Abstract
Background:
Adolescents with single ventricle heart disease (SVHD), who have undergone the Fontan procedure, show cognitive/memory deficits. Mammillary bodies are key brain sites that regulate memory; however, their integrity in SVHD is unclear. We evaluated mammillary body (MB) volumes and their associations with cognitive/memory scores in SVHD and controls.
Methods:
Brain MRI data were collected from 63 adolescents (25 SVHD; 38 controls) using a 3.0-Tesla MRI scanner. Cognition and memory were assessed using Montreal Cognitive Assessment (MoCA) and Wide Range Assessment of Memory and Learning 2. MB volumes were calculated and compared between groups (ANCOVA, covariates: age, sex, and total-brain-volume [TBV]). Partial correlations and linear regression were performed to examine associations between volumes and cognitive scores (covariates: age, sex, and TBV).
Results:
SVHD group showed significantly lower MoCA and WRAML2 scores over controls. MB volumes were significantly reduced in SVHD over controls. After controlling for age, sex, and TBV, MB volumes correlated with MoCA and delayed memory recall scores in SVHD and controls.
Conclusion:
Adolescents with SVHD show reduced MB volumes associated with cognitive/memory deficits. Potential mechanisms of volume losses may include developmental and/or hypoxic/ischemic-induced processes. Providers should screen for cognitive deficits and explore possible interventions to improve memory.
INTRODUCTION
Adolescents with single ventricle heart disease (SVHD) require at least three staged palliative surgeries before the age of four, culminating with the Fontan procedure. Many SVHD children experience a variety of cognitive symptoms, including memory, attentional, and behavioral impairments (i.e. intellectual deficits, learning difficulties, pragmatic language, social interaction, visuospatial, motor, and executive function deficits) that persist into adulthood (1). With significant improvements in fetal detection, surgical strategies, and perioperative care, survival rates have improved in SVHD patients (2). However, neurodevelopmental/cognitive outcomes have only modestly improved (3). Such deficits can limit academic achievement, lower quality of life, and ultimately diminish the ability for self-care (4,5).
Approximately 60% of children with SVHD show brain injury, commonly reported as changes in white or gray matter or cerebral thromboembolic events (1, 6). Cognitive deficits, in particular memory, have been reported in SVHD and other complex congenital heart disease (CHD). Brain changes have been assessed and documented in several memory regulatory areas, including the hippocampus and anterior thalamus, in SVHD (7). However, due to their small size and location adjacent to bony structures and cerebral spinal fluid (CSF), mammillary bodies have not been assessed in SVHD.
In addition to other memory circuitry structures (hippocampus, anterior thalamus, and fornix fibers), mammillary bodies, located in the inferior aspect of the brain, are important regions associated with spatial and episodic memory. These bodies are divided into medial and lateral nuclei and consist solely of projection cells that receive both excitatory and inhibitory inputs, while delivering only excitatory outputs. The mammillary bodies receive inputs from the hippocampus through fornix fibers, and project to anterior thalamus and then cortical sites. Injury to the mammillary bodies has resulted in poor performance in spatial and working memory tasks in animal studies (8). Furthermore, mammillary body injury has been linked to memory deficits in multiple conditions, such as obstructive sleep apnea, heart failure, congenital central hypoventilation syndrome, children with epilepsy, preterm infants and Korsakoff’s syndrome (9, 10, 11, 12). However, mammillary body injury has not been reported in SVHD patients.
Therefore, the purpose of this study was to evaluate mammillary body volumes in SVHD and controls using high-resolution T1-weighted magnetic resonance imaging (MRI) procedures, and to correlate mammillary body volumes with various cognitive/memory scores.
MATERIAL AND METHODS
Participants
This comparative and correlational study was approved by the Institutional Review Boards at the University of California Los Angeles (UCLA) and Children’s Hospital Los Angeles (CHLA) prior to implementation. A total of 63 adolescents were enrolled (25 SVHD, [mean age 15.88 ± 1.24 years, 15 males]; 38 controls [mean age 15.97 ± 1.10 years, 19 males]). Adolescents (ages 14–18 years) with SVHD, who have undergone the Fontan procedure, were recruited via flyers or physician referrals from UCLA and CHLA Medical Centers. Healthy controls were recruited from local high schools, word of mouth, and from the surrounding Los Angeles community. Exclusion criteria for SVHD and controls were claustrophobia, non-removable metal (such as braces, pacemakers), previous head injury, severe developmental delay (e.g. cerebral palsy or severe hypoxic-injury) precluding active study participation, diagnosis of depression, premature birth (<37 weeks gestation), history of previous extracorporeal membrane oxygenation (ECMO) use, documented stroke and cardiac arrest. In addition, control participants were screened and excluded for any chronic medical or psychiatric conditions. The SVHD participants were matched to a control for age (±1 year), sex, and ethnicity. All SVHD and control participants completed a demographic information form. Clinical data were collected from medical records of the SVHD patients. For all participants under 18 years of age, parental permissions and subject assents were obtained, and written and informed consents were obtained from participants age 18 and above before data collection.
Data Collection Instruments
Cognitive Assessment.
We used the Wide Range Assessment of Memory and Learning, Second Edition (WRAML2), which is a well-validated and reliable measure of various aspects of memory and learning in children and adults (e.g. 5–90 years of age) (13). The WRAML2 consists of 6 subtests, involving verbal and visual memory and attention/concentration, which yields the General Memory Index (GMI) score, and working memory, verbal and visual memory recognition, which produce the General Recognition Index (GRI) scores (normal, 100±15; ≤ 85 considered mild to moderate memory impairment). The alpha reliabilities for the core subtests range from 0.85–0.94 (16). The WRAML2-derived GMI score has been widely used to assess memory in the adolescent population both with and without CHD (14).
The Montreal Cognitive Assessment (MoCA) is an administered screener used to measure multiple domains of cognitive function, such as visuospatial, attention/concentration, executive function, language, delayed memory recall, and naming. The visuospatial and executive function tasks are written items (i.e. 3-dimensional cube, Watson Clock Drawing Task, and alternating Trail Making Task Part B), and the remaining are scored based on verbal response (15). The total MoCA score ranges from 0–30, and a score <26 is considered abnormal. The MoCA has been validated in adolescents both with and without CHD and has a Cronbach’s alpha of 0.8 (15, 16).
Socioeconomic Status
Annual household income reflects socioeconomic status (SES) and is derived from each subject’s residential postal zip code. SES was calculated for each participant from the American Community Survey data available on Population Studies Center, Institute for Social Research (https://www.census.gov/programs-surveys/acs/).
Magnetic Resonance Imaging
Brain images from SVHD patients and controls were acquired using a 3.0-Tesla MRI scanner (Siemens, Magnetom Prisma, Erlangen, Germany). Participants were placed in the supine position in the scanner with foam padding on both sides of their head to minimize movement. If movement artifact was visually identified on images, the scan was repeated. High resolution T1-weighted images were collected using a magnetization prepared rapid acquisition gradient-echo (MPRAGE) sequence [repetition time (TR) = 2200 ms; echo time (TE) = 2.4ms; inversion time = 900 ms; flip angle (FA) = 9°; matrix size 320 × 320; field of view (FOV) = 230 × 230 mm; slice thickness = 0.9 mm). Proton density (PD) and T2-weighted images (TR = 10000 ms; TE1,2 = 12, 124 ms; FA = 130°) were collected with a dual-echo turbo spin-echo pulse sequence in the axial plane (230 × 230 mm FOV, 256 × 256 matrix size, 3.5 mm slice thickness, and no interslice gap). In addition, all MRIs were formally interpreted by a pediatric neuroradiologists, who was blinded by group assignment, to visually assess for any serious brain structural injury.
MRI Data Processing
Brain images were preprocessed using the statistical parametric mapping (SPM12) package (Wellcome Department of Cognitive Neurology, London, UK; http://www.fil.ion.ucl.ac.uk/spm/), and MATLAB-based (The MathWorks Inc, Natick, MA) custom software.
Both high-resolution T1-weighted image volumes of each subject were realigned and averaged to improve signal-to-noise ratios. The averaged images were bias-corrected for any potential image signal intensity variation using the unified segmentation approach, reoriented into Montreal Neurological Institute (MNI) common space (rigid-body affine transformation), and resampled to voxel size 0.7×0.7×0.7 mm.
Total Brain Volume (TBV) Calculation
A unified segmentation approach was used to partition the reoriented T1-weighted images into gray matter, white matter, and cerebral spinal fluid (CSF) tissue types (17). The TBV volumes were computed using gray matter and white matter probability maps. Voxels with a probability values >0.5 for gray and white matter in each subject were counted and TBV values were calculated.
Mammillary Body Tracings and Volume Quantification
Using the reoriented images, the mammillary body areas were oversampled to a resolution of 0.2 × 0.2 × 0.2mm and manual region of interest (ROI) measures of the left and right structures were performed separately using MRIcron (McCausland Center for Brain Imaging, University of South Carolina, Columbia, SC). One investigator, blinded to subject group assignment, performed the left and right mammillary body tracings in all participants, as described in our previous reports (9, 10, 11). Briefly, sagittal and coronal views were used to establish the brain midline and the medial borders of both bodies. Coronal views were used to determine the superior and inferior mammillary body edges, and consecutive sagittal slices were used to outline the entire structure with coronal and axial views to confirm the borders. Voxels were counted from each ROI, and the mammillary body volumes were calculated by summing all voxels and multiplying by voxel volume. Figure 1 shows the mammillary body volume tracing method used.
Figure 1.
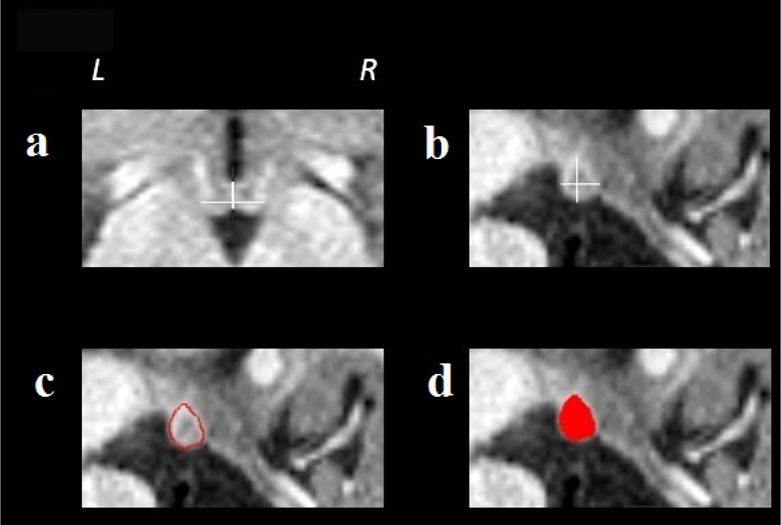
Tracing method used to calculate mammillary body volumes. Midline borders for the left and right mammillary bodies were determined using coronal (a) views; these views also determine the superior and inferior right mammillary body edges (b) and consecutive sagittal slices were used to outline the entire structure in red (c) with coronal and axial views used to confirm the border. The right mammillary body is filled in red (d). The total volume of each mammillary body was calculated by counting the number of voxels in the traced red area.
Intra- and Inter-tracing Reliability Analyses
Intra-tracer reliability for outlining mammillary body structures was examined by re-outlining in 8 randomly-selected SVHD and 8 control participants by the same investigator who outlined both left and right bodies in all SVHD and controls. A second investigator, blinded to the first reviewer’s results and subject group membership, traced mammillary bodies in the same participants (8 SVHD and 8 control), from which inter-tracing reliability was calculated. Inter-and intra-tracing reliabilities were calculated using intra-class correlation (ICC).
Statistical Analysis
The Statistical Package for the Social Sciences software (IBM SPSS Statistics for Windows, v24, IBM Corp., Armonk, N.Y., USA) was used for data analyses. Demographic and cognitive data of SVHD and controls were evaluated using independent samples (two-tailed) t-tests for normally distributed data, Mann-Whitney U test for non-normally distributed data, and Chi-square for categorical variables. The normality of each variable for SVHD and control subjects were evaluated using Shapiro-Wilk test. The mammillary body volumes between SVHD and controls were assessed using ANCOVA, with covariates of age, sex, SES and TBV. Correlations between cognitive scores and mammillary body volumes in combine SVHD and controls were assessed using partial correlations (covariates; age, sex, and TBV). Linear regression analyses were conducted to examine possible confounding factors contributing to mammillary body volume reduction (age, sex, and TBV as independent variables, and cognitive scores as dependent variables). All tests were two-tailed, with p < 0.05 considered statistically significant.
RESULTS
Demographic, Clinical, and Cognitive Characteristics
Demographic and cognitive scores from SVHD and control participants are summarized in Table 1. No statistically significant differences appeared in age, sex, ethnicity, body mass index, or handedness between groups. However, SES was significantly lower in the SVHD group compared to controls.
Table 1.
Demographic and Cognitive Scores of Single Ventricle Heart Disease and Controls.
| Variables | SVHD n=25 | Control n=38 | p values |
|---|---|---|---|
| Age, median (IQR) | 16.0 (15.0 – 17.0) | 16.0 (15.0 – 17.0) | 0.67 |
| Sex Male (%) | 15 (60%) | 19 (50%) | 0.54 |
| Ethnicity (%) | 0.67 | ||
| White | 13 (52%) | 20 (50%) | |
| Hispanic | 10 (40%) | 16 (42%) | |
| Other | 2 (8%) | 2 (8%) | |
| Socioeconomic Status, median (IQR) (Annual Household Income) | $73167.5 ($54068-$96758) (n=24) | $85696.0 ($74317.5-$116633.0) (n=37) | 0.02 |
| Handedness Right (%) | 23 (92%) | 34 (92%) | 0.74 |
| BMI (kg/m2), median (IQR) | 21.1 (19.7 – 23.0) | 21.9 (19.6 – 24.9) | 0.45 |
| Total Brain Volume (unit, L; mean ± SD) | 1.13 ± 0.10 | 1.24 ± 0.10 | <0.001ǂ |
| SVHD Diagnosis: | - | - | |
| Hypoplastic Left Heart Syndrome | 6 (24%) | ||
| Double Outlet Right Ventricle, unbalanced AVC | 6 (24%) | ||
| Tricuspid Atresia | 4 (16%) | ||
| Pulmonary Atresia / unbalanced AVC | 4 (16%) | ||
| Pulmonary Atresia / IVS / HRV | 3 (12%) | ||
| Double Inlet Left Ventricle | 2 (8%) | ||
| Ventricle Type (Right) | 16 (67%) | - | - |
| Fontan Type (Extracardiac) | 21 (78%) | - | - |
| Fontan Fenestration (Yes) | 7 (26%) | - | - |
| Residual Cyanosisa | 7 (26%) | - | - |
| Number of Surgeries [mean (range)] | 3 (2–4) | - | - |
| MoCA Total, median (IQR) | 23 (20.5 – 25.0) | 29 (27.8 – 30.0) | <0.001 |
| Visuospatial / EF (MoCA), median (IQR) | 4.0 (3.0 – 5.0) | 5.0 (5.0 – 5.0) | <0.001 |
| Naming (MoCA) ), median (IQR) | 3.0 (3.0 – 3.0) | 3.0 (3.0 – 3.0) | 0.03 |
| Attention (MoCA), median (IQR) | 4.0 (3.0 – 5.0) | 6.0 (6.0 – 6.0) | <0.001 |
| Language (MoCA), median (IQR) | 2.0 (1.0 – 2.0) | 3.0 (2.0 – 3.0) | <0.001 |
| Abstraction (MoCA), median (IQR) | 1.0 (1.0 – 2.0) | 2.0 (2.0 – 2.0) | <0.001 |
| Delayed Memory Recall (MoCA), median (IQR) | 2.0 (0.5 – 3.0) | 4.0 (4.0 – 5.0) | <0.001 |
| Orientation (MoCA), median (IQR) | 6.0 (6.0 – 6.0) | 6.0 (6.0 – 6.0) | 0.49 |
| WRAML2 (GMI) Total, median (IQR) | 83.0 (79.0 – 92.5) | 111 (104.8 – 118) | <0.001 |
| Verbal Memory Index (GMI), median (IQR) | 88.0 (82.0 – 95.5) | 111 (99.3 – 114) | <0.001 |
| Visual Memory Index (GMI) | 98.7 ± 11.3 | 107.3 ± 11.2 | 0.005 |
| Attention/Concentration (GMI) | 83.5 ± 10.7 | 109.6 ± 10.6 | <0.001 |
| WRAML2 (GRI) Total | 93.8 ± 11.6 | 112.0 ± 11.5 | <0.001 |
| Working Memory Index (GRI), median (IQR) | 90.0 (79.5 – 97.5) | 113.5 (102.0 – 122.0) | <0.001 |
| Verbal Recognition Index (GRI), median (IQR) | 93.0 (86.5 – 104.0) | 108.0 (96.0 – 115.0) | 0.001 |
| Visual Recognition Index (GRI), median (IQR) | 90.5 (84.3 – 108.3) | 109 (102.3 – 115.8) | <0.001 |
SVHD = Single Ventricle Heart Disease; BMI = Body Mass Index; AVC = Atrioventricular Canal; IVS = Intact Ventricular Septum; HRV = Hypoplastic Right Ventricle;
oxygen saturation; WRAML2 = Wide Range Assessment of Memory and Learning, Second Edition; GMI = General Memory Index; GRI = General Memory Recognition Index; MoCA = Montreal Cognitive Assessment; L=liters;
Corrected for age and sex.
Clinical characteristics of SVHD patients are listed in Table 1. The majority had a diagnosis of single right ventricle (67%), had an extracardiac Fontan (78%). Many had a Fontan fenestration (26%) and/or residual cyanosis (26%) defined as oxygen saturations < 93%.
The total MoCA score and all subscales, except for naming and orientation, were significantly lower in SVHD compared to controls (p<0.04). In addition, WRAML2-derived GMI and GRI scores were significantly reduced, as well as sub scores, including verbal and visual memory, attention/concentration, working memory, and visual and verbal recognition, in SVHD compared to controls (p<0.006; Table 1). In addition, the mean TBV was significantly smaller in the SVHD over controls (1.13±0.10 vs. 1.24±0.10 Liter; p<0.001; covariates; age and sex).
Brain MRI Findings
Abnormal brain MRI findings appeared in 8 SVHD subjects out of 25 (32%) compared to 2 out of 38 (5%) in the control group. Cerebral lesions were detected in 5 out of 25 (20%) in the SVHD group which consist of white mater changes, old infarctions / strokes (incidental finding in all participants), and periventricular volume loss. Incidental developmental abnormalities in both the SVHD and control groups consisted of Rathke’s cleft, pterygoid, or perivascular region cysts.
Tracing Reliability
Intra-tracer reliability was high for mammillary body tracings (ICC = 0.99, p<0.001, 95% confidence interval), indicating consistent tracings across all participants. Inter-tracing reliability for mammillary bodies was in agreement between the two investigators (left mammillary body, ICC = 0.98, p<0.001; right mammillary body, ICC = 0.97, p<0.001, 95% confidence interval).
Mammillary Body Volumes
An example of the mammillary bodies in a SVHD and age- and sex-matched control is shown in figure 2, and scatterplot with mean left and right mammillary volumes between groups presented in figure 3. Both left and right mammillary bodies showed reduced volume in SVHD compared to controls (left, 40.24±19.18 mm3 vs. 53.59±18.68 mm3, p = 0.01; right, 42.57±17.26 mm3 vs. 52.87±16.81 mm3, p = 0.03; covariates, age, sex, and TBV). Both left and right mammillary body volumes were reduced in SVHD subjects with identified cerebral lesions (n=5) compared to participants without, but reductions were not statistically-significant (left, 33.76±18.48 mm3 vs. 37.51±17.43 mm3, p = 0.695; right, 31.30±19.75 mm3 vs. 40.12±18.62 mm3, p = 0.391; covariates, age, sex, and TBV). Mammillary body volume differences continue after controlling for SES in addition to age, sex and TBV.
Figure 2.
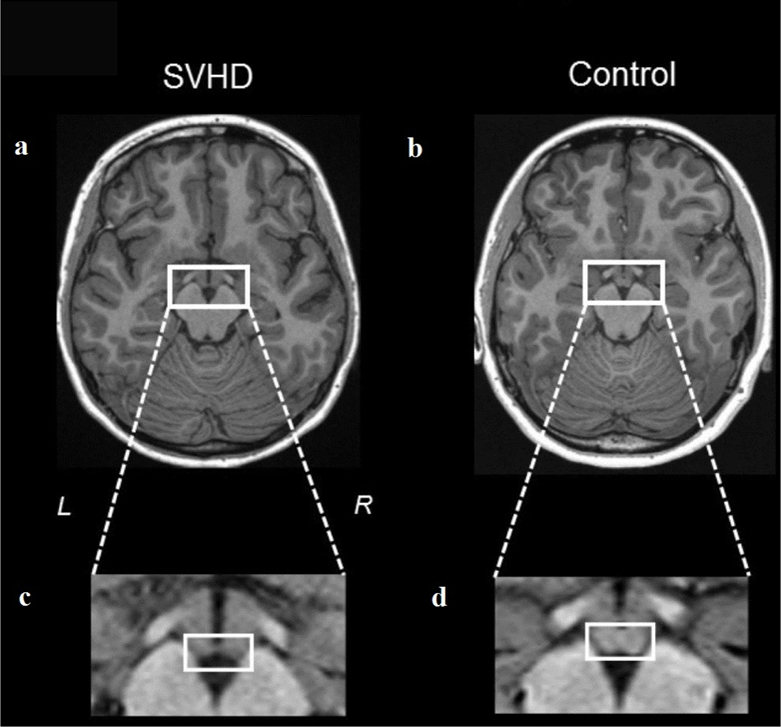
High-resolution T1-weighted images show mammillary bodies in a SVHD (a) and Control (b) subject (white rectangles). All images are in neurological convention (L = Left, R = Right). Brain images (c) and (d) show magnified areas within the rectangle of the SVHD (a) and Control (b) participants. The right and left mammillary bodies in the SVHD subject are almost absent compared to the control subject (c vs. d, white circles).
Figure 3.
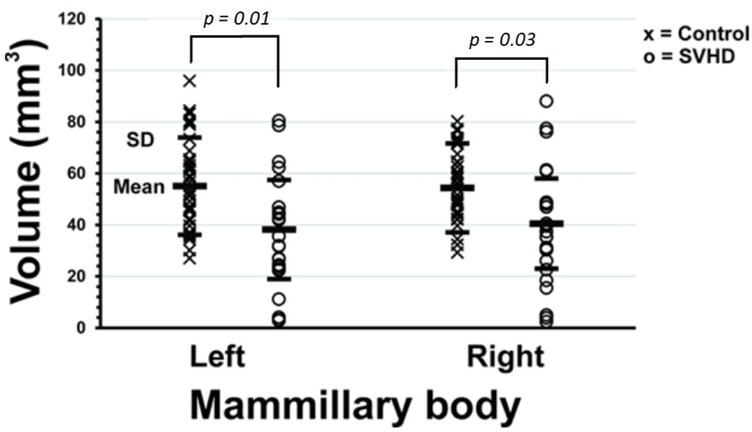
Scatter plot shows smaller mean left (40.24 ± 19.18 mm3 vs. 53.59 ± 18.68 mm3, p=0.01) and right (42.57 ± 17.26 mm3 vs. 52.87 ± 16.81 mm3, p=0.03) mammillary body volumes in SVHD (O) vs. Controls (X), controlling for age, sex, and TBV.
Correlations between Mammillary Body Volumes and Cognitive Scores
Significant positive correlations appeared between the left mammillary body volumes and WRAML2-derived verbal memory scores, and total MoCA and delayed memory recall sub-scores showed significant associations with left and right MB volumes in combined SVHD and control subjects (Table 2). Furthermore, linear regression analyses to predict mammillary body volumes (separate left and right) were performed for each functional outcome (dependent variables) listed in Table 3. Both left and right mammillary body volumes were significantly associated with total MoCA, and subscale delayed memory recall and these associations were independent of age, sex, and TBV (Table 3). The GMI subscale, verbal memory index correlates with the left mammillary body volume and visuospatial / executive function with the right mammillary body volume independent of age, sex, and TBV (Table 3).
Table 2.
Partial correlations between mammillary body volumes and cognitive scores in combined SVHD and controls.
| Variables | Left Mammillary Body Volume | Right Mammillary Body Volume | ||
|---|---|---|---|---|
| WRAML2 GMI | r = 0.23 | p = 0.07 | r = 0.22 | p = 0.09 |
| Verbal Memory Index (GMI) | r = 0.26 | p = 0.04 | r = 0.25 | p = 0.06 |
| Visual Memory Index (GMI) | r = 0.13 | p = 0.34 | r = 0.20 | p = 0.12 |
| Attention/Concentration (GMI) | r = 0.16 | p = 0.22 | r = 0.12 | p = 0.37 |
| WRAML2 GRI | r = 0.18 (n=62) | p = 0.17 | r = 0.19 (n=62) | p = 0.16 |
| Working Memory Index (GRI) | r = 0.04 | p = 0.75 | r = 0.04 | p = 0.79 |
| Verbal Recognition Index (GRI) | r = 0.05 (n=62) | p = 0.71 | r = 0.10 (n=62) | p = 0.47 |
| Visual Recognition Index (GRI) | r = −0.02 | p = 0.91 | r = −0.06 | p = 0.64 |
| MoCA Total | r = 0.28 | p = 0.03 | r = 0.26 | p = 0.04 |
| Visuospatial/Executive Function (MoCA) | r = 0.20 | p = 0.13 | r = 0.24 | p = 0.07 |
| Naming (MoCA) | r = 0.02 | p = 0.90 | r = 0.05 | p = 0.70 |
| Attention (MoCA) | r = 0.13 | p = 0.32 | r = 0.16 | p = 0.23 |
| Language (MoCA) | r = 0.13 | p = 0.33 | r = 0.14 | p = 0.30 |
| Abstraction (MoCA) | r = 0.16 | p = 0.22 | r = 0.19 | p = 0.14 |
| Delayed Memory Recall (MoCA) | r = 0.34 | p = 0.008 | r = 0.29 | p = 0.03 |
| Orientation (MoCA) | r = −0.11 | p = 0.42 | r = −0.13 | p = 0.31 |
MoCA = Montreal Cognitive Assessment; GMI = General Memory Index; WRAML2 = Wide Range Assessment of Memory and Learning, Second Edition; Covariates age, sex, and TBV
Table 3.
Mammillary body volume in relation to cognitive outcomes for SVHD and controls.
| Left mammillary body volume | Right mammillary body volume | |||||
|---|---|---|---|---|---|---|
| Variables | Corrected R2 | Beta | p values | Corrected R2 | Beta | p values |
| WRAML2 GMI | 0.29 | 0.22 | 0.07 | 0.29 | 0.23 | 0.09 |
| Verbal Memory Index (GMI) | 0.18 | 0.28 | 0.04 | 0.17 | 0.27 | 0.06 |
| Visual Memory Index (GMI) | 0.13 | 0.13 | 0.34 | 0.15 | 0.22 | 0.12 |
| Attention/Concentration (GMI) | 0.19 | 0.16 | 0.22 | 0.18 | 0.13 | 0.37 |
| WRAML2 GRI | 0.22 | 0.18 | 0.17 | 0.22 | 0.20 | 0.16 |
| Working Memory Index (GRI) | 0.22 | 0.04 | 0.75 | 0.22 | 0.04 | 0.79 |
| Verbal Recognition Index (GRI) | 0.02 | 0.06 | 0.71 | 0.26 | 0.11 | 0.47 |
| Visual Recognition Index (GRI) | 0.11 | −0.02 | 0.91 | 0.12 | −0.07 | 0.64 |
| MoCA Total | 0.45 | 0.24 | 0.03 | 0.44 | 0.24 | 0.04 |
| Visuospatial/Executive Function (MoCA) | 0.38 | 0.18 | 0.13 | 0.39 | 0.23 | 0.05 |
| Naming (MoCA) | 0.06 | 0.02 | 0.90 | 0.06 | 0.06 | 0.70 |
| Attention (MoCA) | 0.45 | 0.11 | 0.32 | 0.45 | 0.14 | 0.23 |
| Language (MoCA) | 0.12 | 0.13 | 0.33 | 0.12 | 0.15 | 0.30 |
| Abstraction (MoCA) | 0.22 | 0.16 | 0.22 | 0.23 | 0.20 | 0.14 |
| Delayed Memory Recall (MoCA) | 0.23 | 0.35 | 0.008 | 0.20 | 0.32 | 0.03 |
| Orientation (MoCA) | 0.01 | −0.12 | 0.42 | 0.02 | −0.16 | 0.31 |
WRAML2 = Wide Range Assessment of Memory and Learning, Second Edition; GRI = General Recognition Index; GMI = General Memory Index; MoCA= Montreal Cognitive Assessment. Linear regression analyses (dependent variable: functional outcomes). Variables included: left and right mammillary body volume, total brain volume, sex, and age.
Risk Factors
No clinical risk factors such as current oxygen saturation level, number of surgeries, ventricular type, and number of medications correlated with smaller mammillary body volumes.
DISCUSSION
This study identified significantly reduced left and right mammillary body volumes in SVHD compared to age- and sex-matched controls. Bilateral mammillary body volumes showed a significant association with cognitive scores, including memory deficits. The underlying etiology for reduced mammillary body volume in SVHD is not completely known, but may include delayed brain development (18), pre- and postoperative brain injury associated with hypoxemia/ischemia, reduced CBF, hemodynamic instability (19, 20), and possible nutritional deficiencies in the condition.
More recently, fetal and neonatal MRI studies have shown lower brain volumes (due to delayed brain maturation by up to one month) most commonly in the third trimester, possibly caused by reduced blood flow and cerebral oxygenation (21), with more defects on left ventricular outflow tract obstruction (18, 20). Additionally, these infants may have lower birth weights, lower gestational ages, and smaller head circumferences, indicating brain developmental issues, compared to the general population. Brain immaturity in SVHD results in delays in myelination, placing increased risk for hypoxic-ischemic structural injury in the pre- and postoperative period (22). Consequently, brain MRI studies have identified new or worsening white matter injury or other ischemic injury after neonatal cardiac surgery (22, 23). As a highly vascular area of the brain, the mammillary bodies are vulnerable to hypoxic injury.
The mammillary bodies relay impulses from the amygdalae and hippocampi via the fiber projections of the mamillo-thalmic tract to the anterior thalamus and may mediate several aspects of spatial and memory formation (24). A recent adolescent SVHD study found reduced gray matter volumes and tissue changes in multiple sites that are part of the mamillo-thalmic tracts and are associated with memory regulation (i.e. bilateral anterior, mid, and posterior thalamus, para hippocampus, and temporal gyrus) (6, 16).
In addition to the effects of surgery, SVHD patients are at risk of malnutrition due to inadequate nutritional intake, insufficient nutrient absorption secondary to decrease mesenteric perfusion (25), and increase metabolic demands that may all affect brain development. Furthermore, feeding difficulties are found to be a significant predictor of worse neurodevelopmental outcomes in infants with SVHD (26). Lower weight-for-age z scores have been well documented after SVHD stage one and two palliation (27), and most likely associated with the imbalance between pulmonary and systemic circulation. Although modest weight improvement is noted after the Fontan procedure (28), many remain underweight into adulthood (29).
Nutritional deficits associated with mammillary body volume loss has been seen in reduced vitamin B intake or absorption, in particular thiamine. The volume loss and thiamine deficiency has been accompanied with anterograde memory loss in Korsakoff’s syndrome, which is associated with chronic alcoholism (30). In animal models, thiamine deficiency has led to extensive damage to the mammillary bodies correlating with worse performance on memory and spatial tasks (31). In addition to dietary issues, thiamine deficiency can develop from use of diuretics or medical conditions associated with malabsorption such as anorexia (32), intestinal bypass, or the need for dialysis (33). One study in infants with ventricular septal defects and associated congestive heart failure treated with loop diuretics identified thiamine deficiency before and after surgical repair, despite adequate dietary intake of thiamine (34). Although we speculate that mammillary body injury is associated with hypoxic-ischemic insult, early nutritional deficits commonly encountered in SVHD may contribute to inadequate neural protection or lead to additional neural injury.
Our findings identified lower SES in the SVHD group compared to controls, with 50% of the SVHD sample of Hispanic ethnicity. Disparities have been identified in population-based studies in children with critical CHD in which SES explains a significant portion of the association between Hispanic ethnicity and worse health outcomes (35). However, controlling for SES in our analysis, there were still significant differences in mammillary body volumes and functional outcomes in the SVHD group compared to controls, and SES did not contribute to those variables. However, other important factors of SES such as maternal age, and education may provide more of an impact than annual salary, as measured in this study.
Lastly, targeted interventions to improve memory are needed to mitigate these deficits in SVHD. Over the years, clinical management changes have been implemented to address delayed brain maturation by allowing the SVHD fetus to remain in utero for the entire 40 week gestation period (36), avoidance of hemodilution (hematocrit < 25%) during cardiopulmonary bypass (37), regional low-flow cerebral perfusion to increase cerebral perfusion during stage one palliation (38, 39), and vigilant single ventricle interstage follow-up to optimize nutrition and growth (40). While these interventions individually have some modest benefit, many are not sufficient to fully alleviate the life-long cognitive outcomes in SVHD patients, and other interventions are needed to focus on ways to promote neurogenesis or regrowth of mammillary bodies to optimize memory processes and subsequent academic achievement and ability for self-care.
Limitations
Despite the small sample size, significant differences in mammillary body volumes emerged between groups. While the extensive exclusion criteria made a more homogeneous SVHD sample (gestation < 37 weeks, no previous stroke, ECMO use, cardiac arrest, pacemaker, protein losing enteropathy), this may reflect a group with better health within their chronic condition, and thus, may not be generalizable to all SVHD participants. Secondary to a small sample size, we combined SVHD and control participants to examine correlations. Further studies are required to assess such correlations in SVHD participants only. Overall IQ was not measured as part of this study and we are unable to discern if the memory impairment is isolated or part of general IQ reduction. In addition, there is the potential for subjective bias related to manual ROI tracings compared to automated programs. However, manual tracing is considered the gold standard due to the small size of the structures and close proximity to related structures. Lastly, without fetal or neonatal brain MRI, we are unable to specify the evolution of altered brain development leading to smaller mammillary body volumes. In addition, we examined adolescents with SVHD at one time point representing the cumulative injury and are unable to determine the timing and mechanism of altered mammillary body volumes in the condition.
Conclusions
Adolescents with SVHD, who have undergone the Fontan procedure, show significantly reduced left and right mammillary body volumes, which may contribute to cognitive deficits. The mechanisms contributing to volume loss are likely multifactorial, including innate, nutritional, hypoxic/ischemic induced processes, impaired CBF, and potential pre- and postoperative hemodynamic instability. Further investigation is needed to explore possible interventions to improve memory / cognition and promote neuroprotection, which could potentially enhance academic achievement, self-care, and quality of life in this high risk population.
Acknowledgements:
Authors would like to thank Dr. Marie Poulsen, Chief Clinical Psychologist, Children’s Hospital Los Angeles, Ms. Patty Chung, Mr. Luke Ehlert, Ms. Paola Moreno, and Ms. Madeline Townsley for assistance with data collection.
Statement of Financial Support: This research and Drs. Pike, Woo, Halnon, Lewis, and Kumar received support by grants from the National Institutes of Health R01NR 013930 and R01NR016463.
Footnotes
Disclosure: All other authors have no conflict of interest to disclose.
Category of Study: Population Study
References
- 1.Bellinger DC, Watson CG, Rivkin MJ, et al. , 2015. Neuropsychological Status and Structural Brain Imaging in Adolescents With Single Ventricle Who Underwent the Fontan Procedure. J Am Heart Assoc 4(12): e002302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marelli AJ, Mackie AS, Ionescu-Ittu R, Rahme E, Pilote L 2007. Congenital heart disease in the general population: changing prevalence and age distribution. Circulation 115:163–172. [DOI] [PubMed] [Google Scholar]
- 3.Gaynor JW, Stopp C, Wypij D, et al. , International Cardiac Collaborative on Neurodevelopment I 2015 Neurodevelopmental outcomes after cardiac surgery in infancy. Pediatrics 135:816–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Atz AM, Zak V, Mahony L, et al. , Pediatric Heart Network I 2017. Longitudinal Outcomes of Patients With Single Ventricle After the Fontan Procedure. J Am Coll Cardiol 69:2735–2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pike NA, Evangelista LS, Doering LV, Eastwood JA, Lewis AB, Child JS 2012. Quality of life, health status, and depression: comparison between adolescents and adults after the Fontan procedure with healthy counterparts. J Cardiovasc Nurs 27:539–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Singh S, Kumar R, Roy B, et al. , 2018. Regional brain gray matter changes in adolescents with single ventricle heart disease. Neurosci Lett 665:156–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Munoz-Lopez M, Hoskote A, Chadwick MJ, et al. , 2017. Hippocampal damage and memory impairment in congenital cyanotic heart disease. Hippocampus 27:417–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vann SD, 2010. Re-evaluating the role of the mammillary bodies in memory. Neuropsychologia 48:2316–2327. [DOI] [PubMed] [Google Scholar]
- 9.Kumar R, Birrer BV, Macey PM, et al. , 2008. Reduced mammillary body volume in patients with obstructive sleep apnea. Neurosci Lett 438:330–334. [DOI] [PubMed] [Google Scholar]
- 10.Kumar R, Woo MA, Birrer BV, et al. , 2009. Mammillary bodies and fornix fibers are injured in heart failure. Neurobiol Dis 33:236–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kumar R, Lee K, Macey PM, Woo MA, Harper RM 2009. Mammillary body and fornix injury in congenital central hypoventilation syndrome. Pediatr Res 66:429–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van Asselen M, Kessels RP, Wester AJ, Postma A 2005. Spatial working memory and contextual cueing in patients with Korsakoff amnesia. J Clin Exp Neuropsychol 27:645–655. [DOI] [PubMed] [Google Scholar]
- 13.Sheslow D, Adams W 2003. Wide Range Assessment of Memory and Learning–Second Edition (WRAML2) Wide range Inc, Delaware. [Google Scholar]
- 14.Pike NA, Woo MA, Poulsen MK, et al. , 2016. Predictors of Memory Deficits in Adolescents and Young Adults with Congenital Heart Disease Compared to Healthy Controls. Front Pediatr 4:117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nasreddine ZS, Phillips NA, Bedirian V, et al. , 2005. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc 53:695–699. [DOI] [PubMed] [Google Scholar]
- 16.Pike NA, Poulsen MK, Woo MA 2017. Validity of the Montreal Cognitive Assessment Screener in Adolescents and Young Adults With and Without Congenital Heart Disease. Nurs Res 66:222–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ashburner J, Friston KJ 2005. Unified segmentation. Neuroimage 26:839–851. [DOI] [PubMed] [Google Scholar]
- 18.Miller SP, McQuillen PS, Hamrick S, et al. , 2007. Abnormal brain development in newborns with congenital heart disease. N Engl J Med 357:1928–1938. [DOI] [PubMed] [Google Scholar]
- 19.Rollins CK, Watson CG, Asaro LA, et al. , 2014. White matter microstructure and cognition in adolescents with congenital heart disease. J Pediatr 165:936–944 e931–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nagaraj UD, Evangelou IE, Donofrio MT, et al. , 2015. Impaired Global and Regional Cerebral Perfusion in Newborns with Complex Congenital Heart Disease. J Pediatr 167:1018–1024. [DOI] [PubMed] [Google Scholar]
- 21.Sun L, Macgowan CK, Sled JG, et al. , 2015. Reduced fetal cerebral oxygen consumption is associated with smaller brain size in fetuses with congenital heart disease. Circulation 131:1313–1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Andropoulos DB, Hunter JV, Nelson DP, et al. , 2010. Brain immaturity is associated with brain injury before and after neonatal cardiac surgery with high-flow bypass and cerebral oxygenation monitoring. J Thorac Cardiovasc Surg 139:543–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mahle WT, Clancy RR, McGaurn SP, Goin JE, Clark BJ 2001. Impact of prenatal diagnosis on survival and early neurologic morbidity in neonates with the hypoplastic left heart syndrome. Pediatrics 107:1277–1282. [DOI] [PubMed] [Google Scholar]
- 24.Santin LJ, Rubio S, Begega A, Arias JL 1999. Effects of mammillary body lesions on spatial reference and working memory tasks. Behav Brain Res 102:137–150. [DOI] [PubMed] [Google Scholar]
- 25.Kelleher DK, Laussen P, Teixeira-Pinto A, Duggan C 2006. Growth and correlates of nutritional status among infants with hypoplastic left heart syndrome (HLHS) after stage 1 Norwood procedure. Nutrition 22:237–244. [DOI] [PubMed] [Google Scholar]
- 26.Goldberg CS, Lu M, Sleeper LA, et al. , Pediatric Heart Network I 2014. Factors associated with neurodevelopment for children with single ventricle lesions. J Pediatr 165:490–496 e498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Williams RV, Ravishankar C, Zak V, et al. , Pediatric Heart Network I 2010. Birth weight and prematurity in infants with single ventricle physiology: pediatric heart network infant single ventricle trial screened population. Congenit Heart Dis 5:96–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ono M, Boethig D, Goerler H, Lange M, Westhoff-Bleck M, Breymann T 2007. Somatic development long after the Fontan operation: factors influencing catch-up growth. J Thorac Cardiovasc Surg 134:1199–1206. [DOI] [PubMed] [Google Scholar]
- 29.Pike NA, Evangelista LS, Doering LV, Eastwood JA, Lewis AB, Child JS 2012. Sex and age differences in body-image, self-esteem, and body mass index in adolescents and adults after single-ventricle palliation. Pediatr Cardiol 33:705–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bilici R, Saridogan GE, Turan C, et al. , 2015. A Case of Wernicke-Korsakoff Syndrome Treated 1 Year After the Onset of Symptoms. Prim Care Companion CNS Disord 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Langlais PJ, Savage LM 1995. Thiamine deficiency in rats produces cognitive and memory deficits on spatial tasks that correlate with tissue loss in diencephalon, cortex and white matter. Behav Brain Res 68:75–89. [DOI] [PubMed] [Google Scholar]
- 32.Khalsa SS, Kumar R, Patel V, Strober M, Feusner JD 2016. Mammillary body volume abnormalities in anorexia nervosa. Int J Eat Disord 49:920–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Harper C 2006. Thiamine (vitamin B1) deficiency and associated brain damage is still common throughout the world and prevention is simple and safe! Eur J Neurol 13:1078–1082. [DOI] [PubMed] [Google Scholar]
- 34.Shamir R, Dagan O, Abramovitch D, Abramovitch T, Vidne BA, Dinari G 2000. Thiamine deficiency in children with congenital heart disease before and after corrective surgery. JPEN J Parenter Enteral Nutr 24:154–158. [DOI] [PubMed] [Google Scholar]
- 35.Peyvandi S, Baer RJ, Moon-Grady AJ, et al. , 2018. Socioeconomic mediators of racial and ethnici disparities in congenital heart disease outcomes: A population-based study in California. J Am Heart Assoc 7:e010342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ohye RG, Schranz D, D’Udekem Y 2016. Current Therapy for Hypoplastic Left Heart Syndrome and Related Single Ventricle Lesions. Circulation 134:1265–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wypij D, Jonas RA, Bellinger DC, et al. , 2008. The effect of hematocrit during hypothermic cardiopulmonary bypass in infant heart surgery: results from the combined Boston hematocrit trials. J Thorac Cardiovasc Surg 135:355–360. [DOI] [PubMed] [Google Scholar]
- 38.Chock VY, Amir G, Davis CR, et al. , 2006. Antegrade cerebral perfusion reduces apoptotic neuronal injury in a neonatal piglet model of cardiopulmonary bypass. J Thorac Cardiovasc Surg 131:659–665. [DOI] [PubMed] [Google Scholar]
- 39.Dent CL, Spaeth JP, Jones BV, et al. , 2006. Brain magnetic resonance imaging abnormalities after the Norwood procedure using regional cerebral perfusion. J Thorac Cardiovasc Surg 131:190–197. [DOI] [PubMed] [Google Scholar]
- 40.Di Maria MV, Glatz AC, Ravishankar C, et al. , 2013. Supplemental tube feeding does not mitigate weight loss in infants with shunt-dependent single-ventricle physiology. Pediatr Cardiol 34:1350–1356. [DOI] [PubMed] [Google Scholar]


