Abstract
Purpose:
Obesity during adolescence has multi-system health consequences. The objective of this work was to determine whether pre-adolescent overweight/obese children’s bones respond to a 9-month physical activity intervention by increasing bone density similar to healthy-weight children.
Methods:
Participants included overweight/obese (BMI >85%) and healthy weight (15% < BMI <85%) preadolescents (8-9years old). Participants in the physical activity group participated in a 9-month physical activity curriculum every day after school. The wait-list control group received no intervention. Both groups had overweight/obese children and healthy-weight controls. Whole body bone mineral content (BMC), area, and bone mineral apparent density (BMAD) were assessed using dual X-ray absorptiometry) at the beginning and end of the 9-month trial in the physical activity and control group.
Results:
Overweight/obese pre-adolescent children had higher BMAD than healthy weight children (p<0.001 for spine, leg, and whole body). However, the density/weight (BMAD/lean mass) was lower in overweight/obese children than in healthy weight children indicating the density of bones in overweight/obese children may not compensate sufficiently for the excessive load due to weight. The change in BMAD over 9 months was greater in healthy weight children than overweight/obese children in the whole body and leg, but not the lumbar spine. Physical activity caused a site-specific increase in bone density, affecting the legs more than the lumbar spine, but there was no significant difference in the effect of exercise between the healthy weight and overweight/obese group.
Conclusions:
The smaller change in BMAD over the 9 months and lower BMAD per unit lean mass in overweight/obese compared to healthy weight children may indicate a slower rate of bone mass accrual, which may have implications for bone health during skeletal growth in obese/overweight children.
Keywords: bone density, adolescents, physical activity, obesity
Introduction:
Childhood obesity has reached epidemic proportions. One in three U.S. children between the ages of 10–17 is obese [1]. In the United States, 90% of children have poor diets; and less than 50% of children engage in the recommended ‘dose’ of daily physical activity [2]. Obesity disproportionately affects children of low socioeconomic status [3] and has lasting health implications including high risk of cardiovascular disease, diabetes, asthma, social and psychological problems, and musculoskeletal problems.
Studies have shown that bone density in children with obesity is lower than in children of normal weight when adjusted for their weight [4,5], which may explain their high rates of fracture [6]. Bone health in adolescents with obesity is a major concern because peak bone density is achieved at skeletal maturity (age 21–23 years old) [7]. This means that childhood obesity may have lasting implications for bone health and osteoporosis later in life. Unlike other organ systems that maybe rescued with obesity treatment programs, if bone health is not addressed in adolescence, it may be too late to recover and lead to high risk of osteoporosis and related fracture later in life [8,9].
The gold standard for clinically assessing bone health is dual x-ray absorptiometery (DXA), which is a projected measure of bone mineral content (BMC, g) or bone mineral density, (BMD = BMC/area, g/cm2). Because it is a projected area measure, BMD measurements of large bones may appear higher than BMD of small bones due to the increased thickness through which the projection is made. Adolescent bones pose a particular challenge for assessing mineral density because the bone is changing size and mineral density as it grows. To normalize the effect of size, bone mineral apparent density (BMAD = BMC/area1.5) can be used and has been proposed as a more appropriate measure for children [10,11].
Numerous studies report that total bone mineral content increases with weight, and obese children have higher BMC and BMD than healthy-weight children [4,12,13]. However, studies that adjust for bone size with BMAD find that obese children have lower bone apparent density [4,14]. This is likely because the bone area increases with obesity [15]. Studies have also found that fat free mass index (fat free mass/height2) is more highly correlated with bone mineral density than body mass index (BMI = mass/height2) for both obese and healthy weight adolescents [4]. Collectively, these studies suggest that though obese children have greater total bone mineral, they have reduced bone mineral density for the mechanical loads placed on them.
There are numerous significant effects of childhood obesity on bone health including slipped capital femoral epiphysis [14], Blout’s disease [16], and general musculoskeletal pain [17]. In addition, many studies report increased risk of fracture and fracture rates with obesity in childhood[6,18]. Both reduced bone mineral density for their weight and greater impact loads during falls are contributors to the higher fracture risk in obese children.
The benefits of impact exercises on bone health of adolescents are well documented [19-21]. Most of these studies are implemented into the school physical education program or into classroom activities, lasting 10–30 minutes 2–3 time/week. Children in impact exercise groups exhibit bone mineral content gains at the femoral neck and/or lumbar spine compared to the control groups [22]. The skeletal location and the magnitude of increased bone mass depends on the type and intensity of the loading with most programs that deliver 3–8 times body weights impact loads demonstrating gains [20,21,23] while those with loads 2–3 time body weight do not [24]. The skeletal benefits of a 7 month jumping intervention in 7 year old children demonstrated sustained skeletal benefits 8 years after the intervention [23], indicating that a small amount of the exercise at critical points in skeletal development may have lasting impacts. However, none of these studies have investigated the effects of obesity on bone accrual during exercise. Studies have shown that adipose tissue has negative effects on skeletal metabolism [25], particularly with visceral fat [26], whereas non-pathologic fat is skeletally protective in adults. Currently there is little understanding of the effects of excessive fat mass on bone health during skeletal development [27], and specifically its ability to respond to impact exercise.
The objective of the current study was to analyze the effect of a 9-month after-school physical activity (PA) intervention on BMAD in healthy weight and obese/overweight pre-adolescent children. We hypothesized that obese/overweight children have lower BMAD for their weight, and that PA training increases BMAD in both groups relative to non-exercise controls.
Methods:
Participants:
A total of 407 children between the ages of 8 and 10 years old were recruited to participate in the FITKids (n = 212) (ClinicalTrials.gov: ), and FITKids2 (n = 195) (ClinicalTrials.gov: ) research trials [28].The present study includes 351 of these children (we did not include 13 underweight children and 43 participants who did not complete post-trial review). The institutional review board at the University of Illinois approved the study protocol. Parents provided written informed consent, and participants provided written assent. Participants included overweight/obese (n=148, BMI: >85 percentile), and healthy weight (n=203, BMI: 15–85 percentile) preadolescents. At the beginning of the study, a modified Tanner staging system questionnaire was administered [29]. Participants and their legal guardians collaborated to complete the questionnaire. All children had Tanner scores of 2 or less. Socio- economic status was determined with a trichotomous index based on participation in free or reduced-price lunch program at school, the highest level of education obtained by the mother and father, and the number of parents who worked full time. [28]. Because of small sample sizes in some race groups, the races were combined into the following categories to create large enough groups: black/ African American (n=79), white (n=171), and other (n=102). Each race was recorded into dummy variables, where the race of interest was coded as 1 and all other races except for white/ Caucasian (which served as the reference group) as 0. This process was repeated for each race grouping. White/ Caucasian was chosen as the reference category because it had the largest sample size.
Anthropometry:
At pre- and post-test, standing height and weight measurements were completed with participants wearing light weight clothing and no shoes. Height and weight were measured using a stadiometer (Seca; model 240) and a Tanita WB-300 Plus digital scale, (Tanita, Tokyo, Japan) respectively. Body mass index (BMI) was calculated by dividing body mass (kg) by height (m) squared. Whole body, leg and lumbar spine bone mineral content (BMC) and area were measured by dual energy radiographic absorptiometry (DEXA) with a Hologic QDR 4500A bone densitometer (software version 12.7.3; Hologic, Bedford, Ma). Bone mineral density(BMD=BMC/area) and bone mineral apparent density (BMAD=BMC/area1.5) were calculated. Precision for DXA measurements of interest are ~1–1.5% in our laboratory. Because previous studies have shown BMD in overweight children is correlated with lean mass, rather than fat mass or whole body mass [27], we also examined BMAD per unit lean mass.
Intervention:
Participants were randomized into two groups: 9-month physical activity intervention group or a wait list control group. The intervention group received a 2-hour after-school physical activity program (5 days/week after school for 9 months) based on the Child and Adolescent Trial for Cardiovascular Health curriculum, which is an evidence-based program that provides moderate-to-vigorous physical activity in a noncompetitive environment. The exercises were based on the Community Access to Child Health (CATCH) program, which has demonstrated effectiveness and sustainability in altering physical activity behaviors [30]. The general daily structure was: instant activities, a snack/educational component, with the majority of time spent in moderate-to-vigorous activity centered around game play. The instant activities were based on the CATCH K-5 physical education curriculum, and included short activities that target aerobic activities, muscular strength and endurance, or movement concepts. It should be noted that FITKids was designed to exceed cardiovascular health recommendations; it was not designed for bone health, but vigorous exercise often includes high impact loading. The control group was asked to maintain their regular after-school routine. Details of the intervention and its effects on cognitive function have been previously reported [28].
Statistical Analysis
All statistical analyses were performed with SPSS 23 (IBMCorp, Armonk, New York) via a family-wise alpha threshold for all tests set at p<0.05.
Demographic variables were compared between BMI groups using univariate ANOVAs. For ordinal demographic data (race and sex), Pearson Chi-square values were used to assess the differences between groups. The means (±1 standard error) for the demographic variables are presented in table 1.
Table 1:
Participant Demographics. Four groups were studied: healthy weight and overweight/obese with and without physical activity.
| Demographics | Healthy Weight Control |
Healthy Weight 9-month physical activity |
Overweight/Obese Control |
Overweight/Obese 9-month physical activity |
|---|---|---|---|---|
| Participants | 103 | 100 | 71 | 77 |
| Females | 50 | 52 | 38 | 43 |
| Socio-Economic Status* | 1.96±0.82 | 1.94±0.89 | 1.62±0.83 | 1.78±0.84 |
| Age (years) | 8.84±0.58 | 8.76±0.57 | 8.81±0.55 | 8.68±0.53 |
| Attendance % | N/A | 80.02±15.53 | N/A | 81.06±14.30 |
| Whole Body Fat%* | 23.85±5.56 | 26.62±5.37 | 35.53±6.01 | 36.10±5.18 |
| Lean Mass (kg)* | 23.07±3.29 | 22.10±2.91 | 28.98±5.81 | 28.01±5.60 |
| Height (cms)* | 135.68±6.63 | 134.20±5.84 | 138.42±8.36 | 138.19±8.35 |
| Weight (kg)* | 30.27±4.52 | 30.00±4.13 | 44.80±10.57 | 44.45±10.15 |
| Race (n, %) | ||||
| White | 58, 56.3% | 50, 50% | 31, 43.7% | 32, 41.6% |
| Black or African American | 20, 19.4% | 18, 18% | 20, 28.2% | 21, 27.3% |
| Other | 25, 24.3% | 32, 32% | 20, 28.2% | 24, 31.2% |
indicates the obese overweight group is statistically different (p<0.05) than the healthy weight group.
Longitudinal analyses of BMAD and BMAD per unit lean mass were conducted using multivariate repeated measures analyses of variance. Analyses included a time factor (pre-test, post-test), a group factor (PA, control), and a BMI group factor (NW, OW/OB). The dependent variables of interest were assessed using separate 2 (Weight Status: healthy weight, obese) x 2 (Group: PA, CON) x 2 (Time: pre-test, post-test).
In order to account for any baseline differences between BMI and treatment groups, change scores (Δ) were computed for BMAD. Subsequent analyses of changes in BMAD (ΔBMAD) were conducted using multivariate repeated measures of variance comparing change in NW and OW/OB children. The dependent variables of interest were assessed using separate 2 (Weight status: NW, OW/OB) x 2 (Group: PA, CON).
The analyses above were then repeated with the inclusion of the following covariates: pre-test height and weight, sex, and race.
Results:
Here we report only BMAD, which adjusts for the greater bone size in obese children. Analysis of BMC, bone area and BMD can be found in the Appendix (see Supplementary Figures 1-8, Supplementary Tables 1-3, results for BMC, area, and BMD). The differences in race and sex between the groups were not statistically significant. Hence, they were not included as co-variates. SES was lower in the obese/overweight group (1.70 ± 0.07) than in the NW group (1.95 ± 0.06), t(348) = 2.71, p=0.007. Because groups were divided by weight, not surprisingly the lean mass and fat mass were higher in the obese/overweight group. We therefore did not adjust for weight in our analysis, but did examine BMAD/lean mass. The obese/overweight children were taller than the healthy weight children and this was considered by reporting BMAD, rather than BMD or BMC to account for bigger bone size. Because all other demographic data were similar across groups, analyses were first conducted without adjusting for confounding variables. A secondary analysis was also conducted with demographic variables that have previously been shown to be important when examining bone density, and included pre-test height, pre-test weight, sex, and race.
BMAD is higher in overweight/obese children:
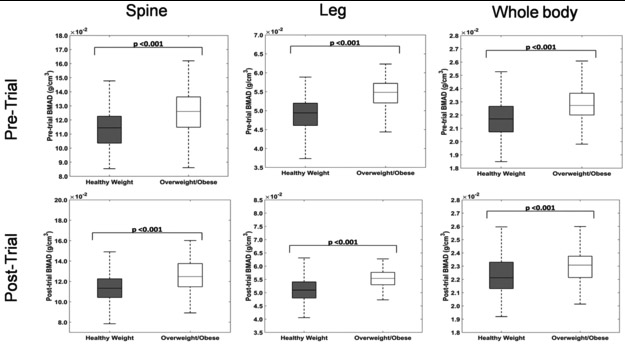
For pre-trial values, BMAD of the legs, lumbar spine, and whole body in overweight/obese children was higher than the healthy weight group (p<0.001). Similarly, post-trial BMAD was higher in children with overweight/obese than in healthy weight children (Figure 1).
Figure 1:
Pre-trial and post-trial mean BMAD of legs, lumbar spine and whole body is higher in overweight/obese children compared to the healthy weight group. The upper and lower boundaries on the box-plot represent the 25th and 75th percentile in the data respectively with the median being represented by the mid-line. The non-outlier extremes in the data are shown by the whiskers.
BMAD per unit lean mass is lower in overweight/obese children:
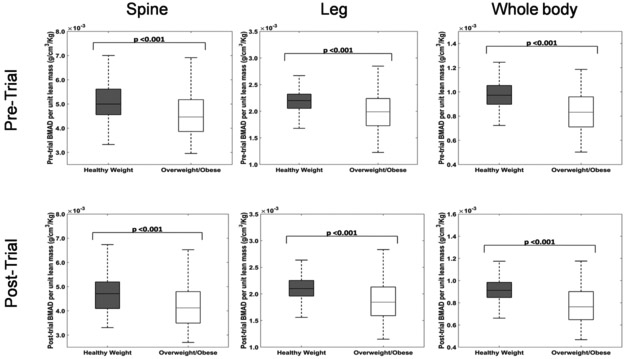
Even though overweight children had higher absolute values of BMAD, pre-trial and post-trial BMAD per unit lean mass of legs (P<0.001), lumbar spine (P<0.001) and whole body (P<0.001) suggests that overweight children have lower bone density for their weight (Figure 2).
Figure 2:
Pre-trial and post-trial mean BMAD per unit lean mass of legs, lumbar spine and whole body is higher in healthy weight children compared to overweight/obese children.
BMAD increases over time:
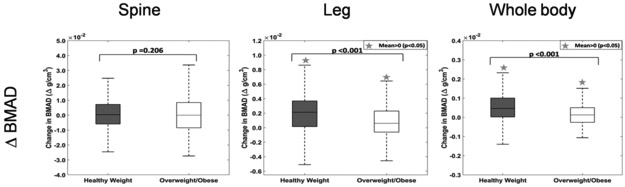
Over a period of 9-months, the change in BMAD (difference of post-trial and pre-trial values) shows a significant increase in leg (p<0.001) and whole body (p<0.001) but not in lumbar spine (p=0.206). The increase in BMAD in healthy weight children is significantly higher than the overweight/obese group in the legs (p<0.001) and whole body (p<0.001). (Figure 3)
Figure 3:
Change in BMAD (a) Lumbar Spine (b) Leg (c) Whole Body over a 9-month period by weight class. Healthy weight children increased their BMAD in leg and whole body more than the overweight/obese group. There is no significant change in the BMAD of lumbar spine in either group.
Exercise increases BMAD:
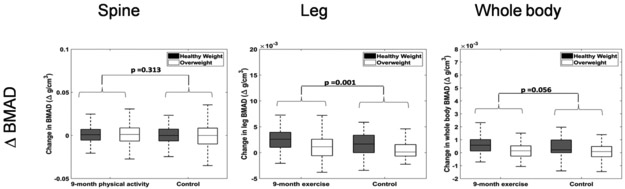
The 9-month physical activity intervention significantly increased BMAD in the leg (p=0.001), compared to the waitlist control group. However, the effect of physical activity on BMAD of the lumbar spine was not significant (p=0.313) and whole body was nearly significant (p=0.056), likely reflecting an average of the increase in the lower limbs and no increase in the spine (Figure 4). The change in BMAD due to the physical activity intervention was not significantly different in the healthy weight and overweight groups. [Weight status x treatment in lumbar spine (p=0.6), leg (p=0.748) and whole body (p=0.535)].
Figure 4:
Change in BMAD over a 9-month period by weight class and treatment for the spine, leg, and whole body. Increase in BMAD is greater in healthy weight than overweight/obese in control group. Physical activity increased the leg BMAD, but not spine or whole body. The effects of physical activity were not different between the healthy weight and obese/overweight groups.
We also examined the results after adjusting for sex, race, pre-height, pre-weight, and pre-test BMAD. Physical activity had a significant effect in the leg, nearly significant effect in the whole body and no significant effect in the spine, similar to the unadjusted results. The change in leg BMAD during the trial is not significantly different between overweight and healthy weight groups after adjusting for weight and pre-test BMAD. This indicates that the amount of bone gained relative to the initial amount of bone is the same between groups (even though the obese group’s change in BMAD was smaller than the healthy group’s).
Discussion:
In this study overweight/obese 8–9-year-oldchildren had higher BMC, BMD, and BMAD than healthy weight children. However, the density per unit lean mass was lower in overweight/obese children than healthy weight children and the amount of bone gained in a 9-month period was less in overweight/obese children compared to healthy weight children. Physical activity increased bone mass in the legs in both groups relative to the control groups.
Previous studies have reported that overweight/obese children have bone density greater [12,13,31], equivalent [4,32] or less [5,33] than healthy weight children. The reason for these discrepancies may be due to difference in participants’ age range, reporting of BMD, BMC, BMAD or values adjusted for height, sex, or lean mass, and differences in sample sizes and populations. The BMC and BMD values for whole body in our study are within the range of other studies [34,35]. Most studies agree that bone mass adjusted for weight (total mass or lean mass) is lower in obese children than in healthy weight children [5,31,33,36]. Previous studies that have measured BMAD in children have found whole body BMAD was lower in obese children compared to healthy children [4,33] (opposite of our results), and lumbar spine BMAD is higher in obese children [31,33] (similar to our results). Our study used a younger population, smaller age range, and a larger sample size than these studies, thereby controlling many of the influencing factors. However, future work should use high resolution peripheral quantitative computed tomography (HRpqCT) to assess 3D structure so that bone size differences do not affect density measures. Previous studies that have used HRpqCT have demonstrated that lean mass affects bone strength in weight-bearing bones [36] of children, and obese children have reduced trabecular thickness compared to healthy weight [37]. Because the relationship between childhood obesity and bone density is still unclear, longitudinal studies with using HRpqCT in representative populations are needed [38].
As children enter puberty, the positive relationship between fat mass and bone appears to attenuate and then reverse [26,27]. Our data indicates that bone mass accrual is reduced in overweight/obese children over a 9-month period compared to healthy weight children, which may be a prediction of these future changes.
Our data indicates a site-specific effect of physical activity on bone mineral apparent density as lumbar spine BMAD values did not significantly differ between the exercise group and the control group while that of the leg was higher in the exercise group. This suggests that high intensity physical activity loaded the legs predominately more than the lumbar spine, which is always loaded in upright posture.
There is significant concern over bone health in obese adolescents because of their high fracture rates [27]. Studies indicate that obese children with low bone density for their weight are at high risk of fracture [5,39] and children who fracture have lower bone density and higher adiposity [40] than those who do not. Our results indicate that despite BMAD being higher in overweight children compared to healthy weight children, they have reduced bone density for their weight and accrue bone more slowly, which may explain the higher fracture rates.
Peak bone mass is achieved at skeletal maturity, making it critical during growth to maximize bone formation in order to prevent osteoporosis. Our data provides a small and early time window into this growth trajectory, but foreshadows poor bone health in later stages. We did not measure nutritional intake or assess diet, which also affect bone mass. Though other organ systems may respond to weight loss therapies after skeletal maturity, it may be too late for bone. It is critical to address bone health before skeletal maturity. We show in this study that physical activity increases bone density in both overweight/obese children and healthy weight children, indicating a cheap, simple, and effective multi-organ therapy that can be used to address bone health in overweight/obese children.
Supplementary Material
Acknowledgements:
The Fitness Improves Thinking in Kids (FITKids) trial was supported by the National Institutes of Health (HD055352 to CH). The Fitness Improves Thinking in Kids (FITKids) 2 trial was supported by the National Institutes of Health (HD069381 to CH and AK). Additional support for this project was provided by the National Institute of Food and Agriculture, US Department of Agriculture, (2011-67001-30101). The results of the study are presented clearly, honestly, and without fabrication, falsification, or inappropriate data manipulation, and statement that results of the present study do not constitute endorsement by ACSM
Footnotes
Conflict of Interest: The authors have nothing to disclose.
References:
- [1].National Survey of Children’s Health - Data Resource Center for Child and Adolescent Health. (n.d.). Retrieved December 21, 2017, from http://www.childhealthdata.org/learn/NSCH.
- [2].Steinberger J, Daniels SR, Hagberg N, et al. Cardiovascular Health Promotion in Children: Challenges and Opportunities for 2020 and Beyond: A Scientific Statement From the American Heart Association. Circulation [Internet]. 2016. [cited 2019 Mar 16];134 Available from: https://www.ahajournals.org/doi/10.1161/CIR.0000000000000441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Rogers R, Eagle TF, Sheetz A, et al. The Relationship between Childhood Obesity, Low Socioeconomic Status, and Race/Ethnicity: Lessons from Massachusetts. Child. Obes. Print 2015;11:691–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Ivuskans A, Lätt E, Mäestu J, et al. Bone mineral density in 11–13-year-old boys: relative importance of the weight status and body composition factors. Rheumatol. Int 2013;33:1681–1687. [DOI] [PubMed] [Google Scholar]
- [5].Goulding A, Taylor RW, Jones IE, et al. Overweight and obese children have low bone mass and area for their weight. Int. J. Obes 2000;24:627. [DOI] [PubMed] [Google Scholar]
- [6].Goulding A, Jones IE, Taylor RW, et al. Bone mineral density and body composition in boys with distal forearm fractures: a dual-energy x-ray absorptiometry study. J. Pediatr 2001;139:509–515. [DOI] [PubMed] [Google Scholar]
- [7].Lu J, Shin Y, Yen M-S, et al. Peak Bone Mass and Patterns of Change in Total Bone Mineral Density and Bone Mineral Contents From Childhood Into Young Adulthood. J. Clin. Densitom. Off. J. Int. Soc. Clin. Densitom 2016;19:180–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Faulkner RA, Bailey DA. Osteoporosis: a pediatric concern? In: Daly RM, Petit MA, editors. Optim. Bone Mass Strength Basel; New York: Karger Publishers; 2007. p. 1–12. [DOI] [PubMed] [Google Scholar]
- [9].Weaver CM, Gordon CM, Janz KF, et al. The National Osteoporosis Foundation’s position statement on peak bone mass development and lifestyle factors: a systematic review and implementation recommendations. Osteoporos. Int 2016;27:1281–1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Smith CM, Coombs RC, Gibson AT, et al. Adaptation of the Carter method to adjust lumbar spine bone mineral content for age and body size: application to children who were born preterm. J. Clin. Densitom 2006;9:114–119. [DOI] [PubMed] [Google Scholar]
- [11].Crabtree NJ, Arabi A, Bachrach LK, et al. Dual-energy X-ray absorptiometry interpretation and reporting in children and adolescents: the revised 2013 ISCD Pediatric Official Positions. J. Clin. Densitom 2014;17:225–242. [DOI] [PubMed] [Google Scholar]
- [12].Ellis KJ, Shypailo RJ, Wong WW, et al. Bone mineral mass in overweight and obese children: diminished or enhanced? Acta Diabetol. 2003;40:s274–s277. [DOI] [PubMed] [Google Scholar]
- [13].Leonard MB, Shults J, Wilson BA, et al. Obesity during childhood and adolescence augments bone mass and bone dimensions. Am. J. Clin. Nutr 2004;80:514–523. [DOI] [PubMed] [Google Scholar]
- [14].Perry DC, Metcalfe D, Costa ML, et al. A nationwide cohort study of slipped capital femoral epiphysis. Arch. Dis. Child 2017;102:1132–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Weiler HA, Janzen L, Green K, et al. Percent body fat and bone mass in healthy Canadian females 10 to 19 years of age. Bone. 2000;27:203–207. [DOI] [PubMed] [Google Scholar]
- [16].Sabharwal S Blount Disease: An Update. Orthop. Clin 2015;46:37–47. [DOI] [PubMed] [Google Scholar]
- [17].Bell LM, Byrne S, Thompson A, et al. Increasing Body Mass Index z-Score Is Continuously Associated with Complications of Overweight in Children, Even in the Healthy Weight Range. J. Clin. Endocrinol. Metab 2007;92:517–522. [DOI] [PubMed] [Google Scholar]
- [18].Kessler J, Koebnick C, Smith N, et al. Childhood Obesity Is Associated With Increased Risk of Most Lower Extremity Fractures. Clin. Orthop. Relat. Res 2013;471:1199–1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Macdonald HM, Kontulainen SA, Petit MA, et al. Does a novel school-based physical activity model benefit femoral neck bone strength in pre- and early pubertal children? Osteoporos. Int 2008;19:1445. [DOI] [PubMed] [Google Scholar]
- [20].Fuchs RK, Bauer JJ, Snow CM. Jumping Improves Hip and Lumbar Spine Bone Mass in Prepubescent Children: A Randomized Controlled Trial. J. Bone Miner. Res 2001;16:148–156. [DOI] [PubMed] [Google Scholar]
- [21].Deere K, Sayers A, Rittweger J, et al. Habitual levels of high, but not moderate or low, impact activity are positively related to hip BMD and geometry: Results from a population-based study of adolescents. J. Bone Miner. Res 2012;27:1887–1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Nguyen VH. School-based exercise interventions effectively increase bone mineralization in children and adolescents. Osteoporos. Sarcopenia 2018;4:39–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Gunter K, Baxter‐Jones AD, Mirwald RL, et al. Impact Exercise Increases BMC During Growth: An 8-Year Longitudinal Study. J. Bone Miner. Res 2008;23:986–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Nichols DL, Sanborn CF, Essery EV, et al. Impact of Curriculum-Based Bone Loading and Nutrition Education Program on Bone Accrual in Children. Pediatr. Exerc. Sci 2008;20:411–425. [DOI] [PubMed] [Google Scholar]
- [25].Kawai M, de Paula FJA, Rosen CJ. New insights into osteoporosis: the bone–fat connection. J. Intern. Med. 2012;272:317–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Dimitri P, Bishop N, Walsh JS, et al. Obesity is a risk factor for fracture in children but is protective against fracture in adults: A paradox. Bone. 2012;50:457–466. [DOI] [PubMed] [Google Scholar]
- [27].Farr JN, Dimitri P. The Impact of Fat and Obesity on Bone Microarchitecture and Strength in Children. Calcif. Tissue Int. 2017;100:500–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Raine LB, Khan NA, Drollette ES, et al. Obesity, Visceral Adipose Tissue, and Cognitive Function in Childhood. J. Pediatr. 2017;187:134–140.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Taylor SJ, Whincup PH, Hindmarsh PC, et al. Performance of a new pubertal self-assessment questionnaire: a preliminary study. Paediatr. Perinat. Epidemiol 2001;15:88–94. [DOI] [PubMed] [Google Scholar]
- [30].Luepker RV, Perry CL, McKinlay SM, et al. Outcomes of a field trial to improve children’s dietary patterns and physical activity. The Child and Adolescent Trial for Cardiovascular Health. CATCH collaborative group. JAMA. 1996;275:768–776. [DOI] [PubMed] [Google Scholar]
- [31].Dimitri P, Wales JK, Bishop N. Adipokines, bone-derived factors and bone turnover in obese children; evidence for altered fat-bone signalling resulting in reduced bone mass. Bone. 2011;48:189–196. [DOI] [PubMed] [Google Scholar]
- [32].Erbayat AE, Serdaroğlu A, Tümer L, et al. Evaluation of Bone Mineral Metabolism in Children Receiving Carbamazepine and Valproic Acid. J. Pediatr. Endocrinol. Metab 2011;13:933–940. [DOI] [PubMed] [Google Scholar]
- [33].Rocher E, Chappard C, Jaffre C, et al. Bone mineral density in prepubertal obese and control children: relation to body weight, lean mass, and fat mass. J. Bone Miner. Metab 2008;26:73–78. [DOI] [PubMed] [Google Scholar]
- [34].van der Sluis IM, de Ridder MAJ, Boot AM, et al. Reference data for bone density and body composition measured with dual energy x ray absorptiometry in white children and young adults. Arch. Dis. Child 2002;87:341–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Kindler JM, Lappe JM, Gilsanz V, et al. Lumbar Spine Bone Mineral Apparent Density in Children: Results From the Bone Mineral Density in Childhood Study. J. Clin. Endocrinol. Metab 2019;104:1283–1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Farr JN, Amin S, LeBrasseur NK, et al. Body Composition During Childhood and Adolescence: Relations to Bone Strength and Microstructure. J. Clin. Endocrinol. Metab 2014;99:4641–4648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Dimitri P, Jacques RM, Paggiosi M, et al. Leptin May Play a Role in Bone Microstructural Alterations in Obese Children. J. Clin. Endocrinol. Metab 2015;100:594–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Sioen I, Lust E, De Henauw S, et al. Associations Between Body Composition and Bone Health in Children and Adolescents: A Systematic Review. Calcif. Tissue Int 2016;99:557–577. [DOI] [PubMed] [Google Scholar]
- [39].Davidson PL, Goulding A, Chalmers DJ. Biomechanical analysis of arm fracture in obese boys. J. Paediatr. Child Health. 2003;39:657–664. [DOI] [PubMed] [Google Scholar]
- [40].Goulding A, Grant AM, Williams SM. Bone and Body Composition of Children and Adolescents With Repeated Forearm Fractures. J. Bone Miner. Res 2005;20:2090–2096. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.