Abstract
Previous evidence suggests that palmitoylcarnitine incubations trigger mitochondrial-mediated apoptosis in HT29 colorectal adenocarcinoma cells, yet nontransformed cells appear insensitive. The mechanism by which palmitoylcarnitine induces cancer cell death is unclear. The purpose of this investigation was to examine the relationship between mitochondrial kinetics and glutathione buffering in determining the effect of palmitoylcarnitine on cell survival. HT29 and HCT 116 colorectal adenocarcinoma cells, CCD 841 nontransformed colon cells, and MCF7 breast adenocarcinoma cells were exposed to 0 μM, 50 μM, and 100 μM palmitoylcarnitine for 24–48 h. HCT 116 and HT29 cells showed decreased cell survival following palmitoylcarnitine compared with CCD 841 cells. Palmitoylcarnitine stimulated H2O2 emission in HT29 and CCD 841 cells but increased it to a greater level in HT29 cells due largely to a higher basal H2O2 emission. This greater H2O2 emission was associated with lower glutathione buffering capacity and caspase-3 activation in HT29 cells. The glutathione-depleting agent buthionine sulfoximine sensitized CCD 841 cells and further sensitized HT29 cells to palmitoylcarnitine-induced decreases in cell survival. MCF7 cells did not produce H2O2 when exposed to palmitoylcarnitine and were able to maintain glutathione levels. Furthermore, HT29 cells demonstrated the lowest mitochondrial oxidative kinetics vs. CCD 841 and MCF7 cells. The results demonstrate that colorectal cancer is sensitive to palmitoylcarnitine due in part to an inability to prevent oxidative stress through glutathione-redox coupling, thereby rendering the cells sensitive to elevations in H2O2. These findings suggest that the relationship between inherent metabolic capacities and redox regulation is altered early in response to palmitoylcarnitine.
Keywords: cancer, glutathione; mitochondria; reactive oxygen species; Warburg effect
INTRODUCTION
It is believed that cancer cell proliferation can be influenced by the interaction between cellular metabolism and redox conditions (reviewed in Refs. 14 and 35). As mitochondria generate reactive oxygen species during oxidative phosphorylation, it follows that the balance between glycolysis and oxidative metabolism may influence the cellular redox environment by altering the demands placed on the glutathione redox couple. This highly regulated antioxidant system is essential for both cancer cell growth and protection from cell death in response to a variety of cellular stressors, including certain chemotherapeutic compounds (reviewed in Refs. 6 and 31). As such, the effectiveness of targeting cancer metabolism to attenuate cancer survival may depend on the efficiency of glutathione buffering mechanisms in response to changes in metabolically derived reactive oxygen species generation.
As many cancers are inherently glycolytic (25, 36, 37), numerous studies have attempted to promote the generation of mitochondrial reactive oxygen species by activating oxidative phosphorylation in various models. For example, redirecting pyruvate away from lactate and toward oxidative phosphorylation through the activation of pyruvate dehydrogenase increases reactive oxygen species production and reduces cell proliferation in lung and tongue cancer cell lines (4). Likewise, resveratrol promotes fat oxidation and triggers reactive oxygen species-induced death in SW620 colon cancer cells (7), and orally administered acetylcarnitine reduces the formation of colorectal neoplastic lesions in a mouse model with no apparent deleterious side effects (32). Additionally, a diet high in butterfat causes an increase in aberrant crypt foci in another mouse model of colon cancer, which is partially prevented by the addition of free carnitine in the diet (10). While it is not fully clear how targeting mitochondrial oxidative phosphorylation decreased survival in these cancer models, several different groups have also demonstrated that the fatty acid-derived mitochondrial substrate palmitoylcarnitine selectively decreased cell survival in colorectal (38) and prostate cancer (1) cells. Specifically, Wenzel et al. (38) reported augmented superoxide production during palmitoylcarnitine oxidation that was lethal to colorectal HT29 cancer cells yet tolerated by preneoplastic NCOL-1 epithelial cells. This approach is intriguing in that palmitoylcarnitine oxidation may be less toxic to healthy cells given it is a natural metabolite oxidized by mitochondria. Collectively, these findings suggest approaches that stimulate mitochondrial bioenergetics may serve as a therapeutic avenue to selectively target cancer cells, but the mechanism for why nontransformed cells may be insensitive remains unclear. It seems possible that heterogeneous effects of palmitoylcarnitine on cell survival could be linked to inherent differences in glutathione redox buffering responses.
The purpose of this investigation was to evaluate the early time course by which palmitoylcarnitine abrogates cell survival in HT29 colorectal cancer cells vs. nontransformed cells as reported by Wenzel et al. (38) and to determine whether cell-specific susceptibilities to palmitoylcarnitine are due to intrinsic differences in the glutathione response. We also determined whether differences in mitochondrial oxidative characteristics are related to the redox response to palmitoylcarnitine. Palmitoylcarnitine elicited HT29-specific decreases in cell survival, concurrent with a decrease in glutathione levels, despite no effect on nontransformed CCD 841 cell survival or glutathione. However, both HT29 and CCD 841 cells increased H2O2 in response to palmitoylcarnitine, despite HT29 cells having approximately twofold greater baseline steady-state H2O2. By pharmacologically depleting glutathione concurrently with palmitoylcarnitine incubations, we rendered CCD 841 cells sensitive to palmitoylcarnitine and further sensitized HT29 cells to the deleterious effects of palmitoylcarnitine. These data support the notion that glutathione is a critical regulator in determining cell survival in HT29 and CCD 841 cells when exposed to palmitoylcarnitine.
EXPERIMENTAL PROCEDURES
Cell Lines
CCD 841 control primary colon epithelial cells were purchased from the American Type Culture Collection (ATCC, Manassas, VA). HT29 and HCT 116 colorectal adenocarcinoma cells and MCF7 breast adenocarcinoma cells were a generous gift from Dr. Samuel Benchimol (York University, Toronto, Canada). CCD 841 cells were grown in Eagle's minimum essential media (Wisent, Saint-Jean-Baptiste, QC, Canada), HT29 and HCT 116 cells were grown in Dulbecco’s modified Eagle’s medium (Wisent), and MCF7 cells were grown in alpha modification of minimum essential media (Wisent). All media were supplemented with 10% fetal bovine serum (Wisent) and 1% penicillin/streptomycin (Wisent) and grown at 37°C in 5% CO2.
Palmitoylcarnitine Incubations
For palmitoylcarnitine treatments, cells were treated with 2 mM l-carnitine (Sigma-Aldrich) and increasing palmitoyl-l-carnitine (0 μM, 50 μM, and 100 μM palmitoylcarnitine; Toronto Research Chemicals) for 24 or 48 h in phenol red-free versions of their respective growth media (Wisent).
Relative Cell Survival Assay
The crystal violet assay was used to determine relative cell survival (13). Following 24 or 48 h of palmitoylcarnitine incubations, with or without and 50 μM L-buthionine-sulfoximine (BSO; Sigma-Aldrich), cells plated in 96-well optical-bottom black-walled plates (ThermoFisher Scientific) were incubated in 10% formalin (Sigma-Aldrich) for 10 min. Formalin was removed, and a 0.5% crystal violet (Sigma-Aldrich) solution in 25% MeOH was added for 10 min. Following crystal violet staining, wells were rinsed clean with water and allowed to dry overnight. Visualization of crystal violet as a marker for relative cell survival was conducted using the Li-Cor Odyssey scanner (Li-Cor Biosciences, Lincoln, NE), and fluorescent density analysis was conducted using Li-Cor software.
XTT As a Measure of NAD(P)H
XTT is a negatively charged tetrazolium salt that changes color and becomes soluble following reduction, particularly in response to NADH (12, 19). Therefore, XTT serves as an indirect measure of either NADH or NAD(P)H concentration, which are collectively referred to as NAD(P)H. In this study, it is assumed that a change in XTT signal in response to palmitoylcarnitine reflects a change in NADH. To perform the assay, cells were treated with increasing amounts of palmitoylcarnitine for 24 or 48 h in 96-well plates. In the final 4 h of each treatment, 50 μL of XTT solution comprised of 1 mg/mL XTT (BioShop Canada) dissolved in media with 25 μM phenazine methosulfate (Sigma-Aldrich) were added directly to each well. Cells were incubated for 4 h at 37°C at 5% CO2, and then absorbance at 450 nm was read using the VICTOR3 1420 Multilabel Counter plate reader (PerkinElmer, Waltham, MA). NAD(P)H absorbance was made relative to 0 μM palmitoylcarnitine. To account for variations in cell number, cells were then digested in-well using 10% RIPA (Sigma-Aldrich,) while protein was assessed with BCA (ThermoFisher Scientific) according to kit instructions.
Live-Cell H2O2 Measurements
Cells were seeded in a 96-well optical-bottom black-walled plate (ThermoFisher Scientific). For H2O2 determination, cells were treated with palmitoylcarnitine for 24 and 48 h. Cells were then incubated with 10 μM Amplex UltraRed and 1 U/mL horseradish peroxidase for 20 min, and then fluorescent intensity (EX568/EM581) was taken using the BioTek Cytation 3 fluorescent plate reader (BioTek, Winooski, VT). A single read after these timepoints reflected the net accumulated fluorescent resorufin product of oxidized Amplex Ultrared and therefore represents the “net emission” or “net pressure” of H2O2 that cells experienced throughout this time period. This measurement was then made relative to a H2O2 standard curve. To account for variations in cell number, cells were then digested in-well using 10% RIPA (Sigma-Aldrich) while protein was assessed with BCA (ThermoFisher Scientific) according to kit instructions.
Glutathione Analysis
Glutathione was measured as previously published (17, 18, 27). Both reduced (GSH) and oxidized (GSSG) glutathione were measured using an Agilent HPLC 1100 (Agilent Technologies, Mississauga, ON, Canada), and separation was achieved using a Zorbax C18 column (Agilent Technologies). Cells were trypsin lifted, washed with PBS, and then resuspended in 50 mM Tris buffer with 20 mM boric acid (Sigma-Aldrich), 2 mM l-serine (Sigma-Aldrich), 20 μM acivicin (Enzo Life Sciences), and 5 mM N-ethylmaleimide (Sigma-Aldrich), pH at 8.0 with HCl. Glutathione concentrations were calculated using standard concentration curves for GSH and GSSG (Sigma-Aldrich) and normalized to total protein.
GSH.
This method was adapted from Giustarini et al. (15). Cells were deproteinated in 10 μL trichloroacetic acid with 100 μL cells in Tris buffer suspension. GSH samples were run under isocratic conditions using a 0.25% glacial acetic acid mobile phase with 6% acetonitrile with a flow rate of 1.25 mL/min. GSH was detected using a modular variable-wavelength detector (Agilent Technologies) at 265 nm wavelength.
GSSG.
This method was adapted from Kand’ár et al. (21). Cells were deproteinated in equal parts 15% perchloric acid to sample volume. Deproteinated sample (100 μL) was added to 500 μL of 0.5 M NaOH. o-Pthalymide (37.5 μL of 0.1%; Sigma-Aldrich) in methanol was added to the 600-μL sample volume and incubated for 15 min rocking in the dark creating a GS-OPA conjugate. Following incubation, samples were transferred to an HPLC autosampler vial for column separation. GSSG mobile phase was 25 mM Na2HPO4 in HPLC-grade water with 15% methanol at a flow rate of 0.5 mL/min. Following column separation, the eluent flowed through a Firefly Sci 8830 flow-through cuvette (Firefly Sci), and GSSG peak was detected using a QuantaMaster 40 spectrafluoromoter (Horiba, Edison, NJ). GS-OPA was excited at 350 nm, and emission was detected at 420 nm.
Caspase Activity Assay
Enzymatic activity of caspase-3 was determined using the substrate Ac-DEVD-AMC (Enzo Life Sciences), as previously described (8, 9). Following palmitoylcarnitine incubations, cells were isolated using lysis buffer containing (in mM): 20 mM HEPES, 10 mM NaCl, 1.5 mM MgCl, 1 mM DTT, 20% glycerol, and 0.1% Triton X-100, pH 7.4, without addition of protease inhibitors and were sonicated for 3 × 3 s. Cells were incubated in 96-well plates with Ac-DEVD-AMC at 37°C. Fluorescence was measured with excitation and emission wavelengths of 360 and 440 nm, respectively. Caspase-3 activity was normalized to total protein content and expressed as fluorescence intensity in arbitrary units per milligram protein.
Intracellular Lactate Determination
Following palmitoylcarnitine incubations, adherent cells were trypsin harvested, washed two times in PBS, resuspended in 0.5 M perchloric acid, vortexed, and freeze-thawed in liquid nitrogen three times. Following centrifugation, 2.2 M KHCO3 was added and centrifuged at 4°C for 15 min at 7,000 rpm, and the supernatant was collected. Supernatant (20 μL) was added to 258 μL lactate buffer [1 M glycine, 500 mM hydrazine sulfate (Sigma-Aldrich), 5 mM EDTA, pH 9.5], 20 μL NAD (Sigma-Aldrich), and 2 μL of heart lactate dehydrogenase (LDH; Sigma-Aldrich). Each sample was rocked briefly, and 300 μL were inserted in a 96-well plate, in triplicate, and compared with a non-LDH added mirroring sample. Absorption of NADH was measured at 340 nm on a BioTek Cytation 3 plate reader and made relative to total protein.
Cell Permeabilization and High-Resolution Respirometry
Cells were seeded in 10-cm dishes for 48 h, trypsin harvested, washed in PBS, and resuspended in mitochondrial respiration media (MiRO5, in mM): 0.5 EGTA, 10 KH2PO4, 3 MgCl2·6 H2O, 60 potassium lactobionate, 20 HEPES, 20 taurine, 110 sucrose, and 1 mg/mL fatty acid-free BSA (pH 7.1) supplemented with 20 mM creatine. Cells were then permeabilized with 10 μg/mL digitonin (Sigma-Aldrich) for 30 min rocking at room temperature. Following centrifugation for 5 min at 2,000 rpm, cells were resuspended in 105 μL MiRO5, with 5 μL subsequently removed to determine protein content, while the remaining 100 μL were used for high-resolution respirometry.
Cells were loaded in the Oroboros Oxygraph-2k (Oroboros Instruments, Innsbruck, Austria) system. Total volume was 2 mL, with spinning at 750 rpm and temperature at 37°C. To determine ADP-stimulated respiration, 5 mM pyruvate and 2 mM malate were added as complex I substrates (NADH), followed by ADP titrations of 25 µM, 500 µM, and 5 mM ADP. Polarographic oxygen measurements were acquired in 2-s intervals, with the rate of respiration derived from 40 data points and expressed as pmol·s−1·mg protein−1.
Statistics
All results are expressed as means ± SE. Significance was determined as P < 0.05 for all measures. Each “N” signifies an individual experiment, with each experiment conducted in triplicate where appropriate. For the comparison of only two groups, unpaired t tests were used. For the comparison of more than two groups, ANOVAs were conducted. Following significance with a one-way ANOVA, a Dunnett’s post hoc analysis was performed, and following a significant two-way ANOVA, a Fisher’s LSD post hoc was performed. All statistics were performed using GraphPad Prism 7 (San Diego, CA).
RESULTS
HT29 Cells Are Sensitive to Palmitoylcarnitine-Induced Cell Death
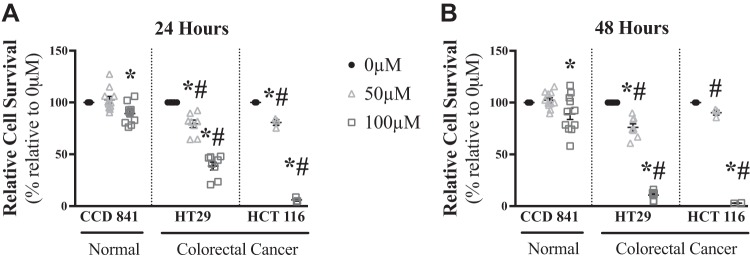
To determine the influence of palmitoylcarnitine on relative cell survival, HT29 and HCT 116 cells and nontransformed colon epithelial CCD 841 cells were incubated for 24 (Fig. 1A) and 48 (Fig. 1B) h with 0 μM, 50 μM, and 100 μM palmitoylcarnitine, similar to concentrations used in previous literature (1, 38). While CCD 841 cells had a modest ~10% decrease in relative cell survival with 100 μM, HT29 and HCT 116 cells had more robust decreases in relative cell survival at 50 (~20%) and 100 (~90%) μM palmitoylcarnitine (P < 0.05), with HT29 and HCT 116 cells showing decreased relative cell survival compared with CCD 841 cells at each palmitoylcarnitine concentration (P < 0.05, Fig. 1, A and B).
Fig. 1.
Palmitoylcarnitine toxicity is greater in colorectal cancer HT29 and HCT 116 cells than normal CCD 841 cells. Relative cell survival was measured in nontransformed CCD 841 cells (N = 11) as well as HT29 (N = 8) and HCT 116 (N = 3) cells for 24 h (A) and 48 h (B). Data are reported as means ± SE. P < 0.05, significant difference relative to 0 µM palmitoylcarnitine within the same cell type (*) and significant difference of the same palmitoylcarnitine concentration relative to CCD 841 (#).
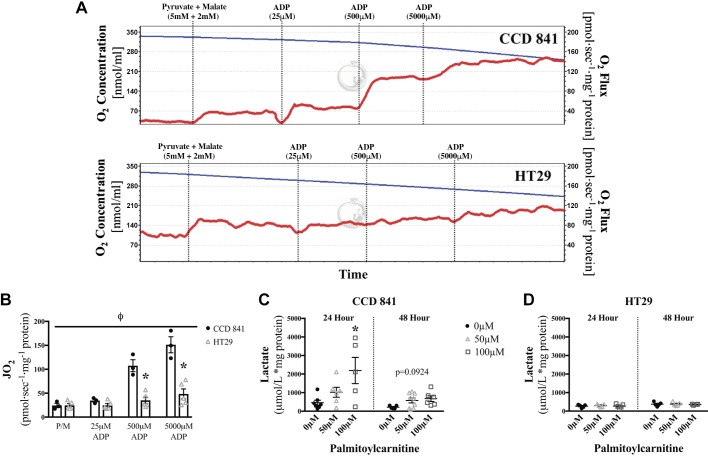
We next determined whether colorectal cancer displayed altered mitochondrial respiratory kinetics and metabolic flexibilities to explain their sensitivity to palmitoylcarnitine. HT29 cells had significantly lower coupled respiratory kinetics (ADP stimulation of ATP synthesis) relative to CCD 841 cells (P < 0.05, Fig. 2, A and B). CCD 841 cells demonstrated increased net lactate levels in response to 24 h of palmitoylcarnitine (P < 0.05), which is in line with the expected redirection of glucose-derived pyruvate away from the mitochondria when excess fatty acids are present (Fig. 2C). At 48 h, there was no difference in intracellular lactate levels. Although difficult to explain, this may suggest that CCD 841 cells had exhausted the exogenously added palmitoylcarnitine supply. This metabolic flexibility was not observed in HT29 cells, given no change in net lactate concentrations was observed in response to palmitoylcarnitine (Fig. 2D).
Fig. 2.
Lower mitochondrial respiratory kinetics and impaired metabolic flexibility in HT29 cells compared with CCD 841 cells. Mitochondrial respiratory kinetics and the cell lactate concentrations were assessed in response to palmitoylcarnitine in CCD 841 and HT29 cells. A: a representative high-resolution respirometric trace showing oxygen consumption (red line) that is calculated by the change in slope of oxygen concentration in the respiratory chamber (blue line; top, CCD 841 cells; bottom, HT29 cells). B: mitochondrial respiratory kinetics of CCD 841 cells compared with HT29 cells across a range of ADP concentrations reflecting low and high metabolic demands. P < 0.05, a significant difference between HT29 and CCD 841 cells of a given substrate (*) and main effect of cell type (Φ) (N = 5). C and D: net intracellular lactate was determined in CCD 841 (N = 8–9) (C) and HT29 (N = 5) (D) cells for 24 and 48 h. *P < 0.05, significant difference relative to 0 µM palmitoylcarnitine of the same time point. Data are reported as means ± SE.
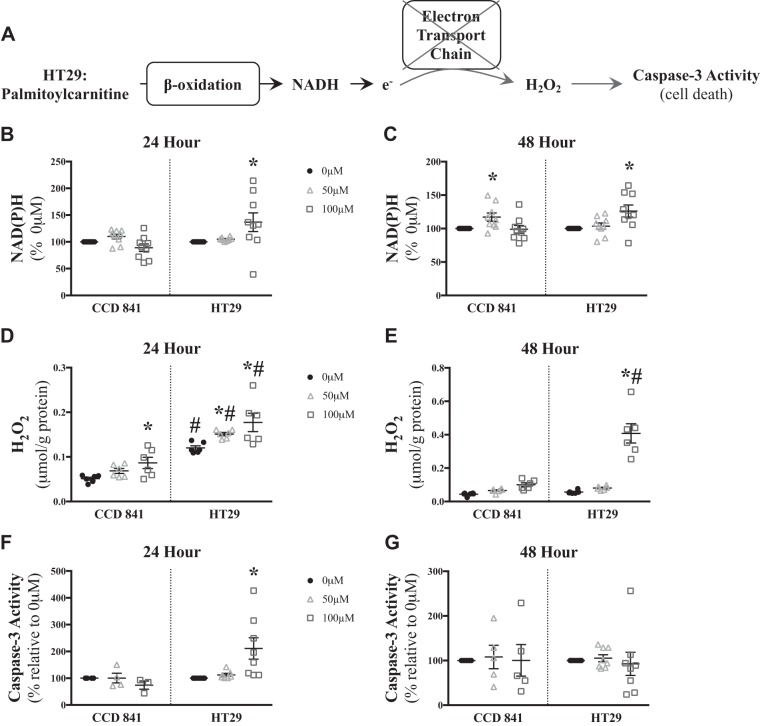
Excessive NADH generation relative to low rates of oxidative phosphorylation can lead to H2O2 production, which can trigger deleterious cellular effects such as caspase-3 activation (Fig. 3A; see Ref. 29). Given the lower respiratory kinetics shown in Fig. 2, we next tested the hypothesis that NADH generation from palmitoylcarnitine remained intact in HT29 cells such that excess NADH provision to the electron transport chain would increase H2O2 emission (Fig. 3A). Indeed, palmitoylcarnitine resulted in greater accumulations in NAD(P)H in HT29 cells at both 24 and 48 h (100 μM: +~35%), with only a modest increase in NAD(P)H observed at 48 h in CCD 841 (50 μM: +~20%) as detected by XTT (P < 0.05, Fig. 3, B and C). This was associated with greater H2O2 emission (P < 0.05, Fig. 3, D and E) and induction of caspase-3 activity (P < 0.05, Fig. 3, F and G) in HT29 cells at 24 h, yet there was no observable change in capase-3 activity at 48 h, suggesting an early activation of caspase-3 resulting in cell death leading to the cessation of caspase-3 activity. This relationship of NAD(P)H to H2O2 emission was not observed in all conditions, such as 50 µM at 24 h (Fig. 3, B and D), but may be related to the difficulty in capturing the precise temporal relationship between NADH and H2O2 kinetics.
Fig. 3.
Elevated NAD(P)H production leads to increased H2O2 emission and caspase-3 induction in low-oxidative-capacity HT29 cells but not high-oxidative-capacity CCD 841 cells. A: proposed schematic of how palmitoylcarnitine may result in elevated caspase-3 activity through H2O2 generation in a state of low oxidative capacity. NAD(P)H levels were measured in CCD 841 (N = 9) and HT29 (N = 9) cells for 24 h (B) and 48 h (C). Total H2O2 emission was measured in CCD 841 (N = 6) and HT29 (N = 6) cells after 24 h (D) and 48 h (E). Caspase-3 activity was measured in CCD 841 (N = 4–5) and HT29 (N = 8) cells for 24 h (F) and 48 h (G). Data are reported as means ± SE. P < 0.05, significant difference relative to 0 µM palmitoylcarnitine of the same time point (*) and significant difference relative to CCD 841 of the same palmitoylcarnitine concentration (#).
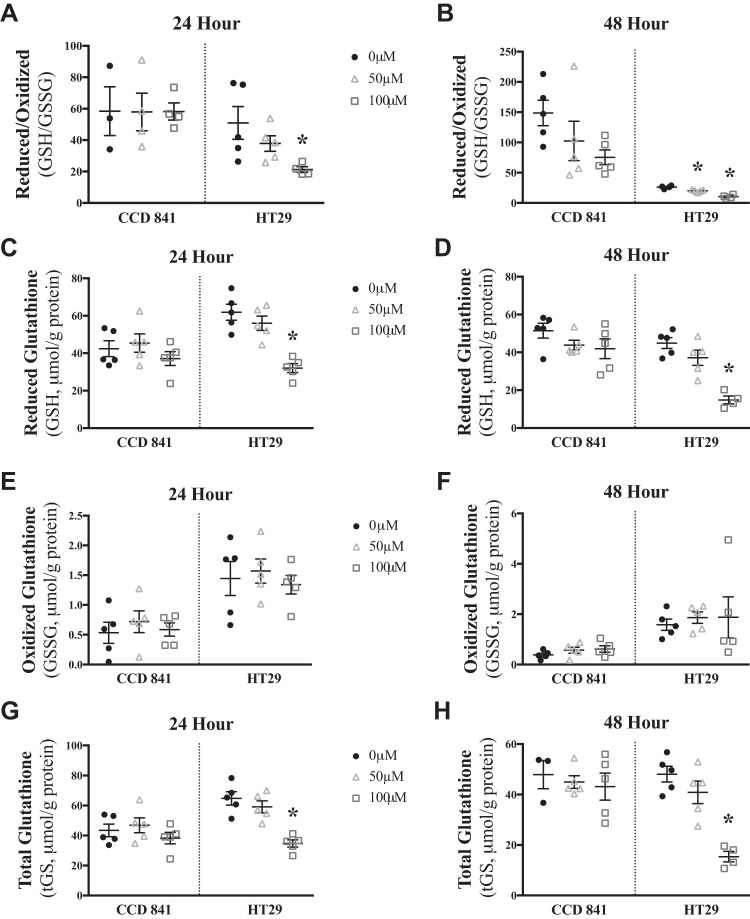
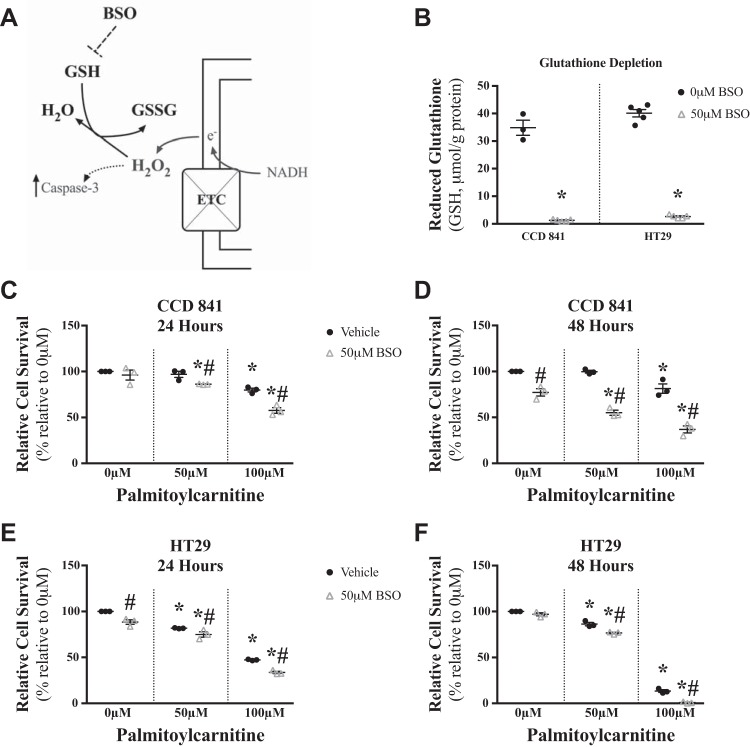
Elevated H2O2 emission in relation to decreased cell survival in HT29 cells suggested that glutathione redox buffering might be insufficient to protect HT29 cells from palmitoylcarnitine-induced stress. In HT29 cells, 24 h of palmitoylcarnitine lowered the reduced-to-oxidized glutathione ratio (P < 0.05, Fig. 4, A and B), reduced (P < 0.05, Fig. 4, C and D) and total (P < 0.05, Fig. 4, G and H) glutathione without changing total oxidized glutathione (Fig. 4, E and F), suggesting a net attenuation of glutathione redox buffering dynamics despite no changes in CCD 841 cell glutathione response to palmitoylcarnitine. Based on these associations, we then determined whether glutathione redox buffering directly determined cell survival in response to palmitoylcarnitine. The glutathione synthesis inhibitor BSO (Fig. 5A) nearly eliminated reduced glutathione in CCD 841 and HT29 cells (P < 0.05, Fig. 5B) and sensitized CCD 841 cells to palmitoylcarnitine-induced decreasing cell survival (P < 0.05, Fig. 5, C and D) while further sensitizing HT29 cells to decreasing cell survival (P < 0.05, Fig. 5, E and F).
Fig. 4.
Palmitoylcarnitine lowers glutathione redox buffering capacity in HT29 cells but not CCD 841 cells. The following parameters of glutathione redox buffering were assessed after 24 and 48 h of palmitoylcarnitine, respectively: ratio of reduced to oxidized glutathione (GSH/GSSG, A and B), reduced glutathione (GSH, C and D), oxidized glutathione (GSSG, E and F), and total glutathione (tGS, G and H) (N = 5). Data are reported as means ± SE. *P < 0.05, significant difference relative to 0 µM palmitoylcarnitine of the same cell type.
Fig. 5.
Glutathione depletion sensitizes CCD 841 and HT29 cells to palmitoylcarnitine-induced decreasing cell survival. A: schematic depicting the influence of buthionine sulfoxomine (BSO, inhibitor of glutathione synthesis) on glutathione levels. ETC, electron transport chain; GSH, reduced glutathione; GSSG, oxidized glutathione. B: GSH was measured in CCD 841 and HT29 cells following 24 h with 0 µM or 50 µM BSO. *Significant difference relative to 0 µM BSO. Following palmitoylcarnitine incubations concurrent with BSO, relative cell survival was measured in CCD 841 cells for 24 and 48 h, respectively (C and D), and in HT29 cells (E and F) (N = 3). Data are reported as means ± SE. P < 0.05, significant difference relative to 0 µM palmitoylcarnitine (*) and significant difference between vehicle and 50 µM BSO of the same palmitoylcarnitine concentration (#).
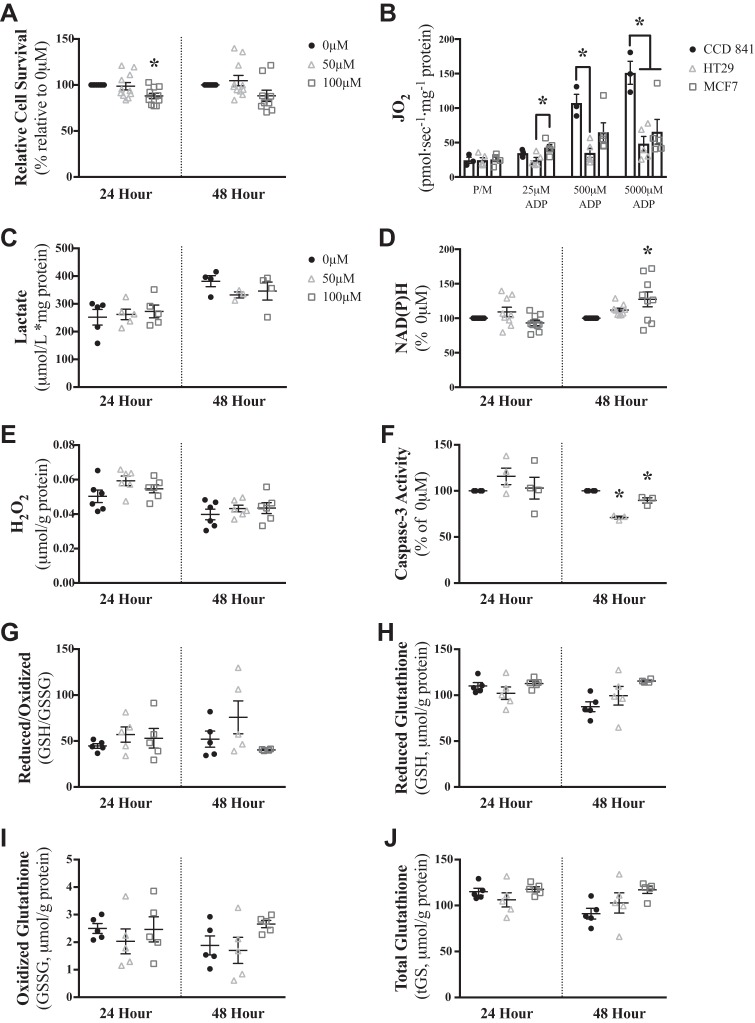
We then explored whether the susceptibility of HT29 cells to palmitoylcarnitine was observed in a cancer line previously shown to be reliant on mitochondrial oxidative phosphorylation (2), the MCF7 breast cancer cell line. In so doing, the role of metabolic and redox flexibility in determining the degree of (in)sensitivity to palmitoylcarnitine could be compared between cell lines. Palmitoylcarnitine had a small effect on cell survival in MCF7 cells after 24 (P < 0.05) but not 48 (Fig. 6A) h, indicating that MCF7 cells are more insensitive to palmitoylcarnitine than HT29 cells. To validate whether MCF7 cells displayed greater mitochondrial respiratory kinetics compared with HT29 cells (consistent with the notion that MCF7 cells are reliant on mitochondrial metabolism), mitochondrial respiration was compared across HT29, MCF7, and CCD 841 cells. MCF7 cell mitochondrial respiration was greater than HT29 cells at physiological 25 µM ADP, yet not statistically different from CCD 841 cells at 25 µM and 500 µM ADP (Fig. 6B). However, no changes in intracellular lactate were observed in response to palmitoylcarnitine (Fig. 6C), suggesting MCF7 cells lack metabolic flexibility in response to fatty acid challenges. Indeed, an increase in NAD(P)H was observed at 48 h (Fig. 6D), similar to that seen previously with HT29 cells, suggesting the generated NADH is not oxidized by the attenuated rates of oxidative phosphorylation. However, this NADH generation was not sufficient to stimulate H2O2 emission (Fig. 6E), which was consistent with no increases in caspase-3 activity (Fig. 6F) and no changes in glutathione redox buffering responses (Fig. 6, G–J).
Fig. 6.
MCF7 cells are insensitive to palmitoylcarnitine. A: MCF7 cells were incubated with palmitoylcarnitine for 24 and 48 h and assessed for relative cell survival (N = 11). B: mitochondrial respiratory kinetics in MCF7 cells were compared with CCD 841 and HT29 cells (from Fig. 2A) (N = 5). MCF7 cells were incubated with palmitoylcarnitine for 24 and 48 h and assessed for net intracellular lactate (N = 5) (C), NAD(P)H (N = 9) (D), H2O2 (N = 6) (E), caspase-3 activity (N = 4) (F) reduced glutathione (GSH)/oxidized glutathione (GSSG, N = 5) (G), reduced glutathione (N = 5) (H), oxidized glutathione (N = 5) (I), and total glutathione (N = 5) (J). Data are reported as means ± SE. *P < 0.05, significant difference relative to 0 µM palmitoylcarnitine of the same time point.
Overall, these results indicate that the susceptibilities of HT29 cells to palmitoylcarnitine are linked to insufficient redox buffering that is not observed in MCF7 breast cancer cells or nontransformed CCD 841 cells.
DISCUSSION
Palmitoylcarnitine caused marked reductions in cell survival in HT29 and HCT 116 colorectal cancer cells, with minor reductions in nontransformed CCD 841 colon cells and MCF7 breast cancer cells. The degree of response was related to oxidative kinetics, H2O2 emission, and the ability to maintain glutathione redox buffering (Fig. 7). Specifically, the greatest H2O2 emission occurred in HT29 cells in association with a collapse in glutathione redox buffering capacity and attenuated cell survival. These results suggest cancer-specific mitochondrial oxidative kinetics and glutathione may influence cell-specific responses to palmitoylcarnitine-induced oxidative stress.
Fig. 7.
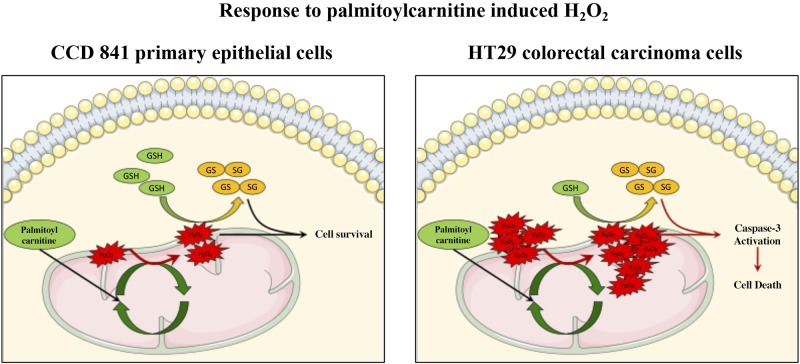
Palmitoylcarnitine-induced decreases in cell survival are dictated by baseline mitochondrial reactive oxygen species and glutathione response. Upon exposure to palmitoylcarnitine, there is an increase in reactive oxygen species production; however, this increase in reactive oxygen species is sufficiently buffered by glutathione, resulting in maintaining CCD 841 cell survival. HT29 cells, however, have significantly higher baseline reactive oxygen species; therefore, upon exposure to palmitoylcarnitine, the additional increase in reactive oxygen species production leads to decreases in glutathione and subsequent caspase-3 activation and cell death. GSH, reduced glutathione; GSSG, oxidized glutathione.
Oxidative Kinetics and Redox Flexibility Determine Susceptibility to Palmitoylcarnitine
In the present study, we considered classic bioenergetic principles that posit that an oversupply of reducing equivalents (NADH, FADH2) from excess fat oxidation relative to the metabolic demands for oxidative phosphorylation leads to increased membrane potential and concurrent electron slip on oxygen to generate superoxide and subsequently H2O2 (29). With these bioenergetic principles in mind, cells with low dependencies on mitochondrial oxidative phosphorylation may generate higher rates of H2O2 emission when provided with excess substrate. Bypassing carnitine palmitoyltransferase-1 in HT29 cells with palmitoylcarnitine increased NADH steady-state levels, possibly because the lower reliance on oxidative phosphorylation in this cell line caused NADH production to exceed its oxidation in the electron transport chain, thereby stimulating H2O2 emission. In contrast, highly oxidative CCD 841 nontransformed epithelial cells may have been able to oxidize the NADH that was generated in response to palmitoylcarnitine, although this is speculative. Because CCD 841 cells demonstrated lower baseline H2O2 emission kinetics, the subsequent increase in H2O2 emission with palmitoylcarnitine reached a lower absolute rate than seen in HT29 cells. The data suggest that this lower H2O2 was effectively buffered by the glutathione redox couple and resulted in no caspase-3 induction and markedly less effects on overall cell survival. Conversely, the higher rates of H2O2 emission generated in HT29 cells were related to a concurrent collapse in glutathione buffering capacity, which further reveals an inferior redox buffering response in this cancer. This is in line with previous literature demonstrating that cells with higher baseline or steady-state reactive oxygen species production are more susceptible to further elevations in reactive oxygen species through glucose starvation, likely resulting in an increased reliance on mitochondrial metabolism (3).
However, several observations are not entirely in agreement with our previous conclusions. For example, while palmitoylcarnitine resulted in an increase in NAD(P)H in MCF7 cells, there was no associated increase in H2O2 emission, perhaps because of glutathione levels successfully buffering changes in palmitoylcarnitine-induced H2O2, preventing any associated change in fluorescent signal. Furthermore, there was no statistical increase in NAD(P)H levels at 24 h following 50 µM palmitoylcarnitine in HT29 cells despite an associated increase in H2O2 emission and a corresponding decrease in cell survival. While difficult to explain, this perhaps could be attributed to the temporal nature of bioenergetics such that an earlier increase in NAD(P)H may have preceded the increase in H2O2 emission. The highly dynamic turnover of NAD(P)H, H2O2, and glutathione, particularly during oxidative phosphorylation, poses a challenge in capturing the precise temporal relationship at any given palmitoylcarnitine challenge and time point. Nevertheless, as noted above, the responses following 100 µM palmitoylcarnitine suggest that the lower cell survival in HT29 cells was related to insufficient glutathione homeostasis.
While palmitoylcarnitine is naturally produced within the cell, exogenous palmitoylcarnitine has been demonstrated to increase intracellular palmitoylcarnitine levels in neuroblastoma NB-2a cells (28), and has previously been demonstrated to cause an adaptive increase in oxidative capacity in mouse embryonic fibroblasts (26). Elevating mitochondrial oxidative phosphorylation has also been mimicked in experiments designed to force pyruvate shuttling toward mitochondria either through inhibiting lactate production (23) or by activating pyruvate dehydrogenase (4). It is therefore possible that similar relative increases in mitochondrial reactive oxygen species between cancerous and nontransformed cells result in different fates, whereby increasing reactive oxygen species in cells with higher baseline levels would cause great attenuations in glutathione, resulting in cell death, whereas cells with lower baseline levels would be able to combat similar relative elevations in reactive oxygen species production through glutathione buffering.
A key finding in this study is that glutathione depletion by BSO amplified the reduction in cell survival following palmitoylcarnitine incubations in HT29 cells and sensitized nontransformed CCD 841 cells. BSO is a selective inhibitor of glutamate cysteine ligase (previously known as γ-glutamylcysteine synthetase), resulting in a rapid decrease in intracellular glutathione levels by preventing its synthesis (11, 16), which was effective at inhibiting growth of a mammary adenocarcinoma cell line in vivo (34). The decreased cell survival in HT29 cells in the present study reveals an insufficient ability of this cancer to maintain glutathione-based redox buffering capacity that is otherwise intact in CCD 841 cells. This suggests that assessment of glutathione redox buffering capacity may serve as a biomarker to predict cancer-specific sensitivity to palmitoylcarnitine as a therapy.
Intriguingly, selective leukemia cell death in acute myeloid leukemia cells was achieved by inhibiting mitochondrial fat oxidation with avocatin B (24), mitochondrial complex I with mubritinib (5), amino acid mitochondrial oxidation (20), and BCL-2-dependent mitochondrial metabolism (22). These findings seem to be opposite to the present data whereby the provision of excess mitochondrial substrates resulted in colorectal cancer cell death. While we cannot fully explain the heterogeneity in cancer cell responses toward mitochondrial-targeted therapies, the response of glutathione to metabolic-targeting therapies may provide insight into determining cell fate. Many current chemotherapies elevate intracellular levels of reactive oxygen species or target redox-homeostasis to elicit anti-cancer effects (39). However, given that we are currently unable to detect in vivo kinetics of reactive oxygen species production in cancer patients, the present results may serve as a foundation to explore the possibility that unique glutathione redox-buffering capacities within tumors may yield predictive value for guiding future therapies that trigger mitochondrial reactive oxygen species by targeting mitochondrial metabolism.
We note two limitations of the present work. First, the respiratory kinetics observed in the present study may be underestimations given the respirometric protocols were performed with cells in suspension rather than in an adherent environment as they are accustomed to given their epithelial origin. While palmitoylcarnitine-supported respiration was not detected in any cell lines possibly because of this reason (data not shown), the increased ADP-stimulated respiration nonetheless demonstrates a robust difference in mitochondrial oxidative phosphorylation between cell lines. Second, the lactate concentrations reflect intracellular lactate rather than efflux, which would otherwise require assessments of acidification rates of local media in combination with lactate efflux assessments. However, changes in intracellular lactate concentrations serve as an index of altered glycolytic flux and support the present interpretation.
Perspectives and Conclusions
Collectively, HT29 cells demonstrate greater reductions in cell survival in response to palmitoylcarnitine in association with lower oxidative kinetics, higher baseline H2O2, and inferior glutathione redox flexibility. These relationships were observed in <48 h, suggesting metabolic and redox processes occur early in response to palmitoylcarnitine challenges. Previous literature demonstrates a paradoxical role of mitochondrial activation in cancer survival, whereby some cancers rely on mitochondrial metabolism to drive cell growth, such as MCF7 cells (2), ovarian cancers (30), and leukemia cells (24), whereas other cancers demonstrate decreased survival if forced to rely on mitochondrial oxidative phosphorylation (3, 23, 33, 38). A striking corollary is the potential for nontransformed cells to tolerate changes in metabolism, as was observed in CCD 841 cells. In contrast, it may be that certain cancers lack such metabolic flexibility by developing a stronger reliance on dedicated metabolic pathways through a myriad of microevolutionary adaptations to maximize growth rates. The metabolic flexibilities of cancers are likely heterogeneous, as seen in the present study whereby HCT 116 and HT29 cell deleterious responses to palmitoylcarnitine were not seen in MCF7 breast cancer cells, which otherwise maintained cell proliferation rates without an increase in H2O2 emission. These findings also guide new directions into the role of H2O2 and glutathione-based redox signaling of specific pathways that regulate cell fate. Finally, MCF7 cells also demonstrated greater oxidative kinetics at physiological levels of ADP relative to HT29 cells and maintained glutathione-buffering capacities throughout palmitoylcarnitine incubations. This finding highlights the possibility that heterogeneous responses of cancers to metabolic therapies may be linked to a dynamic relationship between their inherent metabolic capacities and redox regulation. This discovery guides additional research to determine whether a combination of lower mitochondrial respiratory kinetics and inferior redox buffering couples may represent a biomarker that predicts whether a cancer will be uniquely susceptible to palmitoylcarnitine-mediated reductions in survival.
GRANTS
This work was supported by National Science and Engineering Research Council (NSERC) Grant 436138-2013 to C. G. R. Perry, with infrastructure supported by Canada Foundation for Innovation, the James. H. Cummings Foundation, and the Ontario Research Fund. P. C. Turnbull and M. C. Hughes were supported by NSERC CGS-PhD scholarships.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
P.C.T. and C.G.R.P. conceived and designed research; P.C.T. and M.C.H. performed experiments; P.C.T., M.C.H., and C.G.R.P. analyzed data; P.C.T., M.C.H., and C.G.R.P. interpreted results of experiments; P.C.T. prepared figures; P.C.T. and C.G.R.P. drafted manuscript; P.C.T., M.C.H., and C.G.R.P. edited and revised manuscript; P.C.T., M.C.H., and C.G.R.P. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. Samuel Benchimol for providing the HT29, HCT 116, and MCF7 cells.
REFERENCES
- 1.Al-Bakheit A, Traka M, Saha S, Mithen R, Melchini A. Accumulation of palmitoylcarnitine and its effect on pro-inflammatory pathways and calcium influx in prostate cancer. Prostate 76: 1326–1337, 2016. doi: 10.1002/pros.23222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andrzejewfski S, Gravel SP, Pollak M, St-Pierre J. Metformin directly acts on mitochondria to alter cellular bioenergetics. Cancer Metab 2: 12, 2014. doi: 10.1186/2049-3002-2-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aykin-Burns N, Ahmad IM, Zhu Y, Oberley LW, Spitz DR. Increased levels of superoxide and H2O2 mediate the differential susceptibility of cancer cells versus normal cells to glucose deprivation. Biochem J 418: 29–37, 2009. doi: 10.1042/BJ20081258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ayyanathan K, Kesaraju S, Dawson-Scully K, Weissbach H. Combination of sulindac and dichloroacetate kills cancer cells via oxidative damage. PLoS One 7: e39949, 2012. doi: 10.1371/journal.pone.0039949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baccelli I, Gareau Y, Lehnertz B, Gingras S, Spinella JF, Corneau S, Mayotte N, Girard S, Frechette M, Blouin-Chagnon V, Leveille K, Boivin I, MacRae T, Krosl J, Thiollier C, Lavallee VP, Kanshin E, Bertomeu T, Coulombe-Huntington J, St-Denis C, Bordeleau ME, Boucher G, Roux PP, Lemieux S, Tyers M, Thibault P, Hebert J, Marinier A, Sauvageau G. Mubritinib targets the electron transport chain complex i and reveals the landscape of OXPHOS dependency in acute myeloid leukemia. Cancer cell 36: 84–99.e8, 2019. doi: 10.1016/j.ccell.2019.06.003. [DOI] [PubMed] [Google Scholar]
- 6.Bansal A, Simon MC. Glutathione metabolism in cancer progression and treatment resistance. J Cell Biol 217: 2291–2298, 2018. doi: 10.1083/jcb.201804161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blanquer-Rosselló MD, Hernández-López R, Roca P, Oliver J, Valle A. Resveratrol induces mitochondrial respiration and apoptosis in SW620 colon cancer cells. Biochim Biophys Acta, Gen Subj 1861: 431–440, 2017. doi: 10.1016/j.bbagen.2016.10.009. [DOI] [PubMed] [Google Scholar]
- 8.Bloemberg D, Quadrilatero J. Mitochondrial pro-apoptotic indices do not precede the transient caspase activation associated with myogenesis. Biochim Biophys Acta 1843: 2926–2936, 2014. doi: 10.1016/j.bbamcr.2014.09.002. [DOI] [PubMed] [Google Scholar]
- 9.Dam AD, Mitchell AS, Quadrilatero J. Induction of mitochondrial biogenesis protects against caspase-dependent and caspase-independent apoptosis in L6 myoblasts. Biochim Biophys Acta 1833: 3426–3435, 2013. doi: 10.1016/j.bbamcr.2013.04.014. [DOI] [PubMed] [Google Scholar]
- 10.Dionne S, Elimrani I, Roy MJ, Qureshi IA, Sarma DR, Levy E, Seidman EG. Studies on the chemopreventive effect of carnitine on tumorigenesis in vivo, using two experimental murine models of colon cancer. Nutr Cancer 64: 1279–1287, 2012. doi: 10.1080/01635581.2012.722247. [DOI] [PubMed] [Google Scholar]
- 11.Drew R, Miners JO. The effects of buthionine sulphoximine (BSO) on glutathione depletion and xenobiotic biotransformation. Biochem Pharmacol 33: 2989–2994, 1984. doi: 10.1016/0006-2952(84)90598-7. [DOI] [PubMed] [Google Scholar]
- 12.Dunigan DD, Waters SB, Owen TC. Aqueous soluble tetrazolium/formazan MTS as an indicator of NADH- and NADPH-dependent dehydrogenase activity. Biotechniques 19: 640–649, 1995. [PubMed] [Google Scholar]
- 13.Feoktistova M, Geserick P, Leverkus M. Crystal violet assay for determining viability of cultured cells. Cold Spring Harb Protoc 2016: pdb.prot087379, 2016. doi: 10.1101/pdb.prot087379. [DOI] [PubMed] [Google Scholar]
- 14.Gatenby RA, Gillies RJ. Why do cancers have high aerobic glycolysis? Nat Rev Cancer 4: 891–899, 2004. doi: 10.1038/nrc1478. [DOI] [PubMed] [Google Scholar]
- 15.Giustarini D, Dalle-Donne I, Milzani A, Fanti P, Rossi R. Analysis of GSH and GSSG after derivatization with N-ethylmaleimide. Nat Protoc 8: 1660–1669, 2013. doi: 10.1038/nprot.2013.095. [DOI] [PubMed] [Google Scholar]
- 16.Griffith OW, Meister A. Potent and specific inhibition of glutathione synthesis by buthionine sulfoximine (S-n-butyl homocysteine sulfoximine). J Biol Chem 254: 7558–7560, 1979. [PubMed] [Google Scholar]
- 17.Hughes MC, Ramos SV, Turnbull PC, Edgett BA, Huber JS, Polidovitch N, Schlattner U, Backx PH, Simpson JA, Perry CGR. Impairments in left ventricular mitochondrial bioenergetics precede overt cardiac dysfunction and remodelling in Duchenne muscular dystrophy. J Physiol. In press. doi: 10.1113/JP277306. [DOI] [PubMed] [Google Scholar]
- 18.Hughes MC, Ramos SV, Turnbull PC, Rebalka IA, Cao A, Monaco CMF, Varah NE, Edgett BA, Huber JS, Tadi P, Delfinis LJ, Schlattner U, Simpson JA, Hawke TJ, Perry CGR. Early myopathy in Duchenne muscular dystrophy is associated with elevated mitochondrial H2O2 emission during impaired oxidative phosphorylation. J Cachexia Sarcopenia Muscle 10: 643–661, 2019. doi: 10.1002/jcsm.12405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ishiyama M, Miyazono Y, Sasamoto K, Ohkura Y, Ueno K. A highly water-soluble disulfonated tetrazolium salt as a chromogenic indicator for NADH as well as cell viability. Talanta 44: 1299–1305, 1997. doi: 10.1016/S0039-9140(97)00017-9. [DOI] [PubMed] [Google Scholar]
- 20.Jones CL, Stevens BM, D'Alessandro A, Reisz JA, Culp-Hill R, Nemkov T, Pei S, Khan N, Adane B, Ye H, Krug A, Reinhold D, Smith C, DeGregori J, Pollyea DA, Jordan CT. Inhibition of amino acid metabolism selectively targets human leukemia stem cells. Cancer Cell 34: 724–740.e4, 2018. doi: 10.1016/j.ccell.2018.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kand’ár R, Záková P, Lotková H, Kucera O, Cervinková Z. Determination of reduced and oxidized glutathione in biological samples using liquid chromatography with fluorimetric detection. J Pharm Biomed Anal 43: 1382–1387, 2007. doi: 10.1016/j.jpba.2006.11.028. [DOI] [PubMed] [Google Scholar]
- 22.Lagadinou ED, Sach A, Callahan K, Rossi RM, Neering SJ, Minhajuddin M, Ashton JM, Pei S, Grose V, O’Dwyer KM, Liesveld JL, Brookes PS, Becker MW, Jordan CT. BCL-2 inhibition targets oxidative phosphorylation and selectively eradicates quiescent human leukemia stem cells. Cell Stem Cell 12: 329–341, 2013. doi: 10.1016/j.stem.2012.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Le A, Cooper CR, Gouw AM, Dinavahi R, Maitra A, Deck LM, Royer RE, Vander Jagt DL, Semenza GL, Dang CV. Inhibition of lactate dehydrogenase A induces oxidative stress and inhibits tumor progression. Proc Natl Acad Sci USA 107: 2037–2042, 2010. doi: 10.1073/pnas.0914433107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee EA, Angka L, Rota SG, Hanlon T, Mitchell A, Hurren R, Wang XM, Gronda M, Boyaci E, Bojko B, Minden M, Sriskanthadevan S, Datti A, Wrana JL, Edginton A, Pawliszyn J, Joseph JW, Quadrilatero J, Schimmer AD, Spagnuolo PA. Targeting mitochondria with avocatin B induces selective leukemia cell death. Cancer Res 75: 2478–2488, 2015. doi: 10.1158/0008-5472.CAN-14-2676. [DOI] [PubMed] [Google Scholar]
- 25.Liberti MV, Locasale JW. The Warburg effect: how does it benefit cancer cells? Trends Biochem Sci 41: 211–218, 2016. doi: 10.1016/j.tibs.2015.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lin Z, Liu F, Shi P, Song A, Huang Z, Zou D, Chen Q, Li J, Gao X. Fatty acid oxidation promotes reprogramming by enhancing oxidative phosphorylation and inhibiting protein kinase C. Stem Cell Res Ther 9: 47, 2018. doi: 10.1186/s13287-018-0792-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mandel ER, Dunford EC, Abdifarkosh G, Turnbull PC, Perry CGR, Riddell MC, Haas TL. The superoxide dismutase mimetic tempol does not alleviate glucocorticoid-mediated rarefaction of rat skeletal muscle capillaries. Physiol Rep 5: e13243, 2017. doi: 10.14814/phy2.13243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nałecz KA, Szczepankowska D, Czeredys M, Kulikova N, Grześkiewicz S. Palmitoylcarnitine regulates estrification of lipids and promotes palmitoylation of GAP-43. FEBS Lett 581: 3950–3954, 2007. doi: 10.1016/j.febslet.2007.07.027. [DOI] [PubMed] [Google Scholar]
- 29.Nicholls DG, Ferguson SJ. Bioenergetics (4th ed.), edited by Nicholls DG, Ferguson SJ. Boston, MA: Academic, 2013, p. ix–x. [Google Scholar]
- 30.Nieman KM, Kenny HA, Penicka CV, Ladanyi A, Buell-Gutbrod R, Zillhardt MR, Romero IL, Carey MS, Mills GB, Hotamisligil GS, Yamada SD, Peter ME, Gwin K, Lengyel E. Adipocytes promote ovarian cancer metastasis and provide energy for rapid tumor growth. Nat Med 17: 1498–1503, 2011. doi: 10.1038/nm.2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ortega AL, Mena S, Estrela JM. Glutathione in cancer cell death. Cancers (Basel) 3: 1285–1310, 2011. doi: 10.3390/cancers3011285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roscilli G, Marra E, Mori F, Di Napoli A, Mancini R, Serlupi-Crescenzi O, Virmani A, Aurisicchio L, Ciliberto G. Carnitines slow down tumor development of colon cancer in the DMH-chemical carcinogenesis mouse model. J Cell Biochem 114: 1665–1673, 2013. doi: 10.1002/jcb.24508. [DOI] [PubMed] [Google Scholar]
- 33.Schulz TJ, Thierbach R, Voigt A, Drewes G, Mietzner B, Steinberg P, Pfeiffer AF, Ristow M. Induction of oxidative metabolism by mitochondrial frataxin inhibits cancer growth: Otto Warburg revisited. J Biol Chem 281: 977–981, 2006. doi: 10.1074/jbc.M511064200. [DOI] [PubMed] [Google Scholar]
- 34.Terradez P, Asensi M, Lasso de la Vega MC, Puertes IR, Viña J, Estrela JM. Depletion of tumour glutathione in vivo by buthionine sulphoximine: modulation by the rate of cellular proliferation and inhibition of cancer growth. Biochem J 292: 477–483, 1993. doi: 10.1042/bj2920477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wallace DC. Mitochondria and cancer. Nat Rev Cancer 12: 685–698, 2012. doi: 10.1038/nrc3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Warburg O. The metabolism of carcinoma cells. J Cancer Res 9: 148–163, 1925. doi: 10.1158/jcr.1925.148. [DOI] [Google Scholar]
- 37.Warburg O. On the origin of cancer cells. Science 123: 309–314, 1956. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- 38.Wenzel U, Nickel A, Daniel H. Increased carnitine-dependent fatty acid uptake into mitochondria of human colon cancer cells induces apoptosis. J Nutr 135: 1510–1514, 2005. doi: 10.1093/jn/135.6.1510. [DOI] [PubMed] [Google Scholar]
- 39.Yang H, Villani RM, Wang H, Simpson MJ, Roberts MS, Tang M, Liang X. The role of cellular reactive oxygen species in cancer chemotherapy. J Exp Clin Cancer Res 37: 266, 2018. doi: 10.1186/s13046-018-0909-x. [DOI] [PMC free article] [PubMed] [Google Scholar]