Abstract
The protozoan parasite Cryptosporidium parvum (CP) causes cryptosporidiosis, a diarrheal disease worldwide. Infection in immunocompetent hosts typically results in acute, self-limiting, or recurrent diarrhea. However, in immunocompromised individuals infection can cause fulminant diarrhea, extraintestinal manifestations, and death. To date, the mechanisms underlying CP-induced diarrheal pathogenesis are poorly understood. Diarrheal diseases most commonly involve increased secretion and/or decreased absorption of fluid and electrolytes. We and others have previously shown impaired chloride absorption in infectious diarrhea due to dysregulation of SLC26A3 [downregulated in adenoma (DRA)], the human intestinal apical membrane Cl−/ exchanger protein. However, there are no studies on the effects of CP infection on DRA activity. Therefore, we examined the expression and function of DRA in intestinal epithelial cells in response to CP infection in vitro and in vivo. CP infection (0.5 × 106 oocysts/well in 24-well plates, 24 h) of Caco-2 cell monolayers significantly decreased Cl−/ exchange activity (measured as DIDS-sensitive 125I uptake) as well as DRA mRNA and protein levels. Substantial downregulation of DRA mRNA and protein was also observed following CP infection ex vivo in mouse enteroid-derived monolayers and in vivo in the ileal and jejunal mucosa of C57BL/6 mice for 24 h. However, at 48 h after infection in vivo, the effects on DRA mRNA and protein were attenuated and at 5 days after infection DRA returned to normal levels. Our results suggest that impaired chloride absorption due to downregulation of DRA could be one of the contributing factors to CP-induced acute, self-limiting diarrhea in immunocompetent hosts.
Keywords: cryptosporidiosis, infectious diarrhea, NaCl absorption, SLC26A3
INTRODUCTION
The water-borne apicomplexan parasite Cryptosporidium species that primarily infects small intestine is a major cause of cryptosporidiosis, a diarrheal disease occurring worldwide (3, 6, 7). Cryptosporidiosis has increasingly been recognized as an alarming global health problem. In the United States alone, ~750,000 cases of cryptosporidiosis have been reported to occur annually (10). Stunted growth and associated high mortality and morbidity have also been reported in two recent multicenter studies [The Global Enteric Multicenter Study (GEMS) (21) and a global network study of malnutrition and enteric diseases (MAL-ED) (27)] that recognized this neglected parasite as one of the four major diarrheal pathogens in infants. Undernourished children are particularly vulnerable to infection by this protozoan parasite; however, whether malnutrition is a trigger to, or consequence of, Cryptosporidiosis is not known. Infection in immunocompetent individuals usually causes self-limiting watery diarrhea and abdominal cramps, but infants can develop chronic, severe diarrhea with high mortality. Furthermore, immunocompromised hosts, such as HIV patients, people under immunosuppressive drugs, and patients with inheritable immunodeficiency syndromes can show profuse chronic diarrhea and life-threatening clinical signs. Indeed, in western countries including the United States, the parasite was initially recognized as an agent responsible for causing chronic diarrhea in AIDS patients, as well as for their association with waterborne outbreaks causing high morbidity and mortality (7, 13, 31, 35). Currently, treatment options for cryptosporidiosis are extremely limited, and no vaccines have been developed to date. The only approved drug nitazoxanide is less effective to those at highest risk for morbidity and mortality, viz. malnourished children and immunocompromised patients (28, 31, 32). Current understanding of the molecular basis of Cryptosporidium interactions with host intestinal epithelial cells (IECs) that lead to this debilitating diarrheal disease is extremely limited. Most of the studies to date have been focused on delineating the mechanisms of parasite adhesion and invasion of IECs and identifying parasite factors that facilitate adhesion and colonization with limited studies emphasizing altered epithelial barrier function and impaired ion transport, key contributing factors for most infectious diarrheal diseases. We have recently demonstrated that decreased expression of specific tight junction and adherens junction proteins could contribute to barrier disruption in cryptosporidiosis (23).
Decreased luminal absorption of Na+ and Cl− and/or increased secretion of Cl− and are common features of diarrheal diseases (11, 14, 18). In the mammalian intestine, NaCl absorption occurs via coupled operation of Na+/H+ exchanger 3 (NHE3; SLC9A3) and Cl−/ exchanger downregulated in adenoma (DRA; SLC26A3) (12, 15). We and others have demonstrated impaired DRA expression and function in response to enteric infections (5, 9, 16, 17). Indeed, in recent years, DRA has emerged as a novel therapeutic target for diarrhea. However, virtually nothing is known about ion transport dysregulation in cryptosporidiosis. The results obtained in our current study have demonstrated substantial downregulation of DRA expression and function in response to C. parvum infection that could be a major factor contributing to the pathophysiology of cryptosporidiosis.
EXPERIMENTAL PROCEDURES
Chemicals and antibodies.
The real-time quantitative RT-PCR kits used to measure relative mRNA levels were obtained from Stratagene (La Jolla, CA). RNeasy kits used for RNA extraction were from Qiagen (Valencia, CA). The DRA antibody used in this study was raised in rabbits at the Research Resource Center of the University of Illinois at Chicago against the COOH-terminal amino acid (745–764) sequence INTNGGLRNRVYEPVETKF of SLC26A3 (accession no. BC025671). The NHE3 antibody was obtained as a kind gift from Dr. Chris Yun of the Emory University, Atlanta, GA (40). Antibodies were validated in knockout models and by using positive and negative controls in immunoblotting.
Preparation of Cryptosporidium oocysts.
Human cryptosporidiosis is caused by two major species of the parasite, viz. C. parvum and C. hominis (39). In the current study, we have utilized C. parvum, as this species, but not C. hominis, infects both human and mice. Live oocysts of C. parvum suspended in PBS were obtained from Waterborne (New Orleans, LA). For treating cell monolayers or enteroid-derived monolayers, oocysts were excysted by incubating the suspension with 20% bleach solution (4:1 vol/vol) for 10 min at room temperature. The pellet obtained by centrifugation was washed three times with HBSS to remove traces of bleach. The washed pellet was then resuspended in appropriate cell culture/enteroid culture media containing 0.2% taurocholate to release infectious sporozoites for treatments.
Cell culture.
Caco-2 and T-84 cells obtained from American Type Culture Collection (ATCC, Manassas, VA) were grown at 37°C in a 5% CO2-95% air environment. Caco-2 cells were grown in MEM supplemented with 50 U/ml penicillin and 50 μg/ml gentamicin, and with 20% fetal bovine serum. The medium for growing T-84 cells was DMEM F-12 and 10% fetal bovine serum was used. For different experiments, cells were seeded on 6, 12, or 24-well plastic supports or 12-well transwell inserts and grown to confluency (12 days postplating) before infection with Cryptosporidium oocysts.
In vivo studies.
For in vivo studies, 4- to 6-wk-old C57BL/6 mice (Jackson Laboratories) were maintained in the animal care facility of the Jesse Brown Veterans Affairs Medical Center (JBVAMC, Chicago, IL). These studies were performed with prior approval by the Animal Care Committee of the University of Illinois at Chicago and JBVAMC. Mice were divided into six groups, three groups for infecton with C. parvum oocysts for 24 h, 48 h, and 5 days each and the other three groups being the respective controls. On day 1, each mouse in the 48 h treatment group was gavaged with 1 × 107 intact (unexcysted) oocysts in 200 μl sterile PBS, whereas mice in the respective 48 h control group received 200 μl of the vehicle. On day 2, mice in the 24 h treatment group were gavaged with 1 × 107 intact oocysts and the control groups with the vehicle. Mice were euthanized on the third day post-C. parvum infection. Intestines were resected, and mucosa was scraped for RNA extraction and tissue lysate preparation. Sections (∼2 μm) of the different regions of intestine (jejunum, ileum, proximal, and distal colon) were immediately snap-frozen in optimal cutting temperature embedding medium (Tissue-Tek OCT compound; Sakura) for immunofluorescence studies.
Preparation of crypt-derived mouse enteroids and enteroid-derived monolayers.
Small intestinal crypt-derived enteroids from C57BL/6 mice were generated and cultured according to Sato et al. (30) with minor modifications as described previously by us (23, 24). For preparing enteroid-derived monolayers, enteroids were mechanically disrupted with a p1000 pipette and cultured as monolayers on Matrigels for 96 h as described previously (37) with minor modifications.
Determination of DRA function (Cl−/ exchange activity).
DRA function (Cl−/ exchange activity) was determined by measuring DIDS-sensitive 125I− uptake in base-loaded cells as previously described by us (1). After terminating uptake via addition of cold PBS, the cells were solubilized by incubation with 0.5 N NaOH for 4 h and protein concentration was measured by Bradford method. Radioactivity was measured by Packard Tri-Carb 1600 TR liquid scintillation analyzer (Packard Instruments; Perkin Elmer). The Cl−/ exchange activity was calculated as DIDS-sensitive 125I− uptake, and the specific activity was expressed as nanomoles per milligram protein per 5 min.
Real-time PCR.
Extraction of total RNA from Caco-2/T-84 cells or homogenized mouse intestinal mucosal samples was performed using RNeasy kits (Qiagen). RNA was reverse transcribed and amplified using a Brilliant SYBR Green qRT-PCR Master Mix kit (Stratagene). Gene-specific primers used to amplify mouse and human DRA and NHE3 gene, utilizing mouse and human GAPDH, respectively, as internal controls, have been described previously by us (22, 33, 34).
Immunoblotting.
Cells (Caco-2 and T-84) and mouse intestinal scraped mucosal samples were used to prepare the lysates as described previously by us (22, 33). Protein concentrations in the lysates were measured by the Bradford reagent (Bio-Rad, Hercules, CA). Total proteins (25 μg) in the extracts were subjected to SDS-PAGE and transferred to nitrocellulose membranes. Levels of DRA/NHE3 proteins were immunodetected using specific antibodies and visualized by enhanced chemiluminescence reagents (ECL Plus; GE Healthcare, London, UK).
Immunofluorescence staining in mouse intestinal tissues.
Different regions of the mouse intestine (jejunum, ileum, proximal, and distal colon) were snap frozen in OCT embedding medium, and 5-µM sections from each group of mice were obtained onto glass slides with the aid of a cryostat microtome (Leica, Wetzlar, Germany). These sections were immunostained for DRA protein using methods described previously by us (20). Briefly, cryosections were fixed with 4% paraformaldehyde in PBS for 10 min followed by permeabilization with 0.3% Nonidet P-40 for 5 min at room temperature. The slides were washed once in PBS and encircled with the water repellent pap pen around tissue sections. These sections were then blocked with 5% vol/vol normal goat serum for 2 h followed by incubation with primary antibody of DRA (UIC RRC synthesized raised in rabbit) and Villin (Invitrogen raised in mouse) at a ratio of 1:100 in 1% NGS for 2 h. After being washed several times in PBS, sections were incubated with Alexa fluor-594-conjugated goat anti-rabbit IgG and Alexa Fluor 488-conjugated goat anti-mouse IgG (Invitrogen) at a ratio of 1:100 in 1% normal goat serum for 1 h at room temperature. Again, the slides were washed with three changes of PBS and mounted with coverslips using Gold-antifade reagent with DAPI (Molecular Probes). The slides were stored at −20°C until imaged. Images were acquired with the aid of Olympus BX51/1X70 fluorescent microscope.
Statistical analyses.
Data presented here are means ± SE of three to eight independent experiments. One-way ANOVA with Tukey's test or Student’s t test was used to analyze the differences between controls versus treated groups. Differences were considered significant at P ≤ 0.05.
RESULTS
C. parvum infection decreased DRA mRNA and protein levels in IECs with no effects on NHE3.
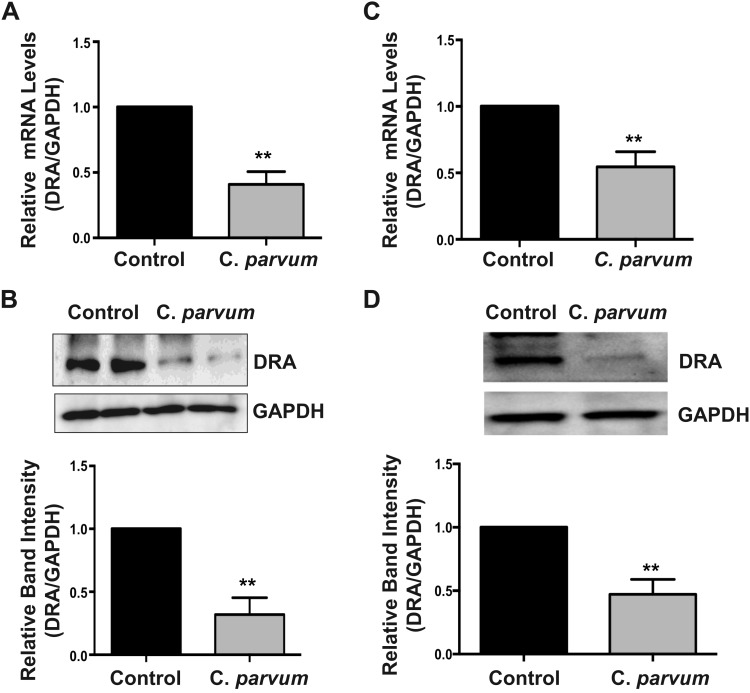
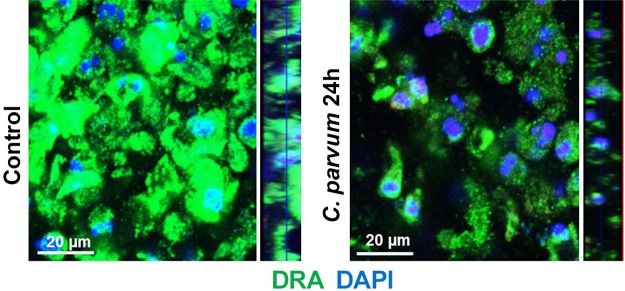
The two intestinal epithelial luminal membrane proteins NHE3 (SLC9A3) and the Cl−/ exchanger DRA (SLC26A3) play key role in mediating electroneutral NaCl absorption in the mammalian intestine (12, 15). Previous studies by us and others have shown extensive downregulation of DRA expression in inflammation (2) or in response to enteric infections (5, 9, 16, 17). Therefore, we examined DRA expression in Caco-2 cells following C. parvum (CP) infection for 24 h by measuring DRA mRNA and protein levels. As shown in Fig. 1, CP significantly downregulated DRA mRNA (Fig. 1A) and protein levels (Fig. 1B) in Caco-2 cells. Similar results showing significant decrease in DRA mRNA (Fig. 1C) and protein (Fig. 1D) levels were also observed in T-84 cells, suggesting that CP effects on DRA were not cell line specific. Immunofluorescence staining of DRA protein in transwell grown mouse enteroid-derived monolayers following CP infection for 24 h also showed substantially decreased immunostaining intensity of DRA (Fig. 2). In contrast to the effects of CP infection on DRA expression, the parasite did not significantly alter the mRNA and protein levels of NHE3, the other member mediating electroneutral NaCl absorption (results not shown).
Fig. 1.
Cryptosporidium parvum infection decreased downregulated in adenoma (DRA) mRNA and protein levels in intestinal epithelial cells (IECs). Postconfluent monolayers of Caco-2 cells grown on 24-well plastic supports were treated with parasite oocysts prepared as described in experimental procedures at a dose of 0.5 × 106/well for 24 h. Total RNA or cell lysates were prepared as described in experimental procedures to measure DRA mRNA and protein levels, respectively. A: relative DRA mRNA abundance in total RNA samples extracted from control or C. parvum-treated Caco-2 cells was determined by real-time RT-PCR using gene-specific primers and utilizing GAPDH as the internal control. Values are means ± SE of 4 separate experiments. B, top: SDS-PAGE analysis of total protein in Caco-2 cell lysates followed by probing with anti-DRA antibody in immunoblotting. GAPDH was used as the internal control. B, bottom: densitometric analysis of relative band intensities of DRA with GAPDH as internal control. Values are means ± SE of 4 separate experiments. C: relative DRA mRNA abundance in total RNA samples extracted from control or C. parvum-treated T-84 cells was determined by real-time RT-PCR using gene-specific primers and utilizing GAPDH as the internal control. Values are means ± SE of 4 separate experiments. D, top: T-84 cell lysate was subjected to SDS-PAGE and probed with anti-DRA antibody in immunoblotting. GAPDH was used as the internal control. D, bottom: densitometric analysis of relative band intensities of DRA with GAPDH as internal control. Values are means ± SE of 3 separate experiments. **P < 0.001, compared with control.
Fig. 2.
Cryptosporidium parvum infection decreased downregulated in adenoma (DRA) immunostaining in mouse enteroid-derived monolayers. Mouse ileal crypt-derived enteroids and enteroid-derived monolayers were prepared as described in experimental procedures. Monolayers treated with C. parvum from apical surface for 24 h were processed for immunofluorescence staining for DRA (green) and DAPI (blue).
CP infection decreased DRA function in Caco-2 cells.
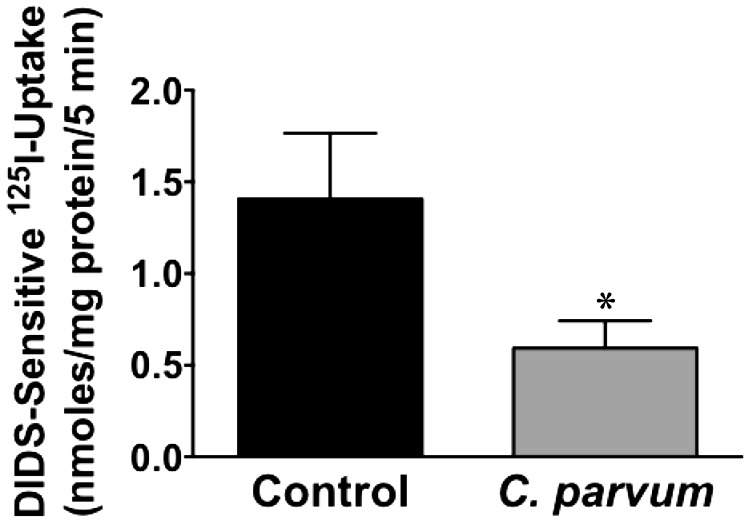
Since DRA expression was substantially decreased following CP infection, we next examined DRA function (Cl−/ exchange activity) in confluent Caco-2 monolayers in response to CP infection. As shown in Fig. 3, parallel to decreased expression, there was also significant decrease in DRA function following CP infection for 24 h.
Fig. 3.
Cryptosporidium parvum infection inhibits Cl−/ exchange activity. Postconfluent Caco-2 monolayers grown on 24-well plastic supports were treated with 0.5 × 106 sporozoites/well for 24 h. DRA function (Cl−/ exchange activity) was measured as DIDS-sensitive (600 μM) 125I− uptake for 5 min. Results are expressed as nmol·mg protein−1·5 min−1 and represent means ± SE of 3 separate experiments performed in triplicate. *P < 0.05, compared with control.
Role of JAK-STAT pathway in mediating C. parvum effects on DRA.
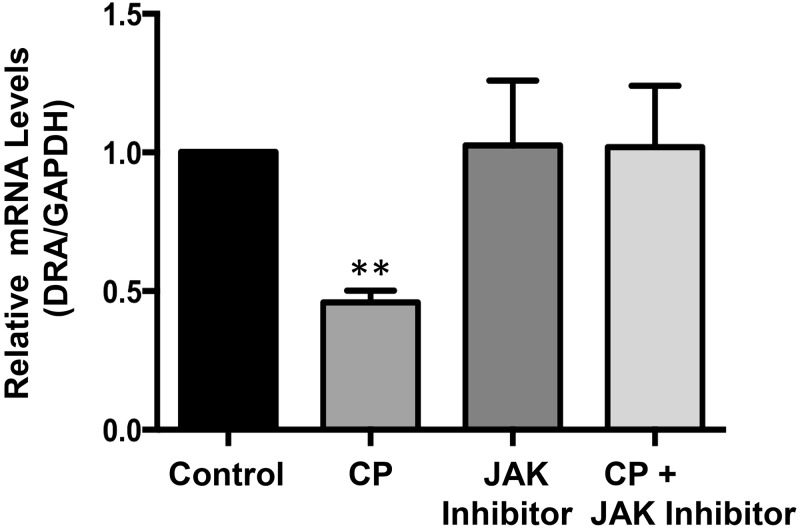
Our previous studies have shown IFNγ-induced decrease in DRA expression via transcriptional mechanism involving JAK-STAT pathway (29). Recently, we have also shown significant increase in IFNγ in vivo in the mouse ileum following CP infection for 24 h (23). Therefore, we next examined the potential role of JAK-STAT pathway in mediating the CP effects on DRA. We utilized pharmacological inhibitor of JAK and examined the effects of CP infection on DRA mRNA in presence or absence of JAK inhibitor. As shown in Fig. 4, JAK inhibitor alone had no effect on DRA but significantly attenuated the CP infection-induced decrease in DRA mRNA in Caco-2 cells.
Fig. 4.
Role of JAK-STAT pathway in mediating Cryptosporidium parvum (CP) effects on downregulated in adenoma (DRA) mRNA. Postconfluent Caco-2 monolayers grown on 24-well plastic supports were treated with 0.5 × 106 sporozoites/well for 24 h in the presence or absence of JAK inhibitor (30 nM). Relative DRA mRNA was determined by real-time RT-PCR. Results represent means ± SE of 3 separate experiments performed in triplicate. **P < 0.001, compared with control.
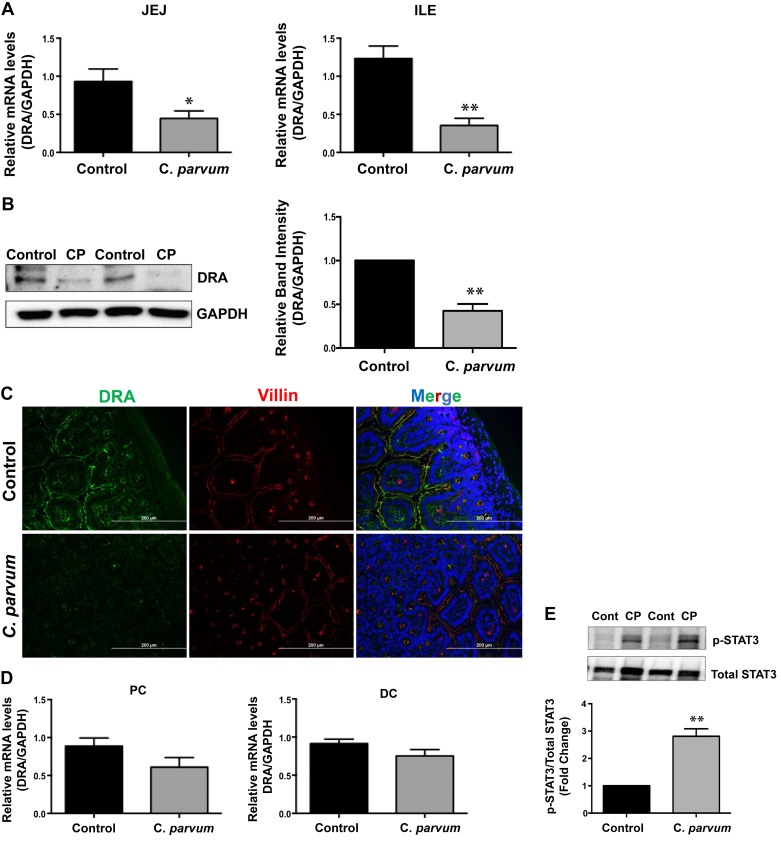
C. parvum infection decreased mouse jejunal and ileal DRA in vivo.
We next utilized in vivo model of CP infection in C57BL/6 mice to examine the effects on the expression of DRA that was substantially downregulated in Caco-2 and T-84 cells and also in mouse enteroid-derived monolayers in response to infection by the parasite. Mice were infected with 1 × 107 oocysts/mouse by oral gavage for 24 and 48 h and 5 days. Similar to our results in vitro and in enteroid-derived monolayers, DRA mRNA was significantly decreased in the mouse jejunum and ileum (Fig. 5A) following CP infection for 24 h, the effect being more pronounced in the ileum. Furthermore, CP infection of mice for 24 h substantially decreased DRA protein levels in the ileum as shown by immunoblotting (Fig. 5B) and immunofluorescence studies (Fig. 5C). On the other hand, there was no significant change in DRA mRNA levels in proximal and distal colon (Fig. 5D). We also observed increased STAT3 phosphorylation in vivo in the ileal mucosa of mice infected with CP for 24 h (Fig. 5E), which could suggest a role of JAK-STAT pathway in mediating CP effects on DRA, as observed in vitro in Caco-2 cells (Fig. 4).
Fig. 5.
Effects of Cryptosporidium parvum (CP) infection on the mRNA and protein levels of downregulated in adenoma (DRA) in mouse small intestine and colon. A: total RNA prepared from scraped mucosa of jejunum (JEJ, left) and ileum (ILE, right) of control or 24 h C. parvum-infected mice were used to measure relative mRNA abundance of DRA by real-time RT-PCR using gene-specific primers. GAPDH was used as an internal control. Values are means ± SE (n = 8; *P < 0.05 vs. control, **P < 0.001 vs. control). B, left: lysates prepared from scraped mucosa of ileum of control or 24 h C. parvum-infected mice were subjected to SDS-PAGE and probed with DRA antibody. B, right: densitometric analysis of relative band intensities with GAPDH as internal control. Values are means ± SE (n = 8; **P < 0.001 vs. control). C: immunofluorescence staining of mucosal sections of ileum of control and 24 h C. parvum-infected mice for DRA (green), villin (red), and DAPI (blue). Representative images of 3 independent experiments are shown. D: relative mRNA abundance of DRA in total RNA samples extracted from scraped mucosa of proximal (PC, left) and distal (DC, right) colon of control or 24 h C. parvum-infected mice was determined by real-time RT-PCR using gene-specific primers. E: C. parvum effects on STAT-3 phosphorylation in mouse ileum. E, top: lysates prepared from scraped mucosa of ileum of control or 24 h C. parvum-infected mice were subjected to SDS-PAGE and probed with STAT-3 antibody. E, bottom: densitometric analysis of relative band intensities with GAPDH as internal control. Values are means ± SE (n = 3; **P < 0.001 vs. control).
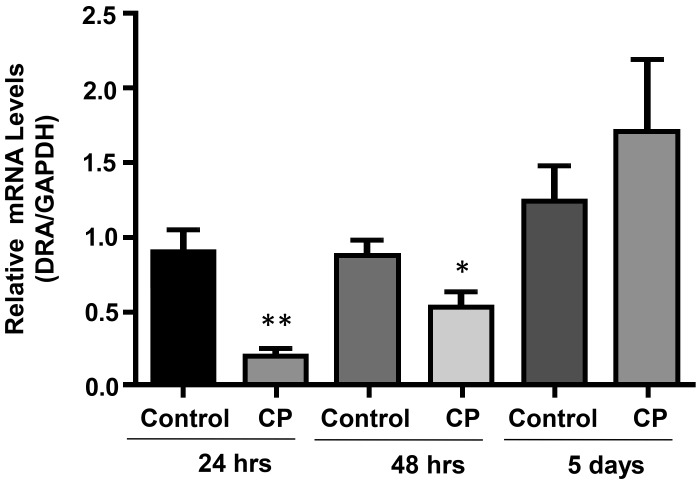
C. parvum effects on DRA in vivo are attenuated at longer infection time.
We next examined the effects of CP infection in vivo for 24 h, 48 h, and 5 days. As shown in Fig. 6, substantial decrease in DRA mRNA in the ileum in response to CP infection for 24 h was attenuated at 48 h postinfection, whereas at 5 days postinfection DRA mRNA level was restored to almost normal level, similar to uninfected controls.
Fig. 6.
Cryptosporidium parvum (CP) effects on ileal downregulated in adenoma (DRA) in vivo are attenuated at longer infection time. Relative mRNA abundance DRA in total RNA samples extracted from scraped mucosa of ileum of control or 24 and 48 h and 5 days C. parvum-infected mice was determined by real-time RT-PCR using gene-specific primers. Values are means ± SE (n = 3; *P < 0.05, **P < 0.001 vs. control).
DISCUSSION
C. parvum, a water-borne intracellular protozoan parasite of the Apicomplexan subfamily, causes acute or recurring diarrhea in children in developing countries, whereas in immunocompromised humans such as HIV patients or patients on immunosuppressant drugs, the parasite is known to cause chronic, profuse, and life-threatening diarrhea (3, 6, 7, 13, 31, 35). However, the pathophysiology of this diarrheal parasite is poorly understood. Our recent studies demonstrated C. parvum infection-induced disruption of barrier function via downregulation of the expression of specific tight junction and adherens junction proteins (23). In the current studies, we have demonstrated that inhibition of chloride absorption via downregulation of DRA (SLC26A3), the key intestinal epithelial apical membrane Cl−/ exchanger protein, could be one of the critical factors contributing to C. parvum-induced diarrhea.
Dysregulated ion and fluid transport is a hallmark of diarrhea. In the mammalian intestine, the major route of NaCl absorption is via coupled operation of NHE3 (SLC9A3) and Cl−/ exchanger DRA (12, 15). Although cryptosporidiosis is a diarrheal disease, there are virtually no studies on ion transport dysregulation in response to infection by this parasite of growing importance. Our current studies, for the first time, demonstrated extensive downregulation of DRA expression in vitro in Caco-2 and T-84 cells with no effects on NHE3 expression. Both cell lines being colon cancer cell lines have limitations as in vitro models to study host-pathogen interactions, as they do not represent normal physiology of a healthy intestinal epithelium. Therefore, we used an ex vivo model of mouse enteroid-derived monolayers and an in vivo mouse model to validate our results on C. parvum infection-induced modulation of DRA expression that showed similar downregulation of DRA following 24 h exposure to C. parvum. Previous studies have demonstrated downregulation of both DRA and NHE3 in diarrheal diseases associated with intestinal inflammation or infection by other pathogens. For example, we have shown extensive downregulation of DRA and NHE3 in murine models of C. rodentium infection (22) that has previously been shown to cause fatal diarrhea in susceptible mice (4). However, we did not observe significant diarrheal phenotype in our current studies following C. parvum infection of immunocompetent C57BL/6 mice. This could suggest induction of specific adaptive host responses in immunocompetent hosts to counteract C. parvum infection-induced dysregulation of ion transport. While DRA expression levels in the mammalian intestine are known to increase from jejunum to distal colon (15), our current studies showed no effects of C. parvum infection on DRA levels in the proximal and distal colon. Therefore, the absence of diarrheal phenotype in our current studies of C. parvum infection in vivo could imply that decreased DRA levels in the small intestine of immunocompetent hosts do not considerably impair fluid homeostasis or that colonic DRA has more critical role in the pathogenesis of diarrheal diseases. Previous studies with human subjects have reported moderate to fulminant diarrhea in response to C. parvum infection in immunocompromised hosts, such as HIV patients (19). Diarrhea has also been reported to be more common and severe in malnourished children (19). Therefore, it will be of critical importance to investigate the role of ion transport dysregulation in the pathophysiology of cryptosporidiosis under immune suppressed or malnourished conditions.
Similar to our current studies showing no effects of C. parvum infection on colonic DRA, we have recently showed that the C. parvum effects in decreasing the expression of specific epithelial tight junction and adherens junction proteins were also primarily confined to ileal and jejunal mucosa with minimal or no changes in the colon (23). This could be partly due to the fact that small intestine is the primary site of human cryptosporidiosis. Extra-intestinal cryptosporidiosis has primarily been reported in AIDS patients with biliary capillaries being the most common extra-intestinal site of infection (26, 38). Few earlier studies also reported infection of gastric mucosa and pancreas by this parasite (19). Recent trials with human subjects have shown increased susceptibility of colorectal cancer patients to Cryptosporidium infection (25, 36). However, this inference was based on increased number of oocysts of the parasite in stool samples of colorectal cancer patients, with no evidence of invasion of the parasite into the colonic mucosa. Therefore, similar to HIV patients, increased susceptibility of colorectal cancer patients to Cryptosporidium infection could also be due to transient or prolonged impairment of the immune system.
Our data also showed a role of JAK-STAT pathway in mediating the parasite effects on DRA expression. Our previous studies have shown IFN-γ inhibition of DRA expression via activation of JAK/STAT pathway (29). Furthermore, our recent study showed significant increase in IFN-γ mRNA levels in Caco-2 cells and in mouse ileum and jejunum at 24 h postinfection with C. parvum (23). In the current study, we also observed an increase in STAT-3 phosphorylation in ileal mucosa following C. parvum infection for 24 h. Taken together, these data suggested a potential role of induction of the JAK-STAT pathway by IFN-γ in mediating C. parvum effects on DRA expression. While IFN-γ has been reported to be of importance in early host response to control C. parvum infection, complete inhibition of the parasite has not been shown to be achieved. Indeed, the parasite itself has been shown to modulate host gene expression via IFN-γ-dependent mechanisms (8).
Cryptosporidium infection of the human with normal immune system commonly causes acute, self-limiting diarrhea. Since the parasite enters via fecal-oral route, however, Cryptosporidium-induced diarrhea in children in developing countries could be recurrent and chronic due to repeated infections resulting from poor hygiene (3). Our current in vivo studies utilizing normal mice also showed that maximum effects in downregulating DRA expression in the ileum were found at 24 h after infection. Inhibition of DRA expression was attenuated at 48 h, whereas at 5 days after infection DRA levels were restored to normal levels. Since DRA is suggested to play a critical role in the pathophysiology of diarrhea, attenuation of the inhibitory effects of the parasite on DRA expression at longer time points appears to be consistent with self-limiting nature of cryptosporidiosis in immunocompetent hosts. It will be of critical importance to examine the effects of C. parvum infection on DRA expression in immunocompromised hosts to further define its role in cryptosporidiosis.
In conclusion, our studies provide the first evidence of impaired chloride absorption in cryptosporidiosis via downregulation of DRA expression and function, which may prove to be an important contributing factor to cryptosporidiosis-related pathology.
GRANTS
These studies were supported by the Department of Veterans Affairs, Veterans Heath Administration, Office of Research and Development, Biomedical Laboratory Research and Development: Merit Review Awards BX002011 (P. K. Dudeja) and BX000152 (W. A. Alrefai); VA Research Career Scientist Award (P. K. Dudeja and W. A. Alrefai); NIH National Institute of Diabetes and Digestive and Kidney Diseases/National Institute of Allergy and Infectious Diseases Grants DK54016, DK81858, DK92441 (P. K. Dudeja), DK109709 (W. A. Alrefai), AI130790 (A. Borthakur); and Bill and Melinda Gates Foundation Grant OPP1058288 (A. Borthakur).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
A.B. and P.K.D. conceived and designed research; A.K., D.J., A.N.A., I.C., S.P., and A.B. performed experiments; A.K., D.J., A.N.A., I.C., S.P., and A.B. analyzed data; A.K., D.J., and A.B. interpreted results of experiments; A.K. and D.J. prepared figures; A.B. drafted manuscript; W.A.A., A.B., and P.K.D. edited and revised manuscript; A.K., A.N.A., I.C., S.P., A.B., and P.K.D. approved final version of manuscript.
REFERENCES
- 1.Anbazhagan AN, Priyamvada S, Alakkam A, Kumar A, Borthakur A, Saksena S, Gill RK, Alrefai WA, Dudeja PK. Transcriptional modulation of SLC26A3 (DRA) by sphingosine-1-phosphate. Am J Physiol Gastrointest Liver Physiol 310: G1028–G1035, 2016. doi: 10.1152/ajpgi.00308.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anbazhagan AN, Priyamvada S, Alrefai WA, Dudeja PK. Pathophysiology of IBD associated diarrhea. Tissue Barriers 6: e1463897, 2018. doi: 10.1080/21688370.2018.1463897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bhalchandra S, Cardenas D, Ward HD. Recent breakthroughs and ongoing limitations in Cryptosporidium research. F1000 Res 7: 1380, 2018. doi: 10.12688/f1000research.15333.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Borenshtein D, Fry RC, Groff EB, Nambiar PR, Carey VJ, Fox JG, Schauer DB. Diarrhea as a cause of mortality in a mouse model of infectious colitis. Genome Biol 9: R122, 2008. doi: 10.1186/gb-2008-9-8-r122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Borenshtein D, Schlieper KA, Rickman BH, Chapman JM, Schweinfest CW, Fox JG, Schauer DB. Decreased expression of colonic Slc26a3 and carbonic anhydrase iv as a cause of fatal infectious diarrhea in mice. Infect Immun 77: 3639–3650, 2009. doi: 10.1128/IAI.00225-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cacciò SM, Chalmers RM. Human cryptosporidiosis in Europe. Clin Microbiol Infect 22: 471–480, 2016. doi: 10.1016/j.cmi.2016.04.021. [DOI] [PubMed] [Google Scholar]
- 7.Checkley W, White AC Jr, Jaganath D, Arrowood MJ, Chalmers RM, Chen XM, Fayer R, Griffiths JK, Guerrant RL, Hedstrom L, Huston CD, Kotloff KL, Kang G, Mead JR, Miller M, Petri WA Jr, Priest JW, Roos DS, Striepen B, Thompson RC, Ward HD, Van Voorhis WA, Xiao L, Zhu G, Houpt ER. A review of the global burden, novel diagnostics, therapeutics, and vaccine targets for cryptosporidium. Lancet Infect Dis 15: 85–94, 2015. doi: 10.1016/S1473-3099(14)70772-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Choudhry N, Korbel DS, Edwards LA, Bajaj-Elliott M, McDonald V. Dysregulation of interferon-γ-mediated signalling pathway in intestinal epithelial cells by Cryptosporidium parvum infection. Cell Microbiol 11: 1354–1364, 2009. doi: 10.1111/j.1462-5822.2009.01336.x. [DOI] [PubMed] [Google Scholar]
- 9.Coffing H, Priyamvada S, Anbazhagan AN, Salibay C, Engevik M, Versalovic J, Yacyshyn MB, Yacyshyn B, Tyagi S, Saksena S, Gill RK, Alrefai WA, Dudeja PK. Clostridium difficile toxins A and B decrease intestinal SLC26A3 protein expression. Am J Physiol Gastrointest Liver Physiol 315: G43–G52, 2018. doi: 10.1152/ajpgi.00307.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cushion MT, Limper AH, Porollo A, Saper VE, Sinai AP, Weiss LM. The 14th International Workshops on Opportunistic Protists (IWOP 14). J Eukaryot Microbiol 65: 934–939, 2018. doi: 10.1111/jeu.12631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Das S, Jayaratne R, Barrett KE. The role of ion transporters in the pathophysiology of infectious diarrhea. Cell Mol Gastroenterol Hepatol 6: 33–45, 2018. doi: 10.1016/j.jcmgh.2018.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dudeja P, Gill RK, Ramaswamy K. Absorption-secretion and epithelial cell function. In: Colonic Diseases, edited by Koch TR. Totowa, NJ: Humana, 2003, p. 3–24. [Google Scholar]
- 13.Efstratiou A, Ongerth JE, Karanis P. Waterborne transmission of protozoan parasites: Review of worldwide outbreaks–an update 2011-2016. Water Res 114: 14–22, 2017. doi: 10.1016/j.watres.2017.01.036. [DOI] [PubMed] [Google Scholar]
- 14.Field M. Intestinal ion transport and the pathophysiology of diarrhea. J Clin Invest 111: 931–943, 2003. doi: 10.1172/JCI200318326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gill RK, Alrefai WA, Borthakur A, Dudeja PK. Intestinal anion absorption. In: Physiology of the Gastrointestinal Tract (5th ed.), edited by Johnson LR, Ghishan FK, Kaunitz JD, Merchant JL, Said HM, Wood JD. London: Elsevier Academic, 2012, p. 1819–1847. [Google Scholar]
- 16.Gill RK, Borthakur A, Hodges K, Turner JR, Clayburgh DR, Saksena S, Zaheer A, Ramaswamy K, Hecht G, Dudeja PK. Mechanism underlying inhibition of intestinal apical Cl/OH exchange following infection with enteropathogenic E. coli. J Clin Invest 117: 428–437, 2007. doi: 10.1172/JCI29625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gujral T, Kumar A, Priyamvada S, Saksena S, Gill RK, Hodges K, Alrefai WA, Hecht GA, Dudeja PK. Mechanisms of DRA recycling in intestinal epithelial cells: effect of enteropathogenic E. coli. Am J Physiol Cell Physiol 309: C835–C846, 2015. doi: 10.1152/ajpcell.00107.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hoque KM, Chakraborty S, Sheikh IA, Woodward OM. New advances in the pathophysiology of intestinal ion transport and barrier function in diarrhea and the impact on therapy. Expert Rev Anti Infect Ther 10: 687–699, 2012. doi: 10.1586/eri.12.47. [DOI] [PubMed] [Google Scholar]
- 19.Hunter PR, Nichols G. Epidemiology and clinical features of Cryptosporidium infection in immunocompromised patients. Clin Microbiol Rev 15: 145–154, 2002. doi: 10.1128/CMR.15.1.145-154.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jayawardena D, Guzman G, Gill RK, Alrefai WA, Onyuksel H, Dudeja PK. Expression and localization of VPAC1, the major receptor of vasoactive intestinal peptide along the length of the intestine. Am J Physiol Gastrointest Liver Physiol 313: G16–G25, 2017. doi: 10.1152/ajpgi.00081.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kotloff KL, Nataro JP, Blackwelder WC, Nasrin D, Farag TH, Panchalingam S, Wu Y, Sow SO, Sur D, Breiman RF, Faruque AS, Zaidi AK, Saha D, Alonso PL, Tamboura B, Sanogo D, Onwuchekwa U, Manna B, Ramamurthy T, Kanungo S, Ochieng JB, Omore R, Oundo JO, Hossain A, Das SK, Ahmed S, Qureshi S, Quadri F, Adegbola RA, Antonio M, Hossain MJ, Akinsola A, Mandomando I, Nhampossa T, Acácio S, Biswas K, O’Reilly CE, Mintz ED, Berkeley LY, Muhsen K, Sommerfelt H, Robins-Browne RM, Levine MM. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): a prospective, case-control study. Lancet 382: 209–222, 2013. doi: 10.1016/S0140-6736(13)60844-2. [DOI] [PubMed] [Google Scholar]
- 22.Kumar A, Anbazhagan AN, Coffing H, Chatterjee I, Priyamvada S, Gujral T, Saksena S, Gill RK, Alrefai WA, Borthakur A, Dudeja PK. Lactobacillus acidophilus counteracts inhibition of NHE3 and DRA expression and alleviates diarrheal phenotype in mice infected with Citrobacter rodentium. Am J Physiol Gastrointest Liver Physiol 311: G817–G826, 2016. doi: 10.1152/ajpgi.00173.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kumar A, Chatterjee I, Anbazhagan AN, Jayawardena D, Priyamvada S, Alrefai WA, Sun J, Borthakur A, Dudeja PK. Cryptosporidium parvum disrupts intestinal epithelial barrier function via altering expression of key tight junction and adherens junction proteins. Cell Microbiol 20: e12830, 2018. doi: 10.1111/cmi.12830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kumar A, Chatterjee I, Gujral T, Alakkam A, Coffing H, Anbazhagan AN, Borthakur A, Saksena S, Gill RK, Alrefai WA, Dudeja PK. Activation of nuclear factor-κB by tumor necrosis factor in intestinal epithelial cells and mouse intestinal epithelia reduces expression of the chloride transporter SLC26A3. Gastroenterology 153: 1338–1350.e3, 2017. doi: 10.1053/j.gastro.2017.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Osman M, Benamrouz S, Guyot K, Baydoun M, Frealle E, Chabe M, Gantois N, Delaire B, Goffard A, Aoun A, Jurdi N, Dabboussi F, Even G, Slomianny C, Gosset P, Hamze M, Creusy C, Viscogliosi E, Certad G. High association of Cryptosporidium spp. infection with colon adenocarcinoma in Lebanese patients. PLoS One 12: e0189422, 2017. doi: 10.1371/journal.pone.0189422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Owen RL. New perspectives on the pathogenesis of Cryptosporidium biliary disease. Hepatology 28: 1159–1160, 1998. doi: 10.1002/hep.510280435. [DOI] [PubMed] [Google Scholar]
- 27.Platts-Mills JA, Babji S, Bodhidatta L, Gratz J, Haque R, Havt A, McCormick BJ, McGrath M, Olortegui MP, Samie A, Shakoor S, Mondal D, Lima IF, Hariraju D, Rayamajhi BB, Qureshi S, Kabir F, Yori PP, Mufamadi B, Amour C, Carreon JD, Richard SA, Lang D, Bessong P, Mduma E, Ahmed T, Lima AA, Mason CJ, Zaidi AK, Bhutta ZA, Kosek M, Guerrant RL, Gottlieb M, Miller M, Kang G, Houpt ER; MAL-ED Network Investigators . Pathogen-specific burdens of community diarrhoea in developing countries: a multisite birth cohort study (MAL-ED). Lancet Glob Health 3: e564–e575, 2015. doi: 10.1016/S2214-109X(15)00151-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ryan U, Hijjawi N. New developments in Cryptosporidium research. Int J Parasitol 45: 367–373, 2015. doi: 10.1016/j.ijpara.2015.01.009. [DOI] [PubMed] [Google Scholar]
- 29.Saksena S, Singla A, Goyal S, Katyal S, Bansal N, Gill RK, Alrefai WA, Ramaswamy K, Dudeja PK. Mechanisms of transcriptional modulation of the human anion exchanger SLC26A3 gene expression by IFN-γ. Am J Physiol Gastrointest Liver Physiol 298: G159–G166, 2010. doi: 10.1152/ajpgi.00374.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sato T, Stange DE, Ferrante M, Vries RG, Van Es JH, Van den Brink S, Van Houdt WJ, Pronk A, Van Gorp J, Siersema PD, Clevers H. Long-term expansion of epithelial organoids from human colon, adenoma, adenocarcinoma, and Barrett’s epithelium. Gastroenterology 141: 1762–1772, 2011. doi: 10.1053/j.gastro.2011.07.050. [DOI] [PubMed] [Google Scholar]
- 31.Shirley DA, Moonah SN, Kotloff KL. Burden of disease from cryptosporidiosis. Curr Opin Infect Dis 25: 555–563, 2012. doi: 10.1097/QCO.0b013e328357e569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shoultz DA, de Hostos EL, Choy RK. Addressing Cryptosporidium infection among young children in low-income settings: the crucial role of new and existing drugs for reducing morbidity and mortality. PLoS Negl Trop Dis 10: e0004242, 2016. doi: 10.1371/journal.pntd.0004242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Singh V, Kumar A, Raheja G, Anbazhagan AN, Priyamvada S, Saksena S, Jhandier MN, Gill RK, Alrefai WA, Borthakur A, Dudeja PK. Lactobacillus acidophilus attenuates downregulation of DRA function and expression in inflammatory models. Am J Physiol Gastrointest Liver Physiol 307: G623–G631, 2014. doi: 10.1152/ajpgi.00104.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Singh V, Raheja G, Borthakur A, Kumar A, Gill RK, Alakkam A, Malakooti J, Dudeja PK. Lactobacillus acidophilus upregulates intestinal NHE3 expression and function. Am J Physiol Gastrointest Liver Physiol 303: G1393–G1401, 2012. doi: 10.1152/ajpgi.00345.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sparks H, Nair G, Castellanos-Gonzalez A, White AC Jr. Treatment of Cryptosporidium: what we know, gaps, and the way forward. Curr Trop Med Rep 2: 181–187, 2015. doi: 10.1007/s40475-015-0056-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sulzyc-Bielicka V, Kuźna-Grygiel W, Kołodziejczyk L, Bielicki D, Kładny J, Stepień-Korzonek M, Telatyńska-Smieszek B. Cryptosporidiosis in patients with colorectal cancer. J Parasitol 93: 722–724, 2007. doi: 10.1645/GE-1025R1.1. [DOI] [PubMed] [Google Scholar]
- 37.VanDussen KL, Marinshaw JM, Shaikh N, Miyoshi H, Moon C, Tarr PI, Ciorba MA, Stappenbeck TS. Development of an enhanced human gastrointestinal epithelial culture system to facilitate patient-based assays. Gut 64: 911–920, 2015. doi: 10.1136/gutjnl-2013-306651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Verdon R, Polianski J, Grodet A, Garry L, Carbon C. Cryptosporidium parvum biliary tract infection in adult immunocompetent and immunosuppressed mice. J Med Microbiol 47: 71–77, 1998. doi: 10.1099/00222615-47-1-71. [DOI] [PubMed] [Google Scholar]
- 39.Ward HD. New tools for cryptosporidium lead to new hope for Cryptosporidiosis. Trends Parasitol 33: 662–664, 2017. doi: 10.1016/j.pt.2017.07.004. [DOI] [PubMed] [Google Scholar]
- 40.Yoo BK, Yanda MK, No YR, Yun CC. Human intestinal epithelial cell line SK-CO15 is a new model system to study Na+/H+ exchanger 3. Am J Physiol Gastrointest Liver Physiol 303: G180–G188, 2012. doi: 10.1152/ajpgi.00069.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]