Abstract
Background:
Complement promotes inflammatory and immune responses and may affect cardiometabolic risk. This study was designed to investigate the effect of complement components C3 and C4 on cardiometabolic risk in healthy non-Hispanic white adolescents.
Methods:
Body mass index (BMI), BMI percentile, waist circumference, and percent body fat were assessed in 75 adolescents. Arterial stiffness was assessed using arterial tomography and endothelial function using reactive hyperemia. Fasting lipids, inflammatory markers, and complement levels were measured and oral glucose tolerance test was performed. A single C3 polymorphism and C4 gene copy number variations were assessed.
Results:
C3 plasma levels increased with measures of obesity. Endothelial function worsened with increased C3 and C4 levels. Triglycerides and LDL increased and HDL and insulin sensitivity decreased with increasing C3 levels but the relationships were lost when body habitus was included in the model. C4 negatively related to HDL and positively to inflammatory markers. Subjects with at least one C3F allele had increased BMI and fat mass index. HDL was significantly related to C4L, C4S, C4A and C4B gene copy number variation.
Conclusions:
C3 levels increase with increasing body mass and increased C4 levels and copy number are associated with increased cardiometabolic risk in healthy adolescents.
INTRODUCTION
Complement is a key part of the immune system and plays a central role in defense against micro-organisms. Prolonged, chronic complement activation, however, may play a detrimental role in the development of cardiometabolic disease (1). Serum complement component C3 is vital to the three complement activation pathways and increased inflammation. C3 is manufactured primarily by hepatocytes but also by adipocytes in adipose tissue, macrophages and endothelial cells (2). Chylomicrons with dietary fat stimulate adipocyte C3 production.(3, 4)
Besides producing complement adipose tissue also responds to complement. Adipocytes have receptors for C3a (C3aR) and C5a (C5aR1 and C5aR2). The C5aR2 is also the only known receptor for C3a-desArg (3). C3a-desArg, also known as acylation stimulation protein, plays an important role in lipid storage and energy metabolism by accelerating adipocyte triglyceride metabolism and increasing plasma triglyceride levels (4). These interactions between adipose tissue and complement strongly suggest that complement may have a significant pathophysiological role in the development of insulin resistance and the metabolic syndrome. In fact, C3 and C3aR knock-out mice are resistant to diet-induced obesity and are more insulin sensitive than control mice (3).
Beyond this endothelial cells, also, have anaphyltoxin receptors for C3a, C5a and other activated complement components that may increase the expression of cellular adhesion molecules and pro-inflammatory cytokines (2). C5a and C5b-9 are associated with increased endothelial dysfunction and e-selectin secretion (2). C4a has been shown to stimulate protease activated receptors 1 and 4 which leads to increased vascular endothelial permeability (5). Thus, the complement system is thought play a dual role in the development of atherosclerosis through removal of cellular debris and amplifying inflammation (2). A wide variety of genetic modifications of the complement system in mice have significant effects on atherosclerosis (6).
Human studies show that increased serum levels of complement C3 are a risk factor for the development of diabetes, and a determinant of cardiometabolic risk (7, 8) in adults. Furthermore, the C3F allele which codes for C3F protein [named because it travels faster than the S-variant (slow) on immunofixation gel (9)] is associated with atherosclerosis in adults (10). For C4 gene copy number variation for its acidic (C4A) and basic (C4B) protein isotypes and for dichotomous gene size, long (C4L) and short (C4S) has, also, been associated with difference in cardiovascular risk and life expectancy (9). Among human subjects with antiphospholipid antibodies, those who experienced thromboses had consistently higher C4 and C3 serum protein levels and higher C4B gene copy number than patients who did not experience thrombosis (11). Moreover, pediatric lupus patients who had hypertension have persistently higher serum C4 and C3 protein levels than normotensive patients (12).
Adult cardiometabolic disease has its origins in childhood and adolescence (13) and several studies have investigated associations between cardiovascular risk and complement in this age group. Wamba et al (14), in Chinese pediatric subjects, found increased C3a-desArg (ASP) levels correlated with serum cholesterol, LDL, and glucose levels in both sexes, and with insulin levels and homeostatic model assessment (HOMA) index of insulin sensitivity in males. Levels were specifically increased in adolescents with the metabolic syndrome. Wei et al (15) in a large population based study of 6–18 year olds found increasing levels of C3 with increasing BMI and an increasing number of metabolic syndrome components within each BMI category. C3a-desArg (ASP) and C3 serum levels are significantly elevated in obese vs non-obese children aged 2–6 years (16). Agostinis-Sobrinho et al (17) found both C3 and C4 levels predicted inflammatory and biochemical cardiovascular risk scores in adolescents. However, the underlying genetic diversity including complement C3 polymorphism and C4 gene copy number variations were not examined. This study was designed to investigate prospectively the relationship of complement C3 and C4 phenotypes and genotypes on both biochemical and functional cardiovascular risk factors in a population of healthy, non-Hispanic white adolescents across a spectrum of body mass indices.
METHODS
Subjects:
Seventy-five, white adolescents who were not of Hispanic or Latino origin, between the ages of 12 to 17 years 11 months years were recruited to participate. Subjects were medication free for at least two weeks except for oral contraceptives in females. Subjects with a history of autoimmune endocrine, connective tissue disease, hematologic disease, renal disease, or malignancy were excluded. The protocol was approved by the Nationwide Children’s Hospital Institutional Review Board and written informed assent from the subject and informed consent from a parent or guardian were obtained.
Protocol
The protocol was registered on Clinical Trials.gov, .
Study Visit:
Subjects arrived at the Clinical Research Center of the Wexner Medical Center at The Ohio State University at 08:00 after an overnight fast beginning at 22:00 the night before the study. Upon arrival a brief history and physical examination was performed.
Anthropometrics:
Height (Stadiometer: 216 Accu-Hite Measuring Device, Seca North America, Chino CA), weight (SECA 1360 Wireless scale) were measured. Waist circumference was measured to the nearest 0.1 cm at the narrowest place between the lowest rib and the iliac crest at the end of normal exhalation with a spring loaded, inelastic measuring tape (18). Body fat percentage was measured using air displacement plethysmography in the BODPOD (COSMED USA Inc., Concord CA). Fat mass index (FMI) was calculated as total body fat weight/height2 and fat free mass index (FFMI) as total nonfat weight/height2.
Vascular function:
Arterial tonometry (SphygmoCor, AtCor Medical, Inc., Itasca IL) was used to determine the augmentation index, as a measure of vascular stiffness. Results were corrected to heart rate of 75 (AIx75).
Endothelial function:
Endothelial function was measured using post-occlusion, shear stress-induced, endothelially mediated vasodilation (reactive hyperemia) after a 20-minute rest period. Strain gauge venous occlusion plethysmography (Hokanson AI6 plethysmograph, DE Hokanson, Inc, Bellevue, WA) was used to measure forearm blood flow (FBF) and forearm vascular resistance (FVR) before and after upper arm occlusion. This method of testing endothelial function assesses resistance vessel function (19).
The reactive hyperemia response was quantified as percent decrease in FVR using the following protocol. Two minutes of baseline FBF were measured after which the upper arm cuff was inflated to 200 mmHg pressure for 5 minutes to occlude arterial inflow. It was then released and FBF was measured for one minute. FVR were calculated by dividing mean arterial blood pressure by FBF. Arterial blood pressure was measured using automated sphygmomanometer. Mean intra-observer coefficient of variation (CV) for FBF before upper arm occlusion is 5.1% and 7.4% after upper arm occlusion.
Blood sampling:
After completion of the test of endothelial function, an intravenous catheter was placed in one arm for initial blood sampling and for the oral glucose tolerance test. Twenty-five ml of blood was taken from each study subject for measurement of fasting plasma glucose, insulin, lipids, high sensitivity c-reactive protein (CRP), interleukin-6 (IL6), plasminogen activator inhibitor-1 (PAI1), endothelin 1, C3, C4, C3a, C4a, and C5a levels, white blood cell and neutrophil counts and for genetic analyses of complement C3 and C4 using genomic DNA. White blood cell and neutrophil counts and IL6 and CRP levels are markers of inflammation which are clearly increased in individuals at increased cardiometabolic risk (15, 20). PAI1 was used to assess clotting risk. Endothelin 1 is an endothelially-produced vasoconstrictor and has been shown to be a biochemical marker for endothelial dysfunction (21).
Insulin Sensitivity and Secretion:
Subjects were given 1.75 grams/kg of oral glucose (up to a maximum of 75 grams). Blood samples for measurement of plasma glucose and insulin levels were drawn every 30 minutes for 120 minutes. Insulin sensitivity (IS) was calculated using the Matsuda Index (IS = 10,000/√ [fasting glucose x fasting insulin] x [mean glucose x mean insulin]) while insulin secretion (SEC) was calculated as the change in insulin divided by the change in glucose from 0 to 30 min (SEC = ∆I0–30/∆G0–30) (22). Disposition index (DI) was calculated by multiplying both these values (DI = IS x SEC) (22). Disposition index is an important predictor of future type 2 diabetes (23).
Laboratory assays: Complement phenotypes:
Protein levels for complement C3 and C4 from EDTA-plasma were determined by single radial immunodiffusion (RID) assays using commercial kits from the Binding Sites (UK). Complement activation peptides C3a, C4a and C5a from EDTA-plasma w ere determined by Enzyme Linked Immunosorbent Assays (ELISA) using OptEIA ELISA kits (cat 550499 for C3a, 550947 for C4a, and 557965 for C5a) from BD Bioscience, San Diego, CA, according to manufacturer’s protocol.
Complement genotypes:
Genomic DNA was prepared from peripheral blood mononuclear cells and used for assessment of C3 genetic polymorphisms and gene copy-number variations of total C4, C4A, C4B, long genes and short genes. C3 genetic variants including the DNA sequence polymorphisms for G102R for fast and slow allotypes were determined through HhaI restriction fragment length polymorphism analyses of PCR amplified products (24). Copy-number variations for total C4, C4A, C4B, long genes and short genes of C4 were elucidated by Southern blot analyses of genomic DNA samples digested by PmeI and resolved by pulsed field gel electrophoresis, and by TaqI or PshAI/PvuII and resolved by 0.8% agarose gel electrophoresis (25). Samples for subjects with ambiguous data were subject to real-time quantitative PCR using five different amplicons and the results were validated as the gene copy-numbers in each subject for total C4=C4A+C4B=long genes+short genes (26).
Cardiovascular measures, glucose and insulin:
Cell differentials (neutrophils, total white blood cells) from EDTA-peripheral blood were measured by a Sysmex Kx-21N cell counter (Sysmex, Lincolnshire, IL). Plasma lipids, glucose, insulin, IL6, CRP, endothelin 1, fibrinogen, plasminogen activator inhibitor antigen (PAI1), were measured in the Clinical Research Center CORE laboratory.
Statistical Analysis:
Robust rank order regression analysis with age and sex included in all models was used to assess relationships between C3 and C4 protein levels to body habitus and lipid, inflammatory measures, carbohydrate metabolism, and clotting factor measures. The cut-off point for outliers was 3 and 100 iterations were performed. Results are presented as 95% confidence intervals (CI). Because many of the cardiometabolic risk factors were related to one or more of the measures of body habitus additional modeling was done including the body habitus measure to which the risk factor was most closely related. Spearman correlation coefficients were used to determine the relationships between cardiovascular risk factor and body habitus measures. Mann-Whitney U was used for between comparisons between subjects with C3F and C3S alleles. All analyses were performed using Systat 13 (Systat Software Inc, San Jose CA).
RESULTS
Demographics:
Thirty-four of the 75 subjects were female. Mean age was 15.0±1.7 years and mean body mass index (BMI) was 22.0±5.8 kg/m2 (mean±SD). Mean BMI percentile was 53.8±28.7. Sixty-two subjects were lean (BMI percentile<85) and 9 were considered obese (BMI percentile≥95).
Complement and body habitus:
EDTA plasma C3, C4, C3a, C4a, and C5a levels did not differ between sexes and were not significantly related to age. C3 levels positively correlated with all measures of body mass and adiposity (Figure 1). When both fat mass index (β=45.7; CI= 28.0–63.4) and fat free mass index (β=10.0; CI: −25.1–44.8) were included in the model only the relationship to fat mass was significant. C4 levels were not significantly related to any measure of body habitus. For C3a, C4a and C5a the only significant relationship was between C4a and FFMI (β=9.82, CI: 1.55–18.1).
Figure 1:
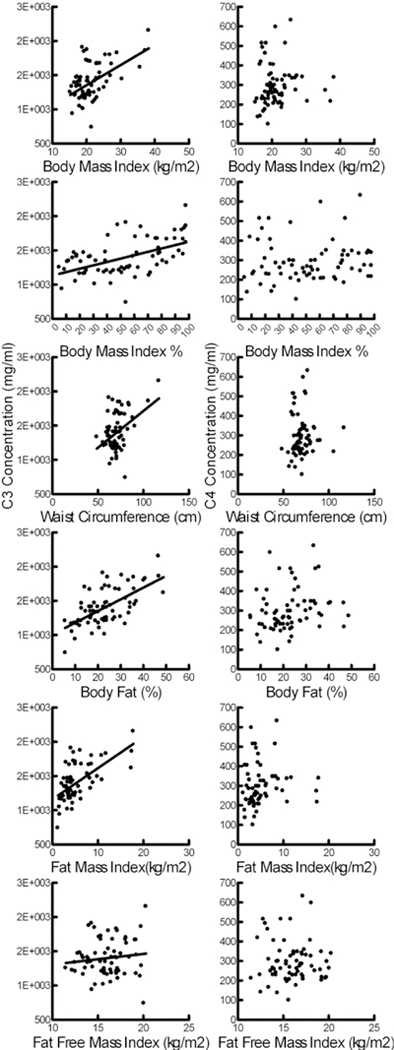
Relationships of C3 and C4 levels to measures of body habitus. Significant relationships were present for C3 [right, BMI: β=36.1; CI: 23.8–48.4; BMI%: β=5.36; CI: 3.2–7.48; Waist circumference: β=15.4; CI: 9.4–21.4; Body Fat %: β=17.3; CI: 10.7–23.9; FMI: β=46.5; CI: 29.2–63.7; FFMI β=60.2; CI: 18.0–102] but not C4 (left).
Complement and cardiometabolic risk:
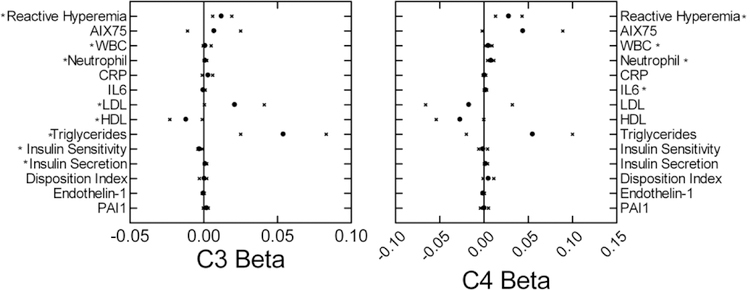
C3 and C4 protein levels were significantly related to several cardiometabolic risk factors (Figure 2). Regarding vascular function, the percent fall in FVR following upper arm arterial occlusion decreased as both C3 and C4 increased. This decrease in reactive hyperemia indicates worsening endothelial function. AIx75 was not significantly related to either C3 or C4. Inflammation increased as both C3 and C4 levels increased, as indicated by significant relationships to white blood cell and neutrophil counts. IL-6 also increased as C4 increased. Triglyceride and LDL levels increased and HDL decreased as C3 levels increased. HDL also negatively correlated with C4 levels. Insulin secretion increased and sensitivity during OGTT decreased as C3 levels increased. Insulin secretion, but not sensitivity, was similarly related to C4 levels. Endothelin 1 and PAI1 levels were not significantly related to either C3 or C4 levels. C3a was positively related to neutrophil count (β=0.012; CI: 0.003–0.021) and to IL-6 (β=0.003; CI: 0.001–0.006). C4a was negatively related to disposition index (β=−0.015; CI: −0.028−-0.002).
Figure 2:
Beta coefficients (mean circle, 95% confidence intervals x) for relationships of cardiometabolic risk factors to C3 (left) and C4 (right) levels. Model included age and sex. *p<0.05.
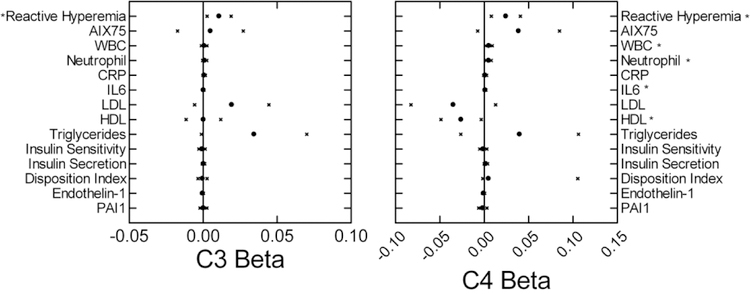
Table 1 shows the Spearman correlation coefficients for relationships between cardiovascular risk factors and body habitus. Figure 3 shows the relationships between C3 and C4 levels after including body habitus measures in the model. The reactive hyperemia response continued to be significantly related to both C3 and C4 levels with lower responses with increasing levels. C4 levels were again positively related to white blood cell and neutrophil counts, IL6 levels and insulin secretion, and negatively related to HDL. The neutrophil count increased with increased C3a levels (β=0.012; CI: 0.003–0.021).
Table 1:
Spearman correlation coefficients for relationship of cardiovascular risk factors to measures of body habitus. Measure with highest (or lowest value if negative) was used in subsequent modeling adjusting relationship of complement levels to cardiovascular risk factors.
| BMI | BMI % | Waist circumference | % Body fat | FMI | FFMI | |
|---|---|---|---|---|---|---|
| Reactive hyperemia | 0.27 | 0.33 | 0.24 | 0.37 | 0.34 | 0.07 |
| AIX75 | −0.06 | 0.01 | −0.09 | 0.13 | 0.14 | −0.15 |
| White blood cell count | 0.16 | 0.14 | 0.12 | 0.22 | 0.21 | 0.10 |
| Neutrophil count | 0.25 | 0.24 | 0.18 | 0.26 | 0.27 | 0.16 |
| IL-6 | 0.25 | 0.20 | 0.28 | 0.16 | 0.16 | 0.25 |
| hs CRP | 0.52 | 0.52 | 0.48 | 0.27 | 0.35 | 0.44 |
| LDL | 0.16 | 0.20 | 0.10 | 0.31 | 0.29 | −0.08 |
| HDL | −0.46 | −0.41 | −0.49 | −0.14 | −0.21 | −0.49 |
| Triglycerides | 0.27 | 0.27 | 0.27 | 0.34 | 0.31 | 0.13 |
| Insulin sensitivity | −0.31 | −0.42 | −0.22 | −0.61 | −0.62 | 0.01 |
| Insulin secretion | 0.37 | 0.37 | 0.26 | 0.54 | 0.54 | 0.08 |
| Disposition index | 0.09 | 0.00 | 0.05 | 0.05 | 0.04 | 0.02 |
| Endothelin 1 | −0.03 | 0.00 | −0.04 | 0.06 | 0.03 | −0.09 |
| Plasminogen activator inhibitor 1 | 0.22 | 0.13 | 0.16 | 0.29 | 0.29 | 0.03 |
Figure 3:
Beta coefficients (mean circle, 95% confidence intervals x) for relationships of cardiometabolic risk factors to C3 (left) and C4 (right) levels. Model included age sex and body habitus. *p<0.05
Complement genetics:
Table 2 shows the subject demographics according to C3F versus C3S genotype and C4 gene copy number. No significant age or sex differences were noted but because of the small number of subjects in some groups, age and sex were included in all analyses.
Table 2:
Age and sex distribution of subjects with at least one C3F gene versus subjects with two C3S genes and according to C4 gene copy number.
| Gene | Characteristic | Genotype | ||||||
|---|---|---|---|---|---|---|---|---|
| C3 | F | S | ||||||
| Male | 13 | 24 | ||||||
| Female | 11 | 22 | ||||||
| Age (yrs) | 15.8±1.6 | 15.0±1.7 | ||||||
| Copy Number | ||||||||
| C4T | 0 | 1 | 2 | 3 | 4 | 5 | 6 | |
| Male | 0 | 10 | 22 | 3 | 3 | |||
| Female | 1 | 8 | 22 | 1 | 1 | |||
| Age (yrs) | 15.4 | 14.7±1.8 | 15.4±1.6 | 14.5±2.0 | 15.8±2.1 | |||
| C4L | ||||||||
| Male | 0 | 1 | 15 | 9 | 11 | 1 | 1 | |
| Female | 1 | 4 | 6 | 14 | 7 | 1 | 0 | |
| Age (yrs) | 17.2 | 16.0±1.6 | 14.7±1.9 | 15.2±1.6 | 15.4±1.7 | 13.8±1.0 | 17.6 | |
| C4S | ||||||||
| Male | 11 | 16 | 10 | 1 | ||||
| Female | 9 | 16 | 6 | 2 | ||||
| Age (yrs) | 15.3±1.8 | 15.1±1.6 | 15.6±2.4 | 15.1±1.4 | ||||
| C4A | ||||||||
| Male | 8 | 16 | 9 | 5 | ||||
| Female | 6 | 20 | 6 | 1 | ||||
| Age (yrs) | 14.8±1.7 | 15.3±1.7 | 15.0±1.5 | 16.0±1.8 | ||||
| C4B | ||||||||
| Male | 1 | 11 | 25 | 1 | ||||
| Female | 1 | 9 | 21 | 2 | ||||
| Age (yrs) | 16.4±0.8 | 14.8±1.5 | 15.4±1.8 | 13.7±1.2 | ||||
Subjects with at least one F allele were slightly older than those with the SS genotype (p=0.034). Subjects with one F allele had a higher BMI (23.0±5.4 versus 20.6±4.1 kg/m2, p=0.042), FMI (6.59±4.40 versus 4.64±3.0 kg/m2, p=0.031) and waist circumference (75.2±12.2 versus 68.9±9.00 cm, p=0.021). Waist circumference, but not BMI or FMI, was positively correlated to age and when adjustment was made for age the difference between subjects with at least one F allele and two S alleles was no longer significant (p=0.063). There were no significant differences in any of the cardiometabolic risk factors.
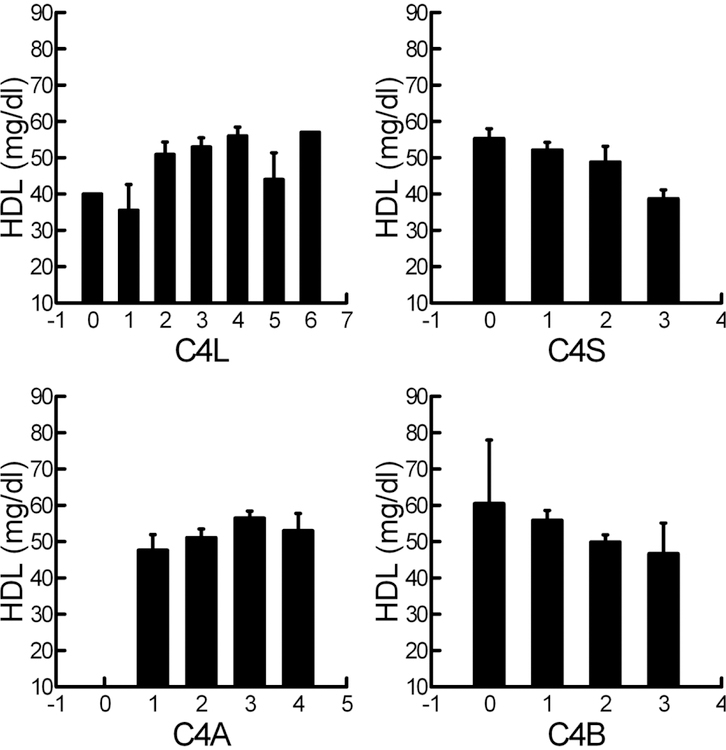
For C4, FFMI decreased as C4T (β=−0.73; CI: −1.24−-0.22) and C4L (β=−0.46; CI: −0.81−-0.10) copy number increased. C4S, C4A, and C4B copy number were not related to any measure of body habitus. C4T gene copy number was not related to any of the cardiovascular risk factors but relationships were found for the various C4 subtypes. Interestingly HDL was significantly related to all 4 subtypes and increased as C4L (β=3.59; CI: 1.19–6.00) and C4A (β=4.33; CI: 1.00–7.66) copy number increased and decreased as C4S (β=0–4.26; CI: −7.50—1.02) and C4B (β=−5.56; CI: −10.3−-0.87) increased (Figure 4). LDL and triglyceride levels were not associated with any of the C4 gene copy numbers. Increased C4L gene copy number in addition to an association with increased HDL, was also associated with decreased insulin secretion (β=−0.24; CI: −0.44−-0.05) and decreased disposition index (β=−0.61; CI: −1.14−-0.08). Increased C4S copy number was also associated with increased insulin secretion (β=0.29; CI: 0.06–0.52) as well as decreased endothelin 1 (β=−0.15; CI: −0.30−-0.01). WBC (β=−0.79; CI: −1.56−-0.02) and neutrophil count (β=−0.87; CI: −1.48−-0.33) both decreased with increasing C4B copy number. Interestingly, the measures of inflammation, IL-6 and CRP were not related to C4 copy number nor were AIX75 or PAI-1.
Figure 4:
Relationship of HDL to C4 type copy number. C4L: β=3.59; CI: 1.19–6.00; C4S: β=−4.26; CI: −7.50−-1.02; C4A: β=4.33; CI: 1.00–7.66; C4B β=−5.56; CI: −10.3--0.87.
DISCUSSION
This study is unique in that we studied the relationships of complement physiology and genetics to a variety of cardiovascular risk factors in healthy, non-Hispanic white adolescents. Importantly, we studied measures of vascular function, lipids, inflammation, clotting, and carbohydrate metabolism. The results clearly demonstrate that C3 protein levels increase with increasing body mass and are most closely related to fat mass. This is in keeping with other studies showing increased serum C3 in men and women and elevated body fat percentage and children with obesity (15, 16, 27). The close relationship to fat mass is not surprising since adipocytes produce C3 (3). Beyond this, both C3 and C4 protein levels were related to several cardiovascular risk factors. Some of these relationships persisted after accounting for obesity.
Genetically, the C3F polymorphism was associated with increased fat mass and possibly waist circumference. This is an important finding since the C3F allele is associated with increased atherosclerosis in adults (10) and several studies have found that C3F allele frequency is significantly higher in patients with severe coronary heart disease who survived myocardial infarction (MI) versus those with no history of MI (28, 29). The increased body fat in adolescents with the C3F polymorphism could well predispose to future cardiovascular disease although we found no significant differences in any of the cardiometabolic risk factors measured beside those of body habitus. Potential differences may appear at later age due to the increased adiposity.
There is strong evidence that increased arterial stiffness and decreased endothelial function precede the development of overt cardiovasclar disease and are present in at risk adolescents (30). Complement has been shown to have pronounced effects on endothelial cells and there is good reason to suspect that complement may play a role in the early development of endothelial dysfunction (2, 5), particularly because of its association with obesity. Our results support this hypothesis since higher C3 and C4 protein levels were associated with poorer reactive hyperemic responses indicating impaired endothelial function. These relationships were present even when controlling for obesity indicating the mechanisms for the associations extend beyond increased adiposity. The reactive hyperemic reponse was not related to C3a, C4a, or C5a so the pathway of the relationships of C3 and C4 concentrations to endothelial function is not clear.
Regarding other traditional and nontraditional cardiovascular risk factors, we found correlations between C3 levels and LDL and triglyceride levels and insulin sensitivity. In light of the previously described ability of adipose to both produce and respond to complement, these relationship may have mechanistic significance. We hypothesized that C3-induced lipolysis may be responsible for the higher triglyceride and LDL levels in obesity (9). Our data do not completely support this hypothesis since the relationships between C3 and lipid levels were lost when measure of body habitus were included in the model.
C4 protein level, on the other hand, was negatively related to HDL even after adjustment for obesity. In addition, we found significant relationships between all four C4 subtypes gene copy number and HDL levels supporting a direct effect of C4 on HDL levels. However, in contrast to previous studies where low C4L or high C4B gene copy number were associated with decreased longevity (9), in regards to HDL our studies would suggest decreased risk with increasing C4L and decreasing C4B gene copy number. These associations have additional significance since HDL has been found to temper immune responses (31). The negative association with FFMI to C4L on the other hand may help explain the association to longevity as FFMI has been related to health maintenance in adolescents (32).
Carbohydrate and lipid metabolism are highly interactive. Thus, it would be suspected that since complement levels play a significant role in predicting lipid levels they might also predict insulin sensitivity. In a study of Pima Indians, fasting C3 concentration was related to adiposity, impaired glucose tolerance, and fasting insulin levels (33). The CODAM study in adults found that plasma C3 levels were independently associated with estimated insulin resistance in muscle, liver, and adipocytes, as well as with glucose tolerance (34). Borne et al (35) found that plasma C3 levels predicted future diabetes in northern European adults. Our data extend these findings to non-Hispanic white adolescents since we found a negative relationship between C3 protein level and insulin sensitivity and a positive relationship to insulin secretion in the group as a whole. However, these relationships were lost when measures of body habitus were included in the model indicating an indirect relationship between C3 and insulin sensitivity mediated by obesity. Interestingly, increased C4L gene copy number was associated with decreased disposition index which suggests an increased future risk of glucose intolerance and possible type 2 diabetes.
Recent studies from our laboratory have revealed that in patients with lupus inflammation is more closely linked to C4 than C3 levels (11, 12). The current study extends this finding to healthy adolescents. Specifically, higher white blood cell count, neutrophil count, and IL6 levels were all associated with elevated C4 protein levels and this remained true after inclusion of body habitus in the model while C3 protein levels correlated with white blood cell count and neutrophil count before, but not after inclusion of body habitus. These relationships are important because atherosclerosis is a chronic inflammatory condition (36). In healthy adults, CRP levels predict future cardiovascular disease and are linked to endothelial dysfunction (37, 38). Our results suggest that increased C4 protein levels may have an important role in the development of chronic inflammation in adolescents. Some additional support may be gained for this by the association of lower white blood cell counts and neutrophil counts to increasing C4B gene copy number. This time the relationship is consistent with the previously reported relationship between low C4B copy number and decreased longevity (9).
Two major limitations to this study are first that we studied only non-Hispanic white adolescents and second that we assessed complement and cardiovascular risk factors at a single time point. The racial differences are particularly important since racial differences in traditional and nontraditional risk factors have been demonstrated (19) and there are clear racial differences in complement genetics (39, 40). Future studies are needed of different races and ethnic groups to determine whether our observations on complement and obesity and cardiovascular risk factors apply to all populations. The cross-sectional correlations between C3 and C4 levels and cardiometabolic risk factors that we found do not demonstrate causation and longitudinal studies are needed to determine whether complement levels and/or genetics predict changes in cardiometabolic risk over time in adolescents. A third limitation is that the small subject number may cause us to miss subtle relationships in what are likely complex interactions between complement and cardiometabolic risk. In addition, because of the small number we did not correct for multiple correlations although studied relationships were planned in advance. Lastly, we did not study physical fitness or lifestyle factors that may confound these relationships.
In conclusion, the results of this study demonstrate important relationships between the complement system and early cardiometabolic risk in non-Hispanic white adolescents. Although C3 was more closely related to obesity, C4 was more closely related to inflammation and cardiometabolic risk factors. This was true both in regards to serum levels and in regard to genetics, particularly, in regard to the consistent relationships between C4 gene copy number and HDL. The fact that both higher C3 and C4 protein levels were both associated with poorer endothelial function independent of obesity suggests that complement may play an important role in the early development of cardiovascular disease.
Acknowledgements:
We would like to thank the nursing staff at the Clinical Research Center at the Wexner Medical Center of The Ohio State University and the staff in the Section of Endocrinology at Nationwide Children’s Hospital for their support in this project.
Funding: The project was supported by a grant from the Great Rivers Affiliate of the American Heart Association (RPH) and NIH grant UL1 TR001070 from the National Center for Advancing Translational Sciences (RPH), and NIAMS grant R01 AR073311 (CYY). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Advancing Translational Sciences or the National Institutes of Health
Footnotes
There are no conflicts of interest.
References
- 1.Koistinen HA, Koivisto VA, Ebeling P 2000. Serum complement protein C3 concentration is elevated in in insulin resistance in obese men. Eur J Int Med 11:21–26. [Google Scholar]
- 2.Hertle E, Stehouwer CD, van Greevenbroek MM 2014. The complement system in human cardiometabolic disease. Mol Immunol 61:135–148. [DOI] [PubMed] [Google Scholar]
- 3.Barbu A, Hamad OA, Lind L, Ekdahl KN, Nilsson B 2015. The role of complement factor C3 in lipid metabolism. Mol Immunol 67:101–107. [DOI] [PubMed] [Google Scholar]
- 4.Onat A, Can G, Rezvani R, Cianflone K 2011. Complement C3 and cleavage products in cardiometabolic risk. Cli Chim Acta 412:1171–1179. [DOI] [PubMed] [Google Scholar]
- 5.Wang H, Ricklin D, Lambris JD 2017. Complement-activation fragment C4a mediates effector functions by binding as untethered agonist to protease-activated receptors 1 and 4. Proc Natl Acad Sci U S A 114:10948–10953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Francescut L, Steiner T, Byrne S, Cianflone K, Francis S, Stover C 2012. The role of complement in the development and manifestation of murine atherogenic inflammation: novel avenues. J Innate Immun 4:260–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Engstrom G, Hedblad B, Eriksson KF, Janzon L, Lindgarde F 2005. Complement C3 is a risk factor for the development of diabetes: a population-based cohort study. Diabetes 54:570–575. [DOI] [PubMed] [Google Scholar]
- 8.Onat A, Hergenc G, Can G, Kaya Z, Yuksel H 2010. Serum complement C3: a determinant of cardiometabolic risk, additive to the metabolic syndrome, in middle-aged population. Metabolism 59:628–634. [DOI] [PubMed] [Google Scholar]
- 9.Copenhaver M, Yu CY, Hoffman RP 2019. Complement Components, C3 and C4, and the Metabolic Syndrome. Curr Diabetes Rev 15–48. [DOI] [PubMed]
- 10.Sørensen H, Dissing J 1975. Association between the C3F gene and atherosclerotic Vascular Diseases. Hum Hered 25:279–283. [DOI] [PubMed] [Google Scholar]
- 11.Savelli SL, Roubey RAS, Kitzmiller KJ et al. 2019. Opposite profiles of complement in antiphospholipid syndrome (aps) and systemic lupus erythematosus (sle) among patients with antiphospholipid antibodies (apl). Front Immunol 10:885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mulvihill E AS, Zhou B, Yu G, et al. 2019. Elevated Serum complement levels and higher gene copy number of complement C4b are associated with hypertension and effective response to statin therapy in childhood onset systemic lupus erythematosus (SLE). Lupus Sci Med In press. [DOI] [PMC free article] [PubMed]
- 13.McGill HC Jr., McMahan CA, Herderick EE, Malcom GT, Tracy RE, Strong JP 2000. Origin of atherosclerosis in childhood and adolescence. Am J Clin Nutr 72:1307S–1315S. [DOI] [PubMed] [Google Scholar]
- 14.Wamba PC, Mi J, Zhao XY et al. 2008. Acylation stimulating protein but not complement C3 associates with metabolic syndrome components in Chinese children and adolescents. Eur J Endocrin 159:781–790. [DOI] [PubMed] [Google Scholar]
- 15.Wei JN, Li HY, Sung FC, Lin CC, et al. 2012. Obesity and clustering of cardiovascular disease risk factors are associated with elevated plasma complement C3 in children and adolescents. Pediatr Diabetes 13:476–483. [DOI] [PubMed] [Google Scholar]
- 16.Cianflone K, Lu H, Smith J, Yu W, Wang H 2005. Adiponectin, acylation stimulating protein and complement C3 are altered in obesity in very young children. Clin Endocrinol (Oxf) 62:567–572. [DOI] [PubMed] [Google Scholar]
- 17.Agostinis-Sobrinho C, Ruiz JR, Moreira C et al. 2018. Ability of nontraditional risk factors and inflammatory biomarkers for cardiovascular disease to identify high cardiometabolic risk in adolescents: results from the labmed physical activity study. J Adolesc Health 62:320–326. [DOI] [PubMed] [Google Scholar]
- 18.Hinriksdottir G, Tryggvadottir A, Olafsdottir AS, Arngrimsson SA 2015. fatness but not fitness relative to the fat-free mass is related to c-reactive protein in 18 year-old. Adolescents. PLoS ONE 10:e0130597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Duck MM, Hoffman RP 2007. Impaired endothelial function in healthy African-American adolescents compared with Caucasians. J Pediatr 150:400–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Onat A, Uzunlar B, Hergenc G et al. 2005. Cross-sectional study of complement C3 as a coronary risk factor among men and women. Clin Sci 108:129–135. [DOI] [PubMed] [Google Scholar]
- 21.Hernandez C, Rodriguez B, Losada E, Corraliza L, Garcia-Ramirez M, Simo R 2009. Normoalbuminuric type 1 diabetic patients with retinopathy have an impaired tubular response to desmopressin: its relationship with plasma endothelin-1. J Clin Endocrinol Metab 94:2060–2065. [DOI] [PubMed] [Google Scholar]
- 22.Utzschneider KM, Prigeon RL, Faulenbach MV, Tong J, Carr DB, Boyko EJ, Leonetti DL, McNeely MJ, Fujimoto WY, Kahn SE 2009. Oral disposition index predicts the development of future diabetes above and beyond fasting and 2-h glucose levels. Diabetes Care 32:335–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bergman RN, Stefanovski D, Kim SP 2014. Systems analysis and the prediction and prevention of Type 2 diabetes mellitus. Cur Opin Biotechnol 28:165–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Botto M, Fong KY, So AK, Koch C, Walport MJ 1990. Molecular basis of polymorphisms of human complement component C3. J Exp Med 172:1011–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chung EK, Wu YL, Yang Y, Zhou B, Yu CY 2005. Human complement components C4A and C4B genetic diversities: complex genotypes and phenotypes. Curr Protoc Immunol Chapter 13:Unit 13 18. [DOI] [PubMed] [Google Scholar]
- 26.Wu YL, Savelli SL, Yang Y, Zhou B, Rovin BH, Birmingham DJ, Nagaraja HN, Hebert LA, Yu CY 2007. Sensitive and specific real-time polymerase chain reaction assays to accurately determine copy number variations (CNVs) of human complement C4A, C4B, C4-long, C4-short, and RCCX modules: elucidation of C4 CNVs in 50 consanguineous subjects with defined HLA genotypes. J Immunol 179:3012–3025. [DOI] [PubMed] [Google Scholar]
- 27.Karkhaneh M, Qorbani M, Mohajeri-Tehrani MR, Hoseini S 2017. Association of serum complement C3 with metabolic syndrome components in normal weight obese women. J Diabetes Metab Disord 16:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Csaszar A, Duba J, Melegh B et al. 2001. Increased frequency of the C3*F allele and the Leiden mutation of coagulation factor V in patients with severe coronary heart disease who survived myocardial infarction. Exp Clinical Immunogenet 18:206–212. [DOI] [PubMed] [Google Scholar]
- 29.Leban N, Jraba K, Chalghoum A et al. 2013. Polymorphism of C3 complement in association with myocardial infarction in a sample of central Tunisia. Diagn Pathol 8:93–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hoffman RP 2017. Nontraditional cardiovascular risk factors in pediatric type 1 diabetes. Curr Diabetes Rev 13:528–532. [PubMed] [Google Scholar]
- 31.Kaji H 2013. High-density lipoproteins and the immune system. J Lipids 2013:684903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Peterson MD, Gordon PM, Smeding S, Visich P 2018. Grip strength is associated with longitudinal health maintenance and improvement in adolescents. J Pediatr 202:226–230. [DOI] [PubMed] [Google Scholar]
- 33.Weyer C, Tataranni PA, Pratley RE 2000. Insulin action and insulinemia are closely related to the fasting complement C3, but not acylation stimulating protein concentration. Diabetes Care 23:779–785. [DOI] [PubMed] [Google Scholar]
- 34.Wlazlo N, van Greevenbroek MMJ, Ferreira I et al. 2014. Complement factor 3 is associated with insulin resistance and with incident type 2 diabetes over a 7-year follow-up period: The CODAM study. Diabetes Care 37:1900–1909. [DOI] [PubMed] [Google Scholar]
- 35.Borne Y, Muhammad IF, Lores-Motta L et al. 2017. Complement C3 associates with incidence of diabetes, but no evidence of a causal relationship. J Clin Endocrinol Metab 102:4477–4485. [DOI] [PubMed] [Google Scholar]
- 36.Heier M, Margeirsdottir HD, Brunborg C, Hanssen KF, Dahl-Jorgensen K, Seljeflot I 2015. Inflammation in childhood type 1 diabetes; influence of glycemic control. Atherosclerosis 238:33–37. [DOI] [PubMed] [Google Scholar]
- 37.Yudkin JS, Stehouwer CD, Emeis JJ, Coppack SW 1999. C-reactive protein in healthy subjects: associations with obesity, insulin resistance, and endothelial dysfunction: a potential role for cytokines originating from adipose tissue? Arterioscl Thromb Vasc Biol 19:972–978. [DOI] [PubMed] [Google Scholar]
- 38.Van Gaal LF, Mertens IL, De Block CE 2006. Mechanisms linking obesity with cardiovascular disease. Nature 444:875–880. [DOI] [PubMed] [Google Scholar]
- 39.Yang Y, Chung EK, Wu YL et al. 2007. Gene copy-number variation and associated polymorphisms of complement component C4 in human systemic lupus erythematosus (SLE): low copy number is a risk factor for and high copy number is a protective factor against SLE susceptibility in European Americans. Am J Hum Genet 80:1037–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen JY, Wu YL, Mok MY et al. 2016. Effects of Complement C4 gene copy number variations, size dichotomy, and C4a deficiency on genetic risk and clinical presentation of systemic lupus erythematosus in east asian populations. Arthritis Rheumatol 68:1442–1453. [DOI] [PMC free article] [PubMed] [Google Scholar]