Abstract
Despite evidence that over 40% of youth in the US have one or more adverse childhood experiences (ACEs), and that ACEs have cumulative, pernicious effects on lifelong health, few primary care clinicians routinely ask about ACEs. Lack of standardized and accurate clinical assessments for ACEs, combined with no point-of-care biomarkers of the “toxic stress” caused by ACEs, hampers prevention of the health consequences of ACEs. Thus, there is no consensus regarding how to identify, screen and track ACEs, and whether early identification of toxic stress can prevent disease. In this review, we aim to clarify why, for whom, when, and how to identify ACEs in pediatric clinical care. To do so, we examine the evidence for such identification; describe the efficacy and accuracy of potential screening instruments; discuss current trends in, and potential barriers to, the identification of ACEs and the prevention of downstream effects; and recommend next steps for research, practice, and policy.
Keywords: screening, social determinants of health, psychosocial factors
Background
Adverse Childhood Experiences (ACEs) are potentially traumatic events that cause overwhelming stress and have lasting negative effects on physical and mental health (1–4). Such experiences include abuse and maltreatment (physical, sexual, emotional/verbal); neglect (physical and emotional/psychological); and household dysfunction (parent mental illness; domestic violence; divorce or separation; incarceration; and alcohol or drug abuse). Other overwhelmingly stressful experiences that were not included in the original ACEs scale, but should be considered possible ACEs due to evidence for their effects on child development and health, include severe economic hardship, hunger, disabilities, medical trauma, war, disaster, homelessness, bullying victimization, and discrimination (5–13).
This overwhelming stress is deemed “toxic” due to its associations with modified gene expression, increased allostatic load, and problems with cognitive and social-emotional development (14–16). Research dating back to the mid-20th-century repeatedly implicates the cumulative effects of ACEs and the resultant effects of toxic stress on health outcomes across the lifespan (17). These effects include higher risk of mental illness, substance abuse, and suicide; diabetes; heart disease; and cancer in adulthood (18–21). Children who have experienced at least one ACE are at increased risk for chronic physical health problems during childhood adolescence (e.g., obesity, learning and developmental delays, and mental, emotional and behavioral problems (22–27).
Pediatric clinicians are increasingly called upon to identify children whose development and health may be at risk due to ACEs. While families may be willing to discuss a child’s exposure to ACEs with a trusted health care professional (28), there is not a clear consensus regarding how to identify, screen, and track these experiences and whether early identification of toxic stress improves outcomes. In this review, we aim to clarify why, for whom, when, and how to identify ACEs in pediatric primary care. To do so, we will examine the evidence in favor of such identification; describe the efficacy and accuracy of potential screening instruments; discuss current trends and potential issues in the identification of toxic stress; and recommend next steps for research and practice.
Rationale for Identification of ACEs in Pediatric Health Care
Universal screening for ACEs in pediatric health care has been proposed as a way to systematically detect and inform interventions to address a variety health, developmental and behavioral problems. For instance, the American Academy of Pediatrics (AAP) has issued policy statements and guidance on screening and surveillance in the medical home that recommends that clinicians identify risk and protective factors for developmental and mental disorders for all children and families in their care (29). However, there remain a number of challenges to fully implementing this AAP policy, including its lack of specificity and relatively shallow evidence base, likely contributing to the only modest shift in practice to proactively identify and treat trauma exposure (30,31). Clinicians do, however, routinely learn about life circumstances that effect children’s health during conversations that take place within high-quality patient-clinician relationships. Given that these relationships and therapeutic conversations are themselves the foundation for trauma-informed care, “surveillance” for ACEs could be a natural part of pediatric health care given appropriate support within clinical systems (32). Similarly, AAP’s 2012 policy and accompanying technical report on ACEs discusses the need for systematic screening of children and their families who are at risk of toxic stress (23). In fact, some ACEs themselves are preventable and/or can be ameliorated by early intervention efforts. For instance, programs such as the Nurse-Family Partnership, Head Start and/or preschool programs, and parenting programs (e.g. Circles of Security, Attachment-Biobehavioral Catch-Up, Positive Parenting Program aka Triple P) show long-term impacts for ameliorating risk among children and families who have experienced risk and ACEs. For children who have experienced ACEs, negative health outcomes may be mitigated in the presence of adequate protective factors, whether internal (such as self-regulation or resilience) or external (such as a consistent, nurturing adult caregiver or strong community connectedness). For example, having a caring and supportive relationship with a teacher and/or adult caregiver helps a child develop problem-solving skills, critical thinking, autonomy, critical consciousness and a sense of purpose. Such relationships provide a foundation for healthy development under stressful conditions (33). Protective factors that buffer against the toxic stress of ACEs thus improve lifelong resilience (34,35). Indeed, the toxic stress of ACEs - such as hypothalamic-pituitary axis perturbations– can be reversed with appropriate early intervention, such as enriched foster care that includes intensive parenting support (36,37).
In pediatric clinical care, ACEs are at least as prevalent as other conditions for which standardized screening is already recommended (e.g., anemia, hypertension, cholesterol, developmental delay, and emotional-behavioral disorders) -- for example, 67% of youth in an urban clinic had at least 1 ACE, and 12% had 4 or more (38). Nationally, findings from the 2016 National Survey of Children’s Health estimate that nearly half (46%) of U.S. children 0–17 have experienced at least one ACE with almost one-third (29.9%) of US children 12–17 years old having experienced two or more ACEs (39). Identifying ACEs in clinical settings could thus be of high yield, yet it remains difficult to implement systemically.
Despite the evidence in favor of identifying ACEs, one-third of AAP members never inquire about ACEs; of those who do, many are not aware of appropriate screening tools or do not use them (30). Most pediatricians who inquire about ACEs are assessing for maternal depression (30) (for which validated screeners exist (40)), whereas other common ACEs such as parental alcohol/drug use, domestic violence, and incarceration are rarely discussed. Clinicians may hesitate to surveil or screen for ACEs for reasons similar to other under-identified conditions such as emotional-behavioral disorders - e.g., lack of training, lack of time, lack of referral resources, and limited awareness of how to address concerns that are raised in the process of screening (i.e., lack of confidence in discussing trauma and resilience) (41–44). Furthermore, pediatric ACE identification through standardized screening may have unique challenges compared to other childhood screenings (Figure 1) – e.g., the medical model focuses on single-outcome “cut-points” to guide the provision of specific biomedical interventions whereas ACE screening identifies social and psychological determinants of health that may be more subjective (lacking evidence of cut-points for “toxicity”) and interventions that current healthcare systems may not yet adequately deliver (45). In childhood lead screening, for example, quantifiable abnormalities are well-established and associated with specific interventions and clinical algorithms. Even for parent-reported screenings, such as for child development and behavior, there are clear best practices, “pass/fail” standards, and interventions. However, these standards and guidelines are not yet available for ACEs, which might defy screening as currently practiced in the medical model due to their psychosocial complexity. For these reasons, ACEs screeners may not fully meet the World Health Organization principles for screening (46).
Figure 1.
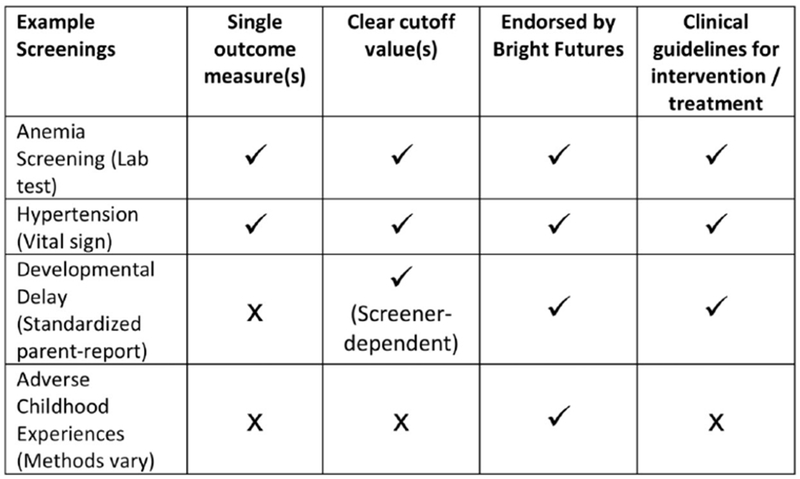
Common Screening Measures in Pediatrics Compared to ACE Identification
Methodological challenges to standardized ACE screening in pediatric health care include differing opinions on which adversities to screen for; when to screen; excessive burden of existing screenings; time constraints within the clinic; and what type of intervention or treatment to provide and how and when to provide it (47–50). Further, because existing ACE screeners provide limited information about the context of any given adverse event (e.g. timing, duration, involvement of protective caregivers), it is difficult to draw accurate conclusions based on a screen in which experiences are endorsed or denied without additional details about context. Furthermore, the perception of whether an ACE is stressful or worthy of intervention differs between individuals and cultures, so patients and families may find such screening intrusive or irrelevant. Additionally, clinics may not have a system in place for referral of mental health/social concerns that are reported in the context of ACE screening, thus placing providers in a difficult position of not knowing what to do with sensitive information revealed by families.
In summary, despite their prevalence, health burden, and effective prevention and treatment strategies, to date there is scant evidence to guide the collection of information about ACEs in pediatric health care. Prevention of ACEs and early interventions for resultant toxic stress thus hinge on identifying ACEs using methods that are feasible, efficient, accurate, actionable, and trackable.
Current Methods for Identifying ACEs
Over the past decade, pediatric researchers increasingly highlighted the importance of identifying child maltreatment in order to better support positive child development (51,52). Through the Bright Futures guidelines for well-child preventive care, the AAP recommends developmental surveillance and psychosocial/behavioral assessments at all well-child checks from newborn to adulthood. While not specific to ACEs, these surveillance touch points are unstructured and potentially vast, covering topics from depression to child abuse. For higher-risk groups such as children in foster care, the AAP has suggested ways in which clinicians can incorporate surveillance for ACEs into routine primary care, including asking questions such as, “Do you know of any really scary or upsetting things that happened to you/your child either before or after he/she came to live with you?” and “Since the last time I saw you/your child, has anything really scary or upsetting happened to you/your child or anyone in the family?” These and other open-ended verbal surveillance questions remain unstudied in terms of their clinical or public health utility, but are likely to complement any efforts towards standardized ACE screening, especially given the current limitations of putative ACE screeners.
Several self-report measures are available to screen youth for trauma (abuse/violence and/or neglect) - including the Childhood Trauma Questionnaire; the Structured Trauma-Related Experiences and Symptoms Screener; the Trauma Symptom Checklist for Children (TSCC); and the Juvenile Victimization Questionnaire-2nd Revision (53–56). While some of these screens such as the JVQ ask about a broad array of experiences and have been used in large epidemiological studies, others are specific to trauma or post-traumatic stress disorder, most have been normed only in small samples of referred youth, and some are not validated for parent-report or for children under age 8, although recent work with adaptations of some measures such as the Traumatic Events Screening Inventory for Children (TESI-C) show promise (57). Some measures, such as the adapted TESI-C or the Survey of Well-Being in Young Children (SWYC) (58), also screen for emotional-behavioral symptoms; others, such as the Center for Youth Wellness Adverse Childhood Experiences Questionnaires (CYW-ACEQ) (59) incorporate symptoms into their guidance to the clinician regarding interpretation of ACE “scores.” Parent-report measures that include other ACEs and may be suitable for screening in pediatrics are summarized in Table 1. Some of these (and others) have recently been reviewed in detail by Oh et al (60).
Table 1.
Examples of Parent-reported Screening Tools for Identifying Adverse Childhood Experiences
| Instrument | Availability | Adverse Experiences Assessed | Validated age range | Informant | Comments |
|---|---|---|---|---|---|
| ACEs Questionnaires | http://www.acestudy.org | 10 items: • Abuse • Neglect • Household dysfunction |
• 18 years | • Adult retrospective • Unspecified for child use |
• Strong population-level evidence for a dose-response gradient in adult health outcomes. • Appropriate for self-report among older youth. • Not validated for parent-report or adolescent self-report • Paper-based instrument • No costs associated with use |
| Center for Youth Wellness Adverse Childhood Experiences Questionnaries (CYW-ACEQ) | https://centerforyouthwellness.org/aceq-pdf/ | 10 items: • Abuse • Neglect • Household dysfunction 7 other negative life event items |
• 0-12 and 13-19 | • Parent-report of child or teen • Teen self-report |
• Provides a total count of items instead of individual item endorsement • Available in Spanish and English • User guide provides tips and workflows for implementation in clinical settings, as well as scoring rubric/algorithm and “scripts” for clinicians to use with patients/families |
| Survey of Well-Being in Young Children (SWYC) | https://www.theswyc.org | 40 items total Subscale of 9 items: • Household dysfunction • Food insecurity |
• Age specific forms for 2–60 months | • Parent-report of child | • Psychometrics assumed to be adequate because all items are from well-validated screeners. • No self-report available for older youth. • Includes items on developmental competence • Includes items on child mental health • Paper based instrument • Available in multiple languages • No costs associated with use, copyright on materials |
| Safe Environments for Every Kid Parent Questionnaire-Revised (SEEK PQ-R) | http://www.seekwellbeing.org | 16 items: • Abuse • Household dysfunction • Parent stress • Food insecurity |
• 0 – 5 years | • Parent-report of child | • Adequate specificity. • Online training recommended before using • Proprietary, fees may apply • Available in multiple languages. • Evidence for preventive effects when used by trained primary care clinicians. • Low sensitivity/positive predictive value • Includes items on household safety • Costs associated with use • Paper-based or electronic based • Recommend at well checks – e.g., 2, 9, and 15-months, 2, 3, 4, and 5-years |
| Well-child care visit; Evaluation; Community resources; Advocacy; Referral; Education (WE-CARE) | Supplement to published RCT60 | 10 items: • Household dysfunction • Parent educational and employment status • Food insecurity • Homelessness • Inadequate child care |
• Not specified | • Parent-report of child | • High test-retest reliability. Face and content validity adequate. Evidence of beneficial effects. • Criterion validity, sensitivity, and specificity not yet established • Includes items to assess motivation for getting help |
| The Yale-Vermont Adversity in Childhood Scale | james.hudziak@med.uvm.edu | 17 items: • Natural disasters • Accidents • Loss • Health • Community violence • Bullying • Criminality • Suicidality • Abuse • Domestic violence • Substance |
• Not specified | • Child self-report • Parent-report • Adult self-report of child • Clinician rating |
• A quantitative approach to adversity assessment. Good initial psychometrics • Not yet published in peer-reviewed form • Improves on the ACE survey by including extra-familial experiences and relying on multiple informants |
| National Child Traumatic Stress Network (NCTSN) Child and Adolescent Needs and Strengths (CANS) Comprehensive-Trauma Version: Exposure to Potentially Traumatic/Adverse Childhood Experiences Module | http://cctasi.northwestern.edu/resources/cans-trauma | 14 items: • Abuse • Neglect • Medical Trauma • Family violence • Community violence • School violence • Natural or manmade disasters • Witness/victim criminal activity • Disruptions in caregiving/attachment losses |
• Not specified | • Interview | • Item anchors are relevant to clinical decision-making. • Psychometrics weak • Includes items on Symptoms Related to Traumatic/ Adverse Childhood Experiences • Includes items on Child Strengths • Includes items on Life Domain Functioning • Includes items on Acculturation • Includes items on Child Behavioral/Emotional Needs • Includes items on Child Risk Behaviors • Includes items on Caregiver Needs and Strengths |
| Children’s PTSD Inventory (CPTSD-I) | http://harcourtassessment.com | 50 items total Subscale assesses potential exposure to traumatic events |
• 6-18 years | • Interview | • Strong psychometric results including ethnically diverse youth. • Available in English, Spanish, French • Interview (18 minutes), costs • Includes items on symptoms related to traumatic/adverse events • Includes items on areas of significant distress |
| Traumatic Events Screening Inventory for Children (TESI-C) | jford@uchc.edu | 15-26 items assessing nonviolent trauma (accidents, disasters, illness), and direct and indirect abuse and violence | • 4-18 | • Interview • Parent-report • Child Self-report |
• Includes structured guidance for collecting details about experiences • Cultural adaptations underway |
| Juvenile Victimization Questionnaire | http://www.unh.edu/ccrc/juvenile_victimization_questionnaire.html | Number of items vary • Conventional crime • Child maltreatment • Peer and sibling victimization • Sexual assault • Witnessing and indirect victimization |
• 8 years old – adult • Caregiver can provide proxy for children younger than 8 years old • Adult retrospective |
Child Self-report • Caregiver report of child • Adult retrospective • Interview format |
• Comprehensive questionnaire • Addresses broad range of victimizations across the full age spectrum of childhood • Modules can be used individually as standalone for more focused assessment • Usable in interview format for children 8 – 17 years old • Can be self-administered for children 12 years old and up • Caregiver as proxy for child • Adult retrospective reporting of childhood events |
Adapted from PICC Toolkit (B. Anthony et al. 2016)
The sensitivity of the original CDC ACE questions may be improved in pediatric settings by adding questions about economic hardship and food insecurity (61); however, such an adaptation of the CDC ACE questions has not yet been studied as a parent-report screener in pediatric clinical settings. The SEEK Parent Questionnaire-Revised (SEEK PQ-R) and WE-CARE screeners seem most promising for use in pediatric clinical care because they have demonstrated positive outcomes in RCTs. Parents who were screened with the SEEK-PQ by primary care clinicians trained to make effective referrals showed reductions in child maltreatment (62), and those screened with WE-CARE showed higher rates of contact with community resources, referrals, and topics discussed with the clinician (63,64). From a psychometric standpoint, there is no current consensus as to what constitutes gold-standard criteria by which to measure the sensitivity and specificity of these or other candidate ACEs screeners, and even trauma-specific screeners lack established norms (65,66). However, responding to the possibility of false-negative screens, the creators of the SEEK PQ-R appropriately point out on their website that “It’s possible that by asking the question(s), [the clinician has] shown [his or her] interest, and sown a seed. [Parents] may disclose in the future. Parents who choose not to disclose are probably not amenable to intervention at this time.”(67) Finally, as noted above, practice barriers to implementing ACE screening are substantial, especially given that broader recommended developmental, behavioral, and mental health screenings are not yet universally implemented.
Emerging Trends and Issues
As mentioned above, concepts of risk and resilience are not new, and evidence has mounted over decades speaking to the multi-level effects of ACEs and other adverse circumstances during childhood. Concurrently, evidence has accumulated that informs prevention of childhood toxic stress, along with treatment for those who have experienced it. Furthermore, the past decade has brought rapid advances to pediatrics: integrated behavioral care; comprehensive and robust electronic health records (EHRs); and inter-professional training and cross-sector collaboration. Taken as a whole, these advances allow clinicians to identify and track risk and/or protective factors during childhood better than could have been done in the past. Nevertheless, the science of ACEs and technological advances alone provide inadequate justification for individual-level ACE screening. Many caveats remain, such as a lack of understanding about the role of the patient-clinician relationship in the assessment of risk and protective factors; potential unintended consequences of individual screening (e.g., loss of patient trust from “false positives” and/or insensitively-delivered screening); whether or how to account for the context of ACEs such as timing and severity; the role of cultural differences; and how screening for ACEs may inadvertently overlook or even perpetuate factors that may underly ACEs such as racism and historical trauma. These factors are outside the scope of this paper, but are reviewed concisely elsewhere (68). Thus, routine and accurate identification of ACEs and related risk factors in pediatrics will require innovative and readily implementable solutions as well as new research; we detail several emerging areas that deserve further attention.
One way to educate about and foster implementation of ACE screening, for example, would be a Maintenance of Certification or Quality Improvement project, which could be accomplished through AAP EQIPP(69) or American Board of Pediatrics Performance Improvement Modules (70). These activities allow clinicians to put knowledge into action, encourage a team approach, and promote incremental practice change. Other trauma-informed projects aimed a practice improvement, such as the Pediatric Integrated Care Collaborative (71), could also produce new knowledge that could inform refinement of existing screening tools or creation of new methods. Provider/clinician training in ACE identification may also vary drastically depending on which role is tasked with screening - e.g. nurse, medical assistant, physician - and whether screening is a review of a previously completed questionnaire (e.g., online) or an in-person conversation. In the case of an in-person conversation, the importance of long-term therapeutic relationships and trauma-informed care cannot be understated - the effects of this kind of care on children deserves ongoing study (72).
To aid in clinical decision making and long-term tracking of ACEs, it is important to consider integration of ACE screening and/or surveillance into the electronic health record (EHR). One promising idea is to use the EHR for more automated ACE identification, using technological solutions such as natural language processing of free-text fields or automatically flagging diagnostic codes indicative of ACEs - however, such approaches may lead to false positives due to technological limitations, while also failing to adequately reflect the true prevalence of ACEs given that clinicians may under-identify ACEs such as child maltreatment in EHRs (73). Potentially, EHRs would provide an alert to the clinician when some form of ACE screen is positive, with suggested language for helping families understand how life experiences shape brain development and health, asking the patient/family if help is wanted, and providing referrals to the appropriate resource or service. Similarly, clinicians might screen for one or more potential ACEs (or proxies for ACEs) that do not require mandated reporting, as do maltreatment or neglect– e.g., hunger/food insecurity, economic hardship or financial stress, housing instability, or family dysfunction - and for those who screen positive, perform expanded screening themselves (e.g., for other ACEs) or refer to social/behavioral clinical team members integrated within their systems of care.
Novel methods of identifying ACEs, such as parent ACEs (74), or even identifying toxic stress itself via altered salivary biomarkers (75), may seem to hold promise based on their correlation with child and adolescent ACEs across numerous studies. Other measures, including various biological moderators of individual differences in sensitivity to experiences (76), may someday be useful (for example, to know which prevention and intervention strategies work best for individual children). However, these methods remain squarely in the domain of research investigation, and for now remain impractical, infeasible, and uninterpretable for individual patients in clinical practice.
Screening for protective factors that help foster resilience in conjunction with screening for ACEs may help clinicians and patients/families feel more empowered to act. Similarly, more in-depth interventions can be integrated into pediatric settings to screen for and address risk and protective factors concurrently (77). For example, the Benevolent Childhood Experiences (BCEs) scale screens for positive experiences in a parallel screen designed to accompany the ACEs (78), presenting an opportunity to briefly incorporate protective factors into the conversation. By screening for protective factors alongside ACEs, there is potential to identify patterns or create clinically relevant tools to aid providers in providing more precise recommendations tailored to the individual situation. By identifying the positive attributes of various protective factors alongside the potentially stressful ACE exposure, there is an opportunity to focus on the strengths of the child and family and tailor recommendations for next steps. The ability to ask about and then track both risk and resilience together can aid in identifying those children and families that are at particular risk due to limited protective factors.
Finally, screening and surveillance are unlikely to be embraced by clinicians until they also feel empowered to do something helpful for patients with the information they gather. In fact, identifying ACEs without having effective interventions available is unethical at best and potentially harmful. Bringing evidence-based practices - including those noted above such as Triple-P, as well as others such as trauma-focused cognitive-behavioral therapy - into pediatric practice settings is potentially one way to do so, and deserving of further study.
Overall, these emerging trends and ongoing questions argue for caution with any form of individual-level screening. A more conservative approach might instead use region-, institution- or clinic-wide aggregate screenings, along with screening for protective factors, and efforts to increase the trauma/resilience-competencies of health care teams so that they can prevent, identify, assess, and treat ACEs for individual patients holistically within the context of ongoing therapeutic relationships - again, this could be accomplished using EHR technologies as described above. Using this approach, aggregate data on the prevalence of ACEs for a given pediatric clinical population could be periodically reported to the health care teams serving that population, along with the prevalence of various protective factors and relevant health outcomes. This information would feed back into the health care teams’ collectively readiness to provide enhanced therapeutic relationships, resources, referrals, and interventions for their population as a whole.
Recommendations
There is widespread agreement that ACEs are highly prevalent and associated with lifelong health risks that begin during childhood and adolescence, and thus important to identify and ameliorate early. There are also valid questions and obstacles regarding ACE screening and surveillance in pediatrics. To answer these questions and overcome these obstacles, we offer the following recommendations.
For Researchers
Answer essential questions about whether individual-level universal ACE screening is effective at preventing chronic conditions, or whether it’s better used at a population level for surveillance and public health. This can be answered via randomized trials - ideally multisite implementation/effectiveness trials in real-world settings.
Establish ACE screeners’ validity, reliability, positive/negative predictive value, and correlation with biomarkers indicative of allostatic load in representative populations, including and/or adapted to cultural and linguistic variables. Such studies should also measure potential harms, downsides, or unintended consequences of ACE screening (e.g., stigmatization, mandated reporting, evoking re-traumatization or other negative feelings in families, and related effects on the clinician-patient relationship(50,79,80)).
Determine when/how often to rescreen ACEs; who screens for which ACEs and where (e.g., behavioral health, primary care, specialty care); how to best track ACEs over time; and how to best collect contextual details about endorsed items (e.g., timing, duration, imminent risk, caregiver involvement).
Determine whether a very limited subset of 1–3 ACEs should be screened for at all pediatric visits (similar to current recommendations regarding domestic violence and depression screening) - i.e., by using “first-level” screening for ACEs that occur most frequently and/or co-occur most often with other ACEs, clinicians might readily identify children who should receive comprehensive “second-level” ACE screening and/or referral.
Examine interactions between ecological factors and ACEs - using clinical trial methodology when possible (including natural experiments and RCTs) - to identify potential areas for systemic or policy-level intervention (e.g., income inequality(81)) that can prevent ACEs and/or improve health outcomes for youth identified with ACEs.
For Clinicians
Use best practices for psychosocial screening implementation and workflows, e.g., using guidelines regarding screening for Social Determinants of Health from the AAP Screening Technical Assistance and Resource Center (STAR) (82).
Consider using ACE screening solely as a population- or clinic-wide measure (e.g., to better assess and address community needs) until more research is done to better understand its risks, benefits, and methods. If piloting individual-level ACE screening while awaiting further research, consider using SEEK PQ-R, CYW-ACEQ, or WE-CARE screeners.
Delineate clinical-care processes for children without ACEs (e.g., offering educational resources on prevention and resilience) and those with ACEs (e.g., referral and follow-up). Concise written and oral information should be readily available in multiple languages to provide guidance about risk and protective factors with all families.
Determine how ACE information can be input into EHRs (e.g., either through a patient portal or during clinic check-in/rooming). Considerations for these systems should include privacy; how to track ACEs longitudinally; how responses can automatically trigger talking points and/or referral information at the point of care; and how to integrate with EHR shortcuts for easy documentation and referral, including local resources and evidence-based programs.
Adopt system-wide, inter-professional trauma-informed approaches to care for children with ACEs (83): Realize that ACEs are important for child health; Recognize signs and symptoms of childhood trauma and stress; Respond using resilience-based language and interventions (84); and Resist Re-traumatization such as restraints for children undergoing medical procedures.
Concurrently identify protective factors and social determinants of health along with ACEs, to better understand strengths and resources. Discuss with all families ways to prevent ACEs, and mitigate/moderate the effects of existing ACEs, using a resilience-informed approach (77).
Consider taking part in care collaboratives that merge screening, early intervention, and prevention in pediatric health care settings, such as Healthy Steps (85).
Clinical training across all pediatric specialties should incorporate trauma- and resilience-informed principles and foster experiential learning in these approaches.
For Policymakers
Support population-level surveillance for ACEs, e.g., by incorporating ACEs into statewide and national surveys of youth and families (e.g., the Youth Risk Behavior Surveillance System).
Promote two-generation initiatives and follow-up strategies for ACEs that build human capital (e.g., Futures Without Violence) (86,87). The efforts can include assessing parents’ exposure to childhood adversity and dyadic interventions that address both parent and child health.
Support cross-sector workforce development in ACE screening and evidence-based trauma-focused treatments (88,89). Successful initiatives have been implemented in many states and communities through home visiting, child welfare, juvenile justice, and educational systems (90–94).
Professional organizations should develop and disseminate practice guidelines for ACE screening and surveillance, in coordination with researchers, clinicians, and educators. These guidelines should take into account, for example, that ACE screening in pediatrics may be viewed favorably by clinicians when framed as universal, confidential, and patient-centered (95) and that trauma screening protocols add little time to a visit (63,96).
Encourage the “unbundling” of service billing to allow reimbursement from insurers for ACE screening occurring in conjunction with a well-child visit. Of note, Early and Periodic Screening, Diagnostic and Treatment covers a wide range of behavioral health screenings designed for early detection and intervention, and federal guidance to Medicaid state plans suggests inclusion of specific screening for adverse childhood experiences and exposure to violence (97). Additionally, certain states (e.g., Colorado, Vermont, Wisconsin) permit coverage of interventions in pediatric settings such as parental education or assessment, so long as these services cannot be considered treatment for the caregiver. These services, however, are often not considered part of routine health care, and thus may not be eligible for financial reimbursement. As such, continued advocacy at the national and state levels is needed to ensure proper payment for the time needed for ACE identification and management.
Increase funding and payer coverage for evidence-based treatments. The availability of such resources may be limited in many communities, calling into question whether screening is justified or ethical when proper treatment cannot be assured. Possible state-level remedies, for example, could include expanding state Medicaid benefit package to increase the number of trauma-focused therapies/therapists that are covered.
Conclusions
In summary, screening for childhood adversity remains under-developed despite evidence that toxic stress during childhood contributes to chronic health conditions. While many pediatric clinicians recognize and understand this, any type of universal, individual-level ACE screening in pediatric health care currently has barriers that need to be addressed. Future ACE screening research must address multiple issues including screening tools and methods; prospective health outcomes across the lifespan; and individual, family, and system-level factors associated with ACEs, resilience, and response to intervention. Clinical identification of ACEs will have to move away from a screening model that relies solely on a pass/fail or cut-off value; instead, novel solutions such as population-level screening and EHRs can be utilized and hold promise for integrating data about these stressors with factors that might include age, family strengths and resources, cultural values, health, and developmental competence. Pediatricians nonetheless are well-positioned to advocate with policymakers about ACEs and resilience as a key step to improving population health. While pediatricians will need alignment with time, evidence, and reimbursement to support ACE identification, they are prevention specialists, and with the help of tools such as EHRs, ready to implement best practices to reduce the societal burden of ACEs.
Acknowledgments:
The authors wish to thank Beth Auslander, PhD, MS, University of Texas Medical Branch Department of Pediatrics, for her contributions to this paper.
Funding Source: Funding for this study was covered in part by the Child Health Research Acceleration Through Multisite Planning Pilot funding and the UC Irvine grant UL1TR001414 and the Children’s National grant UL1TR001876.
Footnotes
Financial Disclosure: The authors have no financial relationships relevant to this article to disclose.
Clinical Trial Registration: Not applicable
Conflicts of Interest: The authors have no conflicts of interest relevant to this article to disclose
References
- 1.Felitti VJ, Anda RF, Nordenberg D, et al. Relationship of Childhood Abuse and Household Dysfunction to Many of the Leading Causes of Death in Adults: The Adverse Childhood Experiences (ACE) Study. Am J Prev Med 1998;14:245–58. [DOI] [PubMed] [Google Scholar]
- 2.Liming KW, Grube WA. Wellbeing Outcomes for Children Exposed to Multiple Adverse Experiences in Early Childhood: A Systematic Review. Child Adolesc Soc Work J 2018;35:317–35. [Google Scholar]
- 3.Felitti VJ, Anda RF, Nordenberg D, et al. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. The Adverse Childhood Experiences (ACE) Study. Am J Prev Med 1998;14:245–58. [DOI] [PubMed] [Google Scholar]
- 4.Anda RF, Butchart A, Felitti VJ, Brown DW. Building a Framework for Global Surveillance of the Public Health Implications of Adverse Childhood Experiences. Am. J. Prev. Med. 2010;39:93–8. [DOI] [PubMed] [Google Scholar]
- 5.Evans GW, Kim P. Childhood Poverty and Health. Psychol Sci 2007;18:953–7. [DOI] [PubMed] [Google Scholar]
- 6.Chilton M, Rabinowich J. Toxic Stress and Child Hunger Over the Life Course: Three Case Studies. J Appl Res Child Informing Policy Child Risk 2012;3:2012. [Google Scholar]
- 7.Richdale A, Francis A, Gavidia-Payne S, Cotton S. Stress, behaviour, and sleep problems in children with an intellectual disability. J Intellect Dev Disabil 2000;25:147–61. [Google Scholar]
- 8.Kazak AE, Kassam-Adams N, Schneider S, Zelikovsky N, Alderfer MA, Rourke M. An Integrative Model of Pediatric Medical Traumatic Stress. J Pediatr Psychol 2006;31:343–55. [DOI] [PubMed] [Google Scholar]
- 9.Thabet AAM, Abed Y, Vostanis P. Comorbidity of PTSD and depression among refugee children during war conflict. J Child Psychol Psychiatry 2004;45:533–42. [DOI] [PubMed] [Google Scholar]
- 10.Durkin MS, Khan N, Davidson LL, Zaman SS, Stein ZA. The effects of a natural disaster on child behavior: evidence for posttraumatic stress. Am J Public Health 1993;83:1549–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barnes AJ, Gilbertson J, Chatterjee D. Emotional health among youth experiencing family homelessness. Pediatrics 2018;141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arseneault L, Walsh E, Trzesniewski K, Newcombe R, Caspi A, Moffitt TE. Bullying Victimization Uniquely Contributes to Adjustment Problems in Young Children: A Nationally Representative Cohort Study. Pediatrics 2006;118:130–8. [DOI] [PubMed] [Google Scholar]
- 13.Ellis BH, MacDonald HZ, Lincoln AK, Cabral HJ. Mental health of Somali adolescent refugees: The role of trauma, stress, and perceived discrimination. J Consult Clin Psychol 2008;76:184–93. [DOI] [PubMed] [Google Scholar]
- 14.Saunderson EA, Spiers H, Mifsud KR, et al. Stress-induced gene expression and behavior are controlled by DNA methylation and methyl donor availability in the dentate gyrus. Proc Natl Acad Sci U S A 2016;113:4830–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McEwen BS. Stress, adaptation, and disease. Allostasis and allostatic load. Ann N Y Acad Sci 1998;840:33–44. [DOI] [PubMed] [Google Scholar]
- 16.Pechtel P, Pizzagalli DA. Effects of early life stress on cognitive and affective function: an integrated review of human literature. Psychopharmacology (Berl) 2011;214:55–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rutter ML. Psychosocial adversity and child psychopathology. Br J Psychiatry 1999;174:480–93. [DOI] [PubMed] [Google Scholar]
- 18.Ports KA, Merrick MT, Stone DM, et al. Adverse Childhood Experiences and Suicide Risk: Toward Comprehensive Prevention. Am J Prev Med 2017;53:400–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sonu S, Post S, Feinglass J. Adverse childhood experiences and the onset of chronic disease in young adulthood. Prev Med (Baltim) 2019;123:163–70. [DOI] [PubMed] [Google Scholar]
- 20.Dimsdale JE. Psychological stress and cardiovascular disease. J Am Coll Cardiol 2008;51:1237–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Godbout JP, Glaser R. Stress-Induced Immune Dysregulation: Implications for Wound Healing, Infectious Disease and Cancer. J Neuroimmune Pharmacol 2006;1:421–7. [DOI] [PubMed] [Google Scholar]
- 22.Kerker BD, Zhang J, Nadeem E, et al. Adverse childhood experiences and mental health, chronic medical conditions, and development in young children. Acad Pediatr 2015;15:510–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Garner AS, Shonkoff JP, Siegel BS, et al. Early Childhood Adversity, Toxic Stress, and the Role of the Pediatrician: Translating Developmental Science Into Lifelong Health. Pediatrics 2012;129:e224–31. [DOI] [PubMed] [Google Scholar]
- 24.Bright MA, Knapp C, Hinojosa MS, Alford S, Bonner B. The Comorbidity of Physical, Mental, and Developmental Conditions Associated with Childhood Adversity: A Population Based Study. Matern Child Health J 2016;20:843–53. [DOI] [PubMed] [Google Scholar]
- 25.Anda RF, Felitti VJ, Bremner JD, et al. The enduring effects of abuse and related adverse experiences in childhood. Eur Arch Psychiatry Clin Neurosci 2006;256:174–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Burns BJ, Phillips SD, Wagner HR, et al. Mental health need and access to mental health services by youths involved with child welfare: A national survey. J Am Acad Child Adolesc Psychiatry 2004;43:960–70. [DOI] [PubMed] [Google Scholar]
- 27.Davis L, Barnes AJ, Gross AC, Ryder JR, Shlafer RJ. Adverse childhood experiences and weight status among adolescents. J Pediatr 2019;204:71–76.e1. [DOI] [PubMed] [Google Scholar]
- 28.Conn A-M, Szilagyi MA, Jee SH, Manly JT, Briggs R, Szilagyi PG. Parental perspectives of screening for adverse childhood experiences in pediatric primary care. Fam Syst Heal 2018;36:62–72. [DOI] [PubMed] [Google Scholar]
- 29.American Academy of Pediatrics Task Force on Mental Health. Appendix S4: The Case for Routine Mental Health Screening. Pediatrics 2010;125:S133–9. [Google Scholar]
- 30.Szilagyi M, Kerker BD, Storfer-Isser A, et al. Factors associated with whether pediatricians inquire About parents’ adverse childhood experiences. Acad Pediatr 2016;16:668–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kerker BD, Chor KHB, Hoagwood KE, et al. Detection and treatment of mental health issues by pediatric PCPs in New York state: An evaluation of Project TEACH. Psychiatr Serv 2015;66:430–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brown JD, King MA, Wissow LS. The Central Role of Relationships With Trauma-Informed Integrated Care for Children and Youth. Acad Pediatr 2017;17:S94–101. [DOI] [PubMed] [Google Scholar]
- 33.Blair C, Raver CC. Poverty, stress, and brain development: New directions for prevention and intervention. Acad Pediatr 2016;16:S30–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chiang JJ, Chen E, Miller GE. Midlife Self-Reported Social Support as a Buffer Against PrematureMortality Risks Associated with Childhood Abuse. Nat Hum Behav 2018;2:261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Masten AS. Ordinary magic: Resilience processes in development. Am Psychol 2001;56:227–38. [DOI] [PubMed] [Google Scholar]
- 36.Slopen N, McLaughlin KA, Shonkoff JP. Interventions to improve cortisol regulation in children: a systematic review. Pediatrics 2014;133:312–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fisher PA, Van Ryzin MJ, Gunnar MR. Mitigating HPA axis dysregulation associated with placement changes in foster care. Psychoneuroendocrinology 2011;36:531–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Burke NJ, Hellman JL, Scott BG, Weems CF, Carrion VG. The impact of adverse childhood experiences on an urban pediatric population. Child Abuse Negl 2011;35:408–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bethell C, Davis M, Gombojav N, Stumbo S, Powers K. Issue Brief: A national and across state profile on adverse childhood experiences among children and possibilities to heal and thrive.
- 40.Kerker BD, Storfer-Isser A, Stein REK, et al. Identifying maternal depression in pediatric primary care. J Dev Behav Pediatr 2016;37:113–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Horwitz SM, Kelleher KJ, Stein REK, et al. Barriers to the identification and management of psychosocial issues in children and maternal depression. Pediatrics 2007;119:e208–18. [DOI] [PubMed] [Google Scholar]
- 42.Williams J, Klinepeter K, Palmes G, Pulley A, Foy JM. Diagnosis and Treatment of Behavioral Health Disorders in Pediatric Practice. Pediatrics 2004;114:601–6. [DOI] [PubMed] [Google Scholar]
- 43.Horwitz SM, Storfer-lsser A, Kerker BD, et al. Barriers to the Identification and Management of Psychosocial Problems: Changes From 2004 to 2013. Acad Pediatr 2015;15:613–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bright MA, Thompson L, Esernio-Jenssen D, Alford S, Shenkman E. Primary care pediatricians’ perceived prevalence and surveillance of adverse childhood experiences in low-income children. J Health Care Poor Underserved 2015;26:686–700. [DOI] [PubMed] [Google Scholar]
- 45.Dube SR. Continuing conversations about adverse childhood experiences (ACEs) screening: A public health perspective. Child Abuse Negl 2018;85:180–4. [DOI] [PubMed] [Google Scholar]
- 46.WIlson JMG, Jungner G. Principles and Practice of Screening for Disease. Geneva: World Health Organization; 1968. p. 26–39. [Google Scholar]
- 47.Finkelhor D, Shattuck A, Turner H, Hamby S. Improving the Adverse Childhood Experiences Study Scale. JAMA Pediatr 2013;167:70–5. [DOI] [PubMed] [Google Scholar]
- 48.Niel C Van Pachter LM, R W Jr Felitti VJ, Stein MT Felitti VJ. Adverse Events in Children: Predictors of Adult Physical and Mental Conditions. 2014;35:549–51. [DOI] [PubMed] [Google Scholar]
- 49.Finkelhor D, Shattuck A, Turner H, Hamby S. A revised inventory of Adverse Childhood Experiences. Child Abuse Negl 2015;48:13–21. [DOI] [PubMed] [Google Scholar]
- 50.Finkelhor D Screening for adverse childhood experiences (ACEs): Cautions and suggestions. Child Abus Negl 2018;85:174–9. [DOI] [PubMed] [Google Scholar]
- 51.Flaherty EG, Stirling J, American Academy of Pediatrics. Committee on Child Abuse and Neglect. Clinical report—the pediatrician’s role in child maltreatment prevention. Pediatrics 2010;126:833–41. [DOI] [PubMed] [Google Scholar]
- 52.Garner AS, Shonkoff JP, Siegel BS, et al. Early Childhood Adversity, Toxic Stress, and the Role of the Pediatrician: Translating Developmental Science Into Lifelong Health. Pediatrics 2012;129:e224–31. [DOI] [PubMed] [Google Scholar]
- 53.Bernstein DP, Stein JA, Newcomb MD, et al. Development and validation of a brief screening version of the Childhood Trauma Questionnaire. Child Abuse Negl 2003;27:169–90. [DOI] [PubMed] [Google Scholar]
- 54.Grasso DJ, Felton JW, Reid-Quiñones K. The Structured Trauma-Related Experiences and Symptoms Screener (STRESS). Child Maltreat 2015;20:214–20. [DOI] [PubMed] [Google Scholar]
- 55.Brier J Trauma Symptom Checklist for Children | The National Child Traumatic Stress Network [Internet]. 1996. [cited 2019 Apr 13];Available from: https://www.nctsn.org/measures/trauma-symptom-checklist-children
- 56.Hamby SL, Finkelhor D, Ormrod R, Turner H, Hamby L. The Juvenile Victimization Questionnaire (JVQ): Administration and scoring manual [Internet]. 2004. [cited 2019 Apr 13];Available from: http://www.unh.edu/ccrc/pdf/CV55_2004.pdf
- 57.Choi KR, McCreary M, Ford JD, Rahmanian Koushkaki S, Kenan KN, Zima BT. Validation of the Traumatic Events Screening Inventory for ACEs. Pediatrics 2019;143:e20182546. [DOI] [PubMed] [Google Scholar]
- 58.Perrin EC, Sheldrick C, Visco Z, Mattern K. The Survey of Well-being of Young Children (SWYC) User’s Manual. version 1. Boston: Floating Hospital for Children at Tufts Medical Center; 2016. [Google Scholar]
- 59.Burke Harris N, Renschler T. Center for Youth Wellness ACE-Questionnaire (CYW ACE-Q Child, Teen, Teen SR). version 7/. San Francisco: Center for Youth Wellness; 2015. [Google Scholar]
- 60.Oh DL, Jerman P, Purewal Boparai SK, et al. Review of Tools for Measuring Exposure to Adversity in Children and Adolescents. J Pediatr Heal Care 2018;32:564–83. [DOI] [PubMed] [Google Scholar]
- 61.Mersky JP, Janczewski CE, Topitzes J. Rethinking the measurement of adversity: Moving toward second-generation research on adverse childhood experiences. Child Maltreat 2017;22:58–68. [DOI] [PubMed] [Google Scholar]
- 62.Dubowitz H, Lane WG, Semiatin JN, Magder LS. The SEEK model of pediatric primary care: Can child maltreatment be prevented in a low-risk population? Acad Pediatr 2012;12:259–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Garg A, Butz AM, Dworkin PH, Lewis RA, Thompson RE, Serwint JR. Improving the Management of Family Psychosocial Problems at Low-Income Children’s Well-Child Care Visits: The WE CARE Project. Pediatrics 2007;120:547–58. [DOI] [PubMed] [Google Scholar]
- 64.Garg A, Toy S, Tripodis Y, Silverstein M, Freeman E. Addressing social determinants of health at well child care visits: A cluster RCT. Pediatrics 2015;135:e296–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tonmyr L, Draca J, Crain J, MacMillan HL. Measurement of emotional/psychological child maltreatment: A review. Child Abuse Negl 2011;35:767–82. [DOI] [PubMed] [Google Scholar]
- 66.Milne L, Collin-Vezina D. Assessment of children and youth in child protective services out-of-home care: An overview of trauma measures. Psychol Violence 2015;5:122–32. [Google Scholar]
- 67.SEEK Safe Environment for Every Kid: SEEK Parent Questionnaire - R (PQ-R) formerly the PQ or PSQ [Internet]. 2018. [cited 2019 Apr 21];Available from: https://www.seekwellbeing.org/the-seek-parent-questionnaire-
- 68.Lingras K, Greifer M, Sheikh K, Fabre B. Adverse childhood experiences (ACEs) and trauma in young children: What we know and what we can do. Child Ment Heal eReview 2019;1–22. [Google Scholar]
- 69.American Academy of Pediatrics EQIPP [Internet]. 2019. [cited 2019 Apr 21];Available from: https://eqipp.aap.org/
- 70.American Board of Pediatrics Practice Improvement Modules [Internet]. 2019. [cited 2019 Apr 21];Available from: https://www.abp.org/content/online-performance-improvement-modules-pims
- 71.The Pediatric Integrated Care Collaborative [Internet]. 2019. [cited 2019 Apr 21];Available from: https://picc.jhu.edu/
- 72.Oral R, Ramirez M, Coohey C, et al. Adverse childhood experiences and trauma informed care: The future of health care. Pediatr. Res. 2016;79. [DOI] [PubMed] [Google Scholar]
- 73.Karatekin C, Almy B, Mason SM, Borowsky I, Barnes A. Documentation of Child Maltreatment in Electronic Health Records. Clin Pediatr (Phila) 2018;57:1041–52. [DOI] [PubMed] [Google Scholar]
- 74.Sun J, Patel F, Rose-Jacobs R, Frank DA, Black MM, Chilton M. Mothers’ Adverse Childhood Experiences and Their Young Children’s Development. Am J Prev Med 2017;53. [DOI] [PubMed] [Google Scholar]
- 75.Purewal Boparai SK, Au V, Koita K, et al. Ameliorating the biological impacts of childhood adversity: A review of intervention programs. Child Abus. Negl. 2018;81:82–105. [DOI] [PubMed] [Google Scholar]
- 76.Shakiba N, Ellis BJ, Bush NR, Boyce WT. Biological sensitivity to context: A test of the hypothesized U-shaped relation between early adversity and stress responsivity. Dev Psychopathol 2019;1–20. [DOI] [PubMed] [Google Scholar]
- 77.Masten A, Barnes A. Resilience in Children: Developmental Perspectives. Children 2018;5:98–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Narayan AJ, Rivera LM, Bernstein RE, Harris WW, Lieberman AF. Positive childhood experiences predict less psychopathology and stress in pregnant women with childhood adversity: A pilot study of the benevolent childhood experiences (BCEs) scale. Child Abus Negl 2018;78:19–30. [DOI] [PubMed] [Google Scholar]
- 79.Van Haeringen AR, Dadds M, Armstrong KL. The child abuse lottery--will the doctor suspect and report? Physician attitudes towards and reporting of suspected child abuse and neglect. Child Abuse Negl 1998;22:159–69. [DOI] [PubMed] [Google Scholar]
- 80.Vulliamy AP, Sullivan R. Reporting child abuse: pediatricians’ experiences with the child protection system. Child Abuse Negl 2000;24:1461–70. [DOI] [PubMed] [Google Scholar]
- 81.Halfon N, Larson K, Son J, Lu M, Bethell C. Income Inequality and the Differential Effect of Adverse Childhood Experiences in US Children. Acad. Pediatr. 2017;17:S70–8. [DOI] [PubMed] [Google Scholar]
- 82.American Academy of Pediatrics Screening Technical Assistance and Resource Center [Internet], 2019. [cited 2019 Apr 21];Available from: https://www.aap.org/en-us/advocacy-and-policy/aap-health-initiatives/Screening/Pages/default.aspx
- 83.Karatekin C, Almy B. Adverse Childhood Experiences 101: A Primer for Clinicians. Creat Nurs (In Press [DOI] [PubMed] [Google Scholar]
- 84.Traub F, Boynton-Jarrett R. Modifiable resilience factors to childhood adversity for clinical pediatric practice. Pediatrics 2017;78:19–30. [DOI] [PubMed] [Google Scholar]
- 85.Kaplan-Sanoff M, Briggs RD. Healthy Steps for Young Children: Integrating Behavioral Health into Primary Care for Young Children and their Families In: Briggs RD, editor. Integrated Early Childhood Behavioral Health in Primary Care. Springer; 2016. p. 71–83. [Google Scholar]
- 86.Flynn AB, Fothergill KE, Wilcox HC, et al. Primary Care Interventions to Prevent or Treat Traumatic Stress in Childhood: A Systematic Review. Acad Pediatr 2015;15:480–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Heckman JJ (James J, Krueger AB, Friedman BM. Inequality in America: what role for human capital policies? MIT Press; 2003. [Google Scholar]
- 88.Lang JM, Ake G, Barto B, et al. Trauma Screening in Child Welfare: Lessons Learned from Five States. J Child Adolesc Trauma 2017;10:405–16. [Google Scholar]
- 89.Lang JM, Campbell K, Shanley P, Crusto CA, Connell CM. Building Capacity for Trauma-Informed Care in the Child Welfare System: Initial Results of a Statewide Implementation. Child Maltreat 2016;21:113–24. [DOI] [PubMed] [Google Scholar]
- 90.Adirim T, Supplee L. Overview of the Federal Home Visiting Program. Pediatrics 2013;132:S59–64. [DOI] [PubMed] [Google Scholar]
- 91.Mckelvey L, Schiffman RF, Brophy-Herb HE, et al. Examing long-term effects of an infant mental health home-based Early Head Start program on family strengths and resilience. Infant Ment Health J 2015;36:353–65. [DOI] [PubMed] [Google Scholar]
- 92.Fox BH, Perez N, Cass E, Baglivio MT, Epps N. Trauma changes everything: Examining the relationship between adverse childhood experiences and serious, violent and chronic juvenile offenders. Child Abuse Negl 2015;46:163–73. [DOI] [PubMed] [Google Scholar]
- 93.Eklund K, Rossi E. Guidance for Trauma Screening in Schools A product of the Defending Childhood State Policy Initiative. 2016.
- 94.Gonzalez A, Monzon N, Solis D, Jaycox L, Langley AK. Trauma exposure in elementary school children: Description of screening procedures, level of exposure, and posttraumatic stress symptoms. School Ment Health 2016;8:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wissow LS, Brown J, Fothergill KE, et al. Universal mental health screening in pediatric primary care: a systematic review. J Am Acad Child Adolesc Psychiatry 2013;52:1134–1147.e23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Dubowitz H, Feigelman S, Lane W, Kim J. Pediatric Primary Care to Help Prevent Child Maltreatment: The Safe Environment for Every Kid (SEEK) Model. Pediatrics 2009;123:858–64. [DOI] [PubMed] [Google Scholar]
- 97.U.S. Department of Health and Human Services: Guidance to State Directors of Medicaid [Internet]. 2013. [cited 2019 Apr 21];Available from: http://www.medicaid.gov/Federal-Policy-Guidance/Downloads/SMD-13-07-11.pdf).


