Abstract
In healthy glomeruli, parietal epithelial cell (PEC)-derived extracellular matrix (ECM) proteins include laminin-β1, perlecan, and collagen type IV-α2 and podocyte-specific ECM proteins include laminin-β2, agrin, and collagen type IV-α4. This study aimed to define individual ECM protein isoform expression by PECs in both experimental and human focal segmental glomerulosclerosis (FSGS) and diabetic nephropathy (DN) and to determine if changes were CD44 dependent. In experimental FSGS induced with a cytotoxic podocyte antibody and in the BTBR ob/ob mouse model of DN, PEC-derived protein staining was significantly increased in PECs. Dual staining also showed de novo expression of the podocyte-specific ECM proteins laminin-β2 and agrin in PECs. Similar findings were observed in biopsies from patients with FSGS and DN. Increases in individual ECM proteins colocalized with CD44 in PECs in disease. To determine the role of CD44, FSGS was induced in CD44−/− and CD44+/+ mice. PEC staining for perlecan, collagen type IV-α2, laminin-β2, and agrin were significantly lower in diseased CD44−/− mice compared with diseased CD44+/+ mice. These results show that in experimental and human FSGS and DN, PECs typically in an activated state, produce both PEC-derived and podocyte-specific ECM protein isoforms, and that the majority of these changes were dependent on CD44.
Keywords: CD44, extracellular matrix protein, glomerular disease, parietal epithelial cell, podocyte
INTRODUCTION
Disease progression in focal segmental glomerulosclerosis (FSGS) and diabetic nephropathy (DN) is largely due to glomerular scarring resulting from the accumulation of extracellular matrix (ECM) proteins produced by podocytes, mesangial cells, and glomerular parietal epithelial cells (PECs) (22, 36, 40, 45).
The differential expression of individual ECM proteins by glomerular cell type has been well described in developing and normal mature kidneys (8, 24–26, 55). In healthy glomeruli, PEC-derived ECM protein isoforms include laminin-β1 (LAMB1) (36, 37), perlecan (22, 36, 44), and collagen type IV-α2 (COL4A2) (36). Perlecan is also present on mesangial and endothelial cells (15–19, 53). Podocyte-specific ECM protein isoforms include laminin-β2 (LAMB2) (1, 3, 37, 49), agrin (1, 6, 17, 19), and collagen type IV-α4 (COL4A4) (1–3). COL4A4 is representative of the COL4A3/4/5 trimer, and COL4A2 is representative of the COL4A1/2/1 trimer.
LAMB2 is also produced by glomerular endothelial cells (49). ECM proteins change in disease, such collagen type IV and heparan sulfate moieties (LKIV69) in FSGS (22, 34, 35, 46), collagen type IV in DN (20), and laminin α1/β1-chain, perlecan, and collagen type IV-α1, -α2, and -α6 in healthy aged kidneys (36).
Although laminin and collagen type IV exist as trimers, we were interested in delineating expression patterns for individual chains specific to PECs, or podocytes. Laminin-α5 and -γ1, and collagen type IV α1- and α3-chains, are neither specific to Bowman’s capsule nor the glomerular basement membrane (GBM). In addition, preliminary testing failed to show localization of the collagen type IV α6-chain to Bowman’s capsule, nor was the α5-chain specific to the GBM. Therefore, taken together, we selected LAMB1, COL4A2, and perlecan as representative of PEC derivation and LAMB2, COL4A4, and agrin as representative of podocyte-specific production.
Given recent evidence for a critical role for PECs in glomerular scarring, the purposes of the current study was to first define the expression pattern by PECs for individual ECM protein isoforms considered to be PEC-derived and podocyte specific in experimental and human FSGS and DN. Second, because CD44 expression is increased in activated PECs in FSGS (11–13, 22, 30) and DN (4, 9, 14, 31), studies were conducted to better understand the role of CD44 in the expression of individual ECM proteins in PECs.
METHODS
Mice
All mice were bred and maintained in the animal care facility at the University of Washington under specific pathogen-free conditions and were provided with ad libitum food and water. At euthanization, all animals were cardiac perfused with 10 mL of ice-cold PBS to remove excessive red blood cells in the kidney tissue. All animal protocols were approved by the University of Washington Institutional Animal Care and Use Committee (2968-04 or 2281-04).
CD44-null mice.
CD44 knockout/LacZ knockin (CD44−/−, stock no. 005085) and wild-type (WT) control (CD44+/+) mice were obtained from The Jackson Laboratory (Bar Harbor, ME) as previously described (32, 35). By disruption of exon 1 and intron 1 using a neomycin/LacZ cassette, CD44 function was ablated and replaced with expression of LacZ.
BTBR ob/ob mice.
BTBR WT mice (n = 6, 4 female and 2 male mice) and BTBR ob/ob mice (n = 6, 3 female and 3 male mice) were retained from heterozygous (ob/WT) breeding pairs obtained from The Jackson Laboratory (stock no. 004824). These mice contain the leptin deficiency mutation ob, which when homozygous manifests as severe and progressive hyperglycemia by 10 wk of age on the BTBR background. BTBR ob/ob mice have been previously described by Attie and colleagues (7, 33). In the present study, BTBR WT and ob/ob mice were euthanized at 24 wk of age. Kidneys were butterflied, embedded in OCT compound, snap frozen in liquid nitrogen, and stored at −80°C as previously described (31, 52).
PEC reporter mice.
Inducible PEC-reverse tetracycline transactivator/LC1/Rosa26 reporter mice aged 10–12 wk, which had been genotyped positive for all three transgenes, were given doxycline hydrochloride via chow at 625 mg/kg for 14 days ad libitum (5, 11, 44). A 1-wk washout period was provided to ensure labeling occurred only within the specific temporal window before the induction of FSGS. PECs therefore would have been permanently genetically labeled by expression of β-galactosidase only within the window of doxycycline administration, precluding the spontaneous expression of the PEC reporter within the glomerular tuft as a result of the induction of FSGS.
Experimental FSGS
Experimental FSGS was induced in both CD44+/+ (n = 8 at baseline and 8 at day 28) and CD44−/− (n = 7–8 at baseline and 5 at day 28) mice by two consecutive intraperitoneal injections, 24 h apart, of sheep anti-podocyte antibody at 11 mg/20 g body weight (29, 57, 58). Mice were then randomly euthanized at days 14 and 28 and processed as previously described (11). Age and sex-matched (all male) CD44+/+ and CD44−/− animals without disease served as baseline controls.
PEC reporter mice underwent the same protocol for the induction of FSGS but at an antibody dose of 10 mg/20 g body weight. Mice were euthanized at day 28 of FSGS and processed as above. For normal baselines, animals were euthanized 7 days after the administration of doxycycline (11). Kidneys were butterflied and fixed in 4% paraformaldehyde solution in PBS (Affymetrix, Santa Clara, CA) for 45 min at room temperature, washed in 30% sucrose at 4°C overnight, patted dry, rinsed briefly, and embedded in OCT compound (Electron Microscopy Sciences, Hatfield, PA) before being frozen in a dry ice 100% ethanol bath and stored at −80°C (11).
Immunostaining and Quantification
Immunofluorescence staining (LAMB1, LAMB2, perlecan, agrin, COL4A2, and COL4A4 staining) was performed on 4-μm tissue sections from stored frozen block specimens.
Sections on slides were thawed and washed in PBS. To avoid nonspecific protein binding, Background Buster (Accurate Chemical & Scientific, Westbury, NY) was used as well as Goat Anti-Rabbit Fab Fragment and Rabbit Fab Fragment (Jackson ImmunoResearch Laboratories, West Grove, PA) if host rabbit primary antibodies were to be used or Goat Anti-Rat Fab Fragment and Rat IgG Fab Fragment (Jackson ImmunoResearch Laboratories, West Grove, PA) if host rat primary antibodies were to be used. Suppression of endogenous biotin activity was performed using the Avidin/Biotin Blocking Kit (Vector Laboratories, Burlingame, CA). All antibodies were diluted in 1% IgG-free BSA in PBS. For a list of primary, secondary, and fluorescent antibodies used, see Supplemental Tables S1 and S2 (all Supplemental Data are available online at https://doi.org/10.6084/m9.figshare.8187638.v2).
To quantitate, we examined 50 glomerular cross-sections per animal using an EVOS FL Cell Imaging System (ThermoFisher, San Diego, CA). For PEC-specific matrix proteins (LAMB1, perlecan, and COL4A2), an injured glomerulus with the presence of increased staining over Bowman’s capsule would be deemed as abnormal, whereas for podocyte-specific matrix proteins (LAMB2, agrin, and COL4A4), an injured glomerulus with de novo staining present along Bowman’s capsule would be deemed as abnormal.
Next, to prove there was an increase in the amount of matrix protein in an abnormal glomerulus, we quantified fluorescence intensity of each matrix protein along Bowman’s capsule and compared it with normal glomeruli. For each matrix protein, we measured 112 normal and 112 abnormal glomeruli from day 28 FSGS mice. Of the 112 glomeruli, half were sampled from CD44+/+ mice and the remaining half were from CD44−/− mice. In the experimental DN model, we measured 30 normal and 30 abnormal glomeruli from diabetic ob/ob mice. Using ImageJ (National Institutes of Health), total raw integrated density, and area were measured by drawing an area of interest around Bowman’s capsule, and fluorescence intensity was calculated thereafter.
Human Kidney Biopsies
Snap-frozen human kidney biopsies used were from the archives of the Department of Pathology, University of Chicago (Chicago, IL). These human kidney biopsies included normal human kidney tissue (n = 4), human FSGS (n = 5), and human DN (n = 5). Before immunostaining, frozen biopsies were thawed and fixed with acetone at −20°C for 10 min. We recognize the limitation of a small number of human samples. As some of the human biopsies had too few glomeruli, we were unable to proceed with quantification analysis.
Statistical Analysis
Statistical analysis was performed using GraphPad Prism 8.0 software (La Jolla, CA). Normal distribution was tested by assessing skewness and kurtosis and using a Shapiro-Wilk normality test. Results are presented as means ± SD. An unpaired t test and one-way ANOVA were used to compare the means of groups. Two-tailed P values of <0.05 were considered to be statistically significant.
RESULTS
PEC-Derived ECM Isoforms Increase in PECs in Mouse Experimental FSGS
Staining for LAMB1, perlecan, and COL4A2 were detected in normal mouse glomeruli along Bowman’s capsule and to a lesser extent in the mesangium, as expected (Fig. 1, A–C). Perlecan was also detected along the GBM, endothelial cells (colocalized with CD31; not shown) and mesangial cells (colocalized with α8-integrin; not shown), but not podocytes (did not colocalize with nephrin; not shown).
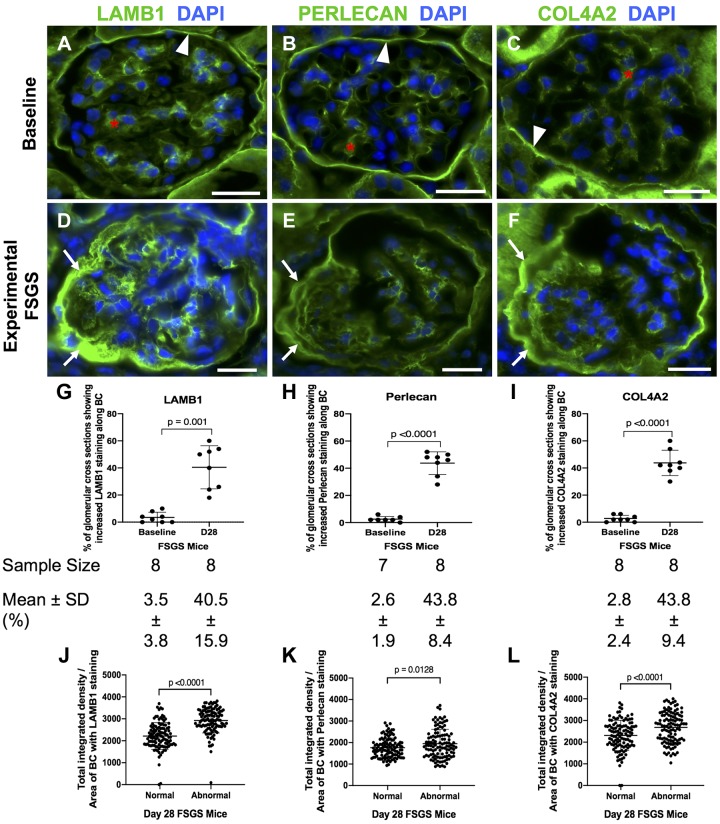
Fig. 1.
Laminin-β1 (LAMB1), perlecan, and collagen type IV-α2 (COL4A2) staining increase in experimental focal segmental glomerulosclerosis (FSGS). A−C: a normal mouse at baseline. Staining (green) for LAMB1 (A), perlecan (B), and COL4A2 (C) was present in parietal epithelial cell (PECs) in mice at baseline. They were detected along Bowman’s capsule (BC; white arrowheads) and in the mesangial matrix (red asterisk) but not along the glomerular basement membrane. Nuclei were stained with DAPI (blue). D−F: FSGS day 28. Staining increased for LAMB1 (D), perlecan (E), and COL4A2 (F) in mice at FSGS day 28 in a PEC distribution in FSGS (white arrows show examples). G−I: graphs of quantification of glomerular cross sections. The percentage of glomerular cross sections with increased staining along BC was increased for LAMB1 (G), perlecan (H), and COL4A2 (I) staining at day 28 FSGS compared with baseline. The number of mice quantitated is shown below each graph. J−L: graphs of quantification of fluorescence intensity. Quantification of fluorescence intensity showed a significant increase in the intensity of LAMB1 (J), perlecan (K), and COL4A2 (L) staining in abnormal glomeruli in FSGS. Original magnification: ×400. Scale bars = 20 μm.
In experimental FSGS, there was a significant increase in the percentage of glomerular cross-sections with increased staining along Bowman’s capsule for LAMB1 (40.5 ± 15.9% vs. 3.5 ± 3.8%, P = 0.001 vs. baseline; Fig. 1, D and G), perlecan (43.8 ± 8.4% vs. 2.6 ± 1.9%, P < 0.0001 vs. baseline; Fig. 1, E and H), and COL4A2 (43.8 ± 9.4% vs. 2.8 ± 2.4%, P < 0.0001 vs. baseline; Fig. 1, F and I). Staining was not detected when primary antibodies were omitted, used as negative controls. Within individual glomeruli in FSGS, there was a significant increase in fluorescence intensity of staining for LAMB1 (2,922 ± 565 vs. 2,217 ± 609, P < 0.0001 vs. normal; Fig. 1J), perlecan (1949 ± 664 vs. 1,759 ± 452, P = 0.0128 vs. normal; Fig. 1K) and COL4A2 (2,680 ± 699 vs. 2,309 ± 699, P < 0.0001 vs. normal; Fig. 1L).
These results show that the percentage of glomeruli with PEC staining for LAMB1, perlecan, and COL4A2 increased in experimental FSGS and, within these glomeruli, so did the intensity of PEC staining for each PEC-derived ECM protein.
Increased PEC-Derived ECM Proteins in Human FSGS
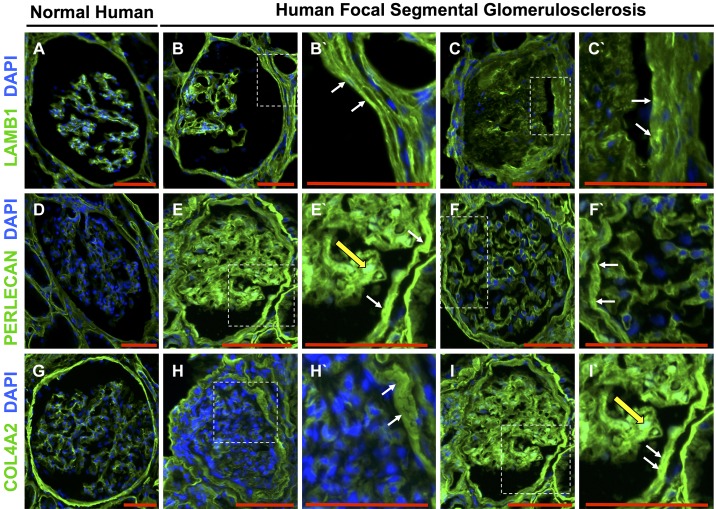
We next determined if the staining of PEC-derived ECM protein isoforms in mouse tissue was reflected in human FSGS. In normal human kidney tissue, staining for LAMB1 (Fig. 2A), perlecan (Fig. 2D), and COL4A2 (Fig. 2G) were detected along Bowman’s capsule and to a lesser degree in the mesangium. In patients with FSGS, staining for LAMB1 (Fig. 2, B and C), perlecan (Fig. 2, E and F), and COL4A2 (Fig. 2, H and I) increased along Bowman’s capsule. These results show similar increases of PEC-derived proteins expression in PECs in both experimental and human FSGS.
Fig. 2.
Laminin-β1 (LAMB1), perlecan, and collagen type IV-α2 (COL4A2) increased in parietal epithelial cells (PECs) in human focal segmental glomerulosclerosis (FSGS). A, D, and G: normal human glomeruli. A: LAMB1 stained (green) Bowman’s capsule and the glomerular mesangium. Nuclei were stained with DAPI (blue). D: perlecan stained (green) Bowman’s capsule and the glomerular mesangium. Nuclei were stained with DAPI (blue). G: COL4A2 stained (green) Bowman’s capsule and the glomerular mesangium. Nuclei were stained with DAPI (blue). B and C: representative examples of two glomeruli from human FSGS showing LAMB1 staining increased along Bowman’s capsule. B′ and C′: higher magnifications of the insets in B and C showing increased LAMB1 staining (white arrows). E and F: representative examples of two glomeruli from human FSGS showing that compared with normal, perlecan staining increased along Bowman’s capsule with occasional increased staining in the glomerular mesangium. E′ and F′: higher magnifications of the insets in E and F showing that perlecan staining increased along Bowman’s capsule (white arrows) and the mesangium (yellow arrow). H and I: representative examples of two glomeruli from human FSGS showing increased COL4A2 staining along Bowman’s capsule and the mesangium. H′ and I′: higher magnifications of the insets in H and I showing increased COL4A2 staining along Bowman’s capsule (white arrows) and the mesangium (yellow arrow). Original magnification: ×200. Scale bars = 100 μm.
PEC-Derived ECM Isoforms Increase in Experimental and Human DN
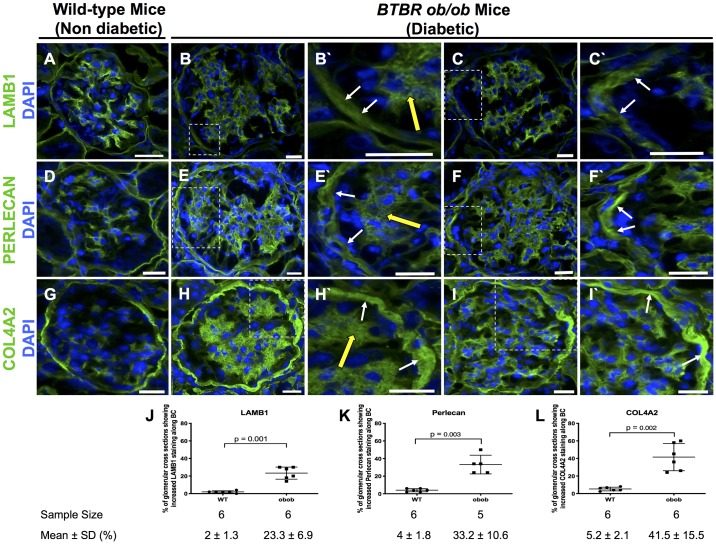
To determine if the above-mentioned changes were also present in other glomerular diseases, we studied archival tissue from the advanced DN model of leptin-deficient BTBR ob/ob mice (31). Compared with nondiabetic WT mice (Fig. 3, A, D, and G), there was an increase in the percentage of glomerular cross-sections in diabetic ob/ob mice with increased staining for LAMB1 (23.3 ± 6.9% vs. 2 ± 1.3%, P = 0.001 vs. WT mice; Fig. 3, B, C, and J), perlecan (33.2 ± 10.6% vs. 4 ± 1.8%, P = 0.003 vs. WT mice; Fig. 3, E, F, and K), and COL4A2 (41.5 ± 15.5% vs. 5.2 ± 2.1%, P = 0.002 vs. WT mice; Fig. 3, H, I, and L). In addition, diseased glomeruli in diabetic ob/ob mice had significantly higher fluorescence intensity of staining for LAMB1 (797 ± 194 vs. 653 ± 121, P = 0.001 vs. normal), perlecan (1,374 ± 255 vs. 1,221 ± 293, P = 0.035 vs. normal), and COL4A2 (1,978 ± 545 vs. 1,665 ± 332, P = 0.0094 vs. normal).
Fig. 3.
Laminin-β1 (LAMB1), perlecan, and collagen type IV-α2 (COL4A2) increased in diabetic ob/ob mice at 24 wk. A, D, and G: nondiabetic wild-type (WT) mice. LAMB1 (A), perlecan (D), and COL4A2 (G) staining (green) was detected along Bowman’s capsule (BC) in nondiabetic WT mice. Nuclei were stained with DAPI (blue). B, C, E, F, H, and I: representative staining in two diabetic ob/ob mouse glomeruli for LAMB1 (B and C), perlecan (E and F), and COL4A2 (H and I) was increased along BC. The insets at higher magnification show increased staining along BC (white arrows) and the mesangium (yellow arrows) for LAMB1 (B′ and C′), perlecan (E′ and F′), and COL4A2 (H′ and I′). J−L: quantification. The percentage of glomerular cross-sections with increased LAMB1 (J), perlecan (K), and COL4A2 (L) along BC was significantly higher in diabetic ob/ob mice than in nondiabetic WT mice. Original magnification: ×400. Scale bars = 20 μm.
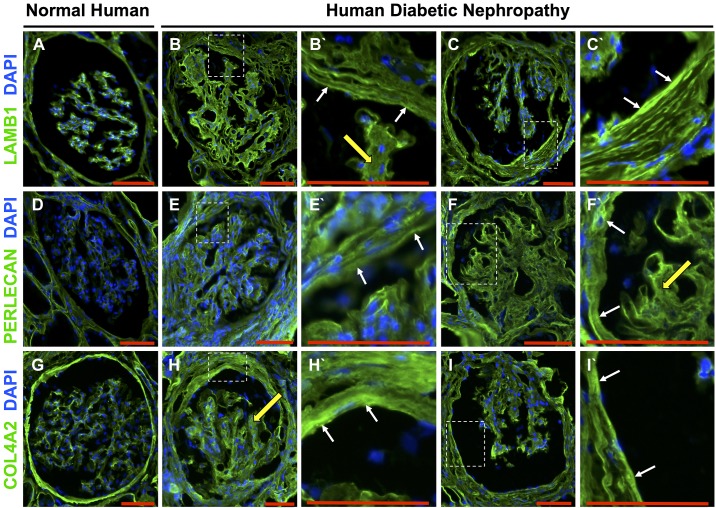
In human DN, staining was also increased along Bowman’s capsule compared with normal kidneys for LAMB1 (Fig. 4, A–C), perlecan (Fig. 4, D–F), and COL4A2 staining (Fig. 4, G–I). All three ECM proteins were also detected in the glomerular mesangium. Taken together, PEC-derived ECM proteins increase along Bowman’s capsule in both experimental and human DN.
Fig. 4.
Laminin-β1 (LAMB1), perlecan, and collagen type IV-α2 (COL4A2) staining patterns in human diabetic nephropathy. LAMB1 (A), perlecan (D), and COL4A2 (G) immunostaining (green) was detected along Bowman’s capsule in normal human kidneys. Nuclei were stained with DAPI (blue). Two representative glomeruli from human diabetic nephropathy showed increased staining for LAMB1 (B and C), perlecan (E and F), and COL4A2 (H and I) along Bowman’s capsule. Higher magnifications of the insets from each glomerulus show staining along Bowman’s capsule (white arrows) and the mesangium (yellow arrows) for LAMB1 (B′ and C′), perlecan (E′ and F′) and COL4A2 (H′ and I′). Original magnification: ×200. Scale bars = 100 μm.
De Novo Expression of Podocyte-Specific ECM Protein Isoforms in PECs in Experimental FSGS
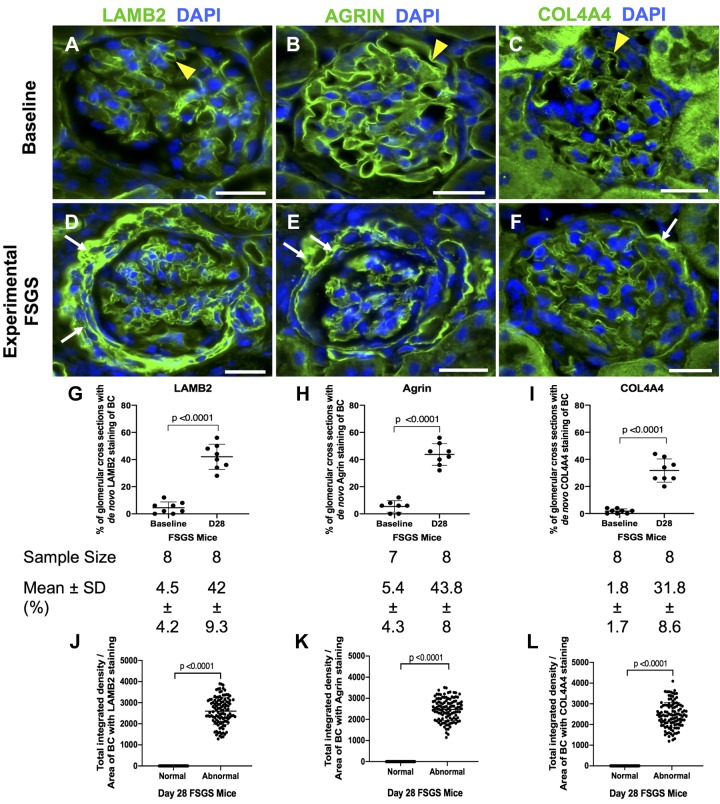
In normal mouse glomeruli, staining for LAMB2, agrin, and COL4A4 were restricted to the GBM, consistent with podocyte secretion (Fig. 5, A–C). In experimental FSGS, the percentage of glomerular cross-sections with de novo staining along Bowman’s capsule increased for LAMB2 (42 ± 9.3% vs. 4.5 ± 4.2%, P < 0.0001 vs. baseline; Fig. 5, D and G), agrin (43.8 ± 8% vs. 5.4 ± 4.3%, P < 0.0001 vs. baseline; Fig. 5, E and H), and COL4A4 (31.8 ± 8.6% vs. 1.8 ± 1.7%, P < 0.0001 vs. baseline; Fig. 5, F and I). Compared with normal glomeruli, the fluorescence intensity for staining in individual glomeruli in experimental FSGS was increased for LAMB2 (2,599 ± 637 vs. 0 ± 0, P < 0.0001 vs. normal; Fig. 5J), agrin (2,474 ± 523 vs. 0 ± 0, P < 0.0001 vs. normal; Fig. 5K), and COL4A4 (2,455 ± 613 vs. 0 ± 0, P < 0.0001 vs. normal; Fig. 5L).
Fig. 5.
Staining for the podocyte extracellular matrix proteins laminin-β2 (LAMB2), agrin, and collagen type IV-α2 (COL4A4) are increased in parietal epithelial cells (PECs) in experimental focal segmental glomerulosclerosis (FSGS). A−C: mice at baseline. LAMB2 (A), agrin (B), and COL4A4 (C) immunofluorescent staining (green) was limited to the glomerular basement membrane (yellow arrowheads). Nuclei were stained with DAPI (blue). D−F: experimental FSGS in mice. De novo immunofluorescent staining for LAMB2 (D), agrin (E), and COL4A4 (F) was detected in PECs at day 28 (D28) FSGS (white arrows). G−I: quantification. The percentage of glomerular cross-sections with de novo staining for LAMB2 (G), agrin (H), and COL4A4 (I) increased at day 28 FSGS compared with baseline. The number of mice quantitated is shown below each graph. J−L: graphs of quantification of fluorescence intensity. Fluorescence intensity was increased for LAMB2 (J), agrin (K), and COL4A4 (L) in abnormal glomeruli in FSGS. Original magnification: ×400. Scale bars = 20 μm.
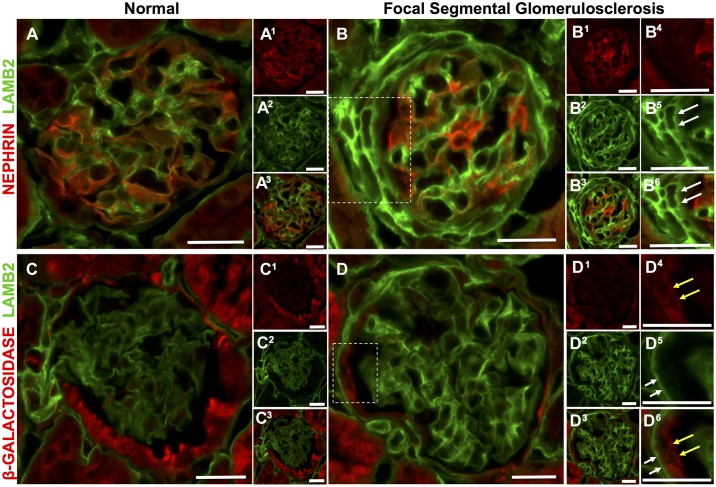
Double staining was then performed to prove that podocyte-specific ECM proteins are indeed coexpressed by a subset of PECs in FSGS. As expected, LAMB2 colocalizes with the podocyte protein nephrin in normal mouse glomeruli (Fig. 6, A–A3). In FSGS, there was de novo expression of LAMB2, but not nephrin, along Bowman’s capsule (Fig. 6, B–B6). To further prove that LAMB2-stained cells along Bowman’s capsule in FSGS were indeed PECs, inducible PEC reporter mice were used, in which β-galactosidase permanently labels PECs (11). Double staining confirmed PEC expression of LAMB2 (Fig. 6, C–D6).
Fig. 6.
Double staining confirmed laminin-β2 (LAMB2) expression in parietal epithelial cells (PECs) in mice with experimental focal segmental glomerulosclerosis (FSGS). A and B: double staining for nephrin (red) and LAMB2 (green). A and A3: a normal mouse glomerulus. The merged image shows staining for LAMB2 (green) in the glomerular basement membrane distribution, with nephrin staining (red) alongside. A1 and A2: staining for nephrin (A1) and LAMB2 (A2) showing their absence along Bowman’s capsule. B and B3: FSGS. The merged image shows de novo LAMB2 but not nephrin staining along Bowman’s capsule. B1 and B2: nephrin (B1) and LAMB2 (B2) staining. Higher magnification of the inset in B showed no staining for nephrin (B4) along Bowman’s capsule in areas staining for LAMB2 (white arrows) (B5). Their merged image is shown in B6. C and D: double staining for β-Gal (red) and LAMB2 (green). C and C3: a normal glomerulus. The merged image shows that there was no overlap of LAMB2 staining in the glomerular basement membrane distribution and β-Gal, which permanently labels PECs. C1 and C2: β-Gal and LAMB2. D and D3: FSGS. The merged image shows de novo staining for LAMB2 in a Bowman’s capsule distribution (D1 and D2). Higher power magnification of the inset D shows that β-Gal (yellow arrows) (D4) is lined by LAMB2 (white arrows) (D5), as easily seen in the merged image (D6). Original magnification: ×400. Scale bars = 20 μm.
Podocyte-Specific ECM Protein Isoforms Increase in PECs in Human FSGS
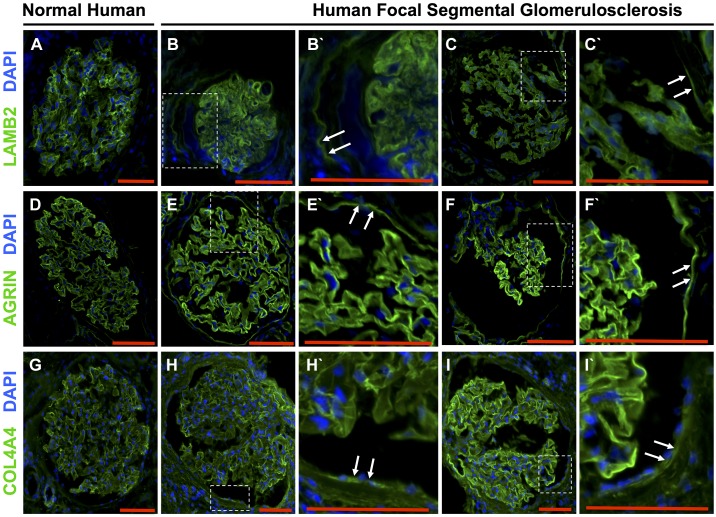
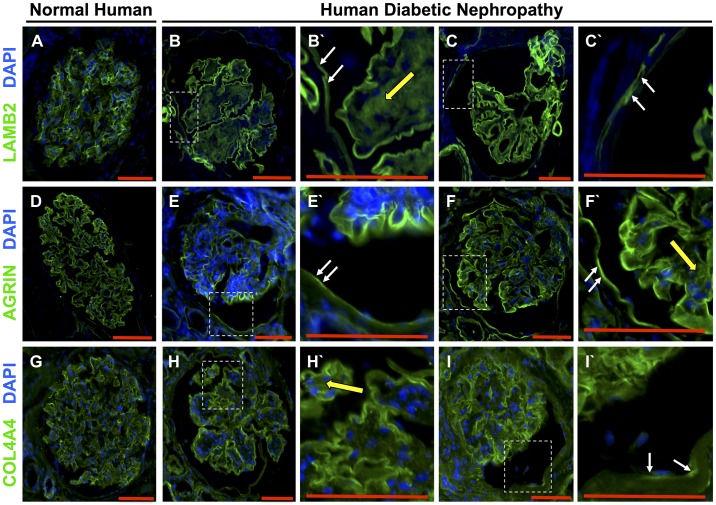
In normal human kidneys, staining for LAMB2 (Fig. 7A), agrin (Fig. 7D), and COL4A4 (Fig. 7G) was restricted to the GBM, with very light staining in the mesangium. In patients with FSGS, de novo staining was detected along Bowman’s capsule for LAMB2 (Fig. 7, B and C) and agrin (Fig. 7, E and F). In contrast, COL4A4 was not as readily detected along Bowman’s capsule (Fig. 7, H and I). Taken together, these results show that three ECM proteins, considered podocyte specific, are de novo expressed by PECs in experimental and human FSGS.
Fig. 7.
The podocyte-specific extracellular matrix proteins laminin-β2 (LAMB2), agrin, and collagen type IV-α4 (COL4A4) increased in parietal epithelial cells (PECs) in human focal segmental glomerulosclerosis (FSGS). A–C: LAMB2 (green) and DAPI (blue). A: LAMB2 localized to the glomerular basement membrane (GBM) in the normal human kidney. B and C: two representative glomeruli from different patients with FSGS showing LAMB2 staining along Bowman’s capsule. B′ and C′: higher magnification of the insets showing LAMB2 staining (white arrows) along Bowman’s capsule. D–F: agrin (green) and DAPI. D: agrin localized to the GBM in a normal human glomerulus. E and F: two representative glomeruli from different patients with FSGS showing agrin staining along Bowman’s capsule. E′ and F′: higher magnification of the insets showing LAMB2 staining (white arrows) along Bowman’s capsule. G–I: COL4A4 (green) and DAPI. G: COL4A4 localized to the GBM in a normal human glomerulus. H and I: two representative glomeruli from different patients with FSGS showing COL4A4 staining along Bowman’s capsule. H′ and I′: higher magnification of the insets showing COL4A4 staining (white arrows) along Bowman’s capsule. These results show that podocyte-specific extracellular matrix proteins are expressed de novo by PECs in human FSGS. Original magnification: ×200. Scale bars = 100 μm.
Podocyte-Specific ECM Isoforms Expressed by PECs in Experimental and Human DN
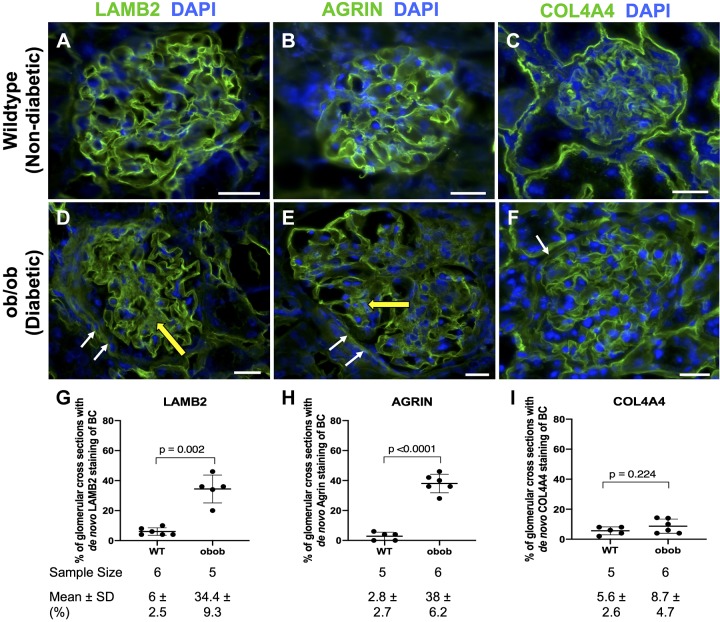
Compared with WT nondiabetic mice, the percentage of glomeruli with Bowman’s capsule staining in the diabetic BTBR ob/ob mouse model of experimental DN was higher for LAMB2 (34.4 ± 9.3% vs. 6 ± 2.5%, P = 0.002 vs. WT mice; Fig. 8, A, D, and G) and agrin (38 ± 6.2% vs. 2.8 ± 2.7%, P < 0.0001 vs. WT mice; Fig. 8, B, E, and H). In contrast, COL4A4 staining did not increase along Bowman’s capsule in diabetic mice (8.7 ± 4.7% vs. 5.6 ± 2.6%, P = 0.224 vs. WT mice; Fig. 8, C, F, and I). Within individual glomeruli, fluorescence intensity was higher in diabetic mice for LAMB2 (1,027 ± 238 vs. 0 ± 0, P < 0.0001 vs. normal), agrin (1,166 ± 266 vs. 0 ± 0, P < 0.0001 vs. normal), and COL4A4 (1,146 ± 327 vs. 0 ± 0, P < 0.0001 vs. normal).
Fig. 8.
Differential immunofluorescent immunostaining in parietal epithelial cells (PECs) for laminin-β2 (LAMB2), agrin, and collagen type IV-α4 (COL4A4) in an experimental diabetic nephropathy model. A−C: nondiabetic wild-type (WT) mice. Immunofluorescent staining for LAMB2 (A), agrin (B), and COL4A4 (C) staining (green) showed a podocyte distribution. Nuclei were stained with DAPI (blue). D−F: diabetic ob/ob mice. De novo staining was detected in PECs (white arrows) for LAMB2 (D), agrin (E), and COL4A4 (F) and in the mesangium (yellow arrows). G−I: quantification. The percentage of glomerular cross sections with de novo expression along Bowman’s capsule (BC) increased in diabetic mice for LAMB2 (G) and agrin (H) but not COL4A4 (I). Original magnification: ×400. Scale bars = 20 μm.
Figure 9 shows de novo staining along Bowman’s capsule in human DN for LAMB2 (Fig. 9, A–C) and agrin (Fig. 9, D–F), with staining also detected in the mesangium. Only occasional de novo staining for COL4A4 along Bowman’s capsule was observed (Fig. 9, G–I). Of note, the number of glomeruli available for study was restricted in these needle biopsies.
Fig. 9.
Laminin-β2 (LAMB2), agrin, and collagen type IV-α4 (COL4A4) staining in human diabetic nephropathy. A–C: LAMB2 staining. A: LAMB2 staining was restricted to the glomerular basement membrane (GBM) in the normal glomerulus. B and C: LAMB2 was detected along Bowman’s capsule in glomeruli from two different patients with diabetic nephropathy. B′ and C′: higher magnification of the insets showing de novo staining for LAMB2 along Bowman’s capsule (white arrows) and in the mesangium (yellow arrow). D–F: agrin staining. D: agrin staining localized to the GBM in the normal glomerulus. E and F: agrin was also detected along Bowman’s capsule in glomeruli from two different patients. E′ and F′: higher magnification of the insets showing staining for agrin along Bowman’s capsule (white arrows) and in the mesangium (yellow arrow). G–I: COL4A4 staining. G: COL4A4 staining localized to the GBM in the normal glomerulus. H and I: COL4A4 was also detected along Bowman’s capsule in glomeruli from two different patients with diabetic nephropathy. H′ and I′: higher magnification showing COL4A4 staining along Bowman’s capsule (white arrows) and in the mesangium (yellow arrow). Original magnification: ×200. Scale bars = 100 μm.
Taken together, PECs de novo expressed podocyte-specific ECM isoforms in experimental and human DN.
Individual ECM Proteins Reduced in PECs in the Absence of CD44
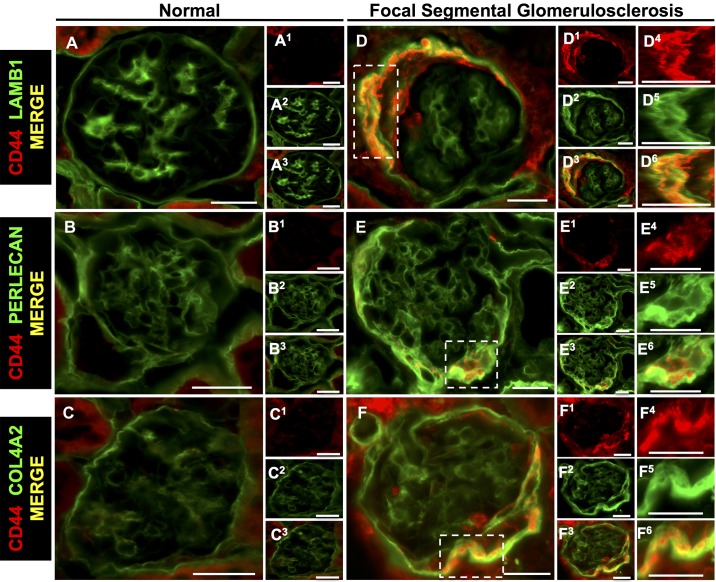
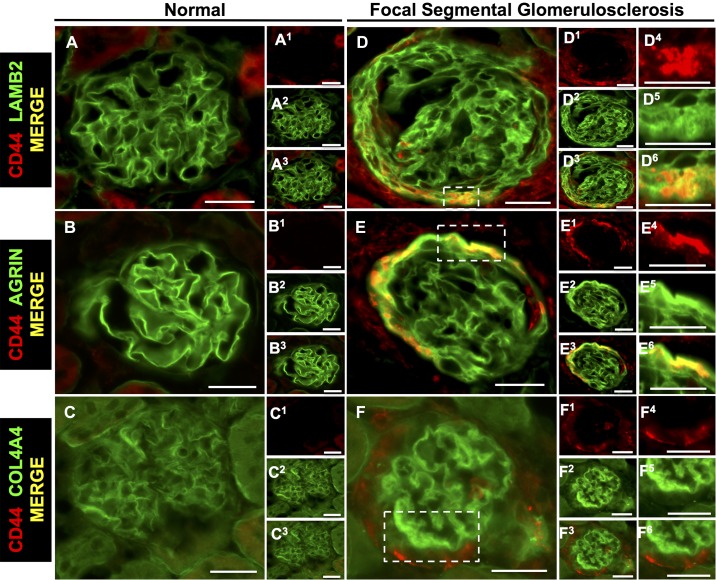
We (35) and others (20, 22, 44) have reported that CD44 levels are markers of activated PECs and regulate ECM proteins in PECs in disease. In the present study, double staining was used to determine the association of CD44 with individual ECM proteins. In experimental FSGS, CD44 staining in PECs colocalized with increased staining for LAMB1 (Fig. 10, A–A3 and D–D6), perlecan (Fig. 10, B–B3 and E–E6), and COL4A2 (Fig. 10, C–C3 and F–F6). In experimental FSGS, CD44 in PECs colocalized with the podocyte-specific ECM proteins LAMB2 (Fig. 11, A–A3 and D–D6) and agrin (Fig. 11, B–B3 and E–E6) but not COL4A4 (Fig. 11, C–C3 and F–F6).
Fig. 10.
Parietal epithelial cell (PEC)-derived proteins colocalize with the activated PEC marker CD44 in mice with experimental focal segmental glomerulosclerosis (FSGS). Double staining was performed for CD44 (red) and PEC-derived extracellular matrix proteins (green). A−C: normal mice. The merged images show staining for LAMB1 (A), perlecan (B), and COL4A2 (C) but not CD44 in normal glomeruli. A1−C3: individual stains for LAMB1, perlecan, and COL4A2. D−F: FSGS mice. Merged images show colocalization (yellow) of CD44 in PECs with LAMB1 (D and D3), perlecan (E and E3), and COL4A2 (F and F3). D1, D2, E1, E2, F1, and F2: corresponding individual stainings for LAMB1 (D1 and D2), perlecan (E1 and E2), and COL4A2 (F1 and F2). Single and merged higher magnifications of the insets are shown in D4–F6. These results are consistent with activated PECs expressing increased LAMB1, perlecan, and COL4A2 isoforms in FSGS. Original magnification: ×400. Scale bars = 20 μm.
Fig. 11.
Podocyte-specific proteins colocalize with the activated parietal epithelial cell (PEC) marker CD44 in mice with experimental focal segmental glomerulosclerosis (FSGS). Double staining was performed for CD44 (red) and podocyte-specific extracellular matrix proteins (green). A−C: normal mice. The merged images show staining for laminin-β2 (LAMB2; A), agrin (B), and collagen type IV-α4 (COL4A4; C) but not CD44 in normal glomeruli. A1−C3: individual stains for LAMB2 (A1−A3), agrin (B1−B3), and COL4A4 (C1–C3). D−F: FSGS mice. The merged images show colocalization (yellow) of CD44 in PECs with LAMB2 (D and D3) and agrin (E and E3) but not COL4A4 (F and F3). D1, D2, E1, E2, F1, and F2: corresponding single stainings for LAMB2 (D1 and D2), agrin (E1 and E2), and COL4A4 (F1 and F2). Single and merged higher magnifications of the insets are shown in D4–F6). These results are consistent with activated PECs expressing the podocyte-specific extracellular matrix proteins LAMB2 and agrin in FSGS. Original magnification: ×400. Scale bars = 20 μm.
Having shown colocalization of CD44 in PECs with both PEC-derived and podocyte-specific ECM isoforms, we next assessed the influence of CD44 on specific ECM isoforms by studying CD44+/+ and CD44−/− mice with experimental FSGS. There were no differences at baseline between these mice for the PEC-derived proteins LAMB1, perlecan, and COL4A2. In contrast, at day 28 of FSGS, staining was lower in CD44−/− mice for perlecan (35.5 ± 6.4% vs. 43.8 ± 8.4%, P = 0.042 vs. CD44+/+ mice) and COL4A2 (34.5 ± 4.2% vs. 43.8 ± 9.4%, P = 0.015 vs. CD44+/+ mice) but not LAMB1 (38 ± 4.3% vs. 40.5 ± 15.9%, P = 0.972 vs. CD44+/+ mice).
There were no differences in staining for the podocyte-specific ECM proteins LAMB2, agrin, or COL4A4 at baseline between CD44+/+ and CD44−/− mice. Compared with CD44+/+ FSGS mice, staining along Bowman’s capsule was lower in FSGS CD44−/− mice for LAMB2 (33.5 ± 4.6% vs. 42 ± 9.3%, P = 0.039 vs. CD44+/+ mice) and agrin (34.8 ± 4.3% vs. 43.8 ± 8%, P = 0.013 vs. CD44+/+ mice) but not COL4A4 (29 ± 4.9% vs. 31.8 ± 8.6%, P = 0.741 vs. CD44+/+ mice).
Taken together, these results show that increases in both PEC-derived and podocyte-specific ECM proteins in PECs colocalize in part with CD44 and that their increases are due to both CD44-dependent and -independent mechanisms, with lower abundance in the absence of CD44.
DISCUSSION
Recent studies have shown that PECs are a major source of increased ECM proteins in FSGS (10, 22, 34, 35, 39, 43, 44, 46, 47) and in DN (20), leading to glomerular scarring and fibrosis (20, 22, 36, 39, 44) and synechial attachments (48). However, the individual ECM protein isoforms that accumulate in PECs is FSGS and DN are not well delineated. Albeit the changes in other matrix proteins like fibrillar collagens or glycoproteins are important, the scope of the present study focused on assessing whether PECs begin to make podocyte-specific GBM matrix proteins as the secretion of “wrong” type of matrix chains likely leads to “wrong” cellular cues, thus limiting glomerular repair after injury.
The selected specific matrix protein chains were based on existing published literature, the results of preliminary test staining, and the availability of well-characterized matrix protein chain-specific antibodies that work well on mouse and human sections to show some differential staining of Bowman’s capsule (made by PECs), mesangial matrix, and the GBM. Under normal (nondiseased) conditions, PECs and podocytes synthesize different isoforms of ECM proteins. PECs constitutively synthesize LAMB1 (36, 37), perlecan (22, 36, 44), and COL4A2 (36), whereas podocytes constitutively synthesize LAMB2 (1, 3, 37, 49), agrin (1, 6, 17, 19), and COL4A4 (1–3). In the present study, we show that in experimental and human FSGS and DN, levels of both PEC-derived and podocyte-specific ECM proteins increase in PECs, that several ECM proteins colocalize with CD44 (activated PEC state), and that their levels decrease in the absence of CD44.
In the present study, we show that the expected staining for the ECM proteins LAMB1, perlecan, and COL4A2 were detected in PECs in healthy mice and healthy human kidneys. The first finding in the present study was an increase in staining for the PEC-derived ECM proteins LAMB1, perlecan, and COL4A2 along Bowman’s capsule in both experimental and human FSGS. Similarly, these three PEC-derived proteins increased along Bowman’s capsule in both experimental and human DN. Taken together, we can now add LAMB1, perlecan, and COL4A2 to the increase in ECM proteins in PECs in disease, which includes heparan sulfate moieties HS4E4, LKIV69, and COL4A2 (10, 43, 46, 47). We are not claiming that all perlecan is PEC derived because of the possibility of contributions by the endothelium and mesangium (18, 21). Groffen et al. (18) described glomerular endothelial cells producing perlecan in the developing glomerulus. Together, these may explain the high perlecan levels in disease.
Of the many ECM proteins comprising the normal GBM, LAMB2, agrin, and COL4A4 are considered podocyte specific. The second finding in the present study was de novo staining for LAMB2, agrin, and COL4A4 along Bowman’s capsule in both experimental and human FSGS. Double staining showed that LAMB2 along Bowman’s capsule did indeed derive from PECs and not from podocytes in that location. Similarly, in diabetic BTBR ob/ob mice, de novo expression for LAMB2 and agrin, but not COL4A4, was detected in PECs. Taken together, these findings are first to show in both FSGS and DN that PECs begin to de novo express ECM proteins considered “podocyte specific.” Studies in DN have described increased collagen type III, IV, and V, laminin, and fibronectin and decreased heparan sulfate proteoglycan (23, 28, 51, 54, 56). That said, collagen type IV has been reported to decrease in DN (51, 54). Holderied et al. (20) have been the only group to demonstrate thickening of Bowman’s capsule and increase of collagen type IV as a result of activated PECs in human DN. The present study shows that in both FSGS and DN, PECs contribute significantly to the ECM protein composition in the glomerulus.
Studies have shown de novo expression of CD44 in PECs in glomerular diseases is a marker and cause for PEC activation (4, 9, 11, 13, 14, 30, 36, 40–42, 46, 50). Increased CD44 underlies PEC-derived ECM production of collagen type IV and agrin (12, 20, 22, 29, 35, 44, 46). A third finding in the present study was that activated PECs (defined by CD44 staining) in FSGS colocalized with increases in both PEC-derived proteins (LAMB1, perlecan, and COL4A2) and podocyte-specific proteins (LAMB2 and agrin). To prove that CD44 was critical in the upregulation of individual ECM proteins, archival tissue from CD44+/+ and CD44−/− mice with FSGS was examined. There were no differences for perlecan, LAMB1, COL4A2, LAMB2, agrin, and COL4A4 at baseline between CD44+/+ and CD44−/− mice. In contrast, PEC-derived perlecan and COL4A2 and podocyte-specific matrix proteins LAMB2 and agrin, which were increased in PECs of FSGS CD44+/+ mice, were consistently lower in FSGS CD44−/− mice. These results are consistent with the increases in individual ECM proteins by PECs to be CD44 dependent and independent and that, when CD44 is not increased in disease, ECM proteins are indeed lower.
We recognize several limitations in our present study. First, we have acknowledged in our earlier study that a global CD44−/− mouse was used instead of PEC-specific deletion of CD44, as no unique PEC gene has been identified yet (35). Even though CD44 has other inflammatory effects in other systems, we demonstrated no differences in inflammatory cell counts (T cells, B cells, macrophages, and neutrophils) within the glomeruli of FSGS CD44+/+ and CD44−/− mice (35). Second, we have yet to explain how activated PECs cause individual ECM protein accumulation.
In summary, in experimental and human FSGS and DN, PECs express higher levels of PEC-derived ECM proteins as well as de novo podocyte-specific ECM proteins. Activated PECs express individual ECM proteins, which are lowered in mice lacking CD44.
GRANTS
This work was supported by 5-R01-DK 056799-10, 5-R01-DK-056799-12, and 1-R01-DK097598-01A1 (to S. J. Shankland). G. C. Chan was supported by the Singapore Ministry of Health's National Medical Research Council (NMRC) under its NMRC Research Training Fellowship (NMRC/FLWSHP/053/2017).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
G.C.C., D.G.E., J.W.P., and S.J.S. conceived and designed research; G.C.C., D.G.E., and J.W.P. performed experiments; G.C.C., J.W.P., and S.J.S. analyzed data; G.C.C., D.G.E., J.W.P., and S.J.S. interpreted results of experiments; G.C.C., J.W.P., and S.J.S. prepared figures; G.C.C., D.G.E., J.W.P., and S.J.S. drafted manuscript; G.C.C., D.G.E., J.H.M., C.E.A., A.C., J.W.P., and S.J.S. edited and revised manuscript; G.C.C., D.G.E., J.H.M., C.E.A., K.L.H., A.C., J.W.P., and S.J.S. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Takako Sasaki for producing the anti-agrin, anti-COL4A4, and anti- laminin-β1 and -β2 antibodies, which have been characterized by the Jeffrey H. Miner laboratory (19, 27, 38). The C4 (mouse anti-human LAMB2) antibody was obtained from the Developmental Studies Hybridoma Bank (deposited by J.R. Sanes), developed under the auspices of Eunice Kennedy Shriver National Institute of Child Health and Human Development and maintained by the Department of Biology, The University of Iowa, Iowa City, IA.
REFERENCES
- 1.Abrahamson DR. Role of the podocyte (and glomerular endothelium) in building the GBM. Semin Nephrol 32: 342–349, 2012. doi: 10.1016/j.semnephrol.2012.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abrahamson DR, Hudson BG, Stroganova L, Borza DB, St. John PL. Cellular origins of type IV collagen networks in developing glomeruli. J Am Soc Nephrol 20: 1471–1479, 2009. doi: 10.1681/ASN.2008101086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abrahamson DR, St. John PL, Stroganova L, Zelenchuk A, Steenhard BM. Laminin and type IV collagen isoform substitutions occur in temporally and spatially distinct patterns in developing kidney glomerular basement membranes. J Histochem Cytochem 61: 706–718, 2013. doi: 10.1369/0022155413501677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andeen NK, Nguyen TQ, Steegh F, Hudkins KL, Najafian B, Alpers CE. The phenotypes of podocytes and parietal epithelial cells may overlap in diabetic nephropathy. Kidney Int 88: 1099–1107, 2015. doi: 10.1038/ki.2015.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berger K, Schulte K, Boor P, Kuppe C, van Kuppevelt TH, Floege J, Smeets B, Moeller MJ. The regenerative potential of parietal epithelial cells in adult mice. J Am Soc Nephrol 25: 693–705, 2014. doi: 10.1681/ASN.2013050481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Borza DB. Glomerular basement membrane heparan sulfate in health and disease: A regulator of local complement activation. Matrix Biol 57-58: 299–310, 2017. doi: 10.1016/j.matbio.2016.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clee SM, Nadler ST, Attie AD. Genetic and genomic studies of the BTBR ob/ob mouse model of type 2 diabetes. Am J Ther 12: 491–498, 2005. doi: 10.1097/01.mjt.0000178781.89789.25. [DOI] [PubMed] [Google Scholar]
- 8.Couchman JR, Beavan LA, McCarthy KJ. Glomerular matrix: synthesis, turnover and role in mesangial expansion. Kidney Int 45: 328–335, 1994. doi: 10.1038/ki.1994.42. [DOI] [PubMed] [Google Scholar]
- 9.Cui C, Cui Y, Fu Y, Ma S, Zhang S. Microarray analysis reveals gene and microRNA signatures in diabetic kidney disease. Mol Med Rep 17: 2161–2168, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dijkman H, Smeets B, van der Laak J, Steenbergen E, Wetzels J. The parietal epithelial cell is crucially involved in human idiopathic focal segmental glomerulosclerosis. Kidney Int 68: 1562–1572, 2005. doi: 10.1111/j.1523-1755.2005.00568.x. [DOI] [PubMed] [Google Scholar]
- 11.Eng DG, Sunseri MW, Kaverina NV, Roeder SS, Pippin JW, Shankland SJ. Glomerular parietal epithelial cells contribute to adult podocyte regeneration in experimental focal segmental glomerulosclerosis. Kidney Int 88: 999–1012, 2015. doi: 10.1038/ki.2015.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eymael J, Sharma S, Loeven MA, Wetzels JF, Mooren F, Florquin S, Deegens JK, Willemsen BK, Sharma V, van Kuppevelt TH, Bakker MA, Ostendorf T, Moeller MJ, Dijkman HB, Smeets B, van der Vlag J. CD44 is required for the pathogenesis of experimental crescentic glomerulonephritis and collapsing focal segmental glomerulosclerosis. Kidney Int 93: 626–642, 2018. doi: 10.1016/j.kint.2017.09.020. [DOI] [PubMed] [Google Scholar]
- 13.Fatima H, Moeller MJ, Smeets B, Yang HC, D’Agati VD, Alpers CE, Fogo AB. Parietal epithelial cell activation marker in early recurrence of FSGS in the transplant. Clin J Am Soc Nephrol 7: 1852–1858, 2012. doi: 10.2215/CJN.10571011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Geng XD, Wang WW, Feng Z, Liu R, Cheng XL, Shen WJ, Dong ZY, Cai GY, Chen XM, Hong Q, Wu D. Identification of key genes and pathways in diabetic nephropathy by bioinformatics analysis. J Diabetes Investig 972–984, 2019. doi: 10.1111/jdi.12986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goldberg S, Harvey SJ, Cunningham J, Tryggvason K, Miner JH. Glomerular filtration is normal in the absence of both agrin and perlecan-heparan sulfate from the glomerular basement membrane. Nephrol Dial Transplant 24: 2044–2051, 2009. doi: 10.1093/ndt/gfn758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Groffen AJ, Hop FW, Tryggvason K, Dijkman H, Assmann KJ, Veerkamp JH, Monnens LA, van den Heuvel LP. Evidence for the existence of multiple heparan sulfate proteoglycans in the human glomerular basement membrane and mesangial matrix. Eur J Biochem 247: 175–182, 1997. doi: 10.1111/j.1432-1033.1997.00175.x. [DOI] [PubMed] [Google Scholar]
- 17.Groffen AJ, Ruegg MA, Dijkman H, van de Velden TJ, Buskens CA, van den Born J, Assmann KJ, Monnens LA, Veerkamp JH, van den Heuvel LP. Agrin is a major heparan sulfate proteoglycan in the human glomerular basement membrane. J Histochem Cytochem 46: 19–27, 1998. doi: 10.1177/002215549804600104. [DOI] [PubMed] [Google Scholar]
- 18.Groffen AJ, Veerkamp JH, Monnens LA, van den Heuvel LP. Recent insights into the structure and functions of heparan sulfate proteoglycans in the human glomerular basement membrane. Nephrol Dial Transplant 14: 2119–2129, 1999. doi: 10.1093/ndt/14.9.2119. [DOI] [PubMed] [Google Scholar]
- 19.Harvey SJ, Jarad G, Cunningham J, Rops AL, van der Vlag J, Berden JH, Moeller MJ, Holzman LB, Burgess RW, Miner JH. Disruption of glomerular basement membrane charge through podocyte-specific mutation of agrin does not alter glomerular permselectivity. Am J Pathol 171: 139–152, 2007. doi: 10.2353/ajpath.2007.061116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Holderied A, Romoli S, Eberhard J, Konrad LA, Devarapu SK, Marschner JA, Müller S, Anders HJ. Glomerular parietal epithelial cell activation induces collagen secretion and thickening of Bowman’s capsule in diabetes. Lab Invest 95: 273–282, 2015. doi: 10.1038/labinvest.2014.160. [DOI] [PubMed] [Google Scholar]
- 21.Kinsella MG, Tran PK, Weiser-Evans MC, Reidy M, Majack RA, Wight TN. Changes in perlecan expression during vascular injury: role in the inhibition of smooth muscle cell proliferation in the late lesion. Arterioscler Thromb Vasc Biol 23: 608–614, 2003. doi: 10.1161/01.ATV.0000063109.94810.EE. [DOI] [PubMed] [Google Scholar]
- 22.Kuppe C, Gröne HJ, Ostendorf T, van Kuppevelt TH, Boor P, Floege J, Smeets B, Moeller MJ. Common histological patterns in glomerular epithelial cells in secondary focal segmental glomerulosclerosis. Kidney Int 88: 990–998, 2015. doi: 10.1038/ki.2015.116. [DOI] [PubMed] [Google Scholar]
- 23.Makino H, Ikeda S, Haramoto T, Ota Z. Heparan sulfate proteoglycans are lost in patients with diabetic nephropathy. Nephron 61: 415–421, 1992. doi: 10.1159/000186959. [DOI] [PubMed] [Google Scholar]
- 24.Miner JH. The glomerular basement membrane. Exp Cell Res 318: 973–978, 2012. doi: 10.1016/j.yexcr.2012.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miner JH. Renal basement membrane components. Kidney Int 56: 2016–2024, 1999. doi: 10.1046/j.1523-1755.1999.00785.x. [DOI] [PubMed] [Google Scholar]
- 26.Miner JH, Patton BL, Lentz SI, Gilbert DJ, Snider WD, Jenkins NA, Copeland NG, Sanes JR. The laminin alpha chains: expression, developmental transitions, and chromosomal locations of α1-5, identification of heterotrimeric laminins 8–11, and cloning of a novel α3 isoform. J Cell Biol 137: 685–701, 1997. doi: 10.1083/jcb.137.3.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miner JH, Sanes JR. Collagen IV alpha 3, alpha 4, and alpha 5 chains in rodent basal laminae: sequence, distribution, association with laminins, and developmental switches. J Cell Biol 127: 879–891, 1994. doi: 10.1083/jcb.127.3.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nerlich A, Schleicher E. Immunohistochemical localization of extracellular matrix components in human diabetic glomerular lesions. Am J Pathol 139: 889–899, 1991. [PMC free article] [PubMed] [Google Scholar]
- 29.Ohse T, Vaughan MR, Kopp JB, Krofft RD, Marshall CB, Chang AM, Hudkins KL, Alpers CE, Pippin JW, Shankland SJ. De novo expression of podocyte proteins in parietal epithelial cells during experimental glomerular disease. Am J Physiol Renal Physiol 298: F702–F711, 2010. doi: 10.1152/ajprenal.00428.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Okamoto T, Sasaki S, Yamazaki T, Sato Y, Ito H, Ariga T. Prevalence of CD44-positive glomerular parietal epithelial cells reflects podocyte injury in adriamycin nephropathy. Nephron, Exp Nephrol 124: 11–18, 2014. doi: 10.1159/000357356. [DOI] [PubMed] [Google Scholar]
- 31.Pichaiwong W, Hudkins KL, Wietecha T, Nguyen TQ, Tachaudomdach C, Li W, Askari B, Kobayashi T, O’Brien KD, Pippin JW, Shankland SJ, Alpers CE. Reversibility of structural and functional damage in a model of advanced diabetic nephropathy. J Am Soc Nephrol 24: 1088–1102, 2013. doi: 10.1681/ASN.2012050445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Protin U, Schweighoffer T, Jochum W, Hilberg F. CD44-deficient mice develop normally with changes in subpopulations and recirculation of lymphocyte subsets. J Immunol 163: 4917–4923, 1999. [PubMed] [Google Scholar]
- 33.Ranheim T, Dumke C, Schueler KL, Cartee GD, Attie AD. Interaction between BTBR and C57BL/6J genomes produces an insulin resistance syndrome in (BTBR x C57BL/6J) F1 mice. Arterioscler Thromb Vasc Biol 17: 3286–3293, 1997. doi: 10.1161/01.ATV.17.11.3286. [DOI] [PubMed] [Google Scholar]
- 34.Razzaque MS, Koji T, Harada T, Nakane PK, Taguchi T. Primary focal segmental glomerulosclerosis is associated with increased intraglomerular type IV collagen synthesis. Clin Chim Acta 235: 121–124, 1995. doi: 10.1016/0009-8981(95)06018-9. [DOI] [PubMed] [Google Scholar]
- 35.Roeder SS, Barnes TJ, Lee JS, Kato I, Eng DG, Kaverina NV, Sunseri MW, Daniel C, Amann K, Pippin JW, Shankland SJ. Activated ERK1/2 increases CD44 in glomerular parietal epithelial cells leading to matrix expansion. Kidney Int 91: 896–913, 2017. doi: 10.1016/j.kint.2016.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Roeder SS, Stefanska A, Eng DG, Kaverina N, Sunseri MW, McNicholas BA, Rabinovitch P, Engel FB, Daniel C, Amann K, Lichtnekert J, Pippin JW, Shankland SJ. Changes in glomerular parietal epithelial cells in mouse kidneys with advanced age. Am J Physiol Renal Physiol 309: F164–F178, 2015. doi: 10.1152/ajprenal.00144.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roediger M, Miosge N, Gersdorff N. Tissue distribution of the laminin beta1 and beta2 chain during embryonic and fetal human development. J Mol Histol 41: 177–184, 2010. doi: 10.1007/s10735-010-9275-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sasaki T, Mann K, Miner JH, Miosge N, Timpl R. Domain IV of mouse laminin β1 and β2 chains. Eur J Biochem 269: 431–442, 2002. doi: 10.1046/j.0014-2956.2001.02663.x. [DOI] [PubMed] [Google Scholar]
- 39.Schneider RR, Eng DG, Kutz JN, Sweetwyne MT, Pippin JW, Shankland SJ. Compound effects of aging and experimental FSGS on glomerular epithelial cells. Aging (Albany NY) 9: 524–546, 2017. doi: 10.18632/aging.101176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shankland SJ, Smeets B, Pippin JW, Moeller MJ. The emergence of the glomerular parietal epithelial cell. Nat Rev Nephrol 10: 158–173, 2014. doi: 10.1038/nrneph.2014.1. [DOI] [PubMed] [Google Scholar]
- 41.Sicking EM, Fuss A, Uhlig S, Jirak P, Dijkman H, Wetzels J, Engel DR, Urzynicok T, Heidenreich S, Kriz W, Kurts C, Ostendorf T, Floege J, Smeets B, Moeller MJ. Subtotal ablation of parietal epithelial cells induces crescent formation. J Am Soc Nephrol 23: 629–640, 2012. doi: 10.1681/ASN.2011050449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Smeets B, Angelotti ML, Rizzo P, Dijkman H, Lazzeri E, Mooren F, Ballerini L, Parente E, Sagrinati C, Mazzinghi B, Ronconi E, Becherucci F, Benigni A, Steenbergen E, Lasagni L, Remuzzi G, Wetzels J, Romagnani P. Renal progenitor cells contribute to hyperplastic lesions of podocytopathies and crescentic glomerulonephritis. J Am Soc Nephrol 20: 2593–2603, 2009. doi: 10.1681/ASN.2009020132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Smeets B, Dijkman HB, Wetzels JF, Steenbergen EJ. Lessons from studies on focal segmental glomerulosclerosis: an important role for parietal epithelial cells? J Pathol 210: 263–272, 2006. doi: 10.1002/path.2051. [DOI] [PubMed] [Google Scholar]
- 44.Smeets B, Kuppe C, Sicking EM, Fuss A, Jirak P, van Kuppevelt TH, Endlich K, Wetzels JF, Gröne HJ, Floege J, Moeller MJ. Parietal epithelial cells participate in the formation of sclerotic lesions in focal segmental glomerulosclerosis. J Am Soc Nephrol 22: 1262–1274, 2011. doi: 10.1681/ASN.2010090970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Smeets B, Moeller MJ. Parietal epithelial cells and podocytes in glomerular diseases. Semin Nephrol 32: 357–367, 2012. doi: 10.1016/j.semnephrol.2012.06.007. [DOI] [PubMed] [Google Scholar]
- 46.Smeets B, Stucker F, Wetzels J, Brocheriou I, Ronco P, Gröne HJ, D’Agati V, Fogo AB, van Kuppevelt TH, Fischer HP, Boor P, Floege J, Ostendorf T, Moeller MJ. Detection of activated parietal epithelial cells on the glomerular tuft distinguishes early focal segmental glomerulosclerosis from minimal change disease. Am J Pathol 184: 3239–3248, 2014. doi: 10.1016/j.ajpath.2014.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Smeets B, Te Loeke NA, Dijkman HB, Steenbergen ML, Lensen JF, Begieneman MP, van Kuppevelt TH, Wetzels JF, Steenbergen EJ. The parietal epithelial cell: a key player in the pathogenesis of focal segmental glomerulosclerosis in Thy-1.1 transgenic mice. J Am Soc Nephrol 15: 928–939, 2004. doi: 10.1097/01.ASN.0000120559.09189.82. [DOI] [PubMed] [Google Scholar]
- 48.Smeets B, Uhlig S, Fuss A, Mooren F, Wetzels JF, Floege J, Moeller MJ. Tracing the origin of glomerular extracapillary lesions from parietal epithelial cells. J Am Soc Nephrol 20: 2604–2615, 2009. doi: 10.1681/ASN.2009010122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.St. John PL, Abrahamson DR. Glomerular endothelial cells and podocytes jointly synthesize laminin-1 and -11 chains. Kidney Int 60: 1037–1046, 2001. doi: 10.1046/j.1523-1755.2001.0600031037.x. [DOI] [PubMed] [Google Scholar]
- 50.Swetha G, Chandra V, Phadnis S, Bhonde R. Glomerular parietal epithelial cells of adult murine kidney undergo EMT to generate cells with traits of renal progenitors. J Cell Mol Med 15: 396–413, 2011. doi: 10.1111/j.1582-4934.2009.00937.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tamsma JT, van den Born J, Bruijn JA, Assmann KJ, Weening JJ, Berden JH, Wieslander J, Schrama E, Hermans J, Veerkamp JH, Lemkes HH, van der Woude FJ. Expression of glomerular extracellular matrix components in human diabetic nephropathy: decrease of heparan sulphate in the glomerular basement membrane. Diabetologia 37: 313–320, 1994. doi: 10.1007/BF00398060. [DOI] [PubMed] [Google Scholar]
- 52.Taneda S, Segerer S, Hudkins KL, Cui Y, Wen M, Segerer M, Wener MH, Khairallah CG, Farr AG, Alpers CE. Cryoglobulinemic glomerulonephritis in thymic stromal lymphopoietin transgenic mice. Am J Pathol 159: 2355–2369, 2001. doi: 10.1016/S0002-9440(10)63085-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Thomas GJ, Shewring L, McCarthy KJ, Couchman JR, Mason RM, Davies M. Rat mesangial cells in vitro synthesize a spectrum of proteoglycan species including those of the basement membrane and interstitium. Kidney Int 48: 1278–1289, 1995. doi: 10.1038/ki.1995.412. [DOI] [PubMed] [Google Scholar]
- 54.van den Born J, van Kraats AA, Bakker MA, Assmann KJ, van den Heuvel LP, Veerkamp JH, Berden JH. Selective proteinuria in diabetic nephropathy in the rat is associated with a relative decrease in glomerular basement membrane heparan sulphate. Diabetologia 38: 161–172, 1995. doi: 10.1007/BF00400090. [DOI] [PubMed] [Google Scholar]
- 55.Virtanen I, Laitinen L, Korhonen M. Differential expression of laminin polypeptides in developing and adult human kidney. J Histochem Cytochem 43: 621–628, 1995. doi: 10.1177/43.6.7769233. [DOI] [PubMed] [Google Scholar]
- 56.Wijnhoven TJ, Lensen JF, Rops AL, van der Vlag J, Kolset SO, Bangstad HJ, Pfeffer P, van den Hoven MJ, Berden JH, van den Heuvel LP, van Kuppevelt TH. Aberrant heparan sulfate profile in the human diabetic kidney offers new clues for therapeutic glycomimetics. Am J Kidney Dis 48: 250–261, 2006. doi: 10.1053/j.ajkd.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 57.Zhang J, Pippin JW, Krofft RD, Naito S, Liu ZH, Shankland SJ. Podocyte repopulation by renal progenitor cells following glucocorticoids treatment in experimental FSGS. Am J Physiol Renal Physiol 304: F1375–F1389, 2013. doi: 10.1152/ajprenal.00020.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhang J, Pippin JW, Vaughan MR, Krofft RD, Taniguchi Y, Romagnani P, Nelson PJ, Liu ZH, Shankland SJ. Retinoids augment the expression of podocyte proteins by glomerular parietal epithelial cells in experimental glomerular disease. Nephron, Exp Nephrol 121: e23–e37, 2012. doi: 10.1159/000342808. [DOI] [PMC free article] [PubMed] [Google Scholar]