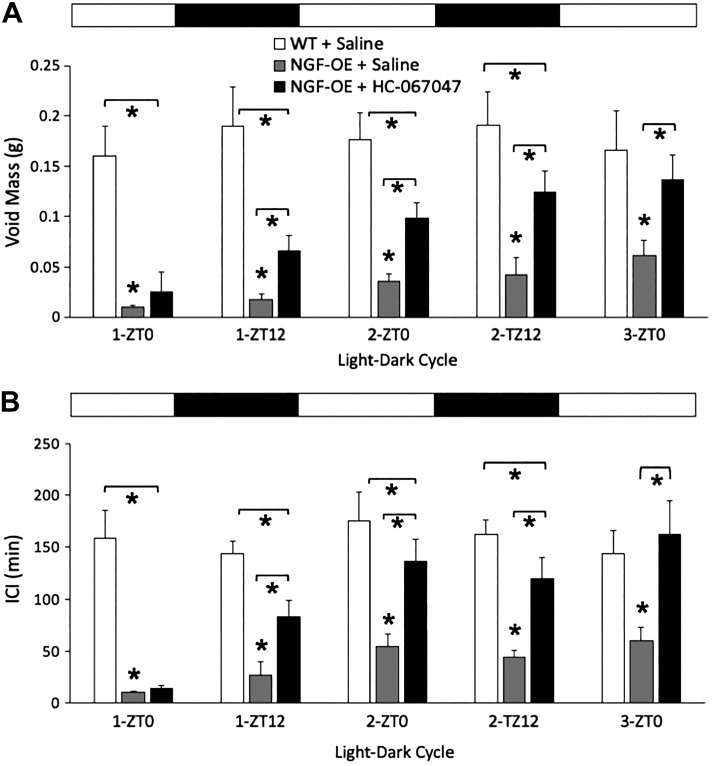
Void mass and ICI were significantly (P ≤ 0.01) reduced in NGF-OE mice intravesically instilled with saline compared with similarly treated littermate WT mice at all 12:12-h light-dark cycles examined. NGF-OE mice treated with a single intravesical instillation of the TRPV4 receptor antagonist HC-067047 (1 µM) exhibited significantly (P ≤ 0.01) increased void mass (Fig. 3A) and ICI (Fig. 3B) compared with NGF-OE mice intravesically instilled with saline beginning with the first 12-h dark cycle (1-ZT12) and continuing for all subsequent 12-h light and 12-h dark cycles examined. Void mass (Fig. 3A) and ICI (Fig. 3B) exhibited by littermate WT mice intravesically instilled with saline and NGF-OE mice treated with a single intravesical instillation of HC-067047 (1 µM) were not different beginning with the second 12-h light cycle and for all subsequent light-dark cycles.
Fig. 3.
Summary histograms of void mass (A) and intercontraction interval (ICI; B) recorded under conscious voiding conditions by the Urovoid system in littermate wild-type (WT) mice and transgenic mice with chronic urothelial overexpression of nerve growth factor (NGF-OE) with intravesical instillation of saline (0.9%) or the transient receptor potential vanilloid family member 4 (TRPV4) receptor antagonist HC-067047 (1 µM). The time (in h) under these conditions acts as a zeitgeber (ZT). ZT0 refers to the beginning of daylight in a cycle, and ZT12 is the beginning of night, under experimental conditions of 12 h in light and 12 h in darkness (black and white bars above histograms). Void mass and ICI data were acquired from mice evaluated during 12:12-h light-dark cycles for 60 h. NGF-OE mice with intravesical instillation of saline exhibited significantly (*P ≤ 0.01) reduced void mass (A) and ICI (B) compared with WT mice with intravesical instillation of saline at all light-dark cycles examined, from the first 12-h light cycle (1-ZT0) through the third 12-h light cycle (3-ZT0). Intravesical instillation of HC-067047 (1 µM) in NGF-OE mice significantly (*P ≤ 0.01) increased void mass compared with NGF-OE mice instilled with saline beginning at the first 12-h dark cycle (1-ZT12) and continuing through 3-ZT0. No differences in void mass between NGF-OE mice with HC-067047 and WT mice with saline were observed at 3-ZT0. Values are means ± SE; n = 6–8 for each group.
Von Frey Filament Testing Demonstrates Reduced Pelvic Somatic Sensitivity in NGF-OE Mice With Intravesical Infusion of the Potent and Selective TRPV4 Antagonist HC-067047
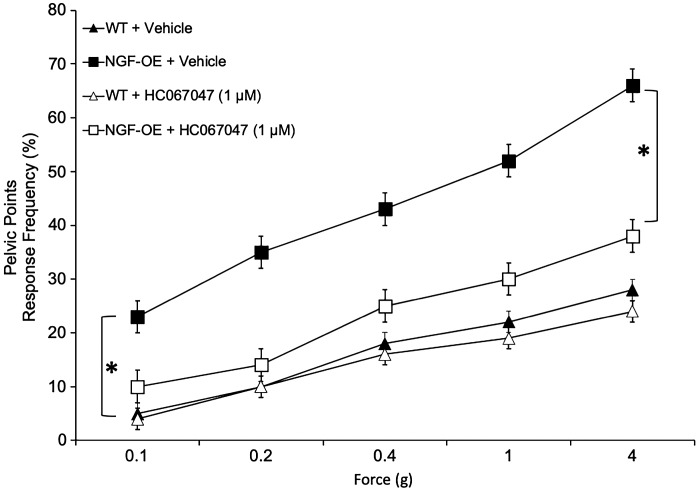
Consistent with a previous study (59), pelvic somatic sensitivity was significantly (P ≤ 0.01) increased in NGF-OE mice compared with littermate WT mice at all monofilament forces (0.1–4 g) evaluated (Fig. 4). Intravesical infusion of HC-067047 (1 µM) significantly (P ≤ 0.01) decreased somatic sensitivity in the pelvic region in NGF-OE mice (Fig. 4). Intravesical infusion of vehicle was without effect on pelvic sensitivity in NGF-OE mice (Fig. 4). In littermate WT mice, intravesical infusion of HC-067047 (1 µM) or vehicle produced no change in somatic sensitivity in the pelvic region (Fig. 4).
Fig. 4.
Pelvic region sensitivity testing with calibrated von Frey filaments was determined in littermate wild-type (WT) mice and transgenic mice with chronic urothelial overexpression of nerve growth factor (NGF-OE) before and after intravesical instillation of the transient receptor potential vanilloid family member 4 (TRPV4) receptor antagonist HC-067047 (1 µM). Stimulation was confined to the lower abdominal area overlying the urinary bladder. NGF-OE mice had a significantly (*P ≤ 0.01) increased pelvic response frequency with all von Frey filaments (0.1–4 g) tested compared with littermate WT mice. In NGF-OE mice, intravesical instillation of HC-067047 (1 µM) significantly (*P ≤ 0.01) reduced pelvic response frequency with all von Frey filaments tested compared with NGF-OE mice treated with vehicle (saline, 0.9%). No changes in pelvic sensitivity were observed in WT mice after intravesical instillation of HC-067047. All somatic testing was performed in a blinded manner. Values are means ± SE; n = 10 for each group.
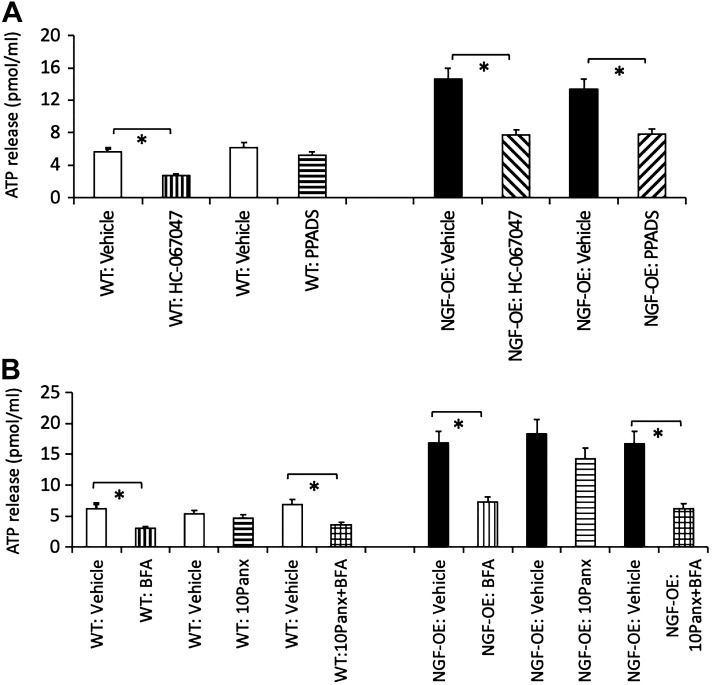
ATP Release Is Decreased in NGF-OE Mice With TRPV4 Blockade Via Intravesical Administration of HC-067047
In littermate WT and NGF-OE mice, TRPV4 blockade with intravesical administration of HC-067047 (1 µM) significantly (P ≤ 0.01) decreased (2.1-fold) distension-evoked urothelial ATP release at 25 cmH2O (Fig. 5A). Continuous intravesical instillation of PPADS (300 µM) did not have a significant effect on urothelial ATP release at 25 cmH2O in WT mice but significantly (P ≤ 0.01) decreased (1.9-fold) distension-evoked urothelial ATP release at 25 cmH2O in NGF-OE mice (Fig. 5A). In littermate WT and NGF-OE mice, continuous intravesical instillation of BFA (10 µM) significantly (P ≤ 0.01) reduced (2.4-fold) distension-evoked urothelial ATP release at 25 cmH2O (Fig. 5B); however, continuous intravesical instillation of 10Panx (50 µM) did not have a significant effect on distension-evoked urothelial ATP release at 25 cmH2O (Fig. 5B) in either NGF-OE or littermate WT mice. Intravesical coadministration of BFA (10 µM) and 10Panx (50 µM) in WT and NGF-OE mice significantly (P ≤ 0.01) reduced (2.3-fold) distension-evoked urothelial ATP release at 25 cmH2O, but the reduction (2.3-fold) was similar to that observed with intravesical administration of BFA (10 µM) alone (Fig. 5B).
Fig. 5.
A: luminal ATP release was increased in transgenic mice with chronic urothelial overexpression of nerve growth factor (NGF-OE) and decreased with intravesical instillation of the transient receptor potential vanilloid family member 4 (TRPV4) receptor antagonist HC-067047 (1 µM) in NGF-OE and littermate WT mice. NGF-OE mice exhibited significantly (*P ≤ 0.01) increased luminal ATP release at 25 mmHg compared with littermate WT mice that was significantly (*P ≤ 0.01) reduced with intravesical treatment with HC-067047 (1 µM). Luminal ATP release in NGF-OE mice was also significantly (*P ≤ 0.01) reduced with intravesical instillation of the purinergic (P2) receptor antagonist pyridoxalphosphate-6-azophenyl-2′,4′-disulfonic acid tetrasodium salt (PPADS; 300 µM). Intravesical PPADS (300 µM) in littermate WT mice was without effect on luminal ATP release. B: luminal ATP release was decreased in NGF-OE and littermate WT mice by intravesical instillation of the general secretory inhibitor brefeldin A (BFA; 10 µM) but unaffected by the inhibitory peptide 10Panx (50 µM) to block pannexin-1 channels. Intravesical instillation of BFA (10 µM) significantly (*P ≤ 0.01) reduced luminal ATP release measured at 25 mmHg from littermate WT and NGF-OE mice. No changes in luminal ATP release were observed in littermate WT or NGF-OE mice with intravesical treatment with 10Panx (50 µM). Intravesical instillation with both BFA (10 µM) and 10Panx (50 µM) reduced luminal ATP release to that observed with BFA treatment alone. Values are means ± SE; n = 6–8 for each group.
DISCUSSION
The present study expands our knowledge of TRPV4 involvement in increased urinary frequency, pelvic pain, and NVCs in our transgenic mouse model with NGF-OE (25, 28, 29, 59). In addition, the present study also extends our understanding of distension-induced luminal ATP release mechanisms and demonstrates an involvement of TRPV4 and vesicular release mechanisms. We used three complementary approaches (i.e., conscious cystometry, natural voiding assays, and void spot assays) to characterize the effects of TRPV4 blockade on urinary bladder function in NGF-OE and WT mice. These results continue to suggest that TRPV4 blockade at the level of the urinary bladder may be a useful target to reduce urinary frequency (21, 24, 29, 48) and somatic sensitivity.
We have previously demonstrated that NGF-OE mice exhibit 1) increased neuronal sprouting in the urinary bladder, 2) local inflammatory changes in the urinary bladder, 3) increased voiding frequency, and 4) referred pelvic hypersensitivity (59). Interestingly, NGF-OE mice display an opposing phenotypic alteration compared with TRPV4 KO mice (24). NGF has been implicated in sensitization of peripheral nociceptors (13, 15, 29, 69), potentially through TRPV4 channels, which have also been implicated in nociceptor sensitization in afferent nerves (3, 4, 37). Growth factors such as glial-derived neurotrophic factor and NGF are known to regulate sensory afferent nerve sensitivity by modulating the function and expression of TRP channels (29, 37, 43), consistent with the finding of increased TRPV4 expression in the urothelium, suburothelium, and suburothelial nerve plexus and lumbosacral DRG of NGF -OE mice (29).
TRPV4 may be involved in bladder disorders such as overactive bladder and BPS/IC (3, 19–21). In the present study, intravesical TRPV4 blockade with HC-067047 (1 µM) significantly reduced voiding frequency and NVCs, as demonstrated by conscious cystometry in NGF-OE mice, consistent with our previous observations of reduced voiding frequency in rats intravesically instilled with HC-067047 (1 µM) and exposed to repeated variate stress (48). In littermate WT mice, intravesical instillation of HC-067047 also significantly reduced voiding frequency, but to a lesser extent than in NGF-OE mice. Void spot assays and subsequent area measurements demonstrated a significant reduction in the number of small and larger area void spots in NGF-OE mice intravesically infused with HC-067047 (1 µM) but no effects in WT mice intravesically instilled with HC-067047. Natural voiding assessment of NGF-OE mice demonstrated that a single intravesical instillation of HC-067047 significantly increased the void mass and intermicturition interval over multiple 12:12-h light-dark cycles. In addition, pelvic sensitivity as assessed by von Frey filament testing was significantly reduced in NGF-OE mice after intravesical infusion of HC-067047 at all forces evaluated.
The long-term effects of a single intravesical instillation of HC-067047 on voiding function were surprising, but to our knowledge, long-term (>2 h) effects of intravesical delivery of HC-067047 (48), or pharmacological antagonists in general, have not been evaluated. A previous study (21) that examined the effects of HC-067047 in WT mice or CYP-treated rats demonstrated reductions in voiding frequency after systemic (i.e., intraperitoneal) administration of HC-067047 (10 mg/kg). A pharmacokinetic study (21) demonstrated that, after intraperitoneal administration (10–100 mg/kg) of HC-067047, levels were significantly increased above the IC50 value for TRPV4 for ≥2 h. Mice that received HC-067047 (50 mg/kg ip) exhibited an average plasma concentration >200-fold the IC50 value of murine TRPV4 at the end of a cystometry session (30 min), suggesting that TRPV4 channel blockade was maximal (21). To our knowledge, studies evaluating the pharmacokinetic properties of intravesical delivery of HC-067047 have not been performed. With the availability of the Urovoid system and natural voiding assessments, long-term effects (12 h to days) of antagonists on voiding frequency, intermicturition interval, and VV in animal models can be evaluated. Effects of intravesical infusion of HC-067047 may include blockade of TRPV4 at the membrane of TRPV4-expressing cells and interference with trafficking and spatial and temporal distribution of TRPV4 as well as downstream cellular functions (6). TRPV4 also regulates inflammation (14) by long-term modulation of cell signaling cascades, including ERK and p38 (5, 53). Thus, intravesical infusion of HC-067047 may elicit short- and longer-term effects on bladder function demonstrated in the present study through a variety of actions. At the level of the urinary bladder, short-term effects of intravesical instillation of HC-067047 may result in blockade of TRPV4 channels on the membrane of TRPV4-expressing bladder cells and subsequent decreased Ca2+ activity, reduced ATP secretion, and decreased bladder afferent activity. Longer-term changes in voiding function after intravesical instillation of HC-067047 may involve effects on trafficking and spatial and temporal distribution of TRPV4 in the membrane (6) and anti-inflammatory effects of TRPV4, including reductions in immune cell infiltration and cytokine and chemokine release (5, 14, 53). It would be of interest in future studies to evaluate the effects of intravesically instilled HC-067047 on mast cell infiltration (59), ERK and p38 signaling pathways, and inflammatory mediators in the urinary bladder of NGF-OE and WT mice and the time course of these effects.
Visceral inflammation, including urinary bladder inflammation, is accompanied by increased sensitivity of somatic structures to noxious stimuli, which is commonly called referred hyperalgesia (33, 34, 39, 59). We hypothesize that the reduction in pelvic sensitivity after intravesical infusion of HC-067047 is a locally mediated effect: the TRPV4 antagonist initiates a chain of events, beginning at the urinary bladder, involving blockade of TRPV4 channels on TRPV4-expressing cells in the bladder, decreased Ca2+ activity in TRPV4-expressing cells, reduced ATP secretion, and decreased bladder afferent activity. Reductions in peripheral bladder afferent activity could affect bladder afferent input to central (i.e., spinal cord) neurons that receive convergent inputs from the urinary bladder and somatic structures [e.g., the pelvic region (viscerosomatic convergence)] (49), resulting in decreased pelvic sensitivity. A previous study (1) has demonstrated that the TRPV4 agonist GSK1016790A increased capsaicin-sensitive bladder afferent activity in the rat, and our present study demonstrated reductions in ICI and VV with intravesical instillation of GSK1016790A in WT and NGF-OE mice. We have previously demonstrated reductions in bladder afferent activity (32, 35) with agents that increase bladder capacity. In the future, it should be determined if bladder afferent activity (32, 35) is reduced with intravesical instillation of HC-067047, consistent with reduced voiding frequency and ATP secretion demonstrated in the present study.
The cell types in the urinary bladder that may be affected by intravesical instillation of the TRPV4 antagonist HC-067047 include urothelial cells and interstitial cells in the lamina propria. Functional TRPV4 expression in urothelial cells (23, 24, 46) has been established after measurements of ionic currents and Ca2+ events induced by TRPV4 agonists (4α-phorbol 12,13-didecanoate and GSK1016790A) or stretch (40, 50, 73). TRPV4 expression has also been examined in DRG neurons innervating the viscera (2, 29, 45, 74), but functional evidence is controversial: there is evidence for and against functional TRPV4 channels in DRG (Ref. 2 and M. A. Vizzard, R. L. Parsons, and J. D. Tompkins, unpublished observations). In contrast, we recently demonstrated functional expression of TRPV4 in lamina propria cells in postnatal rat pups (36). In whole mount tissue preparations isolated from rat pups at or before postnatal day 21, TRPV4 immunoreactivity was observed in cells in the lamina propria that exhibited morphology similar to those expressing platelet-derived growth factor receptor-α immunoreactivity (36). Application of the TRPV4 agonist GSK1016790 increased the time during which lamina propria cells were active and increased the number of cells that exhibited active Ca2+ events, as evidenced by the rate of integrated Ca2+ activity (36). Future studies will include evaluation of Ca2+ events in urothelial and lamina propria cells in WT and NGF-OE mice in response to TRPV4 agonist and antagonist application (35, 36).
Because of its diverse actions within the bladder, aberrant ATP signaling has been suggested to play a significant role in several functional lower urinary tract pathologies (e.g., overactive bladder and IC/BPS) (57, 61, 65). Pathological conditions of the bladder that elevate luminal ATP presumably activate mucosal purinoceptors to increase nerve excitability (7, 32, 70, 76). One mechanism that may account for this effect is autocrine purinoceptor activation of the urothelium, stimulating continual ATP release (65). This aberrant signaling of ATP could be limited by controlling extracellular release from the urothelium and/or its binding to purinergic receptors within deeper layers of the bladder wall. Several mechanisms of urothelial ATP release, such as vesicular exocytosis (32, 44, 64, 72), pannexin and/or connexin ion channels (7, 44, 64), and nucleoside transporters (41, 72), have been demonstrated. These experiments focused on the pharmacological manipulation of vesicular exocytosis and pannexin-1 channels. We intravesically instilled the general secretory inhibitor BFA for vesicular release inhibition. BFA has been shown to decrease mechanical- and stretch-evoked ATP release from the urothelium (32, 44, 64, 71). We also intravesically instilled the inhibitory peptide 10Panx to block pannexin-1 channels, which have been implicated in distension-evoked ATP release from the urothelium with pharmacological or genetic manipulation (7, 32, 54, 68).
ATP release from urothelial cells and subsequent binding to purinergic receptors regulate a variety of actions in the urinary bladder and micturition reflexes, including sensory nerve activity, detrusor smooth muscle contraction, synaptic transmission, and nociception (11, 64, 72). Intravesical instillation of ATP enhanced spinal bladder neuron excitability (52) and increased bladder activity (55). In contrast, NVCs and voiding frequency were decreased when luminal ATP release was attenuated with the inhibition of vesicular (62) or pannexin-1 channel (8, 68) release mechanisms, respectively. Additionally, previous studies have suggested a functional role for TRPV4 in ATP release, such that TRPV4 activation, by mechanical stretch or agonist stimulation, leads to an increase in Ca2+ and ATP that is attenuated in TRPV4 KO mice or by a TRPV4 antagonist (24, 50). Our experiments with BFA, 10Panx, or coadministration of BFA and 10Panx in NGF-OE and WT mice with urinary bladder distension to 25 cmH2O demonstrated that vesicular exocytosis contributed to the majority of the distension-evoked luminal ATP release, consistent with a previous study (32). We quantified luminal ATP after functionally inhibiting known mechanisms of ATP release via vesicular exocytosis and pannexin channels as well as through TRPV4 (24, 50) and purinoceptor P2X (64, 66, 70) blockade. Upon intravesical inhibition of TRPV4 or inhibition of vesicular release in the urinary bladder, distension-induced luminal ATP release was decreased in both WT and NGF-OE mice, although the magnitude of reduction was increased in NGF-OE mice. Inhibition of pannexin channels locally in the urinary bladder (32) did not cause a decrease in the luminal release of ATP in WT or NGF-OE mice. These data suggest that, within the urinary bladder, ATP is released by vesicular exocytosis and TRPV4 has a functional role in this release. While these results suggest a vesicular secretory mechanism, the effects of BFA may also generalize to inhibit the transport of cell surface proteins, such as hemichannels, to attenuate release (71); thus, additional studies are needed to separate the effects of vesicle release and hemichannel inhibition. In addition, future studies using an ex vivo bladder-nerve preparation will evaluate the effects of blocking distension-induced luminal ATP release with TRPV4 antagonism or BFA on bladder afferent activity (35).
GRANTS
This work was funded by National Institute of Diabetes and Digestive and Kidney Diseases Grants DK-051369, DK-060481, DK-065989, and DK-120108 (to M. A. Vizzard) and R37-DK-05382 (to M. T. Nelson). This publication was also supported by National Center for Research Resources Grant 5-P30-RR-032135) and National Institute of General Medical Sciences Grant 8-P30-GM-103498.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
B.M.G. and M.A.V. conceived and designed research; B.M.G., S.E.C., M.P., C.D., G.W.H., T.J.H., and M.A.V. performed experiments; B.M.G., S.E.C., M.P., G.W.H., T.J.H., and M.A.V. analyzed data; B.M.G., S.E.C., G.W.H., T.J.H., and M.A.V. interpreted results of experiments; B.M.G. and M.A.V. prepared figures; B.M.G., M.P., H.H., K.T., G.W.H., and M.A.V. drafted manuscript; B.M.G., S.E.C., M.P., H.H., K.T., G.W.H., T.J.H., M.T.N., and M.A.V. edited and revised manuscript; B.M.G., S.E.C., M.P., H.H., K.T., C.D., G.W.H., T.J.H., M.T.N., and M.A.V. approved final version of manuscript.
REFERENCES
- 1.Aizawa N, Wyndaele JJ, Homma Y, Igawa Y. Effects of TRPV4 cation channel activation on the primary bladder afferent activities of the rat. Neurourol Urodyn 31: 148–155, 2012. doi: 10.1002/nau.21212. [DOI] [PubMed] [Google Scholar]
- 2.Alexander R, Kerby A, Aubdool AA, Power AR, Grover S, Gentry C, Grant AD. 4α-Phorbol 12,13-didecanoate activates cultured mouse dorsal root ganglia neurons independently of TRPV4. Br J Pharmacol 168: 761–772, 2013. doi: 10.1111/j.1476-5381.2012.02186.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andersson KE, Gratzke C, Hedlund P. The role of the transient receptor potential (TRP) superfamily of cation-selective channels in the management of the overactive bladder. BJU Int 106: 1114–1127, 2010. doi: 10.1111/j.1464-410X.2010.09650.x. [DOI] [PubMed] [Google Scholar]
- 4.Araki I, Yoshiyama M, Kobayashi H, Mochizuki T, Du S, Okada Y, Takeda M. Emerging families of ion channels involved in urinary bladder nociception. Pharmaceuticals (Basel) 3: 2248–2267, 2010. doi: 10.3390/ph3072248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Balakrishna S, Song W, Achanta S, Doran SF, Liu B, Kaelberer MM, Yu Z, Sui A, Cheung M, Leishman E, Eidam HS, Ye G, Willette RN, Thorneloe KS, Bradshaw HB, Matalon S, Jordt SE. TRPV4 inhibition counteracts edema and inflammation and improves pulmonary function and oxygen saturation in chemically induced acute lung injury. Am J Physiol Lung Cell Mol Physiol 307: L158–L172, 2014. doi: 10.1152/ajplung.00065.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baratchi S, Keov P, Darby WG, Lai A, Khoshmanesh K, Thurgood P, Vahidi P, Ejendal K, McIntyre P. The TRPV4 agonist GSK1016790A regulates the membrane expression of TRPV4 channels. Front Pharmacol 10: 6, 2019. doi: 10.3389/fphar.2019.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beckel JM, Daugherty SL, Tyagi P, Wolf-Johnston AS, Birder LA, Mitchell CH, de Groat WC. Pannexin 1 channels mediate the release of ATP into the lumen of the rat urinary bladder. J Physiol 593: 1857–1871, 2015. doi: 10.1113/jphysiol.2014.283119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Birder LA. TRPs in bladder diseases. Biochim Biophys Acta 1772: 879–884, 2007. doi: 10.1016/j.bbadis.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Birder LA, Nakamura Y, Kiss S, Nealen ML, Barrick S, Kanai AJ, Wang E, Ruiz G, De Groat WC, Apodaca G, Watkins S, Caterina MJ. Altered urinary bladder function in mice lacking the vanilloid receptor TRPV1. Nat Neurosci 5: 856–860, 2002. doi: 10.1038/nn902. [DOI] [PubMed] [Google Scholar]
- 11.Chen CC, Akopian AN, Sivilotti L, Colquhoun D, Burnstock G, Wood JN. A P2X purinoceptor expressed by a subset of sensory neurons. Nature 377: 428–431, 1995. doi: 10.1038/377428a0. [DOI] [PubMed] [Google Scholar]
- 12.Cheppudira BP, Girard BM, Malley SE, Dattilio A, Schutz KC, May V, Vizzard MA. Involvement of JAK-STAT signaling/function after cyclophosphamide-induced bladder inflammation in female rats. Am J Physiol Renal Physiol 297: F1038–F1044, 2009. doi: 10.1152/ajprenal.00110.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cruz CD. Neurotrophins in bladder function: what do we know and where do we go from here? Neurourol Urodyn 33: 39–45, 2014. doi: 10.1002/nau.22438. [DOI] [PubMed] [Google Scholar]
- 14.Dalsgaard T, Sonkusare SK, Teuscher C, Poynter ME, Nelson MT. Pharmacological inhibitors of TRPV4 channels reduce cytokine production, restore endothelial function and increase survival in septic mice. Sci Rep 6: 33841, 2016. doi: 10.1038/srep33841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dang K, Lamb K, Cohen M, Bielefeldt K, Gebhart GF. Cyclophosphamide-induced bladder inflammation sensitizes and enhances P2X receptor function in rat bladder sensory neurons. J Neurophysiol 99: 49–59, 2008. doi: 10.1152/jn.00211.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Deruyver Y, Weyne E, Dewulf K, Rietjens R, Pinto S, Van Ranst N, Franken J, Vanneste M, Albersen M, Gevaert T, Vennekens R, De Ridder D, Voets T, Everaerts W. Intravesical activation of the cation channel TRPV4 improves bladder function in a rat model for detrusor underactivity. Eur Urol 74: 336–345, 2018. doi: 10.1016/j.eururo.2018.05.020. [DOI] [PubMed] [Google Scholar]
- 17.Duh K, Funaro MG, DeGouveia W, Bahlani S, Pappas D, Najjar S, Tabansky I, Moldwin R, Stern JNH. Crosstalk between the immune system and neural pathways in interstitial cystitis/bladder pain syndrome. Discov Med 25: 243–250, 2018. [PubMed] [Google Scholar]
- 18.Ernsberger U. Role of neurotrophin signalling in the differentiation of neurons from dorsal root ganglia and sympathetic ganglia. Cell Tissue Res 336: 349–384, 2009. doi: 10.1007/s00441-009-0784-z. [DOI] [PubMed] [Google Scholar]
- 19.Everaerts W, Nilius B, Owsianik G. The vanilloid transient receptor potential channel TRPV4: from structure to disease. Prog Biophys Mol Biol 103: 2–17, 2010. doi: 10.1016/j.pbiomolbio.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 20.Everaerts W, Vriens J, Owsianik G, Appendino G, Voets T, De Ridder D, Nilius B. Functional characterization of transient receptor potential channels in mouse urothelial cells. Am J Physiol Renal Physiol 298: F692–F701, 2010. doi: 10.1152/ajprenal.00599.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Everaerts W, Zhen X, Ghosh D, Vriens J, Gevaert T, Gilbert JP, Hayward NJ, McNamara CR, Xue F, Moran MM, Strassmaier T, Uykal E, Owsianik G, Vennekens R, De Ridder D, Nilius B, Fanger CM, Voets T. Inhibition of the cation channel TRPV4 improves bladder function in mice and rats with cyclophosphamide-induced cystitis. Proc Natl Acad Sci USA 107: 19084–19089, 2010. doi: 10.1073/pnas.1005333107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Franken J, Uvin P, De Ridder D, Voets T. TRP channels in lower urinary tract dysfunction. Br J Pharmacol 171: 2537–2551, 2014. doi: 10.1111/bph.12502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gevaert T, Vandepitte J, Hutchings G, Vriens J, Nilius B, De Ridder D. TRPV1 is involved in stretch-evoked contractile changes in the rat autonomous bladder model: a study with piperine, a new TRPV1 agonist. Neurourol Urodyn 26: 440–450, 2007. doi: 10.1002/nau.20343. [DOI] [PubMed] [Google Scholar]
- 24.Gevaert T, Vriens J, Segal A, Everaerts W, Roskams T, Talavera K, Owsianik G, Liedtke W, Daelemans D, Dewachter I, Van Leuven F, Voets T, De Ridder D, Nilius B. Deletion of the transient receptor potential cation channel TRPV4 impairs murine bladder voiding. J Clin Invest 117: 3453–3462, 2007. doi: 10.1172/JCI31766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Girard B, Peterson A, Malley S, Vizzard MA. Accelerated onset of the vesicovesical reflex in postnatal NGF-OE mice and the role of neuropeptides. Exp Neurol 285: 110–125, 2016. doi: 10.1016/j.expneurol.2016.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Girard BM, Malley SE, Braas KM, May V, Vizzard MA. PACAP/VIP and receptor characterization in micturition pathways in mice with overexpression of NGF in urothelium. J Mol Neurosci 42: 378–389, 2010. doi: 10.1007/s12031-010-9384-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Girard BM, Malley SE, Mathews MM, May V, Vizzard MA. Intravesical PAC1 receptor antagonist, PACAP(6-38), reduces urinary bladder frequency and pelvic sensitivity in NGF-OE mice. J Mol Neurosci 59: 290–299, 2016. doi: 10.1007/s12031-016-0764-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Girard BM, Malley SE, Vizzard MA. Neurotrophin/receptor expression in urinary bladder of mice with overexpression of NGF in urothelium. Am J Physiol Renal Physiol 300: F345–F355, 2011. doi: 10.1152/ajprenal.00515.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Girard BM, Merrill L, Malley S, Vizzard MA. Increased TRPV4 expression in urinary bladder and lumbosacral dorsal root ganglia in mice with chronic overexpression of NGF in urothelium. J Mol Neurosci 51: 602–614, 2013. doi: 10.1007/s12031-013-0033-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Girard BM, Tompkins JD, Parsons RL, May V, Vizzard MA. Effects of CYP-induced cystitis on PACAP/VIP and receptor expression in micturition pathways and bladder function in mice with overexpression of NGF in urothelium. J Mol Neurosci 48: 730–743, 2012. doi: 10.1007/s12031-012-9834-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gonzalez EJ, Girard BM, Vizzard MA. Expression and function of transforming growth factor-β isoforms and cognate receptors in the rat urinary bladder following cyclophosphamide-induced cystitis. Am J Physiol Renal Physiol 305: F1265–F1276, 2013. doi: 10.1152/ajprenal.00042.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gonzalez EJ, Heppner TJ, Nelson MT, Vizzard MA. Purinergic signalling underlies transforming growth factor-β-mediated bladder afferent nerve hyperexcitability. J Physiol 594: 3575–3588, 2016. doi: 10.1113/JP272148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guerios SD, Wang ZY, Bjorling DE. Nerve growth factor mediates peripheral mechanical hypersensitivity that accompanies experimental cystitis in mice. Neurosci Lett 392: 193–197, 2006. doi: 10.1016/j.neulet.2005.09.026. [DOI] [PubMed] [Google Scholar]
- 34.Guerios SD, Wang ZY, Boldon K, Bushman W, Bjorling DE. Blockade of NGF and trk receptors inhibits increased peripheral mechanical sensitivity accompanying cystitis in rats. Am J Physiol Regul Integr Comp Physiol 295: R111–R122, 2008. doi: 10.1152/ajpregu.00728.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Heppner TJ, Hennig GW, Nelson MT, May V, Vizzard MA. PACAP38-mediated bladder afferent nerve activity hyperexcitability and Ca2+ activity in urothelial cells from mice. J Mol Neurosci 68: 348–356, 2019. doi: 10.1007/s12031-018-1119-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Heppner TJ, Hennig GW, Nelson MT, Vizzard MA. Rhythmic calcium events in the lamina propria network of the urinary bladder of rat pups. Front Syst Neurosci 11: 87, 2017. doi: 10.3389/fnsys.2017.00087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Homma Y, Nomiya A, Tagaya M, Oyama T, Takagaki K, Nishimatsu H, Igawa Y. Increased mRNA expression of genes involved in pronociceptive inflammatory reactions in bladder tissue of interstitial cystitis. J Urol 190: 1925–1931, 2013. doi: 10.1016/j.juro.2013.05.049. [DOI] [PubMed] [Google Scholar]
- 38.Hu VY, Zvara P, Dattilio A, Redman TL, Allen SJ, Dawbarn D, Stroemer RP, Vizzard MA. Decrease in bladder overactivity with REN1820 in rats with cyclophosphamide induced cystitis. J Urol 173: 1016–1021, 2005. doi: 10.1097/01.ju.0000155170.15023.e5. [DOI] [PubMed] [Google Scholar]
- 39.Jaggar SI, Scott HC, Rice AS. Inflammation of the rat urinary bladder is associated with a referred thermal hyperalgesia which is nerve growth factor dependent. Br J Anaesth 83: 442–448, 1999. doi: 10.1093/bja/83.3.442. [DOI] [PubMed] [Google Scholar]
- 40.Kullmann FA, Shah MA, Birder LA, de Groat WC. Functional TRP and ASIC-like channels in cultured urothelial cells from the rat. Am J Physiol Renal Physiol 296: F892–F901, 2009. doi: 10.1152/ajprenal.90718.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lazarowski ER, Boucher RC, Harden TK. Mechanisms of release of nucleotides and integration of their action as P2X- and P2Y-receptor activating molecules. Mol Pharmacol 64: 785–795, 2003. doi: 10.1124/mol.64.4.785. [DOI] [PubMed] [Google Scholar]
- 42.Lee H, Koh BH, Peri LE, Corrigan RD, Lee HT, George NE, Bhetwal BP, Xie Y, Perrino BA, Chai TC, Sanders KM, Koh SD. Premature contractions of the bladder are suppressed by interactions between TRPV4 and SK3 channels in murine detrusor PDGFRα+ cells. Sci Rep 7: 12245, 2017. doi: 10.1038/s41598-017-12561-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Malin S, Molliver D, Christianson JA, Schwartz ES, Cornuet P, Albers KM, Davis BM. TRPV1 and TRPA1 function and modulation are target tissue dependent. J Neurosci 31: 10516–10528, 2011. doi: 10.1523/JNEUROSCI.2992-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McLatchie LM, Fry CH. ATP release from freshly isolated guinea-pig bladder urothelial cells: a quantification and study of the mechanisms involved. BJU Int 115: 987–993, 2015. doi: 10.1111/bju.12954. [DOI] [PubMed] [Google Scholar]
- 45.Merrill L, Girard BM, May V, Vizzard MA. Transcriptional and translational plasticity in rodent urinary bladder TRP channels with urinary bladder inflammation, bladder dysfunction, or postnatal maturation. J Mol Neurosci 48: 744–756, 2012. doi: 10.1007/s12031-012-9867-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Merrill L, Gonzalez EJ, Girard BM, Vizzard MA. Receptors, channels, and signalling in the urothelial sensory system in the bladder. Nat Rev Urol 13: 193–204, 2016. doi: 10.1038/nrurol.2016.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Merrill L, Malley S, Vizzard MA. Repeated variate stress in male rats induces increased voiding frequency, somatic sensitivity, and urinary bladder nerve growth factor expression. Am J Physiol Regul Integr Comp Physiol 305: R147–R156, 2013. doi: 10.1152/ajpregu.00089.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Merrill L, Vizzard MA. Intravesical TRPV4 blockade reduces repeated variate stress-induced bladder dysfunction by increasing bladder capacity and decreasing voiding frequency in male rats. Am J Physiol Regul Integr Comp Physiol 307: R471–R480, 2014. doi: 10.1152/ajpregu.00008.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Milne RJ, Foreman RD, Giesler GJ Jr, Willis WD. Convergence of cutaneous and pelvic visceral nociceptive inputs onto primate spinothalamic neurons. Pain 11: 163–183, 1981. doi: 10.1016/0304-3959(81)90003-8. [DOI] [PubMed] [Google Scholar]
- 50.Mochizuki T, Sokabe T, Araki I, Fujishita K, Shibasaki K, Uchida K, Naruse K, Koizumi S, Takeda M, Tominaga M. The TRPV4 cation channel mediates stretch-evoked Ca2+ influx and ATP release in primary urothelial cell cultures. J Biol Chem 284: 21257–21264, 2009. doi: 10.1074/jbc.M109.020206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mullins C, Bavendam T, Kirkali Z, Kusek JW. Novel research approaches for interstitial cystitis/bladder pain syndrome: thinking beyond the bladder. Transl Androl Urol 4: 524–533, 2015. doi: 10.3978/j.issn.2223-4683.2015.08.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Munoz A, Somogyi GT, Boone TB, Smith CP. Lumbosacral sensory neuronal activity is enhanced by activation of urothelial purinergic receptors. Brain Res Bull 86: 380–384, 2011. doi: 10.1016/j.brainresbull.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 53.Nayak PS, Wang Y, Najrana T, Priolo LM, Rios M, Shaw SK, Sanchez-Esteban J. Mechanotransduction via TRPV4 regulates inflammation and differentiation in fetal mouse distal lung epithelial cells. Respir Res 16: 60, 2015. doi: 10.1186/s12931-015-0224-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Negoro H, Urban-Maldonado M, Liou LS, Spray DC, Thi MM, Suadicani SO. Pannexin 1 channels play essential roles in urothelial mechanotransduction and intercellular signaling. PLoS One 9: e106269, 2014. doi: 10.1371/journal.pone.0106269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pandita RK, Andersson KE. Intravesical adenosine triphosphate stimulates the micturition reflex in awake, freely moving rats. J Urol 168: 1230–1234, 2002. doi: 10.1016/S0022-5347(05)64631-9. [DOI] [PubMed] [Google Scholar]
- 56.Pelegrin P, Surprenant A. Pannexin-1 mediates large pore formation and interleukin-1β release by the ATP-gated P2X7 receptor. EMBO J 25: 5071–5082, 2006. doi: 10.1038/sj.emboj.7601378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ruggieri MR., Sr Mechanisms of disease: role of purinergic signaling in the pathophysiology of bladder dysfunction. Nat Clin Pract Urol 3: 206–215, 2006. doi: 10.1038/ncpuro0456. [DOI] [PubMed] [Google Scholar]
- 58.Sant GR, Hanno PM. Interstitial cystitis: current issues and controversies in diagnosis. Urology 57, Suppl 1: 82–88, 2001. doi: 10.1016/S0090-4295(01)01131-1. [DOI] [PubMed] [Google Scholar]
- 59.Schnegelsberg B, Sun TT, Cain G, Bhattacharya A, Nunn PA, Ford AP, Vizzard MA, Cockayne DA. Overexpression of NGF in mouse urothelium leads to neuronal hyperinnervation, pelvic sensitivity, and changes in urinary bladder function. Am J Physiol Regul Integr Comp Physiol 298: R534–R547, 2010. doi: 10.1152/ajpregu.00367.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shimizu N, Wada N, Shimizu T, Suzuki T, Takaoka EI, Kanai AJ, de Groat WC, Hirayama A, Hashimoto M, Uemura H, Yoshimura N. Effects of nerve growth factor neutralization on TRP channel expression in laser-captured bladder afferent neurons in mice with spinal cord injury. Neurosci Lett 683: 100–103, 2018. doi: 10.1016/j.neulet.2018.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Silva-Ramos M, Silva I, Oliveira O, Ferreira S, Reis MJ, Oliveira JC, Correia-de-Sá P. Urinary ATP may be a dynamic biomarker of detrusor overactivity in women with overactive bladder syndrome. PLoS One 8: e64696, 2013. doi: 10.1371/journal.pone.0064696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Smith CP, Vemulakonda VM, Kiss S, Boone TB, Somogyi GT. Enhanced ATP release from rat bladder urothelium during chronic bladder inflammation: effect of botulinum toxin A. Neurochem Int 47: 291–297, 2005. doi: 10.1016/j.neuint.2005.04.021. [DOI] [PubMed] [Google Scholar]
- 63.Studeny S, Cheppudira BP, Meyers S, Balestreire EM, Apodaca G, Birder LA, Braas KM, Waschek JA, May V, Vizzard MA. Urinary bladder function and somatic sensitivity in vasoactive intestinal polypeptide (VIP)−/− mice. J Mol Neurosci 36: 175–187, 2008. doi: 10.1007/s12031-008-9100-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sui G, Fry CH, Montgomery B, Roberts M, Wu R, Wu C. Purinergic and muscarinic modulation of ATP release from the urothelium and its paracrine actions. Am J Physiol Renal Physiol 306: F286–F298, 2014. doi: 10.1152/ajprenal.00291.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sun Y, Chai TC. Augmented extracellular ATP signaling in bladder urothelial cells from patients with interstitial cystitis. Am J Physiol Cell Physiol 290: C27–C34, 2006. doi: 10.1152/ajpcell.00552.2004. [DOI] [PubMed] [Google Scholar]
- 66.Sun Y, Keay S, Lehrfeld TJ, Chai TC. Changes in adenosine triphosphate-stimulated ATP release suggest association between cytokine and purinergic signaling in bladder urothelial cells. Urology 74: 1163–1168, 2009. doi: 10.1016/j.urology.2009.02.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Thorneloe KS, Sulpizio AC, Lin Z, Figueroa DJ, Clouse AK, McCafferty GP, Chendrimada TP, Lashinger ES, Gordon E, Evans L, Misajet BA, Demarini DJ, Nation JH, Casillas LN, Marquis RW, Votta BJ, Sheardown SA, Xu X, Brooks DP, Laping NJ, Westfall TD. N-((1S)-1-{[4-((2S)-2-{[(2,4-dichlorophenyl)sulfonyl]amino}-3-hydroxypropanoyl)-1-piperazinyl]carbonyl}-3-methylbutyl)-1-benzothiophene-2-carboxamide (GSK1016790A), a novel and potent transient receptor potential vanilloid 4 channel agonist induces urinary bladder contraction and hyperactivity. Part I. J Pharmacol Exp Ther 326: 432–442, 2008. doi: 10.1124/jpet.108.139295. [DOI] [PubMed] [Google Scholar]
- 68.Timóteo MA, Carneiro I, Silva I, Noronha-Matos JB, Ferreirinha F, Silva-Ramos M, Correia-de-Sá P. ATP released via pannexin-1 hemichannels mediates bladder overactivity triggered by urothelial P2Y6 receptors. Biochem Pharmacol 87: 371–379, 2014. doi: 10.1016/j.bcp.2013.11.007. [DOI] [PubMed] [Google Scholar]
- 69.Vizzard MA. Changes in urinary bladder neurotrophic factor mRNA and NGF protein following urinary bladder dysfunction. Exp Neurol 161: 273–284, 2000. doi: 10.1006/exnr.1999.7254. [DOI] [PubMed] [Google Scholar]
- 70.Vlaskovska M, Kasakov L, Rong W, Bodin P, Bardini M, Cockayne DA, Ford AP, Burnstock G. P2X3 knock-out mice reveal a major sensory role for urothelially released ATP. J Neurosci 21: 5670–5677, 2001. doi: 10.1523/JNEUROSCI.21-15-05670.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wang EC, Lee JM, Ruiz WG, Balestreire EM, von Bodungen M, Barrick S, Cockayne DA, Birder LA, Apodaca G. ATP and purinergic receptor-dependent membrane traffic in bladder umbrella cells. J Clin Invest 115: 2412–2422, 2005. doi: 10.1172/JCI24086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang L, Olivecrona G, Götberg M, Olsson ML, Winzell MS, Erlinge D. ADP acting on P2Y13 receptors is a negative feedback pathway for ATP release from human red blood cells. Circ Res 96: 189–196, 2005. doi: 10.1161/01.RES.0000153670.07559.E4. [DOI] [PubMed] [Google Scholar]
- 73.Xu X, Gordon E, Lin Z, Lozinskaya IM, Chen Y, Thorneloe KS. Functional TRPV4 channels and an absence of capsaicin-evoked currents in freshly-isolated, guinea-pig urothelial cells. Channels (Austin) 3: 156–160, 2009. doi: 10.4161/chan.3.3.8555. [DOI] [PubMed] [Google Scholar]
- 74.Yamada T, Ugawa S, Ueda T, Ishida Y, Kajita K, Shimada S. Differential localizations of the transient receptor potential channels TRPV4 and TRPV1 in the mouse urinary bladder. J Histochem Cytochem 57: 277–287, 2009. doi: 10.1369/jhc.2008.951962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yoshiyama M, Mochizuki T, Nakagomi H, Miyamoto T, Kira S, Mizumachi R, Sokabe T, Takayama Y, Tominaga M, Takeda M. Functional roles of TRPV1 and TRPV4 in control of lower urinary tract activity: dual analysis of behavior and reflex during the micturition cycle. Am J Physiol Renal Physiol 308: F1128–F1134, 2015. doi: 10.1152/ajprenal.00016.2015. [DOI] [PubMed] [Google Scholar]
- 76.Yu Y, de Groat WC. Sensitization of pelvic afferent nerves in the in vitro rat urinary bladder-pelvic nerve preparation by purinergic agonists and cyclophosphamide pretreatment. Am J Physiol Renal Physiol 294: F1146–F1156, 2008. doi: 10.1152/ajprenal.00592.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]