Abstract
Cl− channels serve as key regulators of excitability and contractility in vascular, intestinal, and airway smooth muscle cells. We recently reported a Cl− conductance in detrusor smooth muscle (DSM) cells. Here, we used the whole cell patch-clamp technique to further characterize biophysical properties and physiological regulators of the Cl− current in freshly isolated guinea pig DSM cells. The Cl− current demonstrated outward rectification arising from voltage-dependent gating of Cl− channels rather than the Cl− transmembrane gradient. An exposure of DSM cells to hypotonic extracellular solution (Δ 165 mOsm challenge) did not increase the Cl− current providing strong evidence that volume-regulated anion channels do not contribute to the Cl− current in DSM cells. The Cl− current was monotonically dependent on extracellular pH, larger and lower in magnitude at acidic (5.0) and basic pH (8.5) values, respectively. Additionally, intracellularly applied phosphatidylinositol 4,5-bisphosphate [PI(4,5)P2] analog [PI(4,5)P2-diC8] increased the average Cl− current density by approximately threefold in a voltage-independent manner. The magnitude of the DSM whole cell Cl− current did not depend on the cell surface area (cell capacitance) regardless of the presence or absence of PI(4,5)P2-diC8, an intriguing finding that underscores the complex nature of Cl− channel expression and function in DSM cells. Removal of both extracellular Ca2+ and Mg2+ did not affect the DSM whole cell Cl− current, whereas Gd3+ (1 mM) potentiated the current. Collectively, our recent and present findings strongly suggest that Cl− channels are critical regulators of DSM excitability and are regulated by extracellular pH, Gd3+, and PI(4,5)P2.
Keywords: chloride, detrusor, ion channel, smooth muscle cell, urinary bladder
INTRODUCTION
Detrusor smooth muscle (DSM) cells are the major cell type that forms the wall of the urinary bladder, a complex organ responsible for repetitive urine storage and voiding cycles. DSM relaxation is critical for urine storage during the bladder-filling phase. In contrast, DSM phasic contractions create a pressure sufficient enough to initiate and maintain urine flow during the bladder-voiding phase (26, 27). Similarly to other muscle cells, DSM cell contraction and relaxation are controlled by the membrane potential via an excitation-contraction coupling mechanism, a process that results in an electrical signal transduction into a muscle contraction (27, 38). In spite of fundamental differences in excitation-contraction mechanisms among skeletal, cardiac, and smooth muscle cells, they all have one common feature: finely orchestrated activities of multiple types of ion channels sensing and controlling membrane potential changes leading to a muscle contraction or relaxation (9, 11, 23, 27, 38).
In DSM cells, several ion channel types have already been identified, and their roles in excitation-contraction coupling have been elucidated (26, 27). Most of them are cationic channels, including K+-, Ca2+-, and Na+-conducting channels [for detailed reviews, see Petkov (26, 27)]. Whereas cation channels remain the most studied in DSM cells, little is known about Cl− channels in this cell type. Most of our knowledge as to the role of Cl− channels comes from results obtained in vascular smooth muscle cells (for a review, see reference 3), which although similar, are not necessarily identical to the DSM cells (26, 27). We recently reported the presence of Cl−-conducting channels in freshly isolated guinea pig DSM cells at single-channel and whole cell levels (37). We documented a strong voltage dependence of the channel with prominent outward rectification with the midpoint of activation at high depolarizing membrane potentials and single-channel conductance of 164 pS (37). The channel was insensitive to intracellular Ca2+ concentrations unlike Ca2+-dependent transmembrane protein-16A/anoctamin-1 (TMEM16A/ANO1) and cGMP and Ca2+-dependent Cl− channels (3, 33, 37). We found that the DSM whole cell Cl− current was inhibited by nonselective Cl− channel blockers 4,4′-diisothiocyano-2,2′-stilbenedisulfonic acid (DIDS) and niflumic acid and had the anionic permeability sequence Cl− > Br− > I−> methanesulfonate (MsO−; 37). Based on these properties, the list of potential molecular candidates for DSM Cl− channels is currently limited to voltage-dependent Cl− channels/antiporters (ClCs) with similar properties (4, 14, 18, 21, 28). Further critical identification of candidates would require examining their sensitivity to extracellular pH and to low osmotic pressure, a surrogate for cell volume regulation. A more complete characterization of Cl− current regulation by extracellular divalent cations and intracellular signaling molecules such as phosphatidylinositol 4,5-bisphosphate [PI(4,5)P2] would greatly advance our present understanding of the roles of Cl− conductance in DSM excitation-contraction coupling and urinary bladder function. A thorough examination of whole cell Cl− current regulation will interrogate our hypothesis that DSM Cl− channels belong to the ClC channel/antiporter family.
Here, we provide strong evidence that whole cell Cl− current outward rectification arises from voltage-dependent gating of Cl− channels rather than the Cl− transmembrane gradient. We also demonstrate negligible sensitivity of Cl− current to osmotic pressure. Importantly, we provide data that the Cl− channel is sensitive to the extracellular pH. Collectively, our findings point to properties of DSM Cl− current that are similar but not identical to known ClC antiporters/channels. Additionally, we demonstrate that the intracellular presence of phosphatidylinositol 4,5-bisphosphate diC8 [PI(4,5)P2-diC8], a PI(4,5)P2 analog, determines the magnitude of the Cl− current resulting in approximately threefold current enhancement over positive membrane potentials independent of the DSM cell membrane potential. We show an intriguing finding that Cl− current magnitude does not depend on DSM cell size regardless of the presence or absence of PI(4,5)P2-diC8. Finally, we find that the Cl− channel has sensitivity to extracellular Gd3+ and La3+ but not to extracellular Ca2+ and Mg2+.
MATERIALS AND METHODS
Animals.
A total of 47 adult male Hartley guinea pigs (strain code 051; Charles River Laboratories) weighing 499–1,136 g (median 930 g, 25th percentile 825 g, and 75th percentile 995 g, N = 47 guinea pigs) were used in this study. Animals were housed at the University of Tennessee Health Science Center (UTHSC) Laboratory Animal Care Unit facility on a 12:12-h light-dark cycle with access to food and water ad libitum. All experiments were carried out in accordance with procedures reviewed and approved by the Institutional Animal Care and Use Committee at UTHSC (protocol no. 17-075.0). Guinea pigs were euthanized humanely by a regulated overdose delivery of isoflurane (Forane; Baxter, Deerfield, IL) followed by thoracotomy.
DSM cell isolation.
DSM tissues were obtained from adult male guinea pigs by following procedures described earlier (29, 37). Guinea pig DSM cells were freshly isolated utilizing a two-step enzymatic digestion with papain and collagenase type II as previously described (37). Nominally Ca2+-free dissection solution (DS) containing (in mM) 80 monosodium glutamate, 55 NaCl, 6 KCl, 10 glucose, 10 2-[4-(2-hydroxyethyl)piperazin-1-yl]ethanesulfonic acid (HEPES), and 2 MgCl2, at pH 7.3 adjusted with NaOH, was used as base solution during all stages of DSM cell isolation. Strips of DSM tissues (mucosa-free), 2–5 mm long and 1–2 mm wide, were placed into a 5-mL plastic tube containing 1 mL of dissecting solution (DS) supplemented with 1 mg/mL bovine serum albumin, 1 mg/mL dithiothreitol, and 1.5 mg/mL papain (Worthington Biochemical, Lakewood, NJ) and incubated for 17–35 min at ~37°C. After the incubation, DSM strips were washed briefly in 1–2 mL ice-cold DS followed by a 17–35-min incubation in DS supplemented with 1 mg/mL bovine serum albumin, 100–200 µM CaCl2, and 2 mg/mL collagenase type II (Sigma-Millipore, St. Louis, MO) at ~37°C. DSM strips were then washed gently several times in 1–2 mL ice-cold DS and triturated with a fire-polished Pasteur pipette until single DSM cells were obtained. DSM cells were either immediately used for experiments or stored on ice (up to 6 h following preparation).
Electrophysiology.
Cl− currents were recorded by using conventional whole cell patch-clamp technique. Data were collected from DSM cells with average capacitance of 46.5 ± 0.9 (SE) pF, smallest and biggest capacitances of 15.1 and 85.5 pF, respectively, and median and 25th and 75th percentile values equal to 46.0, 38.0, and 52.5 pF, respectively (n = 182 cells, N = 47 guinea pigs). DSM cells were plated on the glass bottom of a rectangular recording chamber for at least 45 min resulting in the adhesion of viable cells to the glass bottom. Debris and damaged nonadhesive DSM cells were removed from the recording chamber by a single wash with the control bath solution consisting of (in mM) 150 tetraethylammonium chloride (TEA-Cl), 1.5 MgCl2, 2 CaCl2, 1 GdCl3, 10 HEPES, 1 µM paxilline, and 10 µM nifedipine, pH 7.4 adjusted with TEA-OH. Patch pipettes were fabricated from borosilicate glass with a trough filament (Sutter Instruments, Novato, CA) and were filled with a pipette recording solution consisting of (in mM) 150 TEA-methanesulfonate, 5 EGTA, and 10 HEPES, pH 7.4 adjusted with TEA-OH. In some cases, PI(4,5)P2-diC8, a soluble analog of PI(4,5)P2 (cat. no. 901; CellSignals, Columbus, OH), was added to the pipette solution to investigate the regulatory role of PI(4,5)P2 in whole cell Cl− currents. Whole cell currents were recorded using an Axopatch 200B amplifier (Molecular Devices, San Jose, CA) and filtered at 1 kHz using an in-line four-pole Bessel filter, and data were digitized at 10 kHz using a DigiData 1440A interface (Molecular Devices, San Jose, CA). We used a 3 M KCl-filled agar bridge as an indifferent electrode to limit the effect of external solution exchange on liquid junction potential alteration. All voltages are reported as patch pipette command potentials. The impact of series resistance (corrective) and whole cell capacitance (predictive) on the whole cell currents was compensated by at least 80% with circuitry of the Axopatch 200B amplifier. DSM cells were voltage-clamped at −100 mV (holding potential).
To examine whether Cl− current can be activated by DSM cell swelling, we perfused the entire recording chamber (global perfusion method) with a hypotonic solution (182 mOsm) consisting of (in mM) 70 TEA-Cl, 1.5 MgCl2, 2 CaCl2, 1 GdCl3, 10 HEPES, 1 µM paxilline, and 10 µM nifedipine, pH 7.4 adjusted with TEA-OH. We compared whole cell current recordings with those obtained in control (normotonic) bath solution (347 mOsm), which consisted of (in mM) 70 TEA-Cl, 1.5 MgCl2, 2 CaCl2, 1 GdCl3, 160 mannitol, 10 HEPES, 1 µM paxilline, and 10 µM nifedipine, pH 7.4 adjusted with TEA-OH.
To investigate the role of extracellular pH on Cl− currents, control bath solution pH was adjusted to 8.5 with TEA-OH and to 6.0 and 5.0 with HCl, respectively. The pH values were periodically checked during the experiment to make sure that they remained constant through the experiment. To examine the modulatory effect of divalent cations on the Cl− current, we removed CaCl2 and MgCl2 from the control bath solution and compared whole cell currents with those recorded in the presence of the divalent cations. Similarly, the GdCl3 concentration in the control bath solution was lowered to 50 µM and 1 µM, respectively, to find out whether Gd3+ regulates the Cl− current. In experiments where the effect of lowering La3+ was examined, the control bath solution contained 1 mM La3+ (instead of Gd3+) and was lowered to 1 µM and returned to 1 mM. In all these experiments, modified extracellular solutions were applied via a local perfusion system delivering a quasi-laminal flow of the solution via a small (1 × 1 mm) square glass tube (VitroCom, Mountain Lakes, NJ) positioned in very close proximity to the DSM cell of interest. Such a local perfusion system was effective in changing the bath solution in the vicinity of the cell in <10 s.
All recordings were obtained at room temperature (21–23°C).
Data analyses.
All analyses of whole cell currents were performed using Igor Pro (Wavemetrics, Lake Oswego, OR), Clampfit10 (Molecular Devices, San Jose, CA), or Microsoft Excel 2016 (Microsoft, Redmond, WA) software programs. For the voltage step-induced protocol, whole cell current values were obtained by averaging step currents at the last seven milliseconds of 0.1-s depolarizing pulses ranging from −60 to +130 mV at 10-mV increments. Then, current density was calculated as a ratio of current value versus cell capacitance. For ramp time course protocol recordings, cells were held at −100 mV, and 1-s ramps to +100 mV were delivered every 6 s at a rate of 0.2 mV/ms. We used the ramp protocol to evaluate the effects of hypotonic solution, extracellular pH, extracellular divalent cation, and extracellular Gd3+ on Cl− currents. A fraction of Cl− current change caused by the control solution switching to a modified one was obtained as described previously (37). Specifically, the fraction of the current change was calculated as 1 − I/ICntl, where I is the current amplitude measured at +100 mV of the ramp protocol at the last time point of the modified bath solution application and ICntl is a calculated control current amplitude that takes into consideration a linear component of current rundown. The ICntl is the arithmetic average of the current amplitude at the time point right before a modified bath solution application and the current amplitude at the washout time point equal to the duration of the modified bath solution substitute application (37). The percentile change is the current obtained by multiplying the fraction of Cl− current change by 100%. Positive values of the percentile of change represent the inhibitory effect, and negative values indicate Cl− current increase.
Statistical analyses.
Data were calculated and are presented as mean ± SE (normal/Gaussian distribution) or median with 25th and 75th percentiles (non-Gaussian distribution). Pearson’s correlation coefficient calculation and normality testing were performed using GraphPad Prism (GraphPad Software, La Jolla, CA). Student’s t test (paired or unpaired, as appropriate) was used to test for differences between two groups. A P value <0.05 was considered statistically significant; n describes the number of cells recorded from, and N represents the number of male guinea pigs used. Statistical determinations were made on n values using Microsoft Excel 2016 software.
Chemicals.
Papain was obtained from Worthington Biochemical (Lakewood, NJ). Paxilline was purchased from Alomone Laboratories (Jerusalem, Israel). All other chemicals were purchased from Sigma-Millipore (St. Louis, MO). Stock solutions of nifedipine (10 mM), paxilline (1 mM), and PIP2-diC8 (10 mM) were prepared in dimethyl sulfoxide (DMSO; Sigma-Millipore) and stored at approximately −20°C until use. Stock solutions of GdCl3 (100 mM) and LaCl3 (1 M) were prepared in type I purified water.
RESULTS
Voltage-dependent gating of Cl− channels in DSM cells underlies Cl− current outward rectification.
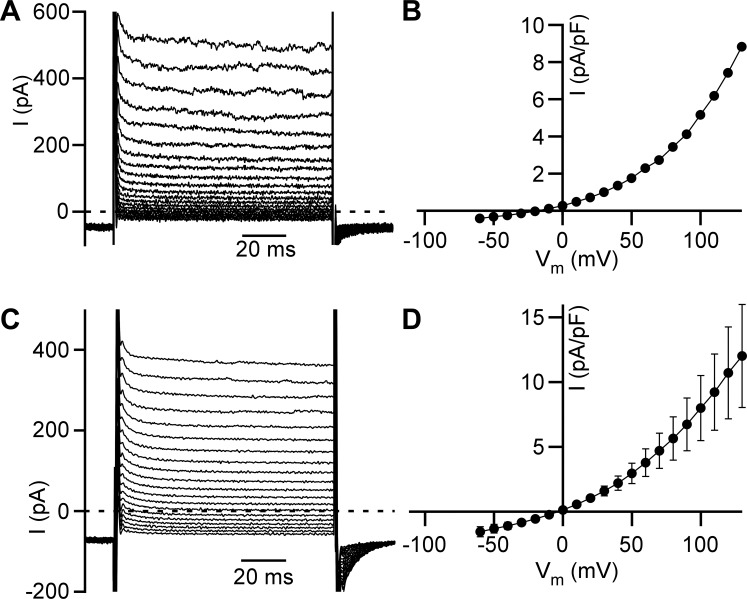
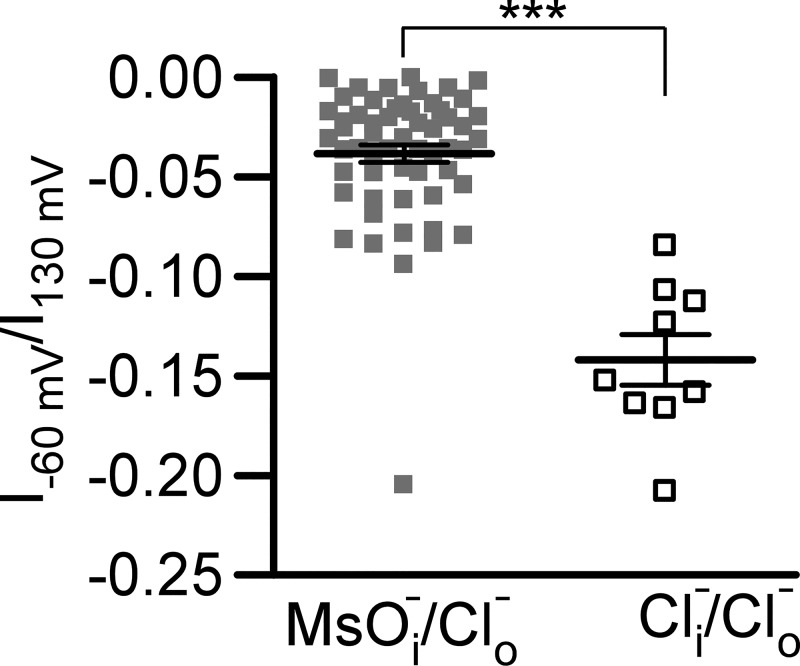
We have recently identified and characterized an outward rectification of the Cl− current in freshly isolated guinea pig DSM cells (37). However, such Cl− current responses (illustrated by representative currents and current density-membrane potential relationships in Fig. 1, A and B, respectively) could be potentially explained by asymmetric Cl− concentrations in the bath (extracellular) and pipette (intracellular) solutions. To investigate the intrinsic nature of the outward rectification of Cl− current, 150 mM TEA-methanesulfonate in the pipette solution was replaced by 150 mM TEA-Cl, and 10 µM PI(4,5)P2-diC8 was also added. We found that the Cl− current preserved the outward rectification in the presence of 150 mM Cl− in the pipette solution (Fig. 1, C and D). The degree of the rectification, calculated as the ratio of the whole cell currents obtained at −60 mV to those obtained at +130 mV, was significantly higher at 0 mM Cl− than at 150 mM Cl−: −0.038 ± 0.004 (n = 57, N = 15) and −0.142 ± 0.013 (n = 9, N = 3, P < 0.001) in the pipette solution, respectively (Fig. 2). However, this difference was small assuming that the loss of rectification would result in values close to 1. Additionally, the reversal potential was close to 0 mV in recordings obtained in the presence of 150 mM Cl− concentration in the pipette (Fig. 1D). Together, these data support a conclusion that Cl− current outward rectification is driven mainly by the voltage-dependent gating of Cl− channels and that Cl− current at physiological membrane potentials may play a role in the control of DSM cell excitability.
Fig. 1.
Cl− channel voltage-dependent gating results in Cl− current outward rectification in detrusor smooth muscle (DSM) cells. A: representative traces of voltage step-induced Cl− currents (I) in DSM cells. Pipette and control bath solutions contained 0 and 160 mM Cl−, respectively. B: current density-membrane potential (Vm) relationship shown for the cell in A. Currents were measured at the end of 100-ms voltage steps. C: representative traces of voltage step-induced Cl− currents in DSM cells in the presence of 150 mM Cl− in the pipette and 160 mM Cl− in the control bath solutions, respectively (n = 57 cells, N = 15 guinea pigs). D: current density-membrane potential relationship obtained as in B in the presence of 150 mM Cl− in the pipette solution and 160 mM Cl− in the control bath solution (n = 9, N = 3).
Fig. 2.
Cl− transmembrane gradient facilitates the outward rectification of Cl− current. A distribution of ratios of Cl− current amplitudes measured at −60 mV (I−60 mV) to those measured at +130 mV (I130 mV) serves as a quantitative comparison of the outward rectification. Values closer to zero support increased outward rectification. Data points obtained in the presence of asymmetrical Cl− solutions [gray squares, n = 57 cells, N = 15 guinea pigs, 150 mM methanesulfonate (MsO−) and 0 mM Cl− in the intracellular solution and 160 mM Cl− in the control extracellular solution] are compared with those obtained in the presence of Cl− in both intracellular and extracellular solutions [open squares, n = 9, N = 3, 150 and 160 mM Cl− in the intracellular pipette and extracellular control solutions, respectively (/)]. Horizontal lines denote means ± SE for each group. ***Statistically significant difference between these two groups (P < 0.001, unpaired Student’s t test).
Cl− current in DSM cells does not increase at low osmotic pressure (cell swelling).
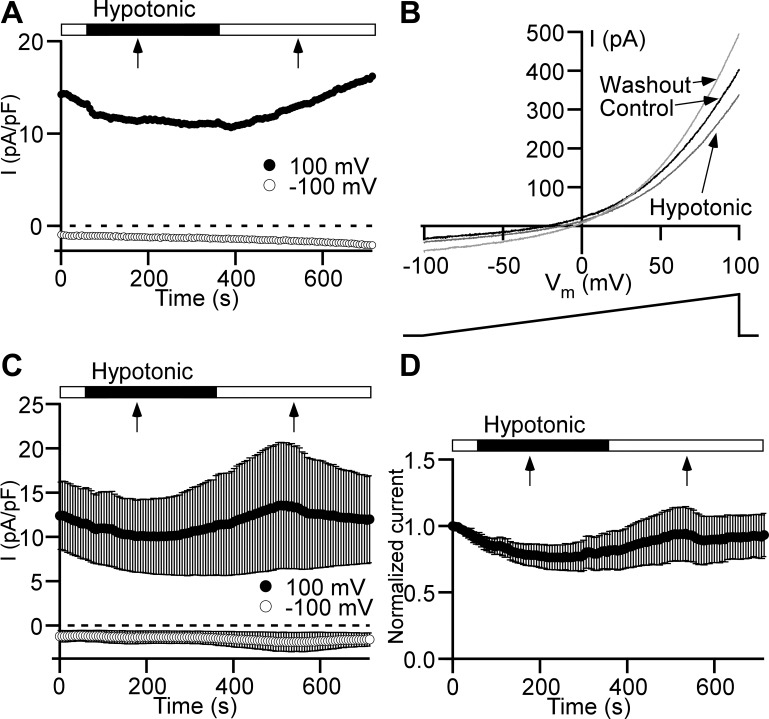
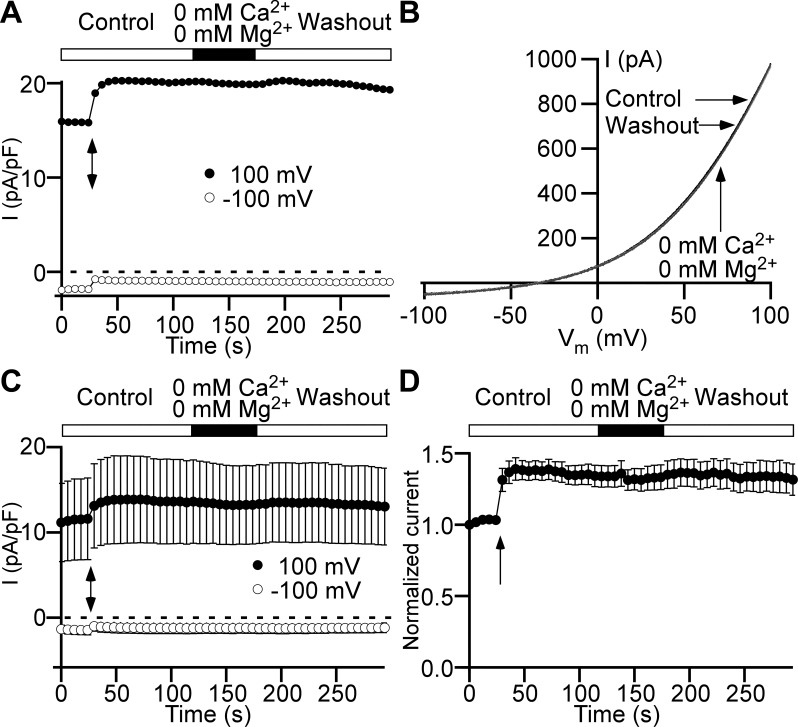
Our recent findings indicate that ClC antiporters are the most likely molecular candidates for the Cl− channels in the plasma membrane of DSM cells (37). ClC-3 channels are thought to be volume regulated, and that cell swelling leads to the activation of these channels (3). Even though the anion permeability profile did not support the contribution of ClC-3 to whole cell Cl− current (Table 1) in DSM plasma membrane (37), these channels may still provide a minor component to the total Cl− current at normotonic conditions. Therefore, we used hypotonic extracellular solution to induce DSM cell swelling and evaluate how the cell volume increase affects Cl− current change. We found that switching from the normotonic (347 mOsm) to the hypotonic solution (182 mOsm) for 5 min did not cause Cl− current increase (Fig. 3). Thus, based on these new data we now rule out all volume-regulated anion channel types, including ClC-3, as potential candidates for Cl− channels in DSM cells.
Table 1.
Summary of properties of ClC channels/antiporters
| Cl− Channel Type | Sensitivity to DIDS | Sensitivity to Niflumic Acid | Permeability Sequence | Sensitivity to Extracellular pH |
|---|---|---|---|---|
| ClC-3 | Yes (1, 7, 8, 36) No (20) |
Yes (1, 5, 12) No (low concentrations) (7) |
I− > Br− > Cl− > gluconate (35) I− > Cl− (8) Cl− > I− (20) SCN− > I− > Br− > Cl− >> aspartate (36) |
pH↓ to 6.35 → ↓ pH↓ to 5.5 → ↑ (21, 22) pH↓ to 6.6 → ↑ pH↓ to 5.8 → no change in (35) |
| ClC-4 | Yes (17) | Yes (15) | I− > Cl− > F− (17) I− = > Br− > Cl− (15) I− >> Br− = Cl− > (15) > Cl− > Br− > I− (10, 15) SCN− >> >> Cl− > Br− ≈ ≈ I− >> aspartate (34) |
pH↓ → ↓ (10, 15, 25, 34) pH↓ → ↑ (15, 17) |
| ClC-5 | No (31) | No (31) |
> Cl− > Br− > I− >> glutamate (31) > Cl− > Br− > I− (10) |
pH↓ → ↓ (6, 10, 28) |
| ClC-6 | Yes (24) | N/A |
> I− > Br− > Cl− > gluconate (4) Cl−>I− (24) |
N/A |
| ClC-7 | Yes (18) | Yes (18) | Cl− > Br− > > I− > gluconate (19) > Cl− > Br− > I− > MsO− (13)* |
pH↓ → ↑ (18) pH↓ → ↓ (19) |
| DSM Cl− channel | Yes (37) | Yes (37) | Cl− > Br− > I− > MsO− (37) | pH↓ → ↑ (present paper) |
In the pH sensitivity column, vertical arrows indicate the direction of pH or Cl− current () change, and horizontal arrows link pH change to the current alteration. ClC, voltage-dependent Cl− channel/antiporter; DIDS, 4,4′-diisothiocyano-2,2′-stilbenedisulfonic acid; DSM, detrusor smooth muscle; MsO−, methanesulfonate; N/A, not available; SCN−, thiocyanate.
Data obtained from Cl− flux assay (13).
Fig. 3.
Low osmotic pressure does not cause Cl− current increase in detrusor smooth muscle cells. A: time course of Cl− current (I) measured with a ramp protocol at +100 mV (●) and −100 mV (○). Hypotonic solution (182 mOsm) was applied via a global perfusion system. The flow started at the beginning of the closed horizontal bar. The flow stopped at the time point indicated by a black arrow. The hypotonic solution application was switched back to the normotonic solution starting at the beginning of the following open horizontal bar. The flow stopped at the time moment indicated by a black arrow. B: current-membrane potential (Vm) relationships obtained from the ramp time course protocol shown in A at the following time points: immediately before starting global perfusion with hypotonic solution (Control, black trace), at the end of the 5-min hypotonic solution application (Hypotonic, dark gray trace), and 5 min after switching back to the normotonic solution (Washout, light gray trace). Arrows link traces with conditions under which they were recorded. Ramp voltage stimulus from −100 to +100 mV and 1-s duration is shown at bottom. C: Cl− current time courses (n = 6 cells, N = 4 guinea pigs) measured at +100 mV (●) and −100 mV (○). All other symbols and marks have the same meaning as in A. D: time course normalized to the first time point of the Cl− current measured at +100 mV shows no statistically significant effect of hypotonic solution (n = 6, N = 4, P > 0.05, paired Student’s t test). All other symbols and marks have the identical meaning as in A and C.
Extracellular pH modulates Cl− current in DSM cells.
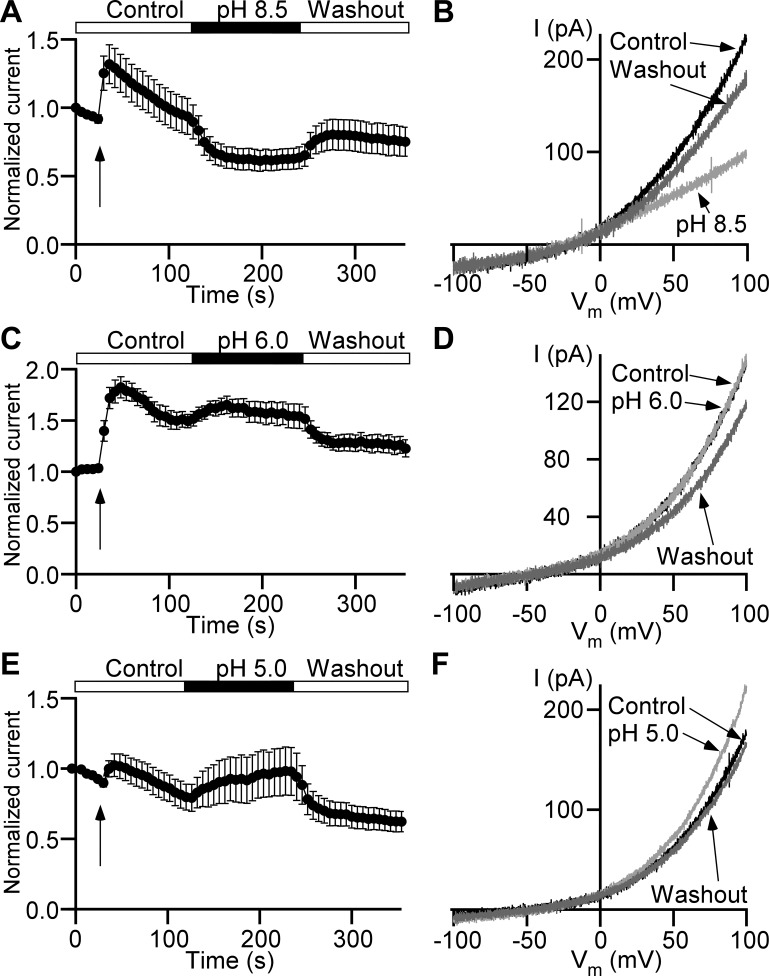
Despite similarities, ClC channels/antiporters demonstrate type-specific responses to alterations in external pH (16). Here we examined the role of extracellular pH in Cl− current regulation by using the ramp time course protocol. The Cl− current measured at the end of the voltage ramp (+100 mV) showed initial increase in all recordings at the onset of the local perfusion (Fig. 4, A, C, and E). We found that switching from the control bath solution with pH 7.4 to a solution with pH 8.5 caused a reversible current decrease of 22.4 ± 4.9% (n = 11, N = 3, P < 0.05; Fig. 4, A and B). Interestingly, the change of the external pH from 7.4 to 6.0 caused a nonsignificant increase of 13.6 ± 8.0% (n = 8, N = 3, P = 0.14; Fig. 4, C and D), but further acidification of the bath solution to pH 5.0 caused a significant increase of 32.4 ± 10.9% (n = 11, N = 3, P < 0.05; Fig. 4, E and F). These data suggest that the Cl− current in DSM cells has a monotonic dependence on the external pH: higher magnitude at pH 5.0 and lower magnitude at pH 8.5.
Fig. 4.
Extracellular pH modulates Cl− current in detrusor smooth muscle cells. A, C, and E: time courses of Cl− currents normalized to their first data points measured at +100 mV. Arrows indicate time points of local perfusion start. The recording begins in control bath solution with pH 7.4 followed by the isochronic application of a solution with pH 8.5 (n = 11 cells, N = 3 guinea pigs; A), pH 6.0 (n = 8, N = 3; C), or pH 5.0 (n = 11, N = 3; E) and return to pH 7.4 (Washout). B, D, and F: representative current (I) recordings evoked by ramps at time points of control (Control, black trace) right before switching to the bath solution with pH 8.5 (B), pH 6.0 (D), or pH 5.0 (F) at the end of modified pH bath solution applications (light gray trace), and same as pH 8.5 (B), pH 6.0 (D), or pH 5.0 (F) application long-duration washout (Washout, dark gray trace). Vm, membrane potential.
PI(4,5)P2-diC8 potentiates Cl− current in DSM cells.
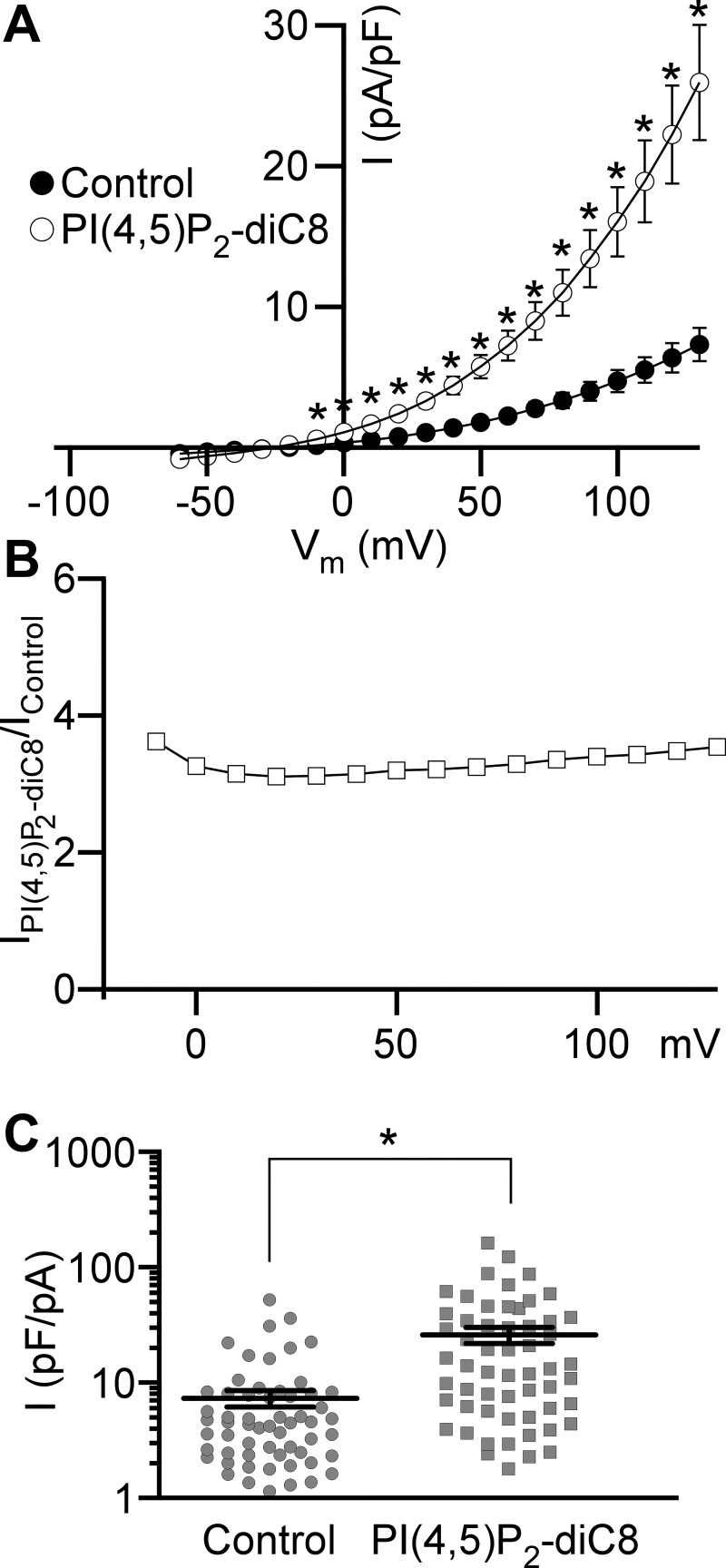
PI(4,5)P2-diC8, an analog of PI(4,5)P2, a regulatory molecule involved in intracellular Ca2+ signaling, was used to investigate whether Cl− channels in DSM cells are regulated by PI(4,5)P2. We found that the intracellular addition of 10 µM PI(4,5)P2-diC8 to the pipette solution (n = 57, N = 15) increased Cl− current density by approximately threefold compared with the control without PI(4,5)P2-diC8 at each positive voltage examined (n = 59, N = 19, P < 0.001; Fig. 5). This illustrates that potentiation of Cl− current was largely voltage independent. The voltage-independent effect of PI(4,5)P2-diC8 indicates that PI(4,5)P2-diC8 likely has a different regulatory role for DSM Cl− channels than for other ion channels (32).
Fig. 5.
Phosphatidylinositol 4,5-bisphosphate diC8 [PI(4,5)P2-diC8] increases Cl− current in detrusor smooth muscle cells. A: whole cell current density (I)-membrane potential (Vm) relationships obtained using voltage step protocol for control and PI(4,5)P2-diC8 applied intracellularly via the patch pipette. Closed and open circles indicate the current densities obtained in the absence (n = 59 cells, N = 19 guinea pigs) or presence (n = 57, N = 15) of 10 µM PI(4,5)P2-diC8, respectively. *Membrane potentials at which two groups are significantly different (P < 0.001, unpaired Student’s t test). B: voltage dependence of a ratio of the average current obtained in the presence of PI(4,5)P2-diC8 (IPI(4,5)P2-diC8) to the average current recorded under control conditions (IControl). C: distributions of current densities measured at the end of 100-ms voltage step from −100 to +130 mV. Circles represent the control group of current densities, and squares depict current densities obtained in the presence of PI(4,5)P2-diC8 in the pipette solution. Mean ± SE values for each group are shown as horizontal lines. *Statistically significant difference (P < 0.001, unpaired Student’s t test).
Cl− current does not depend on DSM cell size.
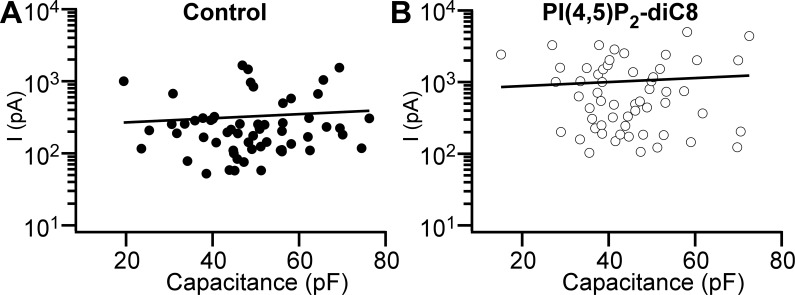
It is expected that larger cells have more ion channels generating larger whole cell current upon activation. In other terms, the Cl− current magnitude should correlate with the DSM cell capacitance. We found, however, that in DSM cells, whole cell Cl− currents paradoxically did not depend on cell capacitance (Fig. 6A). The Pearson’s correlation coefficient for currents caused by step depolarization to +130 mV versus cell capacitance was r = 0.072 (n = 59, N = 19, P = 0.587), suggesting an extremely weak linear association between DSM cell size and the Cl− current magnitude. One explanation is that a sizable fraction of Cl− channels were “silent” at our experimental conditions. If this is true, it would increase the variability for Cl− current amplitude. DSM cells patched with PI(4,5)P2-diC8 added to the pipette solution (Fig. 5)—while showing more than threefold larger currents—also displayed a negligible linear relationship between the Cl− current amplitude and DSM cell capacitance with a Pearson’s correlation coefficient of r = 0.069 (n = 57, N = 15, P = 0.608; Fig. 6B) arguing against the presence of silent Cl− channels in DSM cells.
Fig. 6.
Cl− current does not depend on detrusor smooth muscle (DSM) cell capacitance (cell size). A and B: Cl− currents (I) measured at the end of 100-ms voltage step from −100 to +130 mV plotted against DSM cell capacitance. The solid line represents a linear fit of data points with slope coefficient 2.1 pA/pF in control (A) and 6.6 pA/pF in the presence of 10 μM phosphatidylinositol 4,5-bisphosphate diC8 [PI(4,5)P2-diC8] (B). Data obtained under control conditions (n = 59 cells, N = 19 guinea pigs) are shown in A. Data recorded with PI(4,5)P2-diC8 added to the pipette solution (n = 57, N = 15) are shown in B.
Cl− current does not depend on the presence of external Ca2+ and Mg2+.
Factors contributing to the Cl− current variability in DSM cells and its independence of the cell size could also include a potential inhibition by extracellular divalent cations present in the recording solution. We used the ramp time course protocol to verify whether the simultaneous removal of 2 mM Ca2+ and 1.5 mM Mg2+ from the control bath solution affects the Cl− current. We found that Cl− current did not change in the simultaneous absence of both divalent cations (n = 7, N = 3, P > 0.05; Fig. 7).
Fig. 7.
At physiological concentrations, extracellular Ca2+ and Mg2+ do not affect Cl− current in detrusor smooth muscle cells. A: representative time courses of currents (I) elicited by 1-s ramps from −100 to +100 mV and measured at +100 mV (●) and −100 mV (○) show no effect of extracellular 2 mM Ca2+ and 1.5 mM Mg2+ removal from the control bath solution on Cl− current. The arrow indicates the starting point of local perfusion. The closed horizontal bar shows the duration of a local perfusion with divalent ion-free bath solution (0 mM Ca2+ and 0 mM Mg2+). B: current-membrane potential (Vm) relationships obtained from the ramp time course protocol shown in A at the following time points: immediately before the local application of divalent ion-free bath solution (Control, black trace), at the end of the application (0 mM Ca2+ and 0 mM Mg2+, black trace), and the same-duration divalent-free solution washout (Washout, light gray trace). Arrows link traces with conditions under which they were recorded. C: Cl− current time courses (n = 7 cells, N = 3 guinea pigs) measured at +100 mV (●) and −100 mV (○). All other symbols and marks have the identical meaning as in A. D: time course of the Cl− current normalized to its first data point measured at +100 mV shows no statistically significant difference in the effect of Ca2+ and Mg2+ removal from the bath solution (n = 7, N = 3, P > 0.05, paired Student’s t test). All other symbols and marks have the identical meaning as in A and C.
Gd3+ and La3+ increase Cl− current at high (1 mM) concentration.
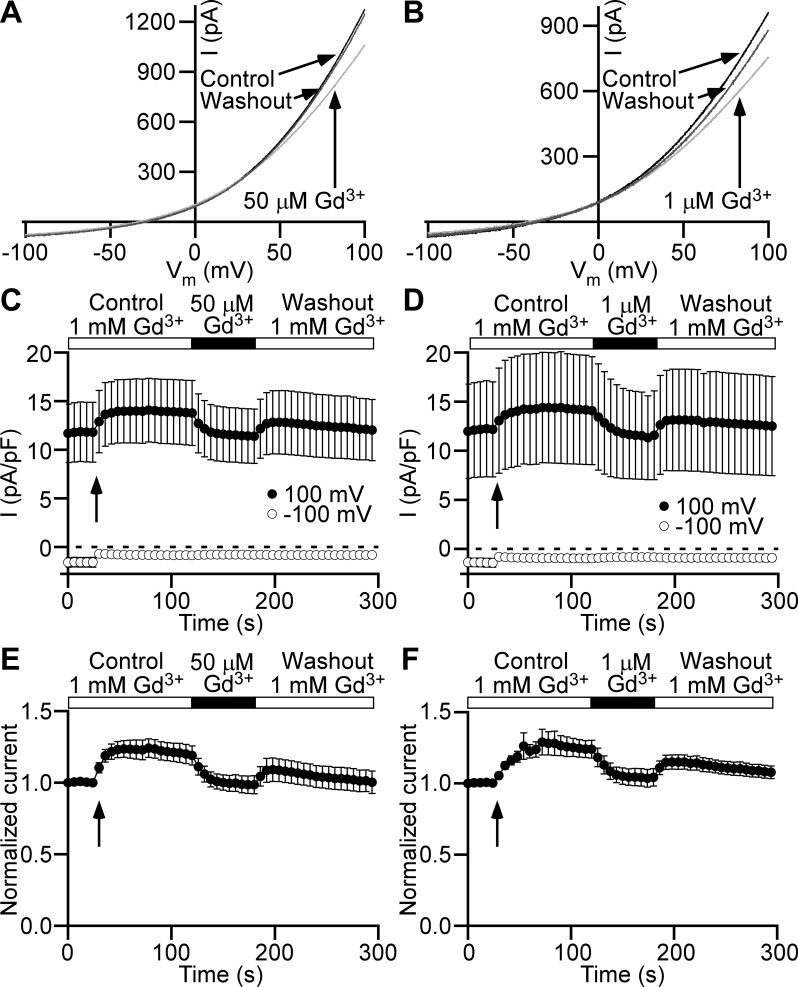
Further, we wanted to find out whether extracellular Gd3+ at 1 mM modulates Cl− current, since at this concentration Gd3+ was used in the control bath solution throughout all experiments described so far. We utilized the ramp time course protocol for the testing of the effect of reduction of extracellular Gd3+ from 1 mM to 1 or 50 µM on the Cl− current. Surprisingly, both applications of very low Gd3+ concentrations decreased Cl− current in a fully reversible manner (Fig. 8). We found that lowering Gd3+ concentration from 1 mM to 1 µM decreased Cl− current measured at +100 mV of the ramp by 12.8 ± 2.3% (n = 7, N = 3, P < 0.05; Fig. 8, B, D, and F), and the decrease to 50 µM caused a 12.5 ± 1.1% reduction in Cl− current (n = 9, N = 3, P < 0.05; Fig. 8, A, C, and E). Hence, the fraction of Cl− current decrease in the presence of 1 µM Gd3+ was not significantly different from the decrease caused by 50 µM Gd3+ (P > 0.05). Upon return to 1 mM GdCl3 from either 1 or 50 µM, there was a substantial recovery in the Cl− current close to the control values at 1 mM Gd3+ (n = 7, N = 3, P < 0.05 and n = 9, N = 3, P < 0.05, respectively). In additional experiments, Gd3+ was replaced with La3+, and the effect of the reduction to 1 µM was determined. The normalized Cl− current showed a decrease of 19.8 ± 3.6 upon La3+ reduction to 1 µM, which was reversible upon return to 1 mM (n = 6, N = 2, P < 0.05; Supplemental Fig. S1; Supplemental Material is available online at https://doi.org/10.6084/m9.figshare.9816875.v1). Therefore, we conclude that high Gd3+ and La3+ concentrations (1 mM) increase the Cl− current.
Fig. 8.
Extracellular Gd3+ increases Cl− current in detrusor smooth muscle cells. A and B: representative current (I)-membrane potential (Vm) relationships obtained from 1-s ramp stimuli from −100 to +100 mV show fully reversible current decrease when extracellular 1 mM Gd3+ concentration (Control, black trace) was reduced to 50 µM (light gray trace in A) or 1 µM (light gray trace in B). Washout of 50 µM Gd3+ (A) or 1 µM Gd3+ (B) traces (Washout, dark gray trace) are shown for time points of recovery equal to the duration of applications of bath solutions with reduced Gd3+ concentrations. Arrows link traces with conditions under which they were obtained. C and D: Cl− current time courses measured at +100 mV (●) and −100 mV (○). Closed horizontal bars show durations of a local perfusion with a bath solution containing Gd3+ at concentrations 50 µM (C) or 1 µM (D). Gd3+ concentration at control conditions (Control) and recovery (Washout) was equal to 1 mM. Arrows indicate the beginning of the local perfusion. E and F: Cl− current time courses normalized to the first time point measured at +100 mV show statistically significant Cl− current decrease upon bath Gd3+ concentration reduction from 1 mM to 50 µM (n = 9 cells, N = 3 guinea pigs, P < 0.05, paired Student’s t test) (E) or 1 µM (n = 7, N = 3, P < 0.05, paired Student’s t test) (F). All other symbols and marks in E and F have the identical meaning as in C and D, respectively.
DISCUSSION
This study used the whole cell patch-clamp technique to further characterize properties of plasma membrane Cl− currents in freshly isolated guinea pig DSM cells that we recently reported (37). Our data showed that voltage-dependent Cl− current had prominent outward rectification and was sensitive to the extracellular pH, high concentrations of Gd3+ and La3+, and PI(4,5)P2-diC8, a stable PI(4,5)P2 analog. We also found that the Cl− current was insensitive to extracellular Ca2+ and Mg2+ and did not increase in hypotonic bath solution, which was used to mimic a cell volume increase.
Our group is the first to have detected and characterized the presence of Cl− channels in freshly isolated guinea pig DSM cells (37). These channels generate voltage-dependent Cl− current with robust outward rectification. The outward rectification of Cl− current could arise from intrinsic voltage-dependent gating of Cl− channels in DSM cells and/or asymmetrical Cl− concentrations in the bath (160 mM) and pipette (0 mM) solutions. Such asymmetry supports only Cl− influx that causes or adds a substantial component to the outwardly rectifying appearance of the Cl− current-membrane potential relationship. Here, we showed that increasing intracellular Cl− concentration from 0 to 150 mM did not eliminate the outward rectification. Thus, our conclusion is that Cl− channel voltage-dependent gating is the major contributor to the outward rectification of the Cl− current. Further, Cl− channels in the plasma membrane of DSM cells must possess a voltage-sensing structure able to generate gating current upon depolarization, as was observed for the ClC-5 channel/antiporter (39).
As we hypothesized recently, voltage-dependent Cl− channels belonging to the ClC family are the most likely candidates for the molecular identity of DSM Cl− channels (37). On the basis of their robust voltage dependence, we initially considered ClC-3, ClC-4, ClC-5, ClC-6, and ClC-7 channels/antiporters as major candidates. The ClC-3 channel/antiporter anion permeability sequence made it the least likely candidate for DSM Cl− current (Table 1; 37). This channel/antiporter is a strong candidate for a volume-regulated anion channel in smooth muscle cells (for review, see reference 3). Thus, hypotonic conditions (cell swelling) should evoke currents via ClC-3 channels, if present (1, 8, 36). Alternatively, unidentified volume-regulated anion channels expressed in the plasma membrane (20) at low levels might also contribute (for review, see reference 16; Table 1). To find out whether volume-regulated anion channels contribute to the Cl− current in the DSM cells, we conducted a series of experiments, which demonstrated no increase of Cl− current under hypotonic conditions, confirming that volume-regulated anion channels, including ClC-3 channels, are not a part of the DSM whole cell Cl− current.
Depending on ClC channel/antiporter type, responses to extracellular pH changes vary uniquely. We observed a monotonic dependence of the Cl− current on extracellular pH, with larger and lower currents under the acidic and basic extracellular environment, respectively. By combining literature findings with our recently reported observations (see Table 1), collectively our data suggest that none of the known ClC channel/antiporter types perfectly fit the DSM Cl− channel profile in terms of anion selectivity and pH sensitivity. Indeed, a consensus has been established now that heterologously expressed ClC-5 and ClC-7 channels can be inhibited by extracellular acidic pH (19, 28). Additionally, the ClC-7 channel/antiporter is mainly localized in lysosomes, and osteoclastogenesis-associated transmembrane protein-1 (Ostm1) serves as an obligatory β-subunit that stabilizes the structure of this channel/antiporter (for review, see reference 16). ClC-7 NH2-terminal leucine-based sorting motifs function in the lysosomal targeting of the ClC-7 channel/antiporter precluding its expression in the plasma membrane (30). Therefore, the presence of the ClC-7 channel in the plasma membrane of DSM cells seems to be very unlikely. The ClC-6 channel/antiporter remains currently one of the least studied. It localizes predominantly in vesicles of the endolysosomal system (for review, see reference 16). However, by tagging the NH2 terminus of ClC-6 channel/antiporter with green fluorescent protein (GFP), a small fraction of GFP-ClC-6 channel/antiporter could reach the plasma membrane in CHO cells or in Xenopus laevis oocytes. Our detailed electrophysiological study revealed that the ClC-6 anion selectivity sequence does not match that of guinea pig DSM cells excluding this subtype as a candidate (Table 1).
We found that PI(4,5)P2-diC8, an analog of the intracellular signaling molecule PI(4,5)P2, potentiated the whole cell Cl− current in DSM cells threefold in a voltage-independent manner. PI(4,5)P2 is well known for its ability to modulate several types of cationic channels, including voltage-gated K+, Na+, and Ca2+ channels, transient receptor potential channels, as well as intracellular cation channels (for review, see reference 32). It was recently reported that PI(4,5)P2 may be involved in a potentiation of Cl− current caused by activation of the Ano6 (TMEM16F) channel in Caco-2 cells (2). Thus, our new finding that PI(4,5)P2-diC8 potentiates the Cl− current in DSM cells complements the emerging body of evidence that PI(4,5)P2 modulates anion channels as well. As in the case of cation channels, the mechanism of modulation by PI(4,5)P2 is likely channel specific and has yet to be elucidated. The voltage-independent potentiation of the Cl− current in DSM cells in the presence of PI(4,5)P2-diC8 indicates that this signaling molecule likely increases the number of active channels rather than affecting the gating mechanisms of the Cl− channel.
We determined that whole cell Cl− current did not depend on DSM cell capacitance. These results were obtained both in the presence or absence of PI(4,5)P2-diC8, a putative potentiator of whole cell Cl− currents. The lack of correlation between the current and cell size along with a high degree of variability in Cl− current magnitude could be due to several reasons: ion channel distribution in clusters rather than uniformly in the plasma membrane resulting in roughly the same number of Cl− channel clusters in smaller and larger DSM cells; a partial blockade by extracellular Ca2+, Mg2+, Gd3+, or unknown intracellular membrane-bound regulatory molecules; DSM cell heterogeneity (bladder location); nonoptimal voltage-dependent activation conditions; and partial cell damage during the enzymatic isolation of single DSM cells. Here, we examined whether extracellular Ca2+ and Mg2+ or Gd3+ could be the cause of the cell size-independent Cl− current. We found that divalent cations had no effect on the Cl− current and that Gd3+ potentiated the current at a high concentration (1 mM). The effect of Gd3+ on Cl− current is unlikely to be due to the plasma membrane surface charge screening, because we did not observe any verifiable voltage shifts in the Cl− current-voltage relationships. Interestingly, La3+ mimicked the potentiating effect of Gd3+ on DSM Cl− currents (see Supplemental Fig. S1). Therefore, some other factor underlies DSM cell size-independent Cl− current.
The isolated single-cell model used here has several limitations relating to the experimental approach utilized. Such limitations include temperature (patch-clamp experiments were done at room temperature), enzymatically obtained single DSM cells lacking cell-cell interactions, species specificity, etc. Follow-up studies at single-cell patch-clamp, isolated DSM strip contractility, isolated whole bladder, and in vivo animal levels are required to further clarify the physiological role of Cl− channels in the control of bladder function.
In conclusion, our study significantly expands the current knowledge about the regulatory mechanisms of Cl− channels in freshly isolated guinea pig DSM cells. This channel is regulated by intracellular PI(4,5)P2 and extracellular pH and trivalent cations (Gd3+ and La3+). The Cl− channel may be a unique critical regulator of DSM cell excitability and, hence, contractility.
GRANTS
This research was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant R01 DK-106964 awarded to G. V. Petkov.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
V.Y., J.M., and G.V.P. conceived and designed research; V.Y. performed experiments; V.Y. analyzed data; V.Y., J.M., and G.V.P. interpreted results of experiments; V.Y. prepared figures; V.Y. drafted manuscript; V.Y., J.M., and G.V.P. edited and revised manuscript; V.Y., J.M., and G.V.P. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Sarah Maxwell for critical evaluation of the manuscript.
REFERENCES
- 1.Al-Nakkash L, Iserovich P, Coca-Prados M, Yang H, Reinach PS. Functional and molecular characterization of a volume-activated chloride channel in rabbit corneal epithelial cells. J Membr Biol 201: 41–49, 2004. doi: 10.1007/s00232-004-0706-5. [DOI] [PubMed] [Google Scholar]
- 2.Aoun J, Hayashi M, Sheikh IA, Sarkar P, Saha T, Ghosh P, Bhowmick R, Ghosh D, Chatterjee T, Chakrabarti P, Chakrabarti MK, Hoque KM. Anoctamin 6 contributes to Cl− secretion in accessory cholera enterotoxin (Ace)-stimulated diarrhea: an essential role for phosphatidylinositol 4,5-bisphosphate (PIP2) signaling in cholera. J Biol Chem 291: 26816–26836, 2016. doi: 10.1074/jbc.M116.719823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bulley S, Jaggar JH. Cl− channels in smooth muscle cells. Pflügers Arch 466: 861–872, 2014. [Erratum in Pflügers Arch 466: 873, 2014.] doi: 10.1007/s00424-013-1357-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buyse G, Voets T, Tytgat J, De Greef C, Droogmans G, Nilius B, Eggermont J. Expression of human pICln and ClC-6 in Xenopus oocytes induces an identical endogenous chloride conductance. J Biol Chem 272: 3615–3621, 1997. doi: 10.1074/jbc.272.6.3615. [DOI] [PubMed] [Google Scholar]
- 5.Coca-Prados M, Sánchez-Torres J, Peterson-Yantorno K, Civan MM. Association of ClC-3 channel with Cl− transport by human nonpigmented ciliary epithelial cells. J Membr Biol 150: 197–208, 1996. doi: 10.1007/s002329900044. [DOI] [PubMed] [Google Scholar]
- 6.De Stefano S, Pusch M, Zifarelli G. A single point mutation reveals gating of the human ClC-5 Cl−/H+ antiporter. J Physiol 591: 5879–5893, 2013. doi: 10.1113/jphysiol.2013.260240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dick GM, Bradley KK, Horowitz B, Hume JR, Sanders KM. Functional and molecular identification of a novel chloride conductance in canine colonic smooth muscle. Am J Physiol Cell Physiol 275: C940–C950, 1998. doi: 10.1152/ajpcell.1998.275.4.C940. [DOI] [PubMed] [Google Scholar]
- 8.Duan D, Winter C, Cowley S, Hume JR, Horowitz B. Molecular identification of a volume-regulated chloride channel. Nature 390: 417–421, 1997. doi: 10.1038/37151. [DOI] [PubMed] [Google Scholar]
- 9.Flucher BE, Campiglio M. STAC proteins: the missing link in skeletal muscle EC coupling and new regulators of calcium channel function. Biochim Biophys Acta Mol Cell Res 1866: 1101–1110, 2019. doi: 10.1016/j.bbamcr.2018.12.004. [DOI] [PubMed] [Google Scholar]
- 10.Friedrich T, Breiderhoff T, Jentsch TJ. Mutational analysis demonstrates that ClC-4 and ClC-5 directly mediate plasma membrane currents. J Biol Chem 274: 896–902, 1999. doi: 10.1074/jbc.274.2.896. [DOI] [PubMed] [Google Scholar]
- 11.Gambardella J, Trimarco B, Iaccarino G, Santulli G. New insights in cardiac calcium handling and excitation-contraction coupling. Adv Exp Med Biol 1067: 373–385, 2017. doi: 10.1007/5584_2017_106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ganapathi SB, Wei SG, Zaremba A, Lamb FS, Shears SB. Functional regulation of ClC-3 in the migration of vascular smooth muscle cells. Hypertension 61: 174–179, 2013. doi: 10.1161/HYPERTENSIONAHA.112.194209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Graves AR, Curran PK, Smith CL, Mindell JA. The Cl−/H+ antiporter ClC-7 is the primary chloride permeation pathway in lysosomes. Nature 453: 788–792, 2008. doi: 10.1038/nature06907. [DOI] [PubMed] [Google Scholar]
- 14.Hebeisen S, Heidtmann H, Cosmelli D, Gonzalez C, Poser B, Latorre R, Alvarez O, Fahlke C. Anion permeation in human ClC-4 channels. Biophys J 84: 2306–2318, 2003. doi: 10.1016/S0006-3495(03)75036-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang L, Cao J, Wang H, Vo LA, Brand JG. Identification and functional characterization of a voltage-gated chloride channel and its novel splice variant in taste bud cells. J Biol Chem 280: 36150–36157, 2005. doi: 10.1074/jbc.M507706200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jentsch TJ, Pusch M. CLC chloride channels and transporters: structure, function, physiology, and disease. Physiol Rev 98: 1493–1590, 2018. doi: 10.1152/physrev.00047.2017. [DOI] [PubMed] [Google Scholar]
- 17.Kawasaki M, Fukuma T, Yamauchi K, Sakamoto H, Marumo F, Sasaki S. Identification of an acid-activated Cl− channel from human skeletal muscles. Am J Physiol Cell Physiol 277: C948–C954, 1999. doi: 10.1152/ajpcell.1999.277.5.C948. [DOI] [PubMed] [Google Scholar]
- 18.Kurita T, Yamamura H, Suzuki Y, Giles WR, Imaizumi Y. The ClC-7 chloride channel is downregulated by hypoosmotic stress in human chondrocytes. Mol Pharmacol 88: 113–120, 2015. doi: 10.1124/mol.115.098160. [DOI] [PubMed] [Google Scholar]
- 19.Leisle L, Ludwig CF, Wagner FA, Jentsch TJ, Stauber T. ClC-7 is a slowly voltage-gated 2Cl−/1H+-exchanger and requires Ostm1 for transport activity. EMBO J 30: 2140–2152, 2011. doi: 10.1038/emboj.2011.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li X, Shimada K, Showalter LA, Weinman SA. Biophysical properties of ClC-3 differentiate it from swelling-activated chloride channels in Chinese hamster ovary-K1 cells. J Biol Chem 275: 35994–35998, 2000. doi: 10.1074/jbc.M002712200. [DOI] [PubMed] [Google Scholar]
- 21.Matsuda JJ, Filali MS, Collins MM, Volk KA, Lamb FS. The ClC-3 Cl−/H+ antiporter becomes uncoupled at low extracellular pH. J Biol Chem 285: 2569–2579, 2010. doi: 10.1074/jbc.M109.018002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matsuda JJ, Filali MS, Volk KA, Collins MM, Moreland JG, Lamb FS. Overexpression of CLC-3 in HEK293T cells yields novel currents that are pH dependent. Am J Physiol Cell Physiol 294: C251–C262, 2008. doi: 10.1152/ajpcell.00338.2007. [DOI] [PubMed] [Google Scholar]
- 23.Misárková E, Behuliak M, Bencze M, Zicha J. Excitation-contraction coupling and excitation-transcription coupling in blood vessels: their possible interactions in hypertensive vascular remodeling. Physiol Res 65: 173–191, 2016. [DOI] [PubMed] [Google Scholar]
- 24.Neagoe I, Stauber T, Fidzinski P, Bergsdorf EY, Jentsch TJ. The late endosomal ClC-6 mediates proton/chloride countertransport in heterologous plasma membrane expression. J Biol Chem 285: 21689–21697, 2010. doi: 10.1074/jbc.M110.125971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Orhan G, Fahlke C, Alekov AK. Anion- and proton-dependent gating of ClC-4 anion/proton transporter under uncoupling conditions. Biophys J 100: 1233–1241, 2011. doi: 10.1016/j.bpj.2011.01.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Petkov GV. Role of ion channels in urinary bladder smooth muscle function. In: Methods in Signal Transduction and Smooth Muscle, edited by Trebak M, Earley S. Boca Raton, FL: CRC Press, Taylor and Francis, 2018, p. 281–304. [Google Scholar]
- 27.Petkov GV. Role of potassium ion channels in detrusor smooth muscle function and dysfunction. Nat Rev Urol 9: 30–40, 2011. doi: 10.1038/nrurol.2011.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Picollo A, Malvezzi M, Accardi A. Proton block of the CLC-5 Cl−/H+ exchanger. J Gen Physiol 135: 653–659, 2010. doi: 10.1085/jgp.201010428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Provence A, Angoli D, Petkov GV. Kv7 channel pharmacological activation by the novel activator ML213: Role for heteromeric Kv7.4/Kv7.5 channels in guinea pig detrusor smooth muscle function. J Pharmacol Exp Ther 364: 131–144, 2018. doi: 10.1124/jpet.117.243162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stauber T, Jentsch TJ. Sorting motifs of the endosomal/lysosomal CLC chloride transporters. J Biol Chem 285: 34537–34548, 2010. doi: 10.1074/jbc.M110.162545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Steinmeyer K, Schwappach B, Bens M, Vandewalle A, Jentsch TJ. Cloning and functional expression of rat CLC-5, a chloride channel related to kidney disease. J Biol Chem 270: 31172–31177, 1995. doi: 10.1074/jbc.270.52.31172. [DOI] [PubMed] [Google Scholar]
- 32.Suh BC, Hille B. PIP2 is a necessary cofactor for ion channel function: how and why? Annu Rev Biophys 37: 175–195, 2008. doi: 10.1146/annurev.biophys.37.032807.125859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thomas-Gatewood C, Neeb ZP, Bulley S, Adebiyi A, Bannister JP, Leo MD, Jaggar JH. TMEM16A channels generate Ca2+-activated Cl− currents in cerebral artery smooth muscle cells. Am J Physiol Heart Circ Physiol 301: H1819–H1827, 2011. doi: 10.1152/ajpheart.00404.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vanoye CG, George AL Jr. Functional characterization of recombinant human ClC-4 chloride channels in cultured mammalian cells. J Physiol 539: 373–383, 2002. doi: 10.1113/jphysiol.2001.013115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang L, Ma W, Zhu L, Ye D, Li Y, Liu S, Li H, Zuo W, Li B, Ye W, Chen L. ClC-3 is a candidate of the channel proteins mediating acid-activated chloride currents in nasopharyngeal carcinoma cells. Am J Physiol Cell Physiol 303: C14–C23, 2012. doi: 10.1152/ajpcell.00145.2011. [DOI] [PubMed] [Google Scholar]
- 36.Yamazaki J, Duan D, Janiak R, Kuenzli K, Horowitz B, Hume JR. Functional and molecular expression of volume-regulated chloride channels in canine vascular smooth muscle cells. J Physiol 507: 729–736, 1998. doi: 10.1111/j.1469-7793.1998.729bs.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yarotskyy V, Malysz J, Petkov GV. Properties of single-channel and whole cell Cl− currents in guinea pig detrusor smooth muscle cells. Am J Physiol Cell Physiol 316: C698–C710, 2019. doi: 10.1152/ajpcell.00327.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zderic SA, Sillen U, Liu GH, Snyder MC 3rd, Duckett JW, Gong C, Levin RM. Developmental aspects of excitation contraction coupling of rabbit bladder smooth muscle. J Urol 152: 679–681, 1994. doi: 10.1016/S0022-5347(17)32679-4. [DOI] [PubMed] [Google Scholar]
- 39.Zifarelli G, De Stefano S, Zanardi I, Pusch M. On the mechanism of gating charge movement of ClC-5, a human Cl−/H+ antiporter. Biophys J 102: 2060–2069, 2012. doi: 10.1016/j.bpj.2012.03.067. [DOI] [PMC free article] [PubMed] [Google Scholar]