Abstract
Organic cation transporters play a critical role in mediating the distribution of cationic pharmaceuticals. Indeed, organic cation transporter (OCT)2 is the initial step in the renal secretion of organic cations and consequently plays a defining role in establishing the pharmacokinetics of many cationic drugs. Although a hallmark of OCTs is their broad selectivity, this characteristic also makes them targets for unwanted, adverse drug-drug interactions (DDIs), making them a focus for efforts to develop models of ligand interaction that could predict and preempt these adverse interactions. This review discusses the molecular characteristics of these transporters as well as the evidence that established the OCTs as key players in the distribution of organic cations. However, the primary focus is the present understanding of the complexity of ligand interaction with OCTs, particularly OCT2, including evidence for the presence of multiple ligand-binding sites and the influence of substrate structure on the affinity of the transporter for inhibitory ligands. This leads to a discussion of the complexities associated with the development of protocols for assessing the inhibitory potential of new molecular entities to perpetrate unwanted DDIs, the criteria that should be considered in the interpretation of the results of such protocols, and the challenges associated with development of models capable of predicting unwanted DDIs.
Keywords: kidney, kinetics, organic cation, transport
INTRODUCTION
Organic cation transporter (OCT)2 (SLC22A2) mediates the initial (basolateral) step in renal secretion of organic cations (OCs; positively charged molecules at physiological pH) by human renal proximal tubules (RPTs). Through this activity, OCT2, working in concert with the apically expressed multidrug and toxin extruder transporters (MATEs), augments glomerular filtration to play a central role in clearing the plasma of many cationic drugs (90), which collectively comprise ∼40% of pharmaceuticals (2, 67). Consequently, renal secretion influences the pharmacokinetics of many clinically significant drugs (56). The broad selectivity of OCTs, including OCT2 (101, 124), also makes them targets for unwanted drug-drug interactions (DDIs) and, therefore, the focus of studies attempting to develop models capable of predicting such interactions (e.g., see Refs. 44 and 85).
This review begins with an outline of the work implicating OCT2 in cationic drug secretion followed by an overview of the molecular characteristics of OCT2 (and its homologs OCT1 and OCT3 as well), preparatory to a discussion of the present understanding of the mechanism of ligand interaction with OCTs. Also discussed is the need to reassess the methods and protocols used to obtain the kinetic parameters required for accurate implementation of both physiologically based pharmacokinetic (PBPK) models of drug clearance and decision tree-based analysis of inhibitory interactions with OCTs (and other multidrug transporters).
OVERVIEW OF THE CELLULAR PHYSIOLOGY OF RENAL OC TRANSPORT
The kidney secretes a diverse collection of small organic electrolytes, which are often divided into two general classes: organic anions and OCs (76, 107). The latter category (OCs) includes primary, secondary, tertiary, or quaternary amines that have a net positive charge on the amine nitrogen at physiological pH. A number of endogenous OCs have been shown to be actively secreted by the proximal tubule [e.g., N1-methylnicotinamide (NMN), choline, histamine, and thiamine; see Ref. 81]. However, renal OC secretion also clears the plasma of many xenobiotic agents, including a wide range of alkaloids and other positively charged, heterocyclic compounds of dietary origin, cationic drugs of therapeutic or recreational use, or other cationic toxins of environmental origin (76, 79, 81). Importantly, the common pathway for OC secretion is also a site of clinically significant interactions between OCs in humans. For example, therapeutic doses of cimetidine retard the renal elimination of amiloride, ranitidine, procainamide, metformin, zidovudine, and triamterene (27, 95–97), and reduced function mutations of OCT2 decrease renal clearance and increase plasma concentration of metformin (e.g., Refs. 16 and 126).
The proximal tubule is the primary site of OC secretion, as shown in studies using stop-flow and micropuncture methods in mammalian, fish, and avian kidneys (79, 81). David et al. (20) and Ullrich and colleagues (109, 112) established the multisubstrate specificity of OC transport pathways in rat kidneys in seminal studies that used the stopped-flow capillary perfusion method. Studies with basolateral and apical membrane vesicles isolated from dog (41, 42, 82), rat (102), and rabbit (57, 94, 119, 122) kidneys showed that OC entry from blood into RPT cells, across the basolateral (peritubular/contraluminal) membrane, is mediated by electrogenic facilitated diffusion, whereas exit from the cells into the tubular filtrate across the apical (luminal brush border) membrane is mediated by a secondary active exchange of cytoplasmic OC for luminal H+. The basolateral entry of OCs into RPT cells was subsequently shown to be mediated by one or more members of the OCT (SLC22A) family of transporters, whereas apical OC exit involves one or more members of the MATE (SLC47A) family of transporters (76). The expression profile of different homologs of both families of transporter is species specific. In humans, basolateral OC transport is dominated by activity of OCT2 (63, 64), whereas sinusoidal OC transport in the human liver involves OCT1 (59). However, in rodents and rabbits, both Oct1 and Oct2 play quantitatively significant roles in renal OC secretion (45, 121). A third homolog, OCT3, has a wider tissue expression profile that includes the human placenta, lung, and brain (70). Interestingly, in the mouse, elimination of Oct2 or Oct1 individually has no effect on renal secretion of tetraethylammonium (TEA), but elimination of both homologs completely eliminates TEA secretion (45), suggesting that the transport capacity of each process is generally sufficient to clear the blood of cationic substrates, at least at low concentrations. In humans, OCT1 is highly polymorphic, with ∼9% of European and white Americans being either homozygous or heterozygous for one of several loss-of-function alleles, whereas OCT2 is less polymorphic and loss of function is rare (60). The allele frequency of the c.808G>T (Ala270Ser) OCT2 polymorph in different populations varies from 7% to 16% (55) but results in only a modest decrease in OCT2 transport activity (55, 130). Nevertheless, individuals homozygous for Ala270Ser show a significant decrease in metformin clearance with a concomitant rise in plasma concentration (98).
MOLECULAR CHARACTERISTICS OF OCTs
OCT1 and OCT2 were cloned from the rat kidney in 1994 (34) and 1996 (71), respectively; the human orthologs were cloned in 1997 (32, 128). OCT3 was cloned from a human kidney carcinoma cell line (Caki-1) and initially described as the “extraneuronal monoamine transporter” (EMT) (88, 123). The human gene for OCT1 (SLC22A1) contains 7 exons (40), whereas the genes for OCT2 (SLC22A2) and OCT3 (SLC22A3) both contain 11 exons (35). OCT1 and OCT2 occur as a pair on chromosome 6 (6q26), ∼90 kb upstream from OCT3 (6q27) (26). Four alternatively spliced isoforms of OCT1 were found in human glioma cells (35), a long (full-length) form and three shorter forms, but only the long form [human (h)OCT1G/L554] supports transport when expressed in human embryonic kidney-293 cells (35). The human kidney expresses at least one splice variant of OCT2. Designated hOCT2-A, it is characterized by the insertion of a 1,169-bp sequence arising from the intron found between exons 7 and 8 of hOCT2 (113). The new open reading frame includes an early stop codon, resulting in a truncated protein that is missing the last three putative TMHs (i.e., see Refs. 10–12). Despite the absence of the last three TMHs, hOCT2-A retains the capacity to transport TEA and cimetidine, although guanidine transport is lost (113).
OCTs (and all members of SLC22A) are in the major facilitator superfamily (MFS; also called the uniporter-symporter-antiporter family; see Ref. 74) and share structural characteristics common to these proteins, including several conserved sequence motifs (10, 120) and 12 TMHs organized as two pseudosymmetrical six-helix bundles (80) with cytoplasmic NH2 and COOH termini. There are other features found in OCTs but not generally in the MFS, including several unique sequence motifs (89), a relatively long cytoplasmic loop between TMHs 6 and 7, and a long extracellular loop between helixes 1 and 2 that contains three NH2-linked glycosylation sites. In OCT2, all three sites are glycosylated, and their elimination is associated with both decreased trafficking of protein to the membrane and changes in apparent affinity for substrate (75). The latter observation suggests that the conformation of the long extracellular loop influences the position of TMHs 1 and 2, which are elements of a hydrophilic “binding cleft” common to OCTs and through which substrate is suspected to pass during the protein’s transport cycle (78, 129). In addition, the loop between TMHs 1 and 2 plays a pivotal role in the oligomerization of OCT complexes within the membrane, although it is not clear whether such protein-protein interactions influence transport function (8, 46).
The focus of the rest of this review is the present understanding of the general mechanism of OCT-mediated transport, with an emphasis on the kidney-specific isoform OCT2, with the intent that such information may assist development of PBPK models of drug clearance and unwanted DDIs. Those interested in the mechanism of MATE-mediated transport are directed to recent reviews on that topic (68, 118).
GENERAL MECHANISM OF OCT UNIPORT
The original report of the cloning of Oct1 from the rat kidney (34) showed that accumulation of TEA, a prototypical cationic substrate used in many of the original studies of renal OC secretion, into Xenopus oocytes that expressed the cloned protein was saturable and blocked by a structurally diverse array of cationic drugs and inhibitors. That study also showed that the process, although insensitive to changes in extracellular pH, was inhibited by elevation in extracellular K+ and to the presence of 10 mM Ba2+, manipulations that should depolarize the membrane potential. Consequently, the authors concluded that rat (r)Oct1 supports the electrogenic (rheogenic) transport of OCs and thus displays a physiological characteristic of basolateral OC transport observed in both intact fish RPTs (93) and in isolated renal basolateral membrane vesicles from the rabbit, rat, and dog (62, 122).
It is appropriate to point out that present understanding of the molecular basis of OCT-mediated transport is derived from studies using different homologs (primarily OCT1 and OCT2) and different orthologs of those proteins (primarily rat and human). In fact, the principal distinguishing characteristic between the three, besides their sites of expression, appears to be relatively modest differences in selectivity. Consequently, I will freely compare results obtained with different OCT homologs and orthologs to create a picture of a general mechanism of OCT-mediated transport.
The electrogenic characteristics of OCT-mediated transport were established in studies that directly measured inward currents produced by transport of OCs into Xenopus oocytes that expressed OCT1 (12), OCT2 (9), or OCT3 (123). The influence of membrane potential on transport was extensively studied for rOct2 (9). Choline flux was examined using excised patches of plasma membrane from rOct2-expressing oocytes (9), thereby allowing characterization of transport that represented either influx (inward current) or efflux (outward current). The two fluxes displayed similar voltage dependencies, and changes in the maximal outward choline-induced currents (Imax) and the associated K50 values (concentration of choline producing half Imax) were nearly linear between −40 and +60 mV (referenced to an extracellular value of 0 mV) (9). Although measurement of current flux provides a useful means to examine mechanisms of transport under well-controlled conditions, net current may not report (only) transport of the primary substrate. Whereas the two fluxes are correlated, Schmitt et al. (86) showed that the stoichiometry of charge-to-substrate coupling for rOct2 need not be 1:1 but instead is both substrate and voltage dependent. With a holding potential of 0 mV, the prototypic OC substrates 1-methyl-4-phenylpyridinium (MPP) and TEA-induced currents exceeded the simultaneously measured flux of the radiolabeled substrates by 4-fold and 3.5-fold, respectively, whereas the charge-to-substrate ratio for guanidinium was ∼1.5. The excess charge appeared to reflect a parallel, Oct2-mediated, nonspecific flux of cationic solutes, including Na+, K+, Ca2+, Mg2+, and even lysine+. Importantly, these linked fluxes did not reflect an energetic coupling such as that in secondary active “cotransport” but instead an opportunistic nonselective interaction of these companion cations with selected anionic residues within the translocation pathway. These differences from ratios of unity largely disappear at a potential of −50 mV. Current flux decreased as membrane potential was depolarized (i.e., rendered more positive in the trans compartment), reflecting changes in both Imax and K50; the Imax for choline efflux decreased by ∼35%, and K50 increased by ∼30% when the potential was depolarized by 60 mV. It was also of interest that the K50 for inward and outward currents (at 0 mV) did not differ significantly, suggesting that the affinity of the extracellular and cytoplasmic faces of Oct2 for choline may be effectively the same (86). Maximal currents were noted under so-called zero-trans conditions (86), i.e., when substrate was restricted to one side of the membrane (see Ref. 99), thereby necessitating that the transporter undergo the conformational changes associated with reorientation to the opposite face of the membrane without substrate bound. Indeed, this is the probable normal, physiological mode of OCT activity. But when substrate was also available in the trans compartment, choline-induced currents decreased (86), suggesting that some transporters “returned” loaded with substrate, resulting in an electroneutral OC-OC exchange and a concomitant reduction in net current flux. However, this exchange activity is mechanistically distinct from the OC/H+ exchange of MATE transporters. Whereas an unloaded OCT can reorient across the membrane, MATEs are “obligatory” exchangers (so-called “no-slippage counterflow”; see Ref. 99), wherein only the fully loaded transporter can undergo the conformational changes that result in substrate translocation. In the case of MATEs, this typically results efflux of an OC+ “coupled” to the influx of H+ (see Refs. 99 and 122).
The presence of substrate in the trans compartment can exert a marked effect on the rate of OCT-mediated transport. When the rate constant for reorientation of a loaded carrier is faster than that for the empty carrier, the presence of substrate in the trans compartment can stimulate the “cis-to-trans” rate of substrate movement, resulting in the so-called trans stimulation of transport so commonly observed with uniporters (99, 100), including OCT1 and OCT2 (e.g., see Refs. 12, 39, 48, 65, 92). Although electrogenic uniport is likely the common mode of OCT-mediated activity, the presence of a substantial intracellular concentration of an OCT substrate can result in electroneutral mediated exchange of OC for OC (9). Indeed, the net reabsorptive flux of choline has been suggested to involve an OCT2-mediated basolateral exchange of cytoplasmic choline for an endogenous cation in the plasma that is undergoing net secretion (19). We recently examined the extent to which substrate transport is stimulated by the presence of saturating concentrations of different OCT2 substrates in the trans compartment (the “infinite” trans condition; see Ref. 100) and found it to be correlated with the maximal rate of transport of the trans substrate (92). For six distinct OCT2 substrates, we also observed a near-linear relationship between increasing values for Vmax (Jmax) (30-fold range) and increasing apparent Km (Kt) values (100-fold range). In light of the theoretical relationship between Km (KD) values and the likely range of transporter turnover numbers (99), we suggested that the rate-limiting step in OCT2-mediated transport is dissociation of substrate from the transporter rather than differences in conformational change of the transport protein associated with translocation of structurally distinct substrates (92).
MULTISUBSTRATE SPECIFICITY OF OCTs
It is common for solute carriers to be restricted to substrates with narrowly defined structural characteristics. Molecular similarity comparisons [e.g., maximum common substructure (MCS)] provide a means to assess the structural characteristics of selectivity (13); an MCS coefficient of 1.0 means that a pair of molecules possess identical structural descriptors, whereas a coefficient of zero means they share no structural features in common. Glucose transporter 1 is representative of a highly selective uniporter, and the average MCS coefficients for a range of substrates (e.g., d-isoforms of glucose, galactose, glucosamine, and 3-0-methylglucose) is ∼0.8. In contrast, OCT2 accepts substrates as structurally diverse as metformin, cimetidine, and lamivudine, with MCS coefficients that average ∼0.2. Indeed, the hallmark of renal OC secretion is, arguably, its broad selectivity (106–108). However, that broad selectivity also makes OCT2 a target for unwanted DDIs that influence the pharmacokinetics of pharmaceutical clearance and increase the risk of associated toxicities (40a). The potential clinical impact of DDIs made OCT2 (and OCT1 as well) the focus of studies that attempted to identify structural characteristics of ligand interaction as part of a growing effort to develop models capable of preempting clinically significant DDIs. This issue is addressed in more detail in an upcoming section. Here, the focus is on the present understanding of OCT selectivity, with an emphasis on OCT2.
Initial efforts to develop models for the functional basis of ligand interaction with OCTs often used the “type I” and “type II” classifications for different structural classes of OCs developed to describe OC secretion in the liver (58). In general, type I OCs are comparatively small (typically <400 Da), monovalent compounds such as TEA, tributylmethylammonium, and procainamide. Importantly, the majority of cationic drugs from a wide array of clinical classes, including antihistamines, skeletal muscle relaxants, antiarrhythmics, and β-adrenoceptor blocking agents, fit the type I criteria. Type II OCs are usually bulkier (typically >500 Da) and frequently polyvalent, including d-tubocurarine, vecuronium, and hexafluorenium (58). Type II OCs are preferential substrates of organic anion-transporting polypeptides (including OATP1B1, OATP1B3, and OATP2B1) (15, 114) and, consequently, are typically excreted by the liver, whereas the smaller, more hydrophilic type I OCs are generally substrates for OCTs and preferentially excreted via the kidneys (see Ref. 37).
In general, all three OCT homologs support transport of a structurally diverse array of type I OCs (120) and interact with a limited number of neutral and even anionic substrates (e.g., see Ref. 3). With respect to the observation that OCTs can interact with (selected) neutral or anionic substrates, Ullrich et al. (110, 111) observed “crossover” interactions of a number of what they referred to as “bisubstrates,” with both cation and anion transport pathways in the rat kidney. Nevertheless, despite the (generally weak) interaction of neutral and anionic substrates with OCTs, the presence of a charged moiety clearly enhances interaction with these transporters, as shown in studies demonstrating more efficient interaction of the weak base cimetidine (pKa = 6.9) with OCT2 when the substrate is protonated (Ref. 4; see also Ref. 115). OCTs typically do interact with type II OCs, but this generally appears to reflect inhibitory binding with modest or no translocation of substrate (51, 115).
Moving beyond the general structural characterization afforded by the type I/II designation, the application of computational methods introduced efforts to identify the molecular determinants that influence the interaction of substrates and inhibitors with OCTs. Using three-dimensional quantitative structure/activity relationships, substrates/inhibitors of OCT1 were shown to commonly share several structural elements, including a positive ion interaction site, one or more hydrophobic surface interaction sites, and hydrogen bond acceptor sites (2, 5, 61). Computational methods also showed that the influence on substrate/inhibitor interaction with OCT2 of the distance between hydrophobic (i.e., aromatic) elements and the center of positive charge (101, 124, 131) and the influence of topological polar surface area (131) are useful criteria for predicting inhibitor interaction with the transporter-binding region. However, the several resulting three-dimensional pharmacophores that identified molecular determinants of ligand-transporter interaction (5, 61, 69, 101, 124, 131) have structurally distinct placements of these structural features. Although the bases of these discrepancies likely include methodological and/or analytical differences used by different research groups, we suggested another possible contributor: the influence of “substrate” on OCT interaction with an “inhibitor” (6). In this regard, it is interesting that the four modeling efforts with OCT2 (and the three modeling efforts with OCT1) were based on inhibition of transport of structurally distinct substrates: MPP (61, 131), TEA (5, 101), metformin (69), or 4-[4-(dimethylamino)styryl]-N-methylpyridinium (ASP+) (2, 49, 124). In other words, the differences in the resulting models may have reflected, at least to some degree, the influence of substrate structure on the inhibitory impact of tested agents with OCTs.
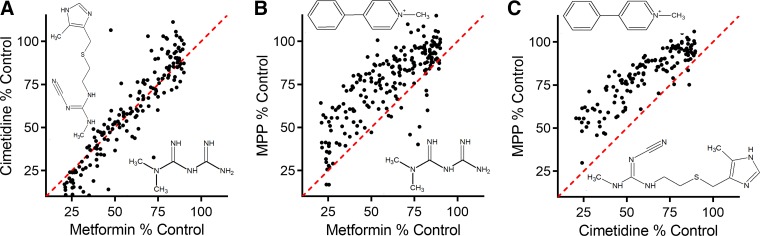
The influence of substrate on inhibition of OCT2-mediated transport produced by structurally distinct ligands was subsequently examined in several studies. Belzer et al. (6) noted that IC50 values for inhibition of MPP transport produced by nine structurally distinct OCs were ∼9-fold greater than those for inhibition of metformin transport, and Hacker et al. (36) subsequently showed that 125 frequently prescribed drugs produced greater inhibition of metformin transport than of MPP transport. The relative insensitivity of OCT2-mediated MPP transport to inhibition was confirmed and extended in a study (85) that determined inhibitory profiles produced by more than 400 structurally distinct inhibitors against OCT2-mediated transport of MPP, metformin, cimetidine, TEA, ASP+, and the fluorescent OC N,N,N-trimethyl-2-[methyl(7-nitrobenzo[c][l,2,5]oxadiazol-4-yl)amino]ethanaminium (NBD-MTMA). While <10% of these compounds (at 20 µM) blocked MPP transport by >50%, ∼20% of them inhibited transport of the other 4 substrates, and IC50 values determined for a subset of 20 of these compounds were ∼6-fold greater for MPP than for the other substrates. The “substrate dependence” of ligand interaction with OCT2 is evident in three paired comparisons of the relative influence of structurally distinct test compounds on inhibition of OCT2-mediated transport of MPP, metformin, and cimetidine (Fig. 1). While metformin and cimetidine transport display very similar inhibitory profiles (Fig. 1A), MPP transport is consistently (and significantly) less sensitive to inhibition than transport of either metformin or cimetidine (Fig. 1, B and C). Other studies also reported different degrees of inhibitory activity for test compounds, depending on the substrate used as an indicator of transport activity (103, 125). For example, IC50 values produced by cimetidine and pyrimethamine are ∼10-fold lower when blocking OCT2-mediated atenolol transport compared with transport of metformin (125).
Fig. 1.
Influence of substrate identity on the degree of inhibition of organic cation transporter 2 (OCT2)-mediated transport. The 3 test substrates were metformin (12 µM), cimetidine (30 nM), and 1-methyl-4-phenylpyridinium (MPP; 12 nM). Each point represents the average of 30-s uptakes of substrate (expressed as %control uptake) from 2 experiments (each measured in triplicate), measured in the presence and absence (control) of a 20 µM concentration of 1 of 150 to 200 structurally distinct test inhibitors. The resulting inhibitory profiles are presented as 3 paired comparisons: inhibition of metformin transport versus inhibition of cimetidine transport (A), metformin transport vs. MPP transport (B), and cimetidine transport vs. MPP transport (C). The lines of unity are represented by dashed red lines (modified from Ref. 85).
Understanding the bases for discrepancies in the inhibitory influence of selected drugs on a process so central to the clearance of pharmaceuticals from the body is of critical importance. Strategies for predicting the potential of “new molecular entities” to perpetrate unwanted DDIs are an important aspect of pharmaceutical development. The initial report by the International Transporter Consortium (40a) proposed using decision trees to identify these potential perpetrators, an idea subsequently included by the United States Food and Drug Administration in their initial Guidance for Industry concerning interaction studies for drug transporters (104). A key element of these decision trees is a comparison of the maximum unbound plasma concentration of a test drug (Cu-max) to its IC50 value for inhibition of activity, typically measured in vitro using cultured cells, of the transporter in question, e.g., OCT2; if the Cu-max-to-IC50 ratio exceeds 0.1, a followup clinical study is generally recommended. In light of the differential sensitivity to inhibition of OCT2-mediated transport of metformin and MPP noted above, it was ironic that the ITC suggested using these two substrates for decision tree-based assessments of potential DDIs with OCTs (40a); a focus on inhibition of MPP transport could underestimate the DDI potential of the compound under study. However, the choice of those substrates was not arbitrary; whereas metformin’s inclusion reflected its widespread clinical importance and evidence that OCT2 plays a quantitatively significant role in mediating its clearance (40a), MPP’s inclusion reflected the recognition (54) that is efficiently transported by OCTs (87), so its use can provide strong transport signals amenable to precise quantitative analysis. However, subsequent modifications of the Federal Drug Administrations’s recommended practices (105) did acknowledge that substrate choice can influence inhibitory efficacy of a test agent. However, the recommended modifications (e.g., using a “probe substrate that usually generates a lower IC50 for known inhibitors to avoid underestimating the interaction potential of the investigational drug”) are not based on an understanding of the mechanism of ligand interaction with OCTs.
MOLECULAR/MECHANISTIC BASIS OF SUBSTRATE-LIGAND INTERACTION
The interpretation of inhibition data for transporters often makes the tacit assumption that substrates and inhibitors compete for a common binding site, but that need not be the case; the mechanisms of inhibition of transport activity are diverse (91). Moreover, in the case of the OCTs, the evidence for complex ligand interaction(s) has existed for many years. Gorboulev et al. (33) identified D475 in rOCT1 (D474 in hOCT1) as a locus for substrate interaction with the transporter. They showed that MPP displays a spatially distinct interaction profile compared with several other substrates; replacing aspartate with glutamate at position 475 reduced Vmax for all tested substrates, but whereas it produced five- to 15-fold decreases in Km values for choline, TEA, and NMN transport, the mutation had no effect on the Km value for MPP transport.
Additional evidence that interaction of ligands (both substrates and inhibitors) with OCTs involves spatially distinct sites arose from studies using site-directed mutagenesis that was informed by homology models of OCT structure. The initial models were based on the first crystal structures of major facilitator superfamily proteins, the glycerol-3-phosphate transporter (GlpT; see Ref. 43) and Lac permease (LacY; see Ref. 1) of E. coli. A model for the structure of rbOCT2 (129) revealed that the glutamate residue at position 447 is within the cleft between the NH2- and COOH-terminal halves common to MFS transporters. While this glutamate is conserved in all OCT2 orthologs (E448 in hOCT2), OCT1 orthologs express glutamine. Interestingly, the E447Q mutant of rbOct2 displayed a 15-fold reduction in affinity for cimetidine to a level similar to that observed for rbOct1. With respect to the issue of distinct spatial interactions of ligands with OCTs, the E447L mutant of rbOct2 eliminated TEA and cimetidine transport but had no effect on MPP transport (129). In addition, whereas MPP transport by the E447L mutant displayed markedly reduced sensitivity to inhibition by TEA (IC50 value of 83 µM increased to 750 µM), inhibition of MPP transport by cimetidine was comparable in both the wild-type and mutant proteins (IC50 values of 16.9 vs. 9.0 µM). A model for rOct1 (78), based on the LacY structure, also revealed that Q448 and L447 are exposed within the “translocation cleft” and that mutations of these sites markedly influenced affinity of the protein for the nontransported inhibitor corticosterone (31). Both sets of observations support the conclusion that ligand interaction with OCTs is not restricted to a single common binding site but can involve multiple discrete sites (or a larger binding surface with multiple, potentially overlapping regions).
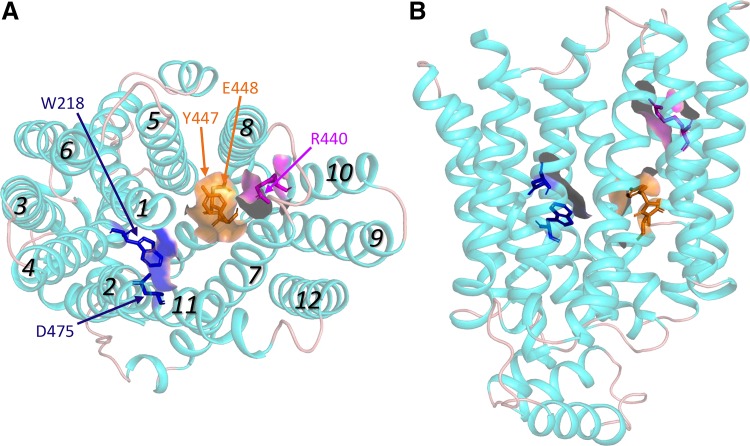
The initial models of OCT structure were based on template structures that were in an inwardly (i.e., cytoplasmic) facing conformation. Subsequent models of the outwardly facing transporters provided further evidence of the complexity of ligand interaction with OCTs. In a study using rOct1, Gorboulev et al. (30) observed altered affinities for substrates and/or inhibitors when F160, W218, R440, L447, or D475 was mutated. The differential influence of mutations to these residues in rOct1 on the kinetic interactions of substrates (e.g., MPP and TEA) and inhibitors (corticosterone, TBuA and TPeA) suggests functional clustering of structurally distinct but overlapping sites. Figure 2 shows a model of hOCT2 (based on the outwardly facing structure of unoccluded glucose transporter 3; see Refs. 18 and 21) demonstrating that the homologous residues in hOCT2 contribute to the solvent-accessible cleft region of the outwardly facing modeled protein (presented in the model as color-coded residues and surfaces).
Fig. 2.
Homology model of human organic cation transporter 2 (OCT2), based on the outwardly facing structure of unoccluded GLUT3 (18, 21), generated using I-Tasser (http://zhanglab.ccmb.med.umich.edu/I-TASSER/). A: face-on view from the extracellular side of the protein. B: side view, with the extracellular aspect toward the top. Italicized numbers indicate transmembrane helices 1 through 12. Amino acid residues experimentally determined to be involved in ligand interaction are shown in stick form (with solvent accessible surfaces) and color-coded to reflect structurally distinct binding interactions. The poorly resolved long extracellular loop between helices 1 and 2 is not displayed. Images shown were prepared using Pymol 2.3.
Recent work with purified and reconstituted transport protein revealed that MPP binds to multiple sites on rOCT1. Keller et al. (47) examined the kinetics of MPP binding to reconstituted “nanodisks” (see Ref. 66) and showed that MPP binds to three spatially distinct binding “sites.” Two of these displayed KD values sufficiently similar (average value of 36 µM) that they could not be distinguished kinetically but could be distinguished through the differential influence on binding of site-directed mutations; one of these sites was associated with W218 and D475, whereas the second site involved interaction with R440 (see Fig. 2A). The third site displayed higher affinity for MPP (KD value of ∼0.2 µM). Although its spatial location was not clear, its presence was sensitive to the lipid composition of the nanodisks. Measurement of MPP transport in reconstituted proteoliposomes containing wild-type and mutant rOct1 showed that, whereas the two low-affinity sites were associated with substrate translocation, the high-affinity site appeared to be restricted to exerting partial inhibition of transport activity.
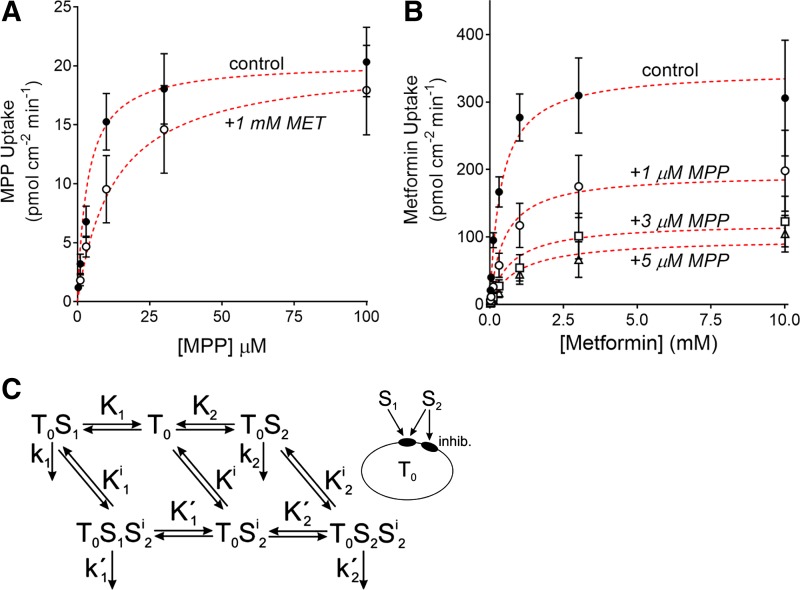
The kinetics of MPP’s interaction with OCT2 also support the conclusion that MPP interacts with multiple sites that exert different effects on transport activity. As noted earlier, OCT2-mediated MPP transport is less sensitive to inhibition than several other, structurally distinct substrates, including metformin. We recently examined the kinetics of MPP and metformin interaction with OCT2, including the kinetic basis for inhibition each displays on transport of the other (84). Whereas metformin’s inhibition of MPP transport is competitive (i.e., producing an increase in the apparent Km value for MPP transport while having no effect on Vmax; Fig. 3A), MPP exerts a “mixed-type” inhibition of metformin transport (i.e., producing both an increase in the apparent Km value and a decrease in Vmax; Fig. 3B). Of particular note, however, was the observation that the IC50 for MPP’s inhibition of metformin transport is significantly lower (<1 µM) than the apparent Km value for MPP transport (5 µM). These observations are inconsistent with either competition for a common binding site or conventional linear mixed-type inhibition (91). The simplest explanation for this collection of observations is that 1) MPP and metformin compete for a common binding site [or, as inferred from the recent observations of Keller et al. (47), a set of identical, noncooperative binding sites] associated with substrate translocation and 2) MPP also interacts with a distinct site not associated with transport but, when occupied, exerts partial inhibition of transport activity. Importantly, this kinetic model (Fig. 3C) can also account for the differential effect of inhibitors on MPP transport compared with that of other substrates (e.g., metformin) (84).
Fig. 3.
The kinetic basis for inhibitory interactions of metformin and 1-methyl-4-phenylpyridinium (MPP) with organic cation transporter 2 (OCT2). A: the kinetics of OCT2-mediated MPP transport determined in the absence (●) and presence (○) of 1 mM metformin. Each point represents the average (± SE) of initial rates of transport, determined from the time courses of net MPP accumulation, measured in 5 separate experiments. B: relationship between increasing metformin concentration and the rate of metformin transport, determined in the absence (0 µM; solid symbols) and presence of unlabeled MPP (1, 3, and 5 µM; ○, △, and □). Each point is the average (±SE) of rates (calculated from 60 s net uptakes) of transport measured in 3 separate experiments, each performed in triplicate. In both A and B, the red dashed lines show the predicted kinetic profiles derived from optimizing the model laid out in C. The configuration of binding sites on the transporter T0 and their binding to substrate 1 (S1; metformin) and substrate 2 (S2; MPP) is shown in the diagram at right. “Inhib.” represents the inhibitory MPP binding site. The reversible transitions between the binding states are represented by double arrows. The associated constants (K1, K2, etc.) represent the ratios of the backward to the forward rate constants for each binding step. The available transport steps for substrate uptake are indicated by single arrows. The associated constants (k1, k2, etc.) represent the rate constants for the corresponding uptake. Because initial uptake rates are considered, cellular release of substrate is not considered (modified from Ref. 84).
EXPERIMENTAL ASSESSMENT OF DRUG INTERACTION WITH OCTs
As noted earlier, the initial decision tree created for identifying potential perpetrators of OCT-based DDIs recommended using transport of MPP or metformin as the measure of OCT activity (40a). Subsequent demonstrations of the impact choice of substrate can have on IC50 values produced by novel test agents (6, 36, 85, 125) contributed to important revisions in decision tree design (105). However, recent insights into the structural (30, 47) and kinetic (84) bases of complex ligand interactions with OCTs (and other multidrug transporters; e.g., see Refs. 28, 83, and 117) underscore the challenges of experimental design and interpretation of in vitro studies that attempt to assess the inhibitory impact of novel drugs on drug clearance. Combined with the acknowledged challenges facing efforts to accurately measure transport kinetics using cultured cell models (77, 84, 127), the difficulty of drawing relevant conclusions concerning the clinical impact of transport on drug clearance is apparent.
Nevertheless, there is great interest in and a need for in vitro/in silico assessment of the quantitative interaction of substrates and inhibitors with drug transporters, including OCTs (17, 22–24). PBPK models are increasingly used to make quantitative predictions of drug clearance and predict DDIs associated with drug metabolizing enzymes in lieu of dedicated clinical studies (17, 72, 73). But for many therapeutically important compounds, the kinetic parameters of transport are critical inputs in PBPK models (e.g., see Ref. 11), so the uncertainty and ambiguity around these values (e.g., see Ref. 7) adversely affect model performance and limit the application of PBPK for predicting the influence of drug transporters on drug disposition and DDIs.
The introduction of experimental protocols that emphasize analysis of the full-time course of net transport (77, 84) may lead to needed improvements in the accuracy of the kinetic assessment of drug transport by revealing initial rates of uptake that are uninfluenced by either unstirred layers or backflux of accumulated substrate (84). However, also required is a more focused approach to the design of studies testing the inhibitory potential of novel drugs (50). The literature now supports a design that involves determining the interaction of a novel test agent with several (often, at least 3) structurally distinct substrates. If available, one of these can be a substrate that is generally more sensitive to inhibition than others (to avoid underestimating the interaction potential of the test agent; see Ref. 105). IC50 values should be determined by employing a range of inhibitor concentrations within the range of unbound concentrations observed clinically. In addition, however, evidence discussed earlier that ligands (substrates or inhibitors) may interact with multiple sites on OCTs supports the suggestion that inhibition of transport activity produced by a novel agent should be examined at several substrate concentrations, including well below (<10-fold) and near the Km value for that substrate (50). Still, the complexity of ligand interaction with the OCTs and other multidrug transporters poses particular challenges for the prospect of developing models of sufficient accuracy to predict a new molecular entity’s likelihood of perpetrating an adverse DDI. Docking studies that use homology models based on related but distinct transporters have provided critical insights into both structures and mechanisms of these processes (e.g., see Refs. 14, 52, and 116), but prediction of binding constants for interactions with OCTs (and other drug transporters) of sufficient accuracy to influence clinical or regulatory decisions will likely require the knowledge of spatial distribution of critical amino acid residues that arise from molecular dynamic studies that use crystal structures of these transporters (52).
Although not presently capable of providing quantitative predictions of ligand interaction with transporters, computational application of machine-learning methods offers the promise of advancing drug discovery (25). For example, Bayesian models can identify structural features most commonly associated with active (i.e., comparatively high-affinity) inhibition of transport activity that could result in adverse DDIs (85), making the approach valuable in early preclinical drug discovery, where the virtual screening of large libraries of novel structures for their probable interaction with transporters can be performed cost effectively to filter out potentially problematic compounds (25).
SUMMARY
OCTs, particularly OCT2, represent the initial step in renal secretion of OCs. Present evidence supports the idea that these proteins can interact effectively with a structurally diverse array of compounds in part because ligand binding does not involve a single binding site but, rather, a suite of several sites. Although this may, at least in part, account for the hallmark “multispecificity” of this family of transporters, it is also the basis for adverse DDIs that complicate the pharmacokinetics of many clinically important drugs. The accurate identification of potential perpetrators of such DDIs will require an experimental design that takes into account the complexity of ligand interaction with OCTs. However, the development of predictive models of such interactions is likely to require elucidation of crystal structures capable of precise localization of ligand-binding sites.
GRANTS
This work was supported by National Institutes of Health Grants 5-R01-DK-058251 and 1-R01-GM-129777.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
S.H.W. conceived and designed research; S.H.W. analyzed data; S.H.W. interpreted results of experiments; S.H.W. prepared figures; S.H.W. drafted manuscript; S.H.W. edited and revised manuscript; S.H.W. approved final version of manuscript.
REFERENCES
- 1.Abramson J, Smirnova I, Kasho V, Verner G, Kaback HR, Iwata S. Structure and mechanism of the lactose permease of Escherichia coli. Science 301: 610–615, 2003. doi: 10.1126/science.1088196. [DOI] [PubMed] [Google Scholar]
- 2.Ahlin G, Karlsson J, Pedersen JM, Gustavsson L, Larsson R, Matsson P, Norinder U, Bergström CA, Artursson P. Structural requirements for drug inhibition of the liver specific human organic cation transport protein 1. J Med Chem 51: 5932–5942, 2008. doi: 10.1021/jm8003152. [DOI] [PubMed] [Google Scholar]
- 3.Arndt P, Volk C, Gorboulev V, Budiman T, Popp C, Ulzheimer-Teuber I, Akhoundova A, Koppatz S, Bamberg E, Nagel G, Koepsell H. Interaction of cations, anions, and weak base quinine with rat renal cation transporter rOCT2 compared with rOCT1. Am J Physiol Renal Physiol 281: F454–F468, 2001. doi: 10.1152/ajprenal.2001.281.3.F454. [DOI] [PubMed] [Google Scholar]
- 4.Barendt WM, Wright SH. The human organic cation transporter (hOCT2) recognizes the degree of substrate ionization. J Biol Chem 277: 22491–22496, 2002. doi: 10.1074/jbc.M203114200. [DOI] [PubMed] [Google Scholar]
- 5.Bednarczyk D, Ekins S, Wikel JH, Wright SH. Influence of molecular structure on substrate binding to the human organic cation transporter, hOCT1. Mol Pharmacol 63: 489–498, 2003. doi: 10.1124/mol.63.3.489. [DOI] [PubMed] [Google Scholar]
- 6.Belzer M, Morales M, Jagadish B, Mash EA, Wright SH. Substrate-dependent ligand inhibition of the human organic cation transporter OCT2. J Pharmacol Exp Ther 346: 300–310, 2013. doi: 10.1124/jpet.113.203257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bentz J, O’Connor MP, Bednarczyk D, Coleman J, Lee C, Palm J, Pak YA, Perloff ES, Reyner E, Balimane P, Brännström M, Chu X, Funk C, Guo A, Hanna I, Herédi-Szabó K, Hillgren K, Li L, Hollnack-Pusch E, Jamei M, Lin X, Mason AK, Neuhoff S, Patel A, Podila L, Plise E, Rajaraman G, Salphati L, Sands E, Taub ME, Taur JS, Weitz D, Wortelboer HM, Xia CQ, Xiao G, Yabut J, Yamagata T, Zhang L, Ellens H. Variability in P-glycoprotein inhibitory potency (IC50) using various in vitro experimental systems: implications for universal digoxin drug-drug interaction risk assessment decision criteria. Drug Metab Dispos 41: 1347–1366, 2013. doi: 10.1124/dmd.112.050500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brast S, Grabner A, Sucic S, Sitte HH, Hermann E, Pavenstädt H, Schlatter E, Ciarimboli G. The cysteines of the extracellular loop are crucial for trafficking of human organic cation transporter 2 to the plasma membrane and are involved in oligomerization. FASEB J 26: 976–986, 2012. doi: 10.1096/fj.11-180679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Budiman T, Bamberg E, Koepsell H, Nagel G. Mechanism of electrogenic cation transport by the cloned organic cation transporter 2 from rat. J Biol Chem 275: 29413–29420, 2000. doi: 10.1074/jbc.M004645200. [DOI] [PubMed] [Google Scholar]
- 10.Burckhardt G, Wolff NA. Structure of renal organic anion and cation transporters. Am J Physiol Renal Physiol 278: F853–F866, 2000. doi: 10.1152/ajprenal.2000.278.6.F853. [DOI] [PubMed] [Google Scholar]
- 11.Burt HJ, Neuhoff S, Almond L, Gaohua L, Harwood MD, Jamei M, Rostami-Hodjegan A, Tucker GT, Rowland-Yeo K. Metformin and cimetidine: Physiologically based pharmacokinetic modelling to investigate transporter mediated drug-drug interactions. Eur J Pharm Sci 88: 70–82, 2016. doi: 10.1016/j.ejps.2016.03.020. [DOI] [PubMed] [Google Scholar]
- 12.Busch AE, Quester S, Ulzheimer JC, Waldegger S, Gorboulev V, Arndt P, Lang F, Koepsell H. Electrogenic properties and substrate specificity of the polyspecific rat cation transporter rOCT1. J Biol Chem 271: 32599–32604, 1996. doi: 10.1074/jbc.271.51.32599. [DOI] [PubMed] [Google Scholar]
- 13.Cao Y, Jiang T, Girke T. A maximum common substructure-based algorithm for searching and predicting drug-like compounds. Bioinformatics 24: i366–i374, 2008. doi: 10.1093/bioinformatics/btn186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Celik L, Sinning S, Severinsen K, Hansen CG, Møller MS, Bols M, Wiborg O, Schiøtt B. Binding of serotonin to the human serotonin transporter. Molecular modeling and experimental validation. J Am Chem Soc 130: 3853–3865, 2008. doi: 10.1021/ja076403h. [DOI] [PubMed] [Google Scholar]
- 15.Chandra P, Brouwer KL. The complexities of hepatic drug transport: current knowledge and emerging concepts. Pharm Res 21: 719–735, 2004. doi: 10.1023/B:PHAM.0000026420.79421.8f. [DOI] [PubMed] [Google Scholar]
- 16.Chen Y, Li S, Brown C, Cheatham S, Castro RA, Leabman MK, Urban TJ, Chen L, Yee SW, Choi JH, Huang Y, Brett CM, Burchard EG, Giacomini KM. Effect of genetic variation in the organic cation transporter 2 on the renal elimination of metformin. Pharmacogenet Genomics 19: 497–504, 2009. doi: 10.1097/FPC.0b013e32832cc7e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Clerbaux LA, Coecke S, Lumen A, Kliment T, Worth AP, Paini A. Capturing the applicability of in vitro-in silico membrane transporter data in chemical risk assessment and biomedical research. Sci Total Environ 645: 97–108, 2018. doi: 10.1016/j.scitotenv.2018.07.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dakal TC, Kumar R, Ramotar D. Structural modeling of human organic cation transporters. Comput Biol Chem 68: 153–163, 2017. doi: 10.1016/j.compbiolchem.2017.03.007. [DOI] [PubMed] [Google Scholar]
- 19.Dantzler WH, Wright SH, Chatsudthipong V, Brokl OH. Basolateral tetraethylammonium transport in intact tubules: specificity and trans-stimulation. Am J Physiol 261: F386–F392, 1991. doi: 10.1152/ajprenal.1991.261.3.F386. [DOI] [PubMed] [Google Scholar]
- 20.David C, Rumrich G, Ullrich KJ. Luminal transport system for H+/organic cations in the rat proximal tubule. Kinetics, dependence on pH; specificity as compared with the contraluminal organic cation-transport system. Pflugers Arch 430: 477–492, 1995. doi: 10.1007/BF00373884. [DOI] [PubMed] [Google Scholar]
- 21.Deng D, Sun P, Yan C, Ke M, Jiang X, Xiong L, Ren W, Hirata K, Yamamoto M, Fan S, Yan N. Molecular basis of ligand recognition and transport by glucose transporters. Nature 526: 391–396, 2015. doi: 10.1038/nature14655. [DOI] [PubMed] [Google Scholar]
- 22.Ekins S. Progress in computational toxicology. J Pharmacol Toxicol Methods 69: 115–140, 2014. doi: 10.1016/j.vascn.2013.12.003. [DOI] [PubMed] [Google Scholar]
- 23.Ekins S, Clark AM, Wright SH. Making transporter models for drug-drug interaction prediction mobile. Drug Metab Dispos 43: 1642–1645, 2015. doi: 10.1124/dmd.115.064956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ekins S, Polli JE, Swaan PW, Wright SH. Computational modeling to accelerate the identification of substrates and inhibitors for transporters that affect drug disposition. Clin Pharmacol Ther 92: 661–665, 2012. doi: 10.1038/clpt.2012.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ekins S, Puhl AC, Zorn KM, Lane TR, Russo DP, Klein JJ, Hickey AJ, Clark AM. Exploiting machine learning for end-to-end drug discovery and development. Nat Mater 18: 435–441, 2019. doi: 10.1038/s41563-019-0338-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eraly SA, Hamilton BA, Nigam SK. Organic anion and cation transporters occur in pairs of similar and similarly expressed genes. Biochem Biophys Res Commun 300: 333–342, 2003. doi: 10.1016/S0006-291X(02)02853-X. [DOI] [PubMed] [Google Scholar]
- 27.Feng B, Varma MV. Evaluation and quantitative prediction of renal transporter-mediated drug-drug interactions. J Clin Pharmacol 56, Suppl 7: S110–S121, 2016. doi: 10.1002/jcph.702. [DOI] [PubMed] [Google Scholar]
- 28.Garrigues A, Loiseau N, Delaforge M, Ferté J, Garrigos M, André F, Orlowski S. Characterization of two pharmacophores on the multidrug transporter P-glycoprotein. Mol Pharmacol 62: 1288–1298, 2002. doi: 10.1124/mol.62.6.1288. [DOI] [PubMed] [Google Scholar]
- 30.Gorboulev V, Rehman S, Albert CM, Roth U, Meyer MJ, Tzvetkov MV, Mueller TD, Koepsell H. Assay conditions influence affinities of rat organic cation transporter 1: Analysis of mutagenesis in the modeled outward-facing cleft by measuring effects of substrates and inhibitors on initial uptake. Mol Pharmacol 93: 402–415, 2018. doi: 10.1124/mol.117.110767. [DOI] [PubMed] [Google Scholar]
- 31.Gorboulev V, Shatskaya N, Volk C, Koepsell H. Subtype-specific affinity for corticosterone of rat organic cation transporters rOCT1 and rOCT2 depends on three amino acids within the substrate binding region. Mol Pharmacol 67: 1612–1619, 2005. doi: 10.1124/mol.104.008821. [DOI] [PubMed] [Google Scholar]
- 32.Gorboulev V, Ulzheimer JC, Akhoundova A, Ulzheimer-Teuber I, Karbach U, Quester S, Baumann C, Lang F, Busch AE, Koepsell H. Cloning and characterization of two human polyspecific organic cation transporters. DNA Cell Biol 16: 871–881, 1997. doi: 10.1089/dna.1997.16.871. [DOI] [PubMed] [Google Scholar]
- 33.Gorboulev V, Volk C, Arndt P, Akhoundova A, Koepsell H. Selectivity of the polyspecific cation transporter rOCT1 is changed by mutation of aspartate 475 to glutamate. Mol Pharmacol 56: 1254–1261, 1999. doi: 10.1124/mol.56.6.1254. [DOI] [PubMed] [Google Scholar]
- 34.Gründemann D, Gorboulev V, Gambaryan S, Veyhl M, Koepsell H. Drug excretion mediated by a new prototype of polyspecific transporter. Nature 372: 549–552, 1994. doi: 10.1038/372549a0. [DOI] [PubMed] [Google Scholar]
- 35.Gründemann D, Schömig E. Gene structures of the human non-neuronal monoamine transporters EMT and OCT2. Hum Genet 106: 627–635, 2000. doi: 10.1007/s004390000309. [DOI] [PubMed] [Google Scholar]
- 36.Hacker K, Maas R, Kornhuber J, Fromm MF, Zolk O. Substrate-dependent inhibition of the human organic cation transporter OCT2: a comparison of metformin with experimental substrates. PLoS One 10: e0136451, 2015. doi: 10.1371/journal.pone.0136451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hagenbuch B. Drug uptake systems in liver and kidney: a historic perspective. Clin Pharmacol Ther 87: 39–47, 2010. doi: 10.1038/clpt.2009.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Harper JN, Wright SH. Multiple mechanisms of ligand interaction with the human organic cation transporter, OCT2. Am J Physiol Renal Physiol 304: F56–F67, 2013. doi: 10.1152/ajprenal.00486.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hayer M, Bönisch H, Brüss M. Molecular cloning, functional characterization and genomic organization of four alternatively spliced isoforms of the human organic cation transporter 1 (hOCT1/SLC22A1). Ann Hum Genet 63: 473–482, 1999. doi: 10.1046/j.1469-1809.2000.6430267.x. [DOI] [PubMed] [Google Scholar]
- 40a.International Transporter Consortium; Giacomini KM, Huang SM, Tweedie DJ, Benet LZ, Brouwer KL, Chu X, Dahlin A, Evers R, Fischer V, Hillgren KM, Hoffmaster KA, Ishikawa T, Keppler D, Kim RB, Lee CA, Niemi M, Polli JW, Sugiyama Y, Swaan PW, Ware JA, Wright SH, Yee SW, Zamek-Gliszczynski MJ, Zhang L. Membrane transporters in drug development. Nat Rev Drug Discov 9: 215–236, 2010. doi: 10.1038/nrd3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Holohan PD, Ross CR. Mechanisms of organic cation transport in kidney plasma membrane vesicles: 1. Countertransport studies. J Pharmacol Exp Ther 215: 191–197, 1980. [PubMed] [Google Scholar]
- 42.Holohan PD, Ross CR. Mechanisms of organic cation transport in kidney plasma membrane vesicles: 2. delta pH studies. J Pharmacol Exp Ther 216: 294–298, 1981. [PubMed] [Google Scholar]
- 43.Huang Y, Lemieux MJ, Song J, Auer M, Wang DN. Structure and mechanism of the glycerol-3-phosphate transporter from Escherichia coli. Science 301: 616–620, 2003. doi: 10.1126/science.1087619. [DOI] [PubMed] [Google Scholar]
- 44.Ivanyuk A, Livio F, Biollaz J, Buclin T. Renal drug transporters and drug interactions. Clin Pharmacokinet 56: 825–892, 2017. doi: 10.1007/s40262-017-0506-8. [DOI] [PubMed] [Google Scholar]
- 45.Jonker JW, Wagenaar E, Van Eijl S, Schinkel AH. Deficiency in the organic cation transporters 1 and 2 (Oct1/Oct2 [Slc22a1/Slc22a2]) in mice abolishes renal secretion of organic cations. Mol Cell Biol 23: 7902–7908, 2003. doi: 10.1128/MCB.23.21.7902-7908.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Keller T, Egenberger B, Gorboulev V, Bernhard F, Uzelac Z, Gorbunov D, Wirth C, Koppatz S, Dötsch V, Hunte C, Sitte HH, Koepsell H. The large extracellular loop of organic cation transporter 1 influences substrate affinity and is pivotal for oligomerization. J Biol Chem 286: 37874–37886, 2011. doi: 10.1074/jbc.M111.289330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Keller T, Gorboulev V, Mueller TD, Dötsch V, Bernhard F, Koepsell H. Rat organic cation transporter 1 contains three binding sites for substrate 1-methyl-4-phenylpyridinium per monomer. Mol Pharmacol 95: 169–182, 2019. doi: 10.1124/mol.118.113498. [DOI] [PubMed] [Google Scholar]
- 48.Keller T, Schwarz D, Bernhard F, Dötsch V, Hunte C, Gorboulev V, Koepsell H. Cell free expression and functional reconstitution of eukaryotic drug transporters. Biochemistry 47: 4552–4564, 2008. doi: 10.1021/bi800060w. [DOI] [PubMed] [Google Scholar]
- 49.Kido Y, Matsson P, Giacomini KM. Profiling of a prescription drug library for potential renal drug-drug interactions mediated by the organic cation transporter 2. J Med Chem 54: 4548–4558, 2011. doi: 10.1021/jm2001629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Koepsell H. Multiple binding sites in organic cation transporters require sophisticated procedures to identify interactions of novel drugs. Biol Chem 400: 195–207, 2019. doi: 10.1515/hsz-2018-0191. [DOI] [PubMed] [Google Scholar]
- 51.Koepsell H. Substrate recognition and translocation by polyspecific organic cation transporters. Biol Chem 392: 95–101, 2011. doi: 10.1515/bc.2011.009. [DOI] [PubMed] [Google Scholar]
- 52.Koldsø H, Grouleff J, Schiøtt B. Insights to ligand binding to the monoamine transporters-from homology modeling to LeuBAT and dDAT. Front Pharmacol 6: 208, 2015. doi: 10.3389/fphar.2015.00208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lazaruk KDA, Wright SH. MPP+ is transported by the TEA(+)-H+ exchanger of renal brush-border membrane vesicles. Am J Physiol 258: F597–F605, 1990. doi: 10.1152/ajprenal.1990.258.3.F597. [DOI] [PubMed] [Google Scholar]
- 55.Leabman MK, Huang CC, Kawamoto M, Johns SJ, Stryke D, Ferrin TE, DeYoung J, Taylor T, Clark AG, Herskowitz I, Giacomini KM; Pharmacogenetics of Membrane Transporters Investigators . Polymorphisms in a human kidney xenobiotic transporter, OCT2, exhibit altered function. Pharmacogenetics 12: 395–405, 2002. doi: 10.1097/00008571-200207000-00007. [DOI] [PubMed] [Google Scholar]
- 56.Lepist EI, Ray AS. Renal drug-drug interactions: what we have learned and where we are going. Expert Opin Drug Metab Toxicol 8: 433–448, 2012. doi: 10.1517/17425255.2012.667401. [DOI] [PubMed] [Google Scholar]
- 57.McKinney TD, Kunnemann ME. Procainamide transport in rabbit renal cortical brush border membrane vesicles. Am J Physiol 249: F532–F541, 1985. doi: 10.1152/ajprenal.1985.249.4.F532. [DOI] [PubMed] [Google Scholar]
- 58.Meijer DKF, Mol WEM, Müller M, Kurz G. Carrier-mediated transport in the hepatic distribution and elimination of drugs, with special reference to the category of organic cations. J Pharmacokinet Biopharm 18: 35–70, 1990. doi: 10.1007/BF01063621. [DOI] [PubMed] [Google Scholar]
- 59.Meyer-Wentrup F, Karbach U, Gorboulev V, Arndt P, Koepsell H. Membrane localization of the electrogenic cation transporter rOCT1 in rat liver. Biochem Biophys Res Commun 248: 673–678, 1998. doi: 10.1006/bbrc.1998.9034. [DOI] [PubMed] [Google Scholar]
- 60.Meyer MJ, Seitz T, Brockmöller J, Tzvetkov MV. Effects of genetic polymorphisms on the OCT1 and OCT2-mediated uptake of ranitidine. PLoS One 12: e0189521, 2017. doi: 10.1371/journal.pone.0189521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Moaddel R, Ravichandran S, Bighi F, Yamaguchi R, Wainer IW. Pharmacophore modelling of stereoselective binding to the human organic cation transporter (hOCT1). Br J Pharmacol 151: 1305–1314, 2007. doi: 10.1038/sj.bjp.0707341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Montrose-Rafizadeh C, Mingard F, Murer H, Roch-Ramel F. Carrier-mediated transport of tetraethylammonium across rabbit renal basolateral membrane. Am J Physiol 257: F243–F251, 1989. doi: 10.1152/ajprenal.1989.257.2.F243. [DOI] [PubMed] [Google Scholar]
- 63.Motohashi H, Nakao Y, Masuda S, Katsura T, Kamba T, Ogawa O, Inui K. Precise comparison of protein localization among OCT, OAT, and MATE in human kidney. J Pharm Sci 102: 3302–3308, 2013. doi: 10.1002/jps.23567. [DOI] [PubMed] [Google Scholar]
- 64.Motohashi H, Sakurai Y, Saito H, Masuda S, Urakami Y, Goto M, Fukatsu A, Ogawa O, Inui K. Gene expression levels and immunolocalization of organic ion transporters in the human kidney. J Am Soc Nephrol 13: 866–874, 2002. [DOI] [PubMed] [Google Scholar]
- 65.Nagel G, Volk C, Friedrich T, Ulzheimer JC, Bamberg E, Koepsell H. A reevaluation of substrate specificity of the rat cation transporter rOCT1. J Biol Chem 272: 31953–31956, 1997. doi: 10.1074/jbc.272.51.31953. [DOI] [PubMed] [Google Scholar]
- 66.Nath A, Atkins WM, Sligar SG. Applications of phospholipid bilayer nanodiscs in the study of membranes and membrane proteins. Biochemistry 46: 2059–2069, 2007. doi: 10.1021/bi602371n. [DOI] [PubMed] [Google Scholar]
- 67.Neuhoff S, Ungell AL, Zamora I, Artursson P. pH-dependent bidirectional transport of weakly basic drugs across Caco-2 monolayers: implications for drug-drug interactions. Pharm Res 20: 1141–1148, 2003. doi: 10.1023/A:1025032511040. [DOI] [PubMed] [Google Scholar]
- 68.Nies AT, Damme K, Kruck S, Schaeffeler E, Schwab M. Structure and function of multidrug and toxin extrusion proteins (MATEs) and their relevance to drug therapy and personalized medicine. Arch Toxicol 90: 1555–1584, 2016. doi: 10.1007/s00204-016-1728-5. [DOI] [PubMed] [Google Scholar]
- 69.Nies AT, Hofmann U, Resch C, Schaeffeler E, Rius M, Schwab M. Proton pump inhibitors inhibit metformin uptake by organic cation transporters (OCTs). PLoS One 6: e22163, 2011. doi: 10.1371/journal.pone.0022163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nies AT, Koepsell H, Damme K, Schwab M. Organic cation transporters (OCTs, MATEs), in vitro and in vivo evidence for the importance in drug therapy. Handb Exp Pharmacol 201: 105–167, 2011. doi: 10.1007/978-3-642-14541-4_3. [DOI] [PubMed] [Google Scholar]
- 71.Okuda M, Saito H, Urakami Y, Takano M, Inui K. cDNA cloning and functional expression of a novel rat kidney organic cation transporter, OCT2. Biochem Biophys Res Commun 224: 500–507, 1996. doi: 10.1006/bbrc.1996.1056. [DOI] [PubMed] [Google Scholar]
- 72.Paini A, Leonard JA, Joossens E, Bessems JGM, Desalegn A, Dorne JL, Gosling JP, Heringa MB, Klaric M, Kliment T, Kramer NI, Loizou G, Louisse J, Lumen A, Madden JC, Patterson EA, Proença S, Punt A, Setzer RW, Suciu N, Troutman J, Yoon M, Worth A, Tan YM. Next generation physiologically based kinetic (NG-PBK) models in support of regulatory decision making. Comput Toxicol 9: 61–72, 2019. doi: 10.1016/j.comtox.2018.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pan Y, Hsu V, Grimstein M, Zhang L, Arya V, Sinha V, Grillo JA, Zhao P. The Application of Physiologically Based Pharmacokinetic Modeling to Predict the Role of Drug Transporters: Scientific and Regulatory Perspectives. J Clin Pharmacol 56, Suppl 7: S122–S131, 2016. doi: 10.1002/jcph.740. [DOI] [PubMed] [Google Scholar]
- 74.Pao SS, Paulsen IT, Saier MH Jr. Major facilitator superfamily. Microbiol Mol Biol Rev 62: 1–34, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pelis RM, Suhre WM, Wright SH. Functional influence of N-glycosylation in OCT2-mediated tetraethylammonium transport. Am J Physiol Renal Physiol 290: F1118–F1126, 2006. doi: 10.1152/ajprenal.00462.2005. [DOI] [PubMed] [Google Scholar]
- 76.Pelis RM, Wright SH. Renal transport of organic anions and cations. Compr Physiol 1: 1795–1835, 2011. doi: 10.1002/cphy.c100084. [DOI] [PubMed] [Google Scholar]
- 77.Poirier A, Lavé T, Portmann R, Brun ME, Senner F, Kansy M, Grimm HP, Funk C. Design, data analysis, and simulation of in vitro drug transport kinetic experiments using a mechanistic in vitro model. Drug Metab Dispos 36: 2434–2444, 2008. doi: 10.1124/dmd.108.020750. [DOI] [PubMed] [Google Scholar]
- 78.Popp C, Gorboulev V, Müller TD, Gorbunov D, Shatskaya N, Koepsell H. Amino acids critical for substrate affinity of rat organic cation transporter 1 line the substrate binding region in a model derived from the tertiary structure of lactose permease. Mol Pharmacol 67: 1600–1611, 2005. doi: 10.1124/mol.104.008839. [DOI] [PubMed] [Google Scholar]
- 79.Pritchard JB, Miller DS. Mechanisms mediating renal secretion of organic anions and cations. Physiol Rev 73: 765–796, 1993. doi: 10.1152/physrev.1993.73.4.765. [DOI] [PubMed] [Google Scholar]
- 80.Reddy VS, Shlykov MA, Castillo R, Sun EI, Saier MH Jr. The major facilitator superfamily (MFS) revisited. FEBS J 279: 2022–2035, 2012. doi: 10.1111/j.1742-4658.2012.08588.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Roch-Ramel F, Besseghir K, Murer H. Renal excretion and tubular transport of organic anions and cations. In: Handbook of Physiology Section 8 Renal Physiology, Vol II, edited by Windhager EE. New York: Oxford University Press, 1992, p. 2189–2262. [Google Scholar]
- 82.Ross CR, Holohan PD. Transport of organic anions and cations in isolated renal plasma membranes. Annu Rev Pharmacol Toxicol 23: 65–85, 1983. doi: 10.1146/annurev.pa.23.040183.000433. [DOI] [PubMed] [Google Scholar]
- 83.Roth M, Timmermann BN, Hagenbuch B. Interactions of green tea catechins with organic anion-transporting polypeptides. Drug Metab Dispos 39: 920–926, 2011. doi: 10.1124/dmd.110.036640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sandoval PJ, Morales M, Secomb TW, Wright SH. Kinetic basis of metformin-MPP interactions with organic cation transporter OCT2. Am J Physiol Renal Physiol 317: F720–F734, 2019. doi: 10.1152/ajprenal.00152.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sandoval PJ, Zorn KM, Clark AM, Ekins S, Wright SH. Assessment of substrate dependent ligand interactions at the organic cation transporter OCT2 using six model substrates. Mol Pharmacol 94: 1057–1068, 2018. doi: 10.1124/mol.117.111443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Schmitt BM, Gorbunov D, Schlachtbauer P, Egenberger B, Gorboulev V, Wischmeyer E, Müller T, Koepsell H. Charge-to-substrate ratio during organic cation uptake by rat OCT2 is voltage dependent and altered by exchange of glutamate 448 with glutamine. Am J Physiol Renal Physiol 296: F709–F722, 2009. doi: 10.1152/ajprenal.90323.2008. [DOI] [PubMed] [Google Scholar]
- 87.Schömig E, Lazar A, Gründemann D. Extraneuronal monoamine transporter and organic cation transporters 1 and 2: a review of transport efficiency. Handb Exp Pharmacol 175: 151–180, 2006. doi: 10.1007/3-540-29784-7_8. [DOI] [PubMed] [Google Scholar]
- 88.Schömig E, Russ H, Staudt K, Martel F, Gliese M, Gründemann D. The extraneuronal monoamine transporter exists in human central nervous system glia. Adv Pharmacol 42: 356–359, 1997. doi: 10.1016/S1054-3589(08)60764-4. [DOI] [PubMed] [Google Scholar]
- 89.Schömig E, Spitzenberger F, Engelhardt M, Martel F, Ording N, Gründemann D. Molecular cloning and characterization of two novel transport proteins from rat kidney. FEBS Lett 425: 79–86, 1998. doi: 10.1016/S0014-5793(98)00203-8. [DOI] [PubMed] [Google Scholar]
- 90.Scotcher D, Jones C, Posada M, Galetin A, Rostami-Hodjegan A. Key to opening kidney for in vitro-in vivo extrapolation entrance in health and disease: part ii: mechanistic models and in vitro-in vivo extrapolation. AAPS J 18: 1082–1094, 2016. doi: 10.1208/s12248-016-9959-1. [DOI] [PubMed] [Google Scholar]
- 91.Segel IH. Enzyme Kinetics. New York: John Wiley & Sons, 1975, p. 1–957. [Google Scholar]
- 92.Severance AC, Sandoval PJ, Wright SH. Correlation between apparent substrate affinity and OCT2 transporter turnover. J Pharmacol Exp Ther 362: 405–412, 2017. doi: 10.1124/jpet.117.242552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Smith PM, Pritchard JB, Miller DS. Membrane potential drives organic cation transport into teleost renal proximal tubules. Am J Physiol 255: R492–R499, 1988. doi: 10.1152/ajpregu.1988.255.3.R492. [DOI] [PubMed] [Google Scholar]
- 94.Sokol PP, McKinney TD. Mechanism of organic cation transport in rabbit renal basolateral membrane vesicles. Am J Physiol 258: F1599–F1607, 1990. doi: 10.1152/ajprenal.1990.258.6.F1599. [DOI] [PubMed] [Google Scholar]
- 95.Somogyi A, Bochner F. Dose and concentration dependent effect of ranitidine on procainamide disposition and renal clearance in man. Br J Clin Pharmacol 18: 175–181, 1984. doi: 10.1111/j.1365-2125.1984.tb02450.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Somogyi A, Gugler R. Clinical pharmacokinetics of cimetidine. Clin Pharmacokinet 8: 463–495, 1983. doi: 10.2165/00003088-198308060-00001. [DOI] [PubMed] [Google Scholar]
- 97.Somogyi A, Stockley C, Keal J, Rolan P, Bochner F. Reduction of metformin renal tubular secretion by cimetidine in man. Br J Clin Pharmacol 23: 545–551, 1987. doi: 10.1111/j.1365-2125.1987.tb03090.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Song IS, Shin HJ, Shim EJ, Jung IS, Kim WY, Shon JH, Shin JG. Genetic variants of the organic cation transporter 2 influence the disposition of metformin. Clin Pharmacol Ther 84: 559–562, 2008. doi: 10.1038/clpt.2008.61. [DOI] [PubMed] [Google Scholar]
- 99.Stein WD. Transport and Diffusion Across Cell Membranes. New York: Academic Press, 1986, p. 1–685. [Google Scholar]
- 100.Stein WD, Litman T. Channels, Carriers, and Pumps: an Introduction to Membrane Transport. San Diego, CA: Academic Press, 2015, p. 406. [Google Scholar]
- 101.Suhre WM, Ekins S, Chang C, Swaan PW, Wright SH. Molecular determinants of substrate/inhibitor binding to the human and rabbit renal organic cation transporters hOCT2 and rbOCT2. Mol Pharmacol 67: 1067–1077, 2005. doi: 10.1124/mol.104.004713. [DOI] [PubMed] [Google Scholar]
- 102.Takano M, Inui K, Okano T, Saito H, Hori R. Carrier-mediated transport systems of tetraethylammonium in rat renal brush-border and basolateral membrane vesicles. Biochim Biophys Acta 773: 113–124, 1984. doi: 10.1016/0005-2736(84)90556-X. [DOI] [PubMed] [Google Scholar]
- 103.Thévenod F, Ciarimboli G, Leistner M, Wolff NA, Lee WK, Schatz I, Keller T, Al-Monajjed R, Gorboulev V, Koepsell H. Substrate- and cell contact-dependent inhibitor affinity of human organic cation transporter 2: studies with two classical organic cation substrates and the novel substrate cd2+. Mol Pharm 10: 3045–3056, 2013. doi: 10.1021/mp400113d. [DOI] [PubMed] [Google Scholar]
- 104.US Food and Drug Administration Guidance for Industry: Drug Interaction Studies - Study Design, Data Analysis, Implications for Dosing, and Labeling Recommendations, edited by Services USDoHaH Rockville, MD: Center for Drug Evaluation and Research, 2012. [Google Scholar]
- 105.US Food and Drug Administration In Vitro Metabolism- and Transporter- Mediated Drug-Drug Interaction Studies Guidance for Industry, edited by Services USDoHaH Rockville, MD: Center for Drug Evaluation and Research, 2017. [Google Scholar]
- 106.Ullrich KJ. Affinity of drugs to the different renal transporters for organic anions and organic cations. Pharm Biotechnol 12: 159–179, 1999. doi: 10.1007/0-306-46812-3_5. [DOI] [PubMed] [Google Scholar]
- 107.Ullrich KJ. Renal transporters for organic anions and organic cations. Structural requirements for substrates. J Membr Biol 158: 95–107, 1997. doi: 10.1007/s002329900247. [DOI] [PubMed] [Google Scholar]
- 108.Ullrich KJ. Specificity of transporters for ‘organic anions’ and ‘organic cations’ in the kidney. Biochim Biophys Acta 1197: 45–62, 1994. doi: 10.1016/0304-4157(94)90018-3. [DOI] [PubMed] [Google Scholar]
- 109.Ullrich KJ, Papavassiliou F, David C, Rumrich G, Fritzsch G. Contraluminal transport of organic cations in the proximal tubule of the rat kidney. I. Kinetics of N1-methylnicotinamide and tetraethylammonium, influence of K+, HCO3-, pH; inhibition by aliphatic primary, secondary and tertiary amines and mono- and bisquaternary compounds. Pflugers Arch 419: 84–92, 1991. doi: 10.1007/BF00373751. [DOI] [PubMed] [Google Scholar]
- 110.Ullrich KJ, Rumrich G, David C, Fritzsch G. Bisubstrates: substances that interact with both, renal contraluminal organic anion and organic cation transport systems. II. Zwitterionic substrates: dipeptides, cephalosporins, quinolone-carboxylate gyrase inhibitors and phosphamide thiazine carboxylates; nonionizable substrates: steroid hormones and cyclophosphamides. Pflugers Arch 425: 300–312, 1993. doi: 10.1007/BF00374180. [DOI] [PubMed] [Google Scholar]
- 111.Ullrich KJ, Rumrich G, David C, Fritzsch G. Bisubstrates: substances that interact with renal contraluminal organic anion and organic cation transport systems. I. Amines, piperidines, piperazines, azepines, pyridines, quinolines, imidazoles, thiazoles, guanidines and hydrazines. Pflugers Arch 425: 280–299, 1993. doi: 10.1007/BF00374179. [DOI] [PubMed] [Google Scholar]
- 112.Ullrich KJ, Rumrich G, Neiteler K, Fritzsch G. Contraluminal transport of organic cations in the proximal tubule of the rat kidney. II. Specificity: anilines, phenylalkylamines (catecholamines), heterocyclic compounds (pyridines, quinolines, acridines). Pflugers Arch 420: 29–38, 1992. doi: 10.1007/BF00378638. [DOI] [PubMed] [Google Scholar]
- 113.Urakami Y, Akazawa M, Saito H, Okuda M, Inui K. cDNA cloning, functional characterization, and tissue distribution of an alternatively spliced variant of organic cation transporter hOCT2 predominantly expressed in the human kidney. J Am Soc Nephrol 13: 1703–1710, 2002. doi: 10.1097/01.ASN.0000019413.78751.46. [DOI] [PubMed] [Google Scholar]
- 114.van Montfoort JE, Hagenbuch B, Fattinger KE, Müller M, Groothuis GM, Meijer DK, Meier PJ. Polyspecific organic anion transporting polypeptides mediate hepatic uptake of amphipathic type II organic cations. J Pharmacol Exp Ther 291: 147–152, 1999. [PubMed] [Google Scholar]
- 115.van Montfoort JE, Müller M, Groothuis GM, Meijer DK, Koepsell H, Meier PJ. Comparison of “type I” and “type II” organic cation transport by organic cation transporters and organic anion-transporting polypeptides. J Pharmacol Exp Ther 298: 110–115, 2001. [PubMed] [Google Scholar]
- 116.Volk C, Gorboulev V, Kotzsch A, Müller TD, Koepsell H. Five amino acids in the innermost cavity of the substrate binding cleft of organic cation transporter 1 interact with extracellular and intracellular corticosterone. Mol Pharmacol 76: 275–289, 2009. doi: 10.1124/mol.109.054783. [DOI] [PubMed] [Google Scholar]
- 117.Westholm DE, Salo DR, Viken KJ, Rumbley JN, Anderson GW. The blood-brain barrier thyroxine transporter organic anion-transporting polypeptide 1c1 displays atypical transport kinetics. Endocrinology 150: 5153–5162, 2009. doi: 10.1210/en.2009-0769. [DOI] [PubMed] [Google Scholar]
- 118.Wright SH. Multidrug and toxin extrusion proteins. In: Drug Transporters, edited by You G and Morris ME. Hoboken, NJ: Wiley, 2014, p. 223–243. [Google Scholar]
- 119.Wright SH. Transport of N1-methylnicotinamide across brush border membrane vesicles from rabbit kidney. Am J Physiol 249: F903–F911, 1985. doi: 10.1152/ajprenal.1985.249.6.F903. [DOI] [PubMed] [Google Scholar]
- 120.Wright SH, Dantzler WH. Molecular and cellular physiology of renal organic cation and anion transport. Physiol Rev 84: 987–1049, 2004. doi: 10.1152/physrev.00040.2003. [DOI] [PubMed] [Google Scholar]
- 121.Wright SH, Evans KK, Zhang X, Cherrington NJ, Sitar DS, Dantzler WH. Functional map of TEA transport activity in isolated rabbit renal proximal tubules. Am J Physiol Renal Physiol 287: F442–F451, 2004. doi: 10.1152/ajprenal.00115.2004. [DOI] [PubMed] [Google Scholar]
- 122.Wright SH, Wunz TM. Transport of tetraethylammonium by rabbit renal brush-border and basolateral membrane vesicles. Am J Physiol 253: F1040–F1050, 1987. doi: 10.1152/ajprenal.1987.253.5.F1040. [DOI] [PubMed] [Google Scholar]
- 123.Wu X, Kekuda R, Huang W, Fei YJ, Leibach FH, Chen J, Conway SJ, Ganapathy V. Identity of the organic cation transporter OCT3 as the extraneuronal monoamine transporter (uptake2) and evidence for the expression of the transporter in the brain. J Biol Chem 273: 32776–32786, 1998. doi: 10.1074/jbc.273.49.32776. [DOI] [PubMed] [Google Scholar]
- 124.Xu Y, Liu X, Li S, Zhou N, Gong L, Luo C, Luo X, Zheng M, Jiang H, Chen K. Combinatorial pharmacophore modeling of organic cation transporter 2 (OCT2) inhibitors: insights into multiple inhibitory mechanisms. Mol Pharm 10: 4611–4619, 2013. doi: 10.1021/mp400423g. [DOI] [PubMed] [Google Scholar]
- 125.Yin J, Duan H, Wang J. Impact of substrate-dependent inhibition on renal organic cation transporters hOCT2 and hMATE1/2-K-mediated drug transport and intracellular accumulation. J Pharmacol Exp Ther 359: 401–410, 2016. doi: 10.1124/jpet.116.236158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Zaharenko L, Kalnina I, Geldnere K, Konrade I, Grinberga S, Židzik J, Javorský M, Lejnieks A, Nikitina-Zake L, Fridmanis D, Peculis R, Radovica-Spalvina I, Hartmane D, Pugovics O, Tkáč I, Klimčáková L, Pīrāgs V, Klovins J. Single nucleotide polymorphisms in the intergenic region between metformin transporter OCT2 and OCT3 coding genes are associated with short-term response to metformin monotherapy in type 2 diabetes mellitus patients. Eur J Endocrinol 175: 531–540, 2016. doi: 10.1530/EJE-16-0347. [DOI] [PubMed] [Google Scholar]
- 127.Zamek-Gliszczynski MJ, Lee CA, Poirier A, Bentz J, Chu X, Ellens H, Ishikawa T, Jamei M, Kalvass JC, Nagar S, Pang KS, Korzekwa K, Swaan PW, Taub ME, Zhao P, Galetin A; International Transporter Consortium . ITC recommendations for transporter kinetic parameter estimation and translational modeling of transport-mediated PK and DDIs in humans. Clin Pharmacol Ther 94: 64–79, 2013. doi: 10.1038/clpt.2013.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Zhang L, Dresser MJ, Gray AT, Yost SC, Terashita S, Giacomini KM. Cloning and functional expression of a human liver organic cation transporter. Mol Pharmacol 51: 913–921, 1997. doi: 10.1124/mol.51.6.913. [DOI] [PubMed] [Google Scholar]
- 129.Zhang X, Shirahatti NV, Mahadevan D, Wright SH. A conserved glutamate residue in transmembrane helix 10 influences substrate specificity of rabbit OCT2 (SLC22A2). J Biol Chem 280: 34813–34822, 2005. doi: 10.1074/jbc.M506342200. [DOI] [PubMed] [Google Scholar]
- 130.Zolk O, Solbach TF, König J, Fromm MF. Functional characterization of the human organic cation transporter 2 variant p.270Ala>Ser. Drug Metab Dispos 37: 1312–1318, 2009. doi: 10.1124/dmd.108.023762. [DOI] [PubMed] [Google Scholar]
- 131.Zolk O, Solbach TF, König J, Fromm MF. Structural determinants of inhibitor interaction with the human organic cation transporter OCT2 (SLC22A2). Naunyn Schmiedebergs Arch Pharmacol 379: 337–348, 2009. doi: 10.1007/s00210-008-0369-5. [DOI] [PubMed] [Google Scholar]