Abstract
The metabolic sensor AMP-activated protein kinase (AMPK) inhibits the epithelial Na+ channel (ENaC), a key regulator of salt reabsorption by the kidney and thus total body volume and blood pressure. Recent studies have suggested that AMPK promotes the association of p21-activated kinase-interacting exchange factor-β1 β1Pix, 14-3-3 proteins, and the ubiquitin ligase neural precursor cell expressed developmentally downregulated protein (Nedd)4-2 into a complex that inhibits ENaC by enhancing Nedd4-2 binding to ENaC and ENaC degradation. Functional β1Pix is required for ENaC inhibition by AMPK and promotes Nedd4-2 phosphorylation and stability in mouse kidney cortical collecting duct cells. Here, we report that AMPK directly phosphorylates β1Pix in vitro. Among several AMPK phosphorylation sites on β1Pix detected by mass spectrometry, Ser71 was validated as functionally significant. Compared with wild-type β1Pix, overexpression of a phosphorylation-deficient β1Pix-S71A mutant attenuated ENaC inhibition and the AMPK-activated interaction of both β1Pix and Nedd4-2 to 14-3-3 proteins in cortical collecting duct cells. Similarly, overexpression of a β1Pix-Δ602–611 deletion tract mutant unable to bind 14-3-3 proteins decreased the interaction between Nedd4-2 and 14-3-3 proteins, suggesting that 14-3-3 binding to β1Pix is critical for the formation of a β1Pix/Nedd4-2/14-3-3 complex. With expression of a general peptide inhibitor of 14-3-3-target protein interactions (R18), binding of both β1Pix and Nedd4-2 to 14-3-3 proteins was reduced, and AMPK-dependent ENaC inhibition was also attenuated. Altogether, our results demonstrate the importance of AMPK-mediated phosphorylation of β1Pix at Ser71, which promotes 14-3-3 interactions with β1Pix and Nedd4-2 to form a tripartite ENaC inhibitory complex, in the mechanism of ENaC regulation by AMPK.
Keywords: AMP-activated protein kinase, epithelial Na+ channel, neural precursor cell expressed developmentally downregulated protein 4-2, p21-activated kinase-interacting exchange factor-β1, 14-3-3 protein
INTRODUCTION
The epithelial Na+ channel (ENaC) is composed of three homologous α-, β-, and γ-subunits assembled as a heterotrimeric channel (48) and is expressed at the apical plasma membrane in many epithelial tissues, including the lung, colon, and kidney (11, 24). By mediating the reabsorption of Na+ in the distal renal tubule, ENaC is a key regulator of total body salt and fluid homeostasis as well as blood pressure. Abnormal ENaC function leads to a number of diseases, including type 1 pseudohypoaldosteronism, Liddle syndrome, and cystic fibrosis (7).
Neural precursor cell expressed developmentally downregulated protein (Nedd)4-2, a member of the E6-associated protein COOH-terminus family of E3 ubiquitin ligases, regulates the expression and activity of ENaC through directly binding to “PY” motifs on the COOH-termini of ENaC subunits, leading to ENaC ubiquitination and degradation. Genetic deletion of Nedd4-2 expression in mice, both globally and specifically in kidney tubules, causes hypertension and progressive kidney disease with increased ENaC retention at the apical membrane in the tubular epithelia, indicating that ENaC is a primary target of Nedd4-2 in vivo (31, 53). Various kinases, including serum and glucocorticoid-regulated kinase 1 (SGK1), PKA, IκB kinase-β, and AMPK, modulate Nedd4-2 function by phosphorylation. Phosphorylation of Xenopus Nedd4-2 (xNedd4-2) on Ser444 (equivalent to Ser328 of mNedd4-2) promotes Nedd4-2 cellular stability and its association with 14-3-3 scaffolding proteins (14, 54).
AMPK, a serine/threonine kinase that exists as a heterotrimer comprised of a catalytic α-subunit and regulatory β- and γ-subunits, has been recognized as a sensor of cellular energy homeostasis. AMPK regulates key metabolic enzymes, cell growth, apoptosis, gene transcription, and protein synthesis (29). In recent years, the roles of AMPK in renal physiology and disease have been investigated extensively (10, 38, 46, 51, 52, 58). We have recently demonstrated that AMPK inhibits ENaC through directly phosphorylating xNedd4-2 at Ser444 and enhancing the binding of Nedd4-2 to ENaC (8, 32). Previous studies from our group and others have indicated that AMPK enhances Nedd4-2-dependent retrieval and ubiquitination of ENaC (3, 8). In AMPKα1 knockout mice, increased ENaC expression was detected in the colon, airways, and kidney, suggesting that AMPK is a physiological regulator of ENaC (3). AMPK also plays a role in the endocytosis and Nedd4-2-dependent degradation of ENaC in hypercapnia caused by acute lung injury (28). Of note, we and others have suggested the importance of AMPK in the regulation of ENaC and other ion transport proteins as a sensitive mechanism for the coupling of ion transport, which is energetically costly to cells, to cellular metabolic status (3, 8, 12, 32, 49).
Small G proteins and guanine nucleotide exchange factors (GEFs) have been implicated in ENaC regulation. p21-activated kinase (PAK)-interacting exchange factor-β βPix is a critical regulatory cofactor in the endothelin-1 (ET-1)-dependent regulation of ENaC via Nedd4-2 and 14-3-3 proteins (50). βPix is a member of the diffuse B cell lymphoma family of Rho GEFs existing in two major isoforms: β1Pix and β2Pix. In the kidneys, β1Pix is the main isoform (37, 56). As a GEF for cell division cycle 42 and/or Rac1, βPix plays an important role in migration, actin cytoskeletal changes, and mitosis (39). Through sequestering c-Cbl ubiquitin ligase and preventing the ubiquitination of various growth factor receptors, β1Pix also mediates cellular transformation and in vivo tumorigenesis (57, 62). The leucine zipper domain on β1Pix is responsible for its dimerization and binding to 14-3-3 proteins (4, 5, 35, 36).
Our recent study (32) has demonstrated that expression of β1Pix is required for the stabilization of Nedd4-2 and thus ENaC inhibition in kidney epithelial cells. The findings indicate that, by facilitating the assembly of β1Pix, Nedd4-2, and 14-3-3 proteins into a complex, AMPK promotes Nedd4-2 stability and increased ENaC ubiquitination by Nedd4-2. Specifically, with shRNA-mediated knockdown of β1Pix in cortical collecting duct (CCD) cells, the inhibition of ENaC currents with AMPK activator treatment was blunted and the interactions of both β1Pix and Nedd4-2 with 14-3-3 proteins were reduced. Moreover, overexpression of a β1Pix-Δ602–611 deletion tract mutant significantly increased ENaC currents in CCD cells and diminished AMPK-dependent ENaC inhibition in Chinese hamster ovary cells. These results suggest a central role of β1Pix and its binding with 14-3-3 proteins in ENaC regulation. However, the underlying molecular mechanisms for this effect are presently unclear. In the present study, we tested the hypothesis that AMPK-mediated phosphorylation of β1Pix promotes Nedd4-2 stability by enhancing interactions with 14-3-3 proteins. The results indicate that direct phosphorylation of β1Pix by AMPK plays an important role in both the inhibition of ENaC and promotion of binding of β1Pix to 14-3-3 proteins caused by AMPK activation. We further show that the association of β1Pix and Nedd4-2 with 14-3-3 proteins is important for ENaC inhibition by AMPK.
MATERIALS AND METHODS
Reagents and chemicals.
All chemicals used were purchased from Sigma or ThermoFisher Scientific unless otherwise noted. [γ-32P]ATP was obtained from MP Biomedicals (Santa Ana, CA). Recombinant active human AMPK holoenzyme (α1-T172D, β1, γ1) was synthesized and purified as previously described (47) and generously provided by D. Neumann (Maastricht University). 5-Aminoimidazole-4-carboxamide-1-β-d-ribofuranoside (AICAR) and A769662 were obtained from Toronto Research Chemicals (North York, ON, Canada) and Selleck Chemicals (Houston, TX), respectively.
Cell culture.
Human embryonic kidney (HEK)-293 and mpkCCDc14 cells were maintained and cultured as previously described (12). mpkCCDc14 cells were grown in defined medium on permeable supports (Costar Transwells, 0.4-μm pore, 24-mm diameter), allowing them to polarize and form monolayers with high resistances and avid Na+ reabsorption.
DNA constructs and transfections.
Myc-tagged rat β1Pix-wild-type (WT) and β1Pix-∆602–611 cDNAs cloned into the pcDNA3.1 plasmid were obtained from A. Staruschenko (Medical College of Wisconsin) and then subcloned into the pMO plasmid followed by tag switching from Myc to V5 epitope to generate a pMO NH2-terminal V5-tagged β1Pix-WT or β1Pix-∆602–611 plasmid (30, 50). V5-tagged β1Pix point mutants were generated using the QuikChange site-directed mutagenesis kit (Agilent Technologies, Santa Clara, CA). Enhanced yellow fluorescent protein (EYFP)-tagged R18 or R18M (mutant) cDNAs were gifts from Haian Fu (Emory University) (40). DNA sequencing was performed to verify all expression constructs. To measure ENaC-equivalent short-circuit currents (Isc) and examine changes in the associations among β1Pix, Nedd4-2, and 14-3-3 proteins with AMPK modulation, mpkCCDc14 cells were seeded and transfected with mammalian expression vectors on Transwells using Metafectene (Biontex, Munich, Germany) according to the manufacturer’s instructions. After transfection, cells were grown for 48 h in growth medium before being treated with AMPK activators. Overexpression levels of various V5-tagged β1Pix constructs were determined by comparing the total cellular β1Pix level to that of empty vector-transfected cells by immunoblot analysis for β1Pix. R18 or R18M transfection efficiency was expressed as the estimated percentage of EYFP-positive cells using fluorescence microscopy.
LC-MS analysis.
To map the AMPK phosphorylation sites of β1Pix, HEK-293 cells were transiently transfected to overexpress V5-tagged β1Pix-WT. β1Pix-WT was immunoprecipitated from precleared lysates using an anti-V5 agarose affinity gel (Sigma) and subjected to in vitro phosphorylation assays using purified recombinant active human AMPK holoenzyme (α1-T172D, β1, γ1), as previously described (2). The phosphorylated β1Pix sample was further reduced, alkylated, and trypsin digested before detergent removal using HiPPR detergent removal resin (Thermo Scientific). Samples were then analyzed by nano-LC (Ultimate 3000, Dionex)-MS/MS (Q Exactive, ThermoFisher Scientific), using a 30-min gradient, as previously described (15); 64% sequence coverage of the protein was obtained. Mass spectra were searched using Proteome Discoverer software (Thermo Fischer Scientific) using the SEQUEST (63) server and rat RefSeq database. Localization and evaluation of identified phosphorylation sites were performed using Proteome Discoverer.
In vitro phosphorylation.
HEK-293 cells were transiently transfected using Lipofectamine 2000 (Invitrogen) to express V5-tagged β1Pix-WT, S71A, or S71D mutants of β1Pix. Cells were lysed in ice-cold RIPA buffer 1–2 days after transfection. Recombinant V5-tagged β1Pix was produced using a eukaryotic cell-free protein expression system (TNT T7 Quick Coupled Transcription/Translation System, Promega) according to the manufacturer’s protocol. V5-tagged β1Pix (WT, S71A, and S71D) was immunoprecipitated using anti-V5 agarose affinity gel. To remove preexisting phosphorylation, purified immunoprecipitated V5-tagged β1Pix was pretreated with λ-protein phosphatase (400 U/reaction, New England Biolabs) at 30°C for 30 min and then subjected to in vitro phosphorylation using purified active AMPK holoenzyme with [γ-32P]ATP labeling, as previously described (8). After SDS-PAGE and transfer to nitrocellulose membranes, immunoblot analysis for the expression of V5-tagged β1Pix was first performed and quantified using Image Studio Lite software (LI-COR). Phosphorylated bands on the membrane were identified by exposure of the same membrane to a phosphoscreen, and the detected bands were quantitated using the same image-analysis software. The intensity of each phosphoscreen band was corrected by subtracting out the local background in the same lane.
Electrophysiology.
A portable epithelial volt-ohmmeter (EVOM, World Precision Instruments, Sarasota, FL) was used to measure equivalent Isc across polarized cell monolayers. The electrode was calibrated by placing into growth medium for 90 min before measurements of the potential difference and resistance across the filter. The equivalent current was calculated by Ohm’s law using the potential difference across the filter divided by the resistance normalized to the surface area to obtain readings measured in μA/cm2 (42). Each data point within each experiment was normalized to the average of the respective control group (β1Pix-WT or R18M). Results are summarized from 3−5 experiments, with a total of 9−22 measurements per condition.
Coimmunoprecipitation assays.
To examine changes in the associations among β1Pix, Nedd4-2, and 14-3-3 proteins with AMPK modulation, cells were harvested and lysed in ice-cold immunoprecipitation lysis buffer [1% Triton X-100 and 2 mM EDTA (pH 8.0) in Dulbecco’s PBS with Ca2+ and Mg2+] after AICAR and A769662 (AA) treatment versus vehicle as indicated for 24 h. Precleared lysates were incubated with pan 14-3-3 antibody (1:100) coupled to protein A/G beads (ThermoFisher Scientific) overnight at 4°C. Immunoprecipitation in the absence of the pan 14-3-3 antibody was also performed as a no antibody control. After three washes with lysis buffer, immunoprecipitation samples were eluted in the sample buffer and, along with the cell lysate samples, subjected to immunoblot analysis to detect β1Pix, Nedd4-2, and 14-3-3 proteins. Relative binding was quantified by normalizing the coimmunoprecipitation signal of β1Pix or Nedd4-2 to the input amount and amount of the relevant immuneoprecipitated 14-3-3 proteins.
Immunoblot analysis.
Lysis and immunoblot analysis of cell lysates for Nedd4-2 (EMD Millipore), phospho-Nedd4-2 (Ser328, Abcam), V5 tag (Cell Signaling Technology), β1Pix (EMD Millipore), phospho-AMPK-α (Thr172, Cell Signaling Technology), AMPK pan α (Cell Signaling Technology), 14-3-3 (Santa Cruz Biotechnology), and β-actin (Sigma) were performed as previously described (50, 58). Gradient gels (4–12%, ThermoFisher Scientific) were used for SDS-PAGE. IRDye goat anti-rabbit and anti-mouse IgG Dylight 800 and 680 were obtained from LI-COR Biotechnology (Lincoln, NE). Membranes were scanned with an Odyssey Fc imaging system (LI-COR). Bands were quantified using Image Studio Lite software (LI-COR) on unprocessed raw data files. To compare immunoblot and coimmunoprecipitation results for specific conditions across multiple replicate experiments, we analyzed the data through the “normalization by sum of the replicate” technique, as previously described (19). Briefly, each data point from an individual group on a blot was divided by the sum of all raw data on the same blot. The data were then further normalized to the average calculated with all of the control group values from all experiments (8, 32).
Statistical analysis.
All summarized data are reported as means ± SE. Statistical analyses were performed using corresponding t-tests and one-way ANOVA with post hoc Bonferroni corrections for multiple comparisons. In all cases, P values of <0.05 were considered significant.
RESULTS
Identifying potential AMPK phosphorylation sites in β1Pix.
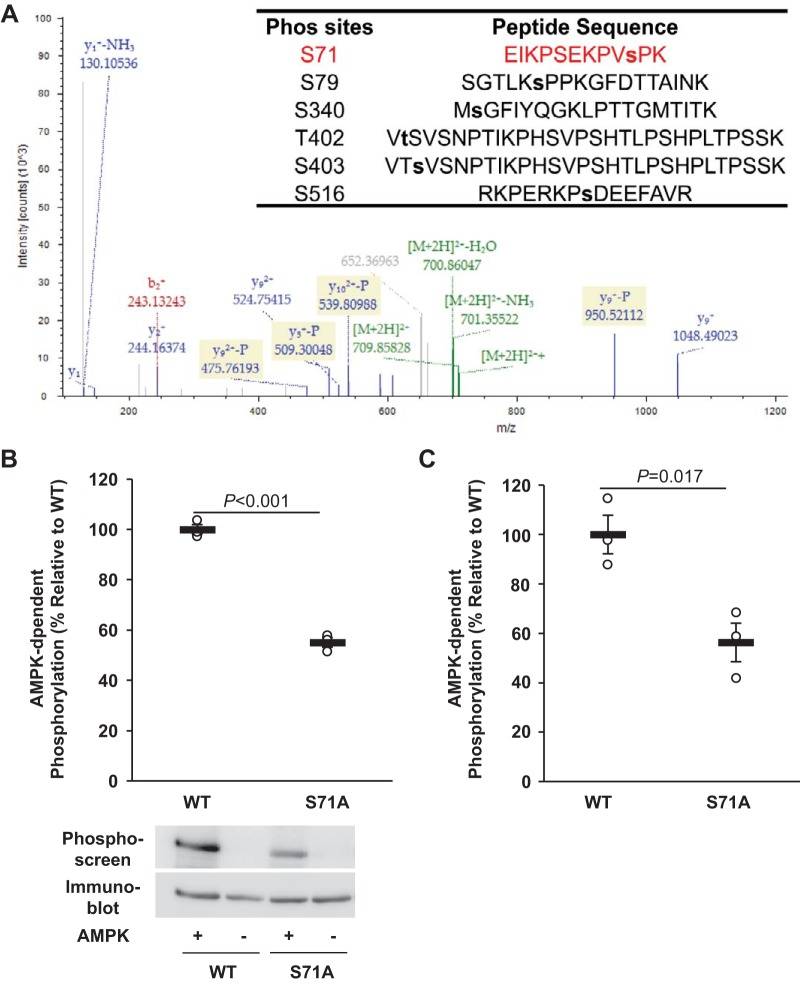
Our previous studies have demonstrated that AMPK enhances ENaC ubiquitination by phosphorylating and stabilizing Nedd4-2, which requires the involvement of β1Pix (32). To investigate whether β1Pix function is also regulated by AMPK through phosphorylation, V5-tagged rat β1Pix was expressed in HEK-293 cells, immunoprecipitated, and subjected to in vitro phosphorylation using purified active AMPK holoenzyme and ATP. Phosphorylation site mapping of tryptic peptides was performed by LC-MS/MS, as previously described (2, 33). We found that AMPK induced detectable phosphorylation of β1Pix at Ser71, Ser79, Ser340, Thr402, Ser403, and Ser516 in vitro (Fig. 1A). To verify AMPK phosphorylation at these sites by site-directed mutagenesis, we then compared [γ-32P]ATP incorporation into β1Pix-WT with that into Ser-to-Ala or Thr-to-Ala β1Pix mutants in vitro. Among these sites, only mutation at Ser71 significantly suppressed the 32P incorporation by ∼45% compared with β1Pix-WT (Fig. 1B). Alanine mutation of each of the other above putative sites identified by LC-MS/MS caused no significant changes in AMPK-dependent phosphorylation (data not shown). Given that Ser71 is also a predicted phosphorylation site for cell division cycle 2 and cyclin-dependent kinase 5 (41), we performed additional in vitro AMPK phosphorylation experiments using in vitro translated β1Pix (WT and S71A) to avoid other potential phosphorylation events that could occur by the presence of other coprecipitated kinases in our in vitro phosphorylation reaction. A similar effect was also observed when we used in vitro translated samples (Fig. 1C), suggesting that Ser71 is a major AMPK phosphorylation site on β1Pix.
Fig. 1.
AMP-activated protein kinase (AMPK) phosphorylates p21-activated kinase-interacting exchange factor-β1 β1Pix at Ser71. A: in vitro AMPK phosphorylation sites on β1Pix were identified using LC-MS/MS analysis. Of the six identified sites, the annotated spectrum for the identification of the peptide containing Ser71 (red) is highlighted. B and C: V5-tagged β1Pix [wild type (WT) or an alanine substitution mutant (S71A)] constructs were transiently transfected into human embryonic kidney-293 cells (B) or translated in vitro (C), immunoprecipitated using an anti-V5 agarose affinity gel, and exposed to purified recombinant AMPK holoenzyme or buffer alone and [γ-32P]ATP. After SDS-PAGE and transfer to a nitrocellulose membrane, immunoblot analysis and exposure to the phosphorimager were performed on the same membrane. Data are means ± SE; n = 3 experiments/condition. P values are shown for the indicated comparisons.
Involvement of β1Pix phosphorylation at Ser71 in AMPK regulation of ENaC.
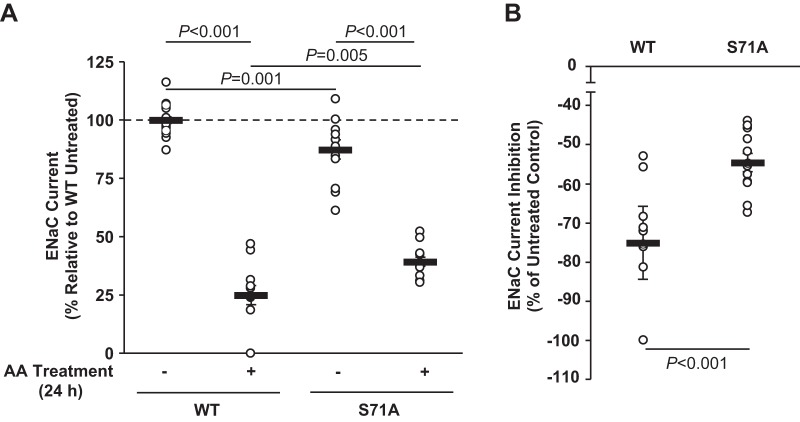
To examine the role of phosphorylation at Ser71 in β1Pix in the regulation of ENaC by AMPK, V5-tagged β1Pix-WT or β1Pix-S71A mutant was transiently transfected into mpkCCDc14 cells and seeded onto permeable supports (Transwells). After 48 h, amiloride-sensitive ENaC currents were measured before and after combined (AA) treatment with the AMPK activators AICAR (1 mM) and A769662 (100 μM) versus vehicle (control) for 24 h. β1Pix-WT and β1Pix-S71A mutant expression levels were 28% and 25% of endogenous β1Pix levels, respectively, as determined by immunoblot analysis with a specific β1Pix antibody (not shown). As shown in Fig. 2A, inhibition of ENaC activity by AMPK was observed in both β1Pix-WT- and β1Pix-S71A-overexpressing cells. However, AMPK-dependent ENaC inhibition was significantly reduced by 27.3% when the β1Pix-S71A mutant was overexpressed (Fig. 2B), suggesting that AMPK phosphorylation of β1Pix at Ser71 plays a significant role in ENaC regulation by AMPK.
Fig. 2.
Mutation at Ser71 attenuates epithelial Na+ channel (ENaC) inhibition by AMP-activated protein kinase (AMPK). A: V5-tagged p21-activated kinase-interacting exchange factor-β1 β1Pix-wild-type (WT) or S71A mutant of β1Pix was transiently expressed in mpkCCDc14 cells. Two days after transfection, measurements of equivalent short-circuit current were conducted in the absence (−) or presence (+) of combined (AA) treatment with the AMPK activators 5-aminoimidazole-4-carboxamide-1-β-d-ribofuranoside (1 mM) and A769662 (100 μM) for 24 h. B: comparisons of the percent change in ENaC current after 24 h of AA treatment between β1Pix-WT- and β1Pix-S71A mutant-expressing cells. Mutation at Ser71 of β1Pix significantly reduced the ENaC current inhibition induced by treatment of AMPK activators. Data shown are means ± SE; n = 5 separate experiments, with a total of 12–22 measurements/condition. P values are indicated for comparisons in the absence or presence of AA treatment and comparison between β1Pix-WT and β1Pix-S71A mutant.
Role of β1Pix phosphorylation and AMPK activation on the interplay of β1Pix, Nedd4-2, and 14-3-3 proteins in polarized mpkCCDc14 cells.
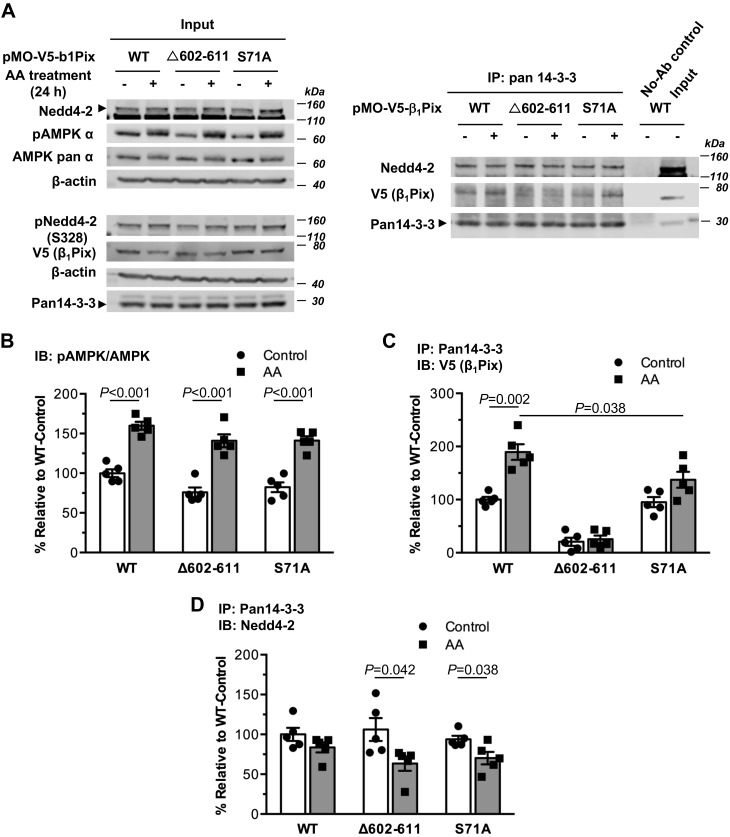
We previously found that ENaC currents were increased with overexpression of dimerization-deficient deletion tract β1Pix-Δ602–611 mutant in both Chinese hamster ovary and CCD cells, whereas overexpression of β1Pix-WT had the opposite effect, suggesting that 14-3-3 protein binding is critical for β1Pix regulation of ENaC (32). To test the role of 14-3-3 protein binding to β1Pix in the association of Nedd4-2 with 14-3-3 proteins, β1Pix-Δ602–611 mutant was expressed in mpkCCDc14 cells followed by AA treatment and coimmunoprecipitation assays. Typical immunoblots for this experiment are shown in Fig. 3A. As shown in Fig. 3, B–D, β1Pix-Δ602–611 mutant failed to bind to 14-3-3 proteins appreciably as expected (13), and this failure significantly reduced the binding of Nedd4-2 to 14-3-3 proteins in cells treated with AMPK activators (AA) compared with control-treated cells. Together, these findings indicate that when AMPK is activated, the association of β1Pix with 14-3-3 proteins plays an important role in regulating the Nedd4-2 interaction with 14-3-3 proteins.
Fig. 3.
AMP-activated protein kinase (AMPK)-dependent phosphorylation of Ser71 and 14-3-3 protein binding of p21-activated kinase-interacting exchange factor-β1 β1Pix are critical for supporting the association of neural precursor cell expressed developmentally downregulated protein (Nedd)4-2 with 14-3-3. A: representative immunoblot (IB; input) analysis and coimmunoprecipitation (co-IP) results of β1Pix, Nedd4-2, and 14-3-3 proteins in total lysates from mpkCCDc14 cells expressing V5-tagged β1Pix-wild type (WT), β1Pix-S71A mutant, or β1Pix-Δ602–611 mutant after 24-h treatment with or without AMPK activators [aminoimidazole-4-carboxamide-1-β-d-ribofuranoside and A769662 (AA)]. B: AMPK activation in cells overexpressing WT, S71A, or Δ602–611 mutant of β1Pix with AA treatment for 24 h. C and D: binding of V5-tagged β1Pix (C) or Nedd4-2 (D) to 14-3-3 proteins under the indicated conditions. The interaction of β1Pix-Δ602–611 mutant with 14-3-3 proteins was significantly reduced compared with that of β1Pix-WT or β1Pix-S71A mutant with or without AMPK activators (P < 0.001). Data are means ± SE; n = 5 experiments/condition. P values are shown for relevant comparisons if significant.
To investigate the potential role of β1Pix phosphorylation by AMPK in the formation of the β1Pix/Nedd4-2/14-3-3 complex, we tested whether overexpression of β1Pix-S71A mutant would alter the associations among these proteins in mpkCCDc14 cells (Fig. 3A). As shown in Fig. 3, B and C, AA treatment significantly activated AMPK and enhanced the binding of 14-3-3 proteins to β1Pix in cells overexpressing both β1Pix-WT and β1Pix-S71A mutant. However, in β1Pix-S71A mutant-overexpressing cells, the degree of enhanced β1Pix/14-3-3 binding was reduced by 27.5% relative to the increase in binding in β1Pix-WT-overexpressing cells. We also found that AMPK activation did not alter the binding of Nedd4-2 to 14-3-3 proteins with β1Pix-WT overexpression, consistent with the results from our previous study (Fig. 3D) (32). In contrast, the association of Nedd4-2 with 14-3-3 proteins was modestly reduced in cells expressing either β1Pix-Δ602–611 or β1Pix-S71A mutants treated with AMPK activators (Fig. 3D), suggesting that AMPK phosphorylation of β1Pix at Ser71 may play an important role in the interaction between β1Pix and 14-3-3 proteins but may also indirectly support the association of Nedd4-2 with 14-3-3 proteins. Of note, phosphorylation of Nedd4-2 at Ser328, a site important for Nedd4-2 stability, was not altered by expression of either S71A or Δ602–611 mutant of β1Pix, suggesting that 14-3-3 binding to β1Pix or Nedd4-2 is not required for Nedd4-2 phosphorylation at this site (Fig. 3A).
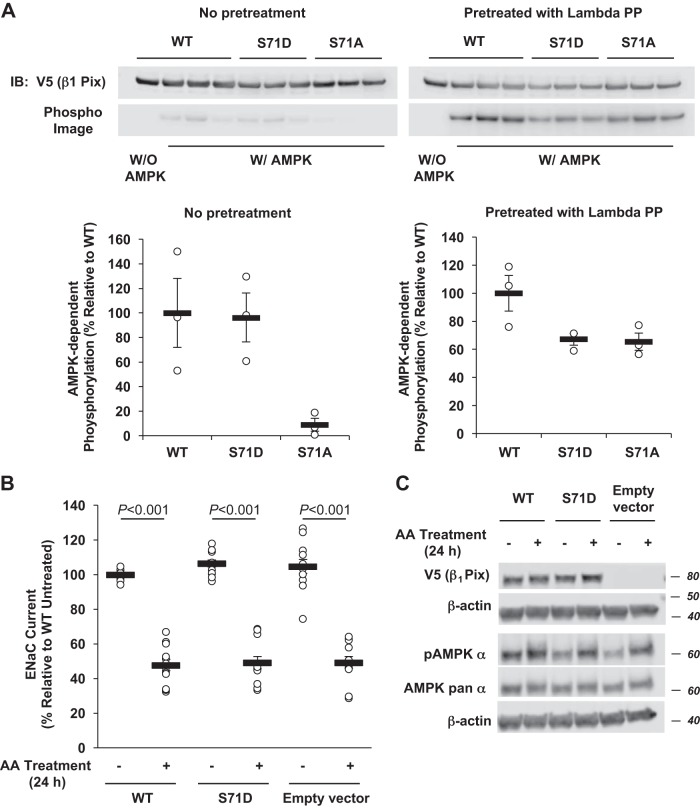
To further examine the role and importance of phosphorylation at Ser71 in β1Pix function, V5-tagged β1Pix-S71D, a presumed phosphomimetic mutant, was expressed in HEK-293 cells, immunoprecipitated, and then subjected to in vitro AMPK phosphorylation assays with purified active AMPK holoenzyme and [γ-32P]ATP. AMPK-dependent phosphorylation of β1Pix-S71D was compared with that of β1Pix-WT and β1Pix-S71A (Fig. 4). To evaluate the effect of any preexisting phosphorylation of the protein in cells, we compared β1Pix phosphorylation in immunoprecipitates from cell lysates that were untreated with those that were treated with λ-protein phosphatase, which removes any preexisting phosphorylation of the protein before in vitro [γ-32P]ATP labeling. We found that, compared with β1Pix-WT, both β1Pix-S71A and β1Pix-S71D mutants had reduced AMPK-mediated 32P incorporation by 30–40% (Fig. 4A, right). However, in the absence of λ-protein phosphatase pretreatment, we observed no significant difference in AMPK-mediated phosphorylation with β1Pix-S71D mutant compared with β1Pix-WT, whereas β1Pix-S71A mutant demonstrated significantly reduced phosphorylation (Fig. 4A, left). Taken together, these results suggest that AMPK-dependent phosphorylation in intact cells that occurs at other sites on β1Pix depends on prior phosphorylation at Ser71, which is prevented by the S71A mutation.
Fig. 4.
Effects of a phosphomimetic p21-activated kinase-interacting exchange factor-β1 β1Pix-S71D mutation on phosphorylation and epithelial Na+ channel (ENaC) regulation by AMP-activated protein kinase (AMPK). A: V5-tagged β1Pix [wild type (WT), S71D, or S71A] constructs were transiently transfected into human embryonic kidney-293 cells, immunoprecipitated using an anti-V5 agarose affinity gel, and treated with (right) or without (left) λ-protein phosphatase (lambda PP) to remove any preexisting phosphorylation. Purified V5-tagged β1Pix proteins were then exposed to recombinant AMPK holoenzyme or buffer alone and [γ-32P]ATP. After SDS-PAGE and transfer to a nitrocellulose membrane, immunoblot (IB) analysis and exposure to the phosphorimager were performed on the same membrane for quantitation. B: V5-tagged β1Pix-WT, β1Pix-S71D, or empty vector was transiently expressed in mpkCCDc14 cells. Two days after transfection, measurements of equivalent short-circuit current were conducted in the absence (−) or presence (+) of combined (AA) treatment with the AMPK activators 5-aminoimidazole-4-carboxamide-1-β-d-ribofuranoside (1 mM) and A769662 (100 μM) for 24 h. C: representative IB result of V5-tagged β1Pix, phospho-AMPK, and AMPK pan α-subunit in total lysates from mpkCCDc14 cells expressing V5-tagged β1Pix-WT, β1Pix-S71D, or empty vector after 24-h treatment with or without AMPK activators. Data are means ± SE; n = 4 separate experiments, with a total of 12 measurements/condition. P values are shown for relevant comparisons if significant.
AMPK activation and the related ENaC inhibition with AA treatment were also observed to a similar extent in mpkCCDc14 cells expressing either β1Pix-WT, β1Pix-S71D, or empty vector (Fig. 4, B and C). Altogether, these results could be explained by Ser71 phosphorylation-dependent conformational changes in β1Pix, which affect the accessibility to phosphorylation at the other sites. Given that protein folding and structure are critical for protein-protein interactions, phosphorylation at Ser71 may affect β1Pix structure and function and thus its role in the regulation of ENaC by AMPK.
Importance of β1Pix and Nedd4-2 interaction with 14-3-3 proteins in ENaC regulation by AMPK.
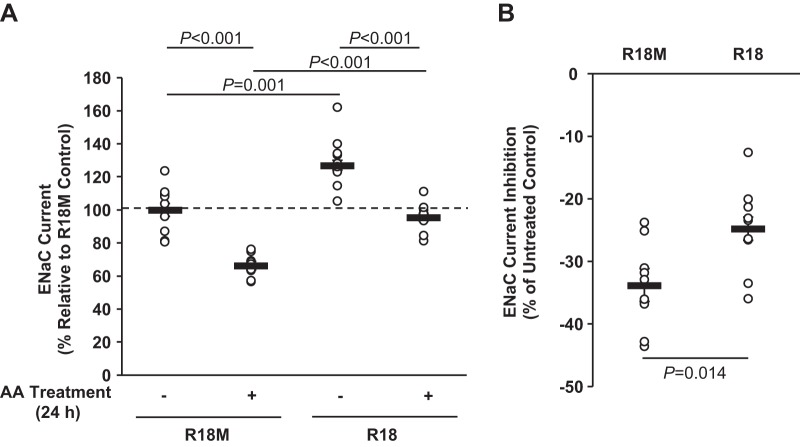
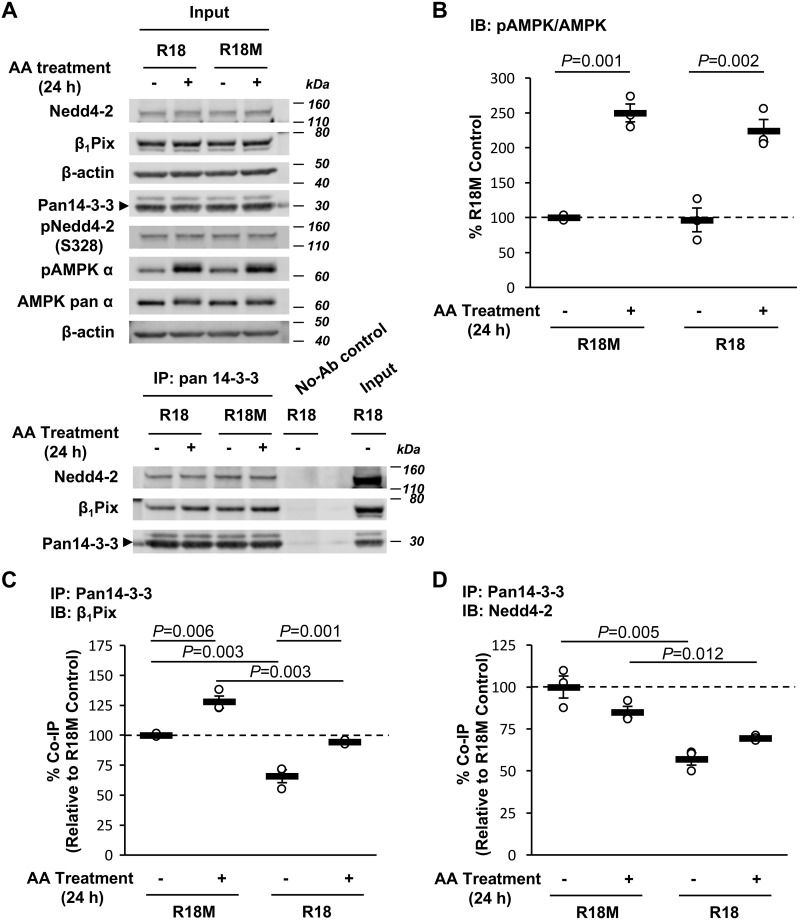
To further confirm the role of 14-3-3 interactions in β1Pix/Nedd4-2-dependent ENaC modulation by AMPK, a generalized inhibitor of 14-3-3-target interactions, the R18 peptide (60), was expressed in mpkCCDc14 cells to disrupt 14-3-3 binding. ENaC currents were measured before and after treatment with AMPK activators. Overexpression of the R18 peptide significantly increased ENaC equivalent Isc in mpkCCDc14 cells by 27% compared with that of R18M-overexpressing cells (Fig. 5A). AA treatment inhibited ENaC activity in both R18- and R18M-overexpressing cells, but in cells with R18 overexpression, ENaC inhibition caused by AMPK activation was significantly reduced by ∼27% relative to R18M-overexpressing cells (Fig. 5B). Coimmunoprecipitation assays were performed to further confirm the interplay of β1Pix, Nedd4-2, and 14-3-3 proteins in mpkCCDc14 cells overexpressing R18 versus R18M peptides (Fig. 6A). As shown in Fig. 6, B–D, R18 overexpression significantly decreased the association of both β1Pix and Nedd4-2 with 14-3-3 proteins without affecting AMPK activation. Phosphorylation of Nedd4-2 at Ser328 was not changed in cells expressing R18, consistent with the results found in β1Pix-S71A or -Δ602–611 mutant-expressing cells. Together, these results suggest that 14-3-3 protein interactions with β1Pix and Nedd4-2 play a central role in AMPK regulation of ENaC.
Fig. 5.
Binding of 14-3-3 is important for epithelial Na+ channel (ENaC) inhibition by AMP-activated protein kinase (AMPK). A: mpkCCDc14 cells were transiently transfected with either wild-type (WT) or mutant (M) enhance yellow fluorescent protein (EYFP)-R18 constructs. Epithelial volt-ohmmeter experiments were performed 2 days after transfection before and after treatment with AMPK activators [5-aminoimidazole-4-carboxamide-1-β-d-ribofuranoside and A769662 (AA)] for 24 h. Transfection efficiencies of R18 and interaction-deficient mutant R18M were ∼25% and 10%, respectively. B: comparisons of the percent change in ENaC current after 24-h AA treatment between R18- and R18M-expressing cells. Inhibition of 14-3-3-target protein interactions by the R18 peptide significantly reduced the inhibition of ENaC current by AMPK. n = 3 separate experiments, with a total of 9–11 measurements/condition.
Fig. 6.
R18 peptide decreases binding of p21-activated kinase-interacting exchange factor-β1 β1Pix and neural precursor cell expressed developmentally downregulated protein (Nedd)4-2 to 14-3-3 without disturbing AMP-activated protein kinase (AMPK) activation. A: representative immunoblot (IB; input) analysis and coimmunoprecipitation (co-IP) results of β1Pix, Nedd4-2, and 14-3-3 proteins in total lysates from mpkCCDc14 cells expressing R18 or R18M after 24-h treatment with or without AMPK activators [5-aminoimidazole-4-carboxamide-1-β-d-ribofuranoside and A769662 (AA)]. B–D: summary graphs of the ratio of phosphorylated (p-)AMPK-α (Thr172) to total AMPK-α (B) and coimmunoprecipitated β1Pix (C) or Nedd4-2 (D) with 14-3-3 proteins under the indicated conditions. n = 3 experiments/condition.
DISCUSSION
In the present study, we demonstrated the functional significance of β1Pix phosphorylation at Ser71 by AMPK in the establishment of an ENaC regulatory complex involving β1Pix, Nedd4-2, and 14-3-3 proteins in the AMPK-dependent regulation of ENaC. It has been previously reported that β1Pix homodimerization, mediated by the leucine zipper domain in the COOH-terminal portion and phosphorylation at Ser516, is required for 14-3-3 binding (13, 36). Our previous work also demonstrates that, through binding to 14-3-3 proteins, β1Pix plays an important role in ENaC inhibition by AMPK (32).
Several potential AMPK phosphorylation sites were found in the present study (Fig. 1), but only mutation at Ser71 significantly inhibited AMPK-dependent phosphorylation. Based on our phosphorylation results obtained in the presence versus the absence of λ-protein phosphatase and the findings that the phosphomimetic β1Pix-S71D mutant affected neither baseline ENaC current nor inhibition of ENaC by AMPK in CCD cells (Fig. 4), we propose that phosphorylation of β1Pix-WT at Ser71 or expression of the β1Pix-S71D mutant in intact cells induces a conformational change in the structure of β1Pix that affects the accessibility of other sites on β1Pix to phosphorylation. This change in the β1Pix structure may thus allow additional AMPK-dependent (and potentially AMPK-independent) phosphorylation events to occur, which are important in the mechanism of ENaC inhibition by AMPK. Of note, AMPK has been previously reported to play a role in the regulation of protein synthesis by sequentially phosphorylating tuberous sclerosis complex 2 together with glycogen synthase kinase 3 (27). Thus, AMPK promotes the association of β1Pix with 14-3-3 proteins at least in part through phosphorylation of β1Pix at Ser71 situated in the NH2-terminal spacer region between SRC homology 3 and Dbl homology domains. Although this region does not harbor a known 14-3-3-binding motif, phosphorylation of Ser71 could potentially induce a conformational change in β1Pix that enhances 14-3-3 binding to a different region of the β1Pix protein, which represents a potential mechanism for further study.
We recently found that Ser444 on xNedd4-2 is an AMPK phosphorylation site that is also targeted by several other kinases, such as SGK1, PKA, and IκB kinase-β (18, 20, 55). Phosphorylation at Ser444 has been revealed to be critical for Nedd4-2 cellular stability and serves as a docking site for 14-3-3 binding. As phospho-serine/threonine-binding proteins, 14-3-3 is involved in stress signaling, cell cycle progression, apoptosis, cytoskeletal structure, transcription, and intracellular trafficking/targeting (1). Reports about the role of 14-3-3 in ENaC regulation have recently escalated. By phosphorylating Nedd4-2, SGK1 increases the association of Nedd4-2 with 14-3-3, likely hindering the interaction of Nedd4-2 and ENaC (6, 34). It has also been reported that ET-1 promotes ENaC ubiquitination and degradation through recruitment of 14-3-3β by β1Pix, which prevents the binding of Nedd4-2 to 14-3-3β (50). However, our previous study (32) showed that AMPK regulates ENaC via β1Pix and Nedd4-2. We found that AMPK activation enhances the association between β1Pix and 14-3-3 proteins in two mouse CCD cell lines: mCCDcl1 and mpkCCDc14 cells. In mCCDcl1 cells, binding of Nedd4-2 to 14-3-3 proteins increased significantly with treatment with AMPK activators. In stable mpkCCDc14 cell lines, binding of Nedd4-2 to 14-3-3 proteins was not inhibited by β1Pix-WT overexpression but appeared to decrease with β1Pix-Δ602–611 mutant overexpression, albeit insignificantly, when AMPK was activated. Similar effects were observed in the present study with the use of transient transfection (Figs. 2 and 3). In mpkCCDc14 cells treated with AMPK activators, expression of β1Pix-WT caused no change in Nedd4-2/14-3-3 association, whereas expression of either β1Pix-S71A or β1Pix-Δ602–611 mutant caused a significant reduction of Nedd4-2 binding to 14-3-3. Moreover, the R18 peptide, which decreases both Nedd4-2- and β1Pix-14-3-3 interactions, blunted AMPK-dependent ENaC inhibition (Fig. 5 and 6). These findings suggest that the recruitment of AMPK-phosphorylated β1Pix into the Nedd4-2/14-3-3 complex is important for maintaining the interaction of Nedd4-2 and 14-3-3 and consequent ENaC degradation. Although β1Pix, Nedd4-2, and 14-3-3 play significant roles in both ET-1 and AMPK-mediated ENaC inhibition, the type of input signal, timing of signaling, or 14-3-3 isoform-specific differences may account for the observed differences in the fate of Nedd4-2 with binding of β1Pix with 14-3-3 proteins.
Rho GTPases are molecular switches that play an important role in membrane-trafficking processes (25, 44). Given that Rho GTPase activation relies on the coordinated action of GEFs, GEFs could play a key role in membrane recruitment of target molecules. As a GEF, βPix has been found to be essential for neuroendocrine exocytosis in pheochromocytoma cells (45). In primary human monocytes, βPix and Rac1 are required for membrane association of nucleotide-binding oligomerization domain-containing protein 2 with a negative regulator (21). Besides membrane trafficking, βPix is also involved in cell polarization by interacting with the Scribble scaffolding protein. Overexpression of inactive βPix or abrogation of the βPix-Scribble interaction interferes with cell polarization and Golgi reorientation (23). It is reasonable to propose that β1Pix, by interacting with 14-3-3, functions as a membrane trafficking factor for Nedd4-2 to target ENaC to the apical membrane. We have demonstrated that β1Pix plays a critical role in supporting the AMPK phosphorylation and stabilization of Nedd4-2 (32). However, results from the present study showed that neither β1Pix-S71A mutant nor R18 peptide overexpression downregulates Nedd4-2 expression levels or phosphorylation of Nedd4-2 at Ser328 (Figs. 3A and 5A). These findings suggest that neither AMPK phosphorylation of β1Pix at Ser71 nor 14-3-3 interaction accounts for Nedd4-2 stability and is consistent with our previous study’s conclusion that 14-3-3 interaction does not contribute to Nedd4-2 stabilization (14). It has been reported that when Nedd4-2 is not interacting with substrates, the PY motif of Nedd4-2 binds weakly to its own WW domains, leading to an autoinhibitory state of Nedd4-2 that prevents self-ubiquitination. However, after targeting its substrates, Nedd4-2 undergoes self-degradation by exposing its PY motif to the WW domain of other Nedd4-2 proteins (9, 61). A previous study of the human ether-à-go-go-related gene (hERG) channel has shown that overexpression of Rab4, a GTPase, promotes the ubiquitination of hERG channels by decreasing Nedd4-2 degradation and increasing expression levels of Nedd4-2 (16). Rab4, localized at the early sorting endosome, is critical for rapid/direct recycling from early endosomes to the cell surface (17, 43). Rab4 tends to interact with monoubiquitinated Nedd4-2, interferes with its degradation, and promotes its recycling (16). One might speculate that β1Pix, as a GEF, could also regulate Nedd4-2 degradation via a similar mechanism, but our data do not fully support this model. Future studies are warranted to investigate the mechanisms by which β1Pix as a GEF regulates Nedd4-2.
One limitation in this study was the moderate efficiency of transient transfection of WT or mutant β1Pix constructs or R18 peptides in mpkCCDc14 cells. Immunofluorescence staining results demonstrated a transfection efficiency of 14–35% after V5-tagged β1Pix transfection into mpkCCDc14 cells and Alexa 488 labeling of V5 protein (data not shown). However, the inhibition of ENaC currents by AMPK and the interactions among β1Pix, Nedd4-2, and 14-3-3 were altered even with modest overexpression of either V5-tagged β1Pix-S71A mutant or R18 peptide, further confirming the critical roles of AMPK phosphorylation of β1Pix and 14-3-3 binding in ENaC regulation. Also of note, it is certainly plausible that additional AMPK phosphorylation events and targets may also be involved in the formation of the β1Pix/Nedd4-2/14-3-3 protein complex and regulation of ENaC in CCD cells.
Although transient overexpression was the main technique used to investigate regulations of the tripartite ENaC inhibitory complex in the present study, similar findings have been shown in nontransfected native cells in our previous study (32). We demonstrated that AMPK regulates ENaC via the β1Pix/Nedd4–2/14-3-3 protein complex in mCCDcl1 cells in the absence of β1Pix knockdown, while demonstrating tripartite interactions among these endogenous proteins. Moreover, knockdown of endogenous β1Pix blunted ENaC regulation by AMPK in these cells and inhibited the AMPK activator-induced promotion of this complex. Future in vivo studies would be helpful to validate our findings.
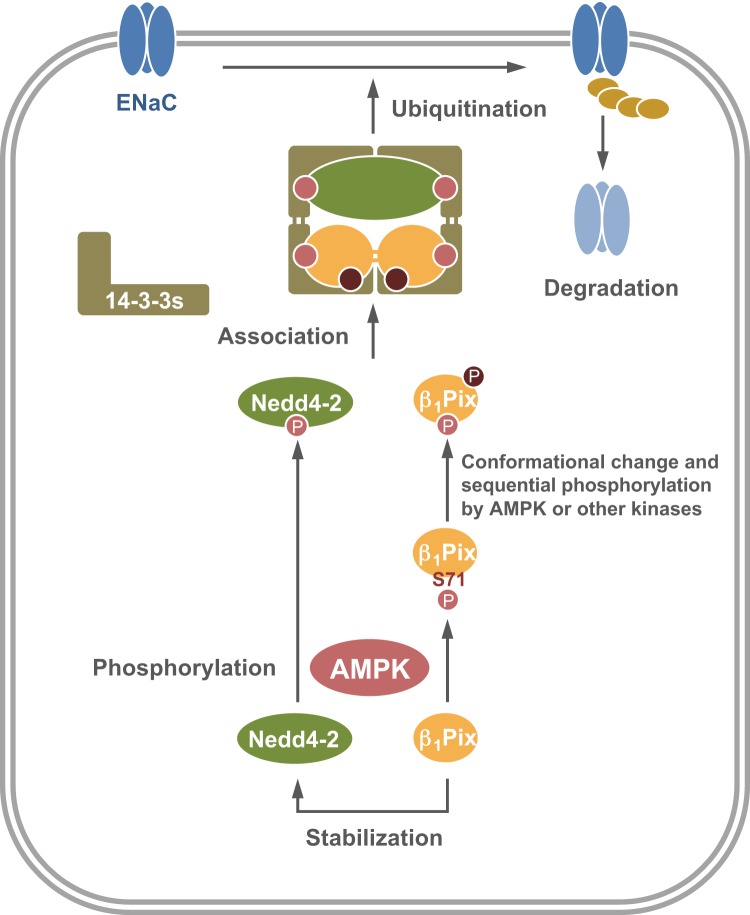
In conclusion, this study elucidates a novel role of β1Pix phosphorylation by AMPK and its involvement in ENaC inhibition by AMPK. Together, the findings from the present study and our previous work demonstrate that AMPK promotes the binding of β1Pix and Nedd4-2 to 14-3-3 proteins by phosphorylating β1Pix and Nedd4-2, which enhances Nedd4-2 binding to ENaC and ubiquitination of the channel. Dimerization of β1Pix appears to be required for ENaC regulation by AMPK, as overexpression of the dimerization-deficient β1Pix-Δ602–611 mutant blocked this regulation (32). Thus, we propose an architecture composed of two pairs of 14-3-3 dimers, one β1Pix dimer and Nedd4-2, as shown in Fig. 7. In this model, each β1Pix molecule could bind to one 14-3-3 protein from each of two dimers, an interaction enhanced by a conformational change of β1Pix resulting from AMPK phosphorylation at Ser71 and potentially other sites. Meanwhile, Nedd4-2 could interact with two different 14-3-3 proteins via two of its phosphorylated sites: Ser328, the AMPK phosphorylation site in mNedd4-2 (32), and another phosphorylated site (e.g., one of the minor sites targeted by PKA and SGK1) (14). Importantly, we expect this mechanism for AMPK regulation of Nedd4-2 function via AMPK-dependent phosphorylation of Nedd4-2 and β1Pix to be relevant to the regulation of a variety of membrane transport proteins, as Nedd4-2-dependent regulation has been found for a growing list of transport proteins, including voltage-gated Na+, K+, and Ca2+ channels, Cl− channels, and organic anion transporters (26). Future work should focus on investigating the mechanisms of how β1Pix promotes Nedd4-2 stabilization, leading to an increase of ENaC or other target transport protein ubiquitination by Nedd4-2.
Fig. 7.
Proposed schematic model for the role of p21-activated kinase-interacting exchange factor-β1 β1Pix in the regulation of epithelial Na+ channel (ENaC) by AMP-activated protein kinase (AMPK). Activation of AMPK promotes AMPK-mediated phosphorylation of neural precursor cell expressed developmentally downregulated protein (Nedd)4-2 and β1Pix at Ser328 and Ser71, respectively, and the subsequent sequential phosphorylation at other sites, events that are all critical for 14-3-3 binding and membrane trafficking. β1Pix is also involved in Nedd4-2 stabilization under an AMPK phosphorylation and 14-3-3 binding-independent mechanism. These actions together lead to an increased association of Nedd4-2, 14-3-3, and β1Pix into a larger complex, with subsequent enhanced targeting and binding of Nedd4-2 to ENaC, which thereby promotes ENaC ubiquitination and degradation.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants R01-DK-075048 (to K. R. Hallows) and R01-DK-091565 (to V. Bhalla) and the Novo Nordisk Foundation, Danish Medical Research Foundation, and Leducq Foundation (to R. Fenton).
DISCLAIMERS
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
P.-Y.H., V.B., R.A.F., and K.R.H. conceived and designed research; P.-Y.H., H.L., and L.C. performed experiments; P.-Y.H., H.L., L.C., R.A.F., and K.R.H. analyzed data; P.-Y.H., R.A.F., and K.R.H. interpreted results of experiments; P.-Y.H. and R.A.F. prepared figures; P.-Y.H. drafted manuscript; H.L., V.B., R.A.F., and K.R.H. edited and revised manuscript; P.-Y.H., H.L., L.C., V.B., R.A.F., and K.R.H. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. Dietbert Neumann (Maastricht University) for the gift of purified active AMPK holoenzyme, Dr. Haian Fu (Emory University) for supplying R18 and R18M plasmid constructs, and Dr. Alexander Staruschenko and Dr. Andrey Sorokin (Medical College of Wisconsin) for providing β1Pix plasmid constructs.
REFERENCES
- 1.Aitken A. 14-3-3 proteins: a historic overview. Semin Cancer Biol 16: 162–172, 2006. doi: 10.1016/j.semcancer.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 2.Al-Bataineh MM, Li H, Ohmi K, Gong F, Marciszyn AL, Naveed S, Zhu X, Neumann D, Wu Q, Cheng L, Fenton RA, Pastor-Soler NM, Hallows KR. Activation of the metabolic sensor AMP-activated protein kinase inhibits aquaporin-2 function in kidney principal cells. Am J Physiol Renal Physiol 311: F890–F900, 2016. doi: 10.1152/ajprenal.00308.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Almaça J, Kongsuphol P, Hieke B, Ousingsawat J, Viollet B, Schreiber R, Amaral MD, Kunzelmann K. AMPK controls epithelial Na+ channels through Nedd4-2 and causes an epithelial phenotype when mutated. Pflugers Arch 458: 713–721, 2009. doi: 10.1007/s00424-009-0660-4. [DOI] [PubMed] [Google Scholar]
- 4.Angrand PO, Segura I, Völkel P, Ghidelli S, Terry R, Brajenovic M, Vintersten K, Klein R, Superti-Furga G, Drewes G, Kuster B, Bouwmeester T, Acker-Palmer A. Transgenic mouse proteomics identifies new 14-3-3-associated proteins involved in cytoskeletal rearrangements and cell signaling. Mol Cell Proteomics 5: 2211–2227, 2006. doi: 10.1074/mcp.M600147-MCP200. [DOI] [PubMed] [Google Scholar]
- 5.Bagrodia S, Bailey D, Lenard Z, Hart M, Guan JL, Premont RT, Taylor SJ, Cerione RA. A tyrosine-phosphorylated protein that binds to an important regulatory region on the cool family of p21-activated kinase-binding proteins. J Biol Chem 274: 22393–22400, 1999. doi: 10.1074/jbc.274.32.22393. [DOI] [PubMed] [Google Scholar]
- 6.Bhalla V, Daidié D, Li H, Pao AC, LaGrange LP, Wang J, Vandewalle A, Stockand JD, Staub O, Pearce D. Serum- and glucocorticoid-regulated kinase 1 regulates ubiquitin ligase neural precursor cell-expressed, developmentally down-regulated protein 4-2 by inducing interaction with 14-3-3. Mol Endocrinol 19: 3073–3084, 2005. doi: 10.1210/me.2005-0193. [DOI] [PubMed] [Google Scholar]
- 7.Bhalla V, Hallows KR. Mechanisms of ENaC regulation and clinical implications. J Am Soc Nephrol 19: 1845–1854, 2008. doi: 10.1681/ASN.2008020225. [DOI] [PubMed] [Google Scholar]
- 8.Bhalla V, Oyster NM, Fitch AC, Wijngaarden MA, Neumann D, Schlattner U, Pearce D, Hallows KR. AMP-activated kinase inhibits the epithelial Na+ channel through functional regulation of the ubiquitin ligase Nedd4-2. J Biol Chem 281: 26159–26169, 2006. doi: 10.1074/jbc.M606045200. [DOI] [PubMed] [Google Scholar]
- 9.Bruce MC, Kanelis V, Fouladkou F, Debonneville A, Staub O, Rotin D. Regulation of Nedd4-2 self-ubiquitination and stability by a PY motif located within its HECT-domain. Biochem J 415: 155–163, 2008. doi: 10.1042/BJ20071708. [DOI] [PubMed] [Google Scholar]
- 10.Cammisotto PG, Londono I, Gingras D, Bendayan M. Control of glycogen synthase through ADIPOR1-AMPK pathway in renal distal tubules of normal and diabetic rats. Am J Physiol Renal Physiol 294: F881–F889, 2008. doi: 10.1152/ajprenal.00373.2007. [DOI] [PubMed] [Google Scholar]
- 11.Canessa CM, Schild L, Buell G, Thorens B, Gautschi I, Horisberger JD, Rossier BC. Amiloride-sensitive epithelial Na+ channel is made of three homologous subunits. Nature 367: 463–467, 1994. doi: 10.1038/367463a0. [DOI] [PubMed] [Google Scholar]
- 12.Carattino MD, Edinger RS, Grieser HJ, Wise R, Neumann D, Schlattner U, Johnson JP, Kleyman TR, Hallows KR. Epithelial sodium channel inhibition by AMP-activated protein kinase in oocytes and polarized renal epithelial cells. J Biol Chem 280: 17608–17616, 2005. doi: 10.1074/jbc.M501770200. [DOI] [PubMed] [Google Scholar]
- 13.Chahdi A, Sorokin A. Protein kinase A-dependent phosphorylation modulates beta1Pix guanine nucleotide exchange factor activity through 14-3-3beta binding. Mol Cell Biol 28: 1679–1687, 2008. doi: 10.1128/MCB.00898-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chandran S, Li H, Dong W, Krasinska K, Adams C, Alexandrova L, Chien A, Hallows KR, Bhalla V. Neural precursor cell-expressed developmentally down-regulated protein 4-2 (Nedd4-2) regulation by 14-3-3 protein binding at canonical serum and glucocorticoid kinase 1 (SGK1) phosphorylation sites. J Biol Chem 286: 37830–37840, 2011. doi: 10.1074/jbc.M111.293233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cheng L, Wu Q, Kortenoeven MLA, Pisitkun T, Fenton RA. A systems level analysis of vasopressin-mediated signaling networks in kidney distal convoluted tubule cells. Sci Rep 5: 12829, 2015. doi: 10.1038/srep12829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cui Z, Zhang S. Regulation of the human ether-a-go-go-related gene (hERG) channel by Rab4 protein through neural precursor cell-expressed developmentally down-regulated protein 4-2 (Nedd4-2). J Biol Chem 288: 21876–21886, 2013. doi: 10.1074/jbc.M113.461715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Daro E, van der Sluijs P, Galli T, Mellman I. Rab4 and cellubrevin define different early endosome populations on the pathway of transferrin receptor recycling. Proc Natl Acad Sci USA 93: 9559–9564, 1996. doi: 10.1073/pnas.93.18.9559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Debonneville C, Flores SY, Kamynina E, Plant PJ, Tauxe C, Thomas MA, Münster C, Chraïbi A, Pratt JH, Horisberger JD, Pearce D, Loffing J, Staub O. Phosphorylation of Nedd4-2 by Sgk1 regulates epithelial Na(+) channel cell surface expression. EMBO J 20: 7052–7059, 2001. doi: 10.1093/emboj/20.24.7052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Degasperi A, Birtwistle MR, Volinsky N, Rauch J, Kolch W, Kholodenko BN. Evaluating strategies to normalise biological replicates of Western blot data. PLoS One 9: e87293, 2014. doi: 10.1371/journal.pone.0087293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Edinger RS, Lebowitz J, Li H, Alzamora R, Wang H, Johnson JP, Hallows KR. Functional regulation of the epithelial Na+ channel by IkappaB kinase-beta occurs via phosphorylation of the ubiquitin ligase Nedd4-2. J Biol Chem 284: 150–157, 2009. doi: 10.1074/jbc.M807358200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eitel J, Krüll M, Hocke AC, N’Guessan PD, Zahlten J, Schmeck B, Slevogt H, Hippenstiel S, Suttorp N, Opitz B. Beta-PIX and Rac1 GTPase mediate trafficking and negative regulation of NOD2. J Immunol 181: 2664–2671, 2008. doi: 10.4049/jimmunol.181.4.2664. [DOI] [PubMed] [Google Scholar]
- 23.Frank SR, Hansen SH. The PIX-GIT complex: a G protein signaling cassette in control of cell shape. Semin Cell Dev Biol 19: 234–244, 2008. doi: 10.1016/j.semcdb.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Garty H, Palmer LG. Epithelial sodium channels: function, structure, and regulation. Physiol Rev 77: 359–396, 1997. doi: 10.1152/physrev.1997.77.2.359. [DOI] [PubMed] [Google Scholar]
- 25.Gasman S, Chasserot-Golaz S, Malacombe M, Way M, Bader MF. Regulated exocytosis in neuroendocrine cells: a role for subplasmalemmal Cdc42/N-WASP-induced actin filaments. Mol Biol Cell 15: 520–531, 2004. doi: 10.1091/mbc.e03-06-0402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goel P, Manning JA, Kumar S. NEDD4-2 (NEDD4L): the ubiquitin ligase for multiple membrane proteins. Gene 557: 1–10, 2015. doi: 10.1016/j.gene.2014.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gwinn DM, Shackelford DB, Egan DF, Mihaylova MM, Mery A, Vasquez DS, Turk BE, Shaw RJ. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol Cell 30: 214–226, 2008. doi: 10.1016/j.molcel.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gwoździńska P, Buchbinder BA, Mayer K, Herold S, Morty RE, Seeger W, Vadász I. Hypercapnia impairs ENaC cell surface stability by promoting phosphorylation, polyubiquitination and endocytosis of β-ENaC in a human alveolar epithelial cell line. Front Immunol 8: 591, 2017. doi: 10.3389/fimmu.2017.00591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hardie DG, Scott JW, Pan DA, Hudson ER. Management of cellular energy by the AMP-activated protein kinase system. FEBS Lett 546: 113–120, 2003. doi: 10.1016/S0014-5793(03)00560-X. [DOI] [PubMed] [Google Scholar]
- 30.Hartley JL, Temple GF, Brasch MA. DNA cloning using in vitro site-specific recombination. Genome Res 10: 1788–1795, 2000. doi: 10.1101/gr.143000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Henshall TL, Manning JA, Alfassy OS, Goel P, Boase NA, Kawabe H, Kumar S. Deletion of Nedd4-2 results in progressive kidney disease in mice. Cell Death Differ 24: 2150–2160, 2017. doi: 10.1038/cdd.2017.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ho PY, Li H, Pavlov TS, Tuerk RD, Tabares D, Brunisholz R, Neumann D, Staruschenko A, Hallows KR. β1Pix exchange factor stabilizes the ubiquitin ligase Nedd4-2 and plays a critical role in ENaC regulation by AMPK in kidney epithelial cells. J Biol Chem 293: 11612–11624, 2018. doi: 10.1074/jbc.RA118.003082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hoffert JD, Fenton RA, Moeller HB, Simons B, Tchapyjnikov D, McDill BW, Yu MJ, Pisitkun T, Chen F, Knepper MA. Vasopressin-stimulated increase in phosphorylation at Ser269 potentiates plasma membrane retention of aquaporin-2. J Biol Chem 283: 24617–24627, 2008. doi: 10.1074/jbc.M803074200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ichimura T, Yamamura H, Sasamoto K, Tominaga Y, Taoka M, Kakiuchi K, Shinkawa T, Takahashi N, Shimada S, Isobe T. 14-3-3 proteins modulate the expression of epithelial Na+ channels by phosphorylation-dependent interaction with Nedd4-2 ubiquitin ligase. J Biol Chem 280: 13187–13194, 2005. doi: 10.1074/jbc.M412884200. [DOI] [PubMed] [Google Scholar]
- 35.Jin J, Smith FD, Stark C, Wells CD, Fawcett JP, Kulkarni S, Metalnikov P, O’Donnell P, Taylor P, Taylor L, Zougman A, Woodgett JR, Langeberg LK, Scott JD, Pawson T. Proteomic, functional, and domain-based analysis of in vivo 14-3-3 binding proteins involved in cytoskeletal regulation and cellular organization. Curr Biol 14: 1436–1450, 2004. doi: 10.1016/j.cub.2004.07.051. [DOI] [PubMed] [Google Scholar]
- 36.Kim S, Lee SH, Park D. Leucine zipper-mediated homodimerization of the p21-activated kinase-interacting factor, beta Pix. Implication for a role in cytoskeletal reorganization. J Biol Chem 276: 10581–10584, 2001. doi: 10.1074/jbc.C000806200. [DOI] [PubMed] [Google Scholar]
- 37.Koh CG, Manser E, Zhao ZS, Ng CP, Lim L. Beta1PIX, the PAK-interacting exchange factor, requires localization via a coiled-coil region to promote microvillus-like structures and membrane ruffles. J Cell Sci 114: 4239–4251, 2001. [DOI] [PubMed] [Google Scholar]
- 38.Lee MJ, Feliers D, Mariappan MM, Sataranatarajan K, Mahimainathan L, Musi N, Foretz M, Viollet B, Weinberg JM, Choudhury GG, Kasinath BS. A role for AMP-activated protein kinase in diabetes-induced renal hypertrophy. Am J Physiol Renal Physiol 292: F617–F627, 2007. doi: 10.1152/ajprenal.00278.2006. [DOI] [PubMed] [Google Scholar]
- 39.Manser E, Loo TH, Koh CG, Zhao ZS, Chen XQ, Tan L, Tan I, Leung T, Lim L. PAK kinases are directly coupled to the PIX family of nucleotide exchange factors. Mol Cell 1: 183–192, 1998. doi: 10.1016/S1097-2765(00)80019-2. [DOI] [PubMed] [Google Scholar]
- 40.Masters SC, Fu H. 14-3-3 proteins mediate an essential anti-apoptotic signal. J Biol Chem 276: 45193–45200, 2001. doi: 10.1074/jbc.M105971200. [DOI] [PubMed] [Google Scholar]
- 41.Mayhew MW, Jeffery ED, Sherman NE, Nelson K, Polefrone JM, Pratt SJ, Shabanowitz J, Parsons JT, Fox JW, Hunt DF, Horwitz AF. Identification of phosphorylation sites in betaPIX and PAK1. J Cell Sci 120: 3911–3918, 2007. doi: 10.1242/jcs.008177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mohan S, Bruns JR, Weixel KM, Edinger RS, Bruns JB, Kleyman TR, Johnson JP, Weisz OA. Differential current decay profiles of epithelial sodium channel subunit combinations in polarized renal epithelial cells. J Biol Chem 279: 32071–32078, 2004. doi: 10.1074/jbc.M405091200. [DOI] [PubMed] [Google Scholar]
- 43.Mohrmann K, van der Sluijs P. Regulation of membrane transport through the endocytic pathway by rabGTPases. Mol Membr Biol 16: 81–87, 1999. doi: 10.1080/096876899294797. [DOI] [PubMed] [Google Scholar]
- 44.Momboisse F, Lonchamp E, Calco V, Ceridono M, Vitale N, Bader MF, Gasman S. betaPIX-activated Rac1 stimulates the activation of phospholipase D, which is associated with exocytosis in neuroendocrine cells. J Cell Sci 122: 798–806, 2009. doi: 10.1242/jcs.038109. [DOI] [PubMed] [Google Scholar]
- 45.Momboisse F, Ory S, Ceridono M, Calco V, Vitale N, Bader MF, Gasman S. The Rho guanine nucleotide exchange factors Intersectin 1L and β-Pix control calcium-regulated exocytosis in neuroendocrine PC12 cells. Cell Mol Neurobiol 30: 1327–1333, 2010. doi: 10.1007/s10571-010-9580-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mount PF, Hill RE, Fraser SA, Levidiotis V, Katsis F, Kemp BE, Power DA. Acute renal ischemia rapidly activates the energy sensor AMPK but does not increase phosphorylation of eNOS-Ser1177. Am J Physiol Renal Physiol 289: F1103–F1115, 2005. doi: 10.1152/ajprenal.00458.2004. [DOI] [PubMed] [Google Scholar]
- 47.Neumann D, Woods A, Carling D, Wallimann T, Schlattner U. Mammalian AMP-activated protein kinase: functional, heterotrimeric complexes by co-expression of subunits in Escherichia coli. Protein Expr Purif 30: 230–237, 2003. doi: 10.1016/S1046-5928(03)00126-8. [DOI] [PubMed] [Google Scholar]
- 48.Noreng S, Bharadwaj A, Posert R, Yoshioka C, Baconguis I. Structure of the human epithelial sodium channel by cryo-electron microscopy. Elife 7: e39340, 2018. doi: 10.7554/eLife.39340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pastor-Soler NM, Hallows KR. AMP-activated protein kinase regulation of kidney tubular transport. Curr Opin Nephrol Hypertens 21: 523–533, 2012. doi: 10.1097/MNH.0b013e3283562390. [DOI] [PubMed] [Google Scholar]
- 50.Pavlov TS, Chahdi A, Ilatovskaya DV, Levchenko V, Vandewalle A, Pochynyuk O, Sorokin A, Staruschenko A. Endothelin-1 inhibits the epithelial Na+ channel through betaPix/14-3-3/Nedd4-2. J Am Soc Nephrol 21: 833–843, 2010. doi: 10.1681/ASN.2009080885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Peairs A, Radjavi A, Davis S, Li L, Ahmed A, Giri S, Reilly CM. Activation of AMPK inhibits inflammation in MRL/lpr mouse mesangial cells. Clin Exp Immunol 156: 542–551, 2009. doi: 10.1111/j.1365-2249.2009.03924.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sharma K, Ramachandrarao S, Qiu G, Usui HK, Zhu Y, Dunn SR, Ouedraogo R, Hough K, McCue P, Chan L, Falkner B, Goldstein BJ. Adiponectin regulates albuminuria and podocyte function in mice. J Clin Invest 118: 1645–1656, 2008. doi: 10.1172/JCI32691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shi PP, Cao XR, Sweezer EM, Kinney TS, Williams NR, Husted RF, Nair R, Weiss RM, Williamson RA, Sigmund CD, Snyder PM, Staub O, Stokes JB, Yang B. Salt-sensitive hypertension and cardiac hypertrophy in mice deficient in the ubiquitin ligase Nedd4-2. Am J Physiol Renal Physiol 295: F462–F470, 2008. doi: 10.1152/ajprenal.90300.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Snyder PM. Down-regulating destruction: phosphorylation regulates the E3 ubiquitin ligase Nedd4-2. Sci Signal 2: pe41, 2009. doi: 10.1126/scisignal.279pe41. [DOI] [PubMed] [Google Scholar]
- 55.Snyder PM, Olson DR, Kabra R, Zhou R, Steines JC. cAMP and serum and glucocorticoid-inducible kinase (SGK) regulate the epithelial Na+ channel through convergent phosphorylation of Nedd4-2. J Biol Chem 279: 45753–45758, 2004. doi: 10.1074/jbc.M407858200. [DOI] [PubMed] [Google Scholar]
- 56.Staruschenko A, Sorokin A. Role of βPix in the kidney. Front Physiol 3: 154, 2012. doi: 10.3389/fphys.2012.00154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stevens BM, Folts CJ, Cui W, Bardin AL, Walter K, Carson-Walter E, Vescovi A, Noble M. Cool-1-mediated inhibition of c-Cbl modulates multiple critical properties of glioblastomas, including the ability to generate tumors in vivo. Stem Cells 32: 1124–1135, 2014. doi: 10.1002/stem.1644. [DOI] [PubMed] [Google Scholar]
- 58.Takiar V, Nishio S, Seo-Mayer P, King JD Jr, Li H, Zhang L, Karihaloo A, Hallows KR, Somlo S, Caplan MJ. Activating AMP-activated protein kinase (AMPK) slows renal cystogenesis. Proc Natl Acad Sci USA 108: 2462–2467, 2011. doi: 10.1073/pnas.1011498108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang B, Yang H, Liu YC, Jelinek T, Zhang L, Ruoslahti E, Fu H. Isolation of high-affinity peptide antagonists of 14-3-3 proteins by phage display. Biochemistry 38: 12499–12504, 1999. doi: 10.1021/bi991353h. [DOI] [PubMed] [Google Scholar]
- 61.Wang J, Peng Q, Lin Q, Childress C, Carey D, Yang W. Calcium activates Nedd4 E3 ubiquitin ligases by releasing the C2 domain-mediated auto-inhibition. J Biol Chem 285: 12279–12288, 2010. doi: 10.1074/jbc.M109.086405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wu WJ, Tu S, Cerione RA. Activated Cdc42 sequesters c-Cbl and prevents EGF receptor degradation. Cell 114: 715–725, 2003. doi: 10.1016/S0092-8674(03)00688-3. [DOI] [PubMed] [Google Scholar]
- 63.Yates JR III, Eng JK, McCormack AL, Schieltz D. Method to correlate tandem mass spectra of modified peptides to amino acid sequences in the protein database. Anal Chem 67: 1426–1436, 1995. doi: 10.1021/ac00104a020. [DOI] [PubMed] [Google Scholar]