Abstract
Successful embryo implantation is a complex and highly regulated process involving precise synchronization between the fetal-derived trophoblast cells and maternal uterine luminal epithelium. Multiple endocrine-driven factors are important for controlling the timely receptivity of the uterus, and this complexity underscores implantation failure as a major cause of recurrent infertility associated with assisted reproductive technologies. One particular cellular structure often hypothesized to promote receptivity is the pinopode or uterodome - a hormonally regulated, large cellular protrusion on the uterine epithelial surface. Recent clinical studies associate pinopodes with favorable fertility outcomes in women, and because they are directly linked to an increase in progesterone levels, the potential utility of these hormone-regulated cell biological structures in predicting or improving implantation in a clinical setting holds promise. In this review, we aim to generate interest in pinopodes from the broader cell biology and endocrinology communities, re-examine methodologies in pinopode research, and identify priorities for future investigation of pinopode structure and function in women’s reproductive health.
Keywords: progesterone, endometrial receptivity, implantation, pinopodes, uterodomes
Graphical Abstract

Introduction
Recurrent implantation failure is a major clinical problem in reproductive medicine, estimated to persist in about 48% of couples undergoing in vitro fertilization (IVF), resulting in extensive physical and emotional trauma (Coughlan et al., 2014; Coughlan et al., 2013). Two main causes of implantation failure include aberrant embryonic development and poor endometrial receptivity (Brosens et al., 2014). Endometrial receptivity is defined as the period during which the uterus undergoes changes to provide an adequate and receptive environment for the incoming fertilized embryo to implant (Teh et al., 2016).
In humans, the endometrial receptivity period occurs during the mid-luteal phase of the menstrual cycle (days 19-23) (Paria et al., 2001). A typical menstrual cycle in women consists of 28 days but can range from 21-35 days (Hawkins and Matzuk, 2008). The menstrual cycle is comprised of two parallel cycles: the ovarian cycle, which includes the follicular and luteal phases, and the uterine cycle consisting of menses and proliferative and secretory phases (Messinis et al., 2014). In the mid-luteal phase, the endometrium undergoes changes in response to a rise in progesterone levels to ensure receptivity of the fertilized embryo (Hawkins and Matzuk, 2008). There is a finite time during which the endometrium becomes receptive for blastocyst attachment, known as the “window of implantation” (WOI) (Harper, 1992).
The events leading up to the WOI are critically important for the establishment of a healthy pregnancy and are of clinical interest to the field of reproductive endocrinology and infertility (Murray et al., 2004). In particular, there has been a strong movement in the reproductive field to better define factors related to endometrial receptivity. The advent of the endometrial receptivity array (ERA) several years ago provided an initial tool to diagnose the molecular status of the receptive endometrium using an expression array panel of approximately 250 genes associated with endometrial receptivity (Diaz-Gimeno et al., 2011). This diagnostic tool also helped elucidate that each woman has a personalized WOI that could be utilized for optimizing timing of embryo transfer (Diaz-Gimeno et al., 2011; Diaz-Gimeno et al., 2013). However, there are limitations to utilizing this tool, including expense, inaccurate results, and the invasive nature of the test (Bassil et al., 2018; Mahajan, 2015). Therefore, identification of additional receptivity markers, including cellular modifications that may enable endometrial receptivity at the WOI and therefore improve clinical outcomes, is highly desirable.
Over the years, a handful of groups have commented on cellular modifications of the uterine luminal epithelium that may be associated with embryo implantation and fertility. Some of the first transmission electron microscope (TEM) studies illustrated surface modifications of the uterine luminal epithelium of rodents following administration of steroid hormones (Bergstrom and Nilsson, 1972; Nilsson, 1966; Warren and Enders, 1964). The observed changes in microvilli and formation of uterine protrusions in the endometrium were believed to be associated with a type of secretory mechanism to nurture the blastocyst at the time of implantation (Nilsson, 1958, 1966; Warren and Enders, 1964). These structures were first described as “mushroom-like” or “sea-anemonae-like” protrusions during the peri-implantation period (Nilsson, 1972; Psychoyos and Mandon, 1971). Originally termed “pinopods” (“drinking foot” in Greek) following the observation that these protrusions took up lead citrate-labeled tracers in vacuoles, these endometrial structures have proven mysterious and controversial for several decades (Enders and Nelson, 1973; Quinn and Casper, 2009). The term “uterodome” has also been used to divorce the name of these protrusions from an implied function. However, in this review, we will refer to these structures as “pinopodes” for the sake of consistency with the majority of the available literature.
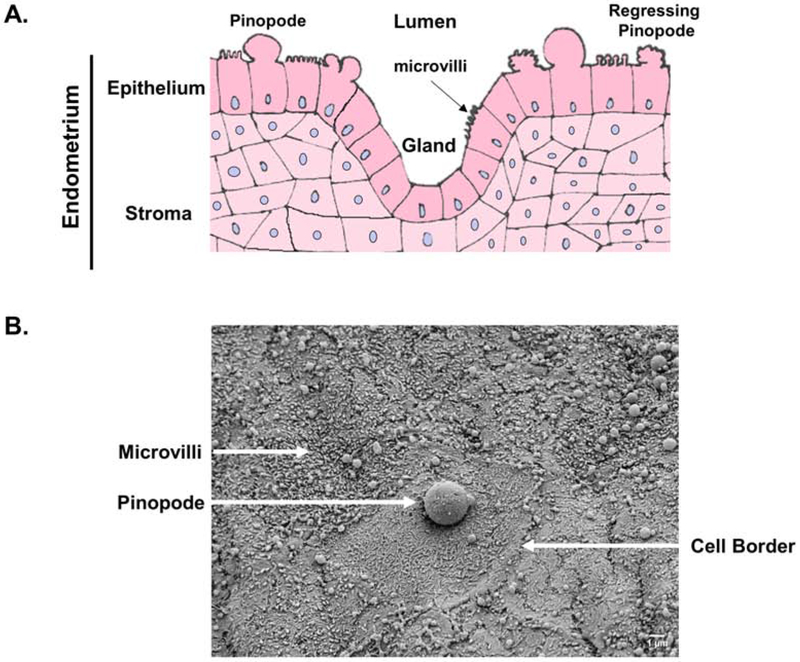
Pinopodes are generally considered to be 5-10 μm cellular protrusions of the apical plasma membrane of uterine epithelial cells (Figure 1). They bear some resemblance to cellular blebs, which are ~2 μm transient plasma membrane extravasations that stochastically appear and disappear within seconds to minutes on the surface of migrating cells. Therefore, in comparison to other epithelial plasma membrane protrusions, including blebs and others like cilia and microvilli, which are approximately 0.2 μm to 90 nm in size, respectively, pinopodes are uniquely large and present during the WOI (Nikas, 2000)— a period of ~1-2 days in rodents and ~4 days in humans.
Fig. 1.
A) Pinopodes are large plasma membrane protrusions of uterine epithelial cells that transiently project toward the uterine lumen during the “window of implantation.” B) Scanning electron micrograph of the murine uterine epithelial surface at pseudopregnancy day 3.5, the peak timing of the “window of implantation.”
Pinopodes occur in multiple species, including mice, rats, and humans, suggesting that they serve an evolutionary conserved purpose. Initially hypothesized to form at the sites of blastocyst attachment (Johannisson and Nilsson, 1972; Nilsson, 1958; Psychoyos and Mandon, 1971), pinopodes have typically been correlated with successful implantation (Nilsson, 1958; Usadi et al., 2003) and are strongly regulated by the presence of the ovarian steroid hormones.
Pinopode Density and Structure: Hormonal Determinants
A strong area of recent research interest has focused on identifying correlations between hormonal and other factors and pinopode development (Table 1) (Nikas et al., 1995; Nikas and Psychoyos, 1997). Indeed, several studies have emphasized the dependence of pinopode formation on hormonal control by the two most studied hormone contributors, estrogen and progesterone (Bentin-Ley, 2000; Lopata et al., 2002; Nardo et al., 2002; Rarani et al., 2018). The majority of literature reports that progesterone is responsible for stimulation of pinopode development, whereas an increase in estrogen parallels pinopode regression (Martel et al., 1991; Psychoyos and Mandon, 1971; Sarantis et al., 1988; Singh et al., 1996). To evaluate pinopodes as a marker of endometrial receptivity, many have tracked pinopode formation during the luteal/secretory phase of the menstrual cycle in women (Figure 2A) (Develioglu et al., 1999; Nikas et al., 1999; Nikas et al., 1995; Nikas and Psychoyos, 1997). Although the timeframe of pinopode formation during the luteal phase varies in the literature, on average, prominent formation occurs on days 20-22 of the natural menstrual cycle and 1-2 days earlier in stimulated cycles, coinciding with the WOI.
Table 1.
Regulators of Pinopodes
| Protein/Treatment | |||
|---|---|---|---|
| Hormones | Species | Summary | Reference |
| Progesterone (P4) | Human | Peak serum P4 levels correlates with increased pinopode numbers. High serum P4 levels results in early pinopode development. |
Develioglu et al., 1999 Stavreus-Evers et al., 2001 |
| Estrogen (E2) | Human Rat |
High doses of E2 inhibit pinopode formation. Centchroman (E2 inhibitor) treatment decreases pinopodes. |
Martel et al., 1991 Singh et al., 1996 |
| Adrenomedullin (AM) | Mouse | Inhibition of AM and haploinsufficiency of Adm reduces pinopodes. Intrauterine delivery of AM increases pinopodes. |
Li et al., 2008 Matson et al., 2017 |
| Gonadotropin releasing hormone agonist (GnRH-a) | Human Mouse |
Treatment of GnRH-a increased pinopode growth in women experiencing infertility. Pinopodes increase and are well-developed at day 4 of pregnancy following treatment of GnRH-a |
Zhou et al., 2017 Guo et al., 2018 |
| Testosterone | Rat | Administration decreases pinopodes. | Mokhtar et al., 2014 |
| Secretion and Transcription Factors | |||
| LIF and LIFR | Human | LIF and LIFR peak between LH days 6 to 9, coinciding with fully developed pinopodes. | Aghajanova et al., 2003 |
| Glycodelin | Human | Increased staining in tissue with pinopodes. | Stavreus-Evers et al., 2006 |
| Hoxa10 | Mouse | Overexpression leads to an increase in pinopodes and blocking Hoxa10 in the uterus reduces pinopode formation. | Bagot et al., 2001 |
| Endocrine Disrupters and Environmental Exposures | |||
| Polychlorinated biphenyls | Mouse | Exposure leads to poorly developed pinopodes. | Qu et al., 2018 |
| Quercetin (polyphenolic compound found in fruits and vegetables) | Rat | Administration causes poorly developed pinopodes. | Shahzad et al., 2017 |
| Cigarette Smoke | Mouse | Decreased pinopode development. | Duran et al., 2014 |
| Cypermethrin (CYP; type II pyrethroids pesticides) | Mouse | In mice with medium and high dosage of CYP, pinopodes are sparse. | Zhou et al., 2018 |
Fig. 2.
A) Representative scanning electron microscopy pinopode images from women experiencing infertility issues. Each image represents a different patient and demonstrates the variety of pinopodes in a given sample. Arrows denote regressing pinopode and cilia. Endometrial biopsies were collected between LH +6 to +10 in a natural menstrual cycle. Figure used from Aunapuu et al., 2018 (CC BY 4.0) under the creative commons attribution license. B) Representative stages of pinopode and microvilli development during pseudopregnancy (pp) in the mouse endometrium. On day 2.5 of pseudopregnancy, pinopodes appear to expand and have punctate holes. By day 3.5 of pseudopregnancy, pinopodes rise above microvilli and become smooth and spherical in shape. Few microvilli are present on day 4.5 of pseudopregnancy with pinopodes demonstrating more of a deflated and elongated appearance. Scale bars, 2 μm.
Based on scanning electron microscopy (SEM) analysis of pinopodes throughout the menstrual cycle, pinopodes are typically classified as developing/immature, fully developed/mature, and regressing according to their ultrastructural morphology (Table 2). However, it is important to reiterate that a specific cycle day does not guarantee that all pinopodes will encompass similar morphology (Aunapuu et al., 2018; Nikas et al., 1995; Stavreus-Evers et al., 2001). Generally, during days 17-19 of the menstrual cycle, cellular bulging increases with the formation of small (1-2 μm) pinopodes. By day 20, fully developed pinopodes, described as spherical, smooth structures with no microvilli, cover the endometrium (Lopata et al., 2002; Quinn and Casper, 2009; Rarani et al., 2018). By the end of the secretory phase in women (days 23-25), pinopodes regress with a wrinkled appearance resembling a raisin or deflated balloon (Nikas, 2000; Nikas et al., 1995). There are discrepancies in the literature regarding pinopode morphology on specific days of the cycle, and of course, these changes may vary between individuals (Table 2) (Nikas, 2000).
Table 2.
Human Pinopode Morphology
| Day of Cyclicity | Microvilli Characteristics | Pinopode Morphology | Reference |
|---|---|---|---|
| 15-16 | Length and density increases | No appearance, cells begin to bulge | Nikas et al., 2000 |
| 17-19 | Tall, long and thick | Small pinopodes appear (1-2 μm), endometrial surface bulging increases | Nikas et al., 2000 |
| 18-19 | Size diminishes with the appearance of swollen tips | Distinct cell bulging with slender pinopodes rising from cell apex | Nikas et al., 2000 |
| 19-21 | Short or no microvilli present | Smooth surface pinopodes develop and fold | Nikas et al., 2000; Stavreus-Evers et al., 2001; Quinn and Casper, 2009 |
| 20-23 | Absent | Full protrusion of pinopodes, some appear with a wrinkled surface | Stavreus-Evers et al., 2001; Aunapuu et al., 2018 |
| 20-23 | Increase in Microvilli | Pinopodes regress with a deflated balloon appearance | Nikas et al., 2000 |
| 23-24 | Microvilli reappear on cell membranes | Pinopodes regress and/or disappear |
Nikas et al., 2000; Stavreus-Evers et al., 2001 |
Variations in pinopode morphology are also observed in the clinic. Women experiencing poor IVF outcomes commonly have alterations in pinopode shape and poor pinopode development (Aunapuu et al., 2018). Previous research has taken into consideration pinopode variance in women by modifying the standard clinical IVF protocol (5 day-old blastocyst transferred on day 7 of progesterone treatment) to include progesterone treatment based on pinopode morphology. When pinopode morphology was evaluated in women undergoing IVF treatment and fully developed pinopodes were synchronized with progesterone treatments, the pregnancy success rate increased, further validating an important stimulatory relationship (Sudoma et al., 2011). Although pinopode development appears to correlate with increasing progesterone, it has yet to be established whether pinopodes themselves secrete hormones. Additional hormones such as adrenomedullin and gonadotropin releasing hormone agonist correlate with increased pinopode growth, whereas testosterone results in a decrease in pinopodes (Matson et al., 2017; Mokhtar et al., 2014; Zhou et al., 2017). Based on these studies, hormonal regulation is an important aspect of pinopode growth and morphology, and further investigation into these correlations could help define the proper period for embryo transfer (Pantos et al., 2004; Sudoma et al., 2011).
Several clinical studies, including two recent publications, demonstrate promising observations of the potential for pinopodes to serve as indicators of endometrial receptivity at the WOI (Jin et al., 2017; Pantos et al., 2004; Qiong et al., 2017; Sudoma et al., 2011). The most recent clinical trials reported that women undergoing IVF with a greater pinopode score exhibited a higher embryo implantation and pregnancy rate compared to those with a lower score (Jin et al 2017; Qiong et al., 2017). Although these studies did not evaluate natural cycles, their extensive patient recruitment and outlining of new strategies for pinopode measurement and quantitation further ratifies pinopodes as a reliable endometrial receptivity marker (Jin et al., 2017; Qiong et al., 2017). Others have reported that women with ample pinopode coverage (greater than 10%) have an increased likelihood of pregnancy success compared to those with sparse or no pinopode coverage (Nikas and Aghajanova, 2002).
However, some argue that although pinopodes are present during the WOI, they do not directly correlate to embryo implantation and pregnancy success in women (Quinn et al., 2007a; Quinn and Casper, 2009). This notion is due to the expanded period of pinopode presence throughout the luteal phase, rather than a specific growth period at the WOI, which would rule out their possible benefit in embryo receptivity (Quinn et al., 2007a). In addition, women with recurrent implantation failure and women experiencing infertility do not exhibit a significant difference in pinopode coverage or morphology (Da Broi et al., 2017; Xu et al., 2012). However, the possibilities remain that there are differences in these patients in pinopode function or in other implantation-related events that are unrelated to pinopodes, underscoring the complexity of the implantation process.
Consistent with human studies, we have also observed changes in pinopode formation and morphology in mice based on the day of pseudopregnancy (Figure 2B). In pseudopregnant mice, the WOI ranges from days 2.0-3.5 and is characterized by an increase in progesterone levels (Ueda et al., 2003). By day 3.5 of pseudopregnancy, an increase in spherical, smooth, “healthy” pinopodes were observed rising above short microvilli. By day 4.5 (indicative of the timeframe of embryo implantation), the pinopodes exhibited an increase in size, yet with a deflated appearance. These findings are consistent with observations in rats and humans, confirming murine pseudopregnancy as a valuable model for studying pinopode formation and functionality in association with hormonal regulation. We have previously reported that mice experiencing impaired embryo implantation and sub-fertility due to haploinsufficiency of the peptide hormone adrenomedullin have fewer pinopodes compared to their wildtype counterparts (Li et al., 2008). In contrast, one group has concluded that infertile mice do not exhibit a difference in pinopode formation, yet this particular cause for infertility may be independent of pinopode function. (Quinn et al., 2007a; Quinn et al., 2007b). Therefore, a direct causal association between pinopodes and embryo receptivity across species is not currently available.
The duration of the pinopode lifespan may be varied, as some have specified a limited lifespan of up to 48 hours, whereas others observe pinopodes persisting beyond 48 hours (Acosta et al., 2000; Usadi et al., 2003). The discrepancies in lifespan could be due to variations in number or location of biopsies collected, in cycle duration, and in individual patient-level physiology. Research has identified a handful of promising molecular markers of endometrial receptivity, but our knowledge of the chronological events establishing endometrial receptivity and how they affect fertility is still naïve. To this extent, it is of utmost importance to further our understanding of factors that define the receptivity period, including establishing better methodologies to study pinopodes. If additional studies are completed to describe how pinopodes correlate with hormonal surges and timely reception, the development of additional tools could advance the clinical reproduction field, especially in IVF patients.
Unfortunately, very little is known about the molecular architecture and organization of pinopodes. One group has identified ezrin, a plasma membrane protein-actin cytoskeleton cross-linker present in ruffled membranes, in pinopode-laden uterine luminal epithelial cells by immunohistochemistry (Tan et al., 2012). Otherwise, whether cell adhesion proteins like integrins are present on the surface of pinopodes, potentially in interaction with the implanting blastocyst, remains to be determined. As described in more detail below, our ability to develop models to study pinopode structure, function, and dynamics comparable to those used to study microvilli, cilia, and blebs has unfortunately been limited. Thus, further investigation into the variation in pinopode morphology is merited.
Pinopode Function
Regulation of Uterine Luminal Contents
As previously discussed, the term “pinopode” was derived from a hypothesized function for these structures in pinocytosis of uterine luminal fluid. Regulation of luminal fluid volume is critically important before and during embryo implantation. Prior to implantation, uterine fluid volume increases to promote embryo transportation and appropriate intrauterine position (Zhang et al., 2017). Following embryo transportation, uterine fluid levels decrease, allowing luminal closure around the embryo to facilitate attachment to the uterus (Yoshinaga, 2013). In rats, endocytic activity peaks between days 4-6 of pregnancy, coinciding with embryo implantation (Parr, 1980). Towards the end of this timeframe, pinopodes begin to regress, a process thought to be functionally important for the movement of material into the apical cytoplasm of epithelial cells (Enders and Nelson, 1973).
Early studies described absorption of trypan blue by epithelial cells of the rat endometrium and subsequent storage in the cytoplasm (athrocytosis), a process that was hypothesized to play a role in establishing an ideal maternal environment for implantation (Sartor, 1972). At the time, pinopodes were not fully classified or named, but this study set the stage for a connection between progesterone, a predominant hormonal driver of pinopodes, and fluid absorption in the endometrium (Bentin-Ley et al., 1999; Sartor, 1972). The process of material migration and removal from the endometrium was then later described as endocytosis and pinocytosis, involving material ingestion into epithelial cells and formation of vacuoles and small vesicles (Enders and Nelson, 1973; Parr and Parr, 1974). This material was often observed to be translocated into lysosomes or dispersed into the uterine stroma (Parr, 1980; Parr and Parr, 1977, 1986; Tung et al., 1988). Additionally, pinopodes were observed to uptake ferritin and horseradish peroxidase into vacuoles in rats and mice. There was therefore speculation that these epithelial projections were important for altering uterine luminal fluid contents to provide an environment conducive to implantation for the blastocyst (Nilsson, 1972).
Another hypothesis suggested that an endocytic function for pinopodes promoted uterine closure (Enders and Nelson, 1973) and the establishment of a proper “runway” for the incoming embryo to adhere to the uterine epithelium (Lessey et al., 2000; Nikas, 1999). A potential endocytic function was supported by a study demonstrating that expression of water channel proteins, aquaporins (AQP), positively correlated with pinopodes in the rat (Lindsay and Murphy, 2007). Some have speculated that pinopodes express AQPs on their surface. Indeed, one group observed localization of AQP2 to pinopodes by confocal microscopy during the mid- to late secretory phase in women (Hildenbrand et al., 2006), and quercetin (a plant-derived polyphenolic compound) causes a decrease in AQPs, which correlates with a decrease in pinopodes (Table 1) (Shahzad et al., 2017). Consistent with a hypothesized role for pinopodes in water transport in the context of embryo attachment and implantation, knockdown of AQP2 decreased embryonic spheroid attachment to endometrial cells in vitro (He et al., 2019). However, the same study also demonstrated that estradiol treatment of human endometrial biopsies increases AQP2 levels, suggesting a pinopode-independent mechanism of AQP2 upregulation (He et al., 2019).
We have also observed a correlation between pinopode formation and uterine fluid transport following treatment with the peptide hormone adrenomedullin (Matson et al., 2017). Intrauterine injection of adrenomedullin during the peri-implantation period in mice stimulates water accumulation correlating with an increase in pinopode size and number and embryo implantation rate. In addition to adrenomedullin, known hormonal drivers of uterine fluid homeostasis include estradiol and progesterone (Zhang et al., 2017). Even a small physiological increase in estradiol in mice can disrupt intrauterine fluid accumulation and disturb efficient embryo implantation (Zhang et al., 2015). Dynamic changes in estradiol and progesterone signaling may result in decreased pinopode formation and thus abnormal fluid accumulation in the uterus prior to implantation, which may impair the establishment of a healthy maternal-fetal environment for embryo attachment (Parr, 1983).
The significance of fluid uptake and secretion and pinopode participation in these processes during pregnancy are continuously debated among researchers, particularly when examining differences between rodents and humans (Adams et al., 2002; Murphy, 2000; Quinn and Casper, 2009). Currently, vacuoles have not been identified in human pinopodes, therefore it is thought that they do not participate in pinocytotic activity and could elicit a different function in the uterus (Adams et al., 2002). However, failure to observe pinopode pinocytotic activity in humans could stem from ethical limitations, causing the distinct activities and the functionality of pinopodes to remain questionable (Adams et al., 2002).
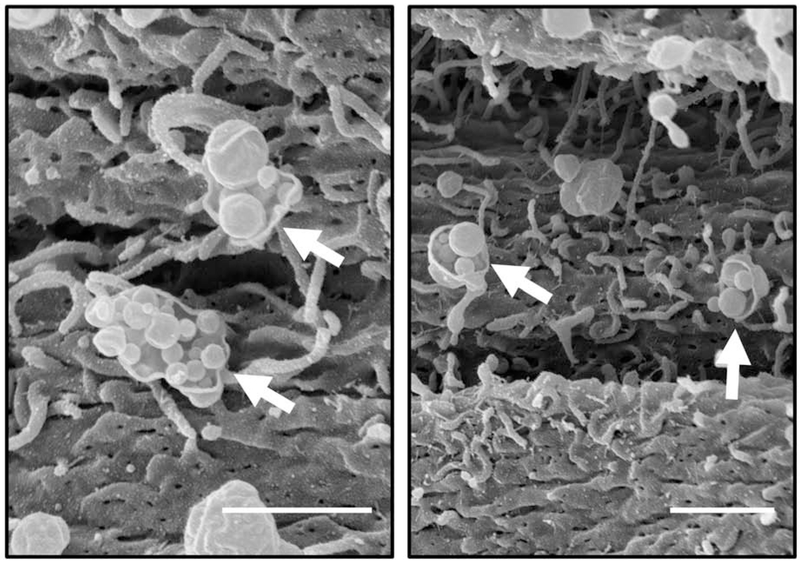
In addition to participation in endocytosis of uterine luminal fluid, pinopodes may participate in exocytosis by moving materials from the stroma to the uterine lumen (Parr, 1980). Parr discovered that administration of horseradish peroxidase as a tracer during day 5 of pregnancy in rats caused localization of the tracer to vesicles close to stromal cells at the basement membrane (Parr, 1980). Later, this tracer was tracked to vesicles adjacent to the apical membrane in uterine epithelial cells. They hypothesized that these vesicles translocate to the luminal surface and fuse with the apical membrane to release material directly into the uterine lumen (Parr, 1980). Unfortunately, they did not specifically determine whether the uterine epithelial cells containing the tracer included pinopodes. However, using SEM, we have observed potential release of vesicular material from pinopodes on day 4 of pregnancy in mice, which may be similar to Parr’s previous findings (Figure 3).
Fig. 3.
Scanning electron microscopy of the mouse endometrium on day 4.5 of pseudopregnancy. Some pinopodes (arrows) appear to rupture and release vesicular contents. Scale bars, 1 μm.
It is possible that this secreted material contains exosomal structures. Much smaller than pinopodes, exosomes are extracellular vesicles ranging in size from 30 nm to 150 nm. They play a role in cell-cell communication through packaging of lipids, proteins, mRNAs, and miRNAs, and their cargo is directly influenced by estrogen and progesterone (Greening et al., 2016; Nguyen et al., 2016). The field of exosomes is quickly evolving, with preliminary evidence demonstrating that exosome release from epithelial cells of the endometrium mediates dialogue between maternal and fetal cell communication during implantation (Machtinger et al., 2016). The process of exocytosis by pinopodes and the hypothesis that pinopodes may release exosomes could be important for communication with the blastocyst or involvement in degradation of the uterine luminal epithelium and remodeling of the endometrial stroma (Enders and Nelson, 1973; Moulton et al., 1978; Parr, 1983). Therefore, pinopode exocytosis at the uterine luminal epithelial surface is an intriguing area of endometrial research, requiring further exploration.
Regulation of Proteins Involved in Implantation
Because pinopodes appear during the WOI, it is not surprising that the majority of hypothesized pinopode markers are related to implantation genes (Bagot et al., 2001; Shimizu et al., 2008; Stavreus-Evers et al., 2002a; Stavreus-Evers et al., 2002b). Unfortunately, few have demonstrated localization of these proteins either on the surface of or within pinopodes. One hypothesis is that pinopodes may express proteins like integrins on their surface that promote blastocyst attachment. For example, in women with high rates of embryonic loss, a decrease in pinopode density was associated with decreased integrin β3 expression (Aghajanova et al., 2003; Liu et al., 2017; Nardo et al., 2003; Xu et al., 2012). Others have hypothesized that pinopodes secrete leukemia inhibitory factor (LIF), which is involved in the epithelial transition to a receptive state (Kabir-Salmani et al., 2005). However, others deny that localization of integrins and LIF parallel that of pinopodes (Creus et al., 2003; Creus et al., 2002; Mikolajczyk et al., 2011).
Another dispute is whether anti-adhesive glycoproteins known as mucins (MUC1 and MUC16), which are thought to disappear and therefore facilitate the unmasking of integrins on epithelial cells (Aplin, 1997), are expressed on or in pinopodes. While one group found that extracellular MUC1 localization is not associated with pinopodes at the time of embryo attachment (Horne et al., 2005; Horne et al., 2002), others have identified MUC1 expression on pinopodes (Gipson et al., 2008). Most recently, MUC1 was identified by SEM in ciliated luminal epithelial cells but not in secretory cells or pinopodes (Wu et al., 2019). Expression of MUC16 also vanishes from pinopodes during endometrial receptivity, which could indicate that pinopode formation is beneficial in embryo adhesion (Gipson et al., 2008).
Additionally, environmental exposures and endocrine disruptors have long been an important topic in female infertility. More recently, these effectors have been an area of investigation with regard to pinopode development and embryo implantation. Studies in mice have demonstrated that environmental exposures or endocrine disruptors can decrease pinopode development and cause poorly developed pinopodes (Table 1) (Duran et al., 2014; Qu et al., 2018; Shahzad et al., 2017; Zhou et al., 2018). Further research pertaining to the association of these effectors with poorly developed pinopodes could provide more insights describing how pinopodes form, resulting in further characterization of pinopodes.
Current Perspectives on Analysis of Pinopodes
Quantitative Evaluation of Pinopodes
Here, we provide insight into the quantitative and qualitative assessment of pinopodes with an emphasis on establishing guidelines for improvement, standardization, and future direction. To date, the vast majority of studies on pinopodes across all species have used SEM to evaluate pinopode coverage in the endometrium (Chen et al., 2016; Jin et al., 2017; Li et al., 2010; Nikas et al., 1995; Qiong et al., 2017). However, there is certainly variability in what each individual observer determines to be the size threshold above which a pinopode is counted. Therefore, investigators should consider developing an overall standard when counting pinopodes regarding both pinopode size and number of fields to be counted. Commonly, the size range of pinopodes varies from 1.0-10.0 μm, with previous average reports around 4.0 μm in the rat and 6.0 μm in the mouse and human (Ljungkvist and Nilsson, 1971; Quinn et al., 2007a; Quinn et al., 2007b). To the second point, Jin et al. recently recommended using a running average of 60 fields in each specimen to reduce sample error and achieve a reproducible result for calculating pinopode coverage (Jin et al., 2017). Therefore, the quantitation methods outlined by Jin et al. stress that increased scientific rigor is required for accurate assessment of pinopode density.
An additional consideration regarding the quantitative evaluation of pinopode size is the qualitative evaluation of pinopode morphology. As previously discussed, pinopodes can be classified into developing/immature, fully developed/mature, and regressing pinopodes based on their morphology, which should be considered when assessing pinopode coverage. For example, Jin et al. calculated a percentage: fully developed pinopodes divided by the total number of pinopodes (including pinopodes in numerous stages) (Jin et al., 2017). A more recent study analyzed both pinopode subpopulations as well as other ultrastructural characteristics of surrounding cells like cilia and microvilli (Wu et al., 2019). An additional caveat to the quantitative evaluation of pinopode size is that differently sized pinopodes may demonstrate different functions. It is certainly possible that women experiencing infertility exhibit pinopodes of various sizes in different ratios and therefore different functions than women who do not experience infertility.
Historically, pinopode density has typically been quantitated by hand counting by a blinded observer (Nikas, 2000; Stavreus-Evers et al., 2001). One should take into consideration that this type of assessment is variable and somewhat imprecise, as the magnification or the subjective criteria of a pinopode could affect how many pinopodes are actually evaluated. Our laboratory recently provided the first to our knowledge automated pinopode quantitation method (Matson et al., 2017). This method eliminates human error and bias from traditional blind-observer counting, thus providing a more accurate pinopode count in a given sample. Briefly, by using the binary plugin in Fiji (Schindelin et al., 2012; Schneider et al., 2012), SEM images can be analyzed based on particle size and circularity to specifically quantitate pinopodes. The use of an automated quantitation method will enable the characterization of specific pinopode populations based on size, ultimately resulting in accurate results and reproducibility between studies.
Imaging Techniques
Because pinopodes are small, transient protrusions dependent on hormonal and potentially osmotic conditions of the extracellular environment, they are often difficult to image in space and time. Imaging pinopodes using confocal microscopy is therefore limited, and interpretations of specific cell staining should be taken with reservation. Uncertainty surrounding several potential markers as well as the overall paucity of markers underscore the importance of using imaging modalities, including SEM and additional tools, to characterize pinopodes. Although SEM and TEM provide superb resolution of pinopode structures, antibodies are often difficult to use in electron microscope imaging strategies (Horne et al., 2005; Stavreus-Evers et al., 2006; Tan et al., 2012). Thus, development and application of super-resolution imaging technologies combined with immunohistochemistry are needed to identify new markers of pinopode structures. In these ways, extracellular and intracellular expression patterns in pinopodes could be elucidated, strongly enhancing our knowledge of pinopode structure and function.
Concluding Remarks and Future Directions
Recently published clinical trials correlating pinopodes with implantation and pregnancy serve to generate renewed interest in pinopode biology (Jin et al., 2017; Qiong et al., 2017). The majority of available literature to date has described and analyzed density of pinopodes without subclassifying pinopodes into their developmental stages. Previous research has indicated that pinopode morphology, rather than absolute numbers, may be more relevant to endometrial receptivity (Jin et al., 2017; Sudoma et al., 2011). Encouragingly, though, studies are beginning to incorporate developmental stage into their analysis of pinopode density (Wu et al., 2019). Optimization of automated techniques to analyze pinopode density and morphology will allow for reproducibility in the field and understanding of the importance of pinopodes during the WOI.
It is still unknown whether pinopode function (e.g. endocytosis, exocytosis, expression of adhesion molecules) may differ between healthy patients and those with recurrent implantation failure. For example, an infertile patient may exhibit the same number of pinopodes as a fertile patient, yet the distribution of pinopodes and the material therein may actually determine embryo spacing and implantation success. We must also not neglect potential interaction of pinopodes with surrounding ultrastructural features of uterine epithelial cells, as some studies report that pinopodes allow for the entrapment of cilia, thereby preventing embryo movement and enabling close contact and adherence of the embryo during implantation (Bentin-Ley, 2000; Bentin-Ley et al., 1995; Bentin-Ley et al., 1999; Bergstrom and Nilsson, 1972).
One of the most pressing quandaries in the pinopode field is the accurate identification of a reliable marker for pinopodes. Identifying this marker could finally confirm the significance of pinopode expression at the WOI and clinically provide a new endometrial receptivity indicator. The WOI requires synchrony between maternal tissues and the embryo to establish appropriate attachment (Valles and Dominguez, 2006). Because there is debate in the literature concerning the formation and development of pinopodes during the WOI, perhaps, a “pinopode window” exists in which certain coverage, morphology, and protein expression is critically important for embryo communication or contact. To examine this hypothesis, pinopode analysis methods must be rigorous and investigators should consider quantifying coverage and morphology in a given sample. Additionally, at least 10 fields (or emphasized by Jin et al., 2017 at least 60 fields) should be examined in a sample to achieve reproducibility. Implementation of a standardized, automated quantitation method would also eliminate variability among publications and could help to establish consistency across the literature.
To further study the function of pinopodes, especially their role in endometrial receptivity, advanced model systems must be implemented. Development of an in vitro culture system would be advantageous in improving our understanding of mechanisms controlling pinopode formation. Prior attempts in our lab and others of in vitro culture systems have identified pinopode-like structures; however, these structures may be easily confused with cellular bleb formation (Bentin-Ley et al., 1999; Fleming et al., 1998; Park et al., 2003). Advances in imaging modalities and the development of a reliable in vitro system will promote the inquiry of appropriate questions regarding pinopode structure and function and help to distinguish pinopodes from cell bleb structures. Future pinopode research will be instrumental to further our broad understanding of factors that mediate implantation failure and may eventually lead to approaches to combat poor pregnancy outcomes in women undergoing natural conception or IVF treatments.
Review of literature regarding the significance of pinopodes in endometrial receptivity and implantation.
Quantitative and qualitative approaches for pinopodes are discussed.
Clinical relevance of pinopodes is revisited.
Acknowledgements
The authors would like to acknowledge The Microscopy Services Laboratory, Department of Pathology and Laboratory Medicine, supported in part by P30 CA016086 Cancer Center Core Support Grant to the UNC Lineberger Comprehensive Cancer Center for their insight in SEM preparation and SEM imaging of pinopodes. We are also grateful to Caron laboratory members for their thoughtful discussions regarding pinopode development and functionality.
Funding
This work was supported in part from The Lalor Foundation Fellowship to KEQ, F30 HD085652 to BCM, AHA 18POST33960353 to MW, and RO1 HD060860 to KMC.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest:
The authors have declared that no conflict of interest exists.
References
- Acosta AA, Elberger L, Borghi M, Calamera JC, Chemes H, Doncel GF, Kliman H, Lema B, Lustig L, and Papier S (2000). Endometrial dating and determination of the window of implantation in healthy fertile women. Fertil Steril 73, 788–798. [DOI] [PubMed] [Google Scholar]
- Adams SM, Gayer N, Hosie MJ, and Murphy CR (2002). Human uterodomes (pinopods) do not display pinocytotic function. Hum Reprod 17, 1980–1986. [DOI] [PubMed] [Google Scholar]
- Aghajanova L, Stavreus-Evers A, Nikas Y, Hovatta O, and Landgren BM (2003). Coexpression of pinopodes and leukemia inhibitory factor, as well as its receptor, in human endometrium. Fertil Steril 79 Suppl 1, 808–814. [DOI] [PubMed] [Google Scholar]
- Aplin JD (1997). Adhesion molecules in implantation. Rev Reprod 2, 84–93. [DOI] [PubMed] [Google Scholar]
- Aunapuu M, Kibur P, Jarveots T, and Arend A (2018). Changes in Morphology and Presence of Pinopodes in Endometrial Cells during the Luteal Phase in Women with Infertility Problems: A Pilot Study. Medicina (Kaunas) 54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagot CN, Kliman HJ, and Taylor HS (2001). Maternal Hoxa10 is required for pinopod formation in the development of mouse uterine receptivity to embryo implantation. Dev Dyn 222, 538–544. [DOI] [PubMed] [Google Scholar]
- Bassil R, Casper R, Samara N, Hsieh TB, Barzilay E, Orvieto R, and Haas J (2018). Does the endometrial receptivity array really provide personalized embryo transfer? J Assist Reprod Genet 35, 1301–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentin-Ley U (2000). Relevance of endometrial pinopodes for human blastocyst implantation. Hum Reprod 15 Suppl 6, 67–73. [PubMed] [Google Scholar]
- Bentin-Ley U, Lindenberg S, Horn T, and Larsen JF (1995). Ultrastructure of endometrial epithelial cells in a three-dimensional cell culture system for human implantation studies. J Assist Reprod Genet 12, 632–638. [DOI] [PubMed] [Google Scholar]
- Bentin-Ley U, Sjogren A, Nilsson L, Hamberger L, Larsen JF, and Horn T (1999). Presence of uterine pinopodes at the embryo-endometrial interface during human implantation in vitro. Hum Reprod 14, 515–520. [DOI] [PubMed] [Google Scholar]
- Bergstrom S, and Nilsson O (1972). Ultrastructural response of blastocysts and uterine epithelium to progesterone deprivation during delayed implantation in mice. J Endocrinol 55, 217–218. [DOI] [PubMed] [Google Scholar]
- Brosens JJ, Salker MS, Teklenburg G, Nautiyal J, Salter S, Lucas ES, Steel JH, Christian M, Chan YW, Boomsma CM, et al. (2014). Uterine selection of human embryos at implantation. Sci Rep 4, 3894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Yan Q, Liu K, Zhou X, Xian Y, Liang D, Zhao X, Guo X, and Quan S (2016). Endometrial Receptivity Markers in Mice Stimulated With Raloxifene Versus Clomiphene Citrate and Natural Cycles. Reprod Sci 23, 748–755. [DOI] [PubMed] [Google Scholar]
- Coughlan C, Ledger W, Wang Q, Liu F, Demirol A, Gurgan T, Cutting R, Ong K , Sallam H, and Li TC (2014). Recurrent implantation failure: definition and management. Reprod Biomed Online 28, 14–38. [DOI] [PubMed] [Google Scholar]
- Coughlan C, Yuan X, Nafee T, Yan J, Mariee N, and Li TC (2013). The clinical characteristics of women with recurrent implantation failure. J Obstet Gynaecol 33, 494–498. [DOI] [PubMed] [Google Scholar]
- Creus M, Ordi J, Fabregues F, Casamitjana R, Carmona F, Cardesa A, Vanrell JA, and Balasch J (2003). The effect of different hormone therapies on integrin expression and pinopode formation in the human endometrium: a controlled study. Hum Reprod 18, 683–693. [DOI] [PubMed] [Google Scholar]
- Creus M, Ordi J, Fabregues F, Casamitjana R, Ferrer B, Coll E, Vanrell JA, and Balasch J (2002). alphavbeta3 integrin expression and pinopod formation in normal and out-of-phase endometria of fertile and infertile women. Hum Reprod 17, 2279–2286. [DOI] [PubMed] [Google Scholar]
- Da Broi MG, Rocha CV Jr., Carvalho FM, Martins WP, Ferriani RA, and Navarro PA (2017). Ultrastructural Evaluation of Eutopic Endometrium of Infertile Women With and Without Endometriosis During the Window of Implantation: A Pilot Study. Reprod Sci 24, 1469–1475. [DOI] [PubMed] [Google Scholar]
- Develioglu OH, Hsiu JG, Nikas G, Toner JP, Oehninger S, and Jones HW Jr. (1999). Endometrial estrogen and progesterone receptor and pinopode expression in stimulated cycles of oocyte donors. Fertil Steril 71, 1040–1047. [DOI] [PubMed] [Google Scholar]
- Diaz-Gimeno P, Horcajadas JA, Martinez-Conejero JA, Esteban FJ, Alama P, Pellicer A, and Simon C (2011). A genomic diagnostic tool for human endometrial receptivity based on the transcriptomic signature. Fertil Steril 95, 50–60, 60 e51–15. [DOI] [PubMed] [Google Scholar]
- Diaz-Gimeno P, Ruiz-Alonso M, Blesa D, Bosch N, Martinez-Conejero JA, Alama P, Garrido N, Pellicer A, and Simon C (2013). The accuracy and reproducibility of the endometrial receptivity array is superior to histology as a diagnostic method for endometrial receptivity. Fertil Steril 99, 508–517. [DOI] [PubMed] [Google Scholar]
- Duran M, Turhan N, Kosus A, Kosus N, Sarac GN, Erdogan D, and Keskin EA (2014). Do cigarette smoke and vitamin E affect the development of endometrial pinopods? An animal study. Eur J Obstet Gynecol Reprod Biol 179, 117–120. [DOI] [PubMed] [Google Scholar]
- Enders AC, and Nelson DM (1973). Pinocytotic activity of the uterus of the rat. Am J Anat 138, 277–299. [DOI] [PubMed] [Google Scholar]
- Fleming H, Condon R, Peterson G, Guck I, Prescott E, Chatfield K, and Duff M (1998). Role of biotin-containing membranes and nuclear distribution in differentiating human endometrial cells. J Cell Biochem 71, 400–415. [DOI] [PubMed] [Google Scholar]
- Gipson IK, Blalock T, Tisdale A, Spurr-Michaud S, Allcorn S, Stavreus-Evers A, and Gemzell K (2008). MUC16 is lost from the uterodome (pinopode) surface of the receptive human endometrium: in vitro evidence that MUC16 is a barrier to trophoblast adherence. Biol Reprod 78, 134–142. [DOI] [PubMed] [Google Scholar]
- Greening DW, Nguyen HP, Elgass K, Simpson RJ, and Salamonsen LA (2016). Human Endometrial Exosomes Contain Hormone-Specific Cargo Modulating Trophoblast Adhesive Capacity: Insights into Endometrial-Embryo Interactions. Biol Reprod 94, 38. [DOI] [PubMed] [Google Scholar]
- Harper MJ (1992). The implantation window. Baillieres Clin Obstet Gynaecol 6, 351–371. [DOI] [PubMed] [Google Scholar]
- Hawkins SM, and Matzuk MM (2008). The menstrual cycle: basic biology. Ann N Y Acad Sci 1135, 10–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He R, Han W, Hu Y, Chen X, Hu X, and Zhu Y (2019). AQP2 is regulated by estradiol in human endometrium and is associated with spheroid attachment in vitro. Mol Med Rep. [DOI] [PubMed] [Google Scholar]
- Hildenbrand A, Lalitkumar L, Nielsen S, Gemzell-Danielsson K, and Stavreus-Evers A (2006). Expression of aquaporin 2 in human endometrium. Fertil Steril 86, 1452–1458. [DOI] [PubMed] [Google Scholar]
- Horne AW, Lalani EN, Margara RA, Ryder TA, Mobberley MA, and White JO (2005). The expression pattern of MUC1 glycoforms and other biomarkers of endometrial receptivity in fertile and infertile women. Mol Reprod Dev 72, 216–229. [DOI] [PubMed] [Google Scholar]
- Horne AW, White JO, and Lalani el N (2002). Adhesion molecules and the normal endometrium. Bjog 109, 610–617. [DOI] [PubMed] [Google Scholar]
- Jin XY, Zhao LJ, Luo DH, Liu L, Dai YD, Hu XX, Wang YY, Lin X, Hong F, Li TC, et al. (2017). Pinopode score around the time of implantation is predictive of successful implantation following frozen embryo transfer in hormone replacement cycles. Hum Reprod 32, 2394–2403. [DOI] [PubMed] [Google Scholar]
- Johannisson E, and Nilsson L (1972). Scanning electron microscopic study of the human endometrium. Fertil Steril 23, 613–625. [PubMed] [Google Scholar]
- Kabir-Salmani M, Nikzad H, Shiokawa S, Akimoto Y, and Iwashita M (2005). Secretory role for human uterodomes (pinopods): secretion of LIF. Molecular human reproduction 11, 553–559. [DOI] [PubMed] [Google Scholar]
- Lessey BA, Castelbaum AJ, Wolf L, Greene W, Paulson M, Meyer WR, and Fritz MA (2000). Use of integrins to date the endometrium. Fertil Steril 73, 779–787. [DOI] [PubMed] [Google Scholar]
- Li L, Xu BF, Chen QJ, and Sun XX (2010). Effects of hydrosalpinx on pinopodes, leukaemia inhibitory factor, integrin beta3 and MUC1 expression in the peri-implantation endometrium. Eur J Obstet Gynecol Reprod Biol 151, 171–175. [DOI] [PubMed] [Google Scholar]
- Li M, Wu Y, and Caron KM (2008). Haploinsufficiency for adrenomedullin reduces pinopodes and diminishes uterine receptivity in mice. Biol Reprod 79, 1169–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsay LA, and Murphy CR (2007). Aquaporins are upregulated in glandular epithelium at the time of implantation in the rat. J Mol Histol 38, 87–95. [DOI] [PubMed] [Google Scholar]
- Liu S, Hua T, Xin X, Shi R, Chi S, and Wang H (2017). Altered expression of hormone receptor, integrin beta3 and pinopode in the endometrium of luteal phase defect women. Gynecol Endocrinol 33, 315–319. [DOI] [PubMed] [Google Scholar]
- Ljungkvist I, and Nilsson O (1971). Ultrastructure of rat uterine luminal epithelium at functional states compatible with implantation. Z Anat Entwicklungsgesch 135, 101–107. [DOI] [PubMed] [Google Scholar]
- Lopata A, Bentin-Ley U, and Enders A (2002). “Pinopodes” and implantation. Rev Endocr Metab Disord 3, 77–86. [DOI] [PubMed] [Google Scholar]
- Machtinger R, Laurent LC, and Baccarelli AA (2016). Extracellular vesicles: roles in gamete maturation, fertilization and embryo implantation. Hum Reprod Update 22, 182–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahajan N (2015). Endometrial receptivity array: Clinical application. J Hum Reprod Sci 8, 121–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martel D, Monier MN, Roche D, and Psychoyos A (1991). Hormonal dependence of pinopode formation at the uterine luminal surface. Hum Reprod 6, 597–603. [DOI] [PubMed] [Google Scholar]
- Matson BC, Pierce SL, Espenschied ST, Holle E, Sweatt IH, Davis ES, Tarran R, Young SL, Kohout TA, van Duin M, et al. (2017). Adrenomedullin improves fertility and promotes pinopodes and cell junctions in the peri-implantation endometrium. Biol Reprod 97, 466–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messinis IE, Messini CI, and Dafopoulos K (2014). Novel aspects of the endocrinology of the menstrual cycle. Reprod Biomed Online 28, 714–722. [DOI] [PubMed] [Google Scholar]
- Mikolajczyk M, Skrzypczak J, and Wirstlein P (2011). No correlation between pinopode formation and LIF and MMP2 expression in endometrium during implantation window. Folia Histochem Cytobiol 49, 615–621. [DOI] [PubMed] [Google Scholar]
- Mokhtar HM, Giribabu N, Muniandy S, and Salleh N (2014). Testosterone decreases the expression of endometrial pinopode and L-selectin ligand (MECA-79) in adult female rats during uterine receptivity period. Int J Clin Exp Pathol 7, 1967–1976. [PMC free article] [PubMed] [Google Scholar]
- Moulton BC, Koenig BB, and Borkan SC (1978). Uterine lysosomal enzyme activity during ovum implantation and early decidualization. Biol Reprod 19, 167–170. [DOI] [PubMed] [Google Scholar]
- Murphy CR (2000). Understanding the apical surface markers of uterine receptivity: pinopods-or uterodomes? Hum Reprod 15, 2451–2454. [DOI] [PubMed] [Google Scholar]
- Murray MJ, Meyer WR, Zaino RJ, Lessey BA, Novotny DB, Ireland K, Zeng D, and Fritz MA (2004). A critical analysis of the accuracy, reproducibility, and clinical utility of histologic endometrial dating in fertile women. Fertil Steril 81, 1333–1343. [DOI] [PubMed] [Google Scholar]
- Nardo LG, Nikas G, Makrigiannakis A, Sinatra F, and Nardo F (2003). Synchronous expression of pinopodes and alpha v beta 3 and alpha 4 beta 1 integrins in the endometrial surface epithelium of normally menstruating women during the implantation window. J Reprod Med 48, 355–361. [PubMed] [Google Scholar]
- Nardo LG, Sabatini L, Rai R, and Nardo F (2002). Pinopode expression during human implantation. Eur J Obstet Gynecol Reprod Biol 101, 104–108. [DOI] [PubMed] [Google Scholar]
- Nguyen HP, Simpson RJ, Salamonsen LA, and Greening DW (2016). Extracellular Vesicles in the Intrauterine Environment: Challenges and Potential Functions. Biol Reprod 95, 109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikas G (1999). Cell-surface morphological events relevant to human implantation. Hum Reprod 14 Suppl 2, 37–44. [DOI] [PubMed] [Google Scholar]
- Nikas G (2000). Endometrial receptivity: changes in cell-surface morphology. Semin Reprod Med 18, 229–235. [DOI] [PubMed] [Google Scholar]
- Nikas G, and Aghajanova L (2002). Endometrial pinopodes: some more understanding on human implantation? Reprod Biomed Online 4 Suppl 3, 18–23. [DOI] [PubMed] [Google Scholar]
- Nikas G, Develioglu OH, Toner JP, and Jones HW Jr. (1999). Endometrial pinopodes indicate a shift in the window of receptivity in IVF cycles. Hum Reprod 14, 787–792. [DOI] [PubMed] [Google Scholar]
- Nikas G, Drakakis P, Loutradis D, Mara-Skoufari C, Koumantakis E, Michalas S, and Psychoyos A (1995). Uterine pinopodes as markers of the ‘nidation window’ in cycling women receiving exogenous oestradiol and progesterone. Hum Reprod 10, 1208–1213. [DOI] [PubMed] [Google Scholar]
- Nikas G, and Psychoyos A (1997). Uterine pinopodes in peri-implantation human endometrium. Clinical relevance. Ann N Y Acad Sci 816, 129–142. [DOI] [PubMed] [Google Scholar]
- Nilsson O (1958). Ultrastructure of mouse uterine surface epithelium under different estrogenic influences. 3. Late effect of estrogen administered to spayed animals. J Ultrastruct Res 2, 185–199. [DOI] [PubMed] [Google Scholar]
- Nilsson O (1966). Structural differentiation of luminal membrane in rat uterus during normal and experimental implantations. Z Anat Entwicklungsgesch 125, 152–159. [DOI] [PubMed] [Google Scholar]
- Nilsson O (1972). Ultrastructure of the process of secretion in the rat uterine epithelium at preimplantation. J Ultrastruct Res 40, 572–580. [DOI] [PubMed] [Google Scholar]
- Pantos K, Nikas G, Makrakis E, Stavrou D, Karantzis P, and Grammatis M (2004). Clinical value of endometrial pinopodes detection in artificial donation cycles. Reprod Biomed Online 9, 86–90. [DOI] [PubMed] [Google Scholar]
- Paria BC, Song H, and Dey SK (2001). Implantation: molecular basis of embryo-uterine dialogue. Int J Dev Biol 45, 597–605. [PubMed] [Google Scholar]
- Park DW, Choi DS, Ryu HS, Kwon HC, Joo H, and Min CK (2003). A well-defined in vitro three-dimensional culture of human endometrium and its applicability to endometrial cancer invasion. Cancer letters 195, 185–192. [DOI] [PubMed] [Google Scholar]
- Parr MB (1980). Endocytosis at the basal and lateral membranes of rat uterine epithelial cells during early pregnancy. J Reprod Fertil 60, 95–99. [DOI] [PubMed] [Google Scholar]
- Parr MB (1983). Relationship of uterine closure to ovarian hormones and endocytosis in the rat. J Reprod Fertil 68, 185–188. [DOI] [PubMed] [Google Scholar]
- Parr MB, and Parr EL (1974). Uterine luminal epithelium: protrusions mediate endocytosis, not apocrine secretion, in the rat. Biology of reproduction 11, 220–233. [DOI] [PubMed] [Google Scholar]
- Parr MB, and Parr EL (1977). Endocytosis in the uterine epithelium of the mouse. Journal of reproduction and fertility 50, 151–153. [DOI] [PubMed] [Google Scholar]
- Parr MB, and Parr EL (1986). Endocytosis in the rat uterine epithelium at implantation. Ann N Y Acad Sci 476, 110–121. [DOI] [PubMed] [Google Scholar]
- Psychoyos A, and Mandon P (1971). Scanning electron microscopy of the surface of the rat uterine epithelium during delayed implantation. J Reprod Fertil 26, 137–138. [DOI] [PubMed] [Google Scholar]
- Qiong Z, Jie H, Yonggang W, Bin X, Jing Z, and Yanping L (2017). Clinical validation of pinopode as a marker of endometrial receptivity: a randomized controlled trial. Fertil Steril 108, 513–517 e512. [DOI] [PubMed] [Google Scholar]
- Qu XL, Ming Z, Yuan F, Wang H, and Zhang YZ (2018). Effect of 2,3’,4,4’,5-Pentachlorobiphenyl Exposure on Endometrial Receptivity and the Methylation of HOXA10. Reprod Sci 25, 256–268. [DOI] [PubMed] [Google Scholar]
- Quinn C, Ryan E, Claessens EA, Greenblatt E, Hawrylyshyn P, Cruickshank B, Hannam T, Dunk C, and Casper RF (2007a). The presence of pinopodes in the human endometrium does not delineate the implantation window. Fertility and sterility 87, 1015–1021. [DOI] [PubMed] [Google Scholar]
- Quinn CE, and Casper RF (2009). Pinopodes: a questionable role in endometrial receptivity. Human reproduction update 15, 229–236. [DOI] [PubMed] [Google Scholar]
- Quinn CE, Detmar J, and Casper RF (2007b). Pinopodes are present in Lif null and Hoxa10 null mice. Fertility and sterility 88, 1021–1028. [DOI] [PubMed] [Google Scholar]
- Rarani FZ, Borhani F, and Rashidi B (2018). Endometrial pinopode biomarkers: Molecules and microRNAs. J Cell Physiol. [DOI] [PubMed] [Google Scholar]
- Sarantis L, Roche D, and Psychoyos A (1988). Displacement of receptivity for nidation in the rat by the progesterone antagonist RU 486: a scanning electron microscopy study. Hum Reprod 3, 251–255. [DOI] [PubMed] [Google Scholar]
- Sartor P (1972). [Trypan blue athrocytosis by rat endometrium. Morphological and dynamic data resulting from changes in the hormonal state]. C R Seances Soc Biol Fil 166, 577–580. [PubMed] [Google Scholar]
- Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, et al. (2012). Fiji: an open-source platform for biological-image analysis. Nat Methods 9, 676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider CA, Rasband WS, and Eliceiri KW (2012). NIH Image to ImageJ: 25 years of image analysis. Nat Methods 9, 671–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahzad H, Giribabu N, Karim K, Kassim N, Muniandy S, Kumar KE, and Salleh N (2017). Quercetin interferes with the fluid volume and receptivity development of the uterus in rats during the peri-implantation period. Reprod Toxicol 71, 42–54. [DOI] [PubMed] [Google Scholar]
- Shimizu Y, Kabir-Salmani M, Azadbakht M, Sugihara K, Sakai K, and Iwashita M (2008). Expression and localization of galectin-9 in the human uterodome. Endocr J 55, 879–887. [DOI] [PubMed] [Google Scholar]
- Singh MM, Trivedi RN, Chauhan SC, Srivastava VM, Makker A, Chowdhury SR, and Kamboj VP (1996). Uterine estradiol and progesterone receptor concentration, activities of certain antioxidant enzymes and dehydrogenases and histoarchitecture in relation to time of secretion of nidatory estrogen and high endometrial sensitivity in rat. J Steroid Biochem Mol Biol 59, 215–224. [DOI] [PubMed] [Google Scholar]
- Stavreus-Evers A, Aghajanova L, Brismar H, Eriksson H, Landgren BM, and Hovatta O (2002a). Co-existence of heparin-binding epidermal growth factor-like growth factor and pinopodes in human endometrium at the time of implantation. Mol Hum Reprod 8, 765–769. [DOI] [PubMed] [Google Scholar]
- Stavreus-Evers A, Mandelin E, Koistinen R, Aghajanova L, Hovatta O, and Seppala M (2006). Glycodelin is present in pinopodes of receptive-phase human endometrium and is associated with down-regulation of progesterone receptor B. Fertil Steril 85, 1803–1811. [DOI] [PubMed] [Google Scholar]
- Stavreus-Evers A, Masironi B, Landgren BM, Holmgren A, Eriksson H, and Sahlin L (2002b). Immunohistochemical localization of glutaredoxin and thioredoxin in human endometrium: a possible association with pinopodes. Mol Hum Reprod 8, 546–551. [DOI] [PubMed] [Google Scholar]
- Stavreus-Evers A, Nikas G, Sahlin L, Eriksson H, and Landgren BM (2001). Formation of pinopodes in human endometrium is associated with the concentrations of progesterone and progesterone receptors. Fertil Steril 76, 782–791. [DOI] [PubMed] [Google Scholar]
- Sudoma I, Goncharova Y, and Zukin V (2011). Optimization of cryocycles by using pinopode detection in patients with multiple implantation failure: preliminary report. Reprod Biomed Online 22, 590–596. [DOI] [PubMed] [Google Scholar]
- Tan O, Ornek T, Fadiel A, Carrick KS, Arici A, Doody K, Carr BR, and Naftolin F (2012). Expression and activation of the membrane-cytoskeleton protein ezrin during the normal endometrial cycle. Fertil Steril 97, 192–199 e192. [DOI] [PubMed] [Google Scholar]
- Teh WT, McBain J, and Rogers P (2016). What is the contribution of embryo-endometrial asynchrony to implantation failure? J Assist Reprod Genet 33, 1419–1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tung HN, Parr EL, and Parr MB (1988). Endocytosis in the uterine luminal and glandular epithelial cells of mice during early pregnancy. Am J Anat 182, 120–129. [DOI] [PubMed] [Google Scholar]
- Ueda O, Yorozu K, Kamada N, Jishage K, Kawase Y, Toyoda Y, and Suzuki H (2003). Possible expansion of “Window of Implantation” in pseudopregnant mice: time of implantation of embryos at different stages of development transferred into the same recipient. Biol Reprod 69, 1085–1090. [DOI] [PubMed] [Google Scholar]
- Usadi RS, Murray MJ, Bagnell RC, Fritz MA, Kowalik AI, Meyer WR, and Lessey BA (2003). Temporal and morphologic characteristics of pinopod expression across the secretory phase of the endometrial cycle in normally cycling women with proven fertility. Fertil Steril 79, 970–974. [DOI] [PubMed] [Google Scholar]
- Valles CS, and Dominguez F (2006). Embryo-endometrial interaction. Chang Gung Med J 29, 9–14. [PubMed] [Google Scholar]
- Warren RH, and Enders AC (1964). An Electron Microscope Study of the Rat Endometrium during Delayed Implantation. Anat Rec 148, 177–195. [DOI] [PubMed] [Google Scholar]
- Wu F, Mao D, Liu Y, Chen X, Xu H, Li TC, and Wang CC (2019). Localization of Mucin 1 in endometrial luminal epithelium and its expression in women with reproductive failure during implantation window. J Mol Histol. [DOI] [PubMed] [Google Scholar]
- Xu B, Sun X, Li L, Wu L, Zhang A, and Feng Y (2012). Pinopodes, leukemia inhibitory factor, integrin-beta3, and mucin-1 expression in the peri-implantation endometrium of women with unexplained recurrent pregnancy loss. Fertility and sterility 98, 389–395. [DOI] [PubMed] [Google Scholar]
- Yoshinaga K (2013). A sequence of events in the uterus prior to implantation in the mouse. J Assist Reprod Genet 30, 1017–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Chen Q, Zhang H, Wang Q, Li R, Jin Y, Wang H, Ma T, Qiao J, and Duan E (2015). Aquaporin-dependent excessive intrauterine fluid accumulation is a major contributor in hyper-estrogen induced aberrant embryo implantation. Cell Res 25, 139–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Wang Q, Wang H, and Duan E (2017). Uterine Fluid in Pregnancy: A Biological and Clinical Outlook. Trends Mol Med 23, 604–614. [DOI] [PubMed] [Google Scholar]
- Zhou WQ, Jiang YL, Tang HB, Gao HY, Zhuang YY, Xia F, Mao CP, and Zhu CR (2017). [Study on the changes of gonadotropin releasing hormone agonist in pinopodes]. Zhonghua Fu Chan Ke Za Zhi 52, 539–544. [DOI] [PubMed] [Google Scholar]
- Zhou YJ, Wang XD, Xiao S, Yu DE, Wang LQ, Wang JH, and Zhu HQ (2018). Exposure to beta-cypermethrin impairs the reproductive function of female mice. Regul Toxicol Pharmacol 95, 385–394. [DOI] [PubMed] [Google Scholar]