Abstract
Cardiac arrhythmias of both atrial and ventricular origin are an important feature of cardiovascular disease. Novel antiarrhythmic therapies are required to overcome current drug limitations related to effectiveness and pro-arrhythmia risk in some contexts. Cardiomyocyte culture models provide a high-throughput platform for screening antiarrhythmic compounds, but comparative information about electrophysiological properties of commonly used types of cardiomyocyte preparations is lacking. Standardization of cultured cardiomyocyte microelectrode array (MEA) experimentation is required for its application as a high-throughput platform for antiarrhythmic drug development. The aim of this study was to directly compare the electrophysiological properties and responses to isoproterenol of three commonly used cardiac cultures. Neonatal rat ventricular myocytes (NRVMs), immortalized atrial HL-1 cells, and custom-generated human induced pluripotent stem cell-derived cardiomyocytes (hiPSC-CMs) were cultured on microelectrode arrays for 48–120 h. Extracellular field potentials were recorded, and conduction velocity was mapped in the presence/absence of the β-adrenoceptor agonist isoproterenol (1 µM). Field potential amplitude and conduction velocity were greatest in NRVMs and did not differ in cardiomyocytes isolated from male/female hearts. Both NRVMs and hiPSC-CMs exhibited longer field potential durations with rate dependence and were responsive to isoproterenol. In contrast, HL-1 cells exhibited slower conduction and shorter field potential durations and did not respond to 1 µM isoproterenol. This is the first study to compare the intrinsic electrophysiologic properties of cultured cardiomyocyte preparations commonly used for in vitro electrophysiology assessment. These findings offer important comparative data to inform methodological approaches in the use of MEA and other techniques relating to cardiomyocyte functional screening investigations of particular relevance to arrhythmogenesis.
Keywords: cardiomyocyte electrophysiology, HL-1 cells, human iPS-derived cardiomyocytes, microelectrode array, neonatal rat ventricular myocytes
INTRODUCTION
Cardiac arrhythmias are an important feature of numerous cardiovascular diseases, including heart failure and ischemic heart disease. Arrhythmias can be of atrial or ventricular origin, disrupting the capacity and stability of the heart pump. Patients with ventricular arrhythmias, including ventricular tachycardia and fibrillation, are especially vulnerable to sudden cardiac death, and immediate defibrillatory intervention is required (42). Atrial fibrillation is the most common sustained arrhythmia, associated with multiple risk factors, including aging, obesity, and genetic predisposition (23, 26, 27). Although atrial fibrillation in isolation may not be lethal, it can lead to thrombotic stroke and heart failure. Implantation of a cardioverter defibrillator can be effective in patients at risk of ventricular arrhythmias but are expensive and associated with significant morbidity (24). Numerous antiarrhythmic treatment strategies are available although risk of side effects and incomplete effectiveness limit their clinical use (19, 24, 25). A better understanding of the subcellular mechanisms underpinning arrhythmias is required for the development of more effective novel antiarrhythmia strategies.
Preclinical experimental models to study cardiomyocyte electrophysiology have proved a useful tool in understanding the basic mechanisms of arrhythmogenesis. Substrate-integrated microelectrode arrays (MEAs) are devices embedded with multiple low-impedance electrodes arranged in a grid formation, designed for noninvasive synchronous multifocal recordings across cardiac slices and cardiomyocyte monolayers.
Cardiomyocytes cultured on MEAs have enabled assessment of electrophysiology and conduction properties and provide a high-throughput platform for screening compounds for antiarrhythmic properties (6). In vitro cardiomyocyte studies have historically relied on a limited number of immortalized cell lines and/or dissociated neonatal rodent ventricular myocytes (5, 28). Recently, inducible pluripotent stem cell preparations have become more widely utilized (37). Comparative benchmarking of these different cell types relative to various functional readouts has not been previously reported.
Commonly used spontaneously beating cardiomyocytes that can be maintained in culture over multiple days/weeks include neonatal rat ventricular myocytes (NRVMs), immortalized mouse atrial HL-1 cell lines, and human induced pluripotent stem cell-derived cardiomyocytes (hiPSC-CMs). NRVMs in culture are minimally proliferative, immature, and able to reestablish gap junction connections when seeded as a dense confluent monolayer (28). Typically, NRVM cultures are sourced from a mixed male/female population, without regard for potential sex-specific cardiac electrophysiological differences. This may be an important oversight, since a growing body of literature describes numerous differences in male and female cardiac physiology, including action potential and Ca2+-handling characteristics, arrhythmia vulnerability, and interventional drug responses (4, 41).
The HL-1 cell line was derived from an atrial tumor excised from an adult female C57BL/6J mouse and is readily commercially available (11). Although immortalized, HL-1 cells substantially maintain their terminally differentiated phenotype in culture even after multiple passages and, like NRVMs, form an electrically coupled syncytia allowing mapping of electrical signal propagation. hiPSC-CMs are generated from induced pluripotent stem cells differentiated toward the cardiac lineage. They can be generated in high quantity, recapitulate the adult human cardiac action potential and ion channel profile to an extent, and can be sourced commercially or produced locally by a defined differentiation protocol (29, 37). Like NRVMs, hiPSC-CMs are immature and beat spontaneously (37). The therapeutic regenerative potential of hiPSC-CMs is intriguing; hence, there is considerable interest in using these human-derived cardiomyocytes as a model to better understand cardiac electrophysiology (38).
All three cell types (hiPSC-CMs, HL-1, and NRVMs) are used for disease modeling and drug screening. However, a direct comparison of electrophysiology/conduction properties across these commonly used cardiomyocyte cultures has not been reported, and there is little information available to assist in determining which cardiomyocyte type is best suited to specific experimental applications and arrhythmia models. The spontaneous pacing properties of these three cell types were hence compared under basal conditions and in the presence of the β-adrenergic agonist, isoproterenol, to assess cardiomyocyte electrophysiology responses in settings known to induce arrhythmias in the intact heart. The aim of this study was to compare the electrophysiological characteristics of three commonly used cardiomyocyte cultures to standardize their use for potential purpose-specific modeling of relevant disease scenarios and aid researchers in the field with the selection of appropriate cultured cardiomyocyte preps for their studies.
Here, we show for the first time that primary cardiomyocytes, immortalized cardiac cell lines, and induced pluripotent stem cell-derived cardiomyocytes (NRVM, HL-1, and hiPSC-CM) exhibit marked difference in relation to beating rates, conduction velocities, rate adaptations of repolarization, and β-adrenergic responsiveness. By describing the distinct electrophysiological characteristics of these cell preparations, these findings provide important new insights for the use of cultured cardiomyocyte MEA as a high-throughput platform for antiarrhythmic drug development.
MATERIALS AND METHODS
Cell Culture
Neonatal rat ventricular myocyte culture.
Experiments were conducted and animals were handled in the manner specified by the National Health and Medical Research Council/Commonwealth Scientific and Industrial Research Organisation Australian Code of Practice for the Care and Use of Animals for Scientific Purposes (2013) and the European Union (EU) Directive 2010/63/EU for animal experiments, with approval and oversight of the project by the University of Melbourne Animal Ethics Committee. For NRVM cultures, cardiomyocytes were isolated from postnatal day 2 Sprague-Dawley litters as previously described (35). Briefly, pups were decapitated, hearts were rapidly excised, and atria were resected. Ventricles were dissected into chunks and gently swirled in 0.5% collagenase II (LS004176; Worthington) for 15 min at 37°C. Single NRVMs were isolated by Trypsin digestion (0.06%, T9201; Sigma) in Hanks’ balanced salt solution + 0.02% DNAse 1 (260913; Calbiochem) and then digestion inhibited with the addition of newborn calf serum. NRVMs were centrifuged at 990 g at 4°C for 10 min, and cell pellets were resuspended in Dulbecco’s modified Eagle’s medium + GlutaMAX (10565–042; ThermoFisher), supplemented with 5% horse serum (16050–122; ThermoFisher), pyruvic acid (3 mM, P-8574; Sigma), bovine serum albumin (2 g/L, A-7030; Sigma), ampicillin (100 µg/mL, A-0166; Sigma), 100× insulin-transferrin-selenium (41400045; ThermoFisher), antibiotic/antimycotic (1%, 15240062; ThermoFisher), linoleic acid (5 µg/mL, L5900; Sigma), and ascorbic acid (100 µM, Sigma; A4544), and then preplated to remove fibroblasts (2 × 30 min, 37°C, 5% CO2). Purified NRVMs were seeded on MEAs precoated with fibronectin (10 µg/mL, F2006; Sigma) by either coating the whole MEA well (1 mL at 1,400,000 cells/mL, final density of 500,000 cells/cm2) or only the central recording matrix with a 75 µl droplet of 300,000 NRVMs. To ensure stable droplet formation critical to establishing a confluent cardiomyocyte monolayer, MEA hydrophobicity was increased by heating to 70°C for ≥2 h before seeding. Droplet-seeded NRVMs were then incubated for 2 h to promote adhesion, with culture media subsequently added to a final volume of 1 mL (300,000 cell/mL of media, final density of ~1,000,000 cells/cm2). Media was changed after 48 h, typically when spontaneously beating monolayers had formed. MEA experiments were performed 5–6 days postisolation, since spontaneous beating rate, field potential amplitude, and conduction velocity have previously been shown to decline from 7 to 8 days postisolation (9, 28).
HL-1 cardiomyocyte culture.
The immortalized atrial cardiomyocyte cell line, HL-1, was purchased from Merck (SCC065). HL-1 cells were cultured in Claycomb media (51800C; Sigma) supplemented with 10% fetal bovine serum (A3160601; ThermoFisher), penicillin/streptomycin (100 U·mL−1·100 µg−1·mL−1, 15140122; ThermoFisher), norepinephrine (0.1 mM, A0937; Sigma), ascorbic acid (0.3 mM, A7631; Sigma), and l-glutamine (2 mM, 25030081; ThermoFisher) and were maintained as per the manufacturer’s instructions. For MEA experiments, 100,000 cardiomyocytes were seeded as a droplet on gelatin- and/or fibronectin-coated MEAs [0.02% gelatin, G9391 (Sigma); 0.5 µg/mL fibronectin, F1141 (Sigma)], similar to the process described for NRVMs. Media were changed daily, and cell recordings were obtained from 2 days postseeding. HL-1 cultures exhibiting spontaneous beating rates in excess of 200 beats/min were excluded from MEA analysis.
Human induced pluripotent stem cell-derived cardiomyocyte culture.
hiPSC-CMs were cultured and differentiated as previously described (1, 13, 14). Briefly, hiPSCs (line PB004.4 female; Murdoch Children’s Research Institute) were cultured in a 75 cm2 tissue culture flask with hiPSC media [DMEM-F-12 (11320033; Gibco), 20% (vol/vol) knockout serum replacement (10828028; Gibco), 1× nonessential amino acids (11140050; Gibco), 1× GlutaMAX (35050061; Gibco), penicillin/streptomycin (15140122; Gibco), 110 µM 2-mercaptoethanol (21985023; Gibco), and 5 ng/mL recombinant human fibroblast growth factor (233-FB-025; R&D)] on irradiated mouse embryonic fibroblasts and passaged using TrypLE Express enzyme (12604013; ThermoFisher). Before differentiation (1 day), 1.6 × 105 cells/cm2 were seeded. To induce differentiation (day 0), cells were treated with basal differentiation media [RPMI (21870076; Sigma), B27 minus vitamin A (12587010; Gibco), and 50 µg/mL ascorbic acid (A8960; Sigma) supplemented with 10 µM CHIR-99021 (4423; Tocris Bioscience) and 80 ng/mL activin A (Peprotech)]. On days 1, 3, and 5, media were changed to basal differentiation media supplemented with 5 µM IWR-1 (I0161; Sigma). Cells were maintained in basal differentiation media from day 7 until used.
For experiments, cells were dissociated with TrypLE, centrifuged (200 g, 4°C, 3 min), carefully resuspended in basal differentiation medium, and replated at 800,000 cells/mL as clusters across Geltrex-coated microelectrode arrays (Geltrex, 1:100, A1413202; Life Technologies). Media was changed after 48 h, and experiments were performed the following day.
Microelectrode Array Configuration
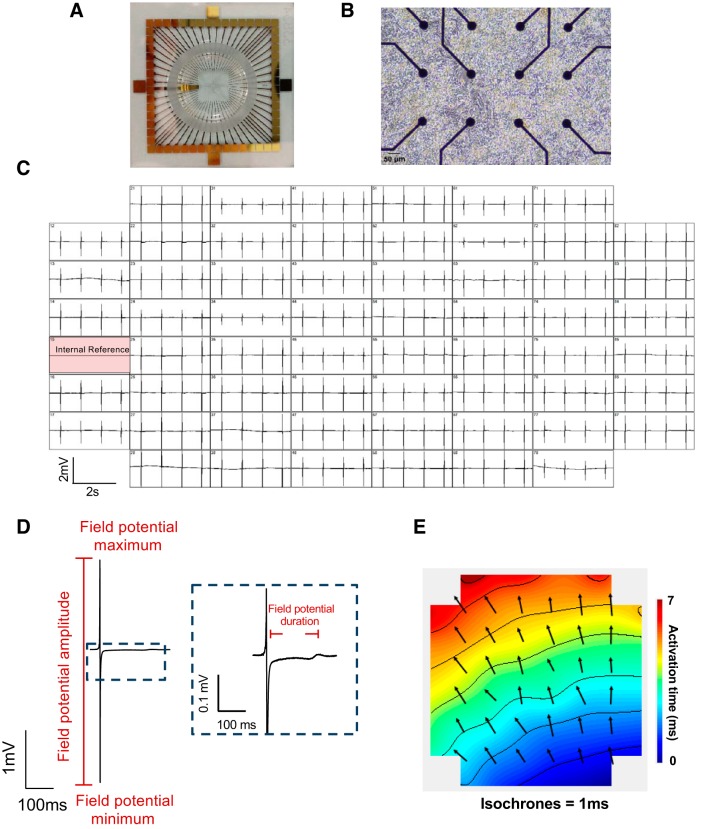
Extracellular electrophysiological field potentials were recorded from cardiomyocyte cultures using the MEA2100 2 × 60 system (Fig. 1; Multichannel Systems, Reutlingen, Germany). MEAs consisted of 60 gold recording electrodes embedded in a glass substrate fabricated with a layer of the conductive polymer poly(3,4-ethylenedioxythiophene) during the manufacturing process to produce the most robust signal amplitudes. Two different electrode configurations were used, either 100 µm diameter with 700 µm spacing or 30 µm diameter with 200 µm spacing (one of the 60 electrodes was used as an internal reference), with data sets comparable across the two configurations.
Fig. 1.
Visual overview of microelectrode arrays (MEAs) and measurable electrophysiological parameters. Exemplar images of MEA chip (200 µm electrode spacing, 30 µm electrode diameter, A), neonatal rat ventricular myocyte (NRVM) seeding on MEA electrodes (B), and multichannel recordings of field potentials (C). Exemplar field potential traces (D) and activation map (E) showing propagation of a single field potential across the NRVM monolayer over 7 ms. Arrows indicate propagation pathway and are situated at approximate electrode positioning; contours indicate 1-ms delay.
Electrophysiology Recordings
Spontaneously beating cultures were maintained at 37°C, and field potential recordings were acquired using Cardio2D software (Multichannel Systems) sampling at 10 kHz (low pass filter: 3,500 Hz, high pass filter: 10 Hz). All experimental recordings began after a 10-min equilibration period. To assess NRVM stability, 2-min recordings were obtained at 0, 15, and 35 min. A subset of NRVM, HL-1, and hiPSC-CM cultures was treated with the β-adrenergic agonist, isoproterenol (1 µM, 3–5 min), which we have shown previously to induce robust maximal electrophysiological responses in NRVM cultures (32).
Electrophysiology Analysis
Spontaneous beating rate was calculated by averaging the interspike interval between 10 consecutive beats. Field potential duration was determined as the time between the peak negative deflection and the peak of the final positive deflection (Fig. 1D; 10, 18). For NRVM and HL-1 cells, this was calculated by averaging across 10 beats at 10 sites across an MEA. Activation maps for NRVMs and HL-1 monolayers were generated in Cardio2D+ software (Multichannel Systems). Delay in activation between neighboring electrodes was computed using a multivector approach, and mean conduction velocity was calculated on a beat-beat basis. Conduction velocity was then averaged across 10 consecutive beats for each MEA. Because of the clustered nature of hiPSC-CMs, field potential duration was averaged from 10 consecutive beats at a single site. All analysis was performed using Cardio2D+, and arrhythmic cultures were excluded.
Statistics
All data are expressed as means ± SE. Statistical analysis was performed and graphs were generated using GraphPad Prism 6.0. Relevant statistical analyses are indicated throughout and P < 0.05 was deemed significant. N denotes the number of cultures, and n denotes the number of MEAs.
RESULTS
Optimizing Methodology to Plate Cardiomyocytes on MEAs
NRVMs seeded on MEAs formed a confluent spontaneously beating monolayer over 5 days in culture, allowing the detection of field potentials across the 59 recording electrodes. Activation maps were derived from detection of the field potential propagation across the monolayer, with isochrones indicating activation latency and arrows showing the dominant regional propagation pathways.
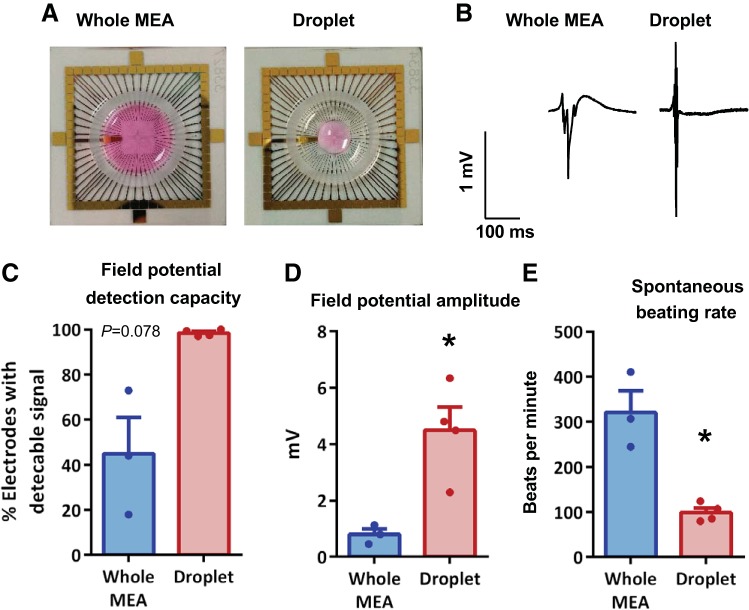
To assess the optimal plating methodology for detecting cardiomyocyte field potentials, mixed-sex NRVMs were either seeded across the whole MEA or as a droplet exclusively over the central recording region without covering the internal reference electrode (Fig. 2, A and B). Following a 10-min stabilization period, basal measurements (time = 0 min) showed plating cardiomyocytes as a droplet-enhanced capacity to detect field potentials (Fig. 2C) and significantly reduced interprep variability (F test, P < 0.001). This was associated with a significantly greater field potential amplitude (4.48 ± 0.83 vs. 0.79 ± 0.20 mV, N = 3–4, P < 0.05; Fig. 2D). The droplet seeding of cells over the central recording region was hence adopted for the remainder of the study. Interestingly, NRVMs plated as a central droplet exhibited a lower spontaneous beating rate (99.1 ± 10.3 vs. 320.5 ± 48.3 beats/min, N = 3–4, P < 0.05; Fig. 2E) with less interprep variability (F test, P < 0.001) compared with those cells plated across the whole MEA.
Fig. 2.
Optimal cell seeding conditions for measuring cultured neonatal rat ventricular myocyte (NRVM) field potentials. Exemplar images of NRVM seeding across the entire microelectrode array (MEA) or as a droplet over the central recording zone of the electrode array (A), with respective exemplar field potential traces (B) and percentage of electrodes detecting field potentials (C). Mean field potential amplitude (D) and spontaneous beating rate from MEAs (E). All data were acquired from mixed-sex NRVMs, immediately after a 10-min stabilization period. *P < 0.05, unpaired t tests, N = 3–4 cultures, n = 3–10 MEAs.
Male and Female NRVM Monolayers Exhibit Similar Electrophysiology
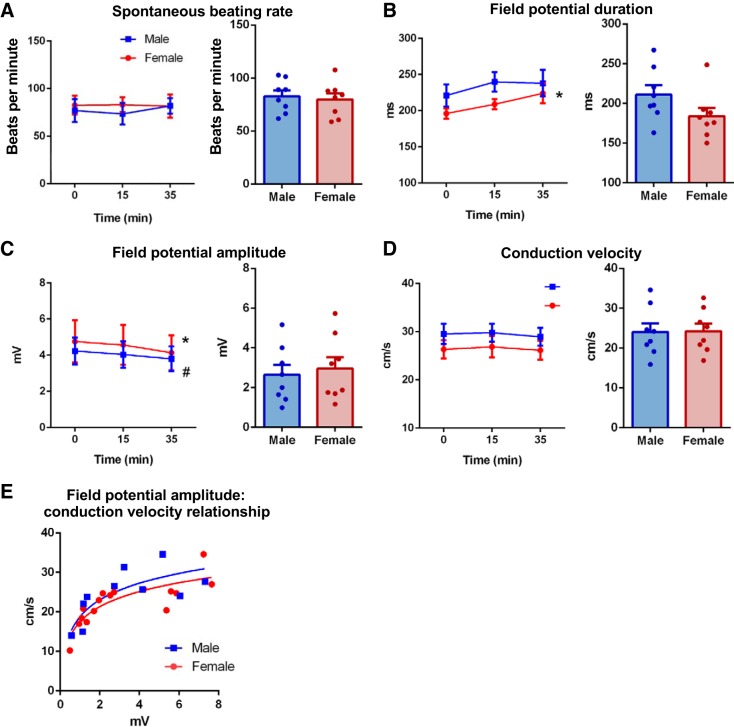
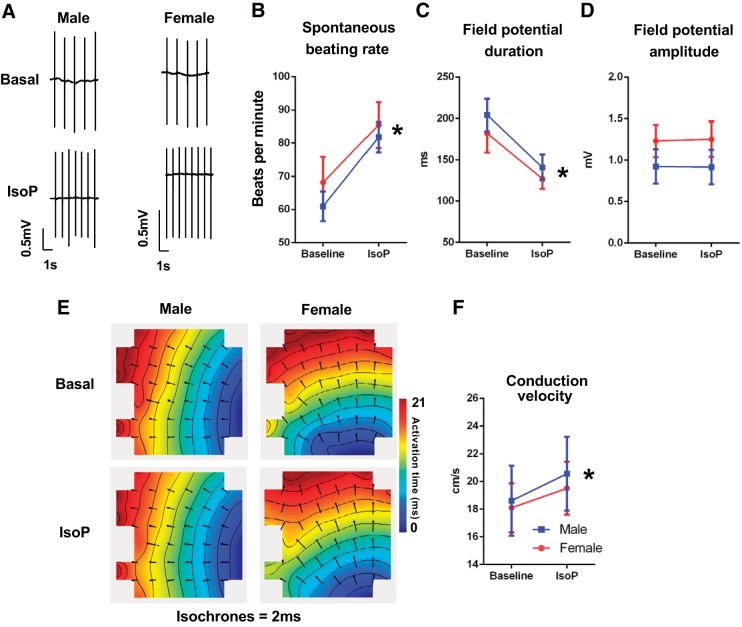
Basal electrophysiology parameters were compared in NRVM monolayers from male and female rat hearts harvested at postnatal day 2. No significant differences in spontaneous beating rate, field potential duration, field potential amplitude, or conduction velocity were evident between male and female NRVM monolayers (Fig. 3, A–D). Both sexes also displayed similar field potential amplitude-conduction velocity relationships (Fig. 3E). MEA recordings of male and female cardiomyocytes were then either continued under basal conditions to assess electrophysiological stability over a 35-min period or treated with 1 µM isoproterenol to determine responsiveness to β-adrenergic stimulation. Spontaneous beating rate did not change in either male or female NRVM monolayers during the 35-min assessment, with evidence of only minor changes in field potential morphology and no change in conduction velocity (Fig. 3, A–D). Male and female NRVMs acutely treated with 1 µM isoproterenol exhibited no significant differences in electrophysiologic response parameters, with both sexes displaying an expected significant increase in spontaneous beating rate, shortening of field potential duration, and no change in field potential amplitude (Fig. 4, A–D, and Supplemental Fig. S1; Supplemental Material is available at https://doi.org/10.26188/5d71e20d43c5e). Conduction velocity increased similarly in cells of both sexes (Fig. 4F and Supplemental Fig. S1).
Fig. 3.
Comparable electrophysiology in cultured male (blue) and female (red) neonatal rat ventricular myocytes (NRVMs). Assessment of prep stability in male and female NRVM electrophysiology with time (left) and direct sex comparison at 0 min (right), including spontaneous beating rate (A), field potential duration (B), field potential amplitude (C), and conduction velocity (D). E: field potential amplitude-conduction velocity relationships in male and female NRVM cultures. P < 0.05 females 0 vs. 35 min (*) and males 0 vs. 35min (#), N = 3–4 cultures, n = 6–9 microelectrode arrays (MEAs). No between-sex differences at any time point. Repeated-measures 2-way ANOVA and Holm-Sidak’s multiple-comparison tests.
Fig. 4.
Comparable isoproterenol (IsoP) response in cultured male (blue) and female (red) neonatal rat ventricular myocytes (NRVMs). A: exemplar field potential traces in male and female NRVMs in basal and 1 µM isoproterenol conditions. Mean spontaneous beating rate (B), field potential duration (C), and amplitude (D), exemplar conduction maps (E), and conduction velocity (F) of male and female NRVMs before (basal) and following an acute treatment of 1 µM isoproterenol. *P < 0.05, baseline vs. isoproterenol for both sexes. No between-sex differences. Repeated-measures 2-way ANOVA and Holm-Sidak’s multiple-comparison tests. N = 5–6 cultures, n = 6–7 microelectrode arrays (MEAs).
Differences in Basal Electrophysiology Depend on Cardiomyocyte Culture Type
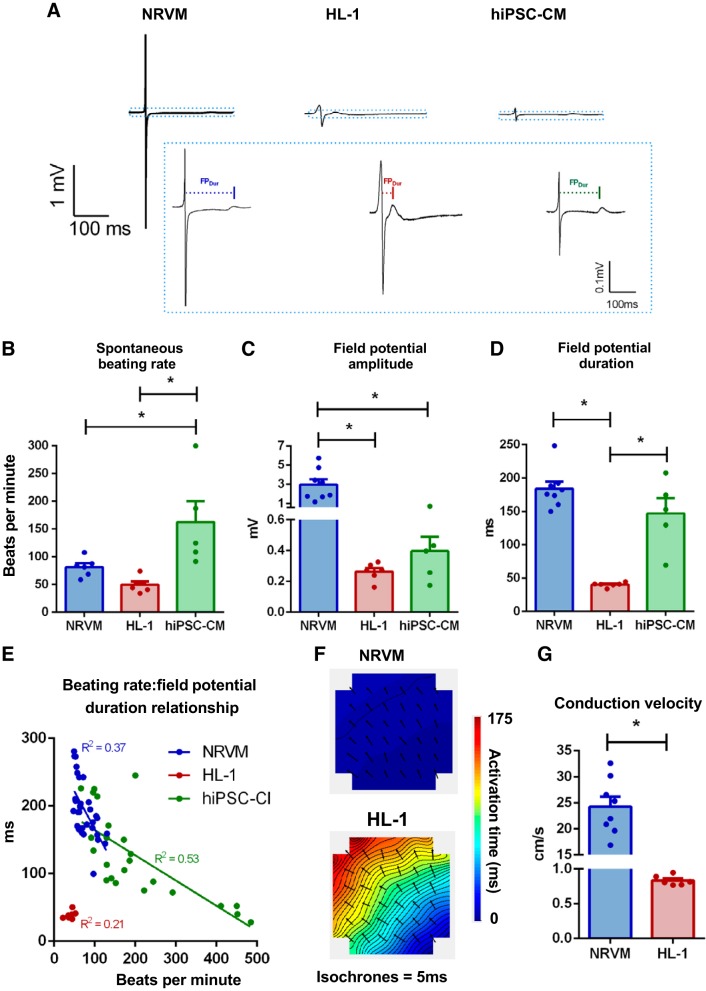
The optimized MEA recording methodology was next used to compare the electrophysiological characteristics of three cardiomyocyte culture types, all of female origin. Significant differences in the electrical properties of female NRVMs, HL-1 cells, and hiPSC-CMs were observed (Fig. 5A). Spontaneous beating rate was significantly faster in hiPSC-CMs compared with both NRVM and HL-1 cells (NRVM vs. HL-1 vs. hiPSC-CM; 81.5 ± 6.9 vs. 49.7 ± 5.6 vs. 162.5 ± 38.0 beats/min, P < 0.05; Fig. 5B). Field potential morphology differed across the three cell types, with NRVMs exhibiting a substantially greater field potential amplitude compared with other cardiomyocyte cultures (2.95 ± 0.58 vs. 0.26 ± 0.02 vs. 0.40 ± 0.09 mV, P < 0.05; Fig. 5C). Field potential duration was similar in NRVMs and hiPSC-CMs but significantly shorter in HL-1 cells (184.0 ± 10.5 vs. 40.2 ± 1.4 vs. 146.9 ± 23.2 ms, P < 0.05; Fig. 5D). Derivation of the beating rate-field potential duration relationship showed that both NRVMs and hiPSC-CMs (but not HL-1 cells) displayed field potential duration rate dependency. Both cell types exhibited faster repolarization at higher beating rates (P < 0.05, Fig. 5E and Supplemental Fig. S2), although the gradient of this relationship differed significantly (P < 0.05). Monolayer conduction rates were assessed in NRVM and HL-1 cells (but not in hiPSC-CMs because of their cluster morphology on plating). Conduction velocity was significantly faster in NRVM vs. HL-1 (24.25 ± 1.91 vs. 0.83 ± 0.03 cm/s, P < 0.05; Fig. 5, F and G).
Fig. 5.
Basal electrophysiology differs among female cardiomyocyte cultures of different origin. A: exemplar field potential traces from neonatal rat ventricular myocytes (NRVMs), HL-1, and human induced pluripotent stem cell-derived cardiomyocytes (hiPSC-CMs) at full scale and cropped (at the dotted lines) to highlight repolarization. Mean spontaneous beating rate (B), field potential amplitude (C), and field potential duration (D) from all 3 cell types. E: spontaneous beating rate-field potential duration relationship was examined on a single microelectrode array (MEA) basis among NRVM, HL-1, and hiPSC-CMs. Representative activation maps (F) and mean conduction velocity (G) in NRVM and HL-1 monolayers. *P < 0.05, unpaired t tests or 1-way ANOVA with Holm-Sidak’s multiple-comparison tests. NRVM: N = 8 cultures, n = 19 MEAs; HL-1: N = 4–6 passages, n = 7–11 MEAs; hiPSC-CMs: N = 5 (3 separate cultures, 5 plating runs), n = 22 MEAs.
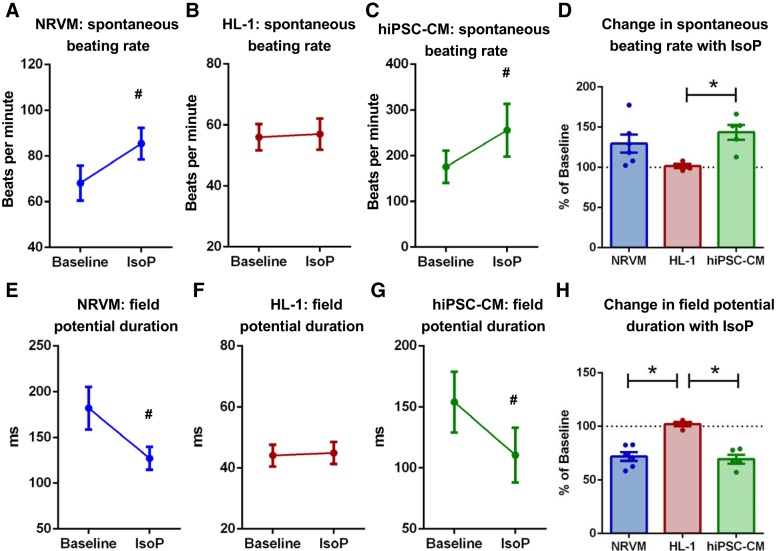
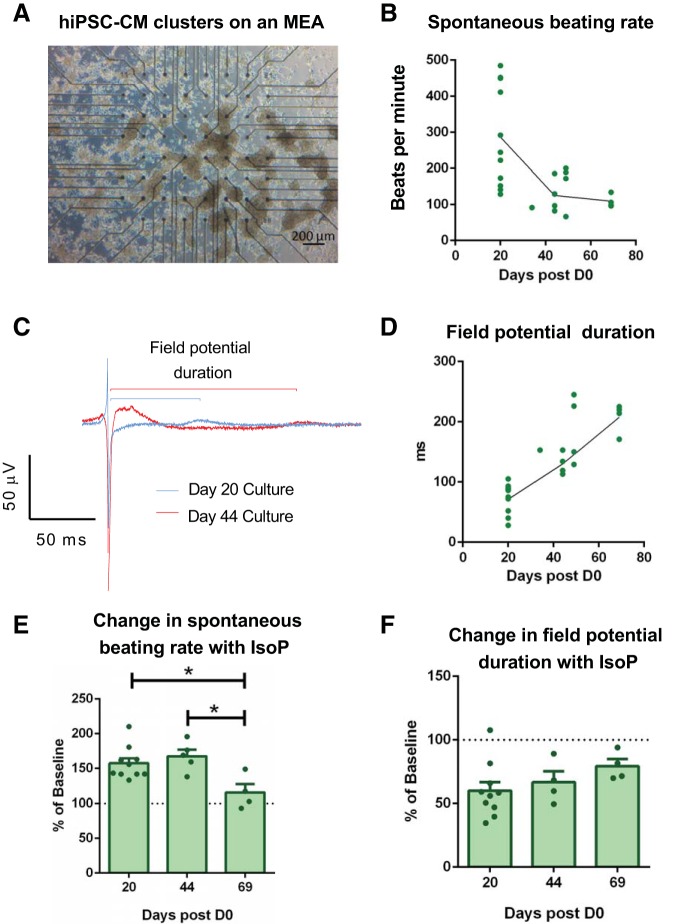
To assess differences in responsiveness to β-adrenergic stimulation among cardiomyocyte cultures, cells were treated with 1 µM isoproterenol for 3–5 min. A significant increase in spontaneous beating rate was detected in both NRVMs and hiPSC-CMs but not HL-1 cells (Fig. 6, A–D, and Supplemental Fig. S3). Concomitant changes in field potential duration were observed, with a significant reduction evident in both NRVMs and hiPSC-CMs, but not HL-1 cells (Fig. 6, E–G, and Supplemental Fig. S3). The percentage decrease in field potential duration was hence significantly greater in both NRVMs and hiPSC-CMs compared with HL-1 cells (71.7 ± 4.0 vs. 102.1 ± 2.0 vs. 69.4 ± 4.0% basal, P < 0.05; Fig. 6H). It was evident that hiPSC-CM displayed greater variability between experimental replicates compared with NRVMs and HL-1 preps, and hence additional analysis was performed to determine whether the time point between differentiation induction (D0) and plating on the MEA was a determining factor (Fig. 7). hiPSCs plated earlier postdifferentiation induction demonstrated faster beating rates and shorter field potential durations (Fig. 7, B–D). hiPSC-CM isoproterenol responsiveness decreased in cells plated at a later time point postdifferentiation induction. An increase in beating rate in hiPSC-CMs treated with isoproterenol was not evident at 69 days postdifferentiation induction, with a similar trend evident in the field potential duration shortening response (Fig. 7, E and F).
Fig. 6.
Isoproterenol-stimulated (IsoP) electrophysiology differs among female cardiomyocyte cultures of different origin. Mean spontaneous beating rate for neonatal rat ventricular myocyte (NRVM, A), HL-1 (B), and human induced pluripotent stem cell-derived cardiomyocytes (hiPSC-CM, C) in response to 1 µM isoproterenol. D: %change in beating rate in response to 1 µM isoproterenol in all cell types. Mean field potential duration for NRVM (E), HL-1 (F), and hiPSC-CM (G) in response to 1 µM isoproterenol. H: %change in field potential duration in response to 1 µM isoproterenol in all cell types. P < 0.05, paired t test (#) and ordinary 1-way ANOVAs with Holm-Sidak’s multiple comparisons (*). NRVM: N = 8 cultures, n = 19 MEAs; HL-1: N = 4 passages, n = 7–11 MEAs; hiPSC-CMs: N = 5 (3 separate cultures, 5 plating runs), n = 22 MEAs.
Fig. 7.
Human induced pluripotent stem cell-derived cardiomyocyte (hiPSC-CM) electrophysiology changes with time postdifferentiation induction. A: exemplar image of hiPSC-CM clusters cultured on a microelectrode array (MEA). Relationship between spontaneous beating rate (B) and field potential duration (C and D) and the duration of the period postdifferentiation induction. n = 22 MEAs (lines plotted through day 22, 44, and 69 means, since these time points had the most data points). Spontaneous beating rate (E) and field potential duration (F) changes induced by 1 µM isoproterenol (IsoP) in cultures examined at different time points postdifferentiation induction. n = 16–22 MEAs. *P < 0.05, 1-way ANOVA with Holm-Sidak’s multiple comparisons.
DISCUSSION
This is the first study to systematically contrast the intrinsic differences in cardiomyocyte culture electrophysiologic function, comparing cultures of different cellular origin and prepared using different seeding methodologies. Our findings with NRVM monolayers showed that field potential detection/amplitude is greatly improved when cardiomyocytes are seeded exclusively over the central recording matrix of MEAs. We demonstrate that NRVM monolayer electrophysiology does not differ in cardiomyocytes isolated from male and female hearts, supporting the systematic use of combined male/female NRVM reported in the literature. Importantly, this study provides novel insight into the electrophysiological characteristics of different cardiomyocyte cultures. Both NRVMs and hiPSC-CMs exhibit a rate-dependent field potential duration and are responsive to β-adrenergic stimulation, contrasting with the relatively slower conduction and shorter field potential duration in HL-1 cells. This highlights the limitations with selecting a cardiomyocyte culture based primarily on cellular origin. Additionally, we report that hiPSC-CM electrophysiological characteristics are dependent on the number of days postdifferentiation at which the cells are plated on the MEA. Together, these findings provide important insights to assist in the selection of cell culture type appropriate for modeling cardiomyocyte conduction, novel comparative information that has not been previously available.
MEAs have been used to assess cardiomyocyte culture conduction properties for over a decade, yet there is a surprising lack of information in the literature with regard to optimizing seeding conditions. We have demonstrated important technical factors that significantly enhance field potential detection in cardiomyocyte monolayers. Seeding cardiomyocytes exclusively over the central recording matrix of the MEA increased the capacity to detect field potentials, with improved field potential morphology and significantly greater amplitude (Fig. 2). This is at least partly attributable to keeping the internal reference electrode free of the cell monolayer, increasing the signal-to-noise ratio, and enhancing the capacity to detect field potentials even at low field potential amplitudes. It is not clear whether the greater cell density over the central recording matrix in “droplet”-seeded MEAs (~1,000,000 vs. 500,000 cells/cm2) is a factor in the capacity to detect field potentials. The considerably lower total number of cells in the 1 mL of media in droplet-seeded MEAs (300,000 vs. 1,400,000) may be important, likely enhancing glucose availability and minimizing accumulation of metabolic by-products. This would be expected to better maintain ATP-dependent ion homeostatic processes that contribute to action potential amplitude/duration.
The use of cardiac cell cultures adds a high-throughput capacity to the MEA that can be exploited for more rapid screening of novel antiarrhythmia drugs not limited by propensity for context-specific proarrhythmic actions. NRVMs, HL-1 cells, and hiPSC-CMs are three of the most commonly used cardiomyocyte cultures for these screening investigations. NRVMs exhibited rapid conduction velocities, particularly compared with HL-1 cells (~20-fold faster than HL-1; Fig. 5, F and G), although slower than the typical conduction velocities of 60–75 cm/s recorded in mouse, guinea pig, and human ventricular slices (8, 33, 44). In addition to the inherent differences between neonatal rat and adult human electrophysiology, the slower conduction of NRVMs in vitro possibly reflects the more disorganized and polymorphic nature of these cells in culture following the cell isolation process.
Clearly, NRVMs provide a useful platform for exploring ventricular conduction properties and drug development. However, a potential oversight in the use of NRVMs in the past has been that these cells are almost exclusively isolated from litters without consideration for the sex of the pups. Cardiovascular disease is the primary cause of death in men and women, although the onset, development, and pathological characteristics of disease differ between sexes (15). Sexual dimorphism in cardiomyocyte electromechanical function is increasingly recognized (3, 34). Our novel NRVM data indicate sex does not influence basal or isoproterenol-induced electrophysiological characteristics in the neonatal rat, contrasting with previous studies in freshly isolated male/female adult cardiomyocytes (7, 17, 41). This suggests either the inherent influence of sex and sex steroids is absent in the neonate and/or that an absence of androgens/estrogens in the media over the 5–6 days of culture may convey transcriptional changes that attenuate fundamental sex differences in cardiomyocyte electrophysiology. Nonetheless, our findings indicate that pooled male and female NRVM cultures provide a valid model for studying at least some aspects of cardiomyocyte electrophysiology on the MEA. The comparison of NRVM, HL-1, and hiPSC-CM electrophysiology in this study used cells derived from females only, allowing for direct sex-controlled comparison of cellular phenotypes. Unfortunately, a parallel comparison of these three cardiomyocyte types in male-derived cells is not possible, since HL-1 cells were developed from an adult female C57BL/6J mouse.
Our hiPSC-CM differentiation protocol has been shown to produce cells expressing ventricular-specific markers (1), indicating the hiPSC-CMs used in this study are primarily of ventricular origin. Accordingly, hiPSC-CMs exhibited similar electrophysiological characteristics to NRVMs, particularly in relation to field potential duration and response to β-adrenergic receptor stimulation with isoproterenol. hiPSC-CMs were redifferentiated separately for each culture (N) from a single pool of stem cells, with reproducibility in the electrophysiological parameters evident between cultures/MEA runs. However, field potential duration and spontaneous beating rate were highly dependent on the number of days postdifferentiation at which the hiPSC-CMs were plated on the MEA. Our data showing that hiPSC-CM electrophysiology was not stable at 20 days postdifferentiation indicate an immature phenotype at this time point and highlight the need to ensure a sufficient period (≥30 days) between hiPSC-CM differentiation induction and MEA plating. Our demonstration of stable beating rates from day 30 and reduced isoproterenol responsiveness in day 60 cultures (vs. day 30 cultures; Fig. 7, F and G) is consistent with previous studies. Paradoxically, β-adrenoreceptor expression has been shown to be increased in hiPSC-CMs at these time points (46). It is not clear why β-adrenoreceptor expression increases at a time when isoproterenol responsiveness is reduced. We speculate the greater β-adrenoreceptor expression reported in hiPSC-CMs could be indicative of extensive β-adrenoreceptor internalization or a compensatory upregulation to overcome potential disruption to downstream signaling pathways, although additional studies would be required to confirm this.
In this study, hiPSC-CMs did not form confluent coupled monolayers, and the capacity to assess conduction velocity was limited. As has been previously observed, the hiPSC-CMs formed clusters when plated, and conduction across the full array could not usually be evaluated (29, 31). There may be differences in culture morphology between commercially sourced and locally generated hiPSC-CMs. Although commercially available hiPSC-CMs can form electrically coupled monolayers, the lack of responsiveness to β-adrenergic stimulation suggests a less mature phenotype (16, 20, 36). Therefore, the hiPSC-CM source may limit the scope of investigation that may be undertaken.
Previous studies performing simultaneous intracellular and extracellular recordings on cell and tissue preparations have shown strong relationships between the action potential and field potential waveforms (10, 18). Our findings show that this action potential-conduction velocity relationship is also evident in the extracellular recording setup, with field potential amplitude correlating strongly with conduction velocity in NRVM monolayers (Fig. 3E). Also, our finding that different cardiomyocyte culture types display different rate adaptation properties has not been reported previously. Both hiPSC-CMs and NRVMs exhibited a rate dependency in relation to field potential duration. When comparing NRVMs and those hiPSC-CMs with a beating rate of ≤150 beats/min, the rate-dependency relationships were comparable (Supplemental Fig. S2). The relationship between pacing rate and action potential duration is a fundamental property of cardiac myocytes (22), as evidenced by the similarities of the hiPSC-CMs used here with neonatal immature rat cardiomyocytes. Given the relationship between beating rate, action potential duration, and arrhythmia occurrence, our findings highlight the utility of these cell types as a model for drug screening.
HL-1 cells in our study did not show rate dependency, at least partly because of the low variability in observed beating rate, which may in turn reflect the minimal murine intrinsic rate adaptation in vivo (30, 46). As was observed for hiPSC-CMs, HL-1 cells exhibited low field potential amplitudes. More strikingly though, were the markedly shorter field potentials and slower conduction velocities compared with the other cell types. The conduction velocities reported here are similar to those reported for HL-1 cells in the literature (5, 39), although this is slower than that reported in NRVMs. It is not clear why conduction velocity is slower in HL-1 cells compared with the other cardiomyocyte cultures studied. HL-1 cells express both connexins (40 and 43 subtypes) and the fast voltage-gated sodium channel (NaV1.5) that are essential for the formation of an electrically coupled excitable syncytium (2, 40, 43). We are not aware of any direct comparison of ion channel expression between cell types. To what extent the immortal nature of HL-1 cells yields a less organized monolayer compared with NRVMs is unknown. Interestingly, shorter field potentials and slower conduction velocities can both be characteristic electrophysiological features of atrial fibrillation. Indeed, several studies have used HL-1 cultures to model reentrant rotors (12, 21) and hence provide a valuable model for determining the underlying mechanisms of these waveforms required to develop new atrial fibrillation therapies.
Our direct comparisons of the three cell types provide novel evidence that β-adrenergic responses differ between cardiomyocyte cultures. HL-1 cell cultures in our study were not responsive to β-adrenergic stimulation with isoproterenol, contrasting with the significant acceleration of beating rate in both NRVM and hiPSC-CMs. This may be related to the presence of norepinephrine in the culture medium specifically formulated for HL-1 cells (45). Prolonged adrenergic stimulation induces a downregulation of adrenergic receptors (47), which may explain the lack of response of HL-1 cells to 1 µM isoproterenol in the current study. Considering the apparent lack of field potential duration rate dependency in the HL-1 cells, this suggests that NRVM and hiPSC-CMs may be better suited for modeling the relationship between heart rate, action potential duration, and arrhythmogenesis.
The direct comparison of the electrophysiological properties of commonly used cardiomyocyte cultures provides phenotypic benchmarking of important criteria for optimizing drug screening and development. Although these in vitro models cannot emulate in vivo characteristics, cardiomyocyte cultures offer clear advantages for electrophysiological investigations compared with in vivo/ex vivo/tissue slice alternatives. The preparations are relatively simple to set up and provide a high-throughput capacity. The differences in cultured cardiomyocyte electrophysiological properties described here may even be exploited, enabling the targeting of specific cellular phenotypes that most closely model specific arrhythmia or drug response scenarios. Our findings suggest that NRVM and hiPSC-CM cultures may be suitable for modeling-triggered arrhythmia scenarios, with HL-1 cells better suited to model conduction rotors and reentrant arrhythmias.
In conclusion, our study provides a methodologic advance as the first study to demonstrate the intrinsic differences in electrophysiology of cultured cardiomyocyte preparations commonly used for in vitro electrophysiology assessment. Considering the interest in the MEA as a drug-screening platform, these differences can be considered for an appropriate experimental design when selecting cardiomyocyte in vitro approaches for modeling triggered and reentrant arrhythmia scenarios. Together with our optimization of the culture-seeding technique, these findings offer important comparative data to inform methodological approaches in the use of MEA and other techniques relating to cardiomyocyte functional screening investigations.
GRANTS
This research was supported by the National Health and Medical Research Council (nos. 1099352 and 1125453; L. M. D. Delbridge, J. R. Bell) and the Australian Research Council (no. DP160102404; L. M. D. Delbridge).
DISCLOSURES
P. Kirchhof receives research support for basic, translational, and clinical research projects from European Union, British Heart Foundation, Leducq Foundation, Medical Research Council (UK), and German Centre for Cardiovascular Research, from several drug and device companies active in atrial fibrillation, and has received honoraria from several such companies in the past. P. Kirchhof is listed as inventor on two patents held by University of Birmingham (Atrial Fibrillation Therapy WO 2015140571, Markers for Atrial Fibrillation WO 2016012783). None of the other authors has any conflicts of interest, financial or otherwise, to disclose.
AUTHOR CONTRIBUTIONS
S.P.W., H.M.W., E.R.P., L.M.D.D., and J.R.B. conceived and designed research; S.P.W., H.M.W., C.B.S., S.Y.L., and G.B.B. performed experiments; S.P.W., H.M.W., and G.B.B. analyzed data; S.P.W., H.M.W., G.B.B., D.P., P.K., E.R.P., L.M.D.D., and J.R.B. interpreted results of experiments; S.P.W., H.M.W., G.B.B., L.M.D.D., and J.R.B. prepared figures; S.P.W., H.M.W., L.M.D.D., and J.R.B. drafted manuscript; S.P.W., H.M.W., C.B.S., S.Y.L., G.B.B., D.P., P.K., E.R.P., L.M.D.D., and J.R.B. edited and revised manuscript; S.P.W., H.M.W., C.B.S., S.Y.L., G.B.B., D.P., P.K., E.R.P., L.M.D.D., and J.R.B. approved final version of manuscript.
REFERENCES
- 1.Anderson DJ, Kaplan DI, Bell KM, Koutsis K, Haynes JM, Mills RJ, Phelan DG, Qian EL, Leitoguinho AR, Arasaratnam D, Labonne T, Ng ES, Davis RP, Casini S, Passier R, Hudson JE, Porrello ER, Costa MW, Rafii A, Curl CL, Delbridge LM, Harvey RP, Oshlack A, Cheung MM, Mummery CL, Petrou S, Elefanty AG, Stanley EG, Elliott DA. NKX2-5 regulates human cardiomyogenesis via a HEY2 dependent transcriptional network. Nat Commun 9: 1373, 2018. doi: 10.1038/s41467-018-03714-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barriga M, Cal R, Cabello N, Llach A, Vallmitjana A, Benítez R, Badimon L, Cinca J, Llorente-Cortés V, Hove-Madsen L. Low density lipoproteins promote unstable calcium handling accompanied by reduced SERCA2 and connexin-40 expression in cardiomyocytes. PLoS One 8: e58128, 2013. doi: 10.1371/journal.pone.0058128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bell JR, Bernasochi GB, Varma U, Raaijmakers AJA, Delbridge LMD. Sex and sex hormones in cardiac stress--mechanistic insights. J Steroid Biochem Mol Biol 137: 124–135, 2013. doi: 10.1016/j.jsbmb.2013.05.015. [DOI] [PubMed] [Google Scholar]
- 4.Bell JR, Curl CL, Harding TW, Vila Petroff M, Harrap SB, Delbridge LMD. Male and female hypertrophic rat cardiac myocyte functional responses to ischemic stress and β-adrenergic challenge are different. Biol Sex Differ 7: 32, 2016. doi: 10.1186/s13293-016-0084-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boyle PM, Franceschi WH, Constantin M, Hawks C, Desplantez T, Trayanova NA, Vigmond EJ. New insights on the cardiac safety factor: unraveling the relationship between conduction velocity and robustness of propagation. J Mol Cell Cardiol 128: 117–128, 2019. doi: 10.1016/j.yjmcc.2019.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Braam SR, Tertoolen L, van de Stolpe A, Meyer T, Passier R, Mummery CL. Prediction of drug-induced cardiotoxicity using human embryonic stem cell-derived cardiomyocytes. Stem Cell Res (Amst) 4: 107–116, 2010. doi: 10.1016/j.scr.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 7.Brouillette J, Rivard K, Lizotte E, Fiset C. Sex and strain differences in adult mouse cardiac repolarization: importance of androgens. Cardiovasc Res 65: 148–157, 2005. doi: 10.1016/j.cardiores.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 8.Camelliti P, Al-Saud SA, Smolenski RT, Al-Ayoubi S, Bussek A, Wettwer E, Banner NR, Bowles CT, Yacoub MH, Terracciano CM. Adult human heart slices are a multicellular system suitable for electrophysiological and pharmacological studies. J Mol Cell Cardiol 51: 390–398, 2011. doi: 10.1016/j.yjmcc.2011.06.018. [DOI] [PubMed] [Google Scholar]
- 9.Chan YC, Tse HF, Siu CW, Wang K, Li RA. Automaticity and conduction properties of bio-artificial pacemakers assessed in an in vitro monolayer model of neonatal rat ventricular myocytes. Europace 12: 1178–1187, 2010. doi: 10.1093/europace/euq120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chowdhury RA, Tzortzis KN, Dupont E, Selvadurai S, Perbellini F, Cantwell CD, Ng FS, Simon AR, Terracciano CM, Peters NS. Concurrent micro- to macro-cardiac electrophysiology in myocyte cultures and human heart slices. Sci Rep 8: 6947, 2018. doi: 10.1038/s41598-018-25170-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Claycomb WC, Lanson NA Jr, Stallworth BS, Egeland DB, Delcarpio JB, Bahinski A, Izzo NJ Jr. HL-1 cells: a cardiac muscle cell line that contracts and retains phenotypic characteristics of the adult cardiomyocyte. Proc Natl Acad Sci USA 95: 2979–2984, 1998. doi: 10.1073/pnas.95.6.2979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Climent AM, Guillem MS, Fuentes L, Lee P, Bollensdorff C, Fernández-Santos ME, Suárez-Sancho S, Sanz-Ruiz R, Sánchez PL, Atienza F, Fernández-Avilés F. Role of atrial tissue remodeling on rotor dynamics: an in vitro study. Am J Physiol Heart Circ Physiol 309: H1964–H1973, 2015. doi: 10.1152/ajpheart.00055.2015. [DOI] [PubMed] [Google Scholar]
- 13.Costa M, Dottori M, Sourris K, Jamshidi P, Hatzistavrou T, Davis R, Azzola L, Jackson S, Lim SM, Pera M, Elefanty AG, Stanley EG. A method for genetic modification of human embryonic stem cells using electroporation. Nat Protoc 2: 792–796, 2007. doi: 10.1038/nprot.2007.105. [DOI] [PubMed] [Google Scholar]
- 14.Elliott DA, Braam SR, Koutsis K, Ng ES, Jenny R, Lagerqvist EL, Biben C, Hatzistavrou T, Hirst CE, Yu QC, Skelton RJ, Ward-van Oostwaard D, Lim SM, Khammy O, Li X, Hawes SM, Davis RP, Goulburn AL, Passier R, Prall OW, Haynes JM, Pouton CW, Kaye DM, Mummery CL, Elefanty AG, Stanley EG. NKX2-5(eGFP/w) hESCs for isolation of human cardiac progenitors and cardiomyocytes. Nat Methods 8: 1037–1040, 2011. doi: 10.1038/nmeth.1740. [DOI] [PubMed] [Google Scholar]
- 15.Garcia M, Mulvagh SL, Merz CNB, Buring JE, Manson JE. Cardiovascular disease in women: clinical perspectives. Circ Res 118: 1273–1293, 2016. doi: 10.1161/CIRCRESAHA.116.307547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goineau S, Castagné V. Electrophysiological characteristics and pharmacological sensitivity of two lines of human induced pluripotent stem cell derived cardiomyocytes coming from two different suppliers. J Pharmacol Toxicol Methods 90: 58–66, 2018. doi: 10.1016/j.vascn.2017.12.003. [DOI] [PubMed] [Google Scholar]
- 17.Graham EL, Balla C, Franchino H, Melman Y, del Monte F, Das S. Isolation, culture, and functional characterization of adult mouse cardiomyoctyes. J Vis Exp 79: e50289, 2013. doi: 10.3791/50289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Halbach M, Egert U, Hescheler J, Banach K. Estimation of action potential changes from field potential recordings in multicellular mouse cardiac myocyte cultures. Cell Physiol Biochem 13: 271–284, 2003. doi: 10.1159/000074542. [DOI] [PubMed] [Google Scholar]
- 19.Heist EK, Ruskin JN. Drug-induced arrhythmia. Circulation 122: 1426–1435, 2010. doi: 10.1161/CIRCULATIONAHA.109.894725. [DOI] [PubMed] [Google Scholar]
- 20.Hernández D, Millard R, Sivakumaran P, Wong RC, Crombie DE, Hewitt AW, Liang H, Hung SS, Pébay A, Shepherd RK, Dusting GJ, Lim SY. Electrical stimulation promotes cardiac differentiation of human induced pluripotent stem cells. Stem Cells Int 2016: 1718041, 2016. doi: 10.1155/2016/1718041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Houston C, Tzortzis KN, Roney C, Saglietto A, Pitcher DS, Cantwell CD, Chowdhury RA, Ng FS, Peters NS, Dupont E. Characterisation of re-entrant circuit (or rotational activity) in vitro using the HL1-6 myocyte cell line. J Mol Cell Cardiol 119: 155–164, 2018. doi: 10.1016/j.yjmcc.2018.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hund TJ, Rudy Y. Rate dependence and regulation of action potential and calcium transient in a canine cardiac ventricular cell model. Circulation 110: 3168–3174, 2004. doi: 10.1161/01.CIR.0000147231.69595.D3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jansen HJ, Moghtadaei M, Mackasey M, Rafferty SA, Bogachev O, Sapp JL, Howlett SE, Rose RA. Atrial structure, function and arrhythmogenesis in aged and frail mice. Sci Rep 7: 44336–44336, 2017. doi: 10.1038/srep44336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei B, Castella M, Diener HC, Heidbuchel H, Hendriks J, Hindricks G, Manolis AS, Oldgren J, Popescu BA, Schotten U, Van Putte B, Vardas P, Group ESCSD; ESC Scientific Document Group . 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J 37: 2893–2962, 2016. doi: 10.1093/eurheartj/ehw210. [DOI] [PubMed] [Google Scholar]
- 25.Lafuente-Lafuente C, Valembois L, Bergmann JF, Belmin J. Antiarrhythmics for maintaining sinus rhythm after cardioversion of atrial fibrillation. Cochrane Database Syst Rev 3: CD005049, 2015. doi: 10.1002/14651858.CD005049.pub4. [DOI] [PubMed] [Google Scholar]
- 26.Lane DA, Skjøth F, Lip GYH, Larsen TB, Kotecha D. Temporal trends in incidence, prevalence, and mortality of atrial fibrillation in primary care. J Am Heart Assoc 6: e005155, 2017. doi: 10.1161/JAHA.116.005155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lubitz SA, Sinner MF, Lunetta KL, Makino S, Pfeufer A, Rahman R, Veltman CE, Barnard J, Bis JC, Danik SP, Sonni A, Shea MA, Del Monte F, Perz S, Müller M, Peters A, Greenberg SM, Furie KL, van Noord C, Boerwinkle E, Stricker BH, Witteman J, Smith JD, Chung MK, Heckbert SR, Benjamin EJ, Rosand J, Arking DE, Alonso A, Kääb S, Ellinor PT. Independent susceptibility markers for atrial fibrillation on chromosome 4q25. Circulation 122: 976–984, 2010. doi: 10.1161/CIRCULATIONAHA.109.886440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meiry G, Reisner Y, Feld Y, Goldberg S, Rosen M, Ziv N, Binah O. Evolution of action potential propagation and repolarization in cultured neonatal rat ventricular myocytes. J Cardiovasc Electrophysiol 12: 1269–1277, 2001. doi: 10.1046/j.1540-8167.2001.01269.x. [DOI] [PubMed] [Google Scholar]
- 29.Mills RJ, Titmarsh DM, Koenig X, Parker BL, Ryall JG, Quaife-Ryan GA, Voges HK, Hodson MP, Ferguson C, Drowley L, Plowright AT, Needham EJ, Wang Q-D, Gregorevic P, Xin M, Thomas WG, Parton RG, Nielsen LK, Launikonis BS, James DE, Elliott DA, Porrello ER, Hudson JE. Functional screening in human cardiac organoids reveals a metabolic mechanism for cardiomyocyte cell cycle arrest. Proc Natl Acad Sci USA 114: E8372–E8381, 2017. doi: 10.1073/pnas.1707316114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mulla W, Gillis R, Murninkas M, Klapper-Goldstein H, Gabay H, Mor M, Elyagon S, Liel-Cohen N, Bernus O, Etzion Y. Unanesthetized rodents demonstrate insensitivity of qt interval and ventricular refractory period to pacing cycle length. Front Physiol 9: 897, 2018. doi: 10.3389/fphys.2018.00897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mummery CL, Zhang J, Ng ES, Elliott DA, Elefanty AG, Kamp TJ. Differentiation of human embryonic stem cells and induced pluripotent stem cells to cardiomyocytes: a methods overview. Circ Res 111: 344–358, 2012. doi: 10.1161/CIRCRESAHA.110.227512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nguyen PD, Hsiao ST, Sivakumaran P, Lim SY, Dilley RJ. Enrichment of neonatal rat cardiomyocytes in primary culture facilitates long-term maintenance of contractility in vitro. Am J Physiol Cell Physiol 303: C1220–C1228, 2012. doi: 10.1152/ajpcell.00449.2011. [DOI] [PubMed] [Google Scholar]
- 33.O’Shea C, Holmes AP, Yu TY, Winter J, Wells SP, Correia J, Boukens BJ, De Groot JR, Chu GS, Li X, Ng GA, Kirchhof P, Fabritz L, Rajpoot K, Pavlovic D. ElectroMap: High-throughput open-source software for analysis and mapping of cardiac electrophysiology. Sci Rep 9: 1389, 2019. doi: 10.1038/s41598-018-38263-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Parks RJ, Howlett SE. Sex differences in mechanisms of cardiac excitation-contraction coupling. Pflugers Arch 465: 747–763, 2013. doi: 10.1007/s00424-013-1233-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Porrello ER, Bell JR, Schertzer JD, Curl CL, McMullen JR, Mellor KM, Ritchie RH, Lynch GS, Harrap SB, Thomas WG, Delbridge LMD. Heritable pathologic cardiac hypertrophy in adulthood is preceded by neonatal cardiac growth restriction. Am J Physiol Regul Integr Comp Physiol 296: R672–R680, 2009. doi: 10.1152/ajpregu.90919.2008. [DOI] [PubMed] [Google Scholar]
- 36.Ribeiro MC, Tertoolen LG, Guadix JA, Bellin M, Kosmidis G, D’Aniello C, Monshouwer-Kloots J, Goumans M-J, Wang YL, Feinberg AW, Mummery CL, Passier R. Functional maturation of human pluripotent stem cell derived cardiomyocytes in vitro--correlation between contraction force and electrophysiology. Biomaterials 51: 138–150, 2015. doi: 10.1016/j.biomaterials.2015.01.067. [DOI] [PubMed] [Google Scholar]
- 37.Sala L, Ward-van Oostwaard D, Tertoolen LGJ, Mummery CL, Bellin M. Electrophysiological analysis of human pluripotent stem cell-derived cardiomyocytes (hPSC-CMs) using multi-electrode arrays (MEAs). J Vis Exp 123: 55587, 2017. doi: 10.3791/55587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sayed N, Liu C, Wu JC. Translation of human-induced pluripotent stem cells: from clinical trial in a dish to precision medicine. J Am Coll Cardiol 67: 2161–2176, 2016. doi: 10.1016/j.jacc.2016.01.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schinner C, Erber BM, Yeruva S, Waschke J. Regulation of cardiac myocyte cohesion and gap junctions via desmosomal adhesion. Acta Physiol (Oxf) 226: e13242, 2019. doi: 10.1111/apha.13242. [DOI] [PubMed] [Google Scholar]
- 40.Severino A, Narducci ML, Pedicino D, Pazzano V, Giglio AF, Biasucci LM, Liuzzo G, Casella M, Bartoletti S, Dello Russo A, Pelargonio G, Santangeli P, Di Biase L, Natale A, Crea F. Reversible atrial gap junction remodeling during hypoxia/reoxygenation and ischemia: a possible arrhythmogenic substrate for atrial fibrillation. Gen Physiol Biophys 31: 439–448, 2012. doi: 10.4149/gpb_2012_047. [DOI] [PubMed] [Google Scholar]
- 41.Trépanier-Boulay V, St-Michel C, Tremblay A, Fiset C. Gender-based differences in cardiac repolarization in mouse ventricle. Circ Res 89: 437–444, 2001. doi: 10.1161/hh1701.095644. [DOI] [PubMed] [Google Scholar]
- 42.Vandenberg JI, Perry MD, Hill AP. Recent advances in understanding and prevention of sudden cardiac death. F1000 Res 6: 1614, 2017. doi: 10.12688/f1000research.11855.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang N, Huo R, Cai B, Lu Y, Ye B, Li X, Li F, Xu H. Activation of Wnt/β-catenin signaling by hydrogen peroxide transcriptionally inhibits NaV1.5 expression. Free Radic Biol Med 96: 34–44, 2016. doi: 10.1016/j.freeradbiomed.2016.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wen Q, Gandhi K, Capel RA, Hao G, O’Shea C, Neagu G, Pearcey S, Pavlovic D, Terrar DA, Wu J, Faggian G, Camelliti P, Lei M. Transverse cardiac slicing and optical imaging for analysis of transmural gradients in membrane potential and Ca2+ transients in murine heart. J Physiol 596: 3951–3965, 2018. doi: 10.1113/JP276239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.White SM, Constantin PE, Claycomb WC. Cardiac physiology at the cellular level: use of cultured HL-1 cardiomyocytes for studies of cardiac muscle cell structure and function. Am J Physiol Heart Circ Physiol 286: H823–H829, 2004. doi: 10.1152/ajpheart.00986.2003. [DOI] [PubMed] [Google Scholar]
- 46.Wu H, Lee J, Vincent LG, Wang Q, Gu M, Lan F, Churko JM, Sallam KI, Matsa E, Sharma A, Gold JD, Engler AJ, Xiang YK, Bers DM, Wu JC. Epigenetic regulation of phosphodiesterases 2A and 3A underlies compromised β-adrenergic signaling in an iPSC model of dilated cardiomyopathy. Cell Stem Cell 17: 89–100, 2015. doi: 10.1016/j.stem.2015.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhao M, Hagler HK, Muntz KH. Regulation of α1-, β1-, and β2-adrenergic receptors in rat heart by norepinephrine. Am J Physiol Heart Circ Physiol 271: H1762–H1768, 1996. doi: 10.1152/ajpheart.1996.271.5.H1762. [DOI] [PubMed] [Google Scholar]